GE OEC UroView 2800, OEC 9800 Plus Digital, OEC MiniView 6800, OEC FlexiView 8800 Mobile C-Arm User manual

GE Healthcare
Pete McCabe
President and CEO
GE Healthcare, Surgery
384 Wright Brothers Drive
Salt Lake City, Utah 84116
U.S.A.
Pete.mccabe@med.ge.com
Certified Mail Return Receipt Requested
URGENT RECALL NOTICE
PLEASE TAKE ACTION TO INFORM ALL USERS OF THE RELEVENT SYSTEM(S) OF
THESE ISSUES AND HOW TO ADDRESS THEM
February 21 2007 |
|
To: |
Hospital Administrator |
|
Director/Manager of Radiology |
Subject: |
Product Safety Issues |
Affected Products: |
OEC UroView 2800, OEC 9800 Plus Digital, OEC MiniView 6800, OEC |
|
FlexiView 8800 Mobile C-Arm |
Our records indicate that your facility has one or more of the following GE Healthcare OEC (GEHC OEC) devices that has received a replacement hard disk drive:
OEC UroView 2800OEC 9800 Plus DigitalOEC MiniView 6800
OEC FlexiView 8800 Mobile C-Arm
GEHC OEC has discovered an issue with the hard disk drives that were installed on the above listed products during service calls. The manufacture dates of the affected hard disk drives are between October 15, 2004 and December 8, 2004
This issue could potentially result in the system failing to store images when the hard disk drive capacity exceeds 170 images instead of the expected 400 images. Systems may also fail to boot or lose patient data as this image capacity is approached.
Page 1 of 2
DOC0287403
Your GEHC OEC Field Service Engineer will be contacting your facility to arrange to have a replacement hard disk drive installed on your system.
Until a GEHC OEC Field Service Engineer has replaced the hard disk drive users should ensure that images on the hard disk drive are stored via an alternate permanent media (film, long term storage) as the hard disk drive approaches the 170 image capacity.
GEHC OEC is actively working to replace the affected hard disk drives in a timely manner. GEHC OEC will, without charge, remedy this issue.
If you have any questions or concerns regarding these issues, please do not hesitate to contact the service team for further information at 800-874-7378 option 8. Information is available at this number 24 hours per day, 7 days a week.
Thank you,
Pete McCabe
President and CEO
Page 2 of 2
DOC0287403
GE Healthcare
Important Device Removal & Product Safety Alert
06 February 2006
Director of Surgical Services
TBD – Hospital Name and address
TBD – Hospital Name and Address
TBD – Hospital Name and Address
Dear Healthcare Provider,
It has come to our attention that there is a potential safety concern associated with the GE Healthcare Navigation Pin Transmitter (GE P/N 1004070) that you might own. Because patient safety is of paramount concern to GE, we ask your immediate attention to help us resolve this matter.
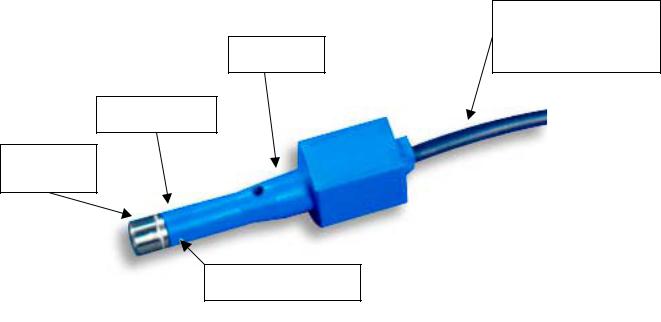
GE Healthcare Navigation Pin Transmitter. (PN: 1004070) [See Figure 1 Below]
Figure 1: Pin Transmitter (1004070)
Casting
Retaining Pin
Stainless
Steel Tip
Cable Attaches to the ENTrak 2500/IT3500 Plus or 9800 C-Arm
Retaining Pin
The housing of the Pin transmitter consists of two main parts: the casting (plastic) and the stainless steel tip that attaches the housing to the clamp/pin which is affixed to the patient (See Figure 1). The design is intended to have a small pin through each side wall of the casting to secure the stainless steel tip with two pins. In a few cases, we have seen that the stainless steel tip of the Pin Transmitter has disconnected from the casting because only one pin of the transmitter was secured. In these cases, there is a potential for this small, unattached retaining pin to fall into the surgical field.
Page 1of 3
GE Healthcare
Figure 2: Pin Transmitter (1004070)
Retaining Pin
Please look for two small pins, one on each side of the Pin Transmitter.
Retaining Pin
Please perform a visual inspection of your GE Healthcare Surgical Navigation Pin Transmitter (PN: 1004070) to make sure that your Pin Transmitter is pinned on both sides of the transmitter (See Figure 2) and follow the instructions below:
If you have a defective Pin Transmitter (pin on one side only):
1)Please discontinue the use of the Pin Transmitter and contact GE Healthcare Customer Service at (800) 874-7378 option 1 to arrange for a part replacement. You may fill out the attached form and FAX it to GE Healthcare Customer Service to help expedite the replacement of the Pin Transmitter.
2)Please convey this information to your OR management along with all users of the ENTrak 2500 Plus, InstaTrak 3500 Plus, or 9800 C-Arm Navigation Systems.
If your Pin Transmitter is not defective (pinned on both sides), you may continue to safely use this device. We simply ask that you contact GE Healthcare Customer Service at (800) 874-7378 option 1 and confirm your inspection with us. You may fill out the attached form and fax it back to GE Healthcare Customer Service at your first available opportunity.
If you have an affected Pin Transmitter, we plan to replace it as soon as possible at no cost to you. A GE Surgery Field Service Engineer will be contacting you to arrange for a part replacement which will enable you to continue using your ENTrak 2500 Plus, IT 3500 Plus and 9800 C-Arm Navigation Systems.
GE appreciates your awareness and cooperation in order to remedy this potential health concern. If you have any questions please contact GE Healthcare Customer Service at (800) 874-7378 option 1.
Sincerely,
Kenneth F. Miles, Ph.D.
GE Healthcare - Interventional Cardiology & Surgery
V.P. Quality & Regulatory
Page 2of 3

GE Healthcare
Pin Transmitter Request Form
Facility:
Contact:
Part Replacement: GE Healthcare Surgical Navigation Pin Transmitter (PN: 1004070)
Please select one of the following:
 I have inspected my GE Healthcare Surgical Navigation Pin Transmitter (PN: 1004070) and it does require replacement.
I have inspected my GE Healthcare Surgical Navigation Pin Transmitter (PN: 1004070) and it does require replacement.
 I have inspected my GE Healthcare Surgical Navigation Pin Transmitter (PN: 1004070) and it does NOT require replacement.
I have inspected my GE Healthcare Surgical Navigation Pin Transmitter (PN: 1004070) and it does NOT require replacement.
Printed Name: ______________________________
Date: __________________________
Please FAX to: (801) 521-3903
Page 3of 3

IMPORTANT |
GE Healthcare |
|
DEVICE REMOVAL & |
OEC Medical Systems, Inc. |
|
PRODUCT ALERT |
Vice President Quality Assurance |
|
|
Kenneth F. Miles, PhD |
|
|
and Regulatory Affairs |
|
|
384 Wright Bros. Drive |
|
May 31, 2006 |
Salt Lake City, UT 84116-2862 |
|
|
||
<TBD – Hospital Name and address> |
T 801-536-4730 |
|
<TBD – Hospital Name and Address> |
||
F 801-517-6459 |
||
<TBD – Hospital Name and Address> |
Kenneth F. Miles@ge.com |
|
Dear Health Care Provider, |
|
It has come to our attention that there is a potential concern associated with the GE Healthcare Navigation Anterior Cervical Post (GE P/N 1006385 or 1006385-NAV an accessory used with the InstaTrak 3500 Plus and 9800 C-Arm Navigation Systems. Because the quality of our products is of paramount concern to GE, we request your immediate attention to help us resolve this matter.
The weld between the post body and the screw was not properly formed in several devices, and the weld can fail even when minimal force is used. Our concern is that if this occurs the screw might have to be removed from the bone by other means and that the scheduled procedure could not continue.
According to our records, your facility purchased this product either in a kit or separately. We will replace it as soon as the product is available at no cost to you. This notification applies to all GE Anterior Cervical Posts (GE P/N 1006385) in commercial distribution and is not limited to any specific lot number. This issue does not affect the GE Healthcare Posterior Threaded Bone Pin (GE PN 1003997).
Please do the following to facilitate this removal:
4” Anterior Cervical Post – Actual Size
1.Identify and discontinue using this product (GE P/N 1006385 or 1006385-NAV)
2.Complete and return attached form
3.Communicate this information to your OR Staff and users of the InstaTrak 3500 Plus, or 9800 C- Arm Navigation Systems.
GE appreciates your awareness and cooperation in order to remedy this potential health concern. Call GE Healthcare Customer Service at (800) 874-7378 Option 1 if you have additional questions.
Sincerely yours,
Kenneth F. Miles, Ph.D.
GE Healthcare - Interventional Cardiology & Surgery
Vice President Quality Assurance and Regulatory Affairs
GE Healthcare |
KFM/SMc |
Internal Ref 06-005R |
VERIFICATION of PRODUCT DISPOSITION
To |
Eric Russell, GE Healthcare |
FAX 801-206-0003 |
From
P r i n t e d n a m e
Date
Subject 4” Anterior Cervical Post Navigation |
Page 1 of ____ |
<TBD – Hospital Name and address> <TBD – Hospital Name and Address> <TBD – Hospital Name and Address>
Please verify the disposition of your four-Inch Anterior Cervical Post (GE P/N 1006385 or 1006385-NAV) by marking the applicable box.
I have removed my cervical post(s) from service. Please credit my account
I have removed my cervical post(s) from service. Please replace it when available
I do not have this instrument
Name _______________________________________________________________________
S i g n a t u r e
Name change?
New facility Name ____________________________________________________________
Address ______________________________________________________________
City ______________________________________ State __________ Zip _______________
Email option: Scan and send completed form to: Sydnee.McMillan@ge.com Subject Line “Cervical Post”

GE Healthcare
OEC Medical Systems, Inc.
384 Wright Bros. Drive
Salt Lake City, Utah 84116
Phone: 801-328-9300
Fax: 801-328-4300
IMPORTANT RADIATION SAFETY NOTICE – 9800 PRODUCT
Date: August 14, 2006
To: |
Facility Administrator or Radiation Safety Officer |
GE Healthcare recently learned that your 9800 C-Arm might exceed the 20 R/minute limit required by the Code of Federal Regulations when in High Level Fluoro (HLF) Pulsed Mode and may pose a serious radiation hazard to the both patient and staff. This could occur if the Default Pulse Rate is set to 30 PPS on the 9800 C-Arm. Only a Field Service or Biomedical Engineer can manually change this value using RUS (Remote Utility Suite Software) or RUT (Remote Utility Tool). The user cannot select it.
Please do the following steps to determine if this issue affects your system:
1.Power the system up.
2.Press the PULSE button on the C-Arm.
3.If 30 PPS is displayed in the status bar on the right workstation monitor, the Default Pulse Rate is set to 30 PPS.
4.If 30 PPS is displayed PLEASE IMMEDIATELY DISCONTINUE any use of the High Level Fluoro Pulsed mode and contact your service team at 800-874- 7378 for a priority service visit to correct this problem.
If 30 PPS is NOT displayed your system complies with the Code of Federal Regulations, however, a GE OEC Field Service Engineer will contact you via phone and determine if a service call is required to check your system.
GE Healthcare will, without charge, remedy the defect or bring the product into compliance with each applicable federal standard. This product correction is being conducted according to Field Modification Instruction 15045.
For Further information, please contact your service team at 800-874-7378.
Sincerely yours,
Kenneth F. Miles, PhD.
Vice President Quality & Regulatory Affairs
GE OEC Medical Systems, Inc.
General Electric Company |
FMI 15045 |
Int. Ref. No. 06-003 |

GE Healthcare
Pete McCabe
President and CEO
GE Healthcare, Surgery
384 Wright Brothers Drive
Salt Lake City, Utah 84116
U.S.A.
Pete.mccabe@med.ge.com
Certified Mail Return Receipt Requested
URGENT RECALL NOTICE
PLEASE TAKE ACTION TO INFORM ALL USERS OF THE RELEVENT SYSTEM(S) OF
THESE ISSUES AND HOW TO ADDRESS THEM
November 8, 2006 |
|
To: |
Hospital Administrator |
|
Director/Manager of Radiology |
Subject: |
Product Safety Issues |
Affected Products: FlexiView 8800 Mobile C-Arm, OEC® UroView 2800, OEC® 9800, OEC® FluoroTrak 9800 Plus, OEC® 9800 Plus, OEC® 9800MD Motorized C-arm System, and OEC® Miniview 6800
Our records indicate that your facility has one or more of the following GEHC OEC products:
FlexiView 8800 Mobile C-ArmOEC® Miniview 6800OEC® UroView 2800OEC® 9800
OEC® FluoroTrak 9800 PlusOEC® 9800 Plus
OEC® 9800MD Motorized C-arm System
GE Healthcare has identified several intermittent potential safety issues that may occur with these products based upon feedback from some customers. The details and symptoms of these issues, as well as the associated interim or permanent solutions, are outlined below.
Page 1 of 6
 Loading...
Loading...