Page 1

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 1 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
REV
NO.
CHANGE SUMMARY
DOCUMENT
OWNER
APPROVER
DATE
APPROVED
112
Updated Document and Fixed Broken Links. Addressed DEKRA NC by
referencing EMG OF as part of AOF. Changed wording to aid clarity in
section 9.6 related to an old system CASPER now Siebel.
Section 8 updated to reflect relationship between AOF and EMG OF.
Section 2.2 Org Chart updated.
Section 4.2. Revised wording related to Infrastructure Org roles and
responsibilities
Kevin Fawl
(BMS Team)
Ted Tucker
13-Nov-2012
Sec#
Topic
ISO9001: 2008 Clause
Page
1
Introduction
Purpose, Background, Reference, Scope
4.2.2
3
2
Organization, Responsibility and Authority
Org. Chart
4.1, 5.2, 5.3, 5.4.2b, 5.5.1, 5.5.2,
5.5.3, 5.6.1, 6.1, 5.3
4-6
3
Business Management System Structure
Business Management System Architecture (process map)
7.2.1c
7-10
4
Business Management
Business Management System (e.g. Quality Policy, Disaster
Recovery, Quality Education, Customer Property, Required
Documents).
Strategic Planning: Acquisitions, Technology development
Management Review
Continual Process Improvement: CPI, Six Sigma
Manage Technical information: TIS
Infrastructure Organizations:
Financial Management
Human Resources
Information Technology (IT)/ ERP
WPS: Plant, Equipment and Facilities Management
4.2.3, 4.2.4, 5.3, 5.6, 6.2.2, 7.2.2,
7.4, 8.1, 8.2.1, 8.2.2, 8.3, 8.5.2,
8.5.3, 7.5.4
4.1c, 4.2.1a, 4.2.1b 5.4.1, 7.1
5.6
5.3b, 5.4.1, 8.2.3, 8.5.1
6.3c, 7.1d, 8.4, 8.5
6.0
5.1, 6.3.c
6.2
6.3, 6.4, 6.1, 6.3
6.3, 6.4
11-16
5
Marketing
Marketing Insight/Scanning
Customer Requirements Definition
Market Product Road Mapping
New Product Launch and Execution
Customer Sales Channel
Outbound Marketing
Field Training
Competitor to Competitor Analysis
5.4, 7.1
5.2, 7.2.1, 8.2.1
7.3
7.2.1
7.2.3
7.2.3
6.2.2
5.4, 8.4
16
6
Research and Development
Product Life Cycle
7.1, 7.3.2, 7.3.5, 7.3.6, 7.3.7
8.2.3, 8.2.4, 8.4, 8.5.1
17
7
Sales Management
Field Sales
Customer Contact Center
EMG Customer Data
7.2.1
7.2.2, 7.2.3
7.2.2, 7.2.3
18-20
Document Control Log
Table of Content
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 2
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 2 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
8
Order Fulfillment
Planning
Materials Purchasing
Manufacturing
Delivery
NPI/Engineering Support
7.2.1a, 7.2.2c, 7.5
7.4, 7.4.1, 7.4.2, 7.4.3
7.3.4, 7.3.6 7.5, 7.6, 8.2.3, 8.3
7.5.1, 7.5.5, 8.2.1
7.3, 7.3.4, 7.5.1, 8.2.3, 8.2.4,
8.5.2, 8.5.3
21-25
9
Customer Satisfaction
Service Solutions Unit/Service Delivery Op: Repair, Cal.
Application Engineering Organization
Professional services and support
Remarketing Solutions Division
Customer Surveys: ACS
Customer Feedback: CFS, VOC, OBD, OTD, Bluebook
Escalations: CIRF, Presidents line, Order Delivery
7.5.1, 7.5.2, 7.5.3, 7.6
6.2.2, 7.2.3
6.3c
6.3c
8.2.1, 8.4
4.1e, 5.6.2b, 7.2.3, 8.2.1, 8.4
7.2.3, 8.2.1
26-30
10
Abbreviations used in this manual
31
11
Documentation Log
32-35
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 3
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 3 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
1. Introduction
1.1 Purpose
This Manual is a summary description of the Electronic Measurement Group (EMG) and its basic policies. It
is intended to be used by all EMG employees, external temporary workers, EMG customers, and Business
Management System auditors (internal and external). This manual is intended to help EMG deploy its
policies, processes and organization to achieve its quality goals and comply with applicable regulations/
standards and understand the overall business processes. To view the EMG BMS Web site go to:
http://epsg.communications.agilent.com/quality/bms/
1.2 Background:
This Manual was written under the direction and leadership of EMG management. The owner of the EMG
Business System Manual is the EMG Business Management System Representative. The controlled copy is
located on the web: http://epsg.communications.agilent.com/quality/bms/040318_docMap.asp
Changes to this Business Management System Manual are made per the “Document Control Requirements”
process. Definitions of special terms, acronyms, and abbreviations used in the EMG Business Management
System are provided at the end of this document.
Dave Packard and Bill Hewlett formed HP in January 1939 developing test and measurement products.
Agilent Technologies was formed in 1999 as a result of a strategic realignment of Hewlett Packard into two
companies. The computing and imaging elements of the business remained with Hewlett Packard while
Agilent Technologies focused on the Communications, Electronics, Life Science and Healthcare Industries.
As of November 1, 1999 Agilent Technologies became its own company separate from Hewlett Packard.
EMG is a major group within Agilent Technologies and is a leading provider of electronic test equipment.
1.3 Scope: (4.2.2)
EMG‟s Business Management System is comprised of all the organization policies, procedures, plans,
resources, processes and the delineation of responsibility and authority, all deliberately aimed at achieving
product or service quality levels consistent with customer satisfaction and organization objectives. These
policies, procedures collectively with our quality objectives and quality policy define how EMG works and how
quality is managed. The EMG Business Management System includes EMG quality-related activities
worldwide. EMG Employees and external temporary workers follow EMG Business Management System
policies, processes and procedures. Product Conformity is measured in alignment with customer feedback
(ACS) and Agilent Technologies‟ Quality Policy. This is a Level 1 document.
EMG sites and entities are registered with, and audited by, and/or certified by regulatory standards agencies
per the agencies rules. This Business Management System Manual defines the Quality philosophy and
System in use at EMG Businesses worldwide. The businesses certified to ISO 9001:2008 are listed in the
ISO9001 certificate addenda: http://www.agilent.com/quality/EMG_ISO9001.pdf.
The EMG Business Management System complies with:
a) Agilent Technologies‟ Policies & Procedures
b) ISO9001:2008
c) ISO/IEC 17025 (EMG complies with this standard as appropriate).
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 4

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 4 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
2. Organization and Responsibility (4.1, 5.2, 5.3, 5.4, 5.4.2b, 5.5.1, 5.5.2, 5.5.3d, 5.6.1, 6.1)
2.1 EMG organization
EMG consists of:
Research and Development, Marketing, Field Sales, Quality, Order fulfillment (Manufacturing &
Procurement), Customer Services & Support and aligned Divisions.
a) EMG Customer Experience & Quality Organization consists of Customer Experience,
Quality Engineering, Quality Information, Business Management System, Education, Data
Quality, Product Solutions, Environmental Compliance, Safety & Regulations and Quality
Process. These areas provide services and tools to help EMG businesses achieve goals
and objectives.
b) Divisions include research and development (R&D) and Marketing. Some businesses
include professional services & support as required.
c) EMG OF includes manufacturing, procurement and NPI-OF areas where they assist R&D by
building prototypes and pilots of the new designs.
d) Field Operations includes Field Sales, the Remarketing Solutions Division and the Customer
Contact Center.
e) Customer Services and Support includes the calibration and repair facilities and the Service
Parts Operation.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 5
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 5 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
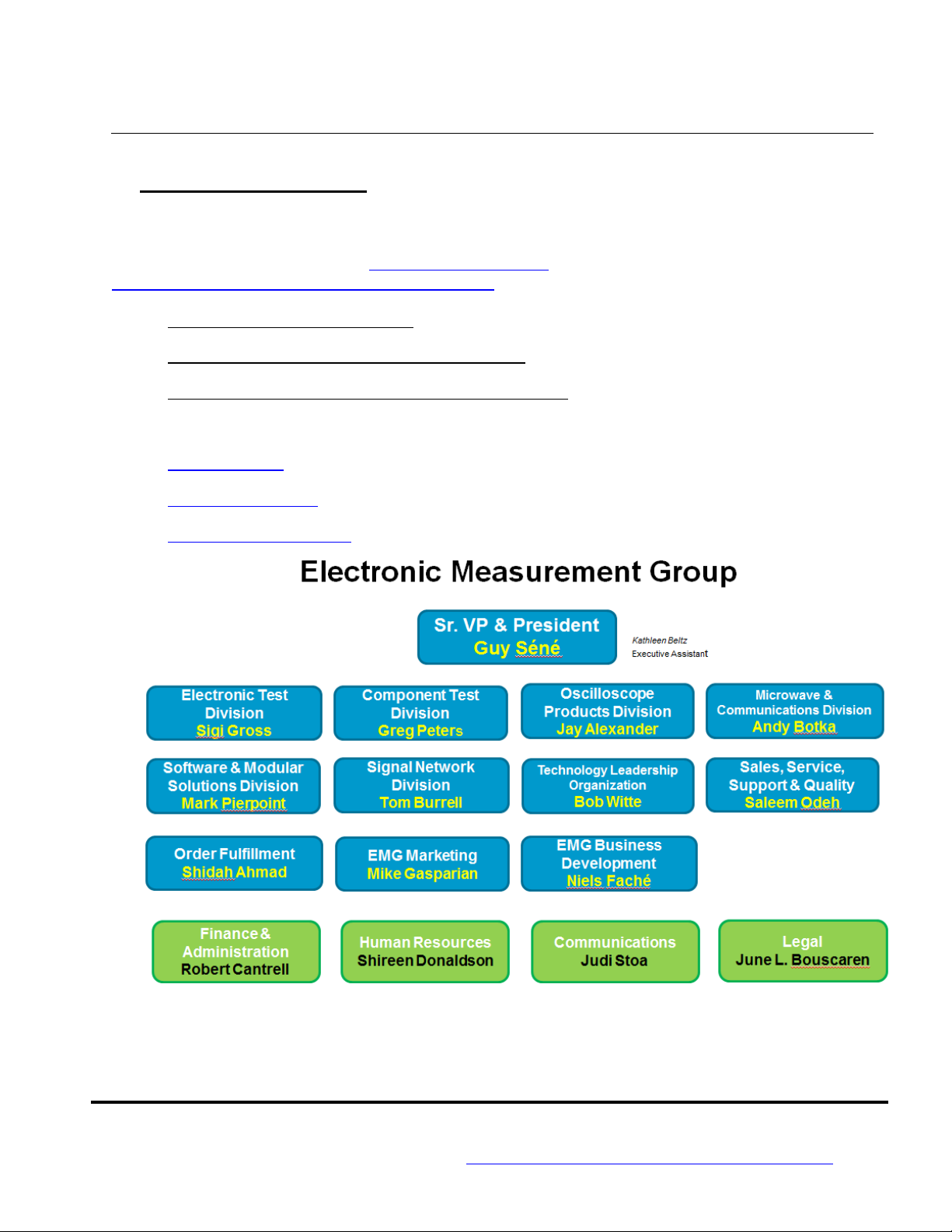
2.2 The EMG organization chart
Depicted below is the management with executive responsibility in EMG and has the authority to
establish or infuence changes to the EMG Quality Policy and Business Management System.
See the following URL for current EMG organization chart:
http://epsg.communications.agilent.com/about/org/
a) EMG President & General Manager jointly owns the BMS with the EMG VP of Customer Experience
& Quality.
b) EMG VP of Customer Experience & Quality (CE&Q) ensures EMG has a robust Business
Management System (BMS) to address customer expectations, quality and regulatory requirements.
c) The EMG Management Representative (BMS Manager) is appointed by EMG Top Management,
reports to the EMG VP of Customer Experience & Quality and has the responsibility and authority to
ensure the EMG Business Management System is effectively established and maintained per the
regulations and standards cited in ISO9001 clause 5.5.2.
d) EMG TAG Team: This team is lead by the EMG Manager Representative (BMS Manager). This team
exists to share best practices/learning, improve quality, and add rigor to the BMS.
e) BMS Representatives assist in the implementation of the BMS and are typically dotted line/support
the Business Management System Manager.
f) Site Management Liaisons are identified to assist the BMS representatives as appropriate.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 6
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 6 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
2.3 Top Management
a) Will ensure that appropriate communications are established within the organization and that
communication takes place regarding the effectiveness of the quality management system.
(E.g. coffee talks, EMG News, video clips, Quality Blue Book...etc) (5.5.3d, 4.1)
b) Will ensure communication throughout the organization regarding customer requirements.
(5.5.2c)
c) Will provide the required resources and training for implementing and maintaining the BMS
and continually improving it effectiveness. (6.1)
d) Will conduct business reviews selecting key quality and business measures to be evaluated
at least on an annual basis and maintain records and action items as per the MRP
(Management Review Process).
e) Will ensure products and services meet specifications. They ensure process effectiveness,
compliance with applicable regulations/standards, and customer loyalty.
f) Will ensure customer requirements are developed and achieved while enhancing customer
satisfaction. (Customer Focus) (e.g. ACS, CFS, OBD) (5.2)
g) Will ensure the Quality Policy meets organizational requirements, focus on improving the
effectiveness of the BMS, ensures quality objectives align with Agilent Quality Policy, and
appropriately communicate and review yearly for continuing sustainability. (5.6.1)
h) Quality planning is established to generate and plan the quality priorities. (e.g. “EMG QLT
review and implementation) (5.4.2)
i) Will ensure quality priorities are established (Measures of Success), measurable and align
with our quality policy (e.g. OBD, OTA, OTS, TAT) (5.4.1)
j) Will ensure changes to the BMS (e.g. strategy, structure, etc.) are planned and maintained
by communicating these changes appropriately (e.g. to BMS Manager, Quality Mgr., etc)
through the GM and ELT. (5.5.3)
k) EMG businesses/entities have the final responsibility and authority for their respective
products‟ design, quality, marketing, manufacturing, distribution, installation and service,
although many activities are common at the Group level.
l) Will ensure that responsibilities and authority are defined and communicated appropriately
within the organization. Responsibilities and authority can be defined written or verbally
depending on the risk and impact to the business.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 7
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 7 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Tiered
Hierarchy
EMG
Level 1
Business Management System Manual
Level 2
Required Documents: Cal Sys Manual, 6 Doc
Level 3
Entity Specific Documents/local procedures
Level 4
Records
3. Business Management System Structure
3.1 Business Management System Architecture
The EMG Business Management System is a set of requirements/policies, and procedures
designed to be effective, simple, uniform, and easy to audit. The architecture of the business
management system and its description in this Business Manual is based on ISO9001: 2008
Standards and “Process Mapping”. See below for process map.
EMG Business Management System requirements, processes and procedures are common
worldwide unless there is a compelling, justifiable, verifiable and documented rationale for
variations that have been reviewed and approved by the cognizant authority. (7.2.1c)
a) EMG Documentation is structured in this tiered hierarchy.
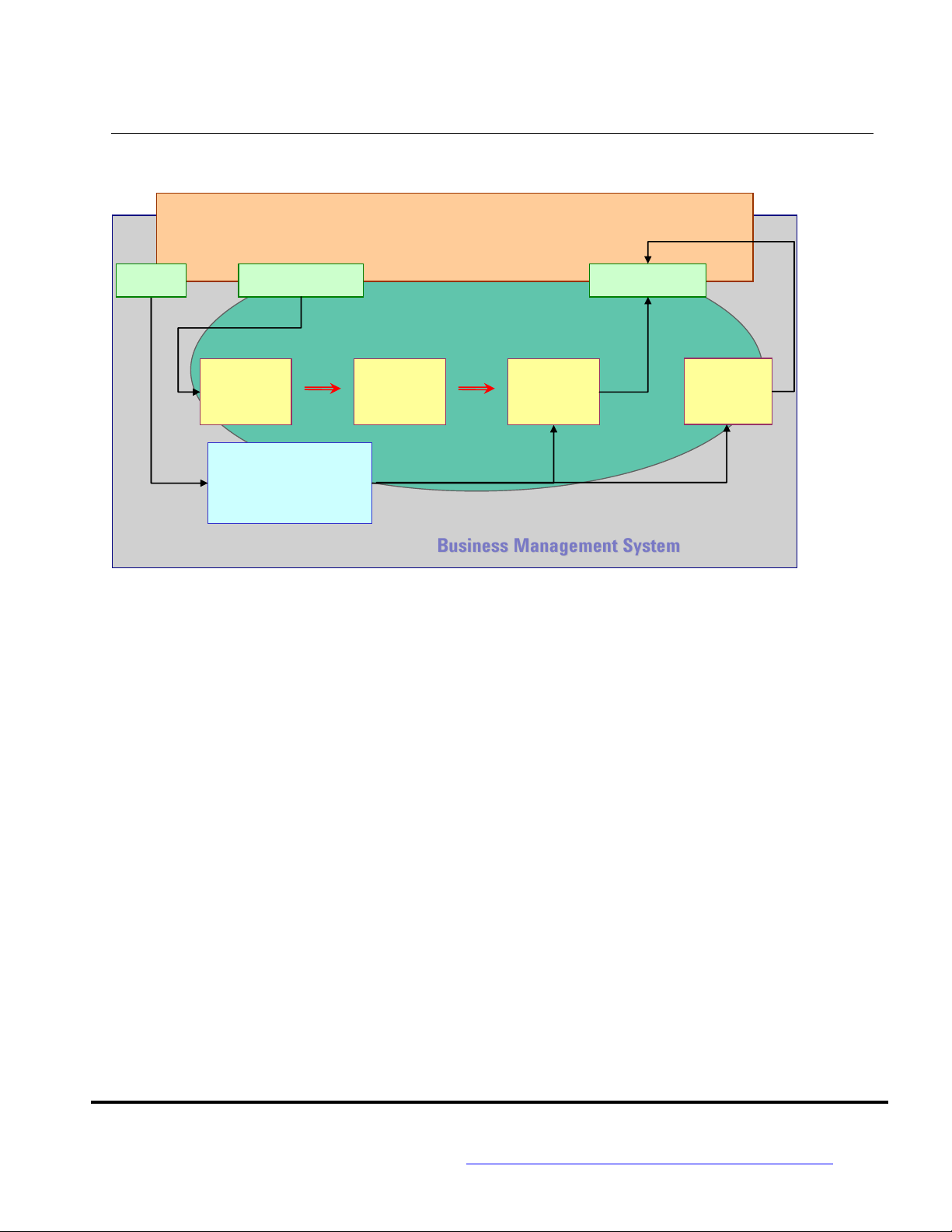
b) EMG‟s input and output map (EMG BMS Relationship Map) depicts the flow of our overall key
processes.
The details of each key process can be found in this BMS Manual and they consist of the following:
Orders: section 7.2
Marketing: section 5.0
R&D: section 6.0
Sales Management: section 7.0
Order Fulfillment: section 8.0
Service Solutions: section 9.1
Business Management: section 4.0
Improvement Activity: This touches all elements of the organization and includes such
program as Escalations, Customer Feedback, CPI, Management Review, etc. Most of
elements can be found in section 4.0.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 8
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 8 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Sales
Management
Customers
Requirements Satisfaction
Business Management System
Business Management System
Orders
R&D OF
Improvement Activity (CPI)
Service
Solutions
Marketing
Sales
Management
Customers
Requirements Satisfaction
Business Management System
Business Management System
Orders
R&D OF
Improvement Activity (CPI)
Service
Solutions
Marketing
EMG BMS Relationship Map
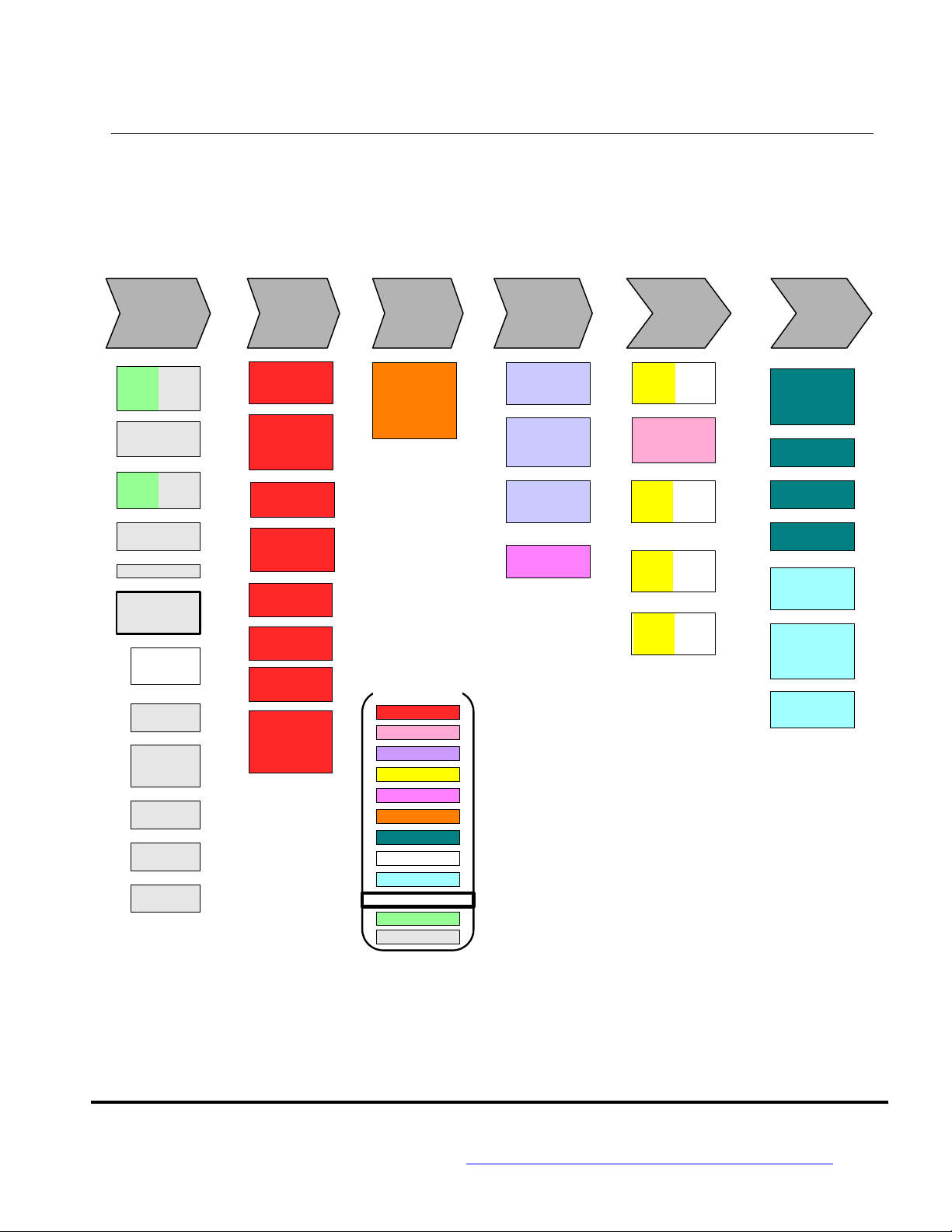
c) EMG Business Process Map:
The top Chevrons are the level-one, key processes.
The blocks below each Chevron are the level-two sub-processes.
Each level-one process is defined in this Business Manual.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 9
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 9 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Funnel &
Forecast
Management
Business
Planning and
Review
R&D
(Product
Generation)
Order
Fulfillment
Customer
Satisfaction
Management
R&D
Marketing
Materials
Sales Mgmt.
Order fulfillment
CCC
Cust. Solutions
Quality
Program Key
Marketing
Order
Generation
Business
Management
Oracle
Execute
Product
Life Cycle
Human
Resource
Management
Financial
Mgmt.
Information
Technology
WPS
Customer
Strategic Bus.
Plan
Service
Solutions
Marketing
Insight/Scanning
Escalation
Processes
Customer
Feedback
Customer
Surveys
Used across map
CPI
Sales
Management
(Order
Generation)
Market and
Product Road
Mapping
New Product
Launch and
Execution
Outbound
Marketing
Field Training
Customer
Requirements
Definition
Competitor to
Competitor
Analysis
TIS
Planning
Customer Sales
Channel
Customer
Contact
Center
Materials
Procurement
Business Mgmt
System
Management
Review
Enterprise
Resource
Planning
Remarketing
Solutions
Infrastructure
Organizations
Professional
Eng. Services
Custom
Solutions
AES
Delivery
Manufacturing
NPI
EMG Business Process Map
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 10
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 10 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
4. Business Management
Business Management contains many high level functions to run our organization. These functions
act as a resource while providing support and direction and are critical to success of our overall
business. EMG adheres to Standards of Business Conduct:
http://sbc.corporate.agilent.com/ch1.htm
4.1 Business Mgmt. System (4.0, 4.2.3, 4.2.4, 5.3, 5.6, 6.1, 6.2.2, 7.2.2, 7.4, 7.5.4, 8.2.1, 8.2,2,
8.3, 8.5.2, 8.5.3)
EMG implements a business management system that ensures customer requirements are fully
met through the consistency in execution and maintenance of our internal operations, which directly
affect EMG‟s ability to produce high quality products and services.
4.1.1 Quality Policy (5.3)
EMG adheres to Agilent Technologies Quality Policy, which can be reviewed at:
http://emg.communications.agilent.com/quality/policy.asp
All EMG managers and employees support the implementation of this Quality Policy in accordance
with their roles and responsibilities in the organization.
4.1.2 Quality Education: (8.5.1, 8.2.1, 5.3b)
EMG Quality Education has the role of establishing the standard course requirements for the
Quality and Six Sigma classes to ensure consistent format and delivery to employees across the
businesses. The standard course requirements include:
Clear Learning Objectives
Available Resources
Employee Learning Assessment
Clear Linkage to Strategic Initiatives and Business Objectives
EMG Quality Education has the responsibility of working with the business units to ensure each
course will build awareness or skills in Quality/Six Sigma which can then be utilized by a business
to achieve their business objectives.
EMG Quality Education measures of success are based on the number of seats in the courses
delivered to EMG employees. Quality of the course, instructor where applicable, and the delivery of
the course is evaluated based on the employee assessment.
http://emg.communications.agilent.com/quality/education/default.asp
4.1.3 Measurement analysis improvement (8.1)
EMG has implemented monitoring, measurement, analysis and improvement of processes and
tools at the appropriate levels to demonstrate conformity, ensure conformity, and continually
improve the effectiveness of the BMS (e.g. CFS; Quality Bluebook; OBD).
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 11

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 11 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
4.1.4 Customer Property
EMG exercises care with customer property while it is under the organization‟s control or being
used. Each area will use local processes to record and track the status of customer property in the
rare instance when EMG receives customer products or property. If customer property is lost,
damaged or otherwise found to be unsuitable for use, this is reported to the customer and records
are maintained locally. (Examples of customer property: government contracts, special handling
specification, product or test equipment).
4.1.5 Core Documents/Processes
EMG‟s quality management system is defined and shown in the EMG Process Map. Details of
specific processes we are required to use can be found in the EMG Documentation in Webdoc or
the Agilent Technologies web site.
http://epsg.communications.agilent.com/quality/bms/040318_docMap.asp
These include-
EMG BMS Manual epsg1026386
Management Review Requirements E106 (5.6) epsg1028737
Management Review Guide
Corrective and Preventative Action Requirements E101 (8.5.2, 8.5.3) epsg1028733
Quality Assessment Program Manual E102 (8.2.2) epsg1028734
Documentation Control Requirements E100 (4.2.3) epsg1028732
Training Requirements E105 (6.2.2) epsg1028736
Control of non-conforming Product/Process Requirements E103 (8.3) epsg1028735
Control of Quality Records Requirements E104 (4.2.4) epsg1028767
EMG ESD Control Manual epsg1039112
Calibration Requirements E108 (7.6) epsg1033182
Calibration System Manual epsg1024153
Measurement Uncertainty Validation Process epsg1059922
EMG Calibration Policy epsg1033182
Option 1A7 & A6J Introduction Guide epsg1038158
HWTC Manual epsg1075658
Anti-virus requirements epsg1115641
The Disaster Recovery Processes/plans can be found at:
http://finance.agilent.com/agrm/organization/index.htm Disaster Recovery (Finance)/Agilent Risk Management.
http://wps.service.agilent.com/drp/site_index.htm -Disaster Recovery (WPS)
http://one.it.agilent.com/security/programs/drp/ - IT Disaster Recovery Plans
CIR (Customer Issue Resolution) Process can be found at: http://emg.communications.agilent.com/quality/cirf/
Learn more about the CIR process in section 9.7 of this manual.
Other Business Management System information can be found at:
http://legal.agilent.com/rim/index.shtm-General Retention Schedule
http://sharedoc.collaboration.agilent.com-Sharedoc
http://epsg.communications.agilent.com/quality/bms/040318_docMap.asp -Documented procedures
http://www.agilent.com/quality/qualityman.pdf -Agilent Quality Manual
http://qes.supplychain.agilent.com/ -Agilent Quality Website
Design and Manufacturing Document List
Design Standards Users Group
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 12

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 12 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
4.2 Strategic Planning (4.1.c, 4.2.1a, 5.4.1, and 7.1)
The strategic plan is developed at the EMG level encompassing all business processes and stating
the direction, leadership, and transformation required to meet specific goals. EMG will improve
customer satisfaction and earn unrivaled loyalty through personalized customer relationships.
Capitalize on our product leadership. Create customer value and intimacy through innovative
systems, high-valued services and support solutions and accelerate EMG business process
transformation.
EMG has yearly strategy reviews at the Group level to ensure plans are consistent with changing
market conditions. EMG focuses on customers, competitors and our offerings. After the strategy
review, our annual financial plan is developed (SCALE: short term commitment and long term
estimate) using the SPR (strategic plan review) as the baseline for profitability and ROIC (return on
invested capital). Tactical reviews are held quarterly to review financial results, NPI revenues,
market share, customer satisfaction, and employee satisfaction.
4.2.1 Acquisitions:
a) Corporate Development (CD): Supports the ongoing assessment of Agilent‟s enterprise business
portfolio to identify candidates for active abandonment or investment. CD insures strong links
between the enterprise and Group strategies, focusing Agilent‟s overall prospects for top line
growth and value creation. This site is designed to support the needs of those involved in
transactions, integration, strategic planning, and new ventures within the businesses and corporate
functions. http://corpdev.agilent.com/
b) EMG has developed an Acquisition Lifecycle (ALC) process that provides the EMG business with
additional acquisition support not specifically covered by corporate development personnel and
processes. ALC Web site, ALC Standard
4.2.2 Discontinuance/Obsolescence strategy is determined in each business. The EMG
Discontinuance Plan is the key document that is used to plan and execute the discontinuance and
final obsolescence of products at the end of their lifecycle. The Discontinuance Plan is split into
several sections, and a cross-functional team is required to address these sections effectively.
The Corporate Discontinuance Process can be found at:
http://wcosedoc.cos.agilent.com/stellent/groups/plc/documents/end_users/019602.doc
4.2.3 Technology Development: (7.1)
a) The Technology Leadership Organization (TLO) as the central technology organization, leads
the creation of EMG Strategy, makes R&D portfolio decisions, delivers breakthrough technology
with clear competitive advantage, executes EMG R&D programs, guides EMG level
architecture, and manages and improves EMG R&D Processes.
http://emg.communications.agilent.com/tlo/
b) EMG also links to Agilent Labs to identify and transfer breakthrough technologies into our
businesses. http://web.labs.agilent.com/
4.2.4 General Outsourcing Requirements
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 13

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 13 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Outsourcing includes: 1) the decision to purchase a product rather than make it internally; 2) the
subsequent selection of a qualified supplier; and, 3) the management of the on-going relationship
with the supplier. The materials purchasing process description in Section 8.2 applies after the
decision, often called the “make versus buy” decision.
The General Manager of each business is ultimately responsible for the decision to outsource any
product in their business that significantly affects conformity with customer, stakeholder, and
regulatory requirements. Once the decision to outsource is made, responsibility for control falls on
the immediate management of the operation responsible for the product, and the supply-chain
management professionals that assist them. The degree of control for the outsourcing depends on
the significance of the outsourcing on product conformity and whether the outsourcing concerns a
new or existing product.
The EMG Product Lifecycle (PLC) process establishes the recommended management
checkpoints and basic considerations for the control of product lifecycle transitions, including
outsourcing for new product introductions and manufacturing, if the new product is taken to market.
The PLC process description is found at URL: http://sharedoc.collaboration.agilent.com/sites/EMG-
PGE/SD/EMG%20PLC/EMG%20PLC%20Rev%205.0/emg_plc_process%20%20epsg1024851.htm
A complementary general framework for the selection and management of EMG contract
manufacturers is found in here: #epsg1043961. Outsourcing procedures are determined at the
local level and typically described in the Quality Procedures Manual and/or other local process and
procedures.
In some cases strategic suppliers are shared across local operations in EMG or its business
divisions. In these cases, one entity may be tasked with managing all or part of the outsourcing on
behalf of the others. The Strategic Supplier Management team is an example. They manage the
overall business relationship with EMG‟s top-level strategic contract manufacturing and component
suppliers.
4.3 Management Review (5.6)
Top management for the business, reviews selected key quality and business measures to be
evaluated on at least on an annual basis and conducts a comprehensive quality review annually.
The Business Managers have the ultimate responsibility for ensuring regular management reviews
are conducted for their organization. As a result of such reviews, changes may be made to any
aspect of the quality management system including the quality policy and/or objectives to improve
suitability, adequacy or effectiveness.
EMG level Management Review
Management Review Process
4.4 CPI (5.3b, 5.4.1, 8.5.1, 8.2.3)
Continual Process Improvement in EMG is based on Management by Objectives. The EMG Quality
Manager and the Executive Leadership Team establish business-wide quality improvement
objectives that align with Agilent Technologies‟ Quality Policy. Objectives are assigned to owners
who are accountable to the Leadership Team for successful accomplishment of the objectives.
These objectives are communicated to all levels of the EMG organization. At each level, plans are
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 14
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 14 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
developed in support of the business-wide objectives, as appropriate, depending on the relevance
of the objective. Periodically, and during management reviews, progress toward completion
measures are reviewed and evaluated by the Executive Leadership Team and course corrections, if
necessary, are initiated. Other process improvement objectives may be established at EMG sites or
organizations and will be managed and monitored by the site/organization management. These
objectives will be based on local needs, criticality, and resource capacity.
a) EMG monitors and measures processes during internal audits, management reviews and
continually improve processes. (8.2.3)
b) Data analysis‟s is performed throughout our business. (e.g. CFS, Blue Book, TQRDCE, RIP,
QIC, MST).
c) Six-Sigma has been adopted by EMG world-wide as a data driven tool for improving the quality
of products and processes and improving business results. The Six Sigma program provides a
highly structured set of tools and methodologies that are applied to a variety of business
processes as appropriate to achieve breakthrough results. EMG Organizations can apply Six
Sigma to R&D, product design and development, manufacturing, sales, services, and support
functions to reduce process complexity, variation, and cost, resulting in increased customer
satisfaction and the elimination of defects. For more information about our Six Sigma program
click on the following URL‟s.
Agilent : http ://sixsigma.quality.agilent.com
Training: http://qes.supplychain.agilent.com/Global_Learning/Index.asp
4.5 Technical Information Systems (TIS): (7.1d, 6.3c, 8.4, 8.5)
Local TIS and the WW EMG BTT TIS Team (Global TIS) are responsible for the management and
implementation of new setups and changes per input from the formal processes including
ECR/ECO and approved Change Requests (CR‟s). TIS creates and maintains the bill of materials
in support of Matrix One and Oracle for the design and manufacture of our products. For more
information click on the following link: http://gtis.is.agilent.com/global/
4.6 Infrastructure Organizations (6.0)
To maximize EMG‟s businesses ability to develop and deliver high quality products and services,
certain infrastructure organizations are in place to deliver internal support services. EMG collectively
refers to these as infrastructure organizations. These organisations are managed at the Agilent
Level and are not subject to EMG Internal audit (findings will be reported if found during an audit
trail) or external audit, but are audited at the Agilent Level to ensure conformance to ISO
9001:2008. EMG shall perform periodical review with Infrastructure organizations to ensure that
they meet EMG BMS needs. Infrastructure web site.
4.6.1 Financial Management (5.1)
Finance‟s primary contribution is business planning and business analysis for decision making.
EMG provides timely, actionable information related to business reporting of process performance.
We ensure sound business controls. EMG publishes a monthly Group Financial “Blue Book” to
report financial, operational performance details and to take action on decisions to improve
operational and financial performance. EMG Finance Web site: http://finance.agilent.com/EMGfin/
Global Financial Service Description can be found at:
http://customer.quality.agilent.com/qual_mgt_systems/serv_descript.shtml
Business Continuity Plan: http://finance.agilent.com/agrm/bcp/
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 15

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 15 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Global Financial Services (GFS) provides efficient, compliant global financial services enabling
businesses to focus on their objectives. The primary areas of responsibility include process and
operations for sales accounting, collections, general accounting, financial planning and controls and
country operations. Accounts Receivable and Collections process maintains short collection
periods, minimizes bad debt through collection efforts, minimizes potential disputes, monitors
customer pay trends, and educates customers on payment terms of the invoice. This function is
managed at the Agilent level.
Agilent Marketing Policy and Contract Solution Organization are responsible for creating contract
documents and terms, which balance Agilent requirements, and customer needs and are
appropriate for the specific business or industry.
The Agilent web site: http://customerfirst.corporate.agilent.com/Contracts/Index.shtml
4.6.2 Human Resource Management (6.2) (HR)
HR‟s role is to be the architect of organizational capability and human potential. HR‟s contributions
aim to increase the productivity and effectiveness of individuals and teams in order for EMG to meet
and exceed business objectives. HR has three primary goals: Build a stronger deeper leadership
bench; Re-enforce EMG as a best place to work; create a simpler, best in class HR function and
HR services. EMG believes, by attaining these goals that HR will create a competitive advantage
for EMG through people practices and a high-performance culture. Success is monitored quarterly
and measured annually at the Group level via external and internal metrics and surveys focused on
our three primary objectives.
HR Web site: http://EMG.communications.agilent.com/toolkit/Decisions/dec35.asp
Click here to see the HR‟s Service Description.
4.6.3 Information Technology (IT) (6.3, 6.4)
IT ensures delivery of all information technology services to enable EMG to be a high growth, high
performance company. Business fundamentals measure the success of IT results.
Reference Web Sites: IT Web site, IT Service Description.
Enterprise Resource Planning (ERP) (6.1, 6.3)
ERP systems (part of IT) integrate departments and functions across the company into single systems that
serve all those different departments and their particular needs. EMG utilises Oracle‟s Enterprise Resource
Planning system as the cornerstone of EMG‟s business process reengineering and transformation initiative.
The documented processes are located in the Knowledge Portal at:
http://knowledgeportal.corporate.agilent.com/
4.6.4 Work Place Services (WPS) Plant, Equipment and Facilities Management (6.3, 6.4)
The Global Work Place Services organization ensures that the facilities used by the Businesses
meet EMG‟s and government standards for safety, that environment regulations are met, and that
the physical plant is adequate to meet the needs of the other functions in performing their
responsibilities. Click here to see WPS Service descriptions manual.
WPS web site can be found at. http://wps.service.agilent.com/
EMG is registered to ISO14001. The Environmental Management System web site can be found at:
https://wps.service.agilent.com/global_ehs/
There are many Health and Safety programs in place within EMG. The Environmental Health and
Safety web site can be found at: https://wps.service.agilent.com/global_ehs/Ergonomics
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 16

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 16 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
5. Marketing
The marketing department has the responsibility to ensure that product designs are based on an
understanding of markets and customer needs and to ensure that the right product reaches our
customer, through the relevant sales channel. Success is measured monthly at the Group level
through orders received. (5.4, 7.1, 5.2, 6.2.2, 7.2.1, 7.2.2, 7.2.3, 7.3, 8.2.1, 8.4, 8.5)
a) Marketing Insight/Scanning (inbound): Monitor market dynamics and identify new opportunities,
technologies, and standards. This also includes determining market size and short/long term growth
rates. (7.2)
b) Customer Requirement Definition (Inbound): Identify key customer requirements and market windows
in targeted market segments. Includes prioritization of these requirements from most to least important.
Meet customer‟s unstated requirements. (7.2.1, 5.2, 8.2.1) EMG Requirements Management web site:
http://www.soco.agilent.com/org/pge/reqman/overview.htm
c) Market and Product Road Map (Inbound/Division): Translate customer requirements into Market
roadmaps and then into multiple product and service roadmaps. The objective of these roadmaps is to
define products and services that meet customer requirements, at the right time, with competitive
differentiation. (7.3)
d) New Product Launch and Execution (Division): Establish new product positioning; launch strategy,
objectives and tactics (pricing, product structure and configuration rules). Set launch criteria. Execute
launch per plan (on time and within budget). (7.2.2)
e) Customer and Sales Channel Support (Division): Provide phone and electronic support to customers,
our sales and service teams. Support content includes technical product and applications insight,
handling of some competitive situations or customer satisfaction issues, and post-sales support of our
customer‟s products. (7.2.3)
f) Outbound Marketing (Outbound): Identify hot customer applications and problems. Set Strategy,
Objectives and tactics that proactively generate awareness, leads, and move customers through the sales
funnel. Vehicles include PR, Advertising, Application notes, technical web and face-to-face seminars,
catalogs, tradeshows, customer visits, special promotions, e-mail notification, etc. Keys to success
include identification of a hot topic, and development of an integrated program tightly linked to the sales
channel. (7.2.3)
g) Field Training (Outbound/Division): Identify and deliver training to EMG and Partner Sales and support
teams on products, application, competitive and service information. (6.2.2)
h) Competitor to Competitor Analysis (Inbound/Division): High level analysis of our key competitors‟
strategies and detailed analysis of their products and services (as they compare to EMG‟s) (5.4).
Marketing Policies: All of the Marketing Policies have been developed for EMG in order to:
Define company or business group wide principles for conducting business with our customers
Establish overall standards of performance and control
Provide a framework for the Business Operations to implement marketing strategy and establish
operational policy.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 17

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 17 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
6. Research and Development
R&D and Divisions have the responsibility of designing products to meet customer requirements
and Agilent design and quality standards. (7.1, 7.3.2, 7.3.5, 7.3.7, 8.2.3, 8.2.4, 8.4, 8.5.1)
6.1 Product Life Cycle (PLC):
The EMG Product Lifecycle (PLC) is a phase review process for cross-organizational and cross
business teams from the point where product development resource involvement begins (CON =
concept) through eventual product obsolescence (CLO = closure)). The PLC focuses on a series of
checkpoints and milestones, along with a specific set of deliverables for each. The PLC embodies
the Shewhart Cycle (Plan, Do, Check, & Act)
The guidelines established here are to set and communicate quality objectives, expectations, and
responsibilities of each organizational area at each phase of product lifecycle culminating in Quality
Sign off** (click on QSO form checklist).
EMG PLC web site: http://www.soco.agilent.com/org/pge/PLC/plc.htm
**Note: Quality Sign-off confirms that the requirements for 1) products regulations, 2) environmental
test, 3) reliability and accelerated life testing, 4) specifications/DFx /Quality Objective setting, 5)
product stewardship 6) Whole Product Support Plans.…etc. have been met.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 18
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 18 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
7. Sales Management
7.1 Field Sales (5.4, 6.2, 7.1, 7.2.2, 7.2.3, 8.2.1)
The purpose of the sales department is to grow orders for EMG within a given cost envelope. The
department applies EMG capabilities in test and measurement to help our customers improve their
business results while maximizing orders for EMG. Working together across functions in the field
and field/factory, EMG create solutions specifically tailored to achieve maximum customer
satisfaction. Customer Loyalty has always been and continues to be our most treasured asset. We
envision being known as the company that it most committed to exceeding customer expectations,
thereby earning their long-term loyalty. Key Metrics: Orders, Expenses, Funnel, Loading, and
Customer Satisfaction.
a) Order Generation: (7.2.1) This process is one of the core customer facing processes, and
includes Account Management, Opportunity Management and Deal Management utilizing web,
phone and face to face (the sales process).
b) Funnel and Forecast Management: (7.2.2) this is the core process for the monthly management
of the Order Generation Process.
c) Business Planning Review: (7.2.3) this process involves all activities related to strategic and
tactical business planning, organizing, and deploying human and other enabling resources for
on-going sales operation and order generation.
d) SSSQ website: http://emg.communications.agilent.com/wwfops/
7.2 Customer Contact Centers
EMG‟s Customer Contact Centers (CCC‟s) are responsible for management of customer
interactions from quotation to invoice processes while adhering to the quality management system.
CCC‟s are a Field Operations entity within EMG SSSQ (Sales, Service, Support and Quality) with
demarcated global presence into four major regions (ie: Americas, Asia Pacific, Europe and Japan).
In each region the Business Centers and Contact Centers make up a CCC regional unit.
The CCC organizations typically consist of four core functions:
- First Contact (FC): Primary contact for all customer interactions with the goal to resolve a
maximum proportion of requests without further handoff.
- Customer Relationship (CR): Manages orders, customer escalations and issue resolution. CR
is an end-to-end bridge between FC and CS.
- Commercial Services (CS): Focuses on non-verbal customer transaction management such
as quotations, order entry and invoicing.
- Contracts Administration (CA): Creates and administrates customer Purchase Agreements
and structuring of pre-sales contracts strategies.
Each region has typically one Business Center focusing on back-office tasks and one to several
Contact Centers focusing on customer interactions.
This model is followed globally except where specifics of each region are taken into account to
better suit customer requirements within that region.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 19

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 19 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
7.2.1 Customer Interaction Management
Customer Interaction processes are managed by First Contact and Customer Relationship teams.
First Contact‟s focus is on the prompt fulfillment of any customer inquiry. Any special customer
requests or resolution of customer issues are managed within the Customer Relationship Team.
The First Contact team is responsible for all initial voice (phone) and non-voice (e-mail, fax, and
web) interaction with customers and business partners. Their objective is to qualify and fulfill the
requests in an expedient manner or when appropriate transfer the request within CCC or to
business partners like sales, technical support or to another business organization for further
resolution. Key FC responsibilities is to help customer in checking product availability or service
capability, providing information on marketing promotions, checking customer entitlement for
service repairs and calibration, and providing order and service status. In general, FC takes
ownership of customer issues to satisfy/delight customers by providing real time help and
responses to a wide variety of customer requests.
The Customer Relationship team is responsible for managing customer interactions that require
escalations. They also make outbound communications related to the management of sales,
support orders and agreements, negotiate changes on orders or service delivery schedules, and
initiate Service Recovery and Customer Feedback.
7.2.2 Lifecycle Management of Orders
The Lifecycle Management of Sales and Service Orders is managed by Commercial Services and
the Customer Relationship teams. Commercial Service‟s focus is on the efficient lifecycle
management of orders. Any exceptions, specific customer requests, and changes in requirements
are owned by the Customer Relationship team.
Job specialization within the Commercial Services team allows focused management of customer‟s
orders:
1. Sales Order Management – Management of sales order lifecycle for hardware and software
products, parts and services sold upfront (e.g. extended warranty, training, consulting). Key
processes performed include quoting, booking, and acknowledgement of customer‟s orders
using the ERP system, fulfilling deliveries, order changes, cancellations, product returns,
corrective transactions and billing.
2. Service Administration – Management of service orders. Service orders are primarily related to
the maintenance, system uptime support, and repair of equipment. Service Administration is
comprised of two functions – Support Agreements Administration and Support Order
Management.
Support Agreements Administration – responsible for the order lifecycle of Agreements for
maintenance, system uptime support, software, and repair of equipment. Key processes
performed include quoting, placing Agreement orders, managing frequency of invoicing,
modifications and cancellations, and Sold Upfront Tracking into the Customer Service System
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 20

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 20 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Support Order Management – responsible for management of the lifecycle of per-incident Trade
and Non-Trade Support Orders from quote to invoice. Key processes performed include quoting,
placing orders into Customer Service System, follow-up on open orders, issue resolution,
invoicing and corrective transactions.
7.3 EMG Customer Data
The EMG Customer Data Team is responsible for managing EMG‟s transactional customer database, which
presents an accurate global view of customers EMG does business with and their company structures. The
team ensures that EMG can derive legal compliance, effective risk management, and operational efficiency
from the customer database through a holistic approach to customer record maintenance. The EMG
Customer Data Team holds the ownership for customer data standards for all EMG customers
worldwide.http://csbarcelona.europe.agilent.com/custmast/Index.htm
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 21

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 21 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
8. Order Fulfillment
EMG Order Fulfillment (OF) is a constituent part of Agilent Order Fulfillment (AOF). The ultimate
vision for the function is “To build a best-in-class order-fulfillment and supply-chain function with
strong gross margins and high customer satisfaction”
EMG OF Org Chart –
http://ofsc.business.agilent.com:81/About%20AOF/EMG%20OF%20Org%20Chart%20(F)Feb.pdf
8.1 Planning (7.2.1a, 7.2.2c, 7.5)
8.1.1 Demand Planning (forecasting) (7.2.1a, 7.2.2.c)
Demand Planning can be one using statistical forecasting tools or sales forecasting and creates a
plan for future customer orders. Forecast accuracy is measured and utilized to improve the
demand plan and processes. Demand planning includes the process to generate product plans,
schedule orders into the manufacturing system (ensuring proper staffing).
8.1.2 Supply Chain Planning and Scheduling (7.4)
Supply Chain planning creates a supply plan that considers supply chain constraints
(i.e., inventory, lead times, etc) and forecasted customer requirements and actual orders to create
and execute shipment plan. Customer orders are promised against the supply chain plan and in
some businesses against customer/service delivery schedules.
8.2 Materials Purchasing (7.4, 7.4.1, 7.4.2, 7.4.3, 7.5.5)
Materials purchasing management in EMG is led out of the manufacturing divisions. Purchasing
departments typically are organized as a function reporting to business unit sub-division
management. The Strategic Supplier Management (SSM) assists the businesses with managing
alliances with top-tier strategic suppliers. The EMG Worldwide Field Operations (WWFO)
organization, which manages sales and support, generally uses the BU organizations for its
materials purchasing needs. The most senior Managers with Procurement responsibility within
the manufacturing divisions are responsible for making sure supplier contracts are complete,
supplier performance reviews are done on time, and performance issues are resolved in a timely
manner.
Four generic processes are used to manage supplier quality:
1. Requirements Specification;
2. Supplier Selection;
3. On-Going Conformance Assurance; and,
4. Performance Evaluation
8.2.1 Requirements Specification
The BU purchasing operations specify the product to be purchased in accordance with the needs of
the operation and the needs of the ultimate Agilent customers. Customer requirements flow into the
purchasing process through the requirement specifications of the manufacturing entity sourcing the
material. Purchasing staff assembles the requirements to be tendered to the supplier, reviews them
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 22

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 22 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
with the division personnel needing the materials, in order to ensure accuracy, and then
communicate the requirements to the supplier.
Quality requirements specifications consist of:
Standard purchase-order and purchase-agreement information that references company-wide
quality requirements, including reference to Agilent‟s Supplier Quality System document 5951-
1665. http://www.agilent.com/quality/supplier_quality_system_rqmt.pdf and ESD requirements.
Business-specific standard terms and conditions, if any are developed at the division level; and,
Specifications dictating the product form, fit, function, etc. and any other specific requirements for
approval of the product, procedures, processes, and equipment.
Current document retention standards only require controlled retention of quotations,
contracts/agreements, confidential non-disclosure agreements, and licensing agreements accepted
from/with suppliers. Where records are retained, it is by the buyer/manager of the supplier and/or
their extended team of internal customers using the supplier. (7.4.2)
8.2.2 Supplier Selection
Suppliers are selected by personnel from the operation where the purchased product will be used,
usually in partnership with materials procurement personnel. Suppliers are asked for information
relative to desired requirements. The type and extent of qualification depends on how critical the
product and supplier is to the continuity of order fulfillment, and the form, fit, and function of the
ultimate product. Where qualification is done it usually consists of ordering and inspecting
prototypes or samples. The entity that establishes the relationship with a new supplier, and/or for a
new item to be purchased, is responsible for maintaining the records from the selection and/or
qualification process, including the criteria and management approval in accordance with the
entity‟s sourcing requirements. (7.4.1)
8.2.3 On-Going Conformance Assurance
The process for ensuring that the purchased product meets the specified purchase requirements is
implemented locally, within the framework of applicable company, BU, and divisional processes and
practices. In general, incoming material shipments are checked to see if any special handling or
inspection is required, otherwise it is assumed fit for use and delivered to the user or storage area.
Material requiring inspection is flagged in the material & order management system. Material not
requiring inspection is delivered to its storage location, which is normally at the point of use. FGI
(finished goods inventory) handling procedures may vary from site to site. If verification is
performed at the supplier‟s location, the purchase order or Agreement will state the arrangements
and method of release. The usual method is to request suppliers to provide outbound quality
control testing reports. Materials procurement personnel usually manage the resolution process if a
supplier fails to meet requirements. (7.4.3)
8.2.4 Performance Evaluation
Supplier performance is managed in real time through the escalation of any observed issues
concerning the delivery, form, fit, and function of the item. In some cases, issues are tracked online,
such as on-line part failure history tracking and engineering alerts. Materials procurement personnel
typically act as the focal point for supplier quality issues. In addition, there is a guideline for
evaluating how well strategic suppliers (critical to business) have met requirements. The technology
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 23

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 23 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
quality, responsiveness, delivery, cost, and environment called TQRDCE. Company-wide guidelines
for the TQRDCE process may be found on this Global Sourcing TQRDCE Site:
http://gsce.supplychain.agilent.com/direct_matls/tqrdce.asp
The businesses determine which suppliers are subject to periodic performance evaluation and
maintain the records from such evaluations
Examples of Strategic Supply Management in EMG: SSM has a supplier performance
measurement survey tool that allows users to enter input and access results. The survey tool,
results, calendar and other SSM TQRDCE information can be found at this link:
http://emg.communications.agilent.com/ssm/
Examples of key metrics: Material cost reduction as a percent of spend, inventory levels in days of
supply on hand, on-time delivery in days early or late, and defect rates in parts per million,
percentage of parts delivered over time, or number of dead-on-arrival (DOA) products.
8.3 Manufacturing (7.3.4, 7.3.6, 7.5, 7.6, 8.2.3, 8.3)
EMG OF manufactures or manages the manufacture of the vast majority of EMG products and has
the responsibility to ensure that products are manufactured to EMG‟s manufacturing and quality
standards and supplied to the customer in a timely, cost-effective manner. EMG OF also provides
replacement assemblies to EMG Service Centers & customers and also performs factory calibration
of customer instruments when required. Example of key metrics: Quality, Delivery and Cost.
8.3.1 Production (7.5, 7.5.5, 7.6, 8.2.3, 8.3)
a) The Material Handlers assigned to the various Production Lines will pull the necessary materials
from stock as part of their daily duties.
b) Assembly & Test: Assemble instrument according to production schedule. Perform functional
test (7.3.6). Non-conforming material is identified as per procedures #E103 Agilent Control of
Non-conforming Product. (8.3)
c) System Integration (where appropriate to business): Assemble system according to customer
order and perform system tests.
d) Calibrate: (8.2.3, 7.6) The process of calibration is defined in related documents:
EMG Calibration Policy:
EMG Calibration System Manual (includes control of subcontracted calibration section 4.5)
e) EMG uses the Agilent level Design & Manufacturing Processes to determine workmanship
criteria. Web site for the list of Agilent‟s Design and Manufacturing documents:
Design and Manufacturing Document List, Design Standards Users Group
f) ESD (Electro Static Discharge) control is a quality requirement in EMG. The EMG ESD Control
Manual contains the minimum control requirements that must be followed. Compliance is
required at all sites where ESD-sensitive electronic devices are manufactured, assembled,
tested, serviced, configured, installed, handled, packaged, stored or supplied by OEMs. Each
entity/business has appropriate local controls to ensure EMG ESD Control requirements are
met." Web site for the EMG ESD Control Manual:
http://sharedoc.collaboration.agilent.com/sites/emg-quality/sd/emgbms/esd%20control%20docs/esd_control_manual%20-%20epsg1039112.pdf
g) Handling and storage of product will be determined by the local area, but will include
identification, packaging, and proper protection of product.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 24

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 24 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
h) Hardware Test Centers exist within EMG to provide environmental testing, failure analysis
services, and regulatory compliance expertise that enable our partners in R&D, Manufacturing,
and Marketing to design and deliver products which meet our trade customers' quality
requirements. This function may reside in OF or a Division.
8.4 Delivery (7.5.1, 7.5.5, 8.2.1)
Delivery is an important part of the overall BMS and assures the product is packaged and shipped
appropriately meeting EMG quality/delivery standards and customer expectations. An EMG On
Time Arrival (OTA) team focuses on achieving OTA goals. The purpose of the EMG On Time
Arrival Corrective Action and Management Review system is to establish a quality improvement
environment that is responsive to EMG‟s promise to customers. The approach is to establish
corrective action ownership, provide consistent data, and establish management reviews.
8.4.1 Shipping (7.5.5) Pack: Pack instrument and accessories according to customer order/requirements. The
Environmental Test Manual outlines several requirements for packaging.
a) Shipping Logistics: Plan and manage worldwide shipping logistics to meet customer
requirements.
8.4.2 Management of customer Complaints: OBD, OTA, OTS: (8.2.1)
Improvements to product and production processes may be initiated in response to information
gained from customer feedback.
a. The OBD (Out-of-Box Defect) system provides information that is analyzed for patterns and
trends in order to prioritize areas for further investigation. Changes to product or process may
be implemented within Order Fulfillment along with involvement from other areas of the
business as required.
b. On Time Arrival (OTA): EMG measures the OTA for all EMG products. The results are
monitored in the EMG monthly Bluebook.
c. On Time Shipment (OTS): http://qes.supplychain.agilent.com/OTD_Metrics/OTD_metrics.asp
EMG measures the OTS for all EMG products. The results are monitored in the EMG monthly
Bluebook.
8.5 New Product Introduction (NPI)/Engineering Support (7.3, 7.3.4, 7.5.1, 8.2.3, 8.2.4)
EMG‟s OF strategy is to have once centralized manufacturing center with a number of smaller
regional Divisions based NPI OF sites. These NPI sites provide New Product Development and
Introduction support for the centralized OF.
Primary Roles: Division NPI OF organizations provides the critical, dynamic, new product
development and introduction linkages between EMG‟s product development labs and centralized
manufacturing. This is a collaborative role that includes influencing product design, and supply
chain design decisions to optimize business results and meet current and future product
development requirements. Specific activities may include validating design performance, providing
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 25

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 25 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
rapid prototyping, characterization and qualification testing, and ensuring smooth new product
introductions and production ramp up execution.
Division NPI OF organizations also provide a business management/leadership linkage between
the Divisions (R&D, Marketing, and Product Planning), it is co-located with, and the manufacturing
center that produce the Division‟s products. This business linkage ensures Division activities link
successfully with Order Fulfillment.
a) NPI‟s & Transfers (7.3, 7.5.1, 7.3.4)
Manage the introduction of new products into manufacturing environment.
Coordinate with Division and Division-OF entities.
b) Engineering /Production Support (8.2.3, 8.2.4)
Provide support for Test Systems (including Test S/W and Measurement
Traceability), Production Processes and Technical Investigations.
c) Customer Feedback – CA/PA (8.5.2, 8.5.3)
Investigate and implement corrective and preventative actions to products in
production phase.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 26
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 26 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
9. Customer Satisfaction
Customer Satisfaction is meeting customers‟ quality, delivery, pricing and features requirements.
Quality is more than hardware reliability. It is all about the customer‟s total experience in interacting
with our products, people, partners, and agents. It is the sum of these interactions over time that
determines customer loyalty.
9.1 Service Solutions Unit (SSU) (7.5.1, 7.5.2, 7.5.3, 7.6)
The Service Solutions Unit‟s mission is to build customer value and intimacy through support services and
solutions. These either extend the usefulness and product life of hardware products, or are unique services
such as consulting and training that provide a whole product solution for the customer
There are two separate entities in SSU from an operational perspective to better serve customers. The SSU
organization will focus on business development, marketing and R&D customer and planning issues while the
SSU SDO, Service Delivery Operation delivers calibration/repair services and parts to EMG business and
trade customers.
9.1.1 Repair: (7.5.3, 7.5.1)
Repair is composed of a number of related processes that return a hardware product back to useful service.
The overall repair process includes sub-processes such as cleaning and safety testing, and information
services such as Service Notes.
http://emg.communications.agilent.com/wcss/internalpages/svcnotes/index.htm
An overall process map is available at: http://bench.service.agilent.com/ (Not applicable to MSD and ASPL)
Examples of key metrics: Turn-around Time (TAT), Ship on time (SOT).
MSD and ASPL provides on-site support (repair and calibration and delivery (site preparation and
installation)) and remote phone technical support to customer.
9.1.2 Calibration: (7.6)
Calibration is the set of operations that establishes, under specified conditions, the relationship between
values indicated by a measuring instrument or measuring system, and the corresponding standard or known
values derived from the standard. The benefit to the customer is to assure that an instrument is operating
within the measurement specification design parameters. Examples of key metrics: TAT and SOT.
The process of calibration is defined in related documents:
EMG Calibration System Manual (includes control of subcontracted calibration section 4.5,
supplier selection.
Audit Program Description
Metrology Policy Manual
Calibration is sometimes a separate step within the repair process and is embedded in the
following production flow diagram located at: http://bench.service.agilent.com/
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 27
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 27 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
9.1.3 Service Parts Operation
The Service Parts Operation works to provide EMG business & trade customers with support parts
to repair customer‟s equipment in a cost effective timely manner with minimum asset levels. SPO
worldwide operations include the functions of 1) Request Management, 2) Planning, 3)
Procurement, and 4) Logistics. MSD manages its own service parts operation and leverages the Global
Service Parts Operation processes.
http://ssuweb.business.agilent.com/SSUQuality/SPO/
9.1.4 Business Planning
SSU business planning provides leadership for Business Development, Marketing and R&D for onsite/mobile cal, meeting customer needs and the development of new products and services.
9.2 Application Engineering Organization (AEO)
EMG offers a flexible range of engineering, training and technical support services specifically
designed to help customers optimize the use of their EMG equipment. We can help them get
started with their new instrument, provide out-of-warranty agreements for technical phone support,
provide continued custom assistance with the optimization of their existing instruments, provide
training based on their specialized requirements, and more. We help customers achieve their
application and measurement goals.
These services can be provided in a variety of ways: face-to-face at the site, remote (via phone and
web collaboration), or through self-guided tutorials. More information about these services is
available:
For Product and Application Services: www.agilent.com/find/consulting
For Education Services: www.agilent.com/find/education
For Technical Support Agreements and contact numbers: www.agilent.com/find/techsupport
9.3 Professional Services & Support
ASPL is in the business of highly distributed monitoring and Quality of Services Solutions focused
on big Telecom operators WW. This business requires specific professional services, such as
project management, deployment & commissioning, acceptance testing and high availability
reactive and proactive support.
9.4 Remarketing Solutions Division (RSD) (6.3c)
RSD is a unit within the Electronic Measurement Group. RSD provides Demo Services for EMG,
Remanufacturing of Demo, Trade-In and Sourced Products and manages the Trade-In/Trade-Up
Program for EMG. Organizationally, the RSD is divided into functional areas: Business
Development / Sourcing, Marketing, Supply Chain, Channel Partners, TQM and Demo. RSD
financial and operational metrics are reviewed on a monthly basis by the management team.
RSD utilizes some 3rd party providers. RSD uses a 120 Day Calibration policy (Products can be
shipped with Calibration Certificates at 120 days or less) (ISO9001:2008 exceptions: 7.3, 7.5 &7.6).
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 28

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 28 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
RSD Home Page web site: http://emg.communications.agilent.com/rsd/
• Remanufacturing is an operation that refurbishes customer pre owned, EMG Demo and factory
used products. Remanufacturing has three channels to sell inventory: Direct sales, Alternate
Channel and scrap mitigation.
• Trade Up is a program that allows EMG customers to leverage a currently owned product for
credit towards a new EMG unit or service. The RSD acts as a facilitator between the EMG sales
force and the original manufacturing division. The pre owned unit traded in by the customer is
disposed of through RSD„s Remanufacturing operations. Trade up web site:
http://tradeup.sales.agilent.com/
• Demo is an organization within RSD that supports sales of EMG products by providing
demonstration units to EMG Sales Representatives (FE, AE, SE) and Distributors.
9.5 Customer Surveys (8.2.1, 8.4)
Survey of EMG Customers is based on the most recent interaction with regional “Touch Points”
(i.e., Business Center, Contact Center, Sales, Service & Support, and Web). EMG has a web based
survey where the Customer can either provide a rating score, select from multiple choices or enter
a comment (verbatim). EMG: http://emg.communications.agilent.com/quality/acs/default.asp
Agilent Customer Satisfaction (ACS) program: Agilent Customer Satisfaction is a customer
satisfaction measurement and improvement program consistent across Agilent business, regions
and touch points. ACS implements an actionable metrics model to track and improve Agilent
Customer Satisfaction (ACS) performance. This includes setting a company-wide metric for
customer satisfaction, setting visible goals to continually improve customer satisfaction, and
monitoring and rewarding improvement.
Agilent: http://customer.quality.agilent.com/customer_satisfaction/acs.shtml
9.6 Customer Feedback (4.1e, 5.62b, 7.2.3c, 8.2.1, 8.4)
9.6.1 The Customer Feedback System provides customer satisfaction and future opportunity
information to EMG regarding product, service, and support offerings. A variety of processes and
tools (Customer Feedback System, Customer visit reports, surveys, etc.) are used to meet the
diverse needs of the organizations within EMG.
Each organization is required to:
Document customer feedback received (solicited and unsolicited)
Take appropriate follow-up action on feedback
o Redirect operational issues requiring immediate response to the appropriate
business process.
o Direct all other feedback to the responsible action/process owner for review, follow-
up and disposition.
Include feedback in their processes to identify systemic customer satisfaction issues and
new product, service, and support opportunities.
Include Customer Feedback as an input for the local management review.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 29

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 29 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Feedback may be submitted using one of the following methods:
Customer Issue Resolution Process (CIR)
The Customer Feedback web site: http://emg.communications.agilent.com/quality/voc/
Email: Customer-feedback,EMG
Voice Mailbox: (719) 590-3855 or Telnet 590-3855
9.6.2 EMG Quality Blue Book (4.1e, 8.4): Provides data about EMG‟s quality performance from our
customers‟ perspective where feasible. It is designed to measure areas which affect customers‟
perception regarding EMG‟s quality. Multiple levels of management review the Quality Blue Book
quarterly to understand EMG‟s overall quality trends to make improvements as appropriate.
EMG Quality Blue Book web site: http://intuition.is.agilent.com/bluebook/Default.aspx
9.6.3 Out of Box defect (OBD): (4.1.e, 8.4)
An OBD is a Performance or Non-performance defect found when the customer opens the box and
turns on the product. Customers expect products to conform to quality expectation and expect the
product to turn on the first time.
Customer reliability expectations: 1) Out of box experience, where Agilent‟s reputation can be
questioned, 2) Early lifetime, where the specific product‟s quality is in doubt, 3) Warranty period,
where reliability perceptions are developed, and 4) Post-warranty period, where service delivery is
measured.
9.7 Escalations and Customer Issue Resolution (8.2.1)
CIR (Customer Issue Resolution)
EMG's worldwide Customer Issue Resolution (CIR) process focuses on resolving crossorganizational customer issues. Timely and effective customer issue resolution is an important
aspect in building customer satisfaction and in sustaining Agilent‟s position as the premier
measurement company. The CIR process was implemented worldwide on February 1, 2007 to
provide a simplified common tool to report and resolve cross-organizational customer issues.
CIRF is a web or Outlook based form that is available to all EMG employees. CIRF provides an
easy way to: Report a customer problem, provide customer feedback/compliment, Request an
escalation, Request a 2nd Level Escalation, and ask for assistance when you don't know where to
go for help.
Key elements of CIR:
Common Process: The field, divisions and SSU will use the CIR process.
Single Entry Point: CIRF (Customer Issue Reporting Form) will be the primary input tool for front-line
employees.
One Platform: Siebel will be the sole content management system.
Single Point of Contact: Each organization has one single point of contact (a specific person, not an email
node).
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 30

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 30 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
Containment Plan Required for Escalations: In addition to initial response time to the issue submitter, a
containment plan is now required.
95% of All Escalations Contained Within 7 Working Days or Less.
9.7.1 The Presidents line: Respond to customers that make complaints to Agilent‟s CEO or a
member of his staff when previous channels used to attempt to resolve the issue have been
either unsatisfactory or unknown to the customer. The Presidents line gives appropriate,
timely responses and follow-up to customer issues and all are treated in a manner that is
respectful and consistent with Agilent corporate objectives. This information provides
visibility to problems so Agilent Technologies can drive process improvement.
Unless a specific resolution plan and milestones negotiated with the customer are in place, it
is expected that the issues will be resolved within 10 working days.
9.7.2 Delivery Escalation Process: The purpose of this process is to avoid customer satisfaction
issues and to ensure the order is delivered as per the correct process. This process will help
manage, prioritize order delivery activities.
9.7.3 Internal escalations: are defined at the entity/business level.
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 31
EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 31 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
List
Examples
ACS
Agilent Customer Satisfaction (Survey)
AEO
Application Engineering Organization
BMS
Business Management System
BMSM
Business Management System Manager
BU
Business Unit
Business Manual
EMG Business Management Systems Manual
CCC
Customer Contact Centers
CIRF
Customer Issue/Input Reporting Form
Comms
Communications
CPI
Continual Process Improvement
CS
Commercial Services
CSG
Communications Solutions Group
DFx
Design for everything
Divisions
Product focused with detailed product definition and a deeper view of the product to meet customer
needs.
ECO
Engineering Change Order
ECR
Engineering Change Request
ELT
Executive Leadership Team reporting to GM
Entity Specific Documents
Quality Procedure Manual
EMG
Electronic Measurements Group
ERP
Enterprise Resource Planning: an automated planning system (Oracle)
ESD
Electro Static Discharge
GM
General Manager
HTC
Hardware Test Center
KP
Knowledge Portal: a documentation vault
Local Processes
Process Documentation
Marketing
Market focus with a system approach, broader view of customer need.
MRP
Management Review Process
MST
Manufacturing Special Test
MTLS
Materials
NPI
New Product Introduction
OBD
Out of Box Defects
OF
Order Fulfillment
OTA
On Time Arrival
OTS
On Time Shipment
QIC
Quality Improvement Cycle
Records
Training records, minutes, contracts etc.
Required Documents
Internal Audit Process, Management Review, etc.
RFI/P
Request for Information, Request for Proposal
RIP
Reliability Improvement Process
ROQ
Renaissance of Quality
RSD
Remarketing Solutions Division
SAP
South Asia Pacific
SPO
Service Parts Organization
TAT
Turn around time
TIS
Technical Information System
VOC
Voice of the Customer
WCSS
Worldwide Customer Service & Support
Webdoc
A web based tool providing a consistent document configuration management tool.
WW
World Wide
WWMat
World Wide Materials
10. Abbreviations used in this manual
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 32

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 32 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
REV
NO.
CHANGE SUMMARY
DOCUMENT
OWNER
APPROVER
DATE
APPROVED
1
As issued.
Diana Clark
Jim Horner
Oct. 1, 2002
2
Updated URL‟s for Disaster Recovery, Common Processes, page 11
Updated URL‟s for Design and Mfging Processes and ESD Page 21
Diana Clark
Jim Horner
Oct 7, 2002
3
Updated Customer Property req.
Diana Clark
Jim Horner
Oct 8, 2002
4
Changed SGDU to DAT & WCS, updated Material Purchasing.
Diana Clark
Jim Horner
Oct 21, 2002
5
Updated section 4.7 EHS
Diana Clark
Jim Horner
Oct 28, 2002
6
Updated Agilent ESD Web Site
Changed KSO to be included in the cert.
Updated Procurement process
Diana Clark
Jim Horner
Nov 14, 2002
7
Clarified support versus supplier within Agilent Technologies, page 4
Diana Clark
Jim Horner
Nov 22, 2002
8
Added copyrights and deleted “public”
Diana Clark
Jim Horner
Dec 9, 2002
9
Updated Commercial Services process
Diana Clark
Jim Horner
Jan 22, 2003
10
Added Product Stewardship information section 6.0 b
Diana Clark
Jim Horner
Feb 13, 2003
11
Changed QMS to BMS, Changed PGU to Division
Diana Clark
Jim Horner
Mar. 17, 2003
12
Updated Commercial Services and added infrastructure organization
heading
Diana Clark
Jim Horner
Mar. 31, 2003
13
Updates resulting from organization changes
Diana Clark
Jim Horner
May 2, 2003
14
Updated FSU process
Diana Clark
Jim Horner
May 7, 2003
15
Updated Quality Education, & Custom Solutions, & moved FSU to
Customer Solutions.
Diana Clark
Jim Horner
June 4, 2003
16
Updated GTLS to SPO, updated FSU URL
Diana Clark
Jim Horner
June 13, 2003
17
Update URL‟s for FSU, HR & Oracle documentation, deleted GSDC,
added AES, updated Marketing and Materials sections.
Diana Clark
Jim Horner
July 18, 2003
18
Update Strategic Planning and FSU, deleted OF website, updated OBD
website information in 8.4, Added MAST and Eesof URL‟s
Diana Clark
Jim Horner
July 25, 2003
19
Fixed page breaks
Diana Clark
Jim Horner
July 30, 2003
20
Fixed pagination again
Diana Clark
Jim Horner
Aug 1, 2003
21
Fixed pagination once again, updated scope
Diana Clark
Jim Horner
Aug 6, 2003
22
Fixed pagination again
Diana Clark
Jim Horner
Aug 8, 2003
23
Fixed pagination (I hope)
Diana Clark
Jim Horner
Aug 8, 2003
24
Updated 2.1 adding Senior VP‟s role
Diana Clark
Jim Horner
Aug 12, 2003
25
Updated Customer Feedback 9.6, Updated Materials Purchasing
information 8.2.
Diana Clark
Jim Horner
Aug 20, 2003
26
Updated 9.2 KSO information
Diana Clark
Jim Horner
Aug 22, 2003
27
Updated 8.3 Manufacturing information about ESD, added Infrastructure
Website in 4.6.
Diana Clark
Jim Horner
Aug 28, 2003
28
Updated FSU 9.4, updated URL‟s
Diana Clark
Jim Horner
Sept 10, 2003
29
Updated Scope page 4.
Diana Clark
Jim Horner
Oct. 1, 2003
30
Commercial Services changes internal processes and changes business
name to Customer Contact Centers (section 7.2)
Diana Clark
Jim Horner
Oct. 10, 2003
31
Update table of contents, Updated GSO to SSO, Deleted AWARD, and
updated business map.
Diana Clark
Jim Horner
Nov. 7, 2003
32
Updated KSO to MAS, updated scope list and abbreviation list.
Diana Clark
Jim Horner
Dec. 18, 2003
33
Updated 9.6 CFS, 9.4 FSU and 2.3.l top management responsibilities
Diana Clark
Jim Horner
Jan. 23, 2004
34
Pagination issue
Diana Clark
Jim Horner
Jan. 26, 2004
35
Updated section 8.4 Delivery
Diana Clark
Jim Horner
Feb 19, 2004
36
Updated section 9.1 SSU, 7.1 Field Sales, updated URL‟s, aligned
Business Process Map, added order delivery escalation process
Diana Clark
Jim Horner
April 9, 2004
37
Update SSU information, and CCC information. Updated URL‟s, typos,
formatting and making the words clear for translation. Added outsourcing
information.
Diana Clark
Jim Horner
April 30, 2004
38
Update Materials Purchasing/Performance Evaluation 8.2.4, and the
scope table adding SQD.
Diana Clark
Jim Horner
June 2, 2004
Document Control Log
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 33

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 33 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
REV
NO.
CHANGE SUMMARY
DOCUMENT
OWNER
APPROVER
DATE
APPROVED
39
Corrected formatting of section numbering, the number “10” appeared
erroneously throughout the document.
Diana Clark
Jim Horner
June 4, 2004
40
Moved the ERP/Oracle information under 4.6.3 Information Technology,
cleaned up typo‟s throughout, added clarity to 4.2.1, 4.2.2. Added
Engineering Services to the scope on page 4.
Diana Clark
Jim Horner
June 29, 2004
41
Updated Materials 8.2.1, 8.2.4, updated the scope page 4.
Diana Clark
Jim Horner
July 9, 2004
42
Updated FSU 9.4, updated TQRDCE URL path 8.2.4 and updated the
scope on page 4.
Diana Clark
Jim Horner
July 23, 2004
43
Updated FSU 9.4 URL‟s
Diana Clark
Jim Horner
July 29, 2004
44
Updated section 3.0 relationship map
Diana Clark
Jim Horner
Aug 5, 2004
45
Updated customer property 4.1.4 and escalations 9.7.
Diana Clark
Jim Horner
Oct 1, 2004
46
Fixed broken link
Diana Clark
Jim Horner
Oct 4, 2004
47
Update ULR for escalation 9.7
Diana Clark
Jim Horner
Oct 21, 2004
48
Fixed typo on front page
Diana Clark
Jim Horner
Oct 28, 2004
49
Added CSTC to scope
Diana Clark
Jim Horner
Nov 5, 2004
50
Updated 9.2: MAS changed their name to AEO
Diana Clark
Jim Horner
Nov 19, 2004
51
Updated 9.4: FSU change their name to RSD (Remarketing Solutions
Division)
Diana Clark
Jim Horner
Dec. 8, 2004
52
Added Agilent Risk Management information (4.1.5)
Diana Clark
Jim Horner
Jan. 6, 2005
53
Updated section 8.2.4 Performance Evaluation.
Diana Clark
Jim Horner
Jan. 18, 2005
54
Added EMG TAG structure and BMS Rep.
Diana Clark
Jim Horner
Jan. 24, 2005
55
Updated broken links
Diana Clark
Jim Horner
Feb. 11, 2005
56
Updated the section 1.3 scope to align business reorganization (deleted
GSBU, added AIBU), added Six Sigma information, updated MR
requirements section 2.3.
Diana Clark
John Herniman
April 22, 2005
57
Updated MR requirements section 4.3 fixed numbering of document,
updated scope in section 1.4.
Diana Clark
John Herniman
June 13, 2005
58
Deleted GSBU
Diana Clark
John Herniman
June 17, 2005
59
Updated section 9.1.4
Diana Clark
John Herniman
June 20, 2005
60
Updated RSD
Diana Clark
John Herniman
June 23, 2005
61
SBM&D update section 8.2
Diana Clark
John Herniman
July 25, 2005
62
Deleted DAT, changed PMT to NMD, updated Packaging and Blue Book
web sites.
Diana Clark
John Herniman
Aug. 12, 2005
63
Updated to reflect organizational change EPSG- EMG and updated
URL‟s.
Diana Clark
John Herniman
Sept. 9, 2005
64
Updated URL‟s and updated TSO business name.
Diana Clark
John Herniman
Dec. 20, 2005
65
Updated section 4.5, page 15: TIS
Diana Clark
John Herniman
Dec. 20, 2005
66
Updated section 4.2.2 discontinuance
Diana Clark
John Herniman
Jan. 10, 2006
67
Updated 9.7.2 Ned line to ECAG
Diana Clark
John Herniman
Jan. 11, 2006
68
Updated 9.1.4 Business Planning for SSU
Diana Clark
John Herniman
Jan. 11, 2006
69
Added the corporate discontinuance process URL at 4.2.2.
Diana Clark
John Herniman
Jan. 25, 2006
70
Updated EPSG to EMG, deleted MIBU, Change AIBU to EIBU.
Diana Clark
John Herniman
March 8, 2006
71
Typo‟s fixed
Diana Clark
John Herniman
March 8, 2006
72
Fixed pagination
Diana Clark
John Herniman
March 9, 2006
73
Added CIRF to 9.7, updated URL in footer, updated table of contents,
and updated 2.3i Quality Objectives.
Diana Clark
John Herniman
April 14, 2006
74
Updated URL in 8.3e
Diana Clark
John Herniman
April 18, 2006
75
Updated URL in the footer, updated typos and formatting throughout the
document.
Diana Clark
John Herniman
April 28, 2006
76
Updated URL 9.7.3, updated 9.1.4 URL.
Diana Clark
John Herniman
May 3, 2006
77
Updated section 7.2 CCC
Diana Clark
John Herniman
May 25, 2006
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 34

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 34 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
REV
NO.
CHANGE SUMMARY
DOCUMENT
OWNER
APPROVER
DATE
APPROVED
78
Updated section 8.2.2 to clarify supplier management requirements
updated 2.2 org chart, updated 1.4 scope and fixed a few typo‟s.
Diana Clark
John Herniman
June 20, 2006
79
Updated section 9.7.3 by adding the ECAG/BillLine overview
presentation URL
Diana Clark
John Herniman
June 28, 2006
80
typo
Diana Clark
John Herniman
June 28, 2006
81
Updated to include ASD and MSD BMS information. Updated 9.7.4 URL
delivery escalation, updated the Process Map 3.1.c. Updated 1.4 scope,
and updated 2.1.e organization.
Diana Clark
John Herniman
July 27, 2006
82
Added Signal Network Division. Updated the URL for packaging (8.4.1).
Diana Clark
John Herniman
August 14, 2006
83
Updated 9.4 RSD
Diana Clark
John Herniman
August 22, 2006
84
Updated the core document links in section 4.1.5
Diana Clark
John Herniman
Sept. 1, 2006
85
Updated the GRS URL at 4.1.5
Diana Clark
John Herniman
Sept. 13, 2006
86
Updated section 8.2 Procurement
Diana Clark
John Herniman
Dec. 8, 2006
87
Updated section 8.2 Procurement
Diana Clark
John Herniman
Dec. 8, 2006
88
Update section 8.2 Procurement by fixing typo
Diana Clark
John Herniman
Dec. 11, 2006
89
Added clarity to section 9.1.3 regarding MSD. Added information about
the HTC in 8.3. Updated broken links.
Diana Clark
John Herniman
Dec. 15, 2006
90
Updated Webdoc again due to corruption error in Webdoc
Diana Clark
John Herniman
Dec. 20, 2006
91
Updated 8.2 Procurement, and updated 1.4 Scope
Diana Clark
John Herniman
Jan. 5, 2007
92
8.2.4 added technology, updated URL‟s and added clarity to section 2.3
top management
Diana Clark
John Herniman
Jan. 16, 2007
93
7.2 updated CCC information and updated table of content
Diana Clark
John Herniman
Feb. 13, 2007
94
8.3-f updated ESD Control requirements information, and updated 9.7.3
changing the name from ECAG to Presidents Line. 6.1 Update QSO
website and deleted the reference to 7.1
Diana Clark
John Herniman
March 23, 2007
95
Updated 9.7 escalations deleting Presto, updated CIR information,
updated 1.4 scope for NMT
Diana Clark
John Herniman
July 1, 2007
96
Updated section 1.4 scope, updated links, updated RSD information
Diana Clark
John Herniman
Aug 8, 2007
97
Updated 4.1.2 Quality Education, updated 4.2.1 Acquisition life cycle,
updated EMG org chart page 6
Diana Clark
John Herniman
Sept. 28, 2007
98
Updated & added URL‟s/links, update section 1.4 Scope, added Anti-
virus requirements document, Deleted AES Infrastructure.,
Diana Clark
John Herniman
April. 14, 2008
99
Updated 4.2.2 EMG Discontinuance URL
Diana Clark
John Herniman
April. 16, 2008
100
Updated URL‟s, updated SBM to SSM in section 8.2, Updated 9.4 RSD,
and updated 1.4 Scope.
Diana Clark
Eric Taylor
June 8, 2008
101
Changed Everest to Oracle, added CIRF update to section 9.7 and 4.1.5,
linked the new DAMDoc list. Updated 2.1 and updated ASD to ASPL.
Diana Clark
Eric Taylor
July 8, 2008
102
Added IT Disaster Recovery website, added OTA and OTS and deleted
OTD in section 8.4.2 b/c.
Diana Clark
Eric Taylor
Oct 24, 2008
103
Updated 4.2.1b to include a link to the Acquisition Standard, cleaned up
some OTA information and updated the EMG BMS Manager reporting as
dotted line.
Diana Clark
Eric Taylor
Oct 28, 2008
104
Updated 1.4 scope to align the latest reorg for NPMD and NMD
Diana Clark
Eric Taylor
Dec. 9, 2008
105
Added section 7.3 Customer Data
Diana Clark
Eric Taylor
Jan. 8, 2009
106
Updated the scope section by adding WWFO as certified, updated
section 7.3 Customer Data information and updated ISO9001:2000 to
ISO9001:2008.
Diana Clark
Eric Taylor
Mar. 20, 2009
107
Updated the footer regarding public use of this document and updated
the copy write year as required
Diana Clark
Eric Taylor
Mar. 30, 2009
108
Updated the scope to align with the organization change
Diana Clark
Eric Taylor
April 23, 2009
109
Updated section 1.4 removing ISO9001:2000 and adding ISO9001:2008,
update section 2.2 to align with the org change.
Diana Clark
Eric Taylor
April 24, 2009
110
Updated document owner name. Removed reference to WBU in Section
4.2.4. Changed TLO to TSO in list of ISO9001 certified businesses
(section 1.4). Reworded section 4.4 (changed we/our to EMG). Updated
Section 7.2 (CCC). Updated ISO9001:2008 reference in Section 4.6.
Edited by Alex Guzman.
Alex Guzman
(BMS Team)
Ted Tucker
31-Aug-2009
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
Page 35

EMG Business Management System Manual
Doc # epsg1026386
Rev. 112
Page 35 of 35
The user of any printed copy of this controlled document is responsible for verifying it is the correct version prior to use. Hard copies are uncontrolled.
This manual contains links to subordinate documents that are restricted to Agilent personnel only
and may not work if accessing this document from a public site.
REV
NO.
CHANGE SUMMARY
DOCUMENT
OWNER
APPROVER
DATE
APPROVED
111
Deleted list of businesses certified to ISO9001 in Section 1.3 (Scope)
and Quality Standards Ownership Matrix (Section 3.2). Reformatted
header/footers. Included TLO in Section 4.2.3 (Technology
Development). Fixed broken URLs. Updated Org Chart descriptions
(Section 2). Edited by Alex Guzman.
Alex Guzman
(BMS Team)
Ted Tucker
24-Sep-2009
112
Updated Document and Fixed Broken Links. Addressed DEKRA NC by
referencing EMG OF as part of AOF. Changed wording to aid clarity in
section 9.6 related to an old system CASPER.
Section 8 updated to reflect relationship between AOF and EMG OF.
Section 2.2 Org Chart updated.
Section 4.2. Revised wording related to Infrastructure Org roles and
responsibilities
Kevin Fawl
(BMS Team)
Ted Tucker
13-Nov-12
The current version is available at the EMG Document Map website: http://emg.communications.agilent.com/quality/bms/040318_docMap.asp
(c) Agilent Technologies, Inc. 2002, AGILENT TECHNOLOGIES
 Loading...
Loading...