Page 1

Service Manual
R Series
®
April 2016 9650-0903-01 Rev. L
Page 2

0123
ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA USA
01824-4105
ZOLL International Holding B.V.
Newtonweg 18
6662 PV ELST
The Netherlands
© 2016 ZOLL Medical Corporation. All rights reserved. R Series, CodeNet, SurePower, OneStep and ZOLL are trademarks or registered trademarks of
ZOLL Medical Corporation in the United States and/or other countries. Masimo is a registered trademark of Masimo Corporation in the United States
and/or other countries. All other trademarks are the property of their respective owners.
Warranty (U.S. Only)
(a) ZOLL Medical Corporation warrants to the original equipment purchaser that beginning on the date of installation, or thirty (30) days after the date of shipment from ZOLL Medical Corporation's facility,
whichever first occurs, the equipment (other than accessories and electrodes) will be free from defects in material and workmanship under normal use and service for the period of one (1) year. During such
period ZOLL Medical Corporation will, at no charge to the customer, either repair or replace (at ZOLL Medical Corporation's sole option) any part of the equipment found by ZOLL Medical Corporation to
be defective in material or workmanship. If ZOLL Medical Corporation's inspection detects no defects in material or workmanship, ZOLL Medical Corporation's regular service charges shall apply. (b)
ZOLL Medical Corporation sh all not be responsible for any equipment defect, the failure of the equipment to perform any functio n, or any other nonconformance of the equipment, caused by or attributable
to: (i) any modification of the equipment by the customer, unless such modification is made with the prior written approval of ZOLL Medical Corporation; (ii) the use of the equipment with any associated
or complementary equipment, (iii) installation or wiring of the equipment other than in accordance with ZOLL Medical Corporation's instructions. (c) This warranty does not cover items subject to normal
wear and burnout during use, including but not limited to lamps, fuses, batteries, patient cables and accessories. (d) The foregoing warranty constitutes the exclusive remedy of the customer and the exclusive
liability of ZOLL Medical Corporation for any breach of any warranty related to the equipment supplied hereunder. (e) Limitation of Liability: ZOLL shall not in any event be liable to Purchaser, nor shall
Purchaser recover, for special, incidental or consequential damages resulting from any breach of warranty, failure of essential purpose, or under any other legal theory including but not limited to lost pr ofits,
lost savings, downtime, goodwill, damage to or replacement of equipment and property, even if ZOLL has been advised of the possibility of such damages.
THE WARRANTY SET FORTH HEREIN IS EXCLUSIVE AND ZOLL MEDICAL CORPORATION EXPRESSLY DISCLAIMS ALL OTHER WARRANTIES WHETHER WRITTEN, ORAL,
IMPLIED, OR STATUT ORY, INCLUDING BUT NOT LIMITED TO ANY WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
For additional information, please call ZOLL Medical Corporation at 1-800-348-9011 (in Massachusetts 1-978-421-9655). International customers should call the nearest authorized ZOLL Medical
Corporation service center.
Software License
Read this License agreement carefully before operating any of the R Series® products.
Software incorporated into the system is protected by copyright laws and international copyright treaties as well as other intellectual property laws and treaties. This software is licensed, not sold. By taking
delivery of and using this system, the Purchaser signifies agreement to and acceptance of the following terms and conditions:
Grant of License: In consideration of payment of the software license fee which is part of the price paid for this product ZOLL Medical Corporation grants the Purchaser a non-exclusive license, without right
to sublicense, to use the system software in object-code form only.
Ownership of Software/Firmware: Title to, ownership of and all rights and interests in the system software and all copies thereof remain at all times vested in the manufacturer, and Licensors to ZOLL
Medical Corporation and they do not pass to Purchaser.
Assignment: Purchaser agrees not to assign, sub-license or otherwise transfer or share its rights under the license without the express written permission of ZOLL Medical Corporation.
Use Restrictions: As the Purchaser, you may physically transfer the products from one location to another provided that the software/firmware is not copied. You may not disclose, publish, translate, release
or distribute copies of the software/firmware to others. You may not modify, adapt, translate, reverse engineer, decompile, crosscompile, disassemble or create derivative works based on the software/
firmware.
No Implied License
Possession or purchase of this device does not convey any express or implied license to use the device with replacement parts which would, alone, or in combination with this device, fall within the scope of
one or more of the patents relating to this device.
Page 3

TABLE OF CONTENTS
Preface
Safety Considerations...................................................................................................................................................i
Additional Reference Material ............................... ... ... ... .... ... ... ... .... ... ... ... ... .... ... ... ... .... ... ... .........................................ii
Conventions..................................................................................................................................................................ii
Service Policy Warranty..............................................................................................................................................iii
Technical Service.........................................................................................................................................................iii
Technical Service Outside of the United States........................................................................................................iv
Ch a p t er 1 Maintenance Tests
Overview.................................................................................................................................................................... 1-1
Before You Begin the Maintenance Tests............................................................................................................... 1-2
Equipment You Need to Perform the Maintenance Tests...................................................................................... 1-2
Physical Inspection of the Unit ............................................................................................................................. 1-4
Front Panel Button Test ....................................................................................................................................... 1-6
3 and 5 Leads Test .............................................................................................................................................. 1-8
Power Supply Test (Optional) .............................................................................................................................. 1-9
Leakage Current Test ........................................................................................................................................ 1-14
Paddles Test ...................................................................................................................................................... 1-15
Heart Rate Display Test ..................................................................................................................................... 1-16
Calibrating Pulses on Strip Chart Test ............................................................................................................... 1-17
Notch Filter Test ................................................................................................................................................ 1-18
Heart Rate Alarm Test ....................................................................................................................................... 1-19
Defibrillator Self Test ......................................................................................................................................... 1-21
Synchronized Cardioversion Test ......................... ....................................... ...................................................... 1-23
Synchronized Cardioversion for Remote ECG Monitoring Test (Optional)........................................................ 1- 24
Shock Test ......................................................................................................................................................... 1-25
Summary Report Test ........................................................................................................................................ 1-27
Advisory Message Test (Manual/Advisory Units) .... ... .... ... ....................................... ... ... ... .... ... ......................... 1-28
Pacer Test ......................................................................................................................................................... 1-29
SpO2 Monitor Test (for SpO2 Option) ............................................................................................................... 1-31
EtCO2 Monitor Test (for EtCO2 Option) ............................................................................................................ 1-33
Barometric Pressure Calibration Check .............................................................................................................. 1-34
CO2 Accuracy Check (for EtCO2 Option) .......................................................................................................... 1-35
9650-0903-01 Rev. L iii
Page 4

NIBP Volume Leak Test ..................................................................................................................................... 1-37
NIBP Transducer Calibration Test ...................................................................................................................... 1-39
NIBP Monitor Test .............................................................................................................................................. 1-41
Ch a p t e r 2 Troubleshooting
Overview.................................................................................................................................................................... 2-1
ZOLL R Series Error Messages............................................................................................................................... 2-1
Ch a p te r 3 Disassembly Procedures
Required Equipment................................................................................................................................................. 3-1
Safety Precautions.................................................................................................................................................... 3-2
Removing the Cable Caddy ............................ ......................................................................................................... 3-3
Removing the Handle............................................................................................................................................... 3-6
Removing the Recorder, 3-
AC Charger and Battery Well....... ... ... .... ...................................... .... ... ... ... ... .... ... ... ... .... ... ... ... .................................. 3-7
Removing the Front Panel Assembly ................................................................................................................... 3-12
Front Panel Disassembly................... .... ... ... ... .... ... ... ... ... ....................................... ... .... ... ... ... ................................ 3-14
Removing the Side Panels........... ... ... .... ... ... .......................................................................................................... 3-16
Removing the Connector Panel and Bezel..................................................................... ... ... .... ... ... ... ................... 3-18
Removing the ECG Input Connector..................................................................................................................... 3-20
Removing the NIBP Assembly............................................................................................................................... 3-21
Removing the System Brick Assembly ................................................................................................................ 3-23
Disassembly of the System Brick Assembly ....................................................................................................... 3-28
Discharging Capacitor............................................................................................................................................ 3-34
Removing the Communication Module ............................................................................................................... 3-35
iv 9650-0903-01 Rev. L
Page 5

TABLE OF CONTENTS
Ch a p te r 4 Replacement Parts
List of Replacement Parts........................................................................................................................................ 4-2
Diagrams...................................................................................................................................................................4-11
Disassembling the System Brick Assembly ........................................................................................................ 4-41
Ch a p t e r 5 Functional Description
AC Charger.......... ... .... ... ... ... ... .... ... ....................................... ... ... ... .... ... ... .................................................................. 5-1
SurePower Battery.................................................................................................................................................... 5-1
Parameter Power Supply (SpO2, EtCO2, NIBP)..................................................................................................... 5-2
Digital System Board................................................................................................................................................ 5-2
Analog System Board................................ ... ... .... ... ... ... ... .... ... ... ... .... ... ... ... ... .... ........................................................ 5-3
Pace/Defib Core Engine ........................................................................................................................................... 5-4
Front Panel Controls .......................... .... ... ....................................... ... ... ... ... .... ........................................................ 5-4
Peripherals ................................................................................................................................................................ 5-4
Accessories............................................................................................................................................................... 5-5
Power Management Support Functions................................................................................................................. 5-5
WiFi ............................................................................................................................................................................ 5-6
Appendix
Interconnect Diagram for the R Series Biphasic Unit ................................................................ ...........................A-2
Delivered Energy at Every Defibrillator
Setting into a Range of Loads.................................................................................................................................A-3
Sync Connector Diagrams.......................................................................................................................................A-4
Maintenance Test Checklist.....................................................................................................................................A-5
9650-0903-01 Rev. L v
Page 6

vi 9650-0903-01 Rev. L
Page 7

R Series Service Manual
Preface
ZOLL® Medical Corporation’ s R Seri es® Service Manual is intended for the service tech nician whose responsibilit y is to maintain and inspect the R Series
defibrillators. The ZOLL R Series Service Manual has five main sections and one appendix.
Preface—Contains safety warnings and an overview of the manual’s contents. Be sure to review this section thoroughly before attempting to use or service
the R Series unit.
Chapter 1—Maintenance Tests explains how to check the defibrillator’s performance using a series of recommended checkout procedures to be
conducted every 12 months.
Chapter 2—Troubleshooting provides a listing of the procedures and error messages to help the service technician detect faults and repair them.
Chapter 3—Disassembly Procedures describes step-by-step procedures for removing assemblies and subassemblies from the R Series unit
Chapter 4—Replacement Parts List displays a complete list of ZOLL part numbers for field replaceable parts available for the R Series unit, allowing
the service person to identify and order replacement parts from ZOLL.
Chapter 5—Functional Description provides technical descriptions for the R Series major subassembly modules.
Appendix—R Series interconnect diagram; table for delivered energy loads; maintenance checklists.
Safety Considerations
The following section describes general warnings and safety considerations for operators and patients. Service technicians should review the safety
considerations prior to servicing any equipment and read the manual carefully before attempting to disassemble the unit. Only qualified personnel should
service the R Series unit.
Federal (U.S.A.) law restricts this unit for use by or on the order of a physician.
Safety and effectiveness data submitted by ZOLL Medical Corporation to the Food and Drug Administration (FDA) under section 510(K) of the Medical
Device Act to obtain approval to market is based upon the use of ZOLL accessories such as disposable electrodes, patient cables and batteries. The use of
external pacing/defibrillation electrodes and adapter units from sources other than ZOLL is not recommended. ZOLL makes no representations or
warranties regarding the performa nce or ef fectivenes s of its product s when used in conjunction with pacing/d efibrillation elect rodes and adapter units fro m
other sources. If unit failure is attributable to pacing/defibrillation electrodes or adapter units not manufactured by ZOLL, this may void ZOLL's warranty.
Only qualified personnel should disassemble the R Series unit.
9650-0903-01 Rev. L i
Page 8

R Series Service Manual
WARNING! This unit can generate up to 2,850 volts with sufficient current to cause lethal shocks.
All persons near the equipment must be warned to STAND CLEAR prior to discharging the defibrillator.
Do not discharge the unit’s internal energy more than three times in one minute or damage to the unit may result.
Do not discharge a battery pack except in a ZOLL SurePower
TM
Battery Charger Station.
Do not use the R Series in the presence of flammable agents (such as gasoline), oxygen-rich atmospheres, or flammable anesthetics. Using the unit near
the site of a gasoline spill may cause an explosion.
Do not use the unit near or within puddles of water.
Additional Reference Material
In addition to this guide, there are several other components to the ZOLL R Series documentation. They include:
• ZOLL R Series Operator’s Guide - describes all the user tasks needed to operate the R S eries.
• ZOLL R Series Configuratio n Guide - describes the R Series features and functions whose operation can be customized by authorized users.
• ZOLL R Series Operator’s Guide - Pulse Oximetry (SpO
• ZOLL R Series Operator’s Guide - Non-Invasive Blood Pressure (NIBP) Insert - describes all the user tasks needed to operate the R Series NIBP
option.
• ZOLL R Series Operator’s Guide - End Tidal Carbon Dioxide (EtCO
option.
) Insert - describes all the user tasks needed to operate the R Series Pulse Oximetry option.
2
) Insert - describes all the user tasks needed to operate the R Series EtCO2
2
Conventions
WARNING! Warning statements describe conditions or actions that can result in personal injury or death.
Caution Caution statements describe conditions or actions that can result in damage to the unit.
Note: Notes contain additional information on using the defibrillator.
ii 9650-0903-01 Rev. L
Page 9

R Series Service Manual
Service Policy Warranty
In North America: Consult your purchasing agreement for terms and conditions associated with your warran ty. Outside of North America, consult ZOLL
authorized representative.
In order to maintain this warranty, the instructions and procedures contained in this manual must be strictly followed. For additional information, please
call the ZOLL Technical Service Department 1-800-348-9011 in North America.
Technical Service
If the ZOLL R Series unit requires service, contact the ZOLL Technical Service Department:
Telephone: 1-978-421-9655; 1-800-348-9011
Fax 1-978-421-0010
Email: techsupport@zoll.com
Have the following information available for the Technical Service representative:
• Unit serial number
• Description of the problem
• Department where equipment is used
• Purchase Order to allow tracking of loan equipment
• Purchase Order for a unit with an expired warranty
• Sample chart recorder strips documenting the problem, if applicable
• Full disclosure file from the unit, if applicable (.FUL extension)
• Ready code file from the unit, if applicable (.DCK extension)
• Activity log file from the unit, if applicable (.RAL extension)
If the unit needs to be sent to ZOLL Medical Corporation, obtain a Service Request number from the Technical Service representative. Return the unit in
its original container to:
ZOLL Medical Corporation
269 Mill Road
Chelmsford, Massachusetts 01824-4105
9650-0903-01 Rev. L iii
Page 10

R Series Service Manual
Attn: Technical Service Department, SR# XXXXXX
Telephone: 1-800-348-9011; 1-978-421-9655 FAX: 978-421-0010
Technical Service Outside of the United States
Customers outside of the United States sh ould return the u nit in its original co ntainer to the nearest authorized ZOLL Medical Corporation Se rvice Center.
To locate an authorized service center, contact the International Sales Department at ZOLL Medical at the above address.
iv 9650-0903-01 Rev. L
Page 11

R Series Service Manual
Chapter 1
Maintenance Tests
Overview
The R Series has two checkout procedures: the R Series Operator’s Guide defibrillator testing checklist and the extensive 12-month maintenance test
checkout procedures.
Because the R Series units must be maintained ready for immediate use, regular readiness testing is required. It can either be performed manually or
automatically. Refer to the R Series Operator’s Guide for details.
A qualified biomedical technician must perform a more thorough maintenance test checkout every 12 months to ensure that the functions of the R Series
unit work properly. This chapter describes the step by step procedures for performing the 12 month maintenance test checkout. Use the checklist at the
back of this document (ZOLL R Series Maintenance Test Checklist) to record your results of the maintenance tests.
This chapter describes the following maintenance tests:
• 1.0 Physical Inspection of the Unit
• 2.0 Front Panel Button Test
• 3.0 3 and 5 Leads Test
• 4.0 Power Supply Test (Optional)
• 5.0 Leakage Current Test
• 6.0 Paddles Test
• 7.0 Heart Rate Display Test
• 8.0 Calibrating Pulses on Strip Chart Test
• 9.0 Notch Filter Test
• 10.0 Heart Rate Alarm Test
• 11.0 Defibrillator Self Test
• 12.0 Synchronized Cardioversion Test
• 13.0 Synchronized Cardioversion for Remote ECG Monitoring Test (Optional)
• 14.0 Shock Test
• 15.0 Summary Report Test
9650-0903-01 Rev. L 1-1
Page 12

R Series Service Manual
• 16.0 Advisory Message Test (Manual/Advisory Units)
• 17.0 Pacer Test
• 18.0 SpO2 Monitor Test (for SpO2 Option)
• 19.0 EtCO2 Monitor Test (for EtCO2 Option)
• 20.0 Barometric Pressure Calibration Check
• 21.0 CO2 Accuracy Check (for EtCO2 Option)
• 22.0 NIBP Volume Leak Test
• 23.0 NIBP Transducer Calibration Test
• 24.0 NIBP Monitor Test
Before You Begin the Maintenance Tests
• Assemble the tools or specialized testing equipment listed in the “Equipment You Need to Perform the Maintenance Tests” section shown below.
• Keep an extra fully charged ZOLL SurePower defibrillator battery available.
• Schedule an hour to conduct the entire maintenance test.
• Photocopy the checklist at the back of this document and use the copy to record your results. As you conduct each step of a procedure, mark the Pass/
Fail/NA check boxes on your checklist and then save it for your maintenance file.
• Perform the tests in the order presented.
• Perform all the steps of each test procedure.
• Complete all the steps of the procedure before evaluating the test results.
Equipment You Need to Perform the Maintenance Tests
This section lists equipment that we use to perform the maintenance tests that we describe in this chapter. You can substitute an equivalent device for a
listed device; however, not all simulators and analyzers will produce the same results. Be sure to follow the manufacturer’s recommendations for
conducting the maintenance tests.
We recommend the use of the following equipment when performing R Series Maintenance Tests
• ZOLL Medical Electrode Adapter from Fluke Biomedical (DNI part number 3010-0378).
• Fluke Impulse 4000 Defibrillator Analyzer with 1.06 software or higher.
• Fluke Biomedical International Safety Analyzer.
• 2 red miniature alligator to miniature alligator leads.
1-2 9650-0903-01 Rev. L
Page 13

• 2 black miniature alligator to miniature alligator test leads.
• DC power supply (15 Amp minimum).
• 0.1resistor (¼W or greater).
• 1000 1% ¼W resistor.
• Fluke 75 multimeter or equivalent.
• Fluke Biomedical
• SpO
• Fluke BP Pump 2 NIBP simulator (if NIBP option is installed).
• CAPNOSTAT 5 Mainstream cable with airway adapter (if EtCO
• Stop watch.
• Paddles.
• Printer paper.
• Battery.
• AC line cord.
• 3 lead adapter (part # 8009-0762-XX) or 3 lead ECG cable, and 5 lead ECG cable.
• Gas Regulator (if EtCO2 option is installed).
• Calibration Gas (if EtCO2 option is installed).
cable and sensor (if option is installed).
2
Index 2PFE SpO2 Simulator or equivalent (if option is installed).
option is installed).
2
R Series Service Manual
9650-0903-01 Rev. L 1-3
Page 14
R Series Service Manual
1.0 Physical Inspection of the Unit
Tools Needed: None.
Test Setup: None.
Observe this... Pass/Fail/NA
1.1 Housing
Is the unit clean and undamaged?
1.2 Does the unit show signs of excessive wear?
1.3 Is the handle undamaged?
1.4 Does the recorder door open and close properly?
1.5 Are input connectors clean and undamaged?
1.6 Are there any cracks in the housing?
1.7 Do the front panel or keypad buttons have any damage or cracks?
1.8 Are there any loose housing parts?
1.9 Do the paddle latches work properly?
1.10 Paddles
Do the adult and pedi plates have major scratches or show signs of damage?
1.11 Do the adult shoes slide on and off easily to expose the covered pedi plates?
1.12 Are the paddles clean (e.g., free of gel) and undamaged? (if applicable)
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
1.13 Cables
Are all cables free of cracks, cuts, exposed or broken wires?
1.14 Are all bend/strain reliefs undamaged and free of excessive cable wear?
1-4 9650-0903-01 Rev. L
ooo
ooo
Page 15
Observe this... Pass/Fail/NA
1.15 Battery
Place battery in battery well.
R Series Service Manual
1.16 Is the battery seated in the battery well correctly?
Record your results on the Maintenance Test Checklist.
ooo
9650-0903-01 Rev. L 1-5
Page 16
R Series Service Manual
2.0 Front Panel Button Test
Tools Needed: Fluke Impluse 4000.
Test Setup:
1. Install strip chart paper into the printer compartment.
2. Install a fully charged battery in the unit or connect the A/C power cord to the unit and then plug the cord into an electrical outlet.
3. Connect the OneStep
(or equivalent).
Do this... Observe this... Pass/Fail/NA
TM
cable and ECG cable (3 lead adapte r (part #8009-0762-XX), 3 lead cable, or 5 lead cable) to the Fluke Biomedical 4000 Analyzer
2.1 Turn the selector switch to MONITOR. (for AED
units, turn the selector switch to ON and select
Manual mode.)
2.2 Press the LEAD button; three times for the 3
lead cable and seven times for the 5 lead cable.
2.3 Set the Impulse 4000 to NSR of 120 BPM.
Press the LEAD button until Lead II displays. To
check the size of the ECG waveform, press the
SIZE button.
2.4 Press the ALARM SUSPEND button. Bell changes from disabled to enabled. If the alarm sounds, press the ALARM
2.5 Press the RECORDER button. The strip chart paper moves out of the unit from the printer compartment.
2.6 Open the printer door.
Press RECORDER button.
Listen for 4 beep tones. PADS and MONITOR display on the monitor.
NOTE: PADS is a factory default setting.
Each time you press the LEAD button, a different lead number appears under
the LEAD heading on the display.
PADS, I, II, III will display if a 3 lead ECG cable is connected or no ECG cable
is connected.
PADS, I, II, III, AVR, AVL, AVF, V1 will display if a 5 lead ECG cable is
connected.
As you press the SIZE button five times (0.5, 1.0, 1.5, 2.0, 3.0), note that the
size of the ECG waveform appropriately changes on the display.
SUSPEND button to turn it off. The alarm will only be suspended for 90
seconds at this point. Press and hold the ALARM SUSPEND button for 3
seconds to disable alarms.
Check that the correct time, date, ECG lead annotation and waveform are
recorded on the paper. (Set Time and Date, if necessary.)
CHECK RECORDER message appears on the monitor.
ooo
ooo
ooo
ooo
ooo
ooo
1-6 9650-0903-01 Rev. L
Page 17
R Series Service Manual
Do this... Observe this... Pass/Fail/NA
2.7 Close the printer door.
Press RECORDER button.
2.8 Press RECORDER button. Strip chart paper stops flowing out of printer compartment.
2.9 Connect A/C current and install a fully charged
battery. Turn the unit off.
2.10 Remove the battery. Observe that the battery LED alternates between green and amber.
2.1 1 Replace the battery and turn the selector switch
to MONITOR (for AED units, turn to ON.)
2.12 Press the ANALYZE button (if available). The SELECT DEFIB MODE message appears on the monitor. (For manual
2.13 Move the selector switch to DEFIB (for AED
units leave in the ON position.) Select 2J. Press
the CHARGE button.
2.14 Press and hold the ENERGY SELECT down
arrow.
2.15 Press and release the ENERGY SELECT up
arrow 18 times.
Strip chart paper flows out of printer compartment. Verify that the CHECK
RECORDER message no longer displays.
The AC Power LED should be illuminated.
The battery LED will be green or amber.
NOTE: If the battery LED alternates between green and amber, no battery is
installed or there is a battery charging fault.
Note that the battery indicator is green or amber.
devices.)
The display shows that the unit is charging. The SHOCK button lights when the
unit is charged. Ready tone for DEFIB sounds.
Unit discharges internally and selected energy decrements to 1J.
Verify the following settings: 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 50, 75, 100,
120, 150, 200J.
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
2.16 Press the CHARGE button. Note the display shows the unit charged up to 200J and the SHOCK button
lights.
2.17 Press the SHOCK button. The unit discharges and the SHOCK button is no longer lit. A 15 second strip
chart automatically prints, displaying the number of joules delivered (if
configured to print post shock).
2.18 (AED UNITS ONLY) Turn selector switch to the
OFF position.Wait a minimum of 10 seconds
and turn selector switch back to the ON
position.
9650-0903-01 Rev. L 1-7
Verify on the front panel that the green AED LED is illuminated.
ooo
ooo
ooo
Page 18

R Series Service Manual
Do this... Observe this... Pass/Fail/NA
2.19 Press the MANUAL MODE softkey, then select
the CONFIRM softkey.
2.20 Turn selector switch to the OFF position. Verify the unit turns off.
Record your results on the Maintenance Test Checklist.
Verify the green AED LED turns off.
ooo
ooo
1-8 9650-0903-01 Rev. L
Page 19
R Series Service Manual
3.0 3 and 5 Leads Test
Tools Needed: Impulse 4000,3 lead adapter (part # 8009-0762-XX) or 3 lead cable, and 5 lead cable. If applicable, test each cable separately.
Test Setup:
1. The R Series unit must be configured to display ECG LEAD OFF message.
2. Connect the lead wires appropriate to each test to the Fluke Biomedical 4000 or equivalent.
Do this... Observe this... Pass/Fail/NA
3.1 Turn the selector switch to MONITOR (for AED
units turn to ON.) Select leads.
3.2 Disconnect one lead from the Impulse 4000. The ECG LEAD OFF message displays within 3 seconds (if configured).
3.3 Reconnect the lead. Repeat step 3.2 with the
remaining leads.
3.4 If applicable, repeat 3.2 and 3.3 for the
remaining cable(s).
Record your results on the Maintenance Test Checklist.
ECG LEAD OFF message is not displayed.
Wait for ECG LEAD OFF message to clear from the display (if configured).
NOTE: If heart rate alarm sounds, press and hold the ALARM SUSPEND
button for 3 seconds to disable the alarms.
ooo
ooo
ooo
ooo
9650-0903-01 Rev. L 1-9
Page 20

R Series Service Manual
4.0 Power Supply Test (Optional)
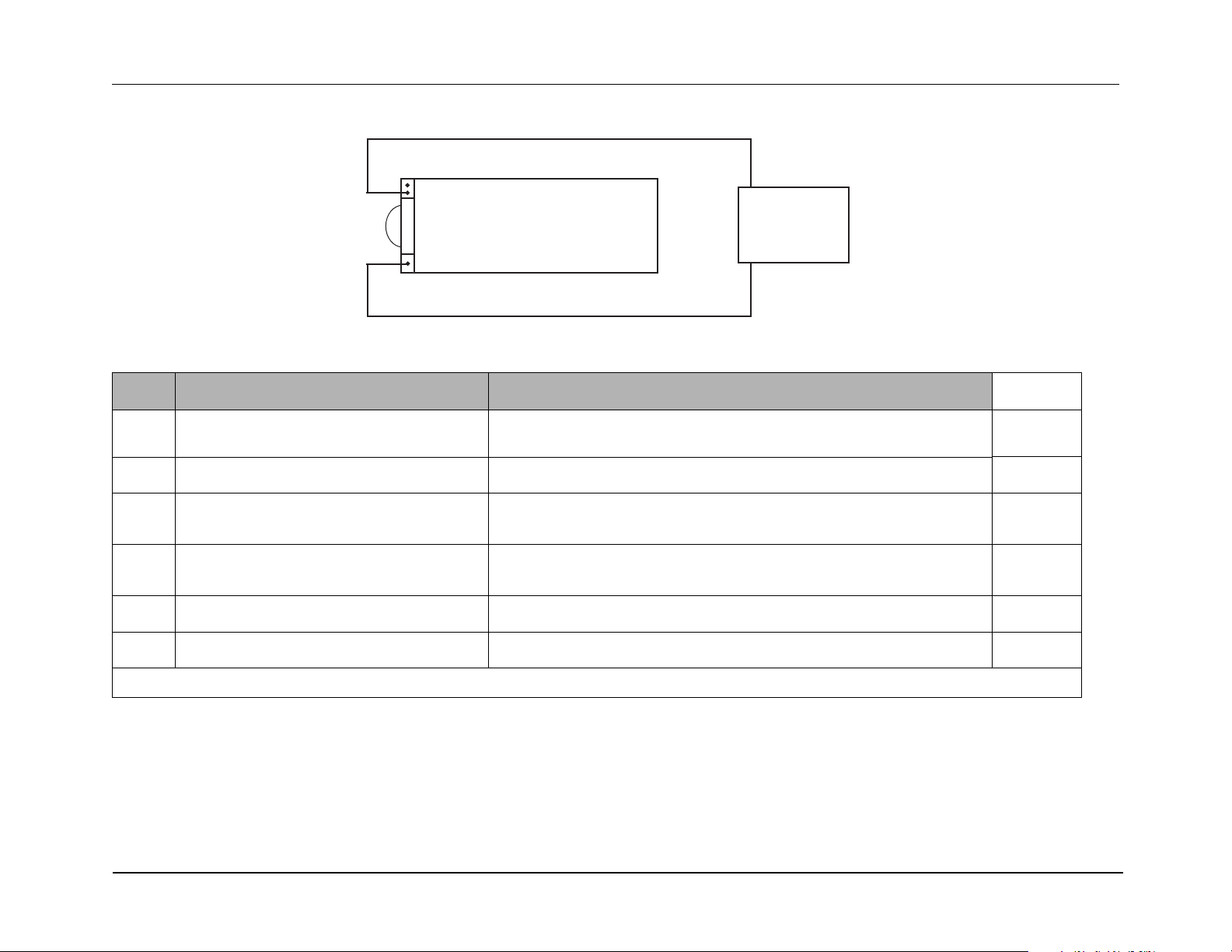
Note: Tests in this section will produce battery errors due to the use of a power supply in place of a SurePower Battery.
Tools Needed:
• 2 red miniature alligator to miniature alligator leads.
• 2 black miniature alligator to miniature alligator test leads.
• DC power supply (15 Amp minimum).
• 0.1resistor (¼W or greater).
• 1000 1% ¼W resistor.
• Fluke 75 multimeter or equivalent.
Test Setup:
1. Disconnect the AC line cord from the unit.
2. Make sure the unit and power supply are turned off.
3. Connect one end of the black lead to the “-” terminal in the battery well.
4. Connect the other end of the black lead to the “-” terminal of the power supply.
5. Connect the red lead to “+” terminal socket of the battery well. Use the middle pin with the plasti c guard around it. Connect t he other end of the red lead
to the “+” terminal of the power supply.
6. Set the power supply voltage to 7V.
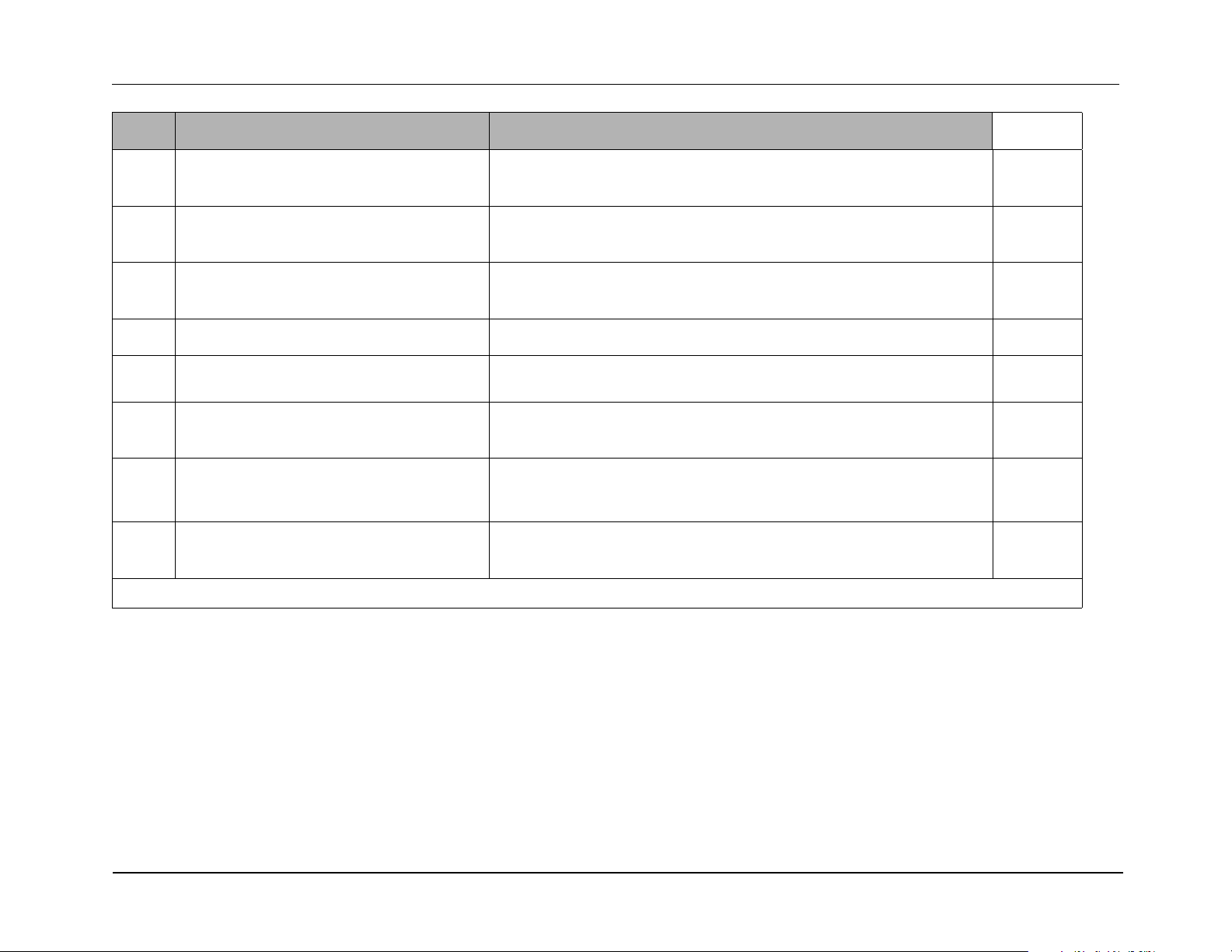
Caution Be sure to connect the power supply properly to the R Series battery well terminals or damage to the unit may result. Do NOT raise the
power supply voltage above 15V.
1-10 9650-0903-01 Rev. L
Page 21
R Series Service Manual
?
Battery Well
15 Amp
Supply
?
Red
Black
Do this... Observe this... Pass/Fail
4.1 Turn the selector switch to MONITOR (for AED
units turn to ON.)
4.2 Turn th e un i t off.
4.3 Adjust the power supply voltage to 10.8V and
turn the selector switch to MONITOR.
4.4 Low Battery Test
Set voltage to 10.5V.
4.5 Set voltage to 10.2V. REPLACE BATTERY message displays within 30 seconds.
4.6 Turn th e un i t off.
Record your results on the Maintenance Test Checklist.
The unit should not turn on.
The unit should turn on.
No LOW BATTERY message displays.
LOW BATTERY message disp lays within 30 seconds.
oo
oo
oo
oo
9650-0903-01 Rev. L 1-11
Page 22
R Series Service Manual
15 Amp
Supply
?
DMM
?
?
Battery Well
Red
Black
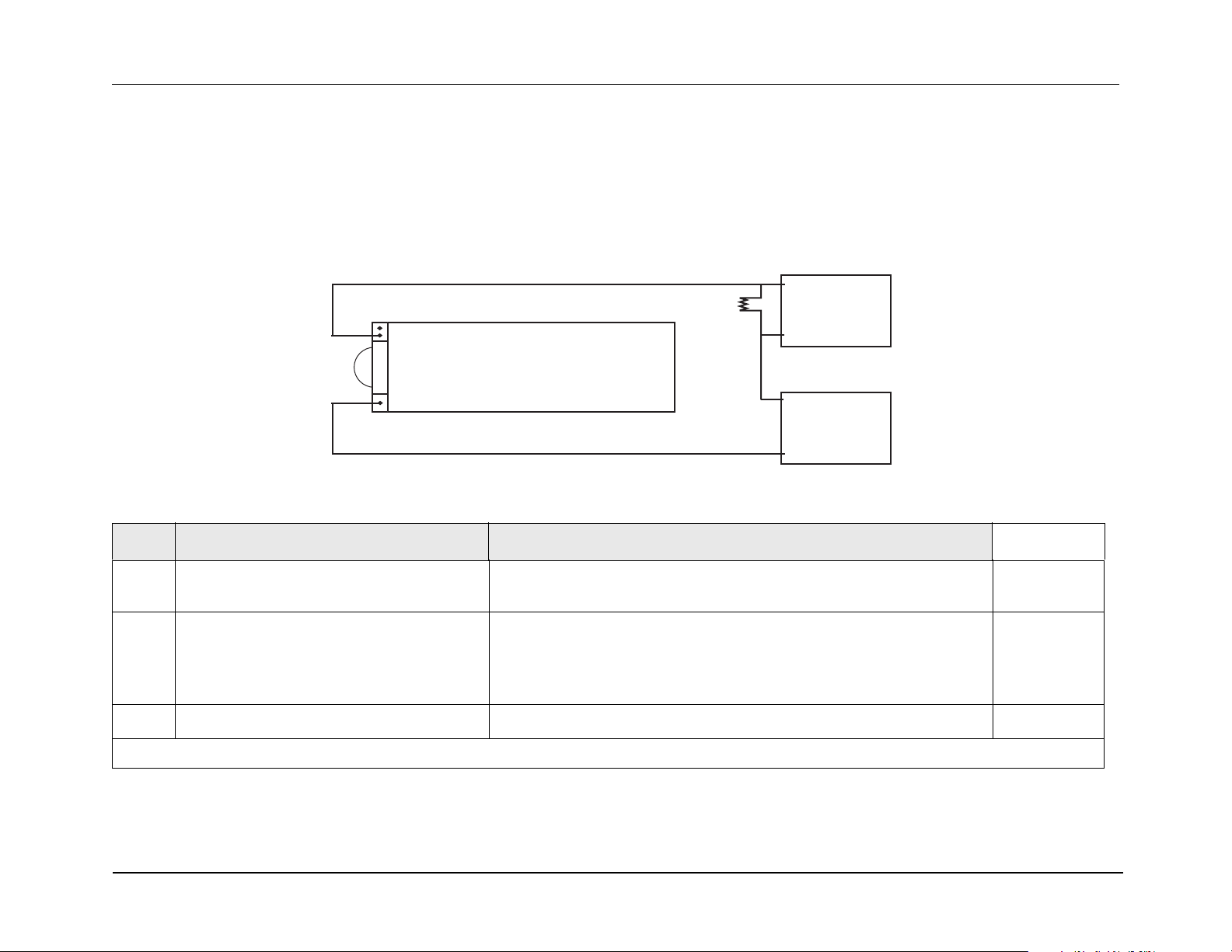
Test Setup:
1. Remove red lead from power supply and connect to 0.1
2.
Connect other end of resistor to “+” terminal of power supply using a second red lead.
resistor.
3. Connect multimeter across the resistor.
4. Set voltage scale (if DVM is not autoranging) to 220 mV.
Do this... Observe this... Pass/Fail/NA
4.7 System Current Test
Set power supply to 10.8V.
4.8 Turn the selector switch to MONITOR. Voltage across resistor should be 145 mV or less (<1.2A of ON current).
NOTE: Without optional parameters.
All devices with SpO
4.9 Turn unit off.
Record your results on the Maintenance Test Checklist.
1-12 9650-0903-01 Rev. L
, EtCO2 or NIBP <160mV
2
ooo
ooo
Page 23
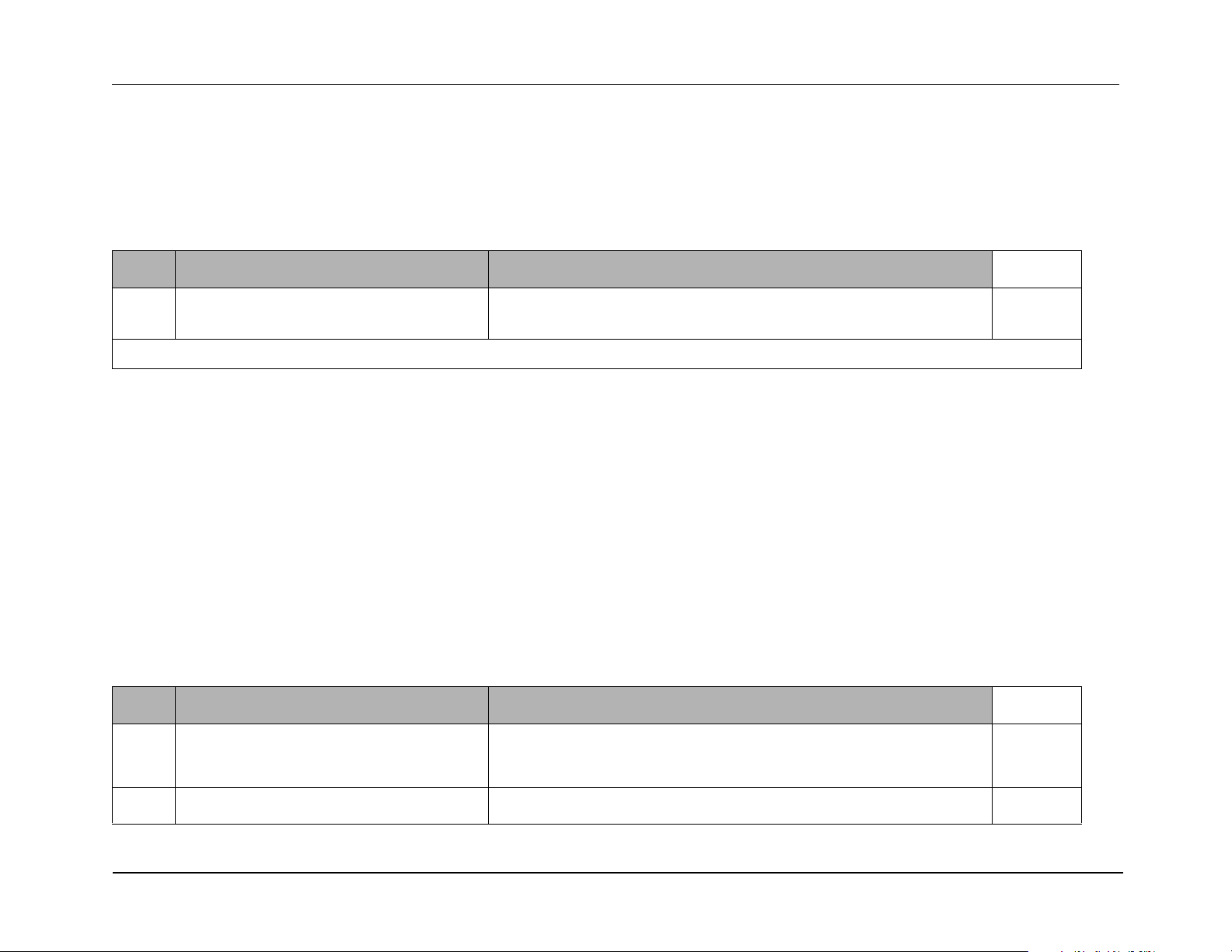
Test Setup for Off Current Test:
R Series Service Manual
1. Remove 0.1
2. Connect DMM across resistor.
3. Set voltage scale to DCV.
4. Measure voltage across resistor.
4.10 Off Current Test
Record your results on the Maintenance Test Checklist.
resistor and replace with 1K.
Do this... Observe this... Pass/Fail
Voltage should be less than 270 mV (<270 A of current).
Measure across resistor with unit turned off.
Charger Test
Tools Needed: 2-Post Battery Fixture (9100-0575-TF), DVM, 2 Test Leads, Impulse 4000, Stopwatch.
Test Setup:
Set DVM to read DC Volts.
Connect Postive lead to positive post of the R Series charger load fixture.
Connect negative lead to negative post of the R Series charger load fixture.
Verify the unit is plugged into AC Power.
oo
Do this... Observe this... Pass/Fail
4.1 1 Set front panel switch to DEFIB or ON, (for BLS
units, press MANUAL MODE, then press
CONFIRM).
4.12 Install Charger T est Fixture.
9650-0903-01 Rev. L 1-13
Page 24
R Series Service Manual
Do this... Observe this... Pass/Fail
4.13 Observe the voltage. Verify the charger voltage is 11.97V-12.43V.
4.14 On the Test Fixture set the switch to 20 Ohms. Verify the charger voltage is 9.50V-11.87V.
4.15 On the Test Fixture set the switch to 27 Ohms. Verify the charger voltage is 11.97V-12.43V.
4.16 Remove the charger load Test Fixture.
4.17 Connect the universal cable to the Impulse
4000.
4.18 Press the ENERGY SELECT UP ARROW
button until 200J is displayed.
4.19 Press the CHARGE button and start timing with
a stopwatch. Stop timing when the SHOCK
button illuminates.
4.20 Press the ENERGY DOWN ARROW button. Verify the unit internally discharged.
Record your results on the Maintenance Test Checklist.
Verify 200J is displayed.
Verify charge time is between 3-10 seconds.
oo
oo
oo
oo
oo
oo
1-14 9650-0903-01 Rev. L
Page 25
R Series Service Manual
5.0 Leakage Current Test
Tools Needed: See the manufacturer’s instructions or supplied specifications for the leakage tester you use.
Test Setup: See the manufacturer’s instructions or supplied specifications for the leakage tester you use. Repeat leakage test with applicable accessories:
OneStep Cable, external paddles, internal paddles, and anterior/posterior paddles.
Maximum Leakage Acceptance Limits
Normal Condition Single Fault Condition*
Lead ECG 10 50
Hands Free Electrodes and Paddles (MFE) 10 100
Earth 500 1000
*Single fault considered AC mains on applied part.
9650-0903-01 Rev. L 1-15
Page 26
R Series Service Manual
6.0 Paddles Test
Tools Needed: None.
Test Setup: If applicable, connect the OneStep cable to the paddles and place the paddles in paddle wells.
Do this... Observe this... Pass/Fail/NA
6.1 Turn the selector switch to DEFIB (for AED
units, turn the selector switch to ON, and
select Manual Mode.) Press and hold the
ENERGY DOWN button on the sternum paddle.
6.2 Press and release the ENERGY UP button on
the sternum paddle for each setting.
6.3 Press and release the RECORDER button on
the sternum paddle.
6.4 Press and release the RECORDER button
again.
6.5 Select 30J using the paddle ENERGY button.
Press the CHARGE button on the Apex paddle.
6.6 Press and release the APEX SHOCK button. No discharge.
6.7 Press and release the STERNUM SHOCK
button.
6.8 Press and hold both paddles SHOCK buttons. The unit discharges. The 30J TEST OK message displays and the red LED
Record your results on the Maintenance Test Checklist.
The energy selection decreases to 1J.
Verify the energy levels increment through the following: 1-10, 15, 20, 30, 50,
75, 100, 120, 150, 200J.
The recorder turns on.
Verify the recorder turns off.
The unit charges to 30J, then the red LED charge indicator illuminates and the
charge tone sounds. (Note that the front panel shock button does not
illuminate).
No discharge.
turns off. The recorder runs.
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
1-16 9650-0903-01 Rev. L
Page 27
7.0 Heart Rate Display Test
Tools Needed:
• Impulse 4000
• ECG Cable (3 or 5 leads).
Test Setup:
1. Turn the selector switch to MONITOR (for AED units turn to ON.) Press LEAD button until “I” displays.
2. Connect the ECG leads to the Fluke Biomedical 4000 or equivalent.
3. Connect the ECG cable to the unit.
Do this... Observe this... Pass/Fail/NA
R Series Service Manual
7.1 Set the Impulse 4000 to 120BPM. The Heart Rate displays as 120 +/- 2 bpm.
Record your results on the Maintenance Test Checklist.
ooo
9650-0903-01 Rev. L 1-17
Page 28
R Series Service Manual
8.0 Calibrating Pulses on Strip Chart Test
Tools Needed: None.
Test Setup: None
8.1 Press the RECORDER button.
8.2 Press and hold SIZE button to activate the
Record your results on the Maintenance Test Checklist.
.
Do this... Observe this... Pass/Fail/NA
calibration signal.
The strip chart displays a signal of 300 ppm with an amplitude of 10 mm
+/- 1 mm. The signal also appears on the video display. (You can verify that the
rate is 300ppm by measuring 5mm from the left edge of one pulse to the left
edge of the following pulse.)
ooo
1-18 9650-0903-01 Rev. L
Page 29
9.0 Notch Filter Test
Tools Needed: Impulse 4000 (or equivalent).
Test Setup:
1. Connect the ECG cable to the Impulse 4000.
2. Connect the ECG cable to the unit.
Do this... Observe this... Pass/Fail/NA
9.1 Turn the selector switch to MONITOR mode (for
AED units turn to ON.)
9.2 Select lead I, size 3x.
Select 60Hz sine wave (or 50 Hz for a 50Hz
unit) on the Fluke 4000.
R Series Service Manual
9.3 Press RECORDER button. Verify that the waveform amplitude on the strip chart is less than 1.5 mm.
9.4 Turn th e un i t off.
Record your results on the Maintenance Test Checklist.
ooo
9650-0903-01 Rev. L 1-19
Page 30
R Series Service Manual
10.0 Heart Rate Alarm Test
Tools Needed:Impulse 4000.
Do this... Observe this... Pass Fail/NA
10.1 Turn the selector switch to MONITOR mode( for
AED units turn to ON.)
Connect the ECG leads to the Impulse 4000.
Set the simulator to 120 BPM and the
defibrillator to lead II.
10.2 Press ALARMS softkey. The alarm menu displays.
10.3 Press NEXT PARAM softkey until ECG HR
displays.
10.4 Press CHANGE VALUE softkey.
10.5 Press INC> softkey for state. Cursor scrolls through ENABLE, AUTO and DISABLE.
10.6 Press DEC< softkey for state. Cursor scrolls through ENABLE, DISABLE, AND A UTO.
10.7 Press INC> softkey until ENABLE displays. ENABLE displays.
10.8 Press ENTER softkey.
10.9 Press NEXT FIELD softkey to select the heart
rate limit.
10.10 Press CHANGE VALUE softkey and press
INC> or DEC< to set low heart rate limit to 30.
10.11 Press ENTER softkey.
10.12 Press NEXT FIELD softkey to select the high
heart rate limit.
10.13 Press CHANGE VALUE and press INC< or
DEC> softkey and set high heart rate limit to
150.
10.14 Press ENTER softkey, then press RETURN
softkey.
10.15 Press ALARM SUSPEND button. No alarm sounds.
Lead II waveform displayed on monitor.
NSR ECG at 120 BPM +/- 2 displayed.
Cursor scrolls through parameters.
Cursor scrolls to Low field.
Cursor scrolls to High field.
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
1-20 9650-0903-01 Rev. L
Page 31

R Series Service Manual
Do this... Observe this... Pass Fail/NA
10.16 Remove a lead wire from the Impulse 4000. The bell symbol flashes and the heart symbol stops flashing. The ECG LEAD
OFF alarm tone sounds and the Heart Rate Value is highlighted. Recorder
prints a stripchart showing a low heart rate, if enabled.
10.17 Reattach ECG Lead wire to Impulse 4000 and
hold the ALARM SUSPEND button on unit for 4
seconds.
10.18 Press the ALARM SUSPEND button. Alarm is enabled. Bell symbol (without “X”) displays.
10.19 Set simulator to 160 BPM or higher. Heart Rate Value is highlighted, alarm tone sounds, the bell and the heart
10.20 Press the ALARM SUSPEND button in the unit. Alarm is suspended for 90 seconds. The bell symbol has an “X” through it. The
10.21 Press and hold ALARM SUSPEND for
3 seconds to disable alarms.
Record your results on the Maintenance Test Checklist.
The bell symbol has an “X” through it.
The heart symbol flashes with each QRS wave.
symbol both flash.
heart symbol flashes with each QRS wave and the Heart Rate Value is
highlighted.
Verify the Heart Rate value is not highlighted, and the bell symbol displays with
an “X” through it and is not flashing.
ooo
ooo
ooo
ooo
ooo
ooo
9650-0903-01 Rev. L 1-21
Page 32

R Series Service Manual
11.0 Defibrillator Self Test
SHOCK HAZARD!
TAKE THE NECESSARY PRECAUTIONS TO GUARD AGAINST SHOCK OR INJURY BEFORE YOU START
CONDUCTING THE DEFIBRILLATO R TESTS.
Keep hands and all other objects clear of the multi-function cable connections an d defibrillator analyzer when discharging
the defibrillator.
Before you discharge the defibrillator, warn everyone near the equipment to STAND CLEAR.
Caution Do NOT internally discharge the unit more than 3 times in 1 minute. Note that multiple rapidly repeating in tern al d ischarges at more than
30 Joules may damage the unit.
Tools Needed:
• ZOLL Medical Electrode Connector (Fluke Biomedical Part Number 3010-0378 or equivalent).
• Impulse 4000 or equivalent defibrillator anal yzer.
• ECG Cable.
• Stop watch.
Test Setup:
1. Ensure the unit is turned off.
2. Ensure the ECG cable is connected to the R Series unit and the defibrillator analyzer.
Note: The OneStep cable should not be connected to any equipment at the beginning of this test.
Do this... Observe this... Pass/Fail
11.1 Turn the selector switch to DEFIB mode (For
AED units, turn to ON, and select MANUAL
MODE.)
Set leads to PADS.
11.2 Connect the OneStep cable to the test port on
the right side of the R Series.
1-22 9650-0903-01 Rev. L
CHECK PADS/POOR PAD CONTACT message displays.
DEFIB PAD SHORT message displays.
oo
oo
Page 33

R Series Service Manual
Do this... Observe this... Pass/Fail
11.3 Select energy level of 100J and press the
CHARGE button.
11.4 Press the SHOCK button. Unit does not discharge. DEFIB PAD SHORT message displays.
11.5 Set energy level to 30J. Unit internally discharges.
11.6 Press the CHARGE button. Unit charges to 30J and displays DEFIB 30J READY. The charge ready tone
11.7 Press and hold SHOCK button. Unit discharges. 30J TEST OK message displays. The message at the top of
Record your results on the Maintenance Test Checklist.
The charge time is >2 second and <10 seconds and SELECT 30J FOR TEST
is displayed.
sounds.
the printed strip chart reads as follows:
30 JOULES TEST OK. TEST_CUR=10-14A DEFIB_IMPED=0.
The impedance value may range from 0 to 5.
oo
oo
oo
oo
oo
9650-0903-01 Rev. L 1-23
Page 34

R Series Service Manual
12.0 Synchronized Cardioversion Test
Tools Needed: Impulse 4000 or equivalent defibrillator analyzer.
Test Setup:
1. Connect the OneStep cable via the adapter (D.N.I #3010-0378) to the defibrillator analyzer.
2. Select cardioversion on analyzer. Input 1mV ECG signal at 60 BPM.
Do this... Observe this... Pass/Fail
12.1 Press LEAD button to select PADS and Size
X1.
12.2 Press the SYNC softkey on the defibrillator.
Enter synchronized cardioversion timing test
mode on the defibrillator analyzer.
NOTE: Press the SYNC softkey again if the unit
is configured for Remote Sync.
12.3 Select 200J.
12.4 Press the CHARGE button. When the SHOCK
button lights, press and hold the SHOCK
button.
Record your results on the Maintenance Test Checklist.
Sync appears on display.
Sync markers display on the monitor. The sync marker appears as a down
arrow over the ECG R-wave peaks on strip chart and display.
Defibrillator discharges.
Observe that the R-wave to energy delivered (sync delay) is less than 60
milliseconds on the analyzer display.
oo
oo
oo
1-24 9650-0903-01 Rev. L
Page 35

R Series Service Manual
13.0 Synchronized Cardioversion for Remote ECG Monitoring Test (Optional)
If applicable, the R Series may be configured to receive defibrillation synchronization pulses from a remote ECG monitoring device (see the R Series
Configuration Manual). Remote Sync should be tested as a complete system that includes the remote monitor and R Series defibrillator. Be sure that the
remote device is connected to the Sync In/Marker Out c onnector on the R Series unit. The remote device must have a sync out connector and you must use
a cable to connect the two devices. Ensure the remote device conf orms with the Sy nc In /Marke r Out sp ecifi catio ns described in Appendix A: Defibrillator
Specifications of the R Series Operator’s Guide.
Tools Needed: Fluke Biomedical 4000 or equivalent defibrillator analyzer.
Test Setup:
1. Connect the OneStep cable via the adapter (D.N.I #3010-0378) to the defibrillator analyzer.
2. Select cardioversion on analyzer. Input 1mV ECG signal at 60 -120 BPM.
Do this... Observe this... Pass/Fail
13.1 Connect the remote monitor’s ECG lead cable
to the defibrillator analyzer. Connect the
R Series OneStep cable via the adapter (D.N.I.
#3010-0378) to the defibrillator analyzer.
13.2 Select Defib Mode. Press the SYNC ON/OFF
softkey. Press the REMOTE SYNC softkey.
13.3 Select 200J on the R Series. Enter
synchronized cardioversion timing test mode on
the defibrillator analyzer.
13.4 Press the CHARGE button. When the SHOCK
button lights, press and hold the SHOCK
button.
Record your results on the Maintenance Test Checklist.
9650-0903-01 Rev. L 1-25
The words REMOTE SYNC are displayed in place of the ECG trace, and a
REMOTE SYNC XXXJ SEL. message appears on the display. The ECG
heartbeat indicator will flash with each synchronization pulse received from the
remote monitoring device. On the remote device’s display, verify that sync
markers coincide with each R-wave as described in the remote device’s user
manual.
The defibrillator discharges. Observe that the R-wave to shock delay (sync
delay) is less than 60 milliseconds on the analyzer display.
oo
oo
oo
Page 36

R Series Service Manual
14.0 Shock Test
Tools Needed: Fluke Biomedical 4000 or equivalent defibrillator analyzer and a stop watch
Test Setup:
1. Connect the OneStep cable via the adapter (D.N.I #3010-0378) to the defibrillator analyzer.
2. Ensure that a fully charged battery is installed in the unit.
3. Select Defib/Energy mode on analyzer.
Do this... Observe this... Pass/Fail/NA
14.1 Turn the selector switch to DEFIB mode.(for
AED units, turn to ON, and select MANUAL
MODE).
14.2 Press the ENERGY SELECT down arrow until
5J displays.
14.3 Press the CHARGE button. Wait for the
SHOCK button to illuminate.
14.4 Press the SHOCK button. Unit discharges 3J-7J into the simulator.
14.5 Press the ENERGY SELECT up arrow until 50J
displays.
14.6 Press the CHARGE button. Wait for the
SHOCK button to illuminate.
14.7 Press the SHOCK button. Verify that the unit discharges 46J-62J into the simulator.
14.8 Press the ENERGY SELECT up arrow until
100J displays.
14.9 Press the CHARGE button. Wait for the
SHOCK button to illuminate.
14.10 Press the SHOCK button. Verify the unit discharges 93J-125J into the simulator.
DEFIB 5J SEL displays.
DEFIB 5J RDY displays.
Verify that DEFIB 50J SEL is displayed
Verify that DEFIB 50J RDY is displayed.
Verify that DEFIB 100J SEL is displayed.
Verify that DEFIB 100J RDY is displayed.
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
1-26 9650-0903-01 Rev. L
Page 37

R Series Service Manual
Do this... Observe this... Pass/Fail/NA
14.11 Press the ENERGY SELECT up arrow until
200J displays.
14.12 Press the CHARGE button. Wait for the
SHOCK button to illuminate.
14.13 Press the SHOCK button. Verify the unit discharges 196J-264J into the simulator.
14.14 Press the CHARGE button and start timing with
a stopwatch. Stop timing when the SHOCK
button illuminates.
14.15 Press the SHOCK button. V erify that the Patient Current is between 23.9-25.9A and Defib Impedance is
14.16 Press the CHARGE button, when the SHOCK
button Illuminates, press the ENERGY SELECT
DOWN ARROW.
14.17 Set Energy level to 200 Joules. Press the
CHARGE button. When the SHOCK button
illuminates, start timer.
14.18 Disconnect the cable from the analyzer. CHECK PADS audio prompt.
Record your results on the Maintenance Test Checklist.
Verify that DEFIB 200J SEL is displayed.
Verify that DEFIB 200J READY is displayed.
Observe and record the value of the charge time on the stop watch.
Charge time 1.0-7.0 sec.
between 46-54 Ohms on the strip chart.
Verify that no fault messages are displayed, and no discharge energy is
displayed on the Impulse 4000.
Verify the unit holds the energy for 60 seconds (15 seconds for AED units), and
that when the unit discharges internally, that there are no fault messages
displayed.
ooo
ooo
ooo
ooo
ooo
ooo
ooo
ooo
9650-0903-01 Rev. L 1-27
Page 38

R Series Service Manual
15.0 Summary Report Test
Tools Needed: Impulse 4000.
Test Setup:
1. Connect the OneStep cable to the Impulse 4000
2. If you are using paddles, place the paddles on the analyzer’s discharge plates.
Do this... Observe this... Pass/Fail
15.1 Press the Report Data * softkey, press Erase,
then Erase All.
15.2 Set selector switch to DEFIB (for AED units turn
to ON, and select MANAUL MODE.) Select
200J using the ENERGY SELECT button, and
press the CHARGE button. When charged,
press the SHOCK button to discharge into the
defibrillator analyzer.
15.3 Wait 18 seconds, then press the Code Marker
softkey. Press the CPR softkey. Wait 20
seconds and turn unit off. (This will allow code
marker to be saved in the summary.)
15.4 Ver if y tha t th e un i t ha s be e n off for a minimum
of 10 seconds and then turn the unit on. Press
the Report Data * softkey, then press Print
Chart, then Print All.
* For software versions 3.0 or earlier, the unit displays Report instead of Report Data.
Record your results on the Maintenance Test Checklist.
ERASING REPORT displays. Wait for the message to clear.
The unit successfully discharges and if configured to do so, prints a strip chart.
The Code Markers display. (After CPR Code Marker is selected, the unit
automatically exits the Code Marker options menu.)
Summary report prints. The report displays the correct date, time, the shock
delivered, and Code Marker event.
oo
oo
oo
oo
1-28 9650-0903-01 Rev. L
Page 39

16.0 Advisory Message Test (Manual/Advisory Units)
Tools Needed: Impulse 4000
Test Setup:
1. Connect the OneStep cable via the adapter (D.N.I #3010-0378) to the defibrillator analyzer.
Do this... Observe this... Pass/Fail
16.1 Connect OneStep cable to the simulator.
Turn the selector switch to DEFIB mode (for
AED units turn to ON, and select MANUAL
MODE.)
R Series Service Manual
16.2 Select VF (ventricular fibrillation) on the Impulse
4000, then press the ANALYZE button.
16.3 Press the SHOCK button. Unit discharges.
16.4 Select the NSR (normal sinus rhythm) on the
simulator, then press the ANALYZE button.
Record your results on the Maintenance Test Checklist.
ANALYZING ECG message displays.
STAND CLEAR message displays.*
SHOCK ADVISED message displays.
PRESS SHOCK message displays*+
*Advisory audio prompts are user configurable.
+If configured for auto charge. PRESS CHARGE message displays if not
configured to auto charge.
ANALYZING ECG message.
STAND CLEAR message.*
NO SHOCK ADVISED message.*
*Advisory audio prompts are user configurable.
oo
oo
oo
9650-0903-01 Rev. L 1-29
Page 40

R Series Service Manual
?
17.0 Pacer Test
Tools Needed: Impulse 4000 Analyzer (software 1.06 or higher) with optional external pl ug in pacing module (TQA-17) or equivalent.
Note: The following tests are to be performed only on R Series units equipped with the optional pacing function.
The pacer output can be measured using an oscilloscope set to DC coupling connected across a load resistor. (See above diagram for OneStep cable
connector polarity.) The load resistor is a 100 ohm, 5 watt or greater. The pacer output is a positive going pulse, 40 +/- 2 ms duration with an amplitude of
0.1 volt per milliamp of selected output (e.g., 4 0 milliamps of sel ected outp ut has an ampl itude of 4 +/- 0.5 volts of t he spec ified tole rance displ ayed on th e
oscilloscope).
If an external non-invasive pacer analyzer is being used, then follow the manufacturer’s guidelines for measuring the frequency (ppm), output (mA) and
the pulse width measured in milliseconds. Note that the analyzer pace load resistor must be less than 250 ohms.
Test Setup:
1. Connect the One Step cable from the R Series to the External Pacer Load (TQA-17) of the Impulse 4000.
2. Turn the Main Selector knob of the R Series to the Pacer mode.
3. Enter the pacer function on the analyzer.
Note: Do not connect ECG cable to Pacer Analyzer or ECG simulator.
Do this... Observe this... Pass/Fail
17.1 Set the PACER OUTPUT to 14 mA and
disconnect MFC connector from the Impulse
4000.
17.2
17.3 Set rate to 180 ppm; output to 0mA. No output appears on the Impulse 4000.
17.4 Increase the output to 40mA. Output on the Impulse 4000 is 40mA +/- 5mA.
1-30 9650-0903-01 Rev. L
Reconnect the
4000. Press Clear Pace Alarm softkey.
universal cable to the Impulse
CHECK PADS and POOR PAD CONTACT messages display . The pace alarm
is active.
CHECK PADS and POOR PAD CONTACT messages disappear. The pace
alarm is cleared.
oo
oo
oo
oo
Page 41

R Series Service Manual
Do this... Observe this... Pass/Fail
17.5 Increase the output to 80mA Output on the Impulse 4000 is 80mA or +/- 5mA.
17.6 Increase the output to 120mA. Output on the Impulse 4000 is 120mA or +/- 6mA.
17.7 Increase the output to 140mA. Output on the Impulse 4000 is 140mA or +/- 7mA. Pulse width is 40mS +/-
2mS. Pacer rate on Impulse 4000 is 177-183 ppm.
17.8 Decrease the output to 60mA.
Decrease the rate to 30 ppm.
17.9 Connect the ECG cable to the R Series and
Impulse 4000. Select the ECG at 60 BPM on
the Impulse 4000. Increase the pacer rate on
the unit to 58ppm.
17.10 Press the Async Pace softkey. ECG at 60 BPM seen on the display with the pace stimulus markers displayed.
17.11 Turn off Impulse 4000. Set Pacer Rate to
100ppm. Press the RECORDER ON button.
17.12 Press and hold 4:1 button. Observe the pace stimulus markers every 60 mm+/- 1.5 mm.
Record your results on the Maintenance Test Checklist.
Pacer rate on Impulse 4000 is 29-31 ppm.
ECG at 60 BPM is seen on the display and no stimulus markers.
Async pace message displays.
Observe the pace stimulus markers every 15mm +/-1mm.
oo
oo
oo
oo
oo
oo
oo
oo
9650-0903-01 Rev. L 1-31
Page 42

R Series Service Manual
18.0 SpO2 Monitor Test (for SpO2 Option)
Tools Needed:
• Masimo
• Masimo
• Fluke Biomedical Index 2XLFE SpO
Test Setup:
1. Connect the OneStep cable to the test port.
2. DO NOT connect the ECG cable to the simulator.
3. Install the Masimo
4. Connect the Masimo
5. Place a fully charged battery into the battery well or connect to AC power.
6. Ensure that the SpO
Reusable Sensor.
Patient Cable.
Simulator (or equivalent) .
2
Patient Cable and attach the Masimo sensor to the patient cable.
sensor to the finger simulation post.
Simulator is off.
2
Do this... Observe this... Pass/Fail
18.1 Turn the selector switch to MONITOR(for AED
units turn to ON, and select MANUAL MODE.)
18.2 Wait ten seconds.
Turn on the SpO
softkey on the Index SpO2 Simulator. Press the
MAN softkey.
18.3 Press the 02+ or 02- softkey of the simulator
until the SpO
18.4
1-32 9650-0903-01 Rev. L
Using the Index
BPM+ or BPM- softkey until the heart rate is
230 BPM.
simulator. Press the SIM
2
output is at 98%.
2
SpO
Simulator, press the
2
The SpO
The SpO
The R Series
Note that you may need to wait up to 2 minutes for the information to appear on
the ZOLL display.
The SpO2 rate 230 BPM displays on the simulator screen.
Note that you may need to wait up to 2 minutes for the information to appear on
the ZOLL display.
The
The heart
saturation percentage appears as a dashed line on the monitor.
2
PULSE SEARCH message displays.
2
SpO
reading of 98 +/- 1% appears on the R Series monitor.
2
SpO
saturation of 96-100% appears on the R Series display.
2
rate of 226-234 BPM displays on the R Series monitor.
oo
oo
oo
oo
Page 43

R Series Service Manual
Do this... Observe this... Pass/Fail
18.5
18.6
18.7 Press the OPTIONS softkey. Press TRACES.
18.8 Press RECORDER. The plethysmographic waveform prints on the strip chart paper.
18.9 Press RECORDER to stop printing. Verify that the recorder stops.
18.10
18.11 Press RECORDER. Verify th at the wavefo rm is printed at the correct rate.
18.12
Record your results on the Maintenance Test Checklist.
Using the Index
BPM- softkey until the heart rate is 50 BPM.
Using the Index
softkey until the SpO
Press TRACE 2. Select
Using the Index
BPM- softkey until the heart rate is at 230 BPM.
Press RECORDER. Remove the Masimo
patient cable.
SpO
Simulator, press the
2
SpO
Simulator, press the 02+
2
output is at 72%.
2
SpO
.
2
SpO
Simulator, press the
2
The SpO2 saturation of 96-100% displays on the unit.
The heart rate of 46-54 BPM displays on the R Series monitor.
The
SpO2 saturation of 69-74% displays on the unit.
The heart rate of 46-54 BPM displays on the R Series monitor.
Plethysmographic waveform appears on the ZOLL display.
The SpO2 saturation rate of 69-74% displays on the unit.
The heart rate in the heart position of 226-234 BPM displays on the monitor.
Press RECORDER to stop printing.
Verify that the recorder stops.
oo
oo
oo
oo
oo
oo
oo
oo
oo
o o
9650-0903-01 Rev. L 1-33
Page 44

R Series Service Manual
19.0 EtCO2 Monitor Test (for EtCO2 Option)
Tools Needed: CAPNOSTAT 5 Mainstream cable with airway adapter.
Test Setup:
1. Install the battery.
Do this... Observe this... Pass/Fail
19.1 Connect the CAPNOSTAT 5 CO2 Mainstream
cable with airway adapter attached to the yellow
connector at the back of the R Series.
19.2 Set the front panel switch to MONITOR or ON.
For AED units, enter Manual Mode.
19.3 When the WARM UP message disappears,
press the Param softkey, then select EtCO2.
19.4 Press the ZERO softkey, then wait for the
ZERO DONE message.
19.5 Press the Return softkey.
19.6 Press the following softkeys to display the CO
waveform, Options, Traces, Trace 2, then
EtCO2,
19.7 Breathe normally into the airway adapter. A capnogram waveform appears.
Record your results on the Maintenance Test Checklist.
NOTE: Make sure the airway adapter is installed in the CO
WARM UP message appears on the display.
NOTE: Warming up may take up to 3 minutes.
The ZERO DONE message appears.
A flat baseline CO2 waveform appears.
2
cable.
2
oo
oo
oo
oo
1-34 9650-0903-01 Rev. L
Page 45

20.0Barometric Pressure Calibration Check
Tools Needed: None.
Test Setup: None
Do this... Observe this... Pass/Fail/NA
R Series Service Manual
20.1 Connect the CAPNOSTAT 5 CO
yellow connector at the back of the R Series
unit, and connect an airway adapter to the
sensor.
20.2 While pressing and holding the second softkey
from the left, turn the selector switch to Monitor
(ON for AED units).
20.3 Wait for the sensor to warm up. The message WARM UP is displayed. (Warming up may take up to 3 minutes.)
20.4 Obtain the local barometric pressure in mmHg.*
20.5 Press the Baro Pr. softkey to enter the
Barometric Pressure Calibration screen.
20.6 Use the Inc> and Dec< softkeys to set the
second value on the pressure display line equal
to your local barometric pressure.
20.7 Press the Return softkey to store the offset and
return to the main EtCO
Record your results on the Maintenance Test Checklist.
*The barometric pressure can be obtained from a calibrated barometer, or from the National Weather Service at www.nws.noaa.gov Note that the barometric
pressure is in inches of mercury, multiply it by 25.4 to convert to mmHg.
Calibration screen.
2
Sensor to the
2
The unit displays EtCO2 Calibration screen.
ooo
9650-0903-01 Rev. L 1-35
Page 46

R Series Service Manual
21.0 CO2 Accuracy Check (for EtCO2 Option)
Tools Needed: Gas regulator, calibration gas (see note on page 37).
Test Setup: None
Do this... Observe this... Pass/Fail/NA
21.1 Connect the CAPNOSTAT 5 CO
yellow connector at the back of the R Series
unit, and connect an airway adapter to the
sensor.
21.2 While pressing and holding the second softkey
from the left, turn the selector switch to Monitor
(ON for AED units).
21.3 Wait for the sensor to warm up. The message WARM UP is displayed (Warming up may take up to 3 minutes.)
21.4 Obtain current room temperature in
Centigrade (Cº).
21.5 Press the Select Gas T emp softkey to enter the
CO
Accuracy screen
2
21.6 Use the Prev, Next, Inc and Dec softkeys to set
each digit of the gas temperature parameter in
the CAPNOSTAT 5 CO2 Sensor until Gas
Degrees C is equal to the room temperature.
21.7 Press the Return softkey to store the
temperature and return to the main EtCO
Calibration screen.
21.8 Press the Zero softkey to zero the mainstream
CAPNOSTAT 5 CO2 Sensor/Airway Adapter.
Sensor to the
2
2
The unit displays EtCO2 Calibration screen.
ooo
ooo
21.9 Attach a regulated flowing gas mixture of 5%
CO2, balance Nitrogen (N2) to the airway
adapter.
1-36 9650-0903-01 Rev. L
The gas flow rate should already be preset to 2 to 5 liters per minute.
Page 47

Do this... Observe this... Pass/Fail/NA
21.10 Set the Gas Balance settings of the
CAPNOSTAT 5 CO2 Sensor to that of the
calibration gas mixture (N
The default gas balance is N2.
, N2O, or He).
2
R Series Service Manual
21.11 Allow a few seconds for the gas mixture to
stabilize and observe the CO2 Percent value.
21.12 Press the Return softkey to return to the main
EtCO2 Calibration screen.
21.13 Turn the device off when calibration is
complete.
Record your results on the Maintenance Test Checklist.
The expected value is 5% ± 0.2%.
ooo
Note: The calibration gas mixture and regulator are available from most medical gas supply companies. You can also try Scott Medical products at
www.scottmedicalproducts.com.
9650-0903-01 Rev. L 1-37
Page 48

R Series Service Manual
22.0 NIBP Volume Leak Test
The volume leak test verifies the integrity of the pneumatic system on the R Series NIBP module. This test should be performed annually or every 10,000
readings, whichever comes first.
Tools Needed: NIBP simulator (the values and procedure provided in this manual are specific to the BP Pump 2)
Test Setup:
1. Connect the simulator hose to the NIBP connector on the R Series unit.
2. Configure the NIBP simulator for the volume leak test. For example, on the BP Pump 2:
• Press the MODE button three times to go into Tests mode.
• Press the SELECT button twice to access the volume leak test.
3. Make sure the ECG cable is not connected to the R Series unit.
4. If the SpO
option is installed, make sure that the SpO2 patient cable is NOT connected to the R Series unit.
2
Do this... Observe this... Pass/Fail
22.1 Turn the Selector Switch to OFF.
After 10 seconds, press and hold the fourth
softkey from the left and turn the Selector
Switch to MONITOR (ON for AED units).
22.2 Press the Leak Test softkey. The R Series displays the NIBP Leak Test Screen.
22.3 On the NIBP simulator, set the pressure
parameter to 200 mmHg.
22.4
1-38 9650-0903-01 Rev. L
On the
R Series unit, press the Close Valves
softkey.
The R Series powers on in the NIBP Service Mode.
The NIBP simulator displays a pressure reading of 200 mmHg.
The Valves status changes from OPEN to CLOSED.
oo
Page 49

R Series Service Manual
Do this... Observe this... Pass/Fail
22.5 On the NIBP simulator, press the START TEST
softkey.
Note: You must press the START TEST
softkey within 30 seconds of closing the
valves on the R Series unit.
22.6 On the NIBP simulator, press the STOP TEST
softkey.
22.7 On the R Series unit, press the EXIT softkey
twice.
Record your results on the Maintenance Test Checklist.
After approximately 1 minute, a number appears in the upper middle area of
the NIBP simulator display.
If the simulator:
• displays a Volume Leak reading <55, then the R Series unit has passed the
test.
• displays a Volume Leak reading >5
test.
• displays no Volume Leak reading, but maintains a stable pressure reading
at or above 200 mmHg, then the R Series unit has passed the test; there is
no volume leak.
In addition, the R Series displays the simulator’s pressure reading in the “Cuff
Pressure” field.
After approximately 3 minutes, the valves on the R Series unit open.
The NIBP simulator terminates the Volume Leak Test.
The R Series returns to the main NIBP Service Mode screen, then to normal
Monitor mode operation.
6
, then the R Series unit has failed the
oo
oo
5
If you are using the Fluke® Biomedical CuftLink Simulator, the volume leak reading should be <10.
6
If you are using the Fluke® Biomedical CuftLink Simulator, the volume leak reading for a failure should be >10.
9650-0903-01 Rev. L 1-39
Page 50

R Series Service Manual
23.0NIBP Transducer Calibration Test
The NIBP module’s pressure transducers are factory-calibrated prior to shipment. However, you can perform a two-point calibration procedure
periodically to ensure accurate pressure measurements.
This test should be performed annually or every 10,000 readings, whichever comes first.
Tools Needed: NIBP simulator (The values and procedure provided in this manual are specific to the BP Pump 2)
Test Setup:
1. Connect the simulator’s hose to the NIBP connector on the R Series unit.
2. Configure the NIBP simulator to simulate cuff pressure. For example, on the BP Pump 2
• Press the MODE button three (3) times to go into Tests mode.
• Press the SELECT button once to access the Pressure Simulator screen.
3. Make sure the ECG cable is not connected to the R Series unit.
4. If the SpO
7
These instructions apply to the BP Pump 2; for equivalent devices, follow the manufacturer's instructions.
option is installed, make sure that the SpO2 patient cable is NOT connected to the R Series unit.
2
7
:
Do this... Observe this... Pass/Fail
23.1 Turn the Selector Switch to OFF.
After 10 seconds, press and hold the fourth
softkey from the left and turn the Selector
Switch to MONITOR.(AED units to ON)
23.2 Press the NIBP Calib softkey. The R Series displays the NIBP Transducer Calibration Screen.
23.3 On the NIBP simulator, set the pressure
parameter to 0 mmHg.
23.4 On the R Series unit, press the Set Low softkey
to calibrate the transducer to a 0 mmHg
pressure reading.
23.5 On the NIBP simulator, set the pressure
parameter to 250 mmHg.
1-40 9650-0903-01 Rev. L
The R Series powers on in the NIBP Service Mode.
The NIBP simulator displays a pressure reading of 0 mmHg.
The NIBP pressure transducer registers its voltage output at a known pressure
of 0 mmHg. The field adjacent to the 0 mmHg value changes to PASS.
Note: If the R Series displays a FAIL reading, verify the NIBP simulator’s
pressure setting and connection to the R Series an d repeat the step.
The NIBP simulator displays a pressure reading of 250 mmHg.
oo
Page 51

R Series Service Manual
Do this... Observe this... Pass/Fail
23.6 On the R Series unit, press the Set High
softkey to calibrate the transducer to a
250 mmHg pressure reading.
23.7 On the NIBP simulator, set the pressure
parameter to stimulate a differ en t cuff pressure
(for example, 205 mmHg).
23.8 On the R Series unit, press the Read Cuff
softkey.
Verify that the value displayed is accurate within
±3 mmHg of the pressure parameter set on the
NIBP simulator.
23.9 On the R Series unit, press the EXIT softkey
twice.
Record your results on the Maintenance Test Checklist.
The NIBP pressure transducer registers its voltage output at a known pressure
of 250 mmHg. The field adjacent to the 250 mmHg value changes to PASS.
Note: If the R Series displays a FAIL reading, verify the NIBP simulator’s
pressure setting and connection to the R Series and repeat the step.
The NIBP simulator displays the specified pressure reading.
The NIBP module measures the pressure from the NIBP simulator and
displays the value in the Cuff Pressure field.
The R Series returns to the main NIBP Service Mode screen, then to normal
Monitor mode operation.
oo
oo
oo
War nin g! NIBP transducer c alibration can affect clinical readings of the NIBP parameter . Ensure that the NIBP Transduc er Calibration
procedure is performed correctly, followed by an NIBP Monitor Test to verify proper operation.
9650-0903-01 Rev. L 1-41
Page 52

R Series Service Manual
24.0NIBP Monitor Test
The NIBP monitor test verifies the repeatability of the systolic, diastolic , and mean bloo d pressu re measurements, as well as the patient pulse rate
calculation.
Tools Needed: NIBP simulator (The values and procedure provided in this manual are specific to the BP Pump 2)
Note The primary propose of an NIBP simulator is to reproduce a pressure profile similar to a live patient to be used for testing repeatability and
functionality of the system. There are many different NIBP simulators on the market, each manufacturer uses a different method to develop their
algorithm, and as such this can cause readings from different simulators to vary. In order to test for repeatability, you should first establish the
1
of your simulator . The offset value should then be used to determi ne the expecte d values. NIBP simulators cannot be used as a source for
offset
testing the accuracy of the non-invasive blood pressure measurements of monitors such as the ZOLL R Series.
Test Setup:
1. Connect the simulator hose to the NIBP connector on the R Series unit.
2. Set the following parameters on the NIBP simulator
Parameter Value
Systolic pressure 120 mmHg
2
:
Diastolic pressure 80 mmHg
Mean pressure
Heart rate 80 bpm
93 mmHg
3
3. Make sure the ECG cable is not connected to the R Series unit.
4. If the SpO
1
NIBP Simulators may produce a reading on the NIBP monitor that is shifted from the simulator's setting. The offset value must be established based on a
statistical sample of monitors and readings. Please contact ZOLL Technical Support if you require assistance establishing the offset of the simulator and test
set-up that you are utilizing.
2
If you are using the Fluke® Biomedical CuftLink, you must change the shift value of the Blood Pressure Envelope to +3 on the Pressure Curve Adjust Menu.
1-42 9650-0903-01 Rev. L
option is installed, make sure that the SpO2 patient cable is NOT connected to the R Series unit.
2
Page 53

3
Not all simulators have a setting of 93mmHg, check the simulators user's manual for recommendations.
Do this... Observe this... Pass/Fail
R Series Service Manual
24.1 Turn the selector switch to MONITOR mode.
(For AED units, turn the selector switch to ON
and select Manual mode.)
24.2 Ensure that the LEADS parameters is set to
PADS (default).
If necessary, press the LEADS button to cycle
through the values to select PADS.
24.3 Press the NIBP button on the R Series front
panel.
24.4 Press the Param softkey.
24.5 Select the Trend softkey, then select the NIBP
Trend softkey.
Record your results on the Maintenance Test Checklist.
4
These values only apply for test set-ups utilizing the BP Pump 2 Simulator. Variations of the test set-up or different simulators may produce readings outside
the provided values and will require end-user facility to establish the appropriate offset and tolerances. Please contact ZOLL Technical Support if you require
assistance establishing the offset of the simulator and test set-up that you are utilizing.
The R Series powers on in MONITOR mode.
The R Series displays PADS in the Lead selection field on the monitor.
The R Series initiates the blood pressure measurement cycle and displays the
following measurements
• systolic pressure (126 ± 5 mmHg)
• diastolic pressure (84 ± 5 mmHg)
• mean pressure (98 ± 5 mmHg)
The R Series displays a summary of the NIBP measurements, including the
pulse rate reading (in the range of 77 - 85 bpm).
4
:
oo
oo
oo
9650-0903-01 Rev. L 1-43
Page 54

R Series Service Manual
1-44 9650-0903-01 Rev. L
Page 55

R Series Service Manual
Chapter 2
T roubleshooting
Overview
This chapter contains a list of error messages that users may see if the unit is not operating properly.
If the problems you encounter are not listed below, call ZOLL Medical Corporation’s Technical Service Department for further assistance. (See page iii for
contact information.)
ZOLL R Series Error Messages
The following is a list of ZOLL R Series error messages that may appear on your display. The “User Advisory” column informs you about an action in
progress or provides feedback on a user correctable situation that typically does not require further technical support. The “Technical Action” column
describes what you as a technician can do to correct the situation. Note that these messages will sometimes overlap part of the waveform display.
First, attempt to clear the message by turning the Selector Switch to OFF fo r ten seconds, then back to the desired operating m ode. If the fault persists, call
ZOLL Technical Service.
Error Message Explanation User
200J MAX BIPHASIC User attempted to set defibrillation energy >200J on
Biphasic Unit. No higher energy is available.
30 J TEST OK Unit successfully passed the 30J defib self-test.
50 J MAX Energy < 50J for internal paddles. No higher energy is
available.
9650-0903-01 Rev. L 2-1
Advisory
Te chnical Acti on
Page 56

R Series Service Manual
Error Message Explanation User
ALARM SET ALARM SET status message when setting alarms.
ANALYSIS HALTED ECG analysis halted due to user interaction such as:
• Lead/size change
• Analyze button was pressed again
• Impedance fault
• Charging error detected in auto defib mode
ASYNC PACE ONLY Posted along with other faults. Indicates that the PD
module cannot detect sync pulses.
ATTACH PADS AED: No pads on Auto Defib power-up.
AUDIO QUEUE FULL Indicates that the audio output queue is full. Additional
voice prompts can't be queued at this time.
BATT HIGH CURRENT Battery is not charged and battery curre nt is greate r than
1.6 A.
BATT HIGH VOLTAGE Indicates that the charger voltage is too high. Replace battery or AC charger.
Advisory
Technical Action
Verify proper OneStep cable/hands-free therapy
electrode connection by disconnecting and
reconnecting the OneStep cable and hands-free
therapy-electrodes.
None.
Replace battery or AC charger.
BATT LOW VOLTAGE Indicates that the charger voltage is too low. Replace battery or AC charger.
BATTERY COMMS ERROR Battery is not communicating with the host. Replace battery interconnect board.
BATTERY FAULT Replace battery.
BATTERY ID FAULT Replace battery.
CAL. BARO. PRESSURE Barometric pressure reading is out of range. Calibrate the barometric pressure.
CALIBRATE NIBP NIBP calibration is incomplete or failed. Cycle power and retry; if problem persist calibrate
NIBP system.
CANNOT CHARGE Cannot charge when charge button pressed. Replace high voltage module or capacitor.
2-2 9650-0903-01 Rev. L
Page 57

R Series Service Manual
Error Message Explanation User
CF TRANSFER FAILED Summary/DVCK/ALOG data file transfer error – either no
CF card or CF card transfer failed
CHANGE LEADS Unit is in Defib Sync mode and heart rate is less than 20
BPM.
CHARGE FAILED Unit failed to perform the requested charge. Replace PD Engineer or Analog Board.
CHECK CO2 ADAPTER Airway adapter is removed, occluded or adapter zeroing
needs to be performed or was performed incorrectly.
CHECK CO2 SENSOR EtCO
CHECK CUFF/HOSE
CHECK ECG CABLE Invalid ECG cable Id is detected.
Sensor is unplugged or defective.
2
• Blood pressure cuff or hose is not installed correctly.
• Cuff or hose is faulty.
• Hose is kinked or disconnected.
• Inflation rate too fast or too slow.
Advisory
Te chnical Acti on
Reseat CF Communication cable or replace
Communication Module.
Replace/Clean airway adapter. Zeroing performed
automatically.
Check that sensor cable is plugged in and seated
properly. Check that sensor is not exposed to
excessive heat. If problem persists, replace the
sensor.
• Verify the hose and cuff is properly connected
and not leaking.
• Replace cuff and hose.
• Replace NIBP module or parameter power
supply.
• Verify that the ECG Cable is property
connected.
• Replace ECG Cable.
• Replace Analog Board.
CHECK ELECTRODE Either Can read ID Chip or the checksum failed. Replace Electrodes or replace Analog board.
CHECK PADS Message displayed in conjunction with either POOR PAD
CONTACT or DEFIB PAD SHORT.
CHECK PATIENT Background ECG analysis detects shockable rhythm.
CHECK PULSE Alternate message for NO SHOCK ADV. message, or
displayed in addition to it according to the configuration
option selected. Also shown after delivering last shock
when Auto Analyze option is enabled.
9650-0903-01 Rev. L 2-3
Ensure pads are coupled to patient. Check/
replace pads and universal cable. Replace system
board.
Page 58

R Series Service Manual
Error Message Explanation User
CHECK RECORDER Produced when paper tray is empty, paper jams or
recorder door is opened.
CHECK SPO2 SENSOR Reposition SpO2 sensor on patient.
CLOCK BATTERY FAULT The RTC coin battery has failed. Replace lithium coin battery or digital board.
CLOCK FAULT 11 Real time clock oscillator failure Replace lithium coin battery or digital board.
CLOCK FAULT 12 Real time clock back-up power supply failure. Found
oscillator stopped at power-up, but oscillator now running
when the system is running (oscillator only runs when
main power is applied).
CLOCK FAULT 13 One of the set time units (seconds, minutes, year, etc.) is
out of range.
CO2 COMM ERROR No or invalid communication from the EtCO
CO2 DEVICE NOT READY There is CO
zero. Zeroing was attempted within 20 seconds of
previous zero operation.
in the airway adapter when attempting to
2
module. Replace EtCO2 module and or system board.
2
Advisory
Technical Action
Replace lithium coin battery or digital board.
Replace lithium coin battery or digital board.
• Remove airway adapter from CO
including the patient’s, and your own exhaled
breaths, and ventilator exhaust valves.
• Wait up to 20 seconds before retrying a
mainstream airway adapter zero, as described
in “Zeroing the Mainstream CAPNOSTAT 5
Sensor/Airway Adapter”
CO
2
source
2
CO2 IN LINE: WAIT Adapter zero attempted with CO
CO2 MODULE NOT VALID Sidestream sensor connected (not supported by the
R Series unit’s operating software)
CO2 OUT OF RANGE The calculated CO
2-4 9650-0903-01 Rev. L
value is greater than 150 mmHg. If error persists, perform a mainstream airway
2
in the adapter.
2
Use mainstream sensor.
adapter zero, as described in “Zeroing the
Mainstream CAPNOSTAT 5 CO
Adapter.”
Sensor/Airway
2
Page 59

R Series Service Manual
Error Message Explanation User
CO2 UNIT ERROR The EtCO2 sensor or module has detected a hardware
error.
CO2 WARM UP The mainstream sensor is warming up. This may take up
to 5 minutes.
CPR FAULT 8 ECG processor not receiving CPR data Replace analog board.
DATA TRANSFERRED Transfer done message
DEFIB DISABLED User prompt issued simultaneously with other faults if defib
is disabled.
DEFIB FAULT 76 PD Defib failure during POST. Replace PD engine.
DEFIB FAULT 77 PD Defib failure while running. Replace PD engine.
Advisory
Te chnical Acti on
Check that the sensor is properly plugged in.
Re-insert the sensor. Turn R Series unit off, then
on again to reset. perform a mainstream airway
adapter or module zero, as described in “Zeroing
the Mainstream CAPNOSTAT 5 CO
Airway Adapter”.
If the problem persists, contact ZOLL Technical
Support.
Wait for sensor or module to warm up.
If the message persists more than 5 minutes,
replace the sensor.
Possible configuration problem. Replace high
voltage module. Call ZOLL Technical Support.
Sensor/
2
DEFIB FAULT 78 PD Defib functional safety error while running. Replace PD engine.
DEFIB FAULT 79 PD module Defib/Pace failure during POST. Replace PD engine.
DEFIB FAULT 80 Undefined error received from the PD module. Replace PD engine.
DEFIB FAULT 94 PD module reset 3 times without being requested to. Replace PD engine.
DEFIB FAULT 95 PD module not communicating. Replace PD engine.
DEFIB FAULT 96 PD module reported an error on the discharge. Replace PD engine.
DEFIB MAINT. REQ. More than 5000 discharges of 200J have occurred.
Maintenance is required.
9650-0903-01 Rev. L 2-5
Replace PD engine.
Page 60

R Series Service Manual
Error Message Explanation User
DEFIB NOT CHARGED Discharge button is pressed in a Defib mode but the unit is
not charged.
DEFIB OVERUSE More than 50 shocks were delivered in less than 20
minutes.
DEFIB PAD SHORT Measured impedance between high voltage leads of MFC.
DISK FORMAT REQ. Report error if any problem with DOC file access occurred. Replace digital board.
ECG DISABLED Persistent Critical Hardware Failure on ECG module. Replace analog board.
ECG FAULT 200 No ECG applicable available. Reload MCU Software and ECG App. Software.
ECG FAULT 3 ECG processor reset failure. Replace analog board.
ECG FAULT 4 Excessive number of missed samples from ECG
processor.
ECG FAULT 5 ECG processor power up failure. Replace analog board.
ECG FAULT 6 ECG processor Hardware failure. Replace analog board.
Advisory
Technical Action
Unit needs to cool down, wait approximately
5 minutes.
Ensure pads are coupled to patient. Check/
replace pads or universal cable. Replace system
board.
Replace analog board.
ECG FAULT 7 ECG processor is not responding. Replace analog board.
ECG LEAD OFF 1 or more ECG leads are not connected when non-MFE
leads are selected as input.
ECG RA LEAD OFF ECG lead RA is disconnected
ECG TOO LARGE Number of intervals with large ECG exceeds threshold.
Issued immediately before RETRY ANALYSIS.
ECG V LEAD OFF ECG lead V is disconnected
ENABLE ETCO2 User attempts to zero EtCO2 sensor while the EtCO2
feature is disabled
2-6 9650-0903-01 Rev. L
Using the softkeys, select Enable EtCO2.
Page 61

R Series Service Manual
Error Message Explanation User
ENERGY INCREMENTED Defib energy has been automatically incremented to the
next configured level after shock 1 or 2 has been delivered
and the unit is configured for Basic Energy Auto
Escalation.
ERASING REPORT The unit is erasing the selected report data.
FULL DISCLS STOPPED Full disclosure data exceeds the storage capacity: 4 hours
or 32Mb.
IF NO PULSE Message displayed upon entered CPR period specified by
AHA protocol.
INSERT CARD No card detected during manual or semi-automatic modes.
INT. DUMP OVERLOAD More than 15 internal discharges in 5 minutes. Unit needs to cool down, wait approximately
INVA LID GAS TEMP:
RETRY
LOW BATTERY Low battery.
The operator has attempted to set a gas temperature
outside the Capnostat’s operating range.
Advisory
Te chnical Acti on
Re-insert CF Card or replace communication
module.
5 minutes.
Recalibrate EtCO
• Replace battery pack with a fully charged
battery pack.
• Plug unit into AC mains.
, verify range.
2
NIBP AR TIFACT The unit is unable to detect systolic, diastolic or mean
blood pressure due to excessive motion or vibration.
NIBP COMM ERR 259 Framing, parity, or fifo error. Replace parameter power supply or NIBP module.
NIBP COMM ERR 260 Received invalid packets. Replace parameter power supply or NIBP module.
NIBP COMM ERR 261 Device not taking action request. Replace parameter power supply or NIBP module.
NIBP COMM ERR 262 No reply from device. Replace parameter power supply or NIBP module.
9650-0903-01 Rev. L 2-7
• Take a single blood pressure measurement.
• Keep patient as still as possible.
• Insulate patient, cuff and hose from vibrations
as much as possible.
Page 62

R Series Service Manual
Error Message Explanation User
NIBP FAULT 263 Fault 90 received from NIBP module. Replace NIBP module.
NIBP FAULT 264 Fault 91 received from NIBP module. Replace NIBP module.
NIBP FAULT 265 Fault 97 received from NIBP module. Replace NIBP module.
NIBP FAULT 266 Fault 98 received from NIBP module. Replace NIBP module.
NIBP FAULT 267 Fault 99 received from NIBP module. Replace NIBP module.
NIBP FAULT 268 Device no response after power up. Replace NIBP module.
NIBP MEAS ABORTED
NIBP NOT READY
• Cuff inflation pressure is set too high for attached cuff.
• Inflation is too fast.
• R Series is unable to find systolic value for 180
seconds.
• Defibrillator is charged or charging.
• User initiated abort.
• The defibrillator is charged or charging in progress.
• NIBP module is performing power-up self-test.
Advisory
Technical Action
• Verify that you are using proper size cuff.
• Check for cuff and hose blockage.\
• Confirm that the unit was not charging.
• If the problem persists, contact ZOLL
Technical Support.
• Wait until the unit discharges before taking the
next measurement.
• Wait for more than 10 seconds after power-up
before taking blood pressure measurements.
NIBP OUT OF RANGE The data from the NIBP module is out of range.
NIBP SIGNAL WEAK There is a weak or no oscillometric signal.
NO DATA TO TRANSFER Summary Report / Device Check / Activity Log data
transfer error.
2-8 9650-0903-01 Rev. L
• Check cuff fit and positioning. Switch cuff to
other arm.
• Measure patient’s blood pressure with other
equipment.
• If the problem persists, contact ZOLL
Technical Support.
• Check cuff fit and positioning.
• Check for kinked hose.
• Increase cuff inflation pressure if clinically
appropriate.
Page 63

R Series Service Manual
Error Message Explanation User
NO QRS DETECT Unit is in sync mode and heart rate is < 20 BPM or QRS
amplitude is too low for proper synchronization.
NO SHOCK ADV. No shock advised. Advisory message when analysis finds
non-shockable rhythm.
NOISY ECG Number of noisy analysis intervals exceeds threshold.
OPEN AIR DISCHARGE Measured Defib Impedance was greater than 1000 ohms
and no energy was delivered.
PACER DISABLED User prompt issued simultaneously with other pace faults if
pacing is disabled.
PACER FAULT 115 PD Pace failure during POST. Replace PD engine.
PACER FAULT 117 PD Pace functional safety failure while running. Replace PD engine.
PACER FAULT 121 PD Pace failure while running. Replace PD engine.
PACER FAULT 122 PD module Defib/Pace failure during POST. Replace PD engine.
PACER WARNING 124 PD Pace warning while running. Replace PD engine.
Advisory
Te chnical Acti on
Increase ECG size and/or change lead.
Stop all patient movement. Check connections.
Press Analyze button again.
Replace high voltage module or system board.
PADDLE FAULT Cannot detect type of accessory attached to the universal
cable.
PEDIATRIC PADS IN USE The one of the Pediatric ID is missing.
PERFORM CPR Message displayed upon entered CPR period specified by
AHA protocol.
POOR LEAD CONTACT One or more ECG leads are poorly connected or not
connected to patient. (User configurable.)
9650-0903-01 Rev. L 2-9
Replace paddles, internal paddles, system board,
high voltage module and/or universal cable.
Check electrode attachment to patient, cable
connector to electrode, and cable to unit
connector.
Page 64

R Series Service Manual
Error Message Explanation User
POOR PAD CONTACT E lectrode impedance exceeds threshold.
PRESS ANAL YZE Alternate message for Check Patient message if this
configuration option is selected.
PRESS CHARGE
PRESS SHOCK Prompt issued in AED auto defib mode when defib is
READING LOG DATA Summary Report Log data printing message.
RECORDER FAULT 142 Strip chart system error. Replace printer assembly, digital board, and/or
RECORDER FAULT 143 Strip chart failed power-up echo test. Replace printer assembly, digital board, and/or
• Discharge button is pressed in Manual Defib mode but
the unit is not charged.
• Advisory message following SHOCK ADVISED in
Manual/Advisory Defib mode with auto-charge
disabled.
charged (ready).
Advisory
Technical Action
• Ensure pads are coupled to patient.
• Check/replace pads or universal cable.
• Check impedance circuit calibration.
• Replace system board.
printer interconnect board.
printer interconnect board.
RECORDER FAULT 147 Strip chart printhead over safe operating temperature . Replace printer assembly, digital board, and/or
printer interconnect board.
RELEASE BUTTONS Simultaneous external paddle button presses detected
before unit reached full defib charge (ready state).
RELEASE SHOCK
REMOVE SYNC Analyze button pressed, or no heart rate detected while in
2-10 9650-0903-01 Rev. L
• Discharge switch(es) closed when pressing charge
button.
• Discharge button pressed before defib reached ready
state.
Defib Sync mode.
Release buttons.
• Release shock button.
• Check paddles.
• Replace controls board.
Page 65

R Series Service Manual
Error Message Explanation User
REPEAT NIBP MEAS
REPLACE BATTERY Battery voltage is less than absolute minimum. Shutdown
REPORT FULL Summary report memory full.
RETRY ANAL YSIS Advisory message in conjunction with noisy ECG. Analysis
RPT NO DISK Internal disk is not formatted and cannot be formatted.
SELECT 30J FOR TEST Attempt to run a self test at an energy other than 30J.
SELECT ASYNC PACE User is prompted to select Async pacing, since the PD
SELECT DEFIB MODE Analyze button pressed in pace or monitor mode.
SELECT LIMB LEADS Paddles or augmented ECG leads selected when
• The unit exceeded the maximum number of inflation
attempts.
• The unit exceeded the 180-second measurement time
limit.
imminent.
halted.
module cannot detect sync pulses.
continuous analysis active or started.
Advisory
Te chnical Acti on
• Check cuff and hose.
• Repeat NIBP measurement.
Replace with charged battery.
Erase summary report.
Turn main selector knob to Defib/ON.
Select limb leads I, II, III or MFE.
SELECT PADS ECG lead (non-MFE Pads) selected when ANALYZE
pressed.
SET CLOCK Real time clock failure: invalid date or time.
SET PACE MA Multiple copy errors are the product of intended software
or memory errors. If error reoccurs other than on entering
pace the first time or after more than 10 minutes in other
mode, the unit could be broken.
9650-0903-01 Rev. L 2-11
• Set time and date information.
• Verify that the internal lithium battery has been
replaced within the last 5 years. Contact ZOLL
Technical Se rvice Department for assistance.
Set pace current. If broken, replace system board.
Page 66

R Series Service Manual
Error Message Explanation User
SHOCK ADVISED Advisory message when Analysis finds a shockable
rhythm. Followed by PRESS SHOCK in Manual Advisory
Defib with auto-charge enabled or in Auto Defib mode, or
by PRESS CHARGE in Manual Advisory Defib with autocharge disabled.
SPO2 AMBIENT LIGHT Ambient light is too bright.
SPO2 COMM ERROR No transmissions from SpO
Communication error or no communication from SpO
module.
SPO2 PULSE SEARCH Pulse search in progress.
STAND CLEAR (Manual Advisory Defib with auto-charge enabled or Semi-
Auto Mode Defib) User pressed Analyze or an analysis
was started automatically as part of the rescue protocol.
Patient rhythm is being analyzed.
SUMMARY TIMEOUT Summary report timeout error.
SYNC DEFIB DISABLED Sync mode active when analyze pressed in defib.
unit received.
2
2
Advisory
Technical Action
• Shield sensor from ambient light.
• Replace sensor.
• Replace SpO2 module
Replace SpO
module and/or system board.
2
SYSTEM FAULT 210 System task have not been activated for 500 ms – ECG
control.
SYSTEM FAULT 211 System task have not been activated for 500 ms – Defib. Replace digital board.
SYSTEM FAULT 212 System task have not been activated for 500 ms – Pace. Replace digital board.
SYSTEM FAULT 212 System task have not been activated for 500 ms – User
Interface.
SYSTEM FAULT 214 System task have not been activated for 500 ms – Display. Replace digital board.
SYSTEM FAULT 215 System task have not been activated for 500 ms – Not
used.
2-12 9650-0903-01 Rev. L
Replace digital board.
Replace digital board.
Replace digital board.
Page 67

R Series Service Manual
Error Message Explanation User
SYSTEM FAULT 36 Filtered sum of all the supply voltages is out of range. Replace analog board or digital board.
SYSTEM FAULT 37 1/2 scale reference voltage is out of range. Replace analog board or PD engine.
SYSTEM FAULT 38 Failure to shutdown after "shutdown order" is written to the
RTC.
TEST FAILED 30J defib self-test failed.
TRANSFERRING DATA Transferring data message.
USE PADDLE DISCHG Defibrillator is charged and front-panel discharge button
pressed when either external paddles or internal spoons
with discharge buttons are connected.
USE PA DS TO PACE MFE accessory other than Pads detected in Pace mode.
USER SETUP REQ. Both copies of configuration data are bad, or software with
a configuration rev older than the current one was loaded.
VF ALARMS OFF Alarms enabled in Pace Mode or when Continuous
Analysis active or started in manual mode but the current
lead is not Pads, Lead I, Lead II, or Lead III. Also displayed
if the Alarm button is pressed but the Heart Rate alarm is
disabled.
Advisory
Te chnical Acti on
Replace digital board.
Replace universal cable, paddles or high voltage
module, capacitor, and/or system board.
Reconfigure unit.
ZERO CO2 ADAPTER Negative CO2 detected. May be caused by a sensor that
was zeroed with CO
blockage of the airway adapter.
ZERO DONE The sensor/adapter zero is finished.
ZEROING CO2 ADAPTER Adapter zeroing is in progress.
9650-0903-01 Rev. L 2-13
in the airway, or by an optical
2
Check the airway adapter and clean if necessary.
Perform a mainstream airway adapter zero as
described in “Zeroing the Mainstream
CAPNOSTAT 5 CO
No action required.
Wait for the adapter zeroing to finish.
Sensor/Airway Adapter.”
2
Page 68

R Series Service Manual
Error Message Explanation User
ZERO FAILED The zero operation did not complete successfully.
Advisory
Technical Action
Clear the occlusion, remove any source of CO2,
and try zeroing again.
If problem persists, contact ZOLL Technical
Support.
2-14 9650-0903-01 Rev. L
Page 69

Chapter 3
Disassembly Procedures
This chapter provides instructions on how to disassemble and reassemble the R Series unit, and includes the following sections:
“Removing the Cable Caddy” on page 3-3
“Removing the Handle” on page 3-6
“Removing the Recorder, AC Charger and Battery Well” on page 3-7
“Removing the Front Panel Assembly” on page 3-12
“Removing the Side Panels” on page 3-16
“Removing the Connector Panel and Bezel” on page 3-18
“Removing the ECG Input Connector” on page 3-20
“Removing the NIBP Assembly” on page 3-21
R Series Service Manual
“Removing the System Brick Assembly” on page 3-23
“Disassembling the System Brick Assembly” on page 3-28
“Discharging Capacitor” on page 3-34
“Removing the Communication Module” on page 3-35
Required Equipment
• #1 Philips head screwdriver
• #2 Philips head screwdriver
• Hex head screwdriver
9650-0903-01 Rev. L 3-1
Page 70

R Series Service Manual
• Wooden stick
• Needlenose pliers
• Exacto knife
• Compressed air
• 1/2” Nut Driver
• 3M Scotch-Weld Hot Melt Adhesive 3779 TC Amber
Safety Precautions
Warning! SHOCK HAZARD!
Caution TAKE THE NECESSARY PRECAUTIONS TO GUARD AGAINST SHOCK OR INJURY BEFORE YOU CONDUCT
DEFIBRILLATOR TESTS OR REPAIRS.
• Only properly trained technicians should service the unit.
• The unit can contain deadly voltages even if the unit is turned off.
• Make sure to discharge the unit before working with it.
• Make sure you take the necessary precautions when working with static sensitive units. For example, you must wear a conductive wrist strap (which
touches your skin) connected to a grounding mat and to the earth ground. You must remove the wrist strap when you discharge high voltage or when
you are working on energized equipment.
• Wear gloves to prevent skin oils from affecting the equipment.
3-2 9650-0903-01 Rev. L
Page 71

R Series Service Manual
Removing the Cable Caddy
1. Unplug all cables from the R Series. 2. There are 6 screws securing the cable caddy (3 at the rear
of the R Series and 3 at the bottom).
9650-0903-01 Rev. L 3-3
Page 72

R Series Service Manual
3. Remove the 3 bottom screws completely. 4. Loosen the 3 screws at the back. It is not necessary to
remove the screws completely or to remove the mesh.
3-4 9650-0903-01 Rev. L
Page 73

5. Unplug the AC Line Cord Extension Cable from the
AC input receptacle when removing the cable
caddy.
R Series Service Manual
9650-0903-01 Rev. L 3-5
Page 74

R Series Service Manual
Removing the Handle
1. Remove the 4 hex screws securing the handle. 2. Lift the handle to remove.
Note: During reassembly, torque to 10 in-lbs.
3-6 9650-0903-01 Rev. L
Page 75

Removing the Recorder,
AC Charger and Battery Well
R Series Service Manual
1. Using a #2 Philips screwdriver, remove the 4
screws securing the AC Charger Assembly.
Note: During reassembly, torque to 10 in-lbs.
2. Remove the AC Charger assembly from the chassis by
lifting straight up. Unplug the cable from the charger to the
PD Engine as shown.
9650-0903-01 Rev. L 3-7
Page 76

R Series Service Manual
Low current harness
High current harness
3. If removing the battery well, disconnect the high and
low current cables from the PD Engine as shown.
4. Remove the label from the battery well as shown (peel
from back to front).
Note: It may be necessary to use a new label upon
reassembly (Part # 9305-0901-01).
3-8 9650-0903-01 Rev. L
Page 77

R Series Service Manual
5. Remove the 3 screws securing the battery well. 6. Lift the battery well from the front of the R Series, tilting
towards the rear. DO NOT disconnect the low and high
current harnesses. Feed them up through the chassis to
remove the battery well.
Note: During reassembly, torque to 6 in-lbs.
9650-0903-01 Rev. L 3-9
Page 78

R Series Service Manual
7. Open the recorder door. Using a wooden stick (or
similar tool) press in on the hinge to release the
door as shown.
8. Remove the two screws that secure the recorder tray.
Note: During reassembly, torque to 6 in-lbs.
3-10 9650-0903-01 Rev. L
Page 79

R Series Service Manual
Recorder
cables
interconnect
Recorder
cables
interconnect
9. Lift up on the recorder tray from the rear of the
R Series to release it.
10.Rotate device so the display is facing you. Lift up on the
paper tray to expose cables. Disconnect the recorder
interconnect cable from the print head and the motor/
sensor board.
Note: During reassembly, observe the labeling on the cable to ensure you
are connecting the cable correctly.
9650-0903-01 Rev. L 3-11
Page 80

R Series Service Manual
Removing the Front Panel Assembly
1. Remove 3 Phillips screws from the top of the rear
Front Panel Assembly.
Note: During reassembly, torque to 6 in-lbs. Note: During reassembly, torque to 6 in-lbs.
2. Remove the 3 hex screws from the bottom front of the
Front Panel Assembly.
3-12 9650-0903-01 Rev. L
Page 81

R Series Service Manual
Control cable
Backlight
cable
LCD display
Recorder
cable
3. Tilt the front panel carefully as shown. 4. Using needlenose pliers, carefully remove the hot melt
glue from the Recorder and Controls cables. Carefully
disconnect the 4 cables shown.
Note: During reassembly, reapply hot melt glue (3M Scotch-
Weld Hot Melt Adhesive 3779 TC Amber.)
9650-0903-01 Rev. L 3-13
Page 82

R Series Service Manual
Front Panel Disassembly
1. Remove 5 screws securing the metal shield. 1. Lift display assembly straight out from the front panel.
(See pages 4-38 thru 4-41 for display assembly parts
breakdown)
Note: During reassembly, torque to 6 in-lbs.
3-14 9650-0903-01 Rev. L
Page 83

R Series Service Manual
2. Remove the main selector and pacer knobs by
pulling them straight out, then remove nuts securing
them to the front panel.
3. Remove 9 screws securing the control board to the front
panel.
Note: During reassembly, torque to 6 in-lbs.
9650-0903-01 Rev. L 3-15
Page 84

R Series Service Manual
Removing the Side Panels
1. Remove the 4 hex screws from right side panel. 2. Remove the 4 hex screws from left side panel.
Note: During reassembly, torque to 10 in-lbs. Note: During reassembly, torque to 10 in-lbs.
3-16 9650-0903-01 Rev. L
Page 85

R Series Service Manual
3. Remove the right panel by pulling towards you. The
speaker may or may not come off with the panel.
4. Remove the left panel by pulling towards you. Remove the
shorting wire from the panel by taking off the nut.
Disconnect the speaker from the Digital System
Board by pulling straight out. Remove the shorting
wire from the panel by taking off the nut.
Note: During reassembly, torq ue nut to 6 in-lbs. Note: During reassembly, torque nut to 6 in-lbs.
9650-0903-01 Rev. L 3-17
Page 86

R Series Service Manual
Removing the Connector Panel and Bezel
5. Remove the label covering the connector panel
bezel. It may be necessary to use a new label upon
reassembly.
6. Using a #1 Phillips screwdriver, remove the 9 screws
securing the connector panel bezel.
Note: During reassembly, torque to 4 in-lbs.
3-18 9650-0903-01 Rev. L
Page 87

R Series Service Manual
7. Remove the connector panel by lifting straight up.
Note: Depending on the options, the RS232 connector may be
attached to the panel. In that case the panel can be draped
over the rear of the unit.
Note: Below is the connector panel with the EtCO2 and NIBP op tions. See
the diagram on page 4-17 for further disassembly.
Note: One or both side panels and/or the front panel may need to be
removed in order to disconnect cabling from some of the connectors
located on the rear panel.
9650-0903-01 Rev. L 3-19
Page 88

R Series Service Manual
Removing the ECG Input Connector
Carefully lift the ECG Connector by tilting the rear of the
connector up first. Then carefully remove the cable
from the connector as shown, using a wooden stick.
Remove the connector from the chassis.
Note: After removing all connectors, clean them with compressed air.
3-20 9650-0903-01 Rev. L
Page 89

Removing the NIBP Assembly
R Series Service Manual
1. Disconnect the hose from the manifold and cut the
cable tie securing the pump to the chassis.
Note: When reassembling, ensure NIBP tubing is not routed
between pump wiring. Secure the NIBP bracket to the
anchor with a cable tie.
2. Disconnect the cable from the NIBP assembly from
parameter power supply by pushing down on latch at
the top of the connector.
Remove the 2 screws securing the NIBP assembly to
the chassis.
Note: During reassembly, torque to 6 in-lbs.
9650-0903-01 Rev. L 3-21
Page 90

R Series Service Manual
3. Slide the NIBP assembly out of the chassis.
3-22 9650-0903-01 Rev. L
Page 91

Removing the System Brick Assembly
Note: See the safety precautions on 3-2 before removing the system brick assembly.
.
1. Remove the printer housing barrier from the chassis. 2. Remove the ECG retainer pad.
R Series Service Manual
Note: During reassembly, the barrier may be reused if the adhesi ve
side is not contaminated.
Note: When reinserting the pad during reassembly, push it down until
it touches the ECG cable connector.
9650-0903-01 Rev. L 3-23
Page 92

R Series Service Manual
3
2
1
3. Carefully disconnect the speaker harness (1), the
sync cable (2), and the patient impedance cable (3).
Note: Disconnect the speaker on the opposite side of the brick
assembly if you have not done this already.
4. Carefully disconnect the sync cable by pulling straight
up from top.
3-24 9650-0903-01 Rev. L
Page 93

R Series Service Manual
7
7
5. From the top of the device, disconnect the USB and
compact flash cables by lifting connector straight up
from the board.
6. Carefully pull back the USB and compact flash
cables and disconnect the MFC cable.
9650-0903-01 Rev. L 3-25
Page 94

R Series Service Manual
7. In applicable, disconnect the EtCO2 cable from the
parameter power supply by pushing down on
connector latch.
8. Carefully pull the brick assembly out slightly through
the front; disconnect the SpO2 connector cable.
3-26 9650-0903-01 Rev. L
Page 95

9. Ensuring that all cables are disconnected from the
brick, slide the entire brick out of the front of the
chassis.
R Series Service Manual
9650-0903-01 Rev. L 3-27
Page 96

R Series Service Manual
Disassembling the System Brick Assembly
Note: See the safety precautions on page 3-2 before removing the system brick assembly.
The system brick assembly consists of three primary boards attached t ogether, Units equipment with SpO
boards.
and/or EtCO2 & NIBP will have two ad ditiona l
2
Top: Digital system board (SpO
Middle: Analog system board
Bottom: Pace defibrillator engine
Use caution when pulling apart the three boards to prevent the EMI suppression plates from scattering.
module and isolated power supply sits on digital board)
2
Removing Isolated Power Supply
1. Remove 5 screws holding the isolated power supply. 2. Peel back glue from connector, and disconnect cable
by pulling straight up. Remove tape holding cable to
isolated power supply, then disconnect cable.
Note: During reassembly torque screws to 4 in-lbs. Note: During reassembly re-apply glue to each side of connector.
3-28 9650-0903-01 Rev. L
Page 97

3. Lift isolated power supply board and shield out of tray.
R Series Service Manual
9650-0903-01 Rev. L 3-29
Page 98

R Series Service Manual
Removing the SpO2 Module
1. Use a prying tool to lift up on the board to release
adhesive. (The SpO2 module is held in place with
adhesive strips.)
Note: Plastic isolator tray, part # 9310-0889, may be damaged
during disassembly and need to be replaced.
3-30 9650-0903-01 Rev. L
Page 99

R Series Service Manual
7
Removing Digital Board
1. Remove 9 screws holding digital board to assembly. 2. Tilt assembly up, and carefully pull board
toward you to disconnect from connectors.
Note: During reassembly, inspect standoffs and corner post to verify they
are not stripped. Replace them if necessary. T orque 4 corner screws
(longer) to 6 in-lbs and the 5 inter screws to 4 in-lbs.
Note: Since ferrite beads on connector are loose, make sure they
are in place during reassembly.
9650-0903-01 Rev. L 3-31
Page 100

R Series Service Manual
7
Removing Pace Defibrillator Engine
1. PD Engine: Remove 9 screws holding PD Engine to
assembly.
Note: During reassembly, inspect standoffs and corner post to verify they
are not stripped, replace them if necessary. Torque 4 corner screws
(longer) to 6 in-lbs, and the 5 inter screws to 4 in-lbs.
2. Carefully remove cable from PD Board.
Note: Use a rocking motion to unseat connector from board.
3-32 9650-0903-01 Rev. L
 Loading...
Loading...