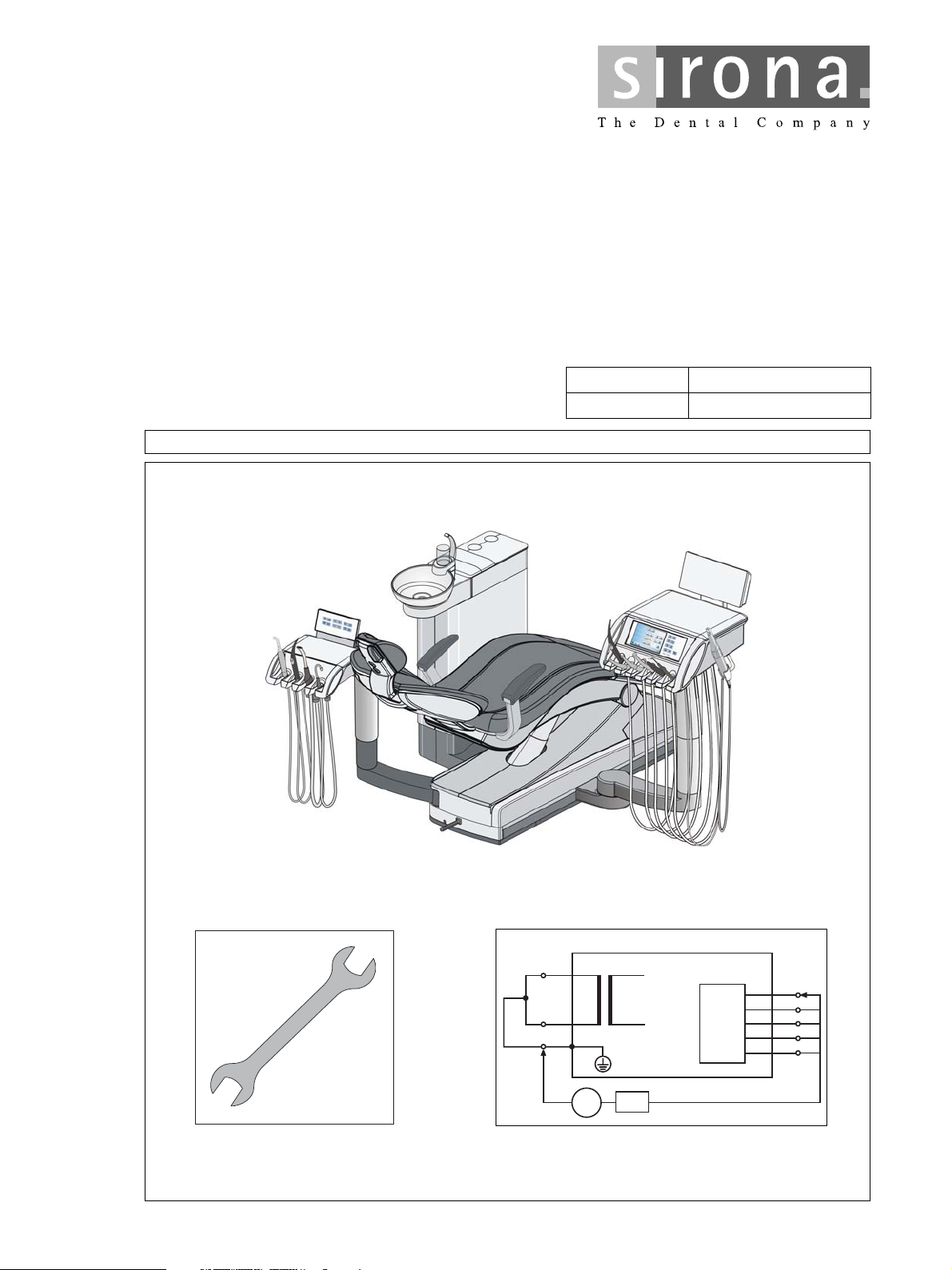
kÉï=~ë=çÑW=
~
qbkbl
j~бенЙе~еЕЙ=j~ем~д
MTKOMMU
qbkbl=L=aPRMV
jçÇÉä pЙкб~д=емгДЙк
bеЦдблЬ
E
N
L
PE
P
fелйЙЕнбзе=~еЗ=г~бенЙе~еЕЙ p~СЙну=ЕЬЙЕвл
AP
VPC
MD


Contents
1 General information ............................................................... 4
1.1 Purpose of the Maintenance Manual ..............................4
1.2 Work to be performed .....................................................4
2 Installation Report / Warranty Passport ................................. 5
2.1 Master data of the unit ....................................................5
2.2 Inspection and maintenance ...........................................5
3 Safety checks ........................................................................ 6
3.1 Visual inspection .............................................................8
3.2 Protective ground wire test............................................10
3.3 Measurement of equivalent leakage currents ...............11
3.3.1 Equivalent device leakage current ........................................ 15
3.3.2 Equivalent patient leakage current ....................................... 17
3.3.3 Final work ............................................................................. 18
3.4 Safety check
(Initial test after initial startup) .......................................18
3.5 Safety check (re-tests) ..................................................19
4 Treatment centers with HF surgical equipment ................... 23
4.1 General information.......................................................23
4.2 List of trained personnel................................................24
4.3 Repair work on the HF module .....................................25
4.4 Effects of malfunctions and repeated, similar operator
errors on the HF module ...............................................27
bеЦдблЬ
61 93 952 D 3509
D 3509.102.01.01.02 01.2008
5 Reporting of incidents to authorities / manufacturers .......... 29
6 Remarks / special issues with regard to the dental treatment
center ................................................................................... 31
3
1 General information
1 General information
1.1 Purpose of the Maintenance Manual
In order to ensure the operational safety and reliability of the system and to
protect the health of patients, users and other persons, inspection and maintenance must be performed at scheduled intervals.
This includes:
• Inspection and maintenance (yearly)
to prevent damage due to natural wear
• Safety tests (every 2 years)
to ensure the technical safety of the system
This document describes the work to be performed by the service engineer.
The performance of the work and the measurement results are documented by the service engineer.
This document must be stored near the dental treatment center.
i
NOTE
For systems with HF surgical equipment, this Maintenance Manual simultaneously serves as a Medical Product Log.
1.2 Work to be performed
By the service engineer: 1. Write the serial number of the dental treatment center (see type label on
the base of the patient chair) on the cover sheet and on the corresponding
pages (header) of the maintenance log.
2. Complete the “Installation Report / Warranty Passport” after the initial installation and file it after chapter 2.
3. Perform inspection and maintenance according to the maintenance
schedule.
Document the performance of these in the “Installation Report / Warranty
Passport".
4. Conduct the safety tests in accordance with chapter 3. Document the results.
5. For systems with HF surgical equipment (see section 4.1), carry out the
documentation as specified in sections 4.2 and 4.3.
Please inform the user of the obligation to carry out points 1 and 2 below.
6. Document comments and special issues regarding the dental treatment
center in chapter 5.
By the user: 1. For systems with HF surgical equipment, carry out the documentation as
specified in sections 4.2 and4.4 .
2. Document the reporting of incidents to authorities / manufacturers in
chapter 5.
61 93 952 D 3509
4 D 3509.102.01.01.02 07.2008

2 Installation Report / W arranty Passport
2 Installation Report / Warranty
Passport
2.1 Master data of the unit
Complete the document “Installation Report / Warranty Passport” and file
the “Customer Copy” after this page.
Unusual occurrences during installation can be additionally noted on the second page of the “Dealer Copy”.
2.2 Inspection and maintenance
To avoid damage due to natural wear, an inspection must be performed every
year.
The dental treatment center automatically recognizes when the regular maintenance is due and indicates this in a timely manner on the user interface.
You'll find more detailed information about the maintenance indicator in the
user manual.
The steps to be performed as well as the parts which must be replaced are
specified in the document “Maintenance Schedule”. The performance of
these is documented there.
A separate maintenance schedule is produced for each maintenance event.
In addition, list the inspection and maintenance events under the maintenance overview in the “Installation Report / Warranty Passport”.
bеЦдблЬ
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
Attachment: Installation Report/Warranty Passport
5
3 Safety checks
3 Safety checks
Medical products are designed in such a way that the first occurrence of a
fault does not create a hazard to the safety of the patient, the user or other
persons. Hence it is important to detect such faults before a second fault
occurs, which might then lead to safety hazards.
For that reason it is essential to perform safety tests aimed particularly at
detecting electrical faults every 2 years. All inspections and measurements
are performed by the authorized service engineer. They are specified in the
following.
Safety tests are performed on the following occasions:
• initial startup (section 3.4)
• regularly every 2 years
• after extensions/upgrades (conversion) of the treatment center
• after repair work
You must document the measured values in section 3.4 and/or 3.5.
CAUTION
When taking measurements, please observe that hazardous voltages might
be present on the system under test.
CAUTION
If the dental treatment center does not pass the safety tests, it must not be operated any longer!
You must advise the user of this fact in your capacity as service engineer.
Corresponding repair work by an authorized service engineer is required before putting the system into service again.
i
NOTE
The safety checks correspond to the standards IEC 62353:2007 and/or
VDE 0751-1:2001.
If you use an automatic tester, you can program it according to these standards.
– Application components Type BF
– Permanently attached unit
– Protection Class I
The measurements to be performed are complex and time-consuming.
Sirona therefore explicitly recommends using an automatic tester.
61 93 952 D 3509
6 D 3509.102.01.01.02 07.2008
3 Safety checks
Measurement according to IEC
60601-1
If you have no possibility of performing the measurements according to
VDE 0751-1:2001, you may also perform them according to IEC 60601-1.
For details on how to perform the measurements, please refer to the standard
IEC 60601-1 and the documents on your measuring device.
i
NOTE
This type of measurement is not recommended by Sirona due to its complexity.
When taking measurements, please observe the following:
Type B application components
Type BF application parts HF surgery handpiece
Micromotors SL, BL BL ISO or
BL implant
Tu rb i n e
Ultrasonic handpiece
Curing light Mini-LED
Sprayvit
SiroCam digital (does not require any
testing)
bеЦдблЬ
Protective ground wire resistance
Earth leakage current N.C. – 5 mA
Patient leakage current N.C. – 0.1 mA
N.C. – normal condition
S.F.C. – single fault condition
When the measurements are performed, the individual treatment instruments
must be operated one after the other. However, the HF surgical instrument
must be measured in an inactive state.
Thus several measurements must be performed one after the other.
Make a note in Section 3.4 or 3.5 stating that you have performed the measurements according to IEC 60601-1 and correct the specified limiting values.
Document the highest measured values.
0.1W
>
S.F.C. – 10 mA (permanent connection)
S.F.C. – 0.5 mA
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
7
3 Safety checks
3.1 Visual inspection
Check the following points:
• Perform a functional test of the dental treatment center in accordance with
the operating instructions.
Are all functions present?
• Are all optical and acoustic warning signals functioning properly?
• Are all housing parts safely attached and intact?
• Are all labels specified in the “Installation Report / Warranty Passport” affixed and legible?
• Are all operating instructions which belong to the treatment center available?
• Is the “Maintenance Manual” for the dental treatment center, which also
serves as the Medical Device Log for systems with HF surgical equipment, available?
• In Germany:
Is the Service Log of the amalgam separator (if applicable) available and
properly maintained?
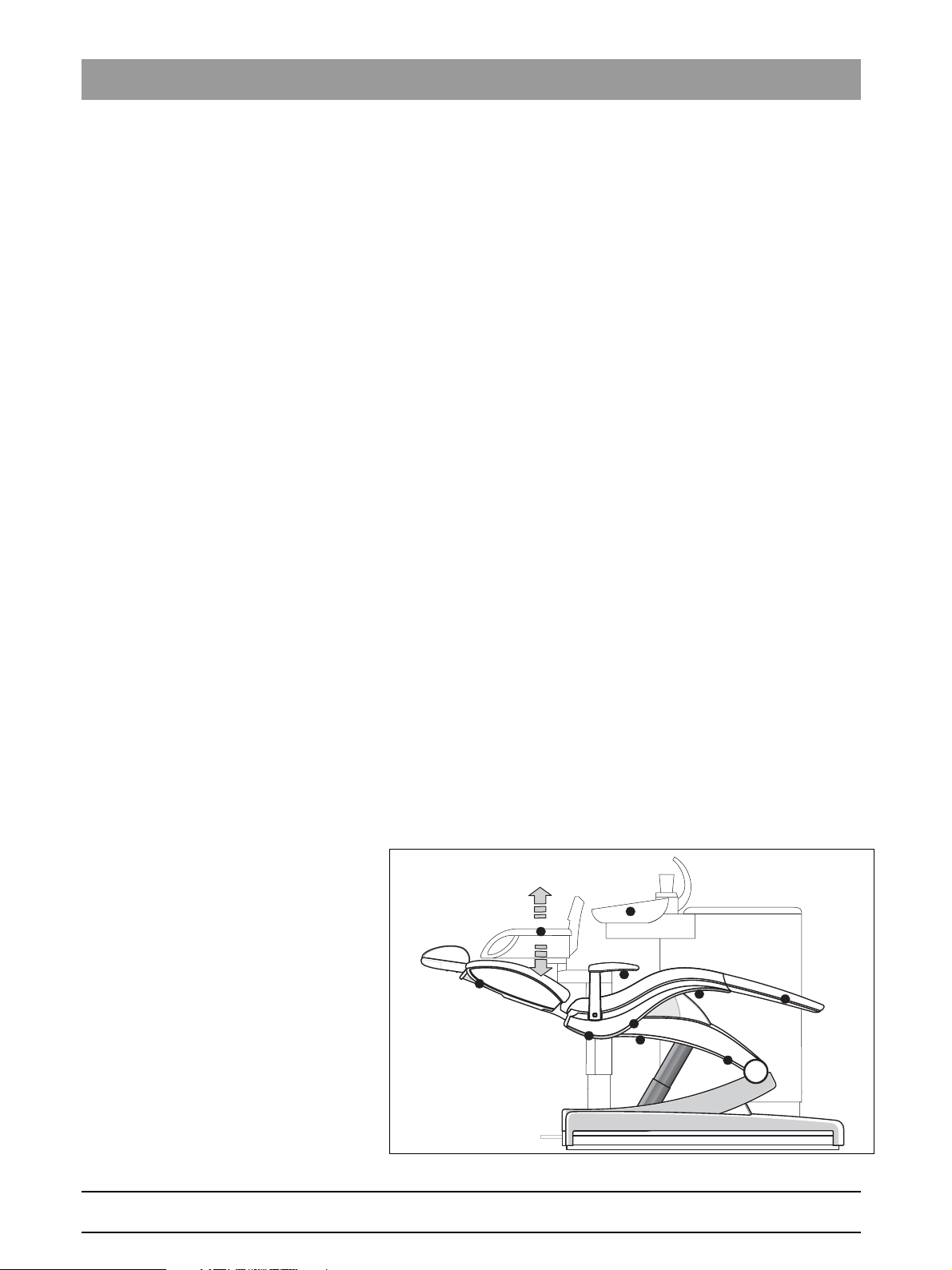
• Are all safety switches functioning?
– Press a key to move the patient chair, e.g. a program key.
– While the patient chair is moving, actuate all safety switches one after
the other and check whether a corresponding error message appears
on the user interface. The positions for triggering the safety switches
are shown in the diagram below.
– Perform the following actions:
1 - Elevate backrest
2 - Elevate footrest
3 - Push rear right and left facing upwards
4 - Push rear right and left elevation frame upwards
5 - Push front right and left elevation frame upwards
6 - Push front right and left manual switch bar on seat support
upwards
7 - Push rear right and left manual switch bar on seat support
upwards
8 - Swivel armrest on the dentist element side outwards
9 - Push the head of the assistant element and pull it upwards
10 - Cuspidor bowl (see below for test)
10
9
8
1
7
3
4
6
2
5
Fig. 3-1 Trigger points for the safety switches
61 93 952 D 3509
8 D 3509.102.01.01.02 07.2008
3 Safety checks
NSA
X1
N
L
X1
– When a safety switch is triggered, the following events occur:
• an acoustic signal sounds
• the patient chair stops moving; a corrective movement in the
opposite direction may take place.
• the triggered safety switch is displayed on the user interface of the
touchscreen
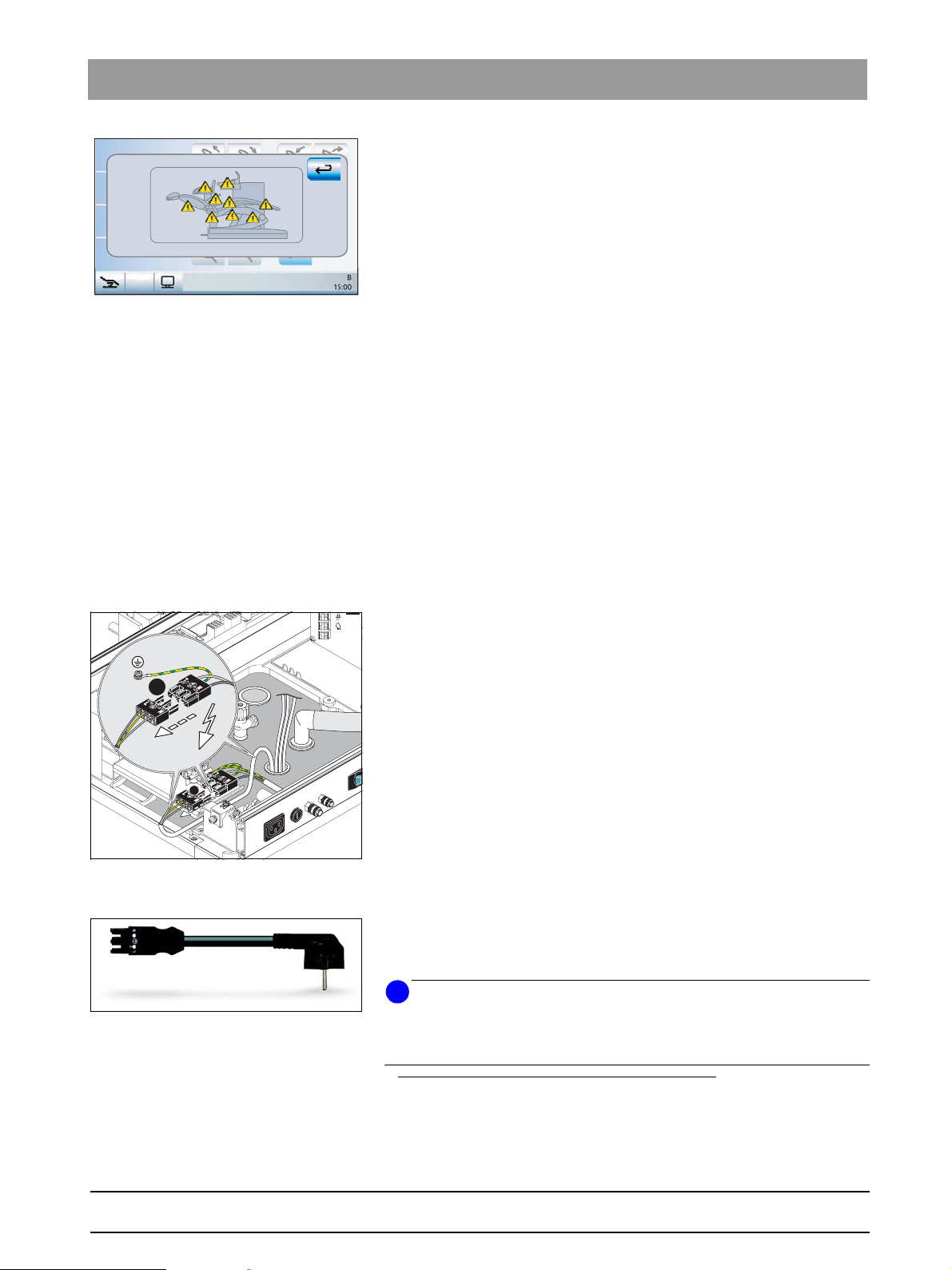
• Does the safety switch of the cuspidor bowl (10) work?
– Move the patient chair to the lowest position.
Fig. 3-2 Display on the EasyTouch user inter-
face
Test preparations Before beginning the safety tests described below, carry out the following
– Swivel the cuspidor bowl towards the patient chair.
– Move the patient chair upwards.
ª The chair can be moved.
However, when the patient chair moves near the cuspidor bowl, the
latter swivels inwards.
preparations:
• Move the dental treatment center to a middle height so that the connection box is easily accessible.
• Shut off the mains power connection to the dental treatment center.
• Remove the seat upholstery (leave the backrest upholstery in place).
• Open the cover of the connection box of the patient chair.
• If a PC is connected: Disconnect the power supply of the PC and remove
all other connections (e.g. shielded signal
cables) which ground the PC.
bеЦдблЬ
A
Fig. 3-3 Disconnect the power supply connec-
tor.
1
2
Fig. 3-4 Test cable
• Disconnect the power supply connector of the dental treatment center
from the building mains (Figure 3-3).
1
• Replace it with the special test connector (1) of the test cable
(Figure 3-4).
• Plug the grounded plug (2) into the test unit.
i
NOTE
If you use an automatic tester, it may be necessary to bring the application
components into contact with the unit before the following protective ground
wire test. Proceed according to section 3.3.1.
1. You can order the test cable, consisting of the test connector, power cable
(2 m) and grounded plug, under Article No. 771-9993/306-201
from WAGO Kontakttechnik GmbH Co. KG, Postfach 2880, 32385 Minden,
Germany, www.wago.com.
You can obtain this test cable free of charge when you attend a training session
for service engineers.
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
9
3 Safety checks
X1
Clean
Setup
Fig. 3-5 Protective ground wire connection
with test cable on the power connection terminal
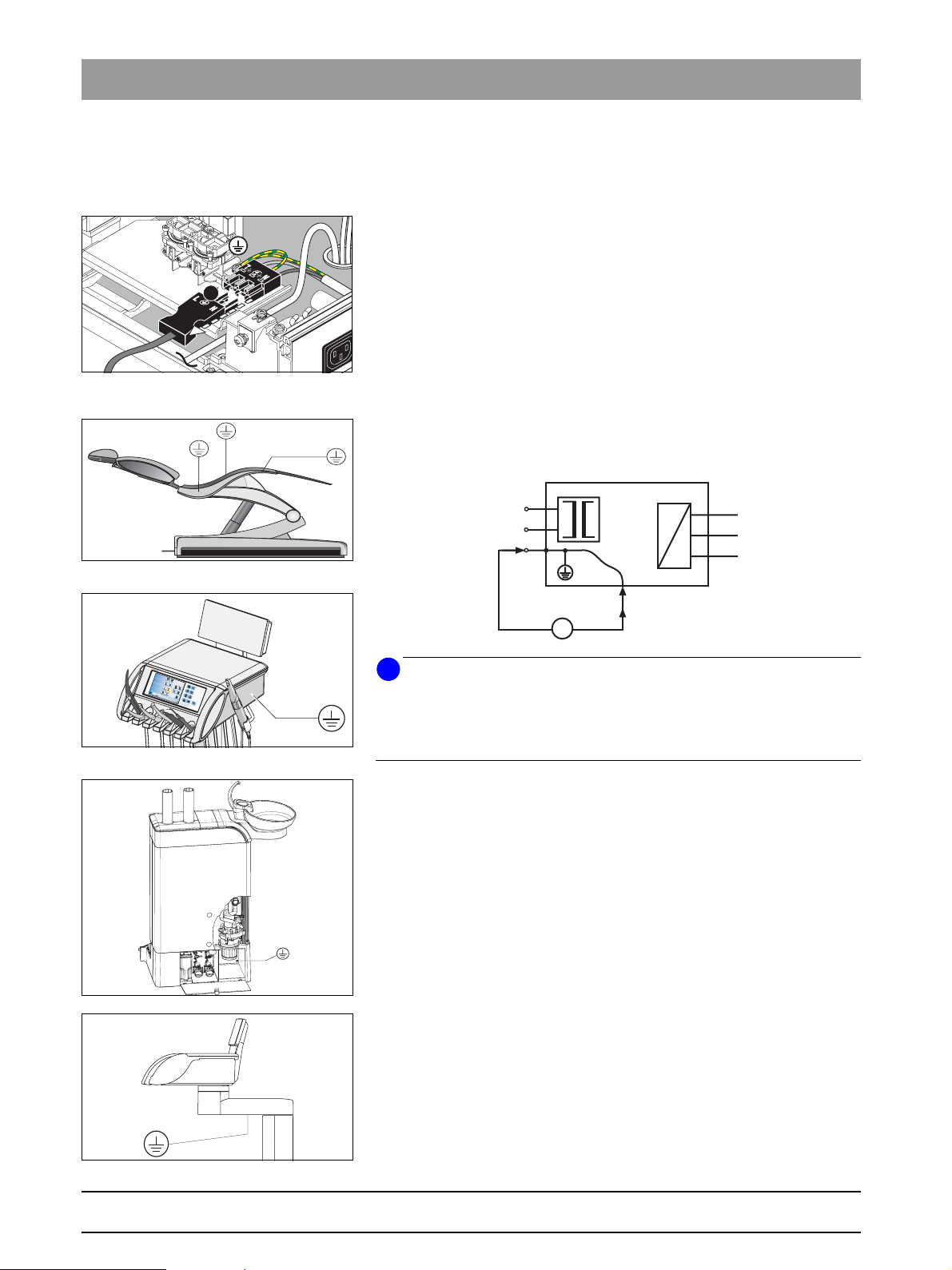
3.2 Protective ground wire test
Before beginning the protective ground wire test, ensure that all protective
ground wire connections are present, firmly attached and intact.
1. Measure the electrical resistance of electrically conductive parts and
parts connected to the protective ground wire on the dental treatment
center to the protective ground wire connected to the power connection
terminal (deduct the resistance of the test cable, Figure 3-5).
Remove the power supply connector of the PC connected to the dental
treatment center and any other network connections that ground the PC.
2. Document the highest measured value.
The measured resistance must not exceed 0.3 W .
I
The measuring current (
The no-load voltage must be between 4 V min. and 24 V max.
Measurements must be performed in accordance with the following measuring set-up as per IEC 62535:2007 or VDE 0751-1.
N
L
) must be between 0.2 A and 25 A.
meas
Fig. 3-6 Measurement points below the seat
upholstery
TENEO
C
C
l
e
a
n
S
e
t
u
p
Fig. 3-7 Measurement point in the dentist ele-
ment
Fig. 3-8 Measurement point in the water unit
Fig. 3-9 Assistant element
PE
M
I
mess
i
Ω
NOTE
If you use an automatic tester as specified in IEC 62353 or VDE 0751, the
aforementioned parameters are automatically guaranteed. Plug the connector
of the test cable into the tester and carry out the measurement in accordance
with the operating instructions for the tester.
The following list provides a selection of possible measuring points (M):
– plate in the connection box on which the power supply connection is
installed
– plate on the base of the patient chair in which the power switch is
installed
– elevation frame and seat support below the seat upholstery
(Figure 3-6)
– backrest support (Figure 3-6)
– screw on the underside of the dentist element (Figure 3-7)
– floor plate of the cable foot control
– screw behind the amalgam separator rotor (open cover, Figure 3-8)
– protective ground wire connection of the monitor (if present)
– cold unit plug socket for additional devices (if present)
– assistant element, screw below the transverse arm (Figure 3-9)
– protective ground wire connection of the external PC on dental treat-
ment centers with PCs (PC power plug disconnected)
Document the measuring results obtained during initial startup in section 3.4.
Document the measuring results obtained during re-tests in section 3.5.
61 93 952 D 3509
10 D 3509.102.01.01.02 07.2008
3 Safety checks
3.3 Measurement of equivalent leakage
currents
Two different equivalent leakage currents are measured:
• Equivalent device leakage current
• Equivalent patient leakage current
i
NOTE
Sirona recommends using an automatic tester that complies with standard
IEC 62353:2007 or VDE 0751 to perform the measurements.
If you do not use an automatic tester, please comply with the instructions on
page 13.
bеЦдблЬ
Use of the VPC measuring point
i
NOTE
The equivalent leakage current measurements also include the applied parts
(treatment instruments).
Since the treatment center is in a non-operating state, the motors of the treatment instruments, their supply cables and lamps are disconnected via relays
and therefore not connected to the potential of the patient circuit.
Thus, under the circumstances insulation defects in the patient circuit may not
be detected.
The following measurements are therefore also measured against the potential of the patient circuit (measuring point in the voltage patient circuit [VPC]).
This circuit is treated like an application component.
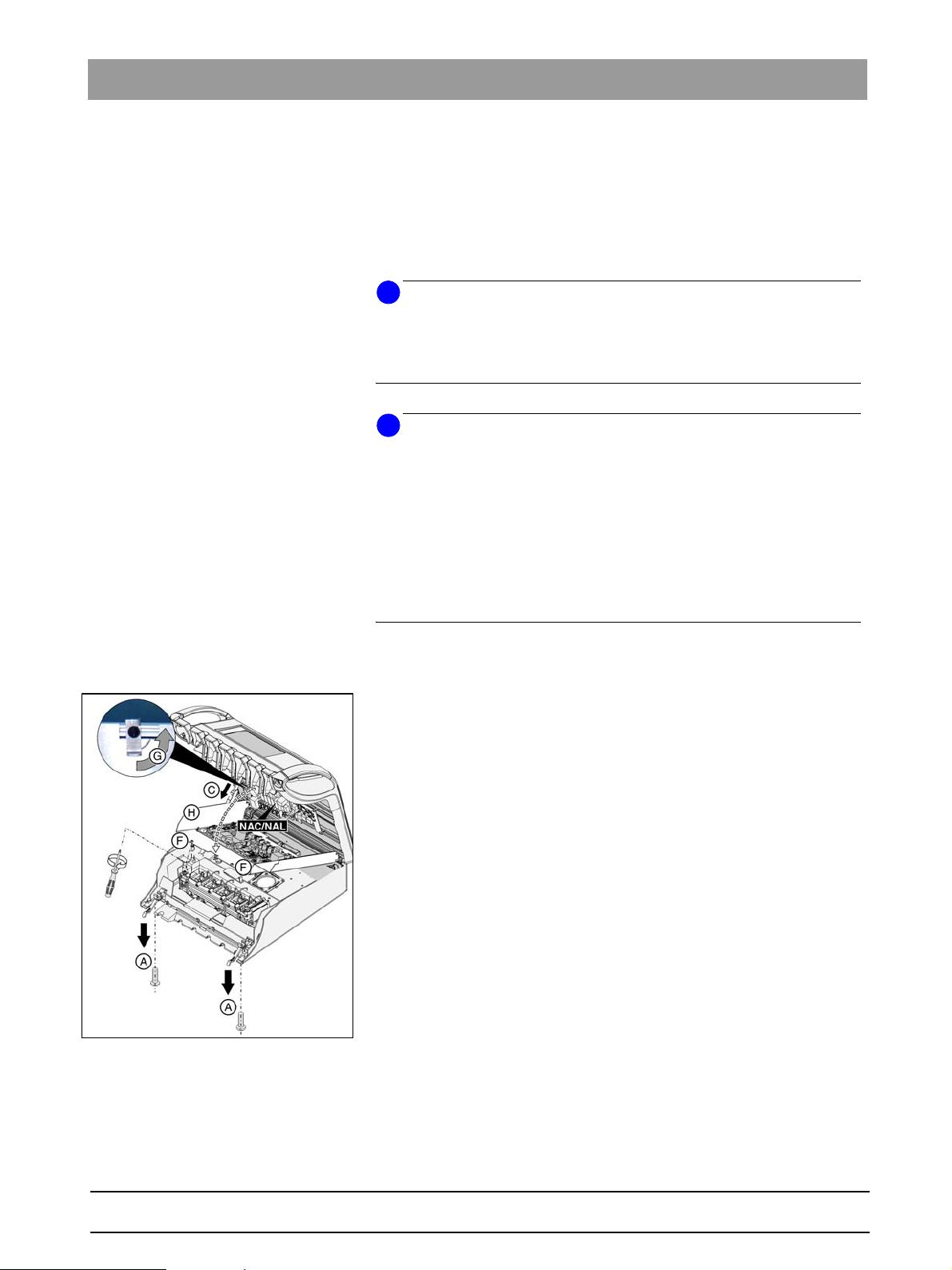
The VPC measurement point is located in the head of the dentist element.
Proceed as follows to establish contact:
1. Open the dentist element by flipping the two locking brackets (A) downwards and removing the two screws with a screwdriver (Figure 3-10).
2. Flip the cover of the dentist element head with the
instrument holder upwards and secure the housing cover with pin C.
The instruments remain in the holder.
3. Remove the two screws (F) on the cover plate.
4. Flip the locking bracket (G) upwards.
5. Now fold the plate (H) downwards.
Fig. 3-10 Opened dentist element
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
6. Attach the test terminal to the VPC measuring point
(see Figure 3-11, 3-12).
7. The VPC measuring point is located on the upper board.
8. Connect the test terminal to the tester like an application component.
11

3 Safety checks
NAL
MP1
VPC measuring point on the SL motor:
NAL board - pin 3 of V 410
Fig. 3-11 NAL board - V 410, pin 3
NAC
Fig. 3-12 NAC board - R533
or
VPC measuring point on the BL, BL ISO, BL Implant motors
NAC board - R533
61 93 952 D 3509
12 D 3509.102.01.01.02 07.2008

3 Safety checks
Measurement without an automatic tester
If you're using an automatic tester, you can skip this page 13
and 14 .
Test conditions
AB
MD
If you are not using an automatic tester, please comply with the following
specifications:
You need a high-resistance, power-frequency, sinusoidal measuring voltage
source for the measurements. The no-load voltage corresponds to the nominal mains voltage.
The short-circuit current must not exceed 3.5 mA (protection of persons).
Since equivalent leakage currents of up to 10mA are permissible, the voltage
of the measuring voltage source must also be monitored during the measurements, and the leakage current must be extrapolated to the nominal line voltage. Please pay attention to the following examples.
Measurements must be performed with the following circuitry in accordance
with IEC 62353:0751 or VDE 1-1
A
=
B
R
1, R2, C1
R1= 10kΩ±5%
= 1kΩ±5%
R
2
: Noninductive components
C
1
= 0,015 µF± 5%
U
1 V = 1 mA
RMS responsive
meter
bеЦдблЬ
Extrapolating the leakage current
for the nominal line voltage
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
I
measure
M
D
U~
Line
P
R
i
I
max
U
Line
R
i
P – Power-frequency measuring voltage source
U
source
– Line voltage
– Internal resistance of measuring voltage source
– Measured source voltage
U
source
V
Test object
13

3 Safety checks
I
max
I
measure
I
leak
– Maximum measuring current 3.5mA
– Measured current
– Leakage current of test object
Example:
U
= 230V AC, I
line
= 230V / 3.5 mA = 65.71 kW
R
i
Selected: R
Case 1:
= 68 kW
i
Measured:
U
source
= 3.5 mA
max
= 162V, I
Leakage current:
I
= 230 V / 162 V = 1.42 x 1 mA = 1.42 mA O. K.
leak
Case 2:
Measured:
U
= 26V, I
source
Leakage current:
I
= 230 V / 26 V = 8.85 x 3 mA = 26.55 mA Error
leak
measure
measure
= 1mA
= 3mA
61 93 952 D 3509
14 D 3509.102.01.01.02 07.2008

3 Safety checks
~
3.3.1 Equivalent device leakage current
Measurements must be performed in accordance with the following measuring set-up as per IEC 62353 or VDE 0751-1
E
N
P
MD
L
PE
AP
VPC
The dental treatment center is disconnected from all pins of the main power
supply.
The power switch at the base of the patient chair must be switched ON.
L, N – Phase, neutral conductor on mains terminal
PE – Protective ground wire on mains terminal
P – Power-frequency measuring voltage source
E – Accessible conductive parts (housing) at pro-
VPC – Measuring point (potential of patient circuit)
AP – Application components
– Connections of measuring device
tective ground potential
CAUTION
The measured leakage current must not exceed 10 mA.
CAUTION
If the measured value deviates considerably from the one obtained during the
first measurement (see section 3.4), find the cause and correct the problem if
necessary.
With automatic tester 1. Using measuring cables, connect all metallic application components and
the VPC measuring point with the tester connections provided.
bеЦдблЬ
Fig. 3-13 Sprayvit contacting
Fig. 3-14 Establishing contact to the heating
cartridge
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
– Sprayvit heating cartridge on the dentist and assistant side
1. Pull off the sheaths of the Sprayvit.
2. Attach a test terminal to the heating cartridge
(Figures 3-13 and 3-14)
– Housing of the SL, BL, BL ISO, BL Implant micromotors
– Turbine housing
– Tip of the US handpiece
– Tip of the HF surgery handpiece
– MiniL.E.D curing light housing
– VPC measuring point on NAL or NAC board
contacting VPC, see section 3.3, Fig. 3-11 or Fig. 3-12.
i
NOTE
The camera is not tested.
2. Connect the power supply connector of the test cable with the tester.
3. Program the tester for the following conditions:
– Type BF application components
– Permanently attached unit
– Protection class I
i
NOTE
Make sure that the tester is programmed for a permanent connection (and not
for 1 mA) (a 10 mA leakage current is permissible).
15

3 Safety checks
4. Perform the measurements according to the operating instructions of the
tester.
i
NOTE
All cables remain on the tester for the measurement of the replacement patient
circuit (see section 3.3.2).
Document the value measured during initial startup in
Section 3.4.
Document the values measured during re-tests in Section 3.5.
Without automatic tester 1. Using measuring cables, connect all metallic application components and
the VPC measuring point with the protective ground wire (Figure 3.17).
– Sprayvit heating cartridge on the dentist and assistant side
1. Pull off the sheaths of the Sprayvit
2. Attach a test terminal to the heating cartridge
(Figures 3.15 and 3.16)
– SL, BL, BL ISO, BL Implant micromotor housing
– Turbine housing
– Tip of the US handpiece
– Tip of the HF surgery handpiece
– MiniL.E.D curing light housing
– VPC measuring point on NAL or NAC board
To establish contact with the VPC, see section 3.3, Fig. 3-11 or
Fig. 3-15 Sprayvit contacting
Fig. 3-12
Fig. 3-16 Establishing contact to the heating
cartridge
Fig. 3-17 Establishing contact of the application
components to the protective ground
wire
i
NOTE
The camera is not tested.
2. Insert the measuring device between the short-circuited mains connections (L and N) and the protective ground wire (PE) connection of the
mains terminal.
3. Measure the current flowing across the insulation and MD (1 V = 1 mA).
4. Remove the connections to the protective ground wire after taking this
measurement.
5. Calculate the leakage current as described on page 13.
Document the value measured during initial startup in Section 3.4.
Document the values measured during re-tests in Section 3.5.
61 93 952 D 3509
16 D 3509.102.01.01.02 07.2008

~
N
L
PE
3 Safety checks
3.3.2 Equivalent patient leakage current
The following measuring set-up according to IEC 62353 or VDE 0751-1.
E
– Connections of measuring device
AP
VPC
L, N – Phase, neutral conductor on mains terminal
PE – Protective ground wire on mains terminal
P – Power-frequency measuring voltage source
E – Accessible conductive parts (housing) at pro-
tective ground potential
VPC – Measuring point (potential of patient circuit)
AP – -Applied parts (type BF)
bеЦдблЬ
P
MD
The dental treatment center is disconnected from all pins of the main power
supply.
The power switch at the base of the patient chair must be switched ON.
CAUTION
The measured leakage current must not exceed 5 mA.
CAUTION
If the measured value deviates considerably from the one obtained during the
first measurement (see section 3.4), find the cause and correct the problem if
necessary.
With automatic tester y The application components, VPC measurement point and the power
supply connector of the test cable are already connected to the tester as
described in section 3.3.1 "With automatic tester" ..
S Perform the measurements according to the operating instructions of the
tester.
Document the value measured during initial startup in Section 3.4.
Document the values measured during re-tests in Section 3.5.
Without automatic tester 1. Connect the short-circuited mains wires (L and N) to the protective ground
wire (PE).
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
2. Successively connect the test device between PE and the different metal
application components. Applied metal parts include:
– The Sprayvit heating cartridge on the dentist and assistant element
side
– Housing of the SL, BL, BL ISO, BL Implant micromotors
– Turbine housing
– Tip of the US handpiece
– Tip of the surgical handpiece
– Mini-LED curing light housing
– VPC measuring point on the NAL/NAC board (see Section 3.3)
17

Model TENEO / D 3509 Serial number
3 Safety checks
3. Measure the current flowing across the insulation and MD (1 V = 1 mA).
4. Calculate the maximum leakage current as described on page13.
Document the values measured during initial startup in Section 3.4.
Document the values measured during re-tests in Section 3.5.
3.3.3 Final work
The safety checks have now been completed!
• Remove the measuring equipment.
• Reattach all covers to the dentist element and close the cover.
• Plug in the power supply connector again.
• Close the connection box cover.
• Switch on the current of the building mains again.
• Complete the documentation.
3.4 Safety check
(Initial test after initial startup)
The values measured during initial startup are documented so that they can
be compared with the values measured during the re-tests.
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
Equivalent device
leakage current
10 mA)
(<
Equivalent patient leakage current
5 mA)
(<
Safety
maintained?
61 93 952 D 3509
18 D 3509.102.01.01.02 07.2008

Model TENEO / D 3509 Serial number
3 Safety checks
3.5 Safety check (re-tests)
The results of the re-tests are documented on these forms.
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Equivalent device
leakage current
10 mA)
(<
Equivalent device
leakage current
10 mA)
(<
Equivalent patient leakage current
5 mA)
(<
Equivalent patient leakage current
5 mA)
(<
Safety
maintained?
Safety
maintained?
bеЦдблЬ
Remarks / Particularities:
Date Name of engineer Depot Signature
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
19

Model TENEO / D 3509 Serial number
3 Safety checks
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
Visual inspection Protective ground wire
resistance
(< 0.3 W)
OK Faults W mA mA yes no
Remarks / Particularities:
Equivalent device
leakage current
10 mA)
(<
Equivalent device
leakage current
(< 10 mA)
Equivalent patient leakage current
5 mA)
(<
Equivalent patient leakage current
(< 5 mA)
Safety
maintained?
Safety
maintained?
Date Name of engineer Depot Signature
Visual inspection Protective ground wire
resistance
(< 0.3 W)
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
Equivalent device
leakage current
(< 10 mA)
Equivalent patient leakage current
(< 5 mA)
Safety
maintained?
61 93 952 D 3509
20 D 3509.102.01.01.02 07.2008

Model TENEO / D 3509 Serial number
3 Safety checks
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Remarks / Particularities:
Equivalent device
leakage current
10 mA)
(<
Equivalent device
leakage current
10 mA)
(<
Equivalent patient leakage current
5 mA)
(<
Equivalent patient leakage current
5 mA)
(<
Safety
maintained?
Safety
maintained?
bеЦдблЬ
Date Name of engineer Depot Signature
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
Equivalent device
leakage current
10 mA)
(<
Equivalent patient leakage current
5 mA)
(<
Safety
maintained?
21

Model TENEO / D 3509 Serial number
3 Safety checks
Visual inspection Protective ground wire
resistance
0.3 W)
(<
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
Visual inspection Protective ground wire
resistance
(< 0.3 W)
OK Faults W mA mA yes no
Remarks / Particularities:
Equivalent device
leakage current
10 mA)
(<
Equivalent device
leakage current
(< 10 mA)
Equivalent patient leakage current
5 mA)
(<
Equivalent patient leakage current
(< 5 mA)
Safety
maintained?
Safety
maintained?
Date Name of engineer Depot Signature
Visual inspection Protective ground wire
resistance
(< 0.3 W)
OK Faults W mA mA yes no
Remarks / Particularities:
Date Name of engineer Depot Signature
Equivalent device
leakage current
(< 10 mA)
Equivalent patient leakage current
(< 5 mA)
Safety
maintained?
61 93 952 D 3509
22 D 3509.102.01.01.02 07.2008

4 T reatment centers with HF surgical equipment
4 Treatment centers with HF surgical
equipment
4.1 General information
In Germany, medical equipment is subject to the provisions of the Ordinance
on the Installation, Operation and Use of Medical Equipment (Medizinprodukte-Betreiberverordnung – MPBetreibV) of June 29, 1998.
According to Section 6, safety tests are required for systems with HF surgical
equipment.
According to Section 7, a “Medical Product Log” must be kept, in which the
measured values as well as the tests conducted must be documented.
These tests for systems with HF surgical equipment are identical to the safety
tests described in chapter 3.
They must be performed every 2 years.
The Maintenance Manual thus simultaneously acts as Medical Product
Log.
The system owner is obliged to keep this Medical Product Log.
bеЦдблЬ
Upon request, the Medical Product Log must be made available to the competent authority for inspection purposes at any time.
The Medical Product Log must be safekept for a period of at least 5 years
after putting the system out of service.
In order to comply with the provisions of the Ordinance on the Installation,
Operation and Use of Medical Devices (MPBetreibV), the following documentation must be maintained for dental treatment centers with HF surgical equipment in Germany:
By the service engineer: • Safety tests conducted (see chapter 3)
• Repair work performed on the HF module (see section 4.3)
• Personnel who have been trained in the use of the HF surgical equipment
according to Section 5 of the MPBetreibV (see section 4.2)
By the user (system owner): • Personnel who have been trained in the use of the HF surgical equipment
according to Section 5 of the MPBetreibV (see section 4.2)
• Effects of malfunctions and repeated, similar operator errors (see section
4.4)
• Reporting of incidents to authorities and manufacturers (see chapter 5)
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
i
NOTE
As a system user outside of Germany, you must observe the legal requirements of your country.
23

Model TENEO / D 3509 Serial number
4 Treatment centers with HF surgical equipment
4.2 List of trained personnel
The treatment center with HF surgical equipment must be operated only by
personnel who have been trained in its use by the manufacturer or supplier.
Trained personnel may train other personnel.
The relevant trainings are documented in the table below.
Date Name,
trainer
Depot Signature Name,
person trained
Signature
61 93 952 D 3509
24 D 3509.102.01.01.02 07.2008

Description of the repair work performed:
Model TENEO / D 3509 Serial number
4 T reatment centers with HF surgical equipment
4.3 Repair work on the HF module
Repair work on the HF module must be performed by authorized service engineers only. After repair, a safety test must be performed and documented in
section 3.5.
The nature of the repair measures must be documented below.
bеЦдблЬ
Date Name of engineer Depot / performing agency Safety test passed? Signature
yes
Description of the repair work performed:
Date Name of engineer Depot / performing agency Safety test passed? Signature
yes
Description of the repair work performed:
Date Name of engineer Depot / performing agency Safety test passed? Signature
yes
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
25

Model TENEO / D 3509 Serial number
4 Treatment centers with HF surgical equipment
Description of the repair work performed:
Date Name of engineer Depot / performing agency Safety test passed? Signature
yes
Description of the repair work performed:
Date Name of engineer Depot / performing agency Safety test passed? Signature
yes
Description of the repair work performed:
Date Name of engineer Depot / performing agency Safety test passed? Signature
yes
61 93 952 D 3509
26 D 3509.102.01.01.02 07.2008

Model TENEO / D 3509 Serial number
4 T reatment centers with HF surgical equipment
4.4 Effects of malfunctions and
repeated, similar operator errors on
the HF module
The nature and effects of malfunctions and repeated, similar operator errors
must be documented here by the user.
i
NOTE
In addition, please comply with the obligation to report incidents according to
chapter 5.
Type of fault:
Date Name of operator Signature
Type of fault:
bеЦдблЬ
Date Name of operator Signature
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
27

Model TENEO / D 3509 Serial number
4 Treatment centers with HF surgical equipment
Type of fault:
Date Name of operator Signature
Type of fault:
Date Name of operator Signature
Type of fault:
Date Name of operator Signature
Type of fault:
Date Name of operator Signature
61 93 952 D 3509
28 D 3509.102.01.01.02 07.2008

Model TENEO / D 3509 Serial number
5 Reporting of incidents to authorities / manufacturers
5 Reporting of incidents to authorities /
manufacturers
Incidents which have led or might have led to the death or a serious deterioration in the state of health of a patient, user or other person must be immediately reported by the user to the competent authority (according to Section
3 of the MPBetreibV).
In addition, reports to the manufacturer can be documented here as well.
These reports must be documented below.
Description of incident:
bеЦдблЬ
Report submitted to:
Date Name Signature
Description of incident:
Report submitted to:
Date Name Signature
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
29

Model TENEO / D 3509 Serial number
5 Reporting of incidents to authorities / manufacturers
Description of incident:
Report submitted to:
Date Name Signature
Description of incident:
Report submitted to:
Date Name Signature
61 93 952 D 3509
30 D 3509.102.01.01.02 07.2008

Model TENEO / D 3509 Serial number
6 Remarks / special issues with regard to the dental treatment center
6 Remarks / special issues with regard
to the dental treatment center
bеЦдблЬ
61 93 952 D 3509
D 3509.102.01.01.02 07.2008
31

Model TENEO / D 3509 Serial number
6 Remarks / special issues with regard to the dental treatment center
61 93 952 D 3509
32 D 3509.102.01.01.02 07.2008


tЙ=кЙлЙкоЙ=нЬЙ=кбЦЬн=нз=г~вЙ=~еу=~днЙк~нбзел=пЬбЕЬ=г~у=ДЙ=кЙимбкЙЗ=ЗмЙ=нз=нЙЕЬебЕ~д=бгйкзоЙгЙенлK
«=pбкзе~=aЙен~д=pулнЙгл=dгДe=OMMM pйк~ЕЬЙW=ЙеЦдблЕЬ= mкбенЙЗ=бе=dЙкг~еу
a=PRMVKNMOKMNKMNKMO===MUKOMMU ûKJkêKW= MMM=MMM fгйкбг¨=Йе=^ддЙг~ЦеЙ
pбкзе~=aЙен~д=pулнЙгл=dгДe=
áå=íÜÉ=rp^W áå=`~å~Ç~W
c~Дкбвлнк~≈É=PN pбкзе~=aЙен~д=pулнЙгл=ii` pбкзе~=`~е~З~
aJSQSOR=_ЙелЬЙбг QUPR=páêçå~=aêáîÉI=pìáíÉ=NMM PORM=oбЗЦЙп~у=aкбоЙ=J=rебн=R lêÇÉê=kç
dÉêã~åó `Ь~кдзннЙI=k`=OUOTP jбллблл~мЦ~I=lен~кбз=iRi=RvS
пппKлбкзе~KЗЙ rp^ `~å~Ç~
SN=VP=VRO=a=PRMV
 Loading...
Loading...