Hanna Instruments HI 83226-01 User Manual
Instruction Manual
HI 83226
Multiparameter Bench
Photometer
for Pool & Spa Applications
w w w . h a n n a i n s t . c o m
1

Dear Customer,
Thank you for choosing a Hanna product. Please read this instruction manual carefully before using the instrument. This manual will provide you with the necessary information for the correct use of the instrument. If you need additional technical information, do not hesitate to e-mail us at tech@hannainst.com.
|
TABLE OF CONTENTS |
PRELIMINARY EXAMINATION .............................................................................................................................................. |
3 |
ABBREVIATIONS ................................................................................................................................................................ |
3 |
GENERAL DESCRIPTION ...................................................................................................................................................... |
3 |
SIGNIFICANCE OF POOL AND SPA TESTING .............................................................................................................................. |
4 |
SPECIFICATIONS ............................................................................................................................................................... |
8 |
PRECISION AND ACCURACY ................................................................................................................................................ |
8 |
PRINCIPLE OF OPERATION ................................................................................................................................................. |
8 |
FUNCTIONAL DESCRIPTION .............................................................................................................................................. |
10 |
TIPS FOR AN ACCURATE MEASUREMENT .......................................................................................................................... |
11 |
HEALTH & SAFETY .......................................................................................................................................................... |
14 |
METHOD REFERENCE TABLE ............................................................................................................................................. |
14 |
OPERATIONAL GUIDE ....................................................................................................................................................... |
15 |
SETUP ........................................................................................................................................................................... |
17 |
HELP MODE ................................................................................................................................................................... |
19 |
ALKALINITY .................................................................................................................................................................... |
20 |
BROMINE ...................................................................................................................................................................... |
22 |
CALCIUM HARDNESS ....................................................................................................................................................... |
24 |
FREE CHLORINE ............................................................................................................................................................. |
27 |
TOTAL CHLORINE ............................................................................................................................................................ |
29 |
FREE COPPER ................................................................................................................................................................ |
31 |
TOTAL COPPER ............................................................................................................................................................... |
33 |
CYANURIC ACID .............................................................................................................................................................. |
35 |
IRON ............................................................................................................................................................................. |
37 |
OZONE .......................................................................................................................................................................... |
39 |
pH ................................................................................................................................................................................ |
42 |
ERRORS AND WARNINGS ................................................................................................................................................. |
44 |
DATA MANAGEMENT ........................................................................................................................................................ |
45 |
STANDARD METHODS ...................................................................................................................................................... |
45 |
ACCESSORIES ................................................................................................................................................................ |
46 |
WARRANTY .................................................................................................................................................................... |
47 |
HANNA LITERATURE ........................................................................................................................................................ |
47 |
All rights are reserved. Reproduction in whole or in part is prohibited without the written consent of the copyright owner, Hanna Instruments Inc., Woonsocket, Rhode Island, 02895 , USA.
2
PRELIMINARY EXAMINATION
Please examine this product carefully. Make sure that the instrument is not damaged. If any damage occurred during shipment, please notify your local Hanna Office.
Each Meter is supplied complete with:
•Four Sample Cuvettes and Caps
•Cloth for wiping cuvettes (1 pcs)
•Scissors
•AC/DC Power Adapter
•Instruction Manual
Note: Save all packing material until you are sure that the instrument works correctly. Any defective item must be returned in its original packing with the supplied accessories.
|
ABBREVIATIONS |
|
|
EPA: |
US Environmental Protection Agency |
°C: |
degree Celsius |
°F: |
degree Fahrenheit |
g/L: |
micrograms per liter (ppb) |
mg/L: |
milligrams per liter (ppm) |
g/L: |
grams per liter (ppt) |
mL: |
milliliter |
HR: |
high range |
MR: |
medium range |
LR: |
low range |
PAN: |
1-(2-pyridylazo)-2-naphtol |
TPTZ: |
2,4,6-tri-(2-pyridyl)-1,3,5-triazine |
GENERAL DESCRIPTION
HI 83226 is a multiparameter bench photometer dedicated for Pool & SPA applications. It measures 11 different methods using specific liquid or powder reagents. The amount of reagent is precisely dosed to ensure maximum reproducibility.
HI 83226 bench photometer can be connected to a PC via an USB cable. The optional HI 92000 Windows® Compatible Software helps users manage all their results.
HI 83226 has a powerful interactive user support that assists the user during the analysis process.
Each step in the measurement process is help supported. A tutorial mode is available in the Setup Menu.
3

SIGNIFICANCE OF POOL AND SPA TESTING
A major family leisure pursuit is the enjoyment of Swimming Pool and Spa facilities world-wide. A basic necessity of Pool water treatment, to ensure such enjoyment, is to maintain the water in a safe and pleasant condition for the bathers.
In order to achieve such an objective, swimming pool water requires testing on daily, and sometimes hourly bases for disinfection residuals and pH. Equally important, Calcium Hardness and Alkalinity parameters should be monitored on weekly bases to ensure the pool water is maintained in a balanced condition, thus to avoid system failure because of corrosion or scale formation.
DISINFECTION RESIDUAL AND pH CONTROL
In terms of swimming pool treatment, disinfection or sanitizing basically means to rid the pool of bather pollution, destroy bacteria, and control nuisance organisms like algae, which may occur in the pool, filtration equipment, and piping.
There are a number of techniques used, namely, chlorine, bromine and ozone dosing systems, of which chlorine is the most common.
Chlorine
Chlorine is a strong oxidizing agent that destroys mostly organic pollutants, bacteria and can combine with nitrogen containing compounds, forming chloramines. Only a part of the original quantity dosed chlorine, remains active and continues its disinfecting action.
From the free chlorine you can distinguish combined chlorine, as that part which combines with nitrogen containing compound and that is less efficient as a disinfectant. The addition of these two parts gives total chlorine. A pool manager needs to aim perfection where free equals total chlorine, and thus to maintain the combined chlorine concentration near zero. The presence of chloramines is not desired because of the distinctive ‘swimming pool’ smell caused by combined chlorines like di-chloramines. Beside this unpleasant odour it does irritate the eyes and the mucous membranes.
Commercially chlorine for disinfection may be available as a gas (Cl2), a liquid like sodium hypochlorite or bleach (NaOCl) or in a solid state like calcium hypochlorite, chloro-hydantoins or chloro-cyanuric acid compounds. These compounds, once dissolved in water do establish equilibrium between the hypochlorous acid (HOCl) and the hypochlorite ions (OCl¯). Although both forms are considered free chlorine, it is the hypochlorous acid that provides the strongest disinfecting and oxidising characteristic of chlorine solutions.
The amount of hypochlorous acid in chlorinated water dependends upon the pH value of the solution. Changes in pH value will effect the HOCl equilibrium in relation to the hydrogen and hypochlorite ion.
As depicted by the curve on the next page, HOCl decreases and OCl¯ increases as pH increases. At a low pH, almost all the free chlorine is in the molecular form HOCl and at a pH of around 7.5, the ratio between HOCl and OCl¯ is 50:50. Since the ionic form OCl¯ is a slow acting sanitizer while the molecular HOCl is a fast acting, it is important to measure regularly the pH. As a general rule a pH of about 7.2 is recommended to maintain fast acting disinfection conditions.
Bromine
In many countries bromine sanitizing has been introduced as an alternative for chlorine, although it is a less strong sanitizer. The advantage of bromine is its stability at higher temperatures (advantageous for hot well pools), and its maintained disinfection power at higher pH. Further it does hardly react with nitrogen compounds, reducing the unpleasant odour,and eye irritation problems. The main disadvantage of bromine is the slower acting disinfecting power, making it less suitable for larger pools.
Ozone
Ozone is a very strong oxidizing agent that does destroy most difficult to oxidize organic compounds and chloramines. It thus allows the pool manager to remove very efficiently combined chlorine without refreshing frequently large amounts of pool water. In general its application is found just before water passes through the filter units. Its sanitizing power is not pH related.
Mainly because of its strong oxidizing power the return water may contain only trace concentrations of ozone. It has to be mentioned that ozone is very unstable and there is anyway the need for low-level chlorination to ensure sanitizing throughout the whole pool.
THE WATER BALANCE AND LANGELIER INDEX (LI)
The pool water characteristics need to be maintained in a balanced condition to avoid system failure. Measuring the water balance is extremely important to predict if the water is corrosive, scaling or balanced.
A saturation index developed by Dr. Wilfred Langelier is widely used to predict the balance of swimming pool waters. It is an estimation of the solutions ability to dissolve or precipitate calcium carbonate deposits. A certain level of this precipitation (filming) is desired to insulate pipes and boilers from contact with water. When no protective filming is formed, water is considered to be corrosive. On the other hand scaling does cause failure because of incrustation problems.
In the treatment and monitoring of pool water, the pool manager must ensure that related parameters as alkalinity, hardness and pH are duly taken into consideration.
4 |
5 |
Calcium Hardness
The presence of calcium in the system is desired to ensure filming on those places where the temperature is relatively high, like in boilers and pipes transporting warm water. Scaling must be avoided because it reduces heat transfer and pump capacity. Beside the calcium carbonate deposits in the pipes, high scaling values do cause cloudy water. It is recommended to maintain the calcium hardness value within the range from 200 to 400 ppm as calcium carbonate (CaCO3).
Alkalinity
Alkalinity is the measure of the total concentration of alkaline substances, mostly bicarbonates, dissolved in the water. The higher the alkalinity the more resistant the water is to pH change, the alkalinity buffers the water. At the same time, high alkaline water is a major contributor to scaling problems like incrustation in filtration equipment, pumps, and piping.
It is recommended to maintain the alkalinity value within the range from 80 to 125 ppm as calcium carbonate (CaCO3).
pH
The pH of the water is an important factor since at lower pH the corrosion rate increases. If the alkalinity values are sufficiently high it will not be difficult to control the pH. Most pools managers do prefer to keep the pH between 7.2 and 7.4, that does ensure low corrosion rates and a sufficient activity of chlorine.
Langelier Index (LI)
The Langelier Index is a powerful tool to calculate the water balance, and to predict corrosion or scaling problems. Theoretically, a LI of zero indicates perfect water condition for swimming pools. If LI>0, scaling and staining of the water is present, and if LI<0 the water is corrosive and highly irritating. A tolerance of ±0.4 is normally acceptable.
The Langelier formula is expressed as:
LI = pH + TF + HF + AF – 12.5
where: |
|
|
LI |
= |
Langelier Index (also called Saturation Index) |
pH |
= |
pH of the water |
TF |
= |
temperature factor |
HF |
= |
hardness factor, log(Ca Hardness, ppm as CaCO3) |
AF |
= |
alkalinity factor, log(Alkalinity, ppm as CaCO3) |
To calculate the exact Langelier Index of your water please use the WATER INDEX reference tables at the end of this chapter to find the Temperature, Hardness and Alkalinity factors.
Recommendations
For most pools, water is balanced if:
•The pH value is maintained within the recommended ranges of pH 7.2 - 7.6
•Ideally the Alkalinity should be maintained within a range of 80 - 125 ppm
•The Calcium Hardness should be maintained within a range of 200 - 400 ppm.
To calculate your water balance three tests are required, measure the Calcium Hardness, the Alkalinity and the pH of the pool water. Find the Hardness and Alkalinity Factor in the WATER INDEX reference tables below. The water temperature is in general controlled between 24oC (76oF) and 34oC (94oF) to ensure pleasant bather comfort. The Temperature Factor in this temperature range has minor importance; therefore an average value of 0.7 may be used.
A simple calculation classifies your water in corrosive, scaling, acceptable or ideal balanced, with treatment recommendations:
Water Balance = pH + TF + HF + AF
Water Balance |
Condition of Water |
Recommendation |
|
11.0 |
– 12.0 |
Corrosive |
Increase pH and/or Alkalinity |
12.1 – 12.3 |
Acceptable Balance |
Retest water frequently |
|
12.4 – 12.6 |
Ideal Balance |
|
|
12.7 – 12.9 |
Acceptable Balance |
Retest water frequently |
|
13.0 – 14.0 |
Scale forming |
Reduce pH and/or alkalinity |
|
|
|
|
|
WATER INDEX REFERENCE TABLES
|
Temperature |
|
|
Calcium Hardness |
|
|
Alkalinity |
||||||||||
|
°C |
|
°F |
|
TF |
|
|
mg/L |
|
HF |
|
|
|
mg/L |
|
AF |
|
|
|
|
|
|
|
|
|
(as CaCO3) |
|
|
|
|
|
|
(as CaCO3) |
|
|
|
0 |
32 |
|
0 |
|
|
5 |
|
0.7 |
|
|
|
5 |
0.7 |
|||
|
4 |
39 |
|
0.1 |
|
|
25 |
|
1.4 |
|
|
|
25 |
1.4 |
|||
|
8 |
46 |
|
0.2 |
|
|
50 |
|
1.7 |
|
|
|
50 |
1.7 |
|||
|
12 |
54 |
|
0.3 |
|
|
75 |
|
1.9 |
|
|
|
75 |
1.9 |
|||
|
16 |
60 |
|
0.4 |
|
|
100 |
|
2.0 |
|
|
|
100 |
2.0 |
|||
|
20 |
68 |
|
0.5 |
|
|
150 |
|
2.2 |
|
|
|
150 |
2.2 |
|||
|
24 |
75 |
|
0.6 |
|
|
200 |
|
2.3 |
|
|
|
200 |
2.3 |
|||
|
28 |
82 |
|
0.7 |
|
|
250 |
|
2.4 |
|
|
|
250 |
2.4 |
|||
|
32 |
90 |
|
0.7 |
|
|
300 |
|
2.5 |
|
|
|
300 |
2.5 |
|||
|
36 |
97 |
|
0.8 |
|
|
400 |
|
2.6 |
|
|
|
400 |
2.6 |
|||
|
40 |
104 |
|
0.9 |
|
|
500 |
|
2.7 |
|
|
|
500 |
2.7 |
|||
|
50 |
122 |
|
1.0 |
|
|
1000 |
|
3.0 |
|
|
|
1000 |
3.0 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EXAMPLE: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
Pool water conditions |
|
|
Factor value |
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
(nearest values) |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
Temperature |
30°C |
|
|
|
TF = 0.7 |
|
|
|||||||
|
|
|
pH |
|
|
|
|
7.2 |
|
|
|
|
pH = 7.2 |
|
|
||
|
|
|
Alkalinity |
80 mg/L |
|
|
|
AF = 1.9 |
|
|
|||||||
|
|
|
Hardness |
230 mg/L |
|
|
|
HF = 2.4 |
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Water Balance = pH + TF + HF + AF = 7.2 + 0.7 + 2.4 + 1.9 = 12.2
Conclusion: the water is acceptable balanced but there is some risk that the water becomes corrosive; frequently testing is recommended.
6 |
7 |
|
SPECIFICATIONS |
|
|
Light Life |
Life of the instrument |
Light Detector |
Silicon Photocell |
Environment |
0 to 50°C (32 to 122°F); |
|
max 90% RH non-condensing |
Power Supply |
external 12 Vdc power adapter |
Auto-Shut off |
built-in rechargeable battery |
Dimensions |
235 x 200 x 110 mm (9.2 x 7.87 x 4.33") |
Weight |
0.9 Kg |
For specifications related to each single method (e.g. range, resolution, etc.), refer to the related measurement section.
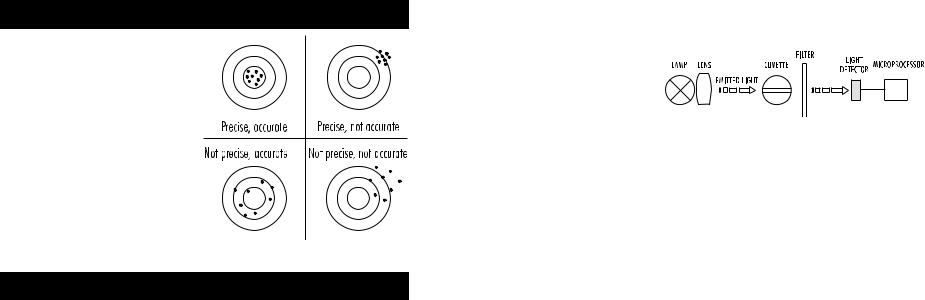
PRECISION AND ACCURACY
Precision is how closely repeated measurements agree with each other. Precision is usually expressed as standard deviation (SD).
Accuracy is defined as the nearness of a test result to the true value.
Although good precision suggests good accuracy, precise results can be inaccurate. The figure explains these definitions.
For each method, the precision is expressed in the related measurement section.
PRINCIPLE OF OPERATION
Absorption of light is a typical phenomenon of interaction between electromagnetic radiation and matter. When a light beam crosses a substance, some of the radiation may be absorbed by atoms, molecules or crystal lattices.
If pure absorption occurs, the fraction of light absorbed depends both on the optical path length through the matter and on the physical-chemical characteristics of substance according to the Lambert-Beer Law:
-log I/Io = ελ c d or
A = ελ c d
Where: |
|
|
-log I/Io= |
Absorbance (A) |
|
Io |
= |
intensity of incident light beam |
I |
= |
intensity of light beam after absorption |
ελ |
= |
molar extinction coefficient at wavelength λ |
c |
= |
molar concentration of the substance |
d |
= |
optical path through the substance |
Therefore, the concentration "c" can be calculated from the absorbance of the substance as the other factors are known.
Photometric chemical analysis is based on the possibility to develop an absorbing compound from a specific chemical reaction between sample and reagents.
Given that the absorption of a compound strictly depends on the wavelength of the incident light beam, a narrow spectral bandwidth should be selected as well as a proper central wavelength to optimize measurements. The optical system of HI 83226 is based on special subminiature tungsten lamps and narrow-band interference filters to guarantee both high performance and reliable results.
Two measuring channels allow a wide range of tests.
Instrument block diagram (optical layout)
A microprocessor controlled special tungsten lamp emits radiation which is first optically conditioned and beamed through the sample contained in the cuvette. The optical path is fixed by the diameter of the cuvette. Then the light is spectrally filtered to a narrow spectral bandwidth, to obtain a light beam of intensity Io or I. The photoelectric cell collects the radiation I that is not absorbed by the sample and converts it into an electric current, producing a potential in the mV range.
The microprocessor uses this potential to convert the incoming value into the desired measuring unit and to display it on the LCD.
The measurement process is carried out in two phases: first the meter is zeroed and then the actual measurement is performed.
The cuvette has a very important role because it is an optical element and thus requires particular attention. It is important that both the measurement and the calibration (zeroing) cuvette are optically identical to provide the same measurement conditions. Most methods use the same cuvette for both, so it is important that measurements are taken at the same optical point. The instrument and the cuvette cap have special marks that must be aligned in order to obtain better reproducibility.
The surface of the cuvette must be clean and not scratched. This is to avoid measurement interference due to unwanted reflection and absorption of light. It is recommended not to touch the cuvette walls with hands. Furthermore, in order to maintain the same conditions during the zeroing and the measurement phases, it is necessary to cap the cuvette to prevent any contamination.
8 |
9 |
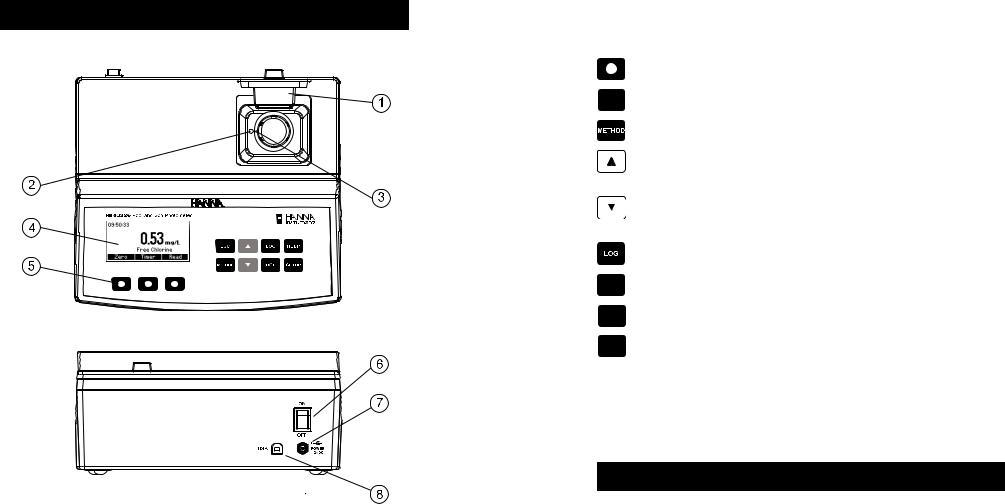
FUNCTIONAL DESCRIPTION
INSTRUMENT DESCRIPTION
1)Open Cuvette Lid
2)Indexing mark
3)Cuvette point
4)Liquid Crystal Display (LCD)
5)Splash proof keypad
6)ON/OFF power switch
7)Power input connector
8)USB connector
KEYPAD DESCRIPTION
The keypad contains 8 direct keys and 3 functional keys with the following functions:
Press to perform the function displayed above it on the LCD.
ESC |
Press to exit the current screen. |
|
|
|
Press to access the select method menu. |
|
Press to move up in a menu or a help screen, to increment a set value, to access second level |
|
functions. |
|
Press to move down in a menu or a help screen, to decrement a set value, to access second |
|
level functions. |
|
Press to log the current reading. |
RCL |
Press to recall the log. |
HELP Press to display the help screen.
SETUP |
Press to access the setup screen. |
TIPS FOR AN ACCURATE MEASUREMENT
The instructions listed below should be carefully followed during testing to ensure most accurate results.
•Color or suspended matter in large amounts may cause interference, and should be removed by treatment with active carbon and filtration.
•Ensure the cuvette is filled correctly: the liquid in the cuvette forms a convexity on the top; the bottom of this convexity must be at the same level as the 10 mL mark.
COLLECTING AND MEASURING SAMPLES
•In order to measure exactly 0.5 mL of reagent with the 1 mL syringe:
(a)push the plunger completely into the syringe and insert the tip into the solution.
(b)pull the plunger up until the lower edge of the seal is exactly on the 0.0 mL mark.
10 |
11 |
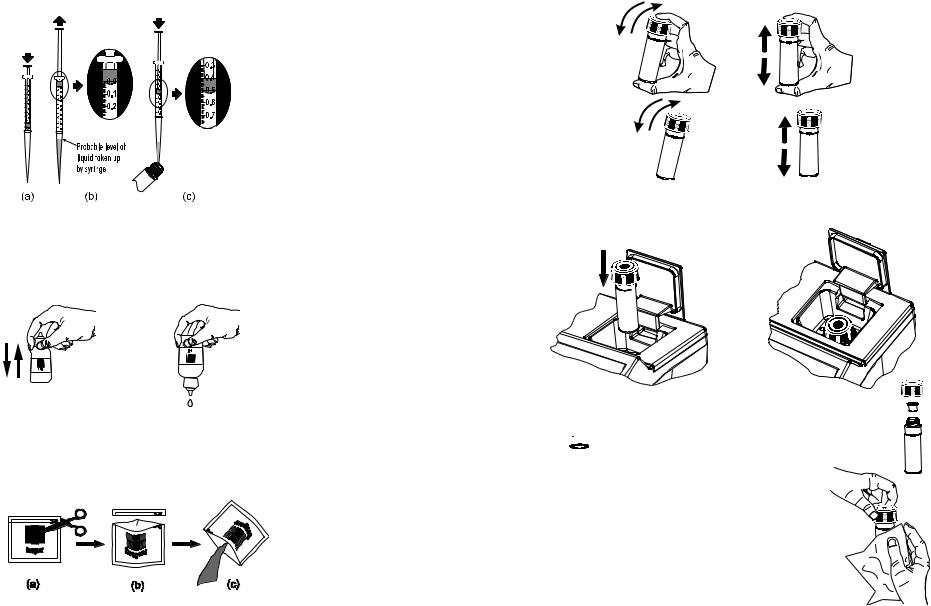
(c)take out the syringe and clean the outside of the syringe tip. Be sure that no drops are hanging on the tip of the syringe, if so eliminate them. Then, keeping the syringe in vertical position above the cuvette, push the plunger down into the syringe until the lower edge of the seal is exactly on the 0.5 mL mark. Now the exact amount of 0.5 mL has been added to the cuvette, even if the tip still contains some solution.
USING CUVETTES
•Proper mixing of the cuvette is done by shaking the cuvette, moving the cuvette up and down. The movement may be gentle or vigorous. This mixing method is indicated with “shake gently” or “shake vigorously”, and one of the following icons:
USING LIQUID AND POWDER REAGENTS
•Proper use of the dropper:
(a)for reproducible results, tap the dropper on the table for several times and wipe the outside of the dropper tip with a cloth.
(b)always keep the dropper bottle in a vertical position while dosing the reagent.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
(b) |
|||
•Proper use of the powder reagent packet:
(a)use scissors to open the powder packet;
(b)push the edges of the packet to form a spout;
(c)pour out the content of the packet.
shake gently shake vigorously
• Pay attention to push the cuvette completely down in the holder and to align the white point on the cap to the indexing mark on the meter. 

• In order to avoid reagent leaking and to obtain more accurate measurements, close the cuvette first with the supplied HDPE plastic
stopper 
 and then the black cap.
and then the black cap.
• Whenever the cuvette is placed into the measurement cell, it must be dry outside, and free of fingerprints, oil or dirt. Wipe it thoroughly with HI 731318 or a lint-free cloth prior to insertion.
• Shaking the cuvette can generate bubbles in the sample, causing higher readings. To obtain accurate measurements, remove such bubbles by swirling or by gently tapping the cuvette.
• Do not let the reacted sample stand too long after reagent is added. For best accuracy, respect the timings described in each
12 |
13 |
specific method.
•It is possible to take multiple readings in a row, but it is recommended to take a new zero reading for each sample and to use the same cuvette for zeroing and measurement when possible (for most precise results follow the measurement procedures carefully).
•Discard the sample immediately after the reading is taken, or the glass might become permanently stained.
•All the reaction times reported in this manual are at 25 °C (77 °F). In general, the reaction time should be increased for temperatures lower than 20 °C (68 °F), and decreased for temperatures higher than 25 °C (77 °F).
INTERFERENCES
•In the method measurement section the most common interferences that may be present in an average sample matrix have been reported. It may be that for a particular treatment process other compounds do interfere with the method of analysis.
HEALTH & SAFETY
•The chemicals contained in the reagent kits may be hazardous if improperly handled.
•Read the Material Safety Data Sheet (MSDS) before performing tests.
•Safety equipment: Wear suitable eye protection and clothing when required, and follow instructions carefully.
•Reagent spills: If a reagent spill occurs, wipe up immediately and rinse with plenty of water.
If reagent contacts skin, rinse the affected area thoroughly with water. Avoid breathing released vapors.
•Waste disposal: for proper disposal of reagent kits and reacted samples, refer to the Material Safety Data Sheet (MSDS).
METHOD REFERENCE TABLE
Method |
Method |
Page |
|
description |
|
|
|
|
1 |
Alkalinity |
20 |
|
|
|
2 |
Bromine |
22 |
|
|
|
3 |
Calcium Hardness |
24 |
|
|
|
4 |
Free Chlorine |
27 |
|
|
|
5 |
Total Chlorine |
29 |
|
|
|
6 |
Free Copper |
31 |
|
|
|
Method |
Method |
Page |
|
description |
|
|
|
|
7 |
Total Copper |
33 |
|
|
|
8 |
Cyanuric Acid |
35 |
|
|
|
9 |
Iron |
37 |
|
|
|
10 |
Ozone |
39 |
|
|
|
11 |
pH |
42 |
|
|
|
OPERATIONAL GUIDE
POWER CONNECTION AND BATTERY MANAGEMENT
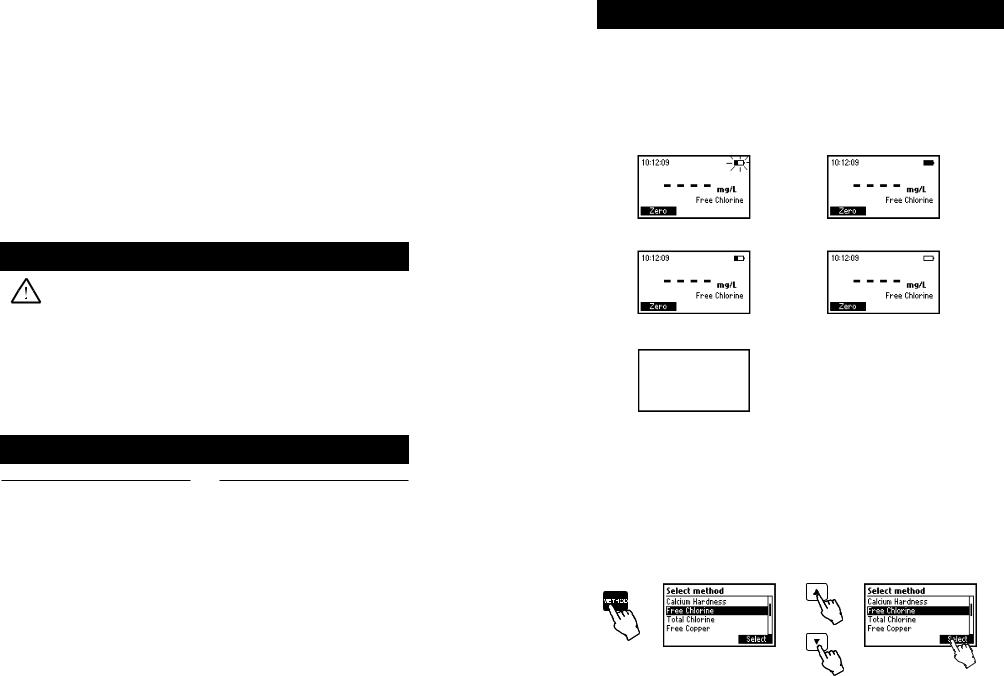
The meter can be powered from an AC/DC adapter (included) or from the built-in rechargeable battery. Note: Always turn the meter off before unplugging it to ensure no data is lost.
When the meter switches ON, it verifies if the power supply adapter is connected. The battery icon on the LCD will indicate the battery status:
- battery is charging from external adapter |
- battery fully charged (meter connected to AC/DC adapter) |
- battery capacity (no external adapter) |
- battery Low (no external adapter) |
- battery Dead (no external adapter)
METHOD SELECTION
•Turn the instrument ON via the ON/OFF power switch.
•The meter will perform an autodiagnostic test. During this test, the Hanna Instrument logo will appear on the LCD. After 5 seconds, if the test was successful, the last method used will appear on the display.
•In order to select the desired method press the METHOD key and a screen with the available methods will appear.
•Press the ▲ ▼ keys to highlight the desired method. Press Select.
14 |
15 |
 Loading...
Loading...