Page 1
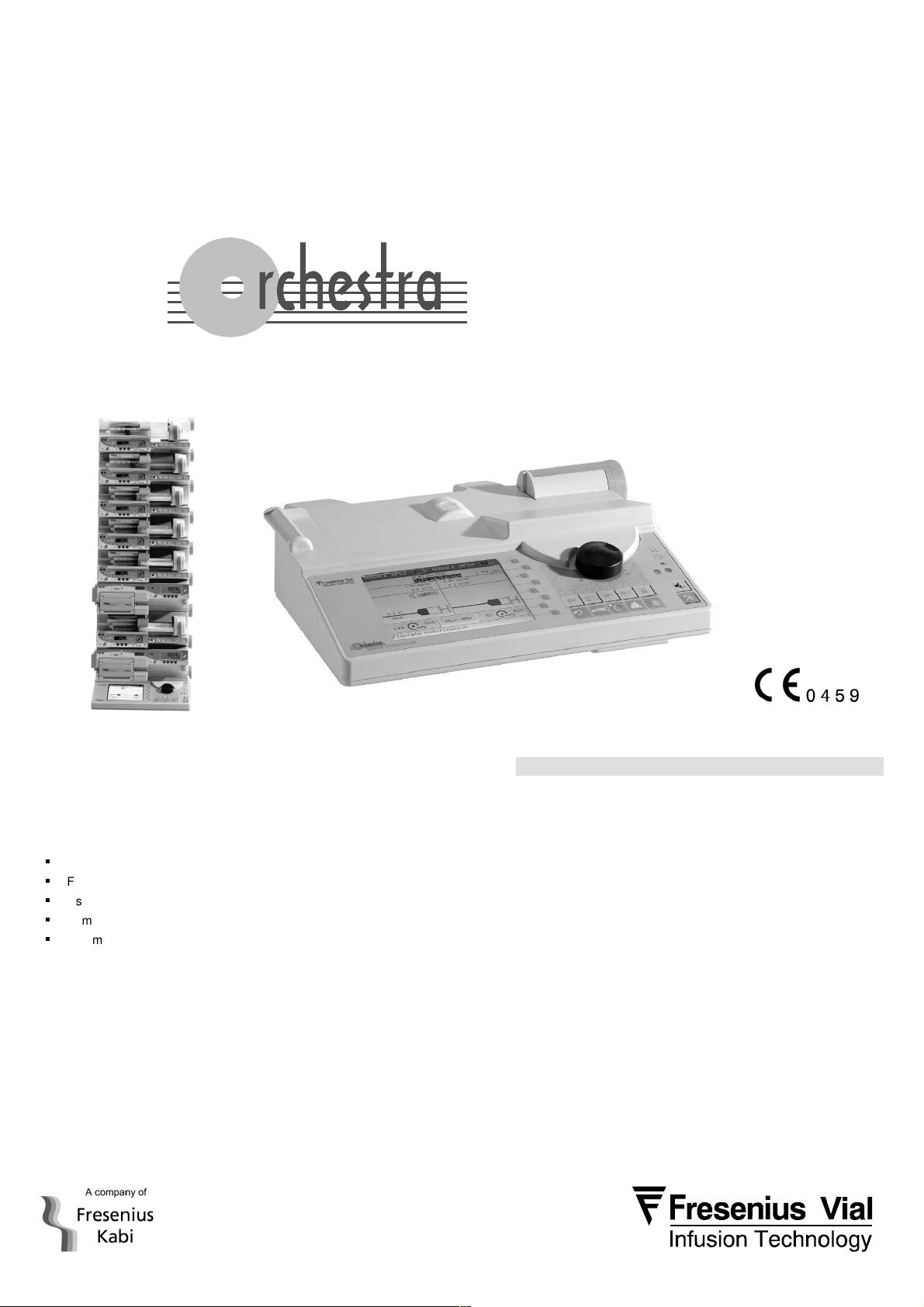
OPERATOR’S GUIDE
Infusion Workstation
®
Base Intensive
Introduction
Base Intensive Orchestra® offers a unique solution in CCU, ICU and anaesthesia.
The Base Intensive Orchestra®:
Brings additional performances.
Facilitates and secures drug programming.
Uses a multi-channel infusion pump potentialities.
Summarises the infusion data at patient bed.
Communicates, via a unique serial cable, parameters to a centralised system of
PDMS (Patient Data Management System).
User interface is underlined by the color screen and an ergonomic command for more
safeties and user friendliness.
According to the number of intravenous infusions needed, at any combination, from 1 to
8 Module MVP or Module MVP+ or Module DPS or Module DPS Visio or Module
DPS+ (up to 14 or 16 Modules if Base Intensive is connected to Base A) can be fitted
onto the Base Intensive Orchestra®.
The use of this material requires great care. The user must be able to handle the instrument properly and must
know how to fully operate.
Please read the operator’s gui de carefully before putting the device into us e.
Prior to any use of Modules with the Base Intensive Orchestra®, please refer to the Module DPS, Module
DPS Visio, Module DPS+, Module MVP and Module MVP+ Orchestra® Operator’s Guides.
Table of contents
Operations for use ................................................................. 2
Infusion modes....................................................................... 6
Two channel relay.................................................................. 7
History.....................................................................................10
OPTIONS menu ...................................................................... 11
Safety features ....................................................................... 14
Base Intensive - Performances.............................................15
Base Intensive – Technical characteristics......................... 16
Precautions to be taken......................................................... 17
Guidance and manufacturer's declaration on electromagnetic
environment ........................................................................... 18
Maintenance recommendations........................................... 20
Function with the internal battery ........................................ 21
Accessories ............................................................................ 21
Conditions of guarantee........................................................22
Useful addresses ................................................................... 24
Page 2
1395-6_nu_base_int_v3_2_is_120606.doc
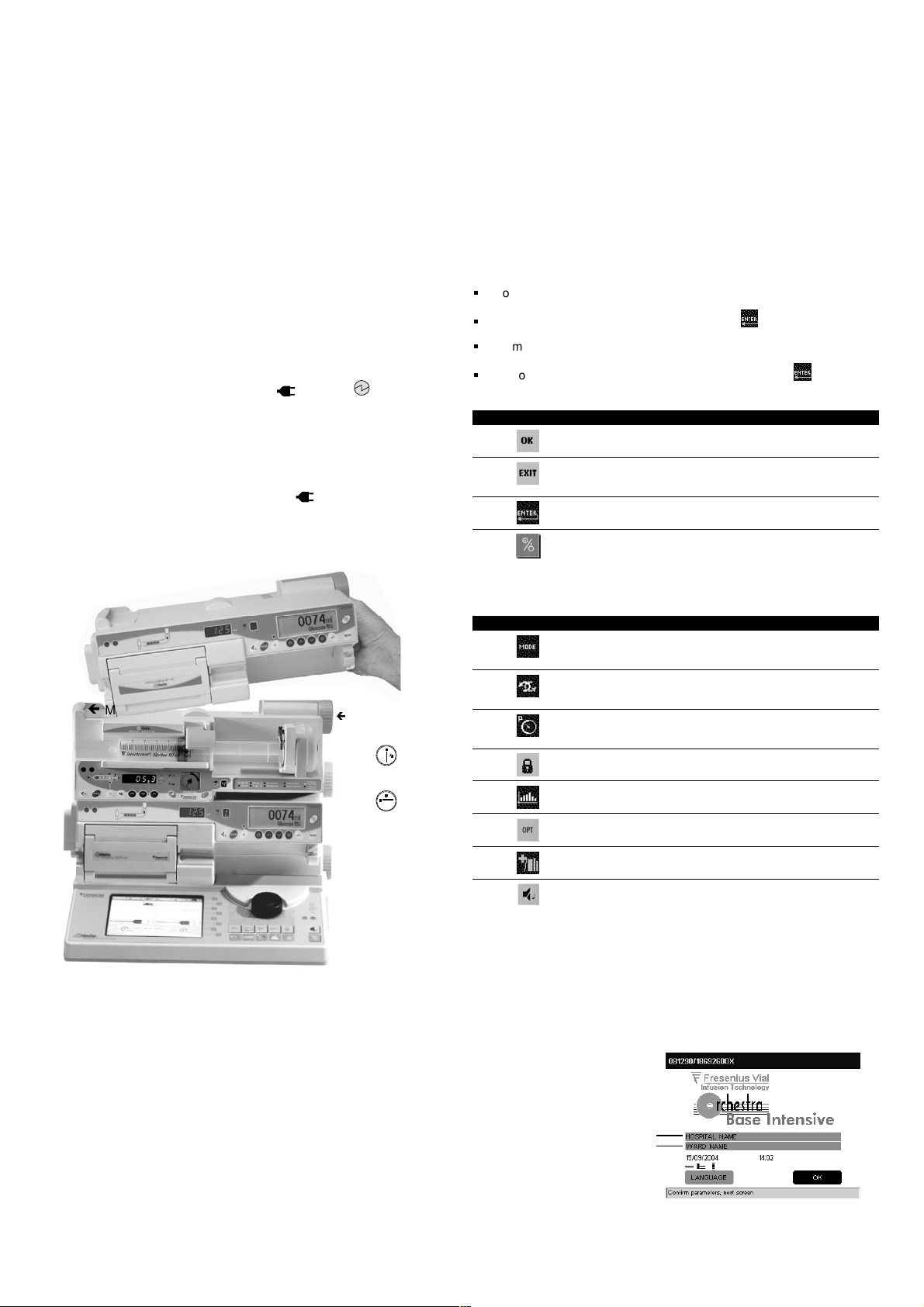
Operations for use
Installation of Modules
(Module
DPS, Module DPS Visio, Module DPS+, Module MVP,
Module MVP+ Orchestra®)
on the Base
Intensive
We recommend you partially or completely recharge the battery when you
receive the devices or in the case of prolonged storage so as to prevent all
risk of premature discharge.
Special attention must be paid to the stability of the Base Intensive when
several Modules are used.
From the 4th Module onwards, the Base must be equipped with a Multifix to
ensure rigidity (see « accessories » page 21).
The Base Intensive can be used on mains or battery (red indication
because looks like an alarm, normal functioning of Base Intensive is on
mains).
Note: connect device to mains as soon as possible to recharge Base and
Modules’ batteries.
1. Connect the power supply cord to the Base Intensive and to the mains
source. The mains power indicator lights up .
2. Install the Modules on the Base Intensive or on another Module already
installed and turn the locking handle to locked position.
Use of keyboard and rotary
knob
Parameter selection and modification
Parameter selections and modifications are done with the rotary knob
and the keyboard selection keys.
To move from an item to another, use the rotary knob.
To select an item, press the rotary knob or the key.
To modify a value of the selected item, use the rotary knob.
To confirm the modification, press the rotary knob or the key.
Symbols Parameter selection and modification
Confirm parameters and go to next screen.
Exit from an item or a screen without saving the
modifications.
Confirm a selection or a value under modification.
Base Intensive power on and off.
Module fixing point
Ex. : Module DPS and Module MVP
3. Quick check: see protocol page 14. This test is recommended before use
or when the device has not been used for a long time. This test allows a
complete alarms and safety features check.
Locking handle
Locked position
Unlocked position
Other functions
Symbols Other available functions
Selection of particular Module DPS infusion modes
functions.
Access to the 2 channel relay program with Module
DPS.
Access to infusion pressure information and
authorized parameters adjustment.
Allows locking of Base Intensive keyboard.
Access to history data.
Access to OPTIONS menu.
Access to the visualisation of drug library parameters.
Silence alarm.
Welcome screen
When Base Intensive is put into use for the first time, complete the following
items:
Free items.
Ex.: Hospital, department
If necessary, select language with the rotary knob.
Page 3

1395-6_nu_base_int_v3_2_is_120606.doc
Patient screen
Note: It is recommended to use codes to identify the patient since the Base
Intensive memorizes the patient infusion history. This allows to guarantee
patient anonymity according to the law in force.
Symbols Function
Patient code
Patient identification with a 30 characters code.
Bed/room number
Bed/room identification with a 30 characters code.
Patient weight
The weight must be entered when you select a new
patient.
This value is used when flow rate units and volume
are calculated by weight.
From 250 g to 1 kg, 10 g increment.
From 1 to 10 kg, 100 g increment.
From 10 to 250 kg, 1 kg increment.
Patient age
Adjustment from 1 to 24 months, 1 month increment
Adjustment from 2 to 150 years, 1 year increment.
Module DPS switching on
When Base Intensive is connected to the mains, Module DPS can be
switched on from their own respective keyboards by pressing the key or
from the Base Intensive by pressing the key.
When Base Intensive is working on its battery, Module DPS can be
switched on only from their own keyboards by pressing the key.
Symbols Module DPS Orchestra® switching on conditions
Module can be switched on from the Base Intensive
Orchestra® or from its keyboard when the Base
Intensive is connected to the mains.
Module can be switched on only from its own keyboard
when the Base Intensive is connected to the mains.
Patient sex
Male
Female
Patient’s height: from 0,20 to 2,50 m, 1 cm increment.
S Calculated body surface.
BMI Body Mass Index.
Note: the hour and date adjustment is possible only when you select a new
patient (ex: summer or winter time change).
Page 4

1395-6_nu_base_int_v3_2_is_120606.doc
Summary screen
When a Module is switched on without any drug program, the message
is displayed. This message means that no drugs have been
programmed via the Base Intensive or that this drug is not identified at the
new connection.
Symbols Modules type
Module DPS
Module MVP
Note: The flashing symbol reminds the user that syringe selection and
confirmation must be done from the Module DPS keyboard.
Drug programming
1. Selecting the drug enables access to the programmed drug library.
2. Select the drug name from the alphabetical list or from the family name.
It is possible, at any time, to recall the drug preprogrammed values from the
library pressing the key.
Symbols Modules status
Infusion
Pause
Alarm
Module DPS awaiting for a 2 channel relay
(See page 8)
Drug programming and
infusion start
To program a drug:
1. Select the channel number with the keys.
2. Select or confirm the installed syringe.
3. Select or confirm the drug.
4. Adjust infusion parameters.
5. Start the infusion.
Note : in order to improve infusion start, it is recommended to prime the
infusion line until a drip appears.
superior limits
usual values
inferior limits
Important:
The Base Intensive integrates a drug library as example. Prior to any
use, the authorized personnel must check that usual drug values
correspond to the protocols used.
Prior to any use of the Base Intensive, it is necessary to carefully read
Drug’Lib Operator’s Guide to modify or confirm the drugs parameters.
Parameters adjustment and infusion start
Values indicated per default are the usual values defined in the drug library.
Syringe selection
To reach the syringe programming screen:
1. Install a syringe.
2. Select and confirm syringe on the Module DPS (Please refer to the
Module DPS Operator’s Guide, page 2).
1. Check that the drug name is the one contained in the syringe of the
selected channel.
2. Confirm or adjust, if necessary, the drug dilution value contained in the
syringe.
3. Confirm or adjust, if necessary, the dose rate value according to the
desired administration.
4. Start infusion pressing the key of the Module DPS.
Note: The symbol and
infusion start is made pressing the key of the Module DPS .
displayed alternatively reminds the user that
Page 5

1395-6_nu_base_int_v3_2_is_120606.doc
Flow rate modifications and
Infusion pressure adjustment
information during infusion
The infusion rate, in the selected unit, can be modified from the Base
Intensive.
Rate (in ml/h) can be modified from the Module keyboard. Information about
rate is automatically updated on Base Intensive.
Flow rate program
To modify the infusion rate from the Base Intensive :
1. Select the channel pressing the key.
2. Press the key or the rotary knob.
3. Adjust flow rate value with the rotary knob.
4. Press the key or the rotary knob to confirm the modification.
5. Press the Module’s key to confirm the new flow rate has been done.
and display
Pressure limit value programming and drops in pressure activation are made
directly from the Base Intensive for a Module DPS only.
For the use of these functions on the Modules, please refer to the Module
Operator’s Guides.
To adjust pressure parameters from the Base Intensive:
1. Select the channel pressing the key.
2. Press the key to have access to the adjustment screen:
It is also possible to modify the pressure alarm threshold, either in 3 pre-set
threshold mode or one variable threshold mode depending on the Module
DPS configuration.
During a rate modification on the Base Intensive, pressing the key will
revert back to the initial value.
Notes:
At syringe change:
the name and concentration of the last selected drug are proposed per
default,
if the selected drug name is not modified, then the total infused volume is
cumulated in the programming screen of the selected channel.
Infusion stop
To stop infusion, press the key from the Module.
Turning off the Modules
When no Module is infusing, turning off the Modules can be done by pressing
the key from the Base Intensive or by pressing the key from the
Module.
When a Module is infusing on the Base Intensive, turning off the Modules is
done by pressing the key from the Module for more than 2 seconds.
Turning off the Base Intensive
Turning off the Base Intensive is done by pressing the key. Some
information messages may be displayed when turning off.
Page 6

1395-6_nu_base_int_v3_2_is_120606.doc
Infusion modes
Continuous mode
Continuous mode is the default mode run by the Base Intensive.
Bolus mode
Bolus programming and activation are possible when a channel is in
continuous infusion or after the injection of a loading dose.
1. Select the channel pressing the key.
2. Press the key to have access to the Modes selection screen.
3. Select the Bolus mode .
4. Adjust the bolus dose value to be infused as well as the duration. The
volume to be infused as well as the infusion flow rate (in ml/h) of the
Module DPS are automatically recalculated.
5. Check bolus parameters.
6. Start bolus infusion selecting the START icon and confirm on the
Module DPS. In case of a pre-program, select the STORE icon to
confirm parameters storage.
When bolus has been delivered, the Module DPS returns to its initial status
(stop or infusion), bolus programming parameters are stored when there is a
new programming.
Loading dose + continuous
mode
Loading dose programming and starting followed by a continuous flow
rate are possible after a drug selection and before starting the
infusion.
1. Select the channel and the drug.
2. Press the key to have access to the Modes selection screen.
3. Select the Loading dose + Continuous Mode .
4. Adjust the loading dose value as well as its duration. The Module DPS
volume to be infused and infusion flow rate (in ml/h) are automatically
recalculated.
5. Adjust the maintenance flow rate value.
6. Start the loading dose infusion selecting the START icon. You can stop
the loading dose infusion, at any time, pressing the STOP key of the
Module DPS or with the STOP icon of the Base Intensive. It can be
reused selecting the START icon.
At the end of the loading dose infusion, the Module DPS infuses at the
programmed maintenance flow rate.
In case you use a drug that does not require any administration adjustment (if
minimum dilution is the same as the maximum dilution and the typical
dilution), the Base Intensive displays automatically the loading dose screen.
Note: Access to CONTINUOUS, BOLUS, LOADING DOSE +
CONTINUOUS modes can be locked or unlocked by user with a code to be
accessed from the summary screen.
Page 7
1395-6_nu_base_int_v3_2_is_120606.doc
Two channel relay
The use of the 2 channel relay is particularly recommended to avoid any infusion discontinuity, for example, in case of infusion of cardio-vascular
or short half-life anaesthetic drugs.
Principle
The 2 channel relay consists in associating two channels that will infuse, either one after the other a same drug at the same administration.
In case of different concentrations for the same drug, the flow rate (in ml/h) calculation will be done automatically to respect the prescribed dose.
Installation of the two syringes can be done simultaneously only if the syringe of the second channel in relay has been validated after the activation of the 2
channel relay.
Pressing the PRIME/BOLUS key is mandatory for the channel in relay so as to improve the two channel relay performances.
In case the second syringe is installed later, the user will preprogram a prealarm « relay not ready » to be informed from 5 minutes to 1 hour before the end of
infusion of the first syringe in order to complete the relay.
Recommendations to operate a 2 channel relay
The respect of these recommendations is mandatory when infusing hemodynamically unstable patients.
Orchestra® Infusion Workstation
installation
Place Base Intensive at the same level or lower than the patient.
Use Module DPS from position 1 to position 6 only.
Syringes to be used
Adapt syringe size to the selected flow rate:
flow rate < 0.5 ml/h : 5 and 10 cc syringes,
0.5 ml/h < flow rate < 1 ml/h : 5, 10 and 20 cc syringes,
flow rate > 1 ml/h : any syringe size, i.e. 5, 10, 20, 50/60 cc.
Please refer to Module DPS syringe list.
2-channel relay syringe installation
Up to 24 hours before the infusion starts.
Always check that the selected syringe corresponds to the installed
syringe.
Make sure that the infusion line is not connected to the patient when
priming.
Prime till a drop appears at the end of the infusion line.
Patient connection
Use catheters with low priming volume and reduced compliance.
Unidirectional valves are authorised while anti-siphon valves are not (the
open pressure can generate infusion delay).
Carefully respect the cautions applying to continuous infusion of vaso-active
drugs (dedicated access route, avoid discontinuous injection, …).
Page 8

1395-6_nu_base_int_v3_2_is_120606.doc
Two channel relay program
1. Program a drug from the library on a channel. Adjust, if necessary, the
infusion parameters and start infusion.
2. Press the key to reach the screen for selection of the channel to be
associated.
3. Power on the channel to be associated :
either from the Base Intensive with the key.
or directly on the Module DPS and select the number of the
channel to be associated with the key.
4. Syringe installation on the channel relay:
a. Install the syringe containing the same drug as the one programmed
during the relay.
b. Select then confirm the syringe type.
e. At the end of the 2-channel relay program, the Module DPS displays
the drug name alternatively with the
Once the 2-channel relay is programmed, the flow rate may be
modified at any time.
The 2 channel relay is now set.
Prealarm will occur at set time to warn of imminent relay.
Relay will occur automatically.
message.
c. "PRIME - Check patient is not connected to the syringe" message
displays on the Base Intensive. On the Module DPS, the
message displays alternatively with the drug name .
Press the PRIME/BOLUS key of the Module DPS to be in prime
mode (
message displays on the Module DPS). Press a
second time and maintain pressed the PRIME/BOLUS key to
make the purge. Prime till a drop appears at the end of the
infusion line.
Important: make sure that the patient is not connected to the syringe
during the priming phase.
d. Confirm the drug name pressing the key of the Module DPS. If
necessary, adjust concentration.
Page 9

1395-6_nu_base_int_v3_2_is_120606.doc
Two channel relay menu
By activating this field with the rotary knob, you have access to this menu.
The following screen displays:
START RELAY
By activating this field with the rotary knob, you start the channel relay and
simultaneously the first channel stops.
CANCEL RELAY
This function allows to cancel the relay mode for the channel in relay.
The manual relay switching off is made stopping one of the two channels and
pressing the key. The following screen is displayed:
Select YES with the rotary knob.
ACCESS TO MANUAL RELAY
By using this function, you command simultaneously the two channels in
relay. You have access to this function only when the warning before relay
has been selected.
Example:
before relay
RELAY MENU
Warning before relay
In this example, the manual relay is authorized with a « warning before relay »
at 1.0 ml before the end of infusion, that is to say 21 minutes before the relay
at a flow rate of 2.8 ml/h.
Activating the manual relay from the rotary knob, the following screen
displays:
From the Base Intensive keyboard, user decides: to start, to stop or to set
the flow rate for each two channels in relay.
Page 10

1395-6_nu_base_int_v3_2_is_120606.doc
History
Base Intensive stores, in real time, infusion data during 96 hours. During this period, user can:
define an observation window from 1 to 96 hours, with one hour increment,
choose the date and hour of this observation window.
Data are updated each ten seconds or each time you use the function.
Access to different information regarding doses, infused volumes and flow rates of each drug as well as the fluid balance display allow, if you think it is
necessary (for example during personnel turnover), to rapidly know the main information about the infusions administered to the patient.
Notes: If several Modules (example: 2 channel relay) infuse the same drug, the Base Intensive will automatically update the drug history.
According to the patient treatment duration and the number of Modules installed on the Base Intensive, it may be possible to have access to the last
4 patients history (if no infusions are running).
Pressing the key, you have access to the selection menu of the different histories.
Please note that when one or several Modules are disconnected from the Base Intensive while they were previously programmed, their history log will be
restored when they are reconnected to the Base Intensive.
Access to history data
When you press the key, you have access to the summary screen for volumes and doses infused per drug in the selected period.
Modification of the
observation window
duration
Pressing the keys gives access to information dedicated to each drug:
Cumulated doses and volumes history
Each segment represents 1 hour. The value displayed on the last segment is
the one of the observation time.
Flow rates history
Access to events list
Horodated events list
Previous events
Next events
From the summary screen for infused volumes and doses, activating the
« fluid balance » option gives access to the summary screen for inputs and
outputs.
Date of beginning
Date of end
It is possible to store 24 balances (ex.: memorisation of a balance every 4
hours during 96 hours).
The fluid balance storage allows resetting the dose and infusing volume
values per drug. It is then possible to evaluate the quantities of infused drugs
on periods of time corresponding to fluid balance period.
Note : the hour of beginning and hour of end of liquid balance must be entire
(ex. : 16 h 00).
Page 11

1395-6_nu_base_int_v3_2_is_120606.doc
OPTIONS menu
Press the key to have access to the following display:
Screen printing
The options from this screen give access to the following information:
AUTONOMY menu
This field gives access to the Modules and Base Intensive battery autonomy.
Example:
autonomy,
maintenance,
time change,
customisation,
screen printing,
detailed printing.
CUSTOMISATION menu
This field gives access to the customisation of the Base Intensive different
configurations:
MAINTENANCE menu
This field gives access to the serial numbers and next maintenance dates for
Modules and Base Intensive.
Example:
TIME CHANGE menu
This field gives access to the daylight change. Example:
INTERFACE CONFIGURATION:
This field gives access to:
This option allows to define the Base Intensive first welcome screen and to
display the flow rate alternating with the drug name on the Module DPS.
CONTRAST:
This field gives access to:
This option allows to tune the contrast of the Base Intensive screen.
Page 12

1395-6_nu_base_int_v3_2_is_120606.doc
ADVANCED CONFIGURATION:
Drug
library : LB1118-1
This option is protected. You only have access to this menu after entering the
00123 code with the rotary knob. If necessary, consult our Maintenance
Service for the code modification.
According to your department practice, this field gives access to:
The following screen is displayed:
In this example, the « priority 5.10 ml/h » field activation will generate the
recalculation of the mass rate necessary to respect the current flow rate in
ml/h. the « priority 5.99 µg/kg/min » field activation will generate the
recalculation of the flow rate necessary to respect the current mass rate in
µg/kg/min.
Programmed bolus mode authorized
Activation/disactivation of the programmed bolus function.
Loading dose mode authorized
Activation/disactivation of the loading dose mode function.
On Module confirmation
If this function is disactivated, any flow rate changes validation will be done
pressing the Base Intensive rotary knob.
Patient weight change authorized
For specific administration protocols, the physician may have to take into
account the patient weight variations to adjust, either infused flow rates (ml/h),
or the administration depending on the new weight setup (example:
µg/kg/min) without stopping the infusion.
This function concern only drugs selected from the Base Intensive with an
administration that takes into account the patient‘s weight.
How to change the patient weight during the infusion:
1. Return to the « patient identification » display:
Display left volume and time
Activation/disactivation of display of the volume to be infused and time.
Supervisor mode
Unique visualisation of the summary screen.
Enable Base A connexion
This function allows to connect one Base A to one Base Intensive and to
monitor up to 8 additionnal Modules. To go from the Base Intensive
summary screen to the one of the Base A, press the key.
Drugs infused from Base A are integrated into Base Intensive history.
Note : Module flow rate adjustment from Base A is performed directly on the
Module.
Connection of Base A to Base Intensive is performed through RS232 port
No 1 using the adequate RS232 cable.
!
"# $
! %
Drug library edition authorized
It is possible to modify drug library directly from the Base Intensive to modify
the adjustment range.
1 – Drug selection :
Drug library : LB1118-1
Author : Fresenius
2. Change patient weight with the rotary knob.
3. Confirm pressing the key. The following screen is displayed:
4. Select the channel(s) where the weight must be modified pressing the
key of each channel. This (these) channel(s) is(are) identified with
symbol.
2 – Different items modification
Author : Fresenius
Note that the access to this function is possible only if no patient is selected
and if the infusions are stopped.
Page 13

1395-6_nu_base_int_v3_2_is_120606.doc
Enable barcode reader
The selection of the drug name and administration can be done directly from
labels stuck on the syringe and with a barcode reader. The labels printing is
made from the Drug'Bar software. Please refer to this software operator's
guide.
Enable display mass flow for 1 ml/h
This function allows to display, in the drug program screen, the
correspondance between a 1 ml/h flow rate and the corresponding mass rate
in chosen unit.
Enable dilution according to dose/volume
This function allows to fill in the dilution indicating the drug dose and the
administration volume.
;<5= >?
45
6786959:
7@ A>5B>?CC?7856
&
'()*(+'+ ,
-
()./ )0 . 1',. '2)0. 1/+3
Activated option Disactivated option
Automatic period history
This function allows to display automatically the doses and volumes infused
during predefined times (4h, 8h, 12h and 24h) since the hour of the last fluid
balance memorisation.
Example : case of a balance every 8 hours.
The fluid balance is stored at 8 h 00.
The history reading is done at 16 h 30 : doses and volumes displayed
correspond to doses and volumes infused from 8 h 00 to 16 h 00.
RELAY OPTIONS:
This option is protected and accessible only after the 00123 code has been
entered with the rotary knob.
According to your department practices, this one allows you to activate or
disactivate the hereunder functions:
Relay configuration
Channel relay authorized
Manual relay authorized
Enable or disable the channel relay
The PRINTING menu
This menu allows the detailed or summarised print out of the history
data:
D
Doses,
D
Total volumes/1 hour,
D
Flow rate graphs,
D
Drugs list.
It is also possible to print the drug parameters of the library.
The choice of data to be printed is done according to the screen you are
consulting.
The printer is connected to the isolated serial port identified by the
symbol.
According to the Base Intensive versions, you can have access to these
options or not.
Page 14

1395-6_nu_base_int_v3_2_is_120606.doc
Safety features
The Base Intensive has a continuous inspection system which functions as soon as the pump is in use.
Any internal failure or anomaly detected will be immediately displayed. Nevertheless, the qualified personnel in your establishment or our AfterSales Department should always be notified of any abnormal function where no specific cause can be found.
Connection / disconnection
The flow rate value limits of the drug selected in the library are stored when the Module is disconnected from its Base. It is impossible to program a flow rate
out of the drug limits.
When you change a syringe on a disconnected Module, the default drug name is the one previously used. Please make sure that the drug concentration in the
new syringe is the same as the previous one before confirming the drug name and its concentration.
If the drug name or concentration is different, select « NO » at the drug selection. The programmed limits for the previous drug are cancelled.
Alarms, pre-alarms and warnings
All Base Intensive alarms, pre-alarms and warnings are displayed and/or are indicated with an audible signal:
D
Mains disconnection pre-alarm.
D
Low battery pre-alarm and alarm.
D
Burned 2 channel relay pre-alarm.
When you press the Base Intensive SILENCE ALARM key , the message is recalled.
Quick check protocol
This test is mandatory when the pump is used in anaesthesia.
It allows a complete alarm and safety features check of the Base Intensive (no patient connected).
Serial numbers (ID/N): Base Intensive: ________________________ Module: ________________________
Date: _____ / _____ / _____Section: ________________________ Name: ________________________
1. Power supply lead not connected, install at least one Module on Base Intensive. Then, switch the
Base on pressing the key. Check that battery indicator illuminates and the information screen
displays.
2. Check indicators’ functionality during auto-test. YES E NO
3. Check audible alarms work. YES E NO
4. Check LCD integrality. YES E NO
Results OK
YES E NO
E
E
E
E
5. Connect the device to the mains source, check mains indicator illuminates. YES E NO
6. Switch the first Module on and check its position number on the summary screen. YES E NO
Note: quick check is OK if answers are “yes” for all items. YES E NO
For the Orchestra® Module’s quick check, please refer to their Operator’s Guides.
E
E
E
Page 15

1395-6_nu_base_int_v3_2_is_120606.doc
Base Intensive - Performances
This Operator’s Guide describes the programming of a Module DPS from the Base Intensive. For the use of functions available from the Module’s
keyboard, please refer to its Operator’s Guide.
Flow rate range available from
the Base Intensive keyboard
for a Module DPS
D
From 0.1 to 1200 ml/h, 0.1 ml/h increment.
Time limit programmable for
the loading dose and the
bolus
D
From 1 second to 60 minutes, 1 second increment.
Volume limit programmable
for the loading dose and the
bolus
D
From 1 ml to 65 ml, 0.1 ml increment.
Flow rate accuracy
Please refer to the Modules Operator’s guides.
Volume variations during the relay setup:
Volumes variations
Syringe types
50 cc 0.100 0.200 125"
20 cc 0.040 0.130 72"
10 cc 0.016 0.034 45"
5 cc 0.018 0.030 34"
Minimum volume is lower than – 0.02 ml, whatever the selected syringe type.
These values are equivalent to volume variations observed for a simple
connection of an IV infusion line on the patient.
∆ V average
(in ml)
∆ V maxi.
(in ml)
Average setup time
(in seconds)
Units and conversion formula
Programmable dilution units are in mg/ml, µg/ml, ng/ml, U/ml, kU/ml, mg, µg,
ng, g, U, kU, mol, mmol, Cal, kCal.
Mass rate units are calculated from mass units (ng, µg, mg, g) or Insulin (U,
kU) or mol, mmol or cal/kcal per time unit (24 h, h, min) pondered or not by
patient’s weight (kg).
Bolus dose or initial dose are calculated from mass units (ng, µg, mg, g) or
Insulin (U, kU) or mol, mmol or cal/kcal pondered or not by patient’s weight
(kg) or in ml.
The used formulas are:
D
Q = D*P/C with pondered weight,
Q = D/C without pondered weight.
D
V = d*P/C if dosis is pondered by weight,
V = d/C if dosis is not pondered by weight.
Q: Volumic rate, in ml, pondered by time unit (24h, h, min)
V: Bolus volume de bolus, in ml.
D: Mass rate.
d : Bolus dose.
C : Drug concentration.
• S = (p
S : Calculated body surface of the patient,
P : Weight in kg,
T : Height in cm or m/100.
• BMI (Body Mass Index) = P / T
P : Weight in kg,
T : Height in m.
0.425 xT0.725
)* 0.007184 unit in m
2
2
Adjustment range, accuracy,
displayed values
Dose rate and concentration adjustment range:
D
Inferior to 1: 0,01 increment.
D
Between 1 and 10: 0,05 increment.
D
Between 10 and 100: 0,5 increment.
D
Between 100 and 1000: 5 increment.
D
Superior to 1000: 50 increment.
Flow rate is calculated according to formula and units described in paragraph
« Units and conversion formula ».
The flow rate order sent to the Module DPS are rounded off and taken into
account at 1/1000 of ml/h. The flow rate value displayed on the Base and on
the Module is at 1/10 of ml/h.
Page 16

1395-6_nu_base_int_v3_2_is_120606.doc
Base Intensive – Technical characteristics
External power supply
Voltage From 95 to 240 Vac
Frequency 50/60 Hz
Max. consumption 1500 mA
Max. power consumption 125 VA
Protective fuse T 1600 mA 250 V IEC 127
Output voltage 7.35 V (mini 7.15 V)
Output current 7 A
Battery
Characteristics 6 V 1,2 to 1,3 Ah - Sealed lead battery
Battery life min. 1 hour
Battery recharging Partial (70% of capacity): 8 hours
Total (100% of capacity): 16 hours
Compliance
Complies with Medical Device Directive 93/42 EEC:
CE mark: CE 0459
Safety of Medical Electrical Equipment:
Complies with EN/IEC 60601-1 and EN/IEC 60601-2-24
Dimensions - Weight
Dimensions Width 32 cm / Depth 30 cm / Height 7.5 cm
Weight approx. 2.6 Kg
Indicators lights
Mains power operation Yellow
Mains disconnection
alarm
Pre-alarm Orange
Alarm Red
LCD screen ¼ VGA color
Flashing red
Symbols
Direct current
Input voltage signal
Output voltage signal
Battery
Fuse
IP34 Protection against splashing fluid
Protection against leakage current: Type CF equipment
Protection against electric shocks: Class II equipment
Electromagnetic Compatibility:
Complies with EN/IEC 60601-1-2 (second edition) and EN/IEC 60601-2-
24. Detailed information is given in paragraph Guidance and
manufacturer's declaration on electromagnetic environment.
Emission compliance:
D
Radiated and Conducted RF: CISPR 11/EN 55011, Group 1 Class B
D
Harmonics: EN/IEC 61000-3-2, Class A
D
Voltage Fluctuations / Flickers: EN/IEC 61000-3-3
Immunity compliance:
D
Electrostatic Discharges (ESD): EN/IEC 61000-4-2
D
Fast Transient / Burst: EN/IEC 61000-4-4
D
Surges: EN/IEC 61000-4-5
D
Voltage dips, variations: EN/IEC 61000-4-11
D
Magnetic Field: EN/IEC 61000-4-8
D
Conducted RF: EN/IEC 61000-4-6
D
Radiated RF: EN/IEC 61000-4-3
Device materials
Lower case:
Upper case:
Locking bloc:
Labels Polyester
LCD glass PMMA
Polycarbonate with glass fiber
ABS
Polycarbonate with glass fiber
Functional earth
Patient’s hand set (not used)
4000 V RS 232
RS 232-1 Additional RS232 connection n°1
RS 232-2 Additional RS232 connection n°2
To use the 3 RS232 and the nurse call connections, please refer to the
corresponding technical file to respect connections.
RS 232 connection insulated 4000 V
To connect one Base A or a device conform
to the IEC 601.1 Standard.
To connect the bar code reader. 5 V voltage.
Nurse call connection
Page 17

1395-6_nu_base_int_v3_2_is_120606.doc
Precautions to be taken
The symbol visible on the condensed instruction guide of the
devices recommends this Operator Guide should be completely
read, in accordance with the EN 60 601-1 standard.
Fresenius Vial will not be liable for any damages or claims,
medical or otherwise, of any nature whatsoever, whether direct or
consequential, caused by improper use of this device.
Special attention must be paid to the stability of Orchestra® when several
modules are used on Base Intensive.
From the 4th Module onwards, Orchestra® must be equipped with the
Multifix accessory to ensure rigidity.
During transportation of Orchestra® with Multifix accessory, only install
maximum 6 Modules on Base Intensive.
Use the device in horizontal position, on a table, or with the Multifix for
use on a pole.
Fresenius Vial recommends not to place the Modules higher than 1,3
meter above patient.
We recommend you partially or completely recharge the battery when
you receive the devices or in the case of prolonged storage so as to
prevent all risk of premature discharge.
To preserve the environment, remove the battery from the device prior to
destruction or at the end of the device life and as during normal
maintenance replacement, return it to a competent recycling
organisation. Proceed in the same way for the device itself (electronic
boards, plastics…).
Avoid short circuit and excessive temperature.
Anaesthetic substances: the device must not be used in the presence of
inflammable anaesthetic agents due to the risk of explosion. It should
always be used away from all risk areas.
Use only disposable proposed in the Module DPS and Module MVP
Operator’s Guide in accordance with local standard operating
procedures and good clinical practices. Using NO recommended
disposable could lead to serious hazards such as free flow or pump
degradation.
Fuses should be replaced by equivalent parts. This should be done by a
qualified technician. Refer to the part list of the technical manual for full
specification.
The Base Intensive may only be connected to the mains with the power
cord supplied by the manufacturer. Check that the supply voltage
corresponds with the value indicated on the label placed underneath the
device.
Do not exceed the permitted voltage whether the supply is from the
mains, an external source or via the different external connections.
Standard precautions should be taken to prevent contamination or
injuries while discarding the associate disposable (e.g.: syringes,
extension sets, needles, etc.).
Only use Luer Lock connection to prevent disconnection due to infusion
pressure.
Check that all equipment connected to Orchestra® resist to a pressure of 2000
HPa.
Do not use in conjunction with positive pressure infusion devices that could
generate back pressure higher than 1 500 Hpa susceptible to damage infusion
disposable and the device.
While in use, negative pressure variation may occur in the syringe, by the
relative height from the device to the injection site or by combined infusion
devices such as blood pump, alternative clamp, etc.
Pressure variation may generate flow discontinuity mainly noticeable at low flow
rates.
When the container is placed higher than the injection site, please pay
attention to correctly manipulate the infusion set only when the extension set is
clamped or disconnected from patient side.
Make sure that infusion line does not hinder moving parts of other devices.
Fresenius Vial recommends the use of one way valves or positive pressure
infusion devices for multi-line infusions. If there is no one way valve on a gravity
infusion line during a multi-line infusion, this will make it impossible to detect
occlusions on the patient side, and could result in accumulation of the drug
being infused in the gravity line, which could later be infused in an uncontrolled
manner when the occlusion is released. Place the connection between the
gravity line and the pump-driver line as near to the start of the set as possible
in order to minimise the dead space and consequently the impact of any
change in flow rate on the gravity line.
One way
valve
For « Y » connections used in 2 channel relay, it is recommended to prime the
lines particularly in case of concentration changes where dead volumes of the
sets may generate a risk of under or over dosage.
This device can be disturbed by environmental pressure or pressure variations,
mechanical shocks, heat ignition sources, etc...
A non-medical electric device connected to the RS232 interface must be in
conformity with the suitable IEC/EN standard (e.g. IEC / EN 60950). In all
cases, the IEC/EN 60601-1-1 international standard must be taken into
account. Both installation and use of Base Intensive via an RS 232 link must
comply with the accompanying document: RS 232 protocol for Base Intensive.
This document is available from our After-Sales Department.
Fresenius Vial will not be responsible whatsoever for use of any interface
communication between the Base Intensive and computer system.
Opening the pump must only be carried out by the qualified personnel in your
establishment, and taking all the necessary technical precautions. W e
recommend you follow the maintenance procedures defined in the Technical
Manual. To obtain a copy of this document, please contact our After-Sales
Department, specifying the identification number of the device. Non-respect of
these procedures is dangerous to the personnel and may damage the pump.
infusion pump
Page 18

1395-6_nu_base_int_v3_2_is_120606.doc
Guidance and manufacturer's declaration on
electromagnetic environment
The Base Intensive has been tested and found to comply with the applicable standards for electromagnetic Compatibility for Medical Devices. These standards
are designed to provide adequate immunity to prevent undesirable operation of the Base Intensive, as well as a limitation of emissions from the device that may
cause undesirable interference to other equipment.
If the Base Intensive is operating in the vicinity of other equipment which causes high levels of interference (e.g. HF surgical equipment, X-rays equipment,
nuclear spin tomography units, mobile telephones, wireless access points, etc... ), maintain the recommended protective distances (see Table 206), reorienting or re-locating the Base Intensive.
The following tables specify the suitable electromagnetic environment for using the device and provide guidance to assure that it is used in such an
environment.
Guidance and manufacturer’s declaration – electromagnetic emissions TABLE 201
The Base Intensive is intended for use in the electromagnetic environment specified below. The user of Base Intensive should make sure it is used in such
an environment.
Emissions test
RF emissions
CISPR 11
Compliance obtained
by the device
Group 1 The Base Intensive uses RF energy only for its internal function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby electronic equipment.
Electromagnetic environment - guidance
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations
Flicker emissions
IEC 61000-3-3
Class B The Base Intensive is suitable for use in all establishments, including domestic and hospital
Complies
Class A
Not applicable Voltage fluctuations / flicker emission are not applicable, because the Base Intensive cannot generate a
establishments and those directly connected to the public low-voltage power supply network that supplies
buildings used for domestic purposes.
The Base Intensive complies by default with Harmonic emissions because input power is lower than the
minimum input power specified in the IEC 61000-3-2.
significant voltage fluctuations and flicker emissions according to IEC 61000-3-3.
Guidance and manufacturer’s declaration – electromagnetic immunity TABLE 202
The Base Intensive is intended for use in the electromagnetic environment specified below. The user of Base Intensive should make sure it is used in such
an environment.
Immunity test
Electrostatic
Discharge (ESD)
IEC 61000-4-2
Electrical fast
Transient / burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations on
power supply input
lines
IEC 61000-4-11
IEC 60601-1-2
IEC 60601-2-24
Test level
+ 8 kV contact
+ 15 kV air
+ 2 kV for power
supply lines
+ 1 kV for input
output lines
+ 1 kV differential
mode
+ 2 kV common
mode
< 5 % Ut
( > 95 % dip in Ut )
for 0,5 cycle
40 % Ut
( 60 % dip in Ut )
for 5 cycles
70 % Ut
( 30 % dip in Ut )
for 25 cycles
Compliance level obtained
by the
device
+ 8kV contact
+ 15 kV air
+ 2 kV for power
supply lines
+ 1 kV for input
output lines
+ 1 kV differential
mode
+ 2 kV common mode
< 5 % Ut
( > 95 % dip in Ut )
for 0,5 cycle
40 % Ut
( 60 % dip in Ut )
for 5 cycles
70 % Ut
( 30 % dip in Ut )
for 25 cycles
Electromagnetic environment – guidance
Coatings of the floors out of wooden, tilings, and concrete, with a relative
humidity level at least 30 %, makes it possible to guarantee the level of
necessary conformity. If it is not possible to guarantee this environment, of the
additional precautions must be taken, such as: anti-static material usage,
preliminary user discharge and the wearing of anti-static clothing.
If the IEC 60417-5134 ESD symbol is adjacent to a connetor, all the
precautions of use must be taken before all handling.
Mains power quality should be that of a typical domestic, commercial or
hospital environment.
Mains power quality should be that of a typical domestic, commercial or
hospital environment. For a very exposed establishments or building with the
lightning, a protection must be installed on mains power.
Mains power quality should be that of a typical domestic, commercial or
hospital environment.
For a short and long interruptions ( < than battery autonomy ) of power mains,
the internal battery provide the continuity of service.
For a very long ( > than battery autonomy ) interruptions of power mains, the
Base Intensive must be powered from an external uninterruptible power
supply ( UPS ).
< 5 % Ut
( > 95 % dip in Ut )
Power frequency
( 50 / 60 Hz )
magnetic field
IEC 61000-4-8
Note: Ut is the a.c. mains voltage prior to application of the test level.
for 5 s
400 A / m 400 A / m If necessary, the power magnetic field should be measured in the intended
< 5 % Ut
( > 95 % dip in Ut )
for 5 s
installation location to assure that it is lower than compliance level.
If the measured field in the location in which the Base Intensive is used
exceeds the applicable magnetic field compliance level above, the Base
Intensive should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as reorienting or re-locating the Base Intensive, or install magnetic shielding.
Page 19

1395-6_nu_base_int_v3_2_is_120606.doc
Guidance and manufacturer’s declaration – electromagnetic immunity TABLE 204
The Base Intensive is intended for use in the electromagnetic environment specified below. The user of Base Intensive should make sure it is used in such
an environment.
Immunity test
Conducted RF
IEC 61000-4-6
IEC 60601-1-2
IEC 60601-2-24
Test level
3 Vrms
150 KHz to 80 MHz
Compliance level
obtained by the
device
10 Vrms
Electromagnetic environment – guidance
Portable and mobile RF communications equipment should be used no closer to
any part of the Base Intensive, including cables, than the recommended
separation distance calculated from the equation applicable to the frequency of
transmitter.
Radiated RF
IEC 61000-4-3
10 V / m
80 MHz to 2,5 GHz
10 V/m
Recommended separation distance:
F
D = 1,2 √ P , for a frequency of 150 KHz to 80 MHz
F
D = 1,2 √ P , for a frequency of 80 MHz to 800 MHz
F
D = 2,3 √ P , for a frequency of 800 MHz to 2,5 GHz
where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer and D is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic
site survey (a), should be less than compliance level.
Interference may occur in the vicinity of equipment marked with the
following symbol:
NOTE 1: these guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and
people.
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio
broadcast and TV broadcast cannot be predicted theoretically with accuracy. To access the electromagnetic environment due to the fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength in the location in which the Base Intensive is used exceeds the applicable
RF compliance level above, the Base Intensive should be observed to verify normal operation. If abnormal performance is observed, additional measures
may be necessary, such as re-orienting or re-locating the Base Intensive, or install magnetic shielding.
Recommended separation distances between portable and mobile RF communication equipment and the Base
Intensive - TABLE 206
The Base Intensive is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The user of the Base Intensive
can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment
(transmitters)and the Base Intensive as recommended below, according to the maximum output power of the communication equipment.
Rated maximum output power
of transmitter
( W )
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
Separation distance according to frequency of transmitter in metres ( m )
150 KHz to 80 MHz
d = 1,2 √√√√ P
80 MHz to 800 MHz
d = 1,2 √√√√ P
800 MHz to 2,5 GHz
d = 2,3 √√√√ P
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using the equation
applicable to the frequency of transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: these guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and
people.
Page 20

1395-6_nu_base_int_v3_2_is_120606.doc
Maintenance recommendations
Cleaning and disinfecting
The Base Intensive is part of the patient’s immediate environment. It is
advisable to clean and disinfect the device’s external surfaces on a daily
basis in order to protect patient and staff.
F
Disconnect the base from its main supply before cleaning.
F
Do not place in an AUTOCLAVE nor IMMERSE the device. Do not let
liquids enter the device’s casing.
F
If the device is placed in a high contamination risk unit, it is advisable to
leave it in the room during disinfecting, after having disinfected it with a
moist cloth.
F
Use a cloth soaked in DETERGENT-DISINFECTANT previously diluted
with water if required, to destroy micro-organisms. Avoid abrasive scrubbing
which could scratch the casing. Do not rinse or wipe surfaces.
F
Do not use: TRICHLOROETHYLENE-DICHLOROETHYLENE AMMONIA - AMMONIUM CHLORIDE - CHLORINE and AROMATIC
HYDROCARBON - ETHYLENE DICHLORIDE-METHYLENE CHLORIDE
- CETONE. These aggressive agents could damage the plastic parts and
cause device malfunction.
F
Take care also with ALCOHOL BASED SPRAYS (20% - 40% alcohol).
They lead to tarnishing of and small cracks in the plastic, and do not provide
the necessary cleaning prior to disinfecting. Disinfecting SPRAYS may be
used, in accordance with the manufacturer recommendation, from a
distance of 30 cm of the device, avoid the accumulation of the product in
liquid form.
Please contact the appropriate service, responsible for cleaning and
disinfecting products, in your establishment for further details.
Environmental conditions
The device should be stored in a dry and cool place. In case of
prolonged storage, the battery should be disconnected via the
battery access flap situated underneath the device. This should be
done by a qualified technician.
F
Storage conditions and carrying.
Quality control
Upon the hospital request, a control check of the device is recommended
every 12 months.
A regular control check (not included in the guarantee) consists of various
inspection operations listed in the Technical manual. These control checks
must be performed by an experienced technician and are not covered by
any contract or agreement provided by Fresenius Vial.
Preventive maintenance
To ensure normal performance of the device, it is recommended that
preventive maintenance is performed every 3 years. This includes battery
replacement and it should be performed by a qualified technician.
The qualified technicians in your establishment or our After-Sales Service
should be informed if the device is dropped or if any of malfunctions occurs.
In this case, the device must not be used.
Caution: Failure to comply with these maintenance procedures can damage
the device and lead to a functional failure. Internal inspection of the device
requires the respect of particular procedures to void damages to the pump
or user.
Servicing
For further information concerning the device servicing or use, please
contact our After-Sales Service or our Customer service.
If a device is returned to our After-Sales Department, it is essential to clean
and disinfect it, then, pack it very carefully, if possible in its original
packaging, before sending it.
Fresenius Vial is not liable for loss or damage to the device during transport
to our After-Sales Department.
At the end of the device life, return it to an organization competent in the
treatment of the electrical and electronic equipment waste. Remove the
battery from the device and return it to a competent recycling organization.
Temperature: 10°C + to 60°C.
Pressure:500 hPa to 1060 hPa.
Humidity: 10% to 90%, no condensation
F
Use conditions:
Temperature: 5°C to 40°C.
Pressure:700 hPa to 1060 hPa.
Humidity: 20% to 90%, no condensation.
Page 21

1395-6_nu_base_int_v3_2_is_120606.doc
Function with the internal battery
❶
❺
❷
❹
❻
❸
The Base Intensive has an internal battery which automatically takes over when the mains supply is disconnected and ensures normal function
with no loss of the programmed data.
Operation from the battery is visualized by the indicator (mains disconnection alarm).
Recharging the battery
To recharge battery, just connect the Modules to the Base Intensive and
then to the mains power supply.
Recharging of the battery is visualised by the main indicator .
Accessories
Fresenius Vial recommends the use of Orchestra® range accessories.
Multifix 4 – Cat # 073804
Multifix 6 – Cat # 073809
Multifix 8 – Cat # 073805
This system simplifies transport and
fastens safety of Orchestra® fitted with 4, 6
or 8 Modules. It can be installed on the
Rolling Stand 180, on the Orchestra
Mobile Stand, on a pole or on two horizontal
rails.
®
Recommendations
The battery should be replaced every three years or according your local
servicing recommendations.
In order to preserve the pump’s memory, it is recommended to recharge
battery at least one time per month even if the pump has never been powered
on.
Orchestra® Mobile Stand
Whatever maybe the architecture of
your department, Orchestra® Mobile
Stand provides the choice of
customized accessories.
➊ Orchestra® Rolling stand
Cat # 073076
Warning: from the 4th Module onwards,
Orchestra® must be equipped with the
Multifix accessory to ensure rigidity.
(eg Multifix 4)
When the Base is used with one or several Modules, Fresenius Vial
recommends the transport be done with the Multifix accessory by the
handle.
During transportation of Orchestra® with Multifix accessory, only install
maximum 6 Modules on the Base.
Mobile hooks for Multifix
(for Multifix cat # 073804/809/805)
Cat # 073800
RS 232 cord
Cat. # 073413 (9m/9f)
Cat. # 073414 (9m/25)
Otherwise, please use a lead whose length
is 3 meters maximum.
Mainy Mod
Cat # 073807 (European plug)
Cat # 073810 (British plug)
External power source.
➋ Fixing part handle for mobile hooks and
mobile arm
Cat # 073079
G
Working table
Cat # 073077
H
I.V pole 4 hooks
Cat # 073078 (*)
I
Mobile arm support for IV pressure
measurement
Cat # 073081 (*)
J
Multichannel stop-cock support - Cat #
073080
(*) Accessories to be used with the Fixing
part accessory cat # 073079.
Page 22

1395-6_nu_base_int_v3_2_is_120606.doc
Conditions of guarantee
Fresenius Vial guarantee that this product is free from defects in material and
workmanship during the period defined by the conditions of sale
accepted, except for the batteries and accessories.
To benefit from the materials and workmanship guarantee from our AfterSales Service or agent authorized by Fresenius Vial, the following conditions
must be respected:
F
The device must have been used according to the instructions in this
Operator’s Guide.
F
The device must not have been damaged when in storage, at the time of
repair, or show signs of improper handling.
F
The device must not have been altered or repaired by non-qualified
personnel.
F
The serial number (ID/N°) must not have been altered, changed, or erased.
F
In case of non-respect of these conditions, Fresenius Vial will prepare an
estimate for repair covering the parts and labor required.
F
When return and repair of a device is necessary, please contact Fresenius
Vial Customer or After-Sales Department.
Page 23

1395-6_nu_base_int_v3_2_is_120606.doc
Notes
Page 24

Useful addresses
All requests for information or documentation (technical files, tubing sets catalogue or brochures) must be sent to:
CUSTOMER SERVICE - AFTER-SALES SERVICE:
Fresenius Vial
Le Grand Chemin
F-38590 BREZINS (France)
Tel: +33 (0)4 76 67 10 10
Fax: +33 (0)4 76 67 11 34
E-mail : customers.vial@fresenius-hemocare.com
Consult our Web site
www.fresenius-vial.fr
This document may contain inaccuraci es or typographical errors.
Modifications may thus be made and will be i ncluded in later edi tions.
As standards and equipment change from time to tim e, the features show n and described
This document may not be reproduced in whole or in part without the written consent of Fresenius V ial.
in this docum ent must be c onfirmed by our departments .
®
Orchestra
is a registered trademark in the name of Fresenius Vial.
Fresenius Vial - Le Grand Chem in - F-38590 BREZINS (FRANCE)
 Loading...
Loading...