Olympus TJF Q180V User manual

Single use soft brush (MAJ-1888)
INSTRUCTIONS
EVIS EXERA II DUODENOVIDEOSCOPE
OLYMPUS TJF TYPE Q180V
Accessories:
•Water resistant cap (MH-553)
•Biopsy valve (MB-358)
•Air/water valve (MH-438)
•Suction cleaning adapter (MH-856)
•Channel cleaning brush (BW-20T)
•Single use channel cleaning brush (BW-201T)
•AW channel cleaning adapter (MH-948)
•Single use soft brush (MAJ-1888)
•Chain for water-resistant cap (MAJ-1119)
•Suction valve (MH-443)
•Mouthpiece (MB-142)
•Channel plug (MH-944)
•Injection tube (MH-946)
•Single use channel-opening cleaning brush (MAJ-1339)
•Single use combination cleaning brush (BW-412T)
MH-553 |
MAJ-1119 |
MB-358 |
MH-443 |
MH-438 |
MB-142 |
MH-856 |
MH-944 |
BW-20T |
MH-946 |
|
MH-948 |
BW-412T |
MAJ-1888 |
BW-201T |
MAJ-1339 |
Refer to the endoscope’s companion manual, the “OPERATION MANUAL” with your endoscope model listed on the cover, for operation information.
USA: CAUTION: Federal law restricts this device to sale by or on the order of a physician.
Revision History
Note: The Revision History shows the latest changes.
Version |
Date |
|
Description of Changes |
|
|
|
|
RC2409 01 |
March, 2015 |
Cover |
Single use soft brush (MAJ-1888) information. |
|
|
Section 1.4 |
Updated WARNING statements for MAJ-1888 and |
|
|
|
using an AER. |
|
|
Sections 2.10, 3.1, and 4.1 |
Updated MAJ-1888 information. |
|
|
Section 4.2 |
Updated MAJ-1888 and AER information. |
|
|
Sections 4.3 and 5.1 |
Updated MAJ-1888 information. |
|
|
Section 5.2 |
Updated “Aspirate water” section. |
|
|
Section 5.3 |
Updated “Detach the endoscope from the light |
|
|
|
source” section. |
|
|
Section 5.4 |
Updated “Equipment needed” chart and CAUTION for |
|
|
|
MAJ-1888, Step 5 on page 69, and added “Brush and |
|
|
|
flush the forceps elevator recess” section. |
|
|
Section 5.5 |
Updated “Flush all channels and around the forceps |
|
|
|
elevator with disinfectant solution” section. |
|
|
Section 5.6 |
Updated “Rinse the endoscope and accessories” |
|
|
|
section; the “Alcohol flush” section. |
|
|
Chapter 7 |
Updated WARNING statements for AER. |
|
|
|
|
GE8415 09 |
February, |
|
|
|
2015 |
|
|
|
|
|
|

Contents
Contents |
|
|
Chapter 1 |
General Policy ......................................................... |
1 |
1.1 |
Instructions...................................................................................... |
1 |
1.2 |
Importance of cleaning, disinfection, and sterilization..................... |
2 |
1.3 |
Signal words ................................................................................... |
2 |
1.4 |
Precautions ..................................................................................... |
3 |
1.5 |
Reprocessing before the first use ................................................... |
8 |
1.6 |
Reprocessing and storage after use ............................................... |
9 |
1.7 |
Reprocessing before patient procedure.......................................... |
9 |
Chapter 2 Function and Inspection of the Accessories for |
|
|
|
Reprocessing........................................................... |
10 |
2.1 |
Water resistant cap (MH-553)......................................................... |
10 |
2.2 |
Channel plug (MH-944)................................................................... |
12 |
2.3 |
Injection tube (MH-946) .................................................................. |
14 |
2.4 |
Channel cleaning brush (BW-20T).................................................. |
16 |
2.5 |
Suction cleaning adapter (MH-856) ................................................ |
18 |
2.6 |
AW channel cleaning adapter (MH-948)......................................... |
19 |
2.7 |
Single use channel cleaning brush (BW-201T)............................... |
20 |
2.8 |
Single use channel-opening cleaning brush (MAJ-1339) ............... |
22 |
2.9 |
Single use combination cleaning brush (BW-412T)........................ |
24 |
2.10 |
Single use soft brush (MAJ-1888)................................................... |
25 |
2.11 |
Chain for water-resistant cap (MAJ-1119) ...................................... |
27 |
Chapter 3 Compatible Reprocessing Methods and |
|
|
|
Chemical Agents .................................................... |
28 |
3.1 |
Compatibility summary.................................................................... |
28 |
3.2 |
Water (for reprocessing) ................................................................. |
30 |
3.3 |
Detergent solution........................................................................... |
31 |
3.4 |
Disinfectant solution........................................................................ |
31 |
3.5 |
Rinse water ..................................................................................... |
31 |
3.6 |
Alcohol ............................................................................................ |
31 |
3.7 |
Ethylene oxide gas sterilization....................................................... |
32 |
3.8 |
Steam sterilization (autoclaving) ..................................................... |
33 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
i |

Contents
Chapter 4 Reprocessing Workflow for the Endoscope |
|
and Accessories ...................................................... |
35 |
4.1Workflow for manually cleaning and disinfecting the endoscope
and accessories .............................................................................. |
36 |
4.2Workflow for cleaning and disinfecting the endoscope and
accessories using an AER .............................................................. |
38 |
4.3Workflow for manually cleaning and sterilizing the endoscope
|
and accessories .............................................................................. |
40 |
Chapter 5 Reprocessing the Endoscope |
|
|
|
(and related reprocessing accessories) ................ |
42 |
5.1 |
Preparing the equipment for reprocessing...................................... |
44 |
5.2 |
Precleaning the endoscope and accessories.................................. |
45 |
5.3 |
Leakage testing of the endoscope .................................................. |
51 |
5.4 |
Manually cleaning the endoscope and accessories........................ |
56 |
5.5 |
Manually disinfecting the endoscope and accessories ................... |
80 |
5.6 |
Rinsing the endoscope and accessories following disinfection....... |
86 |
5.7 |
Sterilizing the endoscope and accessories..................................... |
94 |
Chapter 6 Reprocessing the Accessories .............................. |
96 |
|
6.1 |
Manually cleaning the accessories ................................................. |
98 |
6.2 |
Manually disinfecting the accessories............................................. |
101 |
6.3 |
Rinsing the accessories following disinfection ................................ |
102 |
6.4 |
Sterilizing the accessories............................................................... |
105 |
Chapter 7 Reprocessing Endoscopes and Accessories |
|
|
|
using an Automated Endoscope Reprocessor..... |
106 |
Chapter 8 Storage and Disposal.............................................. |
108 |
|
8.1 |
Storing the disinfected endoscope and accessories ....................... |
109 |
8.2 |
Storing the sterilized endoscope and accessories .......................... |
111 |
8.3 |
Disposal .......................................................................................... |
111 |
ii |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 1 General Policy
Chapter 1 General Policy
1.1Instructions
•This manual contains the cleaning, disinfection, and sterilization methods recommended by Olympus for the endoscopes and accessories listed on the front cover.
•This instruction manual contains essential information on reprocessing endoscopes and accessories safely and effectively.
•Before reprocessing, thoroughly review this manual and the manuals for the reprocessing equipment and chemicals that will be used for reprocessing. Reprocess all the devices as instructed.
•Note that the complete instruction manual set for the endoscope and accessories consists of this manual and the “OPERATION MANUAL” with your endoscope model listed on the cover. Both manuals accompanied the endoscope at shipment.
•Keep this manual and all related manuals in a safe and accessible location (e.g., in the reprocessing area).
•If you have any questions or comments about any information in this manual, or if a problem occurs while reprocessing that cannot be solved, contact Olympus.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
1 |

Chapter 1 General Policy
1.2Importance of cleaning, disinfection, and sterilization
The medical literature reports incidents of cross-contamination resulting from improper cleaning, disinfection, or sterilization. It is strongly recommended that all individuals engaged in reprocessing closely observe all instructions given in this manual and the manuals for all ancillary equipment, and have a thorough understanding of the following items:
•Professional health and safety policies of your hospital
•Instruction manuals for the endoscope, accessories, and all the other reprocessing equipment
•Structure and handling of endoscope and accessories
•Handling of pertinent chemicals
When selecting appropriate methods and conditions for cleaning and disinfection and sterilization, follow the policies at your institution, applicable national laws and standards, and professional society guidelines and recommended practices, in addition to the instructions given in this manual.
1.3Signal words
The following signal words are used throughout this manual:
Indicates a potentially hazardous situation which, if not avoided, could result in death or serious injury.
Indicates a potentially hazardous situation which, if not avoided, may result in minor or moderate injury. It may also be used to alert against unsafe practices or potential equipment damage.
Indicates additional helpful information.
2 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 1 General Policy
1.4Precautions
•An insufficiently cleaned, disinfected, or sterilized endoscope and/or accessories may pose an infection control risk to the patients and/or operators who contact them.
•All disinfection methods (whether performed manually or by an automated endoscope reprocessor), and all sterilization methods (whether performed by ethylene oxide gas or steam) require thorough prior cleaning of the instrument being reprocessed. If the equipment is not adequately cleaned prior to disinfection/sterilization, these processes will be ineffective. Immediately after each patient procedure and before disinfection/sterilization, thoroughly clean the endoscope and the accessories used with the endoscope.
•All channels of the endoscope, including the instrument channel and all accessories used with the endoscope during the patient procedure, such as all valves, must be cleaned and high-level disinfected or sterilized after each patient procedure, even if the channels or accessories were not used during the patient procedure. Insufficient cleaning and disinfection or sterilization of these components may pose an infection control risk to patients and/or operators.
•Disinfectant solutions are hazardous. After disinfection, rinse all external surfaces and channels of the endoscope and accessories thoroughly with water to remove residual disinfectant solution.
•The results of sterilization depend on various factors. These factors include how the equipment was packaged, and the placing and loading of the package in the sterilization device. Verify the sterilization process using biological and/or chemical indicators. Follow the guidelines for sterilization issued by national authorities, professional organizations and infection control professionals, as well as the instruction manual for the sterilization device.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
3 |

Chapter 1 General Policy
•Establish an internal system of identifying contaminated versus reprocessed endoscopes and accessories to prevent both mix-ups and cross-contamination. Touching a reprocessed endoscope and/or accessories with contaminated gloves or placing them on a contaminated hanger or surface, including letting them touch the floor, will recontaminate them.
•Prior to each patient procedure, confirm that the endoscope and accessories have been properly reprocessed and stored. If there are any doubts or questions, reprocess them again before the patient procedure, following the instructions given in this manual.
•Perform a leakage test on the endoscope after each precleaning procedure. Do not use the endoscope if a leak is detected. Use of an endoscope with a leak may cause a sudden loss of the endoscopic image, damage to the bending mechanism, or other malfunctions. Use of a leaking endoscope may also pose an infection control risk.
Leakage tester |
Figure 1.1
•Store alcohol in an airtight container. Alcohol stored in an open container may cause a fire hazard and may result in a loss of efficacy due to evaporation.
4 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 1 General Policy
•Do not use the AW channel cleaning adapter (MH-948) for patient procedures. It will cause continuous insufflation and could result in patient injury.
AW channel cleaning adapter (MH-948)
Figure 1.2
•The accessories listed on the front cover of this manual are consumables, meaning that these accessories cannot be refurbished or repaired and are intended to be replaced once they show any signs of wear. Should any irregularity be observed, use a replacement accessory instead. Using defective accessories may cause equipment malfunction, reduce the efficacy of reprocessing, present a risk to patients and/or operators, or damage the endoscope and/or accessories.
•Single-use brushes, such as the single use channel cleaning brush (BW-201T), the single use combination cleaning brush (BW-412T), the single use channel-opening cleaning brush (MAJ-1339), and the single use soft brush (MAJ-1888), are designed for cleaning only one endoscope and its related accessories. Dispose of the single-use brush immediately after use. Using a single-use brush to clean multiple endoscopes and/or accessories may reduce its cleaning efficacy and may damage the brush leading to brush breakage or endoscope and/or accessory damage.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
5 |

Chapter 1 General Policy
•Patient debris and reprocessing chemicals are hazardous. To guard against contact with dangerous chemicals and potentially infectious material, wear appropriate personal protective equipment during cleaning, disinfection, and sterilization. Such protective equipment should include appropriate eyewear, face mask, cap, moisture-resistant clothing, shoe covers, and chemical-resistant gloves that fit properly and are long enough to prevent skin exposure.
•The reprocessing room must be adequately ventilated. Adequate ventilation protects against the buildup of toxic chemical fumes.
•Always remove contaminated personal protective equipment before leaving the reprocessing area to prevent contamination from spreading.
•Only Olympus-recommended or Olympus-endorsed automated endoscope reprocessors (AERs) have been validated by Olympus. When using an AER that is not recommended by Olympus, the manufacturer of the AER is responsible for validating compatibility of the AER with each Olympus endoscope and accessory.
•Before using an AER, confirm that it is capable of reprocessing the endoscope including all channels, the forceps elevator recess, and accessories. Be sure to attach all required connectors. Otherwise, insufficient reprocessing may pose an infection control risk. If you are uncertain as to the ability of your AER to reprocess the endoscope including all channels, the forceps elevator recess, and accessories, contact the manufacturer of the AER for specific instructions and information on compatibility and required connectors. When you use an AER which allows you skip some steps in precleaning and manual cleaning of endoscopes, confirm with the AER manufacturer that such skip is applicable to this endoscope and establish detailed precleaning and manual cleaning procedures of this endoscope according to both instructions of this manual and the AER manufacturer.
•Put the forceps elevator in intermediate position of the range of movement by turning the elevator control lever and set it in your AER.
6 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 1 General Policy
•Instructions provided in this manual are not valid for Olympus devices repaired by a non-Olympus facility. The Olympus recommended reprocessing procedures have not been validated for reprocessing devices repaired by a non-Olympus facility. In the event that your device has been repaired by a non-Olympus facility, contact that repair facility for instructions regarding reprocessing.
•Prions, which are the pathogenic agent of the Creutzfeldt-Jakob disease (CJD), cannot be destroyed or inactivated by the cleaning, disinfection, and sterilization methods stated in this instruction manual. When using the endoscope and accessories on patients with CJD or variant Creutzfeldt-Jakob disease (vCJD), be sure to use them for such patients only, or immediately dispose of them after use in an appropriate manner to prevent the usage of exposed devices on other patients. For methods to handle CJD, follow the respective guidelines in your country.
•The endoscope and accessories may be damaged by published methods for destroying or inactivating prions. For information on the durability of Olympus equipment against a particular reprocessing method, contact Olympus. In general, Olympus cannot guarantee the effectiveness, safety, and durability of cleaning, disinfection, or sterilization methods not described in this reprocessing manual. If you chose to use a reprocessing method not recommended in this manual, the local institution and/or physicians must assume responsibility for its safety and efficacy. Make sure to carefully inspect each piece of endoscopic equipment for irregularities (damage) prior to each patient procedure. Do not use the equipment if any irregularity is found.
•Good quality control practices typically require appropriate documentation. Items such as local SOPs (standard operating procedures), confirmation of operator training, routine testing of the disinfectant’s MEC (minimal effective concentration), confirmation of the disinfectant’s use-life, etc., should be documented as performed.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
7 |

Chapter 1 General Policy
•When reprocessing the endoscope, confirm that the water resistant cap (MH-553) is securely attached to the electrical connector before immersing the endoscope in reprocessing fluids. If the water resistant cap is not securely attached, the reprocessing fluids could enter the endoscope and damage the endoscope.
Electrical connector |
Water resistant |
|
cap (MH-553) |
||
|
Figure 1.3
•When aerating or irrigating the endoscope channels, the air or water pressure must not exceed 0.5 MPa (5 kgf/cm2,
71 psig). Higher pressures may cause damage to the endoscope.
•Store spare accessories in their original packaging to prevent damage.
•To prevent damage, do not apply excessive force to the endoscope and accessories during reprocessing.
•Vapors from disinfectant solutions and alcohol may damage electronic devices such as computers.
1.5Reprocessing before the first use
New endoscopes, repaired endoscopes, accessories, and the carrying case for endoscopes are not cleaned, disinfected, or sterilized prior to shipping from Olympus, regardless of whether those instruments are for new purchase, demo or loaner purposes. Reprocess all such endoscopes and accessories received from Olympus according to the instructions given in this manual before storage and before using them in a patient procedure.
8 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 1 General Policy
1.6Reprocessing and storage after use
•Do not reuse rinse water.
•High-level disinfectant solutions are only effective when used according to the disinfectant manufacturer’s instructions. Follow the manufacturer’s instructions regarding activation (if required), concentration, temperature, contact time and use life required to achieve high-level disinfection.
•If the disinfectant solution is reused, check its efficacy with a test strip according to the disinfectant manufacturer’s recommendations prior to use.
•Do not reuse alcohol.
•Alcohol is not a sterilant or high-level disinfectant.
•To maintain sterility of equipment following sterilization, use sterile packaging and wraps according to national guidelines.
1.7Reprocessing before patient procedure
•Improper storage practices, such as not thoroughly drying external and internal surfaces (lumens) including the forceps elevator recess prior to storage, will lead to an infection control risk.
•Improper handling, such as touching a reprocessed endoscope and/or accessories with contaminated gloves, placing a reprocessed device on a contaminated hanger or surface, allowing devices to touch the floor, etc., will recontaminate the device.
Some national or professional guidelines recommend reprocessing endoscopes prior to their first use of the day.
Confirm that the endoscope and accessories have undergone proper reprocessing following their last use and that they have been stored properly. Check the storage period of reprocessed endoscopes, and check for surface contamination (e.g., dust). Check the sterilization expiration date(s) of all items so marked and for tears or breaches in sterile packaging. If there are any doubts or questions concerning whether a device is contaminated, reprocess it again following the instructions given in this manual.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
9 |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
Chapter 2 Function and Inspection of
the Accessories for
Reprocessing
Certain accessories are required for reprocessing the endoscope. This chapter describes the function of these accessories. It also describes how to inspect these accessories before using them to reprocess the endoscope.
2.1Water resistant cap (MH-553)
Groove |
|
Seals |
Venting connector |
|
Figure 2.1
10 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
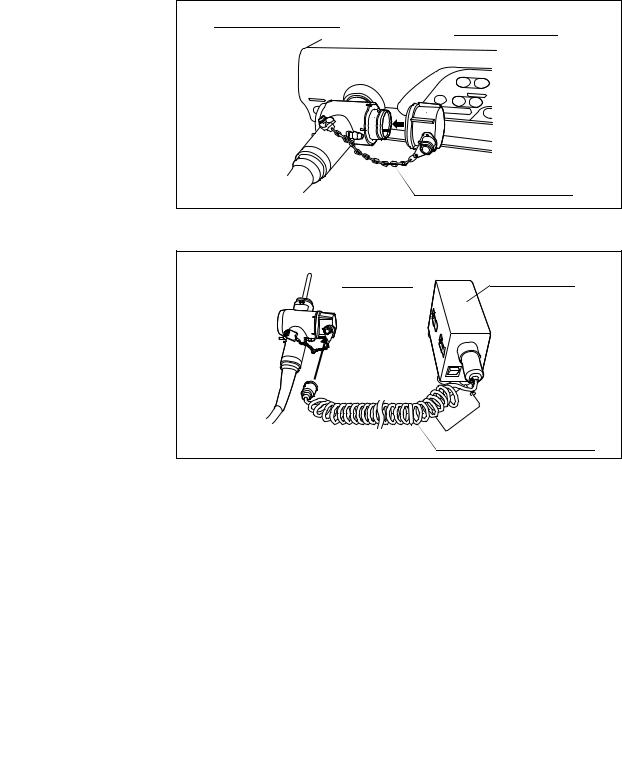
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Function
The water resistant cap is attached to the electrical connector on the endoscope to protect the connector and the endoscope from water penetration during reprocessing. During leakage testing, the leakage tester (MB-155) is attached to the venting connector of the water resistant cap.
Electrical connector |
Water resistant |
|
cap (MH-553) |
||
|
||
|
Chain for water-resistant |
|
|
cap (MAJ-1119) |
Figure 2.2
Venting |
Maintenance |
connector |
unit (MU-1) |
Leakage tester (MB-155)
Figure 2.3
The water resistant cap must be attached to the electrical connector of the endoscope whenever the endoscope is immersed in reprocessing fluids. It is detached from the connector whenever the endoscope is used for patient procedures, being sterilized by ethylene oxide gas, or stored in an endoscope storage cabinet.
Always use a dry water resistant cap. Any water remaining inside the cap may cause damage to the endoscope.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
11 |
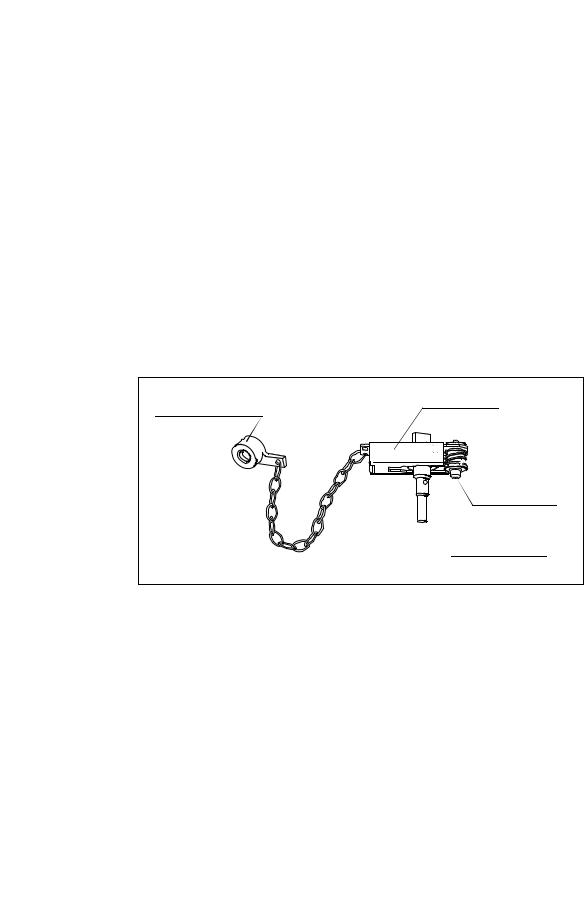
Chapter 2 Function and Inspection of the Accessories for Reprocessing
Use the chain for water-resistant cap (MAJ-1119) to connect the water resistant cap to the endoscope. The water resistant cap can remain connected to the endoscope by the chain at all times (including during patient procedures, reprocessing, and storage of the endoscope).
Inspection
1.Confirm that the inside of the cap is dry and free from debris. Wipe with a dry, lint-free cloth if the inside of the cap is wet or if debris is detected.
2.Confirm that the seals inside the cap are free from scratches, cuts, and debris.
3.Check to ensure that the venting connector on the cap is not loose.
2.2Channel plug (MH-944)
Biopsy valve cap |
Plug frame |
|
|
|
Suction plug |
|
Air/water plug |
Figure 2.4
12 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
Function
The channel plug is used to plug the openings of the instrument channel port and the suction and air/water cylinders of the endoscope whenever the injection tube (MH-946) is used to flush the suction and air/water channels of the endoscope with reprocessing fluids.
Channel plug
Instrument channel port
Figure 2.5
When attached to the endoscope, the channel plug is designed to allow a small amount of fluid to exit from the openings of the endoscope. This enables reprocessing fluids to contact the endoscope openings.
Inspection
Confirm that the suction plug, air/water plug, and the biopsy valve cap of the channel plug are free from cracks, scratches, and debris.
The channel plug does not need to be cleaned, disinfected, or sterilized prior to its first use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
13 |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
2.3Injection tube (MH-946)
Information |
Suction channel tube |
|
card |
|
Connector plug |
|
|
|
Suction |
|
Air pipe port |
channel port |
|
|
Air/water |
|
|
channel port |
|
|
|
|
Air/water channel tube |
Suction port |
|
Filter tube |
|
|
|
(including the filter mesh) |
Filter mesh |
|
Figure 2.6
Function
The injection tube is used to inject reprocessing fluids into the instrument channel, suction channel, and air/water channels of the endoscope. It is also used to flush air through these channels to expel fluids.
Syringe |
Air/water channel tube |
Suction channel tube |
|
Suction port |
|
|
Air pipe port |
Basin |
|
|
Reprocessing |
Connector plug |
fluids |
|
Figure 2.7
Inspection
1.Confirm that all components of the injection tube are free from cracks, scratches, flaws, and debris (see Figure 2.6).
2.Confirm that the filter mesh is in the suction port of the injection tube.
14 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
3. Attach a clean 30 ml syringe to the suction channel port of the injection tube. With the suction port of the injection tube immersed in the water referred to in Section 3.2, withdraw the syringe plunger and confirm that the water is drawn into the syringe. Depress the plunger and confirm that the water is emitted from the suction channel tube of the injection tube. Confirm that the water is not emitted from the suction port when removing the suction port from the water.
Suction channel |
Suction channel |
tube |
port |
|
Syringe |
|
Suction port |
Figure 2.8
4. Move the syringe to the air/water channel port of the injection tube. With the suction port of the injection tube immersed in the water, withdraw the syringe plunger and confirm that the water is drawn into the syringe. Depress the plunger and confirm that the water is emitted from the air pipe port of the injection tube. Confirm that the water is not emitted from the suction port when removing the suction port from the water.
Suction |
Air/water channel port |
channel tube |
|
|
Syringe |
|
Suction port |
Air pipe |
|
port |
|
Figure 2.9
The injection tube does not need to be cleaned, disinfected, or sterilized prior to its first use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
15 |
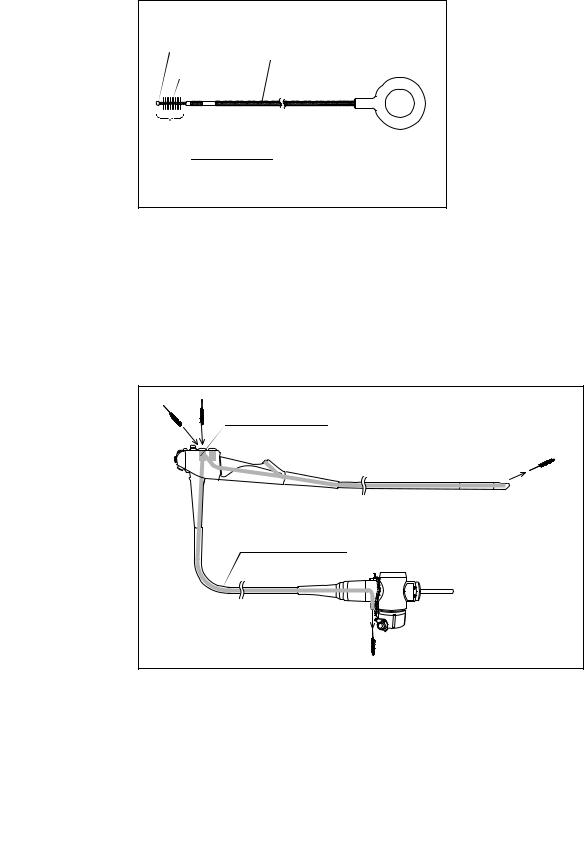
Chapter 2 Function and Inspection of the Accessories for Reprocessing
2.4Channel cleaning brush (BW-20T)
Metal tip |
|
|
Shaft |
|
|
Bristles |
|
|
|
|
|
|
|
|
Brush head
Figure 2.10
Function
The channel cleaning brush is used to brush the inside of the instrument channel and suction channel of the endoscope, and the interior and openings of the suction valve (MH-443), the air/water valve (MH-438) and the biopsy valve (MB-358).
Suction cylinder |
Instrument channel |
Suction channel |
Figure 2.11
16 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
Depress
Suction valve |
Air/water valve |
Biopsy valve (MB-358) |
(MH-443) |
(MH-438) |
|
Figure 2.12
Inspection
1.Confirm that the brush head and the metal tip of the distal end are securely attached. Check the brush head for loose or missing bristles.
2.Check the bristles for damage. If the bristles are crushed, gently straighten them with your gloved fingertips.
3.Check for bends, scratches, and other damage to the shaft.
4.Visually check for debris on the shaft and/or the bristles of the brush head. If there is debris on the brush, immerse the brush in the water referred to in Section 3.2 and clean the brush until no debris is observed on the brush.
The channel cleaning brush does not need to be cleaned, disinfected, or sterilized prior to its first use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
17 |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
2.5Suction cleaning adapter (MH-856)
Connecting end
Weighted end
Figure 2.13
Function
The suction cleaning adapter is used to aspirate reprocessing fluids through the instrument channel port of the endoscope.
Suction cylinder |
Connecting end |
Suction cleaning |
adapter |
Instrument |
channel port |
Weighted end |
Suction pump |
Figure 2.14
Inspection
Check for debris, cracks, scratches, and other damage.
The suction cleaning adapter does not need to be cleaned, disinfected, or sterilized prior to its first use.
18 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
2.6AW channel cleaning adapter (MH-948)
|
|
Information |
Slider |
Button |
card |
|
One-way valve |
|
|
Piston |
|
Figure 2.15
Function
During precleaning of the endoscope, the AW channel cleaning adapter is attached to the air/water cylinder of the endoscope. When the button of the adapter is depressed, the water in the water container is fed through the air/ water nozzle of the endoscope to clean the nozzle and air/water channels of the endoscope. Air is continuously fed through the air/water channels when the button is not depressed.
|
AW channel |
Depress |
|
cleaning adapter |
|
|
|
|
Air/water |
Air channel |
|
nozzle |
|
|
|
|
|
Water |
Air/water cylinder |
|
container |
|
|
|
|
Figure 2.16
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
19 |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
Inspection
Check for debris, cracks, scratches, and other damage.
The AW channel cleaning adapter does not need to be cleaned, disinfected, or sterilized prior to its first use.
2.7Single use channel cleaning brush (BW-201T)
Tip |
Bristles |
Caution sticker |
|
||
|
Brush head |
Shaft |
Figure 2.17
Function
The single use channel cleaning brush is used to brush the inside of the instrument channel and suction channel of the endoscope and the interior and/or openings of the suction valve (MH-443), the air/water valve (MH-438), and the biopsy valve (MB-358).
Suction cylinder |
Instrument channel |
Suction channel |
Figure 2.18
20 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
Depress
Suction valve |
Air/water valve |
Biopsy valve (MB-358) |
(MH-443) |
(MH-438) |
|
Figure 2.19
Inspection
1.Remove the brush from its packaging just prior to use.
2.Confirm that the tip and brush head at the distal end are securely attached. Check the brush head for loose or missing bristles.
3.Check the bristles for any damage. If the bristles are crushed, gently straighten them with your fingertips.
4.Check for bends, scratches, and other damage to the shaft.
The single use channel cleaning brush does not need to be cleaned, disinfected, or sterilized prior to use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
21 |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
2.8Single use channel-opening cleaning brush (MAJ-1339)
Brush head |
Handle |
Figure 2.20
Function
The single use channel-opening cleaning brush is used to brush the suction cylinder, the instrument channel port, the distal end, the forceps elevator, and the forceps elevator recess.
Suction |
|
cylinder |
Instrument |
|
|
|
channel port |
Figure 2.21
22 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |

Chapter 2 Function and Inspection of the Accessories for Reprocessing
Forceps elevator
elevator
(a) Brushing when forceps elevator is lowered.
Forceps elevator recess
(b) Brushing when forceps elevator is raised.
Figure 2.22
Inspection
1.Remove the brush from its packaging just prior to use.
2.Check the brush head for loose or missing bristles.
3.Check the bristles for any damage. If the bristles are crushed, gently straighten them with your fingertips.
4.Check for bends, scratches, and other damage to the shaft.
The single use channel-opening cleaning brush does not need to be cleaned, disinfected, or sterilized prior to use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
23 |
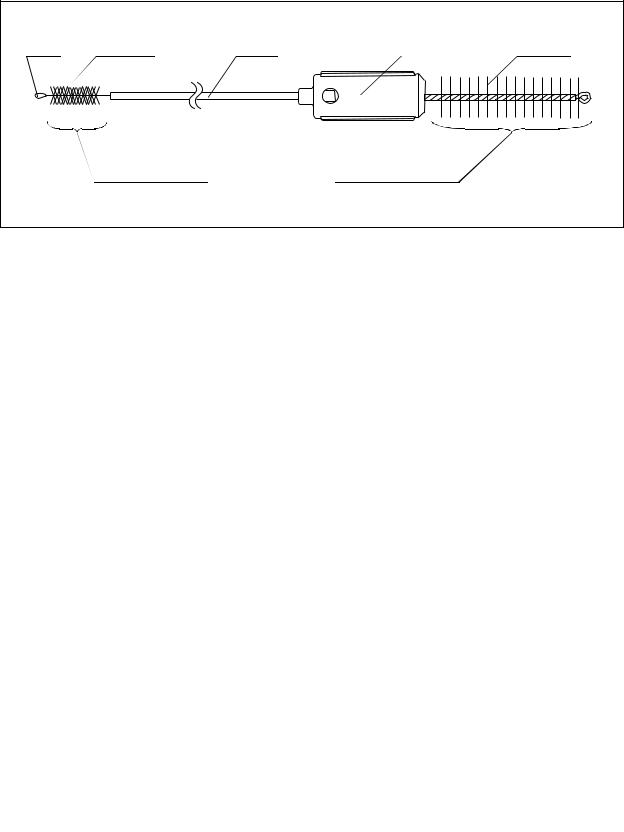
Chapter 2 Function and Inspection of the Accessories for Reprocessing
2.9Single use combination cleaning brush (BW-412T)
Tip |
Bristles |
Shaft |
Handle |
Bristles |
|
Channel cleaning |
|
Channel-opening |
|
|
brush part |
|
cleaning brush part |
|
Figure 2.23
Function
The channel cleaning brush part of the single use combination cleaning brush is used to brush the inside of the instrument channel and suction channel of the endoscope, and the interior and/or openings of the suction valve (MH-443), the air/water valve (MH-438), and the biopsy valve (MB-358). The channel-opening cleaning brush part of the single use combination cleaning brush is used to brush the suction cylinder, the instrument channel port, the distal end, the forceps elevator, and the forceps elevator recess of the endoscope.
Inspection
1.Remove the brush from its packaging just prior to use.
2.Confirm that the channel cleaning brush part and tip at the distal end are securely attached.
3.Check the channel cleaning brush and the channel-opening cleaning brush parts for loose or missing bristles.
4.Check the bristles of the channel cleaning brush and the channel-opening cleaning brush parts for any damage. If the bristles are crushed, gently straighten them with your fingertips.
5.Check for bends, scratches, and other damage to the shaft.
The single use combination cleaning brush does not need to be cleaned, disinfected, or sterilized prior to use.
24 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
Chapter 2 Function and Inspection of the Accessories for Reprocessing
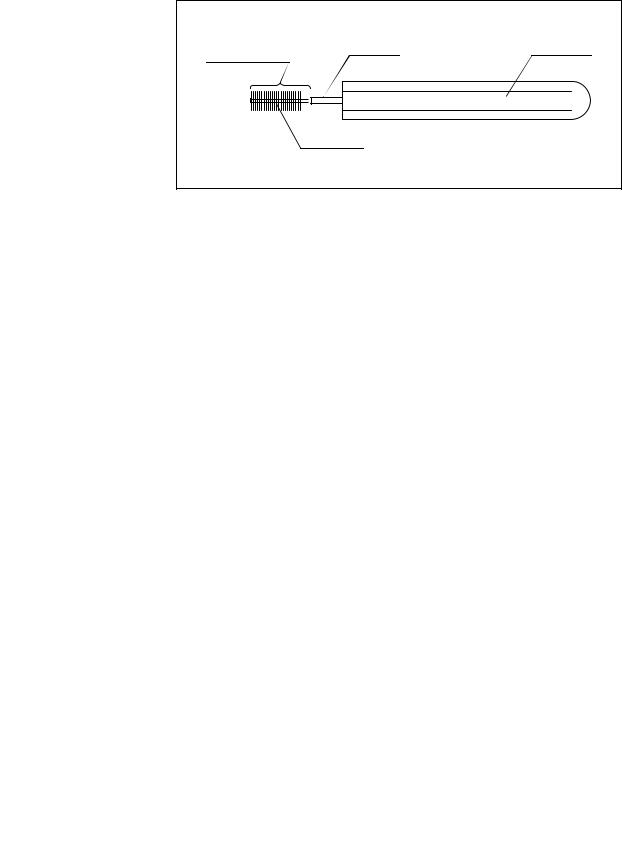
2.10 Single use soft brush (MAJ-1888)
Brush head |
Shaft |
Handle |
|
|
|
|
Bristles |
|
Figure 2.24
Function
The single use soft brush is used to brush around the forceps elevator.
Inspection
1.Check the brush head for loose, missing bristles, and other damage. If the bristles are crushed and/or bent, gently straighten them with your fingers.
2.
3.
Check for bends, scratches, and other damage to the shaft.
Check for debris on the shaft and or in the bristles of the brush head.
The single use soft brush does not need to be cleaned, disinfected, or sterilized prior to use.
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
25 |
Chapter 2 Function and Inspection of the Accessories for Reprocessing
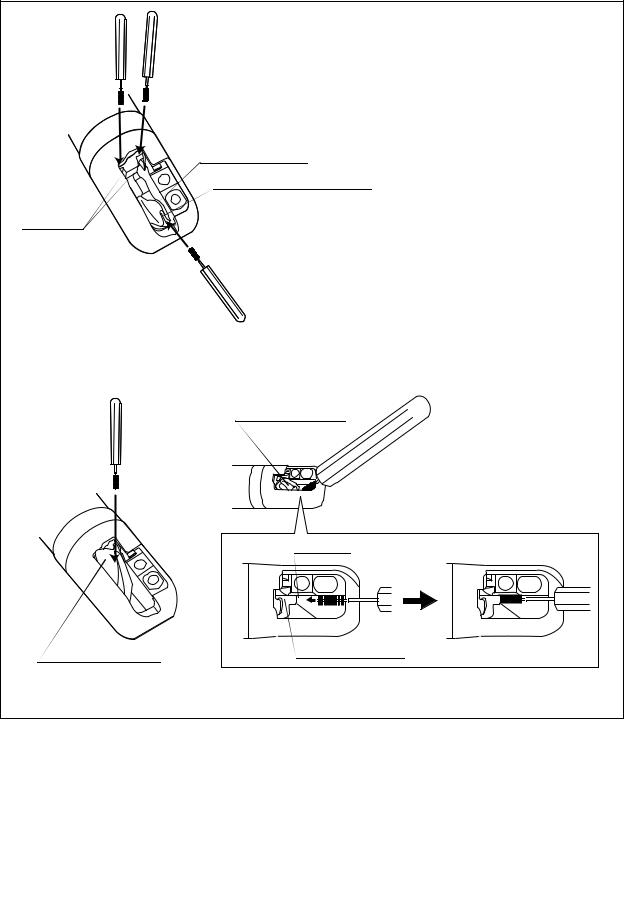
Forceps elevator
Guidewire-locking groove
Groove
(a) Brushing when forceps elevator is lowered.
Forceps elevator
|
Groove |
elevator |
Forceps elevator |
|
(b) Brushing when forceps elevator is raised.
Figure 2.25
26 |
EVIS EXERA II TJF TYPE Q180V REPROCESSING MANUAL |
 Loading...
Loading...