Bio-Rad Experion Protein Analysis Kits User Manual

Experion™
Pro260 Analysis Kit
Instruction Manual
Catalog #700-7101

Bio-Rad Technical Support
For help and advice regarding products from the Experion™ automated electrophoresis system, please contact the Bio-Rad Technical Support department, which in the United States is open Monday–Friday, 5:00 AM–5:00 PM, Pacific Time.
Phone: 1-800-4BIORAD (1-800-424-6723)
Fax: 1-510-741-5802
Email: LSG_TechServ_US@bio-rad.com (for U.S. and international customers)
Online technical support and worldwide contact information are available at www.consult.bio-rad.com.
Legal Notices
No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage or retrieval system, without permission in writing from Bio-Rad Laboratories.
Bio-Rad reserves the right to modify its products and services at any time. This user guide is subject to change without notice.
Although prepared to ensure accuracy, Bio-Rad assumes no liability for errors, or for any damages resulting from the application or use of this information.
Bio-Lyte, Criterion, Experion, Precision Plus Protein, and ReadyPrep are trademarks of Bio-Rad Laboratories. B-PER is a trademark of Pierce. BugBuster is a trademark of Novagen. Coomassie and Pluronic are trademarks of BASF Aktiengesellschaft. Excel is a trademark of Microsoft Corporation. Triton is a trademark of Union Carbide. Tween is a trademark of ICI Americas, Inc. Zwittergent is a trademark of EMD Chemicals Inc.
The dyes in Experion kits are manufactured by Molecular Probes, Inc. and are licensed for research use only.
LabChip and the LabChip logo are trademarks of Caliper Life Sciences, Inc. Bio-Rad Laboratories, Inc. is licensed by Caliper Life Sciences, Inc. to sell products using the LabChip technology for research use only. These products are licensed under U.S. patents 5,863,753; 5,658,751; 5,436,134; and 5,582,977, and pending patent applications, and related foreign patents, for internal research and development use only in detecting,
quantitating, and sizing macromolecules, in combination with microfluidics, where internal research and development use expressly excludes the use of this product for providing medical, diagnostic, or any other testing, analysis, or screening services, or providing clinical information or clinical analysis, in any event in return for compensation by an unrelated party.
Copyright © 2010 Bio-Rad Laboratories, Inc.

|
Contents |
|
|
|
|
|
||
|
Chapter 1: Experion™ Pro260 Analysis Kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
. |
1. |
. . . . . . . . . . |
|||
|
1.1 |
Product Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
|
2 |
|
|
|
|
1.2 |
Kit Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
. |
3 |
|
|
|
|
1.3 |
Storage Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
|
3 |
|
|
|
|
1.4 |
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
. |
3 |
|
|
|
|
1.5 |
Additional Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
|
4 |
|
|
|
|
Chapter 2: Essential Practices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
. |
5. |
. . . . . . . . . . |
|||
|
2.1 |
Storing and Preparing Samples and Reagents . . . . . . . . . . . . . . . . . . |
. |
|
6 |
|
|
|
|
2.2 |
Priming and Loading the Chip . . . . . . . . . . . . . . . . . . . . . . . . |
. |
|
6 |
|
|
|
|
2.3 |
Running the Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
. |
7 |
|
|
|
|
2.4 |
General Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
. |
7 |
|
|
|
|
2.5 |
Experion Video Tutorials . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7 |
|
|
|||
|
Chapter 3: Experion Pro260 Assay Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
|
.9 |
. . . . . . . . . . |
|||
|
3.1 |
Set Up the Electrophoresis Station . . . . . . . . . . . . . . . . . . . . . . |
. |
10 |
|
|
||
|
3.2 |
Equilibrate the Kit Reagents . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
10 |
|
|
||
|
3.3 |
Filter the Gel and Prepare the Gel-Stain Solution . . . . . . . . . . . . . . . . . |
. |
10 |
|
|
||
|
3.4 |
Prepare the Sample Buffers . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
11 |
|
|
||
|
3.4.1 |
Reducing Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
11 |
|
|
||
|
3.4.2 |
Nonreducing Conditions . . . . . . . . . . . . . . . . . . . . . . . . . |
|
11 |
|
|
||
|
3.5 |
Prepare the Samples and the Pro260 Ladder . . . . . . . . . . . . . . . . . . |
. |
11 |
|
|
||
|
3.6 |
Prime the Chip . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
12 |
|
|
||
|
3.7 |
Load the Chip . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
13 |
|
|
||
|
3.8 |
Run the Pro260 Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
14 |
|
|
||
|
3.9 |
Clean the Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
15 |
|
|
||
|
3.10 Evaluate the Run . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
16 |
|
|
|||
|
Chapter 4: Data Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
17. . . . . . . . . . . |
|||||
|
4.1 |
Viewing Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
18 |
|
|
||
|
4.1.1 |
Managing Run Files and Project Folders in the Tree View . . . . . . . . . . . . |
. |
18 |
|
|
||
|
4.1.2 |
General Display Controls . . . . . . . . . . . . . . . . . . . . . . . . . |
|
19 |
|
|
||
|
4.1.3 |
Electropherogram View . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
19 |
|
|
||
|
4.1.4 |
Gel View . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
23 |
|
|
||
|
4.1.5 |
Results and Settings . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
23 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

4.2 |
Changing the Fluorescence Intensity Scale . . . . . . . . . . . . . . . . . . . |
. 24 |
4.3 |
Using Results and Settings to View and Annotate Data . . . . . . . . . . . . . . . |
25 |
4.4 |
Comparing Data from Different Runs . . . . . . . . . . . . . . . . . . . . . . |
26 |
4.5 |
Saving, Exporting, and Printing Data . . . . . . . . . . . . . . . . . . . . . . |
27 |
4.5.1 Saving Data Files . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
27 |
|
4.5.2Exporting Data Files to Other Applications . . . . . . . . . . . . . . . . . . . 27
4.5.3Printing Data Files . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Chapter 5: Protein Quantitation Methods . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
.29. . . . . . . . . . |
|
5.1 |
Protein Quantitation Methods . . . . . . . . . . . . . . . . . . . . . . . . . |
|
30 |
5.2 |
Performing Percentage Determination . . . . . . . . . . . . . . . . . . . . . |
. |
30 |
5.3 |
Performing Relative Quantitation . . . . . . . . . . . . . . . . . . . . . . . |
. |
31 |
5.4Performing Absolute Quantitation . . . . . . . . . . . . . . . . . . . . . . . . 32
5.4.1Absolute Quantitation Using the Upper Marker as Standard . . . . . . . . . . . . 32
5.4.2Absolute Quantitation Using a User-Defined Internal Standard . . . . . . . . . . . 34
Chapter 6: Changing Analysis Settings and Parameters . . . . . . . . . . . . . . . . . . . . |
. |
.35. . . . . . . . . . |
||
6.1 |
Designating and Searching for Specific Proteins . . . . . . . . . . . . . . . . . |
. |
36 |
|
6.2 |
Changing Protein Quantitation Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
36 |
|
6.3 |
Manually Setting a Marker . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
36 |
|
6.4 |
Excluding a Peak from Analysis . . . . . . . . . . . . . . . . . . . . . . . . |
|
36 |
|
6.5 |
Changing Peak Finding Parameters . . . . . . . . . . . . . . . . . . . . . . |
|
37 |
|
6.6 |
Changing General Settings . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
37 |
|
6.7 |
Baseline Modification . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
38 |
|
6.8 |
Turning Analysis Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
39 |
|
6.9 |
Manual Peak Integration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
40 |
|
Chapter 7: Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
. 41. . . . . . . . . . . |
||
7.1 |
Electrophoresis and Priming Stations . . . . . . . . . . . . . . . . . . . . . |
. |
42 |
|
7.2 |
Experion Pro260 Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
42 |
|
7.3 |
Contacting Technical Support . . . . . . . . . . . . . . . . . . . . . . . . . 46 |
|||
Appendices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. |
.47. . . . . . . . . . |
||
Appendix A: How the Experion System Works . . . . . . . . . . . . . . . . . . . . |
|
48 |
||
Appendix B: Deep Cleaning Procedure . . . . . . . . . . . . . . . . . . . . . . . |
|
53 |
||
Appendix C: Chemical Compatibility . . . . . . . . . . . . . . . . . . . . . . . . |
|
54 |
||
Appendix D: Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58 |
||||
Appendix E: Bibliography . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
60 |
||
Appendix F Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . |
|
61 |
||
|
|
|
|
|
|
|
|
|
|

1 Experion™ Pro260
Analysis Kit
1
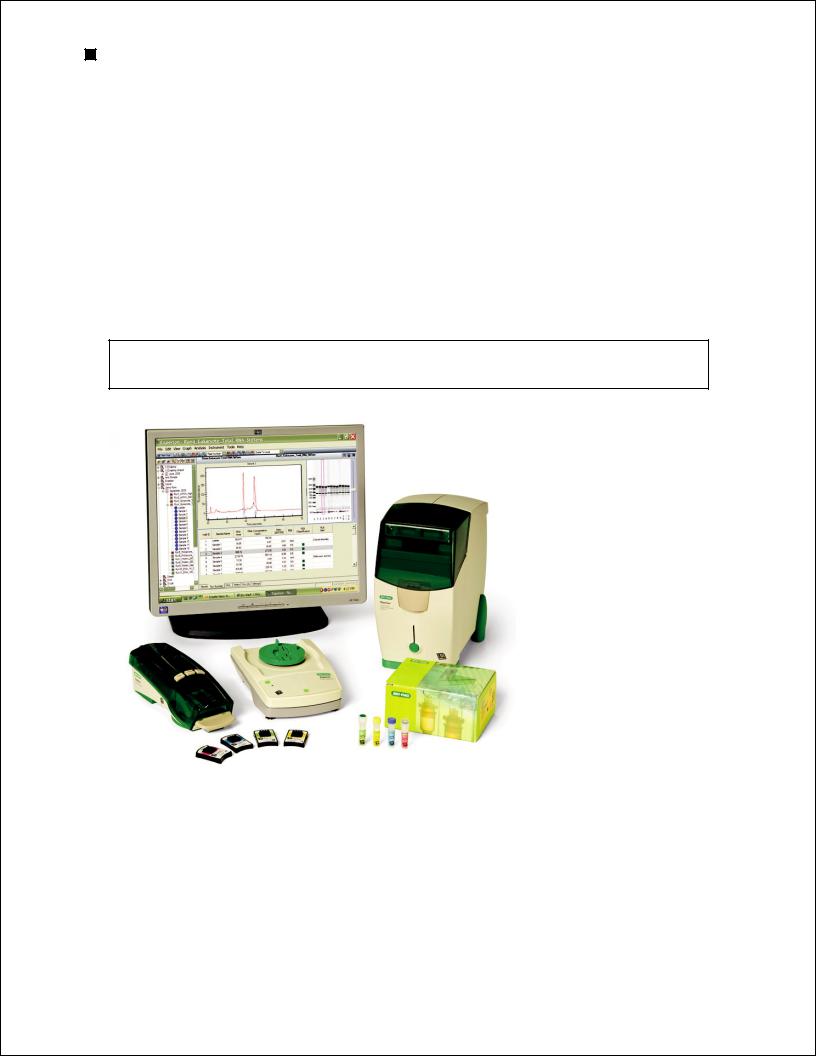
Experion Automated Electrophoresis System
1.1 Product Description
The Experion Pro260 analysis kit is used for protein analysis with the Experion automated electrophoresis system (Figure 1.1). The Experion system employs LabChip microfluidic technology to automate protein and nucleic acid electrophoresis by integrating separation, detection, and data
analysis within a single platform. The Experion automated electrophoresis system uses smaller sample and reagent quantities than standard analysis methods, and it accomplishes analysis in a single 30-minute, automated step.
The Experion Pro260 analysis kit supplies the microfluidic chips, reagents, and instructions required to separate and analyze 10–260 kD proteins under denaturing conditions. The sensitivity of the Experion Pro260 analysis kit is comparable to (though sometimes more sensitive than) colloidal Coomassie Blue staining of SDS-PAGE gels. Each Experion Pro260 chip can analyze up to 10 samples.
For details about how the Experion Pro260 kit analyzes proteins, refer to Appendix A in this manual.
Register your Experion system in order to ensure you receive important updates on software, tech notes, and manuals. Upon installation, a dialog will provide registration instructions.
4
1
2
3
5b
5a
Fig.. 1..1.. The Experion system.. The system includes the following components: 1) automated electrophoresis station, 2) priming station, 3) vortex station used for nucleic acid analysis only, 4) system operation and data analysis tools (software), and 5) analysis kits, which include the (a) chips and (b) reagents for protein (Pro260 kit), standardsensitivity RNA (StdSens kit), high-sensitivity RNA (HighSens kit), and DNA (DNA 1K and 12K kits) analyses.
2 Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com

Experion Pro260 Analysis Kit
1.2 Kit Components
Table 1.1. Components of the Experion Pro260 analysis kit.
Item |
Description |
Volume (per Vial) |
10-Chip Kit |
|
|
|
|
Pro260 chip |
Microfluidic chips used for protein separation |
|
10 chips |
Cleaning chip |
Chip used for cleaning electrodes |
|
1 chip |
Pro260 gel |
Proprietary polymeric sieving matrix |
520 µl |
3 vials |
Pro260 stain |
Proprietary fluorescent dye |
45 µl |
1 vial |
Sample buffer |
Buffer for protein sample preparation; contains a 1.2 kD |
400 µl |
1 vial |
|
lower marker and 260 kD upper marker for alignment of |
|
|
|
samples to the Pro260 ladder |
|
|
Pro260 ladder |
Protein standard containing 9 purified recombinant proteins |
60 µl |
1 vial |
|
of 10–260 kD and optimized for automated electrophoresis |
|
|
|
on the Experion system |
|
|
Spin filters |
Used for filtering reagents during sample preparation |
|
3 filters |
|
|
|
|
1.3 Storage Conditions
Table 1.2. Storage conditions.
Item |
Storage |
Shelf Life |
|
|
|
Experion Pro260 reagents |
4ºC |
See expiration date on packaging |
Experion Pro260 chips |
Ambient |
See expiration date on packaging |
Gel-stain solution (GS, prepared) |
4ºC |
1 month from filtration (can be refiltered once) |
Gel (G, prepared) |
4ºC |
1 month from filtration (can be refiltered once) |
|
|
|
1.4 Specifications
Number of sample wells per chip Sample volume required
Total run time Protein sizing range Limit of detection
Linear dynamic range
10
4 µl
~30 min per chip 10–260 kD
2.5 ng/µl carbonic anhydrase (10 ng total) in 1x PBS; similar to colloidal Coomassie Blue G-250 stain
5–2,000 ng/µl (bovine serum albumin in 1x PBS)
Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com |
3 |

Experion Automated Electrophoresis System
1.5 Additional Requirements
Experion automated electrophoresis station Experion priming station
Microcentrifuge (1,000–10,000 x g)
Heating block or water bath set at 95–100°C Benchtop vortexer
Aluminum foil
Calibrated pipets and narrow-bore tips (for example, VWR #87001-688 or Rainin #L-10F) Microcentrifuge tubes, 0.5 or 1.5 ml
Deionized water, 0.2 µm-filtered (ReadyPrep™ proteomics grade water, catalog #163-2091) β-Mercaptoethanol (catalog #161-0710)
Benchtop centrifuge (optional, catalog #166-0612)
Extra spin filter for additional filtration steps (as needed, catalog #700-7254) Experion electrode cleaner (catalog #700-7252)
4 Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com

2 Essential Practices
5

Experion Automated Electrophoresis System
2.1 Storing and Preparing Samples and Reagents
Store all Experion™ Pro260 reagents at 4°C when not in use. Do not store reagents at room temperature for >2 hr, as this will shorten their shelf life.
Before use, allow all kit reagents to equilibrate to room temperature (~15–20 min). Once thawed, gently vortex all kit reagents before use. Before opening the tubes, quickly centrifuge them to collect solution to the bottoms of tubes.
If the Pro260 gel has frozen, discard it.
Protect the stain, sample buffer, and gel-stain solution (GS) from light: store these solutions in a dark place and keep them covered with foil when using them.
The Pro260 stain contains DMSO, which is hygroscopic. Cap tightly.
Use GS and filtered gel (G) for up to 1 month. After that, GS and G can be refiltered once again.
Prepare samples in either reducing or nonreducing sample buffer, but always prepare Pro260 ladder in reducing sample buffer.
Do not use coated or treated pipet tips or microcentrifuge tubes (for example, siliconized polypropylene) for preparation of kit reagents or samples. Use of treated tips or tubes may cause separation artifacts.
Use 0.2 μm-filtered or ReadyPrep™ proteomics grade water. Do not use autoclaved water for sample or reagent preparation.
If possible, use sample protein concentrations (before preparation with the Pro260 sample buffer) that are near the middle of the linear dynamic range.
2.2 Priming and Loading the Chip
To avoid contamination, wear gloves and handle chips by the edges. Never touch the glass portions of the chip.
Load the chip on a benchtop or in the priming station. Never load a chip in the electrophoresis station.
Avoid sources of dust and other contaminants when preparing samples and loading the chip. Foreign particles in reagents, samples, or the wells of the chip interfere with separation. Remove chips from their packaging immediately before use.
It may be easier to load the chip on a white background. Tilt the chip to look for bubbles.
Use narrow-bore pipet tips for loading the chip (for example, VWR #87001-688 or Rainin #L-10F).
To avoid introducing air bubbles, do the following (for more help with chip loading, refer to the Experion Training Video in the Experion software Help section under Contents and Index > Contents > Appendices > Technical Videos):
nInsert the pipet tip all the way to the bottom of the chip well when dispensing liquids (this reduces the possibility of trapping air)
nHold the tip vertically, perpendicular to the chip surface. Holding the tip at an angle may trap air bubbles at the bottom of the well
nWhen expelling liquid, dispense slowly and only to the first stop on the pipet. Using the second stop introduces air and bubbles into the liquid. Reverse pipetting is acceptable
6Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com

Experion Pro260 Analysis Kit
Dislodge bubbles at the bottom of a well with a clean pipet tip, or remove the solution and load it again.
Use a primed and loaded chip within 5 min of loading. When chips are not used within this time, reagents may evaporate, leading to poor results or a chip performance error.
Fill all the chip wells when running an analysis. Use blank samples (prepared with water instead of sample) or replicates if necessary. All 16 electrode pins must be in contact with liquid; otherwise, an IV (current voltage) check failure error will occur.
2.3 Running the Analysis
Place the electrophoresis station on a stable surface, where it will not be subjected to vibrations or other movement, and away from direct sunlight and all other potential sources of extreme heat.
Power on the electrophoresis station before launching Experion software.
The first time that the Experion electrophoresis station is used, confirm that communication has been established between the software and electrophoresis station before preparing the reagents.
Do not open the lid of the electrophoresis station during a run. The run will abort if the lid is opened.
2.4 General Maintenance
For recommendations on general instrument maintenance, refer to the Experion system manual (bulletin 10001312).
Clean the electrodes after each run (routine cleaning). Cleaning maintains the instrument in optimum condition and prevents buildup and cross-contamination of reagents and samples.
Perform the deep cleaning procedure described in Appendix B to clean the electrodes:
nPrior to first use of the Experion electrophoresis station
nWhenever contamination is suspected or visible (for example, salt deposits or other precipitates) on the electrodes
nWhenever a chip has been left in the electrophoresis station for an extended period of time (for example, overnight)
Never store the cleaning chip inside the electrophoresis station. Store the empty cleaning chip covered to keep the wells clean. A new cleaning chip is included with every box of chips.
2.5 Experion Video Tutorials
For additional information, view the video tutorials available online at www.bio-rad.com:
North America: Home > Life Science Research > Support > Tutorials > Electrophoresis and Blotting > Experion System Training
Other: |
Home > Life Science Research > Electrophoresis > Automated Electrophoresis > |
|
Experion Training Videos |
Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com |
7 |

Experion Automated Electrophoresis System
8 Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com

3 Experion™ Pro260 Assay
Procedure
For an abbreviated version of this protocol, refer to the Quick Guide provided with the kit.
9

Experion Automated Electrophoresis System
3.1 Set Up the Electrophoresis Station
1.If needed, perform a deep cleaning of the electrodes (see Appendix B for instructions).
2.Power on the computer and then power on the Experion electrophoresis station by pushing the green button in the center of the front panel. The steady green LED above the button indicates that the unit is on.
3.Launch Experion software. If the instrument and computer are communicating properly:
nA green dot and the last 4 digits of the instrument serial number appear in the lower right corner of the software screen
nThe electrophoresis station icon appears in the upper left corner
When there is no connection, these indicators are absent and a grayed-out instrument icon appears in the upper left corner of the software screen.
3.2 Equilibrate the Kit Reagents
1.Set a heating block or water bath to 95–100°C. You will use this heating block to denature the samples and Pro260 ladder later in the protocol.
2.Equilibrate the following kit reagents to room temperature for ~15–20 min:
nPro260 stain (blue cap)
nPro260 sample buffer (yellow cap)
nPro260 ladder (red cap)
n2 tubes Pro260 gel (green cap)
3.Vortex the contents of each tube and briefly centrifuge the solutions to the bottoms of the tubes. Make sure the Pro260 stain solution (blue cap) is thawed before proceeding.
If the gel-stain solution (GS) and filtered gel (G) were prepared previously, equilibrate them to room temperature. Use the GS and G within 1 month of preparation. After 1 month, refilter them before use. Keep both the G and GS at room temperature and covered until ready for use.
3.3 Filter the Gel and Prepare the Gel-Stain Solution
1.Prepare the GS by adding 20 µl Pro260 stain (blue cap) to a tube of Pro260 gel (green cap, 520 µl). Vortex the GS for 10 sec at the highest setting and then spin it down briefly in a microcentrifuge.
2.Transfer the GS to a spin filter, and transfer the contents of the other Pro260 gel (green cap) into another spin filter. Label and date the tubes.
3.Centrifuge both spin filters for 5 min at 10,000 × g. Inspect the tubes to ensure all of the gel passed through the filters, and then discard the filters. Cover the GS with foil.
10 Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com
Experion Pro260 Analysis Kit
3.4 Prepare the Sample Buffers
Separate protein samples under either reducing or nonreducing conditions, but always use reducing conditions for separation of the Pro260 ladder. Reduced and nonreduced samples can be run on the same chip. Prepare fresh sample buffer daily.
3.4.1 Reducing Conditions
For each chip, combine 1 µl β-mercaptoethanol and 30 µl sample buffer (yellow cap). Vortex and spin down briefly. Protect the sample buffer from light.
At pH <7, reducing agents such as β-mercaptoethanol and DTT are less effective; use 5–7.5 mM tributylphosphine (TBP) or tris(2-carboxyethyl)phosphine (TCEP) instead. Otherwise, neutralize the buffer or use buffers of higher pH before preparing samples for Experion runs.
3.4.2 Nonreducing Conditions
Prepare two stocks of sample buffer: one reducing (for the Pro260 ladder) and one nonreducing (for the protein samples).
1.For each chip, transfer 30 µl sample buffer (yellow cap) to 2 microcentrifuge tubes.
2.Add 1 µl β-mercaptoethanol to one tube (reducing sample buffer) and 1 µl deionized water to the other (nonreducing sample buffer). This generates enough nonreducing sample buffer for
10 samples. Vortex the tubes and cover them with foil to protect them from light.
3.5 Prepare the Samples and the Pro260 Ladder
Each chip can analyze up to 10 protein samples. All wells of a chip must be filled for the electrophoresis station to operate properly.
The total protein concentration, including any added user-defined internal standards, in all starting samples should be toward the middle of the linear dynamic range of the assay, if possible.
1.Prepare the Pro260 ladder by combining 4 µl Pro260 ladder (red cap) and 2 µl sample buffer with β-mercaptoethanol (reducing sample buffer) in a microcentrifuge tube.
2.Prepare the samples by combining 4 µl sample and 2 µl sample buffer (Section 3.4) in a microcentrifuge tube.
3.Vortex all tubes briefly and spin down in a microcentrifuge for a few sec.
4.Heat the samples and Pro260 ladder at 95–100°C for 3–5 min. Spin down the tubes in a microcentrifuge for a few sec.
5.Add 84 µl deionized water to each tube and vortex briefly to mix. Do not modify this step to adjust sample concentration.
Both the diluted samples and the Pro260 ladder are stable for several hours when stored at room temperature and protected from light.
Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com |
11 |

Experion Automated Electrophoresis System
3.6 Prime the Chip
Start the run within 5 min of priming and loading the chip. For help with chip loading, refer to the Experion Training Video: Chip Loading, available in the Experion software Help section under
Contents and Index > Contents > Appendices > Technical Videos.
1.Pipet 12 µl GS into the top right well of the chip (highlighted and labeled GS, gel priming well) (Figure 3.1). Insert the pipet tip vertically and to the bottom of the well when dispensing. Dispense slowly to the first stop on the pipet, and do not expel air at the end of the pipetting step.
2.On the priming station, set the pressure setting to B and the time setting to 3, as specified by the alphanumeric code on the chip (Figure 3.1).
Gel priming well
Priming code
Fig.. 3..1.. Experion Pro260 chip.. The locations of the gel priming well (GS, highlighted) and alphanumeric priming code are indicated.
3.Open the Experion priming station and place the chip on the chip platform, matching the arrow on the chip with the alignment arrow on the chip platform. A post on the chip prevents insertion in the wrong position. Do not force the chip into position.
4.Close the priming station by pressing down on the lid. The lid should snap closed.
5.Press Start. A “Priming” message appears on the screen of the priming station, and the timer counts down. Priming requires approximately 60 sec. Do not open the priming station during countdown.
6.An audible signal and “Ready” message indicate that priming is complete. Open the priming station and remove the chip. If the lid sticks, press down on it while pressing down on the release lever.
7.Turn the chip over and inspect the microchannels for bubbles or evidence of incomplete priming. If the chip is primed properly, the microchannels will be difficult to see (it may be helpful to compare a primed chip to a new, unused chip). If you detect a problem, such as a bubble or incomplete priming, prime a new chip.
8.Place the chip on a clean surface for loading.
Bubbles forced into microchannels during priming take the shape of the microchannel and are elongated, not round.
12 Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com

Experion Pro260 Analysis Kit
3.7 Load the Chip
1.Using a pipet, remove and discard any remaining GS from the gel priming well. Pipet 12 µl GS into all 4 wells labeled GS (including the gel priming well, Figure 3.2).
2.Pipet 12 µl filtered gel (G) into the well labeled G (Figure 3.2).
3.Pipet 6 µl of each diluted sample into sample wells 1–10.
4.Pipet 6 µl diluted Pro260 ladder into the ladder well labeled L (Figure 3.2). Use the Pro260 ladder within 8 hr of preparation. Every chip must have Pro260 ladder loaded into the ladder well labeled L.
Load 6 µl sample into wells 1–10 |
Load 12 µl GS into all 4 wells labeled GS |
Load 12 µl G into well labeled G
Load 6 µl ladder into well labeled L
Fig.. 3..2.. Experion Pro260 chip.. Wells for loading GS, G, samples, and ladder are indicated.
5.Inspect all wells for bubbles by holding the chip above a light-colored background and looking through the wells (Figure 3.3). Dislodge any bubbles at the bottom of a well with a clean pipet tip or by removing and reloading the solution.
6.Place the loaded chip into the Experion electrophoresis station and start the run within 5 min.
Fig.. 3..3.. Bubble formation during loading of Experion Pro260 chips.. Surface bubbles do not generally cause problems during a run, but bubbles at the bottoms of wells must be removed. Left, bubbles trapped at the bottom of wells. The GS and G wells and sample wells 1, 3, and 4–6 contain no solution. Wells 8, 10, and L are filled properly and have no bubbles, but large bubbles have formed at the bottoms of wells 7 and 9 (note the difference in the diameter of the light-colored circles in wells 8 and 9). Right, bubbles have formed at the surface of the three GS wells on the right side of the chip; the rest of the wells have no bubbles.
Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com |
13 |

Experion Automated Electrophoresis System
3.8 Run the Pro260 Analysis
1.Open the lid of the electrophoresis station by pulling the release latch. Place the primed and loaded chip on the chip platform and close the lid.
2.In the Experion software toolbar, click New Run  . In the New Run screen (Figure 3.4), from the Assay pull-down list, select Protein > Protein 260.
. In the New Run screen (Figure 3.4), from the Assay pull-down list, select Protein > Protein 260.
3.Either select a project folder for the run from the Project pull-down list or create a new project folder by entering a name in the Project field or by selecting File > Project > New. The project folder appears in the project tree.
4.Enter a name for the run in the Run Prefix field and click Start Run  .
.
Fig.. 3..4.. Details of the New Run screen.. The green dot in the lower right corner indicates that communication between the electrophoresis station and Experion software has been established.
5.In the New Run dialog (Figure 3.5), select the number of samples to be analyzed. Though all wells are filled, the Experion system stops the analysis when it reaches the number of samples entered.
Fig.. 3..5.. New Run dialog.. The Experion system stops analysis when it reaches the number of samples entered.
14 Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com

Experion Pro260 Analysis Kit
6.Click Start. The green LED in the center of the front panel on the electrophoresis station blinks, and the system performs a number of checks: it confirms that a chip has been inserted, that all wells contain liquid, that electrical connections are made, etc. A calibration counter marks the progress of these calibrations at the upper right of the screen.
Do not open the lid of the Experion electrophoresis station until the run is complete. The lid does not lock. Opening the lid aborts the run.
An “IV Check Error” message indicates the system cannot make electrical contact in one of the wells. This often means there is a bubble at the bottom of the well. Abort the run, and check the chip for bubbles or empty wells. Refill the affected well(s), and start the run again.
7.During separation, the sample name is highlighted in the project tree and the electropherogram trace, and virtual gel bands appear in real time:
nThe electropherogram of the sample being separated appears in the electropherogram view
nThe lane corresponding to that sample is outlined in pink and has a dark background
To display the electropherogram of another sample, click on either the sample name in the project tree or on a lane in the virtual gel.
8.When analysis is complete (after ~30 min), the instrument beeps and a window opens indicating the end of the run. Select OK and remove the chip from the chip platform.
9.Clean the electrodes using deionized water within 30 min of each Pro260 chip run.
3.9 Clean the Electrodes
1.Fill a cleaning chip with 800 µl deionized water (0.2 µm-filtered). Gently tap the side of the cleaning chip to remove any trapped bubbles from the wells.
2.Place the cleaning chip on the chip platform in the electrophoresis station, close the lid, and leave it closed for 1 min.
Never store the cleaning chip inside the electrophoresis station. Store the empty cleaning chip covered to keep the wells clean. A cleaning chip is included with each box of chips.
3.Open the lid, remove the cleaning chip, and allow the electrodes to dry for 1 min. Close the lid.
4.Replace the water in the cleaning chip after use to avoid contamination. For storage, remove the water from the cleaning chip and store the chip in a clean location.
Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com |
15 |

Experion Automated Electrophoresis System
3.10 Evaluate the Run
When a run is complete, evaluate the run and the analysis of the data by Experion software.
1.Ensure all lanes (ladder and samples) are visible in the virtual gel. The markers (indicated by pink triangles) should be visible in and aligned across all lanes. If the marker peaks are not properly assigned, you may need to include or exclude peaks, or manually set the marker (see Section 6.3).
2.Evaluate the separation of the Pro260 ladder. To display the ladder electropherogram, click the ladder well in the project tree, or click on the lane labeled L in the virtual gel. The electropherogram should resemble the one shown in Figure 3.6 and should have the following features (if your ladder does not have these features, see Chapter 7, Troubleshooting for more information):
nTwo marker peaks and a set of system peaks
nEight Pro260 ladder peaks between the system peaks and upper marker
nFlat baseline
nMarker peaks at least 20 fluorescence units above the baseline
Pro260 ladder peaks
Lower marker
System peaks
Upper marker
Fig.. 3..6.. Separation of the Pro260 ladder.. Note the flat baseline and well-resolved peaks. All identified peaks are numbered, and the lower and
Baseline upper markers are indicated by green asterisks (*). The 260 kD protein in the Pro260 ladder is labeled as the upper marker in the Results table.
3.Examine the separation of at least one sample. Click on the sample name in the project tree or on the lane in the virtual gel to view the electropherogram, which should have the following features:
nTwo well-resolved marker peaks and a set of system peaks
nSample peaks located between the system peaks and upper marker
nFlat baseline
If the upper and lower marker peaks are not properly assigned, you may need to include or exclude peaks, or manually set the marker (see Section 6.3).
4.Evaluate the data analysis performed by Experion software (see Chapter 4, Data Analysis). If necessary, change the analysis settings and parameters — including protein quantitation methods
— by following the instructions in Chapters 5 and 6.
16 Technical Support: 1-800-4BIORAD • 1-800-424-6723 • www.bio-rad.com

4 Data Analysis
This chapter outlines the basic steps and software features used to view and analyze data. For a more detailed description of Experion™ software and its various functions, refer to the software Help menu. For information on how to customize analysis parameters, refer to Chapter 6, Changing Analysis Settings and Parameters.
17
 Loading...
Loading...