Page 1
multiFiltrate
Service Manual
Technical Manual
Edition: 6/03.07
Part no. M28 003 1
Page 2

Page 3

Table of Contents
1 Important Information
1.1 Organization of the Technical Manual ..................................................................................... 1-1
1.2 How to Use the Technical Manual............................................................................................ 1-2
1.3 Precautions for Working on the System ................................................................................. 1-3
1.4 Addresses .................................................................................................................................. 1-4
2 Functional Description
2.1 Extracorporeal Circuit............................................................................................................... 2-1
2.1.1 Pumps ......................................................................................................................................... 2-1
2.1.2 Heaters ........................................................................................................................................ 2-2
2.1.3 Pressure Transducer ................................................................................................................... 2-2
2.1.4 Air Detector and Venous Clamp .................................................................................................. 2-2
2.1.5 Optical Detector (Non-Opaque/Opaque Fluid Detector).............................................................. 2-3
2.1.6 Blood Leak Detector .................................................................................................................... 2-3
2.1.7 Heparin Pump.............................................................................................................................. 2-3
2.2 Weighing Units .......................................................................................................................... 2-3
2.3 Ci-Ca Module (Option)............................................................................................................... 2-4
2.4 Functional Test (T1 Test) and Error Messages....................................................................... 2-4
2.4.1 Battery Test, Part 1...................................................................................................................... 2-5
2.4.2 Scales Test .................................................................................................................................. 2-5
2.4.3 Pump Test ................................................................................................................................... 2-6
2.4.4 Pressure Transducer ................................................................................................................... 2-8
2.4.5 Optical Detector........................................................................................................................... 2-9
2.4.6 Air Detector................................................................................................................................ 2-10
2.4.7 Blood Leak Detector .................................................................................................................. 2-11
2.4.8 Heater ........................................................................................................................................ 2-12
2.4.9 Battery Test, Part 2.................................................................................................................... 2-15
2.4.10 Audible Alarm ............................................................................................................................ 2-16
2.4.11 Heparin Pump ............................................................................................................................ 2-17
2.4.12 multiDataLink............................................................................................................................. 2-18
2.4.13 Ci-Ca Module (Option)............................................................................................................... 2-18
2.5 Error Messages........................................................................................................................ 2-20
2.5.1 Alarm Messages........................................................................................................................ 2-20
2.5.2 Warning Messages.................................................................................................................... 2-22
2.5.3 Fatal Errors ................................................................................................................................ 2-30
2.5.4 Error Codes of Scales Lo-Level Routines ............................................................................... 2-33
2.5.5 Error Codes of Pressures Lo-Level Routines.......................................................................... 2-35
2.5.6 Error Codes of Heparin Pump Lo-Level Routines................................................................... 2-36
2.5.7 Error Codes of Pumps Lo-Level Routines............................................................................... 2-37
2.6 Overview of Display Identification Numbers ........................................................................ 2-37
Fresenius Medical Care multiFiltrate TM 6/03.07 0-1
Page 4

3 Installation
3.1 Preface........................................................................................................................................ 3-1
3.2 Important Information on Initial Start-Up ................................................................................ 3-2
3.3 Initial Start-Up Report multiFiltrate.......................................................................................... 3-3
3.4 Explanations on the Initial Start-Up Report ............................................................................ 3-8
3.5 Installing the Ci-Ca Module (Option)...................................................................................... 3-16
4 TSC / TMC / Maintenance
4.1 Important Information Regarding the Procedure ................................................................... 4-1
4.2 TSC / MA Report multiFiltrate................................................................................................... 4-2
4.3 multiFiltrate TSC Report ........................................................................................................... 4-8
4.4 Explanations on the TSC / MA Report ................................................................................... 4-12
5 Adjustment Instructions and Tests
5.1 Service Tools ............................................................................................................................. 5-1
5.2 Service Program ........................................................................................................................ 5-2
5.2.1 Start ............................................................................................................................................. 5-2
5.2.2 Selecting the Language ............................................................................................................... 5-3
5.2.3 System Messages ....................................................................................................................... 5-3
5.2.4 Deactivating and Activating the Heparin Pump ........................................................................... 5-3
5.2.5 Filtrate Bag Monitoring Limit ........................................................................................................ 5-4
5.2.6 Dialysate Tubing Arrangement .................................................................................................... 5-4
5.2.7 Option MultiDataLink (mDL) ........................................................................................................ 5-5
5.2.8 Taring and Calibrating the Scales................................................................................................ 5-7
5.2.9 Calibrating the Pressures ............................................................................................................ 5-8
5.2.10 Calibrating the Blood Leak Detector .......................................................................................... 5-10
5.2.11 Verifying the System Values...................................................................................................... 5-11
5.2.12 Events Memory.......................................................................................................................... 5-13
5.2.13 Program Treatment Modes........................................................................................................ 5-15
5.2.14 Programming the Ci-Ca Data (Option) ...................................................................................... 5-16
5.3 Extracorporeal Components .................................................................................................. 5-17
5.3.1 Tightness of the Venous Occlusion Clamp................................................................................ 5-17
5.3.2 Checking the Pressure Transducers ......................................................................................... 5-17
5.3.3 Venous Pressure Transducer (P.C.B. LP 450-3)....................................................................... 5-18
5.3.4 Air Detector (P.C.B. LP 450-3) .................................................................................................. 5-20
5.3.5 Optical Detector Sensing Opaque / Non-Opaque Fluid (P.C.B. LP 450-3) ............................... 5-21
5.3.6 Arterial / Filtrate / PHF Pressure Transducers (P.C.B. LP 343-1) ............................................. 5-21
5.3.7 Blood Leak Detector .................................................................................................................. 5-22
5.3.8 Setting the Heparin Pump.......................................................................................................... 5-23
5.4 Programming the Processors ................................................................................................ 5-25
5.4.1 Display Processor (DP) ............................................................................................................. 5-26
5.4.2 Operating and Safety Processors (OP and SP) ........................................................................ 5-26
5.4.3 Interchangeability of Safety and Operating Processor .............................................................. 5-30
5.5 Ci-Ca Module (Option) ............................................................................................................. 5-31
0-2 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 5

5.5.1 Preparing the Functional Test ................................................................................................... 5-31
5.5.2 Functional Test of the Drip Counter........................................................................................... 5-32
5.5.3 Functional Test of the Hall Sensor ............................................................................................ 5-32
5.5.4 Functional Test of the Insertion Switch...................................................................................... 5-32
5.5.5 Functional Test of the Line Occlusion ....................................................................................... 5-33
6 PC Service Software MFT
6.1 Organization of the Quick Guide.............................................................................................. 6-1
6.2 General Information .................................................................................................................. 6-1
6.3 Preparation................................................................................................................................. 6-1
6.3.1 System Requirements ................................................................................................................. 6-1
6.3.2 Software Installation .................................................................................................................... 6-2
6.3.3 Hardware Installation................................................................................................................... 6-3
6.3.4 ServiceCard Description .............................................................................................................. 6-3
6.3.5 Starting the Software ................................................................................................................... 6-3
7 Block Diagrams and Component Layouts
7.1 Block Diagram ........................................................................................................................... 7-2
7.2 AC Wiring ................................................................................................................................... 7-3
7.3 P.C.B. LP 122 Heater Control ................................................................................................... 7-4
7.3.1 Block Diagram ............................................................................................................................. 7-4
7.3.2 Description................................................................................................................................... 7-4
7.3.3 Component Layout ...................................................................................................................... 7-5
7.4 P.C.B. LP 123 Pump Control..................................................................................................... 7-6
7.4.1 Description................................................................................................................................... 7-6
7.4.2 Component Layout ...................................................................................................................... 7-7
7.5 P.C.B. LP 124 Motherboard ...................................................................................................... 7-8
7.5.1 Description................................................................................................................................... 7-8
7.5.2 Component Layout ...................................................................................................................... 7-9
7.6 P.C.B. LP 125 Blood Leak Detector ....................................................................................... 7-10
7.6.1 Component Layout .................................................................................................................... 7-10
7.7 P.C.B. LP 127 Scales Board.................................................................................................... 7-11
7.7.1 Description................................................................................................................................. 7-11
7.7.2 Component Layout .................................................................................................................... 7-11
7.8 P.C.B. LP 128, Power Supply Unit.......................................................................................... 7-12
7.8.1 Block Diagram ........................................................................................................................... 7-12
7.8.2 Description................................................................................................................................. 7-12
7.8.3 Component Layout .................................................................................................................... 7-13
7.9 P.C.B. LP 129 User Interface .................................................................................................. 7-14
7.9.1 Component Layout .................................................................................................................... 7-14
7.10 P.C.B. LP 244 Operating and Safety Processors.................................................................. 7-15
7.10.1 Block Diagram ........................................................................................................................... 7-15
7.10.2 Jumper Description.................................................................................................................... 7-15
7.10.3 Component Layout .................................................................................................................... 7-17
Fresenius Medical Care multiFiltrate TM 6/03.07 0-3
Page 6

7.11 P.C.B LP 343-4 Pressure Transducer .................................................................................... 7-18
7.11.1 Component Layout .................................................................................................................... 7-18
7.12 P.C.B. LP 450-3 multiFiltrate Air Detector Control ............................................................... 7-19
7.12.1 Component Layout .................................................................................................................... 7-19
7.13 P.C.B. LP 950, Control Board (Heparin Pump)...................................................................... 7-20
7.13.1 Component Layout .................................................................................................................... 7-20
7.14 Ci-Ca Module............................................................................................................................ 7-21
7.14.1 Block Diagram ........................................................................................................................... 7-21
7.14.2 Component Layout (P.C.B. LP MS 0407) .................................................................................. 7-22
0-4 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 7

1 Important Information
1.1 Organization of the Technical Manual
Page identification Page number 1-3 is to be interpreted as: Chapter 1, page 3.
Editorial information The current edition of this Technical Manual is
6/03.07 = 5th edition, September 2006
In case of updates, the chapters concerned will be replaced.
Refer to the table below to verify that the Technical Manual is up-todate.
Chapter Current version
1 6/03.07
2 6/03.07
3 6/03.07
4 6/03.07
5 6/03.07
6 6/03.07
7 6/03.07
Chapter 1: Important Information
Changes Manual changes will be released as new editions and supplements. In
general - subject to change without notice.
Fresenius Medical Care multiFiltrate TM 6/03.07 1-1
Page 8

Chapter 1: Important Information
1.2 How to Use the Technical Manual
Purpose This Technical Manual is intended for service technicians and is to be
used for first studies (to acquire a basic knowledge) and for reference
purposes (for TSC, maintenance and repair). The Technical Manual,
however, does not replace the training courses offered by the
manufacturer.
Requirements Knowledge of the current Operating Instructions for the respective
system.
Background experience in mechanics, electrical and medical
engineering.
Specifications For the specifications of the respective system, refer to the current
Operating Instructions.
Circuit diagrams and
component layouts
Explanation of the Note
and Caution symbols used
The identification on the PCB permits the operator/technician to verify if
the circuit diagram/component layout matches the PCB actually
installed in the system.
Note
Informs the operator that in case of a failure to follow the steps as
described, a specific function will be executed incorrectly or will not be
executed at all, or will not produce the desired effect.
Caution
Advises the operator against certain procedures or actions that could
cause damage to the equipment or may have adverse effects on
operators and patients.
1-2 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 9

1.3 Precautions for Working on the System
Authorized persons Assembly, extensions, adjustments, modifications or repairs may only
be carried out by the manufacturer or persons authorized by him.
Chapter 1: Important Information
Test equipment and
accessories
Precautions When working on the open system, the following precautions must be
ESD precautions When repairing and when replacing spare parts, observe the applicable
The activities described in the Technical Manual require the availability
of the necessary technical test equipment and accessories.
respected:
Protect the components against ingress of fluids.
Do not touch live parts.
All plugs, connections and components may only be disconnected or
connected if de-energized.
ESD precautions (e.g. EN 100 015-1).
Fresenius Medical Care multiFiltrate TM 6/03.07 1-3
Page 10
Chapter 1: Important Information
1.4 Addresses
Manufacturer Fresenius Medical Care AG & Co. KGaA
Please address any inquiries to:
D-61346 Bad Homburg
Germany
+49 (0)6172/609-0
www.fmc-ag.com
Service
Central Europe
International
service
Local service
Fresenius Medical Care
Deutschland GmbH
Geschäftsbereich Zentraleuropa
Kundendienst / Servicecenter
Steinmühlstrasse 24b
61352 Bad Homburg
Germany
Phone: +49 6172 609-7100
Fax: +49 6172 609-7102
E-mail: ServicecenterD@fmc-ag.com
Fresenius Medical Care
Deutschland GmbH
Service Support International
Hafenstrasse 9
D-97424 Schweinfurt
Germany
Phone: +49 9721 678-333 (hotline)
Fax: +49 9721 678-130
1-4 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 11

2 Functional Description
2.1 Extracorporeal Circuit
The elements for maintaining and monitoring the extracorporeal circuit
of the multiFiltrate are as follows:
Pumps
Heaters
Pressure transducer
Air detector
Venous clamp
Non-opaque/opaque fluid detector
Blood leak detector
Heparin pump
Chapter 2: Functional Description
2.1.1 Pumps
Altogether, the multiFiltrate is provided with four pumps:
Pump Delivery rate
Blood pump 10 500 ml/min
Filtrate pump 10 180 ml/min
Substituate pump 10 170 ml/min
Dialysate pump 10 170 ml/min
The pumps are driven by direct-current geared motors. To control the
speed, these motors are each provided with a clock pulse generator,
which is directly connected to the motor shaft. The pump processors for
controlling the individual pumps (P.C.B. LP 123) are fitted on the motor
housing.
All pumps are supplied with 24 V. The nominal voltage of the blood
pump motor is 20 V. The nominal voltage of the other pumps, however,
is 24 V.
A Hall sensor in the pump housing and a permanent magnet in each
pump door monitor the state of the pump door.
The line inserting position of the pump rotors for inserting and removing
the pump segments is detected by a combination of a reed switch (in
the pump housing) and a permanent magnet (in the rotor).
The functional test covers a check of all pumps.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-1
Page 12

Chapter 2: Functional Description
2.1.2 Heaters
2.1.3 Pressure Transducer
To allow heating of replacement fluids, two heaters, which are activated
and monitored independently of each other, are installed in the
multiFiltrate.
The heater foil is applied to the outside of the heater rod and supplied
with approx. 28 V. The temperatures are controlled and monitored by
altogether four NTC sensors (two for the operating processor and two
for the safety processor). This is implemented on P.C.B. LP 122.
Both heaters are tested during the functional test.
Four pressures are measured at the multiFiltrate:
Arterial pressure,
Measuring transducer (P.C.B. LP 343-1) between the patients
arterial access and the blood pump
Measuring range: 280 300 mmHg
Pre-hemofilter pressure
Measuring transducer (P.C.B. LP 343-1) between the blood pump
and the filter inlet
Measuring range: 0 750 mmHg
Venous pressure,
Measuring transducer (P.C.B. LP 450-3) between the filter outlet
and the patients venous access,
Measuring range: 80 500 mmHg.
Filtrate pressure or dialysate pressure
Measuring transducer (P.C.B. LP 343-1) between the filter
connector and the filtrate pump
Measuring range: 300 300mmHg.
All pressure transducers are subjected to the functional tests.
2.1.4 Air Detector and Venous Clamp
The air detector (P.C.B. LP 450-3) serves for the detection of air in the
extracorporeal blood circuit and operates on the ultrasound principle .
Both the transmitter and the receiver are integrated in the drip chamber
holder. Once the level in the venous drip chamber has fallen below a
certain threshold, the venous clamp is closed. This function is executed
independently of the operating or safety processor. The additional
board AD 28 increases the transmitter voltage during Preparation to
ensure that the level of saline solution is reliably detected.
The air detector is subjected to the functional test.
2-2 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 13

2.1.5 Optical Detector (Non-Opaque/Opaque Fluid Detector)
The optical detector (P.C.B. LP 450-3) detects, according to the infrared
principle, whether saline solution or blood is present in the tubing
system.
The optical detector is subjected to the functional test.
2.1.6 Blood Leak Detector
The blood leak detector (P.C.B. LP 125) is provided for the detection of
a potential blood loss through the membrane. It is operated applying a
two-color measuring section. In the course of this, red and green light is
alternately transmitted to a reference receiver or, through the filtrate
line, to a measuring receiver.
Both the transmitter and the receiver are integrated in the line holder.
The blood leak detector is subjected to the functional test.
2.1.7 Heparin Pump
Chapter 2: Functional Description
2.2 Weighing Units
The heparin pump is used for continuous heparinization of the blood.
A syringe plunger is moved by means of a carriage bar. The carriage
bar is connected to a threaded spindle via a slide. A microprocessorcontrolled stepper motor causes the spindle to rotate. Depending on the
activation, the piston will move up or down.
One Hall sensor each signals when the piston has reached its upper
and lower end of travel. The safety system of the pump comprises a
speed monitoring device (slotted disc with optical sensor) and a motor
current monitoring function. The syringe types are set via a coding
switch (HEX switch).
The weighing units are used for managing the fluid balance during
treatment.
The weighing cells are operated according to the strain gauge principle.
Signal conditioning, including analog-to-digital conversion, is achieved
per weighing cell on the P.C.B. LP 127. The actual weights are
produced by the operating processor.
Scale 1 and scale 2 each have a useful load of approx. 12 kg.
The scales are subjected to the functional test. To test the scales, a test
weight (ball) of defined value must be taken off each scale. Proper
functioning of the scales can be concluded from the correct difference
between the weights before and after lifting.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-3
Page 14

Chapter 2: Functional Description
2.3 Ci-Ca Module (Option)
The Ci-Ca module is intended for regional citrate anticoagulation in the
CVVHD treatment therapy.
Turning power on The Ci-Ca module requires a supply voltage of 24VDC. This voltage is
provided by the multiFiltrate system's power supply unit via the
connector in the lower IV pole support of the IV pole located on the right
of the system.
After the multiFiltrate system was turned on by pressing the power
switch on its rear, the supply voltage of the module is connected. The
operating processor of the Ci-Ca module switches into the standby
mode.
If the multiFiltrate systems is then turned on via the I/O key on the front
of the system, the operating processor of the Ci-Ca module will perform
an internal processor test. This test is performed simultaneously to the
processor test of the multiFiltrate system.
The Ci-Ca module communicates with the multiFiltrate basic system via
a serial interface.
Processor test If a test is not passed successfully, the module will not establish
communications with the multiFiltrate system. It is not possible to
perform a treatment with citrate anticoagulation. The multiFiltrate
system recognizes this problem and displays a message which
proposes to use an alternative anticoagulation equipment (e.g. heparin
pump) and which has to be confirmed by the operator.
T1 test The Ci-Ca module performs its own T1 test, independent of the T1 test
of the multiFiltrate system. This test will be started automatically and
simultaneously to the multiFiltrate T1 test after the prompt whether the
starting conditions are met was confirmed with [OK].
This test cannot be skipped or deselected.
System errors If it is still possible, system errors in the Ci-Ca module are shown on the
multiFiltrate display with the indication that citrate anticoagulation is not
available.
2.4 Functional Test (T1 Test) and Error Messages
After it has been turned on the rear and the [I/O] key has been pressed,
the system automatically starts the processor test. After completion of
the processor test, the display test will be performed. In this test, the
numerical characters are represented for 2 seconds in all of the three
fonts used. After this test, the functional test (T1 test) is started
automatically. Depending on the configuration in the SETUP (SETUP
automatic), the test is running in the background or (SETUP detailed)
the test steps are represented separately on the display.
2-4 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 15
Chapter 2: Functional Description
2.4.1 Battery Test, Part 1
At the beginning of the T1 test, the system assumes the terminal voltage
and loads the battery with a defined resistance over the entire test.
2.4.2 Scales Test
In the first step, the temperature compensation and calibration factors
are tested. In the second step, the scales are tared. The linearity test
follows in step three. The 4 test weights are lifted, and the weight (44.3
g) is tested for a tolerance of ±0.5 g. The offset drift is tested in step four.
The test weights are lowered again; the system expects a weight of 0.0
g with a tolerance of ±0.5 g. The time out for each step (2 to 4) is 10 sec.
In the event of an error, messages are emitted. Here, the error
messages section only relates to scales I. The messages are identical
for scales II to IV.
Error message Causes Action required
Scale I
Cal. factor missing, error.
Acknowledge with
[START/RESET] key.
Scale I
Temp. coeff. factors missing, error.
Acknowledge with
[START/RESET] key.
Scale I
Movement detected on Scale I.
Acknowledge with
[START/RESET] key.
Lithium battery on P.C.B. LP 244OP discharged.
Defect of the EPROM on P.C.B.
LP 127 of the scales specified.
The system is not in a stable
position. The scales are subjected
to a draft. Bags with tubing system
are placed on the scales; this is
especially applicable to filtrate
scales.
The +12 V and/or 12 V voltage(s)
is (are) missing.
Scales mechanics not smooth. Check for smoothness and adjust,
Replace the battery and execute
the calibration step in the Service
program.
Replace the P.C.B. and the
weighing bar and execute the
calibration step in the Service
program.
Set up the system in a stable
position. Remove the system from
the draft, or close the window.
Relieve the scales.
Check the fuses on the PSU board
LP 128 and replace the P.C.B.
LP 128, if necessary.
Caution: All pressures must be
checked and calibrated, if
necessary.
if necessary. Weight check in the
Service program.
Connector not fitted or defective
ribbon cable.
P.C.B. LP 244-OP defective. Replace P.C.B. LP 244-OP.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-5
Fit the connector to the
motherboard and/or to the P.C.B.
LP 127. Replace the ribbon cable.
Caution: All scales and pressures
must be calibrated.
Page 16
Chapter 2: Functional Description
Error message Causes Action required
Scale I
Test weight outside tolerance.
Acknowledge with
[START/RESET] key.
Scale I
Zero offset outside tolerance.
Acknowledge with
[START/RESET] key.
The system is not in a stable
position. The scales are subjected
to a draft. Bags with tubing system
are placed on the scales; this is
especially applicable to filtrate
scales.
Lifting magnet for ball not
connected or defective.
Ball mechanics not smooth. Check for smoothness and adjust,
The system is not in a stable
position. The scales are subjected
to a draft. Bags with tubing system
are placed on the scales; this is
especially applicable to filtrate
scales.
Scales mechanics not smooth. Check for smoothness and adjust,
Test weight fails to drop back. Ball
mechanics not smooth.
Set up the system in a stable
position. Remove the system from
the draft, or close the window.
Relieve the scales.
Fit the connector to the
motherboard. If defective, the lifting
magnet must be replaced.
if necessary. Weight check in the
Service program.
Set up the system in a stable
position. Remove the system from
the draft, or close the window.
Relieve the scales.
if necessary. Weight check in the
Service program.
Check for smoothness and adjust,
if necessary. Weight check in the
Service program (ESC Service).
2.4.3 Pump Test
In the first step, the stop-by-OP function is tested, i.e. the pumps must
stop running. In this step, the safety processor releases the pumps, and
the operating processor does not activate the pumps. In the second
step, all of the four pumps are activated with a rate of 100 ml/min. The
safety processor checks whether the pumps are running at the correct
rate and whether the reed contact (line inserting position) is actuated. In
the third step, the operating processor activates the pumps and the
safety processor disables the pump activation, i.e. the pumps may not
be running. In the event of an error, messages are emitted. Here, the
error messages section only relates to the blood pump. The messages
are identical for the other pumps, i.e. the filtrate, substituate and
dialysate pumps.
2-6 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 17
Chapter 2: Functional Description
Error message Causes Action required
Blood pump
Blood pump door open.
Acknowledge with
[START/RESET] key.
Blood pump
Not stopped by operating
processor.
Acknowledge with
[START/RESET] key.
Blood pump
Wrong speed, rate.
Acknowledge with
[START/RESET] key.
Blood pump door open. Close the blood pump door.
Magnet in blood pump door
missing.
Hall sensor in pump housing not
connected or defective.
Control electronics (P.C.B. LP 123)
of the specified pump defective.
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
Control electronics (P.C.B. LP 123)
of the specified pump defective.
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
Clock pulse generator of motor on
P.C.B. LP 123 not connected.
Control electronics (P.C.B. LP 123)
of the specified pump defective.
Replace the blood pump door.
Plug the connector onto the
appropriate P.C.B. LP 123 or
replace the pump housing.
Replace P.C.B. LP 123.
Caution: The BLD must be
calibrated.
Replace P.C.B. LP 123.
Caution: The BLD must be
calibrated.
Connect the clock pulse generator
socket.
Replace P.C.B. LP 123.
Blood pump
Reed contact line threading
position
Acknowledge with
[START/RESET] key.
Defect of the clock pulse generator
on the geared motor.
Magnet in rotor missing, defective. Replace the rotor.
Reed switch in pump housing not
connected or defective.
Control electronics (P.C.B. LP 123)
of the specified pump defective.
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
Replace the geared motor.
Caution: Blood pump
20 V/3300 rpm; all other pumps
24 V/3300 rpm.
Plug the connector onto the
appropriate P.C.B. LP 123 or
replace the pump housing.
Replace P.C.B. LP 123.
Caution: The BLD must be
calibrated.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-7
Page 18
Chapter 2: Functional Description
Error message Causes Action required
Blood pump
Stop.
Acknowledge with
[START/RESET] key.
Blood pump
Not stopped by safety processor.
Acknowledge with
[START/RESET] key.
2.4.4 Pressure Transducer
+24-V supply voltage missing. Check the fuse on the PSU board
LP 128. If necessary, replace
P.C.B. LP 128.
Caution: All pressures must be
checked and calibrated, if
necessary.
Control electronics (P.C.B. LP 123)
of the specified pump defective.
P.C.B. LP 244-OP defective.
(The pumps are activated serially,
i.e. the operating processor is
defective only if all pumps fail to be
activated.)
Control electronics (P.C.B. LP 123)
of the specified pump defective.
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
Replace P.C.B. LP 123.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
Replace P.C.B. LP 123.
Caution: The BLD must be
calibrated.
In the first step, the zero point, amplification and detuning factors are
tested. In the second step, the zero points of the pressure transducers
are checked with a tolerance of ±20 mmHg. In the third step, Part and
Pven are detuned to 300 mmHg. The values are checked with a
tolerance of ±20 mmHg. In the fourth step, PPHF and PFil are detuned
to 300 mmHg. The values are checked with a tolerance of ±20 mmHg.
In the event of an error, messages are emitted. The following table only
shows the error messages for "arterial pressure". The messages are
identical for the venous, pre-hemofilter and filtrate pressures.
Error message Causes Action required
Arterial pressure
Factors / Offset lost.
Acknowledge with
[START/RESET] key.
Lithium battery on P.C.B. LP 244OP discharged.
Replace the battery and execute
the calibration step in the Service
program.
2-8 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 19
Chapter 2: Functional Description
Error message Causes Action required
Arterial pressure
Zero outside tolerance.
Acknowledge with
[START/RESET] key.
Arterial pressure
Detuning outside tolerance
Acknowledge with
[START/RESET] key.
Zero point drifted off. Check the setting and calibrate the
pressure in the Service program.
P.C.B. LP 343-1 (for venous
pressure: P.C.B. LP 450-3)
defective.
P.C.B. LP 244-OP defective. Replace P.C.B. LP 244-OP.
Amplification drifted off. Check the setting and calibrate the
P.C.B. LP 343-1 (for venous
pressure: P.C.B. LP 450-3)
defective.
Replace P.C.B. LP 343-1 (LP 450-
3) and calibrate the pressure in the
Service program.
Caution: When replacing the
P.C.B. LP 450-3, check / adjust the
air detector, optical detector and
venous pressure transducer.
Caution: All scales and pressures
must be calibrated.
pressure in the Service program.
Replace P.C.B. LP 343-1 (LP 450-
3) and calibrate the pressure in the
Service program.
Caution: When replacing the
P.C.B. LP 450-3, check / adjust the
air detector, optical detector and
venous pressure transducer.
2.4.5 Optical Detector
P.C.B. LP 244-OP defective. Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
In the first step, the non-opaque state is checked. In the second step,
the optical detector is detuned and the opaque state is checked. In the
event of an error, messages are emitted.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-9
Page 20

Chapter 2: Functional Description
Error message Causes Action required
Opt. detector
Senses opaque fluid.
Acknowledge with
[START/RESET] key.
Opt. detector
Fails to sense opaque fluid after
attenuation.
Acknowledge with
[START/RESET] key.
Blood present in the system or
objects inserted in the OD.
OD adjusted improperly. Check the setting of the OD.
P.C.B. LP 450-3 defective. Replace the P.C.B. LP 450-3.
Measuring head of OD defective. Replace and adjust the OD
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
OD adjusted improperly. Check the setting of the OD.
P.C.B. LP 450-3 defective. Replace the P.C.B. LP 450-3.
P.C.B. LP 244-OP defective. Replace P.C.B. LP 244-OP.
Remove the blood line or the
objects from the OD.
Caution: When replacing the
P.C.B. LP 450-3, check / adjust the
air detector, optical detector and
venous pressure transducer.
measuring head.
Caution: The BLD must be
calibrated.
Caution: When replacing the
P.C.B. LP 450-3, check / adjust the
air detector, optical detector and
venous pressure transducer.
2.4.6 Air Detector
Caution: All scales and pressures
must be calibrated.
AD 28 defective. Replace AD 28-1.
In the first step, it is checked whether fluid is present in the drip
chamber. if YES, the clamp is activated (opened). In the second step,
the air detector is detuned and the alarm state checked. In the event of
an error, messages are emitted.
2-10 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 21

Chapter 2: Functional Description
Error message Causes Action required
Air detector
LDA1 not in alarm mode.
Acknowledge with
[START/RESET] key.
Air detector
Clamp does not close.
Acknowledge with
[START/RESET] key.
LD adjusted improperly. Check the setting of the LD.
P.C.B. LP 450-3 defective. Replace the P.C.B. LP 450-3.
Caution: When replacing the
P.C.B. LP 450-3, check / adjust the
air detector, optical detector and
venous pressure transducer.
Ultrasonic detector defective. Replace and adjust the ultrasonic
detector.
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
LD adjusted improperly (LDA2). Check the setting of the LD.
P.C.B. LP 450-3 defective. Replace the P.C.B. LP 450-3.
Caution: When replacing the
P.C.B. LP 450-3, check / adjust the
air detector, optical detector and
venous pressure transducer.
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
2.4.7 Blood Leak Detector
In the first step, the blood leak detector is checked for being in an
acceptable state. In the second step, the blood leak detector is detuned
and the alarm state checked. In the event of an error, messages are
emitted.
Error message Causes Action required
Blood leak detector
Calibration values incorrect
or missing.
Acknowledge with
[START/RESET] key.
Lithium battery on P.C.B. LP 244SP discharged.
Replace the battery and execute
the calibration step in the Service
program.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-11
Page 22
Chapter 2: Functional Description
Error message Causes Action required
Blood leak detector
Outside acceptable range.
Remove filtrate line.
Acknowledge with
[START/RESET] key.
Blood leak detector
Alarm-free after signal attenuation.
Acknowledge with
[START/RESET] key.
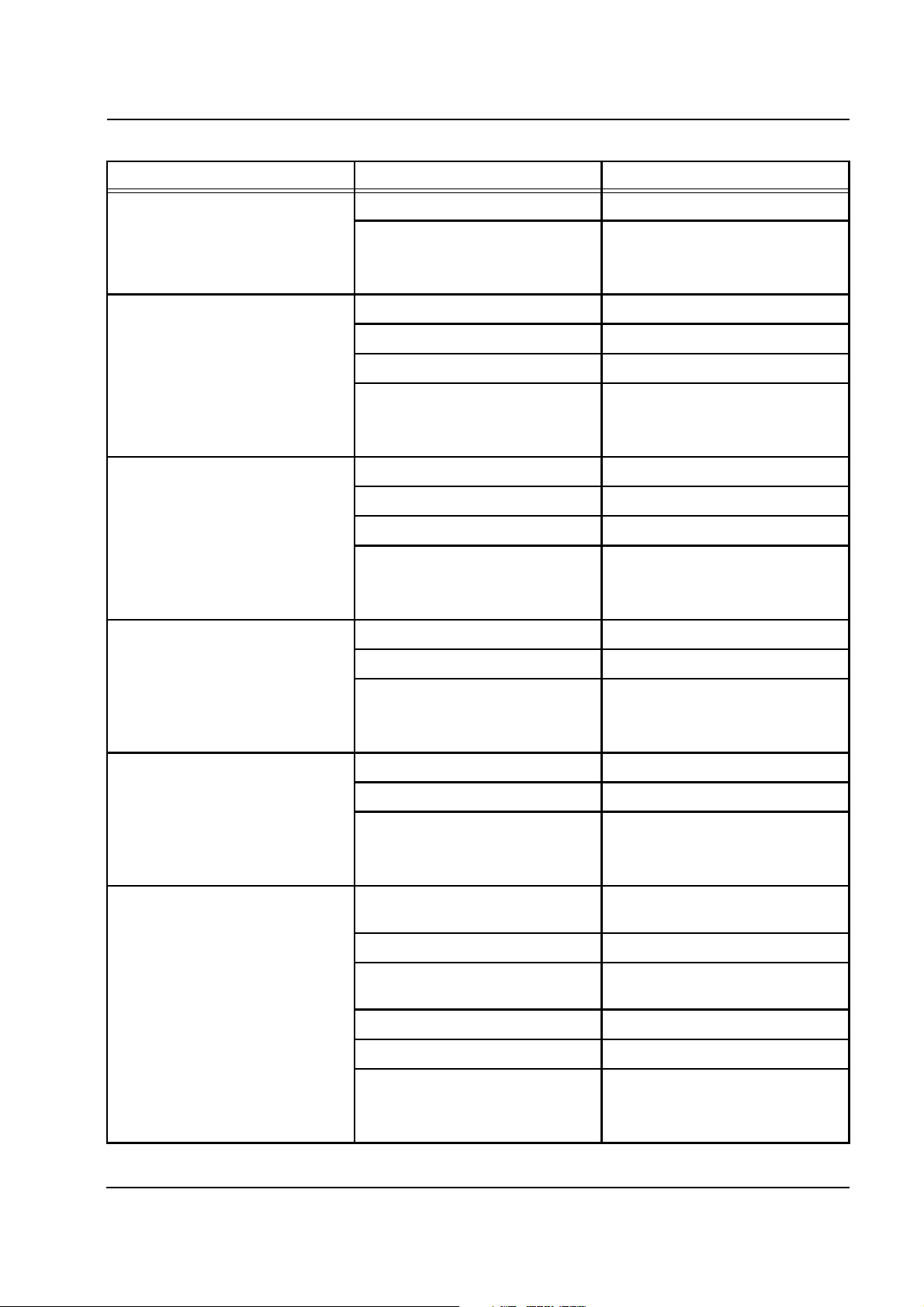
2.4.8 Heater
Empty filtrate line or objects
inserted in the BLD sensor head.
P.C.B. LP 125 and/or BLD sensor
head defective.
P.C.B. LP 244-SR defective. Replace P.C.B. LP 244-SR.
P.C.B. LP 125 and/or BLD sensor
head defective.
P.C.B. LP 244-OP defective. Replace P.C.B. LP 244-OP.
In the first step, the safety relay is checked for being in the open state.
In addition, the sensors are checked for interruption and short-circuit. In
the second step, the safety relay is activated and checked for being in
the closed state. In the third step, the bag and foil sensors of OP and
SP are checked for being synchronized. Tolerances are ±1 °C. In the
fourth step, the foil sensor is detuned to >120 °C and, thus, the safety
shutoff mechanism (fire protection) is checked. In the fifth step, the bag
and foil sensors are detuned to >41 °C to check whether the heater is
switched off in case of an overtemperature of the solutions. In the sixth
step, it is checked whether the heater can be activated. In the event of
an error, messages are emitted. Here, the error messages section only
relates to the lower heater. The messages are identical for the upper
heater.
Remove the filtrate line or the
objects.
Replace P.C.B. LP 125 and the
BLD sensor head, and calibrate the
BLD.
Caution: The BLD must be
calibrated.
Replace P.C.B. LP 125 and the
BLD sensor head, and calibrate the
BLD.
Caution: All scales and pressures
must be calibrated.
Error message Causes Action required
Lower heater
Switch-off path SP defective
(Sub. relay not open)
Voltage missing.
Fuse defective.
Acknowledge with
[START/RESET] key.
2-12 Fresenius Medical Care multiFiltrate TM 6/03.07
Safety relay on P.C.B. LP 122
defective.
Activation on P.C.B. LP 244-SR
defective.
Supply voltage for heater not
connected.
Fuse on P.C.B. LP 122 defective. Replace the fuse.
Replace P.C.B. LP 122.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Fit the socket on P.C.B. LP 122.
Page 23
Chapter 2: Functional Description
Error message Causes Action required
Lower heater
Control FET short-circuit
Acknowledge with
[START/RESET] key.
Lower heater
Bag sensor broken.
Acknowledge with
[START/RESET] key.
Lower heater
Foil sensor broken.
Acknowledge with
[START/RESET] key.
Lower heater
Control FET defective. Replace P.C.B. LP 122.
Activation on P.C.B. LP 244-OP
defective.
Socket of sensors not fitted. Fit the socket on P.C.B. LP 122.
Sensor interrupted. Replace the heater.
P.C.B. LP 122 defective Replace P.C.B. LP 122.
Converter on P.C.B. LP 244-SR
defective.
Socket of sensors not fitted. Fit the socket on P.C.B. LP 122.
Sensor interrupted. Replace the heater.
P.C.B. LP 122 defective Replace P.C.B. LP 122.
Converter on P.C.B. LP 244-SR
defective.
Bag sensor short-circuit. Replace the heater.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Bag sensor short-circuit.
Acknowledge with
[START/RESET] key.
Lower heater
Foil sensor short-circuit.
Acknowledge with
[START/RESET] key.
Lower heater
Sub. relay not closed,
foil defective.
Acknowledge with
[START/RESET] key.
P.C.B. LP 122 defective Replace P.C.B. LP 122.
Converter on P.C.B. LP 244-SR
defective.
Bag sensor short-circuit. Replace the heater.
P.C.B. LP 122 defective Replace P.C.B. LP 122.
Converter on P.C.B. LP 244-SR
defective.
Supply voltage for heater not
connected.
Fuse on P.C.B. LP 122 defective. Replace the fuse.
Heater foil not connected. Connect the heater foil to P.C.B.
Heater foil interrupted. Replace the heater.
Safety relay defective. Replace P.C.B. LP 122.
Activation on P.C.B. LP 244-SR
defective.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Fit the socket on P.C.B. LP 122.
LP 122.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-13
Page 24
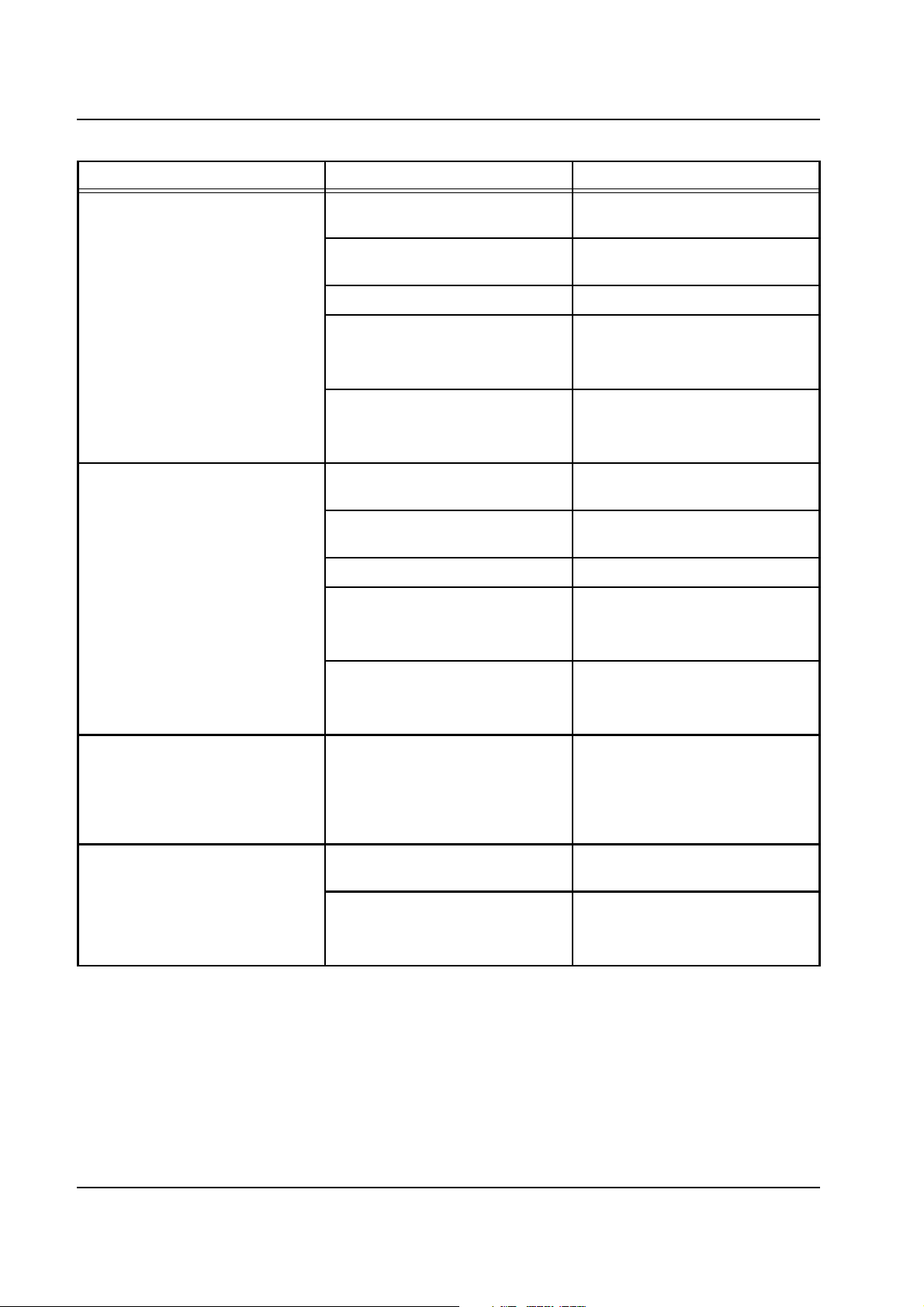
Chapter 2: Functional Description
Error message Causes Action required
Lower heater
Bag sensors,
OP-SP not synchronous
Acknowledge with
[START/RESET] key.
Lower heater
Foil sensors,
OP-SP not synchronous
Acknowledge with
[START/RESET] key.
Heater still warm after last
treatment or Service program.
Sensor board (P.C.B. LP 1220)
defective.
P.C.B. LP 122 defective Replace P.C.B. LP 122.
Converter on P.C.B. LP 244-SR
defective.
Converter on P.C.B. LP 244-OP
defective.
Heater still warm after last
treatment or Service program.
Sensor board (P.C.B. LP 1220)
defective.
P.C.B. LP 122 defective Replace P.C.B. LP 122.
Converter on P.C.B. LP 244-SR
defective.
Allow the heater to cool down.
Replace the heater.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
Allow the heater to cool down.
Replace the heater.
Replace P.C.B. LP 244-SR.
Caution: The BLD must be
calibrated.
Lower heater
Foil sensor detuning,
no overtemperature cutoff.
Acknowledge with
[START/RESET] key.
Lower heater
Bag sensor detuning,
no overtemperature.
Acknowledge with
[START/RESET] key.
Converter on P.C.B. LP 244-OP
defective.
DIL relay on P.C.B. LP 122
defective.
DIL relay on P.C.B. LP 122
defective.
Activation on P.C.B. LP 244-OP
defective.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
Replace P.C.B. LP 122.
Replace P.C.B. LP 122.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
2-14 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 25
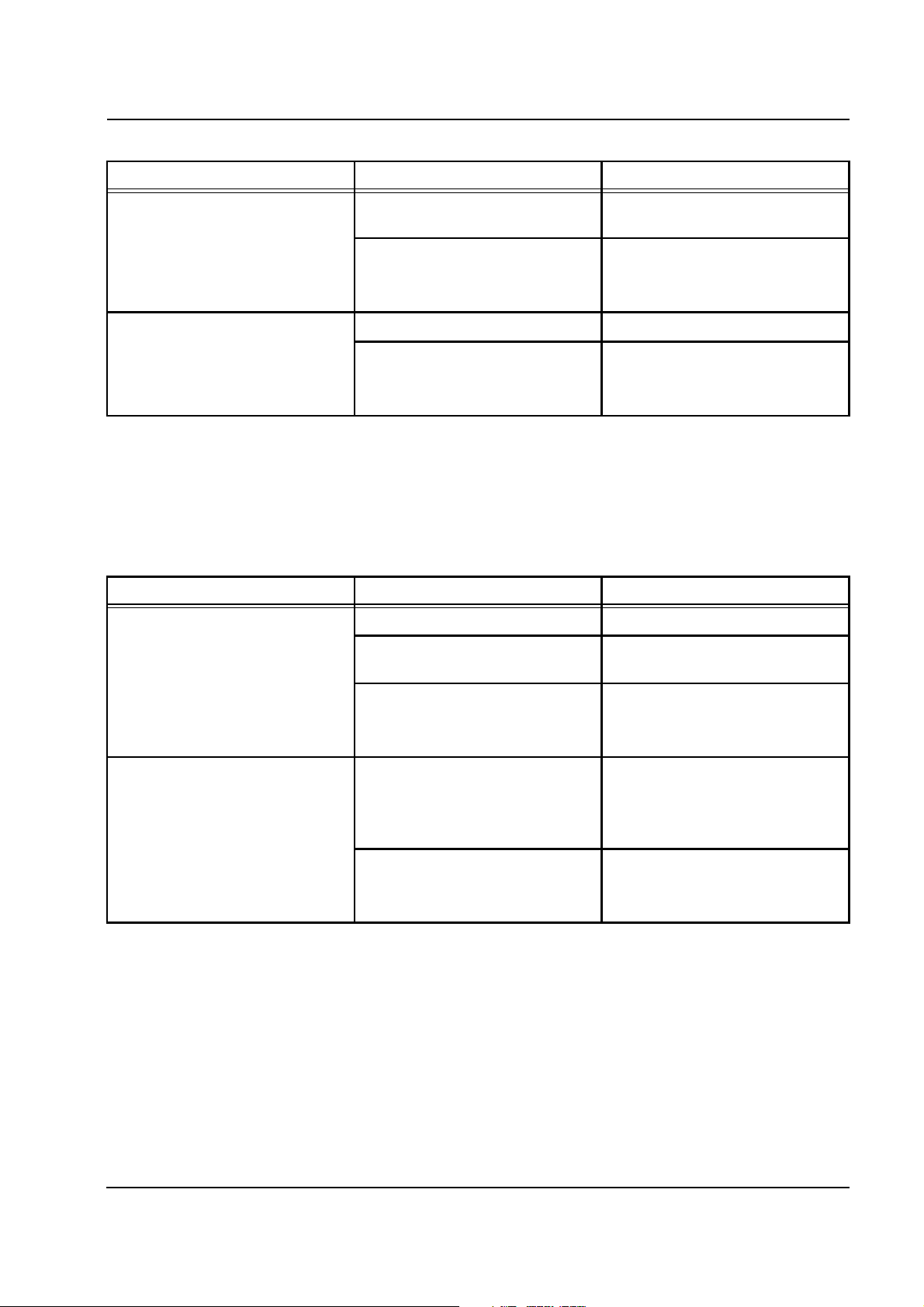
Chapter 2: Functional Description
Error message Causes Action required
Lower heater
Foil sensor detuning,
no overtemperature.
Acknowledge with
[START/RESET] key.
Lower heater
FET control defective
Acknowledge with
[START/RESET] key.
DIL relay on P.C.B. LP 122
defective.
Activation on P.C.B. LP 244-OP
defective.
FET on P.C.B. LP 122 defective. Replace P.C.B. LP 122.
Activation on P.C.B. LP 44-OP
defective.
Replace P.C.B. LP 122.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
2.4.9 Battery Test, Part 2
The first step was carried out at the beginning of the functional test, with
acceptance of the starting value and loading of the battery. In the
second step, the voltage is checked after loading and is compared with
the starting value. In the event of an error, messages are emitted.
Error message Causes Action required
Battery test
Battery not connected, defective.
Acknowledge with
[START/RESET] key.
Battery not connected. Connect the rechargeable battery.
Battery terminal voltage without
load <<18 V.
Converter on P.C.B. LP 244-OP
defective.
Replace the battery.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
Battery test
Load test failed.
Acknowledge with
[START/RESET] key.
Fresenius Medical Care multiFiltrate TM 6/03.07 2-15
Resistor or load relay on the PSU,
P.C.B. LP 128, defective.
Converter or activation on P.C.B.
LP 244-OP defective.
Replace P.C.B. LP 128.
Caution: All pressures must be
checked and calibrated, if
necessary.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
Page 26
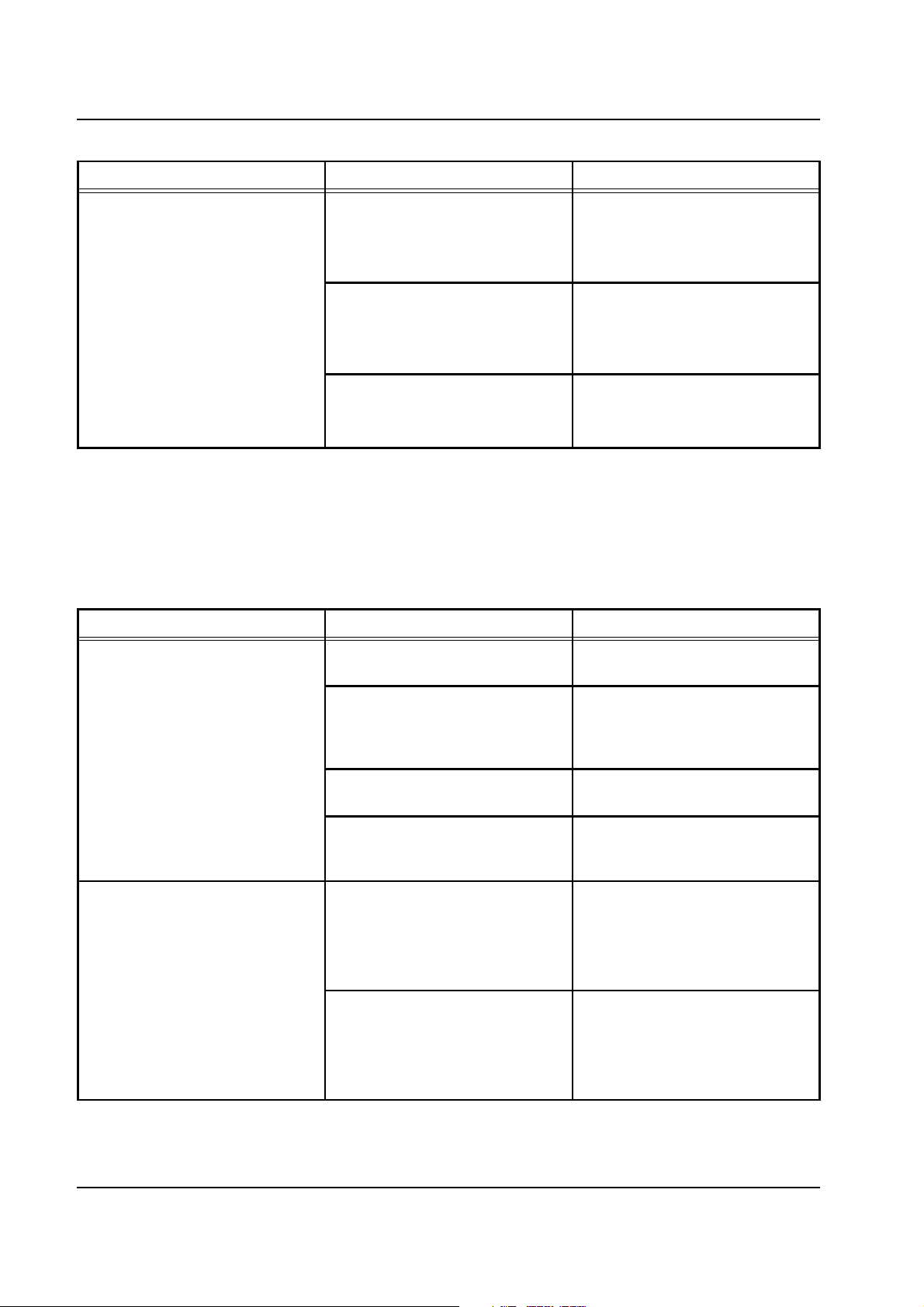
Chapter 2: Functional Description
Error message Causes Action required
Battery test
Insufficient capacity.
Acknowledge with
[START/RESET] key.
System not used for a prolonged
period or frequent power failure
(preparation).
Battery defective.
Charging circuit on the PSU board,
P.C.B. LP 128, defective.
Converter on P.C.B. LP 244-OP
defective.
Load the battery from the mains for
a minimum of 10 hours.
Replace the battery.
Replace P.C.B. LP 128.
Caution: All pressures must be
checked and calibrated, if
necessary.
Replace P.C.B. LP 244-OP.
Caution: All scales and pressures
must be calibrated.
2.4.10 Audible Alarm
In the first step, it is checked whether the audible alarm is silenced. In
the second step, the audible alarm is generated and checked for proper
functioning by means of a microphone. In the event of an error,
messages are emitted.
Error message Causes Action required
Audible alarm
Not silenced.
Acknowledge with
[START/RESET] key.
Constant ambient noise. Repeat the test after the noise has
stopped.
Loud humming noise on the
loudspeaker.
Check the route of the loudspeaker
cable. Replace the loudspeakermicrophone unit or the
motherboard P.C.B. LP 124.
The microphone line is disturbed. Check the route of the microphone
cable.
Microphone or amplifier defective. Replace the loudspeaker-
microphone unit or the
motherboard P.C.B. LP 124.
Audible alarm
Not active.
Acknowledge with
[START/RESET] key.
2-16 Fresenius Medical Care multiFiltrate TM 6/03.07
Alarm tone not audible. Loudspeaker cable connected to
P.C.B. LP 124?
Replace the loudspeakermicrophone unit or the
motherboard P.C.B. LP 124.
Error message despite audible
alarm.
Microphone cable connected to
P.C.B. LP 124?
Replace the loudspeakermicrophone unit or the
motherboard P.C.B. LP 124.
Page 27
Chapter 2: Functional Description
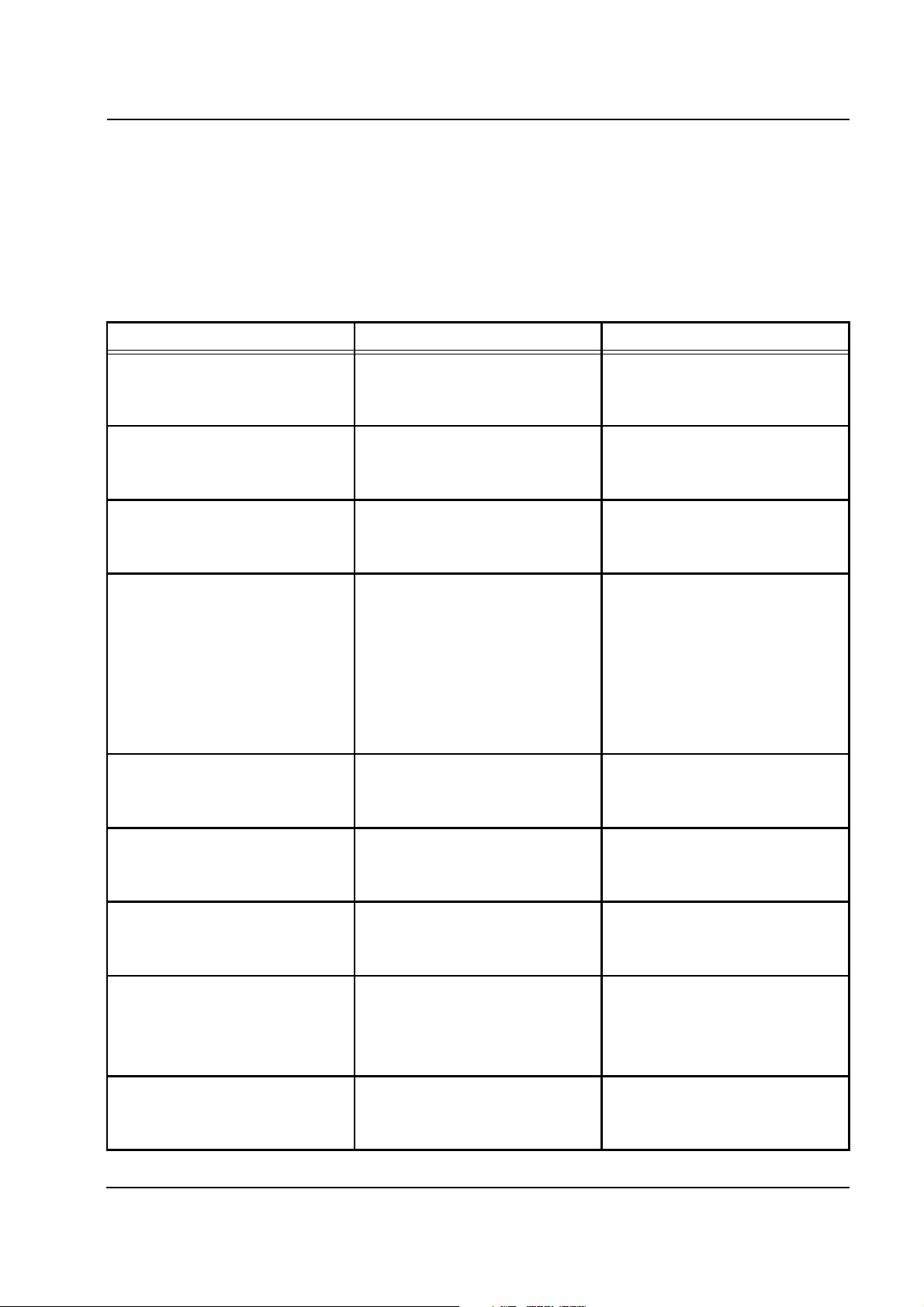
2.4.11 Heparin Pump
After turning power on to the multiFiltrate, a self-test of the heparin
pump will be performed. Immediately before the multiFiltrate self-test,
the operating processor will query the heparin pump status. In the event
of an error, messages are emitted. If the heparin pump is not installed,
not connected to the power supply or deactivated via Service option, no
error message will be displayed.
Error message Causes Action required
Heparin pump
Heparin pump not ready.
No communication.
Heparin pump
Heparin pump optionally
deactivated in Service mode
Heparin pump
Heparin pump detects internal error
Heparin pump
Wrong / unauthorized syringe type
detected
Heparin pump
Unknown hardware error
Heparin pump does not respond. Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
Heparin pump manually
deactivated in the Service mode
Internal heparin pump error without
definite cause.
Wrong syringe type set. Acknowledge with
Heparin pump detects an unknown
hardware error.
Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
[START/RESET] key.
Heparin pump deactivated.
Select a valid syringe type.
0 = 50 ml P syringe
1 = 30 ml heparin syringe
2 = 50 ml Injectomat syringe
Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
Heparin pump
Heparin pump hardware error
Gate array error
Heparin pump
Spike on reset line
Heparin pump
Wrong HEX switch position
Heparin pump
Powerdown
Fresenius Medical Care multiFiltrate TM 6/03.07 2-17
Heparin pump detects an error in
the gate array.
A reset occurred during operation. Acknowledge with
Wrong HEX switch position set. Acknowledge with
Powerdown without 24 V-cutoff. Acknowledge with
Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
[START/RESET] key.
Heparin pump deactivated.
[START/RESET] key.
Heparin pump deactivated.
Set a valid HEX switch position.
[START/RESET] key.
Heparin pump deactivated.
Page 28
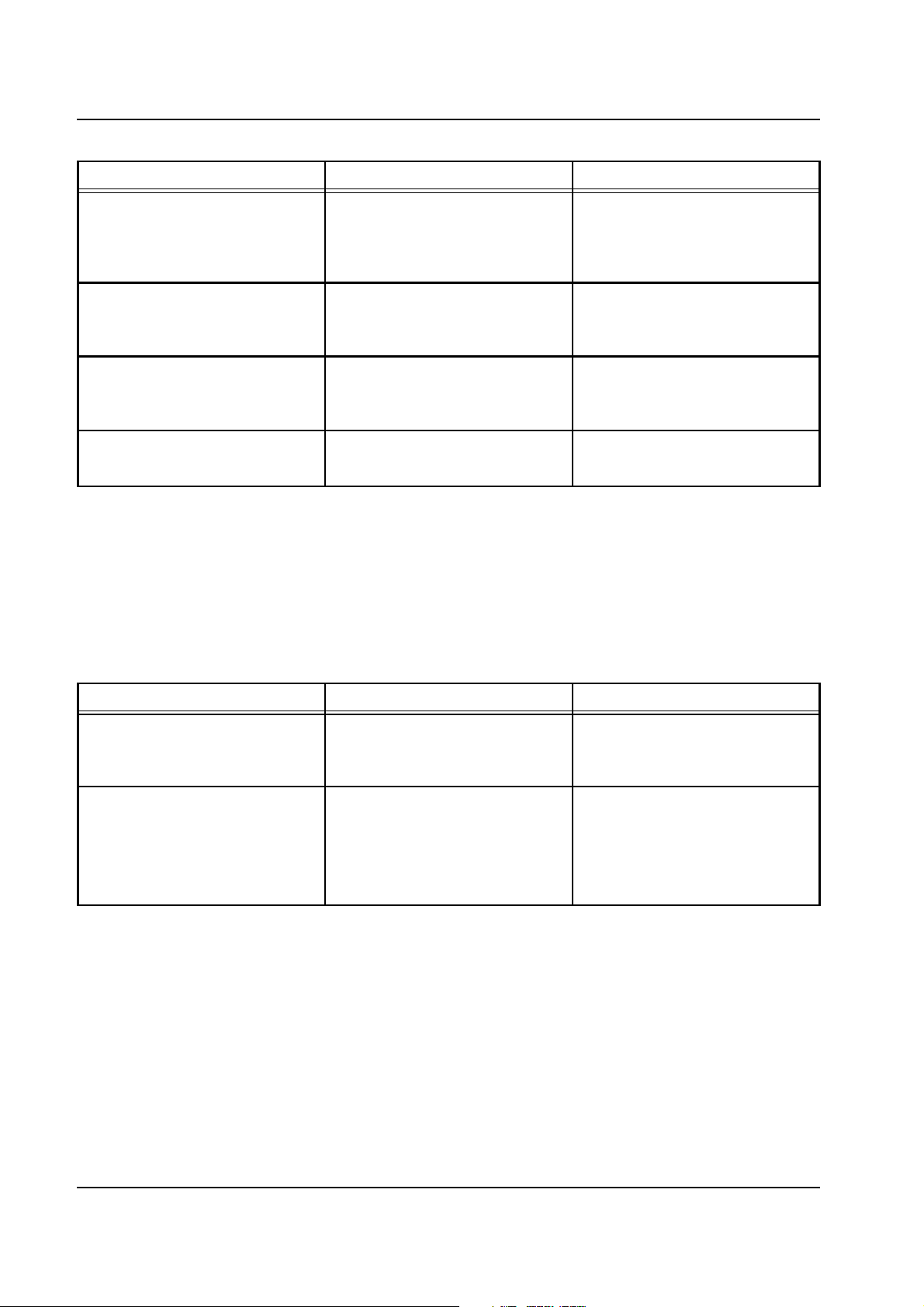
Chapter 2: Functional Description
Error message Causes Action required
Heparin pump
Operating processor internal
communication error with heparin
pump
Heparin pump
Watchdog error
Heparin pump
NOVRAM error
Heparin pump
CAMUS transmission error
2.4.12 multiDataLink
Erroneous data transmission.
Wrong or missing characters.
Check sum error.
The watchdog is not able to
interrupt the 24 V control voltage
for the stepper motor.
The CRC protection of the data
saved in the E2PROM is not
correct.
Not used in the multiFiltrate. Not used in the multiFiltrate.
Immediately before the multiFiltrate self-test, the operating processor
will query the multiDataLink version identifications. A response given by
the mDL is an indicator for its operability. In the event of an error,
messages are emitted. If the multiDataLink is not installed, not
connected to the power supply or deactivated via Service option, no
error message will be displayed.
Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
Acknowledge with
[START/RESET] key.
Heparin pump deactivated.
Error message Causes Action required
multiDataLink
multiDataLink not standby
No communication
multiDataLink
multiDataLink optionally
deactivated in Service mode
multiDataLink does not respond. Acknowledge with
[START/RESET] key.
multiDataLink deactivated.
multiDataLink manually
deactivated in the Service program.
Acknowledge with
[START/RESET] key.
multiDataLink deactivated.
Activate multiDataLink in the
Service program.
2.4.13 Ci-Ca Module (Option)
The test for the Ci-Ca module can not be enabled if the processor test
of the module and the multiFiltrate was not passed successfully. If these
processor tests are performed successfully, the T1 test will be started
automatically as soon as the operator has confirmed that the starting
conditions are met.
The following functions of the Ci-Ca module are tested:
2-18 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 29
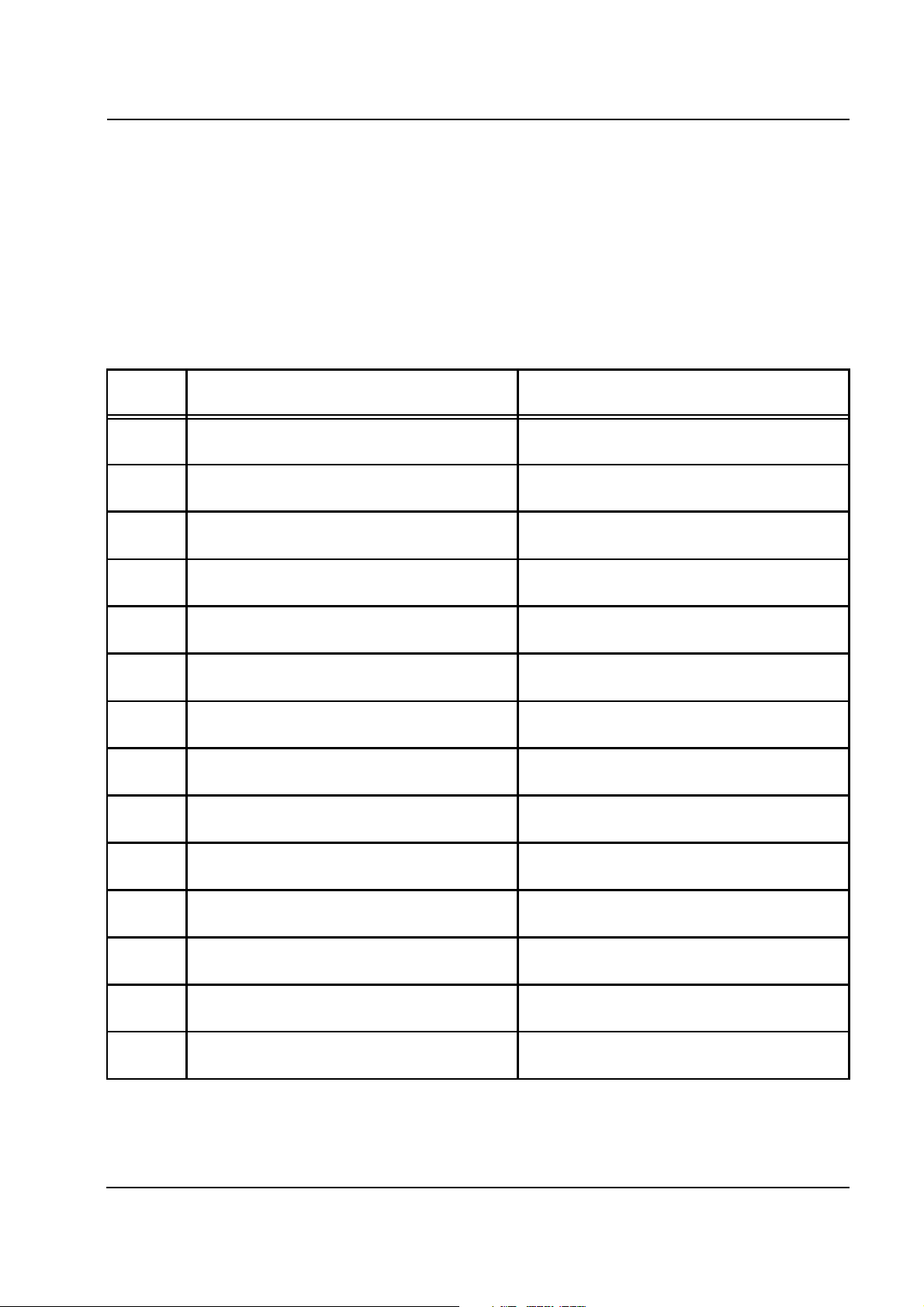
Chapter 2: Functional Description
Stop of the citrate and the calcium pump by the module's operating
processor
Stop of the citrate and the calcium pump by the system's watchdog.
Function of the citrate pump and the insertion switch
Function of the calcium pump and the insertion switch
If a test could not be passed successfully, a warning indicating an error
number appears on the multiFiltrate display. The test can be repeated
any number of times, however, it cannot be skipped.
The T1 test of the multiFiltrate system is performed simultaneously to
the T1 test of the Ci-Ca module.
Error
number
281 Lacking Ci Hall impulse after test was started
282 Ci Hall impulse too early during pump stop test
283 Ci Hall impulse too late during pump stop test
285 Ci Hall impulse too early during pump stop test
286 Ci Hall impulse too late during pump stop test
288 Ci Hall impulse too early during insertion switch
289 Ci Hall impulse too late during insertion switch
291 Lacking Ca Hall impulse after test was started
292 Ca Hall impulse too early during pump stop test
Description Possible cause
(>5 s).
(<7 s).
(>9 s).
(<7 s).
(>9 s).
test (<7 s).
test (>9 s).
(>5 s).
(<7 s).
The rotor of the citrate pump is loose, jammed or
blocked.
Insertion switch pressed --> citrate line already
inserted.
The rotor of the citrate pump is loose, jammed or
blocked.
Insertion switch pressed --> citrate line already
inserted during the test.
The rotor of the citrate pump is loose, jammed or
blocked.
Insertion switch pressed --> citrate line already
inserted during the test.
The rotor of the citrate pump is loose, jammed or
blocked.
The rotor of the Ca pump is loose, jammed or
blocked.
Insertion switch pressed --> calcium line already
inserted.
293 Ca Hall impulse too late during pump stop test
(>9 s).
295 Ca Hall impulse too early during pump stop test
(<7 s).
296 Ca Hall impulse too late during pump stop test
(>9 s).
298 Ca Hall impulse too early during insertion switch
test (<7 s).
299 Ca Hall impulse too late during insertion switch
test (>9 s).
Fresenius Medical Care multiFiltrate TM 6/03.07 2-19
The rotor of the Ca pump is loose, jammed or
blocked.
Insertion switch pressed --> calcium line already
inserted during the test.
The rotor of the Ca pump is loose, jammed or
blocked.
Insertion switch pressed --> calcium line already
inserted during the test.
The rotor of the Ca pump is loose, jammed or
blocked.
Page 30
Chapter 2: Functional Description
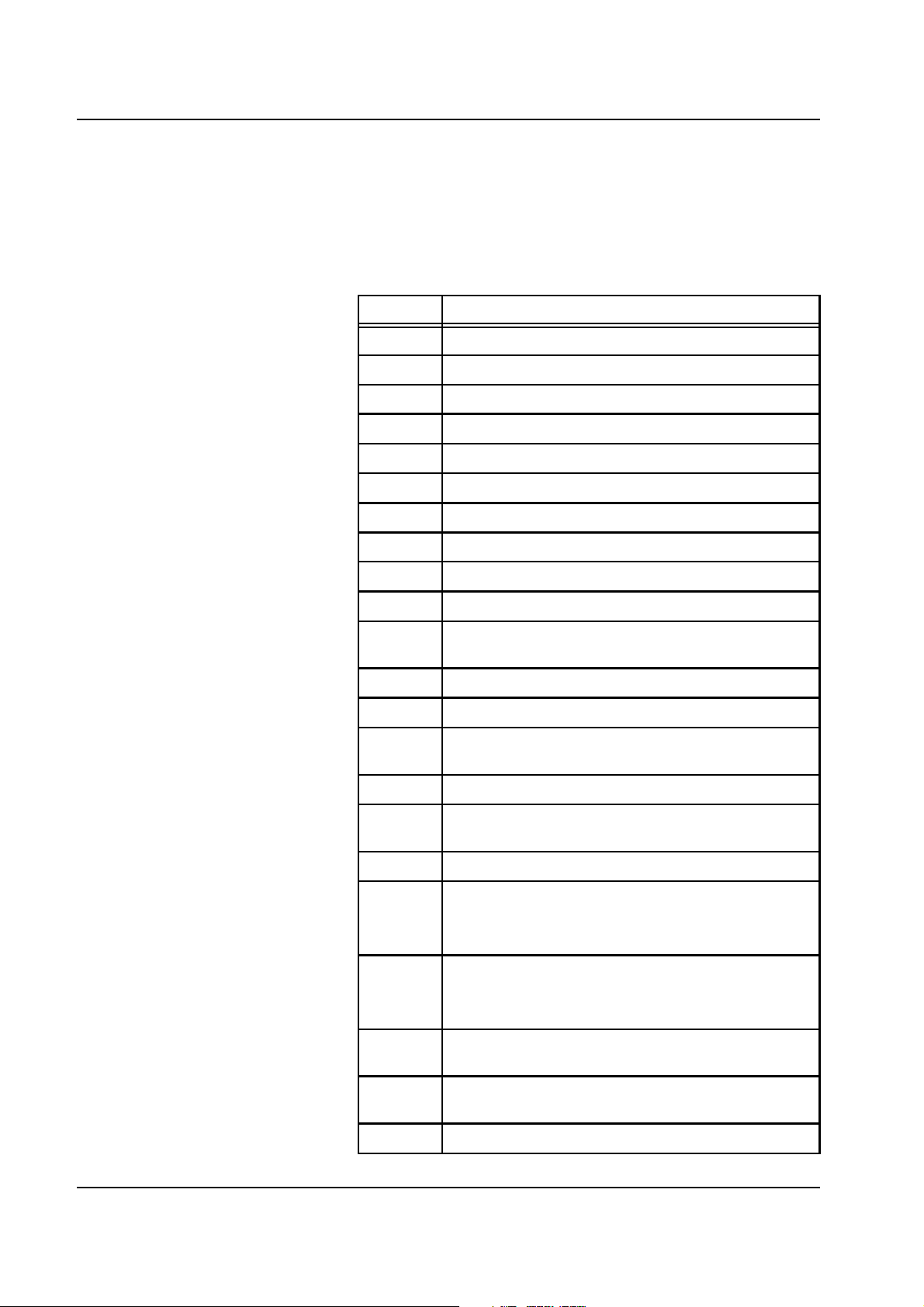
2.5 Error Messages
2.5.1 Alarm Messages
These messages cause all pumps to stop
Code Description
E10 Blood pump: Blood pump door open
E11 Blood pump: Stop
E12 Blood pump: Wrong speed, rate
E13 Arterial pressure, arterial pressure too low
E14 Arterial pressure, arterial pressure too high
E15 Venous pressure, venous pressure too low
E16 Venous pressure, venous pressure too high
E17 TMP, TMP too low
E18 TMP, TMP too high
E19 System stopped, Stop key has been pressed
E20 Pre-filter pressure/ Pre-filter pressure too low (for
hemoperfusion)
E21 Pre-filter pressure, pre-filter pressure too low
E22 Pre-filter pressure, pre-filter pressure too high
E23 Non-opaque/opaque fluid detector, venous detector
senses non-opaque or opaque fluid
E24 Air detector, air and/or microbubbles
E25 Blood leak detector, blood leak, hemolysis, membrane
rupture
E26 Blood leak detector, 2 min override active
E27 Power failure, emergency operation available for max.
15 min.
Converted by OP from SP code 130.
E28 End of emergency operation, the unit will turn itself off
automatically after 5 minutes.
Converted by OP from SP code 189.
E29 Pre-filter pressure/ Pre-filter pressure too high (for
hemoperfusion)
E30 Blood lines: Blood line jammed / Hydrophobic filter
P_pHF wet
E31 Blood lines: Rotor broken
2-20 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 31
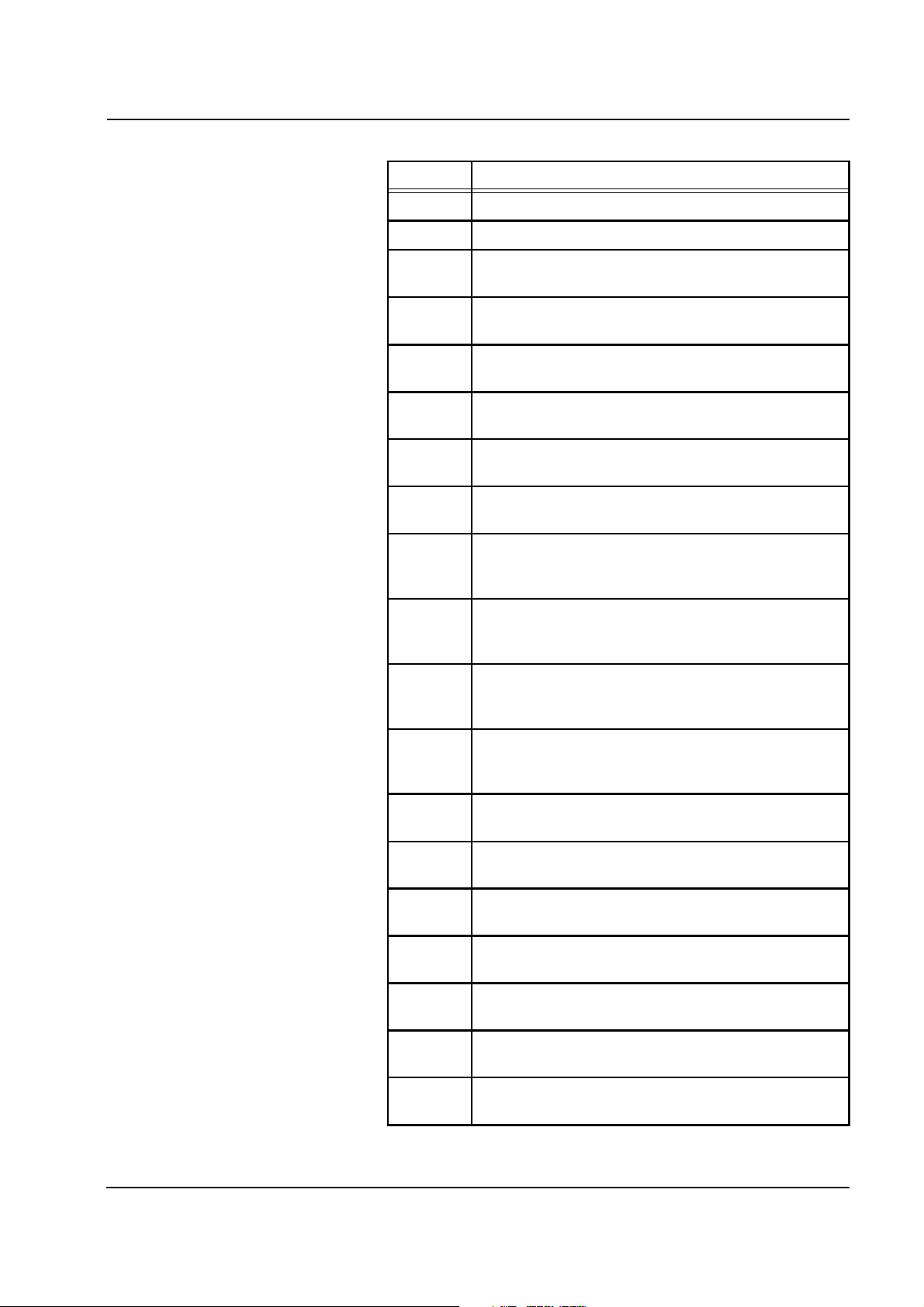
Code Description
Chapter 2: Functional Description
E32 Blood lines: Hydrophobic filter P
E33 Blood lines: Hydrophobic filter P
art
ven
wet
wet
E34 Air detector: Transmitting voltage on the air detector too
high
E35 Ci-Ca module: Citrate pump turning too slowly (volume
monitoring)
E36 Ci-Ca module: Citrate pump turning too fast (volume
monitoring)
E37 Ci-Ca module: Calcium pump turning too slowly
(volume monitoring)
E38 Ci-Ca module: Calcium pump turning too fast (volume
monitoring)
E39 Ci-Ca module: Dialysis is started although the primed
Ci/Ca lines are not free from air
E40 Ci-Ca module: Drop counter citrate insufficient
number of drops; check citrate bag, drip chamber and
tubing system!
E41 Ci-Ca module: Number of drops counted by citrate drop
counter too high; check citrate drip chamber and tubing
system!
E42 Ci-Ca module: Drop counter calcium insufficient
number of drops; check calcium bag, drip chamber and
tubing system!
E43 Ci-Ca module: Number of drops counted by calcium
drop counter too high; check calcium drip chamber and
tubing system!
E44 Ci-Ca module: Citrate pump turning too slowly; (motor
steps)
E45 Ci-Ca module: Citrate pump turning too fast; (motor
steps)
E46 Ci-Ca module: Calcium pump turning too slowly (motor
steps)
E47 Ci-Ca module: Calcium pump turning too fast (motor
steps)
E48 Ci-Ca module: Citrate insertion switch without
contact,Ci pump segment inserted properly?
E49 Ci-Ca module: Calcium insertion switch without
contact,Ca pump segment inserted properly?
E50 Ci-Ca module: The citrate bag change takes more than
2 min; finish bag change!
Fresenius Medical Care multiFiltrate TM 6/03.07 2-21
Page 32
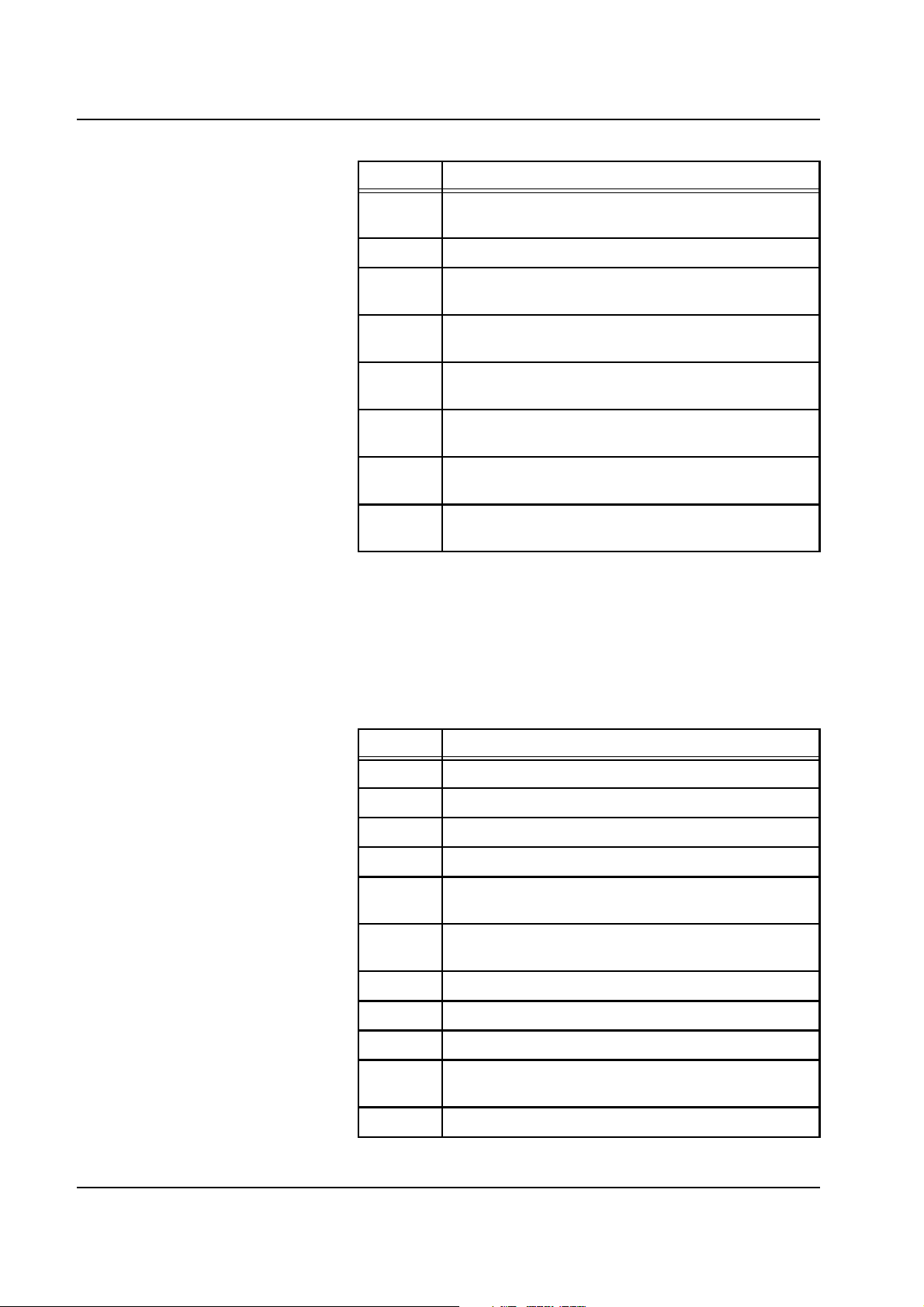
Chapter 2: Functional Description
Code Description
E51 Ci-Ca module: The Ca bag change takes more than
2 min; finish bag change!
E52-54 Not used
E55 Ci-Ca module: MPS treatment is permitted for a
maximum of 4 hours!; initiate reinfusion!
E56 Ci-Ca module: HP treatment is permitted for a
maximum of 4 hours!; initiate reinfusion!
E57 Ci-Ca module: Balance switched off; Ca supply
interrupted!
E58 Ci-Ca module: System error of Ci-Ca module:
Anitcoagulation with citrate completed!
E59 Ci-Ca module: Drop counter citrate insufficient
number of drops; Ci bag change required?
E60 Ci-Ca module: Drop counter calcium insufficient
number of drops; Ca bag change required?
2.5.2 Warning Messages
These messages cause the balancing to stop. The blood circuit will be
maintained.
For error messages no. 132 through 144 the scales must be
recalibrated.
Code Description
W62 Blood pump: Blood pump door open
W63 Filtrate pump: Cover open
W64 Sub pump: Cover open
W65 Dialysate pump: Cover open
W66 Prime mode: No weight loss on sub-scale-post
(HVCVVH)
W67 Prime mode: No weight loss on sub-scale-pre
(HVCVVH)
W68 Bag change: Overload on sub-scale-post (HVCVVH)
W69 Bag change: Sub-scale post underload (HVCVVH)
W70 Opt. detector: is opaque
W71 Opt. detector: Fails to sense opaque fluid after
attenuation
W72 Scale 1: Temp. coeff. factors missing, incorrect
2-22 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 33
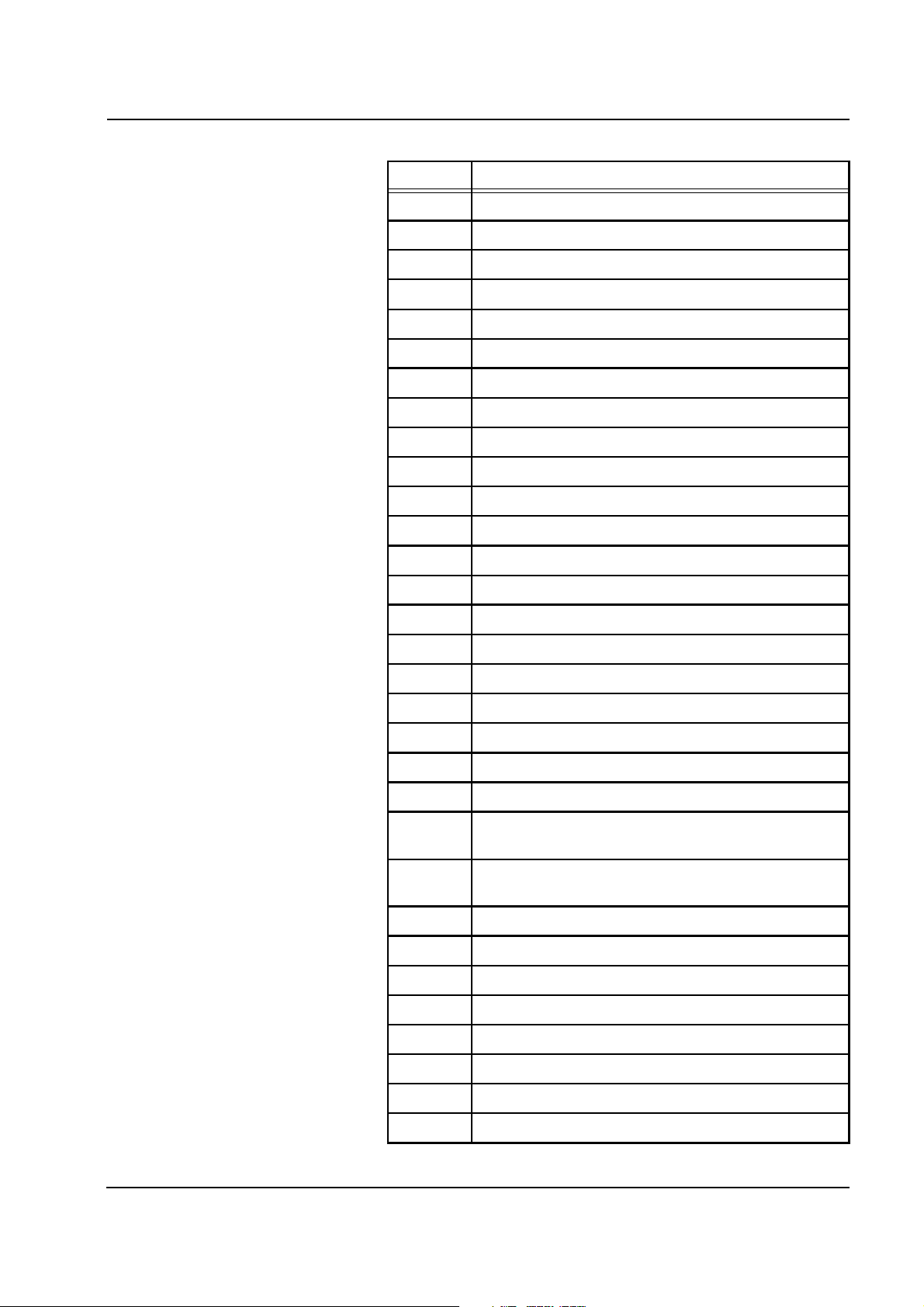
Chapter 2: Functional Description
Code Description
W73 Scale 2: Temp. coeff. factors missing, incorrect
W74 Scale 3: Temp. coeff. factors missing, incorrect
W75 Scale 4: Temp. coeff. factors missing, incorrect
W76 Scale 1: Calibration factor missing, incorrect
W77 Scale 2: Calibration factor missing, incorrect
W78 Scale 3: Calibration factor missing, incorrect
W79 Scale 4: Calibration factor missing, incorrect
W80 Scale 1: Movement detected on Scale 1
W81 Scale 2: Movement detected on Scale 2
W82 Scale 3: Movement detected on Scale 3
W83 Scale 4: Movement detected on Scale 4
W84 Scale 1: Test weight outside tolerance
W85 Scale 2: Test weight outside tolerance
W86 Scale 3: Test weight outside tolerance
W87 Scale 4: Test weight outside tolerance
W88 Scale 1: Test weight fails to drop back
W89 Scale 2: Test weight fails to drop back
W90 Scale 3: Test weight fails to drop back
W91 Scale 4: Test weight fails to drop back
W92 Air detector: LDA1 not in alarm mode
W93 Air detector: Clamp fails to close
W94 Lower heater: Switch-off path SP defective (Sub-relay
not open)
W95 Upper heater: Switch-off path SP defective (dial. relay
not open)
W96 Lower heater: Control FET short-circuit
W97 Upper heater: Control FET short-circuit
W98 Lower heater: Bag sensor broken
W99 Lower heater: Foil sensor broken
W100 Upper heater: Bag sensor broken
W101 Upper heater: Foil sensor broken
W102 Lower heater: Bag sensor short-circuit
W103 Lower heater: Foil sensor short-circuit
Fresenius Medical Care multiFiltrate TM 6/03.07 2-23
Page 34
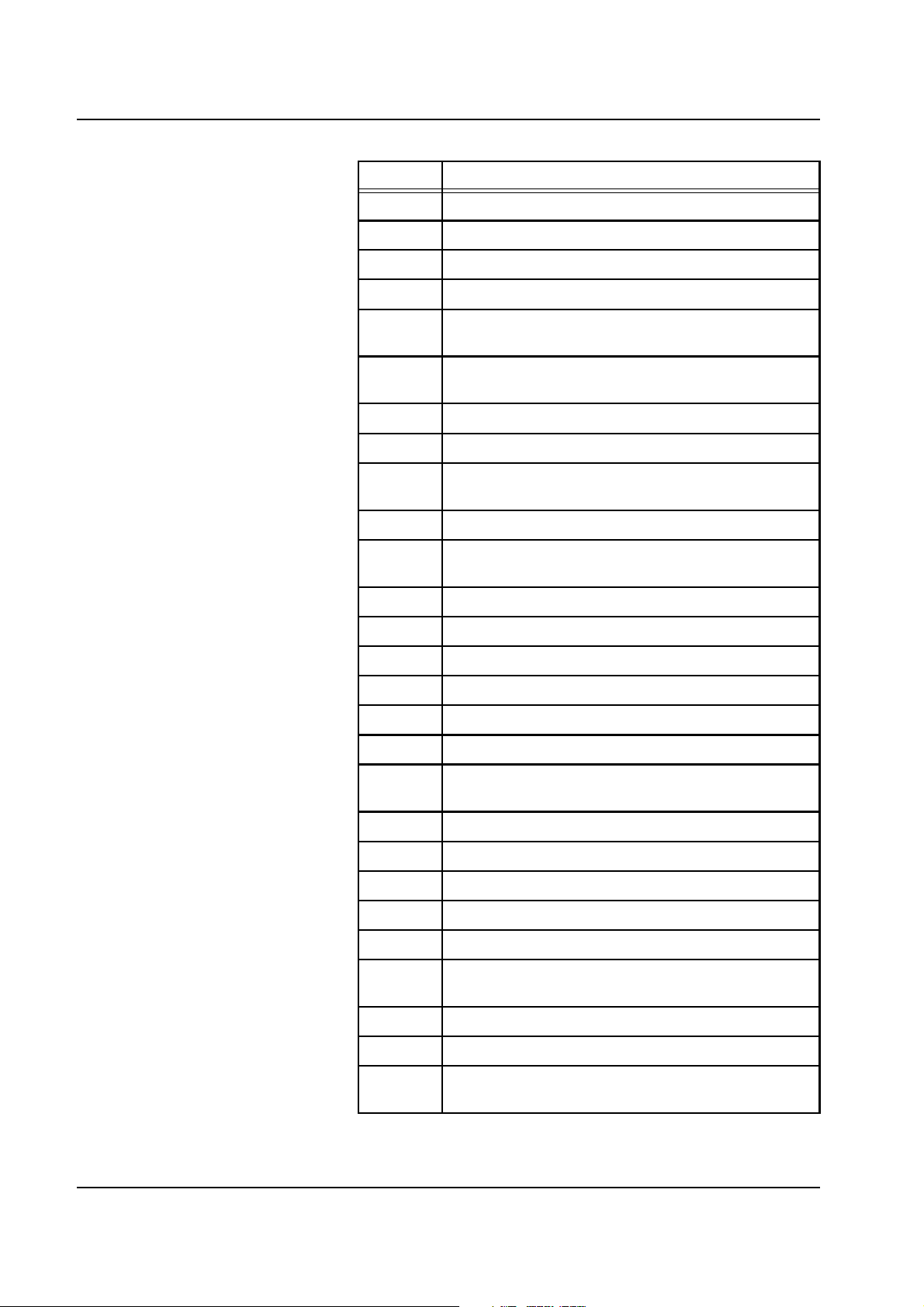
Chapter 2: Functional Description
Code Description
W104 Upper heater: Bag sensor short-circuit
W105 Upper heater: Foil sensor short-circuit
W106 Lower heater: Sub. relay not closed, foil defective
W107 Upper heater: Dial. relay not closed, foil defective
W108 Lower heater: Foil sensor detuning, no over-
temperature cutoff
W109 Upper heater: Foil sensor detuning, no over-
temperature cutoff
W110 Lower heater: Bag sensors, OP-SP not synchronous
W111 Upper heater: Bag sensors, OP-SP not synchronous
W112 Lower heater: Bag sensor detuning, no
overtemperature
W113 Lower heater: Foil sensor detuning, no overtemperature
W114 Upper heater: Bag sensor detuning, no
overtemperature
W115 Upper heater: Foil sensor detuning, no overtemperature
W116 Lower heater: FET control defective
W117 Upper heater: FET control defective
W118 Battery test: Battery not connected, defective
W119 Battery test: Load test failed
W120 Battery test: Insufficient capacity
W121 Blood leak detector: Outside acceptable range, remove
filtrate line
W122 Blood leak detector: Alarm-free after attenuation
W123 Prime mode: No weight loss on SUB scale
W124 Prime mode: No weight loss on DIA scale
W125 Prime mode: No weight loss on SUB scale (CVVHDF)
W126 Prime mode: No weight loss on DIA scale (CVVHDF)
W127 Blood leak detector: Calibration values missing,
incorrect
W128 Audible alarm: Not silenced
W129 Audible alarm: not active
W130 Power failure: Emergency operation available for max.
15 minutes (OP generates code 27)
2-24 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 35
Chapter 2: Functional Description
Code Description
W131 Incorrect allocation of scales, repeat Preparation (very
unusual)
W132 Load on scale, incorrect load on scale I (initial weight)
W133 Load on scale, incorrect load on scale II (initial weight)
W134 Load on scale, incorrect load on scale III (initial weight)
W135 Load on scale, incorrect load on scale IV (initial weight)
W136 Balancing, Scale I, leakage from tubing system, objects
W137 Balancing, Scale II, leakage from tubing system, objects
W138 Balancing, upper scales, leakage from tubing system,
objects
W139 Balancing, filtrate bag full, replace or empty filtrate bag
W140 Balancing, filtrate scales moved, leakage from tubing
system
W141 Scale 1, overload or underload, check load, scale
jammed?
W142 Scale 2, overload or underload, check load, scale
jammed?
W143 Scale 3, overload or underload, check load, scale
jammed?
W144 Scale 4, overload or underload, check load, scale
jammed?
W145 Bag change: Overload on SUB scale (CVVHDF)
W146 Bag change: Overload on SUB scale 1 (CVVH, HF)
W147 Bag change: Overload on SUB scale 2 (CVVH, HF)
W148 Bag change: SUB on wrong scale (CVVHDF)
W149 Bag change: Insufficient substituate (CVVH, HF)
W150 Bag change: Insufficient substituate (CVVHDF)
W151 Bag change: Insufficient saline solution on scale 1
(MPS)
W152 Bag change: Overload on plasma scale 1 (MPS)
W153 Bag change: Overload on plasma scale 2 (MPS)
W154 Bag change: Overload on dialysate scale (CVVHDF)
W155 Bag change: Overload on dialysate scale 1 (CVVHD)
W156 Bag change: Overload on dialysate scale 2 (CVVHD)
W157 Dialysate on wrong scale (tubing arrangement test after
bag change HDF)
Fresenius Medical Care multiFiltrate TM 6/03.07 2-25
Page 36
Chapter 2: Functional Description
Code Description
W158 Bag change: Insufficient dialysate (CVVHD)
W159 Bag change: Insufficient dialysate (CVVHDF)
W160 Bag change: Overload on filtrate scale
W161 TMP/MPS TMP >100mmHg
W162 Lower heater: MPS temperature > 37°C
W163 Upper heater: MPS temperature > 37°C
W164 Lower heater: Temperature too high
W165 Upper heater: Temperature too high
W166 Bag change: Overload SUB pre (HVCVVH)
W167 Bag change: Insufficient SUB pre (HVCVVH)
W168 Balancing: Ultrafiltration or substitution rate too high
W169 Lower heater: Foil sensors, OP-SP not synchronous
W170 Upper heater: Foil sensors, OP-SP not synchronous
W171 Balancing: Aborted because maximum allowable
deviation of 500g was exceeded
W172 Lower heater: MPS foil temperature too high, risk of
hemolysis
W173 Upper heater: MPS foil temperature too high, risk of
hemolysis
W174 Balancing: Balancing stopped, balancing was switched
off
W175 Battery capacity: Charging voltage too high, battery
deactivated, no power failure
W176 Filtrate scale: Test weight outside tolerance (T0 test)
W177 Filtrate scale: Test weight fails to drop back (T0 test)
W178 Balancing: UF goal reached, ultrafiltration was set to 0
W179 Balancing: Substituate bag empty, replace substituate
bag
W180 Balancing: Dialysate bag empty, replace dialysate bag
W181 Balancing: Movement detected on filtrate scales,
leakage from tubing system
W182 Filtrate scale: Overload or underload, check load, scale
jammed?
W183 Balancing: Plasma bag empty, open outlet line(s) on
plasma bags
W184 Balancing: Plasma pump delivers from wrong scale (I)
2-26 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 37
Chapter 2: Functional Description
Code Description
W185 Balancing: Plasma pump delivers from wrong scale (II)
W186 Balancing: Sub-postdilution bag empty, replace
substituate bag
W187 Balancing: Sub-predilution bag empty, replace
substituate bag
W188 End of treatment: Plasma infused (MPS only)
W189 End of emergency operation: The unit will turn itself off
automatically after 5 minutes
W190 Power failure end
W191 Bag change: Insufficient plasma on scale 2
W192 Prime mode: Overload on dialysate scale (CVVHDF)
W193 Prime mode: Overload on SUB scale (CVVHDF)
W194 Prime mode: Insufficient substituate (CVVHDF)
W195 Prime mode: Insufficient dialysate (CVVHDF)
W196 Prime mode: Overload on sub-scale-pre (HVCVVH)
W197 Prime mode: Overload on sub-scale-post (HVCVVH)
W198 Prime mode: Insufficient SUB pre (HVCVVH)
W199 Prime mode: Insufficient SUB post (HVCVVH)
W200 Blood pump: Not stopped by operating processor
W201 Filtrate pump: Not stopped by operating processor
W202 Sub pump: Not stopped by operating processor
W203 Dialysate pump: Not stopped by operating processor
W204 Blood pump: Wrong speed, rate
W205 Filtrate pump: Wrong speed, rate
W206 Sub pump: Wrong speed, rate
W207 Dialysate pump: Wrong speed, rate
W208 Blood pump: Reed contact line threading position
W209 Filtrate pump: Reed contact line threading position
W210 Sub pump: Reed contact line threading position
W211 Dialysate pump: Reed contact line threading position
W212 Blood pump: Stop
W213 Filtrate pump: Stop
W214 Sub pump: Stop
Fresenius Medical Care multiFiltrate TM 6/03.07 2-27
Page 38
Chapter 2: Functional Description
Code Description
W215 Dialysate pump: Stop
W216 Blood pump: Not stopped by safety processor
W217 Filtrate pump: Not stopped by safety processor
W218 Sub pump: Not stopped by safety processor
W219 Dialysate pump: Not stopped by safety processor
W220 Arterial pressure: Factors / offset missing, incorrect
W221 Venous pressure: Factors / offset missing, incorrect
W222 PHF pressure: Factors / offset missing, incorrect
W223 Filtrate pressure: Factors / offset missing, incorrect
W224 Arterial pressure: Zero outside tolerance
W225 Venous pressure: Zero outside tolerance
W226 PHF pressure: Zero outside tolerance
W227 Filtrate pressure: Zero outside tolerance
W228 Arterial pressure: Detuning outside tolerance
W229 Venous pressure: Detuning outside tolerance
W230 PHF pressure: Detuning outside tolerance
W231 Filtrate pressure: Detuning outside tolerance
W232 Heparin pump: does not respond
W233 Heparin pump: manually deactivated in Service
program
W234 Heparin pump: detects an internal problem
W235 Heparin pump: Wrong/invalid syringe type
W236 Heparin pump: No anticoagulation
W237 Heparin pump: Anticoagulation stopped
W238 Heparin pump: Anticoagulant Used Up
W239 Heparin pump: Syringe not changed
W240 Heparin pump: Non-registered error code
W241 Heparin pump: Gate-array error
W242 Heparin pump: Restart after spike reset
W243 Heparin pump: Hex switch position error
W244 Heparin pump: Powerdown without 24V cutoff
W245 Heparin pump: Slotted disc error
2-28 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 39
Chapter 2: Functional Description
Code Description
W246 Heparin pump: Slotted disc not found
W247 Heparin pump: Communication sumcheck error
W248 Heparin pump: No heparin pump STOP
W249 Heparin pump: Communication error with OP
W250 Heparin pump: Watchdog error
W251 Heparin pump: NOVRAM error
W252 CAMUS transmission error
W253 Not used
W254 Not used
W255 mDL not ready/no communication
W256 mDL deactivated via Service option
W257 The BP rate is < 30 ml/min; no rotor breakage
monitoring; closure of pressure transducer is not
detected.
W259 Lower heater: Switch-off path SP defective (Sub-relay
not open), voltage not present, SI defective (for P.C.B.
LP122-B)
W260 Upper heater: Switch-off path SP defective (dial. relay
not open), voltage not present, SI defective (for P.C.B.
LP122-B)
W261 Lower heater: Control FET short circuit (for P.C.B.
LP122-B)
W262 Upper heater: Control FET short circuit (for P.C.B.
LP122-B)
W263 Air detector: Transmitting voltage for air detector not
present / nonconforming (for AD28)
W264 Ci-Ca module: Ci-Ca module does not respond, select
alternate anticoagulation if necessary!
W265 Ci-Ca module: Switch-off path "sleep" does not react,
select alternate anticoagulation if necessary!
W266 Balancing: HF bag change aborted
W281 Ci-Ca module: Citrate pump - test F1, Rotor slack or
stuck?
W282 Ci-Ca module: Citrate pump - OP test F2, rotor slack?
W283 Ci-Ca module: Citrate pump - OP test F3, rotor slack or
stuck?
W285 Ci-Ca module: Citrate pump - WD test F5, rotor slack?
Fresenius Medical Care multiFiltrate TM 6/03.07 2-29
Page 40
Chapter 2: Functional Description
Code Description
W286 Ci-Ca module: Citrate pump - WD test F6, rotor slack or
stuck?
W288 Ci-Ca module: Citrate pump - insertion switch test F8,
line pump segment inserted too early or rotor slack?
W289 Ci-Ca module: Citrate pump - insertion switch test F9,
rotor slack or stuck?
W291 Ci-Ca module: Calcium pump test F11, rotor slack or
stuck?
W292 Ci-Ca module: Calcium pump - OP test F12, rotor
slack?
W293 Ci-Ca module: Calcium pump - OP test F13, rotor slack
or stuck?
W295 Ci-Ca module: Calcium pump - WD test F15, rotor
slack?
W296 Ci-Ca module: Calcium pump - WD test F16, rotor slack
or stuck?
2.5.3 Fatal Errors
W298 Ci-Ca module: Calcium pump - insertion switch test
F18, line pump segment inserted too early or rotor
slack?
W299 Ci-Ca module: Calcium pump - insertion switch test
F19, rotor slack or stuck?
W330 Ci-Ca module: Balance switched off!, Ca supply
interrupted!
W331 Ci-Ca module: Filtrate rate too low for set Ca dose!,
Increase dialysate or substituate or Ca dose if
necessary!
W332 Ci-Ca module: Filtrate rate too high for set Ca dose!,
Reduce dialysate or substituate or Ca dose if
necessary!
W333 Ci-Ca module: Blood flow too low for set citrate dose!,
Increase blood flow or citrate dose if necessary!
W334 Ci-Ca module: Blood flow too high for set citrate dose!,
Reduce blood flow or citrate dose if necessary!
These messages cause the treatment to stop.
2-30 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 41
Chapter 2: Functional Description
Code Description
E500 Scale 1: (factors missing during treatment)
E501 Scale 2: (factors missing during treatment)
E502 Scale 3: (factors missing during treatment)
E503 Scale 4: (factors missing during treatment)
E504 Memory error: (double values incorrect)
E505 Blood pump: (does not respond)
E506 Substituate pump: (does not respond)
E507 Dialysate pump: (does not respond)
E508 Filtrate pump: (does not respond)
E509 Overvoltage +5V
E510 Overvoltage +12V
E511 Processor test OP: CPU defective,
E512 Processor test OP: Incorrect CRC interrupt vectors
E513 Processor test OP: CRC CRC area defective
E514 Processor test OP: CRC Code area defective
E515 Processor test OP: RAM, bank B defective
E516 Processor test OP: RAM, bank 8 defective
E517 State transition
E518 Program sequence
E519 Self-Test
E520 Balancing
E521 SP does not respond
E522 DP does not respond
E523 Program flow OP: Watchdog
E524 Processor test OP: Watchdog does not respond
E525 Processor test SP: CPU
E526 Processor test SP: Program memory vectors
E527 Processor test SP: Program memory - CRC area
E528 Processor test SP: Program memory - code area
E529 Processor test SP: RAM - bank B
E530 Processor test SP: RAM - bank B
E531 Processor test SP: Watchdog does not respond
Fresenius Medical Care multiFiltrate TM 6/03.07 2-31
Page 42
Chapter 2: Functional Description
Code Description
E532 Invalid software combination: OP, DP, SP
E533 Ci-Ca module: Logoff of the option by the MFT
E534 Ci-Ca module: Alarm of the option not followed by MFT
alarm
E535 Ci-Ca module: Timeout of the status message of the
MFT (>2.5 s)
E536 Ci-Ca module: Too many status messages of the MFT
(2x<0.5 s)
E537 Ci-Ca module: Receipt of message not confirmed
E538 Ci-Ca module: unknown identifier
E539 Ci-Ca module: Write buffer full
E540 Ci-Ca module: Read buffer full
E541 Ci-Ca module: Unable to write on TXe
E542 Ci-Ca module: Missing setup values from the MFT (at
end of line installation)
E550 Ci-Ca module: Too many extra Hall pulses from the Ca
pump
E551 Ci-Ca module: Ca pump could not be stopped
E560 Ci-Ca module: Too many extra Hall pulses from the
citrate pump
E561 Ci-Ca module: Citrate pump could not be stopped
E570 Ci-Ca module: Error on checking variable replication
(Int)
E571 Ci-Ca module: Error on checking variable replication
(Float)
E572 Ci-Ca module: Error on checking variable replication
(Long)
E573 Ci-Ca module: Error on checking software diversity
2-32 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 43
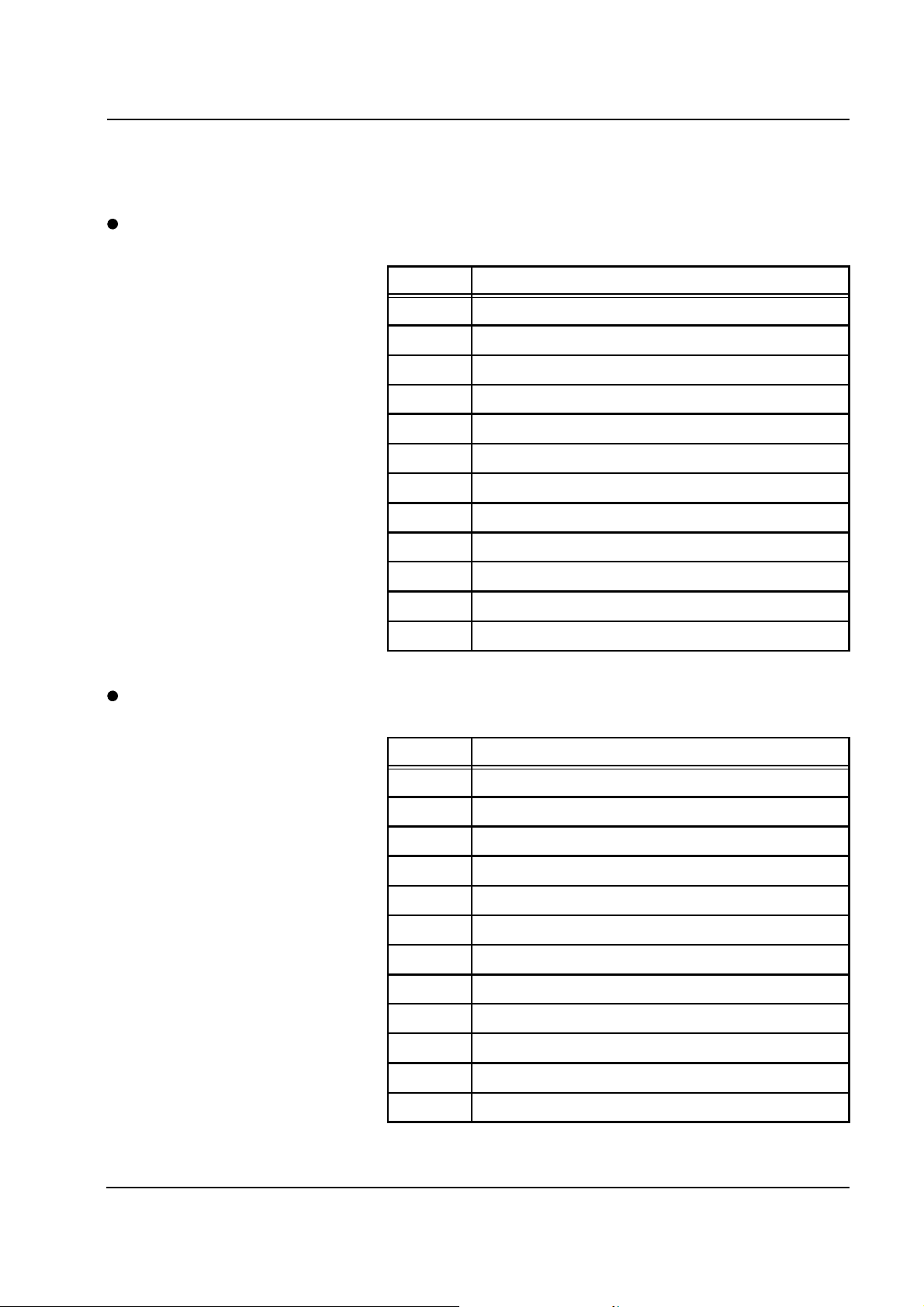
2.5.4 Error Codes of Scales Lo-Level Routines
Scale no. 1
Code Description
E30001 Standstill not possible (timeout after 10 sec)
E30002 Weight value out of range (overload or underload)
E30003 Incorrect calibration weight value
E30004 Negative initial weight
E30005 Wrong initial weight
E30006 ACT_WGT 65535
E30007 Alarm limits permanently violated
E30008 INF_WGT invalid
E30050 Calibration factor missing or incorrect
Chapter 2: Functional Description
Scale no. 2
E30051 Temp. coeff. factors missing or incorrect
E30052 Double fuse defective (zero value)
E30053 Temperature sensor missing / defective
Code Description
E30101 Standstill not possible (timeout after 10 sec)
E30102 Weight value out of range (overload or underload)
E30103 Incorrect calibration weight value
E30104 Negative initial weight
E30105 Wrong initial weight
E30106 ACT_WGT 65535
E30107 Alarm limits permanently violated
E30108 INF_WGT invalid
E30150 Calibration factor missing or incorrect
E30151 Temp. coeff. factors missing or incorrect
E30152 Double fuse defective (zero value)
E30153 Temperature sensor missing / defective
Fresenius Medical Care multiFiltrate TM 6/03.07 2-33
Page 44
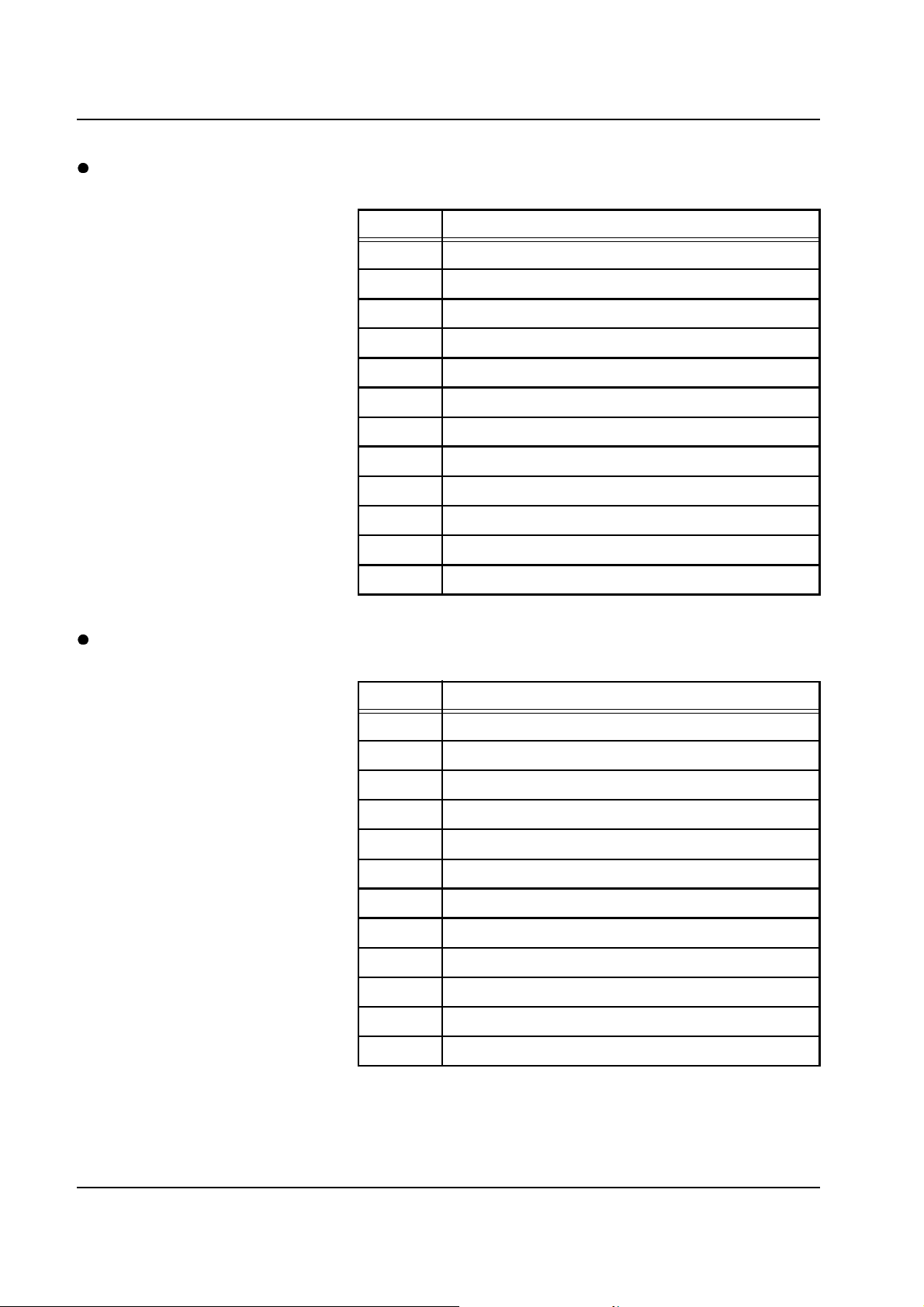
Chapter 2: Functional Description
Scale no. 3
Code Description
E30201 Standstill not possible (timeout after 10 sec)
E30202 Weight value out of range (overload or underload)
E30203 Incorrect calibration weight value
E30204 Negative initial weight
E30205 Wrong initial weight
E30206 ACT_WGT 65535
E30207 Alarm limits permanently violated
E30208 INF_WGT invalid
E30250 Calibration factor missing or incorrect
E30251 Temp. coeff. factors missing or incorrect
E30252 Double fuse defective (zero value)
Scale no. 4
E30253 Temperature sensor missing / defective
Code Description
E30301 Standstill not possible (timeout after 10 sec)
E30302 Weight value out of range (overload or underload)
E30303 Incorrect calibration weight value
E30304 Negative initial weight
E30305 Wrong initial weight
E30306 ACT_WGT 65535
E30307 Alarm limits permanently violated
E30308 INF_WGT invalid
E30350 Calibration factor missing or incorrect
E30351 Temp. coeff. factors missing or incorrect
E30352 Double fuse defective (zero value)
E30353 Temperature sensor missing / defective
2-34 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 45
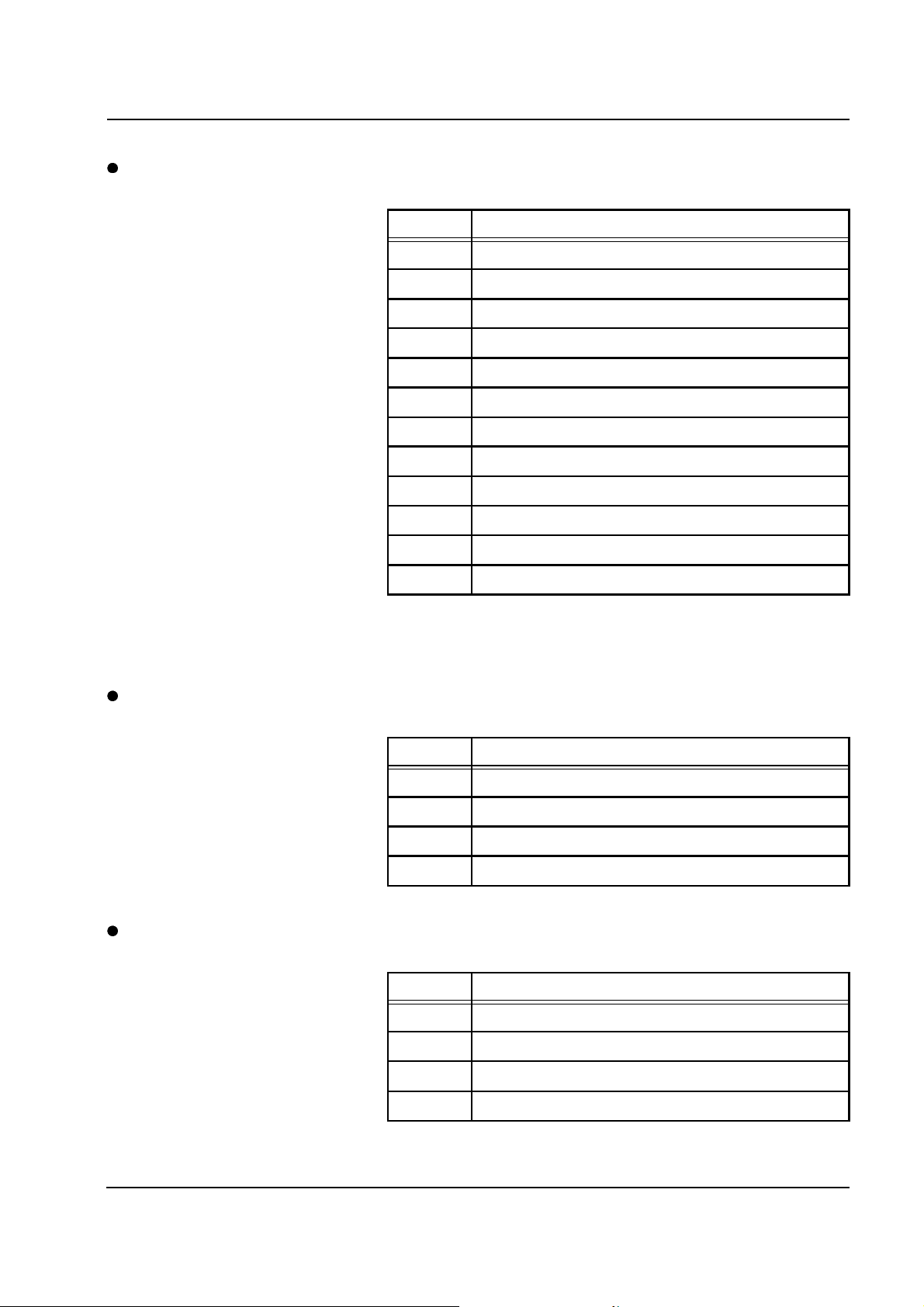
Scales nos. 3 and 4 (both)
Chapter 2: Functional Description
Code Description
E30401 Standstill not possible (timeout after 10 sec)
E30402 Weight value out of range (overload or underload)
E30403 Incorrect calibration weight value
E30404 Negative initial weight
E30405 Wrong initial weight
E30406 ACT_WGT 65535
E30407 Alarm limits permanently violated
E30408 INF_WGT invalid
E30450 Calibration factor missing or incorrect
E30451 Temp. coeff. factors missing or incorrect
E30452 Double fuse defective (zero value)
E30453 Temperature sensor missing / defective
2.5.5 Error Codes of Pressures Lo-Level Routines
PART
Code Description
E30551 Calibration factor missing, incorrect
E30552 Offset factor missing, incorrect
E30553 Offset > measurement value
E30557 DAC setting missing, incorrect
PPHF
Code Description
E30651 Calibration factor missing, incorrect
E30652 Offset factor missing, incorrect
E30653 Offset > measurement value
E30657 DAC setting missing, incorrect
Fresenius Medical Care multiFiltrate TM 6/03.07 2-35
Page 46

Chapter 2: Functional Description
PVEN
PFIL
Code Description
E30751 Calibration factor missing, incorrect
E30752 Offset factor missing, incorrect
E30753 Offset > measurement value
E30757 DAC setting missing, incorrect
Code Description
E30851 Calibration factor missing, incorrect
E30852 Offset factor missing, incorrect
E30853 Offset > measurement value
E30857 DAC setting missing, incorrect
2.5.6 Error Codes of Heparin Pump Lo-Level Routines
Code Description
E31000 BCC error
E31001 Handshake error to HP
E31002 Overflow sensitivity: buffer
E31003 Invalid control character
E31004 No end character received
E31005 HP string 3 answered with NAK
E31006 Wrong unknown command from HP
E31008 HP not ready
E31009 HP by option deactivated in Service mode
E31010 HP detects internal error
E31011 Wrong / unauthorized syringe type detected
E31012 HP failed to stop
E31013 Syringe empty
E31014 HP hardware error #1, GA error
2-36 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 47

Code Description
E31015 ditto #4, spike on reset line
E31016 ditto #5, wrong HEX switch position
E31017 ditto #8, power down
E31018 ditto #11, slotted disk error
E31019 ditto #55, slotted disk not found
E31020 Unknown hardware error
E31021 Internal HP communication error (group error)
2.5.7 Error Codes of Pumps Lo-Level Routines
Code Hex Description
E64256 0xFB00 Invalid tubing type
Chapter 2: Functional Description
E64767 0xFCFF No response from blood pump
E65023 0xFDFF ditto Filtrate pump
E65279 0xFEFF ditto Substituate pump
E65535 0xFFFF ditto Dialysate pump
2.6 Overview of Display Identification Numbers
Fresenius Medical Care multiFiltrate TM 6/03.07 2-37
Page 48

Chapter 2: Functional Description
A number is displayed in the lower left corner of the screen. This
number is the identification number. When an error occurs, this
numbers permits to identify the machine status.
No. Description
M1 Start-up screen
M2 Self-test window with fonts used
M3 Treatment modes, Selection: Previous/New treatment
M4 Treatment modes, Selection: Delete/Retain balance
data
M5 Treatment modes, Selection of treatment modes
M6 Tubing arrangement (self-test already complete)
M7 Tubing arrangement (self-test not yet complete)
M8 Empty self-test window
M9 Preparation for self-test, with confirmation button "All
conditions fulfilled? "OK" to confirm!"
M10 Preparation, Rinse, with confirmation button "Enter
treatment parameters? "OK" to confirm!"
M11 Preparation, Prime/Rinse tubing system, with
confirmation button "Start priming? "OK" to confirm!"
M12 Preparation, Prime tubing system
M13 Preparation, Treatment parameters menu, depending
on the type of therapy, the DP enters the allowed
parameter fields. With confirmation button All treatment
parameters entered? "OK" to confirm!"
M14 Preparation, Waiting for Patient
M15 Preparation, UF volume, with confirmation button Start
UF rinse? "OK" to confirm!"
M16 Preparation, with count-down UF volume display
M17 Preparation, Waiting for patient, with confirmation
button Start connection? "OK" to confirm!"
M18 Preparation, "Connect patient"
M19 Preparation, Connect patient, with confirmation button
"Start treatment? "OK" to confirm!"
M20 Treatment, Home page, depending on the type of
therapy, the DP enters the allowed parameter fields.
M21 Treatment, Treatment data menu, depending on the
type of therapy, the DP enters the allowed parameter
fields.
2-38 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 49

Chapter 2: Functional Description
No. Description
M22 Treatment, Treatment menu, depending on the type of
therapy, the DP enters the allowed parameter fields. All
treatments, except for HP, are identical.
M23 Treatment, Balance data, depending on the type of
therapy, the DP enters the allowed balance fields.
M24 Treatment, Alarm limits menu Identical for all treatment
modes, for HP, the TMP is renamed into pre-filter
pressure (pF)
M25 Heparin pump syringe change
M26-M28 not used
M29 Bag change
M30-M33 not used
M34 Preparation, Prime sub-pre-tubing system (HV-CVVH)
M35 Treatment, Balance data, Confirmation for Delete
balance data (Yes/No), rest like M23
M36-M39 not used
M40 End of treatment, Reinfusion volume, with confirmation
button "Start disconnection? "OK" to confirm!"
M41 End of treatment, Reinfusion volume, with confirmation
button "View treatment parameters? "OK" to confirm!".
The reinfusion volume counts down to "Zero"
M42 End of treatment, with 2 confirmation buttons "Continue
reinfusion / Terminate reinfusion"
M43 End of treatment, Removing tubing system, with
confirmation button "View treatment parameters? "OK"
to confirm!"
M44 End of treatment, View previous treatment parameters
=> Final screen.
M45 End of treatment, view previous treatment parameters
with confirmation button "Previous screen? "OK" to
confirm!"
M46 End of treatment, Reinfusion volume and remaining
time
M47 End of treatment (MPS, plasma return not completed)
M48 End of treatment, confirmation for 'Disconnect without
blood return'
M49 not used
M50 Preparation, Fill plasma
Fresenius Medical Care multiFiltrate TM 6/03.07 2-39
Page 50

Chapter 2: Functional Description
No. Description
M51 Preparation, Connect patient, with confirmation button
"Start connection? "OK" to confirm!"
M52-M59 not used
M60 System parameters, System parameters menu
M61 System parameters, Default treatment settings, with
confirmation button "All parameters entered? "OK" to
confirm!"
M62 System parameters, Program treatment modes, with
confirmation button "Desired treatment mode selected?
"OK" to confirm!"
M63 System parameters, Software versions, with
confirmation button "Terminate? "OK" to confirm!"
M64 System parameters, Ci-Ca settings, with confirmation
button "All parameters entered? "OK" to confirm!"
M65-M69 not used
M70 Service, Password entry
M71 Service, Calibration main menu
M72 Service, Calibrate/Tare scales
M73 Service, Select pressure
M74 Service, with confirmation button "Pressure sensor
open? "OK" to confirm!"
M75 Service, with pressure value and confirmation button
"Entered? "OK" to confirm!"
M76 Service, with confirmation button "Tubing inserted?
"OK" to confirm!"
M77 System parameters, Event memory, with confirmation
button "Terminate? "OK" to confirm!"
M78 Help menu for M72
M79 Service, Pressure, Weights, Temperatures, Voltages
M80 Service, language selection
M81 mDL parameter assignment
M82-M87 not used
M88 Preparation, Starting conditions Ci-Ca with confirmation
button "Return to treatment selection" and "Conditions
fulfilled"
M89 End of treatment, remove Ci-Ca lines with confirmation
button "Patient disconnected? Start removal of Ci/Ca
lines"
2-40 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 51

Chapter 2: Functional Description
No. Description
M90 Preparation, Ci-Ca anticoagulation with confirmation
button "On" and "Off"
M91 Preparation, with prompt "Please wait for completion of
functional test", after prompt was displayed
confirmation button "All conditions fulfilled? "OK" to
confirm!"
M92 Preparation, with note H108. After Z3 protocol the
confirmation button "Ci-Ca clamps opened? Ci-Ca drip
chambers filled? "OK" to confirm!"
M93 Preparation, with note H109. After Z3 protocol
confirmation buttons "Refill citrate", "Refill calcium" and
"Ci-Ca lines primed and free from air? "OK" to confirm!"
M95 Preparation, Deselect Ci-Ca anticoagulation with
confirmation button "No" and "Yes"
M96 Preparation, Deselection Ci-Ca, with note H105 with
confirmation button "All conditions fulfilled? "OK" to
confirm!"
M97 Treatment, Ci-Ca bag change menu, concentrations
and bag volumes displayed, after Z3 protocol 5
confirmation buttons for the Ci-Ca bag exchange
appear.
M98 Preparation, Deselection Ci-Ca, with note H107 with
confirmation button "All conditions fulfilled? "OK" to
confirm!"
Fresenius Medical Care multiFiltrate TM 6/03.07 2-41
Page 52

Chapter 2: Functional Description
2-42 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 53

3 Installation
3.1 Preface
Chapter 3: Installation
Instructions for all technicians who are authorized to commission
our systems.
We, as manufacturers, permanently aim at delivering systems of
highest quality.
To reach this aim, we need your support.
Please commission our systems uniformly using the enclosed "initial
start-up report" and enter the values determined in the columns
provided.
The following is applicable:
Corrections are necessary only if the measured values are outside
of the specified tolerances!
We will then evaluate the initial start-up reports, which will enable us to
monitor the quality of our systems on their delivery.
After initial start-up, please asap send by mail or by fax the
completed form (Initial Start-Up Report) back to the following address:
Thank you very much for your help!
Service
Central Europe
International
service
Fresenius Medical Care
Deutschland GmbH
Geschäftsbereich Zentraleuropa
Kundendienst / Servicecenter
Steinmühlstraße 24
61352 Bad Homburg
Germany
Phone: +49 6172 609-7100
Fax: +49 6172 609-7102
E-mail: ServicecenterD@fmc-ag.com
Responsible Regional Organization
(Technical Service)
Fresenius Medical Care multiFiltrate TM 6/03.07 3-1
Page 54

Chapter 3: Installation
3.2 Important Information on Initial Start-Up
For initial start-up only This technical document is intended for initial start-up only. It is not
intended for restarting systems that have been shut down or have been
put out of service temporarily.
Tester's qualification The initial start-up must be performed by the Technical Service of
Fresenius Medical Care or a person authorized by them.
The initial start-up procedure may only be performed by persons who
are qualified to properly perform the specified checks owing to their
educational background and training, their knowledge and experience
gained in practice. Furthermore, the persons performing the tests must
not be bound by any directives when performing this activity.
Test equipment and
accessories
Environmental conditions Variations in temperature during transport may lead to condensation
TSC/TMC/MA intervals The TSC/TMC/MA procedures for this system are to be performed after
The activities described in this technical document require the
availability of the necessary technical test equipment and accessories.
Any information on the specifications must be observed.
water developing on live parts. In the event of major variations in
temperature, allow sufficient time for the system to adjust to the ambient
temperature before start-up.
12 months.
3-2 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 55

Chapter 3: Installation
3.3 Initial Start-Up Report multiFiltrate
Technician's name: System type including option(s) / software version:
Customer/customer no.: System no.: Inventory no.:
Service report number: Operating hours: Equipment code:
No. Description Meas.
value
1 Unpacking
1.1 Unpack the system.
1.2 Check that all parts of the system have been accounted for.
1.3 System without visible shipping damage.
2 Preparing for operation
2.1 Place the scales trays on the scales plates.
2.2 Install the IV pole.
2.3 Connect the power cable and secure it to prevent improper replacement.
3 Visual inspections
3.1 Fuses accessible from the outside comply with the indicated values.
3.2 Labels and labeling are present and legible.
3.3 The mechanical condition permits further safe use.
3.4 There are no signs of damage or dirt.
3.5 The power cable shows no signs of damage.
4 Functional check
4.1 Pump checked for stopping with open door.
Blood pump
Filtrate pump
Substituate pump
Dialysate pump
4.2 The venous occlusion clamp closes after an air detector alarm.
Fresenius Medical Care multiFiltrate TM 6/03.07 3-3
Page 56

Chapter 3: Installation
No. Description Meas.
value
4.3 The pressure of 2 bar applied in the venous bubble catcher may not drop by more
than 0.1 bar within 3 minutes.
4.4 Air detector operation/calibration checked.
Jumper J1-P.C.B. LP 450 set to the operation position. Place the checking block into
the air detector. LEDs DI 5 and DI 10 are light.
Jumper J1-P.C.B. LP 450 set to the operation position. Place the adjusting block
into the air detector. LEDs DI 5 and DI 10 are dark.
4.5 Pressure transducers: Zero and gain checked
Arterial pressure (red)
Zero point: Display within the range of ± 5mmHg
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
Venous pressure (blue)
Zero point: Display within the range of ± 5mmHg
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
PHF pressure (white)
Zero point: Display within the range of ± 5mmHg
________
________
________
________
________
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
Filtrate pressure (yellow)
Zero point: Display within the range of ± 5mmHg
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
4.6 Scales: calibration with 5000 g test weight
Scale I
Calibration: Test load on scales: display 5000 g ±1 g
Scales II
Calibration: Test load on scales: display 5000 g ±1 g
Scales III
Calibration: Test load on scales: display 5000 g ±1 g
Scales IV
Calibration: Test load on scales: display 5000 g ±1 g
________
________
________
_______g
_______g
_______g
_______g
3-4 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 57

Chapter 3: Installation
No. Description Meas.
value
5 Ci-Ca module option: visual inspections
5.1 Colored markings of drop counters must be present.
5.2 Adhesive labels of citrate and calcium pumps are present and legible.
5.3 The mechanical condition permits further safe use.
5.4 There are no signs of damage or dirt.
5.5 Line holders must be present and undamaged.
6 Ci-Ca module option: extracorporeal components
6.1 Citrate drop counter checked for proper function
6.2 Calcium drop counter checked for proper function
6.3 Line occlusion of the pumps checked.
Citrate pump (pressure loss max. 10 mmHg/min)
Calcium pump (pressure loss max. 10 mmHg/min)
________
________
Fresenius Medical Care multiFiltrate TM 6/03.07 3-5
Page 58

Chapter 3: Installation
No. Description Meas.
value
7 Check of the electrical safety
In Germany according to DIN VDE 0751-1, edition 10/2001.
In other countries, observe the local regulations!
7.1 Visual inspections performed as specified under 3 and 5.
7.2 Protective earth resistance max. 0.3 (with power cable) ______
7.3 Leakage current measurement (device leakage current)
Differential current measurement according to figure C.6
or
Direct measurement according to figure C.5
Nominal voltage of power supply: _______ V
Device leakage current mains polarity 1: _______ A
With line voltage: _______ V
Scaled to nominal voltage
(maximum 500 A, see Additional conditions)
Device leakage current mains polarity 2: _______ A
With line voltage: _______ V
_____ A
Scaled to nominal voltage
(maximum 500 A, see Additional conditions)
7.4 Patient leakage current measurement
7.4.1 For degree of protection type "BF" according to fig. C.8
Nominal voltage of power supply: __________ V
Patient leakage current __________ A
for line voltage __________ V
scaled to nominal voltage (maximum 480 A, see Additional conditions): _____ A
7.4.2 For degree of protection type "CF" according to fig. C.8
Nominal voltage of power supply: __________ V
Patient leakage current __________ A
for line voltage __________ V
scaled to nominal voltage (maximum 34 A, see Additional conditions): _____ A
_____ A
3-6 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 59

Chapter 3: Installation
No. Description Meas.
value
8 Functional test
8.1 Functional test (T1 test) checked for proper performance.
8.2 Ci-Ca option:
After successful completion of the test, screen M86 'Starting conditions' is
displayed.
8.3 Power failure alarm executed.
9 Final check
9.1 Entries made in the Medical Device Register.
9.2 Operating Instructions and accessories package complete and match the system.
Test equipment used:
Temperature, pressure
(type, serial number):
________________________________________________________________________________________
Protective earth resistance, leakage current
(type, serial number):
________________________________________________________________________________________
Comments:
Date: Signature: Stamp:
The system has been released for its intended use.
(Attach inspection sticker.)
Next
inspection date:
Comments:
Date: Signature: Stamp:
Yes
No
Fresenius Medical Care multiFiltrate TM 6/03.07 3-7
Page 60
Chapter 3: Installation
3.4 Explanations on the Initial Start-Up Report
Identification
Technician's name:
Technician's first name and surname.
Customer/customer no.:
Number of the final customer.
Service report number:
Number of the service call.
System type including option(s):
System name with possible options and extras.
System no.:
Serial number indicated on the type label.
Inventory no.:
Inventory number assigned to the system.
Operating hours:
Operating hours, if a time meter is installed.
Equipment code:
Equipment code indicated on the system.
(e.g. EC xxx, E-code xxx)
Re: 1.1
Remove the system from its transport packaging and save the
packaging for possible future return shipments.
Re: 1.2
Check that all parts of the system have been accounted for. Inform your
contact at Fresenius immediately of any missing parts.
Re: 4 - Functional check
For detailed information refer to chapter 5 "Adjustment Instructions and
Tests"
Re: 5 - Check of the electrical safety
In Germany according to DIN VDE 0751-1, edition 10/2001.
In other countries, observe the local regulations!
(Use DIN VDE 0751-1, edition 10/2001, for medical products. Use DIN
VDE 0701-1 for electrical production equipment.)
3-8 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 61

Re: 5.2 Protective earth resistance measurement points
Chapter 3: Installation
1
2
3
Legend
1 Luer locks
2 Venous clamp
3 Filtrate bag hook
4 IV pole and second IV pole (Ci-Ca module option)
5 Potential equalization
6 RS232 screwed connection
4
5
6
Fresenius Medical Care multiFiltrate TM 6/03.07 3-9
Page 62
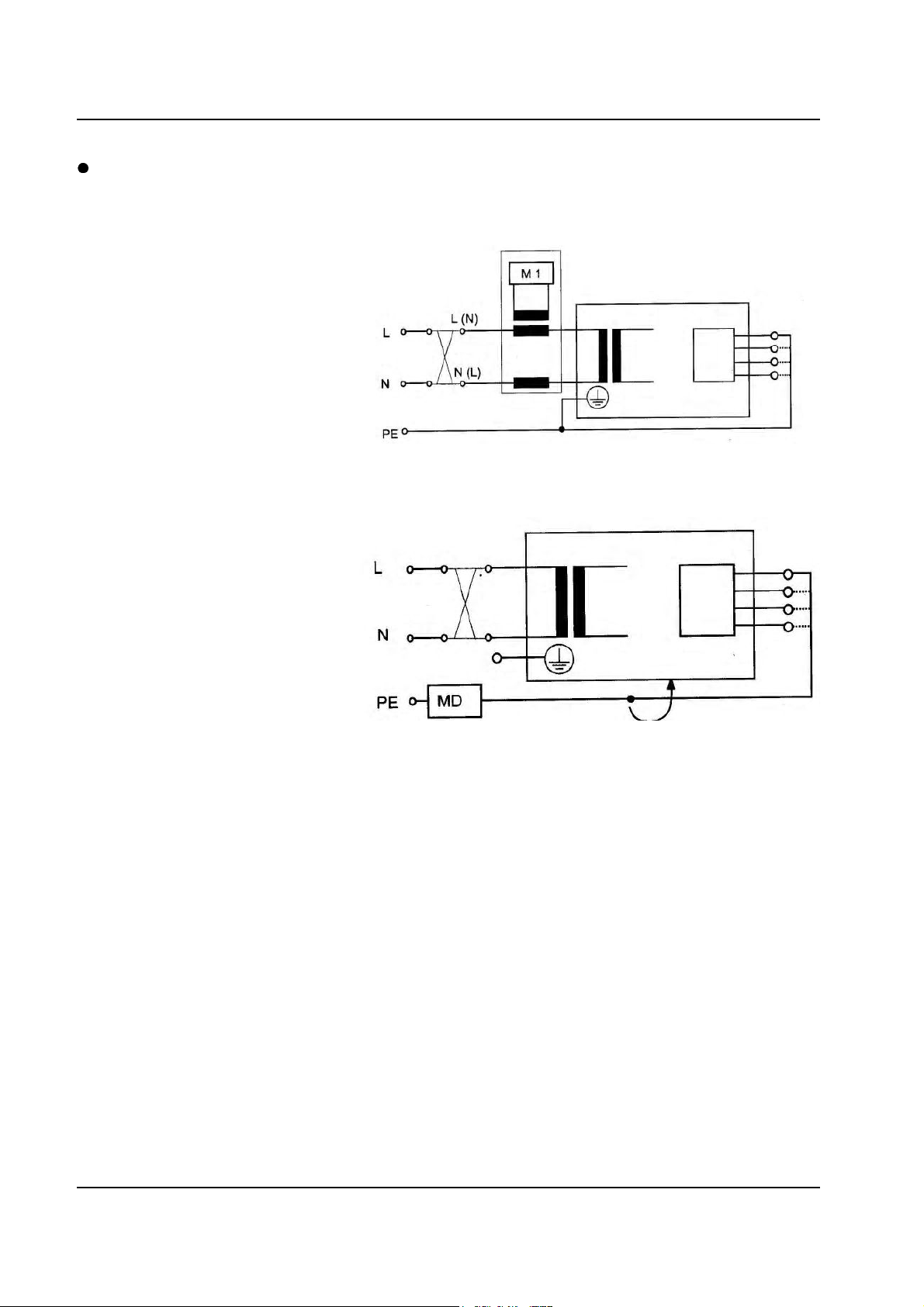
Chapter 3: Installation
Re: 5.3 Leakage current measurement (device leakage current)
Fig.: Differential current measurement according to fig. C.6
Fig.: Direct measurement according to fig. C.5
Basic requirements:
Measurement of the protective earth resistance performed.
The system is in standby operating mode (system connected to the
mains).
The same measurement kit (M28 060 1) as for the patient leakage
current measurement is used for the measurement. Place the
measuring plates on both bag trays and insert the heater bags filled
with NaCl. Measurement setup, see appendix.
When performing a direct measurement, the following precautions
also must be observed:
The system must be insulated when installed. All external
connections and the potential equalization must have been removed
from the system.
3-10 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 63

Chapter 3: Installation
Documentation covers the nominal voltage during the measurement
and the device leakage current at line voltage, scaled to the nominal line
voltage.
Example:
Line voltage during measurement: 225 V
Device leakage current
for mains polarity 1: 38 µA
for mains polarity 2: 57 µA
Maximum value of both mains polarities: 57 µA
Nominal voltage of the power supply: 230 V
Scaled to nominal voltage: 58 µA
(58 µA: 225 V 230 V = 58 µA)
Device leakage current < 500 µA: OK
Additional conditions:
If the device leakage current is higher than 90 % of the admissible alarm
limit (450 µA), the last measured value or the first measured value must
additionally be considered for the rating.
If the device leakage current considerably increased since the last
measurement or continuously increased since the first measurement
(creeping deterioration of the insulation), or if the sum composed of the
current value plus the difference since the last measurement is
> 500 µA, the measurement has not been passed.
Example 1:
Leakage current: 470 µA
Last measured value: 450 A
470 + (470 450) = 470 + 20 = 490
OK
Example 2:
Leakage current: 470 µA
Last measured value: 390 µA
470 + (470 390) = 470 + 80 = 550
Not passed
Fresenius Medical Care multiFiltrate TM 6/03.07 3-11
Page 64

Chapter 3: Installation
Re: 5.3 and 5.4 Patient and device leakage current measurement
Fig.: Setup of the measurement kit for measuring the patient leakage
current and the device leakage current
1
2
Legend
1 Saline bag with 0.9 % NaCl solution for heater
2 Measuring plates for bag trays, left and right
3 Heater bag with measuring probe
4 Patient leakage current measurement: "AWT A" connector
Device leakage current measurement: Protective earth terminal
Symbol:
3
4
3-12 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 65

Note on item 3 concerning device leakage measurement
The upper heater bag must be completely filled with NaCl solution so
that the electrode of the measuring probe is situated inside the solution.
Note on item 4 concerning device leakage measurement
The connection line to the measurement kit must be inserted in the
protective earth socket of the SECUTEST unit. If the measuring
instrument fails to provide this arrangement, the connection line must
be connected to a part of the multiFiltrate that is connected to the
protective earth, e.g. the venous clamp.
If the steps are not followed as described, a specific function will be
executed incorrectly or will not be executed at all, or will not produce the
desired effect.
Re: 5.4.1 Patient leakage current measurement for degree of protection (BF)
Degree of protection type BF is applying to systems with ceramic
heater, operated at a line voltage of 220 V to 240 V and at a line
frequency of 60 Hz.
Chapter 3: Installation
Fig.: Patient leakage current measurement according to figure C.8
Basic requirements:
Measurement of the protective earth resistance performed.
The system is in standby operating mode (system connected to the
mains).
Neither the system nor the test equipment must be touched during
the measurement nor must a cable be connected to the serial
interface.
The patient leakage current measurement kit (M28 060 1) is used for
the measurement. Place the measuring plates on both bag trays and
insert the heater bags filled with NaCl. Measurement setup, see
appendix.
Documentation covers the nominal voltage during the measurement
and the patient leakage current at line voltage, scaled to the nominal
line voltage.
Fresenius Medical Care multiFiltrate TM 6/03.07 3-13
Page 66

Chapter 3: Installation
Re: 5.4.2 Patient leakage current measurement for degree of protection (CF)
Example:
Measurement value: 48 µA, measured at 225 V
Nominal voltage: 230 V
Scaled to nominal voltage: 49 µA
(48 µA: 225 V x 230 V = 49 µA)
Degree of protection type CF is applying to systems with ceramic
heater, operated at a line voltage of 100 V to 240 V and at a line
frequency of 50 Hz or at a line voltage of 100 V to 127 V and at a line
frequency of 60 Hz.
Fig.: Patient leakage current measurement according to figure C.8
Confirming the test
Basic requirements:
Measurement of the protective earth resistance performed.
The system is in standby operating mode (system connected to the
mains).
Neither the system nor the test equipment must be touched during
the measurement nor must a cable be connected to the serial
interface.
The patient leakage current measurement kit (M28 060 1) is used for
the measurement. Place the measuring plates on both bag trays and
insert the heater bags filled with NaCl. Measurement setup, see
appendix.
Documentation covers the nominal voltage during the measurement
and the patient leakage current at line voltage, scaled to the nominal
line voltage.
Example:
Measurement value: 28 µA, measured at 244 V
Nominal voltage: 230 V
Scaled to nominal voltage: 26 µA
(28 µA: 244 V x 230 V = 26 µA)
Test equipment used:
Type and serial number of the test equipment used.
3-14 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 67

Assessing the test
Chapter 3: Installation
Comments:
Irregularities encountered during the test will be recorded in this section.
Date, signature, stamp
Performance of the test has to be confirmed by indicating date, tester's
signature and stamp.
The system has been released for its intended use.
(Attach inspection sticker.)
During the intended use of the system it must be ensured that the
system does not present a hazard to patients, employees or other third
parties.
Within the scope of the overall assessment, the tester must make a
definite decision whether the system may be used or not. The
responsible organization must immediately be informed of any defects
detected.
Next inspection date:
The next inspection date has to be entered in the report.
The intervals specified by the manufacturer have to be respected!
Comments:
Irregularities encountered during the assessment will be recorded in
this section.
Date, signature, stamp:
Assessment of the initial start-up has to be confirmed by indicating date,
tester's signature and stamp.
Fresenius Medical Care multiFiltrate TM 6/03.07 3-15
Page 68

Chapter 3: Installation
3.5 Installing the Ci-Ca Module (Option)
Caution
Before the Ci-Ca module is installed, the power plug of the system must
be disconnected.
The Ci-Ca module is provided with spring pressure pieces, which make
the module click into place at the IV pole on the left side of the
multiFiltrate system. If the securing lever on the rear of the module has
not been unlocked yet, it has to be done before installing the module by
removing the locking screw. The securing lever has to point up when
the module is connected. Place the module onto the lower IV pole
bearing and slide it along the multiFiltrate housing panel, over the action
point of the pressure pieces and onto the IV pole, until the pressure
pieces click into place. Make sure that the electrical connectors easily
slide into each other.
3-16 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 69

Chapter 3: Installation
When the module is locked in place at the IV pole, the securing lever
with pressure roll has to be thrown and then secured with the locking
screw so that it cannot be opened. In this case, another action point
must be overcome.
Fresenius Medical Care multiFiltrate TM 6/03.07 3-17
Page 70

Chapter 3: Installation
3-18 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 71

Chapter 4: TSC / TMC / Maintenance
4 TSC / TMC / Maintenance
4.1 Important Information Regarding the Procedure
Tester's qualification The initial start-up must be performed by the Technical Service of
Fresenius Medical Care or a person authorized by them!
The tests may only be performed by persons who are qualified to
properly perform the specified checks owing to their educational
background and training, their knowledge and experience gained in
practice. Furthermore, the persons performing the tests must not be
bound by any directives when performing this activity.
Test equipment and
accessories
TSC/TMC/MA intervals The TSC/TMC/MA procedures for this system are to be performed after
The activities described in this technical document require the
availability of the necessary technical test equipment and accessories.
Any information on the specifications must be observed.
12 months.
Fresenius Medical Care multiFiltrate TM 6/03.07 4-1
Page 72
Chapter 4: TSC / TMC / Maintenance
4.2 TSC / MA Report multiFiltrate
Technician's name: System type including option(s) / software version:
Customer/customer no.: System no.: Inventory no.:
Service report number: Operating hours: Equipment code:
Type No. Description Meas.
value
1 Visual inspections
TSC 1.1 Fuses accessible from the outside comply with the indicated values.
TSC 1.2 Labels and labeling are present and legible.
TSC 1.3 The mechanical condition permits further safe use.
TSC 1.4 There are no signs of damage or dirt.
TSC 1.5 The power cable shows no signs of damage.
MA 1.6 Replace the lithium battery of operating and safety processors (P.C.B. LP
244) every 4 years. ________
2 Extracorporeal components
TSC 2.1 Pump checked for stopping with open door.
Blood pump
Filtrate pump
Substituate pump
Dialysate pump
TSC 2.2 Pump rotors checked for damage and rolls for smooth running.
Blood pump
Filtrate pump
Substituate pump
Dialysate pump
TSC 2.3 The venous occlusion clamp closes after an air detector alarm.
TSC 2.4 The pressure of 2 bar applied in the venous bubble catcher may not drop
by more than 0.1 bar within 3 minutes.
4-2 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 73

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
MA 2.5 Air detector operation/calibration checked.
Jumper J1-P.C.B. LP 450 set to the operation position. Place the checking
block into the air detector. LEDs DI 5 and DI 10 are light.
Jumper J1-P.C.B. LP 450 set to the operation position. Place the adjusting
block into the air detector. LEDs DI 5 and DI 10 are dark.
MA 2.6 Pressure transducers: zero point and amplification as well as tightness
checked.
Arterial pressure (red)
Zero point: Display within the range of ± 5mmHg
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
Tightness: 600 mmHg, drop max. 10 mmHg within 1 min
Venous pressure (blue)
Zero point: Display within the range of ± 5mmHg
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
________
________
________
________
________
Tightness: 600 mmHg, drop max. 10 mmHg within 1 min
PHF pressure (white)
Zero point: Display within the range of ± 5mmHg
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
Tightness: 600 mmHg, drop max. 10 mmHg within 1 min
Filtrate pressure (yellow)
Zero point: Display within the range of ± 5mmHg
Amplification: 300Apply 300 mmHg, tolerance ±5 mmHg
Tightness: 600 mmHg, drop max. 10 mmHg within 1 min
MA 2.7 Blood leak detector values checked for red coloration and dimness.
________
________
________
________
________
________
________
Fresenius Medical Care multiFiltrate TM 6/03.07 4-3
Page 74

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
3 Mechanical components
MA 3.1 Mechanical parts of scales checked for tight seat, parallelism and
smoothness.
Scale I
Scales II
Scales III
Scales IV
MA 3.2 Zero load, calibration and ball weight of scales checked.
Scale I
Zero load: display within a range from 60 g to 4500 g
Calibration: Test load on scales: display 5000 g ±1 g
Ball weight: display within a range from 43.8 g to 44.8 g
Scales II
Zero load: display within a range from 60 g to 4500 g
_______g
_______g
_______g
_______g
Calibration: Test load on scales: display 5000 g ±1 g
Ball weight: display within a range from 43.8 g to 44.8 g
Scales III
Zero load: display within a range from 60 g to 4500 g
Calibration: Test load on scales: display 5000 g ±1 g
Ball weight: display within a range from 43.8 g to 44.8 g
Scales IV
Zero load: display within a range from 60 g to 4500 g
Calibration: Test load on scales: display 5000 g ±1 g
Ball weight: display within a range from 43.8 g to 44.8 g
MA 3.3 Rotary selector checked for easy movement and tight fit
MA 3.4 Brakes of wheel assy. checked
_______g
_______g
_______g
_______g
_______g
_______g
_______g
_______g
4-4 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 75

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
4 Ci-Ca module option: visual inspections
TSC 4.1 Colored markings of drop counters must be present.
TSC 4.2 Adhesive labels of citrate and calcium pumps are present and legible.
TSC 4.3 The mechanical condition permits further safe use.
TSC 4.4 There are no signs of damage or dirt.
TSC 4.5 Line holders must be present and undamaged.
5 Ci-Ca module option: extracorporeal components
TSC 5.1 Citrate drop counter checked for proper function
TSC 5.2 Calcium drop counter checked for proper function
TSC 5.3 Line occlusion of the pumps checked.
Citrate pump (pressure loss max. 10 mmHg/min)
Calcium pump (pressure loss max. 10 mmHg/min)
________
________
Fresenius Medical Care multiFiltrate TM 6/03.07 4-5
Page 76

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
6 Check of the electrical safety
In Germany according to DIN VDE 0751-1, edition 10/2001.
In other countries, observe the local regulations!
TSC 6.1 Visual inspections performed according to item 1.
TSC 6.2 Protective earth resistance max. 0.3 (with power cable) ______
TSC 6.3 Leakage current measurement (device leakage current)
Differential current measurement according to figure C.6
or
Direct measurement according to figure C.5
Nominal voltage of power supply: _______ V
Device leakage current mains polarity 1: _______ A
With line voltage: _______ V
Scaled to nominal voltage
(maximum 500 A, see Additional conditions)
Device leakage current mains polarity 2: _______ A
With line voltage: _______ V
_____ A
Scaled to nominal voltage
(maximum 500 A, see Additional conditions)
TSC 6.4 Patient leakage current measurement
TSC 6.4.1 For degree of protection type "BF" according to fig. C.8
Nominal voltage of power supply: __________ V
Patient leakage current __________ A
With line voltage: _______ V
scaled to nominal voltage (maximum 480 A, see Additional conditions): _____ A
TSC 6.4.2 For degree of protection type "CF" according to fig. C.8
Nominal voltage of power supply: __________ V
Patient leakage current __________ A
With line voltage: _______ V
scaled to nominal voltage (maximum 34 A, see Additional conditions): _____ A
_____ A
4-6 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 77

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
7 Functional test
TSC 7.1 Functional test (T1 test) checked for proper performance.
TSC 7.2 Ci-Ca module option
After successful completion of the test, screen M86 'Starting conditions' is
displayed.
TSC 7.3 Power failure alarm executed.
8 Final check
TSC 8.1 Entries made in the Medical Device Register.
Test equipment used:
Pressure
(type, serial number):
________________________________________________________________________________________
Protective earth resistance, leakage current
(type, serial number):
________________________________________________________________________________________
Comments:
Date: Signature: Stamp:
The system has been released for its intended use.
(Attach inspection sticker.)
Next
inspection date:
Comments:
Date: Signature: Stamp:
Yes
No
Fresenius Medical Care multiFiltrate TM 6/03.07 4-7
Page 78

Chapter 4: TSC / TMC / Maintenance
4.3 multiFiltrate TSC Report
Technician's name: System type including option(s) / software version:
Customer/customer no.: System no.: Inventory no.:
Service report number: Operating hours: Equipment code:
Type No. Description Meas.
value
1 Visual inspections
TSC 1.1 Fuses accessible from the outside comply with the indicated values.
TSC 1.2 Labels and labeling are present and legible.
TSC 1.3 The mechanical condition permits further safe use.
TSC 1.4 There are no signs of damage or dirt.
TSC 1.5 The power cable shows no signs of damage.
2 Extracorporeal components
TSC 2.1 Pump checked for stopping with open door.
Blood pump
Filtrate pump
Substituate pump
Dialysate pump
TSC 2.2 Pump rotors checked for damage and rolls for smooth running.
Blood pump
Filtrate pump
Substituate pump
Dialysate pump
TSC 2.3 The venous occlusion clamp closes after an air detector alarm.
TSC 2.4 The pressure of 2 bar applied in the venous bubble catcher may not drop
by more than 0.1 bar within 3 minutes.
4-8 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 79

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
3 Ci-Ca module option: visual inspections
TSC 3.1 Colored markings of drop counters must be present.
TSC 3.2 Adhesive labels of citrate and calcium pumps are present and legible.
TSC 3.3 The mechanical condition permits further safe use.
TSC 3.4 There are no signs of damage or dirt.
TSC 3.5 Line holders must be present and undamaged.
4 Ci-Ca module option: extracorporeal components
TSC 4.1 Citrate drop counter checked for proper function
TSC 4.2 Calcium drop counter checked for proper function
TSC 4.3 Line occlusion of the pumps checked.
Citrate pump (pressure loss max. 10 mmHg/min)
Calcium pump (pressure loss max. 10 mmHg/min)
________
________
Fresenius Medical Care multiFiltrate TM 6/03.07 4-9
Page 80

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
5 Check of the electrical safety
In Germany according to DIN VDE 0751-1, edition 10/2001.
In other countries, observe the local regulations!
TSC 5.1 Visual inspections performed according to item 1.
TSC 5.2 Protective earth resistance max. 0.3 (with power cable) ______
TSC 5.3 Leakage current measurement (device leakage current)
Differential current measurement according to figure C.6
or
Direct measurement according to figure C.5
Nominal voltage of power supply: _______ V
Device leakage current mains polarity 1: _______ A
With line voltage: _______ V
Scaled to nominal voltage
(maximum 500 A, see Additional conditions)
Device leakage current mains polarity 2: _______ A
With line voltage: _______ V
_____ A
Scaled to nominal voltage
(maximum 500 A, see Additional conditions)
TSC 5.4 Patient leakage current measurement
TSC 5.4.1 For degree of protection type "BF" according to fig. C.8
Nominal voltage of power supply: __________ V
Patient leakage current __________ A
With line voltage: _______ V
scaled to nominal voltage (maximum 480 A, see Additional conditions): _____ A
TSC 5.4.2 For degree of protection type "CF" according to fig. C.8
Nominal voltage of power supply: __________ V
Patient leakage current __________ A
With line voltage: _______ V
scaled to nominal voltage (maximum 34 A, see Additional conditions): _____ A
_____ A
4-10 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 81

Chapter 4: TSC / TMC / Maintenance
Type No. Description Meas.
value
6 Functional test
TSC 6.1 Functional test (T1 test) checked for proper performance.
TSC 6.2 Ci-Ca module option
After successful completion of the test, screen M86 'Starting conditions' is
displayed.
TSC 6.3 Power failure alarm executed.
7 Final check
TSC 7.1 Entries made in the Medical Device Register.
Test equipment used:
Pressure
(type, serial number):
________________________________________________________________________________________
Protective earth resistance, leakage current
(type, serial number):
________________________________________________________________________________________
Comments:
Date: Signature: Stamp:
The system has been released for its intended use.
(Attach inspection sticker.)
Next
inspection date:
Comments:
Date: Signature: Stamp:
Yes
No
Fresenius Medical Care multiFiltrate TM 6/03.07 4-11
Page 82

Chapter 4: TSC / TMC / Maintenance
4.4 Explanations on the TSC / MA Report
Identification
Technician's name:
Technician's first name and surname.
Customer/customer no.:
Number of the final customer.
Service report number:
Number of the service call.
System type including option(s):
System name with possible options and extras.
System no.:
Serial number indicated on the type label.
Inventory no.:
Inventory number assigned to the system.
Operating hours:
Operating hours, if a time meter is installed.
Equipment code:
Equipment code indicated on the system.
(e.g. EC xxx, E-code xxx)
Re: 2 - Extracorporeal components
For detailed information refer to chapter 5 "Adjustment Instructions and
Tests"
Re: 3 - Mechanical components
For more detailed information on item 3.2, please refer to the Quick
Guide on the PC Service Software MFT.
4-12 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 83

Re: 4 - Check of the electrical safety
In Germany according to DIN VDE 0751-1, edition 10/2001.
In other countries, observe the local regulations!
(Use DIN VDE 0751-1, edition 10/2001, for medical products. Use DIN
VDE 0701-1 for electrical production equipment.)
Re: 4.2 Protective earth resistance measurement points
Chapter 4: TSC / TMC / Maintenance
1
Legend
2
3
4
5
6
1 Luer locks
2 Venous clamp
3 Filtrate bag hook
4 IV pole and second IV pole (Ci-Ca module option)
5 Potential equalization
6 RS232 screwed connection
Fresenius Medical Care multiFiltrate TM 6/03.07 4-13
Page 84

Chapter 4: TSC / TMC / Maintenance
Re: 4.3 Leakage current measurement (device leakage current)
Fig.: Differential current measurement according to fig. C.6
Fig.: Direct measurement according to fig. C.5
Basic requirements:
Measurement of the protective earth resistance performed.
The system is in standby operating mode (system connected to the
mains).
The same measurement kit (M28 060 1) as for the patient leakage
current measurement is used for the measurement. Place the
measuring plates on both bag trays and insert the heater bags filled
with NaCl. Measurement setup, see appendix.
When performing a direct measurement, the following precautions
also must be observed:
The system must be insulated when installed. All external
connections and the potential equalization must have been removed
from the system.
4-14 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 85

Chapter 4: TSC / TMC / Maintenance
Documentation covers the nominal voltage during the measurement
and the device leakage current at line voltage, scaled to the nominal line
voltage.
Example:
Line voltage during measurement: 225 V
Device leakage current
for mains polarity 1: 38 µA
for mains polarity 2: 57 µA
Maximum value of both mains polarities: 57 µA
Nominal voltage of the power supply: 230 V
Scaled to nominal voltage: 58 µA
(58 µA: 225 V 230 V = 58 µA)
Device leakage current < 500 µA: OK
Additional conditions:
If the device leakage current is higher than 90 % of the admissible alarm
limit (450 µA), the last measured value or the first measured value must
additionally be considered for the rating.
If the device leakage current considerably increased since the last
measurement or continuously increased since the first measurement
(creeping deterioration of the insulation), or if the sum composed of the
current value plus the difference since the last measurement is
> 500 µA, the measurement has not been passed.
Example 1:
Leakage current: 470 µA
Last measured value: 450 A
470 + (470 450) = 470 + 20 = 490
OK
Example 2:
Leakage current: 470 µA
Last measured value: 390 µA
470 + (470 390) = 470 + 80 = 550
Not passed
Fresenius Medical Care multiFiltrate TM 6/03.07 4-15
Page 86

Chapter 4: TSC / TMC / Maintenance
Re: 4.3 and 4.4 Patient and device leakage current measurement
Fig.: Setup of the measurement kit for measuring the patient leakage
current and the device leakage current
1
2
Legend
1 Saline bag with 0.9 % NaCl solution for heater
2 Measuring plates for bag trays, left and right
3 Heater bag with measuring probe
4 Patient leakage current measurement: "AWT A" connector
Device leakage current measurement: Protective earth terminal
Symbol:
3
4
4-16 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 87

Chapter 4: TSC / TMC / Maintenance
Note on item 3 concerning device leakage measurement
The upper heater bag must be completely filled with NaCl solution so
that the electrode of the measuring probe is situated inside the solution.
Note on item 4 concerning device leakage measurement
The connection line to the measurement kit must be inserted in the
protective earth socket of the SECUTEST unit. If the measuring
instrument fails to provide this arrangement, the connection line must
be connected to a part of the multiFiltrate that is connected to the
protective earth, e.g. the venous clamp.
If the steps are not followed as described, a specific function will be
executed incorrectly or will not be executed at all, or will not produce the
desired effect.
Re: 4.4.1 - Patient leakage current measurement for degree of protection (BF)
Degree of protection type BF is applying to systems with ceramic
heater, operated at a line voltage of 220 V to 240 V and at a line
frequency of 60 Hz.
Fig.: Patient leakage current measurement according to figure C.8
Basic requirements:
Measurement of the protective earth resistance performed.
The system is in standby operating mode (system connected to the
mains).
Neither the system nor the test equipment must be touched during
the measurement nor must a cable be connected to the serial
interface.
The patient leakage current measurement kit (M28 060 1) is used for
the measurement. Place the measuring plates on both bag trays and
insert the heater bags filled with NaCl. Measurement setup, see
appendix.
Documentation covers the nominal voltage during the measurement
and the patient leakage current at line voltage, scaled to the nominal
line voltage.
Fresenius Medical Care multiFiltrate TM 6/03.07 4-17
Page 88

Chapter 4: TSC / TMC / Maintenance
Re: 4.4.2 Patient leakage current measurement for degree of protection (CF)
Example:
Measurement value: 48 µA, measured at 225 V
Nominal voltage: 230 V
Scaled to nominal voltage: 49 µA
(48 µA: 225 V x 230 V = 49 µA)
Degree of protection type CF is applying to systems with ceramic
heater, operated at a line voltage of 100 V to 240 V and at a line
frequency of 50 Hz or at a line voltage of 100 V to 127 V and at a line
frequency of 60 Hz.
Fig.: Patient leakage current measurement according to figure C.8
Confirming the test
Basic requirements:
Measurement of the protective earth resistance performed.
The system is in standby operating mode (system connected to the
mains).
Neither the system nor the test equipment must be touched during
the measurement nor must a cable be connected to the serial
interface.
The patient leakage current measurement kit (M28 060 1) is used for
the measurement. Place the measuring plates on both bag trays and
insert the heater bags filled with NaCl. Measurement setup, see
appendix.
Documentation covers the nominal voltage during the measurement
and the patient leakage current at line voltage, scaled to the nominal
line voltage.
Example:
Measurement value: 28 µA, measured at 244 V
Nominal voltage: 230 V
Scaled to nominal voltage: 26 µA
(28 µA: 244 V x 230 V = 26 µA)
Test equipment used:
Type and serial number of the test equipment used.
4-18 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 89

Assessing the test
Chapter 4: TSC / TMC / Maintenance
Comments:
Irregularities encountered during the test will be recorded in this section.
Date, signature, stamp
Performance of the test has to be confirmed by indicating date, tester's
signature and stamp.
The system has been released for its intended use.
(Attach inspection sticker.)
During the intended use of the system it must be ensured that the
system does not present a hazard to patients, employees or other third
parties.
Within the scope of the overall assessment, the tester must make a
definite decision whether the system may be used or not. The
responsible organization must immediately be informed of any defects
detected.
Next inspection date:
The next inspection date has to be entered in the report.
The intervals specified by the manufacturer have to be respected!
Comments:
Irregularities encountered during the assessment will be recorded in
this section.
Date, signature, stamp:
Assessment of the initial start-up has to be confirmed by indicating date,
tester's signature and stamp.
Fresenius Medical Care multiFiltrate TM 6/03.07 4-19
Page 90

Chapter 4: TSC / TMC / Maintenance
4-20 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 91

Chapter 5: Adjustment Instructions and Tests
5 Adjustment Instructions and Tests
5.1 Service Tools
Part no. Description
M28 489 1 Dongle ICD cable for programming the OP and SP
M28 060 1 Measuring kit for measuring the patient leakage
current
M28 098 1 Crosstalk Reduction Adapter
510 130 1 ESC tester with measuring adapter for patient leakage
current
M30 770 1 HMED pressure measuring instrument with case (set)
631 064 1 Secutest VDE test device (without printer module)
630 652 1 Printer module for secutest
630 648 1 Carrying bag for secutest
630 387 1 ESD Service Kit
670 004 1 Multimeter Fluke 75
M28 486 1 RS232 null-modem cable, 9-pin D-SUB
M28 487 1 Gender Changer connector connector, 9-pin D-SUB
M28 488 1 Connectorsocket extension, 1.5 m, 25-pin D-SUB
M28 497 1 CD-ROM with programming software for ICD dongle
for programming the OP and SP
M36 234 1 Service Software, complete set
(incl. card reader, null-modem cable, Gender
Changer)
M36 235 1 Service Software, software set without hardware
M28 498 1 Square wrench, housing door
675 845 1 Hook test weight 5 kg / M1
630 360 1 Equipotential bonding cable
M36 067 1 Calibration kit for air detector
640 560 1 Neutral filter for calibrating the optical detector
Fresenius Medical Care multiFiltrate TM 6/03.07 5-1
Page 92

Chapter 5: Adjustment Instructions and Tests
5.2 Service Program
5.2.1 Start
The system must be turned off via I/O. The power switch on the rear of
the system must be turned on.
Press and hold the Start/Reset key. Simultaneously press the I/O
until the yellow status indicator lights up.
Using the rotary selector, select the numerical code and confirm
each number by pressing the OK key.
5-2 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 93
5.2.2 Selecting the Language
5.2.3 System Messages
Chapter 5: Adjustment Instructions and Tests
After the password has been checked positively, all revisions are
displayed to the left of the screen. The menu fields of the Service
program are displayed to the right of the screen.
Use the rotary selector to select the desired menu field and press
OK.
Use the rotary selector to select the desired language and press OK.
Turn off the system via the I/O key immediately thereafter.
Once again turn on the system via the I/O key and restart the Service
program.
Only the error messages (E XX) and warnings (W XX) of the previous
treatments are filtered out of the events memory (see below) and
displayed on the screen.
5.2.4 Deactivating and Activating the Heparin Pump
Permits activation and deactivation of the optional heparin syringe.
Screen display Machine status
Deactivate heparin syringe The heparin syringe is currently
Activate heparin syringe The heparin syringe is currently
Note
If the system does not contain any installed heparin syringe, the
machine status should be set to Heparin syringe currently not active.
If the setting fails to reflect the proper machine status, a warning is
displayed during the T1 test.
active.
not active.
Fresenius Medical Care multiFiltrate TM 6/03.07 5-3
Page 94

Chapter 5: Adjustment Instructions and Tests
5.2.5 Filtrate Bag Monitoring Limit
Permits to set the monitoring limit to a value between 10 kg and 20 kg
on the filtrate scales.
Screen display Machine status
Filtrate bag max. 5 kg Currently, the monitoring limit is
Filtrate bag max. 10 kg Currently, the monitoring limit is
Filtrate bag max. 20 kg Currently, the monitoring limit is
5.2.6 Dialysate Tubing Arrangement
Permits to switch from old to "Fin" dialysate tubing arrangement and
vice versa in the preparation mode.
set to 5 kg.
set to 10 kg.
set to 20 kg.
old Tubing system with heater bag downstream of the pump segment
"Fin" Tubing system with heater bag upstream of the pump segment
Screen display Machine status
Tubing arrangement dialysate
old
Tubing arrangement dialysate
"Fin"
Display of the old tubing
arrangement. Heater bag for
dialysate downstream of the
dialysate pump.
Display of the new tubing
arrangement. Heater bag for
dialysate upstream of the
dialysate pump.
5-4 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 95

5.2.7 Option MultiDataLink (mDL)
Chapter 5: Adjustment Instructions and Tests
This screen permits to set the multiDataLink parameters.
Activating and
deactivating mDL
DHCP active / inactive This soft key informs the mDL of whether a DHCP server is active in the
This soft key permits activation and deactivation of the optional mDL.
Screen display Machine status
Deactivate mDL mDL is currently active.
Activate mDL mDL is currently not active.
Note
If the system does not contain any installed mDL, the machine status
should be set to mDL currently not active. If the setting fails to reflect
the proper machine status, a warning is displayed during the T1 test.
connected network. If this is the case, the mDL receives its IP
addresses from this server. If no, the IP addresses must be set
manually.
DHCP active DHCP inactive
mDL-IP-address Automatic 0...255 adjustable
mDL-IP-subnet Automatic 0...255 adjustable
Gateway-address Automatic 0...255 adjustable
Server-IP-address Automatic 0...255 adjustable
Fresenius Medical Care multiFiltrate TM 6/03.07 5-5
Page 96
Chapter 5: Adjustment Instructions and Tests
DHCP active DHCP inactive
Activating/deactivating the
Patient/Case ID
Server-port 0...65535 adjustable
Default value: 700
Host-port 0...65535 adjustable
Default value: 2512
Technician's port 0...65535 adjustable
Default value: 2511
Note
Before setting the mDL parameters, please clarify with the responsible
hospital network administrator whether a DHCP server is available for
automatically assigning the IP addresses. If no, the administrator has to
specify defined addresses which will then have to be entered manually.
The port numbers depend on the application which further processes
the multiFiltrate data. The administrator of this software will have to
specify the appropriate values if these are different from default values.
Use the rotary selector to select the Terminate? [OK] to confirm!
field and press OK.
This soft key permits activation and deactivation of the optional
patient/case ID.
0...65535 adjustable
Default value: 700
0...65535 adjustable
Default value: 2512
0...65535 adjustable
Default value: 2511
Screen display Machine status
Deactivate patient/case ID The patient/case ID is currently
active
Activate patient/case ID The patient/case ID is currently
not active
5-6 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 97

5.2.8 Taring and Calibrating the Scales
Chapter 5: Adjustment Instructions and Tests
The screen displays the zero load weights of the scales 1 to 4. Upon
start of the Service program, the scales are not tared. Hence, a display
value within a range from 2500 g to 3500 g is expected for scales 1 and
2. A display value within a range from 250 g to 350 g is expected for
scales 3 and 4. Each of the scales (1 to 4) is provided with its own menu
field for taring and calibrating purposes. The tare and calibrate
procedures are described by the example of scale 1. The same
procedures apply to scales 2 to 4.
Taring the scales
Requirement The system must be turned on via I/O for at least 5 minutes to bring the
scales to operating temperature.
Using the rotary selector, select the Tare field under Scale I and
press OK.
While taring is in progress, the field selected is represented light-green.
After a time out of approx. 10 seconds, the weight of the scale is
indicated to be 0 g.
Calibrates the scales
Test equipment Test weight of 5000 g of accuracy class M1 or better.
Requirement The scales must be unloaded!
Tubing sets may not be inserted.
The scales must have been tared beforehand.
Each scale must be tared separately!
Fresenius Medical Care multiFiltrate TM 6/03.07 5-7
Page 98

Chapter 5: Adjustment Instructions and Tests
To calibrate the scales 1 and 2, place the test weight centrally on the
To calibrate the scales 3 and 4, suspend the test weight from a hook.
Use the rotary selector to select the Calibrate field of the loaded
While calibration is in progress, the field selected is represented lightgreen. The time out is approx. 10 seconds.
5.2.9 Calibrating the Pressures
rear part of the scale pan.
scale and press OK.
The menu fields for calibration of the pressures (arterial, venous,
prehemofilter and filtrate) are displayed to the right of the screen.
Performance of the calibration procedure of the pressure transducers is
described by example of the arterial pressure. The three other
pressures are calibrated in the same manner.
Zero offset of pressure transducers
Requirement The pressure transducers must have ambient pressure (pressure
transducers open).
Use the rotary selector to select the P arterial field and press OK.
Use the rotary selector to select the Pressure sensor open? OK
to confirm! field and press OK.
5-8 Fresenius Medical Care multiFiltrate TM 6/03.07
Page 99

The zero offset is made automatically. It is completed when the next
screen is displayed.
Calibrating the pressure transducers
Chapter 5: Adjustment Instructions and Tests
Test equipment Pressure gauge with a measurement range from 0350 mmHg
(0500 mbar), disposable syringe, artery forceps
Measuring instrument
Fresenius Medical Care multiFiltrate TM 6/03.07 5-9
Page 100

Chapter 5: Adjustment Instructions and Tests
Connect the measuring instrument to the pressure transducer.
Using the syringe, build up a pressure of 300 mmHg. (tolerance
Using the rotary selector, set the value indicated by the pressure
Use the rotary selector to select the Entered? OK to confirm!
After calibration is completed, the system returns to the previous
screen.
Determining the setting of the DAC for detuning in the self-test
Requirement The pressure transducers must have ambient pressure.
50 mmHg) Then, using the artery forceps, occlude to the direction
of the syringe so that the pressure transducer and the pressure
gauge are still under load.
gauge on the screen, i.e. in the Parterial field.
field and press OK.
Use the rotary selector to select the Pressure sensor open? OK
to confirm! field and press OK.
While the self-test is in progress, the field selected is represented lightgreen. The time out is approx. 10 seconds.
Note
If an error occurs during calibration, check and, if necessary, calibrate
the corresponding evaluation board (P.C.B. LP 450-3 or P.C.B. LP 343-
1).
5.2.10 Calibrating the Blood Leak Detector
5-10 Fresenius Medical Care multiFiltrate TM 6/03.07
 Loading...
Loading...