Page 1

Operating Instruction Manual
6400
Calorimeter
534M
Page 2

6400 Calorimeter Instruction Manual
TABLE OF CONTENTS
TABLE OF CONTENTS ................................................................................................ 1-1
PREFACE......................................................................................................................1-1
Scope.........................................................................................................................1-1
Purpose......................................................................................................................1-2
Explanation of Symbols..............................................................................................1-2
Safety Information......................................................................................................1-3
INSTALLATION.............................................................................................................2-1
Consumables, Utilities and Power Requirements......................................................2-2
Electrical Connection .................................................................................................2-2
Water Connection ......................................................................................................2-3
Gas Connection .........................................................................................................2-4
Bomb Exhaust Connections.......................................................................................2-5
Communication Connections.....................................................................................2-5
Printer Connections....................................................................................................2-7
Balance Connections.................................................................................................2-7
Mettler 011/012 Interface .....................................................................................2-8
Sartorious Interface .............................................................................................2-8
Generic Interface..................................................................................................2-8
Bar Code Port ............................................................................................................2-8
Computer Connections ..............................................................................................2-9
QUICK START...............................................................................................................3-1
OPERATION..................................................................................................................4-1
Menu System.............................................................................................................4-1
Menu Keys.................................................................................................................4-1
Control Keys...............................................................................................................4-2
Programming..............................................................................................................4-2
Default Settings..........................................................................................................4-2
Sample Preparation ...................................................................................................4-3
Sample Size...........................................................................................................4-3
Particle Size and Moisture Content........................................................................4-3
Sample Types ........................................................................................................4-4
Combustion Aids....................................................................................................4-6
Combustion Capsules............................................................................................4-6
Test Process..............................................................................................................4-7
Loading the sample................................................................................................4-7
Closing the bomb...................................................................................................4-7
Fill Cycle.................................................................................................................4-8
Pre-Period..............................................................................................................4-9
Bomb Firing..........................................................................................................4-10
Post-Period ..........................................................................................................4-10
Cool / Rinse..........................................................................................................4-11
Drain.....................................................................................................................4-12
MENU DESCRIPTIONS.................................................................................................5-1
Main Menu .................................................................................................................5-1
Calorimeter Operation Menu......................................................................................5-1
Temperature vs. Time Plot.........................................................................................5-2
Temperature Plot Setup Menu...................................................................................5-2
Page 3

6400 Calorimeter Instruction Manual
Operating Controls Menu...........................................................................................5-3
Program Information and Control Menu.....................................................................5-6
Calibration Data and Controls Menu..........................................................................5-8
Thermochemical Corrections Menu.........................................................................5-11
Standardization Corrections.............................................................................5-11
Determination Corrections................................................................................5-11
Calculation Factors Menu ........................................................................................5-12
Dry Calculation Menu...............................................................................................5-13
Data Entry Controls Menu........................................................................................5-14
Reporting Controls Menu.........................................................................................5-16
Communication Controls Menu................................................................................5-16
File Management Menu ...........................................................................................5-19
Run Data File Manager............................................................................................5-19
Diagnostics Menu.....................................................................................................5-20
CALCULATIONS...........................................................................................................6-1
Corrections.................................................................................................................6-1
Manual vs. Fixed Corrections.....................................................................................6-2
Definitions ..................................................................................................................6-3
ASTM, ISO and Other Methods.................................................................................6-6
Conversion to Other Moisture Bases.........................................................................6-7
Conversion to Net Heat of Combustion......................................................................6-7
REPORTS......................................................................................................................7-1
MEMORY MANAGEMENT ............................................................................................8-1
MAINTENANCE.............................................................................................................9-1
Inspection of Critical Sealing Surfaces.......................................................................9-1
Bomb Removal...........................................................................................................9-2
Fuses .........................................................................................................................9-2
Daily Maintenance......................................................................................................9-2
Quarterly Maintenance...............................................................................................9-2
50 to 100 Test Maintenance.......................................................................................9-3
500 Test Maintenance................................................................................................9-3
5000 Test Maintenance..............................................................................................9-4
TROUBLESHOOTING.................................................................................................10-1
Bomb Exhaust Troubleshooting...............................................................................10-1
Jacket Temperature Troubleshooting ......................................................................10-3
Error List...................................................................................................................10-3
TECHNICAL SERVICE................................................................................................11-1
Contact Us ...............................................................................................................11-1
Return for Repair......................................................................................................11-1
PARTS LIST................................................................................................................12-1
DRAWINGS.................................................................................................................13-1
1138 Parts Diagram Key..........................................................................................13-2
TABLES.......................................................................................................................14-1
Page 4
6400 Calorimeter Instruction Manual
PREFACE
Scope
This manual contains instructions for installing and operating the Parr 6400 Calorimeter.
For ease of use, the manual is divided into 13 chapters.
Installation
Quick Start
Operation
Menu Descriptions
Calculations
Reports
Memory Management
Maintenance
Troubleshooting
Technical Service
Parts Lists
Drawings
Tables
Subsections of these chapters are identified in the Table of Contents.
To assure successful installation and operation, the user must study all instructions
carefully before starting to use the calorimeter to obtain an understanding of the
capabilities of the equipment and the safety precautions to be observed in the operation.
Additional instructions concerning the installation and operation of various component
parts and peripheral items used with the 6400 calorimeter should be made a part of
these instructions. Additional instructions for the optional printer are found in the
respective printer package and should be made a part of this book.
No. Description
201M Limited Warranty
207M Analytical Methods for Oxygen Bombs
230M Safety in the Operation of Laboratory and Pressure Vessels
483M Introduction to Bomb Calorimetry
Note:
The unit of heat used in this manual is the International Table (IT) calorie,
which is equal to 4.1868 absolute joules.
1-1
Page 5
6400 Calorimeter Instruction Manual
Purpose
Heats of combustion, as determined in an oxygen bomb calorimeter such as the 6400
Isoperibol Calorimeter, are measured by a substitution procedure in which the heat
obtained from the sample is compared with the heat obtained from a standardizing
material. In this test, a representative sample is burned in a high-pressure oxygen
atmosphere within a metal pressure vessel or “bomb”. The energy released by the
combustion is absorbed within the calorimeter and the resulting temperature change is
recorded.
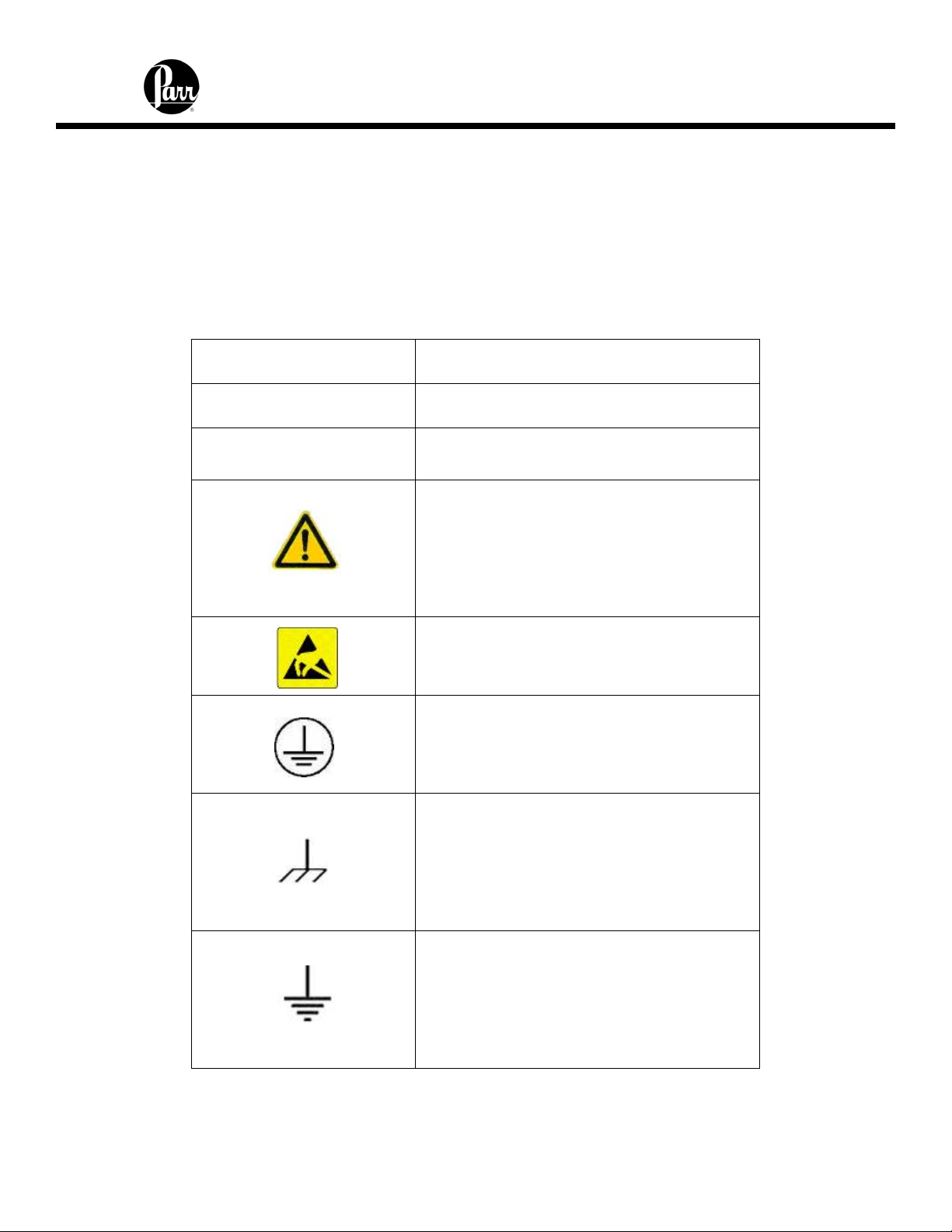
Explanation of Symbols
I
O
~
On position
Off position
Alternating Current (AC)
This CAUTION symbol may be present on
the Product Instrumentation and literature.
If present on the product, the user must
consult the appropriate part of the
accompanying product literature for more
information.
ATTENTION, Electrostatic Discharge
(ESD) hazards. Observe precautions for
handling electrostatic sensitive devices.
Protective Earth (PE) terminal. Provided
for connection of the protective earth
(green or green/yellow) supply system
conductor.
Chassis Ground. Identifies a connection
to the chassis or frame of the equipment
shall be bonded to Protective Earth at the
source of supply in accordance with
national and local electrical code
requirements.
Earth Ground. Functional earth
connection. NOTE: This connection shall
be bonded to Protective earth at the
source of supply in accordance with
national and local electrical code
requirements.
1-2
Page 6

6400 Calorimeter Instruction Manual
Safety Information
To avoid electrical shock, always:
1. Use a properly grounded electrical outlet of correct voltage and current handling
capability.
2. Ensure that the equipment is connected to electrical service according to local
national electrical codes. Failure to properly connect may create a fire or shock
hazard.
3. For continued protection against possible hazard, replace fuses with same type
and rating of fuse.
4. Disconnect from the power supply before maintenance or servicing.
To avoid personal injury:
1. Do not use in the presence of flammable or combustible materials; fire or
explosion may result. This device contains components which may ignite such
material.
2. Refer servicing to qualified personnel.
Intended Usage
If the instrument is used in a manner not specified by Parr Instrument Company, the
protection provided by the equipment may be impaired.
General Specifications
Electrical ratings
115VAC, 5.0 Amps. 50/60 Hz
230VAC, 3.0 Amps, 50/60 Hz
Before connecting the calorimeter to an electrical outlet, the user must be certain that
the electrical outlet has an earth ground connection and that the line, load and other
characteristics of the installation do not exceed the following limits:
Voltage: Fluctuations in the line voltage should not exceed 10% of the rated nominal
voltage shown on the data plate.
Frequency: Calorimeters can be operated from either a 50 or 60 Hertz power supply
without affecting their operation or calibration.
Current: The total current drawn should not exceed the rating shown on the data plate
on the calorimeter by more than 10 percent.
Environmental Conditions
Operating: 15ºC to 30ºC; maximum relative humidity of 80% non-condensing.
Installation Category II (overvoltage) in accordance with IEC 664.
Pollution degree 2 in accordance with IEC 664.
Altitude Limit: 2,000 meters.
Storage: -25ºC and 65ºC; 10% to 85% relative humidity.
1-3
Page 7
6400 Calorimeter Instruction Manual
INSTALLATION
Note:
Some of the following manual sections contain information in the form of
warnings, cautions and notes that require special attention. Read and
follow these instructions carefully to avoid personal injury and damage to
the instrument. Only personnel qualified to do so, should conduct the
installation tasks described in this portion of the manual.
Each Parr 6400 Calorimeter was completely assembled and thoroughly tested prior to
shipment. Unpack the calorimeter and carefully check the individual parts against the
packing list. If shipping damage is discovered, save the packing cartons and report it
immediately to the delivering carrier.
This apparatus is to be used indoors. It requires at least 4 square feet of workspace on a
sturdy bench or table in a well-ventilated area with convenient access to an electric
outlet, running water and a drain. The supply voltage must be within ± 10% of marked
nominal voltage on the apparatus. The supply voltage receptacle must have an earth
ground connection.
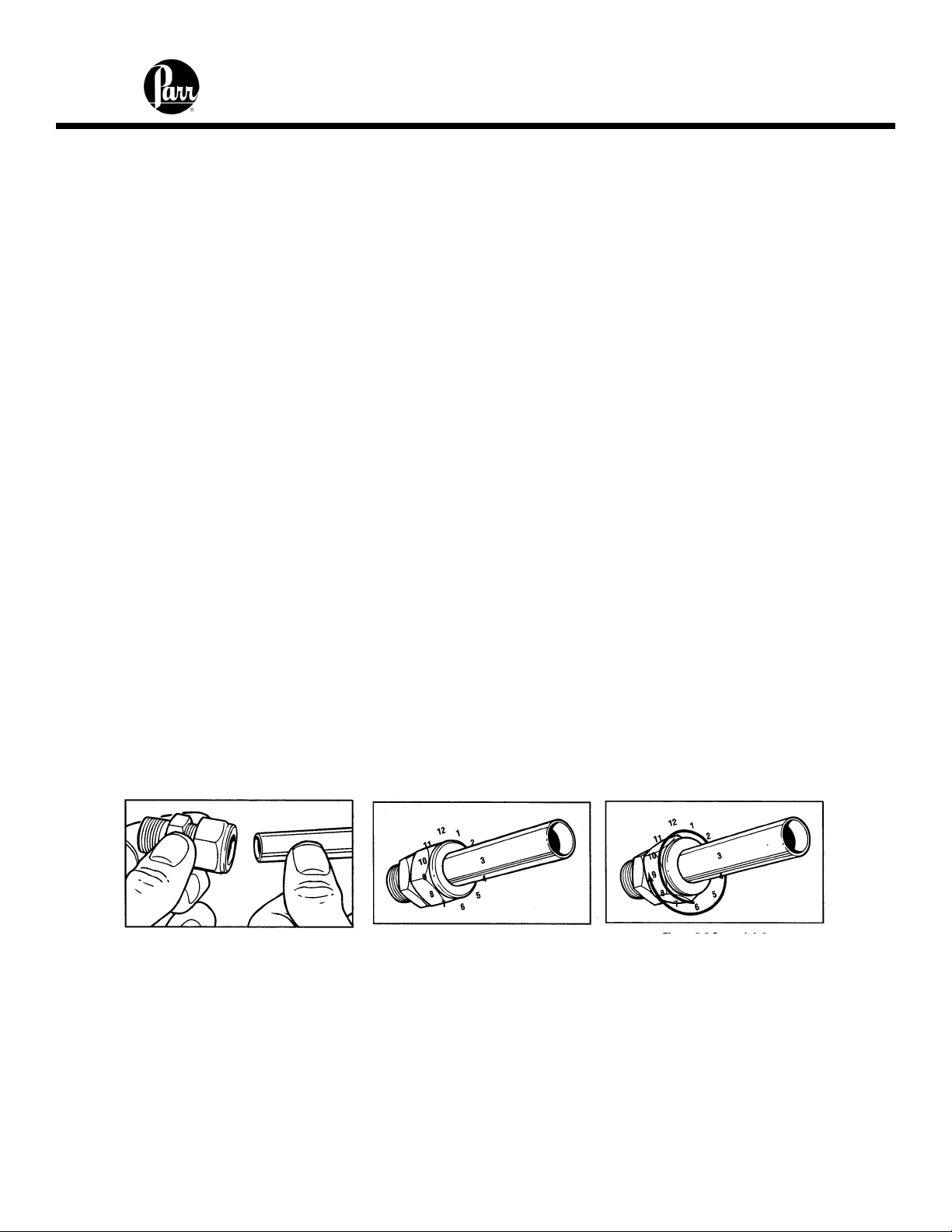
When Swagelok Tube Fittings are used, the instructions for installation are:
1. Simply insert the tubing into the Swagelok Tube Fitting. Make sure that the
tubing rests firmly on the shoulder of the fitting and that the nut is fingertight.
2. Before tightening the Swagelok nut, scribe the nut at the 6 o’clock position.
3. While holding the fitting body steady with a back-up wrench, tighten the nut
1-1/4 turns. Watch the scribe mark, make one complete revolution and
continue to the 9 o’clock position.
4. For 3/16" and 4mm or smaller tube fittings, tighten the Swagelok nut 3/4 turns
from finger-tight.
Swagelok tubing connections can be disconnected and retightened many times. The
same reliable leak-proof seal can be obtained every time the connection is remade using
the simple two-step procedure.
1. Insert the tubing with pre-swaged ferrules into the fitting body until the front
ferrule seats.
2. Tighten the nut by hand. Rotate the nut to the original position with a wrench.
An increase in resistance will be encountered at the original position. Then
2-1
Page 8

6400 Calorimeter Instruction Manual
tighten slightly with a wrench. Smaller tube sizes (up to 3/16” or 4mm) take less
tightening to reach the original position than larger tube sizes.
The type of tubing and the wall thickness also has an effect on the amount of tightening
required. Plastic tubing requires a minimal amount of additional tightening while heavy
wall metal tubing may require somewhat more tightening. In general, the nut only needs
to be tightened about 1/8 turn beyond finger tight where the ferrule seats in order to
obtain a tight seal.
Over tightening the nut causes distortion (flaring) of the lip of the tube fitting where the
ferrule seats. This in turn causes the threaded portion of the body to deform. It
becomes difficult to tighten the nut by hand during a subsequent re-tightening when the
fitting body becomes distorted in this manner.
Consumables, Utilities and Power Requirements
The 6400 Calorimeter requires availability of oxygen, 99.5% purity, with CGA 540
connection, 2500 psig, maximum.
The 6400 Calorimeter requires availability of nitrogen or air, oil and water free, with CGA
580 connection, 2500 psig, maximum.
Approximately 16L of distilled water are required to fill the external pressurized rinse
tank.
Approximately 2L of tap water, with a total hardness of 85 ppm or less, are required for
filling the internal cooling reservoir.
The power requirements for the sub-assemblies of the 6400 Calorimeter are:
Calorimeter
5A @ 120 VAC
3A @ 230 VAC
Printer
(100 to 240 VAC, 50/60 Hz) 0.35 A
Electrical Connection
Plug the power line into any grounded outlet providing proper voltage that matches the
specification on the nameplate of the calorimeter. Grounding is very important not only
as a safety measure, but also to ensure satisfactory controller performance. If there is
any question about the reliability of the ground connection through the power cord, run a
separate earth ground wire to the controller chassis.
Turn the power switch to the ON position. After a short time, the Parr logo will appear on
the LCD display followed by a running description of the instrument boot sequence.
When the boot sequence is complete, the Main Menu is displayed.
2-2
Page 9
6400 Calorimeter Instruction Manual
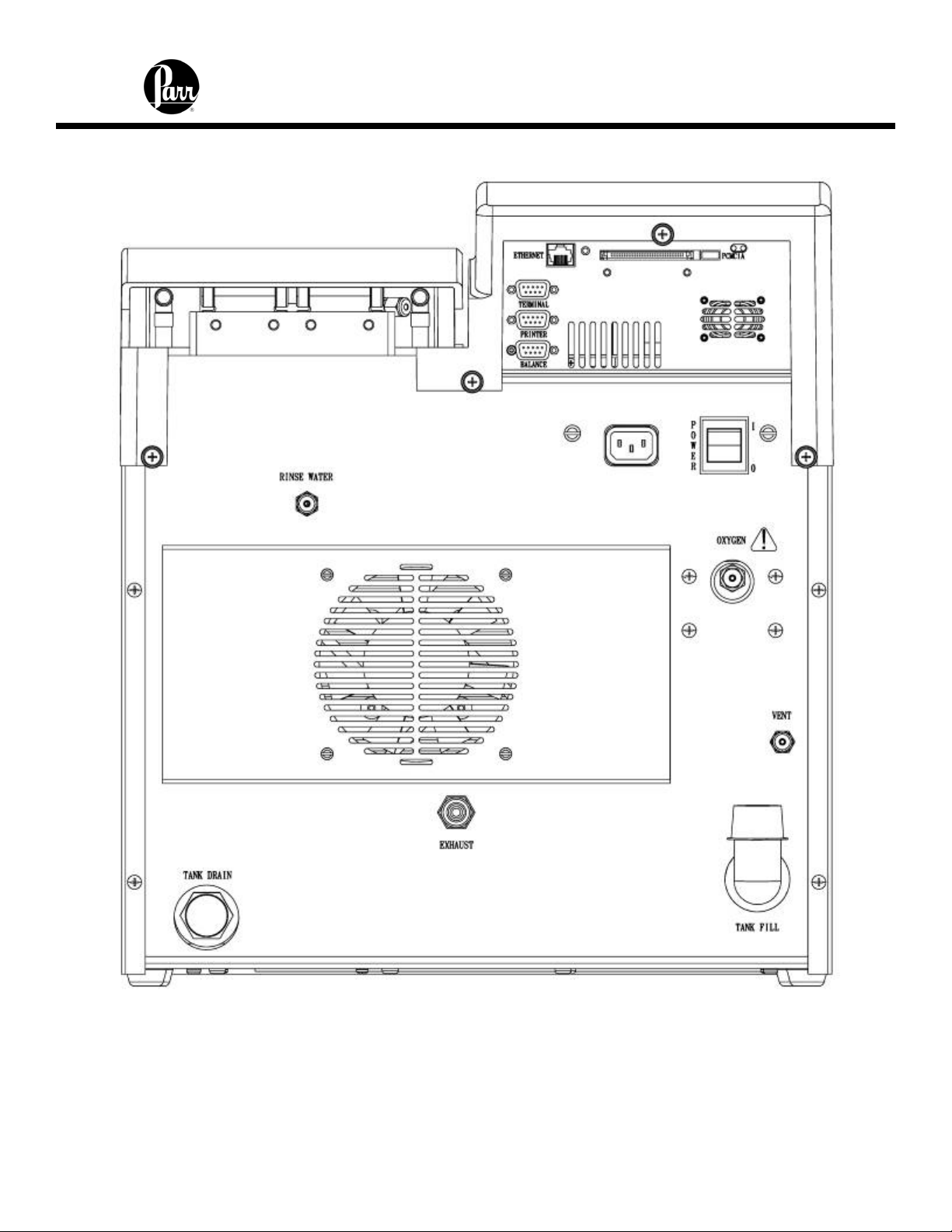
Figure 1 - 6400 Calorimeter Back Panel - Label
Water Connection
Remove the cap plug on the water filling elbow and fill the internal reservoir tank with
water having a total hardness of 85 ppm or less, until the water level is at the bottom of
the filling elbow. The calorimeter water tank will initially accept about 2 liters.
2-3
Page 10
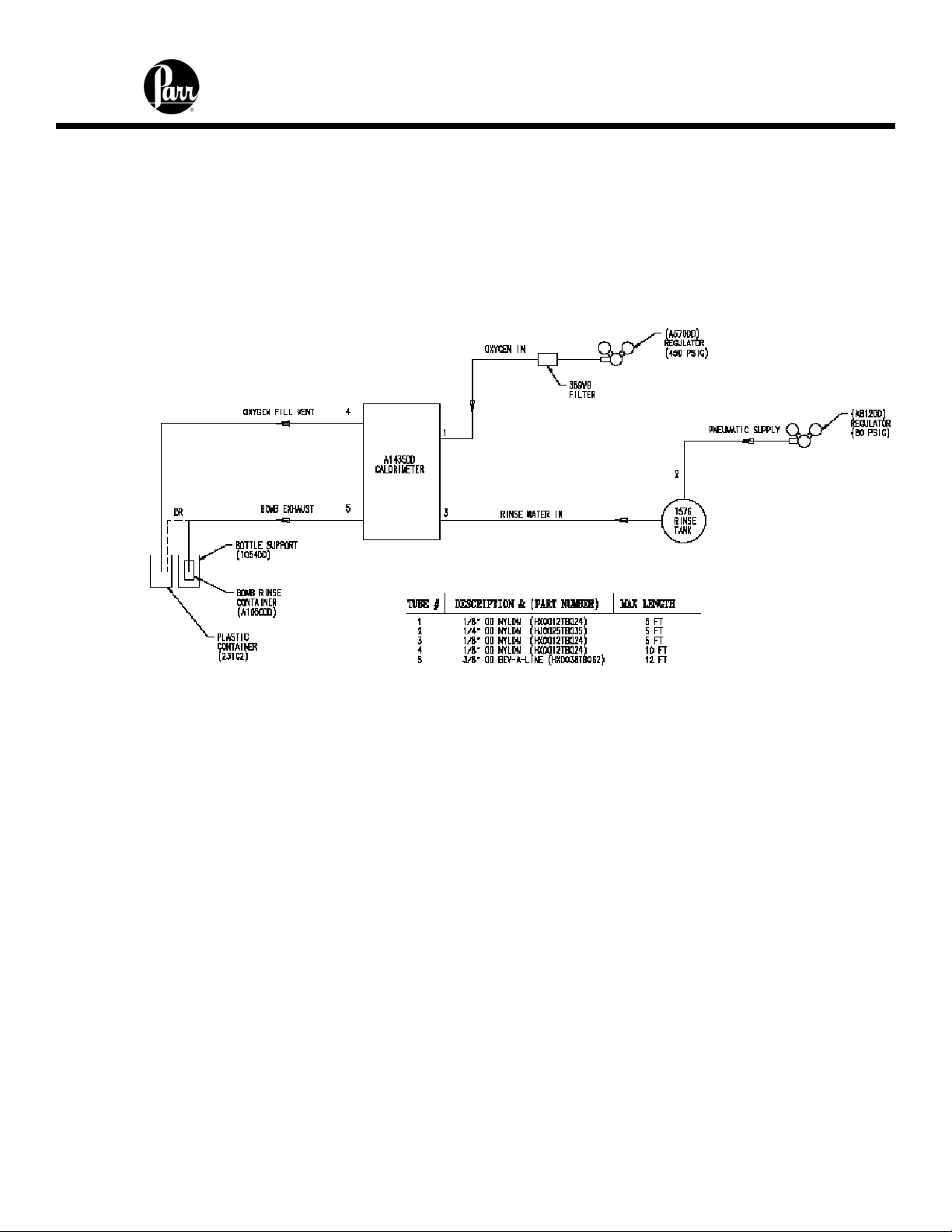
6400 Calorimeter Instruction Manual
Fill the external rinse tank with about 16 liters of distilled water through the large opening
at the top of the tank. The cover for this opening is removed by lifting up on the handle,
pushing down on the lid, tilting and removing. Replace and close the cover after filling.
The two connections between the calorimeter and the 1576 Rinse Tank should be made
with two pieces of 1/8” nylon pressure hose (HX0012TB024).
Figure 2 - 6400 External Plumbing
Gas Connection
Make the connections to the oxygen supply at this time. Refer to Figure 2. 1/8” O.D.
nylon pressure hose (HX0012TB024) is used to connect the oxygen supply. The inlet
connection incorporates a flow restrictor just behind the inlet connection. When making
the oxygen connection, a back-up wrench should be placed on the restrictor to insure a
secure connection and to prevent over tightening the flow restrictor. The delivery
pressure for oxygen should be set to 450 psig. To install the regulator, unscrew the
protecting cap from the tank and inspect the threads on the tank outlet to be sure they
are clean and in good condition. Place the ball end of the regulator in the tank outlet and
draw up the union nut tightly, keeping the gages tilted slightly back from an upright
position. Open the tank valve and check for leaks. The bomb must never be filled to
more than 600 psig (40 atm).
Make the connections to the nitrogen supply at this time. Refer to Figure 2. 1/4” O.D.
nylon pressure hose (HJ0025TB035) is used to connect the nitrogen supply. When
making the nitrogen connection, a back-up wrench should be placed on the restrictor to
insure a secure connection and to prevent over tightening the flow restrictor. The
delivery pressure for nitrogen should be set at 80 psig. To install the regulator, unscrew
the protecting cap from the tank and inspect the threads on the tank outlet to be sure
they are clean and in good condition. Place the ball end of the regulator in the tank
outlet and draw up the union nut tightly, keeping the gages tilted slightly back from an
upright position. Open the tank valve and check for leaks.
2-4
Page 11

6400 Calorimeter Instruction Manual
Note:
A hissing sound will occur while the rinse tank is being pressurized. This
is normal. Adjust the pneumatic supply regulator to 80 psig as needed.
During extended periods of inactivity, close the tank valve to prevent depleting the tank
in the event of a leak. Close the tank valve prior to removing the regulator when
changing tanks. Do not use oil or combustible lubricants in connection with any part of
the oxygen filling system. Keep all threads, fittings and gaskets clean and in good
condition.
Bomb Exhaust Connections
The exhaust and vent connections at the rear of the calorimeter, are made with the dual
tube A1006DD assembly. The end of the assembly with the bomb exhaust diffuser
should be placed into the 10 liter carboy (231C2). The carboy should be placed at or
below the level of the calorimeter to facilitate complete draining of these lines.
Alternatively:
The A1050DD Bomb Rinse Container Assembly is provided as an accessory to the 6400
Calorimeter. See Figure 28. This device allows for complete and systematic recovery of
the bomb combustion products. These combustion products include the initial line
exhaust after the fill cycle and the portion expelled during the bomb rinse cycle. The
Bomb Rinse Container Assembly is connected to the rear of the calorimeter, in place of
the portion of the waste tube assembly that is connected to the bomb exhaust fitting.
Combustion products are discharged from the bomb in two steps. The first step occurs
during the initial rapid release of the residual bomb gases. The 1053DD bottle has
sufficient strength and volume to deal effectively with this sudden pressure release. Gas
is expelled from the four holes on the perimeter of the 1052DD bottle cap, leaving any
discharged liquid in the bottle. As an additional safety measure, the bottle is supported
in a 1054DD acrylic cylinder which serves to keep the bottle upright and contained in the
unlikely event the bottle ruptures. At the end of the bomb exhaust step the aqueous
combustion products reside in the bomb, associated tubing as well as the 1053DD
bottle. The bomb rinse step flushes these combustion products from the bomb and the
tubing into the 1053DD bottle. The bottle can then be unscrewed from the assembly and
capped, until the sample is to be analyzed. Some users find it useful to add the contents
of the rinsed combustion capsule to the washings collected in the bottle. Three 1053DD
bottles are provided with the assembly. Additional bottles may be ordered separately
from Parr.
Communication Connections
There are three RS-232 serial ports at the rear of the calorimeter. These ports are
designated Terminal, Printer and Balance. The pin-out of these three ports are identical
and can be found in Table 1.
2-5
Page 12
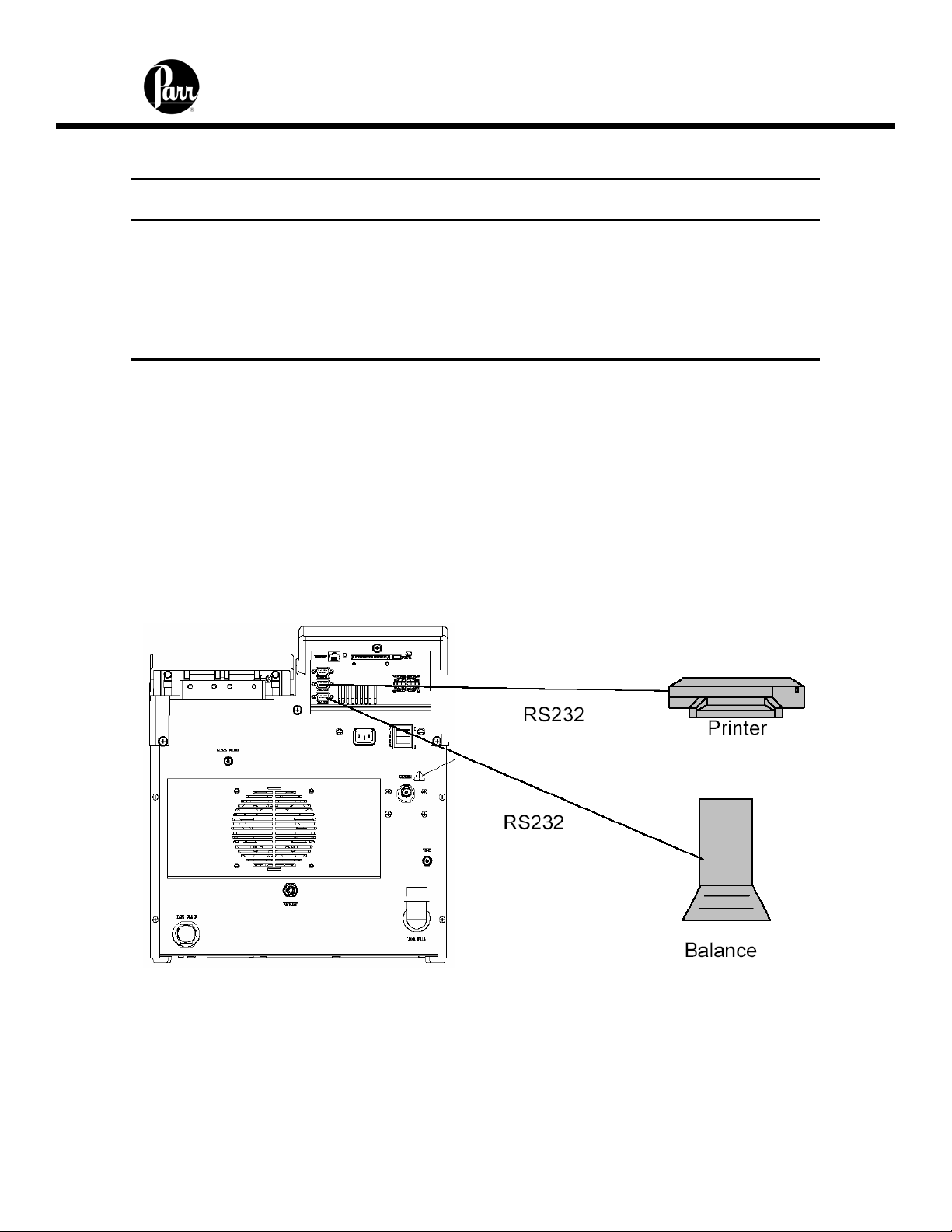
6400 Calorimeter Instruction Manual
Table 1 - 6400 Calorimeter Serial Ports Pin-Out
9 pin D Connector Pin # Description Direction
(6400 – External Device)
2 Received Data
3 Transmitted Data
4
5 Signal Ground
6
7 Ready to Send (RTS)
8 Clear to Send (CTS)
The RS232 balance port is a female port whereas the RS232 printer port is a male port.
The 6400 Calorimeter is also equipped with an RJ45 Ethernet port for connection to a
computer. Before making any of these connections, the data transmission rate of the
Calorimeter and the printer, balance or computer must be matched. Generally the baud
rates on either device can be changed to achieve this match.
The 6400 will also allow the user to specify the IP addresses of one or more Balance
Interface devices on the network by selecting the Network Data Device menu in the
Communications Controls menu. Balance Interface devices are polled from device 1 to
15 for sample and / or spike weights when the weight entry mode is set to Network.
Figure 3 - 6400 Calorimeter Peripherals
Í
Î
Í Î
Î
Í
2-6
Page 13
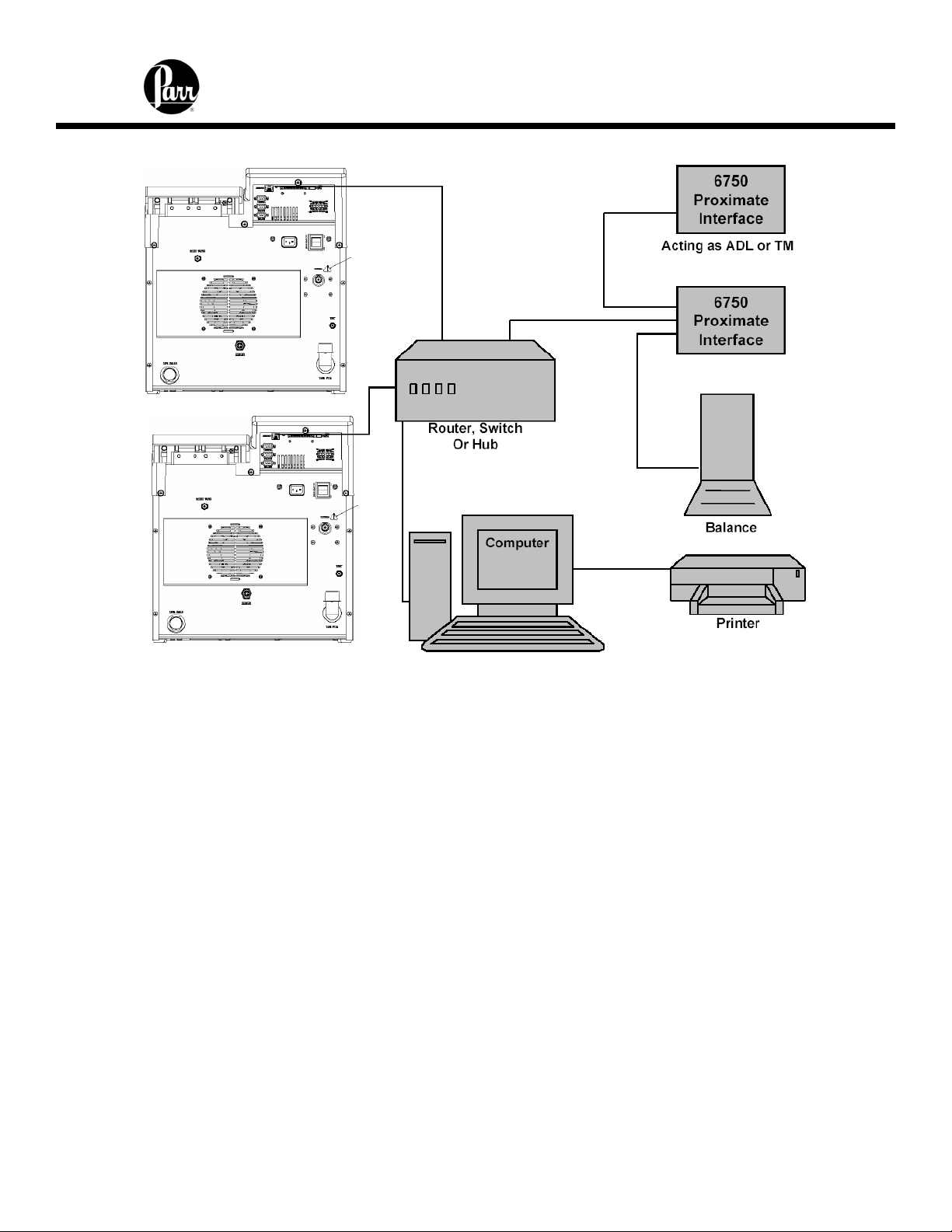
6400 Calorimeter Instruction Manual
Multiple Alternate Configurations
Printer Connections
The printer port settings are on the Communication Controls Menu: Printer Port
Communications Menu. The default parameters for the 6400 are set up for use with the
Parr 1757 Printer. Table 1 identifies and describes the pin-out for the RS-232 port.
Balance Connections
The 6400 Calorimeter supports input from the multiple balance types. Additionally, a
generic input driver is provided for communications with balances that do not conform to
the eight supported protocols. A new feature supported by all balance input drivers is the
ability to change the expected number of characters in the data field. The number of data
characters indicated for each of the drivers, below, are default values. This feature virtually
eliminates the need for balance input drivers to be re-written in the event the balance
manufacturer elects to alter the output string of a balance when new models are
introduced.
The format of an unknown balance can be determined by logging the balance output to
the printer attached to the calorimeter. Those protocols which send a command string to
the balance will do so while logging is active. In order for the logging to produce
meaningful results, the cable connecting the balance to the balance input port of the
calorimeter must be correctly wired or configured. In addition, the specifics of the data
frame, such as the baud rate, # of data bits, parity, # of stop bits and handshaking (if
used) must be the same for both the balance and the calorimeter.
2-7
Page 14

Mettler 011/012 Interface
The ID field must contain “S_” to
indicate a stable mass. The data field
contains the current mass, right
justified, with a decimal point. The
balance should be configured to send
continuously.
Sartorious Interface
The polarity field must contain either a
“+” or a space. Leading zeros in the
data field are blanked, except for the
one to the left of the decimal point.
The stability field must contain “g_” for
the calorimeter to accept a mass. The
balance should be configured to
transmit data upon receipt of the
following command string:
[ESC] P [CR] [LF]
Note:
The automatic data output option should not be used.
6400 Calorimeter Instruction Manual
Field Length
ID 2
space 1
data 9
space 1
g 1
CR 1
LF 1
Field Length
polarity 1
space 1
data 8
space 1
stability 2
CR 1
LF 1
Generic Interface
The data field should consist of 9
numeric characters (0 through 9, +, and space) terminated with a carriage
return (CR). Leading zeros may be blanked as spaces and are counted.
Non-numeric characters are ignored and will reset the input buffer if the data
field has not been filled. Any characters received after filling the data field
and before the carriage return are ignored.
Field Length
data 8
CR 1
Bar Code Port
The use of barcodes in the laboratory has become a highly accurate, rapid and
inexpensive way to identify samples. When purchasing this feature, the user must
supply Parr with the MAC address of the calorimeter (found in the Software & Hardware
Info menu screen). This allows Parr to activate the feature key. In order to enable the
calorimeter to use the bar code feature, the feature key needs to be entered into the
instrument. Select the “Program Information and Control” key from the Main Menu.
Next, select “Feature Key” and enter the feature key purchased from Parr Instrument
Company into the instrument by using the touchpad. Pressing the key labeled “ABC”
allows the user to switch from upper case letters, to lower case letters, to numerals, and
finally to symbols. A CD containing all the necessary documentation and setup
information for using both the scanner and the printer is provided at the time of
purchase. A PC based program used for printing bar coded labels is also provided on
this CD.
2-8
Page 15

6400 Calorimeter Instruction Manual
Computer Connections
If the 6400 Calorimeter is to be
connected to a computer, the Ethernet
connection should be used. Test data
can be transferred to an Ethernet
network connected computer using the
FTP File Transfer Protocol. First, you
must know the IP address of the
network-connected calorimeter. The
network DHCP (Dynamic Host
Configuration Protocol) server
provides this address shortly after the
calorimeter is turned on. The address
can be seen on the Software &
Hardware Info” screen, under Program
Info and Control Menu (see the example screenshot). Users who don’t have a network
infrastructure can create a simple network by connecting a router with DHCP server
capability to the calorimeter using an ordinary CAT 5 network cable. The calorimeter
should be connected to LAN side of the router. The PC in turn is also connected to the
LAN side of the router using a similar CAT 5 cable. A D-Link 614+ router is
recommended for this purpose. For this router, operated without a WAN connection, the
primary DNS address of the router (WAN setup) must be set to the IP address of the
router found on the LAN setup page. Other routers behave differently in the absence of
a WAN connection. Providing an active upstream connection to the WAN port of most
routers generally minimizes the use of any obscure setup configurations. An FTP
enabled web browser can be used to access stored test data. The URL is of the
following form:
ftp://root:rootroot@192.168.0.125/../flash/data/
In this case, 192.168.0.125 is the IP address of the calorimeter.
2-9
Page 16
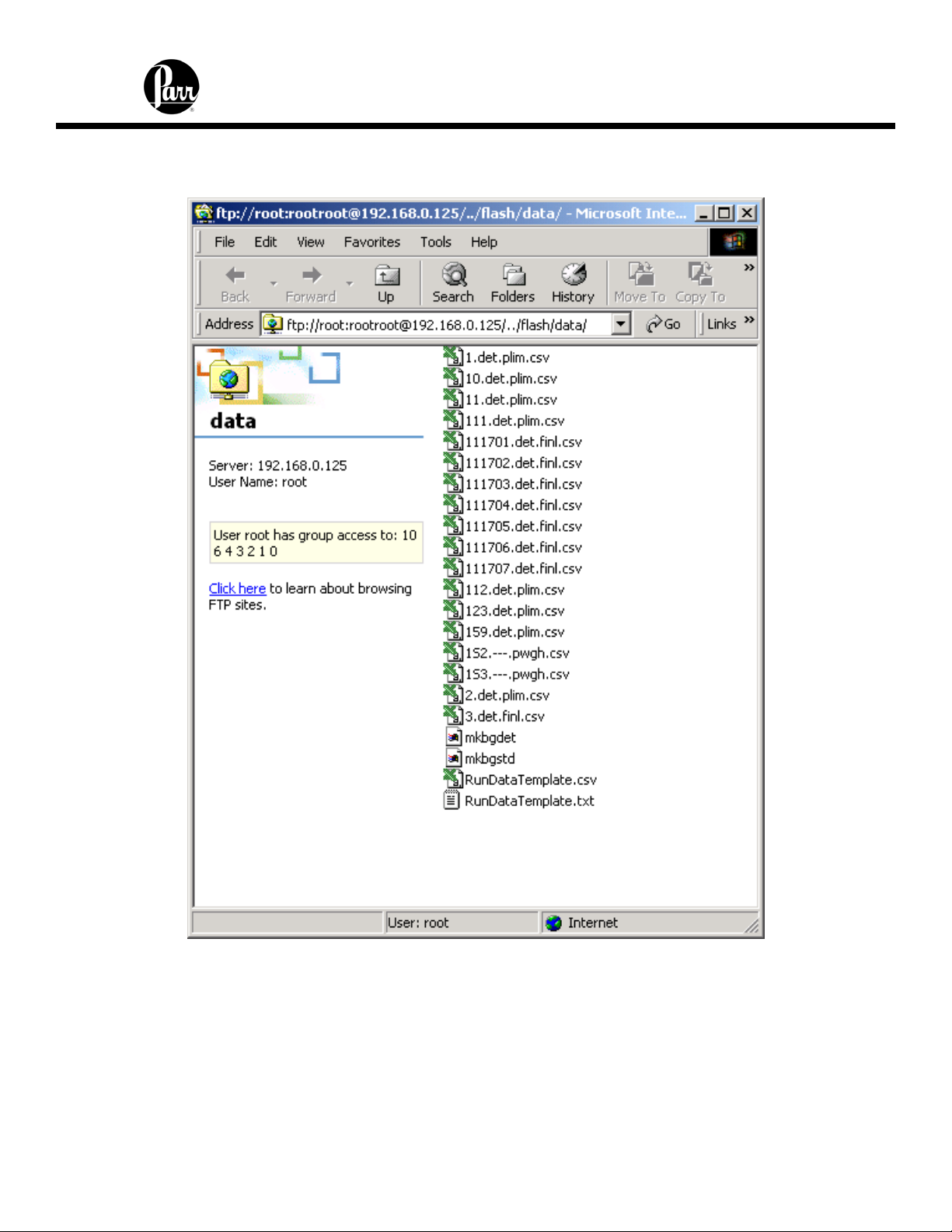
6400 Calorimeter Instruction Manual
The following screenshot illustrates the contents of the calorimeter data directory as
presented by a web browser.
You can drag and drop or copy and paste test data files (with the csv suffix) from the
web browser window to any convenient folder or directory on the PC.
2-10
Page 17
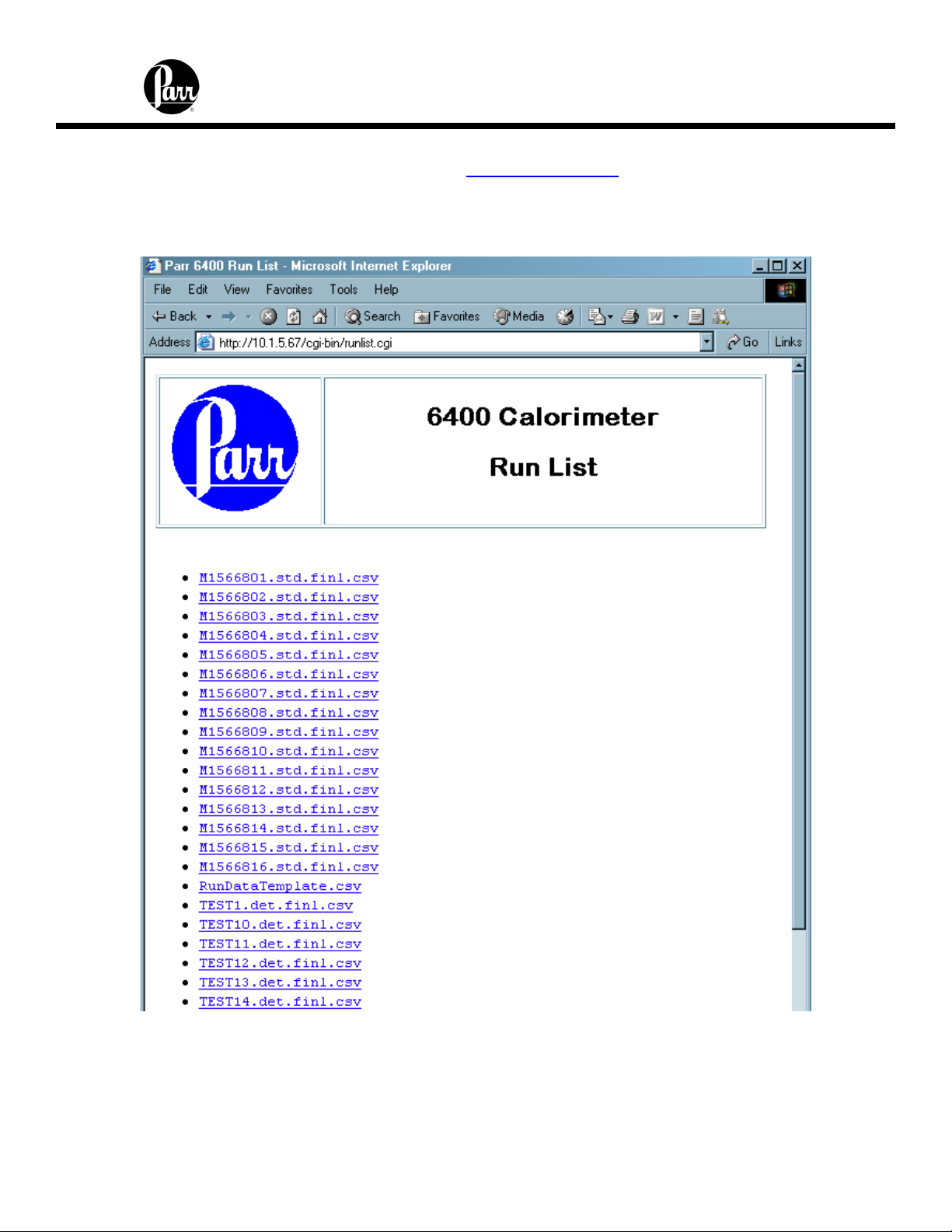
6400 Calorimeter Instruction Manual
The calorimeter offers a web server service. Test reports can be viewed with a web
browser using a URL of the following form: http://192.168.0.125
the IP address of the calorimeter.
Clicking on the Sample Data tab displays a list of reports currently in the instrument
memory.
, where 192.168.0.125 is
2-11
Page 18
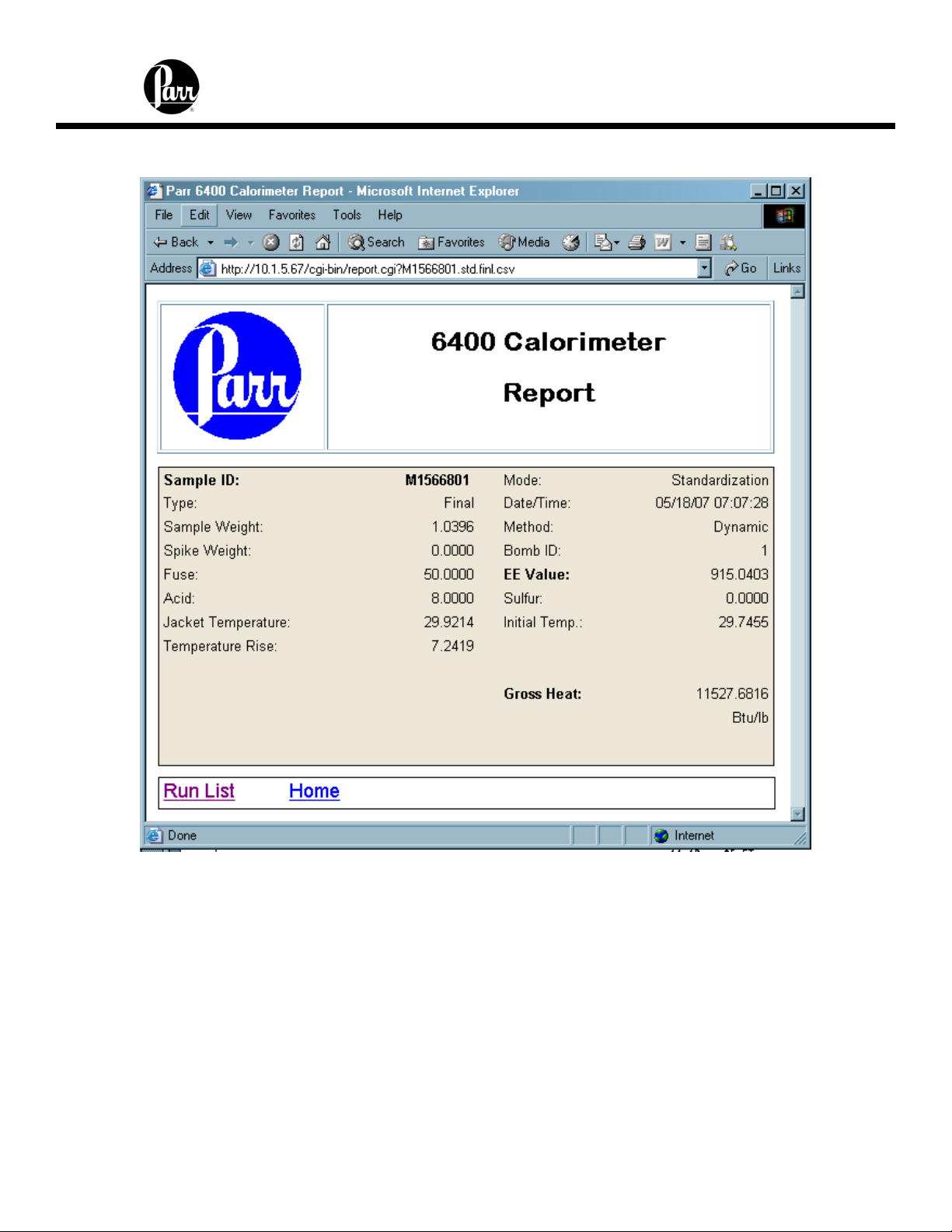
6400 Calorimeter Instruction Manual
Clicking on any given report will provide a display similar to the following:
2-12
Page 19

6400 Calorimeter Instruction Manual
QUICK START
1. Turn on the heater and pump in the Calorimeter Operation menu. Allow at least 20
minutes for the calorimeter to warm up.
2. Initiate a pretest to run the calorimeter through the fill and cool/rinse cycles. This
function is used to pre-condition the calorimeter if it has been sitting idle for an extended
period of time (greater than 15 minutes).
3. Prepare and weigh the sample to 0.0001g.
4. Gently tap capsules that contain powdered samples to compact the material. (Pellets
are easier to handle than loose samples and they burn slower in the bomb, thereby
reducing the chances for incomplete combustion).
5. Carefully place the capsule into the capsule holder, attach 10 cm of ignition thread and
install the bomb head in the calorimeter.
6. Close the calorimeter cover making sure that the latch is engaged.
7. Select determination or standardization as appropriate on the Calorimeter Operation
page, by toggling the operating mode key. Press the Start Key. The calorimeter will
now prompt the operator for Bomb ID number, sample ID number, sample weight and
spike weight in accordance with the instructions set into the operating controls page.
8. The calorimeter will now take over and conduct the test. During the time it is establishing
the initial equilibrium, it will display PREPERIOD on the status bar. Just before it fires
the bomb, it will sound a series of short beeps to warn the user to move away from the
calorimeter. Once the bomb has been fired, the status bar will display POSTPERIOD.
The calorimeter will check to make certain that a temperature rise occurs and will then
look for the final equilibrium conditions to be met. If it fails to meet either the initial or
final equilibrium conditions, or if it fails to detect a temperature rise within the allotted
time, the test will terminate and advise the user of the error.
9. At the conclusion of the test, the calorimeter will signal the user.
10.
Open the cover and remove the head. Examine the interior of the bomb for soot or other
evidence of incomplete combustion. If such evidence is found, the test will have to be
discarded.
11. Titrate the bomb washings with a standard sodium carbonate solution using methyl
orange, red or purple indicator. A 0.0709N sodium carbonate solution is recommended
for this titration to simplify the calculation. This is prepared by dissolving 3.76 grams of
Na
2CO3
normality may be used.
12. Analyze the bomb washings to determine the sulfur content of the sample if it exceeds
0.1%. Methods for determining sulfur are discussed in Analytical Methods for Oxygen
Bombs, No. 207M.
in the water and diluting to one liter. NaOH or KOH solutions of the same
3-1
Page 20

6400 Calorimeter Instruction Manual
OPERATION
Menu System
All configurations and operations are
handled by a menu-driven system
operated from the bright touch screen
display. The settings and controls are
organized into ten main sections as
displayed on the MAIN MENU.
Note:
Keys with a “double box” in the
upper left hand corner lead to
sub-menus.
Menu Keys
The controls that change the data field information in the menus will be one of the
following:
1. Toggles. These data fields contain ON/OFF or YES/NO choices. Simply touching
the key on the screen toggles the choice to the other option. The current setting is
displayed in the lower right corner of the key.
2. Option Selection. These data fields contain a list of options. Touching the key on
the screen steps the user through the available choices. The current setting is
displayed in the lower right corner of the key.
3. Value Entry Fields. These data fields are used to enter data into the Calorimeter.
Touching the key on the screen brings up a sub-menu with a key pad or similar
screen for entering the required value.
clear the current value before entering a new value. Once entered the screen will
return to the previous menu and the new value will be displayed in the lower right
corner of the key.
4. Data Displays. Most of these keys display values that have been calculated by the
calorimeter and are informational only. Certain ones can be overridden by the user
entering a desired value through a sub-menu. The value is displayed in the lower
right corner of the key.
Note:
Some keys will respond with an opportunity for the user to confirm the
specified action to minimize accidental disruptions to the program and/or
stored data.
Some keys lead to multiple choices. Always
4-1
Page 21

6400 Calorimeter Instruction Manual
Control Keys
There are five control keys which always appear in the right column of the primary
displays. These keys are unavailable when they are gray instead of white.
1. Escape. This key is used to go up one level in the menu structure.
2. Main Menu. This key is used to return to the main menu touch screen from
anywhere in the menu structure.
3. Start. This key is used to start a test.
4. Report. This key is used to access the test results stored in the calorimeter, to enter
thermochemical corrections, and to initiate a report on the display, printer or attached
computer.
5. Help. This key is used to access help screens related to the menu currently
displayed on the touch screen.
Programming
The program in the 6400 Calorimeter can be extensively modified to tailor the unit to a
wide variety of operating conditions, reporting units, laboratory techniques, available
accessories and communication modes. In addition, the calculations, thermochemical
corrections and reporting modes can be modified to conform to a number of standard
test methods and procedures. Numerous provisions are included to permit the use of
other reagent concentrations, techniques, combustion aids and short cuts appropriate for
the user’s work.
Note:
Changes to the program are made by use of the menu structure. Any of
these items can be individually entered at any time to revise the operating
program.
Default Settings
Units are preprogrammed with default settings. See Table 2 for a listing of the factory
default settings. A more in-depth explanation of these parameters is found on the
corresponding parameter group help pages. These default settings remain in effect until
changed by the user. Should the user ever wish to return to the factory default settings,
go to the Program Info and Control Menu, User/Factory Settings, touch Reload Factory
Default Settings and YES. Non-volatile memory is provided to retain any and all
operator initiated program changes; even if power is interrupted or the unit is turned off.
If the unit experiences an intentional or unintentional “Cold Restart”, the controller will
return to the last known settings.
The default parameters of the 6400 calorimeter can be changed to guarantee that the
calorimeter, when cold restarted, will always be in the desired configuration before
beginning a series of tests. Users who wish to permanently revise their default settings
may do so using the following procedure:
• Establish the operating parameters to be stored as the user default settings.
• Go to the Program Info and Control Menu, User/ Factory Settings, User Setup ID,
and enter the desired User Setup ID.
• Select Save User Default Settings
4-2
Page 22

6400 Calorimeter Instruction Manual
To re-load the user default setting, go to the Program Info and Control Page,
User/Factory Settings, Re-load User Default Settings, and YES.
Sample Preparation
Sample Size
To stay within safe limits, the bomb should never be charged with a sample which will
release more than 8000 calories when burned in oxygen. The initial oxygen pressure is
set at 30 atmospheres (450 psig). This generally limits the mass of the combustible
charge (sample plus benzoic acid, gelatin, firing oil or any combustion aid) to not more
than 1.1 grams. To avoid damage to the bomb and calorimeter, and possible injury to
the operator, it should be a standing rule in each laboratory that the bomb must never be
charged with more than 1.5 grams of combustible material.
When starting tests with new or unfamiliar materials, it is always best to use samples of
less than 0.7 grams with the possibility of increasing the amount if preliminary tests
indicate no abnormal behavior and sample will not exceed the 8000 calorie limit.
Samples containing sulfur should contain no more than 50 mg of sulfur and have a
calorific value of at least 9000 BTU/lb.
Samples containing chlorine should be spiked to insure that sample contains no more
than 100 mg of chlorine and liberates at least 5000 calories
Particle Size and Moisture Content
Solid samples burn best in an oxygen bomb when reduced to 60 mesh, or smaller, and
compressed into a pellet with a 2811 Parr Pellet Press. Large particles may not burn
completely and small particles are easily swept out of the capsule by turbulent gases
during rapid combustion.
Note:
Particle size is important because it influences the reaction rate.
Compression into a pellet is recommended because the pressure
developed during combustion can be reduced as much as 40% when
compared to the combustion of the material in the powder form. In
addition to giving controlled burn rates, the formation of pellets from
sample material keeps the sample in the fuel capsule during combustion.
Materials, such as coal, burn well in the as-received or air-dry condition, but do not burn
completely dry samples. A certain amount of moisture is desirable in order to control the
burning rate. Moisture content up to 20% can be tolerated in many cases, but the
optimum moisture is best determined by trial combustions. If moisture is to be added to
retard the combustion rate, drop the water directly onto the loose sample or onto a pellet
after the sample has been weighed. Then let the sample stand to obtain uniform
distribution. Low volatile samples with high water content, such as urine or blood, can
be burned in an open capsule by absorbing the liquid on filter paper pulp or by adding a
combustion aid, such as ethylene glycol.
4-3
Page 23

6400 Calorimeter Instruction Manual
Sample Types
Because of the difference in combustion characteristics of the many different materials
which may be burned in an oxygen bomb, it is difficult to give specific directions which
will assure complete combustions for all samples.
The following fundamental conditions should be considered when burning samples:
• Some part of the sample must be heated to its ignition temperature to start the
combustion and, in burning, it must liberate sufficient heat to support its own
combustion regardless of the chilling effect of the adjacent metal parts.
• The combustion must produce sufficient turbulence within the bomb to bring
oxygen into the fuel cup for burning the last traces of the sample.
• A loose or powdery condition of the sample which will permit unburned particles
to be ejected during a violent combustion.
• The use of a sample which contains coarse particles will not burn readily. Coal
particles which are too large to pass a 60 mesh screen may not burn completely.
• The use of a sample pellet which has been made too hard or too soft can cause
spalling and the ejection of unburned fragments.
• The bottom of the cup should always be at least one-half inch above the bottom
of the bomb or above the liquid level in the bomb to prevent thermal quenching.
• If the moisture, ash and other non combustible material in the sample totals
approximately 20% or more of the charge, it may be difficult to obtain complete
combustion. This condition can be remedied by adding a small amount of
benzoic acid or other combustion aid.
Foodstuffs and Cellulosic Materials
Fibrous and fluffy materials generally require one of three modes for controlling the burn
rate. Fibrous materials do not pelletize readily and generally require either moisture
content or a combustion aid such as mineral oil to retard the burn rate and avoid
development of high pressures. Partial drying may be necessary if the moisture content
is too high to obtain ignition, but if the sample is heat sensitive and cannot be dried, a
water soluble combustion aid such as ethylene glycol can be added to promote ignition.
Material such as Napthalene should not be burned in loose powder form but should be
formed into a pellet.
Coarse Samples
In most cases it may be necessary to burn coarse samples without size reduction since
grinding or drying may introduce unwanted changes. There is no objection to this if the
coarse sample will ignite and burn completely. Whole wheat grains and coarse charcoal
chunks are typical of materials which will burn satisfactorily without grinding and without
additives or a special procedure.
Corrosive Samples
The 1138 bomb is made from alloy 20; a special niobium stabilized stainless steel
selected for its resistance to the mixed nitric and sulfuric acids produced during the
combustion process. The 1138CL is made from the halogen resistant Hastelloy G30™.
Hastelloy 30™ is an alloy rich in cobalt and molybdenum and is able to resist the
corrosive effects of free chlorine and halogen acids produced when burning samples
with significant chlorine content. While no alloy will completely resist the corrosive
atmospheres produced when burning samples containing halogen compounds; users
4-4
Page 24
6400 Calorimeter Instruction Manual
who intend to test these materials are urged to select the 1138CL Bomb. These bombs
are 250 mL in volume and are rated to a maximum working pressure of
bombs are hydrostatically tested to 3000 psi and the sample range is ~1g or 5000 – 8000
calories.
Explosives and High Energy Fuels
Materials which release large volumes of gas which detonate with explosive force or
burn with unusually high energy levels, should not be tested in this calorimeter. Rather,
they should be tested in a model 6100 or 6200 Calorimeter which can be equipped with
an 1104 High Strength Oxygen Bomb designed specifically for these types of samples.
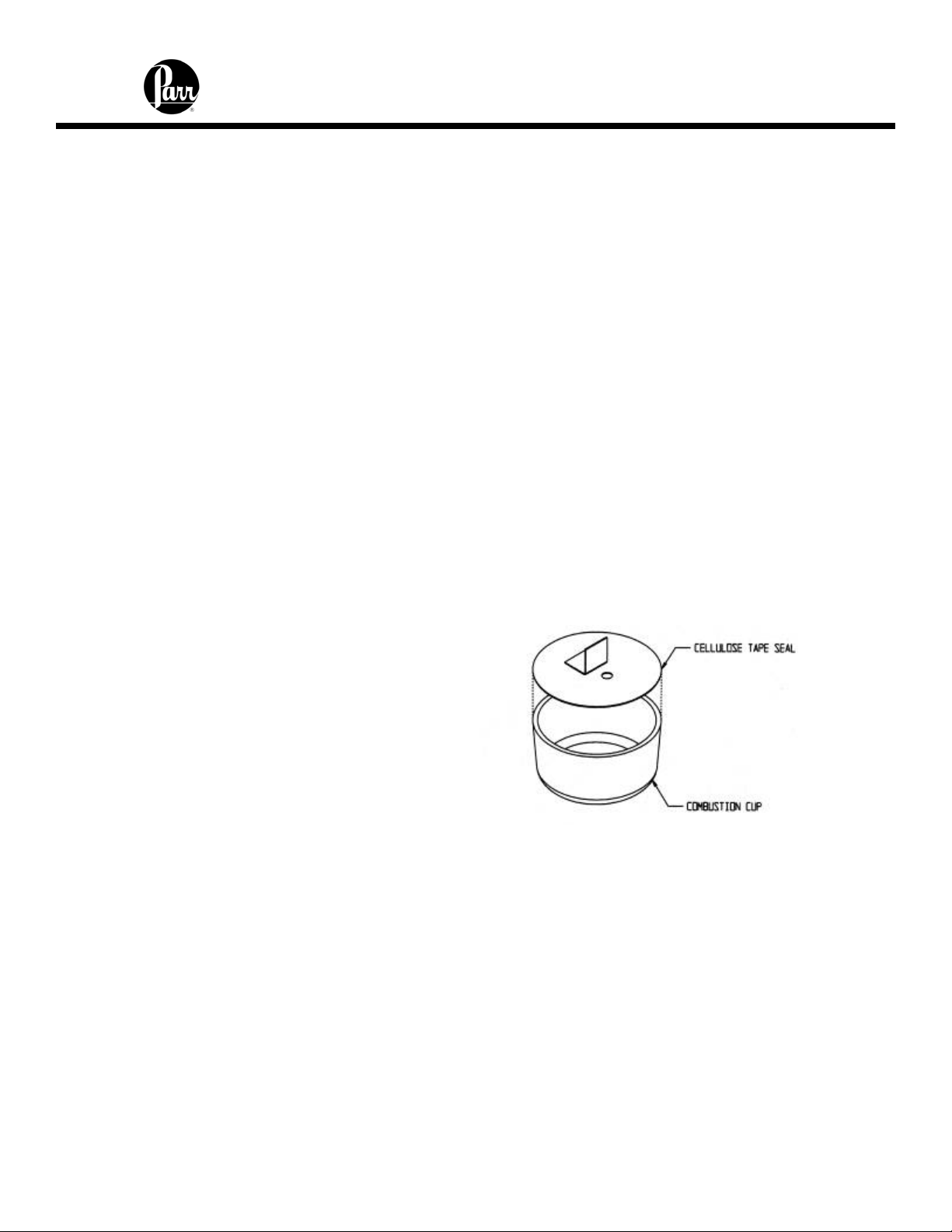
Volatile Sample Holders
Volatile samples are defined as one with an initial boiling point below 180ºC per ASTM
D-2. Volatile samples can be handled in a Parr 43AS Alloy Capsule which has a sturdy
wall with a flat top rim. These holders can be sealed with a disc of plastic adhesive tape
prepared by stretching tape across the top of the cup and trimming the excess with a
sharp knife. The seal obtained after pressing this disc firmly against the rim of the cup
with a flat blade will be adequate for most volatile samples. The tape used for this
purpose should be free of chlorine and as low in sulfur as possible. Borden Mystic Tape,
No. M-169-C or 3M Transparent Tape, No. 610, are recommended for this purpose. The
3M Transparent Tape can be ordered through Parr, Part No. 517A.
Figure 4 - Volatile Sample Technique
The weight of the tape disc must be
determined separately and a correction
applied for any elements in the tape
which might interfere with the
determination. The approximate Heat of
Combustion of the tape is 6300 cal/g. An
actual amount should be determined by
running a blank test with tape alone using
a sample weighing 1.0 gram. The
compensation for heat of tape may be
done through the spike option; see Spike
Controls, Heat of Combustion of Spike.
Note:
Tape should always be stored in a sealed container to minimize changes in
its moisture and solvent content.
Use the following procedure when filling and handling any of these tape-sealed sample
holders:
1. Weigh the empty cup or capsule; then cover the top with tape, trim with a knife
and press the trimmed edge firmly against the metal rim. Also cut and attach a
small flag to the disc (see Figure 4).
2. Puncture the tape at a point below the flag, then re-weigh the empty cup with its
tape cover.
3. Add the sample with a hypodermic syringe; close the opening with the flag and
re-weigh the filled cup.
2000 psi. The
4-5
Page 25

6400 Calorimeter Instruction Manual
4. Set the cup in the capsule holder and arrange the auxiliary fuse so that it touches
the center of the tape disc.
5. Just before starting the test, prick the disc with a sharp needle to make a small
opening which is needed to prevent collapse of the disc when pressure is
applied.
6. Fill the bomb with the usual oxygen charging pressure.
7. The calorimeter will fire the bomb and complete the test in the usual manner.
Combustion Aids
Some samples may be difficult to ignite or they may burn so slowly that the particles
become chilled below the ignition point before complete combustion is obtained. In such
cases white oil or other suitable material of known purity can be mixed with the sample.
Ethylene glycol, butyl alcohol or decalin may be used for this purpose.
Note:
It must be remembered, that a combustion aid adds to the total energy
released in the bomb and the amount of sample may have to be reduced
to compensate for the added charge.
When benzoic acid is combusted for standardization runs, it should be in the form of a
pellet to avoid possible damage to the bomb which might result from rapid combustion of
the loose powder.
Combustion Capsules
Non-volatile samples to be tested in Parr oxygen bombs are weighed and burned in
shallow capsules measuring approximately 1" diameter and 7/16" deep. These are
available in stainless steel, fused silica and platinum alloyed with 3-1/2% rhodium.
Stainless steel capsules (43AS) are furnished with each calorimeter. The stainless steel
capsules will acquire a dull gray finish after repeated use in an oxygen bomb due to the
formation of a hard, protective oxide film. This dull finish not only protects the capsule,
but it also promotes combustion and makes it easier to burn the last traces of the
sample. New capsules are heated in a muffle furnace at 500ºC for 24 hours to develop
this protective coating uniformly on all surfaces. This treatment should be repeated after
a capsule has been polished with an abrasive to remove any ash or other surface
deposits. Heating in a muffle is also a good way to destroy any traces of carbon or
combustible matter which might remain in the capsule from a previous test. Capsules
should be monitored for wear. Do not use the capsule if the wall or base thickness is
less than 0.025”.
Note:
After heating, place the capsules in a clean container and handle them
only with forceps when they are removed to be weighed on an analytical
balance.
When combusting samples that contain metal particles such as aluminum or
magnesium, the non-metallic (fused silica) 43A3 Capsule is required.
4-6
Page 26
6400 Calorimeter Instruction Manual
When superior corrosion resistance is needed, the Platinum Rhodium 43A5 Capsule is
required.
Test Process
Loading the sample
Prepare and weigh the sample to 0.0001g. Gently tap capsules that contain powdered
samples to compact the material. (Pellets are easier to handle than loose samples and
they burn slower in the bomb, thereby reducing the chances for incomplete combustion).
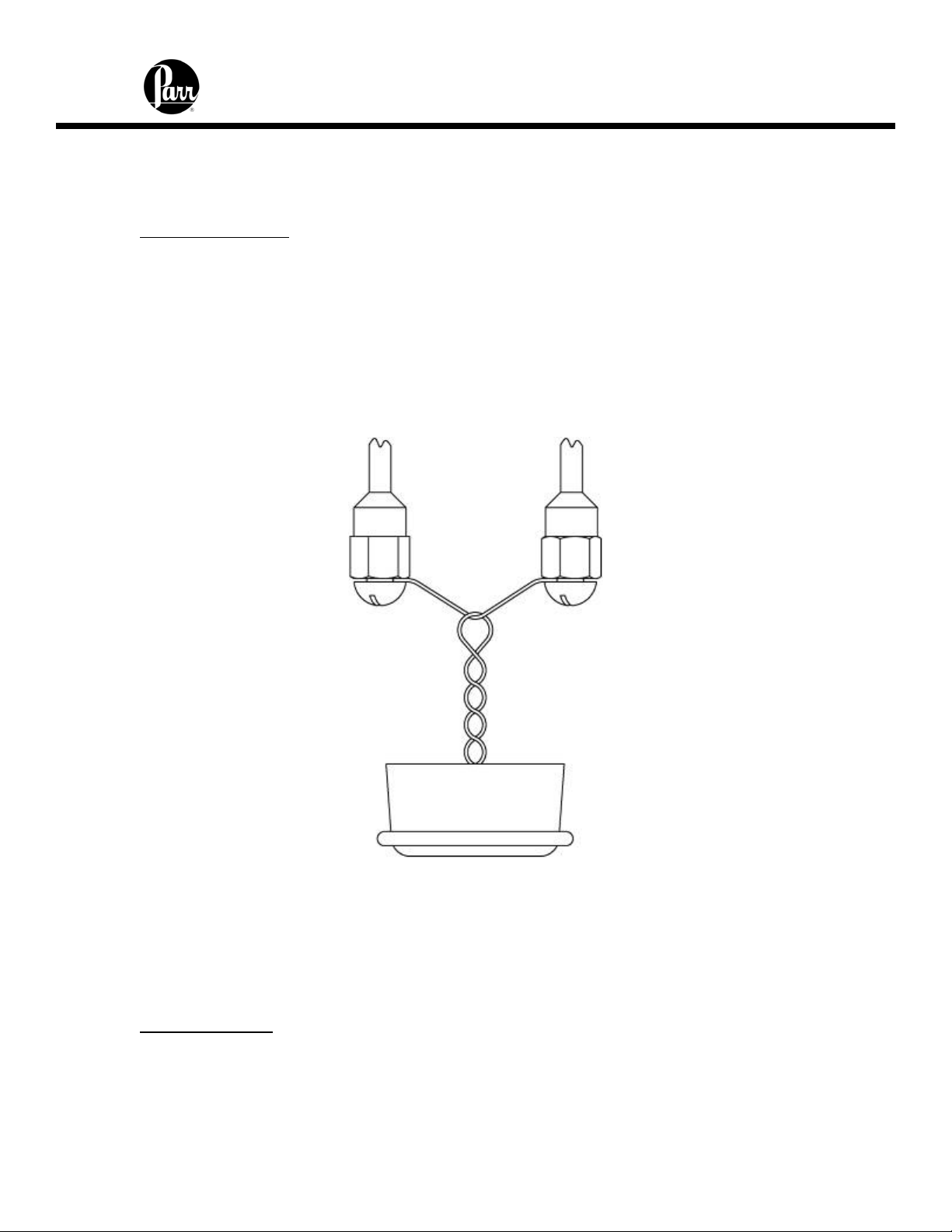
Carefully place the capsule into the capsule holder. A cotton thread (845DD2) is used
as an auxiliary fuse to ignite the sample. Remove any moisture from the heating wire
prior to attaching the cotton thread.
Figure 5 - Cotton Thread Assembly
Four inches of thread is recommended for this auxiliary thread which is looped over the
heating wire, doubled on itself, twisted to form a single strand and fed into the sample
cup to lay on the sample. When contact is made through the heating wire, the thread
will ignite, drop into the sample cup and ignite the sample. One spool of thread, part
number 845DD, is 563 yards. Part number 845DD2 contains approximately 1000 pieces
of thread pre-cut to 4 inches.
Closing the bomb
Care must be taken not to disturb the sample when moving the bomb head from the
support stand to the bomb cylinder in the calorimeter. Check the sealing ring to be sure
that it is in good condition and moisten it with a bit of water so that it will slide freely into
the cylinder.
4-7
Page 27
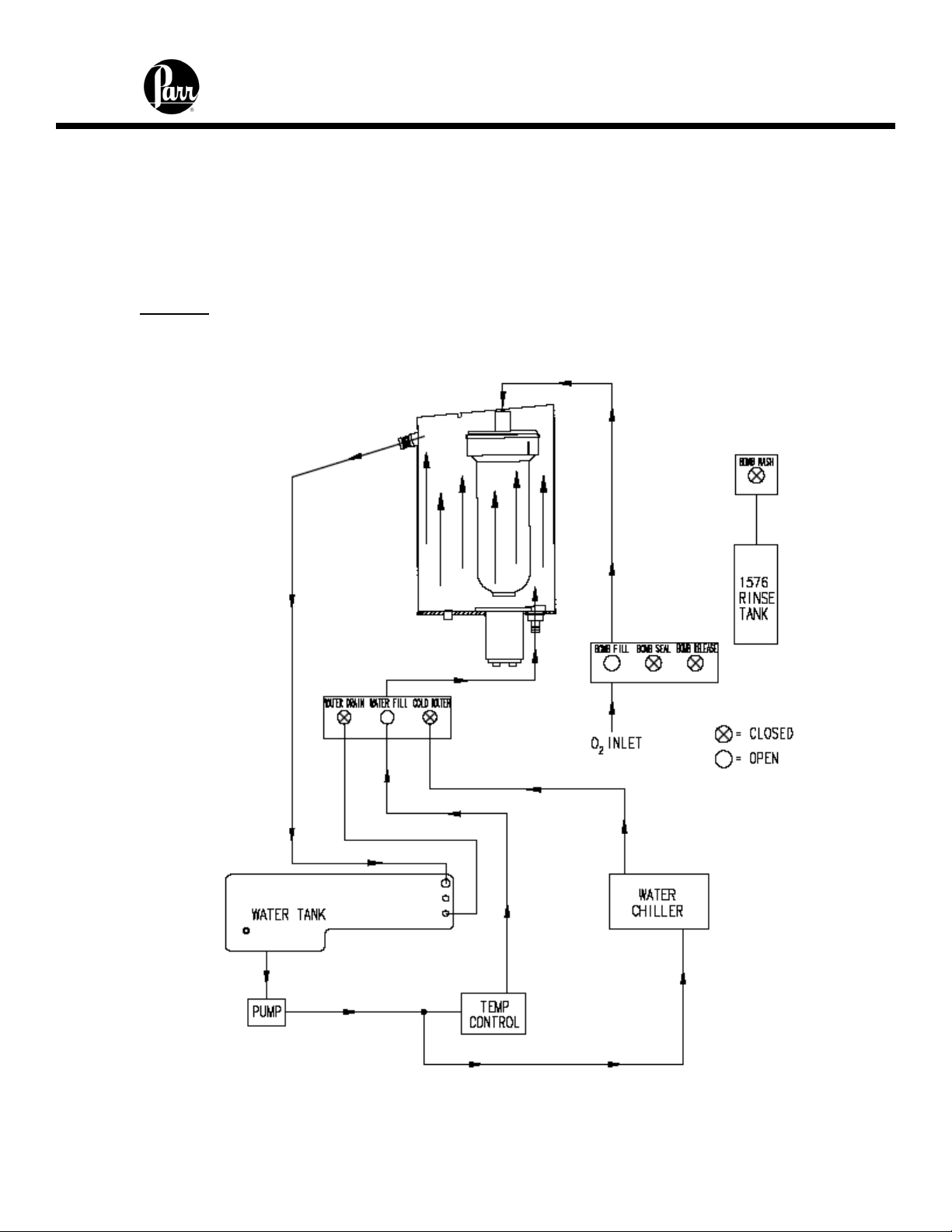
6400 Calorimeter Instruction Manual
Notice that the bomb head grounding lug extends beyond the outside diameter of the
bomb head. A slot for this lug is cut into the top of the calorimeter bucket which holds
the bomb cylinder. Position this lug approximately 20 degrees to the operators right and
slide the head into the cylinder and push it down as far as it will go. Now rotate the
bomb head 20 degrees to the left until the lug contacts the left edge of the cut out and is
pointed to the front of the calorimeter.
Fill Cycle
Once the calorimeter is started and the cover is closed, the fill sequence begins.
Figure 6 - Bucket Fill Flow Diagram
1. The calorimeter checks the bomb ignition circuitry for continuity.
4-8
Page 28
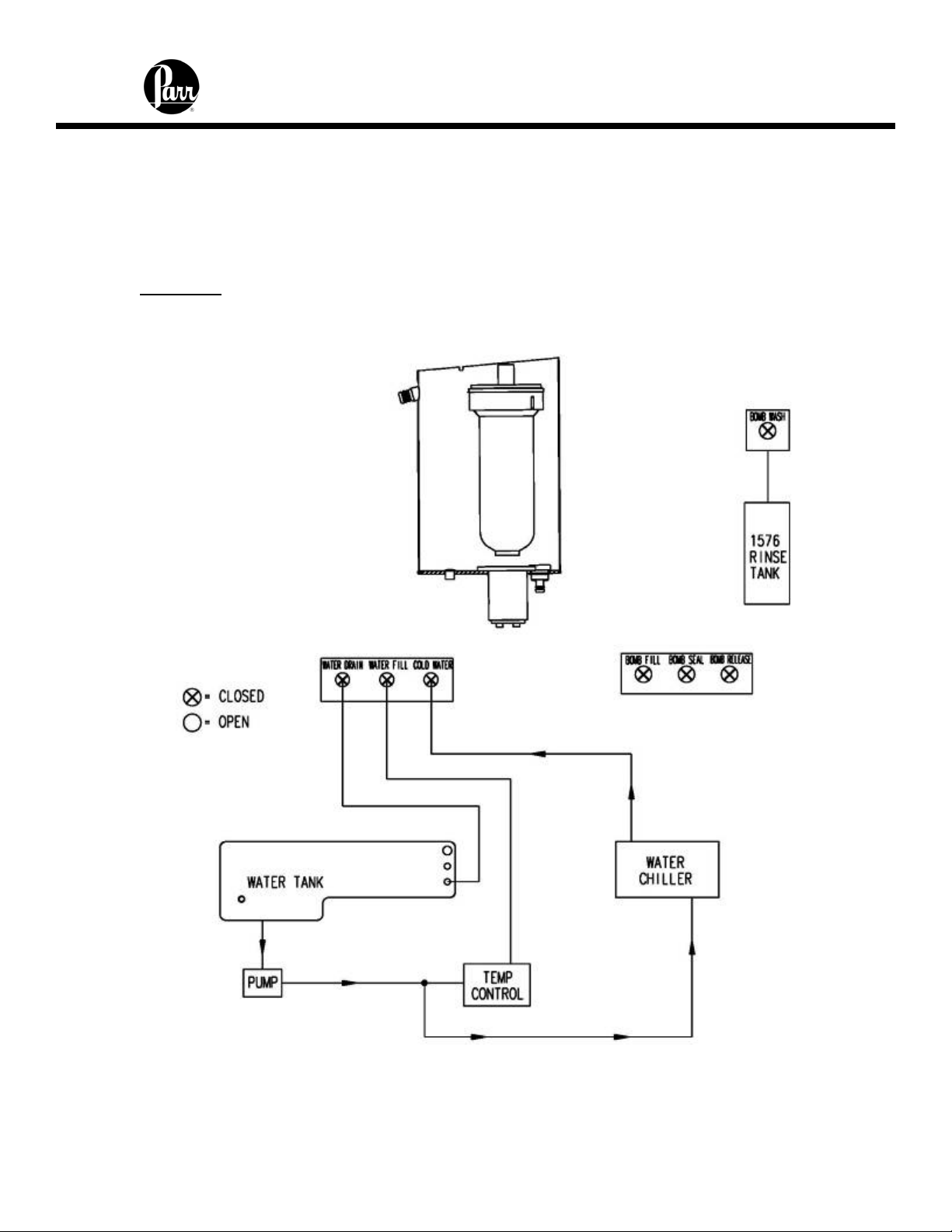
6400 Calorimeter Instruction Manual
2. The water fill solenoid opens and water is pumped from the internal tank into and
through the bucket that surrounds the bomb. Overflow from the bucket is
returned to the closed water tank. Because the jacket and bucket are both filled
with water from the closed water tank, initial equilibrium will be reached quickly.
3. The oxygen fill solenoid is opened and oxygen is added slowly to the bomb to
bring its pressure to approximately 30 atm.
Pre-Period
At the completion of the fill sequence, the pre-period begins.
Figure 7 - Pre-Period / Post-Period Flow Diagram
1. The water fill solenoid valve closes and isolates the water in the bucket from the
rest of the system. Water within this bucket is circulated by the stirrer. Water
4-9
Page 29

6400 Calorimeter Instruction Manual
continues to circulate from the closed water system through the jacket
surrounding the bucket.
2. The oxygen filling valve closes and the pressure in the filling line is vented. The
automatic check valve at the top of the bomb closes and isolates the bomb from
the oxygen filling line.
3. The controller monitors the operating temperature until it confirms that the initial
equilibrium has been established.
Bomb Firing
Once the initial equilibrium is confirmed, the controller initiates the firing sequence.
There are no changes to the circulation pattern, as shown in Figure 7, from the preperiod through the bomb firing and post-period. A warning of short beeps is sounded
indicating the bomb is about to be fired.
Post-Period
A minimal temperature rise will confirm that the sample has ignited. After this verification,
the post-period begins. See Figure 7.
1. The controller monitors the temperature rise and determines the final temperature
2. Once the final temperature rise is determined, it is recorded with the test results.
rise by either the dynamic or equilibrium criteria as established by the user.
4-10
Page 30
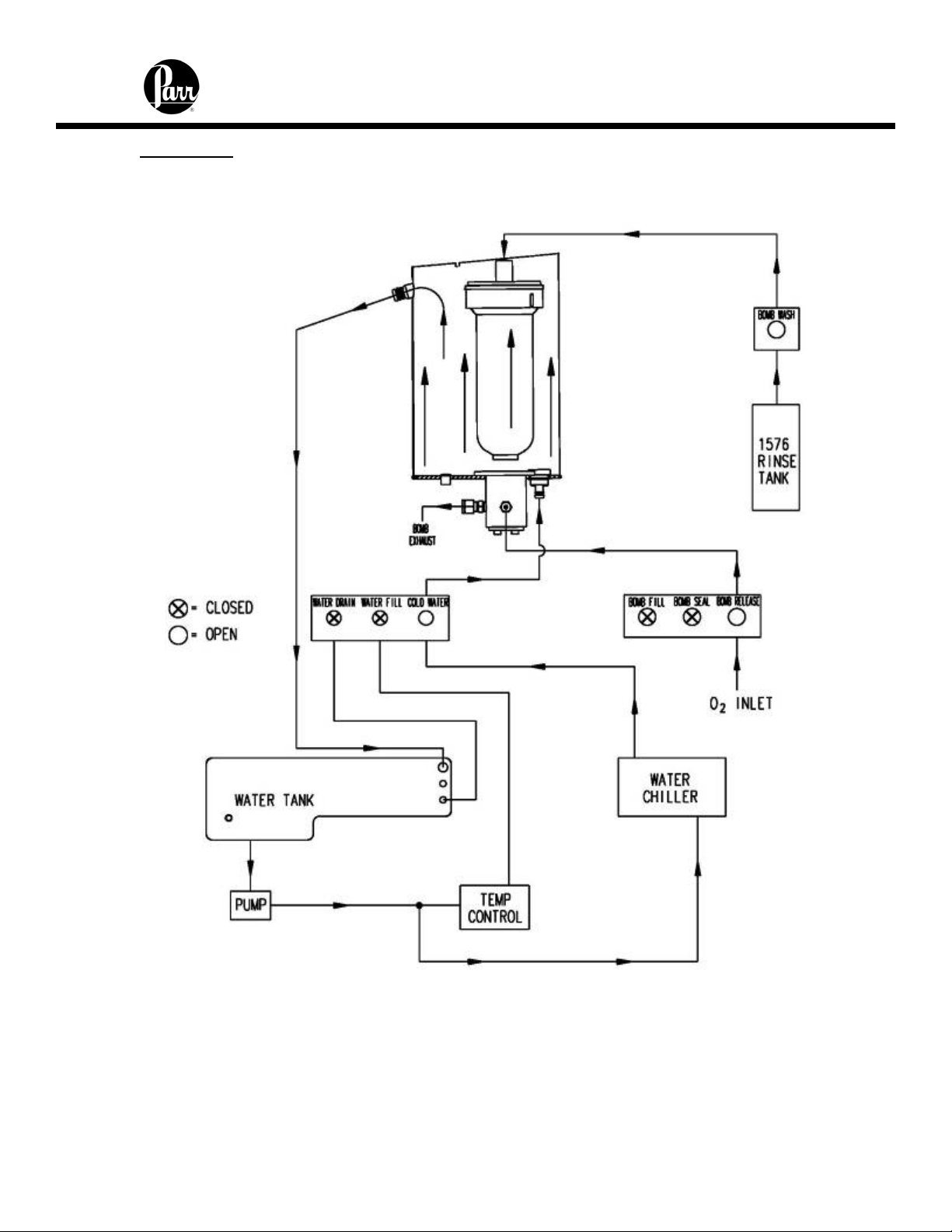
6400 Calorimeter Instruction Manual
Cool / Rinse
At the completion of the post-period, the rinse and cool sequence begins.
Figure 8 - Rinse & Cool Flow Diagram
1. Chilled water is circulated through the bucket to cool the bomb to the starting
temperature.
2. The release valve in the bottom of the bomb is opened and the residual pressure is
released through the bomb exhaust line.
3. Once the excess oxygen is vented, the bomb rinse water from the rinse water tank is
admitted through the bomb rinse solenoid valve and the check valve at the top of the
bomb. The bomb rinse water is released to the wash bottle.
4-11
Page 31

6400 Calorimeter Instruction Manual
Note:
Several rinse patterns may be configured by the user to meet various
operational and analytical requirements.
4. The bomb is filled one more time with oxygen to help flush the water residue from the
interior of the bomb.
Drain
At the completion of the bomb rinse sequence, the drain sequence begins.
4-12
Page 32

6400 Calorimeter Instruction Manual
Figure 9 - Drain Flow Diagram
The water in the bucket is drained out of the bucket and routed to the drain connection.
Once the bucket is drained, the calorimeter may be opened to remove the bomb head
and load the next sample. The test result will then print or be displayed.
4-13
Page 33

6400 Calorimeter Instruction Manual
MENU DESCRIPTIONS
Note:
Keys which make global changes to the setup of the calorimeter contain a
YES or NO response to make certain that the user wishes to proceed.
This two step entry is intended to prevent inadvertent global program
changes.
Main Menu
Escape Key:
Selecting the Escape key on any
menu will return you to the menu one
level up.
Main Menu Key:
Selecting the Main Menu key on any
menu will return you to the screen
pictured on the right of this page.
Start Key:
Press the Start key to begin any
Determination or Standardization run.
Report:
Press the Report key to begin the reporting process.
Help:
Press the Help key on any screen to display the explanation text for that screen.
Calorimeter Operation Menu
The Calorimeter will normally be operated from the Calorimeter Operation Menu, although
tests can always be started from any menu screen.
Operating Mode:
Sets the operating mode by toggling
between Standardization (for
instrument calibration) and
Determination (for test runs).
Temperature Graph:
Press this key to display a real-time
plot of the bucket and / or jacket
temperature on the Temperature vs.
Time Plot screen.
Bomb / EE:
Used to identify the bomb presently
installed in the Calorimeter and its EE
value.
5-1
Page 34

6400 Calorimeter Instruction Manual
Start Preweigh:
This key is used to start the sample pre-weigh process. The user is presented with or
prompted for a sample ID. Next, the user is asked to key in the associated sample mass
or alternatively the mass is retrieved from a connected balance.
Heater and Pump:
The heater and pump must only be turned on after the calorimeter water tank is filled
with water.
Note:
The heater and pump must be turned ON to bring the jacket to the correct
starting temperature before testing can commence.
Start Pretest:
This key is used to initiate a pretest cycle.
A pretest will cycle the calorimeter through
the fill and cool/rinse process. This function
is used to pre-condition the calorimeter.
Temperature vs. Time Plot
Press the Setup key to access the
Temperature Plot Setup Menu, which has
many keys that permit the user to fully
customize both the x (time) axis and the
scaling of the y axis.
Temperature Plot Setup Menu
Enable Bucket: Toggles ON/OFF.
Bucket Autoscale: Toggles ON/OFF.
Enable Jacket: Toggles ON/OFF.
Jacket Autoscale: Toggles ON/OFF.
Time Mode: Toggles between Autoscale, Window, and Range.
Bucket Plot Symbol: Toggles between:
• No Point
• Small Dot
• Round
• Square
• Up Triangle
• Down Triangle
• Diamond
Press this key to access its numeric dialog
box to set a minimum bucket value.
5-2
Page 35

6400 Calorimeter Instruction Manual
Bucket Min Value: Press this key to access its numeric dialog box to set a minimum bucket
value.
Jacket Plot Symbol: (same as Bucket Plot Symbol, above).
Jacket Min Value: Press this key to access its numeric dialog box to set a minimum jacket
value.
Time Window: Sets the time scale for the X-axis
Time Units: Toggles between minutes and seconds.
Bucket Plot Color: Toggles between:
• Red
• Green
• Yellow
• Blue
• Magenta
• Cyan
• White
• Black
Bucket Max Value: Press this key to access its numeric dialog box to set a maximum
bucket value.
Jacket Plot Color: Toggles between:(same as Bucket Plot Color, above).
Jacket Max Value: Press this key to access its numeric dialog box to set a
maximum jacket value.
Time Minimum: Press this key to access its numeric dialog box to set the least
amount of time for the display.
Time Maximum: Press this key to access its numeric dialog box to set the greatest
amount of time for the display.
Operating Controls Menu
Method of Operation:
Offers an operating mode of either
dynamic or equilibrium. In most cases,
the dynamic mode with its curve
matching capability will save
approximately 3-4 minutes per test and
will produce the same operating
precision as the slower equilibrium
mode.
Reporting Units:
Offers a choice of Btu/lb, cal/g, J/kg, or
MJ/kg for the reporting units. A user
selected set of reporting units may be
chosen by selecting “other”.
5-3
Page 36

6400 Calorimeter Instruction Manual
Spiking Correction:
Accesses the Spike Controls sub-menu:
“Spiking” is the addition of material, such as benzoic acid or mineral oil, to samples
which are difficult to burn in order to drive the combustion to completion.
Use Spiking
weight of the spike added and will compensate for the heat of
combustion in the
calculations.
Heat of Combustion of
Spike. The heat of
combustion of spike is
entered on sub-menu
keyboard in cal/g.
Use Fixed Spike. When
set to ON, a constant
amount of spike is to be
added to each test.
Weight of Fixed Spike.
The weight of the fixed
spike is entered on sub-menu keyboard.
Note:
The precision of tests with fixed spikes can be no better than the
accuracy of the spike weight.
Prompt for Spike before Weight. When set to ON, the calorimeter will
prompt the user for the weight of the spike and the weight of the sample.
Normally the calorimeter will prompt the user for the weight of the sample
and then the weight of the spike.
Bomb Rinse Tank Controls:
Accesses the Rinse Tank Controls submenu:
Wash water for the bomb is drawn from the Bomb Rinse Tank.
. When set to ON, the calorimeter will prompt for the
Report Rinse Tank Empty.
turned on the calorimeter will
notify the user when it believes
the rinse tank is empty based
upon capacity of tank and
number of tests.
Rinse Tank Capacity.
Sets the
number of tests available from a
container refill. If the rinse timing
controls have been changed,
then the value must be changed
proportionally.
When
5-4
Page 37

6400 Calorimeter Instruction Manual
Reset Rinse Tank Counter.
been refilled. This counter must be reset after the rinse tank is refilled.
Rinse Time.
is turned ON for each rinse cycle. When the rinse water solenoid is ON,
distilled water from the rinse tank is pumped, under pressure, into the
bomb cylinder. This rinses the cylinder walls and the bomb head. These
rinsings are then pooled and collected at the exhaust port of the
calorimeter. The factory default value is 2.5s. This value, along with the
# of rinse cycles, determines the total volume of recovered rinse. These
default values will yield a total of 50 ml (approx.) of bomb washings.
# Rinses Left.
in the tank. This number is simply a counter not an actual measurement
of the volume in the tank.
Rinse Flush Time.
cycles. During this time the rinse solenoid is turned OFF. This off time
permits the rinse water to drain out before the next rinse cycle begins.
The factory default value is 2s.
Clear Time
time for the bomb. This step is used to clear the lines and valves of any
residual rinse water prior to the next test. The factory default value is
10s.
# of Rinse Cycles
cycles used to rinse the bomb. The factory default value is 3 rinse cycles
“Other” Multiplier:
Press this key to display the Other Multiplier dialog box, where the user can enter a final
multiplier to be used when the reporting units are set to “Other”.
Calibrate Touchscreen:
This key prompts the user to touch the screen at predefined points in order to facilitate
touch screen calibration. It is important that a touch screen stylus, rather than a finger,
be used in order to realize an accurate calibration.
LCD Backlight Timeout:
The unit is equipped with an automatic circuit to shut off the backlight when it is not
being used. The back light will shut off if there is no keyboard activity for the number of
seconds entered. Pressing any key will automatically turn the back lighting ON. A
setting of 0 will keep the backlight ON at all times.
LCD Contrast:
This key accesses a sub-menu with a slide control which adjusts the contrast on the
LCD display for optimum viewing.
This value establishes the time that the rinse water solenoid
This value provides an estimate of how many rinses are left
This value is used to establish a time between rinse
. This time value is used to establish a post-rinse oxygen filling
. This value establishes the number of distinct rinse
Resets the counter when the rinse tank has
5-5
Page 38

6400 Calorimeter Instruction Manual
Print Error Messages:
When turned ON, all error messages will be printed on the printer as well as displayed
on the screen. When turned OFF, messages will only display on the screen.
Language:
Steps the Calorimeter through the installed operating languages.
Program Information and
Control Menu
Date:
Displays current date and accesses
sub-menu on which date is set in
(YY/MM/DD) format.
Time:
Displays current time and accesses
sub-menu on which time is set in
(HH:MM) format.
Software and Hardware Info:
This screen displays important
information such as the main
software version, I/O board
information, CPU information, and
Controller IP address assigned by
the network DHCP server.
Settings Protect:
Provides protection for the program
options and settings on the menus.
If this is turned ON, the user will be
warned that enumeration keys are
locked when a key is pressed.
Enumeration Keys either toggle a
value (ON / OFF) or select from a
predefined list. This feature is used
primarily to protect the instrument settings from accidental changes if one were to
inadvertently touch or bump up against the touchscreen.
User/Factory Settings:
This key leads to a sub-menu that allows the user to save or recall user defined
instrument settings. Additionally, factory preinstalled settings supporting different
bombs or special operating modes can also be recalled.
5-6
Page 39

6400 Calorimeter Instruction Manual
User Setup ID
Used to enter a unique identifier for recalling user settings. Parr offers
a unique program within the 6400 identified as “63-FAST”. The
program will “overlay” the factory settings and shorten the run time by
approximately 2 minutes however the user should be aware that a loss
of precision will occur.
Reload Factory Default Settings:
Used to erase all of the settings and restore the factory default
settings.
Reload User Default
Settings:
Used to restore the
user’s setup should
the program in the
instrument be
corrupted for any
reason.
Save User Default
Settings:
Used to record the
setup to the memory
once the user has
configured the
instrument to their operating requirements.
Feature Key:
This key displays a screen which allows the user to input a code to access special
calorimeter features such as the bar code capabilities or remote calorimeter
operation.
Bomb Type Select:
This key toggles through the different bomb models available for the calorimeter.
When the user chooses a bomb, the instrument must be re-booted to load the
correct version of the software. (Note that the calorimeter will not let you exit this
function without re-booting the system).
User Function Setup:
This key leads to sub menus that support the configuration of five factory / user
definable function keys. The function keys are accessible from the Diagnostics page.
Cold Restart:
This is essentially the same as cycling power on the unit. All valid test data will be
retained during this cold restart procedure.
5-7
Page 40

6400 Calorimeter Instruction Manual
Calibration Data and Controls Menu
Calibration Run Limit:
Displays the maximum number of runs
that will be included in determining the
EE value of a bomb and bucket
combination and accesses the submenu on which this limit is set. Most
test methods suggest 10 tests. Tests
in excess of the most recent ones
used are still available but are not
used in the calculation of the EE
value. For example if 11
standardization tests have been run,
the calorimeter will only use the most
recent 10. The 11
memory and is available for viewing or
printing. Only runs that are at final status will be used in this calculation.
EE Max Std Deviation:
Displays the maximum relative standard deviation in percent that will be
permitted for any EE value calculated by the Calorimeter and accesses the submenu on which this limit is set. If this value is exceeded, the user will be warned
to take corrective action before proceeding with testing. A setting of zero
disables this check.
Heat of Combustion of Standard:
Displays the heat of combustion in calories per gram for the material used to standardize
the calorimeter and accesses the sub-menu on which this value is set. For benzoic acid,
this value is 6318.4 calories per gram.
Bomb Service Interval:
Displays the maximum number of times a bomb may be fired before it is flagged as due
for service and accesses the sub-menu on which this limit is set. Parr recommends 500
firings for this service interval. (Parts may need to be replaced on a more frequent basis
depending upon the nature of the sample).
Control Chart Parameters:
A control chart is a graphical way to interpret test data. In its simplest form, a selected
reference sample is measured periodically and the results are plotted sequentially on a
graph. This key will define the charted value; either the heat of combustion of the
standard or the Energy Equivalent. It also defines the process sigma.
Use Bomb:
Displays the ID of the bomb currently being used in the Calorimeter and toggles through
the four possible bomb numbers.
th
is still stored in the
5-8
Page 41

6400 Calorimeter Instruction Manual
Bomb 1:
Leads to sub-menu, Bomb 1. Displays standardization information for bomb and bucket
combinations. While only one bomb and bucket is installed in the calorimeter at a time, a
spare may be used for servicing and for more rapid turn-around. The respective EE
values for each bomb can be stored in memory.
Note:
For rapid turn around between tests, the user may wish to use an extra
head. Each head should be assigned a bomb ID. On the Data Entry
Controls Menu, set the Prompt for Bomb ID to “ON”.
The following four values are displayed for the Bomb # shown in the title on top of the
screen.
Bomb EE Value. Displays the calculated EE value.
# Runs, EE Val. Displays how many runs have been used to determine the EE
value.
Rel. Std. Dev. Displays the relative standard deviation for the series of tests used
to determine the current EE value in percent of the EE value.
Bomb Fire Count. Displays the current bomb firing count or the number of times
the bomb has been fired since it was last serviced. When this count matches the
limit set by Bomb Service Interval (on the Calibration Data and Controls screen),
the user will be informed that the bomb is ready for service.
Protect EE Value:
Toggles between OFF and
ON. When set to OFF, the
6400 automatically updates
the EE value as new tests
are run. When set to ON, it
keeps the EE value
protected, whether it has
been revised manually via
the Manual EE Entry key or
calculated by the instrument.
Update Statistics:
If the Protect EE Value is set
to OFF, pressing this key will
cause the EE Value for this Calorimeter to be updated using all standardization
runs currently in memory to the limit established in the Calibration Data and
Controls menu. If the Protect EE value is set to ON, this key is not functional.
Manual EE Entry:
This key allows the user to manually enter an EE or calibration factor for a given
calorimeter ID or bomb head. If an EE value is manually entered, it is necessary
to turn the Protect EE Value ON in order to prevent this value from being
overwritten by an automatic update.
5-9
Page 42

6400 Calorimeter Instruction Manual
Print Standardization Runs:
This key will print all of the tests that have been incorporated into the calculated EE
value. This will be helpful in evaluating a series of tests which fail to produce a
satisfactory EE value and relative standard deviation.
Reset Bomb Fire Count:
After bomb service, press this key to reset the fire count to zero.
Control Chart Plot:
Each data point represents a Standardization run used in the calculation of the EE
Value for the Bomb ID being displayed. The left side of the graph contains the
oldest runs and the right represents the most recent. To view the run data used for
a particular data point, click on the data point with a stylus and the run data for that
point is displayed.
5-10
Page 43

6400 Calorimeter Instruction Manual
Thermochemical Corrections Menu
The Thermochemical Corrections Menu
permits three types of fixed corrections
for standardization (instrument
calibration) runs, and the same three
types for determination (test) runs.
Pressing the LEFT side of each key
toggles the correction ON or OFF.
Press the RIGHT side of each key to
access the specific numeric dialog box
where that fixed value can be set. Each
value entered for these fixed corrections
is used in all preliminary reports.
When any fixed correction is set to ON,
the specified value will be used in the
final reports, and the 6400 will not prompt for actual corrections to be entered. (If all
corrections are fixed, only a final report will be generated).
When any fixed correction is set to OFF, during the data entry reporting steps the user
will be prompted to enter an appropriate desired value which will be used in the final
report.
Standardization Corrections
Fixed Fuse Correction:
Press this key on the LEFT side to toggle ON or OFF the fixed fuse correction for
standardization runs. Press it on the RIGHT side to access the Fixed Fuse numeric
dialog box on which the value can be set. An appropriate fixed fuse value is 50 calories.
Fixed Acid Correction:
Press this key on the LEFT side to toggle ON or OFF the fixed acid correction for
standardization runs. Press it on the RIGHT side to access the Fixed Acid numeric dialog
box on which the value can be set. An appropriate fixed acid (nitric acid) value is 8
calories when one-gram benzoic acid pellets are used to calibrate the instrument.
Fixed Sulfur Correction:
Press this key on the LEFT side to toggle ON or OFF the fixed sulfur correction for
standardization runs. Press it on the RIGHT side to access the Fixed Sulfur
numeric dialog box on which the value can be set. When benzoic acid is used as
the calibrant, a fixed sulfur value of 0 should be used.
Determination Corrections
Fixed Fuse Correction:
Press this key on the LEFT side to toggle ON or OFF the fixed fuse correction for
determination runs. Press it on the RIGHT side to access the Fixed Fuse numeric dialog box
on which the value can be set. An appropriate fixed fuse value is 50 calories.
5-11
Page 44

6400 Calorimeter Instruction Manual
Fixed Acid Correction:
Press this key on the LEFT side to toggle ON or OFF the fixed acid correction for
determination runs. Press it on the RIGHT side to access the Fixed Acid numeric dialog box
on which the value can be set.
Fixed Sulfur Correction:
Press this key on the LEFT side to toggle ON or OFF the fixed sulfur correction
for determination runs. Press it on the RIGHT side to access the Fixed Sulfur
numeric dialog box on which the value can be set.
Note:
When fixed corrections are turned ON, the value in the specified field will be used in
both the preliminary and final reports. The calorimeter will not prompt for actual
corrections. If all corrections are fixed, only final reports will be generated. If any
correction value is entered and the toggle is set to OFF, then the preliminary report
will use the displayed fixed value, but the final report will use the value entered when
prompted during the reporting process.
Calculate Net Heat of Combustion:
Toggles ON or OFF the calculations for the net heat of combustion for materials with
significant hydrogen content. When set to ON, the 6400 will prompt for entry of the
hydrogen content during the reporting data entry step.
Calculation Factors:
Accesses the Calculation Factors sub-menu, which provides for setting a number of options
for the way the thermochemical corrections are applied.
Calculation Factors Menu
Acid Value is Nitric Acid Only:
When set to ON, the acid value is
nitric acid only. When set to OFF, it
represents both nitric and sulfuric
acid.
Acid Multiplier:
This multiplier is the normality of the
sodium carbonate used during the
acid correction titration. The default
value of 0.0709 allows for direct entry
of the acid correction in calories. If
the bomb rinses are titrated in order
to determine the acid correction,
press this key to display the Acid Multiplier numeric dialog box, where you can change the
multiplier to represent the concentration of the base (equivalents/L) or normality used for
titration. If this is the case, the acid correction is entered as milliliters of base used to titrate
the bomb rinses.
5-12
Page 45

6400 Calorimeter Instruction Manual
Sulfur Value is Percent:
When set to ON, the sulfur value is being entered as weight percent sulfur. If another
system is to be used, this must be turned OFF and the sulfur multiplier set accordingly.
Sulfur Multiplier:
Values entered by the user to be used for the sulfur correction are multiplied by this value
to get the product into units of milliequivalents. The default number (0.6238) requires that
the sulfur value be entered in weight percent.
Fuse Multiplier:
The fuse corrections represent the number of calories liberated by the burning fuse wire used
to ignite the sample. If another measurement is used, the correction factor must be entered
here. Press this key to access the Fuse Multiplier numeric dialog box and enter this multiplier
value.
Use Offset Correction (ISO):
The thermochemical calculations used for treatment of nitric acid and sulfuric acid corrections
in the ISO and B. S. methods require an offset correction to compensate for the back titration
that is made. To use these calculations, toggle this to ON and enter the appropriate value as
the offset value.
Offset Value:
The value used when Offset Correction is turned ON. Press this key to access the Offset
Value numeric dialog box and change its value.
Fixed Hydrogen:
Press the LEFT side to toggle this setting On / Off. Press the RIGHT side to
display the Fixed Hydrogen numeric dialog box and change its value.
Hydrogen Multiplier:
This value is associated with the net heat of calculation. It is related to the heat of formation
of water. Press this key to display the Hydrogen Multiplier numeric dialog box and change its
value.
Dry Calculation:
Displays whether set to ON or OFF. Press this key to display the Dry Calculations sub-menu
to toggle this setting and update Fixed Moisture and Moisture Multiplier data.
Dry Calculation Menu
Dry Calculation:
Toggles the dry calculation ON or
OFF.
Fixed Moisture:
Press the LEFT side to toggle ON or
OFF whether to use the entered
moisture correction. Press the
RIGHT side to access the Fixed
Moisture numeric dialog box and set
the value. Units are weight %.
5-13
Page 46

6400 Calorimeter Instruction Manual
Moisture Multiplier:
Press to access the Moisture Multiplier numeric dialog box and set the value. This value is
associated with the net heat calculation. It is related to the heat of vaporization of water.
Data Entry Controls Menu
Prompt for Bomb ID:
Toggles ON or OFF. In the ON
position the controller will prompt for
a Bomb ID (1-4) when a test is
started.
Weight Entry Mode:
This key steps through the options
for entering sample weights either
manually through the touch screen,
balance port or through the network.
Acid Entry Mode:
This key steps through the options
for entering acid correction value either manually through the touch screen or
automatically through the balance port.
Hydrogen Entry Mode:
This key steps through the options for entering hydrogen content for calculating the net
heat of combustion either manually through the touch screen or automatically through
the balance port.
Auto Sample ID Controls:
Accesses sub-menu for controlling the automatic assignment of sample identification
numbers.
Automatic Sample ID:
When set to ON the unit will
automatically assign sample
identification numbers in
accordance with parameters set
by the other three keys on this
menu. When set to OFF, the
user manually enters each
sample ID when prompted to do
so.
Auto Sample ID Prefix:
An entry here will be used as a
prefix for all sample IDs, if the
Automatic Sample ID is set to
ON. Press this key to access a
sub-menu for entering an alphanumeric prefix.
5-14
Page 47

6400 Calorimeter Instruction Manual
Next Auto Sample ID Number:
Establishes the initial sample number for a series of tests and then shows the
next sample ID which will be assigned. Used when the Automatic Sample ID is
set to ON. Press this key to access a sub-menu for entering a numeric
increment.
Auto Sample ID Increment:
Establishes the increment between sample numbers; used when the Automatic
Sample ID is set to ON. Press this key to access a sub-menu for entering a
numeric increment.
Sample Weight Warning Above:
This key displays and leads to a sub-menu used to set the maximum allowable sample
weight (including spike) in grams. A warning will be given if sample weights above this
value are entered.
Spike Weight Entry Mode:
This key steps through the options for entering spike weights either manually through the
touch screen, balance port or network.
Sulfur Entry Mode:
This key steps through the options for entering the sulfur correction value either
manually through the touch screen or automatically through the balance port
Moisture Entry Mode:
This key steps through the options for entering the moisture correction value either
manually or through the touch screen or automatically through the balance port.
Auto Preweigh ID Controls:
Accesses sub-menu, used to automatically assign Sample ID numbers when a series of
samples are preweighed ahead of the time they are actually tested.
Automatic Preweigh ID:
ON/OFF toggle for this feature.
Automatic Preweigh ID Prefix:
An entry here will be used as a prefix for all pre-weigh sample IDs.
Next Automatic Preweigh ID Number:
Shows the next Sample ID which will be assigned and is used to enter the
beginning Sample ID of any series
Automatic Preweigh ID Increment:
Establishes the increment between samples.
5-15
Page 48

6400 Calorimeter Instruction Manual
Reporting Controls Menu
Report Width:
Toggle this key to set the column
width of the printer to either 40 or 80
columns. Select 40 when the 1757
Printer is used.
Automatic Reporting:
Toggles the automatic reporting
ON/OFF. When ON, preliminary
reports will be generated at the
conclusion of the test and final
reports will be generated as soon as
all of the thermochemical corrections
are available. When OFF, reports
will only be generated by selecting
the Report key.
Automatic Report Destination:
Toggles to direct the reports to the Printer port or the screen display.
Individual Printed Reports:
When set to ON, will generate header information for each report printed. In the OFF
position, only one header will be printed for a series of tests.
Edit Final Reports:
When set to ON, enables the user to revise sample weight and thermochemical
corrections of finalized reports from the report menu.
Recalculate Final Reports:
When set to ON, causes a recalculation of stored final reports using calibration data and
menu settings currently in the Calorimeter.
Use New EE Value in Recalculation:
When set to ON, any recalculation
made will use the most recent EE
value in the calculations. In the OFF
position, all calculations will be made
using the EE value which was
effective when the test was originally
run.
Communication Controls Menu
Accesses sub-menus which set the
communications protocols for the
printer and balances.
5-16
Page 49

6400 Calorimeter Instruction Manual
Printer Port (RS232):
Accesses sub-menu, Printer Port Communications. Sets the communication parameters
for the RS232 ports used for the printer port. Standard options for data bits, parity, stop
bits, handshaking, baud rate and balance type are provided to match any devices that
might be connected to these ports.
• Number of Data Bits.
Standard options for data
bits. Toggles between 7
and 8.
• Parity. Standard options for
parity. Choose from None,
Odd or Even.
• Number of Stop Bits.
Standard options for stop
bits. Toggles between 1
and 2.
• Handshaking. Standard
options for handshaking.
Choose from Xon/Xoff,
RTS/CTS and None.
• Baud Rate. Standard options for baud rate. Choose from
19.2K, 9600, 4800, 2400, 1800, 1200, 600, 300, 150, 134.5,
110, and 75.
• Printer Type. Toggles between a Parr 1757 and a generic
printer. When set for the 1757 Printer, all of the features of
this printer, such as bold printing, will be activated.
• Printer Port Loop Back Test. Used for factory testing of the
printer port.
Balance Port (RS232):
Accesses sub-menu, Balance Port
Communications.
Balance Type
Toggles through the
available balance templates.
Customize Balance Settings
Sets the communication
parameters for the RS232
port used for the balance
port. Standard options for
data bits, parity, stop bits,
handshaking, baud rate and balance type are provided to match any devices that
might be connected to these ports.
• Number of Data Bits. Standard options for data bits. Toggles
between 7 and 8.
5-17
Page 50

6400 Calorimeter Instruction Manual
• Parity. Standard options for parity. Choose from None, Odd or
Even.
• Number of Stop Bits. Standard options for stop bits. Toggles
between 1 and 2.
• Handshaking. Standard options
for handshaking. Choose from
Xon / Xoff, RTS/CTS and None
• Baud Rate. Standard options for
baud rate. Choose from 19.2K ,
9600, 4800, 2400, 1800, 1200,
600, 300, 150, 134.5, 110, and
75.
• Data Characters from Balance.
This setting is only used when
the generic balance format is
selected. This value determines
the number of numeric data
characters (0-9 . + -) to accept.
Any additional characters after this value and before the string
terminating <CR> are discarded.
• Data Precision. This key allows the user to establish the
number of digits to the right of the decimal point that are
passed from the balance handler.
• Transfer Timeout (seconds). This value determines how long
the interface will wait before giving up on a weight transfer.
The value is entered in seconds.
• Balance Handler Strings. This key leads to a submenu that
allows balance template to be customized for unique balances
or needs.
Log Balance to Display
Directs the incoming data stream from the balance to a display buffer. This
function can be used to determine the data format from an unknown balance
type. The display buffer is 40 characters in length. The balance must be forced
to issue at least 40 characters before the contents of the buffer are displayed.
Balance Port LoopBack Test
Initiates a loopback test on the port. A special loopback plug is required in order
to perform this test.
Parr offers the following communication cables:
25 pin D (male)
A1837E 9-pin DP 25-pin DP S-T
A1838E 9-pin DP 25 pin DP Null
9 pin D (male)
A1892E 9-pin DP 9-pin DP S-T
A1893E 9-pin DP 9-pin DP Null
5-18
Page 51

6400 Calorimeter Instruction Manual
Further information on establishing communications for the Printer Port, Balance
Port, Network Interface, Bar Code Port and other Network Data Devices can be
found in the Installation section of this manual.
File Management Menu
Run Data File Manager:
This key activates the File Manager.
The File Manager is used to delete or
rename test report files. It is also
used to convert file types.
Format the CompactFlash:
This key allows the user to format an
installed CF card in a manner that is
compatible with the calorimeter.
Note: Formatting will erase all
files on the card!
Copy Run Data to CompactFlash:
This key copies all test data to a Compact Flash (CF) card inserted into the rear of the
calorimeter controller. This feature is used as a means of either archiving data or
transferring it to a PC.
Note:
Subsequent use of the same CF card will overwrite the data currently on the card.
Copy User Settings to CompactFlash:
This key copies all previously saved user setups to CF.
Copy User Settings From CompactFlash:
This key copies all user setups previously saved to CF back to the calorimeter controller
memory. This feature can be used to configure multiple calorimeters in an identical manner.
Run Data File Manager
The white upper portion of the Run
Data File Manager screen presents
all tests in memory in a scrollable
window. Test attributes include
filename (sample ID), test type,
status, and date. Touching
anywhere in the column related to a
given test attribute will sort the file list
by that attribute. Successive touches
will toggle between an ascending and
descending sort.
5-19
Page 52

6400 Calorimeter Instruction Manual
Select:
This key is used to begin the file selection process. The up / down and page up / page
down keys are used to scroll up and down the file list. Pressing the select key when a file
is highlighted blue will highlight the file with a cyan color. This indicates that it is
selected. Multiple files throughout the list can be selected in this fashion.
Extend Sel:
This key selects all files between the last file selected and the file that is highlighted in
blue.
Desel All:
This key deselects all files previously selected.
Rename:
This key allows the user to rename the blue highlighted filename.
Delete:
This key deletes all selected files.
Convert Type:
This key allows one or more selected tests to be converted from determinations to
standardizations and vice versa.
Diagnostics Menu
Provides the user with the means to
test many of the components and
subsystems of the calorimeter.
These capabilities should be used in
conjunction with this instruction
manual in order to obtain the
maximum benefits from these
capabilities.
Pretesting Cycle:
This key initiates a pretest to run the
calorimeter through the fill and
cool/rinse cycles. This function is used to pre-condition the calorimeter if it has been
sitting idle for an extended period of time (greater than 15 minutes).
Test Ignition Circuit:
Activates the ignition circuit. A volt
meter can be placed across the
firing connections to ensure that the
actual firing charge is reaching these
contacts.
5-20
Page 53

6400 Calorimeter Instruction Manual
Data Logger:
Displays ON/OFF status and accesses the Data Logger Controls Menu for setting
the specific logging controls.
Data Logger:
This key toggles the data logging function ON / OFF.
Data Log Interval:
This key displays the interval of which the selected data is logged. The interval
in seconds is defined in the Select Data Items sub-menu (normally 12 seconds).
This roughly matches the update interval for the bucket temperature.
Data Log Destination:
Options are logfile, printer or both. When the logfile option is selected, the logfile
is located at /flash/datalog.csv. The maximum allowed size for this file is roughly
one megabyte. If the file reaches this size, logging is halted.
Select Data Log Items:
Press this key to access the
Data Log Items sub-menu,
which provides keys for
fifteen items that can be
individually selected for
logging. By default, both the
bucket and jacket
temperatures are logged. All
records are date and time
stamped. Helpful items to log
are:
D0 - Corrected calorimeter
Tsum - Accumulated
T1 - Extrapolated temperature rise
C0 - Temperature conversion counter
Data Log Format:
Toggles between Text Format and Data Format (csv). Data is either logged with the
supporting tag information (text) or in a comma separated variable (csv) data format
as selected by the user. The text setting is useful if the data log destination is a
printer. The data (csv) format is especially useful if the data is ultimately transferred
to another computer for post processing, graphing, etc. The log file can be
transferred to another computer via FTP.
Delete Data Log File:
When this key is pressed the contents of the data log file are deleted.
drift rate
temperature rise
5-21
Page 54

6400 Calorimeter Instruction Manual
View System Log:
This key accesses its sub-menu which displays the contents of
/flash/log/messages. This file is used primarily to log application program debug
messages. Press the Print key to print these messages.
User Defined Functions:
This key leads to a sub-menu that offers five special purpose user/ factory definable
function keys.
Combine Det. Reports:
Pressing this key combines all
determination reports into a
single file named
/tmp/bigdetfile.txt.
Combine Std. Reports:
Pressing this key combines all
determination reports into a
single file named
/tmp/bigstdfile.txt.
Rinse Bomb:
This key initiates a bomb rinse. This
function can be used to clean out the cylinder in the event a sample is spilled inside the
cylinder.
Instrument Monitor:
This key accesses its sub-menu screen which provides a summary of most of the important
instrument parameters. This screen is used to detail the course of a test or to observe the
heating / cooling performance of the calorimeter.
View System Info:
Press this key to display a screen with current operating system information / statistics such
as:
Press the Print key to print this information.
View Instrument Log:
Press this key to display a screen with contents of the /tmp/instlog file. It contains a
sequential log of the instrument’s processing. Press the Print key to print this log.
• Processes and their associated PIDs
• Memory
• Mass Storage
• Network
5-22
Page 55

6400 Calorimeter Instruction Manual
I/O Diagnostics:
Press this key to display the I/O
Diagnostics sub-menu, which allows the
user to manipulate digital outputs for
troubleshooting. The I/O Diagnostics
screen is used to display the digital
outputs at a basic level for
troubleshooting. Both the bucket and
jacket temperatures are also displayed
on this screen. Any output can be
selected using the left and right arrow
keys. The selected output is turned ON
(1) or OFF (0) using the 1 and 0 keys.
Prior to entering the Diagnostics Menu,
the controller stores the present state of
the outputs. This state is restored when you exit this screen. Digital outputs cannot be
manipulated while a test is in progress.
5-23
Page 56

6400 Calorimeter Instruction Manual
CALCULATIONS
Corrections
Fuse Correction
There are two components to the fuse correction in the 6400 Calorimeter:
The heat introduced by heating the wire used to ignite the cotton thread.
The heat of combustion of the cotton thread used to ignite the sample.
= (fuse value) (fuse multiplier)
e
3
The semi-permanent heating wire is heated by dissipating an electrical charge from a
capacitor. Since this charge is controlled by the size of the capacitor and the charging
voltage, and because the capacitor is fully discharged for each test, the energy released
can be calculated. In the 6400 Calorimeter this is a correction of 10 calories per test.
Cotton has a heat of combustion of 4000 calories per gram. The actual thread being
used should be weighed to see how much is being burned. Ten centimeters of a fine
thread will weigh approximately 0.003 grams which would release 12 calories as it
burns. Heavier threads weigh up to 0.010 grams per 10 centimeters and increase this
correction to 40 calories per test. The finer the thread, the smaller errors will be if the
thread is not exactly ten centimeters in length. Polyester thread is not recommended for
use in the bomb because it has a tendency to melt and fall away from the heating wire
before it ignites.
Using the fine thread mentioned above, the fuse correction for the calorimeter would be
the 10 calories from electrical heating plus 12 calories from the burning thread for a total
of 22 calories per test. The thread supplied by Parr has a mass of approximately 1
milligram per centimeter. This results in a total fuse correction of 50 calories. Fuse
corrections can be entered when Fixed Fuse in the Thermochemical Corrections menu,
is set to OFF. Total errors of more than 5 calories will seldom occur when using a fixed
fuse correction and the fuse wire and cotton thread supplied by Parr.
Spiking Correction
It is sometimes necessary to add a spiking material to samples which are very small,
have a low heat of combustion, or have a high moisture content to add sufficient heat to
drive the combustion to completion. Benzoic acid is an excellent material for spiking for
all of the same reasons it is a good standard material. White oil is also an excellent
material. The 6400 calorimeter can automatically compensate for the addition of spiking
materials to these samples. The calculations are modified in these cases as follows:
H
Where:
H
m
−−−−
=
c
is equal to the Heat of combustion of the spiking material in calories per
cs
321
m
))((
mHeeeWT
scs
gram
is equal to the mass of the spiking material
s
6-1
Page 57

6400 Calorimeter Instruction Manual
This factor is added to the calculations when Spike Controls, Use Spiking is set to ON.
The Heat of Combustion of the spiking material is entered as calories per gram. The
calorimeter will prompt the user to enter the weight of spiking material. Fixed spikes can
also be used. To do this, set Use Fixed Spike to ON and enter the mass of the spike
(Weight of Fixed Spike key).
Nitric Acid Correction
In the high pressure oxygen environment within the oxygen bomb, nitrogen that was
present as part of the air trapped in the bomb is burned to nitric oxide which combines
with water vapor to form nitric acid. All of this heat is artificial since it is not a result of
the sample burning. The nitric acid correction removes this excess heat from the
calculation. Users may find it convenient to enter a fixed value for the acid correction
and avoid the need to determine this correction for each test. Use of a fixed value for
the acid correction is highly recommended. Fixed acid corrections can be entered when
Fixed Acid - Thermochemical Corrections, is set to ON. A correction of 8 calories is a
good number for the fixed nitric acid value. For most work, it is recommended to set
“Acid Value is Nitric Acid Only”, in Calculation Factors to ON. Total errors of more than
3 calories will seldom occur when using fixed nitric acid corrections.
Sulfur Correction
In the oxygen rich atmosphere within the bomb, sulfur in the sample is oxidized to
sulfur trioxide which combines with water vapor to form sulfuric acid. This liberates
additional heat over the normal combustion process which converts sulfur to sulfur
dioxide. The sulfur correction removes this excess heat from the calculation.
Fixed sulfur corrections can be entered if a series of samples contain a constant
amount of sulfur. Fixed sulfur corrections can be entered when Fixed Sulfur Thermochemical Corrections, is set to ON. Enter percent sulfur as indicated on
this line. Any errors will be proportional to the difference between the actual and
assumed value for sulfur.
Note:
For ordinary work where benzoic acid is used for standardizing the
calorimeter, the Fixed Sulfur Correction for Standardizations should be
ON applying a fixed value of 0.0 to all standardization tests. Benzoic acid
contains no sulfur.
Manual vs. Fixed Corrections
If fixed values for fuse, acid and sulfur are turned OFF on the Thermochemical
Corrections Menu, then the user must manually enter the values at the prompt. Final
reports for each test can be obtained whenever the operator is prepared to enter any
required corrections for fuse, acid and sulfur. When entering corrections, the user can
choose either of two methods. These are:
• Manual Entry
• Fixed Corrections
6-2
Page 58

6400 Calorimeter Instruction Manual
Manual Entry
During the reporting process, the controller will prompt the user to enter the following
values:
• Fuse Correction Key in the Fuse/Heat Wire Correction and press the ENTER
key. The default setting for this value is to be entered in
calories.
• Acid Correction Key in the Acid Correction and press the ENTER key. The
default setting for this value is to be entered in milliliters of
standard alkali required to titrate total acid or calories.
• Sulfur Correction Key in the Sulfur Correction and press the ENTER key. The
default setting for this value is to be entered as percent sulfur
in the sample.
Note: If the Spiking Correction is used, a spiking correction must be
entered before obtaining a Final Report. After the last entry has been
made, the Calorimeter will automatically produce a Final Report. If values
for these corrections are not available, the operator can use the SKIP key
to bypass any of the corrections, however, a Final Report will not be
printed until an entry is made for fuse, acid and sulfur.
Fixed Corrections
In many cases, fixed values for fuse and acid can be used without introducing a
significant error since the corrections are both relatively small and constant. Fixed sulfur
corrections can also be used whenever a series of samples will be tested with a
reasonably constant sulfur content. Any value set-up as a fixed correction will be
automatically applied and the calorimeter will not prompt the user for this value.
Definitions
Total acid is the amount of base required to titrate the bomb
washings (milliliters).
Nitric acid is that portion of the total acid in the bomb washings that
result when the nitrogen in the air that is trapped in the
bomb is burned at high pressure. Since this nitric acid
does not result from the sample, and the combustion
conditions are reasonably constant from test to test, the
amount of nitric acid formed is also constant.
Acid multiplier is multiplied by the user entered acid value to arrive at the
number of milliequivalents of acid. This value is usually
the concentration (normality) of the base in equivalents per
liter.
Percent sulfur is the concentration of sulfur in the sample (weight %).
Molecular weight of sulfur is 32.06.
Equivalent weight of sulfur in H
weight).
Heat of formation of nitric acid is 14.1 calories / milliequivalent.
is 16.03 (one half of the molecular
2SO4
6-3
Page 59

6400 Calorimeter Instruction Manual
Heat of formation of sulfuric acid (from SO2) is 36.1 calories / milliequivalent.
Sample mass is the mass of sample burned in the bomb (grams).
Sulfur multiplier is multiplied by the product of the user entered sulfur value
and the sample mass to arrive at the number of
milliequivalents of sulfuric acid in the bomb washings.
Example 1:
The following assumptions are made for the factory default settings of the
calorimeter
1) The acid value is that of nitric acid only
2) The concentration of the titrant used is 0.0709N
3) 1 calorie (I.T.) is equal to 4.184 Joules
4) The heat of formation of HNO
5) The heat of formation of H
e
=
⎛
1.140709.0
=
=
⎜
⎝
cal 6.9
⎛
)6.9(
ml
meq
ml
cal
⎞
⎜
⎟
⎜
meq
⎠
⎝
⎞
⎟
⎟
⎠
is 14.1 cal/meq
3
is 36.1 cal/meq
2SO4
)HNO mult)(H acid)(acid of (ml
3f1
=
e
⎛
1.360.6238meq
cal
⎛
=
=
H
=
c
⎛
⎜
⎜
⎝
=
=
087g)(2.16)(0.5
⎜
cal
⎝
cal 24.7439
e-e-e-Wt
321
m
5971.937
cal
⎞
⎟
o
⎟
C
⎠
⎞
⎜
⎟
⎜
meq
⎠
⎝
o
5087.0
g
MJ/kg 42.8523 or cal/g 10241.9489
SO4)H mult)(Hf urmass)(sulf le(%S2)(samp
22
⎞
⎟
⎟
⎠
0000.507439.246.9)6468.5(
−−−
calcalcalC
6-4
Page 60

6400 Calorimeter Instruction Manual
×
Example 2:
The following assumptions are made and outlined in D240.
1) The acid value is ALL nitric
2) The concentration of the titrant used is 0.0866M
3) 1 calorie (I.T.) is equal to 4.1868 Joules
4) The heat of formation of HNO3 is 57.8 kJ/mol
5) The heat of formation of H2SO4 is 301.4 kJ/mol
The calorimeter must be set accordingly. (The calorimeter performs ALL
calculations in calories, milliequivalents and degrees Celsius)
1) Set “Acid Value is Nitric Acid Only” to OFF
2) Set the “Acid Multiplier” to 0.0866
3) Set the “Sulfur Value is Percent” to ON
4) Set the “Sulfur Multiplier” to 0.6238
5) Leave the Fixed Fuse correction set to ON (50 cal = 209.34 J)
e
[]
=
1
⎡
=
⎢
⎣
0.2
=
cal
ml
0866.0
meq
⎛
)6.9(
⎜
ml
⎝
⎞
()
−
⎟
⎠
⎤
×
meqg
⎥
⎦
1.14mult) urMass)(sulf le(%S2)(Samp-mult) acid)(acid of (ml
1.14)6238.0)(5087.0(%16.2
=
e
⎛
0.360.6238meq
cal
⎛
=
=
H
=
c
⎛
⎜
⎜
⎝
=
=
087g)(2.16)(0.5
⎜
cal
⎝
cal 24.675
e-e-e-Wt
321
m
5971.937
cal
⎞
⎟
o
⎟
C
⎠
⎞
⎜
⎟
⎜
meq
⎠
⎝
o
5087.0
g
MJ/kg 42.9441 or cal/g 10257.0244
SO4)H mult)(Hf urmass)(sulf le(%S2)(samp
22
⎞
⎟
⎟
⎠
0000.50675.240.2)6468.5(
−−−
calcalcalC
6-5
Page 61

6400 Calorimeter Instruction Manual
ASTM, ISO and Other Methods
Current ASTM, ISO, and British Standard Methods differ on their treatment of the nitric
and sulfuric acid thermochemical corrections. ASTM Methods call for titrating the bomb
washings to determine the total acid present. This is assumed to be all nitric acid with a
heat of combustion of -14.1 Kcal per mole. The amount of sulfur is then determined and
converted to equivalents of sulfuric acid. The difference between the heat of formation of
sulfuric acid (-72.2 Kcal per mole or -36.1 calories per milliequivalent) and nitric acid is
then subtracted as the sulfur correction.
Most other test methods treat nitric and sulfuric acid corrections as entirely separate
values instead of combined values. This eliminates the requirement for a total acid
determination and permits the nitric acid correction to be handled in a variety of ways,
including the assumption of a fixed nitric acid correction.
The 6400 Calorimeter can be set up to apply the acid correction by either the ASTM
or ISO convention, as the user prefers. Care must be used to ensure the proper
corrections are applied, and the calculations made are consistent with the procedure
used. See Table 4.
ASTM
In the ASTM treatment, the correction for acid formation assumes that all the acid titrated is
nitric acid. Obviously, if sulfur is present in the sample, which in turn produces sulfuric acid,
part of the correction for the sulfuric acid formed is already included in the ASTM nitric acid
correction (e
of the sample. An additional correction of 1.37 Kcal must be applied for each gram of sulfur
converted to sulfuric acid from sulfur dioxide. This is based upon the heat of formation of
sulfuric acid, from sulfur dioxide, under bomb conditions, which is -72.2 Kcal per mole or -
36.1 calories per milliequivalent. But remember, a correction of 14.1 calories per
milliequivalent of sulfuric acid is already included in the ASTM nitric acid correction (e
Therefore the additional correction which must be applied for sulfur will be the difference
between 36.1 and 14.1 or 22.0 calories per milliequivalent (44.0 Kcal per mole). For
convenience, this is expressed, in the ASTM e
each percentage point of sulfur per gram of sample.
ISO
Both the ISO 1928 and BSI 1016: Part 5 methods for testing the calorific value of coal
and coke, deal with acid and sulfur corrections in a manner which is somewhat different
than ASTM procedures. The analysis of bomb washings in these methods call for a
titration, first using 0.1N barium hydroxide (V2) followed by filtering, and a second
titration using 0.1N HCl (V1) after 20 ml of a 0.1N sodium carbonate has been added to
the filtrate. Table 3 gives the settings which allow the results of the two titrations, V1 and
V2, to be entered into the controller directly for the calculation of the total acid correction.
V1 should be entered at the prompt for acid and V2 is entered at the prompt for sulfur.
The settings in Table 3 assume that the same procedure is carried out for both
standardization and determination. The offset value is the product of -1, the Heat of
Formation of Nitric Acid, the acid multiplier, and the 20 ml of 0.1 N sodium carbonate
used in the analysis. The formula used to get the total correction in calories is as
follows:
V1 (Acid Multiplier) (Heat of Formation of Nitric Acid) V2 (Sulfur Multiplier) (Heat of Formation of Sulfuric Acid) + offset value
). This is adjusted by a separate computation based upon the sulfur content
1
).
1
formula, as 13.7 calories (44.0/32.06) for
2
6-6
Page 62

6400 Calorimeter Instruction Manual
The values for fixed acid and sulfur, which are used in preliminary reports, will reflect a
sulfur correction of 0, and a nitric acid correction of 10 calories.
Conversion to Other Moisture Bases
The calculations described previously give the calorific value of the sample with moisture
as it existed when the sample was weighed. For example, if an air-dried coal sample
was tested, the results will be in terms of heat units per weight of air-dry sample. This
can be converted to a moisture-free or other basis by determining the moisture content
of the air-dry sample and using conversion formulae published in ASTM Method D3180
and in other references on fuel technology.
Conversion to Net Heat of Combustion
The calorific value obtained in a bomb calorimeter test represents the gross heat of
combustion for the sample. This is the heat produced when the sample burns, plus
the heat given up when the newly formed water vapor condenses and cools to the
temperature of the bomb. In nearly all industrial operations, this water vapor
escapes as steam in the flue gases and the latent heat of vaporization, which it
contains, is not available for useful work. The net heat of combustion obtained by
subtracting the latent heat from the gross calorific value is therefore an important
figure in power plant calculations. If the percentage of hydrogen H, in the sample is
known, the net heat of combustion, H
H
= 1.8Hc - 92.7H
net
(Solid fuels, ASTM D2015)
H
= 1.8Hc - 91.23H
net
(Liquid fuels, ASTM D240)
Btu per pound can be calculated as follows:
net
6-7
Page 63

6400 Calorimeter Instruction Manual
REPORTS
The 6400 Calorimeter can transmit its stored test data in either of two ways. The Auto
Report Destination key on the Reporting Controls Menu toggles the report destination
between the display and an optional printer connected to the RS232 printer port of the
calorimeter. This menu also selects the type of reports that are generated automatically
by the calorimeter.
There are two kinds of reports: Preliminary and Final.
• Preliminary Reports are generated at the conclusion of a test. They are
intended to confirm to the operator that the results of the test fell within the
expected range.
• Final reports are generated once all of the thermochemical corrections have
been entered into the file. If fixed corrections are used for all of the
thermochemical corrections, a preliminary report will not be generated. The
report will automatically become finalized.
Thermochemical corrections are entered by using the following steps to select and edit
preliminary reports. Test results are stored as files using the sample ID number as the file
name. A listing of the stored results is accessed by pressing the REPORT command key.
The REPORT command key brings up a sub-menu on which the operator specifies.
Select From List
two keys.
Run Data Type
only standardization runs, only solution runs (not applicable to the 6400) or all
runs.
Run Data Status
reports, only final reports, only preweighed samples reports, all stored reports or
preliminary and final reports.
Prompt For Final Values
enter any missing corrections for fuse, sulfur and acid in any selected preliminary
reports. When turned off preliminary reports will be displayed as entered.
The displayed files can be sorted by sample ID number, by type, by status or by date of
test by simply touching the appropriate column. Individual files can be chosen by
highlighting them using the up and down arrow keys to move the cursor. Press the
SELECT key to actually enter the selection. Once selected the highlight will turn from
dark blue to light blue. A series of tests can be selected by scrolling through the list and
selecting individual files. The double up and down keys will jump the cursor to the top or
bottom of the current display. If a range of tests is to be selected, select the first test in
the series, scroll the selection bar to the last test in the series and press EXTEND SEL
to select the series.
This key displays the stored results specified with the following
This key enables the operator to display only determination runs,
This key enables the operator to display only preliminary
When turned on, the controller will prompt the operator to
7-1
Page 64

6400 Calorimeter Instruction Manual
The DESEL ALL key is used to cancel the current selection of files.
To bring the selected report or series of reports to the display, press the
DISPLAY key. To send the reports to the printer press the PRINT key.
The EDIT key brings up a sub-menu which enables the operator to edit any of
the data in the report or add thermochemical corrections to convert preliminary
reports to final reports. Final reports can only be edited if Edit Final Reports on
the Reporting Controls Menu is turned on.
To have the Net Heat of Combustion print as part of preliminary and final
reports, go to the Thermochemical Corrections Menu and turn ON Calculate Net
Heat of Combustion. During the reporting process, the controller will prompt for
the hydrogen (H) value.
7-2
Page 65

6400 Calorimeter Instruction Manual
MEMORY MANAGEMENT
The 6400 Calorimeter will hold data for 1000 tests in its memory. These tests may be
pre weights, preliminary or final reports for either calibration or determination runs. Once
the memory of the controller is filled, the controller will not start a new analysis until the
user clears some of the memory.
The FILE MANAGEMENT key on the
main menu leads to the file management
sub-menu. The RUN DATA FILE
MANAGER key leads to a listing of the
files. Single files can be deleted by
highlighting the file and pressing the
DELETE key. The controller will then ask
the user to confirm that this file is to be
deleted. A series of files can be deleted
by selecting the first file in the series and
then the last file in the series using the
EXTEND SEL key and then pressing the
DELETE key.
The controller of the 6400 Calorimeter can accept compact flash memory cards. These
cards can be used to:
• Copy test file data for transfer to a computer
• Copy user settings for back up
• Reload user settings to the controller to restore or update the controller’s
operating system.
Compact flash memory cards are inserted into the slot on the back of the control section of
the Calorimeter. Keys are provided on the FILE MANAGEMENT sub-menu to initiate each
of the above three actions.
8-1
Page 66

6400 Calorimeter Instruction Manual
MAINTENANCE
Note:
Some of the following manual sections contain information in the form of
warnings, cautions and notes that require special attention. Read and
follow these instructions carefully to avoid personal injury and damage to
the instrument. Only personnel qualified to do so, should conduct the
maintenance tasks described in this portion of the manual.
Caution – Risk of Electrical Shock
Disconnect the electrical power before servicing or replacing any
components!
Inspection of Critical Sealing Surfaces
The sealing grooves and related surfaces for most of the Parr bombs are machined to
tolerances as small as +/- 0.001" (0.03mm). As a result, any imperfection in a sealing
surface resulting from either normal use or carelessness in handling the bomb can
cause the bomb to leak. If the damage or accumulated wear is much less than 0.001"
(0.03mm), then careful polishing will restore the bomb sealing to an as new condition.
Imperfections that penetrate the sealing surface more than one or two thousandths of an
inch (0.03-0.06mm) may render the seal surface unserviceable.
Any surface that comes in contact with an elastomer seal should be carefully examined
for imperfections that would compromise its ability to seal. A freshly sharpened pencil
can be used to probe the metal sealing surfaces for significant imperfection. If the pencil
point hangs up in the imperfection, further attention is warranted. An attempt should be
made to polish (remove) any significant imperfections. This operation generally requires
the use of a lathe in order to guarantee that the sealing surface to be repaired remains
concentric with the mating surface. Knowledge of the dimensional tolerances and the
ability to accurately measure or gauge the affected area is required in order to insure
that too much polishing (metal removal) has not taken place. We recommend that
bombs with significant imperfection of this nature be serviced at Parr.
Caution! Do not pry elastomer seals (o-rings and quad-rings) from seal
grooves with metallic tools.
The use of dental picks and other metallic tools to pry the seals from their grooves must
be avoided. These hard steel tools, if misused, can leave permanent tool marks on the
sealing surface, which are difficult or impossible to remove. These blemishes are hidden
by the seal during normal use and as a result, are not readily apparent as the cause of a
leaking bomb.
Larger size seals (0.8" or 20 mm O.D.) typically used to seal the bomb head can be
extracted from its groove using either of the following two methods:
1. Grasp the outer circumference of the seal with the thumb and forefinger and
slide them together while applying sufficient pressure on the seal to cause it
to pucker out of the groove. With the other hand, grab the exposed, pinched
section and pull the seal from the groove.
9-1
Page 67

6400 Calorimeter Instruction Manual
2. Use a non-metallic object, such as the rounded corner of a plastic credit card,
to simply pry the seal from its groove.
Smaller diameter seals usually require a different approach. A portion of the seal should
be carefully pulled, not pried, from the groove with a small pair of pliers or a hemostat.
The exposed portion of the seal can then be cut, or pulled further to remove the seal.
The pliers or hemostat should never contact the sealing surface, only the seal.
Bomb Removal
To service or remove the bomb cylinder from the bucket assembly, remove the 668DD
Check Valve from the bomb cylinder. Remove the 941DD Wedge with needle nose
pliers. Remove the two SA1632RD18 Machine Screws (see Figure 27), then remove
the 942DD plastic bushings and the 1071DD Quad-ring.
The entire bucket can be removed by disconnecting the bucket probe at the quick
disconnect. Carefully lift the bucket and bomb assembly out of the air chamber and
position horizontally on the calorimeter to remove the 925DD Oxygen Bomb Retainer
Nut (see Figure 23). Now the cylinder can be removed from the bucket assembly. Note
the position of the locating pin.
To replace, follow these steps in reverse.
Fuses
Lines protective fuses rated: Fast-Acting 15A, 250VAC (Parr number 139E23).
The replacement of protective fuses for the 6400 Calorimeter should be performed by
qualified personnel.
Daily Maintenance
Clean the 1444DDJB o-ring that seals the bomb head and cylinder by wiping with a
tissue. Wet this sealing area with water prior to starting a series of tests. Clean the
corresponding sealing area in the cylinder in a similar fashion. Both surfaces must be
free of any accumulated foreign matter, such as unburned sample material or
combustion by-products. Wet the hole in the center of the head which contains the
check valve.
With a tissue, clean the head where the large bucket quad-ring (1071DD) contacts the
head perimeter. Wet this sealing area with water prior to starting a series of tests.
Remove, inspect and clean the cylinder check valve (668DD) and corresponding sealing
area in the bomb cylinder. In extreme cases, i.e. a spilled sample, use soap and water
to clean the area.
Quarterly Maintenance
Change water in the Water Tank and replace the 149C water filters.
Periodic cleaning may be performed on the exterior surfaces of the instrument with a
damp cloth. All power should be disconnected when cleaning the instrument.
9-2
Page 68

6400 Calorimeter Instruction Manual
50 to 100 Test Maintenance
Replace the heating wire, with 2” of 840DD2. Wind the wire 360 degrees
clockwise around screws. Clean the 986DD Electrode Contact Pins with a mild
abrasive, such as a pencil eraser. Clean the bomb head electrode points in a
similar fashion and tighten the screws holding the heating wire in place.
500 Test Maintenance
Clean the ignition contacts.
Under normal usage Parr oxygen bombs will give long service if handled with
reasonable care. However, the user must remember that these bombs are continually
subjected to high temperatures and pressures which apply heavy stresses to the sealing
mechanism. The mechanical condition of the bomb must therefore be watched carefully
and any parts that show signs of weakness or deterioration should be replaced before
they fail.
For your convenience, these parts may be purchased as kit number 6038, Firing
Maintenance Kit.
Parr recommends that the following parts on the oxygen bomb be changed every 500
tests or six months whichever comes first: 840DD2, 1374HCJV (2), 394HC, 821DD (1),
1071DD, 1444DDJB, 659DD, 519AJV, 694DD. See Figures 10 and 26 for parts
locations. When reassembling the bomb head, take care not to roll the 694DD o-ring as
this will cause an oxygen leak.
Note:
Samples that contain chlorine or are abrasive may require this
maintenance to be performed on a more frequent interval such as every
250 tests.
The 882DD and 969DD o-rings should also be replaced. See Figures 23 and 24 for oring locations.
The 1140DD Seal/Release mechanism should be serviced with the same frequency as
the bomb head. This includes the replacement and lubrication of the 659DDJU (2),
1138DD, 1143DD and 357HCJB o-rings with 811DD lubricant. See Figure 24 and
Figure 25 for o-ring locations. The tools required are: screwdriver, snap ring pliers and
needle nose pliers.
1. Turn off the gas supply to the calorimeter. Disconnect the oxygen connection to the
back of the calorimeter. Go to the Diagnostics Screen and turn on the bomb seal
command. These steps are necessary to release the gas pressure in the seal/release
mechanism before disassembling.
2. Turn off the calorimeter.
3. Insert the bomb head into the cylinder and lock into place.
4. Disengage the screws, SA163X2RD018 that hold the bucket in the air can. Remove
the 941DD plastic wedge that secures the front of the air can assembly.
5. Lift the bomb and bucket as a unit from the calorimeter air can chamber and
disconnect the bucket thermistor probe. Set this unit aside.
6. Remove the vessel spacer, 964DD and the associated o-ring, 969DD.
9-3
Page 69

6400 Calorimeter Instruction Manual
7. Remove the cylinder spacer, 1141DD, which sits on top of the snap ring, 1137DD.
8. Remove the snap ring that retains the cylinder insert in the release mechanism at the
bottom of the air can. Withdraw both the insert and the release pin as a unit using
needle nose pliers.
9. If present, remove any scoring on the 966DD2 release pin, above the smaller o-rings, with
crocus cloth. Replace the 659DDJU o-rings on the release pin as well as the 1138DD oring that seals the cylinder insert. Replace the 659DDJU and 357HCJB o-rings.
10. Reverse the above procedure to reinstall the cylinder insert/pin as well as the bomb
bucket assembly. Make sure that the large side hole in the 1139DD insert is oriented
toward the left side of the instrument. The insert is keyed to the cylinder and can not
be fully inserted unless it is properly oriented
5000 Test Maintenance
To deal with the realities of today’s test loads and cycle times, the ASTM Committee
recommends in method E144-94 that “all seals and other parts that are recommended
by the manufacturer be replaced or renewed after each 5000th firing or a more frequent
interval if the seals or other parts show evidence of deterioration.” Oxygen bombs
returned to Parr for service will be tested in accordance with ASTM E144-94. A test
certificate is provided with each repair.
This service includes:
• Disassembly, cleaning and inspection of all parts
• Re-polishing of the inner surfaces of the bomb
• Re-assembly with new insulators, and seals, sealing rings, and valve
seats
• Proof testing
• Hydrostatic testing
The hydrostatic and proof testing of the oxygen bomb should be performed after every
5000 firings or if:
• The bomb has been fired with an excessive charge
• The ignition of any of the internal components has occurred
• There have been any changes in the threads on the bomb cylinder
• The bomb has been machined by any source other than Parr Instrument
Company
After repeated use with samples high in chlorine (over 1%), the inner surfaces of the
bomb will become etched to the point where appreciable amounts of metal salts will be
introduced during each combustion. Any bomb which is being used for chlorine
determination should be polished at more frequent intervals to prevent the development
of deep pits. If the interior of the bomb should become etched or severely pitted, the
resistance of the metal to further attack can be improved by restoring the surface to its
original polished condition.
There are no user serviceable parts inside the product other than what is specifically
called out and discussed in this manual. Advanced troubleshooting instructions beyond
the scope of this manual can be obtained by calling Parr Instrument Company in order to
determine which part(s) may be replaced or serviced.
9-4
Page 70

6400 Calorimeter Instruction Manual
TROUBLESHOOTING
Bomb Exhaust Troubleshooting
The bomb exhaust and sealing is controlled by movement of the 966DD2 piston inside of
the 1140DD bomb seal/release cylinder. This assembly is mounted on the bottom of the
calorimeter air can. The piston is driven to the up position (bomb exhaust) by applying
oxygen at 30 atm to the 1/8 male connector (344VB). The piston is driven down (bomb
seal) by applying pressure to the 376VB elbow. The application of the oxygen pressure
is controlled by the A1251DD three station solenoid valves. There is a flow restrictor,
part 527VB, on the inlet side of this solenoid which limits the maximum flow rate of
oxygen and in turn creates a gradual increase in pressure at the 1140DD bomb
seal/release cylinder when the solenoid is turned on. Failure of the bomb to exhaust in a
timely fashion can have more than one cause. Certain causes can be eliminated
systematically by checking the bomb exhaust diffuser, at the end of the bomb exhaust
line, for any restrictions in the six small cross drilled holes. This fitting should be
removed from the tubing, inspected thoroughly and cleaned as required.
Confirm Correct Operation of the A1251DD Solenoid Valve
If the piston does not move, it is worthwhile at this point to confirm that both sections of
the A1251DD are working properly (Figure 15). For the location of the A1251DD
assembly, Figure 12.
Disconnect the 1/8 nylon pressure hose at the elbow connection nearest the back panel
by using a 7/16 wrench. Apply power to the unit and re-enter the I/O diagnostics. Turn
the exhaust output on. Oxygen should flow from the elbow connection on the A1251DD.
Turn the bomb exhaust output off and re-connect the nylon pressure hose. Disconnect
the 1/8 nylon pressure hose at the middle connection. Activating the bomb seal output
should produce a flow of oxygen at the elbow.
Turn off the bomb seal output and reconnect the nylon pressure hose. If neither
solenoid produces a flow of gas when activated and the O2 Fill key does not produce a
flow of gas, then, in all likelihood, the 527VB flow restrictor is plugged and should be
replaced. If only one of the solenoids sources gas when activated, then the problem
must be further diagnosed as either being electrical (I/O board, solenoid coil or external
wiring) vs. mechanical (in the valve) and dealt with in an appropriate manner.
If either solenoid sources gas when it is off (i.e. leaks) then replacement of the entire
A1251DD solenoid assembly is indicated. For reference purposes, the normal upward
thrust generated by the 966DD2 piston is 50 pounds. The downward thrust is 20
pounds. Far less than 20 pounds are required to move the piston in either direction
when the bomb is not pressurized.
Service the O-rings on the 966DD2 Piston
This process is described in the 500 test maintenance section.
10-1
Page 71

6400 Calorimeter Instruction Manual
Confirm function of the 966DD2 piston
In order to reduce the amount of time it takes to duplicate and troubleshoot this type of
situation, the I/O diagnostics can be used to pressurize and exhaust the bomb without
having to run lengthy combustion or pre-tests.
WARNING: This screen allows unconditional and arbitrary output control
for testing purposes. Be aware that all user and instrument protection is
disconnected while on this screen. This is very important and you should
take proper pre-caution.
1. Make sure the 668DD check valve is installed at the bottom of the cylinder.
2. Lock the head into the cylinder and close the calorimeter lid.
3. Confirm bomb release is off.
4. Turn bomb seal on then off to retract the 966DD2 piston.
5. Turn on O
minute, at which time O
head which in turn seals the contents of the bomb.
6. The calorimeter lid can be unlocked at this time.
7. Activating bomb release should initiate a bomb exhaust within two seconds. If it takes
much longer than two seconds before the bomb begins to vent, then at least one of the
two following conditions outlined below exist.
8. If the bomb exhaust is initiated in a timely manner but fails to complete in 10
seconds, a blockage or restriction in the bomb exhaust circuit is indicated. This must
be investigated and corrected.
If the bomb fails to exhaust, the 1454DD funnel and 1453DD bomb cap adapter can
slowly be removed to release the pressure in the bomb. See Figure 26.
If the piston moves properly with no applied bomb pressure, but still fails to initiate an
exhaust of a pressurized bomb in a timely fashion, at least one of the following
conditions exist:
1. The 527VB restrictor is partially blocked.
2. The exhaust line is blocked.
3. There is a gas leak between the outlet of the solenoid and the 1140DD cylinder.
This also includes the 357HCJB o-ring seal on the piston inside of the cylinder.
The first condition can be eliminated by cleaning or replacing the 527VB restrictor.
The second condition can be eliminated by replacing the tubing and clearing all
connections.
The third condition can be eliminated by following the procedure outlined in the section
servicing the o-rings on the 966DD2 piston and carefully inspecting the 1/8 nylon
pressure hose and associated compression fittings for leaks while this circuit is
maintained at operating pressure, using the calorimeter I/O diagnostics. A minute leak
will result in a significant reduction in upward thrust.
fill to begin filling the bomb. The bomb will be completely filled in one
2
fill should be turned off. This seats the check valve in the
2
10-2
Page 72

6400 Calorimeter Instruction Manual
Jacket Temperature Troubleshooting
The jacket temperature is monitored with the use of a thermistor installed in the
A1448DD temperature control assembly. This assembly is heated by a heater cartridge,
A1459DD. In the Diagnostics Menu, select Instrument Monitor. If the heater PID is ON
and reading 100%, yet the jacket is at ambient temperature, check the following possible
causes.
If the heater PID is OFF, the heater and pump must be turned on in the Calorimeter
Operation screen to perform the troubleshooting steps.
WARNING: Turn off the power to the calorimeter prior to attempting to reset the
thermostat. The temperature control assembly can become very hot. Use
caution when servicing this area of the calorimeter.
If line voltage (115V or 230V) is present across the heater cartridge connection, check
the resistance across the heater cartridge. Approximately 70 ohms will be seen with a
115V calorimeter. Approximately 140 ohms will be seen with a 230V calorimeter. If the
resistance is not correct the heater may have failed.
If the voltage is not present, then examine the 2040E thermostat reset button. If the
reset button extrudes this means that the temperature in the temperature control
assembly has exceeded 75ºC. Confirm that water is flowing through the system, turn off
the power and then reset the switch by depressing the button. If the thermostat
continues to trip even though water is flowing through the system, refer to the error code
“There Is A Problem With The Jacket Thermistor”
If there is no voltage present, and the reset button on the thermostat is not tripped, refer
to the error code “There Is A Problem With The Jacket Thermistor”
troubleshooting. There may also be a problem with the calorimeter controller, A1250DD,
and Parr service should be contacted.
Error List
The calorimeter will run a number of diagnostic checks upon itself and will advise the
operator if it detects any error conditions. Most of these errors and reports will be selfexplanatory. The following list contains errors that are not necessarily self-evident and
suggestions for correcting the error condition.
A Misfire Condition Has Been Detected
This error will be generated in the event the total temperature rise fails to exceed 0.5°C
after the first minute of the post-period. Possible causes for this error are listed below.
• Fuse wire not intact, too long or improperly formed
• Breakdown of the insulator and o-ring on the insulated electrode
assembly
• Bomb not filled with oxygen
• A bomb leak
• Poor bucket stirring
• Ignition lead wires have broken internal strands
for further troubleshooting.
for further
10-3
Page 73

6400 Calorimeter Instruction Manual
A Preperiod Timeout Has Occurred
The calorimeter has failed to establish an acceptable initial temperature, prior to firing
the bomb, within the time allowed. Possible causes for this error are listed below:
• A bomb leak
• Poor bucket stirring
• Lid not tight
The Current Run Has Aborted Due To Timeout
The calorimeter has failed to establish an acceptable final temperature within the time
allowed. Possible causes for this error are listed below:
• A bomb leak
• Poor bucket stirring
There Is A Problem With The Bucket Thermistor
Possible electrical open. These errors will result if the temperature probe response is
not within the expected range. Probe substitution can be useful in determining the
cause of the problem (probe or electronics). The valid working range of the probe
resistance is 1000 to 5000 ohms
• Check connection to the board
• Check quick disconnect
• Replace probe
• Replace board
There Is A Problem With The Jacket Thermistor
Possible electrical open. These errors will result if the temperature probe response is
not within the expected range. Probe substitution can be useful in determining the
cause of the problem (probe or electronics). The valid working range of the probe
resistance is 1000 to 5000 ohms
• Check connection to the board
• Check quick disconnect
• Replace probe
• Replace board
A/D Initialization Failed
Shortly after power is applied to the calorimeter controller and the operating system has
started, the CPU attempts to read the unique I/O board calibration information from the
I/O board. If the I/O board is not connected to the CPU, or the information on the board
is not valid, this error will be issued.
Bomb ID – Has Been Fired – Times Which Exceeds the Bomb Service Interval
The calorimeter controller keeps track of how many times the bomb has been fired.
When this count exceeds a preset limit (usually 500) this message will be issued each
time the bomb is used for a test. Perform bomb maintenance and reset counter on
Calibration Data and Control page for appropriate bomb number.
10-4
Page 74

6400 Calorimeter Instruction Manual
You Have Exceeded the Run Data File Limit (1000 Files)
The memory set aside for test runs has been filled. Use the memory management
techniques to clear out non-current tests.
Bomb EE Standard Deviation Warning
The relative standard deviation for the calibration runs in memory for the indicated bomb
exceeds the preset limit.
Sample Weight Warning
The entered sample mass exceeds the value entered via the Sample Weight Warning
Above key on the Data Entry Controls page. This warning threshold is normally 2
grams.
The Lid has Failed to Lock or is not Closed Properly
This error will be reported when the controller fails to detect continuity through the bomb
ignition circuit. The most probable cause will be either a poor electrical connection
between the bomb’s internal electrodes and the fuse wire, carbon build up on the
electrodes or a fuse wire that has burned out. This will also occur if the lid is not down
before the pretest or test is initiated.
The heater loop break limit has been detected. The heater will now be shutdown
This error means that the calorimeter is trying to heat the water in the unit for an
extended period of time. The calorimeter suspects that there is a short and shuts the
system down in order to “save” itself. This is a fairly normal occurrence if the lab
temperature is very cool at night. It is acceptable practice to ignore the warning and restart the unit. However, if this error occurs more than three times in a row, then it may
be a true thermistor problem. Please refer to the Jacket Temperature Troubleshooting
section of the manual.
Could Not Cool the Bomb Successfully
The calorimeter has failed to establish the desired cool down temperature within the time
allowed. Check the flow of cooling water to see that it is not restricted or the filter
plugged. It may also be that the water is not cold enough.
Rinse Tank Level May Be Low
The controller decrements the rinse tank counter each time the bomb is rinsed. This
message will be issued when the counter is at or below zero when the bomb rinse
sequence is executed. This message is a reminder that the rinse tank needs refilling,
followed by a manual resetting of the bomb rinse counter.
.
10-5
Page 75

6400 Calorimeter Instruction Manual
TECHNICAL SERVICE
Contact Us
Should you need assistance in the operation or service of your instrument, please
contact the Technical Service Department.
Telephone: (309) 762-7716
Toll Free: 1-800-872-7720
Fax: (309) 762-9453
Email: parr@parrinst.com
Any correspondence must include the following basic information:
1. The model and serial # of the instrument.
2. Date purchased.
3. Software version(s) shown on the “Software and Hardware Information” page.
4. Help system revision. This is displayed by pressing the <MAIN MENU> key and
then the <HELP> key.
When calling by phone, it is helpful if the person is close to the instrument in order to
implement any changes recommended by the Technical Service Department.
Return for Repair
To return the instrument for repair, please call the Technical Service Department for
shipping instructions and a RETURN AUTHORIZATION NUMBER. This number must
be clearly shown on the outside of the shipping carton in order to expedite the repair
process.
If you have not saved the original carton and traps, please request a packaging return
kit.
We prefer the calorimeter to be shipped in our cartons and traps to prevent shipping
damage.
Ship repair to:
Parr Instrument Company
Attn: Service Department, RMA# XXXX
211- 53rd Street
Moline, Illinois 61265
11-1
Page 76

PARTS LIST
Principal Assemblies in Calorimeter
Item Description
1138/1138CL
1795E
1796E
897E
A1250DD
A1251DD
A1447DD
A1448DD
A1449DD
A1254DDEB
A1254DDEE
A1255DD
1433DD
A1449DD
A1456DD
A1275DDEB
A1275DDEE
139E23
WARNING: For continued protection against possible hazard, replace
A1250DD Controller Assembly Parts List
Item Description
1217DD
1803E
A1876E
A1792E
A1933E6
A1794E
A1806E
Oxygen Combustion Vessel
Power Supply, 24V
Power Supply, 5/12V
Capacitor, Ignition
Controller Assembly
Oxygen Solenoid Assembly
Water Solenoid Assembly
Temp Control Sub-Assy
Water Chiller Assembly
Pump Assembly Circulating 115V
Pump Assembly Circulating 230V
Propeller Assembly, stirrer
Tank, Water (A1435DD / 6400)
Water Chiller Assembly
Rinse Valve Assembly
Cartridge, Heater Assembly, 120V
Cartridge, Heater Assembly, 230V
Fuse Fast/ Act 15 AMP 250V
fuses with same type and rating of fuse.
Gasket for Display
Backlight, Inverter
Touchscreen LCD w/ cable
Transition Display Board
CPU Board 6400
Input / Output Board
Cable, Backlight Inverter
6400 Calorimeter Instruction Manual
12-1
Page 77

6400 Calorimeter Instruction Manual
A1457DD Accessory / Installation Kit Parts List
Item Description
231C2
3415
356HCW
43AS
811DD
840DD2
845DD2
876DD
1005DD
A1006DD
A38A
TX03SK
TX14SK
149C
1344DD
1889E
356HCW
HJ0025TB035
HX0012TB024
Container, PP 10L Foldable
Benzoic Acid Pellets 100 Gram Bottle O-Ring,
Pliers for Snap Ring
Capsule, SS
Lube/Sealant
Heating Wire
Ignition Thread, 4”
Cutter, Plastic Tubing
Forceps
Waste Tube Assembly
Bomb Head Support Stand
1/32 Socket Screw Key
9/64 Socket Screw Key
In-line Filter
LCD Stylus
LCD Screen Protector
Pliers for Snap Ring (356HC)
Tubing, Nylon 1/4 OD X .35W
1/8 OD Tubing, Pressure Nylon
A1265DD Bucket and Stirrer Tube Assembly Parts List
Item Description
944DD
946DD
1129DD
1416E
1462E2
A940DD
A1255DD
A1255DD Bucket Stirrer Assembly Parts List
Item Description
682DD
683DD
684DD
690DD
715HC
954DD
1029DD
1242DD2
SA1140RD04
SN1140HLHJ
O-Ring, Buna-N .237 ID
Seal ¼ SS
Pin, Anti-rotating (A940DD)
Thermistor, Bucket
Thermistor Cable
Tube Assy, Bucket; Soldered
Bucket Stirrer Assembly
Snap Ring, Internal .50
Wave Spring, .50 OD
Ball Bearing, .50 OD
V-Seal, Nitrile
O-Ring NBR 1-1/4 ID
Propeller
Baffle Assembly
Pulley, Timing
4-40 X ¼ RHMS 18-8 SS
Nut, 4-40 Hex Lock
12-2
Page 78

A1266DD Cover Assembly Parts List
Item Description
Contact Pin Assembly
986DD
987DD
988DD
989DD
1021DD
SN1332HX
A1400DD
1218DD
1229DD3
1237DD2
1396DD
1324DD2
A1230DD2
Pin Contact
Block Contact Pin
Spring Compression
Snap Ring External .219”
Bushing 0.125 ID
6-32 Hex Nut 18-8 SS
Plunger / Knob Assembly
Cover, Air
Plate
Friction Hinge
Latch Block
Post, Latch Locking
Cover / Tubing Assembly
6400 Calorimeter Instruction Manual
12-3
Page 79

DRAWINGS
6400 Calorimeter Instruction Manual
Figure 10 - 1138 Parts Diagram
13-1
Page 80

6400 Calorimeter Instruction Manual
1138 Parts Diagram Key
Key No. Item Description
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
A1450DD
888DD2
889DD
821DD
668DD
882DD
925DD
SA1632RD06 (2)
902DD
899DD
1454DD
904DD (2)
905DD
SA1632FT06 (2)
898DD
SN1632HX
1374HCJV (2)
394HC
663DD
1071DD
1444DDJB
1452DD
1452DDCL
655DD (2)
1095DD
PA1332RD04 (2)
656DD
653DD
43AS
906DD
658DD
654DD
1094DD
643DD
645DD
647DD
SC1332SC02 (3)
SA1332FP04
659DD
840DD2
694DD
1453DD
519AJV
Item Complete Assemblies
A1450DDCL
A890DD2
A890DD2CL
Cylinder
Outer Ring
O-Ring 1/16 ID NBR
Check Valve
O-Ring 1/2 ID NBR
Bomb Retainer
8-32 x 3/8 RHMS
Ground Stud Head
Head Handle
Funnel
Standoff 8-32 x 5/8 M-F
Standoff 8-32 x 3/8 M-F
8-32 x 3/8 FHMS
Locator Cap
8/32 Hex Nut
O-Ring 1/8 ID FKM
O-Ring 3/8 ID FKM
Contact Bushing
Quad Ring 2.88 ID NBR
O-Ring 1-5/8 ID NBR
Head
Head for Chlorine Service
Electrode Spacer
Electrode
6-32 x 1/4 RHMS
Reducer Bushing
Electrode Nut
Capsule
Capsule Holder
Insulator
Electrode Washer
Electrode
Check Valve
Water Diffuser
Anti-Rotator
6-32 x 1/8 SHSS
6-32 x 1/4 FHMS
O-Ring 5/32 ID NBR
60” Ignition Wire (2” per use)
O-Ring 5/16 ID NBR
Adapter, Bomb Cap
O-Ring, FKM 5/64 ID x 1/16CS
Oxygen Bomb Head Assembly
Oxygen Bomb Head Assembly for Chlorine Service
Cylinder Assembly
Cylinder Assembly for Chlorine Service
13-2
Page 81

6400 Calorimeter Instruction Manual
Figure 11 - 6400 Cutaway Right
13-3
Page 82

6400 Calorimeter Instruction Manual
Figure 12 - 6400 Cutaway Left
13-4
Page 83

6400 Calorimeter Instruction Manual
Figure 13 - 6400 Cover Open
13-5
Page 84

6400 Calorimeter Instruction Manual
Figure 14 - A1250DD Control Schematic
13-6
Page 85

6400 Calorimeter Instruction Manual
Figure 15 - A1251DD Oxygen Solenoid Assembly
Figure 16 – A1447DD Water Solenoid Assembly
13-7
Page 86

6400 Calorimeter Instruction Manual
Figure 17 – A1435DD Rinse Valve Assembly
13-8
Page 87

6400 Calorimeter Instruction Manual
Figure 18 - 6400 Internal Plumbing Diagram
13-9
Page 88

6400 Calorimeter Instruction Manual
Figure 19 - 6400 Water Tank and Jacket Cooling Solenoid
13-10
Page 89

6400 Calorimeter Instruction Manual
Figure 20 – A1455DD Propeller Assembly
Apply thread sealant (Locktite or equivalent) to set screw in 1242DD2 pulley
before installing.
Figure 21 – A1448DD Temperature Control Assembly
13-11
Page 90

6400 Calorimeter Instruction Manual
Figure 22 - A1268DD Stirrer motor and Mount
13-12
Page 91

6400 Calorimeter Instruction Manual
Figure 23 – 6400 Bucket Assembly
13-13
Page 92

6400 Calorimeter Instruction Manual
Figure 24 - 6400 Air Can Assembly, Cutaway Left
13-14
Page 93

6400 Calorimeter Instruction Manual
Figure 25 - Air Can Assembly, Cutaway Front
13-15
Page 94

6400 Calorimeter Instruction Manual
Figure 26 - A1450DD Bomb Head Assembly, View 1
13-16
Page 95

6400 Calorimeter Instruction Manual
Figure 27 - A1450DD Bomb Head Assembly, View 2
13-17
Page 96

6400 Calorimeter Instruction Manual
Figure 28 – A1050DD Rinse Collection Assembly
13-18
Page 97

6400 Calorimeter Instruction Manual
Figure 29 - Wiring Diagram
13-19
Page 98

6400 Calorimeter Instruction Manual
Figure 30 - Wiring Diagram
13-20
Page 99

6400 Calorimeter Instruction Manual
Figure 31 – Fuse Diagram
13-21
Page 100

TABLES
Table 2 - Default Settings
Calorimeter Operation
Operating Mode Determination
Bomb Installed/EE 1/940
Heater and Pump OFF
Operating Controls
Method of Operation Dynamic
Reporting Units btu/lb
Use Spiking Correction OFF
“OTHER” Multiplier 4.1868
Calibrate Touchscreen
LCD Backlight Timeout(s) 1200 S
LCD Contrast 30%
Print Error Messages ON
Language English
Spike Controls
Use Spiking OFF
Heat of Combustion of Spike 6318.4
Use Fixed Spike OFF
Weight of Fixed Spike 0.0
Prompt for Spike before Weight OFF
Bomb Rinse Tank Control
Report Rinse Tank Empty ON
Rinse Tank Capacity 150
# Rinses Left 150
Reset Rinse Tank Counter
Rinse Time 25
Rinse Flush Time 20
Clear Time 100
# of Rinse Cycles 3
Program Information and Controls
Date XX/XX/XXXX
Time XX:XX
Software and Hardware Info
Settings Protect OFF
User/Factory Settings
Feature Key
Bomb Type Select
User Function Setup
Cold Restart
User Factory Settings
User Setup ID 64-1138
Reload Factory Default Settings
Reload User Default Settings
Save User Default Settings
6400 Calorimeter Instruction Manual
Calibration Data & Controls
Calibration Run Limit 10
EE Max Std Deviation 0.0
Heat of Combustion
of Standard 6318.4
Bomb Service Interval 500
Use Bomb 1
Bomb 1 Through 4
EE Value 940
Protected EE Value OFF
Thermochemical Corrections
Standardization
Fixed Fuse Correction ON 50.0
Fixed Acid Correction ON 8.0
Fixed Sulfur Correction ON 0.0
Determination
Fixed Fuse Correction ON 50.0
Fixed Acid Correction ON 8.0
Fixed Sulfur Correction OFF 0.0
Calculate Net Heat of Combustion OFF
Calculation Factors
Acid Value is Nitric Acid Only ON
Acid Multiplier 0.0709
Sulfur Value is Percent ON
Sulfur Multiplier 0.6238
Fuse Multiplier 1.0
Use Offset Correction (ISO) OFF
Offset Value 0.0
Fixed Hydrogen OFF 0.0
Hydrogen Multiplier 50.68
Dry Calculation OFF
Dry Calculation
Dry Calculation OFF
Fixed Moisture OFF 0.0%
Moisture Multiplier 5.83
14-1
 Loading...
Loading...