GE MAC 1200 User manual

MAC® 1200
Operator’s Manual
Software Version 6
2012250-022 |
Revision A |

127( The information in this manual only applies to MAC 1200 software version 6. It does not apply to earlier software versions. Due to continuing product innovation, specifications in this manual are subject to change without notice.
Listed below are GE Medical Systems Information Technologies trademarks. All other trademarks contained herein are the property of their respective owners.
900 SC, ACCUSKETCH, AccuVision, APEX, AQUA-KNOT, ARCHIVIST, Autoseq, BABY MAC, C Qwik Connect, CardioServ, CardioSmart, CardioSys, CardioWindow, CASE, CD TELEMETRY, CENTRA, CHART GUARD, CINE 35, CORO, COROLAN, COROMETRICS, Corometrics Sensor Tip, CRG PLUS, DASH, Digistore, Digital DATAQ, E for M, EAGLE, Event-Link, FMS 101B, FMS 111, HELLIGE, IMAGE STORE, INTELLIMOTION, IQA, LASER SXP, MAC, MAC-LAB, MACTRODE, MANAGED USE, MARQUETTE, MARQUETTE MAC, MARQUETTE MEDICAL SYSTEMS, MARQUETTE UNITY NETWORK, MARS, MAX, MEDITEL, MEI, MEI in the circle logo, MEMOPORT, MEMOPORT C, MINISTORE, MINNOWS, Monarch 8000, MULTI-LINK, MULTISCRIPTOR, MUSE, MUSE CV, Neo-Trak, NEUROSCRIPT, OnlineABG, OXYMONITOR, Pres-R-Cuff, PRESSURE-SCRIBE, QMI, QS, Quantitative Medicine, Quantitative Sentinel, RAC RAMS, RSVP, SAM, SEER, SILVERTRACE, SOLAR, SOLARVIEW, Spectra 400, Spectra-Overview, Spectra-Tel, ST GUARD, TRAM, TRAM-NET, TRAM-RAC, TRAMSCOPE, TRIM KNOB, Trimline, UNION STATION, UNITY logo, UNITY NETWORK, Vari-X, Vari-X Cardiomatic, VariCath, VARIDEX, VAS, and Vision Care Filter are trademarks of GE Medical Systems Information Technologies registered in the United States Patent and Trademark Office.
12SL, 15SL, Access, AccuSpeak, ADVANTAGE, BAM, BODYTRODE, Cardiomatic, CardioSpeak, CD TELEMETRY®-LAN, CENTRALSCOPE, Corolation, EDIC, EK-Pro, Event-Link Cirrus, Event-Link Cumulus, Event-Link Nimbus, HI-RES, ICMMS, IMAGE VAULT, IMPACT.wf, INTER-LEAD, IQA, LIFEWATCH, Managed Use, MARQUETTE PRISM, MARQUETTE® RESPONDER, MENTOR, MicroSmart, MMS, MRT, MUSE CardioWindow, NST PRO, NAUTILUS, O2SENSOR, Octanet, OMRS, PHiRes, Premium, Prism, QUIK CONNECT V, QUICK CONNECT, QT Guard, SMART-PAC, SMARTLOOK, Spiral Lok, Sweetheart, UNITY, Universal, Waterfall, and Walkmom are trademarks of GE Medical Systems
Information Technologies.
© GE Medical Systems Information Technologies, 2003. All rights reserved.
T-2 |
MAC 1200 |
Revision A |
|
2012250-022 |
31 March 2003 |

CE Marking Information
CE Marking Information
Compliance
The MAC 1200 bears CE mark CE-0459 indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices and fulfills the essential requirements of Annex I of this directive. The product is in radio-interference protection class A in accordance with EN 55011.
The country of manufacture can be found on the equipment labeling.
The product complies with the requirements of standard EN 60601-1-2 “Electromagnetic Compatibility - Medical Electrical Equipment”.
The safety and effectiveness of this device has been verified against previously distributed devices. Although all standards applicable to presently marketed devices may not be appropriate for prior devices (i.e. electromagnetic compatibility standards), this device will not impair the safe and effective use of those previously distributed devices. See user’s information.
Exceptions
The MAC 1200 EMC: Immunity Performance
Users should be aware of known RF sources, such as radio or TV stations and hand-held or mobile two-way radios, and consider them when installing a medical device or system.
Be aware that adding accessories or components, or modifying the medical device or system may degrade the EMI performance. Consult with qualified personnel regarding changes to the system configuration.
Revision A |
MAC 1200 |
CE-1 |
|
2012250-022 |
|

CE Marking Information
General Information
The device is designed to comply with IEC 60601 requirements. It is a protection class I device.
The CE mark covers only the accessories listed in the chapter “Order Information”.
The information contained in this manual describes software version 6.
CE-2 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

1
2
Contents
The Basics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
About This Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Revision History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Manual Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
MAC 1200 Resting ECG Analysis System Option Codes . . . . . . . . . . . . . . . . . . . |
1-4 |
Intended Use and Functional Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-5 |
Intended Audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-7 |
Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-7 |
Illustrations and Names . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-7 |
Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8
Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
Underwriters Laboratories, Inc. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-12
Biocompatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-13
Literature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-13
Service Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-14
Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-14
Controls and Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-1 |
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
MAC 1200 Control Panels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
MAC 1200 Keyboard . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-6 |
3 |
Operating and Performance Tests . . . . . . . . . . . . . . . . . |
3-1 |
|
Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-3 |
|
Installation and Mains Connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-4 |
|
Performance Check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-5 |
|
Contrast Adjustment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 3-6 |
|
System Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-7 |
|
|
|
Revision A |
MAC 1200 |
i |
|
2012250-022 |
|

4
5
Connecting Peripheral Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
Preparing for ECG Recording . . . . . . . . . . . . . . . . . . . . . 4-1
Connecting the Patient Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Electrode Application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Applying Plate (Limb) Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5 Applying Suction Electrodes (Chest) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5 Electrode Placement for Standard Leads (l, II, III, aVR, aVL, aVF, V1...V6) . . . . . 4-6 Artifact Due to Poor Electrode Application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
Entering Patient Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-10 |
New Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-12 |
Last Name, First Name . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-12 |
Date of Birth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-12 |
Patient ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-12 |
Chest Pain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-13 |
Pacemaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-13 |
Gender/Race . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-13 |
Height/Weight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-13 |
Systolic BP/Diastolic BP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-13 |
Ordering Physician / Referring Physician / Technician . . . . . . . . . . . . . . . . . . . . |
4-14 |
Phone Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-14 |
Medication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-14 |
Comments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-14 |
ID Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-14 |
Secondary ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-14 |
Secondary ID Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-15 |
Last/First Name Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-15 |
Location Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-15 |
Room . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-15 |
Order Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-15 |
Prompts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-15 |
Recording in 12 Lead Mode . . . . . . . . . . . . . . . . . . . . . . . 5-1
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
The Storage Program . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-8
Report Formats . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
Detailed Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11
ECG Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-12
ii |
MAC 1200 |
Revision A |
|
2012250-022 |
|

6
7
8
9
General Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-12 Transmission via Modem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-12 Batch Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-15 Transmission Log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-15 Transmitting Data to a MUSE CV System Via Modem . . . . . . . . . . . . . . . . . . . . 5-16 Receiving Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-19 Cart to Cart Communication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-20 Modem Setup (for Modem Æ Other) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-20
Adjusting Measurement Points/QT Dispersion . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-22 |
Global Measurement Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-22 |
Local T Offset Measurement Point/QT Dispersion . . . . . . . . . . . . . . . . . . . . . . . |
5-24 |
Brief Operating Instructions – 12 Lead Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-26 |
Recording in 6 Lead Mode . . . . . . . . . . . . . . . . . . . . . . . . 6-1
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Arrhythmia Mode Recording . . . . . . . . . . . . . . . . . . . . . . 7-1
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
During the Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-6
Final Report . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-8
Pacemaker Patients / Recording During Defibrillation . 8-1
Recording ECGs of Pacemaker Patients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-3 ECG Recording During Defibrillation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-4
System Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-1 |
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
12 Lead Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Report Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Rhythm Leads . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Gain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Revision A |
MAC 1200 |
iii |
|
2012250-022 |
|

Report Format . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Detailed Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
Continuous Rhythm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-6
Muscle Filter/AC Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-6
Muscle Filter Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-6
Manual Copy To . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-7
Number of Copies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-7
Interpretation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-7
Print Interpretation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-7
Override Function [no] . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-8
6 Lead Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-9
Report Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-9
Gain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
Speed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
Muscle Filter/AC Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
Muscle Filter Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-10
Anti-Drift System (ADS) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-11
Auto Paper Feed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-11
Arrhythmia Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
Report Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
Gain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
Muscle Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
AC Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-12
Muscle Filter Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-13
Trend Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-13
Arrhythmia Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-13
Episodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-13
Pharma . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-14
Patient Data Customization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-14
Project Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-15
Trial ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-15
Investigator ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-15
Visit Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-16
Visit Type . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-16
Dose Type . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-16
Extra Questions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-16
High Security . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-17
Device Password . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-17
Don’t Allow Record Edits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-18
Delete Only After Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-18
System Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-19
Ordering Physician/Referring Physician/Technician . . . . . . . . . . . . . . . . . . . . . . 9-19
Institution Name . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-19
Cart Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-19
Site Number * . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-19
Location* . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-20
iv |
MAC 1200 |
Revision A |
|
2012250-022 |
|

Date/Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-20
Lead Fail Beep . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-20
High HR Beep . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-20
Lead Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-20
Pace Enhancement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-20
Baseline Roll Filter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-20
Date . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
Units . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
Mains . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
LCD Light Off After . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
Low Battery Beep . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
Default mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
Language . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-21
Enable Password Protection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-22
Test Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-22
Restore Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-22
Print Configuration Lists . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-22
Transmission Log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-23
Communication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-24
Baud Rate (PC) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-24
Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-24
Modem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-24
PIN Dialing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-25
Patient Data Menu Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-26
Required Data Fields . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-27
Extra Questions 1 to 4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-27
Option Code . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-28
ECG Transmission via Modem . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-29
Selecting the Communication Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-29
Direct ECG Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-30
Selecting the Communication Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-30
10 Loading Chart Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3
End-of-Paper Indication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-5
Aging Stability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-6
11 Cleaning, Disinfection and Maintenance . . . . . . . . . . . 11-1
Cleaning and Disinfecting the Recorder Housing . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Revision A |
MAC 1200 |
v |
|
2012250-022 |
|

12
13
Cleaning and Disinfecting the Patient Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3 Cleaning and Disinfecting the Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Checks Before Each Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Technical Inspections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
12-1 |
Troubleshooting Chart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. 12-3 |
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . 13-1
Recording . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-3
Printer Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-3 Paper Transport . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-3 Membrane Keypad . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-4 Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-4 Indicators (LEDs) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-4 Lead Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-4 Automatic Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-4 Detection of Pacer Pulses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-5 Heart Rate Indication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-5 Signal Inputs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-5 Data Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-6 Transfer of ECGs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-6 Receiving Data with the CSI Communication Protocol from the Following Units 13-6 Sending ECGs to the Following Units with the A5 Protocol . . . . . . . . . . . . . . . . . 13-6 Remote Start (Hardware) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-7 Signal Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13-8
14 Order Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
14-1 |
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
General Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14-3
Entering Special Characters . . . . . . . . . . . . . . . . . . . . . . .A-1
Special Characters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
A-3 |
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Index-1
vi |
MAC 1200 |
Revision A |
|
2012250-022 |
|

1 The Basics
Revision A |
MAC 1200 |
1-1 |
|
2012250-022 |
|

For your notes
1-2 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

The Basics: About This Manual
About This Manual
Revision History
This manual has a revision letter, located at the bottom of each page. This revision letter changes whenever the manual is updated. Revision A is the initial release of the document.
Revision |
Date |
Comments |
|
|
|
A |
31 March 2003 |
Initial release of manual, describes version 6.0. |
|
|
|
Manual Purpose
This manual describes the safe and effective operation of the MAC 1200 unit.
127(
This document describes the functionality of the U.S. interface for the MAC 1200 unit.
Information in this manual differs from operating information for MAC 1200 units developed for use internationally. Please refer to PN 2012250-021 for information on using the international interface.
Revision A |
MAC 1200 |
1-3 |
|
2012250-022 |
|

The Basics: MAC 1200 Resting ECG Analysis System Option Codes
MAC 1200 Resting ECG Analysis System Option
Codes
In addition to the software supplied with the unit, optional programs may be purchased to upgrade the MAC 1200 performance features. In order to use a new option, you need to activate it by entering the option code number (refer to Chapter 9, “Option Code” section for details). The option codes are entered into the MAC 1200 prior to shipping.
Software Package |
Functionality |
Option Code |
|
|
|
MEAS |
Measurement (measurement of the 10-second resting ECG) |
_ _ _ _ _ _ _ _ _ _ _ _ |
|
|
|
DIAG |
Interpretation (interpretation of the 10-second resting ECG) |
_ _ _ _ _ _ _ _ _ _ _ _ |
|
|
|
MEMO |
Memory (storage of a maximum of 40 10-second resting ECGs) |
_ _ _ _ _ _ _ _ _ _ _ _ |
|
|
|
C100 |
Activates the three options MEAS, DIAG, MEMO for a maximum of 100 ECGs |
_ _ _ _ _ _ _ _ _ _ _ _ |
|
|
|
C500 |
Activates the three options MEAS, DIAG, MEMO for a maximum of 500 ECGs |
_ _ _ _ _ _ _ _ _ _ _ _ |
|
|
|
EVAL |
Activates the three options MEAS, DIAG, MEMO for a maximum of 4 weeks |
_ _ _ _ _ _ _ _ _ _ _ _ |
|
|
|
|
|
|
Serial No: |
_ _ _ _ _ _ _ _ _ |
|
|
|
|
1-4 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

The Basics: MAC 1200 Resting ECG Analysis System Option Codes
Intended Use and Functional Description
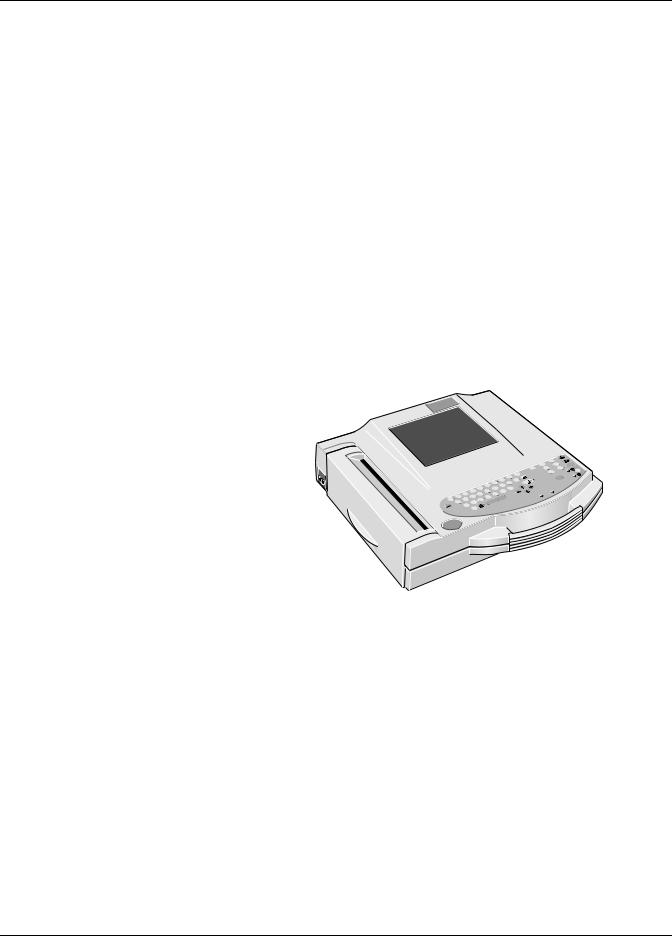
The MAC 1200 is an ECG acquisition and recording system designed and manufactured by GE Medical Systems Information Technologies.
It is intended to be used for resting ECG recording and realtime ECG recording with or without arrhythmia detection.
It is not intended for use as a vital signs physiological monitor.
The arrhythmia detection portion of the MAC 1200 is provided to the customer for the convenience of automatic documentation. It is not designed to provide alarms for arrhythmia detection.
The MAC 1200 offers no diagnostic opinion to the user. Instead it provides analytical statements when configured with the appropriate options.
It is intended to be used by trained operators under direct physician supervision when ECG records are required.
It is not suitable for intracardiac application.
It is designed for continuous operation.
It is not intended for home use.
The MAC 1200 is designed as a portable device and can easily be moved from one patient to another or to different locations. It is not intended to be used during patient transport.
Equipped with the standard software, the MAC 1200 supports the following operating modes.
12 Lead Mode (acquisition of 12 leads of ECG for a period of 10 seconds),
6 Lead Mode (realtime recording of 6 ECG leads), and
Arrhythmia Mode (continuous ECG analysis for arrhythmias).
The graphics display shows 3 leads at a time.
Resting ECGs can be transferred to the CardioSys/CardioSoft or MUSE
CV Information System via the RS232 interface.
The device operates from both AC and DC (rechargeable batteries) power sources.
Revision A |
MAC 1200 |
1-5 |
|
2012250-022 |
|
The Basics: MAC 1200 Resting ECG Analysis System Option Codes
The unit’s performance features can be upgraded with the following optional programs.
MEAS — measurement (measurement of the 10-second resting ECG)
DIAG — interpretation (interpretation of the 10-second resting ECG)
MEMO — memory (storage of a maximum of 40 10-second resting ECGs)
C100 — activates the three options MEAS, DIAG, MEMO for a maximum of 100 ECGs
C500 — activates the three options MEAS, DIAG, MEMO for a maximum of 500 ECGs
EVAL — activates the three options MEAS, DIAG, MEMO for a period of 4 weeks
The MAC 1200 resting ECG analysis system has a setup menu to customize the system parameters.
Patient and user data can be entered for reliable and safe archiving of patient records. The patient name is annotated on each printed report page. All other data is printed on request.
1
|
C1200 |
A |
|
M |
|
102A
The MAC 1200 units are designed to comply with IEC 60601 / EN 60601 requirements. They are protection class I devices/devices with an internal power source. They are classified as MDD class IIa devices. They are designed for continuous operation. The units are not suitable for intracardiac application. The units are not intended for use as vital signs physiological monitors.
1-6 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

The Basics: MAC 1200 Resting ECG Analysis System Option Codes
Intended Audience
This manual is geared for clinical professionals. Clinical professionals are expected to have working knowledge of medical procedures, practices, and terminology as required for monitoring of critically ill patients.
&$87,21
PATIENT HAZARD — Medical technical equipment such as the MAC 1200 must only be used by qualified and trained personnel.
Definitions
|
The following formats are used in this manual to highlight various web |
|
viewer features and functions. |
Black text |
Indicates keys on the keyboard, text to be entered, or hardware items such as buttons or |
|
switches on the equipment. |
Italicized text |
Indicates software terms that identify menu items, buttons, or options in various windows. |
Ctrl+Esc |
Indicates a keyboard operation. A (+) sign between the names of two keys indicates that |
|
you must press and hold the first key while pressing the second key once. |
|
For example, “Press Ctrl+Esc” means to press and hold down the Ctrl key while pressing |
|
the Esc key. |
<Space> |
Indicates you must press the space bar. When instructions are given for typing a precise |
|
text string with one or more spaces, the point where the space bar must be pressed is |
|
indicated as: <Space>. The purpose of the < > brackets is to ensure you press the space |
|
bar when required. |
Enter |
Indicates you must press the “Enter” or “Return” key on the keyboard. Do not type “enter”. |
Illustrations and Names
All illustrations in this manual are provided as examples only. They may not necessarily reflect your monitoring setup or data displayed on your monitor.
In this manual, all names appearing in examples and illustrations are fictitious. The use of any real person’s name is purely coincidental.
Revision A |
MAC 1200 |
1-7 |
|
2012250-022 |
|

The Basics: Safety Information
Safety Information
This manual is an integral part of the device. It should always be kept near the device. Close observance of the information given in the manual is a prerequisite for proper device performance and correct operation and ensures patient and operator safety. Please note that information pertinent to several chapters is given only once. Therefore, carefully read the manual once in its entirety.
The symbol 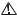 means: Consult accompanying documents. It indicates points which are of particular importance in the operation of the device.
means: Consult accompanying documents. It indicates points which are of particular importance in the operation of the device.
This manual is in conformity with the device specifications and standards on safety of electromedical equipment valid at the time of printing. All rights are reserved for devices, circuits, techniques, software programs, and names appearing in this manual.
On request GE will provide a service manual.
The GE quality management system complies with the standards DIN
EN ISO 9001 and EN 46001.
To ensure patient safety, the specified measuring accuracy, and interference-free operation, we recommend to use only original GE components. The user is responsible for application of accessories from other manufacturers.
The warranty does not cover damage resulting from the use of unsuitable accessories and consumables from other manufacturers.
GE is responsible for the effects on safety, reliability, and performance of the device, only if
assembly operations, extensions, readjustments, modifications, or repairs are carried out by GE or by persons authorized by GE, and
the device is used in accordance with the instructions given in this operator’s manual.
1-8 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

The Basics: Safety Information
Definitions
The terms danger, warning, and caution are used throughout this manual to point out hazards and to designate a degree or level of seriousness. Familiarize yourself with their definitions and significance.
Hazard is defined as a source of potential injury to a person.
'$1*(5 indicates an imminent hazard which, if not avoided, will result in death or serious injury.
:$51,1* indicates a potential hazard or unsafe practice which, if not avoided, could result in death or serious injury.
&$87,21 indicates a potential hazard or unsafe practice which, if not avoided, could result in minor personal injury or product/property damage.
127( provides application tips or other useful information to assure that you get the most from your equipment.
The safety information given in this manual is classified as follows.
'$1*(5
EXPLOSION HAZARD — The device is not designed for use in areas of medically used rooms where an explosion hazard may occur. An explosion hazard may result from the use of flammable anesthetics, skin cleansing agents and disinfectants.
Revision A |
MAC 1200 |
1-9 |
|
2012250-022 |
|

The Basics: Safety Information
:$51,1*6
SHOCK HAZARD — Strictly observe the following warnings. Failure to do so may endanger the lives of the patient, the user and bystanders.
Before using the device, the operator must ascertain that it is in correct working order and operating condition. In particular, all connectors, electrodes as well as sensors and probes must be checked for signs of damage. Damaged parts must be replaced immediately, before use.
When disconnecting the device from the power line, remove the plug from the wall outlet first, before disconnecting the cable from the device. Otherwise there is a risk of coming in contact with line voltage by inadvertently introducing metal parts in the sockets of the power cord.
The mains plug must be connected to an appropriate power supply with a non-fused grounded-to-earth wire. If these requirements cannot be met, operate the device on battery power.
Do not use multiple portable socket outlets (MPSO) to connect the device to the power line.
Devices may be connected to other devices or to parts of systems only when it has been made certain that there is no danger to the patient, the operators, or the environment as a result. In those instances where there is any element of doubt concerning the safety of connected devices, the user must contact the manufacturers concerned or other informed experts as to whether there is any possible danger to the patient, the operator, or the environment as a result of the proposed combination of devices. Standards IEC 60601-1-1/EN60601-1-1 must be complied with in all cases.
All devices of a system must be connected to the same electric circuit. Devices which are not connected to the same circuit must be electrically isolated (isolated RS232 interface).
1-10 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

The Basics: Safety Information
:$51,1*6
EQUIPMENT FAILURE — Magnetic and electrical fields are capable of interfering with the proper performance of the device. For this reason make sure that all peripheral devices operated in the vicinity of the recorder comply with the relevant EMC requirements. X- ray equipment, MRI devices, radio systems (cellular telephones) etc. are possible sources of interference as they may emit higher levels of electromagnetic radiation. Keep the recorder away from these devices and verify the recorder performance before use.
SUFFOCATION HAZARD — Dispose of the packaging material, observing the applicable waste-control regulations. Keep the packaging material out of children's reach.
&$87,216
EQUIPMENT DAMAGE — Devices intended for emergency application must not be exposed to low temperatures during storage and transport to avoid moisture condensation at the application site. Wait until all moisture has vaporized before using the device.
EQUIPMENT DAMAGE — Before connecting the device to the power line, verify that the ratings of your local power line are those indicated on the device nameplate.
RESTRICTED SALE — U.S. federal law restricts this device to sale by or on the order of a physician.
Revision A |
MAC 1200 |
1-11 |
|
2012250-022 |
|

|
The Basics: Safety Information |
|
Classification |
|
|
|
The unit is classified, according to IEC 60601-1, as: |
|
|
|
|
Type of protection against electrical shock |
Class I internally powered equipment |
|
|
|
|
Degree of protection against electrical |
Type CF defibrillation-proof applied part |
|
shock |
|
|
|
|
|
Degree of protection against harmful |
Ordinary Equipment (enclosed equipment without protection against ingress of |
|
ingress of water |
water) |
|
|
|
|
Degree of safety of application in the |
Equipment not suitable for use in the presence of a flammable anesthetic mixture |
|
presence of a flammable anesthetic |
with air or with oxygen or nitrous oxide |
|
mixture with air or with oxygen or nitrous |
|
|
oxide |
|
|
|
|
|
Method(s) of sterilization or disinfection |
Not applicable |
|
recommended by the manufacturer |
|
|
|
|
|
Mode of operation |
Continuous operation |
|
|
|
|
Underwriters Laboratories, Inc.
Medical Equipment
With respect to electric shock, fire and mechanical hazards only in accordance with UL 2601-1, and CAN/CSA C22.2 NO. 601.1.
4P41
1-12 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

The Basics: Biocompatibility
Biocompatibility
The parts of the product described in this operator manual, including all accessories, that come in contact with the patient during the intended use, fulfill the biocompatibility requirements of the applicable standards. If you have questions in this matter, please contact GE Medical Systems
Information Technologies or its representatives.
Literature
Medical Device Directive of August 2, 1994
EN 60601-1: 1990 + A 1: 1993 + A 2: 1995
Medical electrical equipment. General requirements for safety.
EN 60601-1-1: 9/1994 + A1: 12/1995
General requirements for safety. Requirements for the safety of medical electrical systems.
IEC-Publication 513/1994: Fundamental aspects of safety standards for medical equipment.
Revision A |
MAC 1200 |
1-13 |
|
2012250-022 |
|
The Basics: Service Information
Service Information
Requirements
Refer equipment servicing to GE Medical Systems Information Technologies’ authorized service personnel only. Any unauthorized attempt to repair equipment under warranty voids that warranty.
It is the user’s responsibility to report the need for service to GE Medical
Systems Information Technologies or to one of their authorized agents.
Every GE Medical Systems Information Technologies device has a unique serial number for identification. The serial number appears on the device label.
A
B
105A
G |
F |
E |
D |
C |
|
|
|
||
|
|
Table 1. Equipment Identification |
||
|
|
|
|
|
Item |
|
|
Description |
|
Aname of device
Bmanufacturer
Clocation code
Dserial number
Eunique product code
Flast digit of year manufactured
Gmonth manufactured
1-14 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

2 Controls and Indicators
Revision A |
MAC 1200 |
2-1 |
|
2012250-022 |
|

For your notes
2-2 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

Controls and Indicators: General Information
General Information
Controls and indicators of the MAC 1200 electrocardiograph are shown in this chapter.
MAC 1200 Control Panels
1 1 |
2 |
3 |
|
|
|
4 |
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C1200 |
A |
|
M |
|
001A
|
Description |
|
|
1 |
Power input |
|
|
2 |
Paper door, windows allows you to check the paper supply |
|
|
3 |
Patient cable connector |
|
|
4 |
Connection for electrode application system KISS (option) |
|
|
5 |
Serial interface (See Chapter 13, “Technical Specifications” for details.) |
|
|
Revision A |
MAC 1200 |
2-3 |
|
2012250-022 |
|
Controls and Indicators: General Information
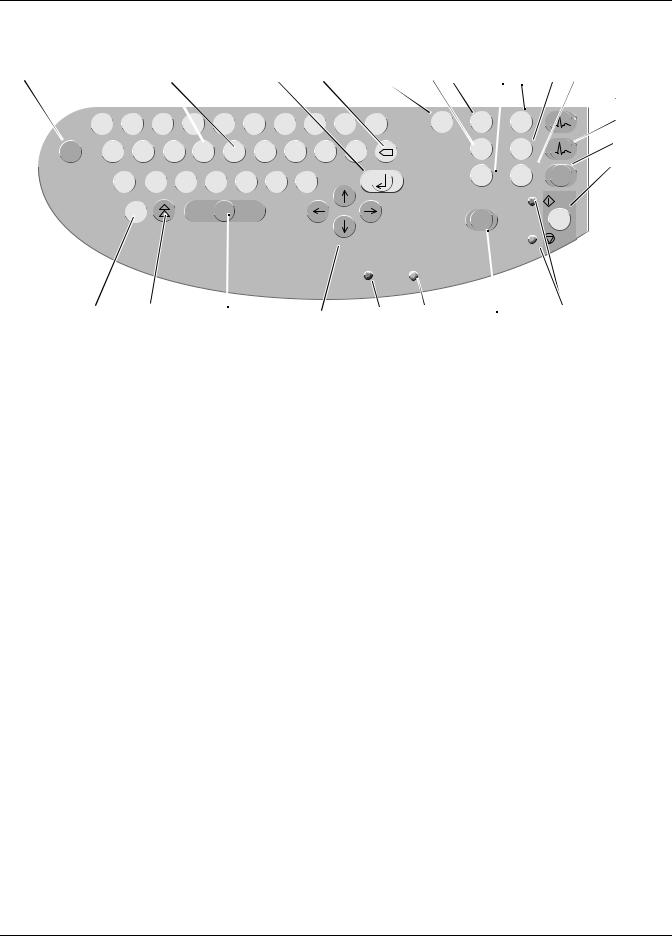
MAC 1200 Keyboard
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12
|
|
Q |
1 |
W |
2 |
E |
3 |
|
R |
4 |
|
T |
5 |
|
Y |
6 |
|
7 |
I |
8 |
|
O |
9 |
|
P |
0 |
setup |
format/ |
copy |
12 |
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
U |
|
|
|
|
|
|
speed |
|
|
|||||||||
|
on |
|
|
! |
|
? |
|
|
= |
|
- |
|
G |
+ |
|
|
* |
|
% |
|
( |
|
|
|
) |
|
|
muscle |
lead |
6 |
|
|
A |
S |
|
D |
F |
|
|
H |
J |
K |
|
|
L |
|
X |
|
|||||||||||||
stdby |
|
|
|
|
|
|
filter |
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Z; |
|
X: |
|
|
C/ |
|
|
V, |
|
|
B. |
|
|
N< |
M> |
|
|
|
|
|
|
|
|
gain |
store/ |
arrhy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
retrieve |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
start |
|
|
|
|
alt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
pat |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
info |
|
|
stop
 13
13
 14
14
 15
15
standby battery low
|
23 |
22 |
21 |
20 |
19 |
18 |
17 |
16 |
098A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Description |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
Power switch (ON/OFF) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
2 |
Keys to select a higher or lower HR alarm limit |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
3 |
Confirms entered data |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4 |
Correction key (entry of data) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
5 |
Displays the configuration menu |
|
|
|
|
|
|
||
|
|
|
|
|
|
||||
6 |
Enables/disables the muscle filter (elimination of muscle artifact) |
|
|
|
|
||||
|
|
|
|
||||||
7 |
Selects the writer speed 25, 50 or 5 mm/s in 6-Lead Mode and the report formats in 12-Lead Mode |
|
|
||||||
|
|
|
|
|
|
|
|
||
8 |
Selects the gain (5, 10, 20, 40 mm/mV) |
|
|
|
|
|
|
||
|
|
|
|
|
|||||
9 |
Press to print the report or additional copies of the ECG, or to send/receive ECGs |
|
|
|
|||||
|
|
|
|
||||||
10 |
Selects the ECG leads displayed and recorded in 6-Lead Mode and displayed 12-Lead Mode |
|
|
||||||
|
|
|
|
|
|
|
|||
11 |
Sends ECG to memory/retrieves ECG from memory |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
12 |
Selects the 12-Lead Mode |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
13 |
Selects the 6-Lead Mode |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14 |
Selects the Arrhythmia Mode |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
15 |
Starts and stops the recorder, clears the setup menu and terminates patient data entry |
|
|
||||||
|
|
|
|
|
|
|
|
|
|
16 |
Indicators: |
|
|
|
|
|
|
|
|
|
Green: recording in selected mode started; |
|
|
|
|
|
|
||
|
Yellow: recording in selected mode stopped |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
2-4 |
MAC 1200 |
Revision A |
|
2012250-022 |
|

|
Controls and Indicators: General Information |
|
|
|
Description |
|
|
17 |
Enables entry of patient data |
|
|
18 |
Indicator lights up when battery needs to be recharged |
|
|
19 |
Indicator is illuminated when unit is connected to the power line |
|
|
20 |
Cursor control keys |
|
|
21 |
Space bar |
|
|
22 |
Shift key |
|
|
23 |
Press to access special characters |
|
|
Revision A |
MAC 1200 |
2-5 |
|
2012250-022 |
|

Controls and Indicators: Symbols
Symbols
Consult accompanying documents
Signal input
Type CF signal input, highly insulated, defibrillation-proof
Start
Stop
2-6 |
MAC 1200 |
Revision A |
|
2012250-022 |
|
 Loading...
Loading...