Page 1
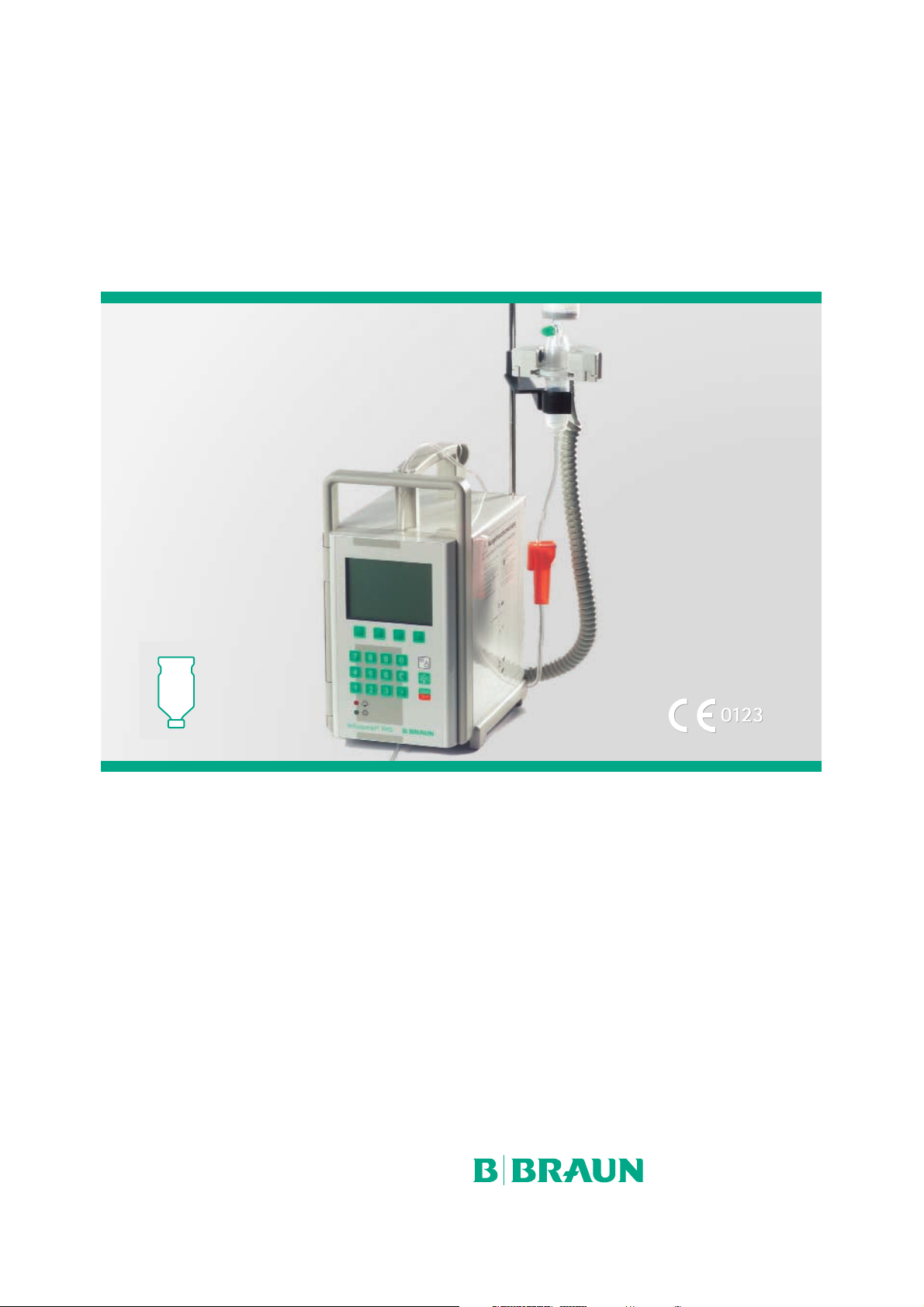
Infusomat®fmS
Instructions for Use
Software IFME/IFMe
Page 2
Attention: Consult accompanying documents!
Operation
➤ Ensure the unit is properly positioned and
secured.
➤ Prior to use check audible and visual alarms
during self test. Also check the device for possible
damage.
➤ If staff call is used we recommend to check the
equipment once after connecting the pump.
➤ Connect to patient only after switching on the
device. Interrupt the connection during changing
administration set(s) to prevent incorrect dose
delivery.
➤ Select cannula/catheter suitable for use with the
intended medical application.
➤ Position the infusion line free of kinks.
➤ Recommended change of disposables after 24 h
(consider national hygiene regulations).
➤ Compare displayed value with entered value.
Start infusion only if values are corresponding.
➤ Installation in medically used rooms must comply
with the appropriate regulations (e.g. VDE 0100,
VDE 0107 or IEC-publications.
➤ Possible explosion hazard if used in presence of
flammable anaesthetics!
➤ Air in line cannot be detected by the air detector
at stop-cocks, infusion ports and additional
administration set components.
Other components
➤ Variations in pressure (e.g. as caused by change
of level) can affect the accuracy of the device.
➤ Where several infusion lines are connected on
one single vascular access the possibility of their
exerting a mutual influence vice-versa cannot be
excluded.
➤ Refer to respective manufacturer’s information
for possible incompatibilities of equipment resp.
drugs.
➤ Use only compatible combinations of equipment,
accessories, working parts and disposables.
➤ It is recommended to use original Infusomat®
infusion lines only.
➤ The use of not recommended resp. incompatible
disposables may influence the technical specification.
➤ Connected electrical equipment must comply
with the relevant IEC/EN-publications
(e.g. IEC/EN 60950 for data-processing equipment).
The user/operator is responsible for the system
configuration if additional equipment is connected.
The international standard IEC/EN 60601-1-1 has to
be taken into account.
Safety Standards
The Infusomat® fmS satisfies all safety standards for
medical electrical devices in compliance with
IEC/EN 60601-1 and IEC/EN 60601-2-24.
➤ The EMC-limits (electro-mangnetic compatibility)
according to IEC/EN 60601-1-2 and IEC/EN
60601-2-24 are maintained. If the equipment is
operated in the vicinity of other equipment which
may cause high levels of interference (e.g. HF surgical
equipment, nuclear spin tomography units, mobile
telephones etc.) maintain the recommended protective distances for these devices. Under certain
conditions malfunctions may occur which lead to a
device alarm with permanent alarm tone (see also
alarm conditions, page 13). Interferences may occur
e.g. at electro-magnetic fields > 10 V/m resp.
electro-magnetic discharges > 8 kV.
Special Function "without drip control”,
see page 10.
Read Instructions for Use prior to use. Application only under regularly supervision by specially trained staff.
Patient Safety
Page 3

Infusomat®fmS
Contents
Infusomat® fmS / Overview Page 4
Operation Page 6
Additional Settings Page 7
Special Functions Page 8
Alarm Conditions Page 13
Start-up Graphs and Trumpet Curves Page 15
Technical Data Page 16
Warranty / TSC*)/ Service / Cleaning Page 18
Ordering Page 19
*
)
Technical Safety Check
The Infusomat® fmS is according to
IEC/EN 60601-1 resp. IEC/EN 60601-2-24 a
volumetric infusion pump for infusion of small
and large volumes at highest accuracy and is
suitable for intravenous and intra-arterial
applications, for blood transfusion and for
enteral nutrition.
The medical specialist has to decide on the
suitability of the application. The decision has
to be made on the basis of the specified
properties and technical data.
For further details please refer to the
Instructions for Use.
3
Page 4

4
Handle
For easy transport.
Holder for Drop Chamber
Prevents unintended
movement (swaying).
Adapter for Drop Sensor
Replaceable, depending on drop
chamber size. Press lateral and pull
off.
Universal Pole Clamp
Attach the Infusomat® fmS from
above, clicking into its place. To
release, press the black button.
Overview
Display
All important information in plain text. Green
background illumination only if connected to
mains or a key is pushed at battery operation.
Correct Input/CLEAR
Display reset to 000.0
Red LED indicates alarm condition
Additionally "AAA.A” is flashing in the display.
Possible alarms: "drop alarm, air alarm, pressure
alarm, pump-door open, battery alarm,
standby-alarm”
Keypad for Input
Operating Indicator
Additional operating control indicator in display.
Page 5

5
Guide for Short Infusion Pole
VOL Infusion Volume
Press key below VOL. Enter volume
(0.1 ... 9999.9 ml). Confirm. Press again key
below VOL.
TIME Infusion Time
Press key below TIME. Enter time e.g. 50 min as
5 0 or 2 h 30 min as 2 3 0. Confirm. Press again
key below TIME.
RATE Delivery Rate
Only active when rate is calculated automatically. The key below RATE confirms the calculated
rate.
SF Special Functions
If activated: dose calculation / bolus function /
standby / drug selection / occlusion pressure /
drop control / piggyback / battery capacity /
data lock / contrast / date, time.
Function Keys
Mains/Power Connection
(protect against ingress of moisture). In case of
mains/power failure, the pump switches to
battery operation. Battery operation time:
> 3.5 h at highest delivery rate. Automatic overload protection. Mains fuse: directly above the
mains/power connector.
Potential Equalisation
To be connected for CF-applications.
Aluminium Housing
Easy-care, drip water protected, resistant to
disinfectants.
Mains/Power Supply
For operation with fluid manager system.
Multi-FunctionConnector (MFC)
Connection for staff
call, ambulances
(12 V DC) and interface for fm anaesthesia/fm intensive.
Optical Interface
Infrared Interface for operation on
"fluid manager system”.
Flow Inhibitor
Opening door clamps off infusion line
automatically.
Peristaltic Pump
For precise and reliable dosage.
Door Opener
Special Functions "SF”
Function Keys (Soft keys)
Mains/Power Switch ON/OFF
Suppress alarm tone for 2 min
Infusion START/STOP
Page 6

Infusion
1. Ensure reliable installation
Never position infusion bottle below pump level.
➤ Connect staff call.
➤ Insert spike vertically into infusion bottle.
Fill lower part of drop chamber to max. 2/3.
➤ Open roller clamp.
2. Filling and Venting
Fill infusion line from bottom to top.
➤ Close roller clamp.
3. Insert Infusion Line
Press door opener.
➤ Insert infusion line: Locate clips first on top,
then on bottom.
➤ Keep infusion line in place at air sensor.
➤ Close door. In the area of the peristaltic
pump segments and free-flow clamp the
infusion line will be positioned self-acting.
➤ Open roller clamp completely. There may not
be a continuous drip.
➤ Place drop sensor on drop chamber
(if necessary, use an appropriate adapter).
4. Switch On with
Green mains/power control or yellow battery
control, alarm tone and display LED’s are briefly
activated.
5. Puncture
6. Setting the Delivery Rate
In the range: 0.1 - 999.9 ml/h and check
(selectable in 0.1 ml/h-increments).
Correction: Press C, then set new rate. Select
additional settings, if desired (see page 7).
7. Press START
Operating symbol appears on the display and
the green operating indicator lights.
8. Stop the infusion
➤ Press STOP. Green operating indicator goes
out
➤ Close roller clamp.
➤ Press door opener. Infusion line will be
clamped off when opening the door.
➤ Remove infusion line. First bottom, then
top.- Replacement: Insert new infusion line as
described. Then press START again.
➤ To end switch off
Press for 2 sec.
Operation
6
Original
Infusomat®
line with
silicone-pump
element
upper clip
free flow clamp
lower clip
air sensor
Page 7

To Change the Delivery Rate
➤ Press START/STOP.
Green operating indicator goes out.
➤ Press C.
Display reset to 000.0
➤ Enter new delivery rate. (No setting possible,
when C has not been pressed.).
➤ Press START/STOP to restart infusion. (Alarm
if no restart within 2 min).
To Change the Rate Without Interrupting the
Infusion (Function can be deactivated by
Service staff)
➤ Enter new rate.
➤ Press key below RATE. Rate is transferred to
the upper level as large numbers.
➤ Pump operates at new rate. (If new rate is
not confirmed within 10 sec, infusion continues
at previous rate.).
Target Volume (Volume Pre-selection)
The target volume will be administered
independent on the infused volume.
➤ Press key below VOL.
➤ Enter target volume via keypad and confirm
with VOL. Values between 0.1 and 9999.9 ml.
After confirming the display shows the residual
volume instead of the target volume.
Note:
When target- / residual volume has been
administered, the device switches to KOR-mode.
Stop pump and press VOL. Clear displayed
residual volume with C (in display appears
target volume ----,- ml) and confirm with VOL.
If desired, enter new target volume.
To continue infusion, the target volume has to
be displayed as ----,- ml or a new target
volume has to be set.
Target Time (Time Pre-selection)
➤ Press key below TIME.
Enter target time via keypad (50 min = 50;
2 h 30 min = 2 3 0).
➤ Confirm: Press key below TIME again,
instead of the target time the residual time is
displayed now.
➤ Correction
Press C. Display ––h––m. New entry.
Note:
When pre-selected time has been expired, the
device switches to KOR-mode. Stop pump and
press TIME. Clear resid. time with C (in display
appears target time ––h––m) and confirm with
TIME.
If desired, enter new target time. To continue
infusion, the target time has to be displayed as
––h––m or a new target time has to be set.
Rate Calculation
(Displayed delivery rate must be 000.0)
➤ Enter volume and time.
The delivery rate is calculated automatically and
displayed (rounded to one decimal place).
➤ Confirm: Press key below RATE.
➤ Start infusion with START/STOP.
Clear Time/Volume
➤ Press STOP, then key below VOL or TIME.
➤ Press C to clear.
1x: Target Vol/Time = ––––.-ml / ––h--m
2x: Infused Volume/Real Time
= 0.0ml / 00h00m
Additional Settings
7
Page 8

KOR-Mode (KVO)
After expiring of the pre-selected time or infusion of the pre-selected volume automatic reduction to keep-open rate (KOR).
➤ KOR and delivery rate flash alternately.
Deactivation and alarm after 30 min.
➤ Switch off with START/STOP.
Status request
Only when infusion is running.
➤ Press 1 x resp. 2 x key below INFO for actual
values.
The display disappears after 10 sec or after all
information has been requested.
➤ Press 3 x key below INFO for battery
capacity in h min and hours of operation.
Additional Settings
8
History Function
In connection with the software “IFME” the
Infusomat fmS is equipped with a history function (memory of events). This permanent
memory records the last 350 events time- and
date related:
- Set delivery rate
- Changes of the rate
- Switching on/off
- Start/Stop of infusion
- Remote Control
- Operating and device alarms
At the moment of an event also the volume
infused from switching on is recorded.
The history function is activated ex works.
After a software-update the function has to be
installed again (service programme: menu 560
calibration history card). With this programme
the function also can be deactivated. A faultless
time-related recording requires exact setting of
time and date.
Recording the data:
The protocol of events only can be transferred
to a computer via interface (MFC interface
lead). For this a terminal programme installed in
the computer has to be selected
(settings: 9600 baud, 1 start – 8 data,
1 stopbit).
For the data transfer the Infusomat® fmS has to
be switched off and connected to mains. The
protocol can be requested with key “##” and
begins with the latest event. Data are screened
in the terminal programme of the computer.
To stop data transfer: Press key below END.
The history function mainly is used for failure
analysis for the technical service. A data
transfer via fm system is not possible.
Special Functions
Page 9

Select Special Mode SF
Depending on the version, several functions may
be deactivated. Contact service.
➤ To set special functions press key below SF
repeatedly until desired special function is
displayed. – Then follow Instructions for Use as
described.
Activate Bolus Function
For additional boli.
➤ Pressing key below ON activates the
function (function is maintained after switching
on again).
T
o change bolus rate:
➤ Press key below RATE. Enter new value and
confirm.
Correction: Press C and re-enter.
Exit bolus function:
➤ Press key below END.
Bolus application during infusion:
Bolus with volume pre-selection
➤ Press key below BOL and release.
Display: BOLUS RELEASE?
➤ Enter bolus. Values between 0.1 and 99.9 ml
(if no entry is made within 10 sec function is
exited automatically).
➤ Press key below YES. Bolus is administered.
After the bolus application the infusion
continues at basic rate.
T
o stop bolus:
➤ Press key below STOP.
Bolus without Volume Pre-selection
➤ Press and hold BOL until a second BOL is
displayed.
➤ Hold both BOL-keys. Bolus is administered
as long as both keys are pressed. Per ml bolus
administered a short audible signal sounds.
Interval bolus
Automatic bolus in set time interval. In case of
manual bolus administration the interval bolus
is skipped.
➤ Select bolus function under SF.
➤ Press key below VOL, TIME or RATE. Enter
values and confirm. After value below TIME has
been confirmed, interval times runs automatically. Exit function with END, set basal rate and
start. Remaining interval time is displayed in
h:min:sec.
A bolus on demand is possible at any time
during infusion at basal rate.
Bolus and dosage calculation
➤ Operation as in volumetric mode.
Depending on the settings in ml (volume),
quantity of active agent (e.g. mg) or in quantity
of active agent per kg weight (mg/kg) the bolus
may be administered. During bolus administration all three values are displayed.
Standby/Pause
In case of extended interruptions set values are
retained.
➤ Press STOP.
➤ Press key below SF until "Standby” is
displayed.
➤ Press key below ON.
➤ Enter length/duration of interruption interval
or confirm time displayed.
Correction: Press C. Display 00h 00m.
New entry.
➤ Confirm TIME. Timer for interruption on
display is running. Alarm at the end of
interruption interval.
➤ End of interruption interval: Press key below
END.
Special Functions
9
Page 10

Drug Display
9 selectable drug names can be stored (input via
service program only).
➤ Select with key below (+). Drug is displayed,
also during infusion.
➤ Key below CLR deletes drug name from
display.
➤ Exit selection: Press key below END.
Occlusion Pressure
Due to variable pressure limits shortened alarm
times are possible. Occlusion pressure
high/medium/low. See Technical Data.
➤ Select pressure with key below (+) or (-).
➤ Exit selection: Press key below END (select
pressure as low as possible).
Switch off Drop Control
Caution:
- No alarm will be emitted if the drop control
is switched off and the roller clamp is closed
(underdosage).
- No alarm will be emitted in case of occlusion within the infusion line and pressure sensor
fails.
- Drop control may only be switched off
where underdosage is not critical for the patient
or where the patient is maintained under
constant observation.
Operation without drop control only with volume pre-selection:
➤ Set infusion volume.
(The volume in the infusion bottle has to be sufficient!).
➤ Press key below SF as many times as
necessary for “drop control” to appear.
➤ Press key below OFF. “no drop control”
appears.
➤ To switch on again: Press key below ON.
➤ Exit from selection: Press key below END.
Check Battery Capacity
Remaining battery life time is displayed,
e.g. battery capacity = 02 h 30 min.
In addition the operating hours are displayed.
To switch off display again: Press key below
END.
➤ With device switched off and mains/power
lead detached: Briefly press . Short display
of remaining battery life time after 3 sec. -
Device switched off and mains/power lead
connected: Permanent display of remaining battery life time.
Battery replacement is recommended if a
remaining life time of less than 2 h is displayed
after 16 h charging time.
Data Lock
Interlocks keys to prevent unauthorised use.
➤ Press key below ON. Keys are interlocked.
➤ Key release: Press decimal point key, then
key below END.
Loudness control (only Software IFME)
The loudness of the audible alarm can be adjusted in 9 steps.
➤ Increase with key below (+) resp. decrease
with key below (-).
➤ Exit selection: Press key below END.
Set Date/Clock
➤ Set date: Press key below DAT, enter date
and confirm with DAT.
➤ Set time: Press key below TIME, enter time
and confirm with TIME.
➤ Exit selection: Press key below END.
Special Functions
10
Page 11

Dosage Calculation (Overview)
The dosage calculation automatically calculates
the delivery rate in ml/h.
Setting parameters:
1. Concentration
- per ml or
- quantity per volume of infusion bottle.
Entry: mcg, mg, IU or mmol, each from 0.001 to
99999 (5-digit, decimal point counts as one
digit).
2. Selection for weight- and time-related or
only time-related dosage. Entry of body weight:
from 0.01 kg to 200 kg.
3. Entry of dosage:
a) Weight- and time-related in mcg/kg,
mg/kg, IU/kg, mmol/kg, each per /min, /h
or /24 h.
b) Only time-related in mcg, mg, IU or mmol,
each per /min, /h or /24 h.
Dosage Calculation (Operation)
➤ Press key below SF repeatedly until DOSAGE
CALC.OFF appears.
➤ Press key below ON.
➤ Select quantity unit.
Select with key below mcg.
Note: After numerical entry change of quantity
unit is not possible anymore.
(Remedy: delete numbers).
➤ Enter concentration by moving flashing star
to desired entry position with arrow keys.
Confirm all numbers with OK.
Entry of concentration per 1 ml or per volume
of infusion bottle.
➤ For dosage by body weight enter body
weight and confirm. Otherwise confirm 0 kg.
➤ Select desired quantity- and time unit.
➤ Enter values (at flashing star as described)
and confirm with OK. Automatically calculated
values (rate or dosage) are displayed.
➤ First check rate displayed for plausibility,
then confirm RATE. Value is displayed.
➤ Start infusion.
Note: Dosage value with unit is displayed (down
on the right). As the automatically calculated
value of the rate is rounded, the dosage value
may change insignificantly.
Info Request
➤ Press key below INFO.
1x: Infused Volume, Run Time
2x: Infused Volume, Actual Dose
3x: Battery Capacity, Operating Hours.
Change Rate / Dosage
➤ Press STOP.
➤ Enter new dosage value and confirm with
RATE.
➤ Start infusion again.
Change Rate or Dosage without Interruption
of Infusion
During infusion a star is flashing down on the
right.
➤ Enter new value and confirm with RATE.
Pump operates with new rate / dosage.
Note: Alternatively the star can be moved with
the arrow key to change the rate in ml/h.
Change Concentration with Dosage
Calculation Activated
➤ Press key below SF repeatedly until DOSAGE
CALC.ON appears and confirm with OK.
➤ Press CLR.
Concentration is cleared.
➤ Enter new value and confirm.
Change dosage
➤ Press key below SF repeatedly until DOSAGE
CALC.ON appears and confirm with OK.
➤ Change values (move flashing star as
described) and confirm entries with OK.
11
Page 12

➤ Check entered or calculated rate for plausi-
bility and confirm with key below RATE. Rate is
displayed.
➤ Start infusion.
Switch off Dosage Calculation
The dosage calculation remains activated until it
is switched off in Special Functions. If the
Infusomat® fmS is switched off in the meantime
all previous values except the body weight are
maintained.
Switch off from Basic Menu
➤ Press key below SF, display DOSISCALC.ON.
➤ Press DOSISCALC.OFF.
Dosage calculation is deactivated.
➤ With key below END back to basic menu.
Piggyback Function
The piggyback-mode offers the possibility to
interrupt the current (primary) infusion temporarily in order to administer a piggyback
(secondary) medication. Above the pump the
piggyback-infusion line (8250740) is connected
with a Y-connector to the administration set
(Infusomat® line 8250715). Close clamp of primary infusion.
All infusion lines must be primed.
➤ Select Special Function Piggyback with key
SF.
➤ Key below ON activates function (remains
active also after switching on the Infusomat®
again).
➤ Enter primary rate and volume and confirm.
The softkey PIGY only will be displayed after a
target volume had been entered before.
➤ Press key below PIGY, enter piggyback rate
and volume and confirm.
➤ Start infusion. Pump delivers the piggyback
volume with the set piggyback rate.
As soon as the programmed piggyback volume
has been infused, delivery continues with “keep
open-rate”(KOR) resp. after 30 min KORoperation the pump stops and activates an
alarm. The operator must switch over to the
primary infusion manually. Close clamp of
secondary medication and re-open clamp of
primary infusion.
Please note: The Piggyback mode requires the
input of a target volume resp. a target time for
the primary as well as for the secondary infusion. It also is possible to start with the primary
infusion (after entering the Piggyback values
switch back to primary with END). In STOP
mode it is always possible to switch over between Piggyback and primary mode.
Special Functions
12
Secondary infusion
e.g. 100 ml
bag volume with
delivery rate 10 ml/h
Primary Infusion
e.g. 1000 ml
bag volume with
delivery rate
25 ml/h
clamp
Piggyback line 8250740
Infusomat line 8250715
clamp
Y-connector
Drop chamber
Roller clamp
Infusion pump
to patient
100 ml
1000 ml
Page 13

Operating alarms
Remedy failure and restart infusion.
Drop Alarm / Pressure Alarm
➤ Infusion bottle empty?
➤ Roller clamp closed?
➤ Flow? – Close roller clamp.
Infusion STOP. There may not be a continuous
drip. Insert new infusion line, if necessary.
➤ Occlusion? – Insert infusion line free of kinks
and check the free flow (consider bolus).
➤ Condensed drop chamber? Shake to remove.
➤ Drop sensor fitted/connected? Replace drop
sensor, if necessary.
Air Alarm
➤ Air in administration set? – Insert infusion
line correctly. Vent and reset fluid level in drop
chamber.
Standby Alarm
➤ Alarm after pre-selected pause?
Switch to Standby with key below SF.
Then end pause with key below OFF or extend
pause with key below ON.
Battery Pre-Alarm
➤ Battery pre-alarm 30 min before battery is
discharged:
- Alternately rate and AAA.A are displayed,
- battery indicator flashes,
- audible alarm every 9 sec.
Alarm can be cleared by pressing -key. The
alarm continues in short intervals until the battery is fully discharged.
➤ Battery Alarm:
- Alternately rate and AAA.A are displayed.
- The text display shows:
"battery discharged, connect to mains”.
- battery indicator flashes,
- red alarm indicator is on,
- audible alarm every 4 sec.,
- operating indicator off,
- staff call.
➤ Switch off device.
Connect to mains/power or 12 V DC power
source.
KOR-Alarm (KVO)
Pre-alarm: audible alarm every 9 sec max.
30 min. End of infusion alarm: Permanent
interval signal tone (also via staff call).
Delay of Alarm Tone
➤ When connected to staff call the alarm tone
on the device can be suppressed for 10 min.
(This function can be activated via service only.)
Further Alarms / Displays
➤ "pump door open”? – Close door.
➤ "invalid rate”? – Enter different value.
Device Alarms
When display indicates "device alarm” an
audible alarm sounds permanently.
➤ Press ON/OFF-key repeatedly until display
indication "do not press any key until display is
off”. Pump switches off automatically after a
few seconds.
➤ Switch on device again.
In case of repeated device alarm inform service.
Alarms
13
Page 14

Mains Operation, 12 V DC or Battery
➤ Check mains voltage as per type plate!
➤ Plug in mains/power lead at rear (screw in
12 V DC lead in ambulance car).
➤ In case of mains/power failure or if 12 V DC
or mains/power is not connected the unit automatically will be switched to integrated
rechargeable battery.
Charge Battery
➤ Charge battery in case of:
- first use
- battery alarm
- non-use > 2 months.
Battery is charged if 12 V DC or mains are
connected – even during infusion.
Charging Time
➤ Approx. 16 hours. Longer charging is not
detrimental.
Capacity
A fully charged battery is sufficient for more
than 3.5 h with highest delivery rate.
Rechargeable Batteries Ageing
After 2 years the original capacity is only
approx. 50 %.
➤ The lifetime of the battery can be extended
by completely discharging from time to time
and recharging afterwards.
Interface
Interface Operation
Connection to interface input via MFC-plug.
Interface descriptions available from B. Braun.
➤ Send Proposal
The delivery rate and a drug can be entered in
the Infusomat® fmS as "proposal” via an
external computer. Both items of data must be
checked on the Infusomat fmS and acknowledged.
➤ Remote Control
Via fm controller possible. When using a commercially available external computer this must
satisfy the requirements acc. to IEC/EN 60601-1
as well as the single-fault fail-safe condition
acc. to IEC/EN 60513.
➤ Documentation
All operating data of the Infusomat® fmS can be
requested and logged via external computer.
Alarms
14
Page 15

Start-up Graphs and Trumpet Curves
15
The graphs show the accuracy/uniformity of flow in relation to
time. Allow for the following:
The delivery behaviour resp. delivery accuracy is essentially
influenced by the types of disposables used.
Significant deviations may be encountered if use is made of
disposables others than those stated in the order data.
Trumpet Curves
Measured values for second and last hour in each case.
Measurement interval ∆t = 0.5 min
Observation interval p x ∆t min
Start-up Graphs
Measurement interval ∆t = 0.5 min
Measurement duration T = 120 min
Flow Q
i
ml/h
Start-up Graphs Trumpet Curves
2 (ml/h) flow
1,5
1
0,5
0
50 (ml/h) flow
37,5
25
12,5
0
(ml/h) flow
150
100
30 60 90 t (min) 120
30 60 90 t (min) 120
Delivery rate = 1 ml/h Delivery rate = 1 ml/h
Delivery rate = 25 ml/h
Delivery rate = 100 ml/h
deviation
50
0
50
2
10 deviation
5
0
-5
2
10 deviation
5
0
Epmax
Epmin
51119p∆t (min) 31
Delivery rate = 25 ml/h
Epmax
Epmin
51119p∆t (min) 31
Delivery rate = 100 ml/h
Epmax
Epmin
50
0
30 60 90 t (min) 120
-5
2
51119p∆t (min) 31
Page 16

16
Technical Data
Type of unit Volumetric infusion pump
Classification (acc. to IEC/EN 60601-1) defibrillator-proof, CF equipment
Protection Class I;
IP 22 (Moisture protection: drip protected)
Class (acc. to Directive 93/42 EEC) II b
Rated voltage / current 230 V AC~ (0.06 A), 50/60 Hz
Mains fuse T 0.16 A or
200 V/230 V/240 V AC~ * (0.06 A), 50/60 Hz
Mains fuse T 0.16 A or
100 V/110 V/120 V AC~ * (0.12 A), 50/60 Hz
Mains fuse T 0.315 A
* Mains voltage can be selected at appliance inlet.
External extra-low voltage 12 V DC
Staff call Max. 24 V / 1 A / 24 VA
Arbitrary connection polarity (VDE 0834)
EMC EN 55011
IEC/EN 60601-1-2 and IEC/EN 60601-2-24
Time of operation 100 % (continuous operation)
Operating conditions
-Relative humidity 30 % ... 90 % (without condensation)
-Temperature + 10 °C ... + 40 °C
-Atmospheric pressure 700 mbar ... 1060 mbar
Storage conditions
-Relative humidity 10 % ... 90 %
-Temperature - 25 °C ... + 55 °C
-Atmospheric pressure 500 mbar ... 1060 mbar
Battery type (rechargeable) NiCd (7.2 V; 1.2 Ah)
Operating time of rech. battery > 3.5 h at highest delivery rate
Recharging time > 16 h
Weight / Dimensions (WxHxD) Approx. 3.1 kg / 140 x 240 x 200 mm
Page 17

Air detector Technical sensitivity
Air bubbles > 0.01 ml
Alarm triggering: With air bubble size
of typ. 0.3 ml
1)
(limit value 0.4 ml) or 1.5 ml/h
2)
(cumulative value of 1 h as of air bubble
volume 0.01 ml)
1)
Can be set from 0.01 to 0.3 ml via service program only
2)
Can be set from 0.5 to 3.5 ml/h via service program only
Accuracy of set delivery rate typ. ± 5 % measured values of second hour acc.
to IEC/EN 60601-2-24
Delivery range 0.1 ... 999.9 ml/h (0.1 ml/h-increments)
Delivery pre-selection 0.1 ... 9999.9 ml (in 0.1 ml-increments)
Occlusion alarm pressures
Alarm reaction time
Max. bolus volume
(measured at 22 °C with OIL infusion set)
Mechanical occlusion pressure limit Occlusion alarm pressure max. 1.6 bar (160 kPa)
under fault conditions max. bolus volume 2 ml
Alarm in case of incorrect dosage In case of incorrect dosage of max. 0.6 ml due
to apparatus malfunction the pump switches off
automatically.
KOR-rate (KVO) Delivery rate > 10 ml/h = 3 ml/h
Delivery rate < 10 ml/h = 1 ml/h
Delivery rate < 1 ml/h = STOP
17
low medium high
rate app. 0.4 bar app. 0.8 bar app. 1.2 bar
1 ml/h 15 min 21 min 30 min
25 ml/h 36 sec 52 sec 72 sec
100 ml/h 9 sec 13 sec 18 sec
0.25 ml 0.35 ml 0.5 ml
Page 18

Responsibility of the Manufacturer
The manufacturer, assembler, installer or
importer considers himself responsible for the
effects on safety, reliability and performance of
the equipment only if:
• assembly operations, extensions, re-adjustments, modifications or repairs are carried
out by persons authorised by him,
• the electrical installation of the relevant
room complies with the appropriate requirements (e.g. VDE 0100, 0107 and/or the IECpublications resp. national requirements),
• the equipment is used in accordance with
the Instructions for Use and
• the Technical Safety Checks are carried out
regularly.
Warranty
B. Braun provides as from the date of delivery a
warranty of 2 years for every Infusomat® fmS.
This covers repair or replacement of parts damaged as a result of design/manufacturing errors
or material defects. Modifications or repairs to
the unit undertaken by the owner or by third
parties invalidate the warranty.
The warranty does not cover the following:
Elimination of faults attributable to
incorrect/inexpert handling, or to normal wear
and tear and rechargeable batteries.
Technical Safety Check*)/ Service
The Technical Safety Check is recommended to
be carried out every 2 years and should be
documented.
Servicing work must be carried out exclusively
by personnel instructed by B. Braun.
Check regularly
Check for cleanliness, completeness and
damage. Use only according to Instructions for
Use. Check each time when switching on: selfcheck, audible alarm, process- and alarm control
indication.
Cleaning
Clean using mild soap suds. Do not use spray
disinfectants at the mains power connection.
Recommended: disinfectant for wiping available
from B. Braun (e.g. Meliseptol®).
Before operation the device allow to vent for at
least 1 min. Do not spray into openings in the
device. Be sure to observe the instructions
provided concerning waste disposal and hygiene
for batteries and disposables.
18
Warranty / TSC
*
)
/ Service / Cleaning
The CE mark confirms that this medical product complies with the "Council Directive on
Medical Devices 93/42/EEC” dated 14
th
June
1993.
B. Braun Melsungen AG
Inspection on Delivery
Despite careful packaging, the risk of transport
damage cannot be entirely prevented. Upon
delivery, please check that nothing is missing.
Do not use a damaged device. Contact the
service department.
Items included
Infusomat® fmS, Power Cord, Drop Sensor, Pole
Clamp, Instructions for Use.
Page 19

19
Ordering
Art.-Nr.
Infusomat® fmS 230 V 871 5548
Infusomat® fmS 200 - 240 V 871 5440
Infusomat® fmS 100 – 120 V 871 5416
Recommended accessories for Infusomat® fmS
Connecting lead for potential equalisation 870 1628
MFC-Connecting lead for staff call 871 1682
MFC-Connecting lead for ambulance cars (12 V DC) 871 1674
MFC-RS 232 interface lead with electrical isolation 871 1661
Short stand with drop chamber holder 870 1644
Original-Infusomat®-infusion set CVP
with line for CVP-measuring, 340 cm 870 0010
Original-Infusomat®-infusion line 250 cm 870 0036
Original-Infusomat®-infusion set 5 µm
with 5 µm-filter, 275 cm 870 0052
Original-Infusomat®-infusion set K
with injection port, 270 cm 870 0087
Original-Infusomat®-infusion line S
black for light-sensitive drugs, 250 cm 870 0125
Original-Infusomat®-infusion set E
for enteral nutrition with bottle connection
total length 250 cm, pressure-proof 873 1934
Original-Infusomat® infusion line
with Y-connector for Piggyback mode 825 0715
Piggyback-connection infusion line 825 0740
Page 20

Material-No. 3891 2341, Drawing No. M671010100F04
05/01 Printed on pulp bleached 100 % chlorine-free
B. Braun Melsungen AG
P.O.Box 11 20
D-34209 Melsungen
Tel. +49 (0) 56 61 – 71-0
Fax + 49 (0) 56 61 – 71-20 44
HOSPITAL CARE
 Loading...
Loading...