Page 1
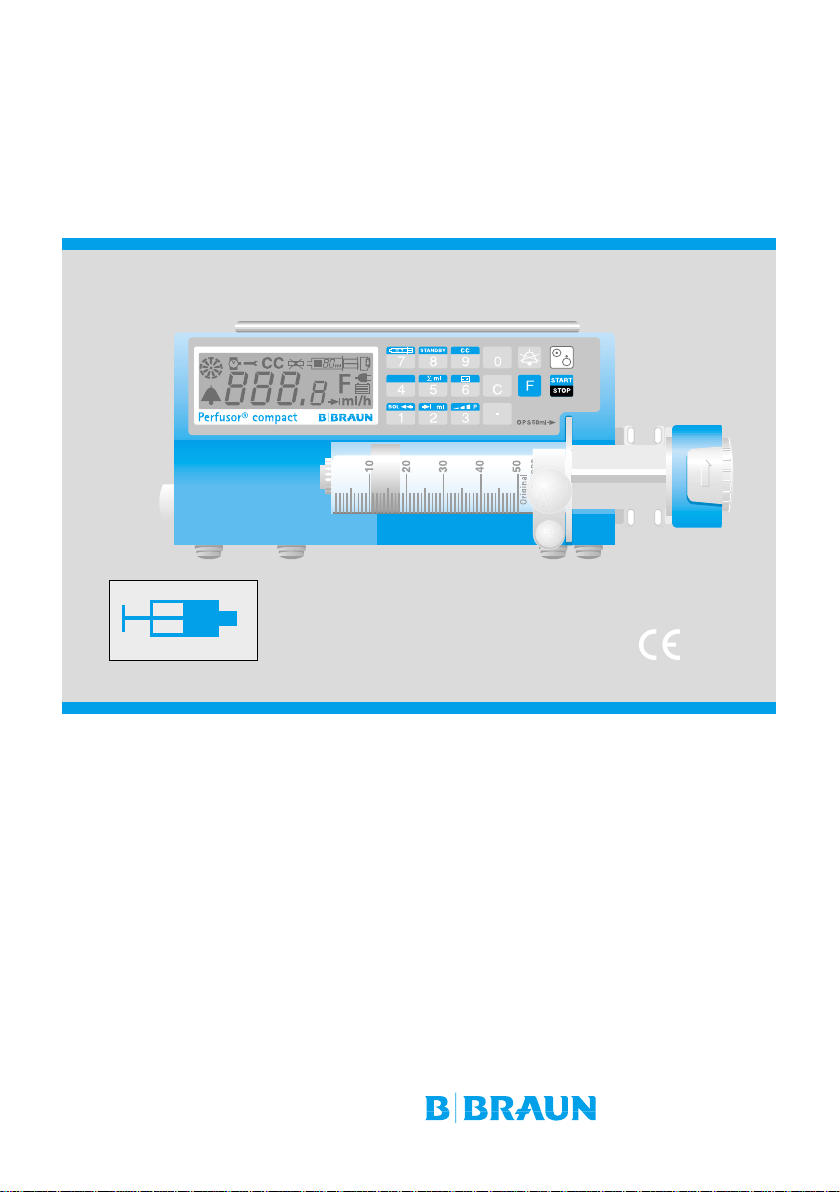
Perfusor® compact
Instructions for use
0123
Software AA
1
Page 2
Patient Safety
Attention: take note of accompanying documents!
▲
!
▲
First read the Instructions for Use.
Use of the equipment presupposes regular
checking by specially-trained staff.
Operation
Make sure that the unit has been positioned
safely and is stable and secure.
Prior to use: always ensure the functioning
of the audible and visual alarms during the
automatic check (see page 6). Also check the
staff call and syringe setting features for
possible damage.
Connection to patient is only permissible
when the device is switched on. Interrupt the
connection to change syringes. There is otherwise a danger of incorrect dosage.
Select syringe/catheter suitable for use with
the connection system and medical application.
Make the connection ensuring that the
infusion line is free of kinks so that a flow is
possible.
Replace disposable articles after 24 hours.
It is presupposed that installation in rooms
used for medical purposes will comply with the
appropriate regulations (e.g. VDE 0100, VDE
0107 or IEC publications). Care must also be
taken to observe regional specifications and
national variations.
Do not use in an area endangered by risk of
explosion.
Compare displayed and input values. Use
only when these match.
Other Components
Work in the area of the pressure shut-down
facility, or variations in pressure (e.g. as caused
by change in level), can affect the accuracy of
the device.
Where several infusion lines are connected,
the possibility of their exerting a mutual
influence on each other cannot be excluded.
Cases of possible incompatibility can be found
in the directions on use of the medicament or
appliances in question.
See also VDE 0753, Part 5, "Application Rules
for Parallel Infusion - Possible Application
Methods", or the BBM application directives
for parallel infusion (38910004).
Only combinations of equipment, accessories,
working parts and disposable parts that have
been shown to be compatible shall be used.
The use of disposable parts that have not
been tested or approved might well exert an
influence on the technical data.
Connected analogue and digital components
must verifiably satisfy the EN specifications
(e.g. EN60950 on data processing devices and
EN60601 on medical electrical devices). Anyone
who additionally connects devices to the signal
input or output part is a system configurator
and is thereby responsible for compliance with
the systems standard EN60601-1-1.
Safety Standards
Perfusor compact satisfies all safety standards
for medical electrical devices in terms of the
IEC 601-1 and IEC 601-2 (-24) publications.
Note: IEC 601-1 corresponds to the European
Standard EN60601.
2
Page 3

Perfusor compact
Contents
Perfusor compact / Overview Page 4
Operation Page 6
Technical Data Page 10
Start-up and Trumpet Curves Page 12
Warranty Page 13
Ordering Page 14
The Perfusor compact is a transportable
infusion syringe pump in accordance with
EN60601-2-24 (draft), Points 2.2.18 and 2.2.23
that is suitable for dispensing liquids in
nutritional and infusion therapy. The medical
specialist must decide on suitability for
application on the basis of the warranted
properties and the technical data.
For further details please refer to these
Instructions for Use.
3
Page 4
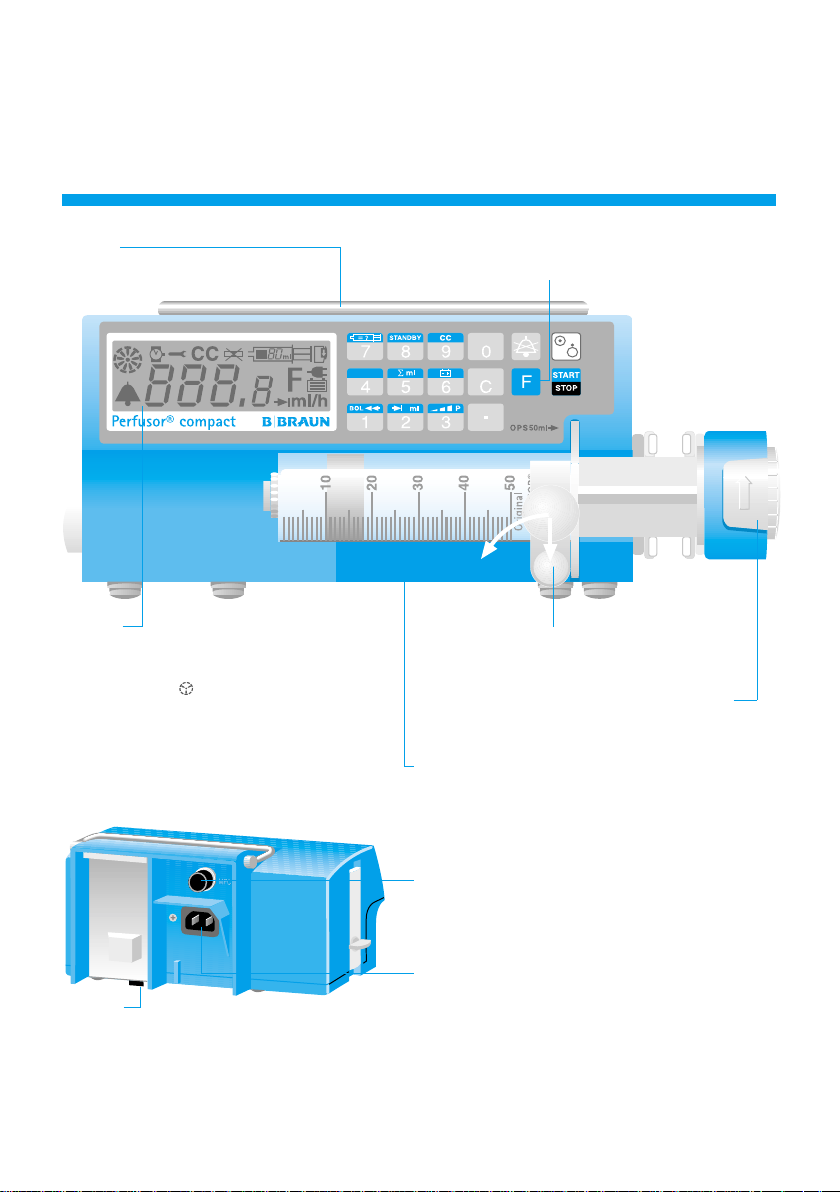
Overview
Handle
Always use the handle when carrying
Display
Shows all important features at a glance: rate,
type of syringe, mains or rechargeable battery,
carriage control
"Alarms" on page 9).
and alarm symbol (see
Operation
For special functions first press the
Syringe holder
Locks in the syringe. To remove, pull and
swing to the left.
The drive unit can be moved by hand after
the locking lever has been opened.
Short instructions for use and Syringe Table
See the underside of the device.
F button.
Battery
Press here to change the battery. Interrupt the
connection to the patient during changing of
the battery. Switch off the device and pull the
cover downwards. Always renew all batteries,
taking care to observe waste disposal regulations.
4
Multi-Function Connector (MFC)
Connection for staff call; ambulance cars (12V)
and interface.
Mains Connection
Connection for the mains supply. In the event of
power failure, automatic switch-over to battery.
Page 5

Input Correction
Interrupt alarm signal for 2 min.
On/Off. Press for 2 sec to switch off.
Start/Stop Infusion
Decimal Point
Inspection on Delivery
Despite careful packaging the risk of transport
damage cannot be entirely excluded. Upon
delivery please check that nothing is missing.
Do not make use of a damaged device! Contact
the service department.
For special functions (green) first press
Transport/Carriage
A maximum of three devices can be connected
together. Special care is required here if a
patient is already connected. Avoid external
mechanical influence!
Locking Devices Together
Place one device on top of the other. Push down
the connecting part until clicks into place. Lock
by turning the vane until it is vertical.
To disconnect, turn until horizontal and then
neu
push upwards.
Stand Clamp/Attachment on Stand
Attach the Perfusor compact from above,
clicking it into place. To release, press the black
button. For safety purposes attach each device
separately to the stand.
Packaging: Reusable, therefore environmentally
acceptable (returns will be accepted).
Extent of Delivery: Perfusor compact, power
lead, pole clamp, instructions for use,
4 batteries.
F.
5
Page 6

Operation
1. Insert Syringe
Raise syringe and prime infusion line.
Tip: an alternative is to evacuate the air using
the bolus button (non-delayed start of infusion).
Switch on using . – Note the automatic
check:
- After switching on, all display elements must
appear for approx. 2 sec and the audible
alarm must be heard (see Display, page 4).
- Then comes the rate display:
111.1 222.2 555.5
- Then the software version: AA
In addition the
point blink.
Open the syringe holder, release and pull out
the drive unit.
Insert the syringe such that the grip and the
pressure plate reach the guide. Lock syringe
holder again. If the syringe has been "correctly"
placed the release catch will snap back on its own.
(The type-of-syringe number displayed must
match that of the syringe inserted - see table.)
Confirm the type of syringe by pressing F .
Connect the patient.
2. Setting the Rate
Value between 0.1 and 99.9 ml/h. Check
display. To correct:
Press C and enter the rate anew.
3. Start Infusion
Press START. Running control is displayed.
4. Stop the Infusion
Press STOP or for 2 sec. Disconnect the
patient.
Open the syringe holder. Remove the syringe.
To switch off, press for 2 sec .
, CC, and decimal
Here, insert only an
Original 50 ml Perfusor
Syringe (OPS).
Here, all others.
Syringe grip plate Release
Change of Syringes
Press STOP.
Disconnect the patient!
Remove the syringe. Fit a new air-extracted
syringe with infusion line.
Confirm the type of syringe using button F.
(Only necessary when using another syringe
type than before.)
Connect the patient and press START.
To Alter the Rate
Press STOP.
Press C and enter the new rate.
Press START.
To Alter the Rate Without Interrupting the
Infusion
While the infusion is taking place: simply press
C and enter the new rate, then confirm this
with F. The new rate will now apply.
(If
F is not pressed the display reverts to the old
rate after 10 sec).
Syringe pressure
plate
6
Page 7

Special Functions
Activate the special functions using the F button (F is shown in the display).
During infusion only the status can be shown; when stopped, changes in value can also be shown.
Use the F button to confirm input values or to interrupt the function.
Syringe Selection
Open the syringe holder, press the symbol for
syringe selection; the syringe code blinks. Press
enter a new number and confirm this using F.
After adjustment check the new syringe number
once while infusion is taking place by pressing
and .
(The bolus rate can be altered by the service unit.)
Checking the bolus rate: Press
each individually.
Initiating the bolus:
First press F, then - while holding this - press
BOL and keep both pressed. An audible signal
will be given for each ml.
Take care not to overdose!
Given a bolus rate of 800 ml/h, e.g. 0.1 ml will
be reached in just 0.45 sec.
A pause in the infusion without a reminder alarm
signal being sounded. Set values are retained.
The display shows
Shows the volume already infused. If this
exceeds 999.
display.
Return the volume to 0,0 ml by pressing C or by
switching the device off.
Bolus
F, then press BOL,
Standby
and F.
Infused Volume
9 ml ---,- will be shown on the
Volume Preselection
Infusion stops automatically when the set
volume is reached. Display of the preselected
C,
volume:
Press first
F
To alter:
Press
For 0 ml the volume limitation is switched off.
Below 1 ml the volume precision can be
reduced. During active volume selection the
symbol displayed blinks. Volume preselection is
erased when the device is switched off.
Shows the actual Dianet address for control by
PC. To change the address:
Press
operation: press START.
Shows the remaining capacity of the
(rechargeable) battery:
The device switches off in the event of line
failure. The switch-off pressure can be set at
from P1 (low) to P3 (high). To alter:
Press
At the sounding of the pressure alarm the bolus
volume built up by the device (approx. 1 ml at
the highest compression phase) is automatically
reduced.
F, then Volume Preselection (ml).
C and then enter the desired volume.
CC Operation
C and enter the new address. To start CC
Battery Capacity
low medium high
Occlusion Pressure
F and then select 1, 2 or 3.
7
Page 8

Mains/Battery Operation
General Information
Check that the mains voltage corresponds to
that on the main label! In the event of power
failure the device switches automatically to
battery operation. Alternatively, a rechargeable
battery pack from B. Braun can optionally be
used.
To ensure safe and reliable operation certain
rules of application must be noted.
The capacity display is a trend display (low,
medium, high).
The actual capacity available can vary from
this due to
- different battery manufacturers
- temperature
- varying load (e.g. frequent bolus input).
Batteries can explode or leak causing
damage if they
- are opened or burned,
- are wrongly poled,
- are used such that new and old batteries are
placed together,
- used together with a different make of
batteries.
Batteries should be removed from the device
during longer period of non-use (storage > 3
months).
The batteries should be renewed when
- a signal such as "battery empty" or "battery
pre-alarm" is given
- breakdown or interruption occurs in
connection with frequent bolus requirements.
- After a period of use of > 2 years, even if the
capacity display indicator shows "full".
The switch-on test checks whether the
internal energy supply is capable of sounding a
power-failure alarm. If the energy source is
exhausted an alarm acknowledgement is
produced. In this case the operator may only
use the device under constant supervision,
since a power failure would remain undetected
by the device.
Only alkali-manganese batteries may be
placed in the battery compartment.
- The alkali-manganese batteries recommended
are free of mercury and cadmium.
- Conventional carbon-cell batteries are give
an incorrect reading on the capacity display
and cannot therefore guarantee reliable
operation.
- NiCd rechargeable batteries must not be
connected to the battery contact points as
their various physical properties disturb the
alarm.
NiCd rechargeable batteries are available as
an accumulator pack (accessories).
- The accumulator pack is charged by the
Perfusor during mains connection.
8
Page 9

Alarms
CC Operation
Causes of Alarm
Audible alarm: the alarm signal blinks in
cases of alarm.
Battery empty, battery pre-alarm beginning
30 min. before the battery is empty.
Pressure alarm because of an occlusion;
automatic bolus reduction.
Pre-alarm 3 min. before syringe is empty
(only black field is blinking) resp. infusion end.
Reminder alarm if the awaited input has
not been received and pre-alarms
Syringe frame pressure plate has not been
correctly positioned.
+ Pressure alarm, automatic bolus
reduction has been interrupted. Bolus has to be
reduced manually.
Syringe catch at the drive head has not
clicked into place.
Volume alarm, quantity reached.
Eliminate the cause of alarm and then
press the start button. If the alarm sounds again,
contact the service unit.
Interrupts the alarm for a period of 2 min.
Interface Operations
Descriptions of interfaces are available
from B. Braun.
Connection to the input interface (MFC). Two
possibilities are envisaged:
Documentation
All operational data can be called up and
recorded via an external PC.
CC Operation
All functions can be specified using an external
computer. This must satisfy the IEC 601-1 safety
standards and must comply with the conditions
of the IEC 513 single-fault safety requirement.
Check Regularly
Check for cleanliness, completeness and damage.
Operate in accordance with the Instructions for
Use. On switching on, check: the self-check,
alarm signal, operating and alarm-control
displays. Check battery contacts once a year for
corrosion and clean them using a soft rubber.
Hygiene / Waste Disposal
Displays
Special function is active
Mains operation
Vol. preselection active
+ Service mode of operation; blinks
when the service interval has elapsed.
Operation / Running control
Clean using mild soap suds. Do not use spray
disinfectant at the mains connection.
Recommended: disinfectant for wiping available
from B. Braun (e.g. Meliseptol). Before operating
the device allow to air for at least 1 min.
Do not spray into openings in the device. Be sure
to observe the instructions provided on waste
disposal and hygiene.
9
Page 10

Technical Data
Type of unit Infusion Syringe Pump
Classification
Moisture protection IP 22; drip protected for horizontal usage
Rated voltage 230/240 V, 50/60 Hz ~ or
Power input 12 VA / 24 VA
External extra-low voltage 12 V d.c. (e.g. ambulance cars)
Staff call Max. 24 V / 1 A / 24 VA
RFI EN55011
EMC EN60601-1-2
Time of operation 100 % (continous operation)
Operating conditions
- Relative humidity 30 % ... 90 % (without condensation)
- Temperature + 5 °C ... + 40 °C
- Atmospheric pressure 500 mbar ... 1060 mbar
defibrillation proof; CF type
Protection class II
110/120 V, 50/60 Hz ~
Arbitrary connection polarity (VDE 0834)
Storage conditions
- Relative humidity 30 % ... 90 %
- Temperature - 20 °C ... + 55 °C
- Atmospheric pressure 500 mbar ... 1060 mbar
Type of battery pack NiCd (rechargeable)
Operating time of rech. battery > 10 h at
Recharging time > 16 h
Battery 4 x 1.5 V alkali manganese
(Duracell recommended)
Operating life of battery > 80 h at
Weight/Dimensions (WxHxD) Approx. 1.5 kg; 190 x 100 x 120 mm
10
< 10 ml/h
< 10 ml/h
Page 11

Selectable B. Braun syringes 20 ml Original Perfusor Syringe
Delivery rate 0.1 - 99.9 ml/h (in 0.1 ml/h increments)
Bolus rate 800 ml/h
Delivery preselection 0.1 - 999.9 ml in 0.1 ml increments
Accuracy of set delivery rate typ. ± 2,5 %, (measuring time > 1 h and
Occlusion alarm pressure 3 settings (low, medium, high; max. 1.2 bar)
50 ml Original Perfusor Syringe
50 ml Omnifix LL
50 ml Proinjekt
infusion volume > 2 ml)
Alarm in the event of incorrect dosage a) Malfunctions of the device
Syringe table showing minimum lifting volume
required to activate the 3-min prior alarm
For incorrect dosages of > 0.015 ml due to
malfunctions of the device the pump
automatically switches off.
b) At shutdown typ. 1 ml bolus volume at
highest compression phase with 50 ml OPS ^=
max. alarm delay time at 5 ml/h = 6:50 min.
Type Code No. Min. Vol.
OPS 50 ml 50 5,6 ml
Proinjekt 50 ml 51 9,8 ml
Omnifix 50 ml 52 9,2 ml
Euroject 50 ml 61 9,3 ml
B-D Plpak 50/60 ml 61 9,3 ml
Terumo 50/60 ml 54 7,0 ml
Terumo (USA) 60 ml 60 7,3 ml
Monoject (USA) 50/60 ml 62 5,4 ml
Monoject (EU) 50/60 ml 55 9,6 ml
OPS 20 ml 20 5,3 ml
Omnifix 20 ml 22 6,3 ml
Terumo 20 ml 23 4,5 ml
B-D Plpak 20 ml 24 5,3 ml
Monoject (USA) 20 ml 26 6,5 ml
Monoject (EU) 20 ml 29 4,4 ml
^
11
Page 12

Start-up and Trumpet Curves
20 ml OPS
Delivery rate = 1 ml/h
1,5
1
0,5
0 30 60 90 t (min) 120
20 (ml/h) flow
15
10
5
0 30 60 90 t (min) 120
2 (ml/h) flow
1,5
1
0,5
0 30 60 90 t (min) 120 2 5 11 19 p∆t (min) 31
50 (ml/h) flow
37,5
25
12,5
0 30 60 90 t (min) 120
20 ml OPS
Delivery rate = 10 ml/h
50 ml OPS
Delivery rate = 1 ml/h
50 ml OPS
Delivery rate = 25 ml/h
The graphs show the accuracy/uniformity of flow in relation to
time. Allow for the following:
The delivery behaviour or delivery precision is essentially
influenced by the types of (disposable) syringe used.
Significant deviations may be encountered if use is made of
(disposable) syringes other than those stated in the order data.
15% deviation 2 (ml/h) flow
10
5
0
5
10
2 5 11 19 p∆t (min) 31
15% deviation
10
5
0
5
10
2 5 11 19 p∆t (min) 31
15% deviation
10
5
0
5
10
15% deviation
10
5
0
5
10
2 5 11 19 p∆t (min) 31
Trumpet Curves
Measured values for second and last hour in each case.
Measurement interval ∆t = 5 min
Observation interval p ·∆t (min)
Start-up Curves
Measurement interval ∆t = 5 min
20 ml OPS
Delivery rate = 1 ml/h
20 ml OPS
Delivery rate = 10 ml/h
50 ml OPS
Delivery rate = 1 ml/h
50 ml OPS
Delivery rate = 25 ml/h
Measurement duration T = 120 min
Flow Q
i
(ml/h)
12
Page 13

Responsibility of the Manufacturer
Warranty
Manufacturer, assembly and installation
personnel or instructors can only be held
responsible for any effects on device safety,
reliability and performance if
- Installation, expansion work, readjustments,
modifications or repairs are carried out by
personnel authorised by the above and
- The electrical wiring in the room concerned
satisfies the requirements of VDE 0100, 0107
and/or the IEC publications and
- The device is operated in line with the
instructions for use.
- The regular technical inspections are carried
out.
Warranty
B. Braun provides 24 months warranty, as from
the date of delivery, for every Perfusor compact.
This covers repair or replacement of parts
damaged as a result of design/manufacturing
errors or material defects. Modifications or
repairs to the unit undertaken by the owner or
by third parties invalidate the warranty.
The warranty does not cover the following:
Elimination of faults attributable to incorrect/
inexpert handling, or to normal wear and tear
incl. normal batteries and rechargeable batteries.
The CE mark confirms that this medical
product complies with the "Council Directive
on Medical Devices 93/42/EEC" dated
14th June 1993.
B. Braun Melsungen AG
Service
A technical inspection must be carried out on
the Perfusor compact every 2 years, with an
entry being made in the medical product book
in accordance with the checklist.
Service work must be carried out exclusively by
personnel instructed by B. Braun.
13
Page 14

Ordering
Copy and send by post or by fax to:
B. Braun Melsungen AG
Sparte Medical
Postfach 1120
34209 Melsungen
Fax +49-(0) 5661 - 71 - 37 98
Telephone orders:
Tel.: +49-(0)5661-71 - 0
Original Perfusor Syringes
Original Perfusor Syringe 50 ml with draw-off cannula ____0872 8810
Original Perfusor Syringe 50 ml without draw-off cannula ____0872 8844
Original Perfusor Syringe 50 ml with draw-off cannula and particle filter, ____0872 8828
with light protection
Original Perfusor Syringe 50 ml with draw-off cannula and particle filter ____0872 8852
Original Perfusor Syringe 20 ml with draw-off cannula ____0872 8623
Original Perfusor Syringe 20 ml without draw-off cannula ____0872 8615
Original Perfusor Syringe 20 ml with draw-off cannula and particle filter ____0872 8631
Original Perfusor Tubing
Original Perfusor tubing N, made of PVC, with Luer lock connectors, 150 cm ____0872 2960
Original Perfusor tubing L, made of PVC, with Luer lock connectors, 200 cm ____0872 2862
Original Perfusor tubing MR, made of PVC, with Luer lock connectors, 75 cm ____0872 2870
Original Perfusor tubing M, made of PVC,
with loose lock nut on patient end, 150 cm ____0872 2994
Original Perfusor tubing PE, made of PE, with Luer lock connectors, 150 cm ____0872 2935
Original Perfusor tubing S, made of PVC, light-protected,
with Luer lock connectors, 150 cm ____0872 2919
Original Perfusor tubing PES, made of PE,
light-protected, with Luer lock connectors, pressure-resistant, 150 cm ____0872 3010
Original Perfusor tubing MK, made of PVC,
with cannula, with Luer lock connectors, 75 cm ____0872 2889
Original Perfusor tubing, made of PVC,
with sterile filter 0.22 µ, with Luer lock connectors, 200 cm
(not for use together with 20 ml syringes) ____0872 3001
Delivery address:
Qty/Art.no.
14
Page 15

Perfusor compact (230 / 240 V) ____0871 4827
Perfusor compact (110 / 120 V) ____0871 4835
Recommended accessories for the Perfusor compact
Connecting lead for staff call ____0871 1682
Connecting lead for ambulance car (12 V) ____0871 1674
Interface lead with electrical isolation ____0871 1661
Rechargeable battery pack ____0871 4991
Y-lead for central mains power supply for 2 Perfusors ____0870 0109
Ordered by (name):
Date / Signature:
15
Page 16

Material no. 3891 1108, Drawing no. M659 01 01 01 F04a
4/97 Printed on pulp bleached 100 % chlorine-free
16
Sparte Medical
B. Braun Melsungen AG
Postfach 1120
D-34209 Melsungen
Tel +49-(0) 5661- 71 - 0
Fax +49-(0) 5661- 71- 20 44
 Loading...
Loading...