Zoll E Service manual

‘
SERVICE MANUAL
ZOLL MEDICAL CORPORATION
9650-1450-01 Rev. A |
October 2008 |
ZOLL, E Series, and RescueNet are registered trademarks, and SurePower Charger Station is a trademark of ZOLL Medical Corporation. All other trademarks and registered trademarks are property of their respective owners.
Copyright © 2008 ZOLL Medical Corporation. All rights reserved.
9650-1450-01 Rev. A

TABLE OF CONTENTS
Preface .............................................................................................................................................................. |
v |
Safety Considerations................................................................................................................................................. |
v |
Additional Reference Material ................................................................................................................................... |
vi |
Conventions ................................................................................................................................................................ |
vi |
Service Policy Warranty............................................................................................................................................ |
vii |
Technical Service....................................................................................................................................................... |
vii |
Technical Service for International Customers ..................................................................................................... |
viii |
Chapter 1 Maintenance Tests ........................................................................................................................ |
1-1 |
Overview.................................................................................................................................................................... |
1-1 |
Before You Begin the Maintenance Tests............................................................................................................... |
1-2 |
Equipment You Need to Perform the Maintenance Tests...................................................................................... |
1-2 |
Equipment You Need for the E Series Options Maintenance Tests ..................................................................... |
1-3 |
Physical Inspection of the Unit............................................................................................................................. |
1-4 |
Front Panel Button Test....................................................................................................................................... |
1-6 |
3, 5, and 12 Leads Test....................................................................................................................................... |
1-9 |
Power Supply Test (Optional)............................................................................................................................ |
1-10 |
Leakage Current Test........................................................................................................................................ |
1-14 |
Paddles Test (If applicable) ............................................................................................................................... |
1-15 |
Heart Rate Display Test..................................................................................................................................... |
1-16 |
Calibrating Pulses on Strip Chart Test............................................................................................................... |
1-17 |
Notch Filter Test ................................................................................................................................................ |
1-18 |
Heart Rate Alarm Test....................................................................................................................................... |
1-19 |
Defibrillator Self Test ......................................................................................................................................... |
1-21 |
Synchronized Cardioversion Test...................................................................................................................... |
1-23 |
Shock Test......................................................................................................................................................... |
1-24 |
Summary Report Test........................................................................................................................................ |
1-26 |
Advisory Message Test ..................................................................................................................................... |
1-27 |
Pacer Test ......................................................................................................................................................... |
1-28 |
SpO2 Monitor Test for SpO2 Option.................................................................................................................. |
1-31 |
EtCO2 Monitor Test (for EtCO2 Option) ............................................................................................................ |
1-33 |
Barometric Pressure Calibration Check.............................................................................................................. |
1-34 |
|
|
9650-1450-01 Rev. A |
i |

|
CO2 Accuracy Check ......................................................................................................................................... |
1-35 |
|
NIBP Monitor Test .............................................................................................................................................. |
1-37 |
|
NIBP Volume Leak Test with Bio-Tek NIBP Analyzer ........................................................................................ |
1-39 |
|
NIBP Volume Leak Test with Fluke Biomedical Cufflink NIBP Analyzer ............................................................ |
1-41 |
|
NIBP Transducer Calibration Test ...................................................................................................................... |
1-43 |
Chapter 2 |
Troubleshooting ............................................................................................................................... |
2-1 |
|
Overview.................................................................................................................................................................... |
2-1 |
|
Troubleshooting........................................................................................................................................................ |
2-2 |
|
ZOLL E Series Error Messages ............................................................................................................................... |
2-5 |
Chapter 3 |
Replacement Parts ........................................................................................................................... |
3-1 |
|
Replacement Parts.................................................................................................................................................... |
3-2 |
|
Field Replacement Parts .......................................................................................................................................... |
3-5 |
Chapter 4 |
Functional Description .................................................................................................................... |
4-1 |
|
Main System Board................................................................................................................................................... |
4-2 |
|
Main System Board Functions ............................................................................................................................... |
4-4 |
|
Power Supply ......................................................................................................................................................... |
4-5 |
|
ECG Front End ...................................................................................................................................................... |
4-6 |
|
Multifunction Electrode (MFE)/PADS (System Board and High Voltage Module) ................................................. |
4-6 |
|
CPU and EPU ........................................................................................................................................................ |
4-6 |
|
High Voltage Module................................................................................................................................................. |
4-7 |
|
Defibrillator Charging and Discharging .................................................................................................................. |
4-7 |
|
Charging .................................................................................................................................................. |
4-7 |
|
Discharging .............................................................................................................................................. |
4-8 |
|
High Voltage Capacitor Monitor ............................................................................................................................. |
4-8 |
|
Pacer/Defibrillator Control Signals ......................................................................................................................... |
4-9 |
|
Internal Discharge Resistor Module ..................................................................................................................... |
4-10 |
|
AC/DC Charger Module .......................................................................................................................................... |
4-10 |
|
System Interconnect Module.................................................................................................................................. |
4-11 |
|
|
|
ii |
|
9650-1450-01 Rev. A |

Stripchart Recorder .............................................................................................................................................. |
4-11 |
PCMCIA Slots ...................................................................................................................................................... |
4-11 |
Front Panel and Controls PWBA ......................................................................................................................... |
4-11 |
Isolated Power Supply Module ............................................................................................................................ |
4-11 |
E Series Options ..................................................................................................................................................... |
4-12 |
12 Lead Option .................................................................................................................................................... |
4-12 |
Pulse Oximetry (SpO2) ........................................................................................................................................ |
4-12 |
End Tidal Carbon Dioxide (EtCO2) ...................................................................................................................... |
4-13 |
Noninvasive Blood Pressure ................................................................................................................................ |
4-14 |
Appendix A Overview .......................................................................................................................................... |
A-1 |
9650-1450-01 Rev. A |
iii |

E Series Service Manual
Preface
ZOLL® Medical Corporation’s E Series® Service Manual is intended for the service technician whose responsibility is to identify malfunctions and/or make repairs at the subassembly level. The ZOLL E Series Service Manual has five main sections and one appendix.
Preface—Contains safety warnings and an overview of the manual’s contents. Be sure to review this section thoroughly before attempting to use or service the E Series unit.
Chapter 1—Maintenance Tests explains how to check the defibrillator’s performance using a series of recommended checkout procedures to be conducted every six months.
Chapter 2—Troubleshooting provides a listing of the procedures and error messages to help the service technician detect faults and repair them.
Chapter 3—Replacement Parts List displays a complete list of ZOLL part numbers for field replaceable parts available for the E Series unit, allowing the service person to identify and order replacement parts from ZOLL.
Chapter 4—Functional Description provides technical descriptions for the E Series major subassembly modules. Appendix A—E Series interconnect diagrams and maintenance checklists.
Safety Considerations
The following section describes general warnings and safety considerations for operators and patients. Service technicians should review the safety considerations prior to servicing any equipment and read the manual carefully before attempting to disassemble the unit. Only qualified personnel should service the E Series unit.
Federal (U.S.A.) law restricts this unit for use by or on the order of a physician.
Safety and effectiveness data submitted by ZOLL Medical Corporation to the Food and Drug Administration (FDA) under section 510(K) of the Medical Device Act to obtain approval to market is based upon the use of ZOLL accessories such as disposable electrodes, patient cables and batteries. The use of external pacing/defibrillation electrodes and adapter units from sources other than ZOLL is not recommended. ZOLL makes no representations or warranties regarding the performance or effectiveness of its products when used in conjunction with pacing/defibrillation electrodes and adapter units from other sources. If unit failure is attributable to pacing/defibrillation electrodes or adapter units not manufactured by ZOLL, this may void ZOLL's warranty.
Only qualified personnel should disassemble the E Series unit.
WARNING! |
This unit can generate up to 2250 volts with sufficient current to cause lethal shocks. |
9650-1450-01 Rev. A |
v |

E Series Service Manual
All persons near the equipment must be warned to STAND CLEAR prior to discharging the defibrillator.
Do not discharge the unit’s internal energy more than three times in one minute or damage to the unit may result.
Do not discharge a battery pack except in a ZOLL SurePower™ Charger Station or compatible ZOLL Battery Charging/Testing unit.
Do not use the E Series in the presence of flammable agents (such as gasoline), oxygen-rich atmospheres, or flammable anesthetics. Using the unit near the site of a gasoline spill may cause an explosion.
Do not use the unit near or within puddles of water.
Note: The E Series is protected against interference from radio frequency emissions typical of two-way radios and cellular phones (digital and analog) used in emergency service/public safety activities. Users of the E Series should assess the unit’s performance in their typical environment of use for the possibility of radio frequency interference from high-power sources. Radio Frequency Interference (RFI) may be observed as shifts in monitor baseline, trace compression, or transient spikes on the display.
Additional Reference Material
In addition to this guide, there are several other components to the ZOLL E Series documentation. They include:
•E Series Operator’s Guide - A comprehensive reference work that describes all the user tasks needed to operate the E Series.
•E Series Configuration Guide - Describes the E Series features and functions whose operation can be customized by authorized users.
•E Series Operator’s Guide Option Insert: 12-Lead ECG Monitoring- Describes using the 12-lead ECG monitoring option with the E Series unit.
•E Series Operator’s Guide Option Insert: End Tidal Carbon Dioxide (EtCO2)- Describes using the EtCO2 option with the E Series unit.
•E Series Operator’s Guide Option Insert: Non-Invasive Blood Pressure (NIBP)- Describes using the NIBP option with the E Series unit.
•E Series Operator’s Guide Option Insert: Pulse Oximetry (SpO2)- Describes using the SpO2 option with the E Series unit.
•E Series Operator’s Guide Option Insert: Non-Interpretive 12-Lead ECG Monitoring- Describes using the non-interpretive 12-lead ECG monitoring option with the E Series unit.
•E Series Operator’s Guide Option Insert:12-Lead Reperfusion Therapy Algorithm - Describes the reperfusion therapy algorithm option for the E Series unit.
Conventions
WARNING! Warning statements describe conditions or actions that can result in personal injury or death.
vi |
9650-1450-01 Rev. A |

E Series Service Manual
Caution Caution statements describe conditions or actions that can result in damage to the unit.
Note: Notes contain additional information on using the defibrillator.
Service Policy Warranty
In North America: Consult your purchasing agreement for terms and conditions associated with your warranty. Outside of North America, consult ZOLL authorized representative.
In order to maintain this warranty, the instructions and procedures contained in this manual must be strictly followed. For additional information, please call the ZOLL Technical Service Department 1-800-348-9011 in North America.
Technical Service
If the ZOLL E Series unit requires service, contact the ZOLL Technical Service Department:
Telephone: 1-978-421-9655; 1-800-348-9011 (US only)
Fax 1-978-421-0010
Have the following information available for the Technical Service representative:
•Unit serial number.
•Description of the problem.
•Department where equipment is used.
•Sample chart recorder strips documenting the problem, if applicable.
•Purchase Order to allow tracking of loan equipment.
•Purchase Order for a unit with an expired warranty.
If the unit needs to be sent to ZOLL Medical Corporation, obtain a Service Request number from the Technical Service representative. Return the unit in its original container to:
ZOLL Medical Corporation 269 Mill Road
Chelmsford, Massachusetts 01824-4105
Attn: Technical Service Department, SR #
9650-1450-01 Rev. A |
vii |
E Series Service Manual
Technical Service for International Customers
International customers should return the unit in its original container to the nearest authorized ZOLL Medical Corporation Service Center. To locate an authorized service center, contact the International Sales Department at ZOLL Medical. See back cover of this manual.
viii |
9650-1450-01 Rev. A |

E Series Service Manual
Chapter 1
Maintenance Tests
Overview
The E Series has two checkout procedures: the operator’s shift checklist and the extensive six-month maintenance tests checkout procedures.
Because the E Series units must be maintained ready for immediate use, it is important for users to conduct the Operator’s Shift Checklist procedure at the beginning of every shift. This procedure can be completed in a few minutes and requires no additional test equipment. (See the ZOLL E Series Operator’s Guide for the Operator’s Shift Checklist.)
A qualified biomedical technician must perform a more thorough maintenance test checkout every six months to ensure that the functions of the E Series unit work properly. This chapter describes the step by step procedures for performing the six month maintenance test checkout. Use the checklist at the back of this document (ZOLL E Series Maintenance Tests Checklist) to record your results of the maintenance tests.
This chapter describes the following maintenance tests:
•1. Physical Inspection of the Unit
•2. Front Panel Button Test
•3. 3, 5, and 12 Leads Test
•4. Power Supply Test
•5. Leakage Current Test
•6. Paddles Test
•7. Heart Rate Display Test
•8. Calibrating Pulses on Strip Chart Test
•9. Notch Filter Test
•10. Heart Rate Alarm Test
•11. Defibrillator Self Test
•12. Synchronized Cardioversion Test
•13. Shock Test
•14. Summary Report Test
9650-1450-01 Rev. A |
1-1 |

E Series Service Manual
•15. Advisory Message Test
•16. Pacer Test
•17. SpO2 Monitor Test
•18. EtCO2 Monitor Test
•19. Barometric Pressure Calibration Check
•20. CO2 Accuracy Check
•21. NIBP Monitor Test
•22. NIBP Volume Leak Test with Fluke Biomedical NIBP Analyzer
•23. NIBP Volume Leak Test with Fluke Biomedical Cufflink NIBP Analyzer
•24. NIBP Transducer Calibration Test
Before You Begin the Maintenance Tests
•Assemble the tools or specialized testing equipment listed in the “Equipment You Need to Perform the Maintenance Tests” section shown below.
•Keep an extra fully charged ZOLL E Series compatible battery available.
•Schedule an hour to conduct the entire maintenance test.
•Photocopy the checklist at the back of this document and use the copy to record your results. As you conduct each step of a procedure, mark the Pass/Fail/NA check boxes on your checklist and then save it for your maintenance file.
•Perform the tests in the order presented.
•Perform all the steps of each test procedure.
•Complete all the steps of the procedure before evaluating the test results.
Equipment You Need to Perform the Maintenance Tests
For testing purposes, you can substitute an equivalent device.
•ZOLL Medical Electrode Adapter from Fluke Biomedical (part number 3010-0378).
•Fluke Biomedical Impulse 4000 Defibrillator Analyzer with 1.06 software or higher.
•Fluke Biomedical 601 Pro Series International Safety Analyzer.
•ECG Simulator; 12 Lead Simulator for 12 Lead test (e.g., Symbio CS1201).
•Stop watch.
1-2 |
9650-1450-01 Rev. A |
E Series Service Manual
•Standard series II PC flash memory cards.
•PCMCIA card reader and PC.
•RescueNet® Code Review V3.31 or higher from ZOLL Data Systems.
•Phillips #1 screwdriver.
•Phillips #2 screwdriver.
•Flatblade screwdriver.
•Needle nose pliers without teeth.
•Wooden cuticle sticks with beveled edges (or similar non-conducting implements).
Equipment You Need for the E Series Options Maintenance Tests
•Fluke Biomedical Index 2PFE SpO2 Simulator or equivalent. (For SpO2 units only.)
•SpO2 cable and sensor (if option is installed).
•EtCO2 cable, and CAPNOSTAT 5 Mainstream cable with airway adapter, or CAPNOSTAT 5 Sidestream cable with cannula (if option is installed).
•Paddles (if used).
•Printer Paper.
•Battery.
•AC line cord.
•3 lead, 5 lead and 12 lead ECG cables. (12 lead cable needed if 12 lead option is installed.)
•Fluke Biomedical BP Pump NIBP Monitor Analyzer (For NIBP units only) with NIBP cable and cuff (if NIBP option is installed), or
•Fluke Biomedical Cufflink Analyzer (if NIBP option is installed)
Note: The Fluke Biomedical BP Pump NIBP Monitor Analyzer and the Fluke Biomedical BP Pump NIBP Analyzer use different technologies for testing NIBP monitors and therefore, the manual provides two different procedures for performing the NIBP Volume Leak test with each of these types of test equipment.
9650-1450-01 Rev. A |
1-3 |
E Series Service Manual
1.0 Physical Inspection of the Unit
Tools Needed: None.
Test Setup: None.
Observe this... |
Pass/Fail/NA |
|||
|
|
|
|
|
1.1 |
Housing |
o |
o |
|
|
Is the unit clean and undamaged? |
|
|
|
|
|
|
|
|
1.2 |
Does the unit show signs of excessive wear? |
o |
o |
|
|
|
|
|
|
1.3 |
Does the handle work properly? |
o |
o |
|
|
|
|
|
|
1.4 |
Does the recorder door open and close properly? |
o |
o |
|
|
|
|
|
|
1.5 |
Are input connectors clean and undamaged? |
o |
o |
|
|
|
|
|
|
1.6 |
Are there any cracks in the housing? |
o |
o |
|
|
|
|
|
|
1.7 |
Do the front panel or selector switches have any damage or cracks? |
o |
o |
|
|
|
|
|
|
1.8 |
Are there any loose housing parts? |
o |
o |
|
|
|
|
|
|
1.9 |
Paddles |
o |
o |
o |
|
Do the adult and pedi plates have major scratches or show signs of damage? |
|
|
|
|
|
|
|
|
1.10 |
Do the adult shoes slide on and off easily to expose the covered pedi plates? |
o |
o |
o |
|
|
|
|
|
1.11 |
Are the paddles clean (e.g., free of gel) and undamaged? (if applicable) |
o |
o |
o |
|
|
|
|
|
1.12 |
Cables |
o |
o |
|
|
Are all cables free of cracks, cuts, exposed or broken wires? |
|
|
|
|
|
|
|
|
1.13 |
Are all bend/strain reliefs undamaged and free of excessive cable wear? |
o |
o |
|
|
|
|
|
|
1.14 |
Battery |
o |
o |
|
|
Is the ZOLL battery fully charged? |
|
|
|
|
|
|
|
|
1-4 |
9650-1450-01 Rev. A |
E Series Service Manual
Observe this... |
Pass/Fail/NA |
|
|
|
|
1.15 |
Is the battery seated in the battery well correctly? |
o o |
|
|
|
Record your results on the Maintenance Test Checklist. |
|
|
|
|
|
9650-1450-01 Rev. A |
1-5 |

E Series Service Manual
2.0 Front Panel Button Test
Tools Needed: None.
Test Setup:
1.Install strip chart paper into the recorder tray.
2.Install the battery in the unit or connect the A/C power cord to the unit and then plug the cord into an electrical outlet.
3.Connect the universal cable and ECG cable (3 lead, 5 lead, or 12 lead) to the ZOLL simulator, or Fluke Biomedical Impulse 4000 Analyzer (or equivalent).
|
Do this... |
Observe this... |
Pass/Fail/NA |
|
||
|
|
|
|
|
|
|
2.1 |
Turn the selector switch to MONITOR. |
Listen for 4 beep tones. PADS and MONITOR display on the monitor. |
o |
o |
|
|
|
(For AED units, turn the selector switch to ON |
NOTE: PADS is a factory default setting. |
|
|
|
|
|
and select Manual mode.) |
|
|
|
|
|
|
|
|
|
|
|
|
2.2 |
Press the LEAD button; three times for the 3 |
Each time you press the LEAD button, a different lead number appears under |
o |
o |
|
|
|
lead cable and seven times for the 5 lead cable. |
the LEAD heading on the display. |
|
|
|
|
|
|
PADS, I, II, III will display a 3 lead ECG cable if connected or no ECG cable is |
|
|
|
|
|
|
connected. |
|
|
|
|
|
|
PADS, I, II, III, AVR, AVL, AVF, V1 will display a 5 lead ECG cable. |
|
|
|
|
|
|
|
|
|
|
|
2.3 |
Connect the 12 lead cable to unit and simulator. |
A 12 Lead cable will display PADS, I, II, III, AVR, AVL, AVF, VI, V2, V3, V4, V4, |
o |
o |
o |
|
|
Press the LEAD button and select the lead for |
V5, V6. |
|
|
|
|
|
each of the 12 lead settings. |
|
|
|
|
|
|
|
|
|
|
|
|
2.4 |
Set the simulator to NSR of 120 BPM. To check |
As you press the SIZE button five times (0.5, 1.0, 1.5, 2.0, 3.0), note that the |
o |
o |
o |
|
|
the size of the ECG waveform, press the SIZE |
size of the ECG waveform appropriately changes on the display. |
|
|
|
|
|
button. |
|
|
|
|
|
|
|
|
|
|
|
|
2.5 |
Press the ALARM SUSPEND button. |
Alarm symbol changes from disabled to enabled. If the alarm sounds, press |
o |
o |
o |
|
|
|
the ALARM SUSPEND button to turn it off. The alarm will only be suspended |
|
|
|
|
|
|
for 90 seconds at this point. Press and hold the ALARM SUSPEND button for |
|
|
|
|
|
|
3 seconds to disable alarms. |
|
|
|
|
|
|
|
|
|
|
|
2.6 |
Press the RECORDER button. |
The strip chart paper moves out of the unit from the paper tray. Check that the |
o |
o |
o |
|
|
|
correct time, date, ECG lead annotation and waveform are recorded on the |
|
|
|
|
|
|
paper. (Set Time and Date, if necessary.) |
|
|
|
|
|
|
|
|
|
|
|
2.7 |
Open the paper compartment door. |
CHECK RECORDER message appears on the monitor. |
o |
o |
o |
|
|
Press RECORDER button. |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
1-6 |
|
9650-1450-01 Rev. A |
||||
E Series Service Manual
|
Do this... |
Observe this... |
Pass/Fail/NA |
||
|
|
|
|
|
|
2.8 |
Close the paper compartment door. |
Strip chart paper flows out of paper tray. Verify that the CHECK RECORDER |
o |
o |
o |
|
Press RECORDER button. |
message no longer displays. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.9 |
Press RECORDER button. |
Strip chart paper stops flowing out of paper tray. |
o |
o |
o |
|
|
|
|
|
|
2.10 |
Press the VOLUME softkey. |
The volume bar graph displays. |
o |
o |
|
|
|
Audible beep when the QRS wave displays. The bar graph increases on the |
|
|
|
|
To increase the volume of the beep, press the |
display indicating an increase in volume. This action does not increase the |
|
|
|
|
volume which is normal. |
|
|
|
|
|
Inc. softkey. |
|
|
|
|
|
Note: The QRS tone is on or off. There is no gradual change in volume. If |
|
|
|
|
|
|
|
|
|
|
|
|
equipped, voice prompts are gradual. Note: The voice volume has 5 settings. |
|
|
|
|
|
Setting 3 is in the mid-range. |
|
|
|
|
|
|
|
|
|
2.11 |
To decrease the volume of the beep, press the |
The bar graph decreases on the display indicating a decrease in volume. The |
o |
o |
|
|
Dec. softkey. |
volume shuts off at the last bar; otherwise, the volume is the same as originally |
|
|
|
|
|
set. |
|
|
|
|
|
|
|
|
|
2.12 |
Press the CONTRAST button. |
Contrast menu displays. |
o |
o |
|
|
|
|
|
|
|
2.13 |
Press the CONTRAST button. |
Background light and characters display. |
o |
o |
o |
|
To increase the contrast of the display, press |
The contrast increases on the monitor display. |
o |
o |
o |
|
the Inc. softkey. |
|
|
|
|
|
|
The bar graph increases on the display indicating an increase in contrast. |
o |
o |
o |
|
|
|
|
|
|
2.14 |
To decrease the contrast of the display, press |
The bar graph decreases on the display indicating a decrease in contrast. The |
o |
o |
|
|
the Dec. softkey. |
display contrast changes. |
|
|
|
|
|
|
|
|
|
2.15 |
Press the SUMMARY button. |
Summary menu displays on the monitor showing the summary report options. |
o |
o |
o |
|
|
|
|
|
|
2.16 |
Press the CODEMARKER button. |
Code marker menu displays. |
o |
o |
o |
|
|
|
|
|
|
2.17 |
Connect a/c current and install the battery. Turn |
The CHARGER ON indicator lights. |
o |
o |
o |
|
the unit off. |
The amber or green lights illuminate. |
|
|
|
|
|
|
|
|
|
|
|
Note: If both lights flash ON/OFF, the unit is defective or no battery is installed. |
|
|
|
|
|
|
|
|
|
9650-1450-01 Rev. A |
1-7 |

E Series Service Manual
|
Do this... |
Observe this... |
Pass/Fail/NA |
||
|
|
|
|
|
|
2.18 |
If applicable, connect d/c current and install the |
The CHARGER ON indicator lights. |
o |
o |
o |
|
battery. Turn the unit off. |
The amber or green lights illuminate. |
|
|
|
|
|
|
|
|
|
|
|
The yellow light indicates the battery is being charged. The green light |
|
|
|
|
|
indicates the battery is fully charged to present capacity. |
|
|
|
|
|
If both lights flash ON/OFF, the unit is defective or no battery is installed. |
|
|
|
|
|
|
|
|
|
2.19 |
Remove the battery. |
Note that both charge lights (green and amber) flash alternately. |
o |
o |
|
|
|
|
|
|
|
2.20 |
Replace the battery and the turn unit on. |
Note that the yellow charge light illuminates. |
o |
o |
|
|
|
|
|
|
|
2.21 |
Press the ANALYZE button. |
The SELECT DEFIB MODE message appears on the monitor. (For manual |
o |
o |
o |
|
|
devices.) |
|
|
|
|
|
|
|
|
|
2.22 |
Move the selector switch to DEFIB. Select 2J. |
The display shows that the unit is charging. The SHOCK button lights when the |
o |
o |
o |
|
Press the CHARGE button. |
unit is charged. Ready tone for DEFIB sounds. |
|
|
|
|
|
|
|
|
|
2.23 |
Press and hold the ENERGY SELECT down |
Unit discharges internally and selected energy decrements to 1J. |
o |
o |
o |
|
arrow. |
|
|
|
|
|
|
|
|
|
|
2.24 |
Press and release the ENERGY SELECT up |
1-10, 15, 20, 30, 50, 70, 85, 100, 120, 150, 200J. |
o |
o |
o |
|
arrow 19 times. |
|
|
|
|
|
|
|
|
|
|
2.25 |
Press the CHARGE button. |
Note the display shows the unit charged up to 200J and the SHOCK button |
o |
o |
o |
|
|
lights. |
|
|
|
|
|
|
|
|
|
2.26 |
Press the SHOCK button. |
The unit discharges and the SHOCK button is no longer lit. A 15 second strip |
o |
o |
o |
|
|
chart automatically prints, displaying the number of joules delivered (if |
|
|
|
|
|
equipped with recorder and configured to print event). |
|
|
|
|
|
|
|
|
|
Record your results on the Maintenance Test Checklist.
1-8 |
9650-1450-01 Rev. A |

E Series Service Manual
3.0 3, 5, and 12 Leads Test
Tools Needed: 3 lead, 5 lead, and 12 lead cables. Test each cable separately.
Test Setup:
1.The E Series unit must be configured to display ECG LEAD OFF message.
2.Connect the lead wires appropriate to each test to the Fluke Biomedical Impulse 4000 or equivalent (Symbio CS1201).
|
Do this... |
Observe this... |
Pass/Fail/NA |
||
|
|
|
|
|
|
3.1 |
Turn the selector switch to MONITOR. Select |
NO ECG LEADS OFF message displayed. |
o |
o |
o |
|
leads. |
|
|
|
|
|
|
|
|
|
|
3.2 |
Disconnect one lead from the simulator. |
The ECG LEAD OFF message displays within 3 seconds (if configured). |
o |
o |
o |
|
|
|
|
|
|
3.3 |
Reconnect the lead. Repeat step 3.2 with the |
Wait for ECG LEAD OFF message to clear from the display (if configured). |
o |
o |
o |
|
remaining leads. |
|
|
|
|
|
|
|
|
|
|
3.4 |
Repeat 3.2 and 3.3 for 5 lead and 12 lead |
NOTE: If heart rate alarm sounds, press and hold the ALARM SUSPEND |
o |
o |
o |
|
cables. |
button for 4 seconds to disable the alarms. |
|
|
|
|
|
NOTE: When testing the 12 lead cable, the ECG LEAD OFF message displays |
|
|
|
|
|
when you pull off a limb lead. When you pull off a V lead, the ECG VX LEAD |
|
|
|
|
|
OFF message displays where “X” is the number between 1 and 6. |
|
|
|
|
|
|
|
|
|
Record your results on the Maintenance Tests Checklist.
9650-1450-01 Rev. A |
1-9 |

E Series Service Manual
4.0 Power Supply Test (Optional)
Tools Needed:
•2 red miniature alligator to miniature alligator leads.
•1 black miniature alligator to miniature alligator test lead.
•DC power supply (15 Amp minimum).
•0.1Ω1% resistor (¼W or greater).
•1000Ω1% ¼W resistor.
•Fluke 75 multimeter or equivalent.
Test Setup:
1.Make sure the unit and power supply are turned off.
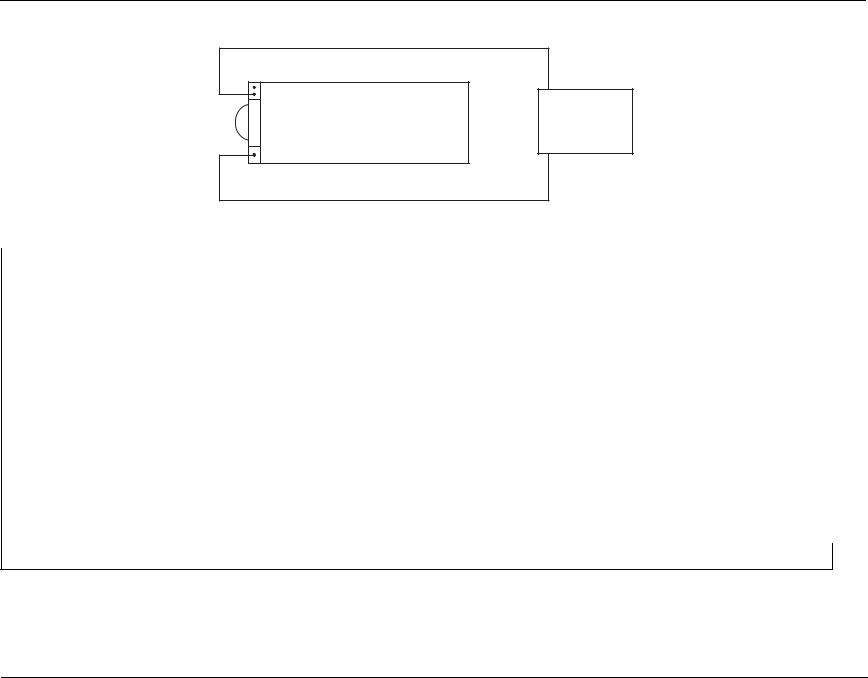
2.Connect one end of the black lead to the “-” terminal in the battery well.
3.Connect the other end of the black lead to the “-” terminal of the power supply.
4.Connect the red lead to “+” terminal socket of the battery well. Use the middle pin with the plastic guard around it. Connect the other end of the red lead to the “+” terminal of the power supply.
5.Set the power supply voltage to 7V.
Caution Be sure to connect the power supply properly to the E Series battery well terminals or damage to the unit may result. Do NOT raise the power supply voltage above 12V.
1-10 |
9650-1450-01 Rev. A |
E Series Service Manual
5HG
|
|
|
|
|
|
|
%DWWHU\ :HOO |
$PS |
|
6XSSO\ |
|
? |
? |
|
|
|
|
%ODFN |
|
|
|
Do this... |
Observe this... |
Pass/Fail |
|
|
|
|
|
|
4.1 |
Turn the selector switch to MONITOR. |
The unit should not turn on. |
o |
o |
|
(For AED units, turn the selector switch to ON |
|
|
|
|
and select Manual mode.) |
|
|
|
|
|
|
|
|
4.2 |
Turn the unit off. |
|
|
|
|
|
|
|
|
4.3 |
Adjust the power supply voltage to 10.3V and |
The unit should turn on. |
o |
o |
|
turn the selector switch to MONITOR (for AED |
|
|
|
|
units, turn the selector switch to ON). |
|
|
|
|
|
|
|
|
4.4 |
Low Battery Test |
No LOW BATTERY message displays. |
o |
o |
|
Set voltage to 9.9V. |
|
|
|
|
|
|
|
|
4.5 |
Set voltage to 9.4V. |
LOW BATTERY message displays within 30 seconds. |
o |
o |
|
|
|
|
|
4.6 |
Shut Down Voltage Test |
Unit should shut off within 30 seconds. |
o |
o |
|
Set voltage to 8.5V. |
|
|
|
|
|
|
|
|
Record your results on the Maintenance Tests Checklist.
9650-1450-01 Rev. A |
1-11 |
E Series Service Manual
Test Setup:
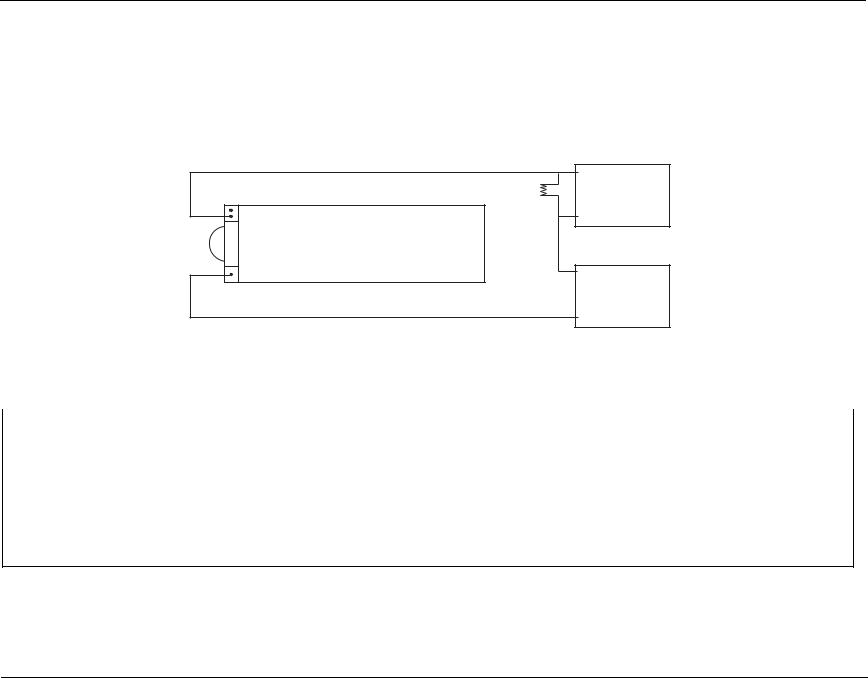
1.Remove red lead from power supply and connect to 0.1Ω resistor.
2.Connect other end of resistor to “+” terminal of power supply using a second red lead.
3.Connect multimeter across the resistor.
4.Set voltage scale (if DVM is not autoranging) to 220 mV
|
? |
5HG |
'00 |
|
|
|
|
%DWWHU\ :HOO
?
|
$PS |
%ODFN |
6XSSO\ |
|
? |
|
Do this... |
Observe this... |
Pass/Fail/NA |
|
|
|
|
4.7 |
System Current Test |
|
|
|
Set power supply to 10.3V. |
|
|
|
|
|
|
4.8 |
Turn the selector switch to MONITOR. |
Voltage across resistor should be 116 mV or less (< 1.16 A of ON current). |
o o o |
|
(For AED units, turn the selector switch to ON |
|
|
|
and select Manual mode.) |
|
|
|
|
|
|
4.9 |
Turn unit off. |
|
|
|
|
|
|
Record your results on the Maintenance Tests Checklist.
1-12 |
9650-1450-01 Rev. A |

E Series Service Manual
Test Setup for Off Current Test:
1.Remove 0.1Ωresistor and replace with 1KΩ.
2.Connect DMM across resistor.
3.Set voltage scale to DCV.
4.Measure voltage across resistor.
|
Do this... |
Observe this... |
Pass/Fail |
|
|
|
|
4.10 |
Off Current Test |
Voltage should be less than 450 mV (<450 μA of current). |
o o |
|
Measure across resistor with unit turned off. |
|
|
|
|
|
|
Record your results on the Maintenance Tests Checklist.
9650-1450-01 Rev. A |
1-13 |
E Series Service Manual
5.0 Leakage Current Test
Tools Needed: See the manufacturer’s instructions or supplied specifications for the leakage tester you use.
Test Setup: See the manufacturer’s instructions or supplied specifications for the leakage tester you use. Repeat leakage test with accessories: MFC, external paddles, and anterior/posterior paddles.
Maximum Leakage Acceptance Limits
|
Normal Condition |
Single Fault Condition* |
|
|
|
ECG |
10μΑ |
50μΑ |
|
|
|
MFC |
100μΑ |
100μΑ |
|
|
|
Earth |
500μΑ |
1000μΑ |
|
|
|
*Single fault considered AC mains on applied part.
1-14 |
9650-1450-01 Rev. A |

E Series Service Manual
6.0 Paddles Test (If applicable)
Tools Needed: None.
Test Setup: If applicable, connect the universal cable to the paddles and place the paddles in paddle wells.
|
Do this... |
Observe this... |
Pass/Fail/NA |
|
|
|
|
|
|
6.1 |
Turn the selector switch to DEFIB. Press and |
The energy selection decreases to 1J. |
o |
o |
|
hold the ENERGY DOWN button on the |
|
|
|
|
sternum paddle. |
|
|
|
|
|
|
|
|
6.2 |
Press and release the ENERGY UP button on |
1-10, 15, 20, 30, 50, 70, 85, 100, 120, 150, 200J. |
o |
o |
|
the sternum paddle for each setting. |
|
|
|
|
|
|
|
|
6.3 |
Press and release the RECORDER button on |
The recorder turns on. Press and release again to turn off. |
o |
o o |
|
the sternum paddle. |
|
|
|
|
|
|
|
|
6.4 |
Select 30J using the paddle ENERGY button. |
The unit charges to 30J, then the red LED charge indicator illuminates and the |
o |
o |
|
Press the CHARGE button on the Apex paddle. |
charge tone sounds. (Note that the front panel shock button does not |
|
|
|
|
illuminate). |
|
|
|
|
|
|
|
6.5 |
Press and release the APEX SHOCK button. |
No discharge. |
o |
o |
|
|
|
|
|
6.6 |
Press and release the STERNUM SHOCK |
No discharge. |
o |
o |
|
button. |
|
|
|
|
|
|
|
|
6.7 |
Press and hold both paddles SHOCK buttons. |
The unit discharges. The TEST OK message displays and the red LED turns |
o |
o |
|
|
off. The recorder runs. |
|
|
|
|
|
|
|
Record your results on the Maintenance Tests Checklist.
9650-1450-01 Rev. A |
1-15 |

E Series Service Manual
7.0 Heart Rate Display Test
Tools Needed:
•Calibrated ECG simulator with 60Hz sine wave output capability.
•Mini-phone plug for measuring output signal from 1 Volt ECG OUT jack (optional).
•ECG Cable (3 or 5 leads).
Test Setup:
1.Turn the selector switch to MONITOR. Press LEAD button until “I” displays.
2.Connect the ECG leads to the Fluke Biomedical Impulse 4000 or equivalent.
3.Connect the ECG cable to the unit.
|
Do this... |
Observe this... |
Pass/Fail/NA |
|
|
|
|
7.1 |
Set the ECG Simulator to 120BPM. |
The Heart Rate displays as 120 +/- 2 bpm |
o o o |
|
|
|
|
Record your results on the Maintenance Tests Checklist.
1-16 |
9650-1450-01 Rev. A |

E Series Service Manual
8.0 Calibrating Pulses on Strip Chart Test
Tools Needed: None
Test Setup: None.
|
Do this... |
Observe this... |
Pass/Fail/NA |
|
|
|
|
8.1 |
Press the RECORDER button. |
|
|
|
|
|
|
8.2 |
Press and hold SIZE button to activate the |
The strip chart displays a signal of 300 ppm with an amplitude of 10 mm +/- 1 |
o o o |
|
calibration signal. |
mm. The signal also appears on the video display. |
|
|
|
|
|
Record your results on the Maintenance Tests Checklist.
9650-1450-01 Rev. A |
1-17 |

E Series Service Manual
9.0 Notch Filter Test
Tools Needed: Fluke Biomedical Impulse 4000 (or equivalent).
Test Setup:
1.Connect the ECG cable to the Fluke Biomedical Impulse 4000 or equivalent.
2.Connect the ECG cable to the unit.
|
Do this... |
Observe this... |
Pass/Fail/NA |
|
|
|
|
9.1 |
Turn the selector switch to MONITOR mode. |
|
|
|
(For AED units, turn the selector switch to ON |
|
|
|
and select Manual mode.) |
|
|
|
|
|
|
9.2 |
Select lead I, size 3x. |
|
|
|
Select 60Hz (or 50 Hz for a 50Hz unit) on the |
|
|
|
Fluke Biomedical Impulse 4000. |
|
|
|
|
|
|
9.3 |
Press RECORDER button. |
Verify that the waveform amplitude on the strip chart is less than 1.5 mm. |
o o o |
|
|
|
|
9.4 |
Turn the ECG simulator off. |
|
|
|
|
|
|
Record your results on the Maintenance Tests Checklist.
1-18 |
9650-1450-01 Rev. A |
E Series Service Manual
10.0 Heart Rate Alarm Test
Tools Needed: Fluke Biomedical Impulse 4000
|
Do this... |
Observe this... |
Pass Fail/NA |
||
|
|
|
|
|
|
10.1 |
Turn the selector switch to MONITOR mode. |
Lead II message displays. |
o |
o |
o |
|
(For AED units, turn the selector switch to ON |
NSR ECG at 120 BPM +/- 2 displayed. |
|
|
|
|
and select Manual mode.) |
|
|
|
|
|
Connect the ECG leads to the Fluke Biomedical |
|
|
|
|
|
Impulse 4000. Set the simulator to 120 BPM |
|
|
|
|
|
and the defibrillator to lead II. |
|
|
|
|
|
|
|
|
|
|
10.2 |
Press ALARMS. |
The alarm menu displays. |
o |
o |
o |
|
|
|
|
|
|
10.3 |
Press SELECT PARAM softkey until ECG HR |
Cursor scrolls through parameters. |
o |
o |
o |
|
displays. |
|
|
|
|
10.4 |
Press INC> for state. |
Cursor scrolls through ENABLE, AUTO and DISABLE. |
o |
o |
o |
|
|
|
|
|
|
10.5 |
Press DEC>for state. |
Cursor scrolls through ENABLE, DISABLE, AND AUTO. |
o |
o |
o |
|
|
|
|
|
|
10.6 |
Press INC> until ENABLE displays. |
ENABLE displays. |
o |
o |
o |
|
|
|
|
|
|
10.7 |
Set LOW limit to 30, HIGH limit to 150 then, |
MONITOR displays. |
o |
o |
o |
|
press the RETURN softkey. |
|
|
|
|
10.8 |
Press ALARM SUSPEND button. |
No alarm sounds. |
o |
o |
o |
|
|
|
|
|
|
10.9 |
Remove a lead wire from the Fluke Biomedical |
The alarm symbol flashes and the heart symbol stops flashing. The ECG LEAD |
o |
o |
o |
|
Impulse 4000. |
OFF alarm tone sounds. Recorder prints a stripchart showing a low heart rate, |
|
|
|
|
|
if enabled. |
|
|
|
|
|
|
|
|
|
10.10 |
Reattach ECG Lead wire to Fluke Biomedical |
The alarm symbol has an “X through it. |
o |
o |
o |
|
Impulse 4000 and hold the ALARM SUSPEND |
The heart symbol flashes with each QRS wave. |
|
|
|
|
button on unit for 4 seconds. |
|
|
|
|
10.11 |
Press the ALARM SUSPEND button. |
Alarm is enabled. Alarm symbol (without “X”) displays. |
o |
o |
o |
|
|
|
|
|
|
10.12 |
Set simulator to 160 BPM or higher. |
Heart Rate Value is highlighted, alarm tone sounds, the alarm and the heart |
o |
o |
o |
|
|
symbol both flash. |
|
|
|
10.13 |
Press the ALARM SUSPEND button in the unit. |
Alarm is suspended for 90 seconds. The alarm symbol has an “X” through it. |
o |
o |
o |
|
|
The heart symbol flashes with each QRS wave. |
|
|
|
9650-1450-01 Rev. A |
1-19 |
 Loading...
Loading...