ZOLL E Series User Manual

October 2007 9650-1212-01 Rev. E
End Tidal Carbon
Dioxide (EtCO
2
)

The issue date or revision level for this Operator’s Guide is shown on the front cover.
ZOLL and E Series are registered trademarks of ZOLL Medical Corporation.
CAPNOSTAT is a registered trademark and LoFlo and CAPNO
Novametrix LLC.
Cidex is a registered trademark of Advanced Sterilization Products, a Johnson & Johnson Company.
Nafion is a registered trademark of DuPont.
System 1 is a registered trademark of the Steris Corporation.
mask are trademarks of Respironics
2
© 2007 by ZOLL Medical Corporation. All rights reserved.

END-TIDAL CARBON DIOXIDE (EtCO2)
General Information
Product Description
ESeries® units equipped with software revision 2.00.000 or higher support two End Tidal Carbon Dioxide (EtCO2)
monitoring options for the continuous measurement of respiratory carbon dioxide (CO2) and respiration rate. These
options use the same connector on the E Series unit and may be used interchangeably.
The first option uses a unique, mainstream, solid-state, infrared sensor called the CAPNOSTAT® 5 Mainstream CO2
Sensor. The CAPNOSTAT 5 CO2 sensor is attached to an airway adapter that connects to an endotracheal (ET) tube
or other airway and measures gases flowing through these breathing circuit components. A disposable mouthpiece
may be connected to the adapter for monitoring non-intubated patients. A CAPNO
with non-intubated patients. This option provides for O
The second option is a sidestream sampling system called the LoFlo™ CO2 Module. The LoFlo module contains a gas
sampling pump, which draws small samples of gas from the patient’s airway via a nasal/oral cannula or airway adapter,
and passes these gases through a solid state infrared sensor (located away from the patient’s airway) that measures
. While the sidestream system is typically used on non-intubated patients, it can also be used for EtCO2
CO
2
measurement on intubated infant, pediatric and adult patients. The sidestream system should not be used, however,
on patients who cannot tolerate the 50ml/min removal of the sample gases from their breathing circuit. The sidestream
module uses specially designed cannulas and airway adapters for sampling airway gases and passing them through
an integrated sample cell, which connects to the LoFlo module’s CO
sample cell, providing maximum filtration of fluids and contaminants, and protecting the system from aspiration of
these fluids.
In both systems, the CO2 sensor generates infrared light and beams it through the airway adapter or sample cell to a
detector on the opposite side. CO2 from the patient, flowing through the mainstream airway adapter or sample cell,
absorbs some of this infrared energy. The ESeries unit determines CO
measuring the amount of light absorbed by gases flowing through the airway or sample cell.
The E Series unit disp lays EtCO
numerical value in millimeters of mercury (mmHg), percent (%), or kilopascals (kPa). In addition, the unit can display a
capnogram. This capnogram is a valuable clinical tool that can be used to assess patient airway integrity and proper
endotracheal (ET) tube placement. The unit calculates respiration rate by measuring the time interval between
detected peaks of the CO
those caused by cardiogenic oscillations and artifact.
(the concentration of carbon dioxide detected at the end of each exhalation) as a
2
waveform. The technology differentiates between waveforms caused by breathing and
2
delivery while monitoring expired CO2.
2
sensor. These cannulas incorporate a filter and
2
concentration in the breathing gases by
2
mask™ is also available for use
2
How to Use This Manual
This section explains how to set up and use the E Series End Tidal Carbon Dioxide option. Important safety
information relating to general use of the E Series End Tidal Carbon Dioxide monitor appears in the “Safety
Considerations” section of this manual.
The E Series Operator’s Guide provides information operators need for the safe and effective use and care of the
ESeries unit. It is important that persons using this device read and understand all the information contained therein.
Please read both safety considerations and warnings sections thoroughly before operating your E Series unit.
All CAPNOSTAT 5 sensor, LoFlo module, airway adapter and cannula questions with regards to the Declaration of
Conformity with European Union Directives should be directed to the authorized representative for Respironics
Novametrix LLC:
Respironics Novametrix LLC
Authorized European Contact
Respironics Deutschland
Gewerbestrasse 17
82211 Herrsching
Germany
+49 8152 93060
9650-1212-01 Rev. E 1
E Series - End Tidal Carbon Dioxide (EtCO2) Option Insert
Safety Considerations
WARNINGS
General
• Carefully read the E Series Operator’s Guide and
these operating instructions before operating the
monitoring option.
EtCO
2
• Ensure that the E Series EtCO2 option is operated
by qualified personnel only.
• Do not use the E S eries EtCO2 option as an apnea
monitor.
• Do not immerse the E Series unit, patient cables, or
sensors in water, solvents, or cleaning solutions.
• If the accuracy of any reading is suspect, first check
the patient’s vital signs by alternate means and then
check the E Series EtCO
option for proper
2
operation.
• If an alarm condition occurs while the alarms are
suspended, the suspended alarm indications will
only be visual displays and symbols. No audio alarm
indications will occur.
CAPNOSTAT and Accessories
• Always ensure the integrity of the patient breathing
circuit after insertion of the airway adapter by
verifying a proper CO
waveform (capnogram) on
2
the monitor display.
• Elevated oxygen levels, nitrous oxide, or
halogenated agents contained in the breathing
gases may degrade the accuracy of measurements
made with the E Series EtCO
oxygen compensation if O
are introduced. Activate N
option. Activate
2
levels in excess of 60%
2
O compensation if nitrous
2
oxide is introduced into the airway circuit. The
presence of Desflurane beyond 5% may positively
bias the carbon dioxide reading by up to 3 mmHg.
• Do NOT use the LoFlo module on patients who
cannot tolerate the removal of 50ml/min of breathing
gases from the airway.
• Carefully route patient cabling to reduce the
possibility of patient entanglement or strangulation.
• Do not touch the bed, patient, or any equipment
connected to the patient during defibrillation. A
severe shock can result. Do not allow exposed
portions of the patient’s body to come in contact with
metal objects, such as a bed frame, as unwanted
pathways for defibrillation current may result.
• Do not use CAPNOSTAT 5 or LoFlo sensors in the
presence of flammable anesthetics or other
flammable gases.
• Do not attempt to open the sensor. An electrical
shock hazard exists internally. Refer servicing to
qualified personnel.
2 9650-1212-01 Rev. E

Safety Considerations
CAUTIONS
• CAUTION: Federal (U.S.A.) law restricts this device
to sale, or use by or on the order of a licensed
medical practitioner.
• Use only ZOLL/Respironics Novametrix
CAPNOSTAT 5 sensors and LoFlo modules, airway
adapters, nasal and nasal/oral cannula sets with the
E Series EtCO
option.
2
• The device is protected against interference from
radio frequency emissions typical of two-way radios
and cellular phones (digital and analog) used in
emergency service/public safety activities. Users
should assess the device’s performance in their
typical environment of use for the possibility of radio
frequency interference from high-power sources.
Radio Frequency Interference (RFI) may be
observed as shifts in monitor baseline, trace
compression, display brightness changes or transient
spikes on the display.
• Do NOT sterilize or immerse the CAPNOSTAT 5
CO
sensor or LoFlo module.
2
• Do NOT reuse or sterilize the disposable airway
adapter, airway adapter with mouthpiece,
CAPNO
sets, or airway adapters, as system performance will
be compromised. These items are intended for
single patient use only.
• Do NOT use a damaged sensor or airway adapter.
• Do NOT use the device if it fails to operate properly.
• Do NOT place the mainstream or sidestream airway
adapters between the ET tube and the breathing
circuit elbow, as this may allow patient secretions to
accumulate in the adapter.
• Position airway adapters with windows in a vertical,
NOT a horizontal, position. This helps keep patient
secretions from pooling on the windows.
• Do NOT insert any object other than the sample cell
into the sample cell receptacle on the LoFlo module.
mask, nasal or nasal/oral sampling cannula
2
• Remove the LoFlo sample cell from the sample cell
receptacle when not in use.
• Clean or replace the airway adapter if excessive
secretions are observed.
• ZOLL Medical Corporation recommends that the
airway adapter be removed from the circuit
whenever aerosolized medication is delivered. The
increased viscosity of the medications may
contaminate the adapter windows, requiring
premature cleaning or replacement of the adapter.
• In order to eliminate the potential build up of CO
2
inside the storage bag, ensure that the LoFlo module
exhaust tube vents gases away from the module
environment.
• To avoid injury to the patient, remove the nasal/oral
cannula from the patient before cutting the oral
cannula tip.
• Do NOT apply tension to the sensor cable.
• Periodically inspect the sampling tubing for the
absence of kinks.
• Monitor the capnogram for an elevated baseline. If
an elevated baseline is observed, verify patient
condition first. If the care giver determines that the
patient condition is not contributing to the elevated
baseline, follow the instructions for zeroing the
sensor or module detailed in this manual.
• Do NOT store sensors, modules, airway adapters, or
cannula sets at temperatures less than -40° C or
greater than 70° C.
• Do not operate CAPNOSTAT sensors at
temperatures less than 0° C or greater than 45° C.
Do not operate LoFlo modules at temperatures less
than 0° C or greater than 40° C.
• Refer servicing to qualified personnel.
• Do not use the LoFlo module on E Series units that
have a software version lower than 2.00.000.
9650-1212-01 Rev. E 3
E Series - End Tidal Carbon Dioxide (EtCO2) Option Insert
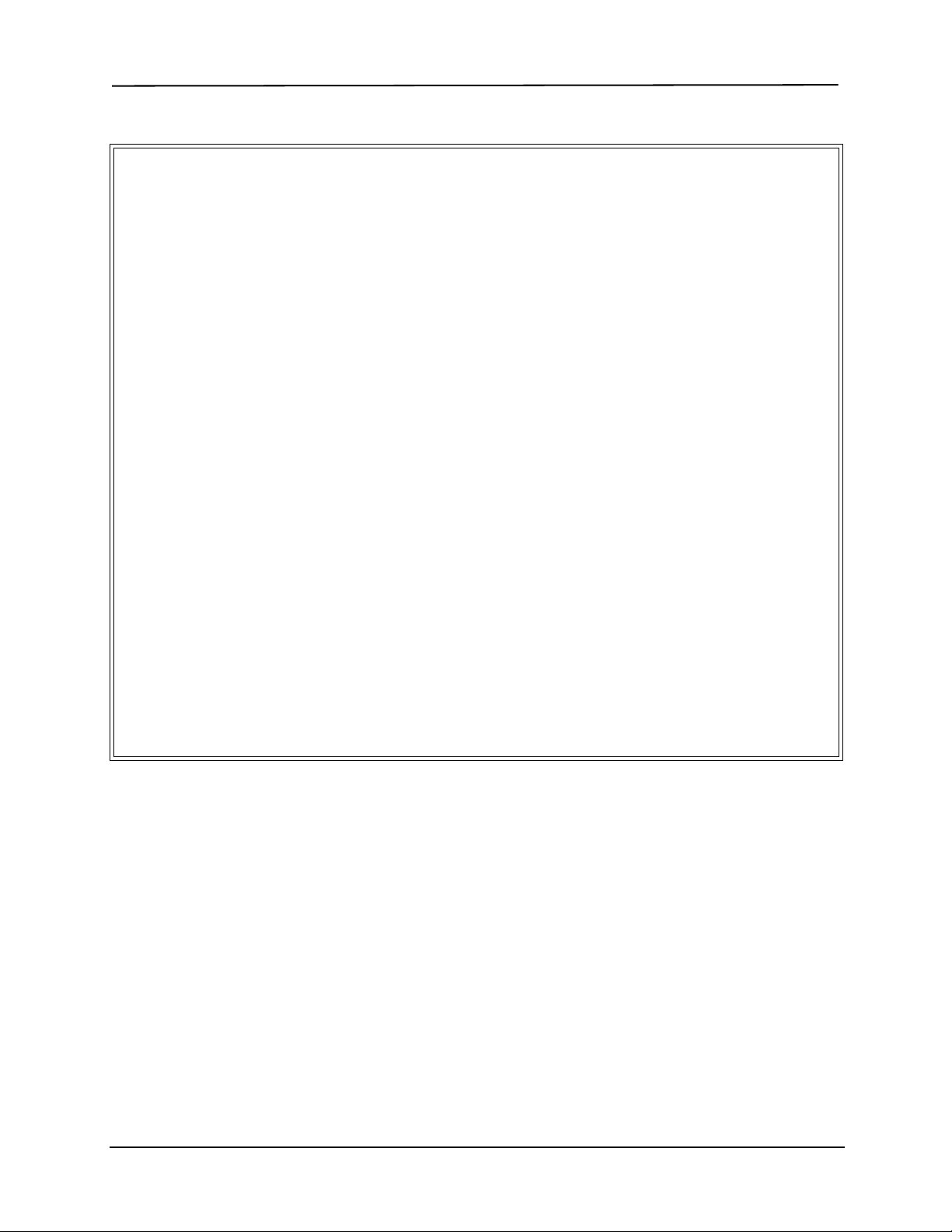
Proper Grasp
Improper Grasp
Figure 1
EtCO2 Indications for Use
The ZOLL E Series EtCO2 option is indicated for the
continuous noninvasive monitoring of end tidal carbon
dioxide (EtCO2) and respiration rate in patients requiring
ventilator support, transport, or anesthesia. The E Series
option with Respironics Novametrix technology
EtCO
2
supports two methods for continuous measurement of
end tidal carbon dioxide (EtCO
The first method uses the CAPNOSTAT 5 Mainstream
CO2 sensor attached to an airway adapter that connects
to an endotracheal tube, mask or disposable
mouthpiece.
The second method uses the LoFlo CO2 module to
monitor both non-intubated and intubated patients using
specially designed sampling cannulas and airway
adapters.
The E S eries EtCO2 option is designed to monitor adult,
pediatric, and neonatal patients.
The following substances can influence CO
measurements made with the CAPNOSTAT 5 CO
sensor:
• elevated oxygen levels
• nitrous oxide
• halogenated agents
The E Series EtCO
nitrous oxide compensation. Halogenated anesthetic
agents alter CO2 readings, but the E Series unit will
monitor CO
2
present at normal clinical levels. The presence of
Desflurane in the exhaled breath beyond normal values
(5-6%) may positively bias measured carbon dioxide
values by up to an additional 2-3 mmHg.
The E S eries EtCO2 option is intended for use only with
the ZOLL/Respironics Novametrix CAPNOSTAT 5
Mainstream CO
mainstream airway adapters, nasal and nasal/oral
sampling cannula sets, and sidestream on-airway
adapters.
option allows high oxygen and/or
2
within specifications when these agents are
Sensor and the LoFlo CO2 Module,
2
) and respiration rate.
2
2
2
back of the E Series unit by matching the key on the
cable to the key on the connector (Figure 1).
Note T o remove the sensor cable from the E Series unit,
grasp the collar surrounding the cable’s E Series
connector and pull up.
Selecting a Mainstream Airway Adapter
Select an airway adapter based on the patient's size, ET
tube diameter and monitoring situation. For more
information refer to the following table or contact ZOLL
Medical Corporation.
Airway Adapter Type ET Tube Diameter
* Pediatric/Adult > 4.0 mm
SPU
Adult Reusable > 4.0 mm
* Neonatal/Pediatric ≤ 4.0 mm
SPU
Neonatal Reusable ≤ 4.0 mm
*SPU = Single Patient Use
Mainstream EtCO2 Setup
There are several steps involved with mainstream
EtCO2 setup. These steps include:
• Attaching the CAPNOSTAT sensor cable.
• Selecting a mainstream airway adapter.
• Attaching the airway adapter to the CAPNOSTAT
sensor.
• Zeroing the CAPNOSTAT sensor/airway adapter .
• Attaching the airway adapter to the airway circuit.
• Applying an airway adapter with mouthpiece.
Attaching the CAPNOSTAT 5 CO
To attach the CAPNOSTAT 5 CO2 sensor cable, plug the
cable’s connector into the yellow CO
Sensor Cable
2
connector at the
2
Attaching the Airway Adapter to the
CAPNOSTAT 5 CO
Before attaching the airway adapter to the
CAPNOSTAT 5 CO
adapter windows are clean and dry. Clean or replace the
adapter if necessary.
CAUTION! The disposable (SPU) Pediatric/Adult and
the Neonatal/Pediatric airway adapters are
intended for single patient use. Do NOT
reuse or sterilize these adapters as system
performance will be compromised.
Attach the airway adapter to the CAPNOSTAT sensor,
as follows:
1. Align the arrow on the bottom of the airway adapter
with the arrow on the bottom of the sensor.
Sensor
2
sensor, verify that the airway
2
4 9650-1212-01 Rev. E
Mainstream EtCO2 Setup
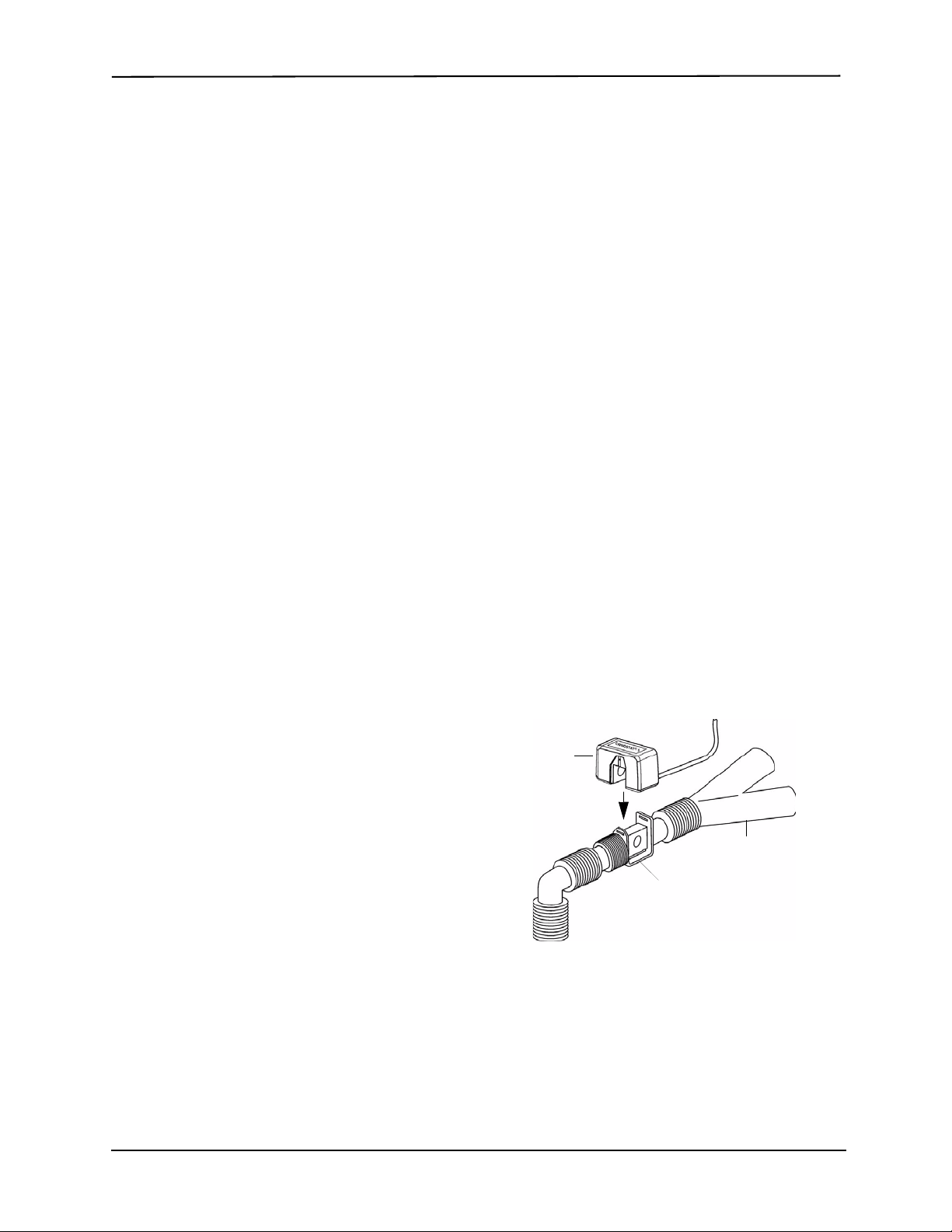
Figure 2
CAPNOSTAT
CO2 Sensor
To Patient
Reusable Adult
Airway Adapter
Ventilator Wye
2. Press the sensor and airway adapter together until
they click.
3. Turn the Selector switch on the E Series unit to
MONITOR (ON for AED units).
4. Wait for the airway adapter and sensor to warm up.
The unit will display a WARM UP message for
approximately one minute while the sensor and
adapter warm to operating temperature. The
message disappears when the sensor is ready to
use.
Note Warm up time varies with ambient temperature of
the sensor.
5. If the unit displays th e CHECK CO2 ADAPTER
message, follow steps a through c.
a. Verify proper connection of the adapter to the
sensor.
b.Verify that the airway adapter windows are clean
and dry.
c. If the adapter is properly connected, and the
windows are clean and dry, then zero the adapter
as described in the next section, “Zeroing the
Mainstream CAPNOSTAT 5 CO
Sensor/Airway
2
Adapter.”
Zeroing the Mainstream CAPNOSTAT 5 CO2
Sensor/Airway Adapter
Adapter zeroing compensates for the optical differences
between airway adapters and should be performed after
switching between single patient use and reusable
airway adapters, in order to obtain accurate readings.
Zeroing is also recommended the first time a particular
CAPNOSTAT 5 CO
1. Place the sensor with the adapter installed away from
all sources of CO
own – exhaled breath and ventilator exhaust valves).
2. Press the Param. softkey and select the EtCO2
menu item, then press Enter.
3. Press the Zero softkey.
The unit zeroes the adapter and displays the
ZEROING CO2 ADAPTER message for 15 to 20
seconds.
The unit displays the message ZERO DONE upon
completion of the zeroing.
Note Do not attempt zeroing for 20 seconds after
removing the adapter from the patient’s airway.
This time allows any CO
to dissipate before zeroing. Do not attempt to zero
the adapter while it is connected to the patient’s
airway. Zeroing with CO
inaccurate measurement and/or other error
sensor is connected to the unit.
2
(including the patient’s – and your
2
remaining in the adapter
2
in the adapter can lead to
2
conditions. If you attempt zeroing while CO
2
remains in the adapter, the time required to zero
the adapter may be increased. If zeroing cannot be
completed, the message ZERO FAILED wil l be
displayed. If this occurs, clear any occlusion in the
adapter, remove any source of CO
, wait 20
2
seconds, and try zeroing again.
Attaching the Airway Adapter to the Airway
Circuit
If you have not yet done so, you must attach the airway
adapter to the CAPNOSTAT 5 CO2 sensor before
attaching the airway adapter to the airway circuit. Refer
to “Attaching the Airway Adapter to the CAPNOSTAT 5
CO2 Sensor” on page 4 if necessary.
Attach the airway adapter to the airway circuit as follows:
1. Place the CAPNOST AT 5 CO
assembly at the proximal end of the airway circuit
between the elbow and the ventilator circuit wye. Do
NOT place the airway adapter between the ET tube
and the elbow, as this may allow patient secretions to
accumulate in the adapter.
Position the airway adapter with its windows in a
vertical, NOT a horizontal, position. This helps keep
patient secretions from pooling on the windows. If
pooling does occur, the airway adapter may be
removed from the circuit, rinsed with water and
reinserted into the circuit. To prevent moisture from
draining into the airway adapter, do NOT place the
airway adapter in a gravity dependent position. See
Figure 2.
2. Check that connections have been made correctly by
verifying the presence of a proper CO
the E Series display.
3. The sensor cable should face away from the patient.
sensor/airway adapter
2
waveform on
2
9650-1212-01 Rev. E 5
 Loading...
Loading...