Page 1
ECG-9620L
ECG-9620M
ECG-9620N
ECG-9620P
ECG-9620S
ECG-9620T
ECG-9620U
SERVICE MANUAL
cardiofax
ELECTROCARDIOGRAPH
ECG-9620
08CK2.782.00516E
Page 2

CONTENTS
Contents
GENERAL HANDLING PRECAUTIONS .................................................................................i
WARRANTY POLICY .............................................................................................................ii
EMC RELATED CA UTION..................................................................................................... iii
Conventions Used in this Manual and Instrument..................................................................iv
Dangers, Warnings, Cautions and Notes.....................................................................iv
Explanations of the Symbols in this Manual and Instrument ........................................v
Section 1 General ...................................................................................1C.1
Introduction ......................................................................................................................... 1.1
General Information on Servicing ....................................................................................... 1.2
Service Policy, Service Parts and Patient Safety Checks ................................................... 1.4
Service Policy ........................................................................................................... 1.4
Service Parts ............................................................................................................ 1.4
Patient Saf ety Checks............................................................................................... 1.5
Maintenance Equipments and Tools ......................................................................... 1.5
General Safety Inf ormation ................................................................................................. 1.6
Specifications.................................................................................................................... 1.11
Panel Descriptions............................................................................................................ 1.14
Front Panel ............................................................................................................. 1.14
Left Side Panel........................................................................................................ 1.14
Operation Panel...................................................................................................... 1.15
Right Side Panel ..................................................................................................... 1.15
Rear Panel.............................................................................................................. 1.16
Composition...................................................................................................................... 1.17
Standard Components............................................................................................ 1.17
Options ................................................................................................................... 1.17
Location ............................................................................................................................ 1.18
Section 2 Maintenance ...........................................................................2C.1
Replacement....................................................................................................................... 2.1
Periodic Replacement Schedule............................................................................... 2.1
Cleaning and Lubrication .................................................................................................... 2.2
Cleaning and Greasing Schedules ........................................................................... 2.2
Cleaning the Paper Mark Sensor and P aper Empty Sensor..................................... 2.2
Cleaning the Motor Rotation Sensor and Lubricating the Motor Gear and Gear
Meshed with Motor Gear .......................................................................................... 2.3
Maintenance Check Sheet.................................................................................................. 2.5
Section 3 Troubleshooting and System Error Message ......................3C.1
Troubleshooting Flowchart .................................................................................................. 3.1
T roub leshooting T able ......................................................................................................... 3.4
Troubleshooting General Operation Problem ............................................................ 3.4
Service Manual ECG-9620 C.1
Page 3

CONTENTS
Troubleshooting Recording Problem......................................................................... 3.6
System Error Message ....................................................................................................... 3.7
Section 4 System Test, Adjustment and Setting ..................................4C.1
System Test ........................................................................................................................ 4.1
Overall ...................................................................................................................... 4.1
Calling up the System Test Level 1 ........................................................................... 4.2
Calling up the System Test Level 2 ........................................................................... 4.3
Entering the System Test Number ............................................................................ 4.4
Executing the System Test........................................................................................ 4.5
Quitting the System Test ........................................................................................... 4.6
Exiting the System Test Mode................................................................................... 4.6
Demonstration .................................................................................................................... 4.7
Recorder ............................................................................................................................. 4.8
Thermal Head ................................................................................................................... 4.10
Key.................................................................................................................................... 4.11
Memory............................................................................................................................. 4.12
Single Memory Test Mode ...................................................................................... 4.13
Continuous Memory Test Mode .............................................................................. 4.13
LCD/LED........................................................................................................................... 4.14
Input Unit .......................................................................................................................... 4.16
Calibration......................................................................................................................... 4.17
Communication................................................................................................................. 4.18
CRO/EXT1........................................................................................................................ 4.20
System Setup Initialization................................................................................................ 4.22
ECG Findings List Recording............................................................................................ 4.23
Recording Resolution Setting ........................................................................................... 4.24
Date and Time Setting ...................................................................................................... 4.25
Setting the Date and Time ...................................................................................... 4.25
Section 5 Board/Unit Description..........................................................5C.1
Block Diagram..................................................................................................................... 5.1
Pow er Unit .......................................................................................................................... 5.2
ECG Control Board ............................................................................................................. 5.2
Section 6 Disassembly...........................................................................6C.1
Before You Begin................................................................................................................. 6.1
Warnings and Cautions ............................................................................................ 6.1
Required T ools.......................................................................................................... 6.1
Cable Connection ............................................................................................................... 6.2
Removing the Upper Casing............................................................................................... 6.4
Removing the Magazine and Recording Paper ........................................................ 6.4
Removing the Battery Pack ...................................................................................... 6.4
Removing the Upper Casing..................................................................................... 6.4
Removing the Thermal Head and Motor Assy .................................................................... 6.5
Removing the Thermal Head.................................................................................... 6.5
C.2 Service Manual ECG-9620
Page 4

CONTENTS
Removing the Motor Assy......................................................................................... 6.6
Removing the ECG Control Board...................................................................................... 6.6
Removing the Power Board ................................................................................................ 6.8
Removing the Power Board ...................................................................................... 6.8
Replacing the Po wer Fuse and Battery Fuse ........................................................... 6.9
Removing the Key Board and LCD Unit............................................................................ 6.10
Section 7 Replaceable Parts List...........................................................7C.1
Instrument........................................................................................................................... 7.2
Section 8 Connector Pin Assignment ..................................................... 8.1
Attaching the Ferrite Core......................................................................................... 8.1
EXT-IN Connector..................................................................................................... 8.2
CRO-OUT Connector ............................................................................................... 8.2
SIO Connector.......................................................................................................... 8.2
Service Manual ECG-9620 C.3
Page 5

GENERAL HANDLING PRECAUTIONS
This device is intended for use only by qualified medical personnel.
Use only Nihon Kohden approved products with this device. Use of non-approved products or in
a non-approved manner may affect the performance specifications of the device. This includes,
but is not limited to, batteries, recording paper, pens, extension cables, electrode leads, input
boxes and AC power.
Please read these precautions thoroughly before attempting to operate the instrument.
1. To safely and effectively use the instrument, its operation must be fully understood.
2. When installing or storing the instrument, take the following precautions:
(1) Avoid moisture or contact with water, dust, extreme atmospheric pressure, excessive humidity and temperatures,
poorly ventilated areas, and saline or sulphuric air.
(2) Place the instrument on an even, level floor. Avoid vibration and mechanical shock, even during transport.
(3) Avoid placing in an area where chemicals are stored or where there is danger of gas leakage.
(4) The power line source to be applied to the instrument must correspond in frequency and voltage to product
specifications, and have sufficient current capacity.
(5) Choose a room where a proper grounding facility is available.
3. Before Operation
(1) Check that the instrument is in perfect operating order.
(2) Check that the instrument is grounded properly.
(3) Check that all cords are connected properly.
(4) Pay extra attention when the instrument is in combination with other instruments to avoid misdiagnosis or other
problems.
(5) All circuitry used for direct patient connection must be doubly checked.
(6) Check that battery level is acceptable and battery condition is good when using battery-operated models.
4. During Operation
(1) Both the instrument and the patient must receive continual, careful attention.
(2) Turn power off or remove electrodes and/or transducers when necessary to assure the patient’s safety.
(3) Avoid direct contact between the instrument housing and the patient.
5. To Shutdown After Use
(1) Turn power off with all controls returned to their original positions.
(2) Remove the cords gently; do not use force to remove them.
(3) Clean the instrument together with all accessories for their next use.
6. The instrument must receive expert, professional attention for maintenance and repairs. When the instrument is
not functioning properly, it should be clearly marked to avoid operation while it is out of order.
7. The instrument must not be altered or modified in any way.
8. Maintenance and Inspection:
(1) The instrument and parts must undergo regular maintenance inspection at least every 6 months.
(2) If stored for extended periods without being used, make sure prior to operation that the instrument is in perfect
operating condition.
Service Manual ECG-9620 i
Page 6

(3) Technical information such as parts list, descriptions, calibration instructions or other information is available for
qualified user technical personnel upon request from your Nihon Kohden distributor.
9. When the instrument is used with an electrosurgical instrument, pay careful attention to the application and/or
location of electrodes and/or transducers to avoid possible burn to the patient.
10. When the instrument is used with a defibrillator, make sure that the instrument is protected against defibrillator
discharge. If not, remove patient cables and/or transducers from the instrument to avoid possible damage.
WARRANTY POLICY
Nihon Kohden Corporation (NKC) shall warrant its products against all defects in materials and workmanship for one year
from the date of delivery. However, consumable materials such as recording paper, ink, stylus and battery are excluded from
the warranty.
NKC or its authorized agents will repair or replace any products which prove to be defective during the warranty period,
provided these products are used as prescribed by the operating instructions given in the operator’s and service manuals.
No other party is authorized to make any warranty or assume liability for NKC’s products. NKC will not recognize any other
warranty, either implied or in writing. In addition, service, technical modification or any other product change performed by
someone other than NKC or its authorized agents without prior consent of NKC may be cause for voiding this warranty.
Defective products or parts must be returned to NKC or its authorized agents, along with an explanation of the failure.
Shipping costs must be pre-paid.
This warranty does not apply to products that have been modified, disassembled, reinstalled or repaired without Nihon
Kohden approval or which have been subjected to neglect or accident, damage due to accident, fire, lightning, vandalism,
water or other casualty, improper installation or application, or on which the original identification marks have been
removed.
ii Service Manual ECG-9620
Page 7

EMC RELATED CAUTION
This equipment and/or system complies with the International Standard IEC60601-1-2 for electromagnetic
compatibility for medical electrical equipment and/or system. However, an electromagnetic environment
that exceeds the limits or levels stipulated in the IEC60601-1-2, can cause harmful interference to the
equipment and/or system or cause the equipment and/or system to fail to perform its intended function or
degrade its intended performance. Therefore, during the operation of the equipment and/or system, if
there is any undesired deviation from its intended operational performance, you must avoid, identify and
resolve the adverse electromagnetic effect before continuing to use the equipment and/or system.
The following describes some common interference sources and remedial actions:
1. Strong electromagnetic interference from a nearby emitter source such as an authorized radio station
or cellular phone:
Install the equipment and/or system at another location if it is interfered with by an emitter source such
as an authorized radio station. Keep the emitter source such as cellular phone away from the
equipment and/or system.
2. Radio-frequency interference from other equipment through the AC power supply of the equipment
and/or system:
Identify the cause of this interference and if possible remove this interference source. If this is not
possible, use a different power supply.
3. Effect of direct or indirect electrostatic discharge:
Make sure all users and patients in contact with the equipment and/or system are free from direct or
indirect electrostatic energy before using it.
4. Electromagnetic interference with any radio wave receiver such as radio or television:
If the equipment and/or system interferes with any radio wave receiver, locate the equipment and/or
system as far as possible from the radio wave receiver.
If the above suggested remedial actions do not solve the problem, consult your Nihon Kohden Corporation
subsidiary or distributor for additional suggestions.
The CE mark is a protected conformity mark of the European Community. The products herewith comply
with the requirements of the Medical Device Directive 93/42/EEC.
The CE mark is applied only to the ECG-9620L/M/N Electrocardiograph.
This equipment complies with EUROPEAN STANDARD EN-60601-1-2 (1993) which requires EN-55011, class
B.
Service Manual ECG-9620 iii
Page 8
Conventions Used in this Manual and Instrument
Dangers, Warnings, Cautions and Notes
Warnings, cautions and notes are used in this manual to alert or signal the reader to specific information.
DANGER
A danger is used to alert the user to a hazardous situation which will cause death or serious injury.
WARNING
A warning alerts the user to possible injury or death associated with the use or misuse of the instrument.
CAUTION
A caution alerts the user to possible injury or problems with the instrument associated with its use or
misuse such as instrument malfunction, instrument failure, damage to the instrument, or damage to other
property.
NOTE
A note provides specific information, in the form of recommendations, prerequirements, alternative
methods or supplemental information.
iv Service Manual ECG-9620
Page 9
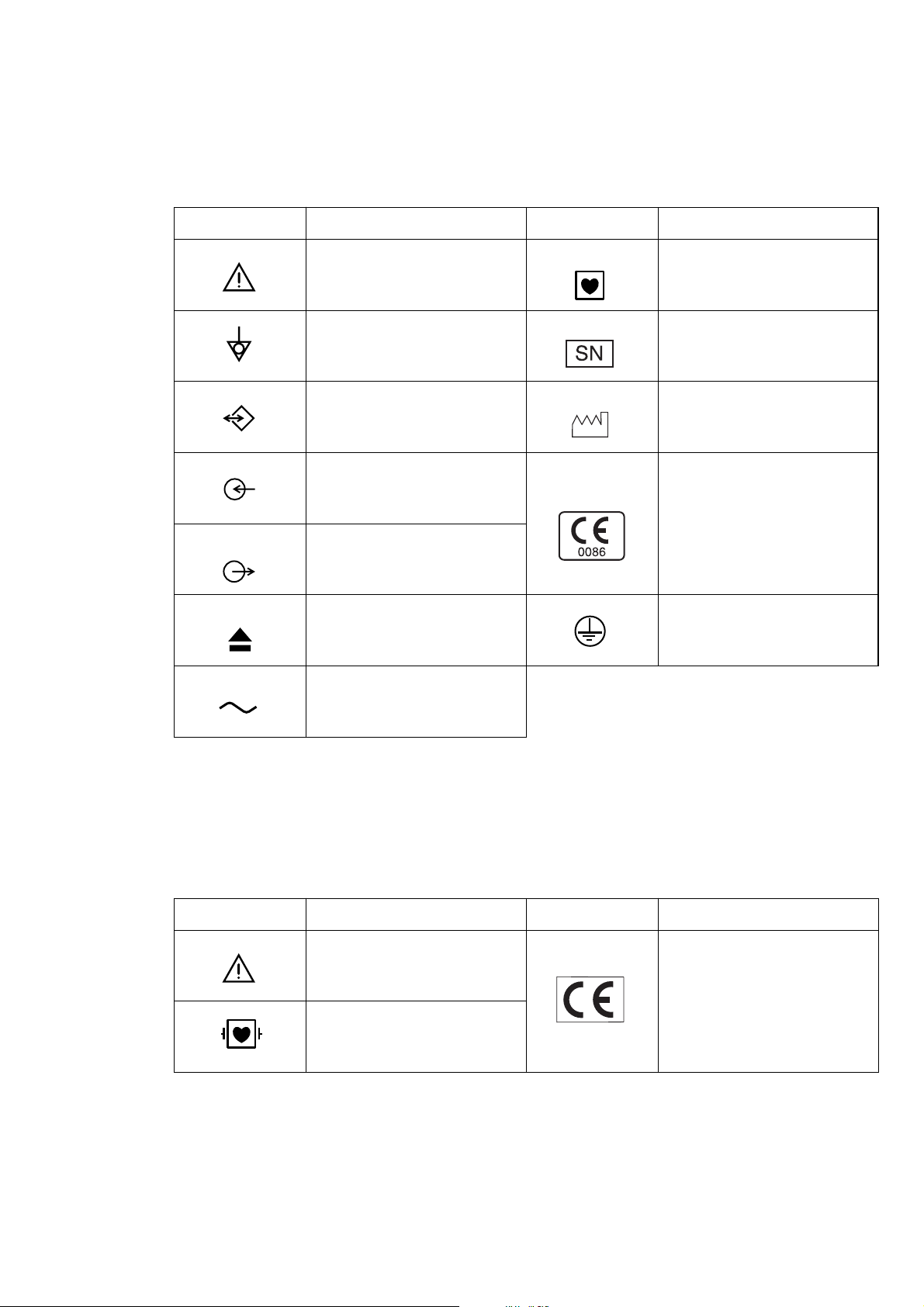
Explanations of the Symbols in this Manual and Instrument
The following symbols found in this manual/instrument bear the respective descriptions as given.
Cardiograph
Symbol Description Symbol Description
Attention, consult operator’s
manual
Equipotential terminal Serial number
Serial input/output terminal
Input terminal for analog signal
Output terminal for analog
signal
Eject (magazine release button)
Alternative current
Type CF applied part
Date of manufacture
The CE mark is a protected
conformity mark of European
Community. The products
herewith comply with the
requirements of the Medical
Device Directive 93/42/EEC.
Protective earth
Patient cable
The CE mark is applied only to the
ECG-9620L/M/N Electrocardiograph.
Symbol Description Symbol Description
Attention, consult operator’s
manual
Defibrillation-proof
Type CF applied par
The CE mark is a protected
conformity mark of European
Community. The products
herewith comply with the
requirements of the Medical
Device Directive 93/42/EEC.
Service Manual ECG-9620 v
Page 10
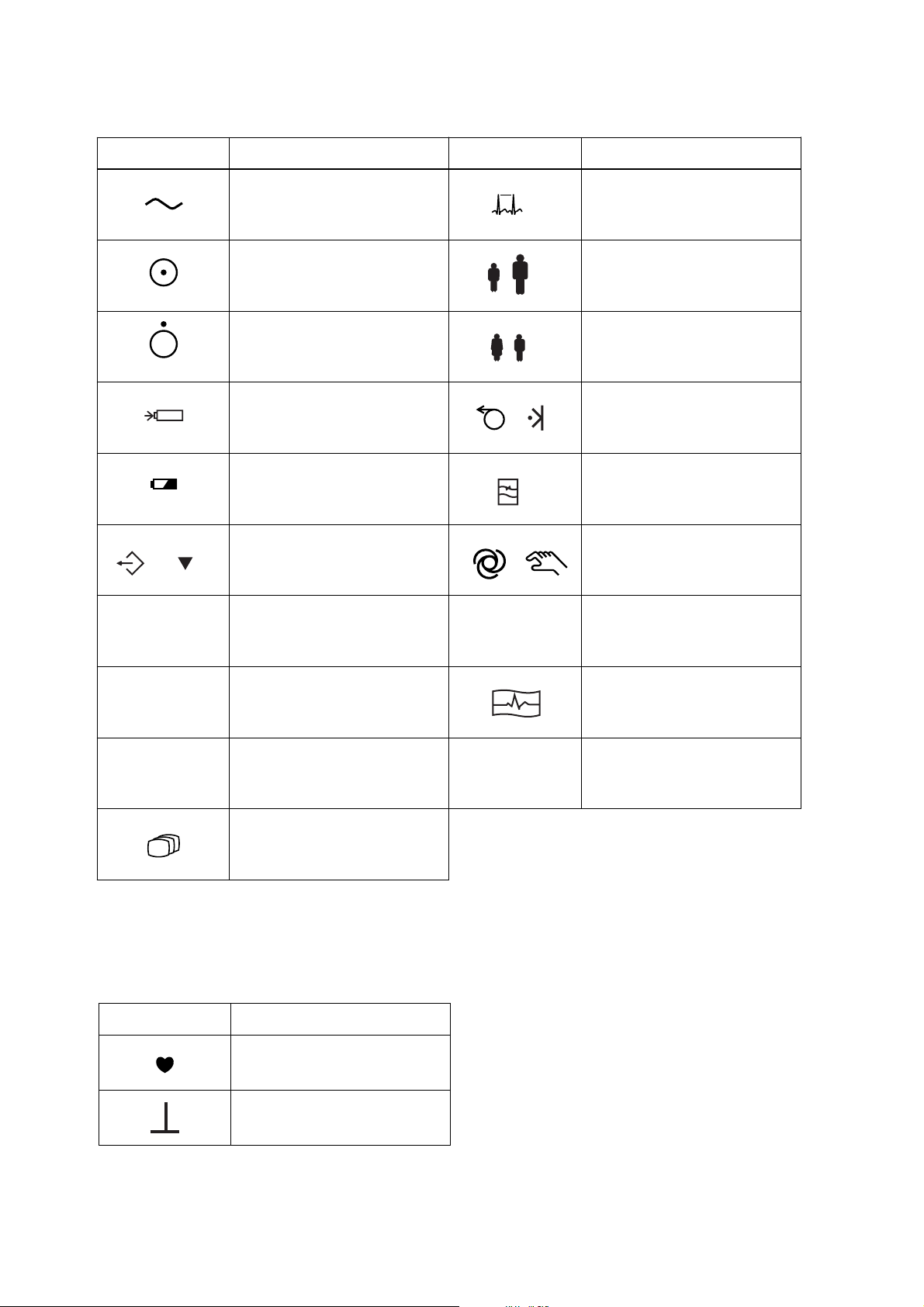
Operation panel
Symbol Description Symbol Description
F1
Alternating current
“On” only for a part of
equipment
“Off” only for a part of
equipment
Battery charging
Battery check
/
Copy / Calibration / Automatic / Manual control
0
F1 function key
1
/
CLR
Rhythm
5
Age
6
Sex
7
Paper feed / Mark
8
Filter
9
Clear
On screen
F2
F3
Symbol Description
F2 function key Start/Stop recording
2
F3 function key
3
Mode
4
QRS sync mark
CAL mark
ENT
Enter
A key with a numeric number is used to enter
numbers in the System Setup screen and paient
information.
vi Service Manual ECG-9620
Page 11

Section 1 General
Introduction ........................................................................................................................ 1.1
General Information on Servicing ...................................................................................... 1.2
Service Policy, Service Parts and Patient Safety Chec ks .................................................. 1.4
Service Policy .......................................................................................................... 1.4
Service Parts ........................................................................................................... 1.4
Patient Safety Checks.............................................................................................. 1.5
Maintenance Equipments and Tools ........................................................................ 1.5
General Safety Inf ormation ................................................................................................ 1.6
Specifications................................................................................................................... 1.11
Panel Descriptions........................................................................................................... 1.14
Front Panel ............................................................................................................ 1.14
Left Side Panel....................................................................................................... 1.14
Operation Panel..................................................................................................... 1.15
Right Side Panel .................................................................................................... 1.15
Rear Panel............................................................................................................. 1.16
Composition..................................................................................................................... 1.17
Standard Components........................................................................................... 1.17
Options .................................................................................................................. 1.17
Location ........................................................................................................................... 1.18
Service Manual ECG-9620 1C.1
Page 12

Introduction
1. GENERAL
This service manual provides useful information to qualified service personnel to
understand, troubleshoot, service, maintain and repair the ECG-9620L/M/N/P/S/T/
U Electrocardiograph (referred to as “the instrument” in this service manual).
The System test, Adjustment and Setting section in this service manual describes
the maintenance that should be performed by qualified service personnel. The
Maintenance section in the operator’s manual describes the maintenance that can
be performed by the user.
The information in the operator’s manual is primarily for the user. However, it is
important for service personnel to thoroughly read the operator’s manual and
service manual before starting to troubleshoot, service, maintain or repair this
instrument. This is because service personnel need to understand the operation of
the instrument in order to effectively use the information in the service manual.
Service Manual ECG-9620 1.1
Page 13

1. GENERAL
General Information on Servicing
Note the following information when servicing the instrument.
Safety
• There is the possibility that the outside surface of the instrument,
such as the operation keys, could be contaminated by contagious
germs, so disinfect and c lean the instrument before servicing it.
When servicing the instrument, wear rubber gloves to protect
yourself from infection.
• There is the possibility that when the lithium battery is broken, a
solvent inside the lithium battery could flow out or a toxic substance
inside it could come out. If the solvent or toxic substance touches
your skin or gets into your eye or mouth, immediately wash it with a
lot of water and see a physician.
CAUTIONS
Liquid ingress
The instrument is not waterproof, so do not install the instrument
where water or liquid can get into or fall on the instrument. If liquid
accidentally gets into the instrument or the instrument accidentally
drops into liquid, disassemble the instrument, clean it with clean
water and dry it completely. After reassembling, verify that there is
nothing wrong with the patient safety checks and function/
performance checks. If there is something wrong with the
instrument, contact your Nihon Kohden representative for repair.
Environmental Safeguards
Depending on the local laws in your community, it may be illegal to
dispose of the lithium battery in the regular waste collection. Check
with your local officials for proper disposal procedures.
Disinfection and cleaning
To disinfect the outside surface of the instrument, wipe it with a nonabrasive cloth moistened with alcohol. Do not use any other
disinfectants or ultraviolet rays to disinfect the instrument.
1.2 Service Manual ECG-9620
Page 14
1. GENERAL
Caution - continued
Transport
• Use the specified shipment container and packing material to
transport the instrument. If necessary, double pack the instrument.
Also, put the instrument into the shipment container after pac king so
that the buffer material does not get inside the instrument.
• When transporting a board or unit of the instrument, be sure to put it
in a conductive bag. Ne ver use an aluminum bag to transport a
board or unit. Also, never use a styrene foam or plastic bag which
generates static electricity to wrap the board or unit of the
instrument.
Handling the instrument
• Because the outside surface of the instrument is made of resin, the
outside surface of the instrument is easily damaged. So when
handling the instrument, remo ve clutter from around the instrument
and be careful to not damage the instrument or get it dirty.
• Because most of the boards in the instrument are multilayer boards
with surface mount electrical devices (SMD), a special tool is
required to remove and solder the electrical devices on it. To avoid
damaging other electrical components, do not remo ve and solder
SMD components yourself.
Measuring and Test Equipment
Maintain the accuracy of the measuring and test equipment by
checking and calibrating it according to the chec k and calibration
procedures.
Service Manual ECG-9620 1.3
Page 15

1. GENERAL
Service Policy, Service Pa rts and P atient Safety Checks
Service Policy Our technical service policy for this instrument is to replace the faulty unit, board
or part or damaged mechanical part with a new one. Do not perform electrical
device or component level repair of the multilayer board or unit. We do not support
component level repair outside the factory for the following reasons:
• Most of the boards are multilayer boards with surface mount electrical
devices, so the mounting density of the board is too high.
• A special tool or high degree of repair skill is required to repair the multilayer
boards with surface mount electrical devices.
Only disassemble the instrument or replace a board or unit in an environment
where the instrument is protected against static electricity.
Service Parts
Refer to “Replaceable Parts List” of this manual for the service parts for technical
service that we provide.
NOTE
When ordering parts or accessories from your Nihon Kohden
representative, please quote the NK code number and part name
which is listed in this service manual, and the name or model of the
unit in which the required part is located. This will help us to
promptly attend to your needs. Always use parts and accessories
recommended or supplied by Nihon Kohden Corporation to assure
maximum performance from your instrument.
1.4 Service Manual ECG-9620
Page 16

1. GENERAL
Patient Safety Checks
Maintenance Equipments
and T ools
Periodic maintenance procedures and diagnostic check procedures are provided in
this manual to ensure that the instrument is operating in accordance with its design
and production specifications. To verify that the instrument is working in a safe
manner with regard to patient safety, patient safety checks should be performed on
the instrument before it is first installed, periodically after installation, and after any
repair is made on the instrument.
For patient safety checks, perform the following checks as described in the
IEC60601-1 “Medical electrical equipment - Part 1: General requirements for
safety”:
• Protective earth resistance check
• Earth leakage current check
• Enclosure leakage current check
• Patient leakage current check
• Withstanding voltage check
Test equipment
When repairing or calibrating the instrument, the following test equipment is
required.
• Oscilloscope: 2 channels or more for input signal, 50 mV to 5 V input range, 1/
10 attenuating probe and 100 MHz or more frequency response characteristic
must be provided.
• Digital voltmeter: standard type (An oscilloscope can be used instead of the
digital voltmeter.)
Service Manual ECG-9620 1.5
Page 17

1. GENERAL
General Safety Information
• Never use this cardiograph in the presence of any flammab le
• Never use this cardiograph in a high-pressure oxygen medical tank.
Using with an electrical surgical unit (ESU)
• Never use this cardiograph near an ESU. The cardiograph may
• When using this cardiograph with an ESU, refer to the instruction
DANGER
anesthetic gas or high-concentration oxygen atmosphere. Failure to
follow this warning may cause e xplosion or fire.
Failure to follow this warning may cause explosion or fire.
WARNING
malfunction due to high-frequency noise from the ESU.
manual for the ESU. Before measurement, check that the return
plate is correctly attached to the patient and check that the
cardiograph operates correctly when using with the ESU. If the
return plate is not attached correctly, it may burn the patient’s skin
where the electrodes are attached.
MRI examination
• Do not install this cardiograph in an MRI examination room. The
cardiograph may not operate properly due to high-frequency
magnetic noise from the MRI.
• When performing MRI tests, remo ve from the patient all electrodes
which are connected to this cardiograph. Failure to follow this
warning may cause serious electrical burn on the patient due to local
heating caused by dielectric electromotive force. For details, refer to
the instruction manual for the MRI.
When performing defibrillation
• Before defibrillation, remo ve all electrodes and gel from the chest of
the patient. If the defibrillator paddle touches electrodes or gel, the
discharged energy may burn the patient’s skin.
• Before defibrillation, all persons must keep clear of the bed and must
not touch the patient or any equipment connected to the patient.
Failure to follow this warning may cause serious electrical burn,
shock or other injury.
1.6 Service Manual ECG-9620
Page 18
1. GENERAL
Warning - continued
Use only the following specified patient cables when using with a
defibrillator or ESU. When the specified patient cable is connected,
the cardiograph is type CF defibrillation-proof compliance. Failure to
follow this warning will cause serious electrical burn where the
electrode is attached and damage the cardiograph due to discharge
energy when defibrillation is performed.
Patient cable: BJ-901D – IEC standard, 3 mm diameter tip
BJ-902D – IEC/DIN standard, 4 mm diameter tip
BJ-903D – IEC/DIN standard, c lip
BA-901D – AHA requirement, 3 mm diameter tip
BA-903D – AHA requirement, color clip
When using an ESU and defibrillator with the cardiograph, use silver
chloride disposable electrodes.
Installation
WARNING
• Only use the 3-prong power cord provided with the cardiograph.
Failure to follow this caution may cause electrical shock to the
patient and operator.
• Only use the specified patient cable and connect the external
instruments with the specified installation procedure. Failure to
follow this warning may cause a serious electrical shoc k to the
patient and operator by leakage current.
CAUTION
• When the provided 3-prong power cord cannot be used, operate the
cardiograph on battery power. When another type of power cord
(especially 2-prong power cord) is used, this may cause electrical
shock to the patient and operator.
• When several medical instruments are used together, ground all
instruments at the same one-point ground to protect the patient and
operator from electrical shock. Any potential difference between
instruments may cause electrical shock to the patient and operator.
• When connecting an external instrument to connectors marked with
, the external instrument and this cardiograph must be connected
according to the IEC60601-1-1 “Medical electrical equipment - Part 11: General requirements for safety - Collateral standard: Safety
requirements for medical electrical systems”. Failure to follow this
warning may cause electrical shock to the patient and operator.
• When inserting or removing the battery from the cardiograph, make
sure that the cardiograph is turned off. Otherwise, the patient and
operator may get an electrical shock.
Service Manual ECG-9620 1.7
Page 19

1. GENERAL
Battery Pack
DANGER
• Keep the battery pack awa y from fire. Do not heat the battery pack.
Otherwise, the substance liquid leaks out and the battery pack
explodes.
• Never short-circuit the + and – terminals on the battery pack with a
wire. Never store or carry the battery pack with metal such as
necklace or hair pins. The battery pack short-circuits and a large
current flows, causing leakage of the substance liquid inside the
battery and battery explosion.
• Never disassemble or modify the battery pack. Never damage or
directly solder the sheath tube. The battery pack short-circuits, the
substance liquid comes out and the battery pack explodes.
• Do not use a battery pack which is damaged, such as from falling.
There is a gas discharge valve inside the battery and if this valve is
damaged, the gas cannot be dischar ged, causing the battery pack to
explode.
• Do not subject the battery pack to a strong mechanical shock. The
susbstance liquid inside the battery leaks and explodes.
• If the battery pack is damaged and substance liquid inside the
battery contacts the eyes or skin, wash immediately and thoroughly
with water and see your physician. Never rub your eyes, otherwise
you may lose your eyesight.
• Only charge the battery pack with the ECG-9620 cardiograph. If any
other battery charger is used, abnormal current flows and the
substance liquid inside the battery leaks and the battery explodes.
• Do not connect the battery pack to an AC outlet or lighter socket in a
car. The substance liquid inside the battery leaks out and the battery
pack explodes.
• The battery has + and – polarity. Make sure that the battery is
installed with the correct polarity direction. Otherwise, the
substance inside the battery leaks out and the battery pack explodes.
• Use only the SB-901D battery pack.
WARNING
• Do not immerse the battery pack in water or seawater. The battery
heats up and rusts and the substance liquid inside the battery leaks.
• Never use a battery pack which is damaged, discolored or has
leakage. A damaged battery pack explodes if used.
• Do not leave the battery pack unused for more than one year. The
battery may leak.
1.8 Service Manual ECG-9620
Page 20
1. GENERAL
CAUTION
• Do not charge the deteriorated battery pack. Otherwise, the
cardiograph cannot operate on battery power.
• Do not expose the battery pack to direct sunlight or leave in a high
temperature place. The life time of the battery pack may be
shortened, the performance of the battery pack may be degraded and
the substance liquid inside the battery may leak.
• Do not leave the battery pack where patients can reach it.
• Before disposing of the battery pack, c heck with your local solid
waste officials for details in your area for rec ycling options or proper
disposal. The battery is recyc lable. At the end of its useful life, under
various state and local laws, it may be illegal to dispose of this
battery into the municipal waste stream.
Operation
CAUTION
• Enter the patient information correctly. Otherwise, the ECG data may
be lost or mixed up with another patient’s ECG data.
ECG recording judgement
• The cardiograph provides automatic ECG analysis function. The
automatic ECG analysis is performed for acquired ECG waveforms
only and does not reflect all conditions of the patient. The results of
the analysis may not correspond to the judgment of a physician.
• Overall judgement must be performed by the ph ysician, referring to
the analysis result, c linical findings, and other examination results.
After the physician’ s overall judgement, the analysis results should
be signed or initialed by the physician.
• Take care when judging the ECG recording because the 25 Hz EMG
filter may cause greater distortion of P-waves and QRS-waves
depending on the waveform shape. The characteristics of the EMG
filter are similar to a conventional analog filter.
• Do not use the output signal from the output connector for a
synchronization signal such as the synchr onized cardioversion
signal. There is a time delay between the input ECG signal and
output signal.
• When the cardiograph operates on battery power and large leakage
current is input from the connected external instrument, ground the
cardiograph or use an isolation transformer for the external
instrument. Failure to follow this caution may cause electrical shock
to patient and operator.
• Use only the KD-103E cart for the cardiograph. When another cart is
used, the cardiograph may fall off or the cart may tip over.
Service Manual ECG-9620 1.9
Page 21
1. GENERAL
Maintenance
Caution - continued
• Never use the cardiograph with its side panel downward. Failure to
follow this caution may cause the cardiograph to fall over or cause
battery liquid leakage.
NOTE
• When using the battery pack and the battery operation lamp is
blinking in orange, measurement results may not be saved.
CAUTION
• Before maintenance (cleaning, disinfection), make sure that the
cardiograph is turned off and the power cord is removed from the AC
outlet and cardiograph. Otherwise, the operator may get an electrical
shock and the cardiograph may malfunction.
• Before battery replacement, make sure that the cardiograph is turned
off and the power cord is removed from the AC outlet and
cardiograph. Otherwise, the operator may get an electrical shock.
• Do not disassemble or repair the cardiograph. Disassembly and
repair must be performed b y qualified service personnel.
1.10 Service Manual ECG-9620
Page 22

Specifications
ECG input
Input impedance 10 MΩ or more
Electrode offset tolerance ±500 mV or more
Input unit protection Isolated and defibrillator protected only when the following specified patient
Standard sensitivity 10 mm /mV ±2%
Common mode rejection ratio 100 dB or more
Frequency response 0.05 to 150 Hz – 3 dB or more
Waveform data processor
Sample rate 500 samples/s (input unit: 8,000 samples/s)
AC line filter 50/60 Hz
High-cut filter 75, 100, 150 Hz
EMG filter 25/35 Hz
Time constant 3.2 s or more
Waveform status detection Electrode detachment (polarization voltage),
Sensitivity selection 5, 10 , 20 mm/mV
1. GENERAL
cable is connected
Patient cable: BJ-901D, BJ-902D, BJ-903D, BA-901D, BA-903D
Noise (high frequency)
LCD
Size 3.8 inch
Number of dots 320 × 240
ECG waveform 6 channel: 2.8 s
Displayed data Waveform, patient information, recording settings, operation mode, heart rate,
QRS sync mark, error message, electrode detachment, noise
Recorder
Printing method High resolution thermal printer head
Printing density 200 dpi (8 dots/mm)
Scanning line density 1 ms
Recording width 56 mm
Number of recording channels 1, 2, 3
Paper speed 25, 50 mm/s
Number of recording lines Up to 14
Printed data Program type, version, date and time, paper speed, sensitivity, lead name, filter,
Patient information (ID number, sex, age zone), timing mark, event mark,
electrode detachment, noise
Mechanical noise 48 dB or less at paper speed 25 mm/s
External input/output
External input 10 mm/0.5 V ±5%, input impedance 100 kΩ or more
Signal output 0.5 V/1 mV ±5%, output impedance 100 Ω or less
Serial I/O Communication method: RS-232C
Baud rate: 2400, 4800, 9600, 19200, 38400,
57600, 115200
Service Manual ECG-9620 1.11
Page 23

1. GENERAL
Power requirement
Line voltage ECG-9620L: 220 V AC ±10%
Line frequency 50 or 60 Hz
Power input 45 VA
Power consumption 45 W or less
Built-in battery (SB-901D) Voltage: 12 V
ECG-9620M: 230 V AC ±10%
ECG-9620N: 240 V AC ±10%
ECG-9620P: 220 V AC ±10%
ECG-9620S: 110 V AC ±10%
ECG-9620T: 120 V AC ±10%
ECG-9620U: 127 V AC ±10%
Current consumption: 6 A or less
Battery operation time: 2 hours or more (when using a new fully charged
battery in manual mode, at 25 mm/s of recording
speed, 3 ch, and in continuous recording.)
Remaining battery power can change depending on
the surrounding temperature and quality of
recording waveform.
Environment
Operating temperature 5 to 40°C (41 to 104°F)
Operating humidity
25 to 85% RH (with battery pack and recording paper)
20 to 85% RH (with battery pack and without recording paper)
25 to 90% RH (with recording paper and without battery pack)
25 to 95% RH (without battery pack and recording paper)
Operating atmospheric pressure 70 to 106 kPa
Storage temperature
Cardiograph: -20 to 65°C (−4 to 149°F)
Battery pack: -20 to 50°C (−4 to 122°F) (within 30 days)
Recording paper: -20 to 50°C (−4 to 122°F)
Storage humidity
Cardiograph: 10 to 95% RH (non-condensing)
Battery pack: 10 to 85% RH (non-condensing) (within 60 days)
Recording paper: 10 to 90% RH (non-condensing)
Storage atmospheric pressure 70 to 106 kPa
-20 to 40°C (−4 to 104°F) (within 90 days)
-20 to 30°C (−4 to 86°F) (within one year)
45 to 85% RH (non-condensing) (more than 60 days)
Electromagnetic compatibility
IEC60601-1-2 (1993), CISPR11 (1990) Group 1 Class B
IEC60601-2-25 Amendment 1 (1999), protection against electrosurgery interference
Other
Indoor portable
1.12 Service Manual ECG-9620
Page 24

Dimensions and weight
Dimensions 280 W × 70 H × 216 D mm (excluding protrusions)
Weight Approx. 3.1 kg (with battery)
Approx. 2.7 kg (without battery)
Safety
Safety standard:
IEC60601-1 (1998)
IEC60601-1 Amendment 1 (1991)
IEC60601-1 Amendment 2 (1995)
IEC60601-2-25 (1993)
IEC60601-2-25 Amendment 1 (1999)
Type of protection against electric shock:
AC power: Class I
Battery power: Internally powered equipment
Degree of protection against electric shock:
Defibrillator proof type CF applied part when patient cable BJ-901D, BJ-902D, BJ-903D, BA-901D or
BA-903D is used
Degree of protection against harmful ingress of water:
Ordinary equipment
Degree of safety of application in the presence of a flammable anaesthetic mixture with air, oxygen or nitrous
oxide:
Not suitable for use in the presence of a flammable anaesthetic mixture with air, oxygen or
nitrous oxide
Mode of operation:
Continuous
1. GENERAL
Service Manual ECG-9620 1.13
Page 25
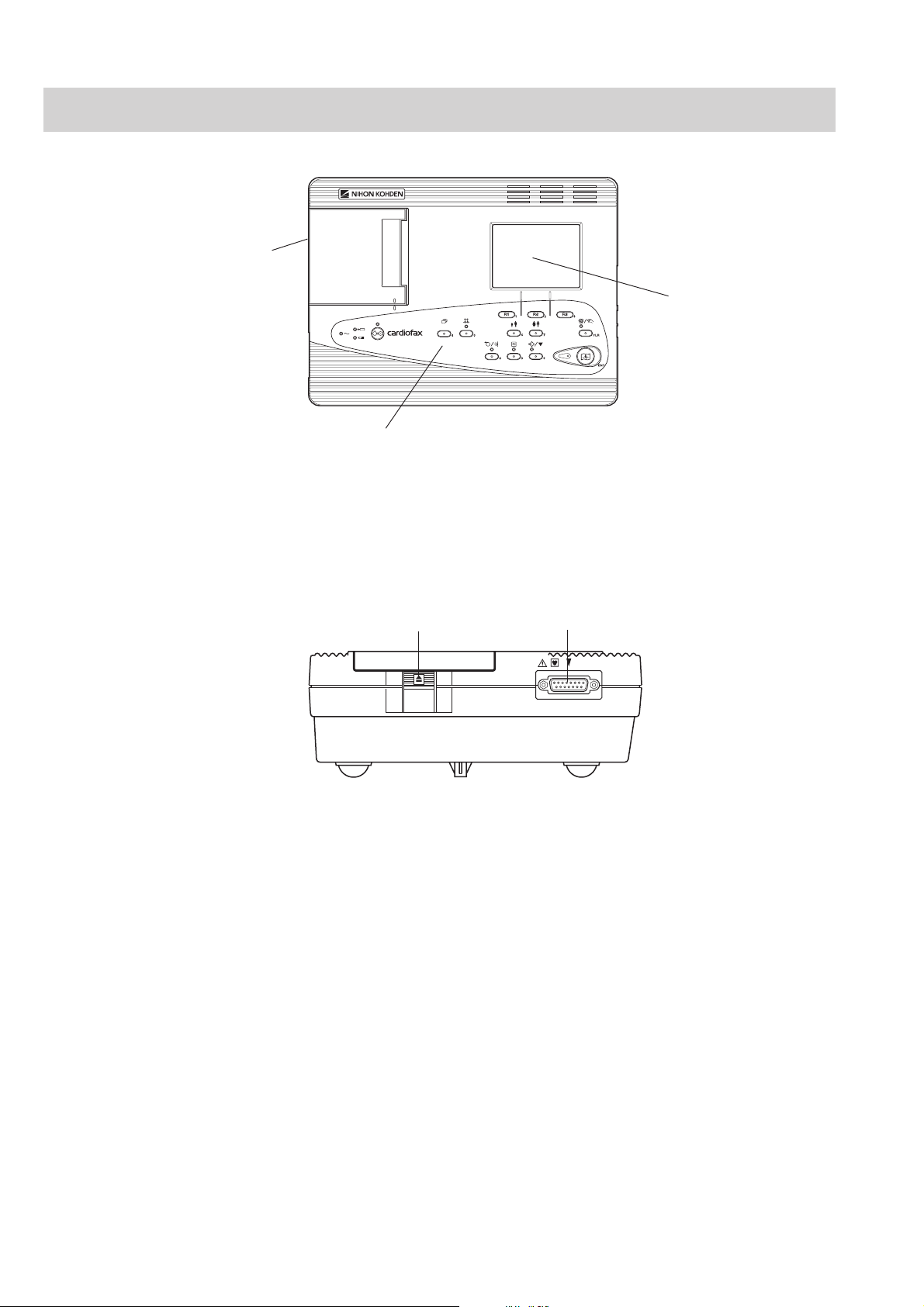
1. GENERAL
Panel Descriptions
Front Panel
2
3
1
Name
1. Operation panel
2. Magazine (paper container)
3. LCD screen
Left Side Panel
1
Name
1. Magazine release button
2. Patient cable connector
2
1.14 Service Manual ECG-9620
Page 26

Operation Panel
1. GENERAL
7
8
3
1
2
4
5
6
11
12
13
9
10
14
Name
1. AC power lamp
2. Battery operation lamp
3. Battery charge lamp
4. Power key/lamp
5. Mode key
6 Rhythm key/lamp
7. F1, F2, F3 function keys
Name
8. Age key
9. Sex key
10. Auto/Manual key/lamp
11. Feed/Mark key
12. Filter key/lamp
13. Copy/CAL key lamp
14. Start/Stop key/lamp
Right Side Panel
CAUTION
• When connecting an external instrument to connectors marked with , the external instrument and this
cardiograph must be connected according to the IEC60601-1-1 “Medical electrical equipment - Part 1-1:
General requirements for safety - Collateral standard: Safety requirements for medical electrical
systems”. Failure to follow this warning may cause electrical shock to the patient and operator.
• Do not use the output signal from the output connector for a synchronization signal such as the
synchronized cardioversion signal. There is a time delay between the input ECG signal and output
signal.
2
1
Name
1. EXT-IN connector
2. CRO-OUT
3. SIO connector
4. AC power cord socket
5. Equipotential ground terminal
Service Manual ECG-9620 1.15
3
4
5
Page 27

1. GENERAL
Rear Panel
The CE mark is applied only to the
ECG-9620L/M/N Electrocardiograph.
Battery
CAUTION
Always install the battery even when the cardiograph operates on AC
power. Otherwise sudden power down occurs when any electrode is
detached during recording.
1.16 Service Manual ECG-9620
Page 28
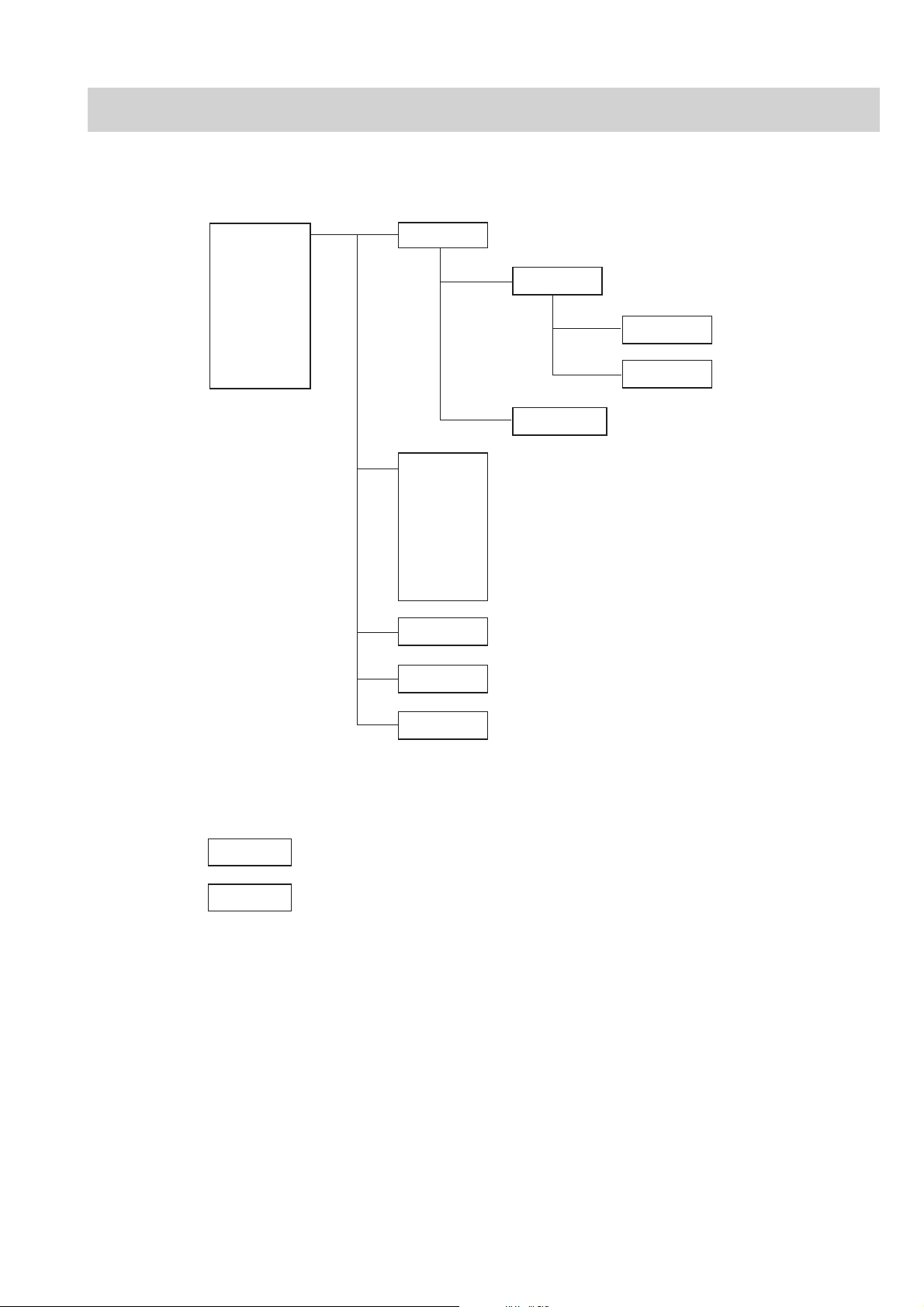
Composition
Standard Components
1. GENERAL
ECG-9620L
ECG-9620M
ECG-9620N
ECG-9620P
ECG-9620S
ECG-9620T
ECG-9620U
RHC-0004 Record Assy
RHC-00041 Motor Assy
UTC-0009 Paper senser board
UTC-0010 Motor sensor board
RHC-00042 Magazine Assy
RKC-0001 Transfer Assy (220 V) for L and P version
RKC-0002 Transfer Assy (230 V) for M version
RKC-0003 Transfer Assy (240 V) for N version
RKC-0004 Transfer Assy (110 V) for S version
RKC-0005 Transfer Assy (120 V) for T version
RKC-0006 Transfer Assy (127 V) for U version
UTC-0006 Key board
UTC-0007 ECG control board
Options
UTC-0008 Power board
KD-103E Cart
KH-801E Patient Cable Hanger
· To order a replacement assembly above, use the Code No.
· To order a replacement component inside an assembly, refer to “Section 7
Replaceablet Parts List”.
Service Manual ECG-9620 1.17
Page 29
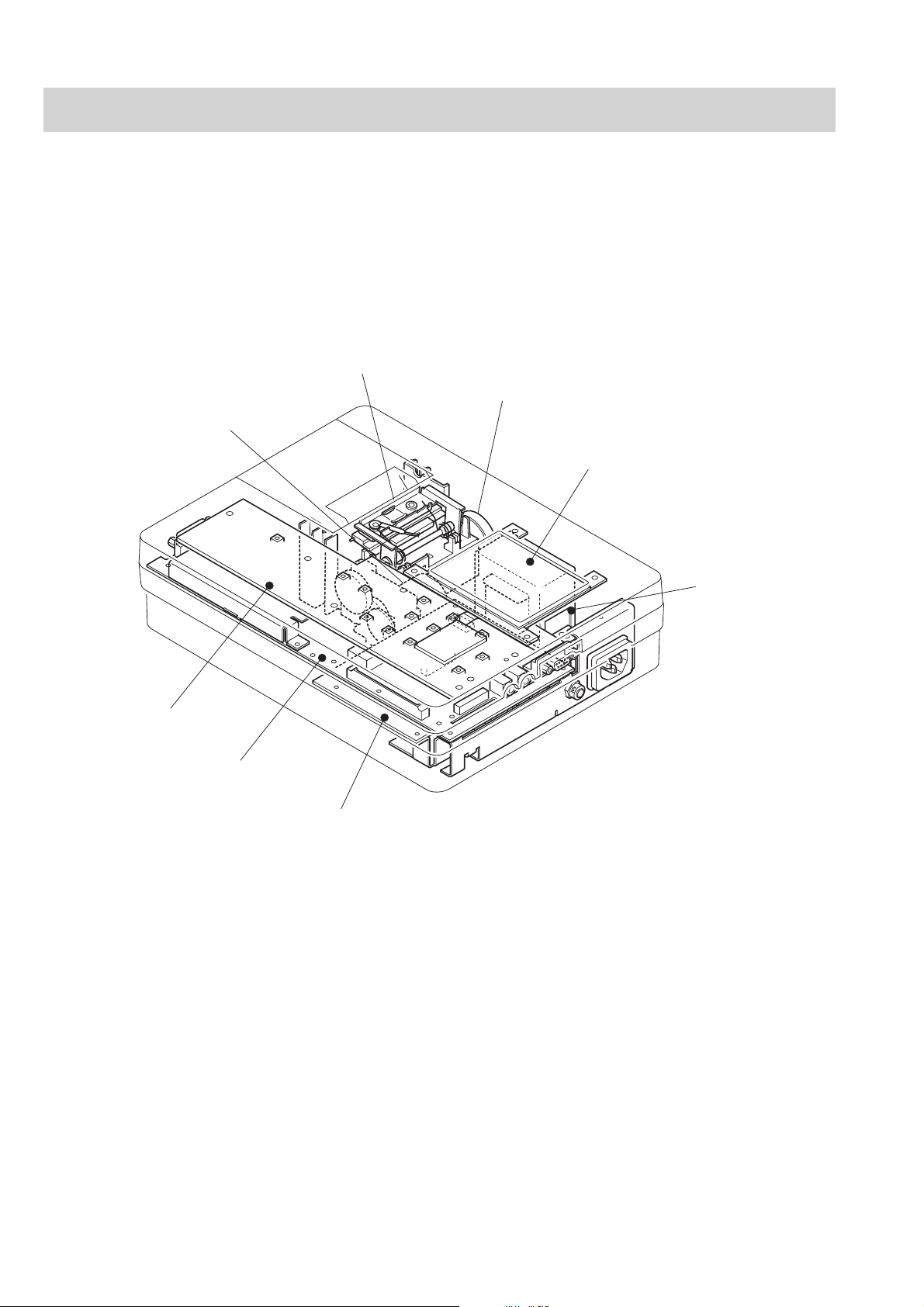
1. GENERAL
Location
Thermal Head Assy
Buzzer
Motor Assy
LCD
Ke y board
ECG control board
Transfer Assy
Power board
1.18 Service Manual ECG-9620
Page 30

Section 2 Maintenance
Replacement...................................................................................................................... 2.1
Periodic Replacement Schedule.............................................................................. 2.1
Cleaning and Lubrication ................................................................................................... 2.2
Cleaning and Greasing Schedules .......................................................................... 2.2
Cleaning the Paper Mark Sensor and P aper Empty Sensor.................................... 2.2
Cleaning the Motor Rotation Sensor and Lubricating the Motor Gear and Gear
Meshed with Motor Gear ......................................................................................... 2.3
Maintenance Check Sheet................................................................................................. 2.5
Service Manual ECG-9620 2C.1
Page 31

Replacement
2. MAINTENANCE
This section describes the periodic replacement and cleaning of parts which are
required to maintain the instrument in good working condition.
This subsection only describes replacement schedule for parts that need to be
periodically replaced. The actual replacement procedures are described in the section
for Disassembly and Assembly. Read the whole “Disassembly and Assembly” section,
especially its Warnings and Cautions, before replacing any of the parts described here.
Periodic Replacement
Schedule
To maintain the performance of the instrument, the parts listed in the table below must
be periodically replaced by qualified service personnel.
Code No. Description Recommendation
SB-901D Battery pack * See below.
08SK3.878.00046 Thermal head, KYT-56-8MPP1-SKH After 50 km recording
RHC-00041 Motor Assy After 1000 hours operation
* Replace the battery pack when it cannot last for 30 minutes during battery operation
at the temperatures between 20 and30°C.
Service Manual ECG-9620 2.1
Page 32

2. MAINTENANCE
Cleaning and Lubrication
This subsection describes the cleaning and lubrication procedures for parts that
must be cleaned and lubricated by qualified service personnel. The cleaning
procedures for parts that can be cleaned by the user are described in the Operator’s
Manual.
Cleaning and Lubricating
Schedules
Cleaning the Paper Mark
Sensor and Paper Empty
Sensor
To maintain the performance of the instrument, the parts listed in the table below
must be regularly cleaned or lubricated.
Part Frequency Performed by
Instrument (external) After each use User
Thermal Head Once a month User
Platen Roller assy Once a year User
Paper Sensor Once a month Qualified service personnel
Motor Sensor Once a year Qualified service personnel
Motor Gear and Gear Once a year Qualified service personnel
Meshed with Motor Gear
1. Remove the magazine. The illustration below shows the location of the paper
sensor.
2. Use a piece of cotton moistened with alcohol to clean both sensors.
Paper sensor
2.2 Service Manual ECG-9620
Page 33

2. MAINTENANCE
Cleaning the Motor
Rotation Sensor and
Lubricating the Motor Gear
and Gear Meshed with
Motor Gear
M3 binding head screw
Thermal head unit
1. Remove the upper casing from the lower casing. Refer to “Removing the
Upper Casing” in Section 6.
2. Remove the two M3 pan screws with washers and spring washers which fasten
the ground leads to the power transformer unit.
Ground lead
M3 pan screw with washer
and spring washer
CNA012 cable
CNA011 cable
Motor assy
3. Disconnect the CNA011 and CNA012 cables from the ECG control board.
4. Remove the three M3 binding head screws which fasten the thermal head unit
to the lower casing and remove the thermal head unit.
5. Remove the two M3 binding head screws which fasten the motor assy to the
thermal head unit and remove the motor assy.
6. Remove the two M3 pan screws with spring washers which fasten the motor
sensor board to the motor assy and remove the motor sensor board.
7. Use a piece of cotton moistened with alcohol to clean the motor rotation
sensor.
8. Use a brush to clean the holes in the gear.
Motor sensor board
Service Manual ECG-9620 2.3
Page 34

2. MAINTENANCE
9. Use grease to lubricate the motor gear and the gear which directly meshes with
the motor gear as shown below.
Top view
Motor
Motor gear
Gear meshed with motor gear
2.4 Service Manual ECG-9620
Page 35

2. MAINTENANCE
Maintenance Check Sheet 1/2
Date:
Customer:
Customer Address:
Service Personnel: Service Company:
Instrument Name: Instrument Model:
Instrument Serial Number: Hardware Revision:
Software Revision:
Overview Outside of instrument is clean. Yes No
No loose screws. Yes No
No physical damage, no bent parts and no contact with liquid. Yes No
Operation panel is not torn or broken. Yes No
All keys, buttons and controls are undamaged. Yes No
Power cord, patient cable are not frayed
and are correctly connected to the instrument. Yes No
Paper magazine opens and closes correctly. Yes No
Thermal head is clean. Yes No
The paper feeding roller is clean. Yes No
Motor rotation sensor is clean. Yes No
Paper detection sensor is clean. Yes No
Motor gear is lubricated properly. Yes No
Accessories Enough electrolyte cream (CardioCream) Yes No
Enough recording paper. Yes No
Installation Instrument is installed in the proper location. Yes No
Specified 3-prong power cord and ground lead are used. Yes No
Battery pack is in the instrument. Yes No
Recording paper is loaded. Yes No
Power on There is no fire, smoke or smell. Yes No
There is no electrical shock when touching the instrument. Yes No
Instrument is not abnormally hot. Yes No
Instrument does not affect surrounding equipment. Yes No
AC lamp lights when the AC power is supplied. Yes No
Battery charge lamp lights when the AC power is supplied. Yes No
Basic operation The screen display is correct. (brightness, no distortion) Yes No
Key lamp indication is correct. Yes No
All keys operate properly. Yes No
All settings are correct. Yes No
The battery is fully charged. Yes No
Electrode detachment functions properly. Yes No
There is no error message or abnormal operation. Yes No
Service Manual ECG-9620 2.5
Page 36

2. MAINTENANCE
Maintenance Check Sheet
Monitoring ECG waveform display is correct. Yes No
The continuity of the ECG connection cable is correct. Yes No
Heart rate display is correct. Yes No
QRS sync mark is displayed and heart rate sync sound generates. Yes No
ECG lead and sensitivity can be changed properly. Yes No
Alarms setting and alarm function is correct. Yes No
Sound volume can be changed properly. Yes No
Recording Paper is fed correctly (no skewing or jam). Yes No
Waveforms and letters are clearly recorded. Yes No
Time printed on the recording paper is correct. Yes No
System T est Recorder Pass Fail
Key Pass Fail
Memory Pass Fail
LCD/LED Pass Fail
Input unit Pass Fail
Calibration Pass Fail
Communication Pass Fail
CRO/EXT1 Pass Fail
2/2
Safety Protective earth resistance Pass Fail
Earth leakage current Pass Fail
Enclosure leakage current Pass Fail
Withstanding voltage Pass Fail
2.6 Service Manual ECG-9620
Page 37

Section 3 T roubleshooting and
System Error Message
Troubleshooting Flowchart................................................................................................. 3.1
T roub leshooting T a ble ........................................................................................................ 3.4
Troubleshooting General Operation Problem ........................................................... 3.4
Troubleshooting Recording Problem........................................................................ 3.6
System Error Message ...................................................................................................... 3.7
Service Manual ECG-9620 3C.1
Page 38

This section describes how to troubleshoot the instrument, using the following:
Is there any response when
any key is pressed?
Check that the LCD cable is
connected to the CNJ201
connector on the key board.
The LCD unit is faulty.
The key board is faulty.
The ECG control board is faulty.
The power LED is on but
there is no LCD display.
No
Yes
Normal
The key board is faulty.
The ECG control board is faulty.
Normal
Check that the CNA013 cable is connected to the
CNJ033 connector on the ECG control board and
CNJ101 connector on the key board.
- flowchart
- troubleshooting table
- system error messages at power-up
If the power is not turned off by pressing the Power key, press and
hold the Power ke y 5 seconds or more.
Troubleshooting Flo wc hart
Use the troubleshooting flowchart to find the possible sources of a problem.
3. TROUBLESHOOTING AND SYSTEM ERR OR MESSAGE
NOTE
Service Manual ECG-9620 3.1
Page 39

3. TROUBLESHOOTING AND SYSTEM ERROR MESSAGE
The power of the
instrument does not turn
on.
Does the instrument
operate during AC
power operation?
No
Does the LED of the AC power
light?
The key board is faulty.
The ECG control board is faulty.
The power board is faulty.
The instrument does not
operate during battery
power operation.
Is the battery charged?
Yes
Yes
No
Yes
No
Charge the battery.
Check the following cable connections
Between transformer unit and power board
CNA014 (between the power board and
ECG control board
CNA013 (between the ECG control board
and key board)
Normal
Check the power fuse in the
fuse holder.
Normal
The power board is faulty.
The power transformer is faulty.
Check the following cable connections
Between transformer unit and power board
CNA014 (between the power board and
ECG control board
CNA013 (between the ECG control board
and key board)
Normal
Is the battery F101 or F102
Yes
fuse on the power board blown?
No
The power unit is faulty.
The power transformer is faulty.
Replace the battery fuse
on the power board.
3.2 Service Manual ECG-9620
Page 40

The recorder does not
feed the recording paper
when the Start key is
pressed.
3. TROUBLESHOOTING AND SYSTEM ERR OR MESSAGE
Does the LED for the Start
Brinks
key light?
Lights
Does the recorder print
when the recording paper
is manually pulled out
from under the thermal
head?
Yes
Check that CNA011 cable is
connected to the CNJ036 connector
on the ECG control board.
Normal
The motor is faulty.
The ECG control board
is faulty.
Is the recording paper set?
No
No
Check that the CNA013 cable is
connected to the CNJ033 connector
on the ECG control board and
CNJ101 connector on the key board.
Normal
The ECG control board
is faulty.
The key board is faulty.
Set the recording paper.
Service Manual ECG-9620 3.3
Page 41

3. TROUBLESHOOTING AND SYSTEM ERROR MESSAGE
T roubleshooting Table
Use the troubleshooting table to locate, identify and solve a problem in the
instrument. The problems are divided into general operation and recording. Each
category has its own troubleshooting table for fast and easy troubleshooting.
How to use the troubleshooting table
1. Determine which troubleshooting table to use.
2. In the “Problem” column find the trouble item that matches the problem.
3. Do the action recommended in the “Corrective Action” column.
4. If the problem is not solved, do the action for the next possible cause or criteria.
5. If none of the actions solve the problem, contact your nearest Nihon Kohden
dealer.
Troubleshooting General
Operation Problem
Problem Possible Cause Action
The power LED lights but nothing is
displayed on the LCD screen.
The instrument does not operate on AC
power.
Faulty cable connection. Check the following cable connection.
CNA013: between the ECG control
board and key board
LCD cable: CNJ201 connector on the
key board.
CNA014: between the power board
and ECG control board
Faulty LCD unit. Replace the LCD unit.
Faulty key board. Replace the key board.
Faulty ECG control board. Replace ECG the control board.
Faulty power fuse. Replace the power fuse.
Faulty cable connection Check the following cable connection.
CNA013: between the ECG control
board and key board
CNA014: between the power board
and ECG control board.
Power cable:between the power board
and power transformer
unit
Faulty power cord Replace the power cord.
Faulty power board. Replace the power board.
Faulty key board. Replace the key board.
Faulty ECG control board. Replace ECG the control board.
Faulty power transformer unit, Replace the power transformer unit.
3.4 Service Manual ECG-9620
Page 42

3. TROUBLESHOOTING AND SYSTEM ERR OR MESSAGE
Problem Possible Cause Action
The instrument does not operate on
The battery is not charged. Charge the battery.
battery power.
Faulty battery fuse. Replace the battery fuse.
Faulty battery. Replace the battery.
Check the following cable connection.
CNA013: between the ECG control
board and key board
CNA014: bet ween the power board
and ECG control board.
Battery cable:CNJ102 connector on the
power board
Faulty power board. Replace the power board.
No key operation Faulty cable connection Check the following cable connection.
CNA013: between the ECG control
board and key board
CNA014: bet ween the power board
and ECG control board.
Faulty key board. Replace the key board.
Faulty ECG control board. Replace ECG the control board.
No ECG waveform appears in a
specific lead or artifact appears on the
waveform.
Faulty electrode attachment. Check that the electrodes are properly
attached to the patient.
Faulty patient cable connection. Check that the patient cable is firmly
connected to the electrodes and
instrument.
Faulty ECG control board. Replace the ECG control board.
No ECG waveform appears in all
channels or artifact appears on the
waveform.
No electrode is attached to the patient
or the RF (RL) electrode is not attached
to the patient.
Check that the electrodes are properly
attached to the patient.
Faulty ECG control board. Replace the ECG control board.
Vertical and horizontal stripes appear
on the LCD screen at constant interval.
Faulty cable connection. Check the following cable connection.
CNA013: between the ECG control
board and key board
LCD cable: CNJ201 connector on the
key board.
Faulty ECG control board. Replace the ECG control board.
Faulty LCD unit. Replace the LCD unit.
No sound Faulty cable connection. Check that the speaker cable is firmly
connected to the CNJ032 connector on
the ECG control board.
Faulty speaker. Replace the speaker.
The date and time is reset to January 1,
1980 and the “Error 09” error message
appears.
The lithium battery is completely
discharged.
Replace the ECG control board. The
lithium battery is in the real time clock
IC on the ECG control board.
Service Manual ECG-9620 3.5
Page 43

3. TROUBLESHOOTING AND SYSTEM ERROR MESSAGE
Troubleshooting Recording
Problem
Problem Possible Cause Action
The recorder does not feed the
recording paper when the Start key is
pressed.
No printing. The thermal head is incorrectly
Sometimes the recorder does not pr int. The thermal head protection circuit
The paper skews. Dirty thermal head. Clean the thermal head.
Dirty paper sensor. Clean the paper sensor.
Faulty cable connection. Check the following cable connection.
CNA013: between the ECG control
board and key board
CNA011: between the ECG control
board and feeding motor,
motor sensor and paper
sensor
Faulty key board. Replace the key board.
Faulty ECG control board. Replace the ECG control board.
Faulty feeding motor. Replace the feeding motor.
Readjust the position of the thermal
positioned.
Faulty cable connection. Check the following cable connection.
Faulty thermal head. Replace the thermal head.
Faulty power board. Replace the power board.
Faulty ECG control board. Replace the ECG control board.
which protects the thermal head from
large artifact, such as AC interference is
rejecting noisy waveforms.
The recording paper is not properly set
in the instrument.
The thermal head is incorrectly
positioned.
Faulty feeding roller. Replace the paper magazine.
head.
CNA013: between the ECG control
board and key board
CNA011: between the ECG control
board and feeding motor,
motor sensor and paper
sensor
Check the electrode attachment. If
necessary, adjust the electrode position
so that clear ECG waveforms are
displayed.
Make sure that the recording paper is
aligned with the lower recording paper
guide.
Readjust the position of the thermal
head.
3.6 Service Manual ECG-9620
Page 44

System Error Message
During power-up and operation the instrument continuously checks itself for
system failure. If a failure is detected, system information and error history are
printed on the recording paper and all operations are stopped. System information
and error history are also displayed or printed due to transient noise. After printing
the system information and error history, the power of the instrument is
automatically turned off.
3. TROUBLESHOOTING AND SYSTEM ERR OR MESSAGE
NOTE
If the same system information appears again after restarting the
instrument, do not use the instrument until service personnel has
corrected the cause of the problem. Sending a copy of the system
information to your nearest Nihon Kohden distributor helps us to
troubleshoot your problem quickly.
System Information
Indicates an error number to identify the problem. To solve the problem, do the
corrective action described below.
Error No. Meaning Corrective Action
00 Input unit error: An interrupt signal of 2 ms
is generated.
01 Input unit error: There is no response to the
host.
02 Input unit error: Communication protocol
error.
03 4 bit CPU error: Initialization error. Replace the ECG control board.
04 4 bit CPU error: “No response” error. Replace the ECG control board.
05 A key on the key board is short-circuited. Replace the key board.
06 RTC error: No interrupt signal of 125 ms. Replace the ECG control board.
07 RTC error: Incorrect data in SRAM. Replace the ECG control board.
09 The lithium battery to back up the date and
time and all system settings is completely
discharged. The system settings other than
the items described in the following note
are returned to the factory initial settings.
10 Bus error. Replace the ECG control board.
11 Address error. Replace the ECG control board.
Replace the ECG control board.
Replace the ECG control board.
Replace the ECG control board.
Replace the ECG control board. The
lithium battery is in the real time clock IC
on the ECG control board. The date and
time is reset to January 1, 1980.
Service Manual ECG-9620 3.7
Page 45

3. TROUBLESHOOTING AND SYSTEM ERROR MESSAGE
Error No. Meaning Corrective Action
12 Illegal command. Replace the ECG control board.
13 Zero division error. Replace the ECG control board.
14 Power off time out. Replace the ECG control board.
15 EEPROM error: This occurs due to the
EEPROM check error, installed language
error or communication error between the
host and EEPROM.
16 Local language flash memory error. Replace the ECG control board.
17 ECG model error. Replace the ECG control board.
18 Local lang uage is not installed. I nstall the local language.
19 Local lang uage is not installed. I nstall the local language.
Error in memory area for local language. Re-install the local language.
20 Local language text file version does not
match the ECG software version.
21 ECG inte r pretation error (Time over). Check the input waveforms. I f any noi se
22 The entered information does not match
the data in the flash memory.
27 Program version error. T he program is
updated.
Replace the ECG control board.
Install the local language text file which is
the same version as the ECG software.
is superimposed on the waveforms, find
and eliminate the cause. If no noise is
superimposed on the waveform, replace the
ECG control board.
Replace the ECG control board.
Turn the power off, then on and check that
the ECG waveforms are displayed
correctly.
NOTE
••
• “Error 05” also appears when any key on the operation panel is
••
pressed and held down.
••
• When “Error 08” appears, the following settings are not reset to
••
the factory initial settings even if the instrument is initialized.
- display language - hum filter
- hospital name - direct/modem connection
- recording resolution setting - elapsed time
- local language font - saved ECG data
3.8 Service Manual ECG-9620
Page 46

3. TROUBLESHOOTING AND SYSTEM ERR OR MESSAGE
Error History
Indicates the latest three errors and the date of the latest error, as in the example
below.
Service Manual ECG-9620 3.9
Page 47

Section 4 System Test, Adjustment,
And Setting
System T est ....................................................................................................................... 4.1
Overall ..................................................................................................................... 4.1
Calling up the System Test Level 1 .......................................................................... 4.2
Calling up the System Test Level 2 .......................................................................... 4.3
Entering the System Test Number ........................................................................... 4.4
Executing the System Test....................................................................................... 4.5
Quitting the System Test .......................................................................................... 4.6
Exiting the System Test Mode.................................................................................. 4.6
Demonstration ................................................................................................................... 4.7
Recorder ............................................................................................................................ 4.8
Thermal Head .................................................................................................................. 4.10
Key................................................................................................................................... 4.11
Memory............................................................................................................................ 4.12
Single Memory Test Mode ..................................................................................... 4.13
Continuous Memory Test Mode ............................................................................. 4.13
LCD/LED.......................................................................................................................... 4.14
Input Unit ......................................................................................................................... 4.16
Calibration........................................................................................................................ 4.17
Communication................................................................................................................ 4.18
CRO/EXT1....................................................................................................................... 4.20
System Setup Initialization............................................................................................... 4.22
ECG Findings List Recording........................................................................................... 4.23
Recording Resolution Setting .......................................................................................... 4.24
Date and Time Setting ..................................................................................................... 4.25
Setting the Date and Time ..................................................................................... 4.25
Service Manual ECG-9620 4C.1
Page 48

System Test
4. SYSTEM TEST, ADJUSTMENT AND SETTING
This section describes:
• how to check the operation of the instrument in the System Test mode.
• how to output the ECG findings list in the System Test mode.
• how to initialize the system in the System Test mode.
• how to adjust the thermal head recording resolution and recording paper cutting
position in the System Test mode.
• how to set date and time in the System Setup mode.
Overall
The instrument has two System Test modes: Test level 1 for operator and Test level
2 for qualified service personnel. The test items marked with “*” perform the same
test in Test levels 1 and 2. Each Test level consists of the following system test
items:
Test level 1 Test level 2
• Demonstration • Recorder
• Recorder • Thermal head
• Key* • Key*
• Memory* • Memory (single)*
• LCD/LED* • Memory (continuous)
• Input unit* • LCD/LED*
• Calibration* • Input unit*
• Communication* • Calibration*
• CRO/EXT1* • Communication*
• System Setup Initialization* • CRO/EXT1*
• ECG Findings List Recording • System Setup Initialization*
• Recording resolution setting
(
NOTE
In the description of some test items in this section, whenever it is
appropriate, a description of the sour ce of problem and its corrective
action will be described in table form for fast and easy
troubleshooting. If none of the actions solve the problem, contact
your Nihon Kohden distributor or representative.
Service Manual ECG-9620 4.1
Page 49

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Calling up the System Test
Level 1
+
1. If the power is on, turn it off.
NOTE
Release the Feed/Mark key immediately after the instrument starts
printing. If you continue to hold the Feed/Mark key for more than 15
seconds, the instrument recognizes that the Feed/Mark key is shortcircuited and prints the system information “Error 05” at the end of
printing.
2. Press the Power key while pressing the Feed/Mark key. Hold the Feed/Mark key
until the instrument begins to print the system test procedure, relationship
between the input number and its corresponding key name on the operation
panel and system test number list as shown below. The Test level 1 is called up
and the instrument is in standby mode for entering the system test number.
To cancel printing the following information, press the Start/Stop key.
.
Printout
System T est Screen
4.2 Service Manual ECG-9620
Page 50

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Calling up the System Test
Level 2
++
Printout
1. If the power is on, turn it off.
NOTE
Release the Feed/Mark key immediately after the instrument starts
printing. If you continue to hold the Feed/Mark key for more than 15
seconds, the instrument recognizes that the Feed/Mark key is shortcircuited and prints the system information “Error 05” at the end of
printing.
2. Press the Power key while pressing the Feed/Mark and Auto/Manual keys
together. Hold the Feed/Mark and Auto/Manual keys until the instrument begins
to print the system test procedure, relationship between the input number and its
corresponding key name on the operation panel and system test number list as
shown below. The Test level 2 is called up and the instrument is in standby
mode for entering the system test number.
To cancel printing the following information, press the Start/Stop key.
System T est Screen
Service Manual ECG-9620 4.3
Page 51

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Entering the System Test
Number
Use the following keys on the operation panel to enter a 2-digit number for
executing the desired system test. The specified system test numbers are indicated
in the [xx] bracket at the right of each system test item on the printout output when
the Test level 1 or 2 is called up. Refer to the “Calling up the Test Level X”
section.
To delete the entered number, press the Auto/Manual key. To delete a 2-digit
number, press the Auto/Manual key twice. At this time, the ones digit number is
deleted before the tens digit number is deleted.
Numeric Key Numeric Key
0 Copy/CAL key 5 Rhyth m ke y
1 F1 fu nction key 6 Age key
2 F2 fu nction key 7 Sex key
3 F3 function key 8 Feed/Mark key
4 Mode key 9 Filter key
Clear Auto/Manual key Enter Start/Stop key
4.4 Service Manual ECG-9620
Page 52

4. SYSTEM TEST, ADJUSTMENT AND SETTING
70
Executing the System Test
Press the Start/Stop key. For some tests, the System Test screen is displayed
during the test as shown below,
System T est Screen
If you entered an unspecified number, 8 repeating “pips” alarm sound and the
“Invalid number. Please re-enter number” error message is displayed as shown
below.
To re-enter the system test number, do either of the following:
• Delete the previously entered number by pressing the Auto/Manual key.
• Enter the system test number by overwriting the previously entered number.
Service Manual ECG-9620 4.5
Page 53

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Quitting the System Test The procedures to quit each system test vary from test to test. Some tests
automatically end after an alarm sound is generated or a printout is output. Refer
to the following explanations for each test. After quitting each test, the instrument
returns to the standby mode for entering the system test number.
After a system test is completed, you can execute other system test without exiting
the System Test mode.
Exiting the System Test
Mode
After all desired system tests are finished, press the Power key.
4.6 Service Manual ECG-9620
Page 54

Demonstration
4. SYSTEM TEST, ADJUSTMENT AND SETTING
This is used to learn or demonstrate instrument operation.
While executing this test item, the instrument generates dummy 12 lead ECG
resting waveforms until the power of the instrument is turned off. The ECG
waveforms can be recorded and also displayed as shown below.
Procedure
Enter the system test number [00] (Test level 1) and press the Start/Stop key.
To quit the test, turn the power of the instrument off by pressing the Power key.
Dummy 12 lead ECG resting waveforms on LCD
Service Manual ECG-9620 4.7
Page 55

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Recorder
This is used to check the condition of the recorder by printing test patterns. The
recording test patterns consist of the following and are printed in the following
order:
1. Diagonal lines
2. Characters H and X (Test level 1 only)
3. Paper speed scales (25 and 50 mm/s)
The recorder test of Test level 1 contains the same recorder test and thermal head
test as Test level 2. With regard to the check procedure for characters H and X,
refer to the “Thermal Head” section.
Procedure
Enter the system test number [01] (Test level 1) or [00] (Test level 2) and press the
Start/Stop key. The following test patterns are printed.
This test automatically ends after the following has been printed. The instrument
returns to the standby mode for entering the system test number.
Printout of Test level 1
Not printed in Test level 2. Refer to the
“Thermal Head” section for this check.
4.8 Service Manual ECG-9620
Page 56

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Check Procedure for Dia gonal Lines
Check that all the diagonal lines are evenly and completely printed.
Possible Source of Problem Corrective Action
A dirty thermal head can cause some
parts to be unevenly or incompletely
printed.
1. Clean the thermal head with the
thermal head cleaner pen.
2. If this does not fix the problem,
replace the thermal head.
A faulty thermal head can cause some
parts at a certain position to be
unevenly or incompletely printed.
1. Clean the thermal head with the
thermal head cleaner pen.
2. If this does not fix the problem,
replace the thermal head.
Check Procedure for Paper Speed Scales
Check that the accuracy of each paper speed during actual recording is within 2%.
The scales for 4 seconds at 10 mm/s and 12.5 mm/s paper speeds and the scales for
2 seconds at 25 mm/s and 50 mm/s paper speeds are consecutively printed. For
example, the length for 4 seconds on the time scale printed at 10 mm/s paper speed
must be within 39.2 mm to 40.8 mm.
Possible Source of Problem Corrective Action
Badly positioned thermal head. 1. Adjust the thermal head position.
2. If this does not fix the problem,
replace the thermal head.
Damaged, deformed or badly
positioned motor gear.
1. Check the motor gear and its
position.
2. If this does not fix the problem,
replace the motor gear.
Dirty motor rotation sensor. Clean the motor rotation sensor as
described in the “Maintenance” section.
Loose or damaged axle. Tighten and check the axle as described
in the “Disassembly and Assembly”
section.
Faulty motor. Replace the motor.
Faulty ECG control board. Replace the ECG control board.
Service Manual ECG-9620 4.9
Page 57

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Thermal Head
This is used to check the condition of the thermal head by printing out the
characters “H” and “X” continually.
Procedure
Enter the system test number [01] (Test level 2) and press the Start/Stop key. The
characters “H” and “X” are printed as follows.
To quit the test, press the Auto/Manual key and the instrument returns to the
standby mode for entering the system test number.
Printout of Thermal Head Test Result
Check Procedure for Character s H and X
Check that all the parts of the characters “H” and “X” are clearly, evenly and
completely printed and that the characters are not printed zigzag or diagonally.
Possible Source of Problem Corrective Action
The thermal head recording resolution
is not set correctly.
Faulty cable connection. Check the following cable connection.
Faulty power board. Replace the power board.
The thermal head unit position is not
correct.
Adjust the thermal head recording
resolution as described in this section.
CNA014: between the ECG control
board and power board
CNA012: CNJ033 connector on the
ECG control board.
Check and adjust the thermal head unit
position.
4.10 Service Manual ECG-9620
Page 58

Key
4. SYSTEM TEST, ADJUSTMENT AND SETTING
This is used to check the condition of the keys on the operation panel.
Procedure
1. Enter the system test number [02] (Test level 1) or [03] (Test level 2) and press
the Start/Stop key.
2. Press the key on the operation panel. The name of the pressed key is printed if
the key is functioning correctly.
To quit the test, press the Auto/Manual key. The instrument returns to the
standby mode for entering the system test number.
NOTE
The Power and Auto/Manual keys cannot be checked by this test. To
check if these two keys are functioning correctly, do the following:
••
• Power key
••
Check that the power of the instrument is on or off when the
Power key is turned on or off.
••
• Auto/Manual key
••
Check that the Key test is stopped by pressing the Auto/Manual
key.
Check Procedure for Operation Panel Key
Check that the name of the pressed key is printed.
Possible Source of Problem Corrective Action
Faulty key board. Replace the key board.
Service Manual ECG-9620 4.11
Page 59

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Memory
This is used to check the condition of the memory by comparing the data of the test
patterns written to and read from each memory area.
The instrument provides two memory test modes: single and continuous. When
single memory test mode is selected, entire memory is tested once. When
continuous memory test mode is selected, the memory is continuously tested until
the Auto/Manual key is pressed. The number of “Count of test” increases by one
each time the entire memory test is tested. One complete memory test takes about
30 seconds.
• Single: [03] Test level 1, [04] Test level 1
• Continuous: [05] Test level 2
If no fault is detected, an “OK” message appears for each memory. If a fault is
detected, an “Error” message appears and the number of “Error count” for each
memory increases by one.
Printout of Memory Test Result
4.12 Service Manual ECG-9620
Page 60

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Single Memory Test Mode This mode tests the entire memory once. You can use this mode to fully check all
memory if a memory problem frequently occurs.
Procedure
Enter the system test number [03] (Test level 1) or [04] (Test level 2) and press the
Start/Stop key.
After the test, the test result is automatically printed. When the test result is
completely printed, a “bing bong” alarm sound is generated and instrument returns
to the standby mode for entering the system test number.
To cancel the test or cancel printing the test result, press the Auto/Manual key.
Check Procedure for Single Memory Test Mode
Check that no “Error” messages appear.
Possible Source of Problem Corrective Action
Faulty memory on the ECG control
board.
Replace the ECG control board. Each
memo ry mounted on the ECG control
board cannot be replaced at memory
component level.
Continuous Memory Test
Mode
This mode continues testing the entire memory until the Auto/Manual key is
pressed. You can use this mode to check an intermittent memory problem.
Procedure
Enter the system test number [05] (Test level 2) and press the Start/Stop key.
To print the test result without quitting the test, press the Start/Stop key. All results
of the tests performed until the Start/Stop key are pressed is printed on one page.
To cancel the test, press the Auto/Manual key. All results of the tests performed
until the Start/Stop key is pressed are printed on one page. When the test result is
completely printed, 8 repeating “pips” alarm sound is generated and the instrument
returns to the standby mode for entering the system test number.
Check Procedure for Continuous Memory Test Mode
Check that no “Error” messages appear.
Possible Source of Problem Corrective Action
Faulty memory on the ECG control
board.
Replace the ECG control board. Each
memo ry mounted on the ECG control
board cannot be replaced at memory
component level.
Service Manual ECG-9620 4.13
Page 61

4. SYSTEM TEST, ADJUSTMENT AND SETTING
LCD/LED
This is used to check all LEDs on the operation panel, LED/LCD control circuit
and all dots on the LCD First, the LCD test starts and after the LCD test is
complete, the LED test starts.
LCD Test
The LCD displays the following four types of test patterns every two seconds in
the following order:
1. Diagonal lines are displayed.
2. Entire LCD lights up.
3. Returns to the System Test screen.
The LCD image for each pattern changes as follow:
12
LED Test
The LEDs on the operation panel light up one by one and remain lit until all LEDs
light up. After all LEDs light up, they go out one at a time.
NOTE
With regard to the LEDs of Battery charge lamp, AC power lamp and
POWER lamp, check if they are in the following condition during the
LED/LCD test:
• Battery charge lamp: Not lit (This lamp indicates the remaining
battery power before and after the LED test
during battery operation.)
• A C power lamp: Lit
• POWER lamp: Lit when AC power is used
Procedure
Enter the system test number [04] (Test level 1) or [06] (Test level 2) and press the
Start/Stop key.
After the test, the instrument returns to the standby mode for entering the system
test number.
To cancel the test, press the Auto/Manual key.
4.14 Service Manual ECG-9620
Page 62

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Check Procedure for LCD Test
Check that all the dots on the screen light up and go out according to the test
pattern.
Possible Source of Problem Corrective Action
Faulty cable connection. Check the following cable connection.
CNA013: between the ECG control
board and key board.
LCD cable: CNJ201 connector on the
key board.
Faulty LCD unit. Replace the LCD unit.
Faulty ECG control board. Replace the ECG control board.
Faulty key board. Replace the key board.
Check Procedure for LED Test
Check that all the LEDs on the operation panel light up.
Possible Source of Problem Corrective Action
Faulty cable connection. Check the following cable connection.
CNA013: between the ECG control
board and key board.
Faulty key board. Replace the key board.
Faulty ECG control board. Replace the ECG control board.
Service Manual ECG-9620 4.15
Page 63

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Input Unit
This is used to check if the input analog signal processing circuit detects the lead
off condition correctly, using the provided input check jig. If each lead is
connected when the input analog signal processing circuit works correctly, the
“Normal” message is printed at the right of each electrode lead name in the test
result. If a lead is not connected, the “Error” message is printed.
The following is an example printout of the input unit test result when the R(RA)
lead is disconnected.
Input check jig
Procedure
1. Connect the electrode lead to the instrument.
2. Attach all tips of each electrode lead to the input check jig.
3. Enter the system test number [05] (Test level 1) or [07] (Test level 2).
4. Disconnect one of the leads from the check jig and press the Start/Stop key.
The disconnected lead name is printed out.
5. Repeat steps 3 and 4 for all leads by one.
To quit the test, press the Auto/Manual key. The instrument returns to the
stanby mode for entering the system test number.
Check Procedure for Input Unit Test
Check that the lead off condition is detected correctly.
Possible Source of Problem Corrective Action
Faulty cable connection. Check the following cable connection.
CNA013: between the ECG control
board and key board.
Faulty key board. Replace the key board.
Faulty ECG control board. Replace the ECG control board.
4.16 Service Manual ECG-9620
Page 64

Calibration
4. SYSTEM TEST, ADJUSTMENT AND SETTING
This is used to check the sensitivity and time constant of the input analog signal
processing circuit. After starting the test, the CAL waveforms for leads I, II and V1
to V6 are printed as shown below. If all the rectangular printed CAL waveforms
have the amplitude of 1 mV and time constant of more than 3.2 seconds, the
sensitivity and time constant of the input analog signal processing circuit are
normal.
Procedure
Enter the system test number [06] (Test level 1) or [08] (Test level 2) and press the
Start/Stop key.
After all CAL waveforms for eight leads are printed, the test automatically ends
and the instrument returns to the standby mode for entering the system test number.
To cancel the test, press the Start/Stop key or Auto/Manual key. The instrument
returns to the standby mode for entering the system test number.
Check Procedure for Calibration Waveforms
Check that all the rectangular printed CAL waveforms match the following
conditions in the illustration below:
• Amplitude when CAL waveform is risen: 10 mm ±2%
• Amplitude of point which is 25 mm : more than 7.3 mm
from the rising point of the CAL waveform
10 mm ±2% more than 7.3 mm
25 mm
Possible Source of Problem Corrective Action
Faulty ECG control board. Replace the ECG control board.
Service Manual ECG-9620 4.17
Page 65

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Communication
This is used to check the external communication input/output line of the
instrument, using the check jig. The instrument has one standard communication
connector (SIO). This test is performed by comparing the original test patterns sent
from the output line with the test patterns received at the input line. If any received
test pattern is different from its original, a “Error” message is printed. A “Normal”
message is printed if the communication line is normal. With regard to TxD-RxD
line, if the same data is printed, the line is normal.
Every time the test of one set is repeated, the number of “Count of test” increases
by one. Every time the error is detected during continuous test, the “Error count”
increases by one. The test of one set takes about 5 seconds.
The following is an example printout of the communication test result.
Preparation
A locally made check jig is required for the test. To make the check jig, shortcircuit the pins as shown below.
Connector Pin Assignment
1. FG
2. TXD
3. RXD
4. RTS
5. CTS
6. DSR
7. GND
8. HS
9. DTR
Mating Connector
Connector: HDEB-9PF (05)
Case: HDE-CTH
4.18 Service Manual ECG-9620
Page 66

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Procedure
1. Connect the check jig to the SIO socket of the instrument.
2. Enter the system test number [07] (Test level 1) or [09] (Test level 2) and press
the Start/Stop key.
The instrument prints the test result of each test if the instrument detects an
“Error”.
To print the test result without quitting the test, press the Start/Stop key. The
results of all tests performed until the Start/Stop key are pressed are printed.
You can see the test number and the number of errors on “Count of test” and
“Error count” on the printout, respectively.
To quit the test, press the Auto/Manual key. The instrument prints the results of
all tests performed until the Auto/Manual key is pressed. When the test result is
completely printed, 8 repeating “pips” sound is generated and the instrument
returns to the standby mode for entering the system test number.
Check Procedure for Serial Communication
Check that no error messages appear.
Possible Source of Problem Corrective Action
Faulty ECG control board. Replace the ECG control board.
Service Manual ECG-9620 4.19
Page 67

4. SYSTEM TEST, ADJUSTMENT AND SETTING
CRO/EXT1
This is used to check the external output/input terminal, using the check jig. The
instrument has the input signal terminal (EXT-IN connector) and output signal
terminal (CRO-OUT connector) at the rear of the instrument.
In this test, the instrument prints the known triangular waveform signals generated
inside the instrument at the lower trace on the recording paper the moment the
instrument outputs the signals from the CRO-OUT terminal to the EXT-IN
connector. At the same time, the instrument prints the triangular waveform signals
input to the EXT-IN connector at the upper trace on the recording paper. There is
no delay time between the printed waveforms on the upper and lower traces.
The following is an example printout of the CRO/EXT1 test result.
Preparation
A locally made check jig is required for the test. To make the check jig, use the
two 3.5φ monaural jacks and leads and solder the signal line and ground line of the
two jacks with leads as shown below.
Signal line
Ground line
4.20 Service Manual ECG-9620
Page 68

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Procedure
1. Connect the check jig to the CRO-OUT/EXT-IN sockets of the instrument.
2. Enter the system test number [08] (Test level 1) or [10] (Test level 2) and press
the Start/Stop key.
To quit the test, press the Start/Stop key or Auto/Manual key. The instrument
returns to the standby mode for entering the system test number.
Check Procedure
Check that the shape of the two printed triangular waveforms are the same and
there is no delay time between them.
Possible Source of Problem Corrective Action
Faulty ECG control board. Replace the ECG control board.
Service Manual ECG-9620 4.21
Page 69

4. SYSTEM TEST, ADJUSTMENT AND SETTING
System Setup Initialization
This is used to reset all the system settings to the factory initial settings.
The following settings are not reset to the factory initial settings even
if the instrument is initialized.
• date and time* • display language**
• recording resolution setting* • hospital name**
• cue mark position* • direct/modem connection**
• elapsed time • local language font
• saved ECG data
For settings marked with *, refer to the following corresponding
subsection in this section; f or settings marked with **, refer to
“Changing Settings Before Measurement (System Setup Screen)” in
the operator’s man ual.
NOTE
Procedure
Enter the system test number [10] (Test level 1) or [12] (Test level 2) and press the
Start/Stop key.
If the initialization is completed, a “System Setup Initialization” message appears
with one “bing bong” alarm sound and the instrument returns to the stanby mode
for entering the system test number.
Refer to the operator’s manual for the factory initial settings.
Following is the LCD display after the system has been initialized.
4.22 Service Manual ECG-9620
Page 70

ECG Findings List Recording
This is used to print out the list of all ECG findings used for the instrument. The
instrument informs you of the ECG finding as a result of the ECG interpretation
when analyzing the ECG.
Procedure
Enter the system test number [11] (Test level 1) and press the Start/Stop key.
When the list is completely printed, one “bing bong” alarm sound is generated and
the instrument returns to the standby mode for entering the system test number.
To cancel printing the list, press the Start/Stop key or Auto/Manual key. The
instrument returns to the standby mode for entering the system test number.
4. SYSTEM TEST, ADJUSTMENT AND SETTING
Service Manual ECG-9620 4.23
Page 71

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Recording Resolution Setting
This is used to adjust the thermal head recording resolution after the thermal head
is replaced with a new one or when the printout is not clearly, evenly and
completely printed.
Normally, the resistor value of the heating element on the thermal head is a specific
value which varies from thermal head to thermal head. Even if the same energy is
applied to the thermal head, the recording quality varies due to the difference of the
thermal head resistor value. Therefore, adjusting the thermal head recording
resolution is required to evenly print regardless of the thermal head resistor value.
Do the following procedure to automatically adjust the thermal head recording
resolution.
Procedure
1. Call up the System Test Level 2.
heating element
Label:
Lot No., thermal head resistance
2. Enter the system test number between [41] and [48] according to the thermal
head resistance value
Thermal head resistance System set No.
935 to 990
991 to 1045
1046 to 1100
1101 to 1155
1156 to 1179
1180 to 1232
1233 to 1265
1266 Ω or more Rec ording resolution setting8 [48]
Current setting Recording re solution setting [49]
3. Press the Start/Stop key.
4. Turn the power off. (Test level 2) and press the Start/Stop key.
Ω
Ω
Ω
Ω
Ω
Ω
Ω
Recording resolution setting1 [41]
Recording resolution setting2 [42]
Recording resolution setting3 [43]
Recording resolution setting4 [44]
Recording resolution setting5 [45]
Recording resolution setting6 [46]
Recording resolution setting7 [47]
4.24 Service Manual ECG-9620
Page 72

Date and Time Setting
4. SYSTEM TEST, ADJUSTMENT AND SETTING
The date and time of the instrument are set in the System Setup mode. In the
System Setup mode, you can also set the entire system settings of the instrument
which determines the operation conditions of the instrument. Refer to the
operator’s manual for details.
The date and time is backed up with the lithium battery in a real time clock IC on
the ECG control board. The life time of the lithium battery is about 7 years. When
the “Error 09” error message appears and the date is reset to January 1, 1980, the
lithium battery is completely discharged. Replace the ECG control board and set
the date and time.
Setting the Date and Time
1. Call up the System Setup mode
1) If the power is on, turn it off.
NOTE
Release the Feed/Mark key immediately after the instrument starts
printing. If you continue to hold the Feed/Mark key for more than 15
seconds, the instrument recognizes that the Feed/Mark key is shortcircuited and prints the system information “Error 05” at the end of
printing.
2) Press the Power key while pressing the Copy/CAL key. Hold the Copy/
CAL key until the instrument begins to print the list of the system setup
settings. The System Setup mode is called up.
To cancel printing the list, press the Start/Stop key.
2. Enter a 3-digit number to call up the stanby mode for entering the new numbers
for year, month/date, hour/minute or second, respectively. Refer to the
“Entering the System Test Number” in this section for entering the numbers.
Following is the LCD screen when the year setting mode is called up.
Service Manual ECG-9620 4.25
Page 73

4. SYSTEM TEST, ADJUSTMENT AND SETTING
Year
Enter the system setup number [318].
Month/date
Enter the system setup number [319].
Hour/minute
Enter the system setup number [320].
Second
Enter the system setup number [322].
To cancel the entered number, press the Auto/Manual key.
NOTE
You cannot enter numbers for “second” setting.
3. Enter a 4-digit number to enter the new numbers of the year, month/date or
hour/minute. The range of the number which is possible to enter is as follows:
Year
[1980] to [2079]
Month/date
[0101] to [1231]
Hour/minute
[0000] to [2359]
To cancel the entered number, press the Auto/Manual key.
Following is the LCD screen when the year “1998” is entered.
4.26 Service Manual ECG-9620
Page 74

4. SYSTEM TEST, ADJUSTMENT AND SETTING
NOTE
• If the Start/Stop key is pressed in the “second” setting mode, the
second is reset to “00” seconds and the instrument starts working
from “00” seconds.
• If the power of the instrument is turned off before the Start/Stop key
is pressed, the newly entered numbers are invalid.
4. Press the Start/Stop key to save the new numbers. The newly entered numbers
are automatically printed.
Following is the LCD screen when the year “1998” is saved.
If you entered an unspecified number, 8 repeating “pips” alarm sound and the
“Invalid number. Please re-enter number” error message is displayed.
To re-enter the system test number, do either of the following:
• Delete the previously entered number by pressing the Auto/Manual key.
• Enter the system test number by overwriting the previously entered
number.
5. Repeat steps 2 to 4 to enter the new numbers for the other settings.
6. Turn the power of the instrument off to exit the System Setup mode.
Service Manual ECG-9620 4.27
Page 75

Section 5 Board/Unit Description
Block Diagram.................................................................................................................... 5.1
Po w er Unit ......................................................................................................................... 5.2
ECG Control Board............................................................................................................ 5.2
Service Manual ECG-9620 5C.1
Page 76

Block Diagram
5. BOARD/UNIT DESCRIPTION
Buzzer
Motor
Motor sensor
Paper sensor
Thermal head
CNJ101 (34 pin)
Key board
CNJ201 (12 pin)
LCD
Patient cable connector
CNA011
CNA012
CNA013
CNJ021 (80 pin)
CNJ032 (2 pin)
CNJ036 (10 pin)
CNJ011 (16 pin)
CNJ033 (24 pin)
CNJ031 (9 pin)
SIO connector
CNJ043
(miniature plug)
External input connector
CNJ041
(miniature plug)
CRO output connector
ECG control board
CNJ091 (D-sub 15 pin)
Battery
(SB-901D)
CNJ035 (30 pin)
CNJ201 (30 pin)
Power board
CNJ102 (3 pin)
CNA014
CNJ101
(2 pin)
Power
transformer unit
AC socket
Service Manual ECG-9620 5.1
Page 77

5. BOARD/UNIT DESCRIPTION
Power Unit
ECG Control Board
The Power unit consists of the power source, battery charging and control circuits.
The Power unit uses the switching regulation method to produce the power
required for the instrument.
• +5 V for digital circuits
• +12 V for analog circuits
• +24 to 30 V for thermal head
The ECG Control board consists of the following components:
Component Description
CPU: MC68EC020 (Operating frequency: 25 MHz)
ROM: For system software, 2 MB
DRAM: Main memory, 4 MB
Flash memory: 16 MB
Real time clock: with 140 B SRAM and back-up battery (lithium battery)
Timer: 1 ms timer
Interrupt request signal ON/OFF: selectable
Operation mode: fixed
Serial interface: Equivelent to RS-232C, 1 channel
Baud rate: 2,400 to 115,200 bps selectable
Speaker circuit: Beep sound, Sound by noise generator
Interrupt request: Auto-vector method
Interface: To ECG input section
Recorder:
LCD:
Controller: For keyboard
A/D converter:
5.2 Service Manual ECG-9620
Page 78

Section 6 Disassembly
Before You Begin................................................................................................................ 6.1
Warnings and Cautions ........................................................................................... 6.1
Required T ools......................................................................................................... 6.1
Cable Connection .............................................................................................................. 6.2
Removing the Upper Casing.............................................................................................. 6.4
Removing the Magazine and Recording Paper ....................................................... 6.4
Removing the Battery Pack ..................................................................................... 6.4
Removing the Upper Casing.................................................................................... 6.4
Removing the Thermal Head and Motor Assy ................................................................... 6.5
Removing the Thermal Head ................................................................................... 6.5
Removing the Motor Assy........................................................................................ 6.6
Removing the ECG Control Board..................................................................................... 6.6
Removing the Power Board ............................................................................................... 6.8
Removing the Power Board ..................................................................................... 6.8
Replacing the Power Fuse and Battery Fuse .......................................................... 6.9
Removing the Key Board and LCD Unit........................................................................... 6.10
Service Manual ECG-9620 6C.1
Page 79

Before Y ou Begin
Warnings and Cautions
6. DISASSEMBLY
The procedures in this section tell how to remove, replace and install major
components in the instrument.
Removing, replacing and installing major components should be done by qualified
service personnel.
WARNINGS
• To avoid the possibility of injury to yourself or damage to the
instrument, do not install or remove any component or change
switch settings while the power is on. Turn the power off and wait 10
minutes before installing to or removing an y component from the
instrument.
• To avoid accidental discharge of static electricity which could
damage the instrument components, use a wrist ground strap when
installing or removing any component of the instrument.
Required T ools
CAUTIONS
• Before connecting or disconnecting any cables, turn off the
instrument, unplug the AC power cord from the instrument and
remove the battery pack.
• Fuses cut off the power when an abnormality occurs in the
instrument. Eliminate the malfunction before replacing the fuse. Use
the correct fuse only. The fuse rating is shown on the holder.
• Removal and replacement of any component in the instrument
should be done by qualified service personnel.
• Use only parts recommended by Nihon Kohden to assure maximum
performance from your instrument.
• Anti-static bench mat
• Wrist ground strap
• Phillips screwdriver (insulated type)
• Flat-blade screwdriver (insulated type)
• Hex (Allen) wrench or hex keys
• Hex driver
• Tweezers
Service Manual ECG-9620 6.1
Page 80

6. DISASSEMBLY
Cable Connection
Buzzer
Motor
Motor sensor
Paper sensor
Thermal head
CNJ101 (34 pin)
Key board
CNJ201 (12 pin)
LCD
Patient cable connector
CNA011
CNA012
CNA013
CNJ021 (80 pin)
CNJ032 (2 pin)
CNJ036 (10 pin)
CNJ011 (16 pin)
CNJ033 (24 pin)
CNJ031 (9 pin)
SIO connector
CNJ043
(miniature plug)
External input connector
CNJ041
(miniature plug)
CRO output connector
ECG control board
CNJ091 (D-sub 15 pin)
Battery
(SB-901D)
CNJ035 (30 pin)
CNJ201 (30 pin)
Power board
CNJ102 (3 pin)
CNA014
CNJ101
(2 pin)
Power
transformer unit
AC socket
6.2 Service Manual ECG-9620
Page 81

6. DISASSEMBLY
No. Code No. Description
CNA011 08SK3.670.00216A ZHR-10 L=120, 90, 130 between motor assy and ECG control board
CNA012 08SK3.670.00181B PHR-16 L=80, between thermal head and ECG control board
CNA013 08SK3.670.00172A PHDR-24VS (W=100), between key board and ECG control board
CNA014 08SK3.670.00199B SHDR-30V-S-B (W=60), between power board and ECG control board
CNA011 cable
CNA012 cable
CNA013 cable
CNA014 cable
Service Manual ECG-9620 6.3
Page 82

6. DISASSEMBLY
Removing the Upper Casing
Removing the Magazine
and Recording Paper
Removing the Battery Pack
1. Press down the magazine release button to open the magazine.
2. Remove magazine and recording paper into the magazine.
1. Remove the two M3 binding head screws from the battery cover.
2. Open the battery cover by sliding it up as shown in the figure.
Removing the Upper
Casing
3. Disconnect the battery cable from the battery pack connector..
4. Remove the battery pack from the battery compartment.
1. Remove the five M3 binding head screws.
2. Open the upper casing in the direction of the arrow.
6.4 Service Manual ECG-9620
Page 83

Removing the Thermal Head and Motor Assy
6. DISASSEMBLY
Removing the Thermal
Head
1. Remove the battery pack and upper casing from the lower casing. Refer to
“Removing the Upper Casing” .
2. Remove the two M3 pan screws with washers and spring washers which fasten
the ground leads to the power transformer unit.
Ground lead
M3 binding head screw
M3 pan screw with washers
and spring washer
Thermal head unit
CNA012 cable
CNA011 cable
Thermal head
3. Disconnect the CNA011 and CNA012 cables from the ECG control board.
4. Remove the three M3 binding head screws which fasten the thermal head unit
to the lower casing and remove the thermal head unit.
5. Remove the two M3 binding head screws washers which fasten the thermal
head to the thermal head unit.
6. Remove the thermal head.
7. Remove the thermal head cable from the thermal head.
Service Manual ECG-9620 6.5
Page 84

6. DISASSEMBLY
Removing the Motor Assy
Motor assy
Motor sensor board
1. Remove the thermal head unit from the lower casing. Refer to step 1 to 6 in
“Removing the Thermal Head Unit”.
2. Remove the M3 pan screw and remove the paper sensor board.
3. Remove the two M3 binding head screws which fasten the motor assy to the
thermal head unit and remove the motor assy.
4. Remove the two M3 pan screws with spring washers which fasten the motor
sensor board to the motor assy and remove the motor sensor board.
6.6 Service Manual ECG-9620
Page 85

Removing the ECG Control Boar d
1. Remove the battery pack and upper casing from the lower casing. Refer to
“Removing the Upper Casing” .
2. Disconnect the CNA011, CNA012, CNA013, CNA014 and speaker cables
from the ECG control board.
6. DISASSEMBLY
ECG control board
CNA011 cable
CNA014 cable
CNA012 cable
CNA013 cable
Speaker cable
3. Remove the six M3 pan screws with washers which fasten the ECG control
board to the lower casing and remove the ECG control board.
Service Manual ECG-9620 6.7
Page 86

6. DISASSEMBLY
Removing the P ower Board
Removing the Power Board
1. Remove the battery pack and upper casing from the lower casing. Refer to
“Removing the Upper Casing” .
2. Remove the ECG control board. Refer to “Removing the ECG Control Board”.
3. Remove the two M3 pan screws with washers and four M3 spacer bolts which
fasten the shield plate to the lower casing and remove the shield plate.
M3 pan screw with spring washer
M3 spacer bolt
Power board
Shield plate
Pow er cable
4. Remove the M3 pan screw with washer which fastens the Power board to the
lower casing and remove the power unit.
5. Disconnect the power cable from the power board.
6.8 Service Manual ECG-9620
Page 87

6. DISASSEMBLY
Replacing the Power Fuse
and Battery Fuse
1. Remove the power board from the lower casing. Refer to “Removing the
Power Board”.
2. Turn the power board over.
3. Replace the battery fuses with a new one.
4. Replace the power fuse with a new one.
Po w er fuse
Battery fuse
Power fuse
Code No. Description
ECG-9620L/M/N/P: 274035 218.250 (0.25 A)
ECG-9620S/T/U: 104665 218.500 (0.5 A)
Battery fuse
Code No. Description
F101: 540657 239.001 (1 A)
F102: 323241 218.008 (8 A)
Service Manual ECG-9620 6.9
Page 88

6. DISASSEMBLY
Removing the Ke y Boar d and LCD Unit
1. Remove the battery pack and upper casing from the lower casing. Refer to
“Removing the Upper Casing”.
M3 pan screws with spring washer and washer
LCD cable
LCD ground plate
LCD ground spring
LCD unit
CNA013 cable
Ke y board
2. Disconnect the CNA013 cable from the key board.
3. Release the connector lock and disconnect the LCD cable from the key board.
4. Remove the six M3 pan screws with spring washers and washers which fasten
the LCD unit to the upper casing and remove the LCD unit, two LCD ground
spring and two LCD ground plates.
5. Remove the four M3 pan screws with spring washers and washers which fasten
the key board to the upper casing and remove the key board.
6.10 Service Manual ECG-9620
Page 89

Section 7 Replaceable P arts List
Instrument.......................................................................................................................... 7.2
Service Manual ECG-9620 7C.1
Page 90

7. REPLACEABLE PARTS LIST
When ordering parts or accessories from your nearest Nihon Kohden Corporation
distributor, please quote the NK code number and part name which are listed in
this service manual, and the name or model of the unit in which the required part
is located. This will help us to promptly attend to your needs. Always use Nihon
Kohden parts and accessories to assure maximum performance from your
instrument.
Service Manual ECG-9620 7.1
Page 91

7. REPLACEABLE PARTS LIST
Instrument
Index Code No. Qty Description
101 08SK8.074.00075 1 ECG-9620 Upper casing
102 08SK8.081.00316 1 Operation panel
103 08SK8.080.00086 1 LCD filter
104 08SK2.929.00047 1 LCD unit, MR604WBE11
105 08SK8.048.00032 2 LCD ground spring
106 08SK8.387.00227 2 LCD ground plate
302 08SK8.223.00066 2 Head collar bearing
303 08SK8.038.00088 1 Thermal head bracket
304 08SK3.878.00046 1 Thermal head, KYT-56-8MPP1-SKH
305 08SK8.300.00099 1 Thermal head shaft
306 08SK8.387.00031 2 Thermal head spring
307 08SK8.387.00218 1 Paper sensor board ground plate
308 107029 1 E ring, E-50
309 08SK8.072.00032 1 Thermal head unit base
310 RHC-00041 1 Motor assy
311 RHC-00042 1 Magazine assy
401 08SK8.615.00089 4 Spacer bolt, L8
402 08SK7.061.00082 1 Heat sink seat
403 08SK8.610.00031 1 Shield plate
404 08SK8.807.00906 2 Protective ground label
501 08SK5.846.00014 1 Speaker assy
502 08SK6.116.00025 1 ECG-9620 lower casing
503 08SK8.080.00095 1 Battery cover
504 08SK8.085.00054 4 Rubber foot
A UTC-0006 1 Key board
B UTC-0007 1 ECG control board
C UTC-0008 1 Power board
D RKC-0001 1 Transfer Assy (220 V) for L and P version
RKC-0002 1 Transfer Assy (230 V) for M version
RKC-0003 1 Transfer Assy (240 V) for N version
RKC-0004 1 Transfer Assy (110 V) for S version
RKC-0005 1 T ransfer Assy (120 V) for T version
RKC-0006 1 Transfer Assy (127 V) for U version
7.2 Service Manual ECG-9620
Page 92

B
301
302
308
311
101
7. REPLACEABLE PARTS LIST
102
103
104
105
304
305
309
303
502
307
310
306
312
402
404
401
C
501
A
106
403
D
404
503
504
Service Manual ECG-9620 7.3
Page 93

Section 8 Connector Pin
Assignment
Connector Pin Assignment ................................................................................................ 8.1
Attaching the Ferrite Core........................................................................................ 8.1
EXT-IN Connector.................................................................................................... 8.2
CRO-OUT Connector .............................................................................................. 8.2
SIO Connector......................................................................................................... 8.2
Service Manual ECG-9620 8C.1
Page 94

Connector Pin Assignment
• When connecting an external instrument to connectors marked with
, the external instrument and this cardiograph must be connected
according to the IEC60601-1-1 “Medical electrical equipment - Part 11: General requirements for safety - Collateral standard: Safety
requirements for medical electrical systems”. Failure to follow this
warning may cause electrical shock to the patient and operator.
• When the cardiograph operates on battery power and large leakage
current is input from the connected external instrument, ground the
cardiograph or use an isolation transformer for the external
instrument. Failure to follow this caution may cause electrical shock
to patient and operator.
8. CONNECTOR PIN ASSIGNMENT
CAUTION
Attaching the Ferrite Core
Connector Ferrite core model Code No.
EXT-IN RI-14-28-6 493182 Wrap the cable once around the ferrite core.
CRO-OUT RI-14-28-6 493182 Wrap the cable once around the ferrite core.
SIO SEC-8 361814 -
Example of Wrapping
NOTE
When connecting an external instrument to the following connecters,
an unwanted radio frequency signal is generated from this
connection. To reduce this unwanted radiofrequency signal, attach
the optional ferrite core to the cable of the external instrument.
NOTE
Attach and fix the ferrite core near the connector of the cable
that connects to the cardiograph.
Service Manual ECG-9620 8.1
Page 95

8. CONNECTOR PIN ASSIGNMENT
EXT-IN Connector
1
2
CRO-OUT Connector
1
2
Unit side: SG-8036 (Code No.: 273446)
Cable side: P-112D 3.5mm ϕ miniature plug (Code No.: 606907)
Pin No. Signal
1 EXT-IN 1
2 GND
Input sensitivity: 10 mm/0.5 V, input impedance 100 kΩ or more
CAUTION
Do not use the output signal from the output connector for a
synchronization signal such as the synchr onized cardioversion
signal. There is a time delay between the input ECG signal and
output signal.
Unit side: SG-8036 (Code No.: 273446)
Cable side: P-112D 3.5 mm ϕ miniature plug (Code No.: 606907)
Pin No. Signal
1 CRO-OUT
2 GND
Output sensitivity: 0.5 V/1 mV, output impedance 100 Ω or less
SIO Connector
5
9
Unit side: JEY-9S-1A2B (Code No.: 390774)
Cable side: Connector, DE-9P (Code No.: 082625)
Cover, DE-C8-J9-F1-1 (Code No.: 336708)
1
6
Pin No. Signal Pin No. Personal conputer
1 Shield 1 Shield
2 TxD 2 TxD
3 RxD 3 RxD
4 RTS 4 RTS
5 CTS 5 CTS
6 DSR 6 DSR
7 GND 7 GND
8 DCD 8 to 19 Not used
9 DTR 20 DTR
21 to 25 Not used
Pin assignment may depend on the shape of connectors for PCs.
To connect a modem, use a dedicated cable 15 m or shorter.
8.2 Service Manual ECG-9620
Page 96

 Loading...
Loading...