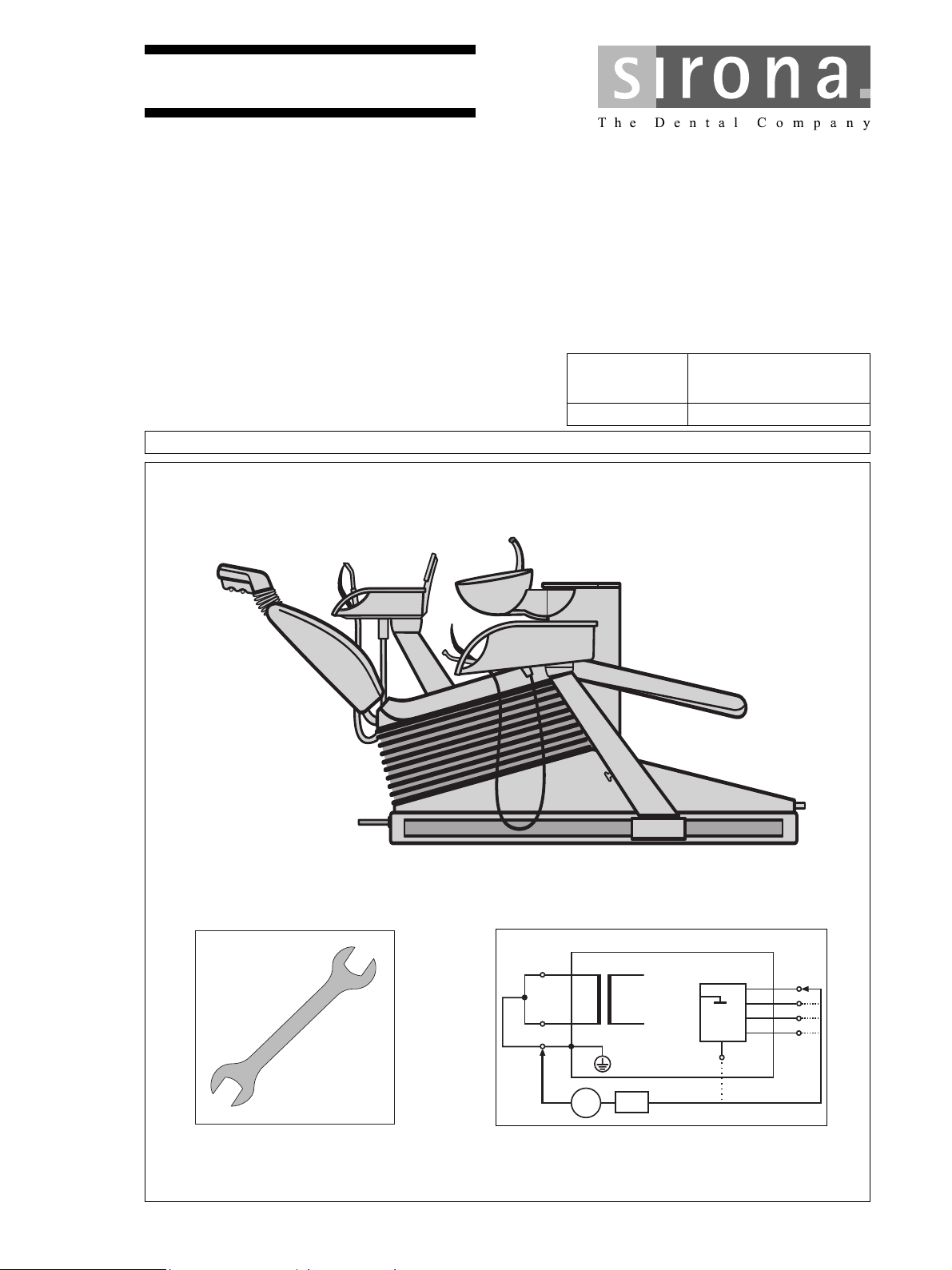
Sirona C4+, C2+, C5+, M1+, C1+ Maintenance manual
...
kЙп=лбеЕЙW=
MQKOMMR
`NHÓ=`RHI=`RHqìêå=I=jN
H
j~бенЙе~еЕЙ=j~ем~д
C ______
M1
Model Serial No. of Chair
+
+
bеЦдблЬ
PE
E
N
L
AMP
MD
~
P
AP

2 D 3264.102.02.04.02 04.2005
59 51 525 D 3264

Table of Contents
1
General information ............................................................... 5
1.1 Purpose of the Maintenance Manual ..............................5
1.2 Work to be performed .....................................................5
2 Installation Report / Warranty Passport ................................. 6
2.1 Master data of the unit ....................................................6
2.2 Inspection and maintenance ...........................................6
3 Safety tests ............................................................................ 7
3.1 Visual inspection .............................................................9
3.2 Protective ground wire test............................................10
3.3 Measurement of equivalent leakage currents ...............11
3.3.1 Equivalent device leakage current ........................................ 13
3.3.2 Equivalent patient leakage current ....................................... 14
3.4 Safety test
(Initial test after initial start-up) ......................................15
3.5 Safety test
(Re-tests) ......................................................................15
4 Treatment centers with HF surgery equipment ................... 20
4.1 General information.......................................................20
4.2 List of trained personnel................................................21
4.3 Repair work on the HF module .....................................22
4.4 Effects of malfunctions and repeated, similar operator
errors on the
HF module.....................................................................24
5 Reporting of incidents to authorities / manufacturers .......... 26
6 Remarks / particularities regarding the treatment center ..... 28
59 51 525 D 3264
D 3264.102.02.04.02 04.2005
3

4 D 3264.102.02.04.02 04.2005
59 51 525 D 3264
1 General information
1.1 Purpose of the Maintenance Manual
In order to guarantee the operational safety and reliability of the system and
to protect the health of patients, users and other persons, inspection and
maintenance must be performed at predetermined intervals.
This includes:
Inspection and maintenance (yearly)
to avoid damage due to natural wear
Safety tests (every 2 years)
to ensure the tecical safety of the system
This document describes the work to be performed by the service engineer.
Its realization and the measurement results are documented by the service engineer.
This document must be stored near the treatment center.
i
NOTE
For units with HF surgery equipment, this Maintenance Manual simultaneously acts as Medical Product Log.
1 General information
bеЦдблЬ
1.2 Work to be performed
By the service engineer: 1. Note the model and the serial number of the chair on the title page and the
relevant pages (headers) of the Maintenance Manual.
2. Complete the “Installation Report / Warranty Passport” and file it after
chapter 2.
3. Perform inspection and maintenance in accordance with the Maintenance
Certificate.
Document their realization on the “Installation Report / Warranty Passport”.
4. Conduct the safety tests in accordance with chapter 3. Document the re-
sults.
5. On units with HF surgery equipment, maintain the documentation re-
quired in section 4.2 and 4.3.
6. Document additional remarks and particularities regarding the treatment
center in chapter 5.
By the user: 1. On units with HF surgery equipment, maintain the documentation re-
quired in section 4.2 and 4.4.
2. Document the reporting of incidents to authorities / manufacturers in
chapter 5.
59 51 525 D 3264
D 3264.102.02.04.02 04.2005
5

2 Installation Report / Warranty Passport
2 Installation Report / Warranty Passport
2.1 Master data of the unit
Complete the document “Installation Report / Warranty Passport” and file
the “Customer Copy” after this page.
Unusual occurrences during installation can be noted down in addition on the
second page of the “Dealer Copy”.
2.2 Inspection and maintenance
To avoid damage due to natural wear, an inspection must be performed every
year.
The treatment center independently recognizes the necessity of regular maintenance and displays this in due time.
You will find further information concerning the maintenance display in the
Operating Instructions.
The steps to be performed as well as the parts which must be replaced are
specified in the document “Maintenance Certificate”. Their realization is
documented there.
For each maintenance event, a separate Maintenance Certificate is produced.
List the inspection and maintenance events also under the maintenance overview in the “Installation Report/Warranty Passport”.
Appendix: Installation Report / Warranty Passport
59 51 525 D 3264
6 D 3264.102.02.04.02 04.2005
3 Safety tests
3 Safety tests
Medical products are designed in such a way that the first occurrence of a
fault does not create a hazard to the safety of the patient, the user or other
persons. Hence it is important to detect such faults before a second fault
occurs, which might then lead to safety hazards.
For that reason it is essential to perform safety tests every 2 years which aim
particularly at detecting electrical faults. All inspections and measurements
are performed by the authorized service engineer. They are specified in the
following.
Safety tests are performed on the following occasions:
Initial start-up (section 3.4)
regularly every 2 years
after extensions/upgrades (conversion) of the treatment center
after repair work
You must document the measured values in section 3.4 and/or 3.5.
ATTENTION
When making measurements, please observe that hazardous voltages might
be present on the system under test.
ATTENTION
If the treatment center does not pass the safety tests, it may no longer be operated!
In your capacity as service engineer, you must advise the user of this fact.
Corresponding repair work by an authorized service engineer is required before putting the system into service again.
i
NOTE
The safety tests are in compliance with the standard VDE 0751-1:2001. It you
use an automatic tester, you can program it according to this standard.
Type BF applied parts
Permanently installed unit
Protection class I
The auxiliary measuring point (see 3.3) is treated like an applied part.
Sirona recommends using an automatic tester.
bеЦдблЬ
59 51 525 D 3264
D 3264.102.02.04.02 04.2005
7
3 Safety tests
Measurement according to IEC 60601-1: If you have no possibility of performing the measurements according to
VDE 0751-1:2001, you may also perform them according to IEC 60601-1.
For details on how to perform the measurements, please refer to the standard
IEC 60601-1 and the documents on your measuring device.
i
NOTE
This type of measurement is not recommended by Sirona due to its
complexity.
When taking measurements, please observe the following:
Type B applied parts Micromotor
Highspeed handpiece
Ultrasound handpieces
Polylight
Sprayvit (peak)
Type BF applied parts Sirocam 3
SIROCAM C (no measurement
required)
HF surgery - handpiece
Protective ground wire resistance
Earth leakage current N.C. – 5mA
Patient leakage current N.C. – 0.1mA
During the measurements, the individual dental instruments must be operated one after the other.
You measure HF surgery however in the inactive condition.
Several measurements in succession may be required.
Make a note in Section 3.4 or 3.5 stating that you have performed the measurements according to IEC 60601-1 and correct the specified limiting values.
Document the highest measured values.
≤ 0.1Ω
S.F.C. – 10mA (permanent connection)
S.F.C. – 0.5mA
NC. – normal condition
S.F.C. – single fault condition
8 D 3264.102.02.04.02 04.2005
59 51 525 D 3264
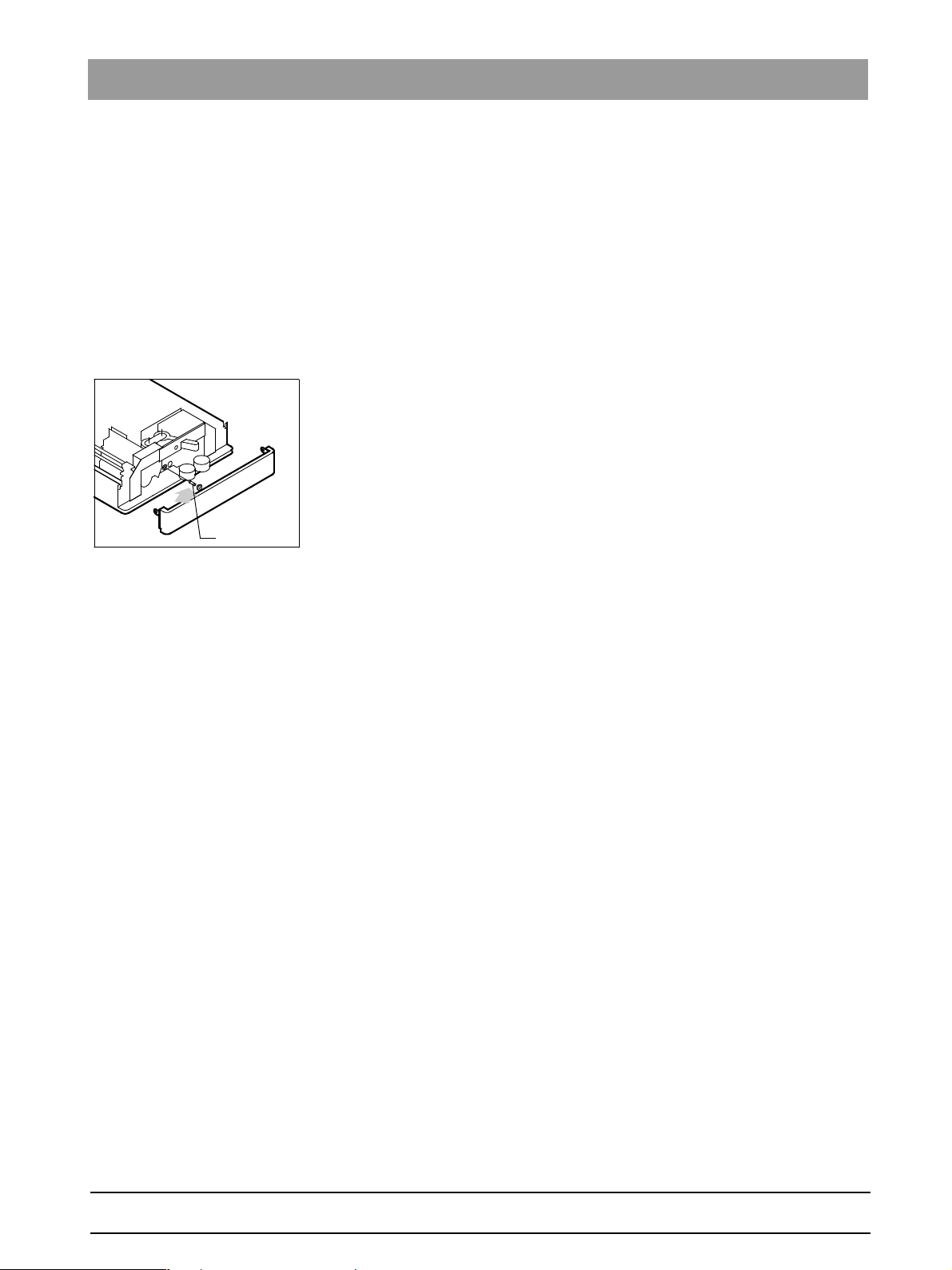
Fig. 3-1 Main fuse
3 Safety tests
3.1 Visual inspection
Check the following details:
Perform a functional test of the treatment center in accordance with the
operating instructions.
Are all functions present?
Are all optical and acoustic warning signals functioning properly?
Are all safety switches functioning?
Are all housing parts safely attached and intact?
Are all protective ground wire connections present, properly attached and
intact?
Does the treatment center have the right main fuse (1)? To check this, un-
screw fuse and compare it to the label next to it.
Are all labels according to the “Installation Report / Warranty Passport” af-
fixed and legible?
Are all operating instructions which belong to the treatment center avail-
able?
Is the document “Care and Maintenance by the Practice Team” available?
1
Is the “Maintenance Manual”, serving also as Medical Product Log on
units with HF surgery equipment, available?
In Germany:
Is the Service Logbook of the amalgam separator (if applicable) available?
bеЦдблЬ
Test preparations Before beginning with the tests described below, make the following prepara-
tions:
The treatment center must be de-energized by means of the building in-
stallation
Open the cover of the connection box in the chair
For a video system connected to a PC:Pull the power plug of the PCs
Disconnect all poles (also PE) of the mains supply at the connection ter-
minal (except protective ground wire PE)
59 51 525 D 3264
D 3264.102.02.04.02 04.2005
9
 Loading...
Loading...