Olympus UCT180 User manual

INSTRUCTIONS
EVIS EXERA II ULTRASOUND GASTROVIDEOSCOPE
OLYMPUS GF TYPE UCT180
CAUTION : Balloons Used with This Product Contain Natural Rubber Latex, Which May Cause Allergic Reactions.
USA: CAUTION: Federal law restricts this device to sale by or on the order of a physician.

Contents
Contents
Symbols |
......................................................................................... |
1 |
Important ....................Information — Please Read Before Use |
3 |
|
Intended ............................................................................................use |
3 |
|
Applicability .............................of endoscopy and endoscopic treatment |
3 |
|
Instruction .....................................................................................manual |
4 |
|
User ....................................................................................qualifications |
4 |
|
Instrument ............................................................................compatibility |
5 |
|
Reprocessing ....before the first use/reprocessing and storage after use |
5 |
|
Spare ......................................................................................equipment |
5 |
|
Maintenance ........................................................................management |
6 |
|
Prohibition ........................................of improper repair and modification |
6 |
|
Signal .............................................................................................words |
6 |
|
Warnings ..............................................................................and cautions |
7 |
|
Examples .........................................................of inappropriate handling |
11 |
|
Chapter 1 ............................Checking the Package Contents |
13 |
|
1.1 ..................................................................... |
Standard components |
13 |
1.2 .............................................................................. |
Ultrasonic cable |
15 |
1.3 ...................................................................... |
Optional components |
15 |
Chapter 2 ......Instrument Nomenclature and Specifications |
16 |
|
2.1 .................................................................................. |
Nomenclature |
16 |
2.2 ....................................................................... |
Endoscope functions |
18 |
2.3 .................................................................................. |
Specifications |
21 |
2.4 ................. |
Attaching the chain for water - resistant cap (MAJ - 1739) |
25 |
Chapter 3 ....................................Preparation and Inspection |
28 |
|
3.1 .......................................................... |
Preparation of the equipment |
29 |
3.2 ........................................................... |
Inspection of the endoscope |
30 |
3.3 ..................................... |
Preparation and inspection of accessories |
34 |
3.4 ........................................ |
Attaching accessories to the endoscope |
38 |
3.5 .......................... |
Inspection and connection of ancillary equipment |
40 |
3.6 .............................................. |
Inspection of the endoscopic system |
46 |
3.7 ....................................... |
Preparation and inspection of the balloon |
52 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
i |

Contents
Chapter 4 |
Operation.................................................................. |
57 |
4.1 |
Insertion .......................................................................................... |
59 |
4.2 |
Observation of the ultrasound image .............................................. |
64 |
4.3 |
Using EndoTherapy accessories .................................................... |
66 |
4.4 |
Withdrawal of the endoscope.......................................................... |
73 |
4.5 |
Removal of the balloon ................................................................... |
74 |
4.6 |
Transportation of the endoscope .................................................... |
75 |
Chapter 5 Reprocessing: General Policy................................ |
76 |
|
5.1 |
Instructions...................................................................................... |
76 |
5.2 |
Importance of cleaning, disinfection, and sterilization..................... |
76 |
5.3 |
Precautions ..................................................................................... |
77 |
5.4Reprocessing before the first use/reprocessing
|
and storage after use ...................................................................... |
80 |
Chapter 6 Compatible Reprocessing Methods and |
|
|
|
Chemical Agents ..................................................... |
81 |
6.1 |
Compatibility summary.................................................................... |
81 |
6.2 |
Detergent solution........................................................................... |
83 |
6.3 |
Disinfectant solution........................................................................ |
84 |
6.4 |
Rinse water ..................................................................................... |
84 |
6.5 |
Ethylene oxide gas sterilization....................................................... |
85 |
6.6 |
Steam sterilization (autoclaving) of accessories ............................. |
87 |
Chapter 7 Cleaning, Disinfection, and Sterilization |
|
|
|
Procedures .............................................................. |
88 |
7.1 |
Required reprocessing equipment .................................................. |
88 |
7.2Cleaning, disinfection, and sterilization
|
procedures for the endoscope ........................................................ |
102 |
7.3 |
Precleaning ..................................................................................... |
103 |
7.4 |
Leakage testing............................................................................... |
108 |
7.5 |
Manual cleaning.............................................................................. |
113 |
7.6 |
High-level disinfection ..................................................................... |
132 |
7.7 |
Rinsing after high-level disinfection................................................. |
135 |
7.8 |
Sterilization ..................................................................................... |
138 |
7.9Cleaning, disinfection, and sterilization procedures for reusable
parts and reprocessing equipment.................................................. |
139 |
7.10 Care of the ultrasonic cable (MAJ-1597)......................................... |
147 |
ii |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

|
|
Contents |
Chapter 8 Cleaning and Disinfection Equipment................... |
148 |
|
Chapter 9 Storage and Disposal.............................................. |
151 |
|
9.1 Storage of the endoscope............................................................... |
152 |
|
9.2 Storage of the balloon..................................................................... |
152 |
|
9.3 Storage of reusable parts and reprocessing equipment ................. |
152 |
|
9.4 Storage of the ultrasonic cable ....................................................... |
153 |
|
9.5 |
Disposal .......................................................................................... |
153 |
Chapter 10 Troubleshooting ...................................................... |
154 |
|
10.1 |
Troubleshooting guide .................................................................... |
154 |
10.2 Withdrawal of the endoscope with an irregularity ........................... |
159 |
|
10.3 Returning the endoscope for repair ................................................ |
161 |
|
Appendix A: System Chart .......................................................... |
163 |
|
Appendix B: Inspection of the endoscope after cleaning, |
|
|
|
disinfection or sterilization in accordance with |
|
|
IEC 60601-2-37 ......................................................... |
170 |
Appendix C: EMC Information..................................................... |
172 |
|
Appendix D: Acoustic Output Information in Accordance |
|
|
|
with the FDA Guidance: |
|
|
“Information for Manufacturers Seeking |
|
|
Marketing Clearance of Diagnostic Ultrasound |
|
|
Systems and Transducers”.................................... |
177 |
Symbol key ............................................................................................... |
177 |
|
Acoustic output table with ALOKA diagnostic ultrasound system ............. |
179 |
|
Acoustic output table when combined with Olympus universal |
|
|
endoscopic ultrasound center EU-ME1 .................................................... |
182 |
|
Clinical measurement accuracy with ALOKA diagnostic |
|
|
ultrasound system ..................................................................................... |
183 |
|
Clinical measurement accuracy when combined with Olympus |
|
|
universal endoscopic ultrasound center EU-ME1 .................................... |
183 |
|
Appendix E: Acoustic Output Information Accordance with |
|
|
|
IEC 60601-2-37 ......................................................... |
184 |
Acoustic output table with ALOKA diagnostic ultrasound system ............ |
184 |
|
Acoustic output table with Olympus universal endoscopic ultrasound |
|
|
center EU-ME1 ......................................................................................... |
195 |
|
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
iii |

Contents
iv |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Symbols
Symbols
The meaning(s) of the symbol(s) shown on the package, the back cover of this instruction manual and/or this instrument are as follows:
|
Refer to instructions. |
|
|
Caution |
|
|
TYPE BF applied part |
|
|
Serial number |
|
IPX7 |
Ingress protection rating (except for connectors) |
|
|
Lock the ultrasound connector |
|
|
Release the ultrasound connector |
|
|
Ultrasonic endoscope |
|
|
Single use only |
|
|
Do not resterilize |
|
|
Use by (expiration date) |
|
|
Sterilized using ethylene oxide |
|
|
Sterilization lot number |
|
|
Lot number |
|
|
|
|
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
1 |
|
Symbols
Nonsterile
Keep away from sunlight
Keep dry
Do not use if package is damaged
Contains or Presence of Natural Rubber Latex
Date of manufacture
Manufacturer
Authorized representative in the European Community
2 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Important Information — Please Read Before Use
Important Information — Please Read
Before Use
Intended use
This instrument has been designed to be used with an Olympus universal endoscopic ultrasound center or a diagnostic ultrasound system (ALOKA CO., LTD), video system center, light source, documentation equipment, monitor, EndoTherapy accessories and other ancillary equipment.
This instrument is designed for endoscopic real-time ultrasound imaging, ultrasound guided needle aspiration and other endoscopic procedures within the upper gastrointestinal tract and surrounding organs.
Do not use this instrument for any purpose other than its intended use.
Applicability of endoscopy and endoscopic treatment
If there is an official standard on the applicability of endoscopy and endoscopic treatment that is defined by the hospital’s administration or other official institutions such as academic societies on endoscopy, follow that standard. Before starting endoscopy and endoscopic treatment, thoroughly evaluate its properties, purposes, effects, and possible risks (their nature, extent, and probability). Perform endoscopy and endoscopic treatment only when its potential benefits are greater than its risks.
Fully explain to the patient the potential benefits and risks of the endoscopy and endoscopic treatment as well as any examination/treatment methods that can be performed in its place, and perform the endoscopy and endoscopic treatment only after obtaining the consent of the patient.
Even after starting the endoscopy and endoscopic treatment, continue to evaluate the potential benefits and risks, and immediately stop the endoscopy/treatment and take proper measures if the risks to the patient become greater than the potential benefits.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
3 |

Important Information — Please Read Before Use
Instruction manual
This instruction manual contains essential information for using this instrument safely and effectively. Before use, thoroughly review this manual and the manuals of all equipment that will be used during the procedure. Then use the equipment as instructed.
Keep this and all related instruction manuals in a safe and accessible location. If you have any questions or comments about any information in this manual, please contact Olympus.
Terms used in this manual
NBI (Narrow Band Imaging) observation mode:
This is an observation mode using narrow band observation light.
Normal light observation (or WLI (White Light Imaging) observation mode):
This is an observation mode using the standard white light illumination.
Elastography:
Mode for displaying the relative elasticity information of a tissue using color images.
For more details, refer to the instruction manual for the ultrasound instrument for which elastography is available.
User qualifications
If there are official standards for user qualifications for performing endoscopy and endoscopic treatment that are defined by the hospital’s medical administrators or other official institutions, such as academic societies on endoscopy, follow those standards. If there are no official qualification standards, the operator of this instrument must be a physician approved by the medical safety manager of the hospital or person in charge of the department (department of internal medicine, etc.).
The physician should be capable of safely performing the planned endoscopy and endoscopic treatment following guidelines set by the academic societies on endoscopy, etc., and considering the difficulty of endoscopy and endoscopic treatment. This manual does not explain or discuss endoscopic procedures.
4 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Important Information — Please Read Before Use
Instrument compatibility
Refer to the “System chart” in the Appendix to confirm that this instrument is compatible with the ancillary equipment being used. Using incompatible equipment can result in patient or operator injury and/or equipment damage.
This instrument complies with EMC standard for medical electrical equipment, edition 3 (IEC 60601-1-2: 2007). However, when connected with an instrument that complies with EMC standard for medical electrical equipment, edition 1 (IEC 60601-1-2: 1993), the whole system complies with edition 1.
Reprocessing before the first use/reprocessing and storage after use
This instrument was not cleaned, disinfected, or sterilized before shipment. Before using this instrument for the first time, reprocess it according to the instructions given in Chapter 7, “Cleaning, Disinfection, and Sterilization Procedures”.
After using this instrument, reprocess and store it according to the instructions given in the endoscope’s companion reprocessing manual. Improper and/or incomplete reprocessing or storage can present an infection control risk, cause equipment damage, or reduce performance.
The balloons are disposable, and are intended for a single use only; a new one must be used for each patient. Do not attempt to reuse or resterilize a balloon.
Spare equipment
Be sure to prepare another endoscope to avoid interruption of the examination due to equipment failure or malfunction.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
5 |

Important Information — Please Read Before Use
Maintenance management
The probability of failure of the endoscope and ancillary equipment increases as the number of procedures performed and/or the total operating hours increase. In addition to the inspection before each procedure, the person in charge of medical equipment maintenance in each hospital should inspect the items specified in this manual periodically. An endoscope with an observed irregularity should not be used, but should be inspected by following Section 10.1, “Troubleshooting guide” on page 154. If the irregularity is still observed after inspection, contact Olympus.
Prohibition of improper repair and modification
This instrument does not contain any user-serviceable parts. Do not disassemble, modify, or attempt to repair it; patient or operator injury and/or equipment damage can result. Equipment that has been disassembled, repaired, altered, changed, or modified by persons other than Olympus’ own authorized service personnel is excluded from Olympus’ limited warranty and is not warranted by Olympus in any manner.
Signal words
The following signal words are used throughout this manual:
Indicates a potentially hazardous situation which, if not avoided, could result in death or serious injury.
Indicates a potentially hazardous situation which, if not avoided, may result in minor or moderate injury. It may also be used to alert against unsafe practices or potential equipment damage.
Indicates additional helpful information.
6 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Important Information — Please Read Before Use
Warnings and cautions
Follow the warnings and cautions given below when handling this instrument. This information is to be supplemented by the warnings and cautions given in each chapter.
•After using this instrument, reprocess and store it according to the instructions given in Chapter 7, “Cleaning, Disinfection, and Sterilization Procedures”. Using improperly or incompletely reprocessed or stored instruments may cause patient cross-contamination and/or infection.
•Before endoscopy, remove any metallic objects (watch, glasses, necklace, etc.) from the patient. Performing high-frequency cauterization treatment while the patient is wearing metallic objects may cause burns on the patient in areas around the metallic objects.
•Move the elevator control lever slowly in the opposite
direction of the “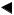 U” direction until it stops and visually confirm that the portion of the elevator wire extending from the distal end of the insertion section is not broken or bent. If the elevator wire is broken or bent, patient injury, bleeding, and/or perforation could result.
U” direction until it stops and visually confirm that the portion of the elevator wire extending from the distal end of the insertion section is not broken or bent. If the elevator wire is broken or bent, patient injury, bleeding, and/or perforation could result.
•Do not strike, hit, or drop the endoscope’s distal end, insertion tube, bending section, control section, universal cord, or endoscope connector. Also, do not bend, pull, or twist the endoscope’s distal end, insertion tube, bending section, control section, universal cord, or endoscope connector with excessive force. The endoscope may be damaged and could cause patient injury, burns, bleeding, and/or perforations. It could also cause parts of the endoscope to fall off inside the patient.
•Never perform angulation control forcibly or abruptly. Never forcefully pull, twist, or rotate the angulated bending section. Patient injury, bleeding, and/or perforation may result. It may also become impossible to straighten the bending section during an examination.
•Never insert or withdraw the endoscope’s insertion section while the bending section is locked in position. Patient injury, bleeding, and/or perforation may result.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
7 |

Important Information — Please Read Before Use
•Never operate the bending section, feed air, perform suction, insert or withdraw the endoscope’s insertion section, or use EndoTherapy accessories without viewing the endoscopic image. Patient injury, bleeding, and/or perforation may result.
•Never operate the bending section, feed air, perform suction, insert or withdraw the endoscope’s insertion section, or use EndoTherapy accessories while the image is frozen. Patient injury, bleeding, and/or perforation may result.
•Never insert or withdraw the insertion section abruptly or with excessive force. Patient injury, bleeding, and/or perforation may result.
•Do not touch the light guide of the endoscope connector immediately after removing it from the light source because it is extremely hot. Operator or patient burns can result.
•That before each use or after a change of viewing modes/settings, check to ensure the view observed through the endoscope provides a live image (rather than a stored one) and has the correct image orientation. Patient injury, bleeding, and/or perforation could result.
•When the endoscopic image does not appear on the monitor, the CCD may have been damaged. Turn the video system center OFF immediately. Continued power supply in such a case will cause the distal end to become hot and could cause operator and/or patient burns.
•Turn ON the diagnostic ultrasound system only when the ultrasonic cable is connected to both the diagnostic ultrasound system and the ultrasonic cable connector on the endoscope. In particular, confirm that the diagnostic ultrasound system is OFF before connecting or disconnecting the ultrasonic cable from the ultrasonic cable connector on the endoscope. Operator injury may result and/or equipment damage may result.
•Do not rely on the NBI observation mode alone for primary detection of lesions or to make a decision regarding any potential diagnostic or therapeutic intervention.
•Never withdraw the endoscope while the balloon is still inflated. Otherwise, the balloon may burst or detach from the distal end of the endoscope. If the balloon cannot be deflated, insert the channel cleaning brush (BW-7L) into the balloon channel. Using slow, short strokes, carefully feed the brush to remove debris.
8 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Important Information — Please Read Before Use
•When withdrawing the endoscope, make sure that the balloon is completely deflated, using the ultrasound image and endoscopic field of view. Withdrawing the endoscope while the balloon is inflated could result in patient injury.
•If it is difficult to insert the endoscope, do not forcibly insert the endoscope; stop the endoscopy. Forcible insertion can result in patient injury, bleeding, and/or perforation.
•If any irregularity in the ultrasound image is observed, turn the ultrasound center OFF immediately. Continued ultrasound radiation will cause the distal end to become hot and could cause operator and/or patient burns.
•Elastography*1 uses the pulsation of a living body. Intentional pressurization is not necessary. Compression onto the tissue by operating the bending section, inserting or withdrawing the endoscope may cause tissue damage, bleeding or perforation.
1 Elastography is not available with the diagnostic ultrasound system (Hitachi Aloka Medical, Ltd.) in Canada.
•After using the endoscope reprocess it according to the instructions given in Chapter 7, “Cleaning, Disinfection, and Sterilization Procedures”. Using improperly or incompletely reprocessed, the endoscope’s distal end damage may result.
•Do not pull the universal cord during an examination. The endoscope connector will be pulled out from the output socket of the light source and the endoscopic image will not be visible.
•Do not coil the insertion tube or universal cord with a diameter of less than 12 cm. Equipment damage can result.
•Do not attempt to bend the endoscope’s insertion section with excessive force. Otherwise, the insertion section may be damaged.
•Do not touch the electrical contacts inside the videoscope cable connector. CCD damage may result.
•Do not apply shock to the distal end of the insertion section, particularly the ultrasound transducer and the objective lens surface at the distal end. Visual abnormalities may result.
•Do not hold the ultrasonic transducer when holding the insertion tube. The ultrasonic transducer damage can result and/or the ultrasonic image will be abnormal.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
9 |

Important Information — Please Read Before Use
•Do not twist or bend the bending section with your hands. Equipment damage may result.
•Do not squeeze the bending section forcefully. The covering of the bending section may stretch or break and cause water leaks.
•Turn the video system center ON only when the videoscope cable is connected to both the video system center and the videoscope cable connector on the endoscope. In particular, confirm that the video system center is OFF before connecting or disconnecting the videoscope cable from the electrical connector on the endoscope. Failure to do so can result in equipment damage, including destruction of the CCD.
•The endoscope’s remote switches cannot be removed from the control section. Pressing, pulling, or twisting them with excessive force can break the switches and/or cause water leaks.
•If remote switch 1 does not return to the OFF position after being pressed strongly from the side, gently pull the switch upwards to return it to the OFF position.
•Do not hit or bend the electrical contacts on the endoscope connector. The connection to the light source may be impaired and faulty contact can result.
•Do not touch the electrical contacts in the ultrasonic cable connector. Equipment damage can result.
•Do not pull, twist or tightly coil the ultrasonic cable. Noise can develop in the ultrasound image.
•Electromagnetic interference may occur on this instrument near equipment marked with the following symbol or other portable and mobile RF (radio frequency) communications equipment such as cellular phones. If electromagnetic interference occurs, mitigation measures may be necessary, such as reorienting or relocating this instrument, or shielding the location.
•To check the electromagnetic interference from other equipment (any equipment other than this instrument or the components that constitute this system), the system should be observed to verify its normal operation in the configuration in which it will be used.
10 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Important Information — Please Read Before Use
•To prevent unnecessary patient exposure to ultrasound radiation, follow the ‘as-low-as-reasonably achievable’ (ALARA) principle when using ultrasound equipment. Freeze the image whenever you are not actively viewing the “live” ultrasound image. When the equipment is in the FREEZE mode, no ultrasound energy is emitted.
•It is highly recommended that a backup ultrasonic cable be available to continue clinical procedures in case of a malfunction.
•This endoscope contains a memory chip that stores information about the endoscope and communicates this information to the video system center CV-160 and CV-180.
Examples of inappropriate handling
Details on clinical endoscopic technique are the responsibility of trained specialists. Patient safety in endoscopic examinations and endoscopic treatment can be ensured through appropriate handling by the physician and the medical facility. Examples of inappropriate handling are described below.
•Over-insufflating the lumen may cause patient pain, injury, bleeding, and/or perforation.
•Applying suction with the distal end in prolonged contact with the mucosal surface, with higher suction pressure than required, or with prolonged suction time may cause bleeding and/or lesions.
•The endoscope has not been designed for use in retroflexed observation in parts of the body other than the stomach. Performing retroflexed observation in a narrow lumen may make it impossible to straighten the angle of the bending section and/or withdraw the endoscope from the patient. Retroflexed observation in parts of the body other than the stomach should be performed only when the usefulness of doing so is determined to be greater than the risk that is posed to the patient.
•Inserting, withdrawing, and using EndoTherapy accessories without a clear endoscopic image may cause patient injury, burns, bleeding, and/or perforation.
•Inserting or withdrawing the endoscope, feeding air, applying suction, or operating the bending section without a clear endoscopic image may cause patient injury, bleeding, and/or perforation.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
11 |

Important Information — Please Read Before Use
•For reasons described below, do not rely on the NBI 1 observation mode alone for primary detection of lesions or to make a decision regarding any potential diagnostic or therapeutic intervention.
NBI has not been demonstrated to increase the yield or sensitivity of finding any specific mucosal lesion including colonic polyps or Barrett’s esophagus.
NBI has not been demonstrated to aid in differentiating establishing the presence or absence of dysplasia or neoplastic changes within mucosa or mucosal lesions.
1 NBI stands for Narrow Band Imaging. For more details, refer to the instruction manual for the video system center CV-180.
Natural rubber latex medical alert
Balloons used with this instrument contain natural rubber latex that may cause allergic reactions.
Do not use the balloon on a latex-sensitive patient. Instead, perform the procedure using “The sterile deaerated water immersion method” described in Section 4.2, “Observation of the ultrasound image” on page 64, in Chapter 4, “Operation”.
12 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Chapter 1 Checking the Package Contents
Chapter 1 Checking the Package
Contents
1.1Standard components
Match all items in the package with the components shown below. Inspect each item for damage. If the instrument is damaged, a component is missing, or you have any questions, do not use the instrument; immediately contact Olympus.
This instrument was not disinfected or sterilized before shipment. Before using this instrument for the first time, reprocess it according to the instructions given in Chapter 7, “Cleaning, Disinfection, and Sterilization Procedures”.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
13 |
Chapter 1 Checking the Package Contents
Ultrasound endoscope
Balloon 3 for the USA (MAJ-249 sterile, 20 pcs.) Balloon for countries other than the USA (MAJ-213, nonsterile, 20 pcs.)
Mouthpiece (MB-142, 2 pcs.)
Cleaning brush (MAJ-1534)
Injection tube Channel plug (MH-946) (MAJ-621)
Single use channel cleaning brush for the USA (BW-201T, 3 pcs. 1)
Channel cleaning brush for countries other than the USA (BW-20T, 1 pcs.)
Single use combination cleaning brush (BW-412T, 3 pcs. 1)
Channel cleaning brush (BW-7L, 2 pcs. 1)
Single use single-ended cleaning brush (BW-400L, 3 pcs. 1)
Single use channel-opening cleaning brush for the USA
(MAJ-1339, 3 pcs. 1)
Channel-opening cleaning brush for countries other than the USA (MH-507, 1 pcs.)
Cleaning adapter for instrument channel port (MAJ-350)
Biopsy valve
(MAJ-853, nonsterile, 10 pcs.)
Balloon applicator |
Water-resistant cap |
Suction cleaning adapter |
Air/water channel cleaning adapter |
(MAJ-675) |
(MH-553, 2 pcs.) |
(MH-856) |
(MAJ-629) |
Washing tube |
Chain for water-resistant cap |
|
|
(MAJ-1739) |
Instruction manual |
||
(MH-974) |
|||
|
|
1 These products may not be available in some areas.
14 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
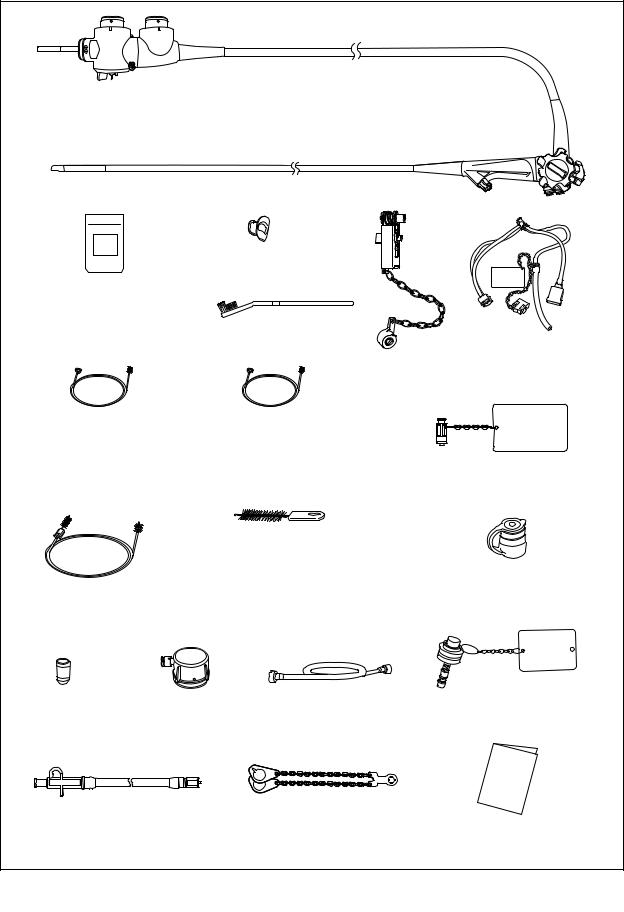
Chapter 1 Checking the Package Contents
1.2Ultrasonic cable
The ultrasonic cable (MAJ-1597) is necessary to use this endoscope (GF-UCT180), but it must be purchased separately (optional) from Olympus.
For Olympus universal endoscopic ultrasound center EU-ME1 and the diagnostic ultrasound system (ALOKA CO., LTD)
Endoscope-side |
connector |
Ultrasound |
connector |
Ultrasonic cable |
(MAJ-1597) |
1.3Optional components
The item listed below is optional for countries other than the USA, and may be purchased from Olympus.
Balloon 3
Balloon 3 is shipped sterile in sets of 20 pieces, enclosed in a resealable package. The correct model to be used with this endoscope is listed in Table 1.1 below.
Endoscope |
Balloon 3 |
|
|
GF-UCT180 |
MAJ-249 |
Table 1.1
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
15 |
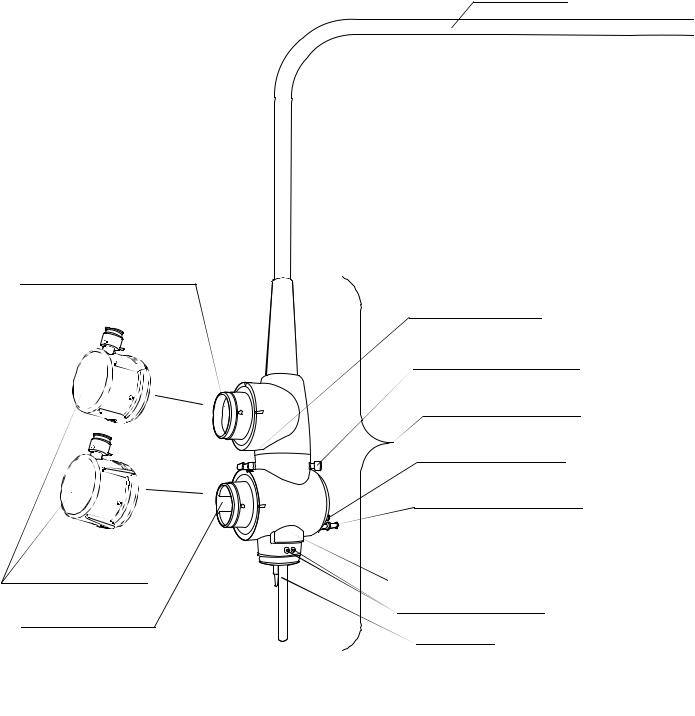
Chapter 2 Instrument Nomenclature and Specifications
Chapter 2 Instrument Nomenclature
and Specifications
2.1Nomenclature
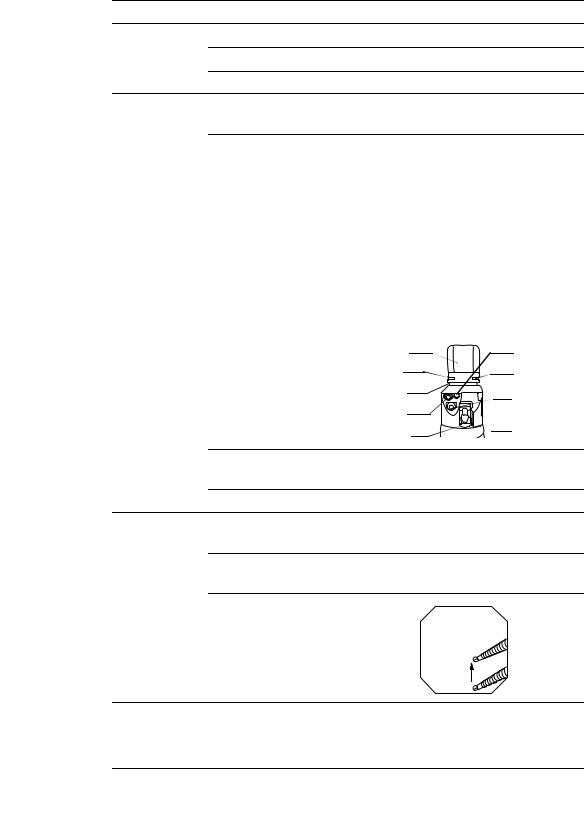
Universal cord
1. Ultrasonic cable connector
|
2. Suction connector |
|
3. S-cord connector mount |
|
4. Endoscope connector |
|
5. Air supply connector |
|
5. Water supply connector |
resistant cap |
Product number and serial number |
|
|
6. Videoscope cable |
Electrical contact points |
connector |
|
|
Light guide |
16 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
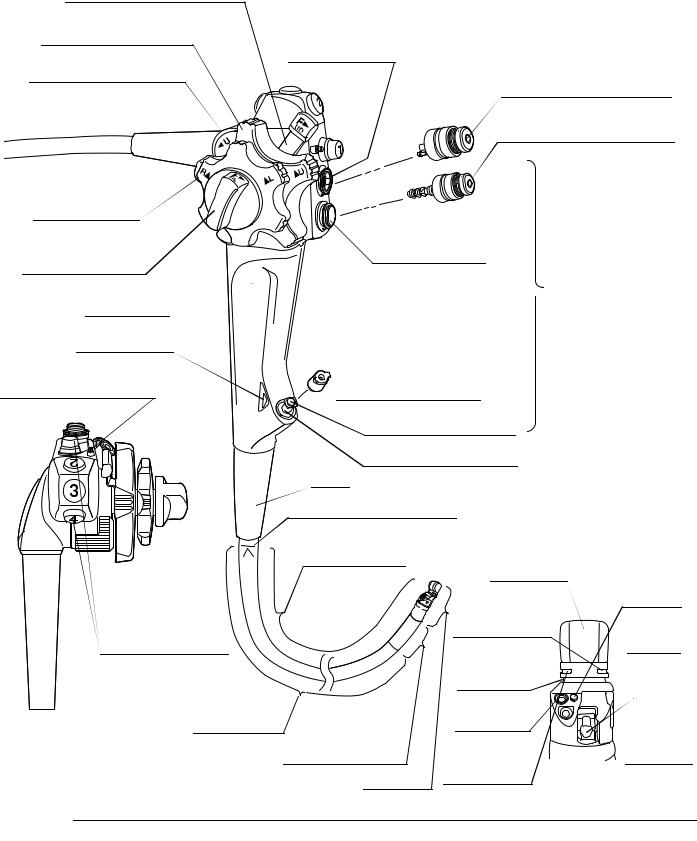
Chapter 2 Instrument Nomenclature and Specifications
9.UP/DOWN angulation lock
8.UP/DOWN angulation control knob
7.Elevator control lever
20. RIGHT/LEFT angulation control knob
19.RIGHT/LEFT angulation lock
Grip section 
18. Color code
17. Elevator channel plug
Suction cylinder
10. Suction valve (MAJ-1443)
11. Air/water valve (MAJ-1444)
Air/water cylinder
Control
 section
section
 Biopsy valve (MAJ-853)
Biopsy valve (MAJ-853)
12. Instrument channel
Instrument channel port
Boot
|
Insertion section limit mark |
|
||
|
Insertion section |
Ultrasound |
|
|
|
|
transducer |
Air/water |
|
|
|
|
||
|
|
Balloon water |
nozzle |
|
|
|
supply port is in |
|
|
16. Remote |
this groove |
Objective |
||
|
switches 1 to 4 |
Balloon |
lens |
|
|
|
|
||
|
|
attachment |
Forceps |
|
|
|
groove |
||
|
|
elevator |
||
|
|
|
||
Top view |
|
Light guide |
|
|
Insertion tube |
lens |
Instrument |
||
|
||||
|
14. Bending section |
Balloon water |
channel |
|
|
outlet |
|||
|
suction port is |
|||
|
Distal end |
in this groove |
|
|
|
Detail of distal end |
|||
|
|
|||
|
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
17 |
||

Chapter 2 Instrument Nomenclature and Specifications
2.2Endoscope functions
1.Ultrasonic cable connector
Connects the ultrasonic cable of the ultrasound diagnostic equipment to the endoscope.
2.Suction connector
Connects the endoscope to the suction tube of the suction pump.
3.S-cord connector mount
Connects the endoscope with the Olympus electrosurgical unit via the S-cord. The S-cord conducts leakage current from the endoscope to the electrosurgical unit. To connect the S-cord, refer to the instruction manual for the electrosurgical unit. Connect the fitting part of the chain for water-resistant cap to this mount, as required (see Section 2.4, “Attaching the chain for water-resistant cap (MAJ-1739)” on page 25).
4.Endoscope connector
Connects the endoscope to the output socket of the light source and transmits light from the light source to the endoscope.
5.Water supply connector and air supply connector
Connects the endoscope to the water container via the water container tube to supply water to the distal end of the endoscope.
6.Videoscope cable connector
Connects the endoscope to the video system center via the videoscope cable. The endoscope contains a memory chip that stores information about the endoscope and communicates this information to the video system center CV-160, CV-180. For more details, refer to the instruction manual for the CV-160, CV-180.
7.Elevator control lever
When this lever is moved in the “ U” direction, the forceps elevator is raised; when the lever is moved in the opposite direction, the forceps elevator is lowered.
U” direction, the forceps elevator is raised; when the lever is moved in the opposite direction, the forceps elevator is lowered.
8.UP/DOWN angulation control knob
When this knob is turned in the “ U” direction, the bending section moves UP; when the knob is turned in the “D
U” direction, the bending section moves UP; when the knob is turned in the “D ” direction, the bending section moves DOWN.
” direction, the bending section moves DOWN.
9.UP/DOWN angulation lock
Moving this lock in the “F ” direction frees angulation. Moving the lock in the opposite direction locks the bending section at any desired position.
” direction frees angulation. Moving the lock in the opposite direction locks the bending section at any desired position.
18 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Chapter 2 Instrument Nomenclature and Specifications
10.Suction valve (MAJ-1443)
The suction valve is depressed to the first stage to activate suction. The valve is also used to remove any fluid or debris adhering to the objective lens.
The suction valve is depressed completely to activate suction of sterile water from the balloon through the balloon channel.
11.Air/water valve (MAJ-1444)
The hole in the air/water valve is covered to insufflate air and the valve is depressed to the first stage to feed water for lens washing. It also can be used to feed air to remove any fluid or debris adhering to the objective lens. The valve is depressed completely to fill the balloon with sterile water through the balloon channel.
12.Instrument channel
The instrument channel functions as:
channel for the insertion of EndoTherapy accessories
suction channel
Fluid feed channel (from a syringe via the biopsy valve)
13.Insertion section limit mark
This mark shows the maximum point to which the endoscope may be inserted into the patient’s body.
14.Bending section
This section moves the distal end of the endoscope when the UP/DOWN and RIGHT/LEFT angulation control knobs are operated.
15.Forceps elevator
The elevator moves EndoTherapy accessories when the elevator control lever is operated.
16.Remote switches 1 to 4
The functions of remote switches 1 to 4 can be selected on the video system center. Refer to the instruction manual for the video system center when setting these functions.
17.Elevator channel plug
This plug is used for connection of the washing tube to clean and disinfect the elevator channel.
18.Color code (orange)
This code is used to quickly determine the compatibility of EndoTherapy accessories. The endoscope can be used with EndoTherapy accessories that have the same color code. For more information on combining the endoscope with particular EndoTherapy accessories, refer to the “System chart” in the Appendix and the instruction manuals for the compatible accessories.
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
19 |

Chapter 2 Instrument Nomenclature and Specifications
19.RIGHT/LEFT angulation lock
Turning this lock in the “F ” direction frees angulation. Turning the lock in the opposite direction locks the bending section at any desired position.
” direction frees angulation. Turning the lock in the opposite direction locks the bending section at any desired position.
20.RIGHT/LEFT angulation control knob
When this knob is turned in the “R ” direction, the bending section moves RIGHT; when the knob is turned in the “
” direction, the bending section moves RIGHT; when the knob is turned in the “ L” direction, the bending section moves LEFT.
L” direction, the bending section moves LEFT.
20 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Chapter 2 Instrument Nomenclature and Specifications
2.3 |
Specifications |
|
|
|
|
Environment |
|
|
|
|
|
|
|
|
|
|
Operating |
Ambient temperature |
10 – 40 C (50 – 104 F) |
|
|
environment |
|
|
|
|
Relative humidity |
30 – 85% |
|
|
|
|
||
|
|
|
|
|
|
|
|
Atmospheric |
700 – 1060 hPa |
|
|
|
pressure |
(0.7 – 1.1 kgf/cm2) |
|
|
|
|
(10.2 – 15.4 psia) |
|
|
|
|
|
|
|
Transportation |
Ambient temperature |
–47 to 70 C (–52.6 to 158 F) |
|
|
and storage |
|
|
|
|
Relative humidity |
10 – 95% |
|
|
|
environment |
||
|
|
|
|
|
|
|
Atmospheric |
700 – 1060 hPa |
|
|
|
|
||
|
|
|
pressure |
(0.7 – 1.1 kgf/cm2) |
|
|
|
|
(10.2 – 15.4 psia) |
|
|
|
|
|
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
21 |
Chapter 2 Instrument Nomenclature and Specifications
Specifications
Endoscope functions
Model |
|
|
GF-UCT180 |
|
Optical system |
Field of view |
|
100 |
|
|
Direction of view |
|
55 forward oblique |
|
|
Depth of field |
|
3 – 100 mm |
|
Insertion tube |
Distal end outer |
|
ø 14.6 mm |
|
|
diameter |
|
|
|
|
|
|
|
|
|
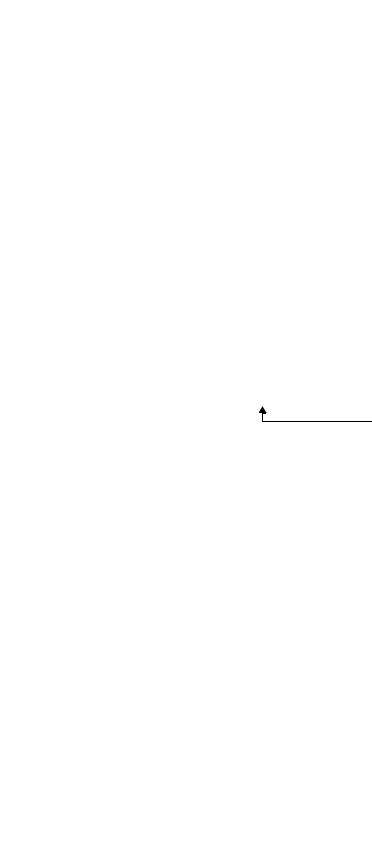
Distal end enlarged |
1. |
Air/water nozzle |
|
|
|
2. |
Objective lens |
|
|
|
3. |
Light guide lens |
|
|
|
4. |
Forceps elevator |
|
|
|
5. |
Instrument channel outlet |
|
|
|
6. |
Ultrasonic transducer |
|
|
|
7. Balloon water supply port |
||
|
|
8. |
Balloon suction port |
|
|
|
9. |
Balloon attachment groove |
|
|
|
|
6. |
1. |
|
|
|
7. |
8. |
|
|
|
9. |
2. |
|
|
|
3. |
|
|
|
|
|
|
|
|
|
5. |
4. |
|
|
|
|
|
|
Insertion tube outer |
|
ø 12.6 mm |
|
|
diameter |
|
|
|
|
|
|
|
|
|
Working length |
|
1250 mm |
|
Instrument |
Channel inner |
|
ø 3.7 mm |
|
channel |
diameter |
|
|
|
|
|
|
||
|
Minimum visible |
|
6 mm from the objective lens |
|
|
distance |
|
||
|
|
|
|
|
|
Direction from which |
|
|
|
|
EndoTherapy |
|
|
|
|
accessories enter |
|
|
|
|
and exit the |
|
|
|
|
endoscopic image |
|
|
|
Airflow rate |
|
|
20 cm3/s |
|
Note: Standard when CLV-180 (high air pressure) is used.
22 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |

Chapter 2 Instrument Nomenclature and Specifications
Bending |
Angulation range |
UP 130 , DOWN 90 , |
section |
|
RIGHT 90 , LEFT 90 |
|
|
|
Total length |
|
1555 mm |
|
|
|
NBI observation mode 1 |
Available |
|
1 For more details, refer to the instruction manual for the CV-180.
Ultrasound function with ALOKA diagnostic ultrasound system SSD10/ProSound 7 1/ProSound F75 1
Operation mode |
B-mode, M-mode, D-mode, Bflow mode, Powerflow mode |
|
|
|
|
Scanning method |
Electronic curved linear array |
|
|
|
|
Scanning direction |
Parallel to the insertion direction |
|
|
|
|
Receiving frequency |
5, 6, 7.5, 10 MHz (SSD10/ProSound F75 1) |
|
|
4, 6.67, 10, 13.3 MHz (ProSound 7) 1 |
|
Scanning range |
180 |
|
|
|
|
Contacting method |
Balloon method, Direct contact method |
|
|
|
|
Transducer surface |
43 C > |
|
max. temperature |
||
|
||
|
|
1 These products may not be available in some areas.
Ultrasound function with Olympus universal endoscopic ultrasound center EU-ME1
Operation mode |
B mode, color flow mode, power flow mode |
|
|
|
|
Scanning method |
Electronic curved linear array |
|
|
|
|
Scanning direction |
Parallel to the insertion direction |
|
|
|
|
Receiving frequency |
5, 6, 7.5, 10, 12 MHz |
|
|
|
|
Scanning range |
180 |
|
|
|
|
Contacting method |
Balloon method, Direct contact method |
|
|
|
|
Transducer surface |
43 C > |
|
max. temperature |
||
|
||
|
|
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
23 |
Chapter 2 Instrument Nomenclature and Specifications
General safety |
IEC 60601-1: 2005 |
Whole of this instrument is possible |
standard for medical |
|
to contact live bodies of operators or |
electrical equipment |
|
patients. |
|
|
|
Medical Devices |
|
This device complies with the |
Directive |
|
requirements of Directive 93/42/EEC |
|
|
concerning medical devices. |
|
|
Classification: Class II a |
|
|
|
EMC |
Applied standards; |
This instrument complies with the |
|
IEC 60601-1-2: 2007 |
standards listed in the left column. |
|
IEC 60601-2-37: 2007 |
|
|
|
CISPR 11 of emission: |
|
|
Group 1, Class B |
This instrument complies with the EMC standard for medical electrical equipment, edition 3 (IEC 60601-1-2: 2007). However, when connecting to an instrument that complies with the EMC standard for medical electrical equipment, edition 1 (IEC 60601-1-2: 1993), the whole system complies with edition 1.
Year of manufacture |
1912345 |
The last digit of the year of manufacture is given in the second digit of the serial number.
Degree of protection |
|
|
against electric |
|
TYPE BF applied part |
shock |
|
|
|
|
|
Ingress protection |
IPX7 |
This instrument complies with the |
rating |
|
standards for medical electrical |
|
|
equipment: IEC 60601-1: 2005 |
|
|
IEC 60601-2-37: 2007. |
|
|
|
24 |
ULTRASOUND GASTROVIDEOSCOPE GF-UCT180 |
 Loading...
Loading...