Page 1

ZOLL Ventilator Operator’s
Guide
Models: EMV+, AEV, Eagle II
906-0731-01-05 Rev. B
Page 2
The issue date for the ZOLL Ventilator Operator’s Guide (REF 906-0731-01-05 Rev. B) is May, 2017.
0123
ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA USA
01824-4105
ZOLL International Holding B.V.
Newtonweg 18
6662 PV ELST
The Netherlands
Copyright © 2017 ZOLL Medical Corporation. All rights reserved. ZOLL, AEV, and EMV+ are registered
trademarks of ZOLL Medical Corporation in the United States and/or other countries. Eagle II and Smart Help
are trademarks of ZOLL Medical Corporation in the United States and/or other countries. All other trademarks
are the property of their respective owners.
Masimo Pulse Oximeter
This device uses Masimo SET® tec hnology to provide conti nuous pulse oximeter and heart rate monitoring and
is covered under one or more of the following U.S.A. patents: 5,758,644, 5,823,950, 6,011,986, 6,157,850,
6,263,222, 6,501,975 and other applicable patents listed at www.masimo.com/patents.htm
.
Limited Copyright Release
Permission is hereby granted to any military/governmental agency to reproduce all materials furnished herein
for use in a military/governmental service training program and/or other technical training program.
Page 3

Chapter 1 General Information
PRODUCT DESCRIPTION .................................................................................................. 1-1
HOW TO USE THIS MANUAL............................................................................................. 1-1
OPERATOR’S GUIDE UPDATES ......................................................................................... 1-2
UNPACKING .................................................................................................................... 1-2
ASSEMBLY ...................................................................................................................... 1-2
SYMBOLS ON THE VENTILATOR1-..................................................................................... 1-2
SYMBOLS ON THE VENTILATOR’S GRAPHICAL USER INTERFACE (GUI)............................... 1-5
CONVENTIONS ................................................................................................................ 1-7
ABBREVIATIONS .............................................................................................................. 1-8
ZOLL VENTILATOR INDICATIONS FOR USE........................................................................ 1-9
WARNINGS.....................................................................................................................1-11
FDA TRACKING REQUIREMENTS .................................................................................... 1-16
SOFTWARE LICENSE...................................................................................................... 1-17
SERVICE....................................................................................................................... 1-19
Chapter 2 Product Overview
ZOLL VENTILATOR MODELS ............................................................................................ 2-2
ZOLL VENTILATOR FEATURES ......................................................................................... 2-3
ZOLL VENTILATOR DEVICE DESCRIPTION ........................................................................ 2-4
CONTROLS AND INDICATORS............................................................................................ 2-6
DISPLAY SCREEN ............................................................................................................ 2-7
PNEUMATIC DESIGN ........................................................................................................ 2-9
FRESH GAS INTAKE AND ATTACHMENTS ......................................................................... 2-10
TOP PANEL ................................................................................................................... 2-10
VENTILATOR CIRCUITS................................................................................................... 2-12
PULSE OXIMETER SENSORS .......................................................................................... 2-14
POWER SOURCES......................................................................................................... 2-14
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1
Page 4

Chapter 3 Setting Up the ZOLL Ventilator
1. ATTACH THE VENTILATOR CIRCUIT ............................................................................... 3-2
2. ATTACH THE HIGH PRESSURE OXYGEN SUPPLY (OPTIONAL) ......................................... 3-3
3. INSPECT FRESH GAS INTAKE FILTERS .......................................................................... 3-3
4. CONNECT FRESH GAS INTAKE ATTACHMENTS............................................................... 3-4
5 SELECT THE VENTILATOR’S POWER SOURCE ................................................................ 3-5
6. POWER ON THE VENTILATOR....................................................................................... 3-7
7. SELECT START UP DEFAULT VALUES ........................................................................... 3-8
8. SELECT OPERATING MODE (OPTIONAL) ..................................................................... 3-10
9. CHANGE PARAMETER VALUES ....................................................................................3-11
10. CHANGE VENTILATOR SETTINGS ...............................................................................3-11
11. PERFORM OPERATIONAL TEST................................................................................. 3-12
12. ATTACH THE PULSE OXIMETER PROBE (OPTIONAL) .................................................. 3-13
13. ATTACH PATIENT ..................................................................................................... 3-13
Chapter 4 Using the ZOLL Ventilator
THE VENTILATOR INTERFACE ........................................................................................... 4-2
PARAMETER BUTTONS..................................................................................................... 4-3
MODE............................................................................................................................. 4-3
BPM (BREATHES PER MINUTE) -- TIMING AND RATE MANAGEMENT .................................. 4-5
VT (TIDAL VOLUME) ........................................................................................................ 4-8
PIP (PEAK INSPIRATORY PRESSURE) -- LUNG PRESSURE MANAGEMENT........................... 4-9
FIO2 (FRACTION OF INSPIRED OXYGEN) -- OXYGEN DELIVERY MANAGEMENT..................4-11
SPO2 -- USING THE PULSE OXIMETER........................................................................... 4-12
HR (HEART RATE) ........................................................................................................ 4-15
MANAGING POP UP MESSAGES..................................................................................... 4-16
MANAGING ALARMS....................................................................................................... 4-17
SILENCING ALARMS....................................................................................................... 4-21
2 www.zoll.com 906-0731-01-05 Rev. B
Page 5

Chapter 5 Alarms
ALARM OVERVIEW .......................................................................................................... 5-2
ALARM PRIORITIES.......................................................................................................... 5-4
MUTING ALARMS ............................................................................................................. 5-5
ALARMS TYPES............................................................................................................... 5-5
ALARM GROUPS ............................................................................................................. 5-6
HIGH PRIORITY ALARMS .................................................................................................. 5-8
MEDIUM PRIORITY ALARMS............................................................................................ 5-12
LOW PRIORITY ALARMS ................................................................................................. 5-18
POP UP MESSAGES ...................................................................................................... 5-26
Chapter 6 Operating Environments
USING THE ZOLL VENTILATOR IN HARSH ENVIRONMENTS ................................................ 6-1
USING THE ZOLL VENTILATOR IN HAZARDOUS ENVIRONMENTS ........................................ 6-4
USING THE ZOLL VENTILATOR IN AN MRI ENVIRONMENT ................................................. 6-7
Chapter 7 Maintenance
INSPECTING THE ZOLL VENTILATOR ................................................................................ 7-1
CLEANING....................................................................................................................... 7-2
GAS INTAKE FILTERS....................................................................................................... 7-3
REPLACING THE ZOLL VENTILATOR’S FILTERS ................................................................. 7-4
BATTERY MAINTENANCE.................................................................................................. 7-5
CALIBRATION CHECKS..................................................................................................... 7-8
TROUBLESHOOTING......................................................................................................... 7-9
Appendix A Specifications
GENERAL........................................................................................................................A-1
PULSE OXIMETER ...........................................................................................................A-3
DEVICE CLASSIFICATION..................................................................................................A-4
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3
Page 6

Appendix B Accessories
Appendix C
Pulse Oximeter Principles
Appendix D Patient Circuits
PEDIATRIC/ADULT, SINGLE-LIMB, WYE PATIENT CIRCUITS .................................................D-2
INFANT/PEDIATRIC, SINGLE-LIMB, WYE PATIENT CIRCUITS ................................................D-8
4 www.zoll.com 906-0731-01-05 Rev. B
Page 7

This chapter provides general information about the ZOLL ventilator and the ZOLL Ventilator
Operator’s Guide, which we provide with this product. Specifically, this chapter provides
• A brief desc ription of the ZOLL Ventilator.
• Information about this manual (ZOLL Ventilator Operator’s Guide).
• A table th at desc ri bes the symbols that appear on the ventilator and in this manual.
• The ZOLL Ventilator’s Indications for Use.
• A list of Warnings and Cautions regarding the use of the ventilator.
• Information regarding FDA tracking requirements, and the product’s warranty and software
license.
• How to contact ZOLL Medical Corporation for service to this product .
Product Description
The ZOLL Ventilator is a small, extremely durable, full-featured portable mechanical ventilator
designed to operate in hospitals or severe and under-resourced environments. It can be used in
prehospital, field hospital and hospital settings.
Chapter 1
General Information
How to Use this Manual
The ZOLL Ventilator Operat or’s Guide provides information that operators need for the safe
and effective use and care of the ventilator. It is import ant that all persons using this device rea d
and understand all the information contained within.
Please throughly read the warnings section.
Procedures for unit care are located in Chapter 7, “Maintenance”.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-1
Page 8
GENERAL INFORMATION
Operator’s Guide Updates
An issue or revision date for this manual is sh own on the front cove r. If more than 3 years have
elapsed since this date, contact ZOLL Medical Corporation to determine if additional product
information updates are available.
All users should carefully review each manual update to understand its significance and then
file it in its appropriate section within this manual for subsequent reference.
Product documentation is available through the ZOLL website at www.zoll.com. From the
Products menu, choose Product Manuals.
Unpacking
Carefully inspect each container for damage. If the shipping container or cushion material is
damaged, keep it until the contents have been checked for completeness and the instrument has
been checked for mechanical and electrical integrity. If the contents are incomplete, if there is
mechanical damage, or if the ventilator doe s not pass its Self Test, U.S.A. customers should call
ZOLL Medical Co rporation (1 -978-421-965 5). Customers out side of the U .S.A. should co ntact
the nearest ZOLL authorized representative. If the shipping container is damaged, also notify
the carrier. If there is no apparent sign of mechanical damage, read instructions contained
within this manual before attempting operation.
Assembly
The unit only requires that you attach the breathing circuit to begin ventilation using either
internal or external power. Both the ventilator and breathing circuit are supplied clean and are
ready for use on a patient.
Symbols on the Ventilator
The following symbols appear on the ventilator or in this manual:
Symbol Description
Off
On
Direct Current: Identifies the location to connect external DC Power.
Mute / Cancel: Identifies button which mutes the active alarms or cancels
the parameter selection.
1-2 www.zoll.com 906-0731-01-05 Rev. B
Page 9
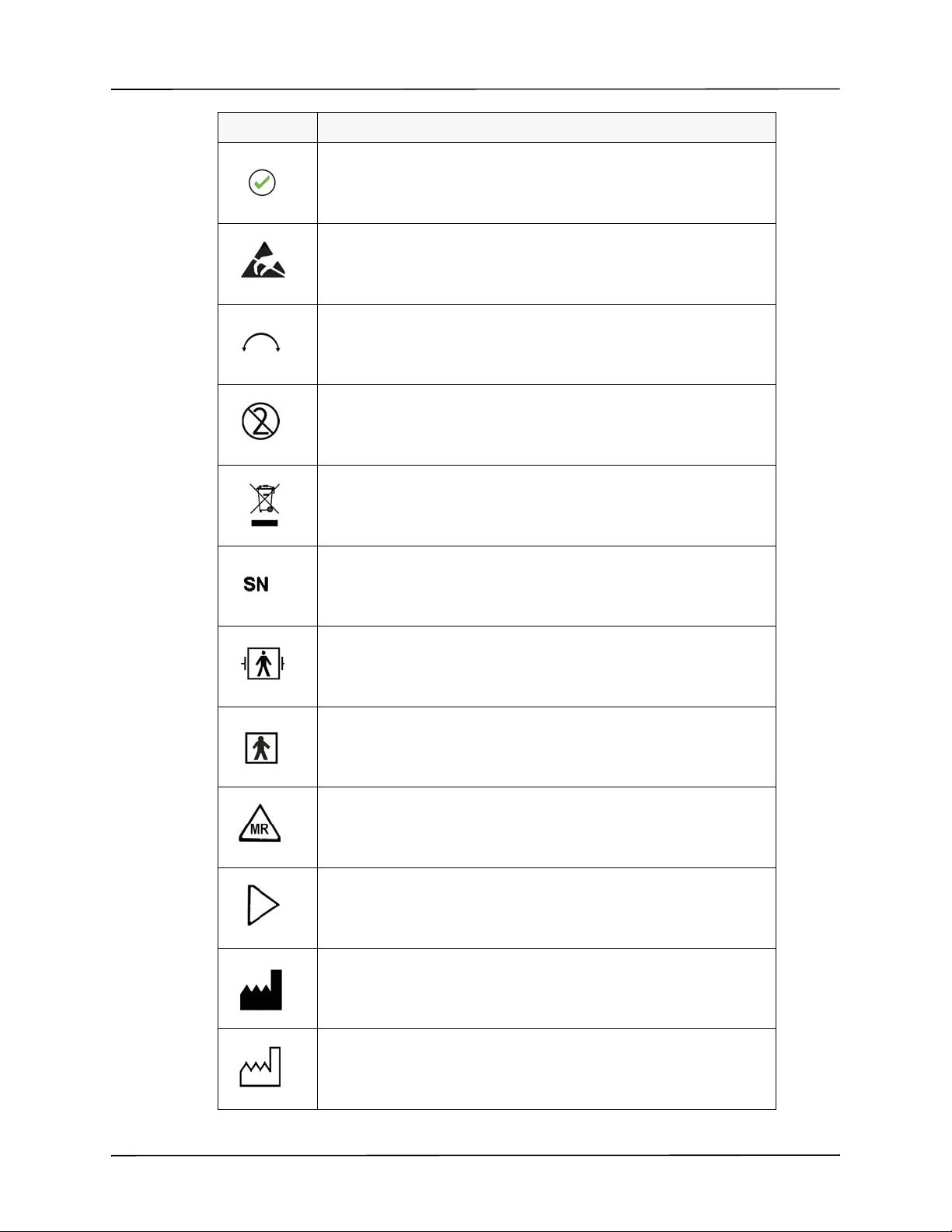
Symbol Description
Accept / Confirm: Identifies button which accepts the parameter
selection.
ESD: Warns that connector pins should not be touched.
Identifies the dial that allows the selection of parameter values.
Do Not Re-Use: This item should not be re-used.
Do Not Discard: Follow all governing regulations regarding the disposal
of any part of this medical device.
Serial Number: Numbers following “SN” indicate the serial number.
Defibrillation Proof: Indicates the degree of protection against electrical
shock.
BF Symbol: Protection against electric shock, Type B with floating
(F-type) parts.
MR Symbol: Identifies the use of the device’s ability to perform in a MRI
environment.
Power Input Orientation: Locates the DC input identifying its point of
insertion.
Manufacturer: This symbol shall be adjacent to the name and address of
the manufacturer.
Manufacturer Date: Manufacturer Date Symbol identifies the device’s
date of manufacture.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-3
Page 10
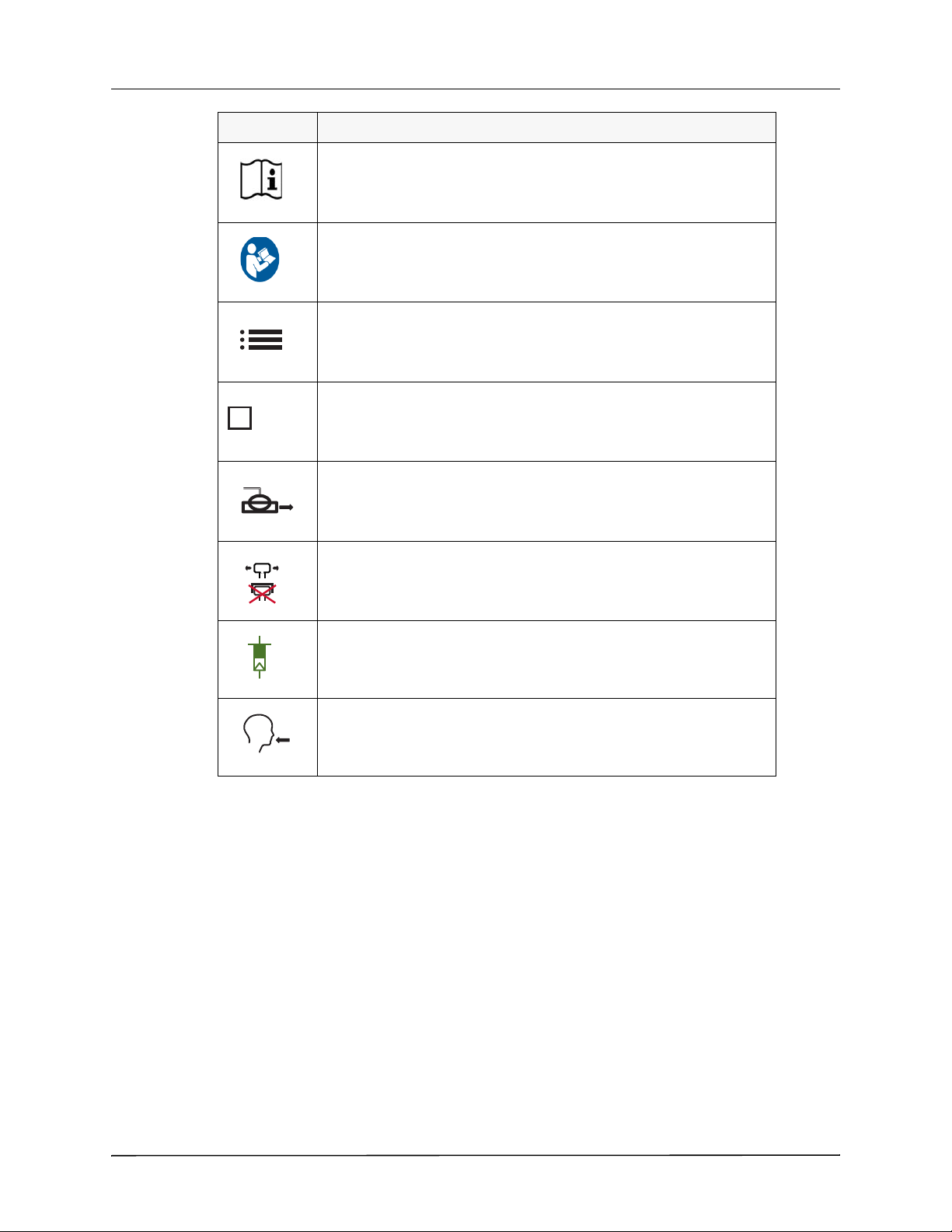
GENERAL INFORMATION
280 - 600 kPa
(40 - 87 PSIG)
O
2
NOT OCC
Symbol Description
Consult Instruction: Consult the instructions for use or operation manual.
Refer to instruction manual.
Menu icon. This icon identifies the button that, when pressed, displays a
menu of options that you can select to configure the ventilator.
High Pressure O
Connector (top faceplate icon).
2
Exhalation Valve (top faceplate icon).
Exhaust Do Not Occlude (top faceplate icon).
Transducer (top faceplate icon).
Gas Output -- Patient Circuit Connector (top faceplate icon).
1-4 www.zoll.com 906-0731-01-05 Rev. B
Page 11

Symbols on the Ventilator’s Graphical User Interface (GUI)
+
LC
LC
_ _ _
_ _
The following symbols appear on the ventilator’s Graphical User Interface (GUI):
Symbol Description
Heart: Provides indication that the pulse oximeter is in use.
Alarm Bell: Identifies the number of off-screen alarms
Alarm Bell Outline: Identifies alarm limit settings; Identifies the on-screen
alarms.
O2 reservoir mode is in use.
Leak Compensation (LC) feature is ON.
Leak Compensation Feature is OFF.
Patient Detect Mode: Backup Ventilation Started.
Not receiving a reading.
Attention: High Priority Alarm Active.
Caution: Medium Priority Alarm Active.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-5
Page 12

GENERAL INFORMATION
EXT
BATT
off
on
Symbol Description
Warning: Low Priority Alarm Active.
Mute: Active Alarm Audible Signal Muted.
Speaker: Active Alarm Audible Signal
Oxygen Supply: Oxygen Supply Connected.
External Power: Indicates the unit is operating using an external power
source.
No External Power: Indicates the unit is operating without an external
power source.
Internal Battery: Provides indication of battery capacity and charging.
Indicates that an external battery is powering the ventilator.
No Internal Battery: Indicates when internal battery is not an available
power source.
Head with Mask: the unit is in Non-invasive Positive Pressure Ventilation
(NPPV) mode.
Feature OFF -- feature or alarm not selected.
Feature ON -- feature or alarm has been selected.
1-6 www.zoll.com 906-0731-01-05 Rev. B
Page 13
Symbol Description
srch
stby
Conventions
This guide uses the following conventions:
Within text, the names and labels for physical buttons and softkeys appear in boldface type (for
example, “Press the CONFIRM/SELECT button”).
This guide uses uppercase italics for text messages displayed on the screen
(for example, LEAD FAULT).
Search
Standby.
Warning! Warning statements alert you to conditions or actions that can result in personal injury
or death.
Caution Caution statements alert you to conditions or actions that can result in damage to the unit.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-7
Page 14

GENERAL INFORMATION
Abbreviations
A/C- Assist/Control I:E- Inverse ratio
AEV- Automatic Electrical Ventilator ID - Internal Diameter
ACLS- Advanced Cardiac Life Support L - Liters
ALS- Advanced Life Support LCD- Liquid Crystal Display
ATLS- Advanced Trauma Life Support LED - Light Emitting Diode
ACV- Assist-Control Ventilation LPM - Liters Per Minute
AMC- Alarm Message Center ml - Milliliters
APOD- Advanced Probe Off Detection mm - Millimeter
ATPD - Atmospheric Temperature and Pressure Dry MRI- Magnetic Resonance Imaging
b/min- Beats Per Minute NPPV – Noninvasive Positive Pressure Ventilation
B/V - Bacterial/Viral Filter O
BiPAP- Bilevel positive airway pressure P
BPM - Breaths per Minute PEEP - Positive End Expiratory Pressure
cm H
O - Centimeters of Water PIP - Peak Inspiratory Pressure
2
CPAP- Continuous Positive Airway Pressure PPV- Positive-Pressure Ventilation
CPR - Cardiopulmonary Resuscitation PS- Pressure support
CPU- Central Processor Unit psig - Pounds per Square Inch Gage
dBA- Decibel RF- Radio Frequency
DISS - Diameter Index Safety System RGA #- Returned-Goods-Authorization number
EMC- Electromagnetic Compatibility RTC- Real time clock
EMV- Emergency Medical Ventilator SIMV- Synchronized Intermittent Mandatory
ESD- Electrostatic Discharge SPM- Smart Pneumatic Module
FIO
Fraction of Inspired Oxygen USP - United States Pharmacopeia
2 -
HME - Heat and Moisture Exchanger VAC - Volts AC
HMEF - Heat and Moisture Exchanger/Bacterial Viral
filter combined
- oxygen
2
- Airway Pressure
aw
Ventilation
VDC - Volts DC
HP O
- High Pressure Oxygen VT -
2
Hz – Hertz (as in frequency, cycles per second) WOB – Work Of Breathing
Tidal Volume
1-8 www.zoll.com 906-0731-01-05 Rev. B
Page 15

ZOLL Ventilator Indications for Use
Ventilation
Each model of the ZOLL 731 Series of Ventilato rs is i ndicated for use in the management of
infant through adult patients weighing greater than or equal to 5 kg with acute or chronic
respiratory failure or during resuscitation by providing continuous positive-pressure
ventilation. ZOLL Ventilators are appropriate for use in hospitals, outside the hospital, during
transport and in severe environments where they may be expo sed to rai n, du st, rough handli ng,
and extremes in temperature and humidity. With an appropriate third-party filter in place, they
may be operated in environments where chemical and/or biological toxins are present. When
marked with an "MRI conditional" label, ZOLL Ventilators are suitable for use in an MRI
environment with appropriate precautions. ZOLL Ventilators are not intended to operate in
explosive environments. ZOLL Ventilators are intended for use by skilled care providers with
knowledge of mechanical ventilation, emergency medical services (EMS) personnel with a
basic knowledge of mechanical ventilation, and by first responders under th e direction of
skilled medical care providers
Pulse Oximetry (SpO2)
The ZOLL Ventilator pulse oximeter with Masimo Rainbow® SET technology is intended for
use for continuous noninvasive monitoring of functional oxygen saturation of arterial
hemoglobin (SpO
use on adult, pediatric, and ne onatal patie nts during both no moti on and motio n conditio ns, and
for patients who are well or poorly perfused, in hospitals, hospital-type facilities, or in mobile
environments.
), and pulse rate. The pulse SpO2 oximeter and accessories are indicated for
2
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-9
Page 16

GENERAL INFORMATION
Features
• Portable ventilator that you can use in the hospital, aeromedical and ground transport, mass
casualty situations, and extreme environments.
• Multiple modes of ventilation for use with acute or chronic respiratory failure in both
intubated and non-intubated patients.
• Intuitive operator interface minimizes operator training and protects existing settings from
inadvertent contact and manipulation.
• Lightweight -- less than 10 lbs (4.4 kg) -- for easy transport.
• Rechargeable battery provides over 10 hours of operation (at factory default with pulse
oximeter operating).
• Operating temperature range for extreme conditions: -25 to 49C.
• Altitude compensation from -2,000 to 25,000 ft.
• Self-contained system able to operate with or without external oxygen.
• Gas manifold design allows operation with both hi gh and low- pressure o xy gen source s. All
oxygen is delivered to the patient breathing circuit.
• Sealed gas path with chemical/biological filter connected to assure safe breathing gas
supply.
• Sealed case and control panel protects components from weather and fluids.
• Smart Help messages guide the operator through on-screen commands when responding to
alarms.
1-10 www.zoll.com 906-0731-01-05 Rev. B
Page 17

Warnings
General
Ventilator
• The ZOLL Ventilator is intended for use by qualified personnel only. You should read this
manual before using the device.
• Before using the ventilator on a patient, you must test the dev ice in its normal co nfiguratio n
to ensure proper operation .
• Do not modify this equipment without authorization of the manufacturer.
• This operator’s guide is not meant to supersede any controlling operating procedure
regarding the safe use of assisted ventilation.
• Follow all governing regulations regarding the disposal of any part of this medical device,
the handling of materials contaminated by body fluids, and shipment of th e Li-ION
batteries.
• The ZOLL Ventilator can operate from its internal battery or from an e xternal po wer source.
When using an external power source, position the supply cables to avoid accidental
disconnect.
• The use of accessories and cables other than those sold by ZOLL may result in increased
emissions or decreased immunity of this device.
• Portable and mobile RF communication equipment may affect the performance of this
device. We describe the EMC performance for this device in the Specifications section of
this operator’s guide.
• The ventilator may cause radio interference or may disrupt the operation of nearby
equipment. It may be necessary to take mitigation measures, such as re-orienting or
relocating of the device or shielding the location.
• Do not connect to an electrical outlet controlled by a wall switch or dimmer.
• The protection against defibrillator depends on the use of accessories (including pulse
taximeter) that are specified by ZOLL.
• Grounding:
• Do not under any circumstances remove t he grounding conductor from the power
plug.
• Do not use extension cords or adapters of any type. The power cord and plug must
be intact and undamaged.
• If there is any doubt about the integrity of the protective earth conductor
arrangement, operate the taximeter on internal battery power until the AC power
supply protective cover is fully functional.
• As with all medical equipment, carefully route the ventilator circuit, patient cabling, and
external power cables to reduce the possibility of patient entanglement or strangulation.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-11
Page 18

GENERAL INFORMATION
• Do not use the unit during magnetic resonance imaging (MRI) scanning unless it has the
appropriate “MRI conditional” label. See “Using the ZOLL Ventilator in an MRI
Environment” for instructions on the use of MRI conditional units, which gives additional
Warnings and Cautions.
• Do not operate the ZOLL Ventilator on a patient when the USB port is connected to any
other device (you use the USB port only for servicing the ventilator).
• The ZOLL-supplied ventilator circuit’s labeling provides the resistance and compliance
values for the circuits under normal operating conditions. If added accessories are used
(e.g. humidification, filters etc.), you should assure they do not degrade the performance of
the device.
Pulse Oximeter
• Do not use the pulse oximeter as an apnea monitor.
• A pulse oximeter should be considered an early warning device. As a trend towards patient
deoxygenation is indicated, blood samples should be analyzed by a laboratory co-oximeter
to completely understand the patient’s condition.
• Measurements: if the accuracy of any measurement does not seem reasonable, first check
the patient’ s vital signs by alternate means and then check the pulse oximeter for proper
functioning.
Inaccurate measurements may be caused by:
• Interfering Substances: carboxyhemoglobin may erroneously increase readings. The level of
increase is approximately equal to the amount of carboxyhemoglobin present. Dyes, or any
substance containing dyes, that change usual arterial pigmentation may cause erroneous
readings.
• Alarms: Check alarm limits each time the pulse oximeter is used to ensure that they are
appropriate for the patient being monitored.
• Incorrect sensor application or use.
• Significant levels of dysfunctional hemoglobin (e.g. carboxyhemoglobin or
methemoglobin).
• Intravascular dyes such as indocyanine green or methylene blue.
• Exposure to excessive illumination, such as surgical lamps (especially ones with a
xenon light source), bilirubin lamps, fluorescent lights, infrared heating lamps, or
direct sunlight (exposure to excessive illumination can be corrected by c overing the
sensor with a dark or opaque material).
• Excessive patient movement.
• Venous pulsations.
• Placement of a sensor on an extremity with a blood pressure cuff, arterial catheter,
or intravascular line.
• The pulse oximeter can be used during defibrillation, but the readings may be
inaccurate for a short time.
1-12 www.zoll.com 906-0731-01-05 Rev. B
Page 19

• Loss of pulse signal can occur in any of the following situations:
• The sensor is too tight.
• Excessive illumination from light sources such as a surgical lamp, a Ru bin lamp, or
sunlight.
• A blood pressure cuff is inflated on the same extremity as the one with an SpO
2
sensor attached.
• The patient has hypotension, severe vascoconstriction, severe anemia, or
hypothermia.
• Arterial occlusion proximal to the sensor.
• The patient is in cardiac arrest or is in shock.
• Sensors:
• Before use, carefully read the LNCS
• Use only Masimo oximetry sensors for SpO
®
sensor directions for use.
measurements. Other oxygen
2
transducers (sensors) may cause improper performance.
• Tissue damage can be caused by incorrect application or use of an LNCS
®
sensor
for example, by wrapping the sensor too tightly. Inspect the sensor site as directed
in the sensor Directions for Use to ensure skin integrity and correct positioning
and adhesion of the sensor.
• Do not damage LNCS
®
sensors. Do not use an LNCS® sensor with exposed optical
components. Do not immerse the sensor in water, solvents, or cleaning solutions
(The sensors and connectors are not waterproof). Do not sterilize by irradiation,
steam, or ethylene oxide. See the cleaning in structions in the direct ions for reusable
®
Masimo LNCS
• Do not use damaged patient cables. Do not immerse the patient cables in water,
sensors.
solvents, or cleaning solutions (the patient cables are not waterproof). Do not
sterilize by irradiation, steam, or ethylene oxide. See the cleaning instructions in
the directions for reusable Masimo patient cables.
• Do not use the pulse oximeter sensor during magnetic resonance imaging (MRI) scanning.
Inducing current could potentially cause burns. The pulse oximeter may affect the MRI
image and the MRI unit may affect the accuracy of the dosimetry measurements.
Batteries
• Only use the Power Supply provided with the device. Use of any other power supp ly could
cause damage or create a fire and/or destroy the battery and unit.
• If you witness a battery or the battery c ompartment starting to balloon, swell up, smoke, or
feel excessively hot, turn off the unit, disconnect external power, and observe it in a safe
place for approximately 15 minutes and send the unit for service. Never punctu re or
disassemble the battery packs or cells.
Operator Safety
• Electric shock hazard: Do not remove equipment covers. You may only perform
maintenance procedures specifically described in this manual. Refer all servicing to
ZOLL or a ZOLL-authorized service center.
• Possible explosion hazard if used in the presence of flammable anesthetics or other
flammable substances in combination with air, oxygen-enriched environments, or ni trous
oxide.
• This device is not intended for use in explosive atmospheres.
• Pins of connectors identified with the ESD warning symbol should not be touched. Always
use precautionary procedures with ESD-sensitive connection s.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-13
Page 20

GENERAL INFORMATION
Patient Safety
• To ensure patient electrical isolation, connect only to other equipment with electronically
isolated circuits.
• Do not place the unit or external power supply in any position that might cause it to fall on
the patient. Do not lift the unit by the power supply cord, ventilator circuit, or pulse
taximeter patient cable.
• Never service the ventilator while in use with a patient.
Ferromagnetic Equipment
• Failure to follow all instructions can result in MRI artifacts, injury to the patient or
operator, or malfunction of the device.
• You must follow all safety procedures that are in effect for the MRI Environment. Do
not use the ventilator in an MRI Environment with greater than 3T magnetic force.
• You must secure the unit to a suitable MRI-compatible cart -- ZOLL MRI Roll
Stand (REF 816-0731-01); Optional IV Arm Assembly (REF 707-0731-09).
• You must place the ventilator behind the 2000 Gauss field line -- approximately 2
meters to the bore opening of the MRI magnet.
• The ventilator must be attended by a person with no other responsibility than
monitoring the device and patient while in the MRI Environment.
• You must visually mo n itor the ventilator for alarms at all times -- during imaging,
the alarms may not be audible beyond the area immediately adjacent to the MRI.
• Danger! Possible Missile Projection.
• DO NOT position any person between the bore entrance and an unsecured cart or
device.
• Lock the wheels when the rolling stand is in place.
• We recommend that you tether the rolling stand in place when in the MRI
Environment.
• Place the ventilator and stand in its position before the patient is positioned on the
scanner table and advanced into the bore.
• Remove the patient from the MRI Environment before removing the ventilator and
roll stand.
• Unapproved device apparatus shall NOT be allowed in the MRI Environment,
including:
• Pulse Oximeters sensors and cabling.
• External AC/DC Power Supply.
• Rolling Cart Breathing Circuit Arm.
• Active Humidification and associated support apparatus.
• Ensure proper configuration of the ventilator.
• DO NOT attach the pulse oximeter sensor to the patient and remove it from the
device.
• The ventilator should run only on battery power in the MRI Environment
-- DO NOT use an external AC/DC power supply.
• The ventilator’s battery should be fully charged before entering the MRI
Environment.
• Oxygen Supply -- an aluminum, non-magnetic cylinder must provide the oxygen
supply.
1-14 www.zoll.com 906-0731-01-05 Rev. B
Page 21

Cautions
• Ensure proper operation of the ventilator’s breathing system.
• 12 ft ventilator circuits are available for use with the ventilator -- the additional
length enables a suitable separation between the ventilator and the bore opening.
(REF 820-130-00 -- Adul t/Ped iatric Wy e Ventilator Circuit; REF 820-131-00 -Pediatric/Infant Wye Ventilator Circuit).
• The extended tubing length of a 12 ft ventilator circuit can result in loss of volume
due to additional compressibility.
-- Set the Tubing Compliance (TC) to OFF and ensure that the patient is
receiving correct tidal volume.
-- Alternatively, calculate the TC as described by the ventilator circuit’s
Instructions For Use (IFU) and adjust the TC value to ensure that the patient is
receiving the correct tidal volume.
• DO NOT use the 12 ft circuit with settings below 5 cmH20.
• Ensure that the ventilator is able to maintain PEEP -- for patients with short
expiratory times, the additional tubing length of the 12 ft circuit may affect system
behavior.
• Inspect the circuit very day to ensure that there is no damage or wear that could affect its
performance. Remove Fluid or other biological material from the circuit or replace the
circuit following the local standard of care.
• Federal law restricts this device to sale by or on the order of a physician.
• Only qualified biomedical equipment technicians should service the device.
• Internal components are susceptible to damage from static discharge. Do not remove device
covers.
• Possession or purchase of this device does not convey any expressed or implied license to
use the device with unauthorized sensors or cables which would, alone, or in combination
with this device fall within the scope of one or more of the patients related to this device.
ZOLL cannot ensure the proper functioning of this device if it is used with unauthorized
sensors, cables, or patient circuits.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-15
Page 22

GENERAL INFORMATION
FDA Tracking Requirements
U.S. Federal Law (21 CFR 821) requires the tracking of ventilators. Under this law, owners
of this ventilator must notify ZOLL Medical Corporation if this product is
• received
• lost, stolen, or destroyed
• donated, resold, or otherwise distributed to a different organization
If any such event occurs, contact ZOLL Medical Corporation in writing with the following
information:
1. Originator's organization – Company name, address, contact name, and contact phone
number
2. Model number, and serial number of the ventilator
3. Disposition of the ventilator (for example, received, lost, stolen, destroyed, distributed to
another organization), new location and/or organization (if known and different from
originator’s organization) – company name, address, contact name, and contact phone
number
4. Date when the change took effect
Please address the information to:
ZOLL Medical Corporation
Attn: Tracking Coordinator
269 Mill Road
Chelmsford, MA 01824-04105
Fax: (978) 421-0007
Telephone: (978) 421-9655
Notification of Adverse Events
As a health care provider, you may have responsibilities under the Safe Medical Devices Act
(SMDA), for reporting to ZOLL Medical Corporation, and possibly to the FDA, the occurrence
of certain events.
These events, described in 21 CFR Part 803, include device-related death and serious injury or
illness. In addition, as part of our Quality Assurance Program, ZOLL Medical Corporation
requests to be notified of device failures or malf unctions. This information is required to ensure
that ZOLL Medical Corporation provides only the highest quality pro ducts.
1-16 www.zoll.com 906-0731-01-05 Rev. B
Page 23

Software License
Note: Read this Operator’s Guide and License agreement carefully before operating any of
the 731 Series Ventilator products.
Software incorporated into the system is protected by copyright laws and international
copyright treaties as well as other intellectual property laws and treaties. This software is
licensed, not sold. By taking delivery of and using this system, the Purchaser signifies
agreement to and acceptance of the following terms and conditions:
1. Grant of License: In consideration of payment of the software license fee which is part of
the price paid for this product, ZOLL Medical Corporation grants the Purchaser a
nonexclusive license, without right to sublicense, to use the system software in object-code
form only.
2. Ownership of Software/Firmware: Title to, ownership of, and all rights and interests in the
system software and all copies thereof remain at all times vested in the manufacturer, and
Licensors to ZOLL Medical Corporation and they do not pass to purchaser.
3. Assignment: Purchaser agrees not to assign, sublicense, or otherwise transfer or share its
rights under the license without the express written permission of ZOLL Medical
Corporation.
4. Use Restrictions: As the Purchaser, you may physically transfer the products from one
location to another provided th at the soft ware/firmware i s not cop ied. You may not disclose,
publish, translate, release, or distribute copies of the software/firmware to others. You may
not modify, adapt, translate, reverse engineer, decompile, crosscompile, disassemble, or
create derivative works based on the software/firmware.
NO IMPLIED LICENSE
Possession or purchase of this device does not convey any express or implied license to use the
device with replacement parts which would, alone, or in combination with this device, fall
within the scope of one or more of the patents relating to this device.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-17
Page 24

GENERAL INFORMATION
Limited Warranty
ZOLL warrants the device to be free from all defects in material and workmanship for a period
of one (1) year from the date of delivery to the original purchaser.
During the warranty period, ZOLL will repair or replace the device or any part which upon
examination is shown to be defective. At its sole discretion, ZOLL may choose to supply a new
or equivalent replacement product or refund the amoun t of th e pu rcha se price (on the date sold
by ZOLL). To qualify for such repair, replacement, or refund, the defective device must be
returned to the ZOLL Service Center within thirty (30) days from the date that the defect is
discovered. This warranty does not apply if the device has been repaired or modified without
the authorization of ZOLL or if the damage was caused by incorrect (off-label) use, negligence,
or an accident.
Batteries, which by their nature are consumable and subjected to environmental extremes, will
be warranted only for a period of ninety (90) days. Accessories, also consumable in usage, such
as connecting hose and breathing circuits, are not warranted.
DISCLAIMER OF IMPLIED & OTHER WARRANTIES:
THE PRECEDING WARRANTY IS THE EXCLUSIVE WARRANTY AND ZOLL
MAKES NO OTHER WARRANTY OR REPRESENTATION OF ANY KIND
WHATSOEVER, EXPRESS OR IMPLIED, WITH RESPECT TO MERCHANTABILITY,
FITNESS FOR A PARTICULAR PURPOSE, OR ANY OTHER MATTER. THE
REMEDIES STATED IN THIS DOCUMENT WILL BE THE EXCLUSIVE REMEDIES
AVAILABLE TO THE CUSTOMER FOR ANY DEFECTS OR FOR DAMAGES
RESULTING FROM ANY CAUSE WHATSOEVER AND WITHOUT LIMITATION.
ZOLL WILL NOT IN ANY EVENT BE LIABLE TO THE CUSTOMER FOR
CONSEQUENTIAL OR INCIDENTAL DAMAGES OF ANY KIND, WHETHER FOR
DEFECTIVE OR NONCONFORMING PRODUCTS, BREACH OR REPUDIATION OF
ANY TERM OR CONDITION OF THIS DOCUMENT, NEGIGENCE, OR ANY OTHER
REASON.
1-18 www.zoll.com 906-0731-01-05 Rev. B
Page 25
Service
If a unit requires service, contact the ZOLL Technical Service Department.
For customers In the U.S.A. For customers outside the U.S.A.
Telephone:
Fax:
1-973-882-1212
1-978-421-0010
Call the nearest authorized ZOLL Medical Corporation
representative.
To locate an authorized service center, contact the
International Sales Department at
ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA 01824
Telephone: 1-978-421-9655
When requesting service, please provide the following information to the service
representative:
• Unit serial number
• Description of the problem
• Department using the equipment and name of the person to contact
• Purchase order to allow tracking of loan equipment
• Purchase order for a unit with an expired warranty
Returning a unit for service
Before sending a unit to the ZOLL Technical Service Department for repair, obtain a service
request (SR) number from the service representative.
The Lithium ion battery should remain inside the unit. Follow directions provided on the return
authorization form. Pack the unit with its cables in the original containers (if availabl e) or
equivalent packaging. Be sure the assigned service request number appears on each package.
For customers Return the unit to
In the U.S.A. ZOLL Medical Corporation
269 Mill Road
Elmsford, MA 01824
Attention: Technical Service Department (SR number)
Telephone: 1-978-421-9655
In Canada ZOLL Medical Canada Inc.
1750 Sismet Road, Unit #1
Mississauga, ON L4W 1R6
Attention: Technical Service Department (SR number)
Telephone: 1-866-442-1011
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 1-19
Page 26
GENERAL INFORMATION
For customers Return the unit to
In other locations The nearest authorized ZOLL Medical Corporation
representative.
To locate an authorized service center, contact the
International Sales Department at
ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA 01824-4105
Telephone: 1-978-421-9655
1-20 www.zoll.com 906-0731-01-05 Rev. B
Page 27
Chapter 2
Product Overview
This chapter provides an overview of the ZOLL Ventil ator, which you can use to manage infa nt
through adult patients with acute or chronic respiratory failure or pat ients that you are
resuscitating by providing continuous positive-pressure ventilation. (See Indications for Use
in Chapter 1.)
This chapter describes the ZOLL Ventilator models, providing a list of common features and
attributes, as well as descriptions of each model. This chapter also provides more detailed
descriptions of the following ventilator features:
• Controls and Indicators
• Display Screen
• Pneumatic Design
• Fresh Gas Intake
• Connector Panel
• Ventilator Circuits
• Pulse Oximeter
• Power Sources
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-1
Page 28

PRODUCT OVERVIEW
ZOLL Ventilator Models
The ZOLL Ventilator is available as the AEV, EMV+, and Eagle II models. The ventilator
offers a range of ventilatory modes to support EMS, military, air transport, and hospital
transport needs.
The AEV ventilator is designed for managing ventilator support patients during ambulance
transport. Its ventilation modes (AC, CPAP with PS, and BL) are specifically chosen to be
consistent with pre-hospital care provider's operating procedures.
The EMV+ ventilator's rugged design makes it ideal for use in emergency vehicle and air
transport of patients. It has a wide range of ventilation modes, such as AC, SIMV, CPAP, and
BL.
The Eagle II ventilator adapts the design of for the EMV+ for use by emergency departments
and intra-hospital transport. Its design also allows it to be mounted on to walls or onto specified
boom arms and roll stands as well as gurneys.
The ZOLL ven tila t o r s have been approved for use in MR I suites. The EMV+ and Eagle II
ventilators have MRI-compatible variants available. The MRI-compatible ventilators can
operate in 3.0 Tesla environments and can be placed approximately 6 1/2 ft. from the bore
opening for easy and safe access to the patient. See Chapter 3 for more information regarding
safe operation in the MRI Environment.
2-2 www.zoll.com 906-0731-01-05 Rev. B
Page 29

ZOLL Ventilator Features
The ZOLL Ventilator models have these common features:
• Rugged design
• Weight: ~10 lbs
• 10 hour battery life
• Rapid charger to achieve 90% battery capacity in 2 hours
• High performance internal compressor
• Smart Help messages
• Integral SpO
• Airworthiness Release
• Daylight visible display
• Oxygen efficient
• Supports infant, pediatric, and adult patients
• Limited 1 year warranty
(Masimo)
2
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-3
Page 30
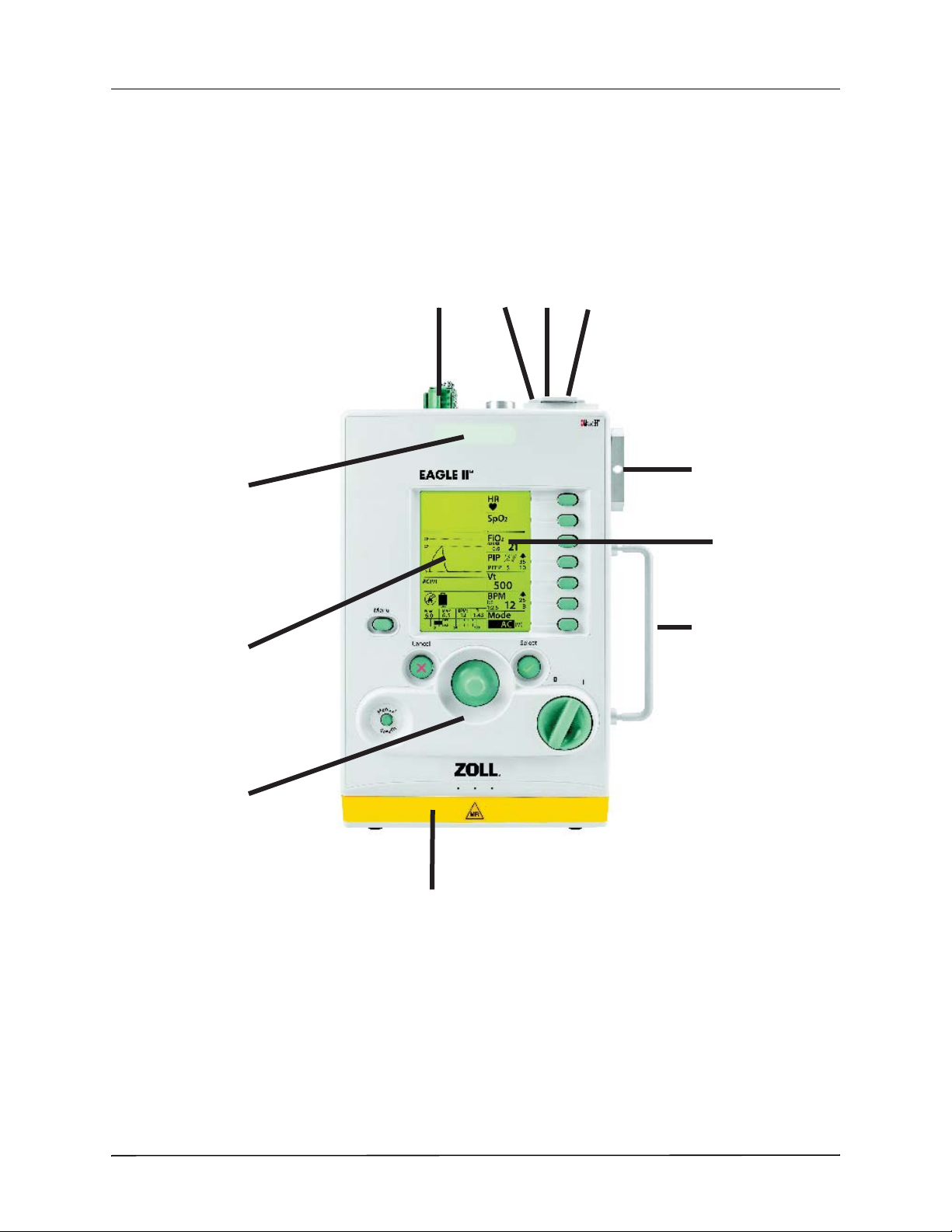
PRODUCT OVERVIEW
1
2
345
10
6
7
8
9
11
ZOLL Ventilator Device Description
The following illustration shows the ZOLL Ventilator’s main features:
2-4 www.zoll.com 906-0731-01-05 Rev. B
Page 31

Item Description
Top
1. Oxygen Inlet Connects the unit to an external oxygen source
2. Status Indicator LED Array Lights up to indicate status of the unit, connected to alarms
3. External Power Input Connector Connects the unit to an external power source
4. USB Connector Connects the unit to a USB drive or USB compatible device
5. Pulse Oximeter Connector Connects the unit to a Pulse Oximeter sensor
Front
6. LCD Display Displays the unit’s settings, patient data, and alarm information
7. Alarm Message Center Displays active alarms and mitigation information
8. Control Panel Access to the unit settings
Bottom
9. Battery Compartment Contains the unit’s rechargeable lithium ion battery
Side
10. Fresh Gas/Emergency Air Intake Allows the unit’s internal compressor to take ambient air and acts as an
anti-asphyxia valve
11. Handle
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-5
Page 32

PRODUCT OVERVIEW
1
6
2
4
7
5
3
8
9
Controls and Indicators
The ZOLL Ventilator has controls and indicators that facilitate ease of use and visibility in all
operating environments.
This ventilator’s control panel includes a display screen (liquid crystal display -- LCD), an LED
array, and the controls that you use to set up and manage the ventilator.
The ventilator’s controls consist of the following:
1. Power On/Off Switch -- turns the ventilator on and off.
2. Parameter buttons -- chooses parameter values.
3. Menu Button -- displays the main menu.
4. Selection dial -- changes the value of the highlighted parameter value.
5. Mute/Cancel button -- mutes audible alarm indicators and cancels parameter entries.
6. Accept/Select button -- accepts parameter value entries, Pop Up conditions or menu
selections.
7. Manual Breath/P Plat (Plateau Pressure) button -- issues a manual breath, and for the
EMV+ and Eagle II models, provides the ability to conduct a plateau pressure maneuver.
The ventilator’s indicators consist of the following:
8. LCD Display -- Brightness and backlight controls are available in the main menu (we
describe the display in more detail later in this chapter).
9. LED Array -- Indicates status of the v entila tor’s operation by lighting red, yellow, or green
LED’s.
2-6 www.zoll.com 906-0731-01-05 Rev. B
Page 33

Display Screen
Message Area
Shared Icon Area
Auxiliary Parameter Boxes
Parameter Windows
The ZOLL Ventilator’s display screen has four functional areas:
Message Area
The display screen’s message area can display the following:
• Airway Pressure and Pleth Waveform Plots -- Under normal operation (as in the example
• Menus -- Displays the Main Menu after you press the menu button on the ventilator’s
• Alarms -- When an alarms occur, the message area displays Smart Help messages that
• Popup Windows -- Display information that assists you when adjusting parameter values.
Parameter Windows
Parameter windows display the measurements, alarm limits, and associated parameters fo r their
labeled parameters. Parameter values that you can adjust, such as alarm limits, appear as solid
text. Parameter values that you cannot adjust, such as measurements taken by the ventilator,
appear as outlined text. We provide information on adjusting parameter values in Chapter 4,
“Using the ZOLL Ventilator.”
above), the message area displays plots for airway pressure and, when the pulse oximeter is
connected, the Pleth waveform.
control panel, or displays a parameter’s context menu (which appears after you press and
hold the associated parameter button on the control panel). When a plot is necessary to
facilitate a parameter adjustment, the message area displays both the plot and the
parameter’s context menu.
identify the alarms and describe possible causes and actions that you can take in response.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-7
Page 34

PRODUCT OVERVIEW
Shared Icon Area
Directly below the message area, the unit displays icons that indicate
• Power source (external power or internal battery)
• Battery Charge Status
• Oxygen Supply attached
• Alarm Muted/Audible
Auxiliary Parameter Boxes
Some parameters have values that the ventilator displays in the parameter boxes at the bottom
of the display screen. You can adjust these values using the parameter’s context menu.
2-8 www.zoll.com 906-0731-01-05 Rev. B
Page 35

Pneumatic Design
The ZOLL Ventilator includes an oxygen valve and a compressor to provide the gas to the
output port. The system includes transd ucers for pressure mea surements includin g input supp ly
pressure and barometric readings.
The wye circuit is part of the ventilator’s pneumatic system. The inspiratory side of the wye
circuit provides gas to the patient. The expiratory side exhausts directly to atmosphere without
returning to the ventilator. The ventilator pneumatically controls the exhalation valve and a
transducer within the ventilator measures the airway pressure.
The following image is a diagram of the ventilator’s pneumatic design.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-9
Page 36

PRODUCT OVERVIEW
External Power Input
Gas Output
High Pressure
Oxygen Input
Transducer
(Patient Airway
Pressure)
Exhalation
Valve
Fresh Gas/
Emergency
Air Intake
Pulse Oximeter Connector
+ USB Connector
Fresh Gas Intake and Attachments
The fresh gas intake, which is located on the side of the ventilator, allows ambient air into the
unit’s internal compressor. The intake also acts as an anti-asphyxia value that enables the
patient to breath ambient air should the ventilator fail.
The fresh gas intake contains a particulate filter and permits the operator to connect either a
bacteria/viral or a chemical/biological filter depending on ambient conditions
ZOLL provides an O2 Reservoir Kit to allow for low flow oxygen supply to the ventilator. An
oxygen concentrator source provides oxygen to the O2 reservoir.
Top Panel
The ZOLL Ventilator’s top panel appears as follows:
The oxygen hose, ventilator circuit, external power, and pulse oximeter attach to the top panel
of the ventilator. The USB port is only used when servicing the unit.
2-10 www.zoll.com 906-0731-01-05 Rev. B
Page 37

Oxygen Input: High Pressure Gas Supply
The external high pressure gas source connects to the device using the high pressure oxygen
input port.
The device attaches to a regulated supply of 40 to 87 PSIG (280 to 600 kPa). The maximum
flow rate of the oxygen supply is 100 liters per minute. This supply can be fro m a medical
grade oxygen system or oxygen cylinder (USP).
The OXYGEN IN fitting has a male oxygen Diameter Index Safety System (D.I.S.S.) thread.
Note: If external oxygen is connected, the gas pressure must be at least 41-psig (± 2 psig) when
the device performs Self-Check after you power on the unit.
High Pressure Oxygen Supply Hose
A standard 6 foot oxygen hose is available to make the connection to the high pressure oxygen
source. The hose is has compatible fittings between the device an the source identified for use.
(Also see Chapter 6 “Operating Environments” ). Hose s are avail abl e from ZOLL, or a suitable
alternative as described below can be used as indicated in the table below.
High Pressure Hoses need to comply with ISO standards
Device Side
Connections
DISS 6 ft (maximum 20ft)
Green or White (as determined by local regulations)
Non-conductive
Hose Attributes Supply Side Connections
Quick Disconnect, DISS, etc.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-11
Page 38

PRODUCT OVERVIEW
Figure 2-1 Ventilator Gas Sources
Ventilator Circuits
The ZOLL Ventilator operates using a standard disposable ventilator circuit.
The Ventilator circuit attaches to the device using three ports on the top of the device.
• Gas Output -- connects to the ventilator circuit using 22 mm ID corrugated hose. The
connector is a 22 mm male conical connection.
• Transducer (Patient Airway Pr essure) -- connects to the ventilator circuit using a
3/16 inch ID transducer tubing. The barb-type connector is colored a green/blue to
distinguish it from the other connector s .
2-12 www.zoll.com 906-0731-01-05 Rev. B
Page 39

• Exhalation Valve -- connects to the ventilator circuit using 1/4 inch ID exhalation valve
tubing. The barb-type con ne cto r is clear anodized aluminum to distinguish it from the oth er
connectors (the 1/4 inch ID ventilator circuit exhalation valve tubing is clear).
Ventilator Circuit Connections
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-13
Page 40

PRODUCT OVERVIEW
Ventilator Circuit Types
The ZOLL Ventilator can use 6 ft or 12 ft ventilator circuits to support adult, pediatric, and
infant patients.
:
Ventilator Circuits
ZOLL provides the following circuit types:
• Pediatric/Adult, 6 ft (REF 820-0106-XX)
Infant/Pediatric, 6 ft (REF 820-0107-XX)
•
•
Pediatric/Adult, 12 ft (REF 820-0130-XX)
Infant/Pediatric, 12 ft (REF 820-0131-XX)
•
Caution Always dispose of the circuit after single patient use following the institutional guidelines for
biologically contaminated material. Reusing the circuit can result in cross contamination
between patients.
2-14 www.zoll.com 906-0731-01-05 Rev. B
Page 41

Pulse Oximeter Sensors
The Masimo Pulse Oximeter is an optional function of the ZOLL Ventilator. When the
appropriate sensor is connected, the pulse oximeter provides continuous noninvasive
monitoring of arterial hemoglobin (SpO
adult, pediatric and infant patients.
The Masimo LCSN series of probes are approved for use with the ventilator. The Accessory
table in Appendix A lists the sensors which are available for use with the ZOLL Ventilator.
Power Sources
The ZOLL Ventilator can operate using external power or it can operate powered by its internal
Lithium Ion Battery.
The external AC/DC Power Supply that ZOLL provides with the ventilator delivers a DC input
to the device of 24V at 4.2A. When this external power source is present, the ventilator
automatically charges its internal battery while operating.
The external AC/DC Power Supply is a universal supply that can operate with an input of
100-240 VAC 50/60 Hz. The external supply can also power the device when provided with a
400 Hz input.
You should only use the extern al power supp ly provided with th e ventilator when connecting to
AC power. This power supply provides both Class I and Class II protection.
) and pulse rate (measured by the SpO2 sensor) for
2
Operating Using External DC Power
The ZOLL Ventilator can also operate using external DC power. When conne cted to a sta ndard
vehicle DC outlet using either the 12 or 28 VDC Power Cable that ZOLL provides, the
ventilator automatically charges its internal battery while operating.
Note: The input connector of the ventilator accepts DC voltages between 11.8 to 30.0 VDC.
Caution When using the standard vehicle DC outlet, do not jump start the vehicle during op eration of the
ventilator.
Operating Using Battery Power
When an external power failure occurs, the ventilator automatically switches to its internal
battery for operating power and activates the EXTERNA L POWER FAILURE alarm; there is no
interruption in operation or loss of any alarms. When external power returns, operating power
automatically switches to the external power sourc e.
In the event that the ventilator needs to be shutdown, turn the POWER switch to the OFF ("O")
position. If this fails to work or puts the patient or operator at possible risk, disconnect the
device from the external power source.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 2-15
Page 42

PRODUCT OVERVIEW
2-16 www.zoll.com 906-0731-01-05 Rev. B
Page 43

Chapter 3
Setting Up the ZOLL Ventilator
This chapter describes how to set up the ZOLL Ventilator. It lists the tasks required to set up the
ventilator for safe, effective use, and describes each task in detail.
Warning ! You must always p r o perly set up the ventila tor before use. Failure to do so can result in
inadequate care or death of the patient.
To set up the ZOLL Ventilator, you must perform the following tasks:
1. Attach the Ventilator Circu it
2. Attach the High Pressure Oxygen Supply (Optional)
3. Inspect Fresh Gas Intake Filters
4. Connect Fresh Gas Intake Attachments
5. Select the Ventilator’s Power Source
6. Power on the Ventilator
7. Select Start Up Default Values
8. Select Operating Mode (Optional)
9. Change Parameter Values
10.Change Ventilator Settings
11. Perform Operational Test
12.Attach the Pulse Oximeter Probe (Optional)
13.Attach Patient
We describe how to perform these tasks in the following sections of this chapter.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3-1
Page 44

SETTING UP THE ZOLL VENTILATOR
Warning! Always follow standard of care, which includes preparations to bag the patient.
DO NOT start up the ventilator with the patient attached.
1. Attach the Ventilator Circuit
Select the correct ventilator circuit for the patient and environment (as we describe in the
previous chapter). Always follow the instructions included with the circuit.
Attach the ventilator circuit to the ventilator’s top panel. Connect
• The 22 mm corrugated hose to the ventilator’s gas output
• The green/blue 3/16 inch ID airway pressure line to the pressure transducer
• The clear 1/4 inch ID exhalation valve control line to the exhalation valve fitting.
Ventilator Circuit Device Connections
3-2 www.zoll.com 906-0731-01-05 Rev. B
Page 45

2. Attach the High Pressure Oxygen Supply (Optional)
Oxygen Inlet
Fresh Gas Intake
Since the ventilator includes an internal compressor, the attachment of a high pressure oxygen
supply is optional.
Review the high pressure supply requirements that we describe in Chapter 2, and use the
oxygen hose to attach the ventilator’s oxygen inlet to the high pressure gas source.
Note: Use only with medical-grade (USP) oxygen. When using with an oxygen cylinder, the
cylinder must be secured.
Note: The O
Hose is either colored green or white, depending on co untry specifications.
2
3. Inspect Fresh Gas Intake Filters
The fresh gas intake is the gas source for the ventilator’s internal compressor. The ventilator
normally operates with two built-in filters:
1. Removable Foam Filter (
2. Fresh Gas Intake Disk Filter (REF 465-0027-00)
Inspect the filters and, if dirty, replace them (See Chapter 7, “Replacing the Ventilator’s
Filters”).
REF 465-0028-00)
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3-3
Page 46

SETTING UP THE ZOLL VENTILATOR
4. Connect Fresh Gas Intake Attachments
The operating environment of the ventilator may require you to connect the following
attachments to the Fresh Gas Intake:
Oxygen Reservoir Bag
If the ventilator will use oxygen from low-flow sources, you may choose to attach an Oxygen
Reservoir Bag Assembly (
Bacterial/Viral (BV) Filter
If the ventilator will operate in an environment where the patient is at risk from cross
contamination or airborne pathogens, you may choose to attach a BV filter (See Chapter 6,
“Using the ZOLL Ventilator in Hazard ous Environments”).
Chemical/Biological C2A1 Filter
If the ventilator will operate in a contaminated environment, you may choose to attach a
chemical/biological C2A1 filter (See Chapter 6, “Using the ZOLL Ventilator in Hazardous
Environments”).
REF 704-0004-00).
3-4 www.zoll.com 906-0731-01-05 Rev. B
Page 47

5. Select the Ventilator’s Power Source
The ZOLL Ventilator can run using one of the following power sources:
1. Internal 14.4V lithium ion (Li Ion) rechargeable battery with 6.75 Ah capacity (fully
charged, the battery provides 10 hours of operation at factory default settings with pulse
oximeter operating at 25C).
2. External AC/DC Power Supply that ZOLL provides (100-240 VAC 50/60 and 400 Hz with
an IEC 320 style AC input connector. The AC/DC Power Supply provides a DC output of
24V at 4.2A.
3. External DC power from a standard vehicle DC outlet using either the 12 or 28 VDC Power
Cable that ZOLL provides to connect the ventilat or to the DC ou tlet. The ZOLL Ventilator’s
input connector accepts DC voltages between 11.8 to 30.0 VDC.
4. An external battery.
The ZOLL Ventilator uses external power when available rather than its internal battery pack.
When an acceptable external power source is present, the ventilator automatically charges the
internal battery while the unit operates. When an external power failure occurs, the unit
automatically switches to its internal ba tte ry for operati ng power and activate s the EXTERNAL
POWER FAILURE alarm; there is no interruption in operation or loss of any alarms. When
external power returns, operating power automatically switches from internal power to the
external source.
In the event that the device needs to be shutdown, turn the POWER switch to the OFF (“O”)
position. If this fails to work or puts the patient or operator at possible risk, disconnect the
device from the mains power.
T o connect t he ventilator to an external power source, c onnect an AC/DC Power Supply plug to
the unit’s External Power Input and an acceptable electrical outlet.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3-5
Page 48

SETTING UP THE ZOLL VENTILATOR
Connecting the Power Supply
Connect the external power cable to the ventilator as follows:
Connecting and Disconnecting the Power Supply
Caution Do not twist the power cable connection plug. Pinch the plug and slide up to release the safety
latches. Failure to do so may damage the power co nnection plug and prevent it fro m functioning.
Warning ! If the power supply, power cable, or power connection plugs are damaged or become
damaged during use, immediately disconnect the power cable from external power and
the power supply assembly.
Power Supply Latching
3-6 www.zoll.com 906-0731-01-05 Rev. B
Page 49

6. Power On the Ventilator
To power on the ventilator, turn the POWER switch to “1”.
After powering on, the unit performs its Self Check procedure, which checks for preexisting
alarm conditions and the operation of the pneumatic system, internal communications, and
power system. After completing the Self Check, the ventilator begins to operate immediately,
and monitors the presence of alarms continuously.
Power Switch
During start-up, the ventilator’s alarms are disabled for 120 seconds to allow you to properly
adjust the patient circuit, pulse oxim eter, and ventilator settings without distraction.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3-7
Page 50

SETTING UP THE ZOLL VENTILATOR
7. Select Start Up Default Values
When you power on the ventilator, the Start Menu appears, through which you choose the
appropriate parameter default values for the patient. You can select the following patient
parameter defaults:
• Adult
• Pediatric
• Mask CPAP -- Continuous Positive Airway Pressure (CPAP)
• Custom -- Values saved in a previous session
• Last Settings -- Values set for the patient last using the device before powering down
Note: The ventilator can may be configured to automatically select the Adult parameter
defaults at start up.
Adult Default Parameter Values
The Adult default parameter values are as follows:
Adult Parameter Default Values
Mode AC (V)
BPM 12
I:E 1:3
VT 450
PEEP 5
PIP Limit 35
FIO2 21
Pediatric Default Parameter Values
The Pediatric default parameter values are as follows:
Pediatric Parameter Start-Up Defaults
Mode SIMV (P)
BPM 20
Ti 0:6
PIP 20
PEEP 4
PIP Limit 30
FIO2 21
3-8 www.zoll.com 906-0731-01-05 Rev. B
Page 51

Mask CPAP Default Parameter Values
The Mask CPAP default parameter values are as follows:
Mask CPAP Parameter Start-Up Defaults
Mode CPAP
Backup BPM 12
Backup I:E 1:3.0
Backup PIP 20
PEEP 4
PIP Limit 30
FIO2 21
To select the unit’s default parameter values, highlight one of the above settings in the Start
Menu and press the ACCEPT/SELECT button. To operate with parameter values that dif fer
from the default values, use the unit’s Parameter buttons (see the “Changing Parameter Values”
section later in this chapter).
Note: You can configure the ventilator to automatically select Adult parameter defaults at
start up.
To adjust the parameter settings, always work from the lowest parameter button, Mode, to the
highest button, HR. The values that you select in a lower parameter window may affect the
values that appear in higher parameter windows
Warning! Never use the Noninvasive Positive Pressure Ventilation (NPPV) modes on a patient
that is NOT spontaneously breathing and/or may stop spontaneously breathing. CPAP
and BL are intended for ventilatory support, NOT ventilation.
When an NPPV mode is in operation, the head with mask icon appears in the location used by
the speaker/mute icons. Low and Medium priority alarms cause this head with mask ico n to
disappear.
It reappears when Low priority alarms are muted.
When Medium priority alarms are muted, the muted speaker icon appears.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3-9
Page 52

SETTING UP THE ZOLL VENTILATOR
8. Select Operating Mode (Optional)
The ventilator offers four operating modes that you can select to optimally manage the patient
(each mode can use either pressure or volume targeting):
1. AC (Assist/Control) -- The patient receives either controlled or assisted breaths. When the
patient triggers an assisted breath, they receive a breath based on either the volume or
pressure target.
2. SIMV (Synchronized Intermittent Mandatory Ventilation)-- The patient receives controlled
breaths based on the set breathing rate. Spontaneous breat hs are eith er unsup ported de mand
flow or supported using Pressure Support. (This mode is not available in the AEV
3. CPAP (Continuous Positive Airway Pressure) -- The patient receives constant positive
airway pressure while breathing spontaneously . Sp ontaneous breaths are either demand flow
or supported using Pressure Support.
4. BL (bilevel) -- the ventilator prov ides two pressure settings to assist patients breathing
spontaneously: a higher inhalation pressure (IPAP) and a lower exhalation pressure (EPAP).
To select the operating mode, press the Mode parameter button, turn the selection dial to
highlight the mode you want to use, and press the Accept/Select button.
When transitioning from active ventilation to NPPV modes, or from NPPV Modes to active
ventilation modes, the following parameter/alarm limit may be adjusted:
®
unit.)
Alarm/Parameter
Low BPM Alarm
High BPM Alarm
Low Airway Pressure Alarm
PEEP
V
High Limit
T
V
Low Limit
T
Rise Time
Pressure Support
Warning! The transition into NPPV automatically sets the rise time to 3, which may be too fast for
infants and small children. Befor e using the ventilator with an infant or small child, you
should always configure the ventilator appropriately before attaching the patient.
Note: An alarm triggers when you connect the patient to the ventilator while the Start Menu
is still active. To resolve the alarm, you must select a mo de of ventilation and configure
the device appropriately for the patient. In addition, you should perform the
Operational Test procedure before reconnecting the patient to the device.
3-10 www.zoll.com 906-0731-01-05 Rev. B
Page 53

9. Change Parameter Values
If the patient requires parameter values that differ from the default values, you can use the
parameter buttons to change these values, as necessary. To change the parameter values, press
the Parameter buttons to display the primary parameter and secondary parameter values, or
Press and hold the parameter button to display the pa rameter’ s contex t menu. Use th e selection
dial to adjust the highlighted parameter. Press the Accept/Select button to implement the
change.
10. Change Ventilator Settings
The Menu button displays the Main Menu, which allows you to change various Ventilator
settings, such as the contrast or brightness of the unit’s Display Screen (LCD Contrast/LCD
Brightness).
When you press the Menu button, the Main Menu appears:
• Alarm Config
• Powerup Settings
• LCD Contrast
• LCD Brightness
• GMT Offset
• Unit Info
• Alarm History
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3-11
Page 54

SETTING UP THE ZOLL VENTILATOR
11. Perform Operational Test
Before attaching the patient to the ventilator, you must perform an Operational Test to ensure
that the breathing circui t is prop erly atta ched an d that the primary pat ient safety alarms, such as
PATIENT DISCONNECT and AIRWAY PRESSURE HIGH are functioning properly.
Operational Test Procedure
Press the MANUAL BREATH button; gas should flow out of the patient connection each time
the button is pressed.
The minimum period between manual breaths is limited by the tidal volume and the time
required to complete a full exhalation based on the I:E ratio.)
Close the patient port with a gloved hand. During inspiratory phase, th e HIGH AIRWAY
PRESSURE LIMIT alarm should activate after 2 breaths that reach the PIP High Limit.
If the AIRWAY PRESSURE HIGH alarm fails to activate, ensure that all of the tubing
connections are secure, the exhalation valve is closing during inhalation, and that the High
Airway Pressure Limit is set to 35 cm H2O or less.
After a breath or two, release the patient port while allowing the ventilator to operate. The
PATIENT DISCONNECT alarm should activate.
Partially close the patient port to reset the PATIENT DISCONNECT alarm. With no other
alarms occurring, remove external power from the ventilator. The EXTERNAL POWER
LOW/DISCONNECT alarms should activate. Reconnect external power to reset alarms.
If either the HIGH AIR WAY PRESSURE, PATIENT DISCONNECT, or EXTERNAL POWER
LOW/DISCONNECT alarms fail to activate, continue to manually ventilate the patient, replace
the ventilator, and send the unit in for service.
If operating using the internal battery, verify that the Battery icon indicates sufficient available
battery capacity remains to support the anticipated duration of operation. If not, begin
ventilation and find an alternate source of power.
The trigger automatically adjusts when the PEEP is changed.
Until you have determined that the ventilator is functioning properly and that the ventilator
parameters are set correctly for the patient, do not connect the patient to the ventilator.
3-12 www.zoll.com 906-0731-01-05 Rev. B
Page 55

12. Attach the Pulse Oximeter Probe (Optional)
The pulse oximeter becomes operational in all ventilator modes when its cable and sensor are
properly attached to the SpO
SpO2 and HR Parameter Windows display stby).
connector (during start up, the p ulse oximeter is o n standby -- the
2
T o operate the pulse oximet er, connect the sensor probe to the patient and the cable to the SpO
connector on the top of the ventilator as shown in the following illustration:
2
Connecting the Pulse Oximeter Sensor
The monitoring function begins automatically when a valid patient signal is detected for > 10
seconds.
For more information about the Masimo pulse oximetry technology that the ZOLL Ventilator
uses, see Appendix C, Pulse Oximeter Principles.
13. Attach Patient
After you confirm that the ventilator is operating correctly, detach the test lung (if used in the
Operational Test) from the ventilator circuit.
Attach the patient to the ventilator using the appropriate connector (tracheal tube or laryngeal
mask) to the ventilator circuit.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 3-13
Page 56

SETTING UP THE ZOLL VENTILATOR
3-14 www.zoll.com 906-0731-01-05 Rev. B
Page 57

Chapter 4
Using the ZOLL Ventilator
This chapter describes how to use the ZOLL Ventilator.
Effective operation of the ventilator requires understanding of the following information:
• The ZOLL Ventilator Interface and Parameter Windows
• Changing Parameter Values
• Selecting Venti lation Mode Options
• Using the Pulse Oximeter
• Managing Pop Up Messages
• Managing Alarms
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-1
Page 58

USING THE ZOLL VENTILATOR
The Ventilator Interface
The ZOLL Ventilator uses a Graphical Use Interface (GUI) to display the parameter settings
and patient readings.
Changing Parameter Values
The ZOLL Ventilator helps you to manage the patient by organizing ventilatory parameters in
parameter windows on the right side of the display screen. These parameter windows display
the primary and secondary parameters and the alarm settin gs for that parameter. In addition, set
values and measurements appear in the auxiliary boxes at the bottom of the display scre en .
Additional settings used to manage t he patient are applied usi ng the context menu fo r parameter
group.
The sections below describe the parameter windows and the associated context menus for each
parameter. A table addresses availability of the parameter and its use in the device models.
The parameter window values are chosen with the parameter button:
Single Press: chooses primary parameter
Multiple Presses: chooses the secondary parameter and alarm limits
Press and Hold: chooses the context menu
T o prevent setting of para meter val ues th at are outside th e typica l clinic al rang e of setting s, the
ZOLL Ventilator displays Pop Up messages that ask if you are sure you would like to set the
parameter beyond the typical range. We describe Pop Up messages in more detail in Chapter 5.
Parameter Buttons
The parameter windows, from lowest to highest, are
• Mode
• BPM (Breaths per Minute)
• Vt (Tidal Volume -- V
• PIP (Peak Inspiratory Pressure)
• FIO2
• SpO2
• HR (Heart Rate)
Mode
The ZOLL Ventilator allows you to select different ventilation modes that you can select to
optimally manage the patient:
• AC (Assist/Control) -- The patient receives either controlled or assisted breaths. When the
patient triggers an assisted breath, they receive a breath based on either the volume or
pressure target.
• SIMV (Synchronized Intermittent Mandatory Ventilation) -- The patient receives
controlled breaths based on the set breathing rate. Spontaneous breaths are either
unsupported demand flow or supported using Pressure Support.
)
T
4-2 www.zoll.com 906-0731-01-05 Rev. B
Page 59

• CPAP (Continuous Positive Airway Pressure) -- The patient receives constant positive
airway pressure while breathing spontaneously. Spontane ous breaths are either demand flow
or supported using Pressure Support.
• BL (B i Leve l) -- the ventilator provides two pressure settings to assist patients breathing
spontaneously: a higher inhalation pressure (IPAP) and a lower exhalation pressure (EPAP).
Press the Mode parameter button to highlight the current ventilation mode. Press the Mode
parameter button again to select volume or pressure targeting which is shown as either “(V)”
for volume or “(P)” for pressure.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-3
Page 60

USING THE ZOLL VENTILATOR
Breath Target
The selected ventilation mode, and the selection of breath target (volume or pressure)
predetermines the parameter availability for the BPM, Vt, and PIP parameter windows.
Volume targeting assures a constant volume is delivered to the patient in the inspiratory time
using a constant flow.
Pressure targeting provides a constant airway pressure for the duration of the inspiratory time
Leak Compensation
Leak Compensation provides flow during the expiratory phase to maintain the baseline
pressure in patients that are breathing spontaneously, but have a leaking airway or facemask
To avoid nuisance alarms in patients with active leaks, Leak Compensation suppresses he
following alarms:
• Incomplete Exhalation (Alarm #3091)
• Insufficient Flow (Alarm #2095)
The following table lists the ventilator modes and their availability in the ZOLL ventilator
models, and gives the options and ranges for the ventilation mode parameters:
Parameter Window Options /
Availability/Notes Models
Range
Primary Value Mode AC All
SIMV EMV+, Eagle II
CPAP All
BL All
Secondary
Target (V) or (P) AC and SIMV modes All
parameter Values
(SIMV not available
with AEV)
LC On or OFF
Default off
AC(P), SIMV(P) modes EMV+, Eagle II
BL modes All
CPAP
Default on
Alarms N/A
Measured Value N/A
Apnea Back Up Context Menu CPAP and BL modes
Apnea Back Up BPM 1 to 80 All
Apnea Back Up PIP 10 to 80 All
Apnea Back Up I:E , Ti 1:1 to
Control selected in con-
All
text BPM Context Menu
1:99, 0.1 to 3
4-4 www.zoll.com 906-0731-01-05 Rev. B
Page 61
BPM (Breathes Per Minute) -- Timing and Rate
Management
The BPM parameter describes the number of breaths-per -minute . The selected ventilation
mode determines when this value is a setting or a measurement.
Assisted and controlled breaths are time-cycled. For spontaneous breaths, the ventilator uses
the percent of the peak flow to terminate the breath being delivered (flow cycled).
Control Parameter
The Ti (Inspiratory Time) parameter adjustment sets the inspiratory time for the control and
assisted breaths (AC and SIMV modes). For volume targeted breaths, the Ti parameter affects
the gas flow rate (the device displays Pop-U p messages when the mini mum and maximum flow
rate values have been reached).
Rise Time
When PS is selected, you can adjust the time it takes to reach PIP. You can specify an index of
1 (shortest) to 10 (longest). The device uses the PIP waveform as a reference when selecting the
Rise Time for the patient.
You should reassess and readjust the Rise Time settings after the patient is placed on the
ventilator and initially stabilized. To minimize patient's work of breathing and potential for
pressure overshoots, you must take the following into consideration when setting the Rise
Time:
• Patient's respiratory pattern
• Patient's comfort
• Patient's flow demand
• Resistance (Mechanical/Physiological)
• Compliance characteristics
The Rise Time for a passive lung is driven primarily by airway resistance, and is fairly
independent of compliance.
Resistance Rise Time
51
20 3
50 5
200 10
An adult patient with high Resistance may benefit from a Rise Time setting of 3 to 4 for
optimal breath delivery. Rise Times of 8 to 10 are optimized for infants and are flow limited.
(The infant circuit is not intended for flows > 60 LPM.)
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-5
Page 62

USING THE ZOLL VENTILATOR
Cycle Off % Parameter
The ZOLL Ventilator transitions from inspiratory to expiratory phase when the flow drops
below a set percentage of the peak flow.
You can adjust the Cycle % value t account for patient leaks.
Note: The longest duration of a spontaneous breath is 5 seconds. At the end of this time, the
ventilator ends flow and opens the exhalation valve.
Clinicians must carefully assess the patient's response when applying the adjusted % -- you
must adjust the % value carefully to optimize patient ventilatory support and comfort.
The Cycle Off % parameter is principally for noninvasive modes where a much higher setting
is required to cycle the breath properly in the presence of a leak. If a higher value is not used
and there is a leak, the system tends to time cycle at 5 seconds instead of flow cycle (if t he leak
flow is higher than 25% of the peak flow, the cycle threshold is never crossed.)
If there is no leak, increasing the Cycle Off % parameter causes breaths to cycle sooner, and
deliver less volume. If you set the Cycle Off % parameter too high, the breath ends early
relative to patient effort, which may lead to the triggering of a second breath.
Spont Ti Limit Parameter
The Spont Ti Limit parameter provides an additional method to operate the delivery of breaths
and maximize patient comfort.
Manual Breath/Plateau Pressure Button
The Manual Breath/Plateau Pressure button delivers a breath only if pressed during the
expiratory phase when the airway pressure drops to the PEEP target.
In AC and SIMV, pressing the Manual Breath/Plateau Pressure button deliv ers a breath defined
by the settings.
In CPAP and BL, pressing the Manual Breath/Pl ateau Pressure button delivers a breath defined
by Apnea Back-Up settings.
Press and hold the Manual Breath/Plateau Pressure button to perform a plateau pressure
maneuver.
4-6 www.zoll.com 906-0731-01-05 Rev. B
Page 63

BPM Parameter Settings
The following table gives the options and ranges for the BPM parameters:
Parameter Window Options /
Range
Primary Value BPM
Breaths per
minute
Secondary
parameter Values
Alarm Limits High breath rate 20 to 99, off
Measured Value Minute Volume
BPM Context Menu
Ti (sec)
|or
I:E
Low breath rate 2 to 40
Vmin (ml)
0 to 80 Volume Target: Control Setting
Ti 0.1 to 3.0
or
1:1 to 1:99
Ti 0.1 to 5.0
or
I:E 4:1 to 1:99
0 to 99.9
Availability/Notes Models
All
Pressure Target: Measured
See BPM context menu: Control Parameter
Inverse I:E EMV+,
All
Eagle II
Control
Parameter
Rise Time - Default # 1 to 10 Auxiliary Box All
Cycle Off %
(% Cycle )
Spont Ti Limit Default
Default I:E 1:1 to
1:99, 0.3 to 3
Ti 0.1 to 5.0
or
I:E $:1 to !:99
Default 25% 10 to 70% Auxiliary Box All
0.30 to 4.00
Adult = 3.00
Infant = 2.00
Mask CPAP =
3.00
The control value is shown in
the Parameter window, the
dependent value is shown in the
Auxiliary Box.
Inverse I:E EMV+,
All
Eagle II
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-7
Page 64

USING THE ZOLL VENTILATOR
Vt (Tidal Volume)
The Vt parameter gives the tidal volume (ml) delivered to the lung. The selected ventilation
mode determines if this value is a setting or a measurement.
In volume targeted modes, pressing the VT parameter button highlights the current set tidal
volume and enables it to be changed.
In pressure targeted breaths, the delivered tidal volume is shown as outlined text and is based
on the patient pulmonary mechanics. The VT High and Low Limits are also available as
secondary parameters.
Warning! In NPPV, a VT that is lower than anticipated given the patient's size may be an
indication that the patient is not able to adequately spontaneously ventilate.
The ventilator circuit is part of the breathing system of the ventila tor. Tubing compliance of the
circuit is a physical property that affects the tidal volume delivered to the patient. The ZOLL
Ventilator allows you to adjust the compliance value of the circuit (see Chapter 6 for more
information).
Note: In the CPAP-NPPV, the V
volume going to the patient when leaks are present. The O
display the O
use, though the amount used is more than if no leak was present.
2
delivered and V
T
may be overestimates of the true
min
Use values accurately
2
Warning! If significant leaks are present during NPPV modes, the VT delivered and V
may be overestimates of what is actua lly being deli vered to the pati ent. The adequacy of
ventilation should be assessed using an alternate method.
min
shown
4-8 www.zoll.com 906-0731-01-05 Rev. B
Page 65

The following table gives the options and ranges for the Vt parameters:
Parameter Window Options /
Range
Primary Value Vt
ml
Secondary
Alarm Limits High Vt 50 to 2000,
Low Vt 5 to 500,
Vt Context Menu
Tubing Compliance (CT)
Adult Default: 1.60 0 to 3.50 The changed value is not
Infant Default: 0.50 0 to 2.00 All
Compliance
Volume (ml)
Default : Off OFF, Adult,
(Measured
value )
50 to 2000 Volume Target: Control Setting
Off
Off
Infant
0 to 349 All
Availability/Notes Models
All
Pressure Target: Measured
Auxiliary Box All
All
retained when the device is
turned OFF
PIP (Peak Inspiratory Pressure) -- Pressure Management
In volume targeted modes, the primary field shows the delivered PIP as outline text. In pressure
targeted modes, the PIP target is displayed and is adjustable. The PIP High Limit, PIP Low
Limit, and PEEP are also available as second ary parame ters.
During the exhalation phase, the ventilator opens the exhalation valve when the pressure is
above the PEEP setting, and closes it when below the setting.
In Bilevel Ventilation Mode, the ventilator provides noninvasive ventilation with the ability to
manage the patient by adjusting the IPAP and EPAP parameters.
Caution Set the trigger level to minimize the work of breathing for the patient and prevent
auto-triggering. Set the Vt alarms to bracket average tidal volume so that the unit detec ts pending
respiratory failure (low tidal volumes) and excessive leaks (h ig h tid al vo lumes).
Spontaneous/Assisted Breath Trigger
The Spontaneous/Assisted Breath Trigger is preset to -2.0 cm H2O and can be adjusted from-
6.0 to -0.5 cm H2O below the baseline (PEEP) pressure. In order to initiate a spontaneous or
assisted breath, the patient must generate -2.0 cm H2O. When the pressure drop is detected, an
assisted breath is delivered.
The trigger automatically adjusts when the PEEP is changed.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-9
Page 66

USING THE ZOLL VENTILATOR
Plateau Pressure
Press and hold the Manual Breath/Plateau Pressure button to perform a plateau pressure
maneauver.
4-10 www.zoll.com 906-0731-01-05 Rev. B
Page 67

Pressure Management
The following table gives the options and ranges for pressure management.
Parameter Window Options /
Range
Primary
Value
Secondary
Value
PIP
cm H2O
PEEP 0 to 30 AC Modes (ACV, SIMV, CPAP,
PS 0 to 60 Spontaneous Breaths
EPAP 3 to 30 Spontaneous Breaths
IPAP 6 to 60
10 to 80 Volume Target: Measurement
3 to 30
Availability/Notes Models
All
Pressure Target: Control Setting
PIP values greater than 60 cm
H2O require the operator to
perform a separate
confirmation.
All
BL Modes
(SIMV and CPAP )
All
BL
Alarm Limits High PIP 20 to 100 PEEP cannot be within 5 cm
H
O of the PIP High Limit
2
setting.
Low PIP 3 to 35, Off All
Measured
Value
PIP Context Menu All
Breath Trigger
Mean Airway Pressure
MAP
Paw Waveform 0 to 100 All
Default : -2
0 to 99.9 All
-6 to -0.5
Adjustment Increments: .5 All
All
(Assisted,
Spontaneous)
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-11
Page 68

USING THE ZOLL VENTILATOR
FIO2 (Fraction of Inspired Oxygen) -- Oxygen Delivery
Management
Pressing the FIO2 parameter button highlights the current FIO2 value and enables you to adjust
it. There are no adjustable secondary parameters. The default values at start up is 21% whether
oxygen is present or not. If an FIO
settings, the unit start ups with that saved FIO
high-pressure oxygen is not present, the unit starts up with FIO
PRESSURE LOW alarm is not activated. The seconda ry di splay i n the parameter wi ndow is O
Use1. This is the flow (liters/min) of high pressure oxygen used by the unit to support the
patient at the current settings. O
sign next to the FIO
oxygen use in the O
The following table lists the options and ranges for the FIO2 parameter:
value when this mode is active. (The “O2 Use” value does not include
2
Reservoir.)
2
value greater than 21% is saved and used for Power Up
2
value if high-pressure oxygen is present. If
2
= 21% and O2 SUPPLY
2
Reservoir mode is indicated on the display with a plus “+”
2
2
Parameter Window
Primary Value FiO2
%
Secondary Values
Alarm Limits
Not Applicable
Not Applicable All
Measured Values O2 Use (L/min)
FiO2 Context Menu
O2 Reservoir Default : off
Options /
Availability/Notes Models
Range
21 to 100 All breaths are delivered from
the compressor at 21%
All breaths are delivered from
the High Pressure O2 Source at
100%
0 to 99.9
Off / On
Shows when High Pressure
Oxygen Supply is present.
“+” icon indicates when “on” for
low flow oxygen.
All
All
All
All
1. O2 Use = ((FIO2-0.21)/0.79)*Minute Volume where FIO2 is represented as a fraction and minute
volume is the actual minute volume (controlled and spontaneous breaths * tidal volume).
4-12 www.zoll.com 906-0731-01-05 Rev. B
Page 69

SpO2 -- Using the Pulse Oximeter
The primary use of the device is as a ventilator -- the pulse oximeter operates only when the
device is providing ventilation.
The following conditions can affect the pulse oximeter reading:
• The sensor is too tight.
• There is excessive illumination from light sources such as a surgical lamp, a bilirubin lamp,
or sunlight.
• A blood pressure cuff is inflated on the same extremity as the one with a SpO2 sensor
attached.
• The patient has hypotension, severe vascoconstriction, severe anemia, or hypothermia.
• There is an arterial occlusion proximal to the sensor.
• The patient is in cardiac arrest or is in shock.
The SpO
standby (and displays stby in the parameter window) when
display is active only when the pulse oximeter is connected. The pulse oximeter is in
2
• No SpO
• The sensor is off the patient during start up
• You place the pulse oximeter in standby
sensor is connected
2
Note: You can place the pulse oximeter in standby only when the probe is disconnected from
the patient. A valid signal automatically brings the pulse oximeter out of standby.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-13
Page 70

USING THE ZOLL VENTILATOR
SpO2 Parameter Values
Pressing the SpO2 parameter button highlights the Low SpO2 Alarm Limit and enables its
value to be changed. The default low SpO
the same Context Menu as the HR parameter.
The following table gives the options and ranges for the SpO2 parameter:
Parameter Window Options /Range Availability/Notes Models
value at start up is 94%. The SpO2 parameter uses
2
Primary Value SpO2
84 to 100
%
Secondary
Not Applicable
Values
Alarm Limits Low Limit
Measured
Values
Pleth
Waveform
86 to 99, Off
SpO2 Context Menu (note same
as HR Context Menu)
Pulse Ox Default :
Standby
Standby, Off,
On
Fast SAT Default : Off Off/On
Measurement
Fast SAT enables rapid tracking of
arterial oxygen saturation changes by
minimizing the averaging. This mode is
clinically applicable during procedures
when detecting rapid changes in SpO
is paramount such as induction,
intubation, and sleep studies.
2
All
All
Sensitivity Norm Max
APOD Off Off, On
Averaging 8 Seconds 2 to 4, 4 to 6,
8, 10, 12, 16
Norm adjusts the pleth signal
sensitivity. Max interprets and displays
data for even the weakest of signals.
Max is recommended during
procedures and when clinician and
patient contact is continuous.
When on, this mode improves detection
of the "probe off patient" condition, but
reduces the ability to acquire a reading
on patients of low perfusion.
Adjusts the SpO2 and HR averaging
durations.
Seconds
4-14 www.zoll.com 906-0731-01-05 Rev. B
Page 71
Signal Strength Measured
Value
0 to 20
Current signal strength value, not
adjustable. A value of zero indicates that
no measurement is available. This value
helps clinicians place sensors on
optimal sites
Signal IQ Measured
Value
Bar Graph
Bar graph displays the relative reliability
of the pulse oximeter signal.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-15
Page 72

USING THE ZOLL VENTILATOR
HR (Heart Rate)
The HR (Heart Rate) parameter window displays the patient’s heart rate when the pulse
oximeter is working and the sensor is attached.
Pressing the HR parameter button highlights the High Heart Rate alarm limit and enables its
value to be changed. Pressing the HR button a second time highlights the current value of the
Low Heart Rate Alarm limit and enables its value to be changed. Both limits are adjustable by
1 b/min. The default value at start up for the high alarm limit is 120 BPM (Beats Per Minute);
the low alarm limit is 40 BPM.
The following table gives the options and ranges for the HR parameter:
Parameter Window Options /
Range
Primary Value HR
Secondary
Values
Alarm Limits High Limit 80 to 240,
Measured
Values
HR Context Menu (note same as SpO2 Context Menu)
%
Not Applicable
Low Limit 30 to 79,
Pleth Waveform
0 to 240 Measurement - Heart Icon blinks
Off
Off
Availability/Notes Models
at the beat rate.
All
4-16 www.zoll.com 906-0731-01-05 Rev. B
Page 73

Managing Pop Up Messages
To prevent the setting of parameter values that are outside the typical cl inica l range of settings,
the ventilator presents Pop Up messages that ask if you are sure you would like to set the
parameter beyond the typical range.
When a message occurs, you are asked to press the Accept/Select button before you can adjust
a parameter beyond the typical range. Pop Up messages are also used to alert you that certain
settings are not permitted. In addition, Pop Up messages can call for you to press Accept/Select
to acknowledge that you are entering configurations where certa in alarms are being suppressed,
turned “off”, and/or canceled.
We provide a comprehensive list of pop up messages in Chapter 5, “Alarms.”
Pop Up Message Example
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-17
Page 74

USING THE ZOLL VENTILATOR
Managing Alarms
The ZOLL Ventilator uses Smart Help™ messages that provide a comprehensive suite of
alarms. Smart Help messages alert operators and guide their action s to resolve alarm conditions
and ensure patient safety.
At the onset of an alarm, the scree n displays the alarm name and then a series of
context-sensitive Smart Help messages, which describe the possible cause and resoluti on of
that alarm. When multiple alarms occur, the unit prioritizes alarms and displays those alarms
that indicate the greatest risk to the patient first.
Smart Help Example
The previous illustration provi des an exa mple of what the device di spla ys when there are
several alarms. The displayed Alarm message corresponds to the dark alarm bell at the bottom
of the display. You can cycle through the various alarms by turning the ventilator’s selection
dial. If there are less than 5 alarms, this alarm list also includes a “plot” icon, where the alarm
screen is replaced by the Pulse Pleth/Time and Pressure/Time plots.
We describe A la rms in det ai l in Chap ter 5, “Alarms” and provide a comprehensive reference.
4-18 www.zoll.com 906-0731-01-05 Rev. B
Page 75
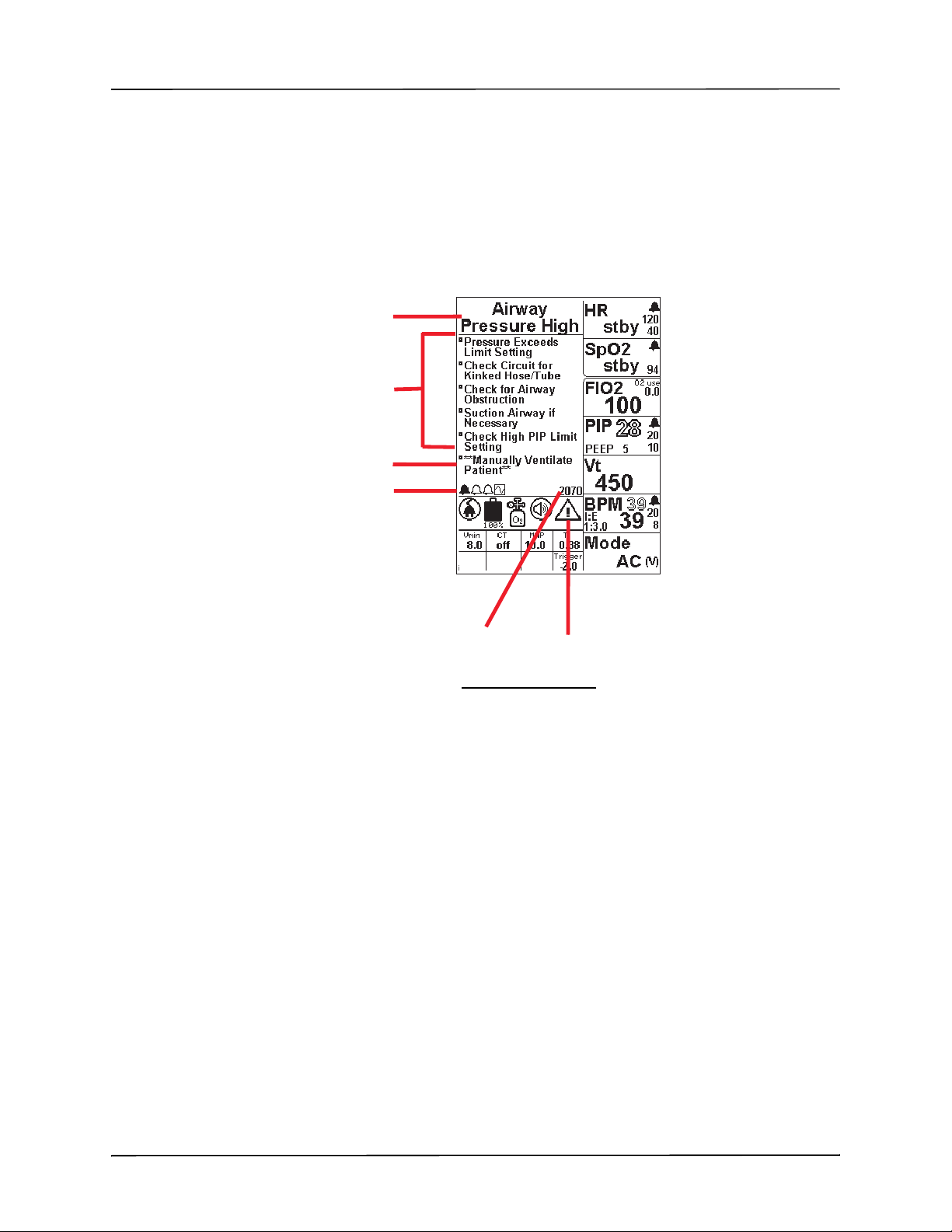
Smart Help Messages
a
b
c
d
e
f
At the onset of an alarm, the Alarm Message Center (AM C) in the upper left-hand corner of the
device’s LCD screen displays a Smart Help message. The Smart Help message displays the
alarm name with a series of messages to help the operator resolve the alarm. The AMC
indicates the number of active alarms as a series of Alarm Bell icons at the bottom with each
bell indicating an active alarm. The ventilator prioritizes alarms and displays the alarm
indicating the greatest risk first. All messages are context-based and suggest what is causing the
condition and how it can be resolved.
Smart Help Display
Smart Help messages contain the information and instructions for all active alarms, such as in
the previous example:
a. Alarm Name: Describes the nature and/or cause of the fault or failure. The Alarm
Name appears at the top of the AMC. When more than one alarm occurs at the
same time, the unit prioritizes them based on patient safety.
b. Mitigation/Resolution Instructions: Instructions for the operator as to how the
alarm state may be resolved.
c. If not Resolved Instructions area: Instructions for the operator on what to do if
they cannot resolve the alarm state. The instruct ion is always shown in the following
format **Message...**.
d. Alarm Icons: For each active alarm, an alarm bell appears. When multip le ala rms
are active, the number of bells corresponds to the number of alarms. The alarm in
the AMC is demonstrated as the solid bell. To view each active alarm, turn the
selection dial to scroll through all active alarms. If there are less then 5 alarms, the
plot icon also appears.
e. Service Code: Each alarm has a 4 digit number associated with it, wh ich helps the
operator indicate the specific alarm when communicating with technical support.
f. Attention W arning Icon: Identifies the severity of the alarm: Low, Medium, or
High priority.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-19
Page 76

USING THE ZOLL VENTILATOR
Alarm Priorities
Alarm priorities define the operational state of the device regarding its ability to provide
mechanical ventilation. The alarm priority determines what effect pressing the
MUTE/CANCEL button has. There are three priorities:
• High Priority: Mechanical ventilation under operator control is no longer possible. This
alarm category requires immediate intervention by the operator. This includes system failure
alarms where the CPU has failed and a backup has taken over to sound the audible and
visual alarms. It also includes when the device is turned on and there is no internal or
external power source. Pressing the MUTE/CANCEL button has no affect on the High
Priority alarm. The alarm can only be silenced by turning off the ventilator.
• Medium Priority: Mechanical ventilation is active or is possible (maybe for a finite period
of time), but there is a failure/fault with the patient, ventilator circuit, a pneumatic
subsystem, or pulse oximeter. This alarm category requires immediate intervention by the
operator. Pressing the MUTE/CANCEL button mutes Medium Priority alarms for 30
seconds. If after 30 seconds the alarm-causing condition still exis ts, the audible alarm recurs
until it is muted again for another 30 second period or resolves.
• Low Priority (Advisory): Safe mechanical ventilation is active, but there is a fault that the
operator must be aware of to ensure safe management of the patient and/or ventilator. Low
Priority alarms present themselves with both an audible and yellow LED alarm signal
alerting the operator to the condition. Pressing the MUTE/CANCEL button cancels the
audible signal. If the alarm is not resolved, the yellow LED remains illuminated to remind
the operator of the fault or failure. You c an cancel some Low Priority alarms to avoid
nuisance alarms.
If the alarms are Low Priority, then the Pleth and Pressure/Time plots appear permanently on
the screen when the alarms are muted. If the alarms are Medium Priority, the unit cycles
through each Medium Priority Alarm for a 20 second period. You can use the selection dial to
select a particular Medium Priority Alarm and/or Plot for 20 seconds, after which the above
cycling rotation resumes. New Alar ms can overwrite the screen at any time.
The first digit in the service code indicates the alarm priority:
1###: High Priority alarms
2###: Medium Priority alarms
3###: Low Priority alarms
4-20 www.zoll.com 906-0731-01-05 Rev. B
Page 77

Silencing Alarms
The operator may decide, based on their clinical assessment, to silence certain alarms that, in
the given situation, are considered “nuisance” alarms and do not assist in the safe management
of the patient. Before any alarms can be silenced, the operator receives a Pop Up message
asking them to confirm their understanding that the alarm is no longer available in the current
operating session.
Alarm Preemptive Mute upon Power up
When the unit is first powered up, certain patient circ uit alarms are preempti vely muted for 120
seconds, to allow the operator time to get the patient circuit properly adjusted without nuisance
alarms.
Note: During this preemptive mute of this audible alarm, the LED alarm light and alarm
message are still indicated.
There is a countdown timer located under the muted alarm symbol, showing how much time of
the 120 seconds is remaining. The alarms that have this preemptive mute are:
Service Code Alarm Name
2062 Exhalation Fault
2070 Airway Pressure High
2071 Low Airway Pressure
2072 High Tidal Volume
2073 Low Tidal Volume
2074 High Breath Rate
2075 Low Breath Rate/Apnea
2076 Apnea
2090 PEEP Leak
2095 Insufficient Flow
2100 Patient Disconnect
2170 Spontaneous Breath-PIP High
2171 Spontaneous Breath-PIP Low
2172 Spontaneous Breath-V
2173 Spontaneous Breath-V
2300 Pulse Ox Module Failed
2301 Internal Communication Failed
2314 SpO2 Sensor Off Patient
2401 SpO2 Low
High
T
Low
T
2410 Heart Rate High
2411 Heart Rate Low (Pulse Rate Low)
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-21
Page 78

USING THE ZOLL VENTILATOR
Service Code Alarm Name
3300 SpO2 Shutdown (MS 11 Failure-Monitor Not In Use)
3301 SpO2 Shutdown (Communication Failure EMV-Pulse Ox-Monitor Not In
Use)
3310 No SpO2 Sensor Connected (No Sensor Detected)
3311 Defective Sensor
3312 SpO2 Pulse Search
3313 SpO2 Signal Interference
3315 Too Much Ambient Light
3316 Invalid SpO2 Sensor (Unrecognized Sensor)
3317 Low SpO2 Perfusion (Low Perfusion)
3318 Low SpO2 Perfusion (Poor SpO2 Signal
Turning Off Alarms at Extreme Range Limits
If the operator sets the following alarm limits to their extreme range, t he ventila tor turns of f the
indicated alarms after Pop Up message confirmation:
1. High Breath Rate (Alarm #2074).
2. PIP Low (Alarms #207 1, 21 71) -- the d evice au tomati cally turn s off these alarm limits
in NPPV mode.
3. V
High (Alarms #2072, 2172) -- the device automatically tu rns off these alarm limits
T
in NPPV mode.
4. V
Low (Alarms #2073, 2173) -- the device automatically turns off these alarm limits
T
in NPPV mode.
5. Low SpO2 (Alarm #2410)
6. High Heart Rate (Alarm #2410)
7. Low Heart Rate (Alarm #2411)
If an alarm has been turned off and is then modified, but is not accepted, then the alarm
parameter is set to the values indicated in the following table. This is done to ensure patient
safety in the event of an inadvertent value change. You can change these values following the
parameter change procedures described above.
High
Breath
PIP Low
VT High VT Low
Low SpO2
Rate
99 BPM 3 cm H2O 2000 ml 0 ml 86% 240 BPM 30 BPM
High
Heart Rate
Low Heart
Rate
4-22 www.zoll.com 906-0731-01-05 Rev. B
Page 79

Alarm Cancellation in Alarm Configuration Menu
There are clinical situations where an alarm occurs, and in the ope rator’s clinical judgment, this
alarm should be canceled for the remainder of the unit’s operating session. The following
constraints apply to alarm cancellation:
1. Only alarms that have occurred in the current operating session can be canceled.
2. Alarms which have not occurred since turn on are indicated with a “--”.
3. Canceled alarms are not be saved in the User Settings for the next session.
4. All canceled alarms reappear (if appropriate) when the unit is next turned on. (As an
example, the Self Check Fault, calibration due Alarm # 3120, reappears in the next
operating session.)
You may cancel the following alarms in the Alarm Configuration Menu:
1. Self Check Fault, calibration due (Alarm #3120)
2. RTC Battery Fault (Battery Low) (Alarm #3110)
3. Incomplete Exhalation (Alarm #3110)
4. PEEP Leak (Alarm #2090)
5. Fresh Gas Intake Fault (Alarm #3031)
• Patient Inspiratory Demand Not Met (Alarm #3092)
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 4-23
Page 80

USING THE ZOLL VENTILATOR
4-24 www.zoll.com 906-0731-01-05 Rev. B
Page 81

Chapter 5
Alarms
This chapter provides a detailed description and comprehensive reference for the ZOLL
Ventilator’s alarms and Pop Up Messages. This chapter
• Describes the display format of ZOLL’s Smart Help messages in detail.
• Describes alarm types and priorities.
• Provides a comprehensive list of alarms and Pop Up messages.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-1
Page 82

ALARMS
Alarm Overview
To safeguard the patient, the ZOLL Ventilator continuously monitors the patient, device, and
environment to ensure that all of the systems are functioning as intended. When device detects
a problem, it triggers an alarm and displays a Smart Help message to alert you.
On the Smart Help message, a multi-line message screen appears in the upper left-hand corner
of the display screen. This screen area is the Alarm Message Center (AMC). The AMC displays
the alarm name with a series of messages to help you resolve the alarm. The device prioritizes
alarms based on the risk to the patient and always presents the alarm with the greatest risk to the
patient first. All messages are context-based and suggest what is causing the alarm and how it
can be resolved.
The Alarm Message Center (AMC) contains the information and instructions for all active
alarms, as in the following example:
Smart Help Alarm Display
a. Alarm Name: describes the type or cause of the alarm. The Alarm Name appears
at the top of the AMC. When more than one alarm occurs at the same time, the
device prioritizes the alarms based on the highest risk to the patient.
b. Mitigation/Resolution Instructions: prioritized instructions that descri be how to
resolve the alarm state.
c. If not Resolved Instructions: Instructions on what to do you cannot resolve the
alarm state. The instruction is always shown in the following format
**Message...**.
5-2 www.zoll.com 906-0731-01-05 Rev. B
Page 83

d. Alarm Icons: For each active alarm, an alarm bell appears. When multip le ala rms
are active, the number of bells corresponds to the number of alarms. The alarm in
the AMC is displayed as the solid bell. To view each active alarm, turn the Dial to
scroll through all active alarms. The plot icon is also in this list. It allows you to see
the current waveform to better assess the nature of the failure. A maximum of six
alarms can be displayed without the plot icon.
e. Service Code: Each alarm has a 4 digit number associated with it, wh ich helps the
operator indicate the specific alarm when communicating with technical support.
The service codes appear in the following format:
1### High Priority Alarm
2### Medium Priority Alarm
3### High Priority Alarm
f. Attention W arning Icon: Identifies the severity of the alarm: Low, Medium, or
High priority . See the Symbols tabl e in Chapter 1 for the appearance of the warning
triangle for each of these three alarms.
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-3
Page 84

ALARMS
Alarm Priorities
Alarm priorities define the operation al status of the device and its abilit y to provide mecha nical
ventilation. The alarm priorities are as follows:
High Priority
Mechanical ventilation under user control is no longer possible. This alarm priority requires
immediate intervention. This includes system failure alarms where the CPU has failed and a
backup has taken over to sound the audible and visual alarms. It also includes when the device
is turned on and there is no internal or external power source.
Pressing the Mute button has no effect on a high priority alarm. The alarm can only be silenced
by turning off the ventilator.
Medium Priority
Mechanical ventilation is active or is possib le (may be for a finite period of time) but, there is a
failure or fault with the patient, ventilator circuit, a pneumatic subsystem, or pulse oximeter.
This alarm priority requires immediate intervention by the user.
Pressing the Mute button mutes medium priority alarms for 30 seconds. If the alarm trigger still
exists after 3 seconds, the audible alarm recurs until you mute it again for another 30 second
period or the alarm is resolved.
Low Priority (Advisory)
Safe mechanical ventilation is active but, there is a fault that you must be aware of to ensure
safe management of the patient or ventilator. Low priority alarms present with both an audible
and yellow LED alarm signal alerting you to the condition. Pressing the Mute button cancels
the audible signal. If the alarm is not resolved, the yellow LED remains illuminated to remind
you of the fault or failure.
Note: Some Low Priority alarms are canceled and the Alarm LED turns green when you
push the Mute button. For others, the audible alarm is canceled but the Alarm LED
stay yellow to remind you that the device is operating in a state that needs careful
monitoring.
Popup Messages
These alerts appear whenever you attempt to adjust that device in a way that is outside clinical
norms or is outside the performance range of the ventilator. Pop Up Messages also appear when
you are required to confirm their action before you proceed.For example, if you try to set the
low breath rate alarm below 4 that would, practically, disable the alarm. If the desired value is
outside the performance range, the Pop Up message alerts you to why cannot make the change.
(Example: trying to set the PEEP level greater than the PIP setting).
5-4 www.zoll.com 906-0731-01-05 Rev. B
Page 85

Muting Alarms
Under most conditions, pressing the Mute button mutes the audible alarm for 30 seconds. As
describe in the previous section, pressing the Mute button when a high priority alarm is active
does not. Using the ZOLL Ventilator, muting functions as follows:
Preemptive Mute -- to prevent excessive noise in the patient care environment patient, safety
alarms, such as Patient Disconnect, PEEP Leak, and so on, can be preemp tively muted for 30
seconds. This enables you to prevent the audible alarm, by pressing the Mute button, before
initiating a procedure that could trigger an alarm.
2-Minute Startup Mute -- at start up, the ventilator suspends active patient safety alarms, with
the exception of those alarms that could affect the performance of the devic e. This prevents
nuisance alarms during start up while you configure the ventilator. When the Start Menu is
used, the 2-minute countdown starts onc e you se lec t a start opt ion. Once the pati ent connected,
the mute cancels automatically after 15 seconds when there are no active alarms.
Use in High Noise Environments -- in high noise environments, you may be inclined not to
mute the alarm while addressing the problem. Not pressing Mute limits the user's ability to
resolve the alarm because with each breath the alarm is retriggered and any parameter changes
you are attempting are canceled as the alarm retriggers
Alarms Types
The ZOLL Ventilator’s alarm types provide a framework for you to see the scope and range of
the alarms that the device uses. The alarm types are:
• Patient Safety -- Patient Safety Alarms address the ventilation of the patient and their
respiratory effort. Pulse oximetry monitoring and circuit/exhalation valve issues are also
part of this group.
• Environment - Environment Alarms address the device inputs: external power, battery,
high pressure O2 supply, and the fresh gas intake. Ambient and device temperature,
barometric pressure, and altitude are also part of this group.
• Self Check -- Self Check Alarms address the performance of the device systems and
include
1.
2. Pneumatic Sensor: faults/failures of the pneumotachographs that measure gas flow or the
3. Pneumatic System: faults/failures of the compressor of O
4. Power System: faults/failures of the power system that render the device unable to operate
5. Pulse Oximeter (Ox) Module: faults/failures of the pulse oximeter module that are not related
6. Preventive Maintenance: alarms that occur when device is due for preventive maintenance.
Internal Communication (Comm): faults/failures of interdevice communication, cyclic
redundancy checking, or processor-related issues
pressure transducers
from external power or charge/operate from the internal rechargeable battery.
to monitoring of the patient, a fault or failure of the module.
.
supply proportional valve.
2
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-5
Page 86

ALARMS
Alarm Groups
The following table displays the ZOLL Ventilator’s Alarm Groups:
Patient Safety Environment Self Check
Alarm Groups
Airway Pressure
-- High/Low
Apnea External Power
AutoPEEP Gas Intake
Breath Rate (BPM)
-- High/Low
Exhalation
-- Fault/Failure
Battery
-- Battery Drained
-- Battery Nearly Drained
-- Battery Low
-- Discharge Fault
-- Too Hot/Cold
-- Current High
-- DC Power Reversed
-- High
-- Low/Disconnect
-- Blocked
-- Restricted
O2 Supply
-- High/Low - Disconnect
Ambient Pressure
-- High/Low
Firmware Mismatch
Internal Comm
-- Fault/Failures
Pneumatic Sensor
-- Airway
-- Autocal
-- Pneumotach
-- Transducer
Pneumatic System
-- Compressor
-- O2 Valve
Heart Rate (HR)
-- High/Low
Inspiratory Demand Preventive Maintenance
Insufficient Flow Pulse Oximeter
Patient Detected Temperature
PEEP Leak
Power Cycle Needed
-- Comm Failure
-- Module Failure
-- High/Low
-- Sensor Failure
5-6 www.zoll.com 906-0731-01-05 Rev. B
Page 87

SpO2
-- Low
-- Disconnect
-- Defective Sensor
-- Not Connected
-- Invalid Sensor
-- Light Contamination
-- Low Perfusion
-- Search
Tidal Volume
-- High/Low
Tubing Compliance
Alarm Groups
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-7
Page 88

ALARMS
High Priority Alarms
Service
Code
1001
1002
Alarm Name/Mitigation/Resolution
Self Check Failure
Alarm triggers when the compressor fails to operate or fails to provide the flow required to deliver a breath
and high pressure O
Mitigation/Info: Pneumatic System: Compressor, Manually Ventilate Patient, Connect 55
psig/380 kPa O2, Restart Ventilator with O2, **Contact Service Center**
Self Check Failure
Alarm triggers when communication between the compressor controller and Smart Pneumatic Module (SPM)
is lost and high pressure O
Mitigation/Info: Pneumatic System: Compressor, Manually Ventilate Patient, Connect 55
is not available to provide ventilation.
2
is not available to provide ventilation.
2
1003
1010 Self Check Failure
1011
1012 Self Check Failure
1020 Low O2 Supply Failure
Self Check Failure
Alarm triggers when the flow from the first breath is ± 20% of the expected flow for the tidal volume at start
up. This unusually low RPM is a symptom of a dirty flow screen which cannot be serviced by the user.
Mitigation/Info: Pneumatic Sensor: Pneumotach, Manually Ventilate Patient, **Contact Service Center**
Alarm triggers when the O
this occurs the device automatically opens the exhalation valve to prevent pressure from accumulating in the
circuit and ventilation stops.
Mitigation/Info: Pneumatic System: O2 Valve, Manually Ventilate Patient, **Contact Service Center**
Self Check Failure
Alarm occurs when the signal to the O
not available to provide ventilation.
Mitigation/Info: Pneumatic System: O2 Valve, Manually Ventilate Patient, **Contact Service Center**
Alarm occurs when the communication between the O
available to provide ventilation.
Mitigation/Info: Pneumatic System: O2 Valve, Manually Ventilate Patient, **Contact Service Center**
Alarm occurs when the O
port ventilation. If the O
the device will not reestablish O
pressure is between 40 and 87 psig (276 to 600 kPa) the user should check the hose connections for leaks.
Occasionally, this alarm can be caused by a regulator that provides a static pressure within range but is not
able to provide the flow necessary to meet the patient flow demand.
Mitigation/Info: Manually Ventilate Patient, Connect 55 psig/380 kPa O2, Restart, Check O2 Supply for
valve fails in the open position which results in continuous inspiratory flow. When
2
valve is not delivering the required flow rate and the compressor is
2
valve and the SPM fails and the compressor is not
2
supply pressure is <35 psig (241 kPa) and the compressor is not available to sup-
2
source can be restored the device should be cycled off then on to reset. By design
2
operation unless the supply pressure is ≥40 psig (276 kPa). If the supply
2
5-8 www.zoll.com 906-0731-01-05 Rev. B
Page 89

1030
Gas Intake Failure
Alarm occurs when the Fresh Gas/Emergency Air Inlet is blocked so that the compressor is not able to
deliver flow sufficient for the current settings and high pressure O
is not available to support ventilation. The
2
user should clear the blockage and restart the ventilator. A false alarm can be triggered in very high vibration
environments.
Mitigation/Info: Manually Ventilate Patient, Clear Blocked Intake, Connect 55 psig/380 kPa O2, Restart Venti-
1041
1051
1052
1060
High O2 Supply Failure
Alarm triggers when the O
supply pressure is >87 psig (600 kPa). Pressures above 87 psig (600 kPa) can
2
result in a catastrophic failure, harm to the patient and/or damage to the device. While the patient is manually
ventilated the user or assistant should seek to reduce the O2 supply pressure. Sometimes this requires
changing the regulator which is not functioning as required. If the pressure cannot be reduced and a low flow
device like a flow meter is available the user can provide supplemental O
via the optional low flow O2 reser-
2
voir. To clear the alarm the device should be turned off and then restarted with supply pressure in the appropriate range (40 to 87 psig, 276 to 600 kPa) or without the high pressure O2 source connected.
Self Check Failure
Alarm triggers when the autocal procedure is not able to zero the airway pressure transducer to ambient
pressure. When this occurs, manually ventilate the patient, replace the ventilator replaced and contact the
service center for additional information. Note: a false alarm can be triggered during operation in very high
vibration environments when the device is not mounted correctly. If this could be the cause, restart the ventilator and continue operation if no alarms are triggered.
Mitigation/Info: Pneumatic Sensor: Autocal, Manually Ventilate Patient, **Contact Service Center**
Self Check Failure
Communication between the airway pressure sensor and SPM is lost. When this happens, manually ven-
tilate the patient, replace the ventilator and contact the service center for additional information. Mitiga-
tion/Info: Pneumatic Sensor: Airway Pressure, Manually Ventilate Patient, **Contact Service Center**
Exhalation System Failure
Alarm occurs when the PIP fails to return to the baseline pressure for 3 consecutive breaths, indicating that
the exhalation control valve has failed. When triggered, the device stops ventilating and attempts to discharge the pressure in the breathing circuit to atmosphere. This failure may be caused by a significant blockage of the exhalation valve or an occlusion/kink in the exhalation valve tube. If possible, the user should
replace the breathing circuit and restart the ventilator. If this does not resolve the failure, replace the ventilator
and contact the service center for additional information.
Mitigation/Info: Patient Can Not Exhale, Manually Ventilate Patient, Check for Kinked Hose/Tube, Replace
1061
Exhalation System Failure
The airway pressure, PIP, is >40 cm H
seconds, or when the PIP is >75 cm H
O, the PIP High Limit (when PIP High Limit is < 35 cm H2O) for > 5
2
O for > 1.5 seconds. When this happens, the device stops ventilating
2
and attempts to discharge the pressure in the breathing circuit to atmosphere. This failure may be caused by
a significant blockage of the exhalation valve or an occlusion/kink in the exhalation valve tube. If possible,
the user should replace the breathing circuit and restart the ventilator. If this does not resolve the problem,
replace the ventilator and contact the service center for additional information.
Mitigation/Info: Patient Can Not Exhale, Manually Ventilate Patient, Check for Kinked Hose/Tube, Replace
1172
Self Check Failure
Alarm occurs when the 5 volt power bus fails to provide the required voltage. If this failure occurs, the user
should manually ventilate the patient, replace the ventilator and contact the service center for additional information.
Mitigation/Info: Pneumatic Sensor: Autocal, Manually Ventilate Patient, **Contact Service Center**
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-9
Page 90

ALARMS
1173
1174
1175
1176
1420 Self Check: Complete Power Failure
Self Check Failure
Alarm occurs when communication fails between one of the subcomponents and the host processor. If this
failure occurs, the user should manually ventilate the patient, replace the ventilator and contact the service
center for additional information.
Mitigation/Info: Intern al COMM, Manually V entilate Patient, Backup Ventilator Started, **Contact Service
Self Check Failure
Alarm occurs when the device is not able to calibrate the one or more transducers and is no longer able to
operate safely. If this failure occurs, the user should manually ventilate the patient, replace the ventilator and
contact the service center for additional information.
Mitigation/Info: Pneumatic Sensor: Transducer, Manually Ventilate Patient, Restart Ventilator, **Contact Service Center**
Self Check Failure
Alarm triggers when the internal communication bus and the host are not able to communicate with the subassemblies. If this failure occurs, the user should manually ventilate the patient, replace the ventilator and
contact the service center for additional information.
Mitigation/Infor: Internal COMM, Manually Ventilate Patient, **Contact Service Center**
Self Check Failure
Alarm triggers when the calibration file fails its integrity check. The user should manually ventilate the patient,
replace the ventilator and contact the service center for additional information.
Mitigation/Info: Internal COMM, Manu a l ly V e n t ila t e Pa t i e nt, **Contact Service Center**
Alarm triggers when power is lost from both the internal battery and an external source during operation.
When this occurs, the LCD blanks (no power for operation), the audible alarm pulses rapidly, and the visual
alarm flashes rapidly. This alarm will last approximately two minutes. If the device can be recharged after the
failure and there are no other issues it can be returned to service. If there are any questions, contact the service center for additional information.
1430 Drained Battery
Alarm triggers when the internal battery power drops below the amount required to provide ventilation and
external power is not connected. When this occurs there is enough power to operate the user interface and
provide information to the user. The user should be manually ventilate the patient while an external source of
power is sought. To cancel the alarm and begin operation with external power the device must be turned off
and then back on.
Mitigation/Info: Manually Ventilate Patient, Connect External Power, **Contact Service Center**
1471
1472 Self Check Failure
Self Check Failure
Alarm triggers when the device is no longer able to communicate with the User Interface Module (UIM) and
the interface controls. When this occurs ventilation continues at the current settings or the backup mode settings and the high priority alarm sounds. The user should manually ventilate the patient, replace the ventilator
and contact the service center for additional information.
Mitigation/Info: Internal COMM, Manually Ventilate Patient, **Contact Service Center**
Alarm triggers when the device is no longer able to communicate with the Smart Pneumatic Module (SPM).
When this occurs ventilation continues at the current settings or the backup mode settings and the high priority alarm sounds. The user should manually ventilate the patient, replace the ventilator and contact the service center for additional information.
Mitigation/Info: Internal COMM, Manually Ventilate Patient, **Contact Service Center**
5-10 www.zoll.com 906-0731-01-05 Rev. B
Page 91

1473 Self Check Failure
Alarm triggers when no valid data is sent from the SPM within 1 second. When this occurs ventilation continues at the current settings or the backup mode settings and the high priority alarm sounds. The user should
manually ventilate the patient, replace the ventilator and contact the service center for additional information.
Mitigation/Info: Internal COMM, Manually Ventilate Patient, **Contact Service Center**
1474
1475
1480
Self Check Failure
Alarm triggers when cyclic redundancy checking between the device and SPM fails. When this occurs ventilation continues at the current setting or the backup mode settings and the high priority alarm sounds. The
user should manually ventilate the patient, replace the ventilator and contact the service center for additional
information.
Mitigation/Infor: Internal COMM, Manually Ventilate Patient, **Contact Service Center**
Self Check Failure
Alarm triggers when the device has lost communication with the contrast control and in most instances the
content of the LCD is not visible. When this occurs ventilation continues at the current settings or the backup
mode setting and the high priority alarm sounds. The user should manually ventilate the patient, replace the
ventilator and contact the service center for additional information.
Mitigation/Info: Intern al COMM, Manually Ventilate Patient, Backup Ventilator Started, **Con tact Service
Center**
Self Check Failure
Alarm triggers when the device and SPM software loads are not compatible. This alarm is typically associated with an SPM change where the technician failed to update the device and SPM to the current software
revision. Ventilation is provided using the backup mode settings. The user should manually ventilate the
patient, replace the ventilator and contact the service center for additional information.
Mitigation/Info: Firmware Mismatch, Manually Ventilate Patient, Software Compatibility Failure,
**Contact Service Center**
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-11
Page 92
ALARMS
Medium Priority Alarms
2001 Self Check Fault
Alarm triggers when the communication between the compressor and the SPM fails and high pressure O
available to provide ventilation. The alarm will continue to sound as a medium priority alarm until the user
acknowledges that ventilation is being provided using O
changes to low priority. While operating in this state the user should ensure an adequate supply of O2. Fail-
ure to maintain the O
Mitigation/Info: Pneumatic System: Compressor, Operation Switched to O2 Supply, Set FIO2 to 100%, Moni-
2002
Self Check Fault
Alarm triggers when the communication between the O
able to provide ventilation. The alarm will continue to sound as a medium priority alarm until the user
acknowledges that ventilation is being provided using the compressor by setting the FIO
the alarm priority changes to low. While operating in this state the user should monitor the SpO2 to ensure
that adequate oxygenation is maintained. If low flow O
Gas/Emergency Air Intake port using the optional O
O
supply up or down to increase or decrease the amount of O2 delivered to the patient.
2
Mitigation/Info: Pneumatic System: Compressor, Operation Switched to O2 Supply, Set FIO2 to 100%, Moni-
2011 Self Check Fault
Alarm triggers when the signal to the O
the compressor is available to provide ventilation. The medium priority alarm will continue until the user
acknowledges that ventilation is being provided using the compressor by setting the FIO2 to 21%. At this time
the alarm priority changes to low priority. While operating in this state the user should monitor the SpO
ensure that adequate oxygenation is maintained. If low flow O
Fresh Gas/Emergency Air Intake port using the optional O
adjusting the O2 supply up or down to increase or decrease the amount of O2 delivered to the patient.
Mitigation: Pneumatic System: O2 Valve, Operation Switched to Compressor, Connect Low Flow O2, Monitor
2012 Self Check Fault
Alarm triggers when the communication between the O2 valve and the SPM fails and the compressor is available to provide ventilation. The alarm will continue to sound as a medium priority alarm until the user
acknowledges that ventilation is being provided using the compressor by setting the FIO2 to 21%. At this time
the alarm priority changes to low. While operating in this state the user should monitor the SpO
that adequate oxygenation is maintained. If low flow O
Gas/Emergency Air Intake port using the optional O
O2 supply up or down to increase or decrease the amount of O2 delivered to the patient.
Mitigation/Info: Pneumatic System O2 Valve, Set FIO2 To 21%, Connect Low Flow O2, Monitor SpO2
supply will result in a high priority alarm.
2
by setting the FIO2 to 100%. At this time the priority
2
valve and the SPM fails and the compressor is avail-
2
to 21%. At this time
2
is available it can be entrained through the Fresh
2
reservoir. Maintain an acceptable SpO2 by adjusting the
2
valve is outside of the calibration range for the required flow rate and
2
to
2
is available it can be entrained through the
2
reservoir. Maintain an acceptable SpO2 by
2
to ensure
2
is available it can be entrained through the Fresh
2
reservoir. Maintain an acceptable SpO2 by adjusting the
2
is
2
5-12 www.zoll.com 906-0731-01-05 Rev. B
Page 93

2020 Low O2 Supply Fault
Alarm triggers when the O
tilation. When this occurs the device begins ventilation using the compressor. The alarm will continue to
sound as a medium priority alarm until the user acknowledges that ventilation is being provided using the
compressor by setting the FIO
The device works with or without external O2. If O2 is connected the device will not continue O2 operation
unless the supply pressure is ≥40 psig (276 kPa). This is done to prevent continuous cycling between alarms
during the inspiratory phase and no alarm during the expiratory phases. If low flow O
entrained through the Fresh Gas/Emergency Air Intake port using the optional O
acceptable SpO2 by adjusting the O2 supply up or down to increase or decrease the amount of O2 delivered
to the patient.
2030 Gas Intake Fault
Alarm triggers when the Fresh Gas/Emergency Air Inlet is blocked so that the compressor is not able to
deliver a breath within ±10% of the current settings and high pressure O
When this occurs the ventilator immediately switches to O
the FIO
set the FIO
to 100% to acknowledge that the patient is being ventilated at 100%, clear the blockage and then
2
back to the original value. Once the blockage has been cleared operation with the compressor
2
will restart. If the blockage cannot be cleared, the alarm will resound, continue ventilation with FIO2 set to
100% and ensure an adequate supply of O
necessary, the user can activate the O
press the alarm.
2053 Self Check Fault
Alarm triggers when the expiratory time is <170 ms for 3 consecutive breaths. When this occurs the device
attempts to reestablish a baseline by momentarily setting PEEP to 0 cm H
breaths. This interruption lasts no longer than 2 breath cycles. The user should also check for leaks in the
hose and tubes, patient airway and exhalation valve. If recalibration is successful the alarm will automatically
cancel. If the device does not reset, manually ventilated the patient, replace the ventilator and contact the
service center for additional information.
Mitigation/Info: Pneumatic Sensor: Airway Pressure, Check Circuit for Leaks/Disconnects, , Check Tube
2062 Exhalation System Fault
Alarm triggers when the airway pressure, PIP, measured at the end of expiration is >5 cm H
baseline pressure, PEEP. This is typically caused by a restriction of the exhalation valve or an occlusion/kink
in one or more of the breathing circuit tubes or hose. If the breathing circuit tubes appear to be intact the circuit should be replaced to eliminate the possibility of a bad exhalation valve. If the condition does not resolve
the user should manually ventilate the patient, replace the ventilator and contact the service center for additional information.
Mitigation/Info: Check Patient Exhalation, Check Circuit for Kinked Hose/Tube, Check for Blocked Exhalation
2070 Airway Pressure High
Alarm triggers when the airway pressure, PIP, is > the high airway pressure limit for 2 consecutive breaths.
When the limit is reached, the flow decelerates to keep the PIP below the airway pressure for the duration of
the breath (inspiratory time). The user should check for kinks or blockage of the breathing circuit, exhalation
valve or patient airway. In some instances the cause can be an accumulation of secretions in the airway
which will require suctioning to clear. The user should also assess if the patient is fighting the ventilator, asynchrony, or if the high airway pressure limit is set too low.
Mitigation/Info: Pressure Exceeds Limit Setting, Check Circuit for Kinked Hose/Tube, Check fo r Airway
supply pressure is <35 psig (241 kPa) and the compressor is able to support ven-
2
to 21%. The alarm will cancel completely when the user sets to 21%. NOTE:
2
is available it can be
2
reservoir. Maintain an
2
is available to support ventilation.
2
powered ventilation. To clear the alarm first set
2
. NOTE: A high vibration environment can trigger this alarm. If
2
Reservoir Mode while continuing to operate normally. This will sup-
2
O and suspending triggered
2
O above the
2
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-13
Page 94

ALARMS
2071 Low Airway Pressure
Alarm triggers when the airway pressure, PIP, is < the low airway pressure limit for 2 consecutive breaths.
The user should check for leaks/disconnects in the breathing circuit, patient airway or a failure of the exhalation valve. The user should also assess if the patient is breathing with the ventilator, the PIP or tidal volume
are set too low, or if the low airway pressure limit is set too high. If a replacement is available the user should
replace the breathing circuit. If these mitigations do not resolve the alarm condition, replace the ventilator and
contact the service center for more information.
Mitigation/Info: Check Patient Connection, Check Circuit For Loose Hose/Tube, Check Exhalation Valve,
2072 High Tidal Volume
Alarm triggers during pressure targeted ventilation when the delivered tidal volume exceeds the user defined
limit for 2 consecutive breaths. This can be caused by a leak in the patient connection or breathing circuit.
When the ventilator is not able to reach the pressure target flow increases to compensate which leads to a
high delivered tidal volume. It is critical to set this alarm with infant and pediatric patients given that the high
resistance airways used with these patients can provide a false airway pressure even when the patient has
extubated or decannulated. The user should check for leaks/disconnects in the breathing circuit, patient airway or a failure of the exhalation valve. Users should also assess if the patient is anxious and breathing
deeply or if the high tidal volume limit is set too low. If a replacement is available the user should replace the
breathing circuit.
Mitigation/Info: Check Patient Connection, Check Circuit For Loose Hose/Tube, Check Exhalation Valve,
2073 Low Tidal Volume
Alarm triggers during pressure targeted ventilation when the delivered tidal volume does not reach the user
defined limit for 2 consecutive breaths. When this occurs flow decelerates to maintain the airway pressure at
airway pressure limit for the duration of the breath (inspiratory time). If the PIP setting is set properly the
breath should be greater than the low limit, provided it is set correctly. The user should check for kinks or
blockage of the breathing circuit or patient airway. In some instances the cause can be an accumulation of
secretions in the airway which will require suctioning to clear. The user should also assess if the patient is
fighting the ventilator, asynchrony, or if the PIP target is set too low.
Mitigation/Info: Check Circuit For Kinked Hose /Tube, Check For Airway Obstruction, Suction Airway If Nec-
2074 High Breath Rate
Alarm triggers when the actual breathing rate (set rate plus spontaneous patient rate) exceeds the high alarm
limit. This can be caused by the patient breathing too fast due to anxiety or pending respiratory failure. It can
also be caused by autotriggering due to a leak or the when the spontaneous/assisted breath trigger is set too
close to the baseline pressure, PEEP. The user should check for leaks/disconnects in the breathing circuit,
patient airway or a failure of the exhalation valve. The user should also assess if the patient is anxious and
breathing deeply or if the high tidal volume limit is set too low. If a replacement is available the user should
replace the breathing circuit.
Mitigation/Info: Check For Loose Circuit Connection, Check Trigger Setting, Check High Alarm Limit Setting,
2075 Low Breath Rate/Apnea
Alarm triggers when the actual breathing rate (set rate plus spontaneous patient rate) is less than the low
alarm limit. This can be caused by the patient not breathing or breathing at a rate less than the limit. If the
spontaneous/assisted breath trigger is not sensitive enough the patient may not be able to trigger breaths.
The user should also determine if the low rate is set too high for the patient.
Mitigation/Info: Check Patient for Spontaneous Breathing, Adjust Breath Trigger, Check Low Alarm Limit Set-
2076 Apnea
ting, Increase Ventilation Support, ** Manually Ventilate Patient**
Alarm triggers when the spontaneous breathing rate is less than the low alarm limit. This alarm only occurs in
noninvasive ventilation, CPAP and BL modes. The alarm can be caused by the patient not breathing or
breathing at a rate less than the limit. The apnea backup ventilation starts automatically when the alarm is
triggered. The user should select and active mode of ventilation, AC or SIMV, to support the patient.
Mitigation/Info: Apnea Backup Ventilation Started, Set Mode to AC or SIMV, Set Rate and Tidal Volume/
Pressure Target, ** Manually Ventilate Patient**
5-14 www.zoll.com 906-0731-01-05 Rev. B
Page 95

2090 PEEP Leak
Alarm triggers when the airway pressure drops below the PEEP setting by 2 cm H
phase of the breath. This can be caused by a leak in the breathing circuit, exhalation valve or patient airway.
The user should check the breathing circuit and exhalation valve to ensure that all connections are tight. If the
circuit appears damaged or is suspect it should be replaced. The user should also check if there is a cuff leak
from the patient’s airway or mask. If these mitigations do not resolve the alarm the user can choose to use
leak compensation to provide additional flow during the expiratory phase to compensate for the leak. If you
still cannot compensate for the leak, consult the attending physician. If this fails replace the ventilator and
contact the service center for more information.
2095 Insufficient Flow
Alarm triggers when the pressure target is not reached during the inspiratory period during pressure targeted
ventilation. Typically this can occur when the Rise Time is set too low for the patient and their respiratory
mechanics. Decrease the Rise Time and check the circuit and exhalation valve for leaks or disconnects. If
the flow cannot be adjusted appropriately then the patient should be ventilated using volume targeted ventilation.
Mitigation/Info: Pressure Target Not Met, Decrease Rise T ime , Press/Hold BPM Button, Consult Physician,
** Ventilate With Volume Target**
2100 Patient Disconnect
Alarm triggers when the airway pressure fails to exceed the PEEP setting by ~7 cm H
the user should quickly check the patient connection, breathing circuit connections and the exhalation valve.
At times this alarm can be caused by the patient breathing with the ventilator during inspiration which prevents the PIP from passing the minimum pressure. While resolving the alarm condition the user should be
sure to manually ventilate the patient.
Mitigation/Info: Check Patient Connection, Check Circuit for Loose Hose/Tube, Check Exhalation Valve,
Check Patient, Replace Circuit, **Manually Ventilate Patient**
O during the expiratory
2
O. When this occurs
2
2110 Patient Detected
An alarm triggers when you connect the patient to the ventilator while the Start Menu is still active. To resolve
the alarm, you must select a mode of ventilation and configure the device appropriately for the patient. In
addition, you should perform the Operational Test procedure before reconnecting the patient to the device.
Mitigation/Info: Backup Ventilation Started, Set Mode (AC, SIMV, CPAP, BL), Configure Other Settings,
**Manually Ventilate Patient and Restart**
2170 Spont. Breath PIP High
Alarm triggers when the airway pressure, PIP, exceeds the High PIP Limit Setting during 2 consecutive spontaneous breaths. The user should quickly check for kinked hoses/ tubes and check for airway obstruction.
Suctioned the patient if necessary. The user should also check if the High PIP limit is set correctly of if the
pressure support (PS) level is set too high. While resolving the alarm condition the user should be sure to
manually ventilate the patient.
Mitigation/Info: Pressure Exceeds Limit Setting, Check Circuit for Kinked Hose/Tube, Check fo r Airway
Obstruction, Suction Airway if Necessary, Check High Limit Setting, **Manually Ventilate Patient**
2171 Spont. Breath PIP Low
Alarm triggers when the airway pressure, PIP, exceeds the Low PIP Limit Setting during 2 consecutive spontaneous breaths. The user should quickly check circuit for loose hoses/ tubes and also check the exhalation
valve and the tube placement/ cuff. The user should also check if the Low PIP Limit is set correctly. While
resolving the alarm condition the user should be sure to manually ventilate the patient.
Mitigation/Info: Check Patient Connection, Check Circuit for Loose Hose/Tube, Check Exhalation Valve,
Check Tube Placement/ Cuff, Check Low Limit Setting, **Manually Ventilate Patient**
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-15
Page 96

ALARMS
2172 Spont. Breath Vt High
Alarm triggers when the high VT Limit is exceeded during 2 consecutive spontaneous breaths. The user
should check: the patient connection, airway placement, breathing circuit for loose hoses/ tubes and also
check the exhalation valve. The user should also check if the High VT Limit is set correctly. While resolving
the alarm condition the user should be sure to manually ventilate the patient.
Mitigation/Info: Check Patient Connection, Check Circuit for Loose Hose/Tube, Check Exhalation Valve,
Check Tube Placement/ Cuff, Check Limit Setting, **Monitor Patient**
2173 Spont. Breath Tt Low
Alarm triggers when the Low VT Limit Setting is not achieved during 2 consecutive spontaneous breaths.
When this occurs, the user should quickly check for kinked hoses/ tubes and check for airway obstruction.
The patient should be suctioned if necessary. The user should also check if the Low VT limit is set correctly.
While resolving the alarm condition the user should be sure to manually ventilate the patient.
Mitigation/Info: Check Circuit f o r Kinked Hose/Tube, Check for Airway Obstruction, Suction Airway if Necessary, Check Low Limit Setting, **Manually Ventilate Patient**
2300 Self Check Fault
Alarm triggers when the pulse oximeter module fails while in use. The user cannot resolve the fault. When the
alarm is active “-- --” displays in the HR and SpO
audible alarm for 30 seconds. To resolve the alarm, remove the probe from the device and put the pulse
oximeter in standby “stby”. Contact the service center for additional information.
Mitigation/Info: Pulse Ox Module, Internal Failure, SpO2/HR Not Available from Pulse Ox, Turn Off Pulse Ox,
windows. Pressing the Mute/Cancel button silences the
2
2301 Self Check Fault
Alarm triggers when the communication between the pulse oximeter module and device fails. When this
occurs the user must turn off the pulse oximeter monitor to end the alarm condition through the SpO
menu while also removing the probe from the device. When this is done “stby“ appears in the parameter windows for SpO
and HR as those parameters are no longer available. When appropriate the user should
2
replace the ventilator and contact the service center for additional information.
Mitigation/Info: Internal COMM: Pulse Ox Module, SpO2/HR Not Available from Pulse Ox, Turn Off Pulse
Ox, Remove SpO2 Cable from Ventilator, **Contact Service Center**
2314 Pulse Ox Sensor Off Patient
Alarm triggers when an operating sensor loses the patient signal. The most common cause is when the sensor disconnects from the patient or is misaligned with the sensor site. This alarm can also be caused by poor
perfusion at the sensor site which doesn’t provide an adequate signal. In these cases try another site.
Replace the sensor if another sensor is available. If the alarm condition cannot be resolved the user should
remove the sensor from the patient and put the pulse oximetry monitor in standby “stby”.
Mitigation/Info: Check Pulse Ox Sensor Site, Check Patient for Peripheral Pulse, Change Placement, Check
Sensor Operation, Replace Sensor,**Turn Off Pulse Ox Monitorin g**
2401 SpO2 Low
Alarm triggers whenever the SpO2 value drops below the Low SpO2 Limit. The default value for the limit is
94%. Corrective actions are increasing oxygenation by increasing the FIO
only be changed based on consultation with the attending physician. When using low flow O2 the user should
increase the flow of O
to the low flow O2 reservoir.
2
Mitigation/Info: SpO2 Below Limit, Increase FIO2, Check O2 Supply, Increase PEEP Per Physician, **Con-
2410 Heart Rate High
Alarm triggers when the heart rate is greater than the High Heart Rate Limit. The default value for the limit is
120 beats/minute. The user should consult with the attending physician on how best to reduce the heart rate
to an acceptable level.
Mitigation/Info: Heart Rate Above Limit, Check High Limit Setting, **Consult Physician**
Context
2
or PEEP settings. PEEP should
2
5-16 www.zoll.com 906-0731-01-05 Rev. B
Page 97

2411 Heart Rate Low
Alarm triggers when the heart rate is less than the Low Heart Rate Limit. The default value for the limit is 40
beats/minute. The user should consult with the attending physician on how best to increase the heart rate to
an acceptable level.
Mitigation/Info: Heart Rate Below Limit, Check Low Limit Setting, **Consult Physician**
2421 Self Check Fault
Alarm triggers when there is a failure of the input protection circuit and the device is able to operate. The
alarm will continue until the device is turned off. The user can mute the alarm for 30 seconds by pushing the
MUTE/CANCEL button. The user should replace the ventilator and contact the service center for additional
information.
Mitigation/Info: Power System, Power System Needs Repa ir, Internal Battery Oper at ion, Monitor B attery %
2423 Self Check Fault
2430 Nearly Drained Battery
Charge, **Contact Service Center**
Alarm triggers when the internal power circuit has failed and external power is connected but cannot be used.
The fault cannot be repaired by the user. Pressing the Mute/Cancel button silences the audible alarm for 30
seconds. Replace the ventilator and contact the service center for additional information.
Mitigation/Info: Power System, Power System Needs Repa ir, Internal Battery Oper at ion, Monitor B attery %
Charge, **Contact Service Center**
Alarm triggers when the device detects that there is ≤5 minutes of battery operation remaining and external
power is not connected. The user should immediately seek a source of external power and/or plan to provide
manual ventilation. Attaching external power will immediately clear the alarm though a low priority alarm will
remain until the internal battery has recharged so that the device can provide 30 minutes of operating time.
This will take approximately 5 to 10 minutes. If recharging the battery does not resolve the issue, contact the
service center for additional information.
Mitigation/Info: <5 Minutes Operation, Connect External Power, Ensure Ability to Manually Ventilate, ** Con-
2450 Battery Discharge Fault
Alarm triggers when the battery temperature reaches 70 °C (158 °F) which is 5 °C from its maximum operating temperature using the internal battery and external power is not connected. When the battery temperature reaches 75 °C (167 °F) the battery will shut down to prevent failure and the device will sound a high
priority alarm and shutdown. If possible the user should provide a source of external power which will allow
operation to continue at the current and higher temperatures. In addition, the device should be removed from
the soft case which acts as insulation. Shading the patient and ventilator from direct sunlight may also help
reduce the battery temperature.
Mitigation/Info: Battery Within 5 °C of High Limit, Remove Padded Case, Ensure External Power Avail able,
2455 Battery Fault
Ensure Ability to Manually Ventilate, **Move To Cooler Location**
Alarm triggers when the device is not able to communicate with the internal battery. When this occurs the
device does not know the current charge of the battery and operation could stop at any time. To continue
operation and the user should connect external power and ensure the ability to manually ventilate the patient.
When external power is connected the alarm priority decreases to Low Priority, replace the ventilator and
contact the service center.
Mitigation/Info: Battery Communication, Connect External Power, Ensure Ability to Manually Ventilate
Patient , **Contact Service Center**
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-17
Page 98

ALARMS
Low Priority Alarms
Service
Code
3001
Alarm Name/Mitigation/Resolution
Self Check Fault
Alarm triggers when the compressor fails to operate or fails to provide the flow required to deliver a breath
within ±10% of the current settings, high pressure O
set the FIO
ure to maintain the O
replace the ventilator and contact the service center for additional information.
Mitigation: Pneumatic System Compressor, Ensure 55 psig/380 kPa O2, O2 Operation Only, **Contact
to 100%. While operating in this state the user should ensure an adequate supply of O2. Fail-
2
2
is available to provide ventilation and the user has
2
supply will result in a high priority alarm. The user cannot repair the compressor,
3002
3011
3012
Self Check Fault
Alarm triggers when communication between the compressor controller and SPM is lost, high pressure O2
is available to provide ventilation and the user has set the FIO
user should ensure an adequate supply of O
. Failure to maintain the O2 supply will result in a high priority
2
to 100%. While operating in this state the
2
alarm. The user cannot repair the device, replace the ventilator and contact the service center for additional
information.
Mitigation: Pneumatic System: Compressor, Ensure 55 psig.380 kPa O2 Supply, O2 Operation Only, **Contact Service Center**
Self Check Fault
Alarm triggers when the signal to the O
valve is outside of the calibration range for the required flow rate,
2
the compressor is available to provide ventilation and the user has acknowledged that ventilation is being
provided using the compressor by setting the FIO2 to 21%. While operating in this state the user should
monitor the SpO
entrained through the Fresh Gas/Emergency Air Inlet port using the optional O
to ensure that adequate oxygenation is maintained. If low flow O2 is available it can be
2
reservoir. Maintain an
2
acceptable SpO2 by adjusting the O2 supply up or down to increase or decrease the amount of O2 delivered
to the patient. The user cannot repair the O
valve, replace the ventilator and contact the service center for
2
additional information.
Self Check Fault
Alarm triggers when communication between the O
able to provide ventilation and the user has set the FIO
valve and SPM is lost, the compressor is avail-
2
to 21%. While operating in this state the user
2
should monitor the SpO2 to ensure that adequate oxygenation is maintained. If low flow O2 is available it can be entrained through the Fresh Gas/Emergency Air Inlet port using the optional O
voir. Maintain an acceptable SpO
amount of O
delivered to the patient. The user cannot repair the O2 valve, replace the ventilator and
2
by adjusting the O2 supply up or down to increase or decrease the
2
reser-
2
contact the service center for additional information.
Mitigation/Info: Pneumatic System: O2 Valve, Compressor Operation Only!, Keep FIO2 at 21%, Con-
5-18 www.zoll.com 906-0731-01-05 Rev. B
Page 99

3030
Gas Intake Fault
Alarm triggers when the Fresh Gas/Emergency Air Inlet is blocked so that the compressor is not able to
deliver breaths within ±10% of the current settings, high pressure O
the user has set the FIO
inal value. If the blockage is cleared operation with the compressor will restart. If the blockage is not
cleared, the alarm will resound, set the FIO2 to 100%, continue ventilation and ensure an adequate supply
. It is possible for this alarm to be a false alarm that is triggered in a very high vibration environment or
of O
2
if the device is not mounted correctly. If the alarm does not resolve contact the service center for additional
information.
Mitigation/Info: O2 Supply Operation, Clear Blocked Intake, Reset FIO2 to Previous, Monitor SpO2, **Con-
3031
Intake Restricted
Alarm triggers when the Fresh Gas/Emergency Air Inlet is blocked but is still capable of delivering breaths
within ±10% of the current settings. This could be caused by an external blockage or a dirty/wet external or
internal filter. If the blockage is cleared the alarm will automatically cancel. Refer to instructions for changing the internal filters. If the problem does not resolve contact the service center for additional information.
On rare occasions, this alarm can be triggered by a patient with a very high inspiratory demand. In this
case increase the rise time or shorten the inspiratory time to increase the inspiratory flow rate.
Mitigation/Info: Clear Fresh Gas Intake, Check Filter for Moisture or Dirt, OR, Manage Settings / Inspiratory
Demand, **Manually Ventilate Patient**
3032 Self Check Fault
Alarm triggers when communication between the Fresh Gas/Emergency Air Inlet pressure sensor is lost.
Normal operation can continue but, if the condition is not cleared by powering off and restarting the device
should be replaced when appropriate as. When used during this alarm condition the user should be sure to
keep the Fresh Gas/Emergency Air Inlet clear and ensure that external filters are checked regularly.
Mitigation/Info: Pneumatic Sensor, Ventilator Operating, Unable to Detect Inlet Obstruction, **Contact Ser-
is available to support ventilation and
2
to 100%. To clear the alarm, clear the blockage and set the FIO2 back to the orig-
2
3041 High O2 Supply Fault
Alarm triggers when the high pressure O
automatically cancels when the supply pressure is <80 psig (552 kPa). Pressure above 87 psig (600kPa) could
result in a catastrophic failure, harm to the patient and/or damage to the device. The user should reduce the
O2 supply pressure, sometimes this requires replacing the regulator that is not functioning correctly. If the
pressure cannot be reduced and a low flow device like a flow meter is available the user can provide supplemental O2 via the optional low flow O2 reservoir. If not, the user should monitor the O2 supply pressure and
ensure that the pressure does not rise further.
Mitigation/Info: Decrease O2 Supply Pressure, Replace Regulator, Connect Low Flow O2, Monitor SpO2
3073 Tubing Compliance Fault
Alarm is triggered when the tubing compliance correction shows that it is >the set tidal volume indicating that
the patient may not be receiving the appropriate tidal volume. In this case the user should assess the patient
and settings. Consult the attending physician if there are questions about how to configure the ventilator correctly to support the patient.
Mitigation/Info: Calculated Compliance Volume Larger than Delivered Volume, Check Tubing Compliance vs.
Circuit
3091 AutoPEEP
Alarm triggers when the exhaled flow from the patient continues throughout the expiratory period causing the
expiratory control valve to cycle throughout the period to maintain the baseline pressure. When this occurs the
user should increase the expiratory period by decreasing the inspiratory time, decreasing the breathing rate or
both. The physician should also be consulted as this alarm is an indication that Auto-PEEP is occurring. Note:
at startup, this alarm is off. The user can choose to activate the alarm if they believe the patient is at risk of
Auto-PEEP using the Alarm Configuration submenu that is access using the Main Menu.
Mitigation/Info: Increase Expiratory Time, Decrease Inspiratory Time, Decrease Respiratory Rate, Disable
supply is ≥80 psig (552 kPa) and <87 psig (600 kPa). The alarm
2
906-0731-01-05 Rev. B ZOLL Ventilator Operator’s Guide 5-19
Page 100

ALARMS
3092 Inspiratory Demand
Alarm triggers when the end-inspiratory pressure is < -1.0 cm H
due to changes in the patient’s status, where the patient attempts to inhale more gas than what is currently set.
When this occurs, the user should note if the patient is breathing or fighting with the vent. The user should
increase the flow rate (by decreasing the inspiratory time) and/or reduce the rise time. The physician should be
consulted.
Mitigation/Info: Patient May be Breath ing with the Ventilator, Increase I Time and/or Decrease Rise Time,
Check Patient and Circuit for Leaks, Disable Alarm, **Consult Physician**
3110 RTC Battery Low
Alarm triggers when the real-time clock (RTC) battery is < ~2.5 volts. The alarm condition is checked at start
up and if this alarm occurs the device is safe to operate but the user should replace the device when appropriate and consult the service center for additional information. The user cannot change the RTC battery. The
RTC battery provides power for the clock that tracks the local time. It is replaced every 4 years during preventive maintenance.
Mitigation/Info: Vent Fully Functional, **Contact Service Center**
3120 PM Due
Alarm triggers at start up when the preselected number of days has elapsed since the last calibration. When
appropriate the device should be replaced and sent for preventive maintenance. The low priority message
serves as a reminder. Calibration is due every 365 days or 730 days for devices configured for stockpile use
(check with your organization regarding the configuration of your device). Users should schedule the device for
service as soon as possible. Users can suspend the yellow alarm notification for the current use by turning the
alarm off using the Alarm Configuration submenu in the Main Menu.
Mitigation/Info: Preventive Maintenance Due, Ventilator Functioning with No Faults, **When Appropriate, Con-
O for 3 consecutive breaths. This can occur
2
3121 Power Cycle Need
This alarm occurs when the device has been running continuously for 30 days. In order to check the flow pneumotach, which is done a startup, the user should manually ventilate the patient and cycle the power. Once this
is done the user can select the Last Settings options from the Start Menu and continue operation if not faults
are detected during the self check. If nonoperating alarms occur, contact the service center for additional information.
Mitigation/Info: Power-on Self Checks are Due, When Appropriate, Power Off Then On, Verify Proper Settings,
**Review Manual For Additional Information**
3130 Self Check Fault
Alarm triggers when the ambient pressure transducer fails. When this occurs, the device is no longer able to
automatically compensate for changes in altitude especially in situations where the ambient pressure could
change rapidly as during transport by air. When this alarm is active during aeromedical transport the user
should ventilate using pressure targeting if the ventilator cannot be replaced. Users should also monitor chest
rise and breath sounds to ensure adequate ventilation.
Mitigation/Info: Sensor: Barometer, Altitude Compensation Disabled, Maintain Airway Pressure, Check Patient
Chest Rise, Avoid Use At Varying Altitude, **Contact Service Center**
3131 Excessive Altitude
Alarm triggers when the ambient pressure transducer detects an altitude >25,000 feet (7620 meters). Beyond
this altitude compensation remains fixed at the 25,000 ft compensation level. The user should monitor the airway pressure and reduce the tidal volume as altitude increases though, there is very little change in performance over this altitude. Where possible cabin pressure should be maintained in the compensated range.
Mitigation/Info: Beyond Altitude Compensation Limit, Maintain Airway Pressure, Check Patient Chest Rise,
Monitor Ventilator/Patient,**Reduce Altitude/ Pre ssurize Ca bin if Possibl e**
5-20 www.zoll.com 906-0731-01-05 Rev. B
 Loading...
Loading...