Page 1
2
C
P
1
3
5
4
6
7
90
8
HEMODIALYSIS
SYSTEM
SEMI-ANNUAL
FRESENIUS 2008K
TIVE
FFE
PREVENTIVE
MAINTENANCE
PROCEDURES
Part Number 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 2

C
TIVE
P
920 Winter St.
FFE
FRESENIUS MEDICAL CARE
NORTH AMERICA
800-227-2572 or 925-295-0200
Fresenius Medical Care North America
Waltham, MA 02451
Manufactured by:
Fresenius USA, Inc.
2637 Shadelands Drive
Walnut Creek, CA 94598
REGIONAL EQUIPMENT SPECIALIST: ___________________________________
rinted 5/18/2012 12:00PM
Page 3

FRESENIUS 2008K
C
P
HEMODIALYSIS SYSTEM
TIVE
FFE
SEMI-ANNUAL
PREVENTIVE MAINTENANCE
PROCEDURES
Part Number 507781 Rev. D
INCLUDING
PREVENTIVE MAINTENANCE CHECKLISTS
SIX (6) MONTH AND ANNUAL/4000 HOUR
http://www.fmcna.com
Copyright ¤ 2000 - 2012 Fresenius Medical Care North America
rinted 5/18/2012 12:00PM
Page 4

C
P
NOTE
Eligibility for semi-annual preventive maintenance program.
Standard preventive maintenance procedures for the Fresenius 2008K
Hemodialysis System are documented in Fresenius P/N 507297 Fresenius
2008K Hemodialysis System Preventive Maintenance Procedures. The
standard PM procedure manual lists maintenance tasks and checks to be
performed at 1000 and 4000 hour intervals.
TIVE
FFE
Fresenius 2008K Hemodialysis Systems manufactured (or refurbished) in
2003 and later may be qualified for the semi-annual preventive maintenance
procedures described in this manual. In addition, 2008K hemodialysis
systems manufactured (or refurbished) in 2002 and earlier may be qualified for
the semi-annual PM procedures if the machine meets the minimum
configuration in the qualification checklist on page 69 and that configuration
is maintained.
Since modules and the power supply are designed to be easily moved from
machine to machine, it is necessary to label machines & modules that meet the
configuration requirements for the semi-annual PM program so that the status
of the machine is known and the minimum configuration of the machine is
maintained.
Page ii
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 5

1.0 INTRODUCTION ............................................................................................................................................1
C
P
2.0 SIX (6) MONTH PREVENTIVE MAINTENANCE ...........................................................................................7
3.0 ANNUAL (4000 HOUR) PREVENTIVE MAINTENANCE.............................................................................17
TIVE
FFE
4.0 REBUILDING THE DIAPHRAGM PUMPS ..................................................................................................49
5.0 SEMI-ANNUAL PREVENTIVE MAINTENANCE PROGRAM INSTRUCTIONS ..........................................56
PREVENTIVE MAINTENANCE CHECKLIST SIX (6) MONTH ............................................................................65
PREVENTIVE MAINTENANCE CHECKLIST ANNUAL/4000 HOUR ..................................................................67
SEMI-ANNUAL PREVENTIVE MAINTENANCE PROGRAM QUALIFICATION CHECKLIST ............................69
PREVENTIVE MAINTENANCE PROCEDURES
TABLE OF CONTENTS
1.1 TEST EQUIPMENT AND SUPPLIES NEEDED .......................................................................................... 1
1.2 OPERATING MODES ................................................................................................................................ 3
1.3 FRONT PANEL CONTROLS ...................................................................................................................... 4
1.4 MEASURING FLUID VOLUMES ................................................................................................................ 6
2.1 FILTERS .................................................................................................................................................... 9
2.2 PRE-UF PUMP FILTER ............................................................................................................................. 9
2.3 CHECK VALVES ...................................................................................................................................... 10
2.4 HIGH VOLTAGE AC CONNECTIONS ...................................................................................................... 10
2.5 UF PUMP ................................................................................................................................................. 11
2.6 CONDUCTIVITY ...................................................................................................................................... 11
2.7 TEMPERATURE ...................................................................................................................................... 12
2.8 ALARM AND PRESSURE HOLDING TESTS ........................................................................................... 13
2.9 POWER FAILURE ALARM ...................................................................................................................... 15
2.10 FINAL CHECKS ....................................................................................................................................... 16
3.1 FILTERS AND O-RINGS .......................................................................................................................... 17
3.2 PRE-UF PUMP FILTER ........................................................................................................................... 18
3.3 CHECK VALVES ...................................................................................................................................... 18
3.4 DEAERATION RESTRICTOR .................................................................................................................. 18
3.5 DIAPHRAGM PUMPS .............................................................................................................................. 18
3.6 HEATER ELEMENT ................................................................................................................................. 19
3.7 HIGH VOLTAGE AC CONNECTIONS ...................................................................................................... 19
3.8 DEAERATION MOTOR BRUSHES .......................................................................................................... 20
3.9 INLET WATER PRESSURE REGULATOR .............................................................................................. 21
3.10 ONLINE CLEARANCE TEST (IF APPLICABLE)....................................................................................... 22
3.11 DEAERATION AND LOADING PRESSURE ............................................................................................ 26
3.12 FLOW RELIEF PRESSURE ..................................................................................................................... 27
3.13 CONCENTRATE AND BICARBONATE PUMPS ...................................................................................... 28
3.14 UF PUMP ................................................................................................................................................. 28
3.15 CONDUCTIVITY ...................................................................................................................................... 28
3.16 TEMPERATURE ...................................................................................................................................... 28
3.17 VOLT HI LO DETECT ............................................................................................................................... 28
3.18 BLOOD LEAK AND DIMNESS ................................................................................................................. 29
3.19 ARTERIAL, VENOUS AND TRANSMEMBRANE PRESSURE ................................................................. 30
3.20 DIALYSATE FLOW .................................................................................................................................. 33
3.21 HEPARIN PUMP ...................................................................................................................................... 33
3.22 BLOOD PUMP ......................................................................................................................................... 36
3.23 LEVEL DETECTOR ................................................................................................................................. 38
3.24 ALARM AND PRESSURE HOLDING TESTS ........................................................................................... 42
3.25 RINSE CHECKS ...................................................................................................................................... 42
3.26 BATTERY TEST AND POWER FAILURE ALARM ................................................................................... 43
3.27 BLOOD PRESSURE MODULE ................................................................................................................ 44
3.28 FINAL CHECKS ....................................................................................................................................... 48
4.1 REBUILDING THE ULTRAFILTRATION PUMP ....................................................................................... 49
4.2 REBUILDING THE CONCENTRATE AND BICARBONATE PUMPS ........................................................ 52
4.3 TESTING CONCENTRATE AND BICARBONATE PUMPS ...................................................................... 54
5.1 BLOOD PUMP REQUIREMENTS ............................................................................................................ 57
5.2 POWER SUPPLY REQUIREMENTS ....................................................................................................... 59
Page iii
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 6

P
WARNINGS
Shock hazard. Refer servicing to qualified personnel.
Failure to install, operate and maintain this equipment according
to the manufacturer’s instructions may cause patient or operator
injury or death.
Never perform maintenance when a patient is connected to the
machine. If possible, remove the machine from the treatment area
when it is being serviced. Label the machine to ensure it is not
accidentally returned to clinical use before the service work is
completed. Always fully test the machine when maintenance is
completed. Confirm dialysate conductivity and pH level before
returning the machine to clinical use.
Page iv
NOTE
This document is written for Fresenius 2008K Hemodialysis
Systems using software versions 2.16 or later.
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 7

1.0 INTRODUCTION
C
P
Preventive Maintenance for the Fresenius 2008K Hemodialysis System is simple and
straightforward. Maintenance is performed in only two intervals: six (6) months, and
annually or after 4000 hours of operation. The maintenance procedures have been
devised to require a minimum of time while ensuring that the machine is maintained
in optimum operating condition.
Included in the Preventive Maintenance procedures are tests to verify normal
machine operation. Should the machine fail to pass any of these tests, repair or recalibrate as needed, then repeat the tests until the specifications are met before
returning the machine to service.
Checklists are provided in the back of this manual to record the work done. Make
copies of these checklists as needed. Your initials on the checklist certifies that each
procedure has been completed and that the machine is performing according to the
specifications given.
TIVE
1.1 TEST EQUIPMENT AND SUPPLIES NEEDED
FFE
A number of small parts must be available to perform the Preventive Maintenance.
Fresenius part number 190098 is a kit of the parts needed, except for the 9-Volt
battery that must be replaced during the annual preventive maintenance. An NEDA
1604AC heavy-duty (alkaline type) battery is required. In addition, the following test
equipment is needed:
Warning! All of the test equipment used must be maintained and calibrated
regularly in accordance with NIST standards. In particular, the conductivity meter
must meet the specifications given below. Failure to do so could result in injury or
death to the patient or to the operator.
Warning! Disinfect the machine internally and externally and check all pressure
transducer protectors for contamination before working on the machine.
Page 1
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 8
C
TIVE
P
x Fresenius Test Kit (Fresenius part number 150034), which contains two gauges
with fittings and hoses for measuring loading pressure and deaeration pressure.
x Dialysate meter to measure dialysate pressure, temperature and conductivity at
the ends of the dialysate lines. The meter must be capable of making pressure
measurements from -250mmHg to +400mmHg with an accuracy of at least
±3mmHg. The temperature function of this meter must be accurate within
0.2°C from 20°C to 45°C and must be capable of measuring dialysate
temperatures up to 85°C with an accuracy of at least ±4.0°C. The conductivity
function of this meter must be accurate to within 0.1mS over a range of 12mS to
17mS at a temperature of 25°C.
x Stopwatch with a resolution to 0.01 second and an accuracy of 0.01% or better.
x Fresenius Buret, 25ml capacity with 0.1ml graduations (Fresenius part number
290104).
x Graduated cylinder: 1000ml capacity with a tolerance of 5.0ml at 1000ml or
better.
x Syringe, 60cc capacity. Tolerance is not important; the syringe is not used for
volume measurements.
FFE
x Tubing, 24” long (Fresenius part number 545325-10). Use on the tip of the
Buret.
x Resistor Plug Set for OLC Testing (Fresenius part number 190168).
The following equipment is also required to test the blood pressure module:
x Test Device (Fresenius part number 370090). The Test Device contains two air
chambers with calibrated volumes.
x Mercury manometer or equivalent pressure meter accurate to within 1mmHg at
pressures up to 330mmHg.
Page 2
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 9

1.2 OPERATING MODES
C
P
TIVE
The following preventive maintenance procedures contain instructions to place the
2008K into Dialysis Mode and Service Mode.
To place the machine in Service Mode, turn the machine power On and wait for the
message, Press CONFIRM for Service Mode to appear. Once it appears, press the
[CONFIRM] key and the message will change to Machine in Service Mode. After
the System Initializing process is complete, the machine will be in Service Mode.
If the [CONFIRM] key is not pressed when the Press CONFIRM for Service
Mode message is on the screen, the screen will change and the message Machine in
Dialysis Mode will appear. After the System Initializing process is complete, the
machine will be in Dialysis Mode.
FFE
Page 3
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 10

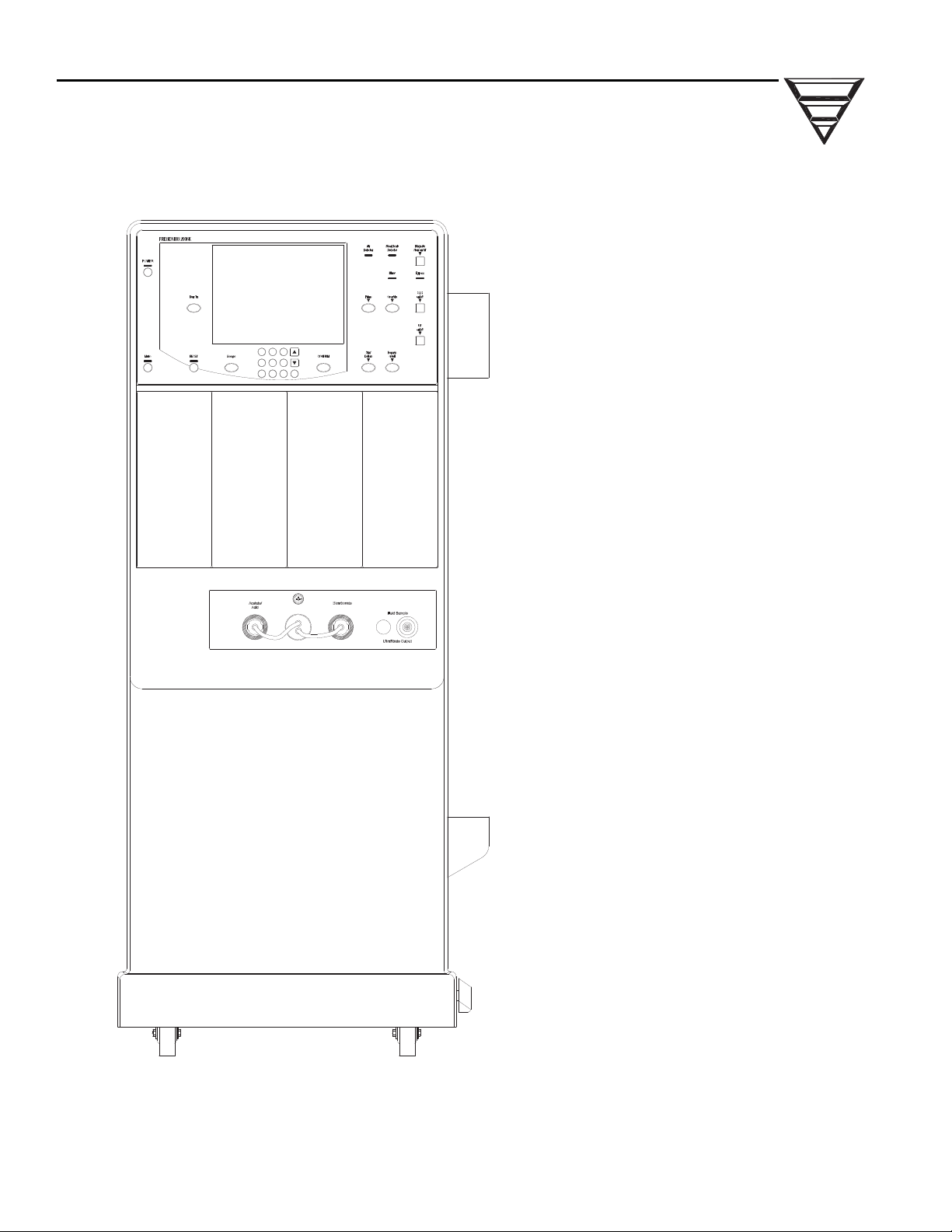
1.3 FRONT PANEL CONTROLS
Control Panel
Touch Screen
C
P
TIVE
The front panel consists of two areas, the touch screen and the control panel. The
touch screen is the area under the glass in the center of the front panel. The control
panel surrounds the touch screen and it contains the membrane keys.
FFE
Figure 1 - 2008K Front Panel
Control Panel Operation
Throughout the preventive maintenance procedures, whenever a control panel key is
to be pressed, the appropriate key name is surrounded by square brackets as in the
following example:
Press the [CONFIRM] key and the screen will change.
In this example, the [CONFIRM] key on the control panel should be pressed.
Page 4
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 11

Data Button
A yellow data button is used to enter a measured volume or
value. When the yellow area of the data button is touched, it
will change to a darker yellow. The data can be changed
using the
[] or []
keys or the value can be entered using
the number keys both on the control panel. Once the data is
entered, press the
[CONFIRM] key and the data button
changes back to light yellow. The
[Escape] key can be
pressed when the data button is dark yellow to abort the data
entry and return it to light yellow. The entered data does not
get stored until the
[CONFIRM] key is pressed.
Some data buttons will change the screen and the data entry
will
be performed on the new screen.
A gray data button means the button is not active and
touching it will have no effect.
Screen Button
Blue rectangles on the touch screen are screen buttons. By
touching the blue area of the screen button the display will
either change to another screen or the selection of an option
will change. A screen button is not active if it is gray.
Data Box
This type of box shows selected data or data the machine is
measuring. During the preventive maintenance process this
type of box is used to verify a value or selection.
C
TIVE
P
Touch Screen Operation
The touch screen is designed to display information and is used to enter data. To
select a button during a procedure, locate the button on the screen. Select a
parameter for which data will be entered using the keys below the screen.
Depending on the type of button, the screen will change. Data boxes are also
displayed on the touch screen. The following describes the type of buttons and data
boxes that will be encountered during the preventive maintenance process.
FFE
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
Page 5
rinted 5/18/2012 12:00PM
Page 12
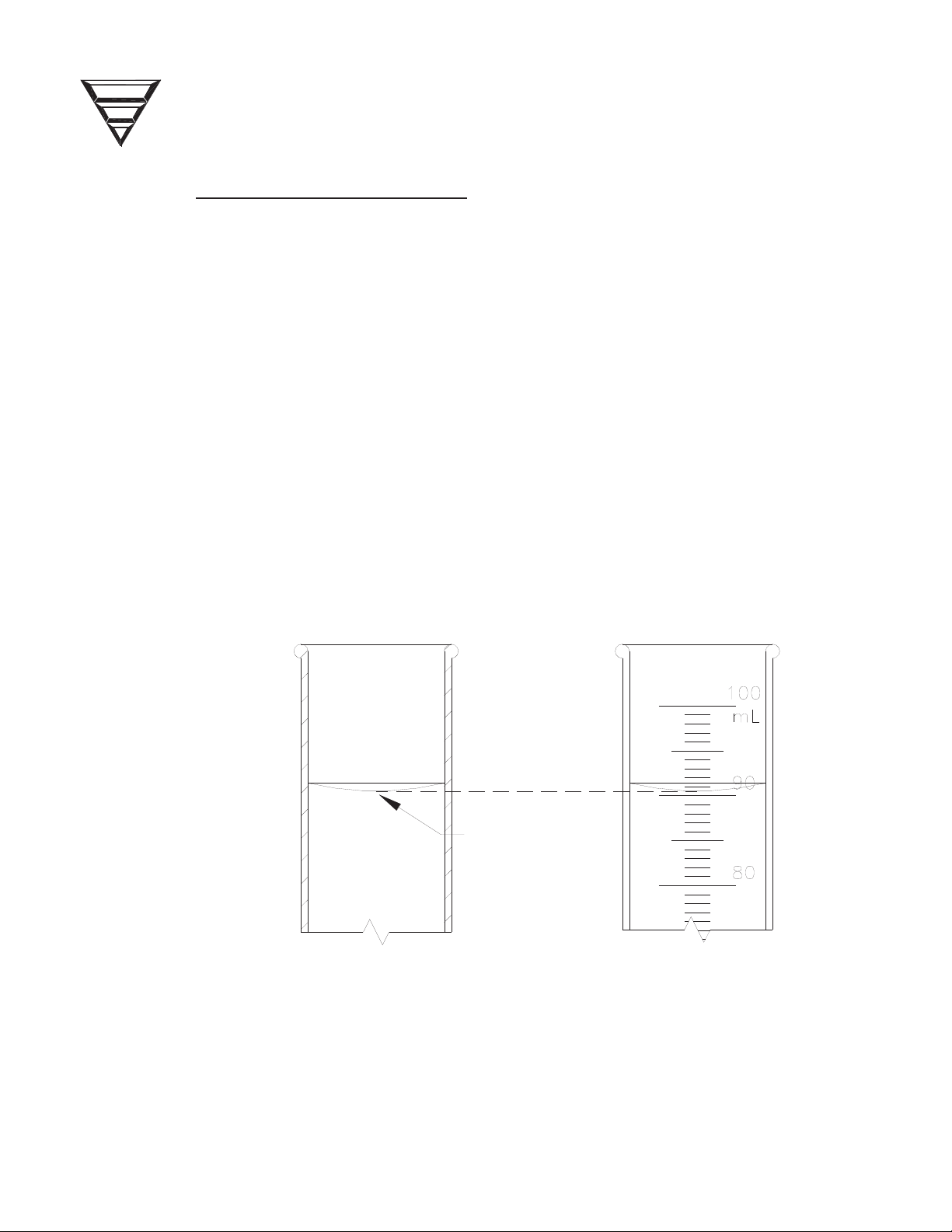
1.4 MEASURING FLUID VOLUMES
C
P
TIVE
Several of the following procedures require measuring fluid volumes using graduated
cylinders and laboratory burets. When making these measurements do the following:
x Make certain the container is clean and dry before collecting the fluid to be
measured. Two drops of fluid are approximately 0.1ml, which is enough to
affect the accuracy of critical measurements.
x Ensure that no items such as thermometers or tubing are allowed to come in
contact with the fluid in the graduate. Such items will change the calibration of
the graduate and affect the accuracy of measurements. Both the total volume
indicated and the amount of fluid indicated by each increment on the graduated
scale will be incorrect. For example, if a graduate is calibrated in 1ml
increments, a piece of tubing in contact with the fluid will cause each increment
to be less than 1ml, depending upon the total volume of the tubing that penetrates
into the fluid.
x Surface tension causes the fluid to curve into a meniscus (See Figure 2).
Measure the volume at the bottom of the meniscus curve as shown.
FFE
Page 6
BOTTOM OF
MENISCUS
CURVE
Figure 2 - Meniscus Curve.
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 13

2.0 SIX (6) MONTH PREVENTIVE MAINTENANCE
C
P
TIVE
Perform the following Preventive Maintenance procedures every six (6) months of
machine operation. Make copies of the Six (6) Month Preventive Maintenance
Checklist provided in the back of this manual and use them to record the
maintenance done.
When performing an Annual Preventive Maintenance, do not perform the six (6)
month procedures below first. Go directly to Section 3 and perform the annual
procedures described there.
While performing the following procedures, check the floor of the hydraulic unit and
all surfaces for moisture that might indicate a leak. Locate and correct any leaks
detected. Clean the floor of the hydraulic unit so that future leaks will be readily
apparent. Also, check all electrical connectors that can be reached to be sure they are
fully seated and there is no strain on the electrical cables.
FFE
Page 7
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 14
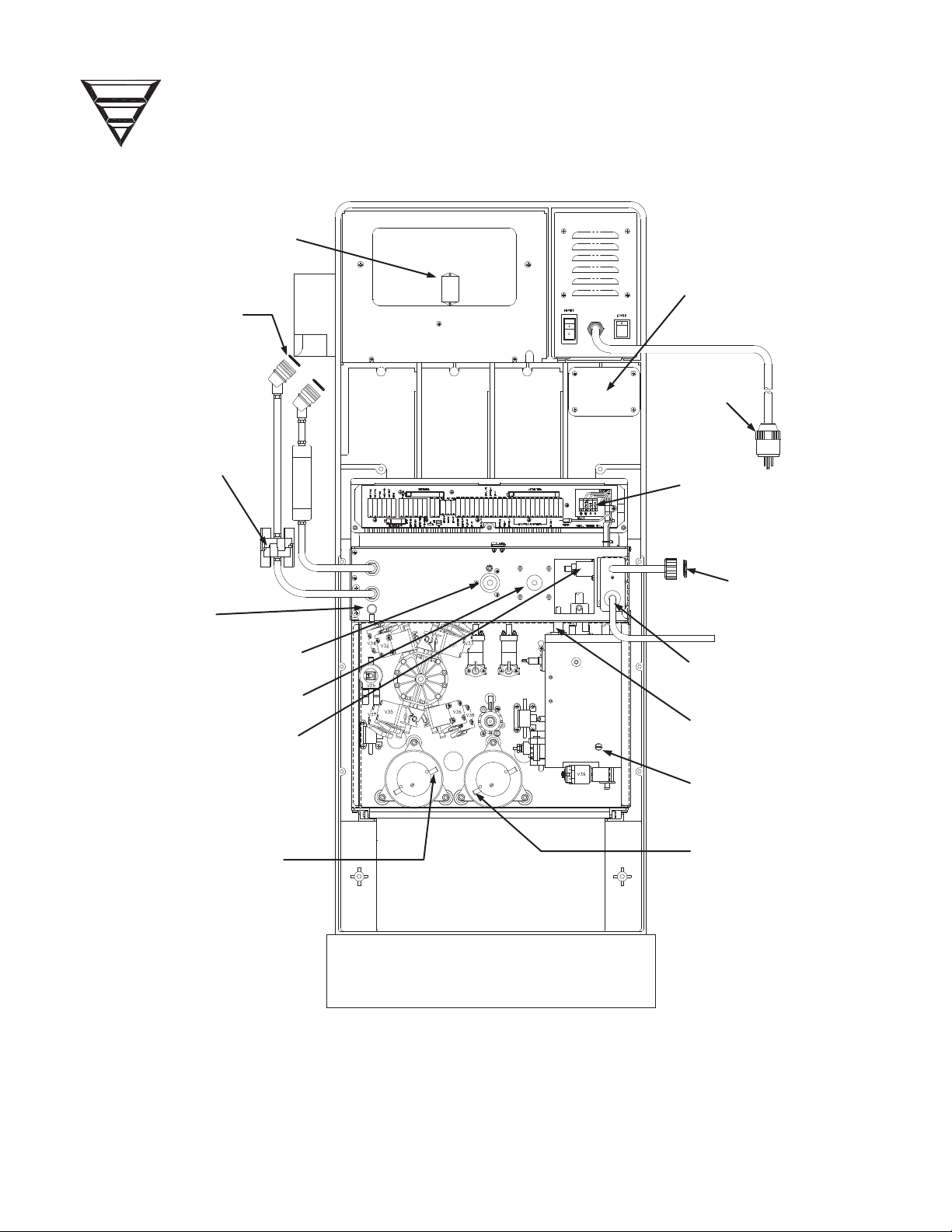
DIALYSATE LINE
CONN. O
-
RINGS
P/N 579097
FLOW PUMP
OUTLET
UF PUMP
ADJUSTMENT
BLOOD PRESSURE
MODULE
INLET PRESSURE
REGULATOR
DRAIN PORT
DEAERATION PUMP
INLET
9-VOLT BATTERY
STRAIN
RELIEF
HEATER
CONNECTIONS
INLET WATER
FILTER P/N 330636
HEATER ELEMENT
P/N 250169
DIALYSATE
INLINE FILTER
P/N 650113
REDUNDANT
GROUND
BICARBONATE PUMP
ACID PUMP BEHIND
INLET PRESSURE
REGULATOR
DEAERATION RESTRICTOR
O
-RINGS P/N 579070
C
P
TIVE
FFE
Page 8
Figure 3 - Fresenius 2008K Hemodialysis System, Rear View.
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 15
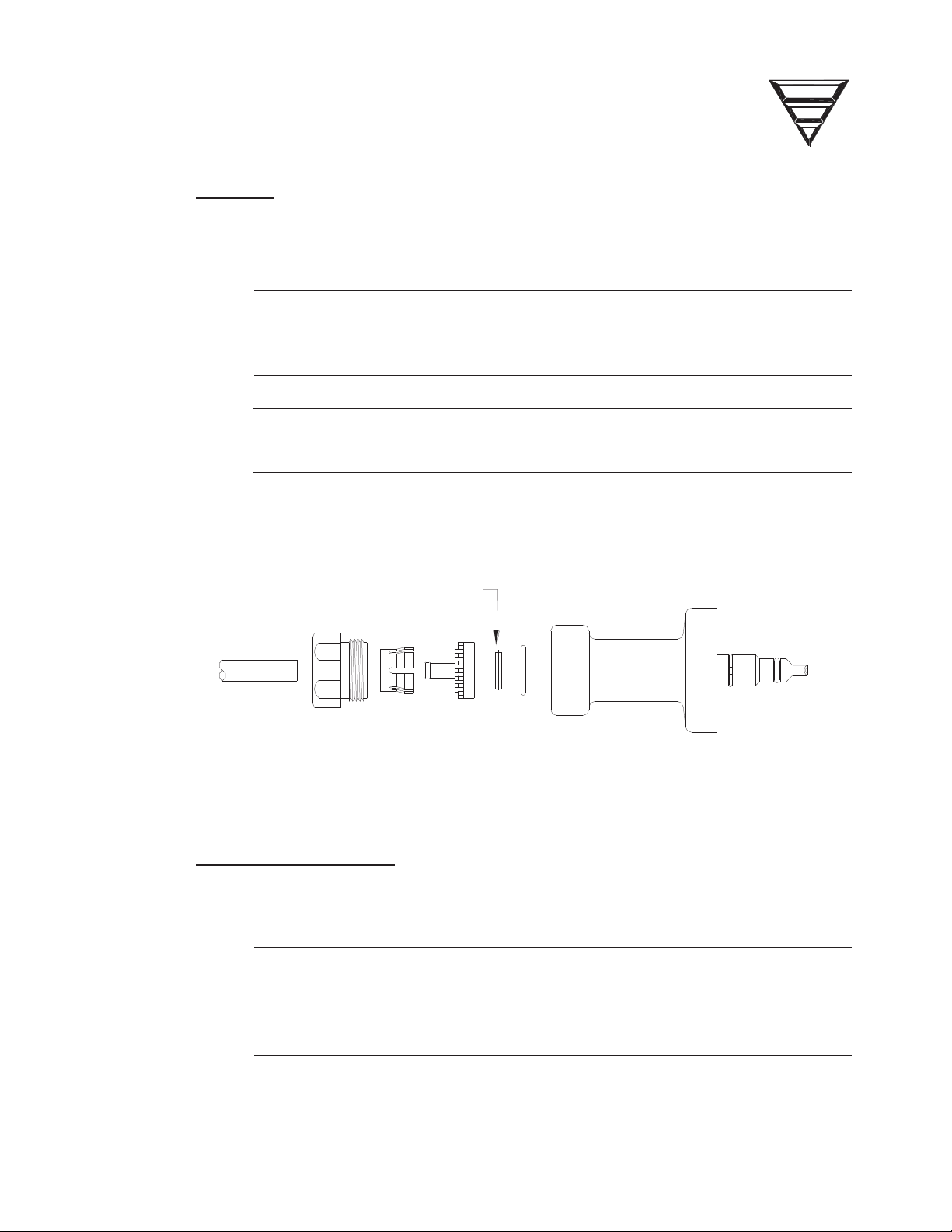
2.1 FILTERS
C
P
Clean filters as follows. Replace any filters that appear damaged or corroded.
1. Inlet Water Filter in the inlet connector of the water supply (See Figure 3, pg 8).
Warning! After cleaning or replacing the inlet filter screen, disinfect the
water inlet line as described in the Operator’s manual and in accordance
with your Unit Policy.
Warning! Do not use excessive O-ring lubricant, as silicone-gel can
damage the hydraulic pressure transducers (P-DIAL and CFS).
2. Filters in the Concentrate and Bicarbonate Connectors (See Figure 4).
3. Dialysate Inline filter (See Figure 3, pg 8).
TIVE
FFE
2.2 PRE-UF PUMP FILTER
FILTER INSERT P/N 566307
Figure 4 - Concentrate and Bicarbonate Connector Assemblies.
4. Replace o-rings in the Dialysate Line Connectors (See Figure 3, pg 8).
Inspect the Pre-UF Pump filter for leaks or distortion. Replace the Pre-UF Pump
Filter if leakage or distortion is found.
Caution: Do not attempt to disassemble the Pre-UF Pump Filter. If not
properly reassembled, the Pre-UF Pump filter may leak. A leak in the
hydraulic system at this location may affect the operation of the machine or
cause fluid loss from the patient.
Page 9
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 16

2.3 CHECK VALVES
C
P
Caution: If a check valve is replaced, ensure it is oriented correctly to
allow fluid flow in the proper direction.
Inspect the UF pump Output check valves (one at the UF pump output and
the other downstream at the UF Sample Port). Replace any that show signs
of wear, damage or leaking.
2.4 HIGH VOLTAGE AC CONNECTIONS
Warning! Dangerous high voltage is present at the connections accessed in
this procedure when the machine is operating. Ensure the machine's power
plug is disconnected from the wall outlet before proceeding.
TIVE
FFE
1. Remove power from the machine then check and tighten the 8-pin heater
connections on the distribution board (See Figure 3, pg 8). Check heater
block AC connections for signs of arcing or melting.
2. If applicable, inspect the power plug for loose or frayed wires. Ensure the
strain relief is securely fastened.
3. Inspect the entire length of the power cord (from plug to strain relief) for
nicks or cuts in the insulation and replace if necessary (Fresenius part
number 150425).
4. Confirm that the strain relief is tightly secured to the power supply chassis.
5. At the strain relief, locate the black, white and green wires from the power
cord (inside the power supply chassis). Follow the black and white wires to
the main power switch. Attached to the main power switch are four (4)
wires (2 black and 2 white). Look for loose connections, cracked insulation,
and signs of overheating, such as discolored or melted insulation. Replace
wires with power supply wire kit (Fresenius part number 190411).
6. Inspect the main power switch and verify that its operation is smooth (no
grinding or sticky operation) and that the wires are not crossed.
Page 10
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 17

7. With a digital multimeter, measure the resistance between the round
P
(ground) pin on the power plug and the redundant ground terminal on the
machine (See Figure 3, pg 8). Verify that the resistance is less than 0.2 ohm.
If the value is above 0.2 ohm, measure the internal resistance of your meter
by shorting the leads together, then subtract this value from the resistance
measured between the power plug ground pin and the redundant ground
terminal on the machine to obtain the true ground resistance.
Warning! Do not operate the machine if the resistance is greater than 0.2
ohm. A shock hazard to operators and patients could exist.
8. Perform the electrical safety checks required by local codes, facility
procedure and the Joint Commission on Accreditation of Healthcare
Organizations.
2.5 UF PUMP
Calibrate the UF Pump Volume (Refer to the 2008K Calibration Procedures –
Fresenius part number 507296.)
2.6 CONDUCTIVITY
Verify that the dialysate conductivity measured by the internal cell in the machine
agrees with an external meter within 0.1mS/cm as follows:
1. Connect an external conductivity meter to the dialysate lines.
2. With the machine in Dialysis Mode and flow ON, compare the value shown
on the external meter with the conductivity shown on the Display screen.
They must be within 0.1mS/cm of each other.
Page 11
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 18

2.7 TEMPERATURE
C
P
TIVE
Verify that the actual dialysate temperature measured by an external meter agrees
with the display screen within 0.5qC at 37qC and 39qC as follows:
1. Connect the dialysate lines to an external meter.
2. Place the machine in Dialysis Mode with concentrate in the system. Clear
any alarms.
3. Select the Temperature button. The button label will change to Temp.
Setting. The value now displayed on this button is the temperature set
point. Adjust the temperature set point to exactly 37.0 then press the
[CONFIRM] key. The button will change back and now reads the actual
temperature of the dialysate again. Wait until this value stabilizes. It will
settle very close to the value set, depending upon inlet water temperature
and other conditions.
4. After the temperature of the dialysate stabilizes, compare the temperature
shown on the Temperature button with the temperature shown on the
external meter connected to the dialysate lines. The two readings must be
within 0.5q of each other.
FFE
5. Repeat steps 3 and 4 with the temperature set to 39.0qC. Verify that the
actual temperature reported on the front panel display screen and the
external meter are within 0.5q of each other after the temperatures stabilize
again.
Page 12
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 19

2.8 ALARM AND PRESSURE HOLDING TESTS
C
P
TIVE
Verify the automatic alarms produce the responses shown in Table 1 and the
machine passes the automatic pressure holding test as follows:
1. Place the dialysate lines in the shunt and close the door.
Warning! The use of a “test drip chamber” or “dummy drip chamber”
must never be used on the treatment floor. It must only be used in a
controlled technical environment.
2. Place a venous chamber filled with water in the holder on the level detector
module.
3. Place the machine in Dialyze mode and start the blood pump. Clear all
blood and water alarms.
4. Select the Test & Options button. On this screen press the Both Tests
button. Press the [CONFIRM] key to start.
5. Observe the machine stepping through the following alarm tests and ensure
that each alarm produces all of the responses shown in Table 1.
FFE
Page 13
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 20
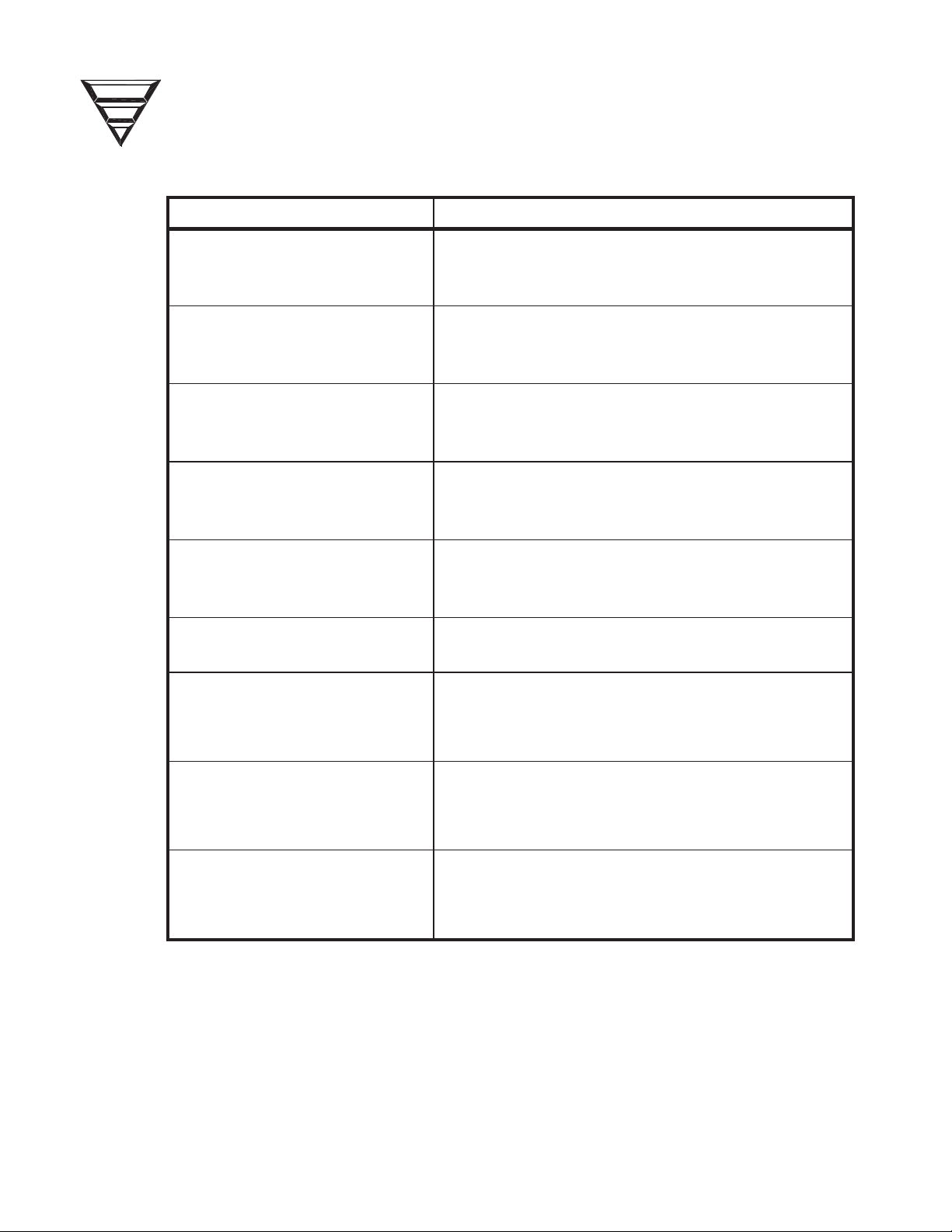
Table 1. Alarm Tests.
ALARM TEST
RESPONSE
Machine in Bypass mode (no flow through the
de (no flow through the
C
P
TIVE
FFE
Air Detector
Blood Leak
Arterial Pressure
Venous Pressure
TMP
9-Volt Battery
Optical Detector
1. Red visual alarm
2. Venous clamp closes
3. Blood pump stops
1 Red visual alarm
2. Venous clamp closes
3. Blood pump stops
1. Red visual alarm
2. Venous clamp closes
3. Blood pump stops
1. Red visual alarm
2. Venous clamp closes
3. Blood pump stops
1. Red visual alarm
2. Venous clamp closes
3. Blood pump stops
Passes if battery voltage is greater than 7.0
volts under a load of 22:.
1. Lower venous alarm limit rises to 0mmHg
causing a venous pressure alarm
2. Venous clamp closes
3. Blood pump stops
Temperature
Conductivity
1. Red visual alarm
2. Yellow visual bypass
3.
dialysate flow indicator).
1. Red visual alarm
2. Yellow visual bypass
3. Machine in Bypass mo
dialysate flow indicator).
Page 14
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 21

C
TIVE
P
6. When the tests shown in Table 1 are complete, the machine conducts a
pressure holding test. When the test ends verify that the display screen
reports TEST COMPLETE, indicating that all the tests were passed
successfully. Press the [RESET] key.
7. Test the UF pump integrity as follows:
x Pull the hydraulics out and remove the output tube from the UF pump.
x Install a 24” tube (Fresenius part number 545325-10) to the empty
output port. Route this tubing out the back of the machine so that it
will not be kinked or pinched when the hydraulics is closed. Close the
hydraulics and place the other end of tube into a collection container.
x Conduct steps 1-6 again.
- If the pressure holding test fails, refer to Section 4.1.
- If the pressure holding test passes, disconnect the 24” tube and
reconnect the original output tube to the UF pump.
FFE
2.9 POWER FAILURE ALARM
8. Test the audible alarm as follows:
x Place a piece of opaque paper inside the housing of the optical detector
to simulate a line containing blood. Close the door of the optical
detector.
x Open the shunt door. Verify that the machine responds with an
audible alarm. Press the [Mute] key and verify that the Mute lamp
lights, and the audible tone stops.
Test the Power Failure alarm by turning the main power switch off on the back of the
power supply with the machine powered on. Verify that the audible alarm sounds. If
no audible alarm occurs, check/replace the 9-Volt battery.
Page 15
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 22

2.10 FINAL CHECKS
C
P
Before returning the machine to clinical use after successful completion of all of the
Preventive Maintenance procedures listed above, complete the following:
x Verify that the machine label with serial number is in place, usually on the back
of the cabinet near the Monitor Control Unit or above the quick connectors of the
open shunt door assembly. Record this serial number on the Preventive
Maintenance Checklist form.
x Verify that no dialysate spills or leaks are visible in the hydraulics or on the
bottom of the cabinet. Clean and dry any spills found and correct the source.
x Verify that all cables are properly routed to prevent pinching or chaffing.
x Verify that all covers are replaced and that all cover screws and mounting
hardware has been replaced.
TIVE
FFE
Caution: Reliable operation of the machine requires that all screws and covers
be properly installed. Ensure that all screws and covers are in place before
returning the machine to clinical use.
x Clean the exterior surfaces of the machine and remove all traces of dirt, oil or
other contaminants.
Caution: Do not use a cleaner containing Dimethyl Benzylammonium
Chloride. This ingredient will damage many plastic surfaces. Certain brands of
cleaners specifically marketed to clinics and hospitals contain this ingredient.
Check the contents of any unknown cleaner before using it.
Page 16
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 23

3.0 ANNUAL (4000 HOUR) PREVENTIVE MAINTENANCE
C
P
3.1 FILTERS AND O-RINGS
TIVE
Perform the following Preventive Maintenance procedures every 12 months or
4000 hours of machine operation, whichever comes first. Perform the procedures
in the order given below to complete the Annual Preventive Maintenance.
Make copies of the Annual Preventive Maintenance Checklist provided at the back
of this manual and use them to record the maintenance done.
Clean filters and replace O-rings as follows. Replace any filters that appear damaged
or corroded.
1. Inlet Water Filter in the inlet connector of the water supply (See Figure 3, pg 8).
Warning! After cleaning or replacing the inlet filter screen, disinfect the
water inlet line as described in the Operator’s manual and in accordance
with your Unit Policy.
Warning! Do not use excessive O-ring lubricant, as silicone-gel can
damage the hydraulic pressure transducers (P-DIAL and CFS).
FFE
2. Filters and O-rings in the Concentrate and Bicarbonate Connectors
(See Figure 5). Replace all three O-rings in each connector.
FILTER INSERT P/N 566307
O-RING P/N 579092
Figure 5 - Concentrate and Bicarbonate Connector Assemblies.
3. Dialysate Inline filter (See Figure 3, pg 8).
4. O-rings in the Dialysate Line Connectors (See Figure 3, pg 8).
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
O-RING
CONCENTRATE CONENCTOR (RED): P/N 641759
BICARBONATE CONNECTOR (BLUE): P/N 579072
O-RING P/N 579070
Page 17
rinted 5/18/2012 12:00PM
Page 24

3.2 PRE-UF PUMP FILTER
C
P
3.3 CHECK VALVES
TIVE
Replace the Pre-UF Pump Filter (Fresenius part number 672574).
Caution: Do not attempt to disassemble the Pre-UF Pump Filter. If not properly
reassembled, the Pre-UF Pump filter may leak. A leak in the hydraulic system at this
location may affect the operation of the machine or cause fluid loss from the patient.
Caution: Ensure each check valve is oriented correctly to allow fluid flow in the
proper direction.
Replace the UF Pump Output check valves. There are two check valves at the UF pump
output. One is at the UF pump itself. The other is downstream at the UF Sample Port.
3.4 DEAERATION RESTRICTOR
FFE
3.5 DIAPHRAGM PUMPS
Note: On newer machines with hydrochamber assemblies the deaeration restrictor is
a glass bead with a fixed orifice built into one of the hydrochamber interconnecting
tubes. Since there are no o-rings in this type of setup, disregard this step. If a screw
is present in the back side of the hydroblock proceed with the following check.
With the machine off, replace the deaeration restrictor O-rings by clamping the
hydroblock vent tube and removing the deaeration restrictor from the hydroblock (See
Figure 3, pg 8). Replace the two O-rings on the deaeration restrictor (Fresenius part
number 579070). Clean any debris that may be present in the angled hole at the tip of
the deaeration restrictor.
Rebuild the UF, Bicarbonate and Concentrate diaphragm pumps as described in
Section 4.1 and 4.2.
Page 18
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 25

3.6 HEATER ELEMENT
C
P
TIVE
Warning! Dangerous high voltage is present at these connections when the
machine is operating. The heater element is hot enough to inflict serious injury
if it is touched while power is applied or shortly after power is removed. Ensure
the machine is disconnected from the wall outlet.
1. Remove the rear access panels and distribution board cover so that the wires of
the heater are accessable.
Note: If the heater element is replaced, replace the heater O-ring as well
(P/N 579075).
2. Remove the heater element from the hydroblock on the machine (See Figure 3,
pg 8). Inspect the heater element for signs of corrosion. If corrosion exists,
replace the heater element.
3. Attach one lead of the voltmeter to the ground (yellow / green wire) and the
other lead to the brown or blue wire. Measure the resistance. Resistance should
be “ OL “ or greater than 19.9 meg-ohms. If this is not the case, replace the
heater element.
FFE
3.7 HIGH VOLTAGE AC CONNECTIONS
4. After completing this check, reinstall the heater and O-ring .
Perform the Six (6) Month Preventive Maintenance procedures in Section 2.4.
Page 19
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 26

3.8 DEAERATION MOTOR BRUSHES
Use Marker to make
marks to ensure proper
reassembling of motor
C
P
TIVE
Replace the deaeration motor brushes every 8000 hrs using the following steps:
1. Remove the complete deaeration motor/pump assembly from the machine.
2. Using a marker, make marks on the motor housing and motor cap as
illustrated below. These marks will ensure the correct alignment when
reassembling the motor case.
Use marker to make hatch
marks to ensure proper
reassembling of motor
Motor Cap
Figure 6 – Deaeration Motor/Pump Assembly with marks.
FFE
3. Using a T-25 Torx screwdriver, remove the two screws holding the motor
cap to the motor housing.
4. Remove the motor cap to gain access to the motor brushes. Replace the
motor brushes.
5. Reassemble the motor, aligning the marks.
6. While holding the pieces together, install and tighten the two T-25 screws.
Note: If the motor case and motor cap are not installed using the marks, the
motor will run in reverse and flow errors will result.
Page 20
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 27

3.9 INLET WATER PRESSURE REGULATOR
C
P
Verify that the regulator provides the proper water pressure as follows:
1. Shut off the water supply to the machine.
2. Install a gauge with a T-fitting to monitor the pressure at the outlet side of
the Inlet Pressure Regulator (See Figure 3, pg 8).
Caution: Use tie wraps or tubing clamps to secure the connections. The
water pressure may be sufficient to blow the lines off the fittings if they are
not secured.
3. Turn the water supply to the machine ON.
4. With the dialysate lines in the shunt, select Rinse Mode. The pressure gauge
will cycle between two readings as water inlet valve opens and closes.
TIVE
FFE
Note: Rinse mode causes all valves, pumps, and motors to run/activate.
While in Rinse Mode inspect for any leaks; paying particular attention to
areas of the hydraulics that were disassembled in previous steps during the
preventive maintenance procedures.
5. Verify that the pressure gauge reads between 18 and 20psi when the pressure
is at its highest value, and reads greater than 8psi when the pressure is at its
lowest value.
Note: Readings below 8psi at the lowest level indicates inadequate inlet
water flow into the machine. This may be caused by a dirty inlet filter
screen or problems with the treated water supply.
6. Turn off the source water supply.
7. Turn the machine OFF, remove the gauge and reconnect the tubing using
clamps to prevent leaks.
8. Turn the treated water supply source ON, turn the machine ON and select
Dialysis Mode. Start dialysate flow and inspect all hoses and connections.
Ensure that there are no leaks.
Page 21
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 28

3.10 ONLINE CLEARANCE TEST (IF APPLICABLE)
C
P
If the machine is equipped with Online Clearance (OLC), conduct the following test:
OLC Self-Test
1. With stable temperature and conductivity, start an OLC Self-Test by
pressing the OLC Self-Test screen button on the Kt/V screen.
TIVE
FFE
Note: Switch to the debug screens by pressing and holding the [
[] keys at the same time for approximately 1 sec. The main display will
change indicating that the machine is in debug mode.
2. When the OLC Self-Test is complete, go to debug screen #5 and confirm that
0 Test (0TST prior to functional software version 3.02) is in the range r20.
Note: If 0 Test (0TST) is out of range, the OLC Self-Test will fail. If
this happens, conduct temperature (PRE & POST) and conductivity
calibrations, then conduct OLC Self-Test again.
OLC Self-Test Troubleshooting
If the OLC Self-Test continues to fail after conducting the temperature (PRE &
POST) and the conductivity calibrations, conduct the following troubleshooting steps:
1. Turn the machine ON and allow the machine to run in Dialysis Mode at
500ml/min flow rate for approximately 10 minutes. This allows the
machine to come up to temperature and conductivity.
Note: On functional software prior to version 3.02, an OLC Self Test will
start automatically as soon as temperature and conductivity are stable. Once
the test starts cancel it by opening the shunt door. The cancel message will
be hidden by the Shunt Door Open banner. Close the shunt door and
confirm that OLC Self Test banner is gone.
] and
2. Once the machine is warmed up, remove X3 (MON-NTC) and X7 (COND)
from the distribution board.
Page 22
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 29

3. Using the Resistor Plug Set for OLC Testing (Fresenius part number 190168),
TPRE
-
=
CPRE
-
=
C
P
plug the 6.04K: plug into X3 (MON-NTC) and the 274: plug into X7
(COND) on the distribution board.
Note: Due to the resistor placement inside the OLC test plugs, the
orientation of the plug when inserted onto the distribution board is irrelevant.
4. Switch to the debug screens by pressing and holding the [] and [] keys at the
same time for approximately 1 sec. The main display will change indicating
that the machine is in debug mode.
TIVE
FFE
5. Use the [
6. On debug screen #5, locate TPRE and observe its value for 1 minute. During
this time, record the highest and lowest values observed in the table below.
7. Calculate the difference between the highest and lowest TPRE value and
record it in the table above.
8. Verify that the difference for TPRE d 2. If the difference for TPRE > 2, then
possible causes are sensor board, motor noise, or -12V problems.
9. Again on debug screen #5, locate CPRE and observe its value for 1 minute.
During this time record the highest and lowest values observed in the table
below.
] and [] keys to go to debug screen #5.
Highest Value Lowest Value Difference
Highest Value Lowest Value Difference
10. Calculate the difference between the highest and lowest CPRE value and
record it in the table above.
11. Verify that the difference for CPRE d 8. If the difference for CPRE > 8, then
possible causes are sensor board, motor noise, or -12V problems.
Page 23
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 30

TPOS
-
=
CPOS
-
=
C
TIVE
P
12. Remove the 6.04K: and 274: plug from the distribution board and
replace the original connectors.
13. Remove X44 (NTC-POST) and X13 (COND-POS) from the distribution
board.
14. Using the Resistor Plug Set for OLC Testing (Fresenius part number
190168), plug the 6.04K: plug into X44 (NTC-POST) and the 274:
plug into X13 (COND-POS) on the distribution board.
15. On debug screen #5, locate TPOS and observe its value for 1 minute.
During this time record the highest and lowest values observed in the
table below.
Highest Value Lowest Value Difference
16. Calculate the difference between the highest and lowest TPOS value and
record it in the table above.
FFE
17. Verify that the difference for TPOS d 2. If the difference for TPOS > 2, then
possible causes are sensor board, motor noise, or -12V problems.
18. Again on debug screen #5, locate CPOS and observe its value for 1 minute.
During this time record the highest and lowest values observed in the table
below.
Highest Value Lowest Value Difference
19. Calculate the difference between the highest and lowest CPOS value and
record it in the table above.
20. Verify that the difference for CPOS d 8. If the difference for CPOS > 8, then
possible causes are sensor board, motor noise, or -12V problems.
Page 24
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 31

C
TIVE
P
21. With the 6.04K: plug on X44 (NTC-POST) and the 274: plug on X13
(COND-POS) of the distribution board, verify that CPOS does not change
more then 10 increments when the dialysate flow is turned OFF. (Note:
Conduct this test for the dialysate flow ON to OFF transition.)
22. Remove the 6.04K: and 274: plug from the distribution board and
replace the original connectors.
23. With stable temperature and conductivity, start an OLC Self Test by
pressing the OLC Self-Test screen button on the Kt/V screen.
24. When the OLC Self Test is complete, go to debug screen #5 and confirm that
0 Test (0TST prior to functional software version 3.02) is in the range r20.
FFE
Page 25
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 32

3.11 DEAERATION AND LOADING PRESSURE
atmospheric
Minimum target deaeration
feet
mmHg
inches of Hg
0
760
-24.0
1000
728
-23.0
2000
697
-22.0
3000
667
-21.0
4000
639
-20.0
5000
612
-19.0
6000
585
-18.5
7000
561
-17.5
8000
537
-16.9
9000
514
-16.2
10000
492
-15.5
C
P
Verify that the deaeration pressure is between –24 and -25 inHg and the loading
pressure is between 18 and 20psi (between 23 and 25psi if a DIASAFEfilter system
is installed) as follows:
1. With dialysate flow OFF, install a gauge with a T-fitting to monitor the pressure
on the inlet (suction) side of the deaeration pump (See Figure 3, pg 8).
2. Connect a gauge equipped with a yellow connector into the red
ACETATE/ACID port.
3. In Dialysis Mode, turn dialysate flow ON and verify that the gauge on the
deaeration pump indicates between -24 and -25 inHg. The needle will be
vibrating somewhat. Verify that it does not go higher than -24 inHg or
lower than -25 Hg. Verify that the gauge in the ACETATE/ACID port
indicates between 18 and 20psi (between 23 and 25psi if a DIASAFE
system is installed).
filter
TIVE
FFE
Note: When the machine is at a different elevation above sea level, it may
be difficult or impossible to achieve -24inHg. The following table will help
in determining the appropriate deaeration pressure calibration point at
different elevations:
Approx.
Elevation
pressure
pressure relative to
atmospheric pressure
Page 26
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 33

3.12 FLOW RELIEF PRESSURE
C
P
TIVE
Verify that the flow relief pressure is between 29 and 30psi (between 35 and 36psi if a
DIASAFEfilter system is installed) as follows:
1. Turn the machine on in Service mode. From the Calibrate Hydraulics
screen, select the Flow Pressure screen button. The screen will change to
the following:
FFE
2. Connect a gauge in line at the output of the flow pump (See Figure 3, pg 8).
Note: The output side of the flow pump is the side with the white
reinforced jacket over the line. The input side has clear plastic line
3. Press the [CONFIRM] key to start the flow pump.
4. Verify the gauge indicates a pressure between 29 and 30psi (between 35 and
36psi if a DIASAFE
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
filter system is installed.
P/N 507781 Rev. D
Page 27
rinted 5/18/2012 12:00PM
Page 34

3.13 CONCENTRATE AND BICARBONATE PUMPS
C
P
3.14 UF PUMP
3.15 CONDUCTIVITY
3.16 TEMPERATURE
TIVE
Test the Concentrate and Bicarbonate diaphragm pumps as described in Sections 4.3.
Calibrate the UF Pump Volume (Refer to the 2008K Calibration Procedures –
Fresenius part number 507296.)
Verify that the dialysate conductivity measured by the internal cell in the machine
agrees with an external meter within 0.1mS/cm as described in Section 2.6.
Verify that the dialysate temperature shown on the front panel Display screen agrees
with an external meter within 0.5qC at 37qC and 39qC as described in Section 2.7.
3.17 VOLT HI LO DETECT
FFE
Verify that the 5-volt supply is operating within 0.2 volts as reported on the debug
screen as follows:
1. Switch to the debug screens by pressing and holding the [
the same time for approximately 1 sec. The main display will change
indicating that the machine is in debug mode.
2. Use the [
3. Locate the 5V value on the display screen. This value must be between
4.8V and 5.2V.
Note: If the 5V value is not within limits, the problem is most likely the
12volt supply out of tolerance. Perform the Volt Hi Lo Detect calibration
procedure. Refer to the 2008K Calibration Procedures – Fresenius part
number 507296.
] or [] keys to go to debug screen #4.
] or [] keys at
Page 28
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 35

3.18 BLOOD LEAK AND DIMNESS
C
P
Verify that the blood leak level is between 4.5 and 5.2 volts and the blood dimness
level is within 5.0 r1.0 volts as follows:
TIVE
FFE
1. Switch to the debug screens by pressing and holding the [
the same time for approximately 1 sec. The main display will change
indicating that the machine is in debug mode.
2. Use the [
3. Locate the LEAK value on the display screen. This value must be between
4.5 and 5.2.
4. Locate the DIMN value on the display screen. This value must be between
4.0 and 6.0.
Note: If these values are outside the limits given, perform a bleach rinse
to clean the glass detector tube inside the machine before considering other
repairs.
] or [] keys to go to debug screen #4.
] and [] keys at
Page 29
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 36

3.19 ARTERIAL, VENOUS AND TRANSMEMBRANE PRESSURE
C
P
ARTERIAL PRESSURE
1. Inspect the internal pressure transducer protector for contamination. If
contamination is found, replace the pressure transducer protector
(Fresenius part number 650158) and remove and disinfect the pressure
port with 1:100 bleach for a minimum of 15 minutes.
TIVE
FFE
2. Open the arterial transducer port P
(atmospheric pressure). Verify that the Arterial Pressure bargraph
indicates 0.
3. Attach a syringe and a calibrated pressure gauge to the P
T-fitting.
4. Push the syringe in to show a pressure of 200mmHg on the external gauge.
Verify that the Arterial Pressure bargraph indicates 200.
5. Switch to the debug screens by pressing and holding the [
the same time for approximately 1 sec. The main display will change
indicating that the machine is in debug mode.
6. Use the [
7. Increase the pressure to a range of 310 – 315mmHg. Clamp off the tubing at
the P
maximum allowable leakage is 2mmHg in 30 seconds.
] and [] keys to go to debug screen #1 and locate the ART value.
port and monitor the debug ART value for 30 seconds. The
.
ART
on the blood pump module to air
.
ART
port using a
.
ART
] and [] keys at
Page 30
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 37

VENOUS PRESSURE
C
P
1. Inspect the internal pressure transducer protector for contamination. If
contamination is found, replace the pressure transducer protector
(Fresenius part number 650158) and remove and disinfect the pressure
port with 1:100 bleach for a minimum of 15 minutes.
TIVE
FFE
2. Open the venous transducer port P
(atmospheric pressure). Verify that the Venous Pressure bargraph
indicates 0.
3. Attach a syringe and a calibrated pressure gauge to the P
T-fitting.
4. Push the syringe plunger in to show 400mmHg on the external pressure
gauge. Verify that the Venous Pressure bargraph indicates 400.
5. Switch to the debug screens by pressing and holding the [
the same time for approximately 1 sec. The main display will change
indicating that the machine is in debug mode.
6. Use the [
value.
7. Decrease the pressure to a range of 310 – 315mmHg. Clamp off the tubing
at the P
maximum allowable leakage is 2mmHg in 30 seconds.
] and [] keys to go to debug screen #1 and locate the VEN
port and monitor the debug VEN value for 30 seconds. The
.
VEN
on the level detector module to air
.
VEN
port using a
.
VEN
] and [] keys at
Page 31
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 38

TRANSMEMBRANE PRESSURE
C
P
1. With dialysate flow ON, ensure the dialysate lines are full of fluid and no air
is visible passing through the flow indicator.
2. Hang a four-way connector to the I.V. pole at normal dialyzer height.
3. Set dialysate flow to 500ml/min, then press the [CONFIRM] key.
TIVE
FFE
4. Open the arterial (P
(atmospheric pressure).
5. Turn the blood pump off.
6. Turn dialysate flow OFF, remove the dialysate lines from the shunt and
attach them to the connector. Close the shunt door.
Note: Filling the lines before removing them from the shunt will avoid
wetting the pressure gauge transducer during the test.
7. Connect a 30cc syringe to one of the four-way connector outlets and a
pressure gauge to the remaining outlet.
8. Switch to the debug screens by pressing and holding the [
the same time for approximately 1 sec. The main display will change
indicating that the machine is in debug mode.
9. Use the [
10. Use the syringe to set the pressure on the external gauge to 0mmHg.
Calculate the dialysate pressure measured by the machine as follows:
] or [] keys to go to debug screen #1.
) and venous (P
.
ART
) transducer ports to air
.
VEN
] and [] keys at
x Note the value shown for TMP on the display screen.
x Subtract the value shown for VEN on the display screen.
11. Verify that the calculated value is between +20 and -10mmHg.
12. Use the syringe to create a pressure of -250mmHg on the pressure gauge.
13. Calculate the dialysate pressure measured by the machine again as
described in step 10. Verify that the calculated value is between
-230 and -260mmHg.
Page 32
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 39

3.20 DIALYSATE FLOW
C
P
TIVE
Verify the dialysate flow is within r3% of the stated rate at 500ml/min as follows:
Note: All flow rates are controlled by the software. Testing the rate at
500ml/min verifies the accuracy of all rates.
1. In Dialysis Mode, turn the dialysate flow ON at 500ml/min. Verify that the
UF pump is OFF and the machine is out of bypass. Allow the dialysate flow
to run for 2 minutes, minimum, before continuing.
Note: Do not collect the spent dialysate from the drain hose in the next
step. The accuracy of the collection will be affected if not collected directly
from the drain port with the drain hose removed.
2. Collect spent dialysate from the drain port on the back of the machine (See
Figure 3, pg 8) for exactly 1 minute. Verify that the amount collected is
between 485 and 515ml.
3.21 HEPARIN PUMP
FFE
If the machine is equipped with a heparin pump module, clean and test the pump as
follows:
1. Remove the heparin pump module from the machine and wipe the lower
edge of the module and the heparin pump cabinet opening to remove any
residual disinfecting agent.
2. Reinstall the heparin pump into the machine cabinet. When installing the
module screws do not use a power screwdriver.
3. Place the machine in Dialysis Mode and clear all alarms.
4. Select the Heparin screen button. On this screen press the Syringe button.
Use the [
[CONFIRM] key.
5. Press the Load Syringe button. Press the [CONFIRM] key and the carriage
will fully retract downward.
6. When the carriage has stopped, press the [Escape] key and clean the
carriage bar (See Figure 7).
] or [] keys to select the syringe being used for this test and press the
Page 33
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 40

7. Extend the syringe plunger past the 10ml mark. Install the syringe into the
SYRINGE BODY HOLDER
CARRIAGE BAR
CARRIAGE / PLUNGER
RELEASE LEVER
PLUNGER HOLDER
CARRIAGE
P
pump and latch the plunger handle into the carriage. If needed, squeeze the
carriage/plunger release lever to slide the carriage up to meet the plunger
handle. The syringe plunger should be at a position greater than 10ml.
Figure 7 - Heparin Pump Module.
8. Press the Heparin Prime button. Press and hold the [CONFIRM] key. The
plunger will start moving towards the 10ml mark. Release the [CONFIRM]
key as soon as the syringe plunger is aligned exactly with the 10ml mark on
the side of the syringe body.
9. Select the Bolus data button. Use the [
] or [] keys to set a value of 5.0ml
then press the [CONFIRM] key.
10. Select the Total Infused data button. Press the [0] (zero) key and then the
[CONFIRM] key.
11. Press the Infuse Bolus button. Press the [CONFIRM] key and start timing
the interval with the stopwatch.
Page 34
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 41

12. When the pump stops, use the table below to verify the time to run a 5ml
Type
Syringe Size
5ml Bolus Time
B-D
10ml
29.0 – 30.0 seconds
Terumo
10ml (12ml)
24.0 – 25.0 seconds
Monoject
10ml (12ml)
24.5 – 25.5 seconds
C
P
Bolus is correct for the selected syringe type.
13. Also, verify that the Total Infused data button reads 5.0 and that the syringe
plunger has moved to between 4.8 to 5.2 on the syringe scale.
14. Move the carriage all the way up to the end of its travel.
TIVE
FFE
15. Select the RATE data button. Use the [
9.9ml/hr then press the [CONFIRM] key
16. Press the [Heparin on/off] key to start the pump. The green lamp above the
key should light
17. Verify that a HEPARIN PUMP ALARM is displayed on the status line
within 2 minutes
Note: If the machine is not alarm free, another message of higher priority
may be displayed on the status line.
] or [] keys to set a value of
Page 35
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 42

3.22 BLOOD PUMP
C
P
TIVE
Clean and test the arterial blood pump module as follows:
1. Remove the blood pump rotor by opening the door, pulling out the handle
and turning the rotor 90 degrees
2. Clean the rollers with a cloth dampened only with water.
3. With the crank lever pulled out, locate and lubricate the crank lever retainer
ball. If the crank lever is hard to pull out, replace the crank lever retainer
assembly (Fresenius part number 564301).
4. Without removing the plastic sleeve, inspect the solid guide post on all four
(4) tubing guides. The solid guide post should not be loose or bent. If any
of the solid guide posts are loose or bent, the rotor assembly needs to be
replaced. (Fresenius part number M30990).
Note: The plastic sleeve will make it feel like the solid guide post is loose.
When inspecting, pay close attention to the movement of the metal shaft.
5. Clean the inside of the blood pump housing with the damp cloth.
Compressed air may be used if it is available.
FFE
6. Remove the blood pump module from the machine and inspect the motor
gearbox. In heavy use, some oil may accumulate on the gearbox housing.
Wipe the housing clean.
7. Wipe the lower edge of the module and the blood pump cabinet opening to
remove any residual disinfecting agent.
8. Reassemble the blood pump module and reinstall it in the machine cabinet.
When installing the module screws do not use a power screwdriver.
9. Place the machine in Dialysis Mode and clear all blood alarms.
10. Start the blood pump if it is not already running.
11. Open the blood pump door. Verify that the red alarm light on the blood
pump module lights within 15 or 30 seconds.
Note: Delay time before the blood pump alarm lights is set by dipswitch 4 on
the LP955 blood pump board.
Page 36
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 43

Clamp
C
TIVE
P
12. Close the blood pump door and press the [RESET] key to clear the alarm.
13. Test the pump as follows:
x Set the Tubing Size to 8mm.
x Insert a bloodline in the pump. Do not change the setting of the
tubing size selector, even if you are not using 8mm tubing.
x Connect a pressure gauge to the bloodline in the pump (See
Figure 8) and allow the rotor to pull up 37qC r 1.5qC water. Let
this fluid flow past the pressure gauge long enough to clear out air.
FFE
Figure 8 - Blood Pump Rotor Occlusion Test
x With the pump running at 600ml/min, clamp the outlet so that the
pressure gauge is between the output of the pump and the clamp
(See Figure 8). The peak pressure on the gauge must be between
25 and 35psi. If the peak pressure is out of range, replace the
blood pump rotor springs (Fresenius part number 650174).
x Set the Tubing Size Selector to agree with the size of bloodline
used in the pump.
Page 37
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 44

3.23 LEVEL DETECTOR
C
P
Caution: In the following steps, the level detector must be removed from the machine
cabinet. Before returning it to the cabinet, wipe the lower edge of the module and the
level detector cabinet opening to remove any residual disinfecting agent. When
installing the module screws do not use a power screwdriver.
ALARM TEST
1. Calibrate level detector (Refer to Section 3.2 of the 2008K Calibration
Procedures – Fresenius part number 507296).
2. Place the machine in Dialysis Mode and turn the blood pump ON. Insert a
water filled venous chamber into the level detector and clear all alarms.
3. Position the Level Detector so you can watch the Channel 1 and Channel 2
LED's on the circuit board (See Figure 10).
TIVE
FFE
4. While watching the Channel 1 and Channel 2 LED's, remove the venous
chamber to create a blood alarm and close the occlusion clamp. Verify that
the Channel 1 LED lights first followed quickly by Channel 2.
OCCLUSION CLAMP TEST
1. Place a venous line into the closed occlusion clamp. Do not connect this
line to the venous chamber in the level detector.
2. Place the lower end of the venous line below the occlusion clamp in a
container of water positioned so that air escaping from the end of the line is
easily seen.
3. Connect a syringe and a pressure gauge to the venous line above the
occlusion clamp.
4. With the syringe, apply a pressure of at least 30psi (1550mmHg) to the
venous line while watching the end of the venous line in the water.
5. Verify that no air escapes from the venous line, indicating that the clamp is
fully occluding the line.
Page 38
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 45

RELAY CONTACT TEST
C
P
A relay prevents the level up and level down membrane keys on the face of the
level detector to function if they are pressed at the same time.
Various module configurations are available. Use the following information to
select the correct method to use to check the relay contacts:
x Use Method 1 if LP450 has LED’s D6 & D19 (See Figure 9)
x Use Method 2 if LP450 does not have relay test LED’s then locate a separate
relay board LP1026 with test LED’s D3 & D4 (See Figure 9).
x Use Method 3 if LP1026 is present and does not have LED’s D3 & D4
(See Figure 10).
Method 1:
1. With the machine in Dialysis Mode and the blood pump turned ON, insert a
water filled venous chamber into the level detector and clear all alarms
TIVE
FFE
2. Locate the relay test LED’s D6 and D19 on the LP450 board.
3. To test the LED’s, press the level down switch on the face of the level
detector and verify that both LED’s D6 and D19 light. The on-board air
pump will also run. If both LED’s light, proceed to step 4.
Note: If either LED doesn’t light during the LED test (step 3), then the results
of the Relay Contact Test (step 4) would be invalid. In this case use Method 3
instead.
While in alarm condition (clamp closed), attach the ground lead of a voltmeter
to TP3 (ground) on the LP450 board. Measure the voltage on the solder side of
pins 1 and 2 of X153 on the LP450 board. Verify that both pins are 0 volts. If
voltage is present, the relay contacts are bad.
4. To test the relay contacts, remove the venous chamber to create a blood alarm
and verify that both LED’s D6 and D19 do not light when the level down
switch is pressed. If either LED lights, the relay contacts are bad.
Page 39
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 46

CHANNEL 1 LED
LIGHTS FIRST
CHANNEL 2 LED
LIGHTS SECOND
X4 ON RELAY
BOARD LP1026
TP3 (GROUND)
RELAY CONTACT TEST
LED’S D19 & D6 ON LP450
BOARD WHEN LP1026 IS
NOT PRESENT
X153 ON LP450
RELAY CONTACT
TEST LED’S D3 & D4
ON LP1026 BOARD.
C
TIVE
P
Figure 9 - Level Detector with Relay Contest Test LED’s.
FFE
Page 40
Figure 10 - Level Detector without Relay Contest Test LED’s.
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 47

Method 2:
C
P
1. With the machine in Dialysis Mode and the blood pump turned ON, insert a
water filled venous chamber into the level detector and clear all alarms.
2. Locate the relay test LED’s D3 and D4 on the LP1026 board.
3. To test the LED’s, press the level down switch on the face of the level
detector and verify that both LED’s D3 and D4 light. The on-board air
pump will also run. If both LED’s light, proceed to step 4.
Note: If either LED doesn’t light during the LED test (step 3), then the results
of the Relay Contact Test (step 4) would be invalid. In this case use Method 3
instead.
4. To test the relay contacts, remove the venous chamber to create a blood
alarm and verify that both LED’s D3 and D4 do not light when the level
down switch is pressed. If either LED lights, the relay contacts are bad
TIVE
FFE
Method 3:
1. With the machine in Dialysis Mode and the blood pump turned ON, insert a
water filled venous chamber into the level detector and clear all alarms
2. Locate the solder side of X4 on the LP1026 (or X153 on the LP450).
3. Attach the ground lead of a voltmeter to TP3 (ground) on the LP450 board.
4. Test for 24 volts at X4 (X153) by pressing the level down switch on the face
of the level detector and verify that both pins 1 and 2 on the solder side of
X4 (X153) are 24 volts.
5. To test the relay contacts, remove the venous chamber to create a blood
alarm and measure the voltage on the solder side of pins 1 and 2 of X4
(X153). Verify that both pins are 0 volts. If voltage is present, the relay
contacts are bad.
Page 41
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 48

3.24 ALARM AND PRESSURE HOLDING TESTS
C
P
Perform the Six (6) Month Preventive Maintenance procedures in Section 2.8.
3.25 RINSE CHECKS
Perform the Rinse Checks as follows:
1. Place the machine in rinse mode, then turn the water supply off. Verify that
the display screen shows NO WATER.
2. Turn water supply on
3. Start rinse mode and watch the flow from the drain line. The water from the
drain line will stop at one point for 15 seconds. At this time put the drain
line in a 1000ml graduated cylinder. Start timing when the flow starts again.
At 30 seconds, remove the line from the graduated cylinder. Verify that a
minimum of 310ml is collected.
TIVE
FFE
4. To avoid problems with pre-rinse, allow the rinse to complete prior to
proceeding to the next step.
5. Open the shunt door and remove the dialysate lines.
6. Connect the dialysate lines to an external meter capable of reading at least
85qC.
7. Close the shunt door.
8. Start the machine in heat disinfect mode with the dialysate lines on the
external meter as follows:
x With the shunt door closed, reach up into the opening at the bottom of the
shunt and find the two spring-loaded switches that protrude through the
holes next to the connectors. Push these switches in with your fingers,
simulating dialysate lines connected to the shunt.
x While holding these switches pushed in, press the Heat Disinfect
button on the startup screen.
x While holding the switches, open the shunt door. Verify the heat
disinfect does not get interrupted.
x Leave the shunt door open; closing it will stop the heat disinfect mode.
Page 42
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 49

3.26 BATTERY TEST AND POWER FAILURE ALARM
C
P
TIVE
Warning! Scalding hot water is passing through the dialysate lines and the
external meter manifold. Allow the dialysate lines and the external meter
manifold to cool before disconnecting the lines.
9. The heat disinfect timer will not be operating. It is not needed. Monitor the
external temperature meter and verify that the temperature rises to between
80qC and 90qC.
Note: Some external temperature meters may not display correctly at higher
temperatures. Consult the appropriate product literature from the meter
manufacturer for applicable conversion charts.
Remove the 9-volt battery located behind the monitor unit (See Figure 3, pg 8). The
battery is inside the plastic housing. Press the cover in and to the left to release it,
then pull it out.
FFE
Note: If the 9-volt battery is swollen, the power logic board is bad and should be
replaced.
With the battery removed, perform an Alarm Test and confirm the Battery Test fails.
Note: If the Battery Test passes with the 9-Volt battery removed, IC9 is bad on the
Power Logic Board and should be replaced.
Replace the 9-volt battery with a new one observing the correct polarity marked
inside the holder. Install the battery/holder assembly into the monitor unit.
With the new battery in place, perform an Alarm Test and confirm the Battery Test
passes.
Test the power failure alarm by removing the machine power plug from the wall
outlet with the machine powered on. Verify that the audible alarm sounds.
Page 43
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 50

3.27 BLOOD PRESSURE MODULE
Module Type
Time to 250
M400
≤ 7.3 seconds
M2000, M2600 or M3600
≤ 10.0 seconds
TM-2910 or TM-2915
≤ 10.0 seconds
SunTech
≤ 11.0 seconds
P
Note: There are seven types of blood pressure modules that can be connected to the
2008K. Since each module type has different test criteria and the 2008K is unable to
detect which module is connected, disregard the displayed Pass/Fail results and use the
appropriate table to determine pass or fail.
Test the blood pressure module (See Figure 3, pg 8) as follows:
Place the machine in Service Mode.
Select the Test BP Module button and the screen will change.
INFLATION SPEED TEST
From the Test BP Module screen, select the Inflation Speed button.
1. Connect the pressure tubing from the module to the 700cc port on the Test
Device. Ensure the tubing fits snugly on the ports.
2. Press the [CONFIRM] key. The screen will change and the test will start.
3. The blood pressure module will pressurize the Test Device and report the
time. When the test is complete, use the table below to verify that the Time
to 250 value is within range dependent upon the module type installed.
Page 44
4. Press the [CONFIRM] key.
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 51

Pressure
Model Type
RATE AT 240
(mmHg/sec)
RATE AT 160
(mmHg/sec)
RATE AT 80
(mmHg/sec)
RATE AT 40
/sec)
Min
Max
Min
Max
Min
Max
Min
Max
M400
4.3
6.2
4.6
6.2
3.3
5.2
2.6
4.4
M2000 or M2600
4.4
6.7
4.4
6.7
3.0
5.2
2.4
4.6
M3600
3.8
6.6
3.8
6.6
2.8
5.5
2.3
4.6
TM2910 or TM-2915
3.5
7.5
3.5
7.5
3.5
7.5
3.5
7.5
SunTech
N/A
N/A
N/A
N/A
N/A
N/A
N/A
N/A
C
TIVE
P
DEFLATION SPEED TEST
Note: The deflation speed test is not applicable to the SunTech module.
From the Test BP Module screen, select the Deflation Speed button.
1. Connect the pressure tubing from the module to the 220cc port on the Test
Device. Ensure the tubing fits snugly on the ports.
2. Press the [CONFIRM] key. The screen will change and the test will start.
3. The blood pressure module will depressurize (deflate) the Test Device. The
screen will change and report the deflation speed at various pressures in mmHg
per second.
4. Use the table below to verify that the values reported are within the following
ranges dependent upon the module type installed.
(mmHg
FFE
5. Press the [CONFIRM] key.
AIR LEAKAGE TEST
From the Test BP Module screen, select the Air Leakage button.
1. Connect the pressure tubing from the module to the appropriate module
specific port on the Test Device (see table at the top of the next page).
Ensure the tubing fits snugly on the ports.
2. Press the [CONFIRM] key. The screen will change and the test will start.
3. The blood pressure module will pressurize the Test Device as indicated by a
rise in the Cuff Pressure displayed to approximately 290mmHg. An
internal timer is then activated automatically. Any change in the pressure
being held will show in the Cuff Pressure display.
Page 45
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 52

4. At the end of the Air Leakage test, the pressure is automatically released,
Module Type
Test Device Port
Leak Rate
M400, M2000 or M2600
15mmHg
M3600
12mmHg
TM
30mmHg
SunT
22mmHg
Module Type
Over Pressure Release Range
M400
between 320 and 330mmHg
TM-2910
between 310 and 330mmHg
C
P
the screen will change and the Leak Rate will be displayed.
5. Use the table below to verify that the Leak Rate value is within range
dependent upon the module type installed.
TIVE
FFE
220cc
220cc
-2910 or TM-2915 700cc
ech 700cc
6. Press the [CONFIRM] key.
CALIBRATION CHECK
For the M400 & TM-2910
1. From the Test BP Module screen, select the Calibrate Mode button.
2. Disconnect the pressure tube from the Test Device and connect it to a mercury
manometer or similar pressure meter accurate to within r1mmHg.
3. Press the [CONFIRM] key. The screen will change and the blood pressure
module will pressurize the line.
4. When the pressure shown on the display screen and on the external meter
stabilizes, verify that they agree within r3mmHg.
d
d
d
d
Page 46
Caution: Do not exceed 330mmHg in the following step. The blood
pressure module may be damaged if this pressure is exceeded.
5. Over-Pressure Relief Check:
Remove the hose from the external meter and connect it to a large syringe
(60cc) that has its plunger pulled back. Use the syringe to over-pressurize
the blood test module while watching the display carefully. Use the table
below to verify that the pressure is automatically released within range
dependent upon the module type installed.
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 53

For the M2000 & SunTech
C
P
1. From the Test BP Module screen, select the Calibrate Mode button.
2. Disconnect the pressure tube from the Test Device and connect it to a mercury
manometer or similar pressure meter accurate to within r1mmHg.
3. Press the [CONFIRM] key. The screen will change and the blood pressure
module will pressurize the line.
4. When the pressure shown on the display screen and on the external meter
stabilizes, verify that they agree within r3mmHg.
5. Over-Pressure Relief Check:
Due to the microprocessor control of the M2000 and SunTech modules, the
over-pressure relief check does not need to be conducted. In fact, an overpressure relief test could permanently damage the module.
For the M2600 & M3600
TIVE
FFE
1. From the Test BP Module screen, select the Air Leakage button.
2. Disconnect the pressure tube from the Test Device and connect it to a mercury
manometer or similar pressure meter accurate to within r1mmHg.
3. Press the [CONFIRM] key. The screen will change and the blood pressure
module will pressurize the line.
4. The blood pressure module will pressurize as indicated by a rise in the Cuff
Pressure displayed to approximately 290mmHg. An internal timer is then
activated automatically.
5. When the pressure shown on the display screen and on the external meter
stabilizes, verify that they agree within r3mmHg.
6. At the end of the Air Leakage test, the pressure is automatically released.
7. Over-Pressure Relief Check:
Due to the microprocessor control of the M2600 and M3600, the overpressure relief check does not need to be conducted. In fact, an overpressure relief test could permanently damage the module.
Page 47
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 54

For the TM-2915
C
P
1. From the Test BP Module screen, select the Air Leakage button.
2. Disconnect the pressure tube from the Test Device and connect it to a mercury
manometer or similar pressure meter accurate to within r1mmHg.
3. Press the CONFIRM key. The screen will change and the blood pressure
module will pressurize the line.
4. The blood pressure module will pressurize as indicated by a rise in the Cuff
Pressure displayed to approximately 290mmHg. An internal timer is then
activated automatically.
5. When the pressure shown on the display screen and on the external meter
stabilizes, verify that they agree within r3mmHg.
6. At the end of the Air Leakage test, the pressure is automatically released.
7. Press the CONFIRM key.
TIVE
FFE
3.28 FINAL CHECKS
Caution: Do not exceed 330mmHg in the following step. The blood
pressure module may be damaged if this pressure is exceeded.
Over-Pressure Relief Check:
8. Remove the hose from the external meter and connect it to a large syringe
(60cc) that has its plunger pulled back.
9. From the Test BP Module screen, select the Calibrate Mode button.
10. From the first Calibrate Mode screen, press the CONFIRM key.
Use the syringe to over-pressurize the blood pressure module while
watching the display carefully. Verify that the pressure is automatically
released between 310 and 330mmHg.
1. Check the machine for any optional components that are not included here.
Perform the preventive maintenance tests provided with those units.
2. Perform the Six (6) Month Preventive Maintenance procedures in
Section 2.10.
Page 48
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 55

4.0 REBUILDING THE DIAPHRAGM PUMPS
C
P
4.1 REBUILDING THE ULTRAFILTRATION PUMP
TIVE
The machine contains two types of diaphragm pumps. The ultrafiltration (UF) pump
is a solenoid coil type. The concentrate and bicarbonate pumps are stepper motor
types.
Figure 11 shows an exploded view of the ultrafiltration pump. When working on the
pump, be especially careful to do the following:
x Do not lose the wear button or the shim washers inside the pump solenoid.
x Count the shim washers when you disassemble the pump. The number of
washers varies as needed to mate the solenoid to the pump properly.
Caution: Replace exactly the same number of shims in the ultrafiltration
pump as were removed. The pump will not operate correctly if the same
number of shims are not replaced.
Examine all components for signs of corrosion. Replace any components that
show signs of excessive rust.
FFE
Note: If the UF pump does not provide output even though the green light is
on and not flashing, inspect the check valves. If a check valve is installed
backwards, the pump will not work.
Page 49
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 56

WEAR BUTTON
C
P
P/N 565219
SHIM
WASHERS
P/N 562343
SEALS
P/N 565279
OUTLET SPRING
P/N 563025
(STRONGER)
O-RINGS
P/N 579058
OUTLET
INLET
TIVE
FFE
SPRING RETAINING CLIP
IS POSITIONED FOR
MAXIMUM COMPRESSION
INLET SPRING
P/N 565278
(WEAKER)
SPACER, P/N 566149
(ON SOME PUMPS THE SPACER
MAY BE MOULDED INTO THE
OUTLET NOZZLE.)
Figure 11 - Ultrafiltration Pump Exploded View.
Rebuild the ultrafiltration pump as follows:
1. Remove the UF pump from the machine (See Figure 3, pg 8). Make a note
of which line goes to which port on the pump. When looking at the end
plate, the arrow pointing into the pump nozzle is the input side. The arrow
pointing away from the pump nozzle is the output side.
2. Hold the two sections of the pump housing and end plate together with one
hand and remove the four long screws from the end plate.
3. Carefully remove the end plate to reveal the input and output nozzles,
springs, seals and O-rings.
4. Replace the inlet and outlet springs.
Caution: Be certain the correct springs are used. The weaker spring goes
on the inlet side. The stronger spring goes on the outlet side.
5. Inspect the seals. Replace them if there is any sign of swelling or wear.
6. Inspect the O-rings. Replace them if there is any sign of wear.
Page 50
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 57

Note: Before reassembling the UF pump, verify nothing has fallen into the
C
P
solenoid opening.
7. Reassemble the pump as follows:
x The outlet seal goes toward the pump membrane.
x The inlet seal faces away from the pump membrane.
x Reinstall the spacer in the outlet nozzle if one was removed from the
pump.
x Ensure the outlet nozzle is oriented so it will be at the top of the pump
solenoid to help avoid air locks in the pump.
8. Replace the long screws into the end plate and tighten.
TIVE
FFE
9. Replace the pump in the machine and reconnect the electrical and hydraulic
lines. Orient the pump so the outlet nozzle is above the inlet nozzle to help
avoid air locks.
10. To displace air and to check for leaks, start a rinse and allow the machine to
complete the entire rinse cycle.
11. Verify proper function of the UF pump by performing the Six (6) Month
Preventive Maintenance procedures in Section 2.5.
Page 51
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 58

4.2 REBUILDING THE CONCENTRATE AND BICARBONATE PUMPS
C
P
Figure 12 is an exploded view of the concentrate and bicarbonate pumps.
TIVE
FFE
OUTLET SPRING
SEALS
P/N 565279
INLET SPRING
P/N 565278
* ON CONCENTRATE (ACID) PUMPS THIS SPRING
IS STRONGER THAN THE INLET SPRING. USE P/N 563025.
ON BICARBONATE PUMPS USE WEAK SPRINGS
FOR BOTH INLET AND OUTLET (P/N 565278).
ON MACHINES WITH ONLINE CLEARANCE (OLC), THE
CONCENTRATE (ACID) PUMP USES WEAK SPRINGS
FOR BOTH INLET AND OUTLET (P/N 565278).
Figure 12 - Concentrate and Bicarbonate Pump Exploded View.
SEE BELOW *
O-RINGS
P/N 579058
IF THE PUMP CONTAINS A PLASTIC SPACER
IN THE OUTLET PORT, BE SURE TO REPLACE
IT WHEN REASSEMBLING THE PUMP. NEWER
PUMPS HAVE THIS SPACER BUILT INTO THE PORT.
OUTLET
INLET
RETAINING PLATE
Rebuild the concentrate pumps as follows:
1. Remove the pump from the machine (See Figure 3, pg 8). Make a note of
which line goes to which port on the pump. When looking at the end plate,
the arrow pointing into the pump nozzle is the input side. The arrow
pointing away from the pump nozzle is the output side.
2. Hold the two sections of the pump housing and end plate together with one
hand and remove the long screws that hold the end plate. On some models
of the pump, all four screws hold the end plate, and on others only two
screws hold the end plate.
If you removed all four screws to free the end plate, replace one or two of
them to avoid having the pump come completely apart. You only need
access to the inlet seals and springs.
Page 52
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 59

3. Carefully remove the end plate to reveal the input and output nozzles,
C
P
springs, seals and O-rings.
4. Replace the inlet and outlet springs.
Caution: Be certain the correct springs are used on concentrate pumps.
See Figure 12 on previous page.
5. Inspect the seals. Replace them if there is any sign of swelling or wear.
6. Inspect the O-rings. Replace them if there is any sign of wear.
7. Reassemble the pump as follows:
x The outlet seal goes toward the pump membrane.
x The inlet seal faces away from the pump membrane.
TIVE
FFE
x Reinstall the spacer in the outlet nozzle if one was removed from the
pump.
x Ensure the outlet nozzle is oriented so it will be at the top of the pump
solenoid to help avoid air locks in the pump.
8. Replace the long screws into the end plate and tighten.
9. Replace the pump in the machine and reconnect the electrical and hydraulic
lines. Orient the pump so the outlet nozzle is above the inlet nozzle to help
avoid air locks.
10. To displace air and to check for leaks, start a rinse and allow the machine to
complete the entire rinse cycle.
11. Test the rebuilt diaphragm pump by performing the procedure described in
Section 4.3.
Page 53
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 60

4.3 TESTING CONCENTRATE AND BICARBONATE PUMPS
C
P
The concentrate (acid) and bicarbonate pumps are tested by measuring the volume of
water they pump and comparing that measurement with the expected value
determined by the machine. The measured pump volume must be within 2% of the
expected value.
Test each pump as follows:
1. Place the machine in Dialysis Mode and clear all alarms.
TIVE
FFE
2. Switch to the debug screens by pressing and holding the [
the same time for approximately 1 sec. The main display will change
indicating that the machine is in debug mode.
3. Use the [
x Locate the debug value for FILACT. In order to turn off dialysis flow
x Locate the debug value for DOUBLE. Certain types of Bicarbonate or
x If the debug values for the Bicarbonate Pump Volume (BMIN and
Note: The AMIN, AMAX, BMIN, BMAX are the expected limits for twenty
pulses of the acid (concentrate) pump volume and the bicarbonate pump
volume, respectively. These volumes are based on the measured volume of
the balancing chamber to produce the correct mixing ratio.
] or [] keys to go to debug screen #1.
later, FILACT must be 0.
higher Bicarbonate levels selected will require the pump to double
stroke. If the debug value for DOUBLE is 1, the pump is double
stroking. Before continuing the test, adjust the Bicarbonate level until
the debug value for DOUBLE is 0.
BMAX) are showing 0 on debug screen #1, the machine is set up for a
concentrate family that does not use bicarbonate. Change the
concentrate selection to one requiring bicarbonate.
] and [] keys at
4. Press the [Dialysate Flow on/off] key to stop dialysate flow.
5. Connect a 25ml buret filled with water to the concentrate connector for the
pump to be tested. Use a short length of tubing forced over the end of the
connector, and make sure there are no leaks in the fitting to the connector or
to the buret.
Page 54
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 61

6. With the machine in Dialysis Mode, press the [Dialysate Flow on/off] key
C
P
to start the flow. Observe that liquid is drawn from the buret in discrete
steps. Allow the machine to run and pump fluid from the buret for about 20
strokes, to prime the pump. Press the [Dialysate Flow on/off] key to stop
the flow.
Note: Do not let the buret run dry so that air can enter the system.
7. Refill the buret exactly to the full mark. Press the [Dialysate Flow on/off] key
to start the flow and count exactly 20 pulses of water drawn from the buret,
then stop the flow.
Note: If DOUBLE=1 on debug screen #1 then count each pulse of the
double stroking bicarbonate pump.
TIVE
FFE
8. Measure the amount of water drawn from the buret carefully to within
0.05ml (within 1/2 division on a buret calibrated in 0.1ml increments).
Verify that this value is between the displayed limits of AMIN and AMAX
for the acid pump volume and BMIN and BMAX for the bicarbonate pump
volume.
9. If a pump volume falls outside of the permitted error, repeat the above
procedure to ensure that you are getting consistent results before recalibrating the machine.
Page 55
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 62

5.0 SEMI-ANNUAL PREVENTIVE MAINTENANCE PROGRAM INSTRUCTIONS
C
P
TIVE
Fresenius 2008K Hemodialysis Systems manufactured (or refurbished) in 2003
and later may be qualified for the semi-annual preventive maintenance procedures
described in this manual. In addition, 2008K hemodialysis systems manufactured
(or refurbished) in 2002 and earlier may be qualified for the semi-annual PM
procedures if the machine meets the minimum configuration in the qualification
checklist on page 69 and that configuration is maintained.
Since modules and the power supply are designed to be easily moved from
machine to machine, it is necessary to label machines & modules that meet the
configuration requirements for the semi-annual PM program so that the status of
the machine is known and the minimum configuration of the machine is
maintained.
Record the Machine Serial Number on the qualification checklist on page 69.
FFE
Page 56
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 63

5.1 BLOOD PUMP REQUIREMENTS
Blood Pump EC Code
LP955 Revision Level
(SP=G shown)
C
P
TIVE
x Record the Blood Pump Serial Number on the qualification checklist on page 69.
x Module Engineering Code is EC004 or greater
1. Locate the Engineering Code (EC) on top of the Blood Pump housing (see
Figure 13).
2. Confirm that it is EC004 or greater. If not, replace the module (P/N
M30656).
x Printed Circuit Board LP955 is Revision SP=G or later
1. Locate the revision label on the back (solder side) of the LP955 board (see
Figure 13).
2. Confirm that the revision level of the LP955 board is SP=G or later. If not,
either replace the LP955 board (P/N 677818) or replace the module (P/N
M30656).
FFE
Note: If LP955 is newer than SP=H, the label may have different letters on
it. For example, if the LP955 is SP=I then the upper letters will be I, J, K, L.
Figure 13 – EC Code and LP955 Revision Level
Page 57
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 64

C
TIVE
P
x Blood Pump Software Version is 1.09 or later
1. During the machine power on procedure the blood pump will display the
software version currently installed.
2. Confirm that the software version is 1.09 or later. If not, the blood pump
software must be upgraded. Use Blood Pump Software Upgrade Kit (P/N
190230) to upgrade the software to the latest version.
x Rotor must be P/N M30990
1. Remove the blood pump rotor and inspect the four (4) tubing guide rollers.
2. The required blood pump rotor must have smooth tops to the metal posts not
a slotted screw type head (see Figure 14).
3. If the rotor has slotted screw type head or there are any signs of damage to
the rotor assembly, replace the complete rotor (P/N M30990).
FFE
Page 58
Obsolete Blood Pump Rotor Required Blood Pump Rotor
for Semi-Annual PM
Figure 14 – Blood Pump Rotor
x Check the freedom of movement of the lever – not stuck or stiff
1. With the rotor still out inspect the lever by unlatching the handle and
pivoting it out.
2. If the lever is stuck or is stiff in operation, replace the rotor assembly (P/N
M30990).
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 65

x Check that the crank handle fits correctly – not too loose or tight
C
P
1. Retrieve the plastic crank handle from the rear of the machine.
2. With the blood pump rotor lever in its upright position, slide the handle
crank onto the lever.
3. Confirm that the crank handle fits correctly and is not too loose or tight. If
not, replace the plastic crank handle assembly (P/N 673615)
5.2 POWER SUPPLY REQUIREMENTS
Note: The following steps require the power supply to be removed from the machine
and disassembled. As an alternative, replacement of the complete power supply (P/N
190011) will eliminate all of the following steps. The replacement meets the SemiAnnual PM requirements.
TIVE
FFE
x Record the Power Supply Serial Number on the qualification checklist on page 69.
Warning! Dangerous high voltage is present at the connections accessed in this
procedure when the machine is operating. Ensure the machine's power plug is
disconnected from the wall outlet before proceeding.
x Inspect power plug & power cord length for damage
1. Inspect the power plug for heat damage and the power cord length (from
plug to strain relief) for cuts, chips, crushing, heat damage, etc.
2. If damage is found, replace the complete power cord (P/N 150425). The
complete power cord includes a molded power plug on one end.
Page 59
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 66

x Inspect main power switch
Main Power
Switch
C
P
1. Inspect the main power switch and verify that its operation is smooth (no
grinding or sticky operation).
Figure 15 – Main Power Switch
TIVE
FFE
2. If switch does not operate smoothly, replace the main power switch (P/N
363020-01).
x Inspect connections to the main power switch
1. Locate the strain relief where the power cord connects to the power supply.
2. On the other side of the strain relief, locate the black, white and green wires
from the power cord (inside the power supply chassis).
3. Follow the black and white wires to the main power switch.
4. Attached to the main power switch are four (4) wires (2 black and 2 white).
Look for loose connections, cracked insulation, and signs of overheating,
such as discolored or melted insulation.
5. If the black or white wires from the power cord to the main power switch
are damaged, replace the complete power cord (P/N 150425)
If the black or white wires from the main power switch to the power control
board show signs of damage, replace them (P/N 190273 for the black wire
and P/N 190274 for the white wire).
Page 60
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 67

Location of
10µF capacitor
C
P
TIVE
x Inspect all internal wiring
1. Inspect all wires inside the power supply assembly for loose connections,
cracked insulation, and signs of overheating, such as discolored or melted
insulation.
2. If any wires appear damaged use power supply wiring kit (P/N 190411).
This kit includes all wire assemblies including P/N 190273 and 190274.
Installation instructions and a diagram are included to assist in the
replacement of each wire.
x Inspect the 10μF capacitor
1. Locate the 10μF capacitor mounted to the chassis of the power supply (see
Figure 16).
FFE
Figure 16 - 10µF Capacitor
2. Inspect the 10μF capacitor for signs of bubbling, swelling, leaking, etc.
3. Replace the 10μF capacitor (P/N 361216-03) if it shows any signs of damage
or if the power supply is more than 5 years old.
Page 61
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 68

x Inspect power control board
C
P
1. Locate resistors R10 and R11 on the power control board (see Figure 17).
TIVE
FFE
Figure 17 – R10 & R11 on Power Control Board
2. Inspect each resistor and the circuit board in the immediate area of R10 and
R11 for heat damage.
3. If heat damage is found replace the power control board (P/N 190019).
Page 62
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 69

NOTES:
C
P
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
TIVE
FFE
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
Page 63
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 70

NOTES:
C
P
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
TIVE
FFE
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
Page 64
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 71

PREVENTIVE MAINTENANCE CHECKLIST
C
P
SIX (6) MONTH
SERIAL NO. _________________________________________________DATE __________________________
MACHINE I.D. NO. ___________________________________________MACHINE HOURS ______________
TECHNICIAN(S) _____________________________________________LABOR HOURS _________________
See the Referenced Section of the Fresenius 2008K Semi-Annual Preventive Maintenance Procedures
(Fresenius part number 507781 Rev D) for detailed instructions to perform the following:
Ref. Section Procedure Completed By:
2.1 Clean Filters (replace if necessary) ..................................................... _________
2.2 Inspect Pre-UF Pump filter .................................................................. _________
2.3 Inspect UF Pump Check Valves (replace if necessary) ..................... _________
2.4 Check High Voltage AC Connections.................................................. _________
2.5 Calibrate UF Pump ............................................................................... _________
2.6 Check Conductivity with External Meter ........................................... _________
TIVE
2.7 Check Temperature with External Meter .......................................... _________
2.8 Check Alarm Operation and Pressure Holding Test ......................... _________
2.10 Final Checks .......................................................................................... _________
FFE
NOTES:
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
2.9 Check Power Failure Alarm ................................................................ _________
Page 65
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 72

NOTES:
C
P
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
TIVE
FFE
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
Page 66
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 73

PREVENTIVE MAINTENANCE CHECKLIST
C
P
ANNUAL/4000 HOUR
SERIAL NO. _________________________________________________DATE __________________________
MACHINE I.D. NO. ___________________________________________MACHINE HOURS ______________
TECHNICIAN(S) _____________________________________________LABOR HOURS _________________
See the Referenced Section of the Fresenius 2008K Semi-Annual Preventive Maintenance Procedures
(Fresenius part number 507781 Rev D) for detailed instructions to perform the following:
Ref. Section Procedure Completed By:
3.1 Clean Filters and Replace O-Rings .................................................. __________
3.2 Replace Pre-UF Pump Filter ............................................................. __________
3.3 Replace UF Pump Check Valves ...................................................... __________
3.4 Check Deaeration Restrictor and Replace O-Rings ....................... __________
3.5 Rebuild UF, Concentrate and Bicarbonate Pumps ......................... __________
3.6 Check Heater Element ....................................................................... __________
3.7 Check High Voltage AC Connections............................................... __________
TIVE
FFE
3.8 Replace Deaeration Motor Brushes (every 8000 hrs) ..................... __________
3.9 Check Inlet Water Pressure Regulator ............................................ __________
3.10 Conduct Online Clearance Test (If Applicable) .............................. __________
3.11 Check Deaeration and Loading Pressure ........................................ __________
3.12 Check Flow Relief Pressure .............................................................. __________
3.13 Test Concentrate and Bicarbonate Pumps ...................................... __________
3.14 Calibrate UF Pump ............................................................................ __________
3.15 Check Conductivity Calibration ....................................................... __________
3.16 Check Temperature Calibration ...................................................... __________
3.17 Check Volt Hi Lo Detect .................................................................... __________
3.18 Check Blood Leak and Dimness Calibration ................................... __________
3.19 Check Arterial, Venous and TMP Calibration ............................... __________
3.20 Check Dialysate Flow ......................................................................... __________
3.21 Check Heparin Pump (If Present) .................................................... __________
3.22 Check Blood Pump ............................................................................. __________
3.23 Check and Calibrate Level Detector ................................................ __________
3.24 Check Alarm and Pressure Holding Tests ....................................... __________
3.25 Rinse Checks ....................................................................................... __________
3.26 Replace Battery and Check Power Failure Alarm ......................... __________
3.27 Check Blood Pressure Module .......................................................... __________
3.28 Final Checks ....................................................................................... __________
Page 67
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 74

NOTES:
C
P
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
TIVE
FFE
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
Page 68
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 75

SEMI-ANNUAL PREVENTIVE MAINTENANCE
C
P
PROGRAM QUALIFICATION CHECKLIST
The blood pump module and the power supply must be removed from the 2008K machine in order to
verify the configuration listed below. Any components that do not meet the minimum revision level
requirements must be upgraded. Contact Fresenius Technical Support for upgrade options and pricing.
Machines that do not meet the minimum configuration are subject to the standard preventive maintenance
requirements; refer to PM manual P/N 507297.
See the Section 5.0 of the Fresenius 2008K Semi-Annual Preventive Maintenance Procedures (Fresenius
part number 507781 Rev D) for detailed instructions to perform the following:
MACHINE SERIAL NUMBER: _________________________________________________________
Blood pump P/N M30656: MODULE SERIAL NUMBER: _______________________________
TIVE
Power Supply P/N 190011: POWER SUPPLY SERIAL NUMBER: ________________________
FFE
I certify that the 2008K hemodialysis system listed above meets the minimum configuration requirements
of this checklist and is qualified for the semi-annual maintenance procedure.
x Module Engineering Code is EC004 or greater ........................................................
x Printed Circuit Board 955 is Revision SP=G or later ............................................... □
x Blood Pump Software Version is 1.09 or later. ........................................................ □
x Rotor must be P/N M30990 ...................................................................................... □
x Check the freedom of movement of the lever – not stuck or stiff. ............................ □
x Check that the crank handle fits correctly – not too loose or tight. .......................... □
x Inspect power plug & power cord length for damage ...............................................
x Inspect main power switch. ....................................................................................... □
x Inspect connections to the main power switch .......................................................... □
x Inspect all internal wiring.......................................................................................... □
x Inspect the 10µF capacitor ........................................................................................ □
x Inspect the power control board ................................................................................ □
□
□
_________________________________ ____________________
Name / Title Date
Page 69
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 76

NOTES:
C
P
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
TIVE
FFE
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
Page 70
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 77

NOTES:
C
P
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
TIVE
FFE
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
Page 71
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 78

NOTES:
C
P
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
TIVE
FFE
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
___________________________________________________________________________________
Page 72
Fresenius 2008®K Semi-Annual Preventive Maintenance Procedures
P/N 507781 Rev. D
rinted 5/18/2012 12:00PM
Page 79

C
TIVE
P
COMMON CONVERSIONS
PRESSURE
1 Bar 29.53 inHg
1 inHg 25.4 mmHg
1 Psi 51.72 mmHg
VOLUME
1 FLUID OUNCE 26.6 MILLILITERS
1 U.S QUART 0.946 LITERS
1 U.S. GALLON 3.8 LITERS
0.034 FLUID OUNCES 1 MILLILITER
1.057 QUARTS 1 LITER
0.26 U.S. GALLON 1 LITER
FFE
MASS
1 OUNCE (avdp.) 28.35 GRAMS
1 POUND (avdp.) 0.45 KILOGRAM
0.035 OUNCE (avdp.) 1 GRAM
rinted 5/18/2012 12:00PM
Page 80

C
TIVE
P
FFE
Fresenius Medical Care North America
Manufactured by:
Fresenius USA, Inc.
2637 Shadelands Drive
Walnut Creek, CA 94598
800 227-2572
http://www.fmcna.com
rinted 5/18/2012 12:00PM
 Loading...
Loading...