
DC-35/DC-40/DC-45/DC-40S/DC-40 Pro
Diagnostic Ultrasound System
Operator’s Manual
[Basic Volume]


Contents
Intellectual Property Statement .......................................................................................................... I
Responsibility on the Manufacturer Party .......................................................................................... I
Warranty ............................................................................................................................................ II
Exemptions ................................................................................................................................... II
Customer Service Department ..................................................................................................... II
Important Information ....................................................................................................................... III
About This Manual ........................................................................................................................... III
Notation Conventions ....................................................................................................................... III
Operator’s Manuals .......................................................................................................................... IV
Manuals on Paper ............................................................................................................................ IV
Software Interfaces in this Manual .................................................................................................... V
Conventions ...................................................................................................................................... V
1 Safety Precautions ..................................................................................................... 1-1
1.1 Safety Classification ............................................................................................................. 1-1
1.2 Meaning of Signal Words ..................................................................................................... 1-2
1.3 Meaning of Safety Symbols ................................................................................................. 1-2
1.4 Safety Precautions ............................................................................................................... 1-3
1.5 Latex Alert ............................................................................................................................ 1-9
1.6 Warning Labels .................................................................................................................. 1-10
2 System Overview ........................................................................................................ 2-1
2.1 Intended Use ........................................................................................................................ 2-1
2.2 Contraindication ................................................................................................................... 2-1
2.3 Product and Model Code ..................................................................................................... 2-1
2.4 Product Specifications .......................................................................................................... 2-1
2.4.1 Imaging Mode ............................................................................................................... 2-1
2.4.2 Power supply ................................................................................................................ 2-2
2.4.3 Environmental Conditions ............................................................................................. 2-2
2.4.4 Size and Weights .......................................................................................................... 2-3
2.5 System Configuration ........................................................................................................... 2-3
2.5.1 Standard Configuration ................................................................................................. 2-3
2.5.2 Probes Available ........................................................................................................... 2-3
2.5.3 Options ......................................................................................................................... 2-4
2.5.4 Peripherals Supported .................................................................................................. 2-5
2.6 Introduction of Each Unit ...................................................................................................... 2-6
2.7 I/O Panel .............................................................................................................................. 2-9
2.8 Power Supply Panel ........................................................................................................... 2-10
2.9 Physiological-signal Panel ................................................................................................. 2-10
2.10 Control Panel ...................................................................................................................... 2-11
2.11 Symbols .............................................................................................................................. 2-16
3 System Preparation .................................................................................................... 3-1
3.1 Move/Posit the System ........................................................................................................ 3-1
3.2 Power Supply ....................................................................................................................... 3-2
3.2.1 Connecting AC Power Supply ...................................................................................... 3-2
3.2.2 Powered by Batteries ................................................................................................... 3-3
3.2.3 Equipotential Terminal .................................................................................................. 3-3
3.3 Power ON/OFF ..................................................................................................................... 3-3
3.3.1 Powering ON the System ............................................................................................. 3-3
i

3.3.2 Powering OFF the System ........................................................................................... 3-5
3.3.3 Standby ......................................................................................................................... 3-5
3.4 Monitor Adjusting .................................................................................................................. 3-6
3.4.1 Monitor Position Adjusting ............................................................................................ 3-6
3.4.2 Adjusting Brightness/Contrast on the Monitor .............................................................. 3-7
3.4.3 Control Panel Position Adjustment ............................................................................... 3-8
3.5 Connecting a Probe.............................................................................................................. 3-9
3.5.1 Connecting a Probe ...................................................................................................... 3-9
3.5.2 Disconnecting a probe .................................................................................................. 3-9
3.6 Connecting Peripheral Devices .......................................................................................... 3-10
3.6.1 Connecting the USB Devices ..................................................................................... 3-10
3.6.2 Connecting a Footswitch ............................................................................................ 3-10
3.6.3 Graph /Text printer ...................................................................................................... 3-11
3.6.4 Installing Analog Video Printer .................................................................................... 3-14
3.6.5 Installing Digital Video Printer ..................................................................................... 3-16
3.6.6 Installing a Wireless Printer ........................................................................................ 3-16
3.6.7 Installing/ uninstalling Probe/Gel Holder ..................................................................... 3-16
3.7 Basic Screen and Operation .............................................................................................. 3-18
3.7.1 Basic Screen ............................................................................................................... 3-18
3.7.2 Basic Operations of Screens ...................................................................................... 3-22
3.7.3 Touchscreen ............................................................................................................... 3-23
3.7.4 Soft keyboard .............................................................................................................. 3-24
4 Exam Preparation ....................................................................................................... 4-1
4.1 To Start an Exam .................................................................................................................. 4-1
4.2 Patient Information ............................................................................................................... 4-1
4.2.1 New Patient Information ............................................................................................... 4-1
4.2.2 Retrieve Patient Information ......................................................................................... 4-5
4.3 Select Exam Mode and Probe ............................................................................................. 4-8
4.3.1 Dual-probe Switch ........................................................................................................ 4-8
4.4 Select the Imaging Mode ..................................................................................................... 4-9
4.5 Activate& Continue an Exam ............................................................................................... 4-9
4.5.1 Activate an Exam .......................................................................................................... 4-9
4.5.2 Continue Exam ............................................................................................................. 4-9
4.6 Pause & End an Exam ......................................................................................................... 4-9
4.6.1 Pause Exam ................................................................................................................. 4-9
4.6.2 End Exam ................................................................................................................... 4-10
5 Image Optimization ..................................................................................................... 5-1
5.1 Imaging Mode ....................................................................................................................... 5-1
5.1.1 Switching Between Imaging Modes .............................................................................. 5-1
5.1.2 Image Adjustment ......................................................................................................... 5-1
5.1.3 Quickly Saving Image Settings ..................................................................................... 5-2
5.2 B Mode Image Optimization ................................................................................................. 5-3
5.2.1 Basic Procedures for B Mode Imaging ......................................................................... 5-3
5.2.2 B Mode Parameters ...................................................................................................... 5-3
5.2.3 B Mode Image Optimization ......................................................................................... 5-4
5.3 M Mode Image Optimization .............................................................................................. 5-10
5.3.1 Basic Procedures for M Mode Imaging ...................................................................... 5-10
5.3.2 M Mode Image Parameters ........................................................................................ 5-10
5.3.3 M Mode Image Optimization ....................................................................................... 5-11
5.4 Color Mode Image Optimization ......................................................................................... 5-13
ii

5.4.1 Basic Procedures for Color Mode Imaging ................................................................. 5-13
5.4.2 Color Mode Image Parameters .................................................................................. 5-13
5.4.3 Color Mode Image Optimization ................................................................................. 5-13
5.5 Power Mode Image Optimization ....................................................................................... 5-18
5.5.1 Basic Procedures for Power Mode Imaging ............................................................... 5-18
5.5.2 Power Mode Image Parameters ................................................................................. 5-18
5.5.3 Power Mode Image Optimization ............................................................................... 5-18
5.6 PW/CW Doppler Mode ....................................................................................................... 5-20
5.6.1 Basic Procedures for PW/CW Mode Exam ................................................................ 5-20
5.6.2 PW/CW Mode Image Parameters .............................................................................. 5-21
5.6.3 PW/CW Mode Image Optimization ............................................................................. 5-22
5.7 Color M Mode ..................................................................................................................... 5-27
5.7.1 Enter Color M Mode.................................................................................................... 5-27
5.7.2 Exit Color M Mode ...................................................................................................... 5-27
5.7.3 Color M Mode Image Parameters .............................................................................. 5-27
5.8 Anatomical M Mode............................................................................................................ 5-28
5.8.1 Free Xros M Imaging (Anatomical M-Mode) .............................................................. 5-28
5.8.2 Free Xros CM (Curved Anatomical M-Mode) ............................................................. 5-29
5.9 TDI ...................................................................................................................................... 5-30
5.9.1 Basic Procedures for TDI Imaging .............................................................................. 5-31
5.9.2 TDI Image Parameters ............................................................................................... 5-31
5.9.3 TDI Image Optimization .............................................................................................. 5-31
5.9.4 TDI Quantitative Analysis (QA) ................................................................................... 5-32
5.10 3D/4D ................................................................................................................................. 5-36
5.10.1 Overview ..................................................................................................................... 5-36
5.10.2 Note Before Use ......................................................................................................... 5-40
5.10.3 Static 3D ..................................................................................................................... 5-42
5.10.4 iLive ............................................................................................................................ 5-52
5.10.5 4D ............................................................................................................................... 5-54
5.10.6 Smart 3D ..................................................................................................................... 5-55
5.10.7 iPage (Multi-Slice Imaging) ......................................................................................... 5-57
5.11 iScape View (Real-time Panoramic Imaging)..................................................................... 5-61
5.11.1 Basic Procedures for iScape Imaging ........................................................................ 5-61
5.11.2 Image Acquisition........................................................................................................ 5-62
5.11.3 iScape Viewing ........................................................................................................... 5-62
5.11.4 Cine Review ................................................................................................................ 5-64
5.12 Elastography ...................................................................................................................... 5-64
5.12.1 Basic Procedure for Elastography .............................................................................. 5-64
5.12.2 Enter/Exit .................................................................................................................... 5-65
5.12.3 Pressure Hint Curve ................................................................................................... 5-65
5.12.4 Cine Review ................................................................................................................ 5-66
6 Display & Cine Review ............................................................................................... 6-1
6.1 Image Display ....................................................................................................................... 6-1
6.1.1 Splitting Display ............................................................................................................ 6-1
6.2 Image Magnification ............................................................................................................. 6-1
6.2.1 Spot ............................................................................................................................... 6-1
6.2.2 Pan ............................................................................................................................... 6-2
6.2.3 iZoom (Full-screen Zooming) ....................................................................................... 6-2
6.3 Freeze/Unfreeze the Image ................................................................................................. 6-2
6.3.1 Imaging Mode Switching When Frozen ........................................................................ 6-2
6.3.2 Imaging Display Format Switching When Frozen ........................................................ 6-3
iii

6.4 Cine Review ......................................................................................................................... 6-3
6.4.1 Entering/ Exiting Cine Review ...................................................................................... 6-4
6.4.2 Cine Review in 2D Mode (B/B+Color/B+Power/B+TVI/B+TEI) .................................... 6-4
6.4.3 Cine Review in M or D Mode ........................................................................................ 6-5
6.4.4 Linked Cine Review ...................................................................................................... 6-5
6.5 Image Compare .................................................................................................................... 6-6
6.5.1 Cine Compare ............................................................................................................... 6-6
6.5.2 Frame Compare ............................................................................................................ 6-6
6.6 Cine Saving .......................................................................................................................... 6-6
6.7 Cine Memory ........................................................................................................................ 6-7
6.7.1 Cine Memory Setting .................................................................................................... 6-7
6.8 Cine Settings ........................................................................................................................ 6-8
7 ECG .............................................................................................................................. 7-1
7.1 ECG Operation Basic Procedures ....................................................................................... 7-2
7.2 Parameter Description ......................................................................................................... 7-2
7.3 ECG Review ......................................................................................................................... 7-3
8 Measurement ............................................................................................................... 8-1
8.1 Basic Operations .................................................................................................................. 8-1
8.2 General Measurements ........................................................................................................ 8-2
8.2.1 2D General Measurements .......................................................................................... 8-2
8.2.2 M General Measurements ............................................................................................ 8-2
8.2.3 Doppler General Measurements .................................................................................. 8-3
8.3 Application Measurement ..................................................................................................... 8-3
8.4 Measurement Accuracy ........................................................................................................ 8-5
9 Comments and Body Marks ...................................................................................... 9-1
9.1 Comments (Annotations) ...................................................................................................... 9-1
9.1.1 Comments Basic Procedures ....................................................................................... 9-1
9.1.2 Touch Screen Display in Comments ............................................................................. 9-2
9.1.3 Adding Comments ........................................................................................................ 9-3
9.1.4 Moving Comments ........................................................................................................ 9-5
9.1.5 Modifying (Editing) Comments ..................................................................................... 9-5
9.1.6 Deleting Comments ...................................................................................................... 9-6
9.1.7 Comment Setting .......................................................................................................... 9-6
9.2 Body Marks .......................................................................................................................... 9-6
9.2.1 Touch Screen Display in Body Mark ............................................................................. 9-6
9.2.2 Adding Body Marks....................................................................................................... 9-7
9.2.3 Moving Body Marks ...................................................................................................... 9-7
9.2.4 Deleting Body Marks..................................................................................................... 9-7
10 Patient Data Management ........................................................................................ 10-1
10.1 Patient Information Management ....................................................................................... 10-1
10.1.1 Enter Patient Information ............................................................................................ 10-1
10.1.2 Patient Information Setting ......................................................................................... 10-1
10.2 Image File Management .................................................................................................... 10-2
10.2.1 Memory Media ............................................................................................................ 10-2
10.2.2 Image File Formats ..................................................................................................... 10-2
10.2.3 Image Storage Preset ................................................................................................. 10-3
10.2.4 Quickly Saving Images to the System ........................................................................ 10-3
10.2.5 Quickly Saving Full Screen Image to the System ...................................................... 10-4
10.2.6 Thumbnails ................................................................................................................. 10-4
iv

10.2.7 Image Review and Analysis ........................................................................................ 10-4
10.2.8 iVision ......................................................................................................................... 10-6
10.2.9 Sending Image File ..................................................................................................... 10-8
10.3 Report Management........................................................................................................... 10-9
10.4 Patient Data Management (iStation) ................................................................................ 10-10
10.4.1 Viewing Patient Information ...................................................................................... 10-10
10.4.2 Searching a Patient .................................................................................................. 10-11
10.4.3 Patient Data View & Management ............................................................................ 10-11
10.5 iStorage ............................................................................................................................ 10-13
10.6 Print .................................................................................................................................. 10-13
10.6.1 Setting ....................................................................................................................... 10-13
10.6.2 Image Print ................................................................................................................ 10-13
10.6.3 Report Print ............................................................................................................... 10-14
10.7 Backup Files through DVD Drive ..................................................................................... 10-14
10.8 Patient Task Management ................................................................................................ 10-15
10.9 Administration ................................................................................................................... 10-16
10.9.1 Access Setting .......................................................................................................... 10-16
10.9.2 Setting Access Control ............................................................................................. 10-16
10.9.3 System Login ............................................................................................................ 10-16
10.9.4 Adding/Deleting a User ............................................................................................. 10-17
10.9.5 Modify Password ...................................................................................................... 10-18
11 DICOM .........................................................................................................................11-1
11.1 DICOM Preset .................................................................................................................... 11-1
11.1.1 Network Preset ........................................................................................................... 11-1
11.1.2 DICOM Local Preset ................................................................................................... 11-2
11.1.3 Service Preset ............................................................................................................ 11-3
11.2 Verify Connectivity .............................................................................................................. 11-8
11.3 DICOM Services ................................................................................................................. 11-8
11.3.1 DICOM Storage .......................................................................................................... 11-8
11.3.2 DICOM Print ............................................................................................................. 11-10
11.3.3 DICOM Worklist ......................................................................................................... 11-11
11.3.4 MPPS ........................................................................................................................ 11-12
11.3.5 Storage Commitment ................................................................................................ 11-12
11.3.6 Query/Retrieve .......................................................................................................... 11-13
11.4 DICOM Media Storage ..................................................................................................... 11-14
11.5 Structured Report ............................................................................................................. 11-15
11.6 DICOM Task Management ............................................................................................... 11-15
12 Setup .......................................................................................................................... 12-1
12.1 System Preset .................................................................................................................... 12-1
12.1.1 Region ........................................................................................................................ 12-3
12.1.2 General ....................................................................................................................... 12-4
12.1.3 Image Preset .............................................................................................................. 12-5
12.1.4 Application .................................................................................................................. 12-6
12.1.5 OB Preset ................................................................................................................... 12-6
12.1.6 Key Configuration ....................................................................................................... 12-6
12.1.7 Admin .......................................................................................................................... 12-7
12.2 Exam Preset ....................................................................................................................... 12-8
12.3 Measure Preset .................................................................................................................. 12-8
12.4 Print Preset ......................................................................................................................... 12-9
12.5 Network Preset ................................................................................................................. 12-10
v

12.5.1 Local TCP/IP ............................................................................................................. 12-10
12.5.2 iStorage ..................................................................................................................... 12-12
12.5.3 MedTouch/MedSight Preset ..................................................................................... 12-13
12.6 Maintenance ..................................................................................................................... 12-13
12.6.1 Option ....................................................................................................................... 12-13
12.6.2 Other Settings ........................................................................................................... 12-14
12.7 System Information .......................................................................................................... 12-14
13 Probes and Biopsy ................................................................................................... 13-1
13.1 Probe .................................................................................................................................. 13-1
13.1.1 Name and Function of Each Part of the Probe ........................................................... 13-2
13.1.2 Orientation of the Ultrasound Image and the Probe Head ......................................... 13-3
13.1.3 Procedures for Operating ........................................................................................... 13-3
13.1.4 Wearing the Probe Sheath ......................................................................................... 13-6
13.1.5 Probes Cleaning and Disinfection .............................................................................. 13-7
13.1.6 Storage and Transportation ...................................................................................... 13-10
13.2 Biopsy Guide .................................................................................................................... 13-11
13.2.1 Basic Procedures for Biopsy Guiding ....................................................................... 13-13
13.2.2 Needle-guided Brackets ........................................................................................... 13-14
13.2.3 Needle-guided Bracket Inspection and Installation .................................................. 13-19
13.2.4 Verifying the Biopsy Guide Line ................................................................................ 13-23
13.2.5 Removing the Needle-guided Bracket ...................................................................... 13-24
13.2.6 Clean and Sterilize the Needle-guided Bracket ........................................................ 13-27
13.2.7 Storage and Transportation ...................................................................................... 13-28
13.2.8 Disposal .................................................................................................................... 13-28
13.3 Middle Line ....................................................................................................................... 13-29
14 Acoustic Output ........................................................................................................ 14-1
14.1 Concerns with Bioeffects .................................................................................................... 14-1
14.2 Prudent Use Statement ...................................................................................................... 14-1
14.3 ALARA Principle (As Low As Reasonably Achievable) ...................................................... 14-1
14.4 MI/TI Explanation ............................................................................................................... 14-2
14.4.1 Basic Knowledge of MI and TI .................................................................................... 14-2
14.4.2 MI/TI Display ............................................................................................................... 14-3
14.5 Acoustic Power Setting ...................................................................................................... 14-3
14.6 Acoustic Power Control ...................................................................................................... 14-4
14.7 Acoustic Output .................................................................................................................. 14-4
14.7.1 Derated Ultrasonic Output Parameters ...................................................................... 14-4
14.7.2 Limits of Acoustic Output ............................................................................................ 14-5
14.7.3 Differences between Actual and Displayed MI and TI ................................................ 14-5
14.8 Measurement Uncertainty .................................................................................................. 14-6
14.9 References for Acoustic Power and Safety ........................................................................ 14-6
15 EMC Guidance and Manufacturer’s Declaration .................................................... 15-1
16 System Maintenance ................................................................................................ 16-1
16.1 Daily Maintenance .............................................................................................................. 16-1
16.1.1 Cleaning the System .................................................................................................. 16-1
16.1.2 Clean the Peripherals ................................................................................................. 16-4
16.1.3 Checking the Probe .................................................................................................... 16-5
16.1.4 Checking the Power Cable and Plug .......................................................................... 16-5
16.1.5 Checking Appearance ................................................................................................ 16-5
16.1.6 Backup of the System Hard Drive .............................................................................. 16-5
16.2 Troubleshooting .................................................................................................................. 16-6
vi

Appendix A Barcode Reader ...................................................................................... A-1
Appendix B Electrical Safety Inspection .................................................................. B-1
Appendix C iScanHelper ............................................................................................ C-1
Appendix D Wireless LAN .......................................................................................... D-1
Appendix E Ultrasound Gel Warmer ......................................................................... E-1
Appendix F Batteries .................................................................................................. F-1
vii


©2017 Shenzhen Mindray Bio-Medical Electronics Co., Ltd. All rights Reserved.
For this Operator’s Manual, the issue date is 2017-04.
Intellectual Property Statement
SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD. (hereinafter called Mindray)
owns the intellectual property rights to this Mindray product and this manual. This manual may
refer to information protected by copyright or patents and does not convey any license under
the patent rights or copyright of Mindray, or of others.
Mindray intends to maintain the contents of this manual as confidential information. Disclosure
of the information in this manual in any manner whatsoever without the written permission of
Mindray is strictly forbidden.
Release, amendment, reproduction, distribution, rental, adaptation, translation or any other
derivative work of this manual in any manner whatsoever without the written permission of
Mindray is strictly forbidden.
, , , , , BeneView, WATO,
BeneHeart,
countries. All other trademarks that appear in this manual are used only for informational or
editorial purposes. They are the property of their respective owners.
are the trademarks, registered or otherwise, of Mindray in China and other
Responsibility on the Manufacturer Party
Contents of this manual are subject to change without prior notice.
All information contained in this manual is believed to be correct. Mindray shall not be liable
for errors contained herein or for incidental or consequential damages in connection with the
furnishing, performance, or use of this manual.
Mindray is responsible for the effects on safety, reliability and performance of this product,
only if:
z all installation operations, expansions, changes, modifications and repairs of this
product are conducted by Mindray authorized personnel;
z the electrical installation of the relevant room complies with the applicable national
and local requirements; and
z the product is used in accordance with the instructions for use.
Note
This equipment must be operated by skilled/trained clinical professionals.
Warning
It is important for the hospital or organization that employs this equipment to carry out a
reasonable service/maintenance plan. Neglect of this may result in machine breakdown or
personal injury.
I

Warranty
THIS WARRANTY IS EXCLUSIVE AND IS IN LIEU OF ALL OTHER WARRANTIES,
EXPRESSED OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY OR
FITNESS FOR ANY PARTICULAR PURPOSE.
Exemptions
Mindray's obligation or liability under this warranty does not include any transportation or
other charges or liability for direct, indirect or consequential damages or delay resulting from
the improper use or application of the product or the use of parts or accessories not approved
by Mindray or repairs by people other than Mindray authorized personnel.
This warranty shall not extend to:
Malfunction or damage caused by improper use or man-made failure.
Malfunction or damage caused by unstable or out-of-range power input.
Malfunction or damage caused by force majeure such as fire and earthquake.
Malfunction or damage caused by improper operation or repair by unqualified or
unauthorized service people.
Malfunction of the instrument or part whose serial number is not legible enough.
Others not caused by instrument or part itself.
Customer Service Department
Manufacturer:
Address:
Website:
E-mail
Address:
Tel: +86 755 81888998
Fax: +86 755 26582680
Manufacturer: Mindray DS USA, Inc.
Address: 800 MacArthur Blvd.
Tel: +1(201) 995-8000
Toll Free: +1 (800) 288-2121
Fax: +1 (800) 926-4275
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Mindray Building,Keji 12th Road South,High-tech industrial
park,Nanshan,Shenzhen 518057,P.R.China
www.mindray.com
service@mindray.com
Mahwah, NJ 07430-0619 USA
II
Important Information
1. It is the customer’s responsibility to maintain and manage the system after delivery.
2. The warranty does not cover the following items, even during the warranty period:
(1) Damage or loss due to misuse or abuse.
(2) Damage or loss caused by Acts of God such as fires, earthquakes, floods, lightning,
etc.
(3) Damage or loss caused by failure to meet the specified conditions for this system,
such as inadequate power supply, improper installation or environmental conditions.
(4) Damage or loss due to use of the system outside the region where the system was
originally sold.
(5) Damage or loss involving the system purchased from a source other than Mindray or
its authorized agents.
3. This system shall not be used by persons other than fully qualified and certified medical
personnel.
4. Do not make changes or modifications to the software or hardware of this system.
5. In no event shall Mindray be liable for problems, damage, or loss caused by relocation,
modification, or repair performed by personnel other than those designated by Mindray.
6. The purpose of this system is to provide physicians with data for clinical diagnosis. It is
the physician’s responsibility for diagnostic procedures. Mindray shall not be liable for the
results of diagnostic procedures.
7. Important data must be backed up on external memory media.
8. Mindray shall not be liable for loss of data stored in the memory of this system caused by
operator error or accidents.
9. This manual contains warnings regarding foreseeable potential dangers, but you shall
always be alert to dangers other than those indicated as well. Mindray shall not be liable
for damage or loss that results from negligence or from ignoring the precautions and
operating instructions described in this operator’s manual.
10. If the manager for this system is changed, be sure to hand over this operator’s manual to
the new manager.
About This Manual
This operator’s manual describes the operating procedures for this diagnostic ultrasound
system and the compatible probes. To ensure safe and correct operations, carefully read and
understand the manual before operating the system.
Notation Conventions
In this operator’s manual, the following words are used besides the safety precautions (refer
to "Safety Precautions"). Please read this operator’s manual before using the system.
CAUTION:
U.S.A. Federal Law restricts this device to sale by or on the order
The diagnostic ultrasound system is not intended for ophthalmic
use. Its use in this clinical specialty is contraindicated.
of a physician.
III
Operator’s Manuals
You may receive multi-language manuals in compact disc or paper. Please refer to English
manual for latest information and register information.
The content of the operator manual, such as screens, menus or descriptions, may be different
from what you see in your system. The content varies depending upon the software version,
options and configuration of the system.
Manuals on Paper
z Operator’s Manual [Basic Volume]: Describes the basic functions and operations of
the system, safety precautions, exam modes, imaging modes, preset, maintenance
and acoustic output, etc.
z Operator’s Manual [Advanced Volume]: Describes measurement preset,
measurements and calculations, etc.
z Operator’s Manual [Acoustic Power Data and Surface Temperature Data]: Contains
data tables of acoustic output for transducers.
z Operation Note: Contains quick guide for basic operations of the system.
NOTE: 1. The manuals in CD are the manuals translated into languages other than
English according to English manuals.
2. When you find that the contents of the manuals in CD are NOT consistent with
the system or English manuals, please ONLY refer to the corresponding English
manuals.
3. The accompanying manuals may vary depending upon the specific system you
purchased. Please refer to the packing list.
IV

Software Interfaces in this Manual
Depending on the software version, preset settings and optional configuration, the actual
interfaces may be different from those in this manual.
Conventions
In this manual, these conventions are used to describe the buttons on the control panel, the
items in menu, buttons in dialog box and some basic operations:
z <Button>: The angular bracket indicates buttons, knobs and other controls on control
panel or keyboard.
z [Item in menu (soft menu) and button in dialog box]: The square bracket indicates
items in menu or buttons in dialog box.
z Click [Item or Button]: Move the cursor to the item or button and press <Set>, or click
it on the menu.
z [Item in Menu]Æ[Item in Submenu]: Select a submenu item following the path.
z [Dyn Rng (Value)]: Indicates menu items with parameter, (value) shows the current
value of the item.
V


1 Safety Precautions
1.1 Safety Classification
According to the type of protection against electric shock:
Class I equipment powered by outer power & equipment powered by inner batteries
According to the degree of protection against electric shock:
Type-BF applied part
According to the degree of protection against harmful ingress of water:
Main unit: IPX0
Probes: IPX7
Footswitch:
FS-81-SP-2 (one-pedal) belongs to IPX8.
971-SWNOM (2-pedal or 3-pedal) belongs to IP68.
According to the degree of safety of application in the presence of a FLAMMABLE
ANESTHETIC MIXTURE WITH AIR or WITH OXYGEN OR NITROUS OXIDE:
EQUIPMENT not suitable for use in the presence of a FLAMMABLE ANESTHETIC
MIXTURE WITH AIR or WITH OXYGEN OR NITROUS OXIDE
According to the mode of operation:
CONTINUOUS OPERATION
According to the installation and use:
MOBILE EQUIPMENT
Safety Precautions 1-1
1.2 Meaning of Signal Words
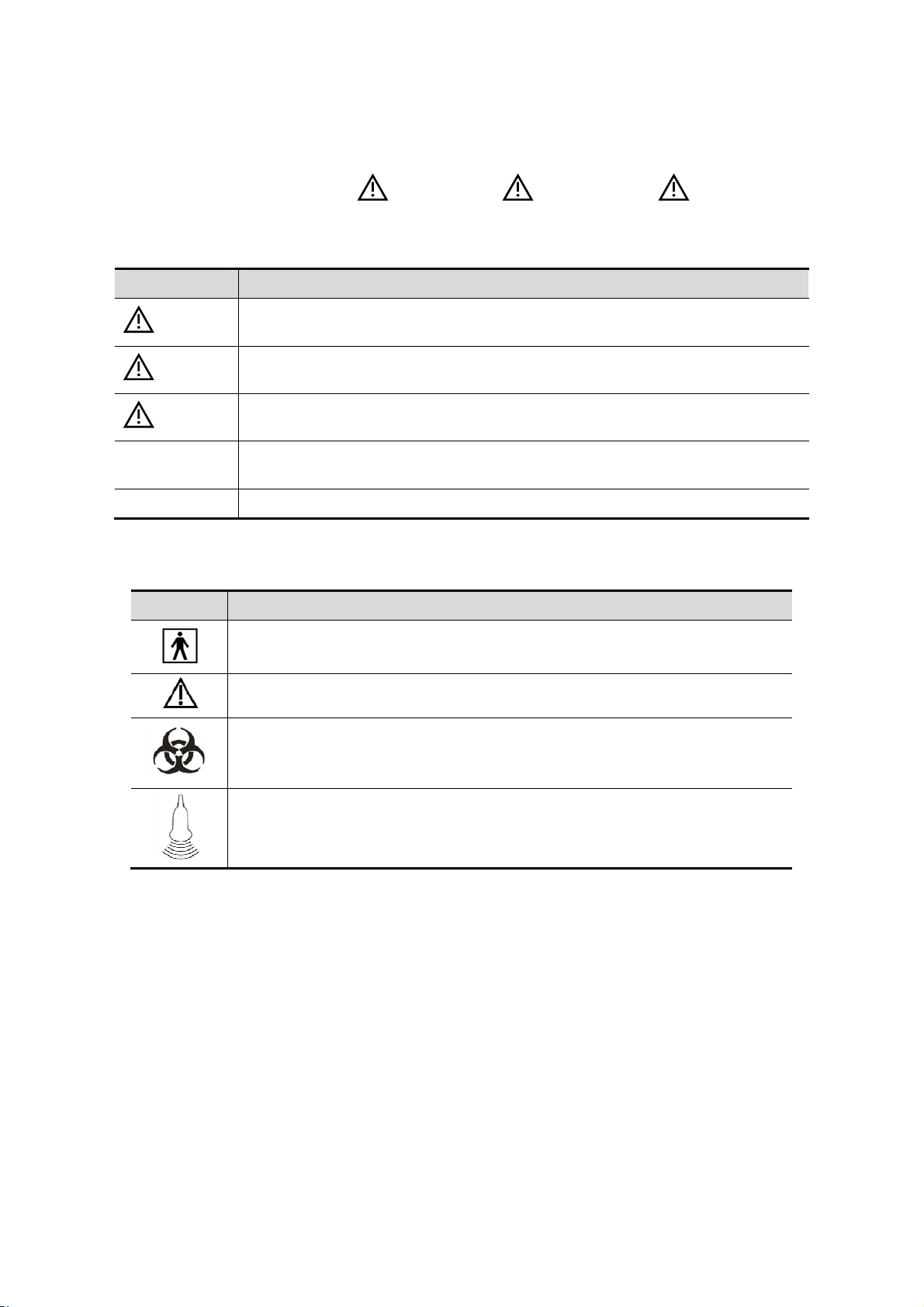
In this manual, the signal words" DANGER”, “ WARNING”, “ CAUTION”,
“NOTE” and "Tips" are used regarding safety and other important instructions. The signal
words and their meanings are defined as follows. Please understand their meanings clearly
before reading this manual.
Signal word Meaning
DANGER
WARNING
CAUTION
NOTE
Tips Important information that helps you to operate the system more effectively.
Indicates an imminently hazardous situation that, if not avoided, will result
in death or serious injury.
Indicates a potentially hazardous situation that, if not avoided, could result
in death or serious injury.
Indicates a potentially hazardous situation that, if not avoided, may result
in minor or moderate injury.
Indicates a potentially hazardous situation that, if not avoided, may result in
property damage.
1.3 Meaning of Safety Symbols
Symbol Description
Type-BF applied part.
The ultrasound probes connected to this system are type-BF applied parts.
General warning, caution, risk of danger.
Patient/user infection due to contaminated equipment. Be careful when
performing the cleaning, disinfection and sterilization.
Patient injury or tissue damage from ultrasound radiation. It is required to
practice ALARA when operating ultrasound system.
1-2 Safety Precautions
1.4 Safety Precautions
Please observe the following precautions to ensure patient and operator’s safety when using
this system.
DANGER:
WARNING:
Do not operate this system and probes in an atmosphere
containing flammable gasses or liquids such as anesthetic
gasses, hydrogen, and ethanol, because there is danger of
explosion.
1.
Do connect the power plug of this system to wall recept acle s
that meet the ratings indicated on the rating nameplate. If
adapters or multifunctional receptacles are used, it may cause
the leakage current to exceed the safety requirement.
2.
In the environment that patient is 1.5 meters around, conn ect
peripherals to the auxiliary power outlet which i s cap able of
isolation protection, or power the peripherals by auxiliary
output cable or isolation transformer complied with IEC 606011-1 or the power input of the same safety level.
3.
DO NOT use power supply of different phases to power
peripherals, like power supply of air-conditioning.
4.
When using peripherals not powered by the auxiliary output
of the ultrasound system, or using peripherals other than
permitted by Mindray, make sure the overall leakage current
of peripherals and the ultrasound system meets the
requirement of the local medical device electrical regulation
(like enclosure leakage current should be no more than
500uA of IEC 60601-1-1), and the responsibility is held by the
user.
5.
Connect the grounding conductor before turning ON the
system. Disconnect the grounding cable af ter turning OFF the
system. Otherwise, electric shock may result.
6.
For the connection of power and grounding, follow the
appropriate procedures described in this operator’s manual.
Otherwise, there is risk of electric shock. Do not connect the
grounding cable to a gas pipe or water pipe; otherwise,
improper grounding may result or a gas explosion may occur.
7.
Before cleaning the system, disconnect the power cord from
the outlet. Otherwise, system failure and electric shock may
result.
8.
This system is not water-proof. Do not use this system in an y
place where water leakage may occur. If any water is sprayed
on or into the system, electric shock may result. If water is
accidentally sprayed on or into the system, contact Mindray
Customer Service Department or sales represe nt ative.
Safety Precautions 1-3
9.
DO NOT use a probe that has a damaged, scratched surface, or
exposed wiring of any kind. Immediately stop using the probe
and contact Mindray Customer Service Dep artment or sa les
representative. There is risk of elect ric shock if using a
damaged or scratched probe.
10.
Do not allow the patient to contact the live parts of the
ultrasound system or other devices, e.g. signal I / O port s.
Electric shock may occur .
11.
Do not use an aftermarket probe other than t hose specified by
Mindray . The probes may damage t he system causing a
profound failure, e.g. a fire in the worst case.
12.
Do not subject the probes to knocks or drops . Use of a
defective probe may cause an electric shock.
13.
Do not open the covers and front panel of the system. Short
circuit or electric shock may result when the system hardware
is exposed and powered on.
14.
Do not use this system when any digit al device such as a highfrequency electrotome, high-frequency therapeutic device or
defibrillator is applied already. Otherwise, there is a risk of
electric shock to the patient.
15.
Only use the ECG leads and PCG transducer provided with the
physiology module; otherwise, electric shock may be resulted.
16.
When moving the system, you should hold the handle;
otherwise, damage may be resulted by abnormal force. Do not
push the system from the left/right side; otherwise, it m ay be
toppled over .
17.
The auxiliary power output outlet in the system is used to
supply power for the recommended peripheral devices. Do not
connect other devices to the outlet, otherwise, the rated out put
power may be exceeded and failure may be resulted. Maximum
output power of the outlet is 240VA (including the auxiliary
output port in the printer compartment).
18.
Accessory equipment (analog or digital) connected to the
ultrasound system must comply with the relevant IEC
standards (e.g., IEC 60950 informa tion technology equipment
safety standard and IEC 60601-1 medical equipment
standard).Furthermore, all configurations must comply wit h the
standard IEC 60601-1-1.It is the resp onsibility of the perso n,
who connects additional equipment to the signal input or
output ports and configures a medical system, to verify that the
system complies with the requirements of IEC 60601-1-1.If y ou
have any questions regarding these requirement s, consult y our
sales representative.
19.
Prolonged and repeated use of keyboards may result in hand or
arm nerve disorders for some individuals. Observe the local
safety or health regulations concerning the use of keyboards.
1-4 Safety Precautions
20.
When using intra-cavity probes, do not activate the probe
outside the p atient’s body .
DO NOT touch the Signal I/O ports if in contact with the patient;
21.
otherwise patient injury may result.
CAUTION:
1. Precautions concerning clinical examination techniques:
This system must be used only by qualified medical
professionals.
This operator’s manual does not describe clinical examination
techniques. The clinician should select the proper examination
techniques based on specialized training and clinical
experience.
2. Malfunctions due to radio wave:
If a radio wave emitting device is used in the proximity of this
system, it may interfere with operations. Do not use or take any
devices transmitting RF signals (such as cellular phones,
transceivers and radio controlled products) in the room placing
the system.
If a person brings a device that generates radio waves near the
system, ask him / her to immediately turn OFF the device.
3. Precautions concerning movement of the system:
Please install the system on a flat plane with casters locked.
Otherwise, damage may be resulted by accidental moving.
Do not move the system laterally, which may result in damage in
case of toppling.
Move the system slowly on the slope by two people, otherwise,
damage may result in case of unexpected sliding.
Do not sit on the system, which may result individual falling in
case of system moving.
Object placed on the monitor may fall and injure an individual.
Fasten and fully secure any peripheral device before moving the
system. A loose peripheral device may fall and injure an
individual.
When move the system on the steps, please take care to prevent
the system from toppling.
4. If the circuit protector is tripped, it indicates that the system or a
peripheral device was improperly shut down and the system is
unstable. You cannot repair the system under this circumstance
and must call the Mindray Customer Service Department or
sales representative.
5. There is no risk of high-temperature burns during normal
ultrasound examinations. It is possible for the surface
temperature of the probe to exceed the body temperature of a
patient due to environmental temperature and exam type
combinations. Do not apply the probe to the same region on the
patient for a long time. Apply the probe only for a period of time
required for the purpose of diagnosis.
6. Do not use the system to examine a fetus for a long period of
time.
Safety Precautions 1-5

7. The system and its accessories are not disinfected or sterilized
prior to delivery. The operator is responsible for the cleaning
and disinfection of probes and sterilization of biopsy brackets
according to the manuals, prior to the use. All items must be
thoroughly processed to completely remove harmful residual
chemicals, which will not only be harmful to the human body,
but also damage the accessory.
8. It is necessary to press <End Exam> to end the current scan
that is in progress and clear the current Patient Information
field. Otherwise, new patient data may be combined with the
previous patient data.
9. Do not connect or disconnect the system’s power cord or its
accessories (e.g., a printer or a recorder) without turning OFF
the system power first. This may damage the system and its
accessories or cause electric shock.
10. If the system is powered off improperly during operation, it may
result in data damage of the system hard disk or system failure.
11. Do not use a USB memory device (e.g., a USB flash drive,
removable hard disk) which has unsafe data. Otherwise, system
damage may result.
12. It is recommended to only use the video devices specified in
this manual.
13. Do not use gel, disinfectant, probes, probe sheath or needleguided brackets that are not compatible with the system.
14. Read the Acoustic Output Principle in the operation manual
carefully before operating this system on clinical examination.
15. The cover contains natural rubber that can cause allergic
reactions in some individuals.
NOTE: 1. DO NOT use the system in the vicinity of strong electromagnetic field (such as
2. Do not use the system in the vicinity of high-frequency radiation source (e.g.
3. When using or placing the system, keep the system horizontal to avoid
4. To avoid damaging the system, do not use it in following environment:
16. Please use the ultrasound gel compliant with the relevant local
regulations.
17. Normal operation may be affected by unstable mains power
supply; it is recommended that our product be powered from an
uninterruptible power supply.
a transformer), which may affect the performance of the system.
cellular phones), which may affect the performance of the system or even lead
to failure.
imbalance.
z Locations exposed to direct sunlight;
z Locations subject to sudden changes in environmental temperature;
z Dusty locations;
z Locations subject to vibration;
z Locations near heat generators;
z Locations with high humidity.
1-6 Safety Precautions

5. Turn ON the system only after the power has been turned OFF for a while. If the
system is turned ON immediately after being turned OFF, the system may not be
rebooted properly and could malfunction.
6. Press <Freeze> key to freeze an image or turn off the power of the system
before connecting or disconnecting a probe. Otherwise, the system and/or
probe can be damaged.
7. Remove the ultrasound gel from the face of the probe when the examination is
completed. Water in the gel may enter the acoustic lens and adversely affect
the performance and safety of the probe.
8. You should properly back up the system to a secure external storage media,
including system configuration, settings and patient data. Data stored to the
system’s hard drive may be lost due to system failure, improper operation or
accident.
9. Do not apply external force to the control panel. Otherwise, the system may be
damaged.
10. If the system is used in a small room, the room temperature may rise. Please
provide proper ventilation and free air exchange.
11. To dispose of the system or any part, contact Mindray Customer Service
Department or sales representative. Mindray is not responsible for any system
content or accessories that have been discarded improperly. Mindray is not
responsible for any system content or accessories that have been discarded
improperly.
12. Electrical and mechanical performance may be degraded due to long usage
(such as current leakage or distortion and abrasion); the image sensitivity and
precision may become worse too. To ensure optimal system operations, it is
recommended that you maintain the system under a Mindray service
agreement.
13. The replaceable fuse is inside the chassis. Refer replacing job to Mindray
service engineers or engineers authorized by Mindray only.
14. Do not turn OFF the power supply of the system during printing, file storage or
invoking other system operations. An interrupted process may not be
completed, and can become lost or corrupted.
15. The iScape feature constructs a single extended image from a series of
individual image frames. The quality of the final image is user-dependent and
requires skill to efficiently apply the feature and technique. Exercise caution
when measurements are performed from an iScape image.
16. Ensure that the current exam date and time are the same as the system date
and time.
Please read the following precautions carefully to ensure the safety of the patient and the
operator when using the probes.
WARNING:
1. The probe is only for use with the specified ultrasonic
diagnostic system. Please refer to the “2.5.2 Probes Available” to
select the proper probe.
2. The ultrasonic probe must be used only by qualified
professionals.
3. Confirm that the transducer and probe cable are normal before
and after each examination. A defective probe may cause
electric shock to the patient.
Safety Precautions 1-7
4. Do not subject the probe to shock. A defective probe may cause
electric shock to the patient.
CAUTION:
5. Do not disassemble the probe to avoid the possibility of electric
shock.
6. Never immerse the probe connector into liquids such as water
or disinfectant because the connector is not waterproof.
Immersion may cause electric shock or malfunction.
7. A probe sheath must be installed over the probe before
performing intra-cavity or intra-operative examination.
1. When using the probe, wear sterile gloves to prevent infection.
2. Be sure to use sterile ultrasound gel. Please use the ultrasound
gel compliant with the relevant local regulations. And manage
the ultrasound gel properly to ensure that it does not become a
source of infection.
3. In normal diagnostic ultrasound mode, there is no danger of a
normal-temperature burn; however, keeping the probe on the
same region of the patient for a long time may cause such a
burn.
4. Do not use the carrying case for storing the probe. If the carrying
case is used for storage, it may become a source of infection.
5. It is required to practice ALARA when operating ultrasound
system. Minimize the acoustic power without compromising the
quality of images.
6. The probe and accessories supplied with it are not delivered
disinfected or sterilized. Sterilization (or high-level disinfect)
before use is required.
7. Disposable components should be packaged sterile and for
single-use only. Do not use if integrity of packaging violated or if
expiration date has passed. Please use the disposable
components compliant with the relevant local regulations.
8. Please use the disinfection or sterilization solution
recommended in this operator’s manual; otherwise Mindray will
not be liable for damage caused by other solutions. If you have
any questions, please contact Mindray Customer Service
Department or sales representative.
9. Do not use pre-lubricated condoms as a sheath. Lubricant may
not be compatible with the probe material and damage may
result.
10. The damage of the probe may be caused by the contact of
improper gel or cleaner:
z DO NOT soak or saturate probes in the strong polar solution
of ethanol, chloride of lime, ammonium chloride, acetone or
formaldehyde.
z DO NOT contact the probe with solutions or ultrasound gels
containing oily medium such as mineral oil or lanoline.
1-8 Safety Precautions
NOTE: Read the following precautions to prevent the probe from malfunction:
z Before connecting or disconnecting the probe, freeze or turn off the
diagnostic ultrasound system.
z Clean and disinfect the probe before and after each examination.
z After the examination, wipe off the ultrasound gel thoroughly. Otherwise,
the ultrasound
Ambient conditions:
1. To prevent the probe from being damaged, do not use it where it will be
exposed to:
z Direct sunlight or X-rays
z Sudden changes in temperature
z Dust
z Excessive vibration
z Heat generators
2. Use probes under the following ambient conditions :
z ambient temperature: 0°C to 40°C
z relative humidity: 30% to 85% (no condensation)
z atmospheric pressure: 700 hPa to 1060 hPa
3. Use D7-2E probes under the following ambient conditions :
z ambient temperature: 10°C to 40°C
z relative humidity: 30% to 85% (no condensation)
z atmospheric pressure: 700 hPa to 1060 hPa
Repeated disinfection will eventually damage the probe, please check the probe
performance periodically.
gel may solidify and the image quality would be degraded.
1.5 Latex Alert
When choosing a probe sheath, it is recommended that you directly contact CIVCO for
obtaining probe sheath, pricing information, samples and local distribution information. For
CIVCO information, please contact the following:
CIVCO Medical Instruments
Tel: 1-800-445-6741
WWW.civco.com
WARNING:
Allergic reactions in latex (natural rubber) sensitive patients may
range from mild skin reactions (irritation) to fatal anaphylactic
shock, and may include difficulty in breathing (wheezing),
dizziness, shock, swelling of the face, hives, sneezing or itching
of the eyes (FDA Medical Alert on latex products, “Allergic
Reactions to Latex-containing Medical Devices”, issued on March
29, 1991).
Safety Precautions 1-9
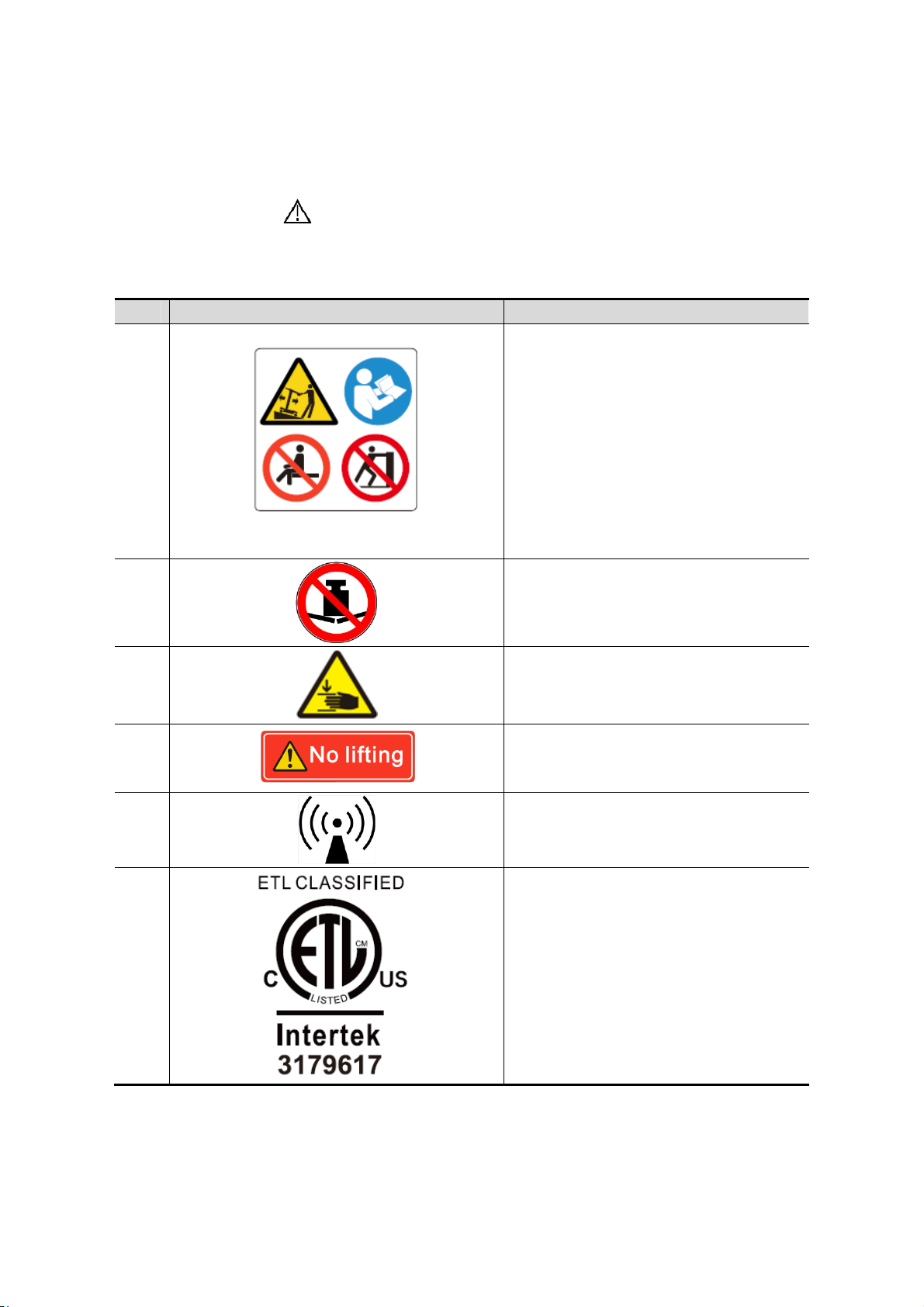
1.6 Warning Labels
The warning labels are attached to this system in order to call your attention to potential
hazards. The symbol on the warning labels indicates safety precautions.
The warning labels use the same signal words as those used in the operator’s manual. Read
operator’s manual carefully before using the system.
The name, pattern and meaning of each warning label are described as follows:
No. Warning Labels Meaning
1.
a
b
d
c
a. Do not place the system on a sloped
surface. Otherwise the system may
slide, resulting in personal injury or the
system malfunction. Two persons are
required to move the system over a
sloped surface.
b. Do not sit on the system.
c. DO NOT push the system when the
casters are locked.
d. Please carefully read this manual
before use system.
2.
3.
4.
5.
6.
Beware of excessive stress exerted to
the system.
Mind your hands.
Please do not lift the hanger or try to
push the ultrasound system by using it.
Non-ionizing radiation
CONFORMS TO AAMI STD 60601-1,
IEC STD 60601-2-37,IEC STD 606012-18;
CERTIFIED TO CSA STD C22.2 NO.
60601-1, 60601-2-37, 60601-2-18
1-10 Safety Precautions
2 System Overview
2.1 Intended Use
The Diagnostic Ultrasound System is intended for adults, pregnant women, pediatric
patients and neonates.It is intended for use in fetal, abdominal, pediatric, small organ(breast,
thyroid, testes), neonatal cephalic,adult cephalic,trans-rectal, trans-vaginal, musculoskeletal(conventional), musculo- skeletal(superficial), cardiac adult, cardiac pediatric and
peripheral vessel exams.
2.2 Contraindication
This system is not intended for ophthalmic use.
2.3 Product and Model Code
DC
-
Model code
Product code
NOTE: The functions described in the operator’s manual may vary depending upon the
specific system you purchased.
2.4 Product Specifications
2.4.1 Imaging Mode
B Mode B
M Mode M
Color M Mode (CM)
C Mode Color
D Mode PW Doppler
CW Doppler
Power(Dirpower)
System Overview 2-1
Special
Imaging
Elastography
Tissue Doppler Imaging
TDI QA
iScape View
Free Xros M/Free Xros CM
3D/4D
Smart 3D
Static 3D
iPage
iLive
2.4.2 Power supply
Voltage
Frequency 50/60Hz
Power
consumption
Fuse 250V~ T10AH
100-240V~
600VA
2.4.3 Environmental Conditions
Operational Conditions Storage and Transportation
Conditions
Ambient
temperature
Relative
humidity
Atmospheric
pressure
WARNING:
10℃~40℃ -20℃~55℃
30%~85% 30%~95%
700hPa~1060hPa 700hPa~1060hPa
Do not use this system in the conditions other than those
specified.
2-2 System Overview
2.4.4 Size and Weights
External dimensions:
z 812mm (L)X600mm (W)X1331 (1515)mm (H)
Net weight: 130.87Kg (without the battery)
2.5 System Configuration
2.5.1 Standard Configuration
Main unit
Accessories
z Operator's manuals
z Ultrasound gel
z Dust-proof cover
z Probe ports dust-proof cover
z Tray assembly
z Left bracket for the intra-cavity probe
z Right bracket for the intra-cavity probe
z Probe holder
z Probe holder for pencil probe
z Cables
z Grounding cable
z Multilingual controls overlay
2.5.2 Probes Available
No. Probe Model Intended Use Region Applied
3C5A Fetal, Abdominal, Pediatric, Neonatal
1.
7L4A Abdominal, Pediatric, Small Organ
2.
3. L7-3
4. D7-2E Fetal, Abdominal Abdomen
5. L14-6NE
Cephalic, Musculo-skeletal
(Conventional), Peripheral vessel
(breast, thyroid, testes), Neonatal
Cephalic, Musculo-skeletal
(Conventional), Musculo-skeletal
(Superficial), Peripheral vessel
Abdominal, Pediatric, Small Organ
(breast, thyroid, testes), Neonatal
Cephalic, Musculo-skeletal
(Conventional), Musculo-skeletal
(Superficial), Peripheral vessel
Pediatric, Small Organ (breast, thyroid,
testes), Musculo-skeletal
(Conventional), Musculo-skeletal
(Superficial), Peripheral vessel
Body surface
Body surface
Body surface
Body surface
6. V11-3 Fetal, Trans-rectal, Trans-vaginal Intracavitary
System Overview 2-3
No. Probe Model Intended Use Region Applied
7. P4-2
Abdominal, Adult Cephalic, Cardiac
Adult, Cardiac Pediatric
Body surface
Some of the probes have matched needle-guided brackets for biopsy, the available probes
and the corresponding needle-guided brackets are listed as follows:
NGB-004
V11-3
Metal/needle un-detachable
/ 16G, 17G, 18G
Plastic/needle detachable:
3C5A
NGB-006 (plastic/needle
detachable, metal/needle
detachable)
25°, 35°, 45°
13G, 15G, 16G, 18G, 20G;
Metal/needle detachable:
14G, 16G, 18G, 20G, 22G
Metal/needle detachable:
14G, 16G, 18G, 20G, 22G;
Plastic/needle detachable:
13G, 15G, 16G, 18G, 20G
7L4A/L14-6NE/
L7-3
NGB-007
plastic/needle detachable
metal/needle detachable
40°, 50°, 60°
NGB-011
P4-2
11°, 23° 13G, 15G, 16G, 18G, 20G
metal/needle un-detachable
2.5.3 Options
No. Item
1 ECG module (including ECG lead)
2 DC-IN cable (configured with ECG module)
3 Ultrasound gel warmer
4 4D module
5 CW module
6 Buit-in battery
7 Built-in Wireless Adapter
8 Pencil probe port
9 Footswitch: (two-pedal/three-pedal)
10 1D barcode reader
11 Nerve Package
12 Emergency&Critical Package
13 Application
software
14 IVF
package
15 Smart OB
IMT
16 Smart NT
17 Smart Bladder
18 Tissue Doppler Imaging
19 iScape View
2-4 System Overview
 Loading...
Loading...