Page 1

OPERATOR’S GUIDE
Syringe pump
3,/27$
Introduction
Pilot A2
The
care. It introduces a new conc ept of control with easy reading of alarm s
and safety features.
The configuration flexibility of the
in the working conditions of medical teams, t hus increasing patient
safety. A choice of easily accessible conf i gurations ensures optimum use
of functions accordi ng to the needs of each department .
The use of this material requires great care. The user must be able to handle the instrument
properly and must know how to fully operate.
Please read the operator’s guide carefully before putting the device into use.
has been designed and manufactured with the greatest
Pilot A2
provides overall improvem ent
Table of contents
Operations for use ......................................................2
Internal safety features...............................................3
Performances...............................................................5
Technical characteristics ...........................................6
Configuration...............................................................8
Operating precautions................................................9
Maintenance...............................................................10
External power source..............................................12
Operation with the internal battery..........................12
Accessories................................................................13
Disposable .................................................................13
Conditions of guarantee...........................................14
Useful addresses.......................................................16
Page 2
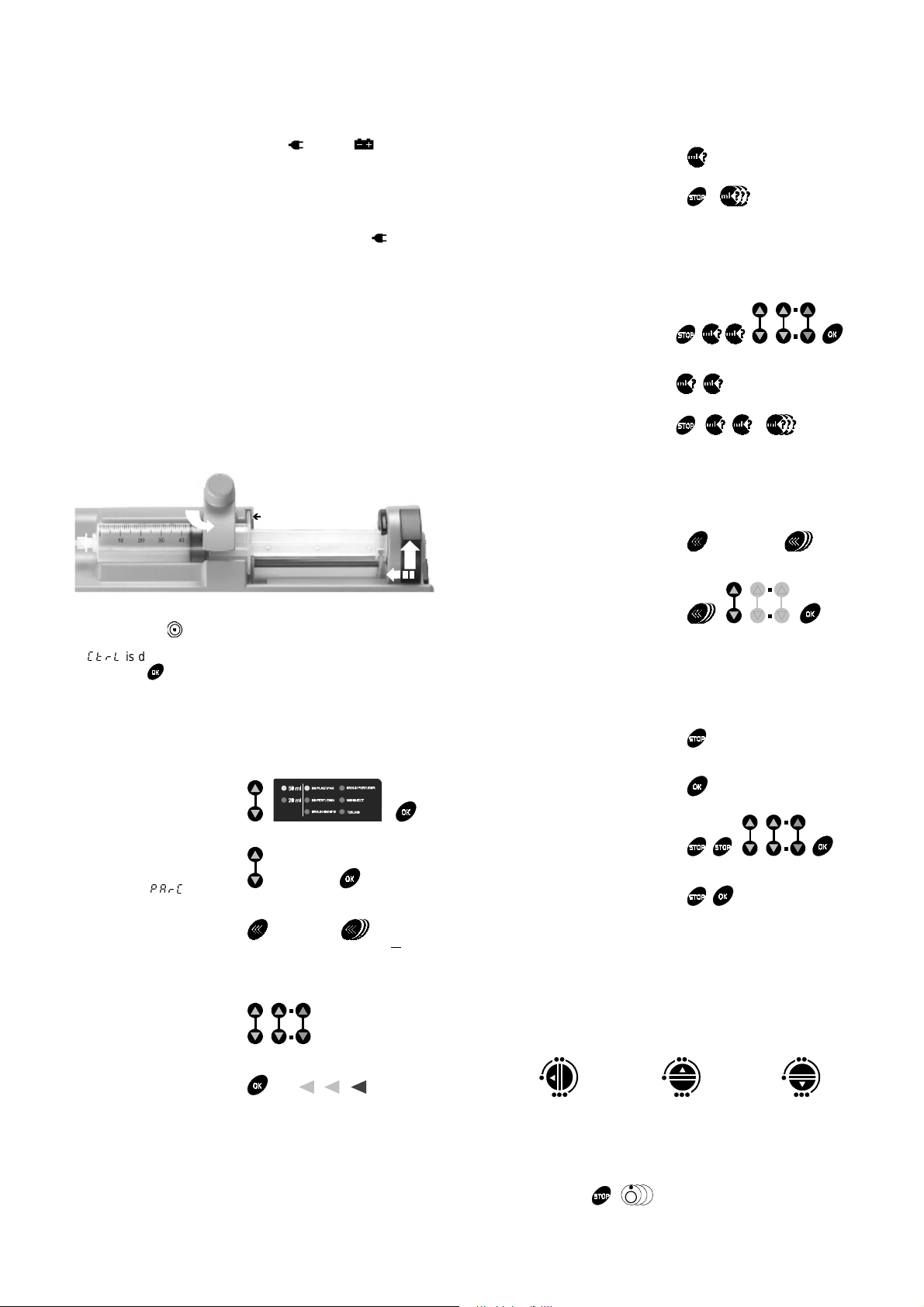
Operations for use
Installation
The syringe pump can be used on mains , battery or external
supply.
Particular attention should be paid to the stability of the device
before it is put into use.
1. Connect the power supply cord to the mains source and to the
syringe pump. The mains power indicator lights up
Note: connect device to mains as often as possible to recharge
battery.
.
Syringe installation
1. Connect the extension set to the syringe according to proper
practices.
2. Place syringe in its c radl e, the flanges correctly in t he provi ded slot.
3. Turn the syringe barrel clasp int o t he closed position and move t he
syringe drive forward the syringe plunger head.
Syringe barrel clasp
Í
Syringe flanges cradle
Infused volume
Consulting the infused volume:
Erasing the infused volume:
Volume limit
Programming a volum e l i mit (ml):
Programmed volume limit recall:
Erasing programmed volume limit:
Bolus function
Administering a bolus (ml):
10 1 0.1
(double press)
(double press)
EROX
(continuous press)
(continuous press)
(continuous press)
Syringe plunger head
4. Press the ON key to turn ON the
&WU/
Note: if
considered. Press
is displayed, preventive m ai ntenance should be
to continue.
Pilot.
Programming infusion
capacity brand and type
1. Syringe brand selecti on:
Drug name selection:
(according to device
configuration:
2. Prime the l i ne:
3. Connect infusi on set to patient and check general i nstallation.
4. Flowrate setting (ml/h):
3$U&
)
10 ml
syringe list given as an example
$GUH
(e.g.: ADRENALIN)
3XU*
10 1 0.1
(continuous nd press)
(e.g.: flow rate 5.2 ml/h)
Changing bolus rate (ml/h):
(continuous press)
STOP and Pause
Stop (sound warning after one
minute):
To resume infusion:
Pause duration selection, from 1 min
to 9 hrs 59 min:
Ending Pause and resuming
infusion:
(double press)
Occlusion alarm threshold setting
3 occlusion pressure limits available. S el ecti on i s adjusted m anual ly
by a safety located on the rear of the syringe drive.
5. Starting the i nf usion:
Important: flow rate may be programmed during infusi on and must be
confirmed within 15 seconds following the change. Proceed as desc ri bed
above to change the syringe.
- 2 -
Lower limit
Middle limit Upper limit
OFF
To turn off the Pilot :
(press for more than 2 seconds)
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
Page 3

Internal safety features
The Pilot device have a continuous inspection system which
functions as soon as the pump is in use.
Nevertheless, the qualified personnel in your establishment or our
After-Sales Department should always be notified of any abnormal
function where no specific cause can be found.
Prealarms and alarms
Checks Infusion
Battery Prealarm
Alarm
Mains Disconnection
Infusion End of infusion prealarm
With visual and audi bl e signal.
Silence alarm Activation Message
Stop
NO YES low battery
YES YES (2 min) discharged battery
NO YES m ai ns disconnected
NO YES 5 m i nutes before end of
infusion alarm or 10% of total
syringe capacity.
In case of single fault condition, an alarm i s acti vated within the limit
of 5% rate deviation. In addition, a secondary control feature
activates an alarm at 1 ml over infusion or 20% rate deviati on,
whichever is shortest.
Note: the battery automatically takes over when the mains supply is
disconnected.
Battery alarm + prealarm indicators
Battery alarm + alarm indicators
Note: memorisation of programmed parameters (10
min). Connect device t o mains.
E$W
messsage displayed.
Press
Note: to use Empty syringe mode press CONFIRM
key.
to acknoledge this warning.
Prealarm + end of infusion indicators
End of infusion alarm
Empty syringe
Volume
limit
Pressure Occlusion alarm
Syringe
installed
Others
alarms
Prealarm
Alarm
Syringe barrel clasp
Syringe flanges insertion
Plunger head position
Anti-siphon systems
Disengaged mechanism
Unconfirmed program or
flow rate = 00.0 ml/hr
YES YES Syringe em pty (theory)
YES YES Total syringe empty
NO YES 5 m i nutes before the volume
limit alarm or 10% of syringe
capacity.
KVO rate YES (2 min) Volume limit reached
YES YES (2 min) Programmed limit reached
YES YES (2 min) Syringe incorrectly positioned
YES YES Syringe incorrectly positioned
YES YES Drive systems not engaged
--- NO No confirm > 15 seconds
Alarm + end of infusion indicators
Note: to use Empty syringe mode press CONFIRM
key.
Prealarm + end of infusion indicators
Prealarm + flashing ml indicators
Alarm + KVO + ml indicators
Flashing alarm + oc clusion indicators
Alarm + syringe barrel clasp
indicators
Alarm + plunger head position
indicators
Alarm + disengaged mechanism
indicators
● Flashing confirm indic ator
No syringe selection
Key disabled
Programmed end of
pause
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
YES YES no syringe selection > 1 min
NO NO Pressing an unauthorised
key
YES NO Programmed end of pause
● Flashing confirm s i gnal + flashing capacity and
brand syringe indicators
Audible signal only
Alternating displays of flow rate value and
6W23
- 3 -
Page 4
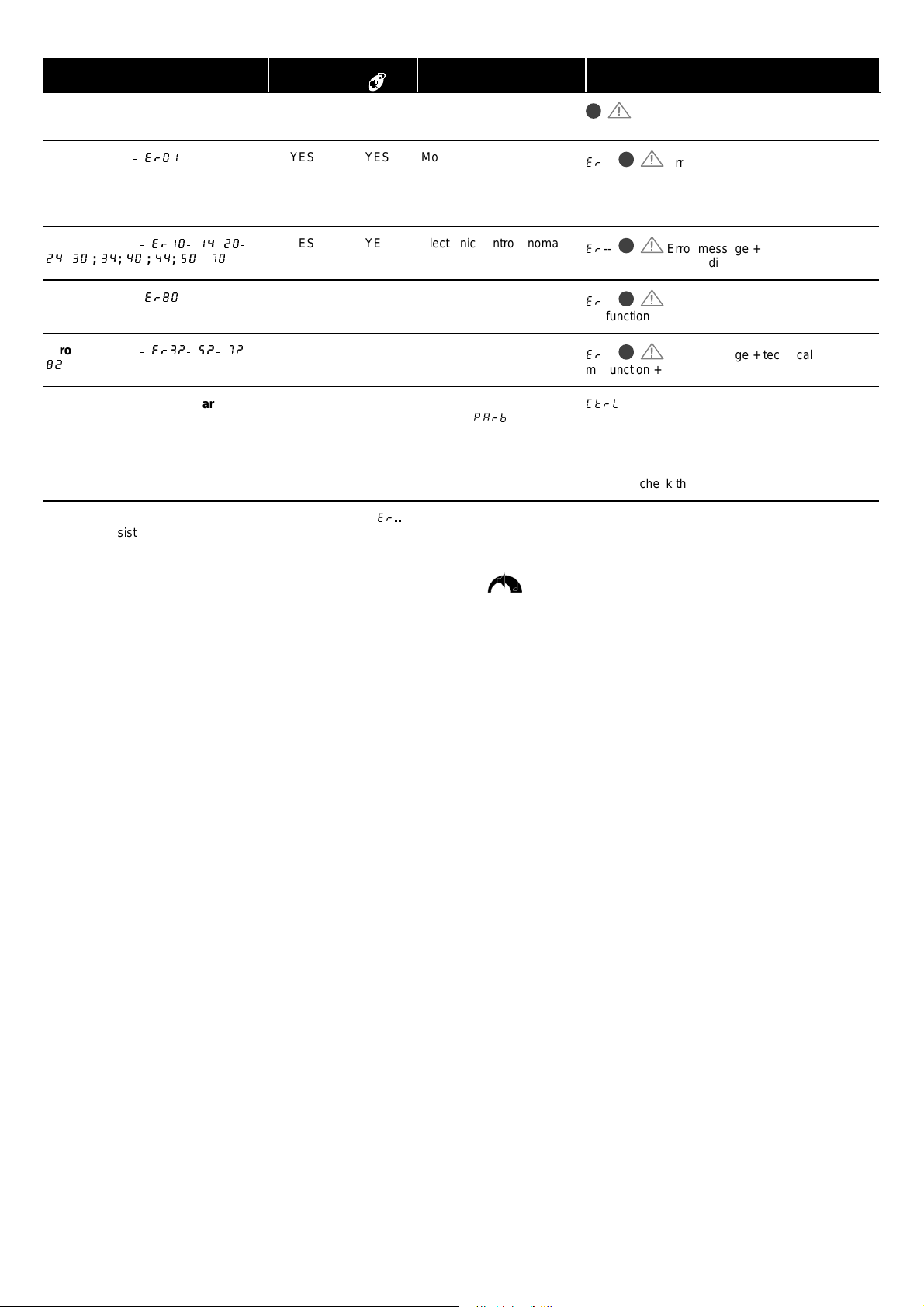
Checks Infusion
Stop
Silence alarm Activation Message
Malfunction alarm
(U
(U
,
;
;
(U
(U
(U
(U
;
(U
;
(U
;
;
;
Error message :
Error messages :
;
;
Error message :
Error messages :
Preventive maintenance warning
Note: in case of mal function alarm, note t he error message (
the alarm persists when the device is switched on again, without use on pat i ent, contact the qualif i ed t echnicians in your establishment or our AfterSales Department.
YES YES Device cannot check the
YES YES Motor rotation control
YES YES Electronic control anomaly
;
YES NO Keyboard anomaly
YES YES 1 ml deviation/ volume to be
;
NO --- Date of maintenance
infusion
anomaly
infused
reached (
(U
) and stop the device by pressing t he OFF key (5 - 10 seconds can be necessary). If
3$UE
Technical malfunc tion + alarm indicators
(U
--
malfunction + alarm indicators
Press STOP to resume the device normal operation
(U
malfunction + alarm indicators
(U
malfunction + alarm indicators
(U
malfunction + alarm indicators
&WU/
)
turned on).
Press CONFIRM to continue.
Warning: chec k the device as soon as possibl e.
Error message + techni cal
--
Error message + techni cal
--
Error message + techni cal
--
Error message + techni cal
message only displayed when the devic e i s
The sound level can be set by rotating the shutter placed underneath the devic e
.
- 4 -
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
Page 5
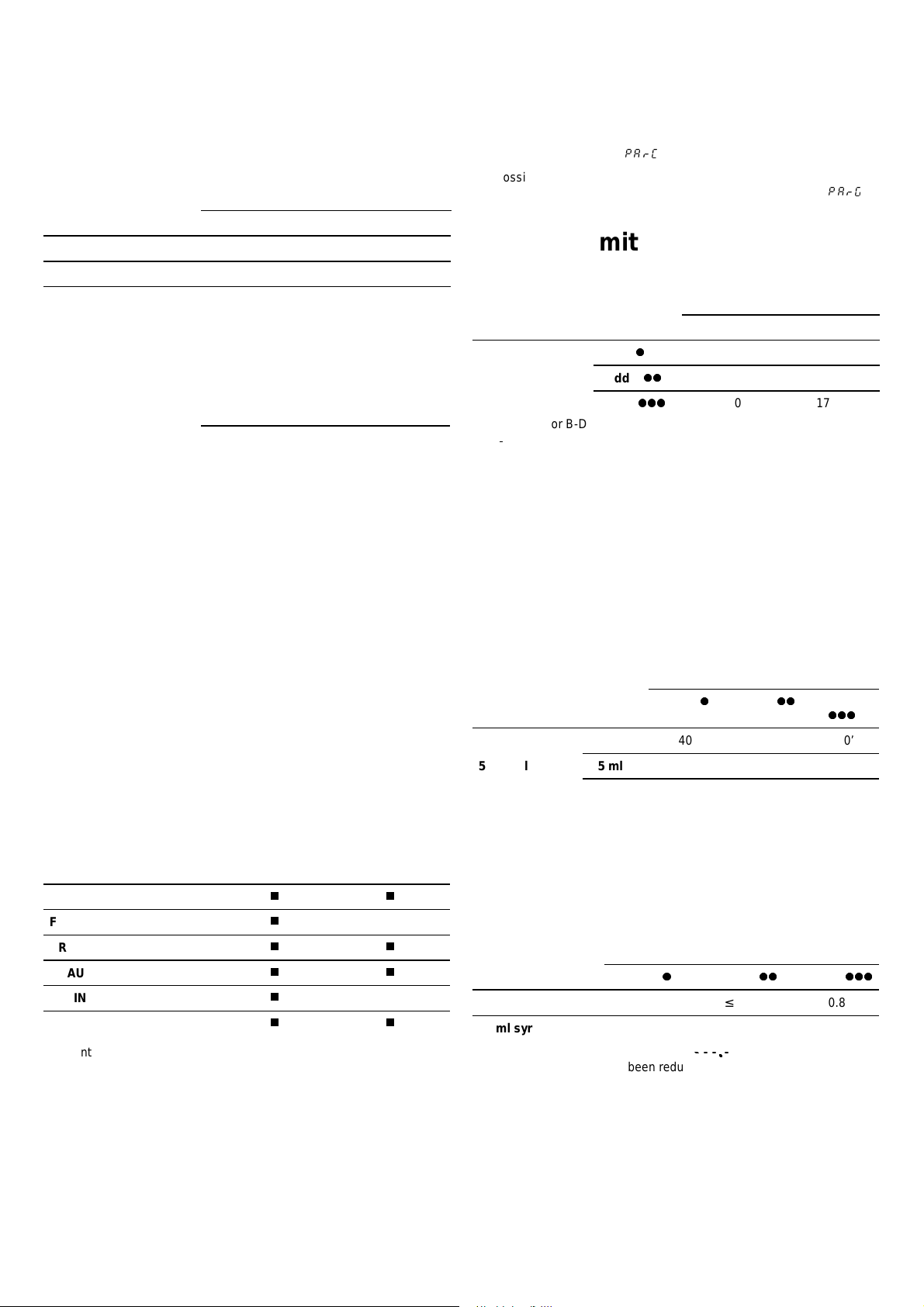
Performances
Flow rates
Display of the name of the drug
The values given in the table below correspond to device
configuration.
Syringes
50/60 ml 20 ml
Infusion flow rate (m l /h)
Bolus rate (ml/h)
Prime rate (ml/h)
0.1 ml/h increments.
from 0.1 to 200.0 from 0.1 to 120.0
from 50.0 to 500.0 from 50.0 to 275.0
500.0 275.0
Volume limit
Volume limit (ml)
KVO rate (Keep Vein Open): 1 m l /h or flow rate originally selected if t hi s
is less than 1 ml / h.
from 1 to 99.9 ml, 0.1 ml increment
from 100 to 999 ml, 1 ml increment
Accuracy
Flow rate accuracy
Device accuracy
Syringe accuracy
± 3 % with pre-programmed syringes
± 1%
± 2%
According to configurat i on (
It is possible to di splay periodically during infusion t he name of the drug
used. 15 names of drugs may be programmed by configurat i on (
Pressure limit
Pilot A2
proposes a choice of 3 occl usion alarm thresholds.
Threshold value lower
(mmHg)
Values given for B-D Plastipak® Luer Lok® syringes.
Note : 1 bar = 750 mmHg = 1000 hP a.
middle
upper
3$U&
z
z
zz
zz
zzz
zzz
).
3$U*
).
Syringes
50/60 ml
300
500
900
20 ml
600
1000
1700
Occlusion alarm response time
versus infusion flow rate
These values are representative of s yringes used during trials with an
and serve as an indication only of t he pump’s overall
Pilot A2
performance.
Pause duration
From 1 minute to 9 hours 59 minutes, 1 min. i ncrements.
Syringe type list
The Pilot A2 recognises the size of the installed syringe. The last
syringe brand used is proposed when the device is turned on.
Brands and types 50/60 ml 20 ml
B-D PLASTIPAK
FRESENIUS INJECTOMAT
BRAUN OMNIFIX
BRAUN PERFUSOR
PIC INDOLOR
TERUMO
Different syringe list s are avai l abl e. For further information, pl ease
contact our Custom er S ervi ce.
Fresenius Vial
modifications of the specifications of the syringes introduced by t he
manufacturer.
cannot accept any responsibility for errors in flow due to
Syringe
50/60 ml
Syringe used: B-D Plastipak® Luer Lok® (B-D Plastipak and Luer Lok are registered trademarks
of Becton Dickinson).
Bolus volume on occlusion
Flow rate lower
1 ml/h
5 ml/h
120 ml/h
Threshold values
z
z
40’
8’
25’’
middle
60’
12’
35’’
zz
zz
upper
zzz
zzz
120’
20’
50’’
release
50/60 ml syringe
20 ml syringe
Note: wait until the Alarm + occlusion +
on, indicating the bolus has been reduced.
lower
0.2 ml
≤
0.2 ml
≤
Threshold value
z
z
middle
zz
zz
0.4 ml
≤
0.4 ml
≤
flashing indicators t urns
upper
≤
≤
zzz
zzz
0.8 ml
0.8 ml
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
- 5 -
Page 6

Technical characteristics
Mains supply
230 V ~ - 50-60 Hz (110V on request)
Mains supply
Maxi. consumption
Maxi. power
consumption
Internal protective fuse
External supply
External supply
Battery
Characteristics
Autonomy
Battery recharging
100mA
23 VA
T 100 mA 250V IEC 127
12 to 15 Volts - Continuous voltage
Power > 15 Watts
6 V 1,1/1,3 Ah - Sealed lead rechargeabl e
min. 7 h av. at 5 ml /hr
Partial (70% of capacity): 8 hours
Total (100% of capacity): 16 hours
Indicators lights
Mains power
operation
Battery power
operation
Confirm signal
Infusion in progress
Prealarm
Alarm
KVO
Programmed volume
limit or infused
volume
Flow rate
On hold duration
KVO
ml/h
ml
min
constant yellow
constant green
flashing green
flashing green
flashing orange
flashing red
flashing red
constant or flashing green
constant or flashing green
constant or flashing green
Compliance
Compliance with EN 60 601-1 and EN 60 601-2-24.
CE 0459 marking in com pl i ance with EEC
CE0454
IP34
Device materials
Casing/ Drive/ Syringe
barrel clasp
Programming keyboard
/ labels
Dimensions - Weight
Height / Width / Depth
Weight
93/42 Medical Device Directi ve
Protection against ingress of liquid
Protection against leakage current: Type
CF equipment
Protection against electrical shocks: Class
II equipment
Polycarbonate/ Polyester alloy / shock
resistant
Polyester
120 x 330 x 155 mm
approx. 2.2 Kg
Display
Syringe list available
(example)
Occlusion
Syringe barrel clasp
Syringe flanges
insertion
Plunger head position
anti- siphon system
Disengaged
mechanism
End of infusion
Battery alarm
Technical malfunction
3 green digits (tens, units)
1 orange digit (decimals)
capacity (ml): constant or
flashing green
brand and type: constant or
flashing green
flashing red
flashing red
flashing red
flashing red
flashing orange
flashing red
constant red
- 6 -
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
Page 7

Trumpet curves
Trumpet curves demonstrate the evolution of the minimum and maximum variance of the Syringe/Syringe-Pump combination.
The test protocol used to obtain these results is described in the EN 60 601-2-24. For further information, please refer to this publication.
This graph is therefore representative of syringes used during trials and serve as an indication only of the pump’s overall performance.
Please contact our After-Sales Department for the others curves.
Syringes used B-D Plastipak® 50 ml Luer Lok®.
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
- 7 -
Page 8

Configuration
Fresenius Vial
help you implement the configuration procedures you wish to choose.
Moving to configuration of the various parameters mode, press , then simultaneously + :
within 2 seconds to confirm start of configuration.
Note: press to cancel modification at any time - Press to leave configuration mode at any time.
Press
recommends the presence of its qualified personnel or of a member of the Technical Department of your establishment to
Configuration mode
: flow rate memorizing
3$U
3$U
: syringe selection type
3$U
3$U
: max. flow rate selectable
3$U
3$U
: selectable syringes
3$U
3$U
syringe list given as an example
: confirming compulsory
3$U
3$U
prime (after syringe confirming)
: KVO rate
3$U
3$U
: empty syringe mode
3$U$
3$U$
: frequency of maintenance
3$UE
3$UE
: drug name
3$U&
3$U&
: syringe flanges
3$UG
3$UG
: bolus rate memorizing
3$U)
3$U)
: drug name entry
3$U*
3$U*
: mains disconnected
3$U-
3$U-
detection
: date and time selection
3$U2
3$U2
Confirm Choice Configuration available on start up
last selection in ml/hr
default value 00.0 ml/hr
automatic confi rmation
manual scrolling
for 50 ml syringe types
for 20 ml syringe types
1st syringe brand 50 ml capac i ty
selectable
not selectable
selection for all syringe l i st
priming compuls ory
priming not compul s ory
KVO rate
no KVO rate
empty syringe mode
no empty syringe mode
from 1 to 9999 hours of
continuous use
drug name selection
no selection
syringe flanges detection
no detection
last selection in ml/h
default value in ml/h
1st name of drug
(15 names programmabl e)
Note: confirm the 15th name to
leave
configuration.
3$U*
disconnection detect i on
no detection
Date (G / 0 / \) and
time (K / Q) selection
Display
0(0
QR0(
6(/
6(/
FF
FF
6(/
QR6(
3XU*
QR3X
.92
QR.9
68,G
QR68
ex : 1230 h
GU8*
QRGU
$,/(
QR$,
0(0
QR0(
$GUH
e.g.:
ADRENALIN
name
6(&W
QR6(
G
: day
0
: month
\
: year
K
: hour
Q
: minute
, or free
from 50 to maxi
next name, or
change the name (
appears on display. Press
3DU
3DU
Press to select
max. flow rate
from 1 to 9999
to accede to the
$=
Confirm
to
)
- 8 -
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
Page 9

Operating precautions
The symbol visible on the condensed instruction guide of the
device, recommends this Operator Guide should be completely read,
in accordance with the EN 60 601-1 Standard.
Fresenius Vial
or otherwise, of any nature whatsoever, whether direct or
consequential, caused by improper use of this device.
Special attention must be paid to the stability of the Pilot. Use the device
in horizontal position, on a tabl e or with the I.V. pole access ories.
We recommend you partially or complet el y rec harge the battery when you
receive the device or in the case of prolonged storage so as to prevent all
risk of premature dis charge.
The device must not be used i n the presence of inflammable anaesthetic
agents due to the risk of explosi on. It should always be used away from all
risk areas.
The recommended tem perature for normal use of the devic e i s between
+10° and +40°C.
The device may only be connected t o the mains with the power cord
supplied by the manufact urer. Check that the supply volt age corresponds
with the value indicated on the label plac ed underneath the device.
Fuses should be replaced by equivalent part s. Refer to the part list of the
technical manual for full specificati on.
Do not exceed the permitted voltage whether the supply is from the m ai ns,
an external source or via the different external connections. DC adapter
should not be used. Only external battery like vehicle battery can be
attached to drive the pump f rom external power.
recommend the used of the external power source cable for Pilot.
To preserve the environment, remove the battery from the devic e pri or to
destruction or at the end of t he devi ce life and as during normal
maintenance replacem ent, return it to a competent recycling organisation.
Proceed in the same way for the device itself (electronic boards,
plastics…).
Avoid short circuit and excessive temperature.
will not be liable for any damages or claims, medical
Fresenius Vial
Standard precaution should be tak en t o prevent contamination or injuri es
while discarding the associate disposable (e.g. syringes, extens i on sets,
needles, etc.).
The device is designed to infus e any medical substance that can be
injected. The physiologic al ef fects of medici ne can be influenced by the
characteristics of the device and disposable syringe. Check that they are
compatible with prescriptions, the characteris tics of trumpet curves and
occlusion alarm s et ting times in relati on t o the programmed flow rate.
While in use, negat i ve pressure variation may occur in the syringe, by the
relative height from the devi ce to the injection sit e or by combined infusion
devices such as blood pump, alternative clamp, etc.
When the device is placed higher than the injection site, please pay
attention to correctl y s ecure the syringe and manipulate the syringe only
when the extension set is clamped or di sconnected from patient side.
High depression may create s yri nge siphoning. In this sit uat i on, you must
check the integrity if the syringe used (possible leak age), and if necessary
insert anti-siphon valves.
Pressure variation may generat e f l ow dis c ontinuity mainly noticeabl e at
low flow rates and depending of the infusion system characteristics such
as friction force, stickiness, c ompliance of syringes and mechanical back
lash. Anti-siphon valves will also eliminate any risk of f ree f low during
syringe changes. An air leakage i n a syringe with a line not equipped with
an anti-siphon valve may generate an uncont rol l ed flow delivery.
Do not use in conjunction with posi tive pressure infusion devices that
could generate back pressure higher t han 2 000 Hpa susceptible to
damage infusion dispos abl e and t he devi ce.
Fresenius Vial
pressure infusion devices for multi-line infusi ons. If there is no one way
valve on a gravity infusion line during a multi-line infusion, this will make it
impossible to detect occlusions on the pat i ent side, and could result in
accumulation of t he drug bei ng i nfused in the gravity line, which could l ater
be infused in an uncontrolled m anner when the oc clusion is released.
Place the connection bet ween the feeder l i ne and the syringe-driver line as
near to the start of the catheter as possible in order to minimise the dead
space and consequently the impact of any change in flow rate on the
feeder line.
recommends the us e of one way valves or positive
This device can be disturbed by a large el ectromagnetic fields, external
electrical influences and electrostatic discharges above the limit s
stipulated by EN 60 601-1-2 and EN 60 601-2-24. It can also be disturbed
by pressure or pressure variations, mechanical shocks, heat ignition
sources, etc.
If you wish to use the device in s pecial conditions, pleas e c ontact our
After-Sales Departm ent.
Only use Luer Lock three-part syringes from the list of pre-programmed
brands. If a syringe is us ed which does not correspond to the syringe lis t
on the device, the specif i ed precision level cannot be guaranteed.
Use only sterile catheter extens i ons which can resist pressures of up to
2000 HPa.
The use of unscrewable extension lines or syringes may result in spillage
if infusions are carried out at high flow rates and/or high pressure. I nfusion
line set up must be done in accordance with local standard operati ng
procedures and good clinical practice.
use of the Luer Lock type infus ion lines proposed in page 13.
Fresenius Vial
recommends the
Do not use this assembly Preferred assembly
Opening the pump or the battery cover must only be carried out by the
qualified personnel in your establi shment, and taking all the necessary
technical precautions. Non-respect of these procedures i s dangerous to
the personnel and may damage the syringe pump. We recommend you
follow the maintenance procedures defined in the technical m ai ntenance
manual. To obtain a copy of t he technical maintenance manual, please
contact our After-Sal es Department or our Comm ercial Department
specifying the identif i cation number of the device.
Non-reflux valve
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
- 9 -
Page 10

Maintenance
Cleaning and disinfection
Servicing
The Pilot is part of the patient’s immediate environment. It is
advisable to clean and disinfect the device’s external surfaces on a
daily basis in order to protect patient and staff.
Disconnect the device f rom its mains s uppl y before starting to clean.
Do not place in an AUTOCLAVE nor IMMERSE the device. Do not let
liquids enter the device’s c asing.
If the device is placed i n a hi gh contamination risk uni t , it is advisable
to leave it in the room during aerial disinfection, aft er having
disinfected it with a moist cloth.
Use a cloth soaked in DETE RGE NT-DISINFECTANT, previously
diluted with water if required, to destroy micro-organisms. A voi d
abrasive scrubbing which could scratch the casing. Do not rins e or
wipe surfaces.
Do not use: TRICHLOROETHYLENE-DICHLOROETHYLENE AMMONIA - AMMONIUM CHLORIDE - CHLORINE and AROMATIC
HYDROCARBON - ETHYLENE DI CHLORIDE-METHYLENE
CHLORIDE - CETONE. These aggressi ve agent s could damage the
plastic parts and caus e devi ce malfunction.
Take care also with ALCOHOL BASED SPRAYS (20% - 40%
alcohol). They lead to tarnishing of and small cracks in the plastic,
and do not provide the necessary cleaning prior to disinfection. Using
disinfecting applies by SPRAYS may be done, in accordanc e with the
manufacturer recom mendations, from a di stance of 30 cm of t he
device, avoid the accum ul ation of the product in liquid form.
Please contact the appropri at e service, handling suitable c l eani ng and
disinfection products, in your establishm ent for further details.
To ensure normal performance of the device, it is recommended to
replace the internal battery each 3 years. This should be done by a
qualified technician.
The qualified technicians i n your es tablishment or our Aft er-S al es Service
should be informed if the devi ce is dropped or if any of malfunction occurs.
In this case, the devi ce must not be used.
For further information c oncerning the pump servicing or it s use, please
contact our After-Sales Service or our Customer service.
If the device has to be returned t o our After-Sales Department , proceed to
its cleaning and desinfection. Then , pack it very carefully, if possible in its
original packaging, before sendi ng i t .
Fresenius Vial
transport to our After-Sal es Department.
is not liable for loss or damage to the device during
Regular inspections
In order to check that the device is functioning optimally, regular
inspections are recommended every 12 months.
A regular control check c onsists of various inspection operations listed i n
the Technical manual. These control checks must be performed by an
experienced technician and are not covered by any cont ract or agreement
provided by
Fresenius Vial
.
Storage
The device should be stored in a dry, cool place. In case of
prolonged storage, the battery should be disconnected via the
battery access flap situated underneath the device. This should be
done by a qualified technician.
Storage temperature: -10°C + 60° C.
Permissive relative humidity: maxi 85%, no condensation.
Note: the pump mus t be checked, serviced and repaired only by
Fresenius Vial
maintenance procedures can damage the device and lead to a funct i onal
failure.
or by a qualified technician. Fai l ure to comply with these
- 10 -
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
Page 11

Quick check procedure - Pilot A2
This protocol allows a quick check of the pump functionality.
Serial number (ID/N): ............................. Date: ..... / ..... / ..... Department: ........................ Name: .............................
1. Check the state of t he devi ce : absence of impact marks and noises (t urn ups i de down the device), presence of all labels as
well as their legibility.
2. Press ON
3. Check the condition of the power lead and connect the device to the mains source : the mai ns
4. Install a syringe.
Auto-control mode : press simultaneously on
&75
1. Press
2. Check the presence of all l uminous indicators and pres s
3. Select
&75
1. Press
2. The DISENGAGED MECHANIS M and ANTI -SIPHON indicators flas h. Disengage pusher block: constant indicators light up.
The confirm signal fl ashes: press
3. The SYRINGE BARREL CLAS P and
light up. The confirm signal flashes: press
(power supply lead not connected): the indicator illuminates.
and keys.
Indicator light test.
to start the test.
.
2.$<
(comply) ; QR (no comply) or
Alarms test.
to start the test :
$/$5
appears on display.
&WU
(return) by pressing , and confirm .
.
+,*+
flash. Position the syringe barrel clasp on upper position: constant indicator and
.
indicator illuminates.
+,*+
YES NO
YES NO
4. The flashing display indicates &&. Turn the syringe barrel clasp int o t he closed position and check the detected capacity by the
device. The confirm s i gnal flashes: press
5. The SYRINGE BARREL CLAS P and
constant indicator and
6. Select
2.$<
or
&75
1. Press
2. Install a 50 or 20 ml syringe filled at 7 cc.
3. Select syringe
FF
4. Select
5. After the validation of
6. Press
Visa : All control result comply: YES
to start the test.
(volume infused) for 50 and 20 m l syringe (check the advance of syringe pl unger: 5 cc ± 0.5 cc).
2.$<
; QR or
to restart device on normal mode.
/28
light up. The confirm signal flashes: press
&WU
(return) by pressing
Pusher block advance test.
and start the test :
&WU
(return) by pressing
2.$<
, the message
.
/28
flash. Remove the syri nge and pos i tion the syringe barrel clasp on lower positi on:
.
and confirm .
UXQ
appears on displays. The end of the t est is signalled by : m essage
and confirm .
(QG
indicates the end of the aut o control test.
2.$<
and
YES NO
YES NO
NO
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
- 11 -
Page 12

12-15 V power source
Operation with the internal
A socket positioned on the rear panel makes it possible to use a 1215 V 15 W supply in rescue vehicles.
Operation from the external power can be recognised by t he mains
indicator
The battery automatical l y rec harges.
.
battery
The Pilot contains an internal battery which automatically takes over
when the mains supply is disconnected and ensures normal
function with no loss of the programmed data.
message is displayed and a
When mains is disconnected, the
warning signal is turned on.
Press
Operation from the battery can be recognised by the battery
indicator
to acknowledge this warning.
.
Recharging the battery
To recharge battery, just connect the Pilot to a mains power supply.
Recharging of the battery is vi sualised by the mains i ndi cator
Battery life indicator
While the pump is running on battery, battery li f e may be displayed.
Battery life displayed t akes care of the current flow rate.
E$W
K
autonomy in h/min
Note: use charging mode for a complete battery life i ndi cator when device
is turned off.
E$W
E$W
.
Charging mode
It includes total duration of charging battery when device is not used.
1. Remove syringe and press
2. Charging mode activation:
Note: to leave the charging m ode, press
.
&+$U
(continuous press)
(continuous press)
- 12 -
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
Page 13

Accessories
Disposable
Fresenius Vial
Transfix
cat # 073416
Composed of transport handle (cat #
073419) and the multi-purpose clamp (cat #
073418), this system enabl es rapid fixation
to a horizontal rail or vertical s upport,
decreases loss of space and provides
perfect stability.
Transport Handle
cat # 073419
Multi-purpose clamp
RS 232 cord
Cat. # 073413 (9m/9f)
Cat. # 073414 (9m/25)
Battery supply cable
cat # 073415
recommends the use of Pilot range accessories.
- cat # 073418
SE 2400Y
Injectomat Line PVC 150 cm
Injectomat Line PE 200 orange
light sensitive drugs or f or drugs not compatible with PVC.
SE 1500 AR
connector equipped with one way valve.
SE 1600 AR AS
anti-siphon valve and Y connector equipped with one way valve.
- 2 channel - Sterile catheter extensi on set in PVC.
– Extension line for infusion.
- Opaque extension line for infusion of
- 1 channel - Sterile catheter extensi on set in PVC with Y
- 1 channel - Sterile catheter extensi on set in PVC with
Power Fix 2
Power Fix 4
1 power cord only to connect 2 or 4 Pilot t o
mains.
Power Fix 2 and 4 : includes mounting
clamps for I.V. pole.
CE marking - complies with EN 60-601.1.
230 V ~ - 50/60 Hz (110 V on request).
Installation with 2 POWER FIX 2, 1 POWER LINK ; 1
ROLLING STAND 180 (cat # 073070)
- cat # : 073428.
- cat # : 073429.
Power Link
073430.
To connect a POWER FIX
2 to a POWER FI X 2 or 4
together.
- cat # :
Note that the expiry date is written on the pac kaging (set can be used for
5 years from the manuf acturing date also written on the peel-open
pouch).
All sets are designed and controlled by Fresenius in order to guarantee
the performances and the s afety features of our pumps. The
manufacturing is done by Fres enius (CE0123, CE0459) or by its qualif i ed
subcontractors (CE0123, CE 0318) for and on behalf of Fresenius in
exclusive distribution. The CE certificates are availabl e on request.
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
- 13 -
Page 14

Conditions of guarantee
Fresenius Vial
materials and workmanship (excluding batteries and accessori es)
for a period of one year from the date of invoice. If you comply to
benefit from the materials and workmanship guarantee from our
After-Sales Service or an agent authorised by
following conditions must be respected:
The device must have been used according to the instructions in
this Operator’s Guide.
The device must not have been damaged when in storage, at the
time of repair, or show signs of improper handling.
The device must not have been alt ered or repai red by non-qual i fied
personnel.
The serial number (ID/N°) must not have been altered, changed, or
erased.
In case of non-respect of t hese conditions,
an estimate for repair c overi ng the parts and labour required.
Where return and repair of a devic e i s necessary, please cont ac t
Fresenius Vial
guarantee that this product is free from defects in
Fresenius Vial
Fresenius Vial
Customer or After-Sal es Department.
, the
will prepare
- 14 -
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
Page 15

Notes
NU PIL A2 TD IS - 1342 rev 3 – 09/12/03
- 15 -
Page 16

Useful addresses
All requests for information or documentation (technical files, tubing sets catalogue or brochures) must be sent to:
CUSTOMER SERVICE - AFTER-SALES SERVICE:
Fresenius Vial
Le Grand Chemin
F-38590 BREZINS (France)
Tel: +33 (0)4 76 67 10 10
Fax: +33 (0)4 76 67 11 34
Consult our Web site
www.fresenius-vial.fr
This Operator’s Guide may contain inaccuracies or typographical errors.
Modifications may thus be made and will be included in later editions.
As standards and equipment change from time to time, the features shown and described in this document
in whole or in part without the written consent of Fresenius Vial.
Fresenius Vial - siège social : Le Grand Chemin - F-38590 BREZINS (FRANCE).
must be confirmed by our departments.
This Operator’s Guide may not be reproduced
 Loading...
Loading...