Philips M3535A, M3536A User Manual

English
Instructions For Use
HeartStart MRx
M3535A/M3536A


About This Edition
Publication number: 989803160421
Edition 1 Printed in the USA
To determine the product level version to which these Instructions for Use are applicable, refer to the version level appearing on the back cover of this book or on the label of the User Documentation CD-ROM that accompanied this device. This information is subject to change without notice.
Edition |
Print Date |
1 |
April 2009 |
|
|
NOTE: To obtain Instructions for Use for previous versions of the HeartStart MRx, visit the Philips Documentation and Downloads web site at http://www.philips.com/ProductDocs and search for resuscitation.
Philips shall not be liable for errors contained herein or for incidental or consequential damages in connection with the furnishing, performance, or use of this material.
Copyright
Copyright © 2009 Koninklijke Philips Electronics N.V.
All rights are reserved. Permission is granted to copy and distribute this document for your organization’s internal educational use. Reproduction and/or distribution outside your organization in whole or in part is prohibited without the prior written consent of the copyright holder.
SMART Biphasic is a registered trademark of Philips.
Rosetta-Lt™, Rosetta-Rx™ and CAREpoint™ are trademarks of General Devices. Microstream® and FilterLine® are registered trademarks of Oridion Medical Ltd. Smart CapnoLine™ is a trademark of Oridion Medical Ltd. Q-CPR® is a registered trademark of Laerdal Medical AS. The HeartStart MRx contains an Ezurio PC Card with Bluetooth® wireless technology. The Bluetooth wordmark and logos are owned by the Bluetooth SIG, Inc. and any use of such marks by Ezurio is under license. Coverage Plus® and Coverage Plus NPD® are registered trademarks of Steris Corp. CidexPlus® is a registered trademark of Advanced Sterilization Products. Nellcor® is a registered trademark of Nellcor Puritan Bennett, Inc. TransPac® IV is a registered trademark of ICU Medical, Inc. TruWave® is a registered trademark of Edwards Lifescience Corp. DTX Plus™ is a trademark of Becton, Dickinson & Co.
Notice
RelyOn and Virkon are registered trademarks or trademarks of E.I. du Pont de Nemours and Company or its affiliates. Other trademarks and trade names are those of their respective owners.
Use of supplies or accessories other than those recommended by Philips may compromise product performance.
THIS PRODUCT IS NOT INTENDED FOR HOME USE. U.S. FEDERAL LAW RESTRICTS THIS DEVICE TO SALE ON OR BY THE ORDER OF A PHYSICIAN.
Medical Device Directive
The HeartStart MRx complies with the requirements of the Medical Device Directive 93/42/EEC and carries the 
 0123 mark accordingly.
0123 mark accordingly.
Manufacturer
Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810 USA
Authorized EU-representative
Philips Medizin Systeme Böblingen GmbH
Hewlett Packard Str. 2
71034 Böblingen
Germany
Canada EMC:ICES-001
U.S. FCC and Industry Canada Radio Compliance:
Contains FCC ID: PQC-WMTS-MODULE
When using the IntelliVue networking option, operation of this equipment requires the prior coordination with a frequency coordinator designated by the FCC for the Wireless Medical Telemetry Service. This device complies with Part 15 of the FCC rules and RSS-210 of Industry Canada. Operation is subject to the following conditions:
•This device may not cause harmful interference.
•This device must accept any interference received, including interference that may cause undesired operation.
i

Notice
Any changes or modifications to this equipment not expressly approved by Philips Medical Systems may cause harmful radio frequency interference and void your authority to operate this equipment.
China:
After Sales Service: Beijing MEHECO-PHILIPS Medical Equipment Service Center.
After Sales Service Address: No. 208, 2nd District, Wang Jing Li Ze Zhong Yuan, Chao Yang District, Beijing. Postal code: 100102.
Telephone: 8008100038.
Registration number: SFDA(I)20083211481. Product Standard number: YZB/USA 1863-2008.
For the Declaration of Conformity Statement, please see the Philips Medical web site at http:// incenter.medical.philips.com/PMSPublic. Scroll over the Quality and Regulatory Tab located in the upper left corner of the window. Click to select Regulatory by Modality. Then click to select Defibrillators and select the entry for Declaration of Conformity (DoC).
Warning
Radio frequency (RF) interference coming from devices other than the HeartStart MRx may degrade the performance of the MRx. Electromagnetic compatibility with surrounding devices should be assessed prior to using the monitor/defibrillator.
ii
These Instructions for Use contain the following conventions:
WARNING Warning statements describe conditions or actions that can result in personal injury or loss of life.
CAUTION Caution statements describe conditions or actions that can result in damage to the equipment or loss of data.
NOTE Notes contain additional information on usage.
"Voice" |
represents voice prompt messages |
Text |
represents messages that appear on the display |
[Soft key] |
represents soft key labels that appear on the display above the |
|
button to which they correspond. |
Images of the HeartStart MRx display and menus appearing in this document are for illustration purposes only. Menu choices on your device are driven by the options you have purchased and selections you make in Configuration Mode.
iii


Table of Contents
|
1 Introduction |
1 |
|
Overview |
1 |
|
Intended Use |
2 |
|
Indications for Use |
3 |
|
AED Therapy |
3 |
|
Manual Defibrillation |
3 |
|
Noninvasive External Pacing Therapy |
3 |
|
Pulse Oximetry |
3 |
|
Noninvasive Blood Pressure Monitoring |
3 |
|
End-tidal CO2 |
3 |
|
12-Lead ECG |
3 |
|
Q-CPR |
4 |
|
Invasive Pressures |
4 |
|
Temperature |
4 |
|
ACI-TIPI |
4 |
|
TPI |
4 |
|
Safety Considerations |
5 |
|
Documentation and Training |
5 |
|
2 Getting Acquainted |
7 |
|
|
|
|
|
|
|
|
|
|
Basic Orientation |
8 |
|
Front Panel |
8 |
|
Side Panels |
9 |
|
Top Panel |
10 |
|
Back Panel |
11 |
|
M3538A Lithium Ion Battery |
12 |
|
Battery Capacity |
12 |
|
Battery Life |
12 |
|
Operating Modes |
12 |
|
Password Security |
13 |
|
Display Views |
13 |
|
General Status |
14 |
|
Wave Sectors |
15 |
|
Parameter Blocks |
16 |
|
Turning Parameters On/Off |
16 |
|
Soft Key Labels |
16 |
|
Menus |
17 |
|
Message Windows |
18 |
|
High Contrast Display |
18 |
v

|
Controls |
19 |
|
Therapy Knob |
19 |
|
General Function Buttons |
20 |
|
Defibrillation Controls |
21 |
|
Soft Keys |
21 |
|
Indicators |
22 |
|
Audio Recording |
23 |
|
Reviewing Recorded Audio |
23 |
|
Alarms |
24 |
|
Responding to Alarms |
24 |
|
Printing on Alarms |
26 |
|
Identifying Your Device |
26 |
|
Entering Patient Information |
27 |
|
Continued Use |
27 |
|
Printing Waveforms |
28 |
|
Return to Owner |
29 |
|
3 Setting Up |
31 |
|
|
|
|
|
|
|
|
|
|
Attaching the Carrying Case and Accessory Pouches |
31 |
|
Storing Accessories |
33 |
|
Connecting the ECG Cable |
35 |
|
Connecting the SpO2 Cable |
36 |
|
Connecting the NBP Interconnect Tubing |
37 |
|
Connecting the Invasive Pressures Cable |
38 |
|
Connecting the Temperature Cable |
39 |
|
Connecting the CO2 FilterLine |
40 |
|
Connecting the Therapy or Pads/CPR cables |
41 |
|
Installing Paper |
42 |
|
50 mm paper |
42 |
|
75mm Printer (optional) |
43 |
|
Installing Batteries |
44 |
|
Charging Batteries |
44 |
|
Battery Safety |
44 |
|
Installing the AC Power Module |
45 |
|
Installing the Data Card |
46 |
|
|
46 |
|
4 ECG and Arrhythmia Monitoring |
47 |
|
|
|
|
Overview |
47 |
|
Monitoring View |
48 |
|
Preparing to Monitor ECG |
49 |
|
Electrode Placement |
51 |
|
Lead Selection |
53 |
|
Lead Choices |
53 |
|
Selecting the Lead |
54 |
|
Arrhythmia Monitoring |
54 |
vi

|
Aberrantly-Conducted Beats |
55 |
|
Intermittent Bundle Branch Block |
55 |
|
Heart Rate and Arrhythmia Alarms |
56 |
|
Arrhythmia Alarm Latching |
56 |
|
INOP Messages |
58 |
|
Setting Alarms |
59 |
|
Changing Heart Rate or VTACH Alarm Limits |
59 |
|
Enabling/Disabling Heart Rate and Arrhythmia Alarms |
59 |
|
Responding to HR and Arrhythmia Alarms |
59 |
|
Displaying an Annotated ECG |
60 |
|
Arrhythmia Learning/Relearning |
61 |
|
Troubleshooting |
61 |
|
5 AED Mode |
63 |
|
|
|
|
|
|
|
|
|
|
Precautions for AED Therapy |
63 |
|
AED View |
64 |
|
Preparation |
65 |
|
Using AED Mode |
66 |
|
Step 1 - Turn the Therapy Knob to AED |
67 |
|
Step 2 - Follow Screen and Voice Prompts |
67 |
|
Step 3 - Press Shock Button, if Prompted |
69 |
|
Using Q-CPR in AED Mode |
69 |
|
Troubleshooting |
69 |
|
6 Manual Defibrillation and Cardioversion |
71 |
|
|
|
|
Overview |
71 |
|
Precautions for Manual Defibrillation Therapy |
72 |
|
Synchronized Cardioversion Therapy |
72 |
|
Code View |
73 |
|
Preparing for Defibrillation |
74 |
|
Using Multifunction Electrode Pads |
74 |
|
Using External Paddles |
75 |
|
Using Pediatric Paddles |
76 |
|
Using Internal Paddles |
76 |
|
Defibrillating (asynchronously) |
77 |
|
Performing Synchronized Cardioversion |
79 |
|
Preparing for Synchronized Cardioversion |
79 |
|
Delivering a Synchronized Shock |
80 |
|
Delivering Additional Synchronized Shocks |
81 |
|
Disabling the Sync Function |
81 |
|
Using Q-CPR in Manual Mode |
81 |
|
Troubleshooting |
81 |
|
7 Noninvasive Pacing |
83 |
|
|
|
|
Overview |
83 |
|
Alarms |
84 |
vii

|
Pacing View |
85 |
|
Demand Mode Versus Fixed Mode |
86 |
|
Preparing for Pacing |
87 |
|
Demand Mode Pacing |
88 |
|
Fixed Mode Pacing |
89 |
|
Defibrillating During Pacing |
91 |
|
Troubleshooting |
91 |
|
8 Pulse Oximetry |
93 |
|
|
|
|
Overview |
93 |
|
Understanding Pulse Oximetry |
94 |
|
Selecting a Sensor |
95 |
|
Applying the Sensor |
96 |
|
Monitoring SpO2 |
97 |
|
Pleth Wave |
98 |
|
SpO2 Alarms |
99 |
|
Changing the SpO2 Alarm Limits |
99 |
|
SpO2 Desat Alarm |
100 |
|
Enabling/Disabling the SpO2 Alarms |
100 |
|
Pulse Rate Alarms |
100 |
|
Enabling/Disabling the Pulse Rate Alarms |
101 |
|
Changing the Pulse Rate Alarm Limits |
101 |
|
Disabling the SpO2 Monitoring Function |
101 |
|
Caring for Sensors |
102 |
|
Troubleshooting |
102 |
|
9 Noninvasive Blood Pressure |
103 |
|
|
|
|
|
|
|
|
|
|
Overview |
103 |
|
Preparing to Measure NBP |
104 |
|
Measuring NBP |
106 |
|
Changing the NBP Schedule |
106 |
|
Alarms |
107 |
|
Changing NBP Alarms |
107 |
|
Enabling/Disabling NBP Alarms |
108 |
|
Troubleshooting |
108 |
10 Monitoring Carbon Dioxide |
109 |
|
|
|
|
|
Overview |
109 |
|
Preparing to Measure EtCO2 |
110 |
|
Selecting the Accessories |
110 |
|
Setting Up Microstream EtCO2 Measurements |
111 |
|
Using the Nasal FilterLine |
111 |
|
Using the FilterLine and Airway Adapter |
111 |
|
Measuring EtCO2 |
112 |
|
EtCO2 and AwRR Alarms |
112 |
|
Changing the EtCO2 Alarm Limits |
113 |
viii

|
Enabling/Disabling the EtCO2 Alarms |
113 |
|
Changing the AwRR Alarm Limits |
113 |
|
Changing the Apnea Time Alarm Limit |
114 |
|
Enabling/Disabling AwRR Alarms |
114 |
|
Disabling the EtCO2 Monitoring Function |
114 |
|
Troubleshooting |
114 |
11 Invasive Pressures |
115 |
|
|
|
|
|
Overview |
115 |
|
Setting up for a Pressure Measurement |
115 |
|
Selecting a Pressure to Monitor |
117 |
|
Pressure Waves |
118 |
|
Zeroing the Pressure Transducer |
119 |
|
Zeroing Using the Menu Select Button |
119 |
|
Zeroing Using a Soft Key in Monitor Mode |
119 |
|
Calibration |
121 |
|
Known Calibration Factor |
121 |
|
Calibrating Reusable Transducer CPJ840J6 |
122 |
|
Calibration Confirmation |
123 |
|
Last Zero/Calibration |
124 |
|
Non-Physiological Artifact Suppression |
124 |
|
Alarms |
125 |
|
Enabling/Disabling alarms |
126 |
|
Viewing/Changing/Setting Source for Alarms |
126 |
|
CPP Alarms |
127 |
|
Wedge |
127 |
|
Pulse |
128 |
|
Pulse Sources |
128 |
|
Changing Pulse Source |
129 |
|
Setting Pulse Alarms |
129 |
|
Enabling/Disabling Pulse Alarms |
129 |
|
Pulse Alarm Limits |
130 |
|
Changing Default Pulse Source and Alarm Limits |
130 |
|
Caring For Your Transducers and Probes |
130 |
|
Troubleshooting |
130 |
12 Temperature |
131 |
|
|
|
|
|
Overview |
131 |
|
Selecting a Temperature Label |
131 |
|
Monitoring Temperature |
132 |
|
Alarms |
132 |
|
Setting Temperature Alarms |
132 |
|
Changing Temperature Alarm Limits |
133 |
|
Enabling/Disabling Temperature Alarms |
133 |
|
Changing Degree Units |
133 |
|
Disabling the Temperature Function |
134 |
ix

|
Caring For Your Temperature Cables and Probes |
134 |
|
Troubleshooting |
134 |
13 12-Lead ECG |
135 |
|
|
|
|
|
Overview |
135 |
|
Preparation |
136 |
|
Preview Screen |
137 |
|
Acquiring a 12-Lead ECG |
138 |
|
Acquiring a 12-lead ECG with ACI-TIPI and/or TPI Analysis |
139 |
|
Critical Values |
143 |
|
Culprit Artery |
145 |
|
12-Lead Report |
146 |
|
Accessing Stored Reports |
148 |
|
Improving Signal Quality |
149 |
|
Adjusting Wave Size |
149 |
|
12-Lead Filters |
150 |
|
Troubleshooting |
150 |
14 Vital Signs Trending |
151 |
|
|
|
|
|
Overview |
151 |
|
Reviewing Trending Data |
151 |
|
About The Data Displayed |
152 |
|
Vital Signs Trending Report Parameter List Order |
153 |
|
Scrolling in the Vital Signs Trending Report |
153 |
|
Vital Signs Trending Report Intervals |
153 |
|
Adjusting Vital Signs Trending Report Interval |
153 |
|
Printing the Vital Signs Trending Report |
154 |
|
Exiting Vital Signs Trending Report |
155 |
|
Troubleshooting |
155 |
15 Q-CPR and Data Capture |
157 |
|
|
|
|
|
Overview |
157 |
|
Preparing to Use Q-CPR |
159 |
|
Connecting the Pads/CPR Cable |
159 |
|
Connecting the CPR Meter to the Pads/CPR Cable |
160 |
|
Applying Multifunction Electrode Pads |
160 |
|
CPR Meter |
161 |
|
Attaching the CPR Meter Adhesive Pad |
162 |
|
Placing the CPR Meter on the Patient |
163 |
|
Starting CPR with the CPR Meter |
164 |
|
CPR Meter Display |
165 |
|
Q-CPR Feedback on the HeartStart MRx |
167 |
|
Advanced View |
167 |
|
Basic View |
170 |
|
Using Q-CPR in Manual Defib Mode |
171 |
|
Using Q-CPR in AED Mode |
171 |
x

|
Feedback Prompts |
172 |
|
Adjusting CPR Feedback Volume |
173 |
|
After Each Use |
174 |
|
Q-CPR Data Capture |
174 |
|
Q-CPR Feedback Setting |
174 |
|
Data and Events Recorded |
175 |
|
Research Storage Setting |
175 |
|
Reviewing Q-CPR Data |
175 |
|
Troubleshooting |
175 |
16 Networking |
177 |
|
|
|
|
|
IntelliVue Networking Display |
178 |
|
Connecting to the Network |
179 |
|
Physical Connections |
179 |
|
Wired Connection |
180 |
|
Wireless Connection |
180 |
|
Combined Connection |
181 |
|
Configuring to Work on the Network |
181 |
|
Using the Device Location Option |
182 |
|
Network Settings |
183 |
|
Admit, Discharge, Transfer of Patients |
184 |
|
Admit |
184 |
|
Discharge |
185 |
|
Transfer |
187 |
|
Transfer Mode |
188 |
|
Sharing Information on the Network |
190 |
|
Patient Information |
190 |
|
Conflict Handling |
192 |
|
Viewing Patient Incident Data |
193 |
|
Alarms |
193 |
|
Printing |
193 |
|
Turning a Networked Device Off |
193 |
|
Leaving a Clinical Mode |
194 |
|
Events Logged |
195 |
|
INOPs, Alarms and Messages at the Information Center |
195 |
|
Troubleshooting |
197 |
17 Configuration |
199 |
|
|
|
|
|
Overview |
199 |
|
Accessing the Configuration Menu |
199 |
|
Setting the Date and Time |
200 |
|
Modifying Settings |
200 |
|
Saving Configuration Settings to a Data Card |
201 |
|
Loading Configuration Settings from a Data Card |
201 |
|
Restoring the Default Settings |
201 |
|
Printing Configuration Settings |
201 |
xi

|
Configurable Parameters |
202 |
18 Working with Data |
225 |
|
|
|
|
|
Overview |
225 |
|
Initiating an Event Summary |
226 |
|
Entering Data Management Mode |
226 |
|
Copying from Internal Memory |
227 |
|
Viewing and Erasing the External Data Card |
228 |
|
Printing During a Patient Event |
229 |
|
Event Summaries |
229 |
|
Vital Signs Trending Reports |
229 |
|
12-Lead ECG Reports |
229 |
|
Printing Individual Events |
230 |
|
Printing from Data Management Mode |
231 |
|
Events Stored in Event Summary |
232 |
|
Marking Events |
237 |
19 Data Transmission |
239 |
|
|
|
|
|
|
|
|
|
|
|
Overview |
239 |
|
Transmitting During a Critical Care Event |
240 |
|
Preparing for Transmission |
241 |
|
Modifying Reference IDs |
241 |
|
Setting up Bluetooth Transmissions |
242 |
|
Setting up Rosetta Transmissions |
245 |
|
Connecting Rosetta-Lt |
246 |
|
Setting Up for RS-232 Transmissions |
247 |
|
Transmitting in 12-Lead Mode |
248 |
|
Transmitting to a Manually Entered Fax Number |
249 |
|
Transmitting to a Personal Computer |
249 |
|
Periodic Clinical Data Transmission |
250 |
|
Transmitting Clinical Values |
250 |
|
PCDT Contents |
250 |
|
Starting a Periodic Clinical Data Transmission |
252 |
|
Ending a Periodic Clinical Values Transmission |
253 |
|
Transmitting Event Summaries Post Event |
254 |
|
Transmitting in Data Management Mode |
255 |
|
Tracking Data Transmission |
257 |
|
Transmission Errors |
257 |
|
Cancelling a Transmission |
257 |
|
Queuing Transmissions |
258 |
|
Finding Transmission Results |
258 |
|
Batch LAN Data Transfer |
259 |
|
Setting Up for Batch LAN Data Transfer |
259 |
|
Transferring Files with BLDT |
260 |
|
Troubleshooting |
261 |
xii

20 Maintenance |
263 |
|
|
Overview |
263 |
|
Automated Tests |
264 |
|
Automated Test Summary |
265 |
|
Ready For Use Indicator |
266 |
|
Shift Checklist |
267 |
|
Weekly Shock Test |
267 |
|
HeartStart MRx Shift Checklist |
268 |
|
Operational Check |
270 |
|
Performing the Operational Check |
271 |
|
Operational Check Report |
277 |
|
Operational Check Summary |
282 |
|
Battery Maintenance |
283 |
|
Battery Life |
283 |
|
Charging Batteries |
284 |
|
Battery Calibration |
284 |
|
Storing Batteries |
286 |
|
Discarding Batteries |
286 |
|
Cleaning Instructions |
287 |
|
Monitor/Defibrillator |
287 |
|
Printer Printhead |
287 |
|
Paddles, Therapy Cable |
288 |
|
ECG Cable |
288 |
|
Carrying Case |
289 |
|
NBP Cuff |
289 |
|
SpO2 Sensor and Cable |
289 |
|
Invasive Pressures Transducer and Cable |
289 |
|
Temperature Probe and Cable |
289 |
|
CPR meter |
289 |
|
HeartStart MRx Disposal |
290 |
|
Empty Calibration Gas Cylinders Disposal |
290 |
|
CPR Meter and Adhesive Pads Disposal |
290 |
21 Supplies & Accessories |
291 |
|
|
|
|
|
Overview |
291 |
22 Troubleshooting |
297 |
|
|
|
|
|
Symptoms |
298 |
|
Audio Tones and Alarm Indications |
318 |
|
Calling for Service |
319 |
23 Specifications and Safety |
321 |
|
|
|
|
|
Specifications |
321 |
|
General |
321 |
|
Defibrillator |
321 |
xiii

ECG and Arrhythmia Monitoring |
324 |
Display |
326 |
Battery |
326 |
Thermal Array Printer |
327 |
Noninvasive Pacing |
327 |
SpO2 Pulse Oximetry |
328 |
NBP |
329 |
Invasive Pressures |
330 |
Temperature |
331 |
EtCO2 |
331 |
AwRR |
332 |
Calibration Gas for CO2 Measurement System |
332 |
CPR Meter |
333 |
Patient Adhesive Pads |
333 |
12-Lead ECG |
333 |
Networking |
333 |
Patient Data Storage |
334 |
Environmental (M3535A) |
334 |
Environmental (M3536A) |
335 |
Bluetooth |
336 |
Symbol Definitions |
337 |
Units and Abbreviations |
340 |
Clinical Performance Summary - Defibrillation |
341 |
Methods |
341 |
Results |
341 |
Conclusion |
341 |
Clinical Performance Summary - Cardioversion |
342 |
Methods |
342 |
Results |
342 |
Conclusion |
343 |
Clinical Performance Summary - Internal Defibrillation |
344 |
Overview |
344 |
Methods |
344 |
Results |
344 |
Conclusion |
344 |
Safety Considerations |
345 |
General |
345 |
Defibrillation |
348 |
Battery |
349 |
Supplies and Accessories |
350 |
Electromagnetic Compatibility |
350 |
Reducing Electromagnetic Interference |
350 |
Restrictions for Use |
351 |
Emissions and Immunity |
351 |
Guidance and Manufacturer’s Declaration |
351 |
xiv

Index |
359 |
xv


1
Introduction
Thank you for choosing the HeartStart MRx monitor/defibrillator. Philips Healthcare welcomes you to its family of resuscitation devices.
The HeartStart MRx is designed to meet your monitoring and resuscitation needs by providing advanced, multi-parameter monitoring functions, a full range of defibrillation therapies, industryleading algorithms and a suite of data transmission options. This guide provides instructions for the safe and proper operation of the device, as well as set-up, configuration, and maintenance information.
Be sure to familiarize yourself with the features and operation of your HeartStart MRx prior to its use.
Overview
The HeartStart MRx is a lightweight, portable, monitor/defibrillator. It provides four modes of operation: Monitor, Manual Defib, AED, and Pacer (optional).
In Monitor Mode you can monitor up to four ECG waveforms, acquired through a 3-, 5-, or 10-lead ECG set. Optional monitoring of pulse oximetry (SpO2), noninvasive blood pressure (NBP), carbon dioxide (EtCO2), temperature, and invasive pressures are also available. Measurements from these parameters are presented on the display. Alarms are available to alert you to changes in the patient’s condition. You can also display a Vital Signs Trending Report to view all key parameters and their measurements at a glance.
Monitor Mode also provides an optional 12-Lead ECG function, enabling you to preview, acquire, store, and print 12-lead ECG reports, with or without analysis/interpretation. In addition, there are several STEMI decision support tools, including STEMI Culprit Artery, Critical Values and the Acute Cardiac Ischemia Time-Insensitive Predictive Instrument (ACI-TIPI) and Thrombolytic Predictive Instrument (TPI) algorithms. You can also transmit 12-Lead reports and Event Summaries via the 12Lead ECG Transmission and Event Summary Data Transfer options. Other transmission options are available. See “Data Transmission” on page 239.
Manual Defib Mode offers simple, 3-step defibrillation. You analyze the patient’s ECG and, if appropriate: 1) select an energy setting, 2) charge, and 3) deliver the shock. Defibrillation may be performed using paddles or multifunction electrode pads. Manual Defib Mode also allows you to perform synchronized cardioversion and internal defibrillation. If desired, use of Manual Defib Mode may be password protected.
In AED Mode, the HeartStart MRx analyzes the patient’s ECG and determines whether a shock is advised. Voice prompts guide you through the 3-step defibrillation process, providing easy-to-follow instructions and patient information. Voice prompts are reinforced by messages on the display.
1

1 Introduction |
Intended Use |
The Manual Defib and AED modes incorporate Philips’ low energy SMART Biphasic waveform for defibrillation. Both modes also offer the Q-CPR® option. Q-CPR offers real-time measurement and corrective feedback on the rate, depth/complete release of compressions (and lack of CPR activity) and ventilation rate. The HeartStart MRx displays a CPR Timer and compression counter to assist with protocol management.
The HeartStart MRx also has an optional Audio function which allows you to record audio during a patient event.
Optional Pacer Mode offers noninvasive transcutaneous pacing therapy. Pace pulses are delivered through multifunction electrode pads, using a monophasic waveform. If desired, use of Pacer Mode may be password protected.
The HeartStart MRx is powered by rechargeable lithium ion batteries. Available battery power is easily determined by viewing the convenient battery power indicators located on the device display or by checking the gauge on the battery itself. Additionally, an external AC or DC Power Module may be applied as a secondary power source and for continual battery charging.
The HeartStart MRx performs Automated Tests on a regular basis. The results of these tests are reported to the Ready For Use (RFU) indicator. The prominently displayed RFU indicator communicates the status of your device, letting you know it is operating correctly, needs attention, or is unable to deliver therapy. In addition, performing the specified Operational Check ensures that the HeartStart MRx is functioning properly.
The HeartStart MRx automatically stores critical event data, such as Event Summaries, 12-Lead Reports and Vital Signs Trending, in its internal memory. The HeartStart MRx also enables you to store data and event information on an optional data card for downloading to Philips’ data management solution, HeartStart Event Review Pro, or you can send the data electronically via several methods to your destination point.
The HeartStart MRx is highly configurable to better meet the needs of diverse users. Be sure to familiarize yourself with your device’s configuration before using the HeartStart MRx. See Chapter 17 “Configuration” for more details.
Intended Use
The HeartStart MRx is intended for use in hospital and pre-hospital settings by qualified medical personnel trained in the operation of the device and qualified by training in basic life support, advanced cardiac life support or defibrillation.
When operating as a semi-automatic external defibrillator in AED Mode, the HeartStart MRx is suitable for use by medical personnel trained in basic life support that includes the use of an AED.
When operating in Monitor, Manual Defib or Pacer Mode, the HeartStart MRx is suitable for use by healthcare professionals trained in advanced cardiac life support.
The SMART Biphasic waveform utilized in the HeartStart MRx has previously undergone clinical testing in adults. These trials support the waveform’s effectiveness for defibrillation of ventricular tachyarrhythmias at 150J.
2

Indications for Use |
1 Introduction |
Indications for Use
The HeartStart MRx is for use for the termination of ventricular tachycardia and ventricular fibrillation.
The device is for use by qualified medical personnel trained in the operation of the device and qualified by training in basic life support, advanced cardiac support, or defibrillation. It must be used by or on the order of a physician.
AED Therapy
To be used in the presence of a suspected cardiac arrest on patients of at least 8 years of age that are unresponsive, not breathing and pulseless.
Manual Defibrillation
Asynchronous defibrillation is the initial treatment for ventricular fibrillation and ventricular tachycardia in patients that are pulseless and unresponsive. Synchronous defibrillation is indicated for termination of atrial fibrillation.
Noninvasive External Pacing Therapy
The pacing option is intended for treating patients with symptomatic bradycardia. It can also be helpful in patients with asystole, if performed early.
Pulse Oximetry
The SpO2 option is intended for use when it is beneficial to assess a patient’s oxygen saturation level.
Noninvasive Blood Pressure Monitoring
The NBP option is intended for noninvasive measurement of a patient’s arterial blood pressure.
End-tidal CO2
The EtCO2 option is intended for noninvasive monitoring of a patient’s exhaled carbon dioxide and to provide a respiration rate.
12-Lead ECG
The 12-Lead ECG function is to provide a conventional diagnostic 12-Lead ECG report, which may include measurements and interpretative statements.
3

1 Introduction |
Indications for Use |
Q-CPR
The Q-CPR option provides feedback designed to encourage rescuers to perform resuscitation in accordance with AHA/ERC guidelines for chest compression rate, depth, and duty cycle and ventilation rate, volume and flow rate (inflation time).
The Q-CPR option is contraindicated as follows:
•The Q-CPR option is contraindicated for use on neonatal and pediatric patients (under 8 years of age or weighing less that 25 kg).
•The Q-CPR option is not for use when CPR is contraindicated.
Invasive Pressures
The Invasive Pressures option is indicated for measuring arterial, venous, intracranial and other physiological pressures on patients.
Temperature
The Temperature option is indicated for measuring temperature in patients.
ACI-TIPI
This device is intended to be an aid to clinicians by focusing their attention on indicators of Acute Cardiac Ischemia.
TPI
Indications for Use: Physiological purpose: To aid the clinician deciding whether to administer thrombolytic therapy or to provide another avenue of treatment; Condition: Patient is a potential candidate for thrombolytic therapy; Patient Population: adult (35 to 75 years) patients diagnosed as having symptoms of Acute Myocardial Infarction; Body or type of tissue interacted with: No body or tissue contact. Prescription versus over-the-counter: TPI is a prescription device.
Contraindications: NOTE: The Thrombolytic Predictive Instrument (TPI) is contraindicated for patients with conditions which mimic acute myocardial infarction. Some of these conditions are: Prinzmetal variant angina, acute pericarditis, acute myocarditis, cardiomyopathy, and primary and secondary cardiac neoplasms.
The Thrombolytic Predictive Instrument (TPI) is also contraindicated for patients with conditions whom the administration of thrombolytics is contraindicated. Some of these conditions are: aortic dissection, acute myocardial infarction due to bacterial endocarditis, vasculitis, intracardiac thrombi, and acute nonsuppurative and suppurative pericarditis mimicking acute myocardial infarction.
These situations, as well as Posterior Acute Myocardial Infarction, were not considered or covered in the development of the predictive instrument calculations.
NOTE See note on page 140 for additional details on contraindications for TPI therapy.
4

Safety Considerations |
1 Introduction |
Safety Considerations
General warnings and cautions that apply to use of the HeartStart MRx are provided in “Specifications and Safety” on page 321. Additional warnings and cautions specific to a particular feature are provided in the appropriate section of this guide.
WARNING Electric shock hazards exist internally. Do not attempt to open the device. Refer servicing to qualified personnel.
WARNING Use only supplies and accessories approved for use with your HeartStart MRx. Use of non-approved supplies and accessories could affect performance and results.
WARNING Use single-use supplies and accessories only once.
Documentation and Training
Available documentation and training for the HeartStart MRx includes:
•HeartStart MRx Instructions for Use
•HeartStart MRx Quick Reference Cards
•HeartStart MRx Battery Application Note
•HeartStart MRx Improving ECG Quality Application Note
•HeartStart MRx Web-based User Training (Located at: www.medical.philips.com/goto/mrxtraining. Enter training access password: meetMRx.)
•HeartStart MRx User Training Videotape and DVD
•To purchase additional copies of the Instructions for Use or Quick Cards, visit the Philips Healthcare eStore at: www.philips.com/healthcarestore.
•Other Application Notes can be found on the Philips website at: www.medical.philips.com/goto/ productdocumentation.
5


2
Getting Acquainted
The HeartStart MRx is designed with your needs in mind. Controls, indicators, and menus are carefully organized to facilitate easy use. Display information is tailored to the current task.
This chapter acquaints you with the HeartStart MRx operational modes, display views, controls, and indicators. It also provides general information on device use.
NOTE If your HeartStart MRx does not have some of the optional functionality listed in this chapter, disregard these controls and the related information described throughout this manual.
7
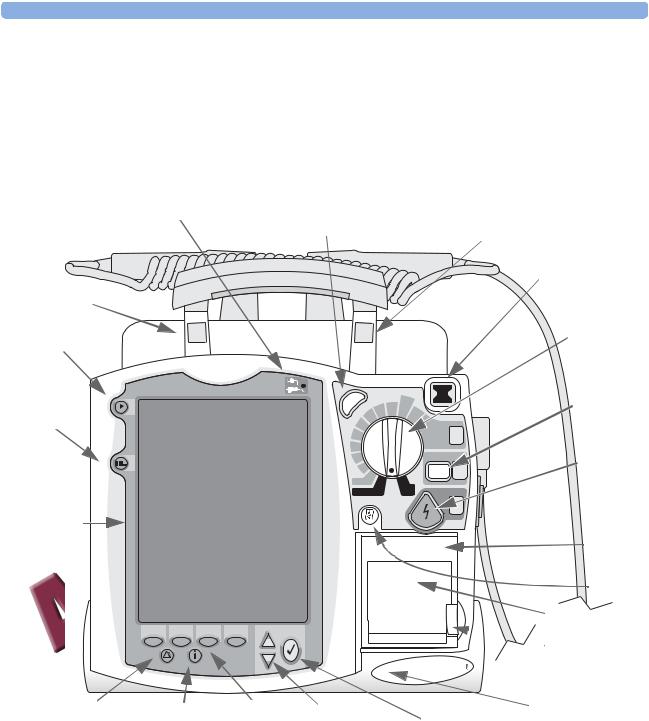
2 Getting Acquainted |
Basic Orientation |
Basic Orientation
HeartStart MRx controls, indicators, and connections are carefully organized.
Front Panel
The front panel contains operational controls and indicators, as shown below.
Figure 1 Basic Orientation (Front)
External Power Indicator
Label Recess
Mark Event button 
Lead Select button
Display
Synchronized
Cardioversion (Sync)
c Syn
|
|
|
|
|
|
|
|
Adult |
|
|
|
|
|
|
|
120 |
Dose |
|
|
|
|
ib |
|
100 |
150 |
|
|
|
|
f |
70 |
|
|
170 |
|
|
l |
D |
e |
|
|
|
|
|
|
|
50 |
|
|
|
|
||
|
a |
|
|
|
|
|
|
|
u |
|
|
|
|
|
|
|
|
n |
|
|
|
|
|
|
|
|
a |
|
|
30 |
|
|
|
|
|
M |
|
|
|
|
|
|
||
|
|
20 |
|
|
|
|
||
|
|
|
15 |
|
|
|
|
|
|
|
|
|
1-10 |
|
|
|
|
|
|
|
Pacer |
On |
Off |
On |
||
Monitor AED
Location of Networking icon
(if device is network enabled)
Ready For Use
(RFU) Indicator
Therapy Knob
|
|
Charge |
|
|
button |
200 |
1 |
|
Select |
|
|
Energy |
Shock |
|
Charge |
2 |
|
|
button |
|
Shock |
3 |
|
Printer (50 mm)
Button
 Printer Door
Printer Door
 Printer Door
Printer Door
Latch

 Speaker
Speaker
Alarm Pause button Summary |
Soft keys |
Navigation |
Microphone |
button |
(4 total) |
buttons |
Menu Select button |
Additional controls and indicators are on the paddles (if used) and batteries.
NOTE A palette of colored decals is included with your HeartStart MRx. These colored decals may be applied to the label recesses located on the device handle to aid in identification. Use an indelible marker to print identification information on the decal.
8

Basic Orientation |
2 Getting Acquainted |
Side Panels
The left side of the HeartStart MRx has ports for monitoring cables, including ECG, pulse oximetry (SpO2), noninvasive blood pressure (NBP), invasive pressure (2), temperature and carbon dioxide (CO2). The ECG port may be used to connect a 3-, 5-, or 10-lead patient cable. The ECG Out jack may be used to connect to an external monitor.
The right side of the HeartStart MRx has a therapy port for paddles (external or internal), or therapy cable and multifunction electrode pads. It also has a slot for a data card to transfer patient information.
Figure 2 Basic Orientation (Right/Left Sides)
CO2 Inlet
Port
CO2
Outlet Port
Temperature 
Port
ECG Out
(Sync) Jack
CO2 |
|
|
|
|
|
|
|
|
™ |
|
|
|
|
a |
|
|
|
t r |
e |
|
r o |
s |
|
|
ic |
|
|
|
|
|
|
|
|
|
M |
|
|
|
|
2
1
ECG
ECG
Therapy
Connector
Data Card slot
Invasive Pressure ports


 NBP Port
NBP Port


 ECG Port
ECG Port


 SpO2 Port
SpO2 Port
9

2 Getting Acquainted |
Basic Orientation |
Top Panel
The top of the HeartStart MRx has a handle and basic operating instructions. If optional external paddles are present, they reside on the top panel as shown.
Figure 3 Basic Orientation (Top - with Optional Paddles)
10

Basic Orientation |
2 Getting Acquainted |
Back Panel
The back panel of the HeartStart MRx has two compartments for lithium ion batteries. Compartment B may also be used to connect an AC power module. Between the battery compartments is a DC Power Input port.
The back panel also has an RS-232 serial port for 12-lead ECG transmission or setting up a wireless connection to the IntelliVue Network. The LAN port is for a wired connection to the IntelliVue Network or for Batch LAN Data Transfer.
Figure 4 Basic Orientation (Back)
|
Battery |
Battery/AC |
Compartment A |
Compartment B |
|
LAN Port 






RS-232
Serial Port
AC Power |
|
Module |
|
|
Input |
Battery |
|
DC Power |
|||||
WARNING The HeartStart MRx LAN port is intended for connection to the IntelliVue Clinical Network or for Batch LAN Data Transfer. It should only be used for connection to devices that comply with IEC 60950-1 and IEC 60601-1. During real-time patient monitoring, the HeartStart MRx wired LAN connector should only be connected to the IntelliVue Network. For post-event Batch LAN Data Transfer, the HeartStart MRx should only be connected to the facility network.
The RS-232 Serial Port is intended for connection to the IntelliVue wireless backpack. It is also for connecting with the Rosetta-Lt and cellphones for data transmission. Improper system operation may result if any other device is connected to this port.
11

2 Getting Acquainted |
M3538A Lithium Ion Battery |
M3538A Lithium Ion Battery
The HeartStart MRx uses the M3538A Lithium Ion Battery. The battery has a fuel gauge with 5 LED indicators, each representing a charge of at least 20% of capacity. Press the fuel gauge button to illuminate the fuel gauge.
CAUTION A battery should be used as the primary power source. AC/DC should be used as a secondary source, if desired. If an AC/DC power module is used as the only power source, the HeartStart MRx takes longer to charge to the desired energy level and, in the event of power loss, all settings reset to the default settings and a new incident is created when power is returned. All stored data remains intact and can be found by retrieving the previous incident. Keep your unit charged.
Battery Capacity
A new, fully-charged M3538A battery, operating at room temperature 25oC (77oF), provides at least 5 hours of monitoring, with ECG, SpO2, CO2, temperature, two invasive pressures monitored continuously, NBP measured every 15 minutes, and 20 200J discharges. A fully charged new battery provides approximately 3.5 hours of monitoring, with ECG, SpO2, CO2, temperature, two invasive pressures monitored continuously, NBP measured every 15 minutes, and pacing at 180ppm at 160mA.
Battery Life
Battery life depends on the frequency and duration of use. When properly cared for, the M3538A Lithium Ion battery has a useful life of approximately 2 years. To optimize performance, a fully (or nearly fully) discharged battery should be charged as soon as possible.
Operating Modes
The HeartStart MRx has four clinical modes of operation, each with a customized display view. The modes are as follows:
Table 1 Operating Modes and Views
Mode of Operation |
Display View |
Description |
|
|
|
Monitor Mode |
Monitoring View, or |
Used to monitor ECG, take an optional 12-lead ECG, and monitor |
|
12-Lead View |
optional parameters such as SpO2, EtCO2, NBP, Invasive Pressures, |
|
|
Temperature and for viewing Vital Signs Trending data. |
AED Mode |
AED View |
Used to analyze ECG and if necessary, perform semi-automatic external |
|
|
defibrillation. Q-CPR available. |
|
|
|
Manual Defib Mode |
Code View |
Used to perform asynchronous and synchronous defibrillation |
|
|
(cardioversion). Q-CPR available. |
|
|
|
Pacer Mode |
Pacing View |
Used to perform demand or fixed mode pacing. |
|
|
|
NOTE Upon returning to a clinical mode from a non-clinical mode such as Configuration or Data Management, all settings reset to the default settings.
12
 Loading...
Loading...