Page 1

®
®
Model 5000
Patient Manual
20
B0060 Rev
A - P
1
Page 2

Contents
i
Restricted sale
Federal (USA) law restricts this device to sale by or on the order of a physician.
Effectivity
This manual describes the LifeVest 5000 wearable defibrillator system.
Disclaimer
Information, operation, specifications, and product appearance may change without notice. Names and
data used in examples are fictitious.
Trademarks
ZOLL, LifeVest, Blue and the color Blue are trademarks and/or registered trademarks of ZOLL Medical
Corporation in the United States and/or other countries.
Copyright notice
© 2016 ZOLL Medical Corporation. All rights reserved.
Patents
Patent: www.zoll.com/patents
Software nonexclusive license
The LifeVest device includes certain software (“Software”). ZOLL grants you a nonexclusive license to use
the Software solely for diagnostic and treatment purposes as part of use of the LifeVest device. You are
prohibited from:
(
i) reproducing the Software;
(
ii) removing or destroying any proprietary markings, copyright notices or other legends which
are part of the Software;
(
iii) modifying or reverse engineering the Software; or
(
iv) removing the Software from the LifeVest device.
Title to the Software will remain at all times with ZOLL. You must keep the Software confidential.
Page 3

LifeVest 5000 Patient Manual
ii
Contact information
ZOLL
Pittsburgh, PA 15238
USA
Phone toll free (USA and Canada
)
Phone outside USA
Fax
Web
1-800-543-3267
1-412-968-3333
1-412-826-9485
www.zoll.co
m
Page 4

Contents
Contents
1: Introduction .............................................................................................................................1-1
About this manual ................................................................................................................................. 1-
1
About the LifeVest system and cardiac defibrillation ........................................................................... 1-
1
How the LifeVest works ........................................................................................................................ 1-
1
Safety information ................................................................................................................................ 1-
2
Environment considerations related to noise and vibration ................................................................ 1-
5
Intended use locations .......................................................................................................................... 1-
5
Essential performance........................................................................................................................... 1-
5
Aircraft use ............................................................................................................................................ 1-
5
Operator profile .................................................................................................................................... 1-
6
Patient profile ....................................................................................................................................... 1-
6
ZOLL authorized representative profile ................................................................................................ 1-
6
Patient training ...................................................................................................................................... 1-
6
Electromagnetic interference ............................................................................................................... 1-
7
Wireless interference ............................................................................................................................ 1-
8
FCC regulatory information ................................................................................................................... 1-
8
2: Meet the LifeVest 5000 system..................................................................................................2-1
Components .................................................................................................................. ........................ 2-
1
Garment and electrode belt .................................................................................................................. 2-
2
Monitor — front view ........................................................................................................................... 2-
3
Monitor — back view ............................................................................................................................ 2-
4
Monitor touchscreen ............................................................................................................................ 2-
5
Help screens .......................................................................................................................................... 2-
7
Charger .................................................................................................................................................. 2-
8
Page 5

3: Using the LifeVest .....................................................................................................................3-1
Daily routine .......................................................................................................................................... 3-
1
Normal startup routine ......................................................................................................................... 3-
2
Battery care ........................................................................................................................................... 3-
3
Charger setup ........................................................................................................................................ 3-
4
Change and charge batteries daily ........................................................................................................ 3-
7
Record your heart rhythm (ECG) ........................................................................................................... 3-
8
Patient menu screen ............................................................................................................................. 3-
9
Voice options ....................................................................................................................................... 3-
10
Sending data manually ........................................................................................................................ 3-
11
Airplane mode ..................................................................................................................................... 3-
13
WiFi ..................................................................................................................................................... 3-
15
System information ............................................................................................................................. 3-
23
Support mode ..................................................................................................................................... 3-
24
Periodically clean and inspect the system .......................................................................................... 3-
26
What family members need to know .................................................................................................. 3-
27
When you are finished with the device .............................................................................................. 3-
28
4: Garment and electrode belt ......................................................................................................4-1
Assemble the garment and electrode belt ............................................................................................ 4-
1
Assembled garment and electrode belt ................................................................................................ 4-
3
Putting on the assembled garment and electrode belt ........................................................................ 4-
4
Connecting and disconnecting the electrode belt ................................................................................ 4-
7
iii
Page 6

LifeVest 5000 Patient Manual
iv
Remove when showering and bathing .................................................................................................. 4-8
Disassembling the garment and electrode belt .................................................................................... 4-9
Laundering the garment ...................................................................................................................... 4-10
5: Responding to alerts .................................................................................................................5-1
Overview of alerts ................................................................................................................................. 5-1
Physiological alerts: vibration or siren sound ....................................................................................... 5-1
Siren with call for help message ............................................................................................................ 5-4
Technical alerts: gong sound ................................................................................................................. 5-5
Informative alerts: gong or high-pitched “train whistle” sound ........................................................... 5-6
6: Using the activities options .......................................................................................................6-1
Introduction to activities ....................................................................................................................... 6-1
Taking the health survey ....................................................................................................................... 6-2
Taking the health survey now ............................................................................................................... 6-3
Taking the WalkTest™ ........................................................................................................................... 6-7
Taking the WalkTest™ now ................................................................................................................... 6-9
Appendix A:
Symbols and Icons ..................................................................................................... A-1
Appendix B :
Software Licensing Statement ................................................................................... B-1
NO WARRANTY ..................................................................................................................................... B-1
Page 7

Introduction
1-1
1:
Introduction
About this manual
This manual:
•
is for patients who are using the LifeVest wearable defibrillator.
•
gives you instructions on the use and care of the device.
•
is intended to supplement the training you received when you were fitted with the
LifeVest device.
About the LifeVest system and cardiac defibrillation
The LifeVest device continuously monitors your heart. If it detects a life threatening rhythm that
is too fast, the device delivers treatment to restore normal rhythm. If you are conscious, you can
prevent a treatment by using the response buttons when the device alerts you that a treatment
is coming.
How the LifeVest works
The round electrodes in the garment monitor your heart. Therefore, it is important to maintain
a snug fit across your chest. If the LifeVest detects a possible life threatening rhythm, the device
will first create a vibration in the middle of your back to warn you of a possible treatment. The
siren alert then occurs to inform you that the LifeVest is about to deliver a treatment, a
therapeutic dose of electrical energy, in an attempt to restore your normal heart rhythm.
If you are
conscious:
To prevent a treatment from occurring, press the
response buttons on the monitor. You may
release the buttons, but must repeat pressing
them each time the siren alert occurs. Pressing
the buttons tells the device that you are
conscious.
If you are
unconscious:
The response buttons will not be pressed, which
tells the LifeVest to prepare to deliver a
treatment. The monitor will then play an auditory
message to inform bystanders to not interfere
while the treatment is delivered.
Page 8

LifeVest 5000 Patient Manual
1 - 2
Safety information
This information helps you safely operate the LifeVest device. Read and understand these
warnings, cautions, and symbols before using the device.
•
Conductive parts of electrodes and associated connectors should not contact other
conductive parts including earth.
•
If the charger or power supply makes unusual noises, sparking, starts to smoke, or
emits a burning smell, unplug it immediately from the wall outlet.
The only way to
completely remove power is by unplugging the AC power plug from the wall outlet.
•
Any medical electrical equipment connected to the patient at the same time as
the LifeVest must be defibrillator proof, as shown by this symbol.
If the equipment is
not defibrillator proof, the equipment may be damaged if the LifeVest delivers a
treatment. Disconnect any non-defibrillator proof equipment from the patient while the
patient is wearing the LifeVest.
Terms used
WARNING:
Warns of possible injury or death that can be caused by misuse of the device.
This includes device failure that could result in the inability of the device to
protect you.
CAUTION:
Advises of possible problems that include damage to the device or other
property, or minor injury.
WARNINGS
Do not use the LifeVest System until you receive training and understand the manual.
If you do
not understand how to use the system, you may damage it and/or cause the system to
malfunction.
Always wear the LifeVest when instructed to do so by a medical professional.
Make sure the
electrode belt is properly connected to the monitor. Inappropriate use of the LifeVest may damage
it and/or cause the system to malfunction.
Do not tamper, alter, drop or abuse any part of the system or labeling.
Altering the equipment in
any way may damage it and/or cause the system to malfunction. Do not disassemble the unit. A
shock hazard exists. Refer all servicing to qualified personnel.
Do not put the monitor, electrode belt, battery or charger in or near water.
Water entering the
device may damage it and/or cause the system to malfunction.
Page 9

Introduction
1-3
WARNINGS
Always operate the system within the range of 0°C to 50°C (32°F to 122°F), up to 95% relative
humidity (non-condensing), and up to 10,000 feet in altitude.
Operating the device outside of this
range may damage it and/or cause the system to malfunction.
Do not use the device in the presence of flammable agents or in an oxygen enriched atmosphere.
This could present an explosion and fire hazard.
If you see Blue™ gel other than during a treatment, this may indicate a damaged electrode belt
and cause the system to malfunction.
Call ZOLL LifeVest immediately.
If the therapy pad Blue™ gel gets into your eyes, flush your eyes immediately with water and
contact your physician.
Your eyes may become irritated from the Blue™ gel.
The LifeVest is magnetic resonance (MRI) unsafe.
Do not use it in an MRI imaging
environment.
Do not stack or place the LifeVest next to other devices.
Doing so could expose the device to EMI
disturbance that may cause the system to malfunction.
Only the patient should press the response buttons.
The patient’s ability to press the response
buttons lets the device know whether or not the patient is conscious and is critical in deciding when
to give the patient a treatment. If anyone other than the patient presses the response buttons,
needed therapy may not be delivered, possibly resulting in serious injury or death.
Do not touch the patient while a treatment is being delivered.
Anyone touching the patient during
a treatment may be shocked.
Do not remove the battery, do not disconnect the electrode belt from the monitor, and do not
loosen the garment while the monitor is broadcasting alert sounds and/or voice prompts.
If the
battery is removed, the electrode belt disconnected from the monitor, or the garment is loosened,
needed therapy may not be delivered, possibly resulting in serious injury or death. CPR can be
performed as long as the monitor is not broadcasting alert sounds and/or voice prompts. If external
defibrillation is available, a decision can be made by a medical professional to remove the device
and monitor/treat the patient with external equipment.
Do not dispose of or incinerate the batteries.
The batteries contain lithium ion and must be
disposed of properly by ZOLL.
Do not force the connector.
Allow the connector to align before pushing it in. Forcing the
connector may damage it and cause the system to malfunction.
Do not use chlorine bleach, bleach alternatives, fabric softener, anti-static sprays or detergents
that include bleach or fabric softener additives when laundering the garment.
Using bleach or any
of these other prohibited agents to launder the garment may damage it and cause the system to
malfunction.
Page 10

LifeVest 5000 Patient Manual
1 - 4
WARNINGS
If you get an alert sound and you are awake, always press the response buttons to prevent
receiving a treatment.
If you fail to press the response buttons, you will get a treatment.
If you receive a treatment while your heart is beating normally and you did not use the response
buttons, the treatment may cause an abnormal rhythm to occur.
There is a small possibility that
the abnormal heart rhythm may not be detected and death may result.
If you get the siren alert, stop walking and press the response buttons.
When performing the WalkTest™, do not continue walking if the monitor broadcasts an alert
sound.
Stop walking and press the response buttons as you normally would. If you continue
walking, you may place yourself at risk for a cardiac arrest, possibly resulting in serious injury or
death.
When performing the WalkTest™, do not continue walking if you experience symptoms such as
shortness of breath, chest pain, or other pain or discomfort.
Stop walking and sit or lie down. If
the symptoms continue or get worse, immediately call your doctor or emergency help. If you
continue walking or ignore the symptoms, you may place yourself at risk for a cardiac arrest or
other health problems, possibly resulting in serious injury or death.
Page 11

Introduction
1-5
Indications for use
The LifeVest system is indicated for patients 18 years of age and older who are at risk for sudden
cardiac arrest and are not candidates for or refuse an implantable defibrillator.
The LifeVest system is indicated for patients under 18 years of age who are at risk for sudden
cardiac arrest and are not candidates for or refuse an implantable defibrillator. Patients must
have a chest circumference of 26 inches (66 centimeters) or greater and a weight of 18.75
kilograms (41.3 pounds) or greater.
Environment considerations related to noise and vibration
Certain environments or situations you encounter that are loud and have high vibration could
affect the LifeVest. A loud environment could make it difficult for you to hear an alert to be able
to appropriately respond. A high vibration environment may result in an inappropriate
treatment. For example, riding a motorcycle or using some lawnmowers may cause vibration
and make it difficult for some patients to hear an alert which may result in an inappropriate
treatment.
If you encounter a loud, high vibration environment while wearing the LifeVest, you should be
attentive to your device to ensure you respond to any alerts. In the unlikely event that vibration
causes you to receive a siren alert, press the response buttons to prevent receiving a treatment
and move away from the source of vibration.
Intended use locations
The intended electromagnetic environments for the LifeVest 5000 wearable defibrillator are
home, small clinic, hospital, and transport.
Essential performance
The essential performance of the LifeVest is that it detects ventricular fibrillation or ventricular
tachycardia, then delivers a treatment. Unacceptable risks include loss of detection and
treatment functionality.
Aircraft use
The LifeVest 5000 was tested to demonstrate compliance to the emissions and immunity
requirements of the following standard: RTCA DO-160F, Environmental Conditions and Test
Procedures for Airborne Equipment, Section 20 (RF immunity) and Section 21 (RF emissions).
Check with the airline for any special restrictions on using personal electronic equipment when
making flight reservations.
The Airplane mode feature disables the LV5000 from communicating wirelessly. Use this option
when on an airplane or in any environment where cellular phone use is prohibited.
Page 12

LifeVest 5000 Patient Manual
1 - 6
Operator profile
As defined by IEC 60601-1-6, the operator profile is a summary of the mental, physical, and
demographic traits of the intended user population.
According to the IEC definition, the operator is defined as the person interacting with the device.
In the case of the LifeVest, there are two operators:
•
The patient wears and interacts with the LifeVest on a daily basis. The patient profile
appears below.
•
ZOLL authorized representatives fit patients with the equipment, and train patients. The
ZOLL authorized representative profile appears below.
Patient profile
The device is appropriate for patients who are at risk for sudden cardiac arrest and meet the
requirements described in the Patient Manual.
ZOLL authorized representative profile
ZOLL authorized representatives are ZOLL-trained professionals. Their job is to program the
equipment according to the patient’s prescription, measure and fit patients with the LifeVest
equipment, train patients and family members, and provide follow-up assistance if necessary.
ZOLL authorized representatives must have the mental and physical capabilities to understand
training given by ZOLL that covers programming the device, measuring and fitting patients, and
training patients in the daily use of the device.
Patient training
It is essential that all patients receive training before wearing the LifeVest. This training is given
by ZOLL authorized representatives. These representatives deliver the LifeVest training in the
patient’s home or in a clinical setting.
Patient training consists of instructions for garment and electrode belt assembly and
disassembly; alert response, including the use of the response buttons; and how to change and
recharge the batteries.
After completing the training, patients sign an agreement that documents receipt of training in
the use and care of the LifeVest.
In addition, phone support is available for patients by calling ZOLL or a ZOLL representative. See
page ii, for the ZOLL phone support number.
Page 13

Introduction
1-7
Electromagnetic interference
Many common devices, including motors and electronic equipment, could produce
electromagnetic interference, also known as EMI, in the LifeVest device that could affect its
operation. The LifeVest device has been tested with a number of common sources of
electromagnetic disturbance, including cellular telephones, airport security systems, and anti-
theft detection systems. This testing, along with clinical trial testing, has demonstrated that in
everyday use the LifeVest device is not normally affected by commonly encountered
electromagnetic disturbances.
Anti-theft detection systems, also known as electronic article surveillance systems, are often
used in department stores and libraries to prevent theft by electronically sensing a special tag
on a piece of merchandise when the tag passes through a detector gate. In the USA, these
detector gates are commonly located near the doorways. In Europe, the detector gates may be
positioned near the checkout areas.
To prevent possible disturbance with the LifeVest device, follow these simple guidelines when
passing through airport security gates or anti-theft detection gates:
•
Walk through the gate at a normal pace.
•
Avoid lingering near or leaning on the gate.
In some occupational and hospital environments, unusually high levels of electromagnetic
disturbance could be encountered. Examples of possible sources of such disturbance include:
Magnetic resonance (MR) imaging equipment, communication equipment such as microwave
transmitters, arc welding equipment, high voltage transmission lines, electrocautery systems,
and electronic muscle stimulators. These environments should be avoided while wearing the
LifeVest device.
In the unlikely event that electromagnetic disturbance causes you to receive arrhythmia alerts,
press the response buttons to prevent being shocked and move away from the source of the
disturbance. The LifeVest device should return to normal monitoring mode in approximately 5
seconds.
Page 14

LifeVest 5000 Patient Manual
1 - 8
Wireless interference
The LifeVest can be susceptible to or cause wireless interference. Follow these instructions:
•
Cell phone use
– When using a cell phone, keep it at least 10.6 inches (27
centimeters) away from the round electrodes on the electrode belt. If you
experience noise alerts while using a cell phone, move the cell phone away from
the electrode belt or stop using the cell phone.
•
Charger use
– The charger contains a cellular device for data transmissions. Keep
the charger at least 10.6 inches (27 centimeters) away from your body to
prevent interference. If you experience noise alerts while using the charger,
move away from the charger. If you take the charger to a hospital, be sure that
the use of cellular devices is permitted. If not, do not use the charger while in the
hospital. If you need to transmit data, use your in-home or supported open WiFi
network.
•
General precaution
– If you experience any interference with the LifeVest when
in the presence of any other wireless device, move away from the device or stop
using the other device. If you continue to have problems, call ZOLL.
FCC regulatory information
This device complies with part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) This device may not cause harmful interference, and (2) this device must accept
any interference received, including interference that may cause undesired operation.
The monitor contains FCC-ID: W56MTPCIE-BW-A.
Refer to charger label for FCC ID.
Changes or modifications to this device not authorized by ZOLL could void the RF compliance
and negate your authority to operate the device.
The available scientific evidence does not show that any health problems are associated with
using low power wireless devices. There is no proof, however, that these low power wireless
devices are absolutely safe. Low power Wireless devices emit low levels of radio frequency
energy (RF) in the microwave range while being used. Whereas high levels of RF can produce
health effects (by heating tissue), exposure of low-level RF that does not produce heating effects
causes no known adverse health effects. Many studies of low-level RF exposures have not found
any biological effects. Some studies have suggested that some biological effects might occur, but
such findings have not been confirmed by additional research. The LV5000 has been tested and
found to comply with FCC radiation exposure limits set forth for an uncontrolled environment
and meets the FCC radio frequency (RF) Exposure Guidelines in Supplement C to OET65. The
maximum SAR levels tested for the LV5000 has been shown to be 0.133 W/kg at Body.
Page 15

Meet the LifeVest 5000 System
2-1
2:
Meet the LifeVest 5000 system
Components
Item
Description
Garment and
electrode belt
The garment fits around your body and the electrode belt connects to
the monitor. The electrode belt contains the therapy pads, round
electrodes and the vibration box. See details on page 2-2.
Monitor
Connects to the electrode belt. Monitors your heart rhythm and
initiates a treatment. See details on page 2-3 and 2-4.
A strap is attached to the monitor for easy mobility while wearing the
device.
Charger and
battery
Recharges the battery, communicates wirelessly with the monitor,
and transmits data for prescriber review. The LifeVest system includes
two batteries so that the monitor can run continuously on battery
power. See details on page 2-8 and 2-9.
2 1 3
Page 16

Page 17

Meet the LifeVest 5000 System
2-3
Monitor — front view
Item
Description
Response
buttons
Two buttons, located on the front and back of the monitor, that light solid red
when the device senses that your heart has a life threatening, irregular
rhythm. If you are conscious, press both response buttons to stop the LifeVest
from delivering a treatment. You can press and release the response buttons;
you don’t have to hold them continuously.
Speaker
Audible voice prompts and alerts.
Status
indicator
lights
Indicate system status.
Green: On solid during normal operation. Off during gong or siren alerts.
Yellow: Flashes during a gong alert to tell you that something requires your
attention. Check monitor screen for any instructions.
Connector
Connection site for the electrode belt cable.
Strap
attachment
Two strap attachment areas on either side of the monitor. The strap is
adjustable to give you two options to comfortably wear the system. For
options on how to wear, see section 3.
Touchscreen
Displays messages about device operation, and allows you to interact by touch
with the device. When the monitor is held upside down, the display flips so
that you can read the screen.
Most of the time during normal monitoring, the touchscreen is dark. To view
the display on the screen, press the response buttons.
For details, see page 2-5.
1
2
3 4
6
5
Page 18

LifeVest 5000 Patient Manual
2 - 4
Monitor — back view
Item
Description
Battery
placement area
Location of battery placement in the monitor.
Battery
Powers the monitor. To recharge the battery, use the charger; see
page 2-8.
Battery release
button
Press to remove the battery from the monitor.
1 2
3
Page 19

Meet the LifeVest 5000 System
2-5
Monitor touchscreen
Shown on the following page is an example of the monitor screen during normal monitoring.
Not all of the symbols, controls, and indicators shown in this example will appear all of the time.
Some symbols are shown only at certain times.
As situations change, the screen will change to advise you and suggest an action to take. Screens
that require you to take some action will have a help screen associated with them. For more on
help screens, see page 2-7.
Most of the time the monitor display screen is darkened to conserve battery power. If you wish
to view the Home screen or complete an action while wearing the LifeVest, press the response
buttons.
For a complete description of how to use the touchscreen in the daily use of the LifeVest, see
section 3.
Page 20

LifeVest 5000 Patient Manual
2 - 6
Item
Description
Patient name
Displays your name so you know this device was programmed for you.
Menu button
Tap to display the Patient Menu, where you can select various options. See
details in section 3.
Electrode belt
status
Round electrodes are connected to the patient and are in normal
monitoring mode.
Belt is not connect
ed. See details in section 5.
Battery status
Shows amount of charge remaining in battery.
Green displays the remaining charge level.
Yellow indicates that the battery needs to be changed. Call ZOLL
for a replacement.
Red indicates a depleted charge.
Airplane mode
Airplane mode is on. For details, see section 3.
Recording heart
rhythm
Indicates that your heart rhythm is being recorded for your prescriber to
revi
ew, see section 3.
Page 21

Meet the LifeVest 5000 System
2-7
Help screens
For any screen on the monitor that has a question mark icon in the lower right corner, you
can tap that icon and see a help screen.
Shown below is an example of a help screen.
Note:
Not all screens will have a help button. This button also appears in different colors such
as
on response button screens where the help button
is
blue
.
Item
Description
Help message
Brief message telling you what to do based on the present condition.
Touch to speak
If the speak option is enabled, touch the message area of the screen to hear the
help message. The message will be spoken in the language displayed. The area to
tap is shown by the dashed line in the example above; the dashed line will not be
visible on screen. To enable this feature, see section 3.
X button
Tap to close the screen.
Translation
button
O
nly appears if more than one language is programmed.
Page 22

LifeVest 5000 Patient Manual
2 - 8
Charger
The charger recharges the battery and communicates wirelessly with the monitor.
The LifeVest system includes two batteries so that the monitor can run continuously on battery
power. For details on battery care, see section 3.
Item
Description
Power supply
Plugs into a standard power outlet to provide power to the charger.
For the charger power supply, any 60601-1 or 60950-1 approved
power supply providing an output of 18 VDC at 1.67 Amps (min) with
a CUI PPM-2-35135-SG connector, see Charger setup in section 3 for
details
.
Battery status
indicators
Indicate charger status. See details on page 2-9.
Battery
Powers the monitor. To recharge the battery, use the charger.
Power supply
connection area
Connects the power supply to the charger.
1 2
3
4
Page 23

Meet the LifeVest 5000 System
2-9
Battery charger indicators
The battery charger indicators are located on the top of the charger. These indicate the charger
status. For details, see section 3.
Icon
Description
Power
Charger is connected to a power source.
Charging
Battery is currently being charged. Light blinks until fully charged
and then turns off.
Charge complete
Battery has full power.
Battery fault
Charger communication may not be functioning properly. Charger
can still be used to charge battery. Solid light and a momentary
beeping sound occurs. Call ZOLL.
Charger fault
Problem with the charger. Light blinks and a beeping sound occurs.
Call ZOLL.
2 1 3 4
5
Page 24

LifeVest 5000 Patient Manual
2 - 10
This page intentionally left blank.
Page 25

Using the LifeVest 5000 System
3 - 1
3: Using the LifeVest
Daily routine
This is an overview of the steps involved in the daily use of the LifeVest. Some details are found
elsewhere in this manual.
1. Wear the assembled electrode belt and garment. For details on wearing the LifeVest,
see section 4.
2. Put a fully-charged battery into the monitor and follow the normal startup routine. See
page 3-2.
3. Change and recharge the battery every 24 hours. See page 3-3, Battery care.
4. Wear the monitor with the strap fastened to the two attachment areas on the monitor.
The strap is adjustable to give you two options to comfortably wear the system, either
around your waist or hanging over your shoulder like a purse.
5. Respond to any alerts. See section 5.
6. Remove the battery from the monitor and then completely remove the LifeVest from
your body when you shower or bathe. Do not reinsert the battery until you are wearing
the fully assembled device. See section 4.
7. Change and wash the garment every 1 or 2 days. Wash only the garment. Do not wash
the electrode belt, monitor, or any other accessories. Follow the instructions in section 4
for laundering the garment.
Page 26

LifeVest 5000 Patient Manual
3 - 2
Normal startup routine
1. Remove the depleted battery from the monitor.
2. Insert a fully-charged battery into the monitor.
•
Make sure the battery is completely inserted.
3. Startup screen appears.
4. When you hear the gong and feel the vibration
on your back, press the response buttons.
•
If you do not hear the gong or feel the
vibration within 30 seconds, remove the
battery. Reinsert the battery and try again.
•
If the monitor still does not operate normally,
contact ZOLL.
5. The monitor displays your name and battery
level.
•
Make sure your name appears on the monitor.
If your name does not appear, contact ZOLL
immediately.
6. During normal monitoring, most of the time the
LifeVest monitor screen displays a dark screen
and the green status indicator is lit solid.
•
To see the display, press the response buttons.
Page 27

Using the LifeVest 5000 System
3 - 3
Battery care
What you need to know
•
You have two batteries, so you can use one
while charging the other.
•
Change and recharge batteries every 24 hours.
•
Recharging the battery takes approximately 4 to
6
hours.
•
Place the charger in a safe place where you can
leave it plugged in. Keep the second battery in
the charger while you use the monitor.
•
Always have a battery in the charger, even when
fully cha
rged.
•
The battery and charger may get warm. This is normal. Place the charger in a well ventilated
place.
•
Use only the batteries and charger supplied with the LifeVest system.
•
Remove the battery from the monitor when not wearing the device (when showering, for
example). Always remove the battery first to ensure the device is not active when you are not
wearing it. A “whistle” alert will sound if the battery has been left in the monitor for over 20
minutes with the electrode belt disconnected.
In the event of a power outage
If there is a power interruption you need to take steps to keep your batteries charged.
•
Notify your electric company that you have a
medical device that needs power. If they believe
the power will be out for 24 hours or more seek
alternatives for powering the batteries, such as a
neighbor or family member not affected by the
outage.
•
Notify your local emergency medical services and
see if they can help.
•
Locate a source of backup power if possible, such as a generator. Plug the battery charger into
the backup power and charge the spare battery continuously. Change the batteries every 24
hours.
If help is not available, and you expect power to be out for more than 24 hours, contact ZOLL
immediately to have spare batteries sent to you.
When the power is restored, continue charging and changing batteries every 24 hours.
Page 28

LifeVest 5000 Patient Manual
3 - 4
Charger setup
If the charger or power supply makes unusual noises, sparking, starts to smoke, or emits a burning
smell, unplug it immediately from the wall outlet.
The only way to completely remove power is by
unplugging the AC power plug from the wall outlet.
Note:
For the charger power supply, any 60601-1 or 60950-1 approved power supply providing
an output of 18 VDC at 1.67 Amps (min) with a CUI PPM-2-35135-SG connector as detailed
below may be used.
Connection
Designation
Inner Contact
+
V
Outer Ring
-
V
1. Place the charger in the room where you
sleep, on a nightstand or end table, near a
power outlet.
•
Place the charger so you can easily get to
the top of the unit to insert and remove the
battery.
•
The charger can actually be placed
anywhere in the house, but we recommend
the room where you sleep so it is
convenient to use every day.
2. Plug the power supply into the back of the
charger, then plug it into a standard power
outlet.
•
Make sure the outlet stays on all the time,
and is not controlled by a light switch.
•
Keep the charger plugged in at all times.
•
When you first plugin the charger, all of the
icons will blink three times and you will hear
a rapid, beeping sound.
•
When the charger is powered on, the blue
icon
remains on
.
Page 29

Using the LifeVest 5000 System
3 - 5
3. Gently insert the spare battery into the
charger.
•
Orient the battery with its connector facing
into the base of the charger.
•
Push the battery in firmly.
•
It takes approximately 4 to 6 hours to
charge a battery.
•
Keep a battery in the charger at all times,
even when fully charged.
4. Look for a symbol along the left of the
charger’s display. These symbols indicate the
battery status.
•
The battery should be charged or
charging.
•
These symbols are explained in the next
section, Charger and battery status
indicators.
Page 30

LifeVest 5000 Patient Manual
3 - 6
Charger and battery status indicators
Icon
What it means
What to do
1
Power
Power is on.
Plug the power supply into the back of
the charger, then plug it into a standard
power outlet. Make sure the outlet stays
on all the time, and is not controlled by a
light switch.
Always leave a battery in the charger,
even when the battery is fully charged.
2
Charging
Battery is charging.
Let the battery charge. This can take 4 to
hours.
6 3
Charge complete
Battery is fully charged,
ready for use in monitor.
Leave battery in the charger until ready to
exchange battery even when the battery
is fully charged.
4
Battery fault
Battery has a problem.
Call ZOLL for a replacement battery.
5 Charger fault
Charger has a problem,
and cannot charge the
battery.
Do not leave a battery in the charger.
Battery is not being charged.
Call ZOLL for a replacement charger.
Page 31

Using the LifeVest 5000 System
3 - 7
Change and charge batteries daily
Leave the electrode belt connected during this
procedure.
1. Remove the existing battery from the monitor
by pressing the release button on top of the
battery, moving it away from the monitor.
•
Leave the electrode belt connected to the
monitor during this procedure.
2. Remove the fully-charged battery from the
charger and place it into the monitor.
•
Push the battery firmly into the monitor until
it clicks to ensure it is fully inserted.
3. Gently place the used battery from the
monitor into the battery charger.
•
Properly orient the battery with the label side
down and firmly push it into the charger
base.
•
Check the battery status on the charger and
verify that the battery is being charged. It
takes approximately 4 to 6 hours to charge a
battery.
Page 32

LifeVest 5000 Patient Manual
3 - 8
Record your heart rhythm (ECG)
At times, you may want to record your heart rhythm
(
ECG) for your prescriber to review.
1. Hold the response buttons for 3 seconds.
2. Release response buttons when you see the
recording indicator and hear a single gong.
On the monitor the ECG recording indicator will
appear in the bottom right corner of the screen. The
recording will take 15 seconds.
Page 33

Using the LifeVest 5000 System
3 - 9
Patient menu screen
Tap
MENU
on the Home screen to open the Patient Menu screen.
Note:
Your prescriber may assign
ACTIVITIES
for you to perform. If the
ACTIVITIES
icon is not
displayed on your monitor your prescriber has not assigned you to do any.
Settings menu
See more information starting on page 3-10.
Send data
See more information in on page 3-11.
Activities menu
See more information in section 6, Using the
activities options.
Note:
If the
ACTIVITIES
icon is not displayed
on your monitor it is inactive. It is not
necessary to have
ACTIVITIES
activated. This
is at your prescriber’s discretion.
Page 34

LifeVest 5000 Patient Manual
3 - 10
Voice options
The LifeVest system provides the option of having Help screen messages spoken to you when
they are displayed on the monitor. Help screens provide information and/or instructions about a
specific topic or aid in resolving a problem. This section explains the voice options and how to
set them so the monitor speaks Help messages.
Note:
Voice options do not affect the volume level of physiological alerts or messages.
1. On the home screen, tap
MENU
.
2. On the Patient Menu, tap
SETTINGS
.
3. On the Settings Menu, tap
VOICE
.
4. On Help Voice Options there are two options:
Volume and Mode.
Volume
Use the arrow keys to increase or decrease the
volume of the messages.
Note:
Volume is not affected for physiological
al
ert
s or mess
ages.
Mode
If you want the messages to
automatically speak to you
when Help screens appear,
select the Always Speak
option.
If you want the messages
spoken to you only when you
tap the screen when Help
screens appear, select the Tap
to Speak option.
If you do not want to hear the
messages spoken to you,
select Mute.
5. Tap
ACCEPT
to confirm your selections and
return to the Settings screen.
To close the screen without making any
changes, tap
X
.
Page 35

Using the LifeVest 5000 System
3 - 11
Sending data manually
Normally, data transmission from the monitor to ZOLL occurs without you having to initiate it.
However, if data is requested by ZOLL after a treatment or for another reason, the Send Data
icon provides the means for you to send the data.
1. On the monitor home screen, tap
MENU
.
•
If the monitor screen is off, press the response
buttons to show the home screen.
2. On the Patient Menu, tap
SEND DATA
.
3. Tap
SEND
to begin the data transfer.
Note:
Look at the WiFi and cell signal strength
icons for network availability. See details on
page A-1.
If you experience problems sending data, call
ZOLL.
4. The device then connects through the charger or
through WiFi to send your data.
•
The gray envelopes on the screen will turn
green as data is successfully sent to ZOLL.
Page 36

LifeVest 5000 Patient Manual
3 - 12
5. Your download is complete once you see all
envelopes turn green and hear a gong alert.
6. Tap
ACCEPT
to close the Send Data screens and
go to the Home screen.
7. You have now successfully sent your data to
ZOLL.
If data is unable to send, you will see this screen
and hear a gong alert.
Tap
TRY AGAIN
.
If you continue to have a problem sending data,
write down the service code (in the bottom right
corner of the screen) and call ZOLL.
If airplane mode is ON you will see this screen.
Tap
CONTINUE
to turn airplane mode off and
continue sending data.
For details, see Airplane mode page 3-13.
Page 37

Using the LifeVest 5000 System
3 - 13
Airplane mode
Airplane mode disables transmitting data capabilities. Use Airplane mode in any situation where
wireless transmission use is prohibited in order to avoid interference with aircraft operation and
other electrical equipment.
1. On the home screen, tap
MENU
.
2. On the Patient Menu, tap
SETTINGS
.
3. On the Settings Menu, tap
AIRPLANE
. 4. Airplane mode is off by default. Tap
ON
to
enable Airplane mode, then tap
ACCEPT
to
confirm and close the screen.
Note:
The monitor will go out of airplane mode
the next time you remove or change the
battery.
Tap
X
to close the screen without making any
changes
.
Page 38

LifeVest 5000 Patient Manual
3 - 14
5. The airplane symbol will appear and remain on
the Home screen when airplane mode is
enabled.
Note:
The monitor will go out of airplane mode
the next time you change the battery.
If you attempt to manually send data, enter
support mode, or set up a WiFi connection while in
airplane mode, you will see this screen:
You have two choices:
1. To turn off airplane mode and continue
with the task, tap
CONTINUE
.
2. To leave airplane mode turned on and
not proceed with the task, tap
X
.
Page 39

Using the LifeVest 5000 System
3 - 15
WiFi
WiFi allows you to transmit data to ZOLL from any location where wireless networks are
available.
Searching for and accessing available WiFi networks
1. On the home screen, tap
MENU
.
2. On the Patient Menu, tap
SETTINGS
.
3. On the Settings Menu, tap
WiFi
.
4. On WiFi Settings, tap
SEARCH AVAILABLE
NETWORKS
to browse for an available WiFi
network.
Note:
If airplane mode is off and no downloads
are taking place, the WiFi Settings screen
displays.
A searching message will appear while the
device looks for an available network.
Page 40

LifeVest 5000 Patient Manual
3 - 16
5. If networks are available they will appear for
selection. Use the up and down arrows to
navigate to additional networks in the list. Each
network displays signal strength and security
status (locked or unlocked). A locked network is
a closed network that is not accessible by the
LifeVest.
6. Tap on the network name you want to use. A
wait screen may appear while your selection is
identified.
Tap
X
at any time to cancel.
Encrypted Network Password required:
If a
password is required when you select a network, a
keyboard appears for entering the password for
that network.
Tap the shift key (up arrow) on the
keypad to shift between
uppercase and lowercase letters.
Tap the numeric button to access
numbers and symbols.
Tap the cancel/backspace key to
delete and retype the password.
After typing the password, tap
ACCEPT
.
A wait screen may appear while
your password is being validated.
Page 41

Using the LifeVest 5000 System
3 - 17
7. If the network is available, its name is displayed
as connected.
Tap
ACCEPT
. Password invalid:
A screen is displayed If the
password is invalid.
Tap
TRY AGAIN
and re-enter the password on the
keypad screen.
Tap
X
to close.
A screen displays to inform you if the network is
not available.
If this occurs, tap
ACCEPT
to go back to the Search
Available Networks menu to choose a new
network.
If Previously Connected:
If the device was
previously connected to the WiFi network selected
then the WiFi network information screen will
appear.
Tap
CONNECT
to proceed to the Enter Password
screen.
Tap
FORGET
for your device to forget all
information for this WiFi network.
To close the screen without making any changes,
tap X .
Page 42

LifeVest 5000 Patient Manual
3 - 18
Tapping
FORGET
on the previous screen displays the
WiFi Network information screen
.
Tapping
FORGET
a second time deletes all network
information on this device.
To go back to the WiFi Network security scre
en,
tap X .
If no networks are available, the “No
WiFi networks
are available” message is displayed.
Tap
ACCEPT
to go back to the WiFi Settings menu.
If you do not tap
ACCEPT
, after 30 seconds a timed
out screen will display.
If you are already connected to a WiFi network or
have previously connected you will bypass the
password screens and go directly into th
is screen.
Network Connected:
If you are connected to a
network
DISCONNECT
appears on the WiFi Network
screen.
Tap
DISCONNECT
to get back to the WiFi settings
menu.
Tap
FORGET
to go to the WiFi Network Forget
screen.
To go back to the WiFi
Settings screen, tap
X
.
Page 43

Using the LifeVest 5000 System
3 - 19
If you are not able to connect to WiFi Settings one of the next two screens will display.
If a download is in progress, this screen
displays.
If airplane mode is on, this screen displays.
Tapping
CONTINUE
turns off airplane mode.
Tap
X
to close this screen and remain in
airplane mode.
For details, see Airplane mode on page 3-13.
Page 44

LifeVest 5000 Patient Manual
3 - 20
Entering a hidden WiFi network
1. On the home screen, tap
MENU
.
2. On the Patient Menu, tap
SETTINGS
.
3. On the Settings Menu, tap
WiFi
.
4. On the WiFi Settings menu, tap
ENTER HIDDEN
NETWORK
.
If
ENTER HIDDEN NETWORK
is selected from
the WiFi Settings screen, a keypad screen
appears to enable entry of the network name.
5. After typing in a Network Name, tap
ACCEPT
.
6. If a
Network
is
available
, the Network Security
screen appears, displaying security level
options. If you are not sure of your network
security, select
WPA2
as your first option.
A “Please wait…” screen may then appear while
your selected network option is being
connected.
If you require assistance with the hidden
network connection, contact ZOLL.
7. If a
Password
is
required
wh
en you select a
network from the list, a keyboard appears.
After typing the password, tap
ACCEPT
. If you
do not tap
ACCEPT
, after 30 seconds a timed
out screen displays.
Page 45

Using the LifeVest 5000 System
3 - 21
If NO password is required
, the connection is
made. Tap
ACCEPT
to continue.
If the network is not available
, a screen is
displayed to inform you of this.
Tap
TRY AGAIN
to try and connect to the network
again. This takes you back to the Enter Network
Name keypad screen.
Tap
X
to close. This takes you back to the Enter
Network Name keypad screen.
Try connecting with the
WPA
security network
option.
If you are still unable to connect, contact ZOLL for
assistance.
Page 46

LifeVest 5000 Patient Manual
3 - 22
Clearing all saved WiFi networks
1. On the home screen, tap
MENU
.
2. On the Patient Menu, tap
SETTINGS
.
3. On the Settings Menu, tap
WiFi
.
4. On the WiFi Settings menu, tap
CLEAR ALL
SAVED NETWORKS
.
5. On the
CLEAR ALL SAVED NETWORKS
screen,
tap
DELETE
to delete all saved WiFi networks or
CANCEL
to go back.
6. Tap
DELETE
to confirm deleting all saved WiFi
networks.
Tap
CANCEL
to go back to the WiFi Settings
menu.
7. The last screen provides confirmation that all
WiFi networks have been deleted.
Tap
ACCEPT
to take you to the WiFi Settings
menu screen.
Page 47

Using the LifeVest 5000 System
3 - 23
System information
If you ever call ZOLL, you may be asked for information about your LifeVest. You may be
instructed to go into the system information screens.
1. On the home screen, tap
MENU
.
2. On the Patient Menu, tap
SETTINGS
.
3. On the Settings Menu, tap
INFO
.
4. The System Information screens display
information associated with the system
components.
Tap on the right arrow to see more
System Information options.
Tap
X
to close and return to the Settings
Menu.
Page 48

LifeVest 5000 Patient Manual
3 - 24
Support mode
This feature lets ZOLL update the settings in your LifeVest monitor while you remain at home.
It is a simple process that should take just a few minutes.
Updating your LifeVest:
•
ZOLL will call you to talk you through the updating process.
•
Remain on the phone with ZOLL until this entire process has been completed.
•
Continue to wear the device during this process.
•
You will interact with the monitor screen, tapping icons and reading back what the
display says.
•
If you are unable or uncomfortable doing this, have someone with you who can talk
with ZOLL and interact with the monitor.
•
Follow along with the procedure below.
Note:
You need to have access to your charger or WiFi to update your settings.
ZOLL will call you to assist you through this process.
1. On the home screen, tap
MENU
.
2. On the Patient Menu, tap
SETTINGS
.
3. On the Settings Menu, tap
SUPPORT
.
4. On the Support Mode screen, tap
CONTINUE
.
5. A “Connecting” screen appears.
Page 49

Using the LifeVest 5000 System
3 - 25
6. When this screen appears, read this number to
the ZOLL representative.
7. A “Please wait…” screen appears.
8. Read this number to the ZOLL representative.
9. Tap
ACCEPT
.
Note:
If you attempt to enter the support mode
while in airplane mode, you will see this screen.
You have two choices:
•
To turn off airplane mode and continue
to enter support mode, tap
CONTINUE
.
•
To leave airplane mode turned on and
not enter support mode, tap
X
and
return to the Settings Menu screen.
For information on Airplane mode, see page 3-13.
Page 50

LifeVest 5000 Patient Manual
3 - 26
Periodically clean and inspect the system
WARNING
Do not put the monitor, electrode belt, battery or charger in or near water.
Water entering the
device may damage it and/or cause the system to malfunction.
If you see Blue™ gel other than during a treatment, this may indicate a damaged electrode belt
and cause the system to malfunction.
Call ZOLL LifeVest immediately.
Clean the electrode belt and inspect equipment when changing garments. Specific details about
laundering the garment are in section 4.
Cleaning non-washable items
Unplug the battery charger and disconnect the power cord from the wall outlet before cleaning.
If necessary non-washable items such as the charger, batteries, cables, and therapy pads may be
cleaned with a soft cloth. Do not use a damp cloth or anything with water, chemicals or cleaning
solutions.
If necessary, clean the monitor touchscreen by wiping it with a soft cloth dampened with
window or glass cleaner. Dampen the cloth directly, do not spray anything directly on the
monitor.
The round electrode metallic surface may be cleaned with a soft cloth dampened with rubbing
alcohol.
•
Don’t apply liquids directly to any of the non-washable items, as they contain electronic
components that can be damaged.
•
Don’t attempt to clean any electrical contacts or connectors.
•
Do not use any cleaning solution on the garment. Specific details about laundering the
garment are in section 4.
Call ZOLL if any additional cleaning is needed for the monitor or charger.
Inspection
Inspect your system when changing garments. If you should notice any of the following
conditions, please notify ZOLL as soon as possible:
•
Cracks in the housing of the monitor, battery, or charger.
•
Cracks or lines showing on the monitor screen.
•
Cracks in the therapy pads.
•
Tears in the garment.
•
Blue™ gel leaking from the therapy pads at any time other than when defibrillation is
about to occur or has just occurred.
Page 51

Using the LifeVest 5000 System
3 - 27
What family members need to know
WARNINGS
Only the patient should press the response buttons.
The patient’s ability to press the response
buttons lets the device know whether or not the patient is conscious and is critical in deciding when
to give the patient a treatment. If anyone other than the patient presses the response buttons,
needed therapy may not be delivered, possibly resulting in serious injury or death.
Do not touch the patient while a treatment is being delivered.
Anyone touching the patient during
a treatment may be shocked.
Do not remove the battery, do not disconnect the electrode belt from the monitor, and do not
loosen the garment while the monitor is broadcasting alert sounds and/or voice prompts.
If the
battery is removed, the electrode belt disconnected from the monitor, or the garment is loosened,
needed therapy may not be delivered, possibly resulting in serious injury or death. CPR can be
performed as long as the monitor is not broadcasting alert sounds and/or voice prompts. If external
defibrillation is available, a decision can be made by a medical professional to remove the device
and monitor/treat the patient with external equi
pment.
Information for family members
Since your family member or friend may be wearing the LifeVest device for a period of time, you
may want to understand the daily routine involving the device, as well as warnings and cautions
directed to the patient. In that case you should read this entire manual.
Anyone associated with the patient should be aware of the following information:
The LifeVest directs a conscious patient to respond to messages and alerts. A patient who has
lost consciousness due to their arrhythmia will be unable to respond, letting the device know it
is time to deliver a treatment. The bystander should not respond to messages and alerts in
place of the patient.
•
If the LifeVest treatment
does not revive the
patient and the patient is unconscious, call for help
and then start CPR.
•
If the patient remains conscious, check the belt to
make sure all round electrodes are pressing
against the patient’s skin.
•
A label on the front of the garment reminds
medical professionals to open the garment before
giving the patient conventional external
defibrillation.
•
Keep the LifeVest device out of the reach of
children.
*1
*1
See indication for patients under 18 years of age.
Page 52

LifeVest 5000 Patient Manual
3 - 28
When you are finished with the device
Call ZOLL and arrange to return the LifeVest system.
Battery recycling
WARNING
Do not dispose of or incinerate the batteries.
The batteries contain lithium ion and must be
disposed of properly by ZOLL.
The lithium-ion batteries used with the LifeVest device are recyclable and should be returned to
ZOLL.
Page 53

Assembling and putting on the garment
4 - 1
4: Garment and electrode belt
Assemble the garment and electrode belt
Lay the electrode belt and garment on a flat
surface as shown in the illustration.
•
Place the garment with the silver mesh fabric
facing up.
•
Place the electrode belt with the silver sides
of the therapy pads facing up.
1. Insert the rear therapy pads into the
garment’s rear pockets.
•
The rear pockets are numbered 1.
•
Insert the pads silver to silver, meaning place
the silver sides of the pads (with the green
labels) facing the silver mesh fabric on the
pockets. Make sure the pads are flat against
the silver mesh fabric.
•
Snap the green tabs to close the pockets,
making sure they are securely fastened.
Page 54

LifeVest 5000 Patient Manual
4 - 2
2. Position and secure the vibration box to the
garment.
•
The vibration box flap is numbered 2 on the
garment.
•
Place the vibration box with the label side
facing up.
•
Make sure wires are not crossed or twisted.
•
Snap the flap over the vibration box to the
blue tabs, making sure all three snaps are
securely fastened.
3. Insert the front therapy pad into the front
pocket.
•
The front pocket on the garment is
numbered 3.
•
Insert the pad with silver to silver, meaning
place the silver side of the pad (with the
green labels) facing the silver mesh fabric on
the pocket.
•
After the therapy pad is fully inserted, snap
the pocket closed, making sure it is fastened
securely.
Page 55

Assembling and putting on the garment
4 - 3
4. Attach the round electrodes to the garment,
matching the colors on the backs of the round
electrodes to the colors of the Velcro on the
garment. Make sure not to cross or twist the
wires.
5. Make sure that you have properly assembled
the electrode belt to the garment.
Assembled garment and electrode belt
The assembled electrode belt and garment should look like the following figures.
Outside view
This side faces away from your body
when worn. The foam sides of the
therapy pads face the back of the
garment.
Inside view
This side is placed against your body when
worn. The silver mesh fabric pockets are
against your skin. The green labels are
visible through the silver mesh fabric.
Page 56

LifeVest 5000 Patient Manual
4 - 4
Putting on the assembled garment and electrode belt
Follow these instructions to put on the assembled garment, then make sure you’re wearing it
properly.
1. Before putting on the garment, remove all
clothing and undergarments from your upper
body.
•
All clothing, including underwear must be
worn OVER the device, NOT under it.
2. If desired, apply unscented hand lotion to the
four round electrodes.
3. Put on the garment, making sure:
•
The garment doesn’t get twisted.
•
The silver mesh fabric pockets touch your bare
skin.
•
The round electrodes are flat against the skin
with no crossed or twisted wires.
If you are a female:
•
Wear a bra
OVER
the assembled electrode
belt and garment.
•
Make sure that the silver side of the front
therapy pad is pressing against your body
underneath your lef
t breast.
Page 57

Assembling and putting on the garment
4 - 5
4. Connect the garment ends together in the
front.
•
Make sure that the clips are fully inserted past
the slight bumps in the clips.
•
There are two clip sets, a looser and tighter
setting allowing for fitting adjustments.
5. Look in a mirror to make sure that:
•
The garment is being worn correctly, fitting snug to your torso.
•
The garment is not twisted. Straps are flat against your skin and tightened.
•
The round electrodes and therapy pads are pressing against bare skin. The silver mesh fabric
pockets and silver side of the therapy pads (with green labels) MUST TOUCH YOUR BODY for the
device to work properly.
•
None of the cables are crossed, twisted together or interfering with the round electrodes.
Your garment should look like these figures.
Page 58

LifeVest 5000 Patient Manual
4 - 6
6. Check the position of the garment on your body
and make sure it is not too high or too low.
To position the garment properly, you may
need to adjust the shoulder straps.
Move the buckle sliders to position the garment
properly, and for a snug fit.
•
The garment
should
cross your body just
below your breastbone.
•
The garment
should not
be as high as your
nipples.
•
The garment
should not
be as low as your
belly button.
Page 59
Assembling and putting on the garment
4 - 7
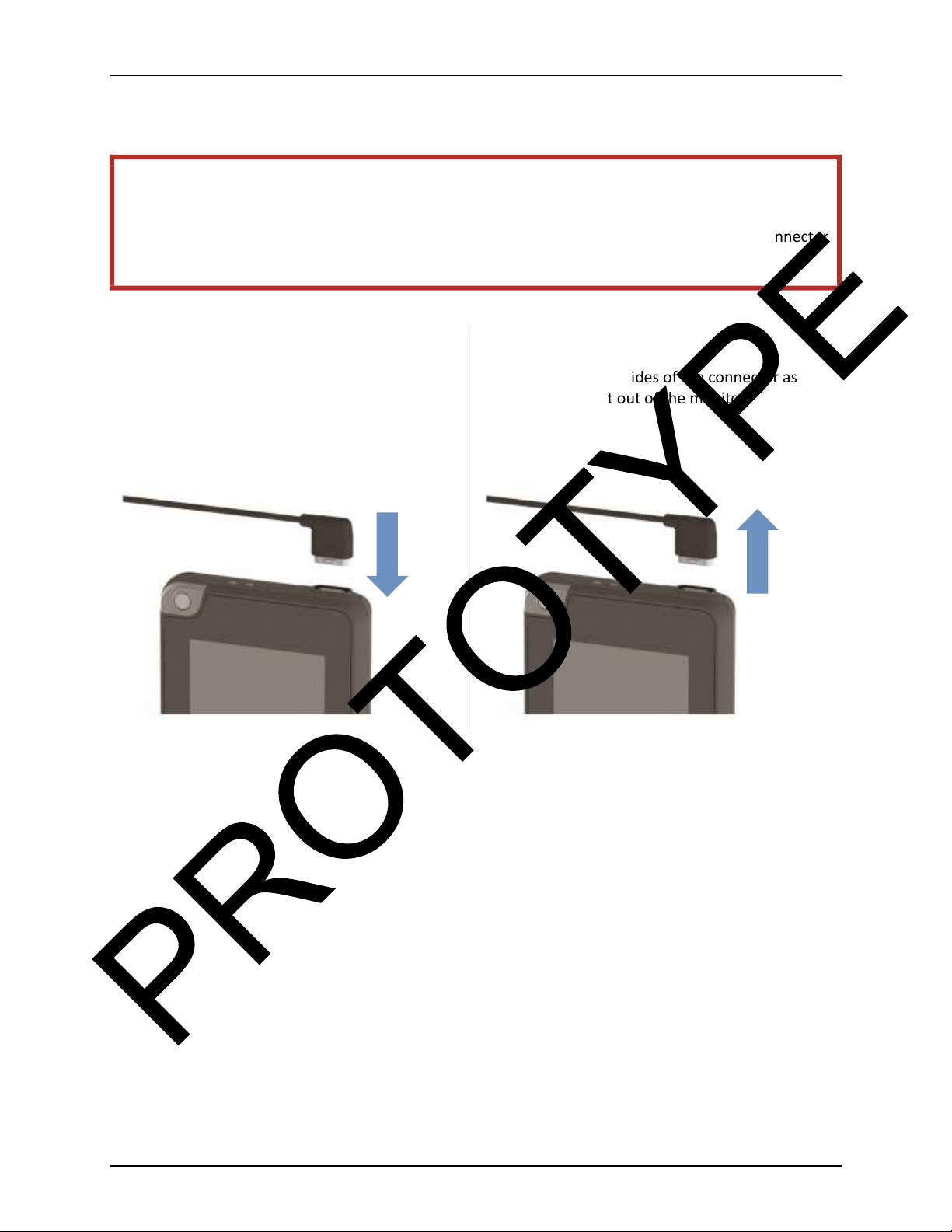
Connecting and disconnecting the electrode belt
WARNING
Do not force the connector.
Allow the connector to align before pushing it in. Forcing the connector
may damage it and cause the system to malfunction.
To connect the belt
Line up the connector with the monitor. The
cable should face toward the center of the
monitor. Gently push the connector straight in
until it locks in place.
To disconnect the belt
Gently squeeze the sides of the connector as
you pull it straight out of the monitor.
Page 60

LifeVest 5000 Patient Manual
4 - 8
Remove when showering and bathing
WARNING
Do not put the monitor, electrode belt, battery or charger in or near water.
Water entering the
device may damage it and/or cause the system to malfunction.
Always completely remove the LifeVest device when you
shower or bathe. Don’t put the monitor, electrode belt,
or battery in or near water.
When you remove the device for any reason, remove
the battery first.
When you remove the LifeVest to bathe or shower, you
are not protected by the device. Bathe or shower
preferably when someone else is home with you.
When you put the LifeVest back on, put the battery in
last.
Note: The battery should be removed if the LifeVest is not
being used.
A loud whistle sound is activated if the LifeVest has been idle for 20 minutes or more with the
electrode belt disconnected. This alerts you to remove the battery.
Removing the LifeVest device before you bathe or shower
1. Remove the battery from the monitor. Keep
the belt connected to the monitor.
2. Unfasten and remove the garment from your
body.
To change the garment, follow the procedure
on page 4-9 and then 4-1.
Page 61

Assembling and putting on the garment
4 - 9
Disassembling the garment and electrode belt
1. Remove the four round electrodes from the
garment by gently separating the Velcro from
the garment. Do not pull on the wires.
2. Unsnap fasteners and remove the vibration
box from the garment.
•
Gently pull vibration box away from the
garment. Pulling on the vibration box wires
can damage the internal wiring and cause
the system to malfunction.
3. Unsnap and remove the therapy pads from
their pockets.
4. Wipe the metallic surfaces of the round
electrodes with a soft cloth dampened with
rubbing alcohol.
Note for reference:
•
Assembling the garment and electrode belt
see page 4-1.
•
Putting on the assembled garment and
electrode belt see page 4-4.
•
For laundering instructions, see page 4-10.
Page 62

LifeVest 5000 Patient Manual
4 - 10
Laundering the garment
WARNING
Do not use chlorine bleach, bleach alternatives, fabric softener, anti-static sprays or detergents
that include bleach or fabric softener additives when laundering the garment.
Using bleach or any
of these other prohibited agents to launder the garment may damage it and cause the system to
malfunction.
Launder the garment every 1 or 2 days.
Before washing the garment:
•
Remove the electrode belt (round electrodes and therapy pads)
to protect it from damage.
Do not wash the electrode belt with
the garment.
•
Attach the clips of the garment together.
•
Wash the garment by itself. Do not wash it with other laundry.
•
The garment may be hand-washed or machine washed, using a
normal wash cycle and warm water, with a maximum water
temperature of 45°C (113°F).
•
Use a mild clothes washing detergent only, such as varieties for
sensitive skin.
•
Use a clothes dryer on medium heat setting to dry the garment.
Do not use the high heat setting. Do not use dryer sheets. (If a
dryer is not available, the garment may be laid out on a flat
surface to air dry.)
Page 63

Responding to alerts
5 - 1
5: Responding to alerts
Overview of alerts
This section is organized by the types of alerts you can experience while wearing the LifeVest:
•
Physiological alerts
, such as in response to a treatment or asystole. See below.
•
Technical alerts
such as a service code. See page 5-4.
•
Informative alerts
, such as a reminder to change the battery or respond to round
electrode noise. See page 5-6.
Physiological alerts: vibration or siren sound
These alerts are in response to a physiological condition or possible heart related activity
detected while wearing the LifeVest.
Vibration
alert
During normal use, if you get a vibration in the back of the electrode
belt, you are being warned that the siren alert will sound within 5
seconds. You will also get a short vibration alert every time you change
the battery.
Press the response buttons to stop the siren alert and the vibration alert.
Siren alerts
The siren alert is a high-pitched two-tone sound that means an abnormal
heart rhythm has been detected. The response buttons will turn red
during this alert.
Press the response buttons to stop this alert. If you do not respond to
this alert, you will probably receive a treatment within the next minute.
The siren alert is accompanied by screen messages and voice prompts.
There are two possible messages:
1. If the alert is accompanied by a “respond” message follow instructions on the following
page. This alert instructs you to respond.
2. If this alert is accompanied by the “call for help” message it will audibly instruct
bystanders to call for help.
Page 64

LifeVest 5000 Patient Manual
5 - 2
Responding to a siren alert
WARNINGS
If you get an alert sound and you are awake, always press the response buttons to prevent
receiving a treatment.
If you fail to press the response buttons, you will get a treatment.
If you receive a treatment while your heart is beating normally and you did not use the response
buttons, the treatment may cause an abnormal rhythm to occur.
There is a small possibility that
the abnormal heart rhythm may not be detected and death may result.
If you are conscious
, press the response buttons to
stop the treatment from occurring. You will see these
red bordered screens when you need to respond to a
siren alert.
•
The siren stops and a voice prompt indicates
that the treatment is being delayed.
•
NO ONE ELSE
should press the response
buttons. Only you, the patient, should press the
response buttons.
•
If you feel dizzy, find a place to sit or lie down.
•
Press the response buttons each time you feel
the vibration alert or hear the siren alert.
If you are not conscious
, you will not be able to press the response buttons.
This allows the device to deliver a treatment.
Voice prompts alert bystanders not to touch you.
Voice prompts also alert bystanders to call for help after you have been given a treatment and
remain unconscious.
Page 65

Responding to alerts
5 - 3
If you receive a treatment
After receiving a treatment, your back, sides and chest may feel wet. This wet feeling is the
Blue™ gel that was released just before the first treatment was delivered. You might also have
some chest soreness.
When any of the following messages appear, tap Help for additional information about what to
do.
1. Call your prescriber’s emergency number
immediately to report your treatment.
2. Unless your prescriber tells you otherwise
,
continue to wear the LifeVest system.
•
Leave the belt connected. Do not remove
the electrode belt or garment, and do not
disconnect the electrode belt from the
monitor.
•
Leave the battery in the monitor. Do not
remove the battery. Continue to change
and recharge the batteries as normal.
•
Leave the Blue™ gel under the therapy
pads.
Do not wipe them dry.
•
Tap
ACCEPT
after reading the message.
3. Call ZOLL and arrange to get a new belt.
Tap
ACCEPT
to acknowledge you have read
the “Call ZOLL” message on the screen.
4. Check for any further instructions that may
be displayed for you to follow.
For additional information,
tap Help.
For more details, see page 5-19.
Page 66

LifeVest 5000 Patient Manual
5 - 4
Siren with call for help message
If you are awake and feel fine
and the monitor shows
this message:
•
Press the response buttons to stop the
message and siren alert.
•
If the alert continues even after you press the
response buttons, remove and replace the
battery to reset the device.
•
If you continue to get this message, call ZOLL.
If you are not conscious
, naturally you will not be able to press the response buttons.
Voice prompts and messages alert bystanders to call for help.
Page 67

Responding to alerts
5 - 5
Technical alerts: gong sound
These alerts may indicate a problem with the device.
Technical alerts are accompanied by a low-pitched “gong” sound that repeats about once a
second.
Note:
This is the same gong sound that sounds for informative alerts,
see page 5-6.
If you get a technical alert, there is a problem that needs your
attention.
Take the following action:
•
Read the message on the monitor’s display.
•
Use the technical alerts list below to see what the message
means and what to do.
Message or
i
con
on monitor
What it means
What to do
System has a problem
that requires servicing.
The device is still able to
be used.
Continue using the
device.
Find the code number
Example: 101) shown on
(
the bottom left side of
the monitor.
Write down the code
number and call ZOLL.
Tap
ACCEPT
to return to
Home screen.
System has a more severe
service problem.
You
CANNOT
use the
device.
Call ZOLL immediately.
Find the code number
(
Example: 202) shown on
the bottom left side of
the monitor.
Write down the code
number and call ZOLL.
Page 68

LifeVest 5000 Patient Manual
5 - 6
Informative alerts: gong or high-pitched “train whistle” sound
These alerts are for informational purposes.
Informative alerts are accompanied by either a low-pitched “gong” sound that repeats about
once a second, or a high-pitched “train whistle” type of sound.
If you get an informative alert there is a problem that needs your attention. Take the following
action:
•
Read the message on the monitor’s display.
•
For specific help with any message, tap Help if it is available on the screen.
•
Use the following lists to see what the message means and what to do.
Informative general gong alerts
The following screens occur along with a gong alert during general functioning of the
device. These alerts may occur at the startup of the device after battery replacement.
Message or
i
con
on monitor
What it means
What to do
Press the response
buttons to test their
function every time the
battery is installed.
At startup, press the
response buttons as a
reminder of what to do
when a siren alert
sounds.
This message may appear
at startup.
You may be holding the
response buttons instead
of pressing and releasing
them.
Release the response
buttons.
If you are not holding the
response buttons, the
device may not be
functioning properly.
Call ZOLL.
Page 69

Responding to alerts
5 - 7
Message or
i
con
on monitor
What it means
What to do
Electrode belt is not
connected to the
monitor.
Monitor is receiving a
poor signal or no signal
from the electrode belt.
Gently connect electrode
belt to monitor.
If belt is already
connected:
1. Remove battery.
2. Disconnect and
reconnect
electrode belt.
3. Reinstall battery.
Note:
Until you connect
the belt, the monitor will
intermittently toggle
between this alert screen
and the Home screen.
The system is performing
maintenance on the belt.
No action required.
Wait while this screen is
displayed, usually about
seconds.
30 The monitor has not
automatically sent data
either after a treatment
was delivered or after a
routine specified length
of time.
Send data manually as
soon as possible.
For details, see page 3-11.
Page 70

LifeVest 5000 Patient Manual
5 - 8
Message or
i
con
on monitor
What it means
What to do
A connectivity issue is
preventing the monitor
from sending data to
ZOLL.
Tap
TRY AGAIN
to
attempt sending again or
tap X to close and try
again later.
Find the code number
Example: 405) shown on
(
the bottom right side of
the monitor.
Write down the code
number and call ZOLL.
A connectivity issue is
preventing the monitor
from connecting to the
chosen network.
Tap
ACCEPT
to attempt
connecting again.
If the connection fails a
second time, find the
code number (Example:
shown on the
601)
bottom left side of the
monitor.
Write down the code
number and call ZOLL.
Page 71

Responding to alerts
5 - 9
Informative electrode belt gong alerts
The following screens occur along with a gong alert when the device detects electrode
belt problems.
Messa
ge or i
con
on monitor
What it means
What to do
Monitor is not receiving a
good signal from the
round electrodes that are
colored red on the
screen.
A voice prompt will tell
you to check round
electrodes.
Adjust your garment and
electrode belt so that
each round electrode is
touching your skin.
Clean the round
electrodes with alcohol
and apply unscented
hand lotion.
The system is checking
the round electrode
placement and their
contact with your skin.
No action required.
Wait while this screen is
displayed, usually about
30
seconds.
The therapy pads are not
making contact with your
skin.
Make sure the therapy
pads are inserted
correctly, with the metal
sides facing the silver
mesh fabric on the
pockets.
Make sure the therapy
pads and mesh pockets
are pressing against your
skin.
Page 72

LifeVest 5000 Patient Manual
5 - 10
Messa
ge or i
con
on monitor
What it means
What to do
Monitor is not receiving a
signal from the electrode
belt.
Adjust your garment and
electrode belt so that
each round electrode is
touching your skin.
Make sure nothing is
covering the metal
surfaces of the round
electrodes.
Put a dab of unscented
hand lotion on the
surface of each round
electrode.
The monitor is not
receiving a good signal
from the electrode belt.
Make sure the electrode
belt is connected to the
monitor.
Remove the battery from
the monitor. Take off the
garment and make sure
nothing is covering the
round electrodes or
therapy pads.
Put a dab of unscented
hand lotion on the
surface of each round
electrode.
Put the garment back on
and reinsert the battery.
If problems continue, call
ZOLL.
For details, see section 4.
Page 73

Responding to alerts
5 - 11
Informative battery gong alerts
The following screens occur along with a gong alert when the device detects possible
battery problems.
Message or i
con
on monitor
What it means
What to do
Yellow battery:
Battery
charge level cannot be
determined.
Remove the battery and
insert the fully charged
battery from the charger
into the monitor.
Continue wearing the
LifeVest.
Call ZOLL for a
replacement battery.
Red battery:
Battery
needs to be changed.
Replace with a fully-
charged battery.
Place discharged battery
into the charger.
Tap
ACCEPT
to return to
Home screen.
A gong sounds if the
battery has been left in
the monitor when not in
use.
If the LifeVest has been
idle for 20 minutes or
more with the electrode
belt disconnected a “train
whistle” alert occurs.
Remove the battery when
the LifeVest is not being
used.
If the LifeVest is being
used, ensure the
electrode belt is properly
connected to the
monitor.
Call ZOLL if the alert
persists.
Page 74

LifeVest 5000 Patient Manual
5 - 12
Informative after treatment gong alerts
The following screens occur along with a gong alert after you have experienced an
abnormal heart rhythm and received a treatment.
Message or i
con
on monitor
What it means
What to do
This message may appear
after detection.
You may be pressing the
response buttons and you
are no longer having a
treatable heart rhythm.
Release the response
buttons.
If you are not pressing the
response buttons, device
may not be functioning
properly.
Call ZOLL.
You have received a
treatment.
Continue to wear the
LifeVest device.
Call your prescriber’s
emergency number
immediately.
Leave the electrode belt
connected; change and
recharge the battery as
normal.
Follow instructions
about what to do after
receiving a treatment,
see page 5-3.
Tap
ACCEPT
to return to
the Home screen.
You have received a
treatment.
Page 75

Responding to alerts
5 - 13
Message or i
con
on monitor
What it means
What to do
Blue™ gel has been
released and the gel is
drying out.
Add gel to the therapy
pads.
Call ZOLL to get a
replacement electrode
belt.
Tap
ACCEPT
to return to
normal operation.
For details, see page 5-19.
Too much gel is on your
skin.
Leave the Blue™ gel that
is under the therapy pads,
but wipe the gel from the
skin that is not under the
therapy pads.
Tap
ACCEPT
to return to
normal operation.
For details, see page 5-20.
Page 76

LifeVest 5000 Patient Manual
5 - 14
Informative gong alerts: additional instructions on what to do
The following are expanded instructions on what to do when certain gong alerts occur.
Electrode belt message
This screen shows which round electrodes are causing problems.
The symbols help you to determine the problem. You may get any combination of these
symbols:
Good signal:
(Green) Situation normal, no
action required.
Poor signal:
(Yellow) Could be muscle noise,
electrical noise, weak signal, lost signal, or
interference.
Off skin:
Red) Round electrode is not
(
making good contact with skin. No signal is
detected.
If you get one or more of the yellow or red symbols, make sure:
•
The round electrodes indicated by the yellow or red symbols are touching your skin.
•
Nothing is between the round electrodes and your skin, such as clothing or one of the
cables.
•
Garment and belt fit snugly, with the round electrodes pressing against your skin.
•
Round electrode cable is securely connected into the monitor.
While you’re wearing the LifeVest, tap Help for reminders about what to do.
Page 77

Responding to alerts
5 - 15
Check round electrodes message
You may get this screen with this voice prompt:
Check electrodes.
The problem may be with
the signal from the round electrodes.
Follow this procedure to correct the problem.
1. Check your electrode belt and garment. Make sure:
•
All four round electrodes are touching your skin, not flipped over or pulled away
from your skin.
•
Nothing is between the round electrodes and your skin, such as clothing or one of
the cables.
•
Garment and belt fit snugly, with the round electrodes pressing against your skin.
•
Round electrode cable is securely connected into the monitor.
2. If you continue to get this message, call ZOLL.
Page 78

LifeVest 5000 Patient Manual
5 - 16
Check belt message
After you get a number of belt problem screens, you may get a remove belt and check assembly
message. It means that the monitor is still not receiving a good signal from the electrode belt.
If you get this message:
1. Remove the battery from the monitor.
2. Disconnect the electrode belt from the monitor.
3. Take off the garment and make sure that nothing is covering the metal surfaces of the
round electrodes.
4. Check that the therapy pads are in the pockets with the silver side touching the silver
mesh fabric.
5. Put a dab of unscented hand lotion on the surface of each round electrode (the round
ones, not the therapy pads).
6. Put the garment on, making sure it fits snugly, and connect the electrode belt to the
monitor. Put the battery back into the monitor.
While you’re wearing the LifeVest, tap Help for reminders about what to do.
7. If problems continue, call ZOLL.
Page 79

Responding to alerts
5 - 17
Replace belt message
After you receive a treatment to correct an abnormal rhythm, you may see a message telling
you to replace the belt.
Continue to wear the belt until you get the replacement belt. The belt is still functional and can
provide additional treatments if needed.
When you receive the new belt, follow the instructions below to replace the belt.
While you’re wearing the LifeVest, tap Help for reminders about what to do.
Tap
ACCEPT
to return to the Home screen.
To replace the electrode belt:
1. Remove the battery from the monitor.
2. Disconnect the electrode belt from the monitor.
3. Remove the electrode belt and garment from your body.
4. Disassemble the electrode belt from the garment, assemble the new electrode belt into
a clean garment, and put on the assembled electrode belt and garment, see section 4.
5. Connect the electrode belt to the monitor.
6. Put the battery into the monitor and follow the normal startup routine.
Page 80

LifeVest 5000 Patient Manual
5 - 18
Therapy pad message
This screen shows when the therapy pads (the large rectangular ones) are causing problems by
not making good contact with your skin.
Remember that there are three therapy pads: one in front and two in back. Any one of them
could be causing the problem, so be sure to check all three if you get a red symbol.
The symbols help you to determine the problem:
Therapy pad off skin:
Metal side is not
making good contact with skin. Check all
three therapy pads, front and back.
Therapy pad on skin:
Situation normal,
no action required.
If you get red symbols, make sure:
•
Therapy pads and mesh pockets are pressing against your skin.
•
Therapy pads are inserted correctly into their pockets, with the metal sides (with
the green stickers) facing the metal mesh.
•
Garment and belt fits snugly, with the metal mesh of the therapy pads pressing
against your skin.
•
Make sure to properly launder the garment, see page 4-10.
While you’re wearing the LifeVest, tap Help for reminders about what to do.
Page 81

Responding to alerts
5 - 19
Add gel to therapy pads
After you receive a treatment to correct an abnormal
rhythm, you may see this message telling you to add
gel.
Follow the instructions below to add gel.
While you’re wearing the LifeVest, tap Help for
reminders about what to do.
Tap
ACCEPT
to return to the
Home
screen.
To add gel:
1. Locate the packets of gel you received with the LifeVest system.
2. Remove the battery from the monitor, then remove the electrode belt and garment
from your body.
3. Add one-half packet of gel to all three therapy pads, directly onto the silver mesh
material of each pocket. Apply to the rear pads and the front pad (but not to the round
electrodes.)
4. Put on the electrode belt and garment, see section 4.
5. Put the battery into the monitor and follow the normal startup routine.
Page 82

LifeVest 5000 Patient Manual
5 - 20
Too much gel on therapy pads
After you add gel to the electrode belt, you may
see this message telling you there is too much gel
between the therapy pads.
Follow the instructions below to remove the
excess gel.
While you’re wearing the LifeVest, tap Help for
reminders about what to do.
Tap
ACCEPT
to return to the Home screen.
To remove excess gel:
1. Remove the battery from the monitor.
2. With the electrode belt and garment on your body, and using a towel or soft cloth, wipe
your skin between the front and back therapy pads on the left side of your body. Be
careful not to remove the gel under the therapy pads.
3. Reinstall the battery into the monitor.
Page 83

Using the activities options
6 - 1
6: Using the activities options
Introduction to activities
Your prescriber can request two activity options that are programmed into the system while you
are wearing the LifeVest. These options are the health survey and the WalkTest™. Before you
perform either of these activities for the first time, read this section of the manual.
If your prescriber did not request the health survey or WalkTest™, you will not be able to access
these options.
These activities allow you to provide some additional information to your prescriber. Your
prescriber may then use that information to help evaluate your condition.
For more about the health survey option see “Taking the health
survey” section below.
For more about the WalkTest™ option see “Taking the
WalkTest™”, page 6-7.
Page 84

LifeVest 5000 Patient Manual
6 - 2
Taking the health survey
If you get the message “Please complete health survey” when you change the battery, you are
to take the health survey. You have the option of taking the health survey now or later.
The LifeVest stores your answers and, during your next download, sends the results to a secure
website where your prescriber can choose to review the results. Your prescriber may have you
repeat the health survey once a day or once a week. The LifeVest will remind you when you are
scheduled to take the health survey.
If you have any questions, please contact your prescriber.
What do I have to do?
You need to answer the health survey questions honestly.
It is important that you wear the LifeVest the entire time that you are taking the health survey
so that it can monitor and protect you. While taking the health survey, follow the on-screen
directions.
Why is it important that I do the health survey?
It is very important that you indicate how you really feel when you answer the questions. Your
prescriber may use the information from the health survey to help evaluate your condition.
Page 85

Using the activities options
6 - 3
Taking the health survey now
If the monitor displays the screen with the message “Please Complete Health Survey” when you
change the battery, you are to take the health survey.
Read through all the health survey steps in this manual before you answer the questions for the
first time.
There are many health related questions that your prescriber can include in the survey. The
questions and answers shown in these instructions may not be the questions and answers you
see on your monitor.
If any alerts are displayed during the health survey, see section 5.
1. Tap
NOW
to start the health survey.
Tap
Help
for details.
2. The message on the screen instructs you to use
the arrow buttons to select your answers.
Tap
ACCEPT.
Note:
To cancel taking the health survey, tap
X
at any time.
Page 86

LifeVest 5000 Patient Manual
6 - 4
3. Read the first question.
4. Use the arrow keys to scroll to the appropriate
answer.
5. Tap
ACCEPT
to choose your answer and
continue to the next question.
The question shown here is just an example.
Your screen may show a different question.
Note:
If you want to hear the question spoken
to you, tap the screen in the question area.
You can change the voice option so you hear
each question screen as soon as it appears,
without tapping the screen. You can also change
the voice volume. For the details about
changing the voice setting, see Voice options in
section 3.
6. Repeat steps 3 - 5 for the remaining questions.
7. Upon completion of the health survey you will
see this screen.
8. Tap
ACCEPT
.
Page 87

Using the activities options
6 - 5
Taking the health survey later
If you tap
LATER
, the system returns to the
Home screen instead of proceeding with the
health survey.
When you decide to complete the health survey, access it by tapping
MENU
on the Home
screen, then
ACTIVITIES
, and then
HEALTH SURVEY
.
Page 88

LifeVest 5000 Patient Manual
6 - 6
Health survey messages
As you take the health survey, you may get one of these messages. If so, here is what the
message means and what you should do.
If you get any other messages, see Section 5, Responding to alerts.
Message
What it means
What t
o do
You are attempting to
take the survey with the
electrode belt
disconnected.
The belt needs to be
connected to take the
health survey.
Wear the LifeVest and
connect the electrode
belt to the monitor.
After connecting the
electrode belt, the
message goes away.
Try again to take the
health survey.
You are attempting to
take the health survey
more frequently than
scheduled.
Tap
ACCEPT
to clear the
message.
Wait until you get this
message: “Please
complete health
survey."
Page 89

Using the activities options
6 - 7
Taking the WalkTest™
WARNINGS
If you get the siren alert, stop walking and press the response buttons.
When performing the WalkTest™, do not continue walking if the monitor broadcasts an alert
sound stop walking and press the response buttons as you normally would.
If you continue
walking, you may place yourself at risk for a cardiac arrest, possibly resulting in serious injury or
death.
When performing the WalkTest™, do not continue walking if you experience symptoms such as
shortness of breath, chest pain, or other pain or discomfort.
Stop walking and sit or lie down. If
the symptoms continue or get worse, immediately call your doctor or emergency help. If you
continue walking or ignore the symptoms, you may place yourself at risk for a cardiac arrest or
other health problems, possibly resulting in serious injury or death.
Your physician prescriber may order the WalkTest™ as one of the ways to use the LifeVest to
gather information about your condition.
How does it work?
The purpose of the WalkTest™ is to monitor your heart and count your steps while you walk for
minutes.
6
Before and after the walk, you will be asked two questions: one about your shortness of breath
level and another about your fatigue level (how tired you feel). After the walk, you will answer
the same two questions again. Look at the screen for the possible answers and pick the one that
most closely describes how you feel at that moment.
During the next scheduled data download, the LifeVest sends your WalkTest™ information to a
secure website where your physician prescriber can review the results.
Your physician prescriber may have you repeat the WalkTest™ once a day or once a week. The
LifeVest will remind you when you are scheduled to take the WalkTest™.
Page 90

LifeVest 5000 Patient Manual
6 - 8
What do I have to do?
It is important that you wear the LifeVest the entire time that you’re doing the WalkTest™ so
that it can monitor your progress and protect you.
If possible, have someone with you while you do the WalkTest™. Try to walk at a pace you can
comfortably maintain for 6 minutes. You can walk in a circle or oval (as walking a track), in a
square or rectangle (around a room), or in a straight line, turning as you reach the ends (walking
back and forth in a hallway).
While you are walking, the LifeVest will give you audible prompts about how much longer you
are to walk. It will also tell you when to stop walking. It is alright if you need to stop and rest at
any time. Continue walking when you are able. After the walk, you will answer the fatigue and
shortness of breath questions again.
It is also important that you only walk for 6 minutes as part of this WalkTest™. Listen for the
voice prompt that tells you to “stop walking” after 6 minutes. When you hear this voice prompt,
please stop walking.
•
Wear comfortable shoes.
•
Walk on a hard, flat surface. Do not walk on a treadmill.
•
Do not run or jog. This test monitors you while you are walking at a normal pace.
•
If you have any walking problems, or if you have an abnormal gait, such as foot
dragging, shuffling, or limping, or if you need assistance while walking, such as by using
a cane, walker or crutches, please tell your physician prescriber as it may produce
inaccurate results.
•
The monitor gives you voice prompts throughout the WalkTest™, so you may prefer to
do the WalkTest™ in a private location. So that you can hear the voice prompts, do not
engage in any other activity like listening to music or talking on a cell phone.
•
If your physician prescriber asks you to do multiple WalkTests™, doing the walk in the
same location gives the most accurate results.
Page 91

Using the activities options
6 - 9
Why is it important that I do the WalkTest™?
Your prescriber may use the information from the WalkTest™ to help evaluate your condition.
It is very important that you indicate the true state of your condition when you answer the
questions. Your physician prescriber may review your answers and use this information to help
evaluate your condition.
Taking the WalkTest™ now
If you get this message “Please Complete WalkTest™” when you change your battery, your physician
prescriber has asked that you perform a WalkTest™.
Throughout the WalkTest™ procedure, you must wear the LifeVest, with it connected normally.
You have the option of taking the WalkTest™ now or later. Make sure the electrode belt is connected to
the monitor before starting the WalkTest™.
During the WalkTest™, the LifeVest monitors your heart and counts your steps. The test takes six
minutes to complete. If you receive an alert while walking, stop walking and press the response buttons.
If you have any questions, please contact your physician.
1. Tap
NOW
to begin.
•
Tap
Help
for details.
Page 92

Page 93

Using the activities options
6 - 11
The monitor will display two pre
-
walk questions
about your shortness of breath and fatigue levels
(
how tired you feel).
4. Select your levels using the arrows to the left
and right of the scale.
5. Tap
ACCEPT
on each screen to confirm and
continue.
6. The WalkTest™ start screen appears. Tap
START
when you are ready
to begin the test.
A voice prompt instructs you to start walking.
What you need to do during the WalkTest™
is described in the “What do I have to do?”
section on page 6-8.
To cancel the WalkTest™, tap
X
. If you
cancel the WalkTest™ you will be asked to
confirm that you really want to cancel.
If you do want to cancel, tap
X
.
If you do not want to cancel, tap
RESUME
.
7. A progress bar is displayed during the
WalkTest™.
You will receive a voice prompt after each
minute of the WalkTest™.
Continue walking for 6 minutes.
Page 94

LifeVest 5000 Patient Manual
6 - 12
8. When the test is completed (after
approximately 6 minutes), you are instructed
to stop.
Tap
ACCEPT
.
9. The shortness of breath and fatigue questions
are displayed again with level rating screens.
Select the numbers that best match your
levels after the walk. Your answers DO NOT
need to match your previous answers.
Tap
ACCEPT
to confirm and continue to the
last WalkTest™ screen.
10. Tap
ACCEPT
on this screen to close the
WalkTest™ and return to the Home screen.
After completing the WalkTest™, sit down and
rest before resuming your normal activities.
If you have any questions about these
instructions, call ZOLL.
If you have any questions about your medical
condition, call your health care
provider.
During the data download, the LifeVest sends your WalkTest™ information to a secure website
where your physician prescriber can review the results.
Your physician prescriber may have you repeat the WalkTest™ at certain intervals. The LifeVest
will remind you when you are scheduled to take the WalkTest™ by displaying a message on the
monitor.
Page 95

Using the activities options
6 - 13
Taking the WalkTest™ later
If you choose to complete the WalkTest™
later, tap
LATER
to return to the Home
screen.
If you tap
LATER
another WalkTest™ prompt
won’t be displayed until you change the
battery.
When you decide to complete the WalkTest™, access it by tapping
MENU
on the Home screen,
then
ACTIVITIES
, and then
WALKTEST™
.
Page 96

LifeVest 5000 Patient Manual
6 - 14
WalkTest™ messages
As you take the WalkTest™, you may get one of these messages. If so, here is what the message
means and what you should do.
If you get any other messages, see Section 5, Responding to alerts.
Message
What it means
What to do
You are attempting to
take the WalkTest™
with the electrode belt
disconnected.
The belt needs to be
connected to take the
WalkTest™.
Wear the LifeVest and
connect the electrode
belt to the monitor.
After connecting the
electrode belt, the
message goes away.
Try again to take the
WalkTest™.
You are attempting to
take the WalkTest™ and
the LifeVest is advising
you not to take the
WalkTest™ at this time.
Tap
ACCEPT
to clear the
message.
Try again later (or wait
until tomorrow).
You are attempting to
take the WalkTest™
more frequently than
scheduled.
Tap
ACCEPT
to clear the
message.
Wait until you get this
message:
“Please
complete WalkTest™.”
Page 97

Cell signal strength: LifeVest is in range of a cell network and can transmit data. Number
of bars indicates signal strength.
Note: These icons also appear on a different color background, such as green when
sending data.
• Registered, connected, data can be transmitted, white bars indicate signal
strength.
• Signal strength is too weak to send data.
• Cell modem is powered but not functioning properly.
• Not connected to ZOLL charger network server.
Appendix A: Symbols and Icons
Appendix A: Symbols and Icons
Page 98

WiFi signal strength: WiFi networks are available for transmitting data.
Note:
These icons also appear on a different color background, such as green when
sending data.
•
Connected to WiFi network, data can be transmitted, white bars indicate signal
strength.
•
Signal strength is too weak to send data.
•
WiFi is powered/turned on but not connected to any access points.
•
WiFi is not functioning properly.
•
WiFi is not powered or turned off.
Page 99

Battery levels that are displayed on the monitor.
Green segments: Depict how much charge is left in the battery.
Yellow battery: Call ZOLL for service.
Red battery: Battery is low, a critical situation. Change battery as soon as possible and
recharge battery.
Electrode belt symbol shows that the electrode belt is connected to the monitor.
The electrode belt is not connected to the monitor. If not corrected, a gong alert sounds
and the “belt not connected” screen is displayed.
Help button: Tap this icon to see help screen information.
X button: Tap this icon to close the screen.
Round electrode good signal: Situation normal, no action required.
Round electrode poor signal: Check round electrodes for cause of poor signal to fix the
problem.
Round electrode off skin: Round electrode is not making good contact with skin. No signal
is detected. See Responding to alerts, section 5.
A-1 LifeVest 5000 Patient Manual
Page 100

Battery: Do not incinerate.
 Loading...
Loading...