Fluke BP Pump 2 User Manual

BP Pump 2
NIBP Simulator and Tester
Operators Manual
PN 2196592
June 2007
© 2007 Fluke Corporation, All rights reserved. Printed in USA
All product names are trademarks of their respective companies.
Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and workmanship for one year from the date of original purchase. During the warranty period, we will repair or at our option replace, at no charge, a product that proves to be defective, provided you return the product, shipping prepaid, to Fluke Biomedical. This warranty covers the original purchaser only and is not transferable. The warranty does not apply if the product has been damaged by accident or misuse or has been serviced or modified by anyone other than an authorized Fluke Biomedical service facility. NO OTHER WARRANTIES, SUCH AS FITNESS FOR A PARTICULAR PURPOSE, ARE EXPRESSED OR IMPLIED. FLUKE SHALL NOT BE LIABLE FOR ANY SPECIAL, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES OR LOSSES, INCLUDING LOSS OF DATA, ARISING FROM ANY CAUSE OR THEORY.
This warranty covers only serialized products and their accessory items that bear a distinct serial number tag. Recalibration of instruments is not covered under the warranty
This warranty gives you specific legal rights and you may also have other rights that vary in different jurisdictions. Since some jurisdictions do not allow the exclusion or limitation of an implied warranty or of incidental or consequential damages, this limitation of liability may not apply to you. If any provision of this warranty is held invalid or unenforceable by a court or other de- cision-maker of competent jurisdiction, such holding will not affect the validity or enforceability of any other provision.
07/07
Notices
All Rights Reserved
♥ Copyright 2007, Fluke Biomedical. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language without the written permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and other printed materials for use in service training programs and other technical publications. If you would like other reproductions or distributions, submit a written request to Fluke Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for damage. If damage is found, stop unpacking the instrument. Notify the carrier and ask for an agent to be present while the instrument is unpacked. There are no special unpacking instructions, but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such as bent or broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email techservices@flukebiomedical.com or call 1-800- 648-7952 or 1-425-446-6945.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical damage is found, retain all packing materials in their original condition and contact the carrier immediately to file a claim. If the instrument is delivered in good physical condition but does not operate within specifications, or if there are any other problems not caused by shipping damage, please contact Fluke Biomedical or your local sales representative.
Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and items bearing a distinct serial number tag) are eligible for partial refund and/or credit. Nonserialized parts and accessory items (e.g., cables, carrying cases, auxiliary modules, etc.) are not eligible for return or refund. Only products returned within 90 days from the date of original purchase are eligible for refund/credit. In order to receive a partial refund/credit of a product purchase price on a serialized product, the product must not have been damaged by the customer or by the carrier chosen by the customer to return the goods, and the product must be returned complete (meaning with all manuals, cables, accessories, etc.) and in “as new” and resalable condition. Products not returned within 90 days of purchase, or products which are not in “as new” and resalable condition, are not eligible for credit return and will be returned to the customer. The Return Procedure (see below) must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of 15 %. Products returned in excess of 30 days after purchase, but prior to 90 days, are subject to a minimum restocking fee of 20 %. Additional charges for damage and/or missing parts and accessories will be applied to all returns.

Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freight-prepaid to our factory location. When you return an instrument to Fluke Biomedical, we recommend using United Parcel Service, Federal Express, or Air Parcel Post. We also recommend that you insure your shipment for its actual replacement cost. Fluke Biomedical will not be responsible for lost shipments or instruments that are received in damaged condition due to improper packaging or handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the following guide for repackaging:
Use a double-walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent material around the instrument.
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our Order Entry Group at 1-800-648-7952 or 1-425-446- 6945.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service, or
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-993-5853
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s manufacturing specifications when it was shipped from the factory. Calibration measurements are traceable to the National Institute of Standards and Technology (NIST). Devices for which there are no NIST calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may result in electrical shock hazards or improper operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.

Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by Fluke Biomedical. Changes made to the information in this document will be incorporated in new editions of the publication. No responsibility is assumed by Fluke Biomedical for the use or reliability of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The BP Pump 2 Non-invasive Blood Pressure Simulator and Tester is manufactured in Everett, Washington by Fluke Biomedical, 6920 Seaway Blvd., Everett, WA, U.S.A.
Table of Contents
Chapter |
Title |
Page |
|
1 |
Introduction and Specifications.............................................. |
1-1 |
|
|
Introduction .......................................................................................... |
|
1-3 |
|
Key Features ......................................................................................... |
|
1-3 |
|
General Safety Considerations.............................................................. |
|
1-4 |
|
Symbols ............................................................................................ |
|
1-4 |
|
Warnings and Cautions..................................................................... |
|
1-5 |
|
Instrument Familiarity .......................................................................... |
|
1-7 |
|
Powering Up the Tester ........................................................................ |
|
1-10 |
|
Specifications........................................................................................ |
|
1-10 |
|
Accessories ........................................................................................... |
|
1-12 |
2 |
Setup, Maintenance, and Support........................................... |
2-1 |
|
|
Setting up the Tester ............................................................................. |
|
2-3 |
|
Printer Output ................................................................................... |
|
2-4 |
|
User-Defined Simulations................................................................. |
|
2-5 |
|
Language .......................................................................................... |
|
2-6 |
|
Units of Measure............................................................................... |
|
2-6 |
|
Self Test............................................................................................ |
|
2-8 |
|
Zero Pressure .................................................................................... |
|
2-8 |
|
Enable ECG Signal ........................................................................... |
|
2-9 |
|
Maintenance and Support ..................................................................... |
|
2-10 |
|
Avoiding Damage ............................................................................. |
|
2-10 |
|
Cleaning............................................................................................ |
|
2-10 |
|
Service and Calibration..................................................................... |
|
2-11 |
|
Packing Instructions.......................................................................... |
|
2-11 |
3 |
Operation .................................................................................. |
|
3-1 |
|
Introduction .......................................................................................... |
|
3-3 |
|
Configurations for Devices Under Test (DUT)................................. |
3-4 |
|
|
Conversion Factors ........................................................................... |
|
3-7 |
|
Initializing Tests and Simulations..................................................... |
3-7 |
|
|
Error Messages ................................................................................. |
|
3-7 |
i

BP Pump 2
Operators Manual
Pressure Tests....................................................................................... |
3-8 |
|
|
Pressure Leak Test............................................................................ |
3-8 |
|
Pressure Relief Test.......................................................................... |
3-10 |
|
Pressure Source Test ........................................................................ |
3-11 |
|
Pressure Gauge Test ......................................................................... |
3-12 |
Simulations........................................................................................... |
3-13 |
|
|
Standard BP...................................................................................... |
3-14 |
|
Patient Conditions ............................................................................ |
3-15 |
|
Arrhythmias...................................................................................... |
3-16 |
|
Respiratory Artifacts ........................................................................ |
3-18 |
|
Neonate ............................................................................................ |
3-19 |
|
Wrist................................................................................................. |
3-20 |
|
User-Defined .................................................................................... |
3-21 |
Auto Sequences.................................................................................... |
3-21 |
|
|
Editing Auto Sequences ................................................................... |
3-22 |
|
Printing Auto Sequences .................................................................. |
3-23 |
|
Running Auto Sequences ................................................................. |
3-24 |
|
Pressure Gauge............................................................................. |
3-25 |
|
Leak Test...................................................................................... |
3-25 |
|
Relief Valve Test.......................................................................... |
3-26 |
|
Pressure Source ............................................................................ |
3-26 |
|
Data Sheet Printout....................................................................... |
3-27 |
|
BP Simulations............................................................................. |
3-27 |
Remote Operation ................................................................................ |
3-29 |
|
|
RS-232 Settings................................................................................ |
3-29 |
|
Ansur Software Control.................................................................... |
3-29 |
Appendices |
|
|
A |
ECG Interface............................................................................... |
A-1 |
B |
Questions and Answers ................................................................ |
B-1 |
C |
Abbreviations ............................................................................... |
C-1 |
D |
Computer Control Commands...................................................... |
D-1 |
ii
List of Tables
Table |
Title |
Page |
1-1. |
Symbols ................................................................................................ |
1-4 |
1-2. Top and Side Panel Components .......................................................... |
1-8 |
|
1-3. |
Number Key Functions......................................................................... |
1-9 |
1-4. |
Standard Accessories ............................................................................ |
1-12 |
1-5. |
Optional Accessories ............................................................................ |
1-12 |
3-1. |
Conversion Factors ............................................................................... |
3-7 |
3-2. Standard Blood Pressure Simulations ................................................... |
3-15 |
|
3-3. |
Patient Condition Simulations .............................................................. |
3-16 |
3-4. |
Arrhythmia Simulations........................................................................ |
3-17 |
3-5. |
Respiratory Artifact Simulations .......................................................... |
3-18 |
3-6. |
Neonate Simulations ............................................................................. |
3-19 |
3-7. |
Wrist Simulations ................................................................................. |
3-20 |
D-1. Computer Control Commands .............................................................. |
D-1 |
|
iii

BP Pump 2
Operators Manual
iv
List of Figures
Figure |
Title |
Page |
1-1. Tester Top and Side Panel Components ............................................... |
1-7 |
|
3-1. |
Tester Pneumatic Block Diagram ......................................................... |
3-3 |
3-2. |
Connecting Tester to Single-hose NIBP Monitor (Int Cuff) ................. |
3-4 |
3-3. |
Connecting Tester to Double-hose NIBP Monitor (Int Cuff)................ |
3-5 |
3-4. |
Connecting Tester to Single-hose NIBP Monitor (Ext Cuff) ................ |
3-5 |
3-5. Connecting Tester to Double-hose NIBP Monitor (Ext Cuff)............... |
3-6 |
|
3-6. |
Connecting Tester to Single-hose NIBP Wrist Monitor (Ext Cuff) ...... |
3-6 |
3-7. |
Sample Auto Sequence Test Printout.................................................... |
3-24 |
3-8. |
Sample Data Sheet Printout .................................................................. |
3-28 |
A-1. |
Optional ECG Interface Adapter........................................................... |
A-1 |
v

BP Pump 2
Operators Manual
vi
Chapter 1
Introduction and Specifications
Contents |
Page |
Introduction .................................................................................. |
1-3 |
Key Features................................................................................. |
1-3 |
General Safety Considerations ..................................................... |
1-4 |
Symbols .................................................................................... |
1-4 |
Warnings and Cautions............................................................. |
1-5 |
Instrument Familiarity.................................................................. |
1-7 |
Powering Up the Tester................................................................ |
1-10 |
Specifications ............................................................................... |
1-10 |
Accessories................................................................................... |
1-12 |
1-1

BP Pump 2
Operators Manual
1-2

Introduction and Specifications 1
Introduction
Introduction
The Fluke Biomedical BP Pump 2 Non-Invasive Blood Pressure Simulator and Tester, hereafter known as the “Tester”, is a multi-purpose test instrument for use with oscillometric Non-Invasive Blood Pressure Monitors (NIBPMs). The Tester provides dynamic blood pressure simulations, static calibration, automated leak testing, and pressure relief valve testing. The following models are available:
•BP Pump 2L (Basic Model)
•BP Pump 2M (High-Accuracy Model)
The Tester allows you to verify the performance claims of different blood pressure monitors. You can quickly recall the fixed onboard simulations or define your own. With its internal pump, the Tester can generate pressures up to 400 mmHg (53.3 kPa) for leak testing, pressure sourcing, and relief valve testing.
In addition, you can define auto sequences that automate the sequencing of tests and NIBP simulations and provide an optional printed report.
Key Features
Key features of the Tester include:
•Pressure leak testing on cuff, tubing, and connections
•Relief valve testing on the patient monitor
•Pressure gauge measurements
•Pressure source capability
•NIBP simulations including adult, neonate, arrhythmias, and respiratory artifacts
•Auto sequences with optional reports
•Internal Adult and Neonatal Cuff simulation
Tester capabilities can be extended with optional accessories that allow:
•ECG synchronization with non-invasive output
•External wrist cuff simulations
1-3

BP Pump 2
Operators Manual
Tester pressure accuracy can be improved by upgrading to a high-accuracy pressure transducer. This is a factory service upgrade and is provided to customers wanting to meet the DIN EN 1060 requirements for pressure measurement accuracy. For more information, refer to “Setup, Maintenance, and Support: Maintenance and Support.”
General Safety Considerations
This Tester complies with safety and technical requirements described in the following directives:
•UL 3101-1, Electrical Equipment for Laboratory Use; Part 1: General Requirements.
•CAN/CSA C22.2 No. 1010.1 (1992), Safety Requirements for Electrical Equipment for Measurement, Control and Laboratory Use, Part 1: General Requirements.
•EC 73/23/EEC (Amended 93/68/EEC) EN61010-1:2001, Safety requirement for electrical equipment for measurement, control and laboratory use, Part 1: General Requirements.
Symbols
Table 1-1 describes symbols used in association with the Tester.
Table 1-1. Symbols
Symbol |
Description |
Symbol |
Description |
|
|
|
|
|
|
W |
Risk of danger. Important |
X |
Hazardous voltage. Risk |
|
|
information. See manual. |
|
of electrical shock. |
|
|
Intertek Electrical Test |
|
|
|
Π|
Laboratory listed. |
P |
Conforms to European |
|
Conforms to relevant |
||||
Union directives |
||||
|
Canadian and U.S. |
|
|
|
|
standards. |
|
|
|
|
|
|
|
|
|
Do not dispose of this |
|
|
|
~ |
product as unsorted |
|
|
|
municipal waste. Contact |
|
|
||
|
Fluke or a qualified |
|
|
|
|
recycler for disposal. |
|
|
|
|
|
|
|
1-4

Introduction and Specifications 1
General Safety Considerations
Warnings and Cautions
Users are advised to read the manual carefully, observing all warnings and cautions, before attempting to set up and operate the Tester.
A Warning identifies hazardous conditions and actions that could cause bodily harm or death.
A Caution identifies conditions and actions that could damage the Tester or the equipment under test, or cause permanent loss of data.
W X Warning
To avoid possible electric shock or personal injury, follow these guidelines:
•Read the Users Manual before operating the Tester.
•Use this Tester only in the manner specified by the manufacturer or the protection provided may be impaired.
•Do not connect the Tester to a patient or equipment connected to a patient. The Tester is intended for equipment evaluation only and should never be used in diagnostics, treatment or in any other capacity where the Tester would come in contact with a patient.
•Do not use the product in wet locations, around explosive gases or dust.
•Never open the Tester case, because dangerous voltages are present. There are no user replaceable parts in the Tester.
•The Tester must be properly earthed. Only use a supply socket that has a protective earth contact. If there is any doubt as to the effectiveness of the supply socket earth, do not connect the Tester.
•Do not use a two-conductor adapter or extension cord; this will break the protective ground connection.
•Ensure that the external power source is properly rated for the system.
1-5

BP Pump 2
Operators Manual
•Always connect the system power cord directly to a three-prong receptacle with a functional ground. Never use a two-prong plug adapter to connect primary power to the Tester, thereby disconnecting the utility ground.
•Disconnect the Tester from the power source before changing the supply voltage. The Tester operates at a range of 100 to 240 volts.
W Caution
To avoid damage to the Tester or adverse affects on its performance, follow these guidelines:
•Allow only qualified technical personnel to service the Tester.
•Do not expose the system to temperature extremes. Ambient temperatures should remain between 15° C and 40° C. System performance may be adversely affected if temperatures fluctuate above or below this range.
•Clean the Tester only by gently wiping down with a clean, lint-free cloth dampened with a mild detergent solution. Do not immerse the unit.
•Do not apply pressures greater than 400 mmHg (53 kPa) to the pressure port.
1-6
Introduction and Specifications 1
Instrument Familiarity
Instrument Familiarity
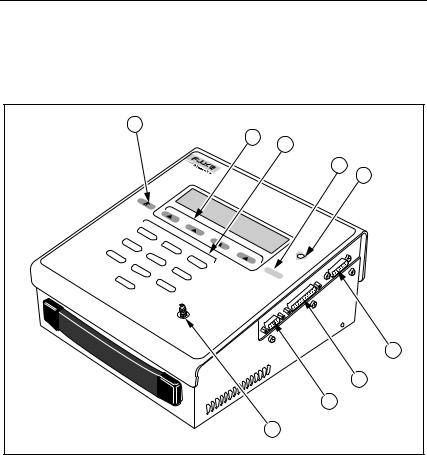
Figure 1-1 shows the Tester. The top and side panel components are described in Table 1-2 and the number key functions are described in Table 1-3.
|
|
1 |
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BP |
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N-I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NVPump |
2 |
|
||
|
|
|
|
|
|
|
|
|
|
|
ASI |
VE |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
BLOOD |
PRESSURE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MON |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ITOR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YZER |
|
|
|
|
|
PRESSURELEAK |
TESTS |
AND |
|
|
|
|
|
|
|
|
|
|
STANDARD |
|
1 |
|
|
SIMULA |
|
|
|
|
|
|||
|
|
BP |
|
|
|
|
|
|
|
|
|
||||
|
|
4 |
|
|
|
RELIEFPRESSURE |
TIONS |
|
|
|
|
||||
RESPIRI |
|
|
|
|
PA |
2 |
|
|
|
|
|
|
|||
ARTIF |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
7 |
ACTORY |
|
|
|
TIENT |
|
|
|
TA |
|
|
|
|
||
T |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
CONDITIONS5 |
|
|
|
TIC |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
PRESSURE |
|
|
|
|
||
|
|
|
NEONA |
|
|
ARRHYTHMIAS6 |
3 |
|
|
|
|
||||
|
|
|
8 |
TE |
|
|
|
|
|
|
|
ENT |
|||
|
USER |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 DEFINED |
|
|
|
|
WRIST |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PRESSURE |
PO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
fas10.eps |
Figure 1-1. Tester Top and Side Panel Components
1-7

BP Pump 2
Operators Manual
Table 1-2. Top and Side Panel Components
Label |
Name |
Function |
|
|
|
|
|
A |
Home Key |
Returns the operator to the Main Menu. |
|
|
|
|
|
B |
Soft Keys 1 - 4 |
Makes dynamic assignments based on |
|
the current screen. |
|||
|
|
||
|
|
|
|
|
Number (Test and |
Allows the operator to perform auto |
|
C |
sequences and simulations using |
||
Simulations) Keys |
|||
|
numeric keys. |
||
|
|
||
|
|
|
|
D |
Enter Key |
Advances to the next menu or |
|
saves/selects options. |
|||
|
|
||
|
|
|
|
|
|
LED blinks in synchronization with |
|
E |
Pulse Indicator |
beeper, indicating that the pump is |
|
generating a simulated blood pressure |
|||
|
|
||
|
|
pulse. |
|
|
|
|
|
F |
ECG Interface Port |
Allows connection of optional ECG |
|
|
|
accessory (refer to Appendix, ECG |
|
|
|
Option). |
|
|
|
|
|
G |
Printer Port |
Provides D-25 female connector for |
|
|
|
external parallel printer. |
|
|
|
|
|
H |
RS-232 Serial Port |
Provides serial D-9 female connector for |
|
|
|
bi-directional computer control. |
|
|
|
|
|
|
|
Connects to the Non-Invasive Blood |
|
I |
Pressure Port |
Pressure Monitor for all pressure |
|
|
|
simulations and tests. |
|
|
|
|
1-8

|
|
Introduction and Specifications |
1 |
|
|
|
Instrument Familiarity |
||
|
Table 1-3. Number Key Functions |
|
|
|
|
|
|
|
|
Number |
Name |
Function |
|
|
|
|
|
|
|
|
|
Pressurizes a pneumatic system to an |
|
|
|
PRESSURE |
operator-defined target pressure up to |
|
|
1 |
400 mmHg (53.3 kPa) and then |
|
|
|
LEAK |
|
|
||
|
measures the loss of pressure over |
|
|
|
|
|
|
|
|
|
|
time. |
|
|
|
|
|
|
|
|
|
Increases the pressure in the |
|
|
|
PRESSURE |
pneumatic system until the relief valve |
|
|
2 |
on the NIBP monitor opens or until the |
|
||
RELIEF |
|
|||
|
Setpoint is reached, whichever occurs |
|
||
|
|
|
||
|
|
first. |
|
|
|
|
|
|
|
|
|
Accessed via the Pressure Gauge |
|
|
|
STATIC |
Test, which enables the Tester to |
|
|
3 |
measure static pressure generated by |
|
|
|
PRESSURE |
|
|
||
|
an external source in the range of 50 |
|
|
|
|
|
|
|
|
|
|
to 400 mmHg 6.7 to 53.3 kPa). |
|
|
|
|
|
|
|
|
|
Provides seven variations of NIBP |
|
|
4 |
STANDARD BP |
simulations for both arm and wrist |
|
|
|
|
cuffs. |
|
|
|
|
|
|
|
|
PATIENT |
Includes simulations for healthy, |
|
|
5 |
geriatric, and obese patients, a well as |
|
||
CONDITIONS |
|
|||
|
various levels of exercise. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Measures erratic heart rhythms, |
|
|
6 |
ARRHYTHMIAS |
including atrial fibrillation and |
|
|
|
|
premature ventricular contraction. |
|
|
|
|
|
|
|
|
RESPIRATORY |
Exhibits a beat-to-beat variation in the |
|
|
7 |
blood pressure caused by intrathoracic |
|
||
ARTIFACT |
|
|||
|
pressure. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tests the ability of the NIBP monitors |
|
|
8 |
NEONATE |
to detect blood pressure on neonatal |
|
|
|
|
patients. |
|
|
|
|
|
|
|
9 |
WRIST |
Tests wrist cuff NIBP monitors. |
|
|
|
|
|
|
|
0 |
USER DEFINED |
Allows the operator to define blood |
|
|
pressure simulations. |
|
|
||
|
|
|
|
|
|
|
|
|
|
1-9

BP Pump 2
Operators Manual
Powering Up the Tester
The Tester is very simple to power up. Follow these steps:
1.Plug in a three-pronged power cord to the back of the unit.
2.Plug the cord into an appropriate socket, ensuring that the external power source is properly rated for the system.
3.Move the power switch above the plug to the on position. After two momentary screens, the Tester displays the Main menu, from which all Tester functions are selected.
Fluke Biomedical BP Bump 2 |
|
|
|
PERFORM |
PRESSURE |
AUTO |
|
SIMULATION |
TESTS |
SEQUENCE |
SETUP |
fas31.eps
Specifications
The following are specifications for the Tester. Please contact your Fluke Biomedical service representative for more information regarding the device specifications.
Mains Voltage
Range......................................................................100 - 240 V ac 50/60 Hz, 60 VA
Environmental Conditions
Operating Temperature |
...........................................15 °C to 40 °C |
Storage Temperature .............................................. |
-20 °C to +65 °C |
Relative Humidity .................................................... |
90 % max |
Pressure Measurement
Units |
........................................................................kPa |
|
mmHg |
|
cmH2O |
|
inH2O |
|
psi |
1-10

Introduction and Specifications |
|
|
|
Specifications |
1 |
Range ..................................................................... |
0 mmHg to +400 mmHg |
|
Resolution............................................................... |
0.1 kPa |
|
|
1 mmHg |
|
|
1 cmH2O |
|
|
1 inH2O |
|
|
0.1 psi |
|
Resolution (High Accuracy Version) ....................... |
0.01 kPa |
|
|
0.1 mmHg |
|
|
0.1 cmH2O |
|
|
0.1 inH2O |
|
|
0.01 psi |
|
Accuracy (Standard Version) |
|
|
0 to 300 mmHg ................................................... |
±0.5 % of reading ±1 mmHg |
|
301 to 400 mmHg .............................................. |
±2 % of reading |
|
High-Accuracy Version ........................................... |
<0.8 mmHg (0.1 kPa) |
|
Pressure Generation |
|
|
Pressure Generator, Static Pressure Range .......... |
50 mmHg to +400 mmHg |
|
Difference between target pressure |
|
|
and actual pressure ................................................ |
±10 mmHg from 100-400 mmHg with |
|
|
a minimum volume of 300 cc |
|
Internal Leak Rate .................................................. |
<2 mmHg per minute, with a minimum |
|
|
volume of 300 cc |
|
Electrical ECG |
|
|
Signals .................................................................... |
RA, LA, RL, LL, V |
|
Waveform ............................................................... |
Lead II |
|
Amplitude................................................................ |
1 mV peak (±10%) |
|
Connections............................................................ |
Signals available via the optional ECG |
|
|
adapter |
|
Heart Rate for NIBP Simulations |
|
|
Heart Rate Accuracy |
|
|
With ECG disabled ............................................ |
±1 BPM |
|
With ECG enabled.............................................. |
±1 BPM |
|
Except for the following Patient Conditions: |
|
|
Weak Pulse, Tachycardia, Obese, Geriatric....... |
±1% ±1 BPM |
|
Patient Condition Mild Exercise.......................... |
±1.5% ±1 BPM |
|
Patient Condition Strenuous Exercise ................ |
±3% ±1 BPM |
|
1-11

BP Pump 2
Operators Manual
Accessories
The following are accessories for the Tester. To order, contact your Fluke Biomedical equipment dealer and use the Fluke Biomedical part numbers provided. Table 1-4 lists standard accessories shipped with the tester. Table 1-5 lists optional accessories that must be ordered separately.
Table 1-4. Standard Accessories
Description |
Quantity Shipped |
Part Number |
|
|
|
|
|
Operator's Manual |
1 |
2196592 |
|
|
|
|
|
Warranty Card |
1 |
2241856 |
|
|
|
|
|
Tubing and Fittings |
1 |
2196394 |
|
|
|
|
|
Country-specific Power Cord |
|
|
|
USA |
1 |
284174 |
|
|
|
|
|
Schuko |
1 |
769422 |
|
|
|
|
|
UK |
1 |
769455 |
|
|
|
|
|
AU |
1 |
658641 |
|
|
|
|
|
|
|
|
|
Table 1-5. Optional Accessories |
|||
|
|
|
|
Description |
|
|
Part Number |
|
|
|
|
Wrist Cuff Mandrel |
|
|
2391875 |
|
|
|
|
ECG adapter |
|
|
2391894 |
|
|
|
|
Carrying Case |
|
|
2222822 |
|
|
|
|
RS-232 Serial Cable (9M-9F) |
|
|
2238659 |
|
|
|
|
BP Pump 2 Ansur Plug-In |
|
|
2755836 |
|
|
|
|
1-12
Chapter 2
Setup, Maintenance, and Support
Contents |
Page |
Setting up the Tester..................................................................... |
2-3 |
Printer Output ........................................................................... |
2-4 |
User-Defined Simulations ........................................................ |
2-5 |
Language .................................................................................. |
2-6 |
Units of Measure ...................................................................... |
2-6 |
Self Test.................................................................................... |
2-8 |
Zero Pressure ............................................................................ |
2-8 |
Enable ECG Signal................................................................... |
2-9 |
Maintenance and Support............................................................. |
2-10 |
Avoiding Damage..................................................................... |
2-10 |
Cleaning.................................................................................... |
2-10 |
Service and Calibration ............................................................ |
2-11 |
Packing Instructions ................................................................. |
2-11 |
2-1
 Loading...
Loading...