GE Proteus XR-a User Manual

GE Medical Systems
Technical
Publications
Direction 2259724-100
Revision 22
Proteus XR/a
Operator Manual
0459
Copyright © 2000~2009
By General Electric Company
Operating Documentation
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
|
This page intentionally left blank |
ii

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
IMPORTANT!...X-RAY PROTECTION
X-ray equipment if not properly used may cause injury. Accordingly, the instructions herein contained should be thoroughly read and understood by everyone who will use the equipment before you attempt to place this equipment in operation. The General Electric Company, Medical Systems Group, will be glad to assist and cooperate in placing this equipment in use.
Although this apparatus incorporates a high degree of protection against x-radiation other than the useful beam, no practical design of equipment can provide complete protection. Nor can any practical design compel the operator to take adequate precautions to prevent the possibility of any persons carelessly exposing themselves or others to radiation.
It is important that everyone having anything to do with x-radiation be properly trained and fully acquainted with the recommendations of the National Council on Radiation Protection and Measurements as published in NCRP Reports available from NCRP Publications, 7910 Woodmont Avenue, Room 1016, Bethesda, Maryland 20814, and of the International Commission on Radiation Protection, and take adequate steps to protect against injury.
The equipment is sold with the understanding that the General Electric Medical Systems, its agents, and representatives have no responsibility for injury or damage which may result from improper use of the equipment.
Various protective material and devices are available. It is urged that such materials or devices be used.
 CAUTION
CAUTION  Federal law restricts this device to sale by or on the order of a physician.
Federal law restricts this device to sale by or on the order of a physician.
i

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
If you have any comments, suggestions or corrections to the information in this document, please write them down, include the document title and document number, and send them to: GENERAL ELECTRIC MEDICAL SYSTEMS
MANAGER - INFORMATION INTEGRATION AMERICAS, X-RAY W-622
P.O. BOX 414
MILWAUKEE, WI 53201-0414
 CERTIFIED ELECTRICAL CONTRACTOR STATEMENT
CERTIFIED ELECTRICAL CONTRACTOR STATEMENT
All electrical installations that are preliminary to positioning of the equipment at the site prepared for the equipment shall be performed by licensed electrical contractors. In addition, electrical feeds into the Power Distribution Unit shall be performed by licensed electrical contractors. Other connections between pieces of electrical equipment, calibrations, and testing shall be performed by qualified GE Medical personnel. The products involved (and the accompanying electrical installations) are highly sophisticated, and special engineering competence is required. In performing all electrical work on these products, GE will use its own specially trained field engineers. All of GE’s electrical work on these products will comply with the requirements of the applicable electrical codes.
The purchaser of GE equipment shall only utilize qualified personnel (i.e., GE’s field engineers, personnel of third-party service companies with equivalent training, or licensed electricians) to perform electrical servicing on the equipment.
ii
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
REGULATORY REQUIREMENTS
This product complies with the regulatory requirements of the following:
Council Directive 93/42/EEC concerning medical devices: the CE label affixed to the product testifies compliance to the Directive.
The location of the CE label on the product is described page 2-4.
EU Authorized Representative:
GE Medical Systems SCS 283 rue de la Minière 78530 BUC, FRANCE
Green QSD 1990 Standard issued by MDD (Medical Devices Directorate, Department of Health, UK).
Quality System Regulation issued by the FDA (Food and Drug Administration, Department of Health, USA).
Underwriter’s Laboratories, Inc. (UL), an independent testing laboratory.
Canadian Standards Association (CSA).
International Electrotechnical Commission (IEC).
The following equipment classifications are applicable to the product:
Equipment classification with respect to protection from electric shock: Class 1
Degree of protection from electric shock: Type B
Degree of protection against ingress of liquids: not classified
Equipment not suitable for use in the presence of a flammable anaesthetic mixture with air or with nitrous oxide; mode of operation: continuous
Mode of operation: continuous with intermittent loading
The Proteus XRa has only level 1 EMC susceptibility immunity responses.
UDI Label
Every Proteus XR/a system has an unique marking for identification. The Unique Device Identification (UDI) marking appears on the product label which is located on system cabinet.
iii
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
This page intentionally left blank |
|
iv

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
ELECTROMAGNETIC COMPATIBILITY (EMC)
This product conforms with IEC 60601-1-2:2001+A1:2004 EMC standard for medical devices.
Note:
Note:
WARNING
This equipment generates, uses, and can radiate radio frequency energy. The equipment may cause or subject to radio frequency interference with other medical and non–medical devices and radio communications. To provide reasonable protection against such interference, the Proteus XR/a System (32, 50, 65, 80kW) complies with emissions limits for a Group 1, Class A Medical Devices and has applicable immunity level as stated in EN IEC 60601-1- 2:2001+A1:2004.
However, there is no guarantee that interference will not occur in a particular installation. Special precautions and other information regarding EMC provided in the accompanying documents of this equipment shall be observed during installation and operation of this equipment.
If this equipment is found to cause interference (which may be determined by switching the equipment on and off), the user (or qualified service personnel) should attempt to correct the problem by one or more of the following measure(s):
Reorient or relocate the affected device(s).
Increase the separating space between the equipment and the affected device.
Power the equipment from a source different from that of the affected device.
Consult the point of purchase or service representative for further suggestions.
Use of accessories, transducers, cables and other parts other than those specified by the manufacturer of this equipment may result in increased emissions or decreased immunity of the equipment. The manufacturer is not responsible for any interference caused either by the use of interconnect cables other than those recommended, or by unauthorized changes or modifications to this equipment. Unauthorized changes or modifications could void the user’s authority to operate the equipment.
v

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
ELECTROMAGNETIC COMPATIBILITY (EMC) (CONT.)
Note: To comply with the regulations applicable to an electromagnetic interface for a Group 1, Class A Medical Device, and to minimize interference risks, the following requirements shall apply:
All interconnect cables to peripheral devices must be shielded and properly grounded. Use of cables not properly shielded and grounded may result in the equipment causing radio frequency interference in violation of the European Union Medical Device directive and FCC regulations.
All of those recommended guidance regarding electromagnetic environment should be followed.
Note: Do not use devices that intentionally transmit RF signals (Cellular Phones, Transceivers, or Radio Controlled Products) in the vicinity of this equipment as it may cause performance outside the published specifications. Keep the power to these type devices turned off when near the equipment. The medical staff in charge of this equipment is required to instruct technicians, patients, and others.
Guidance and manufacturer’s declaration – Electromagnetic Emissions
The Proteus XR/a system is suitable for use in the specified electromagnetic environment. The purchaser or user of the Proteus XR/a system should assure that it is used in an electromagnetic environment as described below:
Emissions Test |
Compliance |
Electromagnetic Environment |
||
|
|
|
|
|
|
|
The Proteus XR/a system uses RF energy only for |
||
RF Emissions |
Group1 |
its internal function. Therefore, its RF emissions are |
||
CISPR11 |
|
very low and are not likely to cause any interference |
||
|
|
in nearby electronic equipment. |
||
RF Emissions |
Class A |
The Proteus XR/a system is suitable for use in all |
||
CISPR11 |
|
establishments other than domestic and those |
||
|
|
directly connected to the public low-voltage power |
||
Harmonic emissions |
Not |
|||
supply network that supplies buildings used for |
||||
IEC 61000-3-2 |
applicable |
|||
domestic purposes. |
|
|||
Voltage fluctuations/ |
Not |
|
||
|
|
|||
flicker emissions |
applicable |
|
|
|
IEC 61000-3-3 |
|
|
|
|
ELECTROMAGNETIC COMPATIBILITY (EMC) (CONT.)
vi

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
Guidance and manufacturer’s declaration - Electromagnetic Immunity (1)
The Proteus XR/a system is suitable purchaser or user of the Proteus XR/a environment as described below:
for use in the specified electromagnetic environment. The system should assure that it is used in an electromagnetic
Immunity Test |
IEC 60601-1-2 |
Compliance |
Electromagnetic Environment |
||||
|
Test Level |
Level |
|
|
|
|
|
Electrostatic |
6 kV contact |
6 kV contact |
Floors are wood, concrete, or ceramic |
||||
discharge (ESD) |
8 kV air |
8 kV air |
tile, or floors are covered with synthetic |
||||
IEC 61000-4-2 |
|
|
material and the relative humidity is at |
||||
|
|
|
least 30 %. |
|
|
|
|
|
2 kV for power |
2 kV for |
|
|
|
|
|
Electrical fast |
supply lines |
power supply |
|
|
|
|
|
transient/burst |
|
lines |
Mains power quality is that of a typical |
||||
IEC 61000-4-4 |
1 kV for |
1 kV for |
commercial and/or hospital environment |
||||
|
input/output |
|
|
|
|
|
|
|
lines |
input/output |
|
|
|
|
|
|
|
lines |
|
|
|
|
|
|
1 kV differential |
1 kV |
|
|
|
|
|
Surge |
mode |
differential |
Mains power quality is that of a typical |
||||
IEC 61000-4-5 |
2 kV common |
mode |
commercial and/or hospital environment. |
||||
|
mode |
2 kV common |
|
|
|
|
|
|
|
mode |
|
|
|
|
|
Voltage dips, |
|
|
Mains power quality is that of a typical |
||||
short |
< 5 % UT |
0 % UT for 5 |
commercial and/or hospital environment. |
||||
interruptions and |
(> 95 % dip in UT) |
sec |
If the user of the Proteus XR/a system |
||||
voltage |
for 0.5 cycle |
|
requires |
continued operation |
during |
||
variations on |
|
|
power mains interruptions, it is |
||||
power supply |
40 % UT |
|
recommended that the Proteus XR/a |
||||
input lines |
(60 % dip in UT) |
|
system |
be |
powered |
from |
an |
IEC 61000-4-11 |
for 5 cycles |
|
uninterruptible power supply or a battery. |
||||
|
70 % UT |
|
|
|
|
|
|
|
(30 % dip in UT) |
|
|
|
|
|
|
|
< 5 % UT |
|
|
|
|
|
|
|
(> 95 % dip in UT) |
|
|
|
|
|
|
|
for 5 s |
|
|
|
|
|
|
Power |
|
|
Power frequency magnetic fields are at |
||||
frequency |
3 A/m |
3 A/m |
levels characteristic of a typical location |
||||
(50/60 Hz) |
|
|
in a typical commercial and/or hospital |
||||
magnetic field |
|
|
environment. |
|
|
|
|
IEC 61000-4-8 |
|
|
|
|
|
|
|
Note: These are guidelines. Actual conditions may vary.
ELECTROMAGNETIC COMPATIBILITY (EMC) (CONT.)
vii

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
Guidance and manufacturer’s declaration - Electromagnetic Immunity (2)
The Proteus XR/a system is suitable for use in the specified electromagnetic environment. The purchaser or user of the Proteus XR/a system should assure that it is used in an electromagnetic environment as described below:
Immunity |
IEC 60601-1-2 |
Compliance |
Electromagnetic Environment |
Test |
Test Level |
Level |
|
|
|
|
Portable and mobile RF communications |
|
|
|
equipment are used no closer to any part of the |
|
|
|
[EQUIPMENT and/or SYSTEM], including cables, |
|
|
|
than the recommended separation distance |
|
|
|
calculated from the equation appropriate for the |
|
|
|
frequency of the transmitter. |
|
|
|
Recommended separation distance |
|
Conducted RF |
3 V |
|
|
|
IEC 61000-4-6 |
150 kHz to |
[V1 =] 3 V |
|
d= 1.2 |
|
80 MHz |
|
|
|
|
|
|
d= 1.2 |
80 MHz to 800 MHz |
|
|
|
d= 2.3 |
800 MHz to 2,5 GHz |
Radiated RF |
3 V/m |
|
|
|
IEC 61000-4-3 |
80 kHz to |
[E1=] 3 V/m |
Note: P is the power rating of the transmitter in |
|
|
800 MHz |
|
||
|
|
watts (W) according to the transmitter |
||
|
|
|
||
|
|
|
manufacturer and d is the recommended |
|
|
|
|
separation distance in meters (m). |
|
|
|
|
Field strengths from fixed RF transmitters, as |
|
|
|
|
determined by an electromagnetic site survey,* |
|
|
|
|
are less than the compliance level in each |
|
|
|
|
frequency range.** |
|
|
|
|
Interference may occur in the vicinity of |
|
|
|
|
equipment marked with the following symbol: |
|
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
*Field strengths from fixed transmitters, such as base stations for cellular telephones and land mobile radios, amateur radio, AM and FM radio broadcast, and TV broadcast cannot be estimated accurately. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be performed. If the measured field strength exceeds the RF compliance level above, observe the Proteus XR/a system to verify normal operation in each use location. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the [EQUIPMENT and/or SYSTEM].
**Over the frequency range 150 kHz to 80 MHz, field strengths are less than 3 V/m.
The Recommended Separation Distances are listed in the next table.
Note: These are guidelines. Actual conditions may vary.
ELECTROMAGNETIC COMPATIBILITY (EMC) (CONT.)
viii

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
Recommended Separation Distances for Portable and Mobile RF Communications Equipment and the Proteus XR/a system
Frequency of |
|
|
|
Transmitter |
150KHz to 80 MHz |
80 MHz to 800 MHz |
800 MHz to 2,5 GHz |
|
|||
|
|
|
|
Equation |
|
|
|
|
d= 1.2 |
d= 1.2 |
d= 2.3 |
|
|
|
|
Rated Power of |
|
|
|
Transmitter |
DISTANCE |
DISTANCE |
DISTANCE |
(W) |
(meters) |
(meters) |
(meters) |
0.01 |
0.12 |
0.12 |
0.23 |
0.1 |
0.38 |
0.38 |
0.73 |
1 |
1.2 |
1.2 |
2.3 |
10 |
3.8 |
3.8 |
7.3 |
100 |
12 |
12 |
23 |
For transmitters rated at a power not listed above, the DISTANCE can be estimated using the equation in the corresponding column, where P is the power rating of the transmitter in watts (W) according to the transmitter manufacturer.
Note: These are guidelines. Actual conditions may vary.
ix
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
|
This page intentionally left blank |
x

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
SAFETY
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
WARNING
ELECTRIC SHOCK HAZARD! DO NOT REMOVE COVERS OR PANELS. GENERATOR CABINET CONTAINS HIGH VOLTAGE CIRCUITS FOR GENERATING AND CONTROLLING X-RAYS. PREVENT POSSIBLE ELECTRIC SHOCK BY LEAVING COVERS AND PANELS ON THE EQUIPMENT. THERE ARE NO OPERATOR SERVICEABLE PARTS OR ADJUSTMENTS INSIDE THE CABINETS UNDER THE TABLE. ONLY TRAINED AND QUALIFIED PERSONNEL SHOULD BE PERMITTED ACCESS TO THE INTERNAL PARTS OF THIS EQUIPMENT.
FOR CONTINUED SAFE USE OF THIS EQUIPMENT, FOLLOW THE INSTRUCTIONS CONTAINED IN THIS OPERATING MANUAL. STUDY THIS MANUAL CAREFULLY BEFORE USING THE EQUIPMENT AND KEEP IT AT HAND FOR QUICK REFERENCE.
RADIOGRAPHIC EQUIPMENT MUST BE OPERATED BY QUALIFIED PERSONNEL AND ONLY AFTER SUFFICIENT TRAINING.
UNITED STATES FEDERAL LAW RESTRICTS THIS DEVICE TO USE BY OR ON THE ORDER OF A PHYSICIAN.
IT IS THE RESPONSIBILITY OF THE OPERATOR TO ENSURE THE SAFETY OF THE PATIENT WHILE THE MACHINE IS IN OPERATION BY CHECKING PROPER PATIENT POSITIONING AND USING THE EQUIPMENT PROTECTIVE DEVICES.
TO AVOID INJURY TO FINGERS AND HANDS OF PATIENT AND OPERATOR CAUSED BY TABLE TOP MOVEMENT, HANDS MUST BE KEPT AWAY FROM TABLE TOP EDGES AT ALL TIMES.
USE A SID AS LARGE AS POSSIBLE IN ORDER TO KEEP THE ABSORBED DOSE TO THE PATIENT AS LOW AS REASONABLY ACHIEVABLE.
IT IS THE RESPONSIBILITY OF THE OPERATOR TO PROVIDE MEANS FOR AUDIO AND VISUAL COMMUNICATION WITH THE PATIENT FROM THE CONTROL ROOM.
PERFORM PERIODIC MAINTENANCE TO ENSURE CONTINUED SAFE USE OF THE EQUIPMENT. (See chapter 11 Planned Maintenance).
IF ANY SAFETY PROBLEM OCCURS, PLEASE STOP USING THIS DEVICE AND CONTACT OUR SERVICE AT ONCE.
RESTRICT ACCESS TO THE EQUIPMENT IN ACCORDANCE WITH LOCAL REGULATIONS FOR RADIATION PROTECTION.
TO AVOID THE RISK OF ELECTRIC SHOCK, THIS EQUIPMENT MUST ONLY BE CONNECTED TO A SUPPLY MAINS WITH PROTECTIVE EARTH.
FOR DIAGNOSTIC X-RAY EQUIPMENT SPECIFIED TO BE USED IN COMBINATION WITH ACCESSORIES OR OTHER ITEMS NOT FORMING PART OF THE SAME, PLEAE PAY ATTENTION TO THE POSSIBLE ADVERSE EFFECT ARISING FROM MATERIALS LOCATED IN THE X-RAY BEAM. REFER TO THE TABLE BELOW FOR MAXIMUM ATTENUATION EQUIVALENT OF POSSIBLE MATERIALS LOCATED IN THE X-RAY BEAM.
xi

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
 CAUTION
CAUTION
 CAUTION
CAUTION
Always be alert to safety when you operate this equipment. You must be familiar enough with the equipment to recognize any malfunctions that can be a hazard. If a malfunction occurs or a safety problem is known to exist, do not use this equipment until qualified personnel correct the problem.
Apply necessary sterilization with 75% medical Alcohol to components which are possible to be contacted with the patients, such as Table top, Wall Stand (including SG120 Wall Stand) front panel, etc.
xii
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
ENVIRONMENTAL PROTECTION
WITH THE DISPOSAL OF WASTE PRODUCTS, RESIDUES AND EQUIPMENT ACCESSORIES THAT ARE OUT OF THEIR EXPECTED SERVICE LIFE, TO AVOID THE IMPACT OF ENVIRONMENT, PLEASE COMPLY WITH LOCAL STATUTE OR CALL GE SERVICE.
ESTABLISH EMERGENCY PROCEDURES
ESTABLISH PROCEDURES FOR HANDLING THE PATIENT IN CASE OF THE LOSS OF RADIOGRAPHIC IMAGING OR OTHER SYSTEM FUNCTIONS DURING AN EXAM.
ESTABLISH PROCEDURES FOR HANDLING THE PATIENT IN CASE OF THE LOSS OF RADIOGRAPHIC IMAGING OR OTHER SYSTEM FUNCTIONS DURING AN EXAM.
POSSIBLE PATIENT INJURY!
TO AVOID POSSIBLE PATIENT INJURY, BE SURE THAT SYSTEM POWER IS APPLIED BEFORE THE PATIENT ENTERS THE ROOM. THE OVER HEAD TUBE SUSPENSION MOVEMENT EM LOCKS AND TABLE LONGITUDINAL TRAVEL LOCKS FUNCTION ONLY WHEN SYSTEM AC POWER IS APPLIED.
IF POWER IS DISCONNECTED, THE OTS AND THE TABLE TOP (LONGITUDINAL) WILL MOVE FREELY, POSSIBLE CAUSING THE PATIENT TO FALL.
DO NOT ALLOW THE PATIENT TO MOUNT OR DEMOUNT THE SYSTEM.
DO NOT ALLOW THE PATIENT TO USE THE OTS AS A SUPPORT. OPERATIONAL CHECKS
Be sure the equipment is functioning properly and safely before each examination:
Verify that the following controls are operating correctly:
Motion controls, and Lock Releases
Audible and visual alarms
Visually inspect the equipment and make sure that:
Equipment is not damaged or missing parts
All cover panels are in place prior to turning on electrical power (hazardous electrical or mechanical parts could be exposed).
APPROVED OPERATING PROCEDURES AND ACCESSORIES
Be sure to use the equipment and the approved accessories according to approved operating procedures:
Perform X-ray tube warm up procedure prior to the exam. Failure to perform this procedure could damage the X-ray Tube assembly.
Do not exceed tabletop rating of a 220 kg (484 lbs.) patient. Excessive loading could damage the tabletop and/or cause the patient to fall.
Accessories should be properly attached to the table and positioned so as not to interfere with system motions.
Avoid unnecessary exposure to radiation. Stay behind the lead glass radiation shield or lead screen. When in unshielded areas, wear protective apparel such as goggles, lead aprons, and gloves.
xiii

|
|
|
|
|
PROTEUS XR/a |
|
GE MEDICAL SYSTEMS |
Operator Manual |
|
||||
REV 22 |
|
|
DIRECTION 2259724-100 |
|||
PLANNED MAINTENANCE |
|
|
||||
|
|
|
|
POSSIBLE PATIENT OR OPERATOR INJURY! |
|
|
|
|
|
|
|
|
|
|
WARNING |
|
TO AVOID POSSIBLE PATIENT OR OPERATOR INJURY, BE SURE TO PERFORM |
|||
|
|
|
|
|||
|
|
|
|
THE PERIODIC INSPECTIONS AND MAINTENANCE PROVIDED IN THIS |
||
|
|
|
|
DOCUMENT. FAILURE TO PERFORM THESE INSPECTIONS COULD ALLOW |
||
|
|
|
|
DETERIORATING CONDITIONS TO DEVELOP WITHOUT BEING DETECTED. THIS |
||
|
|
|
|
DETERIORATION COULD RESULT IN EQUIPMENT FAILURES WHICH COULD |
||
|
|
|
|
CAUSE SERIOUS INJURY EQUIPMENT DAMAGE. |
|
|
|
|
|
RADIATION SAFETY |
|
|
|
Always use proper technique factors for each procedure to minimize x-ray exposure and to produce the best diagnostic results. In particular, you must be thoroughly familiar with safety precautions before operating this System.
It is not always possible to determine when some components, such as x-ray tubes, are nearing the end of their operating lives. These components could stop operating during a patient examination.
KNOW THE EQUIPMENT
Read and understand all the instructions in the operating manuals before attempting to use the product and request training assistance from GE Medical System if needed.
Keep the operating manuals with the equipment at all times and periodically review the procedures and safety precautions.
This system contains operating safeguards to provide maximum safety. Before calling for service, be certain proper operating procedures are being used.
Satisfactory equipment performance requires the use of service personnel specially trained on x-ray apparatus. GE Medical Systems is responsible for the effects on safety, reliability, and performance only if the following conditions are met:
The electrical wiring of the relevant rooms complies with all national and local codes as well as the Regulations for the electrical equipment of buildings published by the Institution of Electrical Engineers.
All assembly operations, extensions, re-adjustments, and modifications or repairs are carried out by GE Medical Systems’ authorized service representatives.
The equipment is used in accordance with the instructions for use.
xiv
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
|
This page intentionally left blank |
xv
|
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
|
REV 22 |
|
DIRECTION 2259724-100 |
|
TABLE OF CONTENTS |
|
CHAPTER |
TITLE |
PAGE NUMBER |
1 |
QUICK START |
1-1 |
1-1 |
Turn System On |
1-1 |
1-2 |
Tube Warm-Up |
1-1 |
1-3 |
Set Technique APR |
1-2 |
1-4 |
Set Manual Technique |
1-3 |
1-5 |
Set AEC Technique |
1-4 |
2 |
SYMBOLS |
2-1 |
2-1 |
Special Notices |
2-1 |
2-2 |
X-ray Tube |
2-1 |
2-3 |
Power ON and OFF |
2-2 |
2-4 |
Electrical Type |
2-2 |
2-5 |
Electrical Current |
2-2 |
2-6 |
Ground |
2-3 |
2-7 |
Proteus XR/a Collimator / Eclipse Proteus Collimator |
2-3 |
2-8 |
Emergency Button |
2-3 |
2-9 |
Warning Signs and Labels |
2-3 |
2-10 |
System Labeling |
2-5 |
3 |
SYSTEM DESCRIPTION |
3-1 |
3-1 |
System Components/Features |
3-1 |
3-2 |
HHS Compliance Compatibilities |
3-3 |
4 |
PROTEUS XR/A SYSTEM START UP AND SHUT DOWN |
4-1 |
4-1 |
Turn the power on |
4-1 |
4-2 |
Turn Power off |
4-1 |
4-3 |
Daily Warm Up Procedures |
4-2 |
4-4 |
System Status Display |
4-2 |
4-5 |
Radiography Control Key |
4-3 |
5 |
PROTEUS XR/A SYSTEM CONSOLE |
5-1 |
5-1 |
Introduction |
5-1 |
5-2 |
Procedure Edit |
5-9 |
5-3 |
Application |
5-13 |
6 |
PROTEUS XR/A TABLE COMPONENTS |
6-1 |
6-1 |
Safe Operation Precautions |
6-1 |
6-2 |
Introduction |
6-3 |
6-3 |
Table Operation |
6-4 |
6-4 |
Cassette Tray Operation |
6-6 |
7 |
PROTEUS XR/A OVERHEAD TUBE SUSPENSION (OTS) |
7-1 |
7-1 |
Introduction |
7-1 |
7-2 |
Overhead Rail System |
7-1 |
7-3 |
Telescopic Column and Carriage |
7-2 |
7-4 |
X-ray Tube Support |
7-4 |
7-5 |
OverHead Tube Suspension User Interface |
7-6 |
7-6 |
Proteus XR/a Automatic Collimator |
7-8 |
7-7 |
Proteus XR/a Manual Collimator (Optional) |
7-14 |
7-8 |
Eclipse Proteus Collimator |
7-15 |
xvi
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
TABLE OF CONTENTS (CONT.)
CHAPTER |
TITLE |
PAGE NUMBER |
8 |
PROTEUS XR/A WALL STAND (GPCP No.: 2260354) COMPONENT |
8-1 |
8-1 |
Introduction |
8-1 |
8-2 |
Operation |
8-3 |
9 |
PROTEUS XR/A SG120 WALL STAND (GPCP No.: 2402562) COMPONENT 9-1 |
|
9-1 |
Safe Operation Precautions |
9-1 |
9-2 |
Introduction |
9-2 |
9-3 |
Applications |
9-4 |
9-4 |
Operation |
9-4 |
10 |
ACCESSORIES |
10-1 |
10-1 |
Introduction |
10-1 |
10-2 |
Accessories |
10-1 |
11 |
PLANNED MAINTENANCE |
11-1 |
11-1 |
General |
11-1 |
11-2 |
HHS Testing |
11-1 |
11-3 |
Qualified Service |
11-2 |
11-4 |
Periodic Maintenance |
11-2 |
11-5 |
Recycling |
11-5 |
12 |
SYSTEM FAULTS |
12-1 |
12-1 |
Introduction |
12-1 |
12-2 |
General Trouble Shooting |
12-1 |
12-3 |
Other Operator Fault Analysis |
12-4 |
12-4 |
Resetting Faults |
12-4 |
13 |
PHYSICAL REQUIREMENTS OF ROOM |
13-1 |
13-1 |
Environmental Requirements/Limitations |
13-1 |
13-2 |
Equipment Heat output |
13-2 |
13-3 |
Radiation Protection |
13-3 |
14 |
SPECIFICATION |
14-1 |
14-1 |
General System Specifications |
14-1 |
14-2 |
Table Specifications |
14-2 |
14-3 |
Generator Specifications |
14-3 |
14-4 |
System Console Specifications |
14-10 |
14-5 |
OTS Specifications |
14-10 |
14-6 |
Collimator Specifications |
14-11 |
14-7 |
Wall Stand (GPCP No.: 2260354) Specifications |
14-14 |
14-8 |
SG120 Wall Stand (GPCP No.: 2402562) Specifications |
14-15 |
14-9 |
X-ray Tube Specifications |
14-16 |
14-10 |
Printer Specifications |
14-18 |
14-11 |
Dose/DAP Specifications |
14-18 |
APPENDIX
xvii
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
|
|
REVISION HISTORY |
|
REV |
DATE |
TYPE OF MODIFICATION |
|
0 |
10/01/2000 |
Initial production Release |
|
1 |
20/07/2000 |
Add system function description and system specification |
|
2 |
05/12/2000 |
Update OTS user’s interface description |
|
3 |
01/02/2001 |
Update the regulatory requirements. |
|
3 |
01/02/2001 |
Add a warning to wall stand operators. |
|
3 |
01/02/2001 |
Update system labeling. |
|
3 |
01/02/2001 |
Add printer information. |
|
3 |
01/02/2001 |
Update wall stand illustration. |
|
3 |
15/02/2001 |
Add notes. |
|
3 |
19/02/2001 |
Add new wall stand. |
|
3 |
19/02/2001 |
Add a maintenance item. |
|
3 |
01/03/2001 |
Add manufacturer’s name |
|
3 |
07/03/2001 |
Add a warning. |
|
4 |
27/09/2001 |
Add notes, change specs. |
|
5 |
14/05/2003 |
Add description of MX100 X-ray tube and SG100 Wall Stand |
|
6 |
12/04/2004 |
Add a caution about the shroud of the elevating table. |
|
7 |
25/06/2005 |
Add EMC and WEEE Rules. |
|
7 |
25/06/2005 |
Add description of SG120 Wall Stand. |
|
8 |
05/12/2005 |
Add description of Eclipse Proteus collimator |
|
8 |
05/12/2005 |
Add a warning |
|
9 |
15/02/2006 |
Add description of Reciprocating Bucky and AID Ion Chamber. |
|
10 |
20/06/2006 |
Update warning label to meet HHS requirements in Chapter 2. |
|
11 |
08/10/2006 |
Add mA and mAs |
|
12 |
12/09/2007 |
Add Dose and DAP calculation descriptions |
|
12 |
12/09/2007 |
Add Hg label description |
|
13 |
30/01/2008 |
Add collimator and tube leakage technique factors |
|
13 |
30/01/2008 |
Add anti-toe pinch warning during table descending. |
|
14 |
25/07/2008 |
Update the table top’s dimensions to 2250mm*880mm in Chapter 6 |
|
15 |
02/06/2009 |
Add warning label in Chapter 2; Update Table minimum height; |
|
|
|
Remove ANTI-TOE-PINCH. |
|
16 |
11/08/2009 |
Minor Update; |
|
17 |
19/08/2011 |
Revise EMC standard version |
|
18 |
24/03/2012 |
Update EU Authorized Representative Contact Information |
|
19 |
28/05/2012 |
Update contents according to the 3rd edition IEC60601 standards |
|
20 |
16/08/2012 |
Update contents due to the console redesign. |
|
21 |
01/07/2015 |
Update “United States Federal law restricts this device to be used by or |
|
|
|
on the order of a physician into “Federal law restricts this device to sale |
|
|
|
by or on the order of a physician” |
|
22 |
01/06/2016 |
Update the cleaning and disinfecting requirement |
|
|
|
Update the UDI Requirement |
|
xviii
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
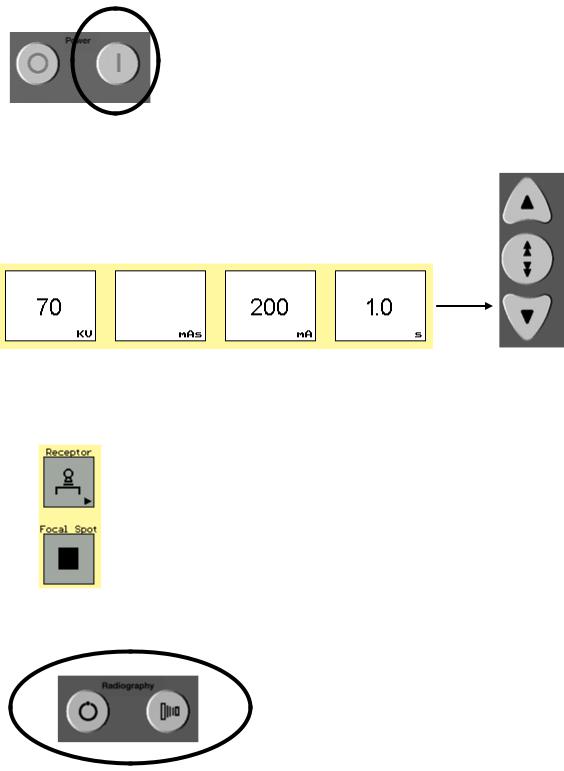
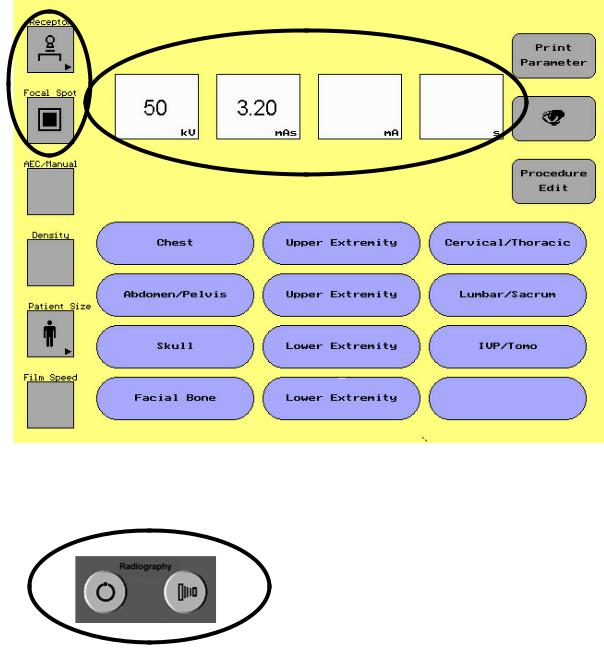
CHAPTER 1 PROTEUS XR/A QUICK START
1-1 Turn System On
1-2 Tube Warm-Up
-Set Technique
-Set Parameters
-Take 2 Exposures 10 sec apart
1-1
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
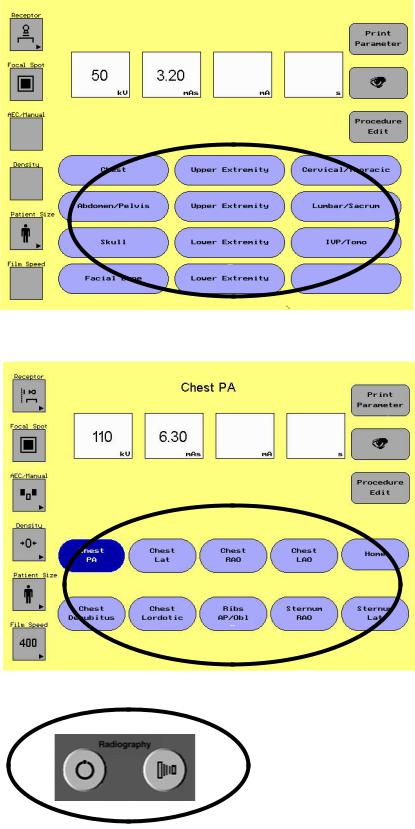
1-3 Set Technique APR
-Select Category
-Select Procedure
-Take Exposure
1-2
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
1-4 Set Manual Technique
-Set Parameters
-Set Technique
-Take Exposure
1-3

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
1-5 Set AEC Technique
-Set Parameters
-Set Technique
-Take Exposure
1-4
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
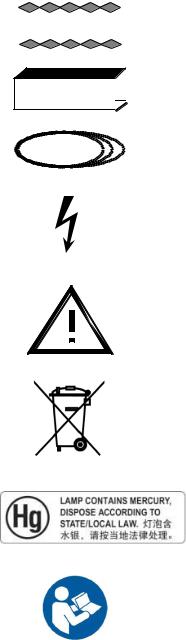
CHAPTER 2 SYMBOLS
2-1 Special Notices
 CAUTION
CAUTION
WARNING
DANGER
Symbols used on this system and in its accompanying documents are shown and explained in this section.
Caution advises of an avoidable condition that could cause minor physical injury, or damage to equipment or data.
Warning advises of an avoidable condition that may allow or cause a personal injury or the catastrophic destruction of equipment or data.
Danger advises of an avoidable condition that will cause serious or fatal injury.
Dangerous Voltage. Indicates an avoidable dangerous high voltage hazard.
This symbol on the equipment means that the operating instructions should be consulted to assure safe operation.
This symbol indicates that waste electrical and electronic equipment must not be disposed of as unsorted municipal waste and must be collected separately. Please contact an authorized representative of the manufacturer for information concerning the decommissioning of your equipment
This product consists of devices that may contain mercury, which must be recycled or disposed of in accordance with local, state, or country laws. (Within this system, the backlight lamps in the monitor display contain mercury.)
Follow instructions for use.
2-1

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
2-2 X-ray Tube
X-ray emission. X-ray tube head is emitting X-rays. Take adequate precautions to prevent the possibility of any persons carelessly, unwisely, or unknowingly exposing themselves or others to radiation.
Identifies controls or indicators associated with the selection of a small focal spot or the connection for the corresponding filament.
Identifies controls or indicators associated with the selection of a large focal spot or the connection for the corresponding filament.
2-3 Power ON and OFF
Power ON switch or switch position that applies mains voltage. Indicated connection to the mains for all mains switches or their positions. This symbol is used in all cases where safety is involved.
Power OFF switch or switch positions that removes mains voltage. Indicated disconnection from the mains for all mains switches or their positions. This symbol is used in all cases where safety is involved.
2-4 Electrical Type
Type B Equipment. Equipment providing a particular degree of protection again electrical shock regarding leakage current and protective grounding per IEC 601-1.
2-5 Electrical Current
Alternating Current. Indicates equipment that is suitable for alternating current only.
Direct Current. Indicates equipment that is suitable for direct current only.
2-2
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
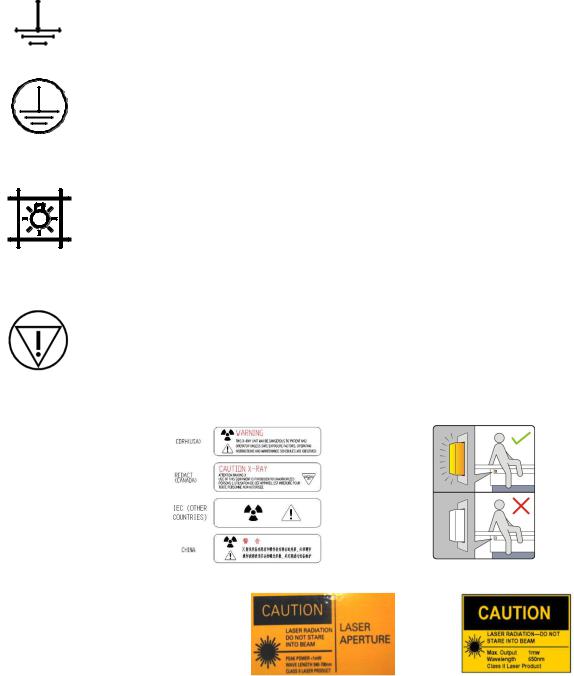
2-6 Ground
Functional Earth (ground) Terminal. Terminal directly connected to a point of a measuring supply or control circuit or to a screening part which is intended to be earthen for functional purposes.
Protective Earth (ground). Identifies any terminal that is intended for connection of an external protective conductor to protect against electrical shock in case of a fault.
2-7 Proteus XR/a Collimator / Eclipse Proteus Collimator
Control for indicating radiation field by using light.
2-8 Emergency Button
Immediately removes power from table.
2-9 Warning Signs and Labels
Label for inhibition button
Laser Warning
Proteus XR/a Collimator |
Eclipse Proteus Collimator |
Note: If have, please confirm the collimator and wall stand you’ve chosen by referring to the next chapter.
2-3

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROTEUS XR/a |
|
|||||||||||||||||||||||
GE MEDICAL SYSTEMS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operator Manual |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
REV 22 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DIRECTION 2259724-100 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 2-1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
MEANINGS OF PROTEUS XR/A SIGNS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Illustration 2-1
PROTEUS XR/A SYSTEM WARNING SIGNS LOCATION
2-4

|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
2-10 System Labelling
The labels for the Proteus XR/a system are found on the side panel of the Proteus XR/a cabinet. This label includes the CE mark for the entire system. See the following sketch.
For other name plate location, see table 2-2.
Table 2-2
PROTEUS XR/a SYSTEM IDENTIFICATION AND COMPLIANCE PLATES
DESIGNATION |
System console |
Wall Stand |
OTS radiographic |
Cabinet |
|
|
|
suspension (2/3 m) |
|
PART NUMBER |
2259976 or 5441870 |
600-0301 |
S3918MD/S3918K |
2259973 |
LOCATION of |
|
|
|
|
Name Plate |
|
|
|
|
|
|
|
|
|
DESIGNATION |
X-ray Tube (RAD-14) |
Proteus XR/a |
Bucky (L/H) |
Jedi Generator |
|
|
Automatic Collimator |
|
|
PART NUMBER |
2259981 |
2259298-54 |
2189553 or |
2268970 or |
|
|
|
5159516-1 |
2244165-2 |
LOCATION of |
|
|
|
|
Name Plate |
|
|
|
|
|
|
|
|
|
DESIGNATION |
X-ray Tube (MX 100) |
Eclipse Proteus |
SG120 Wall Stand |
|
|
Collimator |
|
PART NUMBER |
D2301R |
2379827 |
2402562 |
LOCATION of |
|
|
|
Name Plate |
|
|
|
|
|
|
|
2-5
|
PROTEUS XR/a |
GE MEDICAL SYSTEMS |
Operator Manual |
REV 22 |
DIRECTION 2259724-100 |
This page intentionally left blank.
2-6
 Loading...
Loading...