Getinge MEERA 720001B2, MEERA 720001B0, MEERA 720001F2, MEERA 710001B2, MEERA 710001B0 Field Safety Notice
...Page 1
Field Safety Notice
GETIFIGE
2019-04-02 I 2018-025
Please forward this information to all relevant users, [biomedical staff for
capital equipment, materials management and/or purchasing for consumables]
and risk management department concerned in your facility
Preventive Corrective Action: mobile OR-Table MEERA
Products affected:
Product
MEERA
MEERA
EU
with Drive Unit
EU
w/o Drive Unit
MEERA US with Drive Unit
MEERA US w/o Drive Unit
MEERA ST
MEERA ST
EU
Drive Unit
EU
w/o Drive Unit
MEERA ST US w/o Drive Unit
Article No.
720001B2
720001B0
720001F2
720001F0
710001B2
710001B0
710001F0
SIN No.
SN 1 to SN 416
SN 1 to SN 1416
SN 1 to SN 91
SN Ito SN 220
SN 1 to SN 8
SN 1 to SN 11
SN 1
Dear Customer
The purpose of this letter is to inform about a potential issue concerning the mobile OR-Table MEERA.
Our records indicate that your facility has received one or more of these devices.
Normal use and indications
The mobile OR-Table MEERA is designed to support and position the patient immediately before, during and
after surgical interventions as well as for examination and treatment.
The following has been discovered
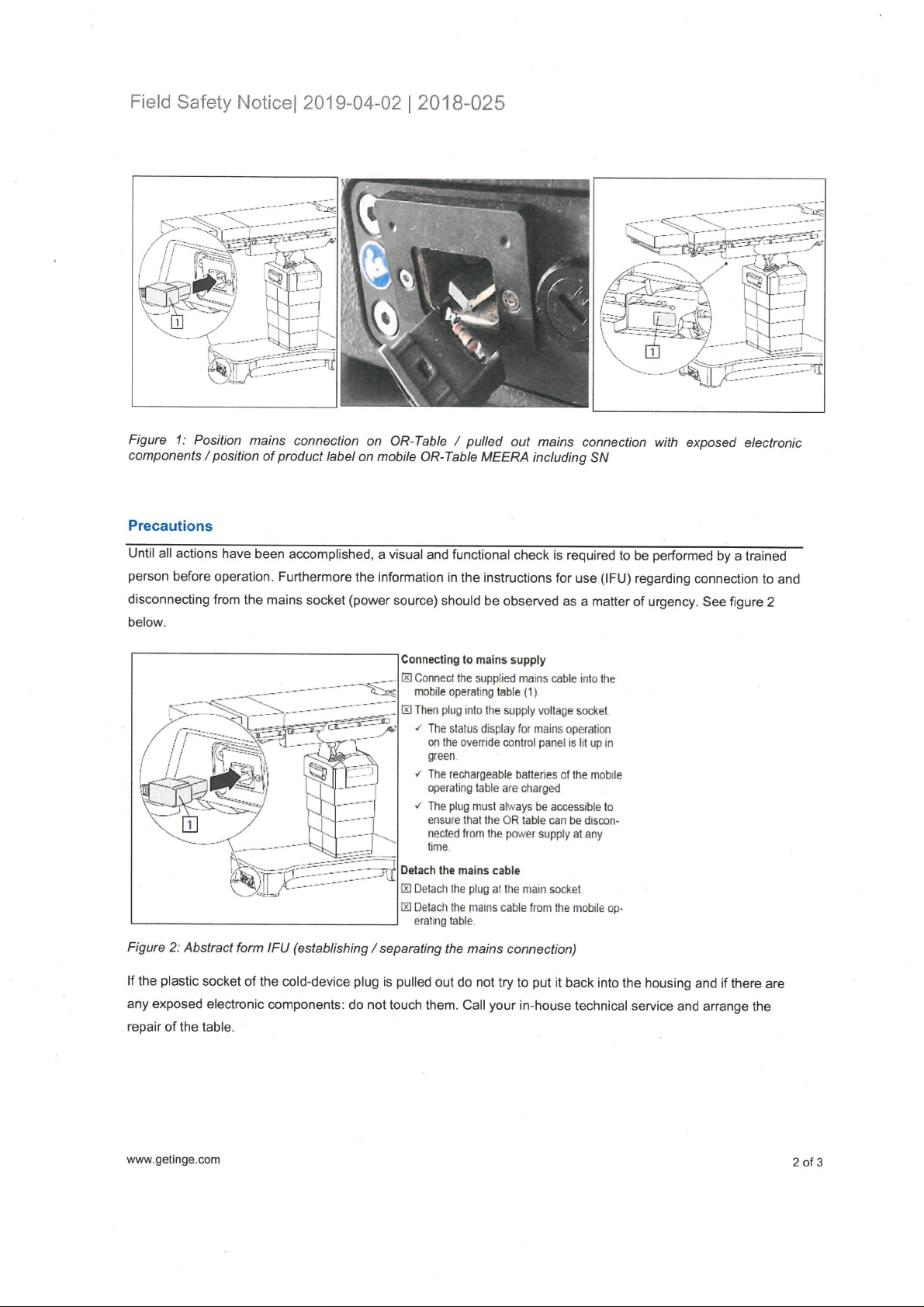
As part of our post market surveillance, cases were reported to Getinge involving a mobile OR-Table MEERA,
and in which the plastic socket of the IEC 60320-1 socket was pulled out of the metal cage and electronic parts
were exposed. See Figure 1 below.
Potential hazards
In cases where the socket was pulled out and main cable is still connected to the main socket (power source),
there is a risk that a user or third person may suffer an electric shock when coming in contact with the exposed
electronic components.
Three cases have been reported to Getinge where the user received an electric shock. No permanent injury was
reported to Getinge in any of these cases.
MAQUET
GmbH
Kehler
Straße
31
76437 Rastatt, Germany
Phone: +49 7222 932-0
Fax: +49 7222 932-868
www.getinge.com
Gesellschaft mit beschränkter Haftung, Sitz: Rastatt
Registergericht Mannheim HRB 715072
Ust.-IdNr. DE 144021332
Steuer-Nr. 39487/29420
Geschäftsführer:
Aufsichtsratsvorsitzender: Frederic Fette
Dr.
Dieter Engel,
Dr.
Benno Bröcher
Sicherheitsbeauftragter.medizinprodukte©maquetde
Phone: +49 7222 932-1647
Fax: +49 7222 932-234
Page 2
Field Safety Notice' 2019-04-02 I 2018-025
Figure 1: Position mains connection on OR-Table / pulled out mains connection with exposed electronic
components/position of product label on mobile OR-Table MEERA including SN
Precautions
Until all actions have been accomplished, a visual and functional check is required to be performed by a trained
person before operation. Furthermore the information in the instructions for use (IFU) regarding connection to and
disconnecting from the mains socket (power source) should be observed as a matter of urgency. See figure 2
below.
Connecting to mains supply
GI Connect the supplied mains cable into the
mobile operating table (1).
E Then plug into the supply voltage socket.
.
7
The status display for mains operation
on the override control panel is lit up in
green
The rechargeable batteries of the mobile
operating table are charged.
.
7
The plug must always be accessible to
ensure that the OR table can be disconnected from the power supply at any
time.
Detach the mains cable
[RI Detach the plug at the main socket.
Cl
Detach the mains cable from the mobile op-
erating table.
Figure 2: Abstract form IFU (establishing / separating the mains connection)
If the plastic socket of the cold-device plug is pulled out do not try to put it back into the housing and if there are
any exposed electronic components: do not touch them. Call your in-house technical service and arrange the
repair of the table.
www.getinge.com
2 of 3
Page 3

Field Safety Notice' 2019-04-02 I 2018-025
Corrective action
A technical solution that will correct this issue has been developed.
Getinge will initiate an immediate field action of all affected MEERA OR-Tables with the serial numbers listed
above. You will be contacted by your Getinge sales or service representative to plan for the update of your
device.
Passing on the information described here
Please ensure that all persons within your organization who use the above-mentioned devices - and anybody
else who needs to know - receive this field safety notice. If you have distributed the product on to third parties,
please forward a copy of this notice or inform the Getinge contact you are aware of.
Please complete & return the attached acknowledgement form and maintain awareness of this notice and related
actions until your mobile OR-Table MEERA has been updated, to ensure effectiveness of the corrective action.
Contact person
Should you have questions or require additional information, please contact your local Getinge representative.
The competent authorities concerned have received a copy of this field safety notice.
We apologize for any inconvenience. However, please consider this action as a preventive action to increase
quality.
With kind regards
MAQUET GmbH
www.getinge.com
3 of 3
 Loading...
Loading...