Bio-Rad 491 Prep Cell and Mini Prep Cell User Manual

Model 491 Prep Cell
Instruction Manual
For Technical Service call your local Bio-Rad office or in the U.S. call 1-800-424-6723.

Note
To ensure the best performance from the Model 491 prep cell, become fully acquanted with these operating instructions before using the cell to transfer samples. Bio-Rad recommends that you first read these instructions carefully. Then assemble and disassemble the cell completely without transferring sample. After these preliminary steps, you should be ready to transfer a sample.
Bio-Rad also recommends that all Model 491 prep cell components and accessories be cleaned with a suitable laboratory cleaner (such as Bio-Rad cleaning concentrate, catalog number 161-0722) and rinsed thoroughly with distilled water, before use.
Model
Catalog No.
Date of Delivery
Serial No.
Invoice No.
Purchase Order No.
Warranty
Bio-Rad Laboratories’ Model 491 prep cell is warranted against defects in materials and workmanship for 1 year from date of purchase. If any defects occur in the instrument during this warranty period, Bio-Rad Laboratories will repair or replace the defective parts free of charge. The following defects, however, are specifically excluded.
1.Damage caused by improper operation, accident, or misuse.
2.Repair or modification done by anyone other than Bio-Rad Laboratories.
3.Use of fittings or other parts supplied by anyone other than Bio-Rad Laboratories.
Note: This warranty does not apply to platinum wire electrodes.
For any inquiry or request for repair service, contact Bio-Rad Laboratories after confirming the model and serial number of your instrument.
|
Table of Contents |
|
Section 1 |
General Information.................................................................................. |
1 |
1.1 |
Introduction................................................................................................ |
1 |
1.2 |
Accessory Equipment................................................................................. |
1 |
1.3 |
Specifications............................................................................................. |
2 |
1.4 |
Chemical Compatability.............................................................................. |
2 |
1.5 |
Safety......................................................................................................... |
2 |
Section 2 |
Description of Major Components.......................................................... |
4 |
2.1 |
Model 491 Prep Cell Components.............................................................. |
4 |
2.2 |
Lower and Upper Buffer Chamber.............................................................. |
5 |
2.3 |
Cooling Core.............................................................................................. |
5 |
2.4 |
Gel Tube Assembly..................................................................................... |
6 |
2.5 |
Elution Chamber Base................................................................................ |
6 |
2.6 |
Casting Stand............................................................................................. |
7 |
Section 3 |
Assembly and Operation.......................................................................... |
8 |
3.1 |
Casting the Preparative Gel........................................................................ |
8 |
3.2 |
Preparing the Frits and Dialysis Membrane............................................... |
10 |
3.3 |
Assembly of the Elution Chamber............................................................. |
10 |
3.4 |
Assembly of Upper and Lower Buffer Chambers...................................... |
12 |
3.5 |
Purge the Elution Chamber of Air Bubbles................................................ |
14 |
3.6 |
Cooling the Gel......................................................................................... |
15 |
3.7 |
Loading the Sample.................................................................................. |
16 |
3.8 |
Elution Rate, Detection, Collection, and Analysis....................................... |
16 |
Section 4 |
Optimizing Running Conditions for Preparative SDS-PAGE................ |
17 |
4.1 |
Gel Pore Size............................................................................................ |
17 |
4.2 |
Optimization Procedures........................................................................... |
18 |
4.3 |
Gel Length Determination for Preparative SDS-PAGE............................... |
19 |
4.4 |
Gel Tube Size Selection............................................................................ |
19 |
4.5 |
Running Conditions.................................................................................. |
21 |
4.6 |
Elution Times for Proteins......................................................................... |
21 |
4.7 |
Examples of the SDS-PAGE Optimization Procedure................................ |
22 |
Section 5 |
Disassembling and Cleaning................................................................. |
26 |
Section 6 |
Troubleshooting Guide........................................................................... |
27 |
Section 7 |
Preparation of Electrophoresis Buffers and Acrylamide |
|
|
Stock Solutions for SDS-PAGE.............................................................. |
28 |
7.1 |
Separating (Resolving) Gel Buffer Stock.................................................... |
28 |
7.2 |
Stacking Gel Buffer Stock......................................................................... |
28 |
7.3 |
Sample Buffer (SDS-Reducing Buffer) Stock............................................. |
28 |
7.4 |
10x Electrode (Running) Buffer, pH 8.3..................................................... |
29 |
7.5 |
30% Acrylamide Stock Solution................................................................ |
29 |
Section 8 |
SDS-PAGE Gel Preparation................................................................... |
30 |
8.1 |
Gel Recipes.............................................................................................. |
30 |
8.2 |
Analytical Separating Gel.......................................................................... |
31 |
8.3 |
Analytical Stacking Gel............................................................................. |
31 |
8.4 |
Preparative SDS-PAGE Separating and Stacking Gels ............................. |
32 |
Section 9 |
A Guide to Preparative Native-PAGE..................................................... |
33 |
9.1 |
Introduction.............................................................................................. |
33 |
9.2 |
How to Choose Native-PAGE Systems..................................................... |
33 |
9.3 |
Discontinuous Native-PAGE...................................................................... |
36 |
9.4 |
Continuous Native-PAGE.......................................................................... |
39 |
9.5 |
References............................................................................................... |
42 |
Section 10 |
Ordering Information.............................................................................. |
43 |
Section 1
General Information
1.1 Introduction
The Model 491 prep cell* is designed to purify proteins or nucleic acids from complex mixtures by continuous-elution electrophoresis. Conventional gel electrophoresis buffer systems and media are used with the prep cell.
During a run, samples are electrophoresed through a cylindrical gel. As molecules migrate through the gel matrix, they separate into ring shaped bands. Individual bands migrate off the bottom of the gel where they pass directly into the patented elution chamber for collection.
The elution chamber consists of a thin polyethylene frit. A dialysis membrane, directly underneath the elution frit, traps proteins within the chamber. Elution buffer enters the chamber around the perimeter of a specially designed gasket. The unique design of the gasket results in an even flow of buffer into the elution frit. Buffer is drawn radially inward to an elution tube in the center of the cooling core. Purified molecules are drawn up through the elution collection tube at the center of the cooling core by a peristaltic pump. The peristaltic pump drives separated proteins through a UV monitor (optional) to a fraction collector (Bio-Rad's Econo™ system).
To assure that separated molecules migrate in compact, parallel bands, temperature gradients across the gel are minimized. The temperatures of the internal and external surfaces of the gel are equalized by continuously pumping lower electrophoresis buffer through the central cooling core by means of the buffer recirculation pump.
Simple procedures are provided for determining optimal running conditions for most purifications. It is recommended that these procedures be performed for each new sample to be purified before proceeding to a preparative run with the Model 491 prep cell.
1.2 Accessory Equipment
Power supply — 500 volt
Warning: Use only power supplies with isolated ground such as Bio-Rad's PowerPac™ HV or PowerPac Universal
Buffer recirculation pump — (provided with the prep cell
Peristaltic pump (elution pump)*
Fraction collector*
UV monitor
Chart recorder**
*U.S. patent number 4,877,510
**The use of a reliable fraction collector is essential for the isolation of the desired component of the sample. It may also be convenient to monitor elution by UV absorbance. To simplify setup and operation of accessory equipment, we recommend use of Econo system low pressure chromatography components, including peristaltic pump, fraction collector, UV monitor, and chart recorder.
1

1.3 Specifications
Construction |
|
Upper buffer chamber |
acrylic |
Lower buffer chamber |
acrylic |
Electrodes |
platinum, 0.010 inch diameter |
Lid |
acrylic |
Gel tube assembly |
glass/acrylic |
Elution chamber base |
acrylic |
Elution frit |
polyethylene |
Support frit |
polyethylene |
Cooling core |
glazed alumina |
Elution tube |
borosilicate glass, 0.06” ID |
Casting stand |
acrylic |
Shipping weight |
6 lb |
Overall size |
7 in. diameter x 14 in. high |
Voltage limit |
500 volts |
Current limit |
40 milliamperes |
Power limit |
20 watts |
Cooling buffer flow rate |
100 ml/min |
Elution buffer flow rate |
60 ml/hour |
Upper electrophoresis buffer volume |
300–600 ml |
Elution buffer chamber volume |
900 ml |
Lower electrophoresis buffer volume |
2–3 L |
1.4 Chemical Compatibility
The Model 491 prep cell is not compatible with chlorinated hydrocarbons (e.g. chloroform), aromatic hydrocarbons (e.g. toluene, benzene), or acetone. Their use will void all warranties.
1.5 Safety
Power to the Model 491 prep cell is to be supplied by an external DC power supply. This power supply must be ground isolated in such a way that the DC voltage output floats with respect to ground. The recommended power supply for this instrument is the PowerPac HV power supply. The PowerPac universal power supply may also be used. The maximum specified operating parameters for the Model 491 prep cell are:
500 VDC |
|
maximum operating voltage |
|
||
40 mA |
|
maximum operating current |
20 W |
|
maximum operating power limit |
Current to the Model 491 prep cell, provided from the external power supply, enters the unit through the lid assembly, providing a safety interlock to the user. Current flow to the cell is broken when the lid assembly is removed. Do not attempt to circumvent this safety interlock, and always turn the power supply off when working with the cell.
2
The buffer recirculation pump is also ground isolated as should be any pump used with this cell. During normal operation, the buffer in the lower buffer chamber is circulated through the cooling core and routed back into the system via the buffer recirculation pump. The buffer flowing through the tubing and the pump is electrically active. For this reason handle the tubing carefully while the power supply is on. Do not touch any exposed liquid when the power supply is on. Tube connections should be made with the power supply turned off. Both the recirculation pump and the recommended power supplies are ground isolated by design to minimize the potential shock hazard. However, working around high voltage equipment in a laboratory environment is potentially dangerous. As a result it is the user’s responsibility to always excercise care in setting up and running electrophoresis instruments. If a liquid leak occurs, always turn off the power supply before correcting the problem.
During operation, do not expose the cell to ambient temperatures above 50 ˚C.
Important
This Bio-Rad instrument is designed and certified to meet IEC1010-1* safety standards. Certified products are safe to use when operated in accordance with the instruction manual. This instrument should not be modified or altered in any way. Alteration of this instrument will:
•Void the manufacturer's warranty
•Void the IEC1010-1 safety certification
•Create a potential safety hazard
Bio-Rad is not responsible for any injury or damage caused by the use of this instrument for purposes other than for which it is intended or by modifications of the instrument not performed by Bio-Rad or an authorized agent.
*IEC1010-1 is an internationally accepted electrical safety standard for laboratory instruments.
3

Section 2
Description of Major Components
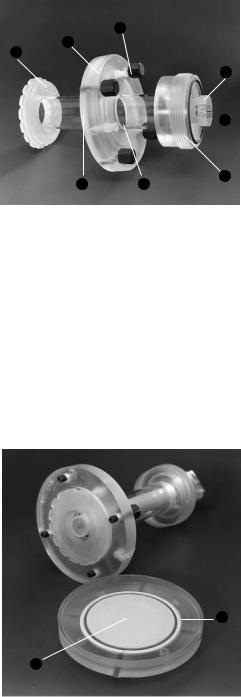
2.1 Model 491 Prep Cell Components
Lid & power cables
Cooling buffer outlet
Elution buffer outlet
Upper buffer chamber
Cooling core
Elution buffer feedline
Gel assembly tube
Elution chamber cap
Fluted gasket
Elution frit
Dialysis membrane Elution chamber
Support frit
O-ring
Elution chamber base
Lower buffer chamber
Cooling buffer inlet
Fig. 1. Exploded view of the Model 491 prep cell.
4

2.2 Lower and Upper Buffer Chamber
The lower buffer chamber forms a stable base for the unit. It houses the anode and contains the lower electrophoresis buffer. The upper buffer chamber holds the upper electrophoresis buffer and the elution buffer, and houses the cathode.
3 4
6
8 |
5 |
|
|
|
7 |
1 |
|
|
2 |
Fig. 2. Upper and lower buffer chamber. Lower buffer chamber: Stopcock for lower electrophoresis buffer inlet (1), and anode (2). Upper buffer chamber: Elution buffer reservoir (3), upper electrophoresis buffer reservoir (4), ring nut (5), cooling buffer line (6), cooling
buffer feedline (7), and elution buffer feedline (8).
2.3 Cooling Core
The cooling core extends to the bottom of the graduated gel tube and houses the elution tube in its center. Cooling is recommended during polymerization as well as during electrophoresis.
1 |
|
2 |
|
3 |
4 |
Fig. 3. Cooling core. Cooling buffer ports (1), elution buffer outlet (2), cooling core placement guides (3), and glazed ceramic cooling core (4).
5
2.4 Gel Tube Assembly
The gel tube assembly holds both the gel and the cooling core. The elution chamber cap and the gasket mounted on the graduated gel column make up the upper part of the elution chamber. Two gel tube assemblies are provided with the Model 491 prep cell: 28 mm ID and 37 mm ID. See Section 4.4 for selecting the appropriate gel tube size for specific applications.
5
4
7
1
 8
8
3 |
6 |
2 |
|
Fig. 4. Gel tube assembly. Cooling core collar (1), upper reservoir attachment, i.e. threaded connector (2), graduated gel column (3), elution chamber cap with thumb screws (4), elution buffer inlet port (5), cooling buffer port (6), gasket (7), and O-ring (8).
2.5 Elution Chamber Base
The elution chamber base with the support frit holds the elution frit and the dialysis membrane directly beneath the gel. The dialysis membrane provided with the Model 491 prep cell has a molecular weight cut off of 6,000 daltons. A continuous flow of elution buffer is directed through the channels of the elution chamber gasket to the perimeter of the elution frit. As bands migrate off the gel, they are washed to the center of the frit, up through the elution tube in the center of the cooling core, and out to the peristaltic pump, UV detector (optional), and fraction collector. (The tracking dye, bromophenol blue (Mr 691), does not pass through the dialysis membrane. The flow of elution buffer through the elution frit overcomes the force of electrophoresis in the downward direction.)
2
1
Fig. 5. Elution chamber base and tube assembly. Elution frit (1) and large O-ring (2).
6
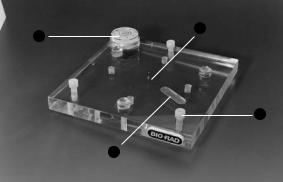
2.6 Casting Stand
Gels are cast with the gel tube assembly mounted directly on the casting stand. The casting stand ensures that gels have perfectly flat lower surfaces. Inserting a spatula in the gel-release slot facilitates the removal of the gel from the casting stand after polymerization by allowing air to enter beneath the gasket and the gel.
2
3
1
4
Fig. 6. Casting stand. Leveling feet (1), center pin (2), leveling bubble (3), and gel release slot (4).
7

Section 3
Assembly and Operation
3.1 Casting the Preparative Gel
1.Place the gel tube assembly on the casting stand, aligning the four screws on the acrylic plate with the holes in the casting stand. Secure the gel tube assembly with the four screws; hand tightening is sufficient. Level the casting stand with the aid of the leveling bubble using the leveling legs.
2.Insert the cooling core so that the two placement guides slide through the grooves of the cooling core collar in the gel tube assembly and the center pin on the casting stand is inserted in the elution tube of the cooling core. Turn the core 90° until it locks into place. This will prevent any vertical or lateral movement of the cooling core.
8
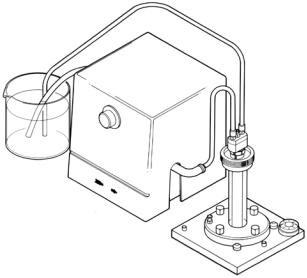
3.It is advisable to cool the gel during polymerization. Cooling prevents excess heat accumulation in the interior of the reaction mixture and aids in the formation of uniform gels. To cool, pump room temperature water (or buffer) from an external source through the cooling core. Ensure that cooling is in progress prior to casting the gel.
Fig. 7. Diagram of cooling path during polymerization. Cooling of gels during polymerization is recommended. This is accomplished by circulating room temperature buffer between a reservoir and the cooling finger using the buffer recirculation pump.
4.Prepare the acrylamide monomer solution. Refer to Section 4 for selecting the appropriate acrylamide gel concentration for a given application. Sections 8 and 9 describe preparative gel formulations for SDS-PAGE and native-PAGE, repsectively.
5.Pour the degassed monomer mixture into the gel tube through the gap between the cooling core and the collar of the gel tube assembly. Avoid trapping air bubbles in the gel. Gently tapping the casting stand (with the gel tube assembly mounted to it) against the bench top will help to dislodge trapped air bubbles. Visually inspect the gel for bubbles immediately after pouring the separating-gel solution into the tube.
Carefully overlay the resolving gel with water-saturated 2-butanol or tert-amyl alcohol using the narrow polytetrafluoroethylene (PTFE) tube affixed to a syringe (provided with the unit). Allow the resolving gel to stand overnight for complete polymerization (catalyst concentration in resolving gel is 0.025% APS/0.025% TEMED). After 1–2 hours polymerization, replace the alcohol overlay with gel buffer. In the case of SDS-PAGE and Ornstein-Davis nondenaturing gels, this should be 0.375 M Tris/Cl, pH 8.8 buffer.
6.Very carefully decant or aspirate the buffer overlay. Cast the stacking gel, approximately twice the sample volume, on top of the resolving gel. Overlay the stacking gel monomer with water-saturated 2-butanol or tert-amyl alcohol. Allow the stacking gel to polymerize for 1–2 hours.
9

3.2 Preparing the Frits and Dialysis Membrane
Soak the elution manifold support frit, elution frit, and dialysis membrane in buffer. The frits must be completely wetted prior to use. To ensure removal of entrapped air in the pores of the frits, place the container in which the frits are soaking in a vacuum chamber for approximately 10 minutes. Alternatively, the frits can be soaked in buffer overnight to completely wet them. To maintain the wetting of the frits, store them in buffer.
The dialysis membrane provided with the Model 491 prep cell has a molecular weight cut off of 6,000 daltons. Dialysis membranes with other pore sizes may be substituted for those provided. The dialysis membrane must be soaked in buffer before use and stored in buffer or water between uses. If the membrane becomes dry between runs, discard it. A properly stored dialysis membrane can be used at least for 5-6 runs. Prior to each run, inspect the membrane carefully. Discard it if any holes or tears are detected.
3.3 Assembly of the Elution Chamber
1.Insert the soaked support frit into the base of the elution chamber. The stepped support frit is the thicker of the two frits. Press the frit all the way into the base to form a flat surface on which to place the dialysis membrane. Place the dialysis membrane on the support frit and the elution frit on the dialysis membrane. Press the large elution O-ring in the groove around the perimeter of the base.
10
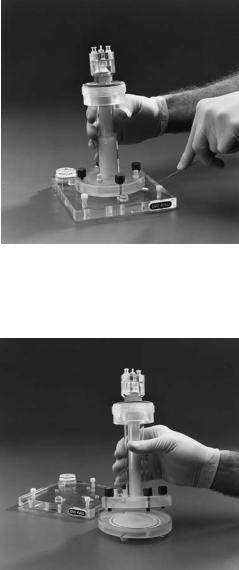
2.Decant the stacking gel overlay, rinse the surface of the stacking gel with water, and loosen the four screws holding the column to the casting stand. Carefully remove the gel tube assembly from the casting stand. Insert a spatula into the gel release slot and use it to gently pry the gel tube assembly off the casting stand. Inspect the lower surface of the gel to make sure it is smooth. Trapped air bubbles may cause a pitted gel surface which will result in uneven elution of proteins from the gel. If a pitted or otherwise uneven gel surface is observed, pour a new gel.
3.Place the gel tube assembly containing the gel on the elution chamber base. Align the four screws with the holes in the elution chamber base and hand tighten them.
11
 Loading...
Loading...