Philips Avalon FM20, Avalon FM30 Service manual

Obstetrical Care
S E R V I C E G U I D E
Avalon Fetal Monitor
FM20
FM30
F E T A L M O N I T O R I N G
Printed in Germany 07/05
*M2703-9000A*
Part Number M2703-9000A
4512 610 10021
S

Table of Contents
1 |
Introduction |
1 |
|
Who Should Read This Guide |
1 |
|
What to Do Next |
1 |
|
Repair Strategy |
2 |
|
Manufacturer’s Information |
2 |
|
Passwords |
3 |
|
Warnings and Cautions |
3 |
2 |
Site Preparation |
5 |
|
|
|
|
Introduction |
5 |
|
Site Planning |
5 |
|
Roles and Responsibilities |
5 |
|
Site Preparation Responsibilities |
5 |
|
Procedures for Local Staff |
6 |
|
Procedures for Philips Personnel |
7 |
|
Site Requirements |
7 |
|
Space Requirements |
7 |
|
Environmental Requirements |
7 |
|
Safety Requirements (Customer or Philips) |
8 |
|
Electrical Requirements (Customer or Philips) |
8 |
|
Connecting Non-Medical Devices |
8 |
|
Cabling Options and Requirements for Connection to OB TraceVue |
9 |
|
Mounting Options |
9 |
|
Input Devices |
10 |
3 |
Installation Instructions |
11 |
|
|
|
|
Initial Inspection |
11 |
|
Mechanical Inspection |
11 |
|
Electrical Inspection |
11 |
|
Claims for Damage |
11 |
|
Repackaging for Shipment or Storage |
12 |
|
Mounting Instructions |
12 |
|
Line Voltage Selection |
12 |
|
Connecting the Monitor to AC Mains |
12 |
|
Connecting the Monitor to Non-Medical Devices |
13 |
|
Checking and Setting Line Frequency |
13 |
|
Checking/Setting Paper Scale |
13 |
|
Checking/Setting Paper Speed |
13 |
|
PS/2 Keyboard/Mouse |
14 |
i

4 |
Theory of Operation |
15 |
|
Monitor Hardware Overview |
15 |
|
Power Supply |
16 |
|
Connector Block |
16 |
|
Bus Master Board |
16 |
|
Main CPU Board |
16 |
|
Fetal Recorder (Thermal Printer Unit) |
16 |
|
Recorder Adapter Board |
17 |
|
Thermal Line Printhead (TLPH) |
17 |
|
Paper Sensor |
17 |
|
Stepper Motor |
17 |
|
LCD Display and Touchscreen |
17 |
|
Noninvasive Blood Pressure Assembly |
17 |
|
SpO2 Assembly |
17 |
|
Input/Output Interface Boards |
18 |
|
Transducer Hardware Overview |
18 |
|
Transducer Types |
19 |
|
Functional Description of the Transducer CPU |
19 |
|
CPU (Micro Controller) |
19 |
|
Analog-to-Digital Converter |
19 |
|
Communication Transceiver (CAN Bus Driver) |
19 |
|
EEPROM |
19 |
|
Toco Transducer Frontend |
19 |
|
Ultrasound Transducer Frontend |
20 |
|
Toco+ Transducer Frontends |
20 |
|
Toco Frontend |
20 |
|
IUP Frontend |
20 |
|
ECG Frontend |
20 |
5 |
Interfaces |
21 |
|
|
|
|
LAN / RS232 Interface |
21 |
|
Dual PS/2 Interface |
21 |
6 |
Testing and Maintenance |
23 |
|
|
|
|
Recommended Frequency |
23 |
|
When to Perform Test Blocks |
24 |
|
Test Reporting |
25 |
|
How to Carry Out the Test Blocks |
25 |
|
Preventive Maintenance Procedures |
26 |
|
Other Regular Tests |
26 |
|
Visual Check |
26 |
|
Fetal Recorder Maintenance |
26 |
|
Testing Transducers and Patient Modules |
26 |
|
Ultrasound Transducer Electrical Check |
26 |
|
Toco Transducer Electrical Check |
28 |
ii

Testing the Patient Module (M2738A)/Toco+ Transducer (M2735A): DECG Mode |
28 |
Testing the Patient Module (M2738A)/Toco+ Transducer (M2735A): MECG Mode |
29 |
Testing the Patient Module (M2738A)/Toco+ Transducer (M2735A): IUP Mode |
30 |
Performance Assurance Tests |
31 |
Noninvasive Blood Pressure Performance Tests |
31 |
Accuracy Test |
31 |
Leakage Test |
32 |
Linearity Test |
32 |
Valve Test |
32 |
SpO2 Performance Test |
32 |
Safety Tests |
33 |
Warnings, Cautions, and Safety Precautions |
33 |
Electrical Isolation Diagram |
34 |
Safety Test Procedures |
34 |
S(1): Sum of Functional Earth and Enclosure Leakage Current Test |
34 |
S(2): Patient Leakage Current - Single Fault Condition (SFC), Mains on Applied Part |
35 |
System Test |
36 |
What is a Medical Electrical System? |
36 |
General Requirements for a System |
36 |
System Example |
36 |
Touchscreen Calibration |
37 |
Disabling/Enabling Touch Operation |
38 |
Checking the Fetal Recorder Offset |
39 |
Setting the Fetal Recorder Offset |
39 |
Fetal Recorder Selftest Report |
40 |
7 Troubleshooting |
41 |
|
|
Who Should Perform Repairs |
41 |
Replacement Level Supported |
41 |
Hardware Revision Check |
41 |
Software Revision Check |
42 |
Obtaining Replacement Parts |
42 |
Troubleshooting Guide |
42 |
Checks for Obvious Problems |
42 |
Checks Before Opening the Instrument |
42 |
Checks with the Instrument Switched On, AC connected |
43 |
Individual Parameter INOPs |
43 |
Initial Instrument Boot Phase |
44 |
Troubleshooting Tables |
44 |
How to Use the Troubleshooting Tables |
44 |
Boot Phase Failures |
45 |
Screen is Blank |
46 |
Touchscreen Not Functioning |
46 |
General Monitor INOP Messages |
47 |
Keyboard/Mouse Not Functioning |
48 |
Alarm Tones |
48 |
iii

|
Alarm Behavior |
48 |
|
Fetal Recorder |
48 |
|
LAN / RS232 |
51 |
|
Transducers |
52 |
|
Status Log |
53 |
|
Troubleshooting with the Support Tool |
54 |
|
Troubleshooting the Individual Measurements or Applications |
54 |
8 |
Disassembly and Reassembly |
55 |
|
|
|
|
Introduction |
55 |
|
Serial Numbers |
56 |
|
Removing the Top Cover Assembly |
57 |
|
Removing the Display Assembly |
59 |
|
Replacing the Display Assembly |
62 |
|
Recorder Disassembly |
64 |
|
Removing the Drawer Assembly |
64 |
|
Replacing the Drawer Assembly |
66 |
|
Removing the Recorder Chassis |
68 |
|
Removing the Thermal Line Printhead (TLPH) |
72 |
|
Replacing the TLPH |
73 |
|
Replacing the Recorder Chassis |
74 |
|
Removing the Paper Sensor Assembly |
75 |
|
Replacing the Paper Sensor Assembly |
75 |
|
Removing the Recorder Adapter Board |
75 |
|
Replacing the Recorder Adapter Board |
77 |
|
Removing the Stepper Motor |
78 |
|
Replacing the Stepper Motor |
79 |
|
Replacing the Top Cover Assembly |
80 |
|
Removing the Power Supply Assembly |
81 |
|
Replacing the Power Supply Assembly |
82 |
|
Removing the Noninvasive Blood Pressure Assembly |
82 |
|
Replacing the Noninvasive Blood Pressure Assembly |
84 |
|
Removing the SpO2 Assembly |
85 |
|
Replacing the SpO2 Assembly |
86 |
|
Removing the Interface Boards |
87 |
|
Removing the Main CPU Board |
88 |
|
Replacing the Main CPU Board |
89 |
|
Exchanging the Loudspeaker |
89 |
|
Exchanging the Transducer Cable |
90 |
|
Exchanging the Transducer Belt Button |
92 |
9 |
Parts |
95 |
|
|
|
|
Monitor |
95 |
|
Transducers |
96 |
|
Patient Modules |
97 |
|
Mounting Hardware |
97 |
iv

Assembly and Kit Contents |
98 |
Bottom Housing Assembly (M2703-64101) |
98 |
Power Supply Assembly (M2703-60001) |
99 |
Top Cover Assembly (M2703-60052) |
99 |
Top Cover Housing (M2703-64102) |
100 |
Stepper Motor Assembly (M2703-60004) |
100 |
Paper Sensor Assembly (M2703-60003) |
100 |
Drawer Assembly (M2703-64651) |
100 |
Display Assembly (M2703-64503) |
101 |
Transducer Cable Assembly (M2735-64201) |
101 |
Small Parts Kit - Top (M2735-64202) |
102 |
Small Parts Kit - Bottom (M2703-64203) |
102 |
Belt Button Kit (M2703-64204) |
102 |
10 Upgrades |
105 |
|
|
11 Understanding Configuration |
107 |
|
|
What is Configuration Mode? |
107 |
Understanding Settings |
108 |
Entering and Leaving Configuration Mode |
108 |
Storing Changes in the User Defaults |
109 |
Loading the Factory Default |
109 |
Loading the User Defaults |
110 |
Loading Configurations Using the Support Tool |
110 |
About Configuration Files (.cfg) |
111 |
Selecting the Correct Configuration |
111 |
12 Configuration Settings Appendix |
113 |
|
|
Documenting Monitor Configurations |
113 |
Using the Configuration Tables |
113 |
Configuration Table Example |
114 |
Understanding Configuration Implications |
114 |
Measurement-Related Settings |
115 |
Color Configuration |
115 |
Configuring FHR (Ultrasound) |
115 |
FHR Configuration Implications |
115 |
Configuring Toco |
116 |
Configuring IUP |
116 |
Configuring DFHR (DECG) |
116 |
DFHR Configuration Implications |
116 |
Configuring MHR (ECG) |
117 |
ECG Configuration Implications |
117 |
Configuring Pulse |
118 |
Configuring SpO2 |
119 |
SpO2 Configuration Implications |
119 |
Configuring Noninvasive Blood Pressure (NBP) |
119 |
v

NBP Configuration Implications |
120 |
Monitor-Related Settings |
121 |
Configuring Alarms |
121 |
Alarm Settings Configuration Implications |
121 |
Configuring the NST Timer |
122 |
NST Timer Configuration Implications |
122 |
Configuring Fetal Recorder Settings |
122 |
Recorder Configuration Implications |
123 |
Configuring User Interface Settings |
123 |
User Interface Configuration Implications |
124 |
Hardware Settings |
124 |
Global Settings |
125 |
Global Settings Configuration Implications |
125 |
vi

1
Introduction
This Service Guide contains technical details for the Avalon FM20 and FM30 Fetal/Maternal Monitors. It provides a technical foundation to support effective troubleshooting and repair. It is not a comprehensive, in-depth explanation of the product architecture or technical implementation. It offers enough information on the functions and operations of the monitoring systems so that engineers who repair them are better able to understand how they work. It covers the physiological measurements and the monitor hardware that acquires and displays them.
The Avalon FM20/FM30 Fetal Monitor Service Guide supplements the maintenance and troubleshooting procedures, carried out by the operator, that are described in the Instructions for Use. Refer to the Instructions for Use for maintenance and troubleshooting procedures that may be performed during normal operation.
Only qualified service personnel should attempt to install the system, disassemble the monitor, remove or replace any internal assemblies, or replace the transducer cable or belt buttons.
Who Should Read This Guide
This guide is for biomedical engineers or technicians responsible for troubleshooting, repairing, and maintaining Philips’ Avalon fetal monitors.
You must:
•understand English
•be familiar with standard medical equipment installation procedures
•be familiar with current conventional technical terms as used throughout this guide
What to Do Next
Familiarize yourself with the contents of this guide and the Instructions for Use before attempting to service or repair the system.
1

1 Introduction |
Repair Strategy |
Repair Strategy
The Service Support Tool software helps you to determine whether a fault is a hardware or software problem. The main replaceable parts are:
•unit exchange for the transducers
•replacement of
–the top cover assembly
–the bottom housing
–the power supply assembly
–the display assembly
–the recorder adapter board
–the paper drawer assembly
–the paper sensor assembly
–the stepper motor assembly
–the SpO2 assembly
–the noninvasive blood pressure assembly
–the main CPU board
–the bus master board
–the socket connector block
–the transducer cable
–the transducer belt button
See Chapter 9, “Parts” for part numbers, and Chapter 8, “Disassembly and Reassembly” for repair details.
Repair or replacement of individual components on the boards is not supported, and should never be attempted.
For tests that you are required to perform after repairs, refer to “When to Perform Test Blocks” on page 24.
Manufacturer’s Information
© Copyright 2003 - 2005. Koninklijke Philips Electronics N.V.
All Rights Reserved.
Philips Medizin Systeme Böblingen GmbH
Hewlett-Packard-Str. 2
71034 Böblingen, Germany
2

Passwords |
1 Introduction |
Passwords
In order to access different modes within the monitor a password may be required. The passwords are listed below.
Monitoring Mode: No password required
Configuration Mode: 71034
Demo Mode: 14432
Service Mode: 1345
Refer to Chapter 11, “Understanding Configuration” before making any changes to the monitor configuration.
Warnings and Cautions
In this guide:
•A warning alerts you to a potential serious outcome, adverse event or safety hazard. Failure to observe a warning may result in death or serious injury to the user or patient.
•A caution alerts you where special care is necessary for the safe and effective use of the product. Failure to observe a caution may result in minor or moderate personal injury or damage to the product or other property, and possibly in a remote risk of more serious injury.
3

1 Introduction |
Warnings and Cautions |
4

2
Site Preparation
Introduction
This section describes the procedures you should follow to plan and prepare a site for an Avalon FM20/FM30 fetal monitor installation.
•Site planning.
•Roles and responsibilities for local and Philips personnel.
Site Planning
The careful planning of the site for the FM20/FM30 monitor is essential for its safe and efficient operation. A consulting schedule should be established between the Customer and Philips Sales and Support Representatives, to ensure that all preparations are completed when the system is delivered.
The site planning phases prior to equipment installation are:
Location: Planning the location of the various system components.
Environment: Confirming and correcting, as necessary, the environment of the proposed installation site(s).
System Capabilities: Explaining the possibilities for system expansion.
Mounting: Referencing the mounting hardware information website for the listing of suitable mounting hardware recommended for use with the various system components, and all details on the available mounts and accessories.
Cabling: Identifying the requirements for the cabling, conduiting and faceplates for connecting the various system components.
Roles and Responsibilities
This section describes the procedures necessary to prepare a site for a system installation. The procedures are grouped into two parts: procedures that local staff or contractors are responsible for, and procedures that Philips personnel are responsible for.
Site Preparation Responsibilities
Local Staff
•Ensure that all safety, environmental and power requirements are met.
•Provide power outlets.
•Prepare mounts, and consult Philips for detailed mounting requirements.
5

2 Site Preparation |
Introduction |
• Pull cables, install conduit, install wallboxes.
Philips Personnel
•Provide the customer with the safety, environmental and power requirements.
•Assemble mounts, as necessary.
•Provide requirements for cabling.
Procedures for Local Staff
The following tasks must be completed before the procedures for Philips personnel may be started.
•Providing Power Outlets
Provide a power outlet in the vicinity (1 m or 3 ft) or any peripheral equipment.
WARNING Only the power cables provided with the system may be used. For reasons of safety, power (mains) extension cables or adapters shall not be used.
•Preparing Mounts
Where ceiling, wall, or shelf mounts are required for mounting the equipment, the customer is responsible for the following:
–Providing and installing all hardware which is required to install the mounting hardware supplied by Philips as detailed in the installation notes.
–Making sure that all ceilings, walls, and mounting rails that supports mounting hardware are suitable for their proposed load.
WARNING It is the customer's responsibility to have the attachment of the mounting hardware to the ceiling, wall, or mounting rail and the construction of the ceiling, wall, or mounting rail evaluated for structural integrity and compliance with all local, state and any other required codes by a registered, professional, structural and/or mechanical engineer.
Although considerable effort has been made to ensure the safety of the ceiling mount installation and or mounting guidelines, it is to be understood that the installation itself is beyond the control of Philips Medical Systems. Accordingly, Philips Medical Systems will not be responsible for the failure of any such installation.
•Providing Conduit
–Providing conduit and/or trunking of a sufficient cross-sectional area for the planned cables and possible future expansion (for additional components or systems).
–Providing and/or installing suitable wall boxes to accommodate the faceplates.
•Pulling Cables
WARNING NEVER run power cables through the same conduit or trunking used for system cables.
• Installing Wall Boxes
6

Site Requirements |
2 Site Preparation |
It is the customer's responsibility to provide and install wallboxes to house faceplates. The customer must notify the Philips installation coordinator of which size is to be used.
Procedures for Philips Personnel
Before you begin the procedures in the installation sections, ensure that the customer has completed all necessary preparations outlined in the previous section, “Procedures for Local Staff.”
Site Requirements
The site requirements are listed in this section.
Space Requirements
The situating of the monitor should be planned such that the nursing staff are able to monitor the patient with relative ease, with all patient connectors and controls readily available and the displays clearly visible. The location should also allow access to service personnel without excessive disruption and should have sufficient clearance all round to allow air circulation.
Dimensions and weight:
Monitor:
Size (W x H x D): 335 x 286 x 133 mm (13.2 x 11.3 x 5.2 in)
Weight; 5.1 kg (11.2 lb)
Transducer:
Size (diameter): 83 mm (3.27 in)
Weight (without cable): 190g (6.7 oz.)
Environmental Requirements
The environment where the FM20/FM30 monitor will be used should be reasonably free from vibration, dust and corrosive or explosive gases. The ambient operating and storage conditions for the FM20/FM30 monitor must be observed. If these conditions are not met, the accuracy of the system will be affected and damage can occur.
.
Monitor (M2702A/M2703A)
Temperature Range |
Operating |
0°C to 45°C (32°F to 113°F) |
|
Storage |
-20°C to 60°C (-4°F to 140°F) |
Humidity Range |
Operating |
<95% relative humidity @ 40°C/104°F |
|
Storage |
<90% relative humidity @ 60°C/140°F |
Altitude Range |
Operating |
-500 to 3000 m/-1640 to 9840 ft. |
|
Storage |
-500 to 3000 m/-1640 to 9840 ft. |
|
|
|
Transducers (M2734A/M2735A/M2736A)
Temperature Range |
Operating |
0°C to 40°C (32°F to 104°F) |
|
Storage |
-40°C to 60°C (-40°F to 140°F) |
|
|
|
7
2 Site Preparation |
Site Requirements |
Transducers (M2734A/M2735A/M2736A)
Humidity Range |
Operating |
<95% relative humidity @ 40°C/104°F |
|
Storage |
<90% relative humidity @ 60°C/140°F |
Altitude Range |
Operating |
-500 to 3000 m/-1640 to 9840 ft. |
|
Storage |
-500 to 3000 m/-1640 to 9840 ft. |
|
|
|
SpO2 Sensors
Operating Temperature Range |
0°C to 37°C (32°F to 98.6°F) |
|
|
Safety Requirements (Customer or Philips)
The monitor is an electrical Class II device in which the protection against electric shock does not rely on basic insulation and a protective earth conductor but on double and/or reinforced insulation.
WARNING • Always use the supplied power cord with the earthed mains plug to connect the monitor to an earthed AC mains socket. Never adapt the mains plug from the power supply to fit an unearthed AC mains socket.
•The protective earth conductor is required for EMC purposes. It has no protective function against electric shock! The protection against electric shock in this device is provided by double and/or reinforced insulation.
•Do not use additional AC mains extension cords or multiple portable socket-outlets. If a multiple portable socket-outlet without an approved isolation transformer is used, the interruption of its protective earthing may result in enclosure leakage currents equal to the sum of the individual earth leakage currents, so exceeding allowable limits.
Electrical Requirements (Customer or Philips)
Line Voltage Connection
The FM20/FM30 monitor uses < 60 W.
Line Voltage: the FM20/FM30 monitor may be operated on ac line voltage ranges of 100 to 240V (50/60 Hz).
Connecting Non-Medical Devices
The standard IEC-60601-1-1 applies to any combination of devices, where at least one is a medical device. Therefore IEC-60601-1-1 must still be met after all devices are connected.
8

Site Requirements 2 Site Preparation
WARNING • Do not use a device in the patient vicinity if it does not comply with IEC-60601-1. The whole installation, including devices outside of the patient vicinity, must comply with IEC-60601-1-1. Any non-medical device, including a PC running an OB TraceVue system, placed and operated in the patient’s vicinity must be powered via an approved isolation transformer that ensures mechanical fixing of the power cords and covering of any unused power outlets.
• Do not connect any devices that are not supported as part of a system.
Whenever you combine equipment to form a system, for example, connecting the monitor to an OB TraceVue system, perform a system test according to IEC 60601-1-1 (see “System Test” on page 36).
Figure 1 Equipment Location in the Patient Vicinity
Cabling Options and Requirements for Connection to OB TraceVue
For cabling options and requirements for connection to an OB TraceVue system, refer to the OB
TraceVue Site Preparation Guide and the OB TraceVue Service Guide.
Mounting Options
See “Mounting Hardware” on page 97 for a list of mounting options. Refer to “Mounting Instructions” on page 12, or contact your local Philips representative for advice on mounting the monitor.
9

2 Site Preparation |
Site Requirements |
Input Devices
The following tables describes the input devices which can be connected to the monitor via the optional PS/2 interface.
Product Option |
Part Number |
12NC Part |
Description |
Number |
|
Number |
|
|
|
|
|
M8024A #A01 |
862454 |
9898 031 24741 |
Slimline Keyboard with integrated Trackball |
M8024A #B01 |
M4046-60104 |
4512 610 00661 |
Optical Mouse USB / PS/2 |
M8024A #C01 |
M4046-60103 |
4512 610 00651 |
Wired Track Ball USB / PS2 |
M8024A #C02 |
M4046-60105 |
4512 610 00671 |
Wireless Track Ball |
M8024A #C03 |
M4046-60106 |
4512 610 00681 |
Wired off table Track Mouse |
|
|
|
|
10

3
Installation Instructions
The information contained in this chapter, in addition to that given in the Instructions for Use, should enable the monitor to be installed ready for use (the preparation and planning should be adhered to as specified in the “Site Preparation” chapter). Safety checks and inspection procedures for mounts are explained in the “Testing and Maintenance” chapter, and configuration of the system is explained in the “Configuration” chapter.
Please keep the packing materials until you have completed the initial inspection, in case there is a defect on arrival.
Initial Inspection
Inspect the delivery on arrival.
Mechanical Inspection
Open the shipping container(s) and examine each part of the instrument for visible damage, such as broken connectors or controls, or scratches on the equipment surfaces. If the shipping carton/container is undamaged, check the cushioning material and note any signs of severe stress as an indication of rough handling in transit. This may be necessary to support claims for hidden damage that may only become apparent during subsequent testing.
Electrical Inspection
The instrument has undergone extensive testing prior to shipment. Safety testing at installation is not required (except in situations where devices are interconnected forming a system, see “Connecting Non-Medical Devices” on page 143). An extensive self check may be performed. This recommendation does not supersede local requirements.
All tests are described in the “Testing and Maintenance” chapter of this manual.
Claims for Damage
When the equipment is received, if physical damage is evident or if the monitor does not meet the specified operational requirements of the patient safety checks or the extended self check, notify the carrier and the nearest Philips Sales/Support Office at once. Philips will arrange for immediate repair or replacement of the instrument without waiting for the claim settlement by the carrier.
11

3 Installation Instructions |
Repackaging for Shipment or Storage |
Repackaging for Shipment or Storage
If the instrument is to be shipped to a Philips Sales/Support Office, securely attach a label showing the name and address of the owner, the instrument model and serial numbers, and the repair required (or symptoms of the fault). If available and reusable, the original Philips packaging should be used to provide adequate protection during transit. If the original Philips packaging is not available or reusable please contact the Philips Sales/Support Office who will provide information about adequate packaging materials and methods.
Mounting Instructions
Every type of compatible mounting solution is delivered with a complete set of mounting hardware and instructions. Refer to the Site prep chapter for a list of mounting options. Refer to the documentation delivered with the mounting hardware for instructions on assembling mounts.
WARNING It is the customer's responsibility to have the attachment of the mounting hardware to the ceiling, wall, or mounting rail and the construction of the ceiling, wall, or mounting rail evaluated for structural integrity and compliance with all local, state and any other required codes by a registered, professional, structural and/or mechanical engineer.
Ensure that this commitment has been met before assembling mounts.
Line Voltage Selection
You do not need to set the line voltage, as this is done automatically by the power supply. The monitor has a wide-range power supply that allows you to operate the monitor from an AC (alternating current) power source of 100 V to 240 V (± 10%) and 50 to 60 Hz (± 5%).
Connecting the Monitor to AC Mains
The monitor is an electrical Class II device in which the protection against electric shock does not rely on basic insulation and a protective earth conductor but on double and/or reinforced insulation.
WARNING • Always use the supplied power cord with the earthed mains plug to connect the monitor to an earthed AC mains socket. Never adapt the mains plug from the power supply to fit an unearthed AC mains socket.
•The protective earth conductor is required for EMC purposes. It has no protective function against electric shock! The protection against electric shock in this device is provided by double and/or reinforced insulation.
•Do not use AC mains extension cords or multiple portable socket-outlets.
12

Connecting the Monitor to Non-Medical Devices |
3 Installation Instructions |
Connecting the Monitor to Non-Medical Devices
Connect the monitor to an obstetrical surveillance system, such as OB TraceVue, via the optional system interface. For cabling requirements, refer to “Cabling Options and Requirements for Connection to OB TraceVue” on page 9. For safety-related information, refer to “Connecting NonMedical Devices” on page 8, and “System Test” on page 36.
Checking and Setting Line Frequency
Before using the monitor, check that the line frequency setting is correct for your location, and change the setting if necessary in Configuration Mode.
WARNING An incorrect line frequency setting can affect the ECG filter, and disturb the ECG measurement. Ensure the line frequency setting is correct.
To set the line frequency:
1Enter the Main Setup menu.
2Select Global Settings.
3Select Line Frequency and select 50Hz or 60Hz from the pop-up list.
Checking/Setting Paper Scale
You can check the paper Scale Type (US for paper with a scale of 30-240, or Europe for paper with a scale of 50-210) in the Fetal Recorder menu. In Monitoring Mode, you can see this setting (grayed out), but you cannot change it. It can be changed in Configuration Mode.
1Enter the Main Setup menu by selecting the SmartKey  .
.
2Select Fetal Recorder.
3Check the current setting for Scale Type. If it is not appropriate, change it in the Fetal Recorder menu in Configuration Mode:
Select Scale Type to toggle between US and Europe.
Checking/Setting Paper Speed
You can choose a paper speed of 1, 2, or 3 centimeters per minute (cm/min). The default setting is 3 cm/min.
As a change in paper speed results in a change in the appearance of a FHR trace, you are advised to ensure ALL monitors in your institution are set to the same speed.
To set the paper speed:
1Enter the Main Setup menu using the SmartKey  .
.
2Select Fetal Recorder.
3In the Recorder menu, you can see the current speed setting. Select Recorder Speed.
4Select the desired speed from the given choices: 1, 2 or 3 cm/min.
13

3 Installation Instructions |
Checking and Setting Line Frequency |
PS/2 Keyboard/Mouse
Switch off the monitor before connecting any PS/2 compatible device.
Connect the PS/2 connector to the PS/2 Interface board in the monitor at the slot indicated by the appropriate symbol.
The default keyboard language setting for all initial configurations is “US”. However, the monitor will not automatically select the best matching language for the keyboard depending on the language of its software. This means that there is no such choice as “Automatic” for the keyboard language.
To configure the keyboard language manually, go to Service Mode, select Main Setup -> Hardware -> Keyboard and then select the proper language. Please note that this setting does not clone.
14
4
Theory of Operation
This chapter describes the functional operation of the monitor and the transducers. It incorporates features of the mechanical design, indicating the physical relationship of the assemblies and components.
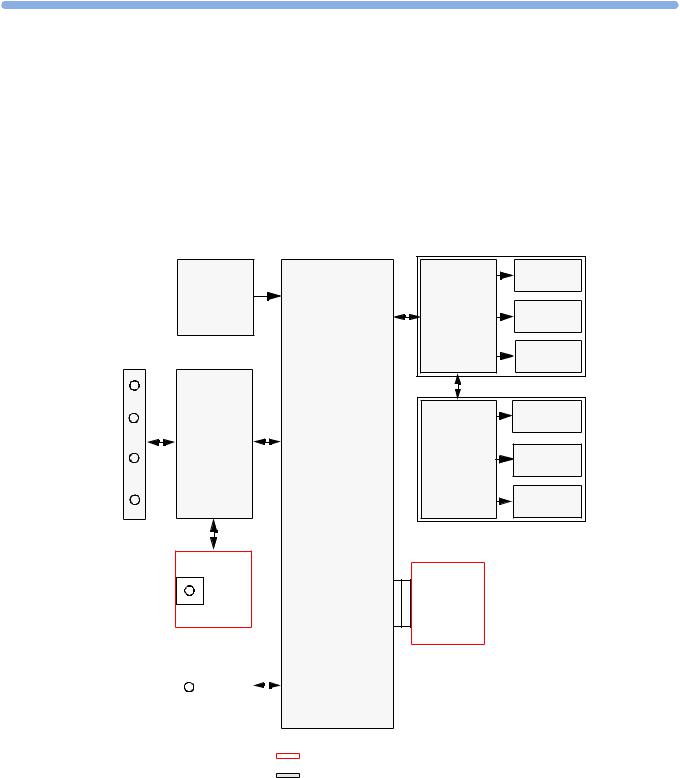
Monitor Hardware Overview
Connector Block
Power |
|
Recorder |
Thermal |
|
Main CPU |
Adapter |
Printhead |
||
Supply |
||||
|
|
|
Paper |
|
|
|
|
Sensor |
|
|
|
|
Stepper |
|
|
|
|
Motor |
|
Bus Master |
|
|
|
|
|
|
|
Touch |
|
|
|
|
Screen |
|
|
|
Display |
Display |
|
|
|
Adapter |
||
|
|
Panel |
||
|
|
|
||
|
|
|
Backlight |
|
|
|
|
Converter |
Fetal Recorder
Display Assembly
SpO2
IF Board 1
|
|
|
|
|
|
|
|
NIBP |
|
|
|
|
|
IF Board 2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
optional boards
standard boards
15

4 Theory of Operation |
Power Supply |
The monitor consists of nine main functional components:
•Power supply M2703-60001
•Connector Block (1253-8415)
•Bus Master Board (M2703-66460)
•Main CPU Board (M2703-66450)
•Fetal Recorder (Thermal Printer Unit)
•Display Assembly (M2703-64503)
•Noninvasive Blood Pressure Board (optional, M2703-64502)
•SpO2 Board (optional, M2703-66453)
•Input /Output Interface Boards (optional):
–LAN / RS232 (M2703-67501)
–Dual PS/2 (M8086-67501)
Power Supply
The power supply is a wide-range input switching unit, with an output of 24V. It is located in the bottom housing assembly.
Connector Block
Any compatible fetal transducer, patient module or remote event marker can be connected in any order to the monitor via the sockets on the Connector Block. The Connector Block is located on the Bus Master Board, and is exchangeable.
Bus Master Board
The signals from the transducers or sensors are conveyed from the sensor sockets on the Connector Carrier Board (M2703-66421) to the Bus Master Board (M2703-66420).
The Bus Master Board is responsible for transducer detection, communicates with the connected transducers via a CAN bus, and communicates parameter data to the Main CPU Board via a serial link for further processing and display.
Main CPU Board
The Main CPU Board controls the monitor’s human interface, and is responsible for the final processing of data from the Bus Master Board. It sends this data to the TFT display, and to the thermal printer unit for recording traces and other patient data. It also controls the optional LAN/RS232 and PS/2 interface boards.
Fetal Recorder (Thermal Printer Unit)
The fetal recorder is located in the Top Cover Assembly. The recorder consists of the following major parts:
16

LCD Display and Touchscreen |
4 Theory of Operation |
•Recorder Adapter Board
•Thermal Line Printhead (TLPH)
•Paper Sensor
•Stepper Motor
Recorder Adapter Board
Recorder signals are handled by the Recorder Adapter Board (M2703-66430), connected to the Main CPU Board. Video signals to the display are also wired through this board, and connection to the Display Adapter Board is made via a silver-colored 50-pin ribbon cable.
The recorder unit, including the TLPH, is connected to the Recorder Adapter Board via a white 50-pin, ribbon cable. The stepper motor and the paper sensor are also connected to the Recorder Adapter Board. The Recorder Adapter Board is connected to the Main CPU Board via a 154-pin connector.
Thermal Line Printhead (TLPH)
The TLPH is located on its own holder in the recorder chassis.
Paper Sensor
The paper sensor hardware consists of a reflective light sensor that detects the black marks on the trace paper, and paper-out. It is attached to the RFI Bracket, and connected to the Recorder Adapter Board via a removable cable connector.
Stepper Motor
The stepper motor is a bipolar motor controlled by a micro-stepping motor driver on the Recorder Adapter Board. The motor is located on the recorder chassis and is connected to the Recorder Adapter Board via a removable cable connector.
LCD Display and Touchscreen
The LCD Display Assembly consists of a four-wire resistive touchscreen, a 6.5” TFT panel, and a backlight inverter, all connected to the Display Adapter Board (M2703-66440) and fitted into the display housing.
The board is connected to the Recorder Adapter Board (M2703-66430) via a 50-pin ribbon cable. The green power LED is incorporated into the Display Adapter Board.
Noninvasive Blood Pressure Assembly
The optional Noninvasive Blood Pressure Assembly (M2703-64602) is located in the front lefthand corner of the bottom housing assembly. It is connected via a serial link to the Main CPU Board.
SpO2 Assembly
The optional SpO2 Assembly (M2703-64603) is physically located on the Bus Master Board, but sends data directly to the Main CPU Board via a serial link.
17
4 Theory of Operation |
Input/Output Interface Boards |
Input/Output Interface Boards
There are two optional interface boards available:
•LAN/RS232 Interface Board, used for connecting to a PC running the Support Tool and to a surveillance and documentation system such OB TraceVue.
•PS/2 Interface Board, used for connecting an external keyboard or mouse.
The interface boards plug into the two interface slots on the underside of the device, and are controlled by the Main CPU Board.
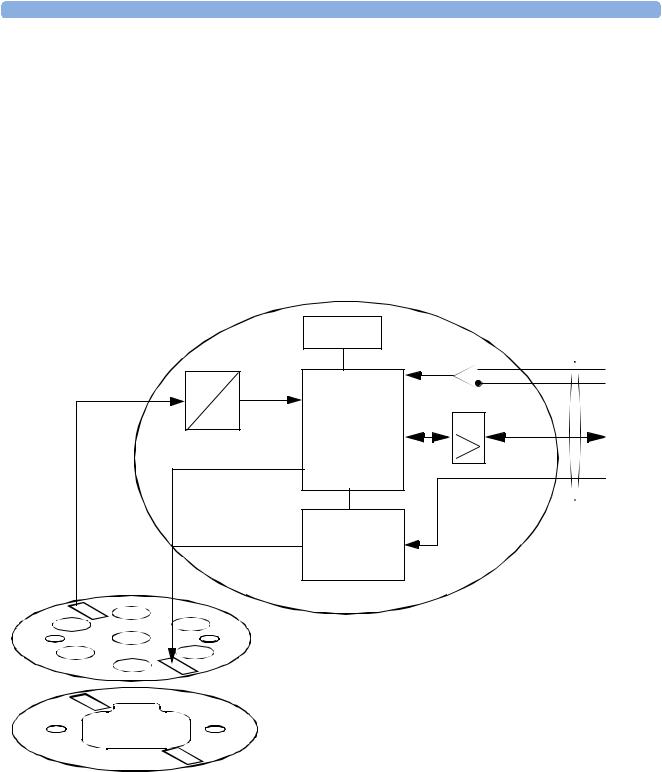
Transducer Hardware Overview
|
EEPROM |
Transducer |
|
|
cable |
Analog/ Digital conversion |
Master Clock recovery |
|
|
||
A |
|
|
Analog Signal |
|
Communication Transceiver |
|
|
|
D |
Signal |
|
|
|
|
|
processor |
|
Timing and Mode Control |
|
|
|
|
Power and |
|
|
Identification |
|
Power supply |
|
|
and reset |
|
|
|
Transducer CPU board |
|
Ultrasound frontend board |
|
|
or alternatively |
|
TOCO/ECG frontend board
1
18

Transducer Types |
4 Theory of Operation |
Transducer Types
Transducers consist of the following types:
•US
•Toco
•Toco+ (includes DECG/MECG and IUP capability)
They all share the same power supply, and analog-to-digital conversion circuitries. The processor software is also the same for all transducers. The frontends, however, are specific to each transducer type.
Functional Description of the Transducer CPU
The CPU section of the transducers is made up of the following main functional blocks:
•CPU (micro controller)
•Analog-to-Digital Converter
•Communication Transceiver (CAN bus driver)
•EEPROM
CPU (Micro Controller)
A single-chip processor is used to control the transducer, generate the frontend control signals, control the analog-to-digital signal conversion, and to perform the signal processing.
Analog-to-Digital Converter
Analog-to-digital (A/D) signal conversion is carried out by the 16-bit AD converter. Digital signals are directly communicated from the A/D converter to the CPU.
Communication Transceiver (CAN Bus Driver)
The communications transceiver (CAN bus driver) communicates directly with the transducer CPU, and allows the transducer to communicate with the Bus Master Board via the CAN bus.
EEPROM
The serial EEPROM stores all non-volatile data required to operate the transducer (for example, calibration and correction factors for frontend gains and offsets, country-specific information, serial numbers and error logs).
Toco Transducer Frontend
Uterine activity is measured by evaluating the hardness of the mother’s abdomen with a pressure sensitive resistor bridge (strain gauge sensor element). The strain gauge sensor element requires an excitation voltage and its differential output signal is proportional to the pressure applied to it. A DC excitation voltage is used, and the resulting output signal is fed directly to an A/D signal converter before being sent to the processor.
19

4 Theory of Operation |
Ultrasound Transducer Frontend |
Ultrasound Transducer Frontend
The ultrasound frontend is a pulsed Doppler system with a 1.0 MHz ultrasound frequency, and a pulse repetition rate of 3 kHz. Seven ultrasound crystals are used as transmitter and receiver.
Toco+ Transducer Frontends
Several parameter frontends are combined on one board. In addition to the Toco frontend, additional supported parameters are DECG, MECG and IUP.
A seven-pin ‘D-type’ socket carries all parameter related inputs and outputs. An external mode resistor, connected to one of the pins, automatically detects which mode to set when an adapter cable is plugged in (whether it is DECG, MECG, or IUP).
Toco Frontend
See “Toco Transducer Frontend” on page 19.
IUP Frontend
Intrauterine pressure (IUP) is measured via a piezo resistive bridge with AC excitation connected to the RA / LA input pins of the ECG amplifier. A/D conversion of the IUP signal is done by the 16-bit A/D converter.
ECG Frontend
The ECG frontend measures both DECG and MECG, using a 3-lead system (RA, LA and reference electrode). The ECG mode is automatically detected when an adapter cable is attached. Input lines are ESD protected.
20

5
Interfaces
There are two interface boards available as options for the Avalon fetal monitors:
•LAN / RS232 system interface
•Dual PS/2 interface
Dual PS/2 system
interface
LAN / RS232 system
interface
The interfaces are “plug-and-play” boards, and fit into dedicated slots on the underside of the monitor. See “Removing the Interface Boards” on page 87 for details of how to remove and fit the boards.
LAN / RS232 Interface
The LAN / RS232 system interface has two fully isolated ports:
•The LAN connection can be used for connecting the monitor to PC for configuration or upgrade using the Support Tool, and for future system expansion.
•The RS232 connection can be used for connecting the monitor to an obstetrical information and surveillance system, such as OB TraceVue.
Dual PS/2 Interface
This interface provides two PS/2 ports to enable the monitor to be connected to off-the-shelf, “plug-and- play” input devices:
•Mouse: any specified PS/2 mouse or trackball may be used for navigation and data entry.
•Computer keyboard: a PS/2 computer keyboard can be used for data entry instead of the on-screen pop-up keyboard.
21

5 Interfaces |
Dual PS/2 Interface |
22
 Loading...
Loading...