Philips Fetal Monitor User manual

Philips Fetal Monitors
Fetal Monitoring Technology
Application Note
This application note explains how Philips fetal monitoring technology supports safe and accurate fetal and maternal monitoring. Four technological aspects are collected together here:
•Precision Signal Track and Hold
•Cross-Channel Verification
•Fetal Heart Rate Baseline Offset
•Fetal Movement Profile
Precision Signal Track and Hold
This technology allows the monitor to track the fetal heart rate signal very closely to ensure an accurate fetal heart rate measurement with almost no gaps.
The signal is monitored with two ultrasound receiver channels so that two overlapping time windows can be monitored (ultrasound travelling time=depth). When the signal is strong in both windows the optimal monitoring depth is found. The measurement window stays in this position while the control window is moved in both directions to check the strength of the signal at different depths. When the control window registers a change in the signal position (due to movement of the fetus or mother) the measurement window is moved to the new optimal position. This adaptation to changing depth happens with each heart beat and so virtually eliminates gaps in the trace due to lost signals.
As the signal is so well tracked, the measurement window around the heart can be made as small as
possible thus reducing the ultrasound energy that mother and fetus are exposed to.
Ultrasound Crystal Placement for Optimal Geometry
The crystals in the Ultrasound transducer are located six around the circumference and one in the center. In this configuration, all lines between crystals are equidistant. If a line were drawn from crystal to crystal, a series of equilateral triangles would be created. This allows the coverage area to be homogenous, or of equal signal strength throughout.
crystals
A homogenous signal eliminates any "dark spots" in the beam, which will reduce the number of times the clinician will need to reposition the transducer. If there are more than 7 crystals within a transducer there are no longer equidistant lines between the crystals and the triangles created are isosceles triangles. This configuration is unlikely to provide a homogenous signal, but rather "dark spots" within the beam.
PAD
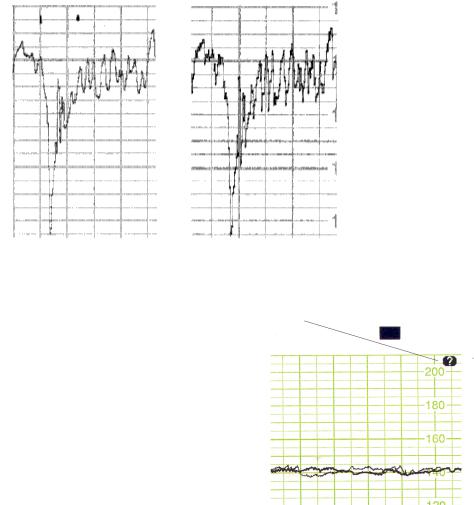
Correlation to Directly Measured FHR
The signal tracking and the homogenous ultrasound signal from the 7-crystal transducer both contribute to a fetal heart rate measured by ultrasound which correlates very highly to the directly measured fetal
fetal trace from ultrasound
Cross-Channel Verification
Cross-Channel Verification indicates when the same heart rate is being recorded by different transducers.
When the maternal heart rate and fetal heart rate are being monitored, Cross-Channel Verification will alert you when the values are the same. This may be an indication that the fetus is deceased and the transducer is picking up a signal from the maternal heart or a large blood vessel.
Cross-Channel Verification can compare all fetal and maternal heart rates and indicates when multiple channels are picking up the same signal. This means when monitoring multiples and maternal heart rate simultaneously Cross-Channel Verification will compare the values from all fetuses and each of these values with the maternal heart rate.
This technology helps reduce potential legal liability associated with continuing to monitor an incorrect heart rate.
When signal “cross-over” occurs, you are alerted within approximately 60 seconds to check the traces and potentially reposition the transducers.
Note: Be aware that a maternal heart rate trace can exhibit features that are very similar to those of a fetal heart rate trace, even including accelerations and decelerations. Do not rely solely on trace pattern features to identify a fetal source.
heart rate. The degree of smoothing caused by autocorrelation signal processing is reduced to a minimum as seen in the following comparison of traces.
original DECG trace
Figure 1 CCV symbol prints on the trace when two channels are recording the same heart beat
Cross-Channel Verification Plus (CCV+) indicator
(Series 50 XMO, not available in the USA and Japan)
To warn you if you accidentally record maternal SpO2 instead of fetal SpO2, (because the sensor is facing the uterine wall instead of the fetus) the monitor compares the heartrate it derives from DECG on the Cardio 1/Combi channel, (or from US on the Cardio 2 channel if DECG is not in use) with the pulse rate it derives from FSpO2. The CCV+ indicator illuminates and  is printed on the trace if the monitor records a pulse rate from FSpO2 and a heart rate from DECG or ultrasound that do not match for more than one minute.
is printed on the trace if the monitor records a pulse rate from FSpO2 and a heart rate from DECG or ultrasound that do not match for more than one minute.

FHR Baseline Offset
When the baselines of multiple FHR traces are very similar, independent trend interpretation can be difficult. To alleviate this, you can offset a baseline by 20 bpm. You can deactivate the offset feature and return the FHR trace to its original baseline anytime you wish.
A section of a twins’ tracing is shown above. It shows the FHR 1 trace before (Line a) and after (Line b) the baseline offset feature is activated. To indicate the recording is in the offset mode, a +20 symbol is repetitively printed at the top of the trace.
Fetal Movement Profile
Study finds Philips’ FMP saves clinicians’ and patients’
time, costs, and undue concern
Fetal movement is recognized as an important indication of fetal condition. Consequently, recordings of fetal movement are increasingly being obtained as part of routine antepartum screenings in obstetricians’ offices, clinics and hospitals.
In use in Europe, the United States and Japan since 1991, Philips Fetal Monitors simultaneously assess fetal heart rate (FHR), fetal gross body movement via the Fetal Movement Profile (FMP) parameter, and uterine activity.
Benefits of the FHR-FMP assessment range from:
•helping clinicians determine the baseline heart rate - especially in difficult-to-interpret traces, to
•predicting and supervising high risk pregnancies which involve a number of fetal disorders, including fetal growth retardation (IUGR).
One of the most important benefits of Philips’ FMP monitoring is its efficiency and cost effectiveness as an early screening tool.
Clinical trials confirm that the use of Philips Fetal Monitors in routine antepartum screenings reduces the number of patients with “suspicious” FHR test results, thus eliminating their need for additional expensive, second-level testing at the hospital. For the patient, this represents significant savings in time, cost and concern. It also means cost savings for the health care system.
Fetal Movement and Fetal Heart Rate
The classic evaluation of fetal movement employs the mother’s own perception. Clinical trials show that the Philips Fetal Monitors detect on average 40 percent more movement than perceived by the mother. Not only can they assure both mother and clinicians of a more accurate level of fetal movement detection, but the FMP, recorded simultaneously with the heart rate, helps in the interpretation of the FHR trace.
Physicians know that heart rate is directly influenced by the physical activity of the fetus and they can assign the baseline heart rate more efficiently with the Fetal Movement Profile recording.
Furthermore, studies confirm that reduced or lack of fetal gross body movement often precedes the change in FHR pattern associated with intrauterine growth retardation (IUGR)
 Loading...
Loading...