Page 1

Corrosion Guide Rev 1.05 Jan 2006
OPTIMASS
•• 11000000 SSeerriieess TTwwiinn SSttrraaiigghhtt TTuubbee CCoorriioolliiss MMaassss FFlloowwmmeetteerr
•• 33000000 SSeerriieess SSiinnggllee ZZ TTuubbee CCoorriioolliiss MMaassss FFllo
owwmmeetteerr
•• 77000000 SSeerriieess SSiinnggllee SSttrraaiigghhtt TTuubbee CCoorriioolliiss MMaassss FFlloowwmmeetteerr
•• 88000000//99000000 SSeerriieess TTwwiinn UU TTuubbee CCoorri
ioolliiss MMaassss FFlloowwmmeetteerr
DDIISSCCLLAAIIMMEERR::
KROHNE does not represent or warrant the accuracy of the information included in this guide for end user applications. The data presented is based on
published data and field experience. However, only the end-user is aware of the specific chemical makeup of their process and must therefore accept the
ultimate responsibility for the suitability of the wetted parts for the process. Responsibility as to suitability and intended use of our instruments rests solely
with the end user!
Corrosion & Abrasion Guidelines for Coriolis Meters
Page 2

2
Corrosion Guide Rev 1.05 Jan 2006
Contents:
1 Corrosion of Wetted Parts
1.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
1.2 Material Compatibility . . . . . . . . . . . . . . . . . . . . . . . . .3
1.3 Material Information . . . . . . . . . . . . . . . . . . . . . . . . . .3
1.3.1 What is Stainless Steel? . . . . . . . . . . . . . . . .4
1.4 NACE Information . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
1.5 Galvanic Corrosion with Titanium . . . . . . . . . . . . . . . .4
1.6 Problem Applications . . . . . . . . . . . . . . . . . . . . . . . . . .5
1.7 Using Material Compatibility Tables . . . . . . . . . . . . . .5
2 Abrasion Guidelines
2.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
2.2 Protecting Flanges with Transition Pieces . . . . . . . . .14
2.3 Managing Fluid Velocity . . . . . . . . . . . . . . . . . . . . . . .14
2.4 Titanium as the standard material . . . . . . . . . . . . . . . .14
2.5 Conditioning the fluid flow . . . . . . . . . . . . . . . . . . . . . .15
2.6 Installation of the Flow Meter . . . . . . . . . . . . . . . . . . .15
2.7 Inclusion of air or gas in the fluid . . . . . . . . . . . . . . . .15
2.8 Consider installing the meter in a by-pass . . . . . . . . .15
2.9 Conclusions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
3 Environmental Protection
3.1 Housing Specifications . . . . . . . . . . . . . . . . . . . . . . . .16
Page 3
Corrosion Guide Rev 1.05 Jan 2006
3
Element Typical Specification
C 0.013 0.035 Max
Mn 1.6 2.0 Max
P 0.028 0.045 Max
S 0.009 0.030 Max
Si 0.38 1.0 Max
Cr 16.84 16.0...18.0
Ni 11. 2 6 10.0...15.0
Mo 2.12 2.0...3.0
Fe Balance Balance
Specifications for SS316L
Element Typical Specification
Al 3.0 2.5...3.5
V 2.5 2.0...3.0
Fe 0.13 0.25 Max
C 0.05 0.08 Max
Ti Balance Balance
Specifications for Titanium Gr9
Stainless Steel
318L
7000 series
All wetted parts: UNS S31803 to
ASTM A789, A790, A479 and A240
(also known as DUPLEX Stainless
Steel SAF2205).
This 318L stainless steel is NACE
approved.
ALL hygienic and aseptic process
connections: 316L UNS 31603 or
equivalent (also NACE approved).
Hastelloy C22
7000 series
All wetted parts: UNS NO6022 to
ASTM B619, B622, B626, B574
and B575
This HC-22 is NACE approved.
Titanium
7000 series
Measuring tube: UNS R56320 to
ASTM B338 titanium grade 9
Flange raised faces: UNS R50400
to ASTM B348 and B265 titanium
grade 2.
Titanium grade 9 is not NACE
approved.
Stainless Steel
316L
3000, 8000 / 9000
series
All wetted parts: 316L UNS 31603
or equivalent.
316L is NACE Approved.
Hastelloy C22
3000, 8000 / 9000
series
All wetted parts: UNS NO6022 to
ASTM B622, B626, B564, B574
and B575.
Hastelloy C22 is NACE approved.
1. Corrosion Of Wetted Parts
1.1 Introduction:
General corrosion guidelines for process vessels,
pipework and parts (such as thermo-wells or MID
flowmeter earthing rings) cannot be applied to Coriolis
mass flowmeters, since they refer to the bulk removal
of material from comparatively thick walled components, and they also tend not to address specific
localised corrosion mechanisms.
Greater care must be taken in the case of mass
flowmeters, since the tube wall thicknesses are in the
region of 0.2 … 2mm (depending on meter size), so
the removal of even a small amount of material can
cause a measurement problem or a meter tube
mechanical failure.
Additionally, localised corrosion effects such as pitting
or stress corrosion cracking can occur even without
corrosion of the overall tube. If severe enough, these
effects may cause a meter tube mechanical failure.
For these reasons you will find that many fluids will be
shown as unsuitable in this guide for use with mass
flowmeters when compared to more general corrosion
guidelines.
1.2 Material Compatibility
1.3 Material information
The Optimass meter range can be supplied with wetted parts (measuring tubes and connections) in the following materials:
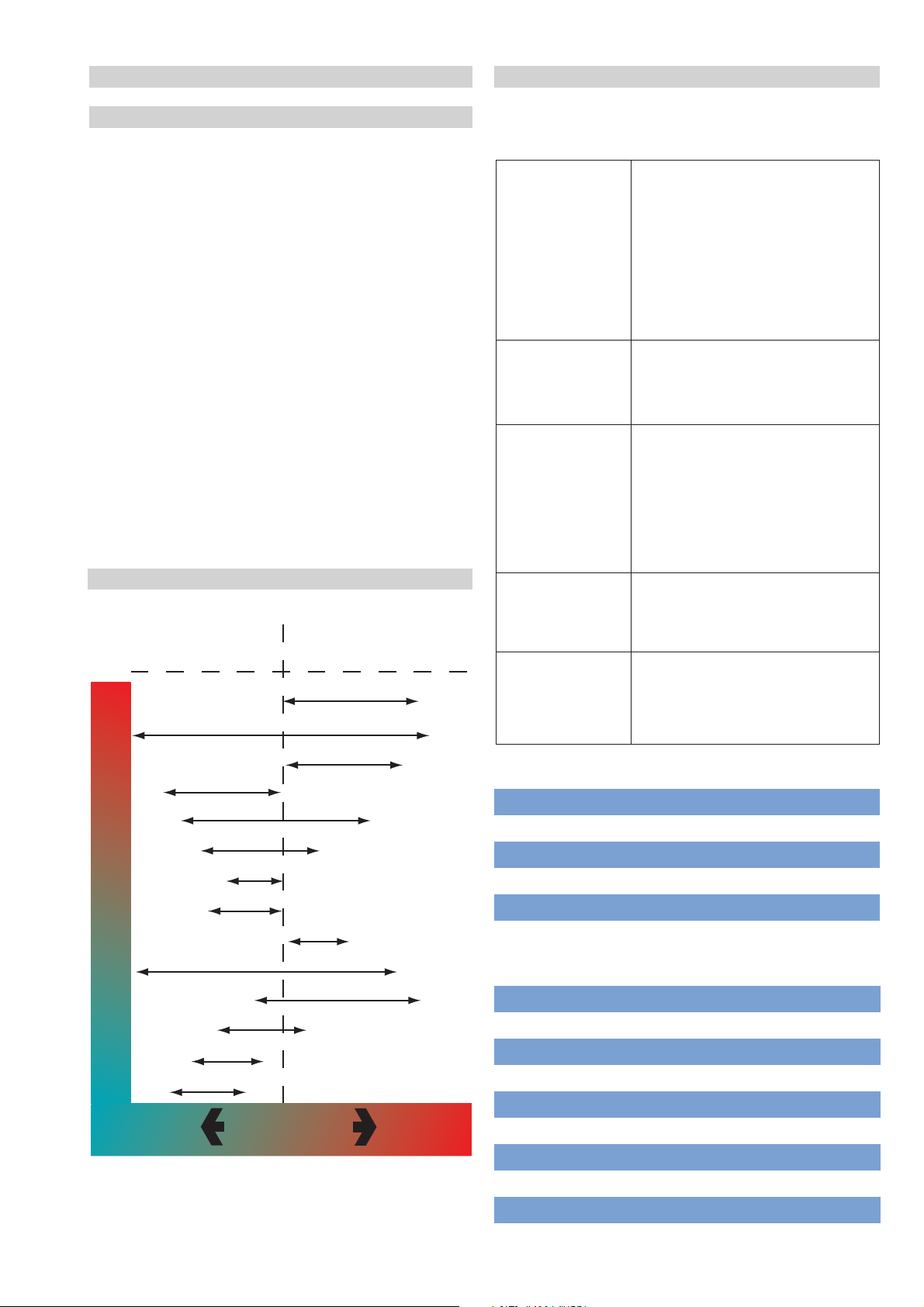
Oxidising
environment
Tantalum
Chlorides
Titanium
C-276
C-4
Ni-Cr-Mo Alloy
No 20 Alloy (Stainless)
Inconel
316 Stainless
Reducing
environment
Zirconium
Ni-Mo Alloy
C-22
Monel
Monel
304 Stainless
Non Chlorides
Oxidizing
Acids
Increasing
aggressivness
Reducing
Agents
Page 4

4
Corrosion Guide Rev 1.05 Jan 2006
Element Typical Specification
C 0.02 0.030 Max
Mn 0.70 2.0 Max
P 0.025 0.030 Max
S 0.001 0.020 Max
Si 0.40 1.0 Max
Cr 22.4 21.0...23.0
Ni 5.8 4.5...6.5
Mo 3.3 2.5...3.5
N 0.16 0.08...0.2
Fe Balance Balance
Specifications for SS318L
Element Typical Specification
Cr 21.6 20.00...22.5
Mo 13.7 12.5...14.5
W 2.9 2.5...3.5
Fe 4.7 2.0...6.0
Co 1.1 2.5 Max
Mn 0.3 0.5 Max
V 0.13 0.35 Max
Ni Balance Balance
Specifications for Hasteloy C22
Please be aware that 318L is a “shorthand” expression
KRONHE have adopted for the material to aid our customers’ understanding that the material is a low carbon
stainless steel of a speciifc composition, in the same
way that 316L is used as “shorthand” for UNS S31603.
318L is more expensive to use than 316L, but we need
to consider the technical benefits that it brings. 318L
has a much higher tensile strength compared to 316L.
We use this property in our meter to manage the stress
caused by lengthening of the tube under thermal
expansion. If we used 316L, the tube would deform
well below the +100
0
C maximum we specify for 318L.
The corrosion resistance of 318L is equal to 316L. The
standard surface roughness is similar and it can be polished to the same fine surface finish of <0.5 um Ra for
hygienic and aseptic applications. 318L is extensively
used worldwide, where customers utilise the higher tensile strength to reduce wall thickness (and so the cost
and weight) of process plant and piping. This means that
there should be no reluctance from our customers in
accepting this material.
KROHNE is the only company to offer a stainless steel
straight tube mass flowmeter.
1.4 NACE Information
National Association of Chemical Engineers (NACE standard MR0175-2000) is a material requirements standard
relating to the general problems of Sulphide Stress
Cracking (SSC) of metals directed towards sour environments.
1.5 Galvanic Corrosion with Titanium
This can occur when a titanium tube meter is placed in
contact with other metals, for instance in a steel
pipeline. As titanium is a “noble” metal, the other material will normally corrode in preference to the titanium.
In the case of stainless steel pipelines, titanium is very
close in the galvanic table therefore galvanic corrosion
is unlikely to be a problem and no precautions are necessary.
If carbon steel pipelines are used, galvanic corrosion of
the carbon steel may occur with certain acids. This corrosion process causes small amounts of hydrogen gas
to be liberated at the wetted metal-to-metal contact
area. This hydrogen gas may then cause embrittlement
in the titanium. This can be avoided by electrically insulating the Optimass from the pipeline using insulating
gaskets, bolt sleeves and washers.
Of course the use of an alternative tube material e.g.
Hastelloy C-22 will prevent the problem entirely.
1.3.1 What is 318L Stainless steel?
Many customers have asked “what is the 318L stainless
steel that you use in the Optimass 7000 single straight
tube meter, and why do you use this material instead of
316L”? Measuring instruments are more normally manufactured with wetted parts from 316L stainless steel,
which is a 100% austenic structure steel, with composition 18% .Chrome, 8% Nickel, 2.5% Molybdenum, with
the balance Iron.
318L is a 50% austenitic / 50% ferritic structure steel
(typically known as "duplex"), with (typical) composition
22% Chrome, 5% Nickel, 3% Molybdenum, with the balance Iron.
This material is defined under the following international codes:
•
UNS S31803
•
ASTM A789, A790, A479, A420
•
DIN 1.4462
Page 5

Corrosion Guide Rev 1.05 Jan 2006
5
Remember in all cases KROHNE cannot accept
responsibility for the final choice of material. The ultimate responsibility lies with the end user. We can only
advise based on our experience!
Explanation of data tables
1. Key to symbols used
Symbol Meaning
Suitable, with a corrosion rate less than
0.05 mm (0.002”) per year
X Unsuitable, due to higher corrosion rate
nd No data is currently available
1.6 Problem Applications
Hydrochloric acid (HCl):
This acid normally contains fluoride and chloride impurities that will promote stress corrosion cracking in all
tube materials. This effect will always cause a meter
failure even if there is no bulk removal of tube material.
For this reason we do not recommend Optimass
meters for any HCl application.
Instead look to alternative instrument technologies such
as capacitive MID (Capaflux) or variable area flowmeters (H250 PTFE).
Methanol:
“Pure” methanol (> 98% purity) tends to cause the
removal of the protective oxide layer on a titanium
measuring tube thus promoting corrosion. Titanium
therefore cannot be used. Stainless steel or Hastelloy
C-22 are suitable alternatives for these applications.
Methanol with a minimum 2%, or greater, water content
does not exhibit this tendency and can be freely used
with all tube materials.
Oxygen gas:
There is a risk of ignition where an oxygen rich (> 35%
O
2
) gas mixture is used with a titanium measuring
tube.Always offer stainless steel as an
alternative.Remember also that the “degreasing” option
for wetted parts should also be offered for use with oxygen gas applications.
Passivation of meters
A “passivation fluid” is normally composed of a mixture
of nitric acid (HNO
3
) and hydrofluoric acid (HF) and is
used to ‘passivate’ (clean) stainless steel pipework in
high purity systems, typically found in the pharmaceutical industry. The process removes weld discoloration,
dirt, grease, metal particles, etc.
The nitric acid has negligible corrosive effect, but
hydrofluoric acid is very aggressive even at levels less
than 0.5% for all wetted tube materials. It is recommended that any meter is removed and replaced with
spool pieces during the passivation process. This is
due to the relatively thin wall of mass flow meter tubes;
the customer’s pipework being relatively thick is tolerant to a small loss of material.
1.7 Using Material Compatibility Tables.
Identify the chemical to be used either by name or by
the Formula.
Check that you know the concentration of the chemical
and that it is within the concentrations listed in column
3.
Identify the best material for the application and then
check that this is acceptable for the customer.
2. Where a temperature is given, this signifies suitability only up to this point (e.g. 212/100 denotes
up to 212
o
F or 100oC). Where no temperature is
given, this signifies suitability up to the lower of
either the boiling point of the product, or the
maximum operating temperature of the Optimass
flowmeter concerned.
The first temperature given is always in Fahrenheit
and the second in Centigrade/Celsius.
3. Always check the relevant technical data sheet
for the maximum operating temperature of the
meter series and wetted material.
4. For the purposes of corrosion resistance, stainless steel grades 316L and 318L can be considered as both being the same.
Page 6

6
Corrosion Guide Rev 1.05 Jan 2006
Fluid Chem. Formula Concentration
Stainless Steel
Max
oF/o
C
Hastelloy C-22
Max
oF/o
C
Titanium
Max
oF/o
C
Acetaldehyde CH3CHO 100% 200/93 200/93 300/149
Acetate CH3CO2CH3 100% 400/204 400/204 400/204
Acetic Acid CH3COOH 20% 400/204 300/149 260/127
Acetic Acid CH3COOH 50% 160/71 210/99 260/127
Acetic Acid CH3COOH 80% 160/71 210/99 260/127
Acetic Acid CH3COOH 95% X 200/93 210/99
Acetic Acid CH3COOH 100% X 200/93 200/93
Acetone CH3COCH3 100% 400/204 140/60 200/93
Acetyl Chloride CH3COCI 100% 70/21 99/37 220/105
Acetylene CH2 100% 400/204 220/105 79/26
Adipic Acid (CH2)5(COOH)2 100% 50/10 200/93 428/220
Air
Aluminium Chloride AlCl3 10% X 220/105 200/93
Aluminium Chloride AlCl3 40% X 220/105 X
Aluminium Chloride AlCl3 100% X 158/70 X
Aluminium Fluoride AlCl3 20% X X X
Aluminium Nitrate AlNO3 100% 302/150 X 208/98
Aluminium Sulphate Al2SO4 6.50% X 285/140 220/105
Aluminium Sulphate Al2SO4 40% X 285/140 220/105
Aluminium Sulphate Al2SO4 100% X nd 200/93
Ammonia (anhydrous) dry NH3 always 100% 248/120 248/120 77/25
Ammonia (aqueous) NH3 + water 30% 158/70 158/70 X
Ammonia (aqueous) NH3 + water 50% 86/30 302/150 X
Ammonium Acetate NH4CH3COO 15% 220/105 220/105 77/25
Ammonium Acetate NH4CH3COO 55% 170/77 nd nd
Ammonium Bicarbonate NH4HCO3 50% X nd 212/100
Ammonium Carbonate (NH4)2CO3 50% X X
Ammonium Chlorate (NH4)ClO3 30% nd nd 122/50
Ammonium Chloride NH4Cl 40% X 221/105 221/105
Ammonium Fluoride NH4F 25% X 170/77 nd
Ammonium Hydroxide NH4OH 45% X X 170/77
Ammonium Nitrate NH4NO3 28% 280/137 80/27
Ammonium Oxalate (NH4)C2O4 10% X 75/24 75/24
Ammonium Perchlorate NH4ClO4 20% 170/77 X 285/140
Ammonium Phosphate (NH4)3PO4 10% X 140/60 248/120
Ammonium Phosphate (NH4)3PO4 100% X 140/60 140/60
Ammonium Sulphate (NH4)2SO4 10% X 220/104 248/120
Ammonium Sulphate (NH4)2SO4 100% X X 248/120
Aniline C6H6NH2 100% 509/265 248/120 230/110
Aniline hydro chloride C6H5NH2xHCl 25% X nd 212/100
Aqua Regia HCl / H2SO4 100% X X X
Argon Ar 100%
Barium Carbonate BaCO3 100% X X 77/25
Barium Chloride BaCl2 25% X 158/70 122/50
Barium Hydroxide Ba(OH)2 10% 225/107 225/107 77/25
Barium Nitrate Ba(NO3)2 20% 150/65 X 77/25
Benzene C6H6 100% X X 240/116
Benzoic Acid C6H5COOH 10% X 180/82 248/120
Benzoic Acid C6H5COOH 100% X 180/82 248/120
Benzyl Chloride C7H7Cl 100% X X 140/60
Boric Acid BH3 10% X 320/160 320/160
Boric Acid BH3 100% nd nd 77/25
Bromine Liquid Br 100% X X X
Butyric Acid CH3(CH2)2COOH 100% X 260/127 225/107
Calcium Carbonate CaCO3 100%
Calcium Chloride CaCl2 75% X 221/105 O 221/105
Calcium Chloride CaCl2 100% X 392/200 X
= Compatible
X = Not Compatible nd = No Data Available
Page 7

7
Corrosion Guide Rev 1.05 Jan 2006
Fluid Chem. Formula Concentration
Stainless Steel
Max oF/oC
Hastelloy C-22
Max oF/oC
Titanium
Max oF/oC
Calcium Hydroxide Ca(OH)2 50% X 212/100 171/77
Calcium Hypochlorite Ca(Ocl)2 15% X 122/50 221/105
Calcium Hypochlorite Ca(Ocl)2 95% X X 221/105
Calcium Sulphate CaSO4 10% nd X nd
Calcium Sulphate CaSO4 100% X nd 140/60
Calcium Hypochlorite CaCl2+6H2O 15% X 122/50 221/105
Calcium Hypochlorite CaCl2+6H2O 95% X X 221/105
Carbon Tetrachloride
(anhydrous)
dry CCl4 100% 140/60 140/60 248/120
Carbonic Acid Saturated X
Chlorine (anhydrous) dry Cl2 any X X X
Chlorine (aqueous) Cl2 + water any X X X
Chloroacetic Acid CH2CICOOH 85% X X 221/105
Chloroform CHCl3 100% 80/27 80/21 221/105
Chlorosulphonic Acid HCISO3 100% X 185/85 X
Chromic Acid H8CrO5 25% X 167/75 221/105
Chromic Acid H8CrO5 50% X 75/24 221/105
Chromic Acid H8CrO5 100% X X 221/105
Citric Acid C6H8O7 25% X 221/105 212/100
Citric Acid C6H8O7 60% X 221/105 X
Copper Nitrate Cu(NO3)2 100% 280/138 X 77/25
Copper Sulphate CuSO4 100% X X 221/105
Cupric Chloride CuCl2 any X X X
Cupric Cyanide CuCN 100% X X 77/25
Cuprous Chloride CuCl 55% nd nd 194/90
Dichloroacetic Acid CHCl2COOH any nd nd X
Diethyl Phthalate C12H14O4 100% 221/105 nd 221/105
Ethyl Alcohol (Ethanol) C2H5OH 100% 221/105 221/105 221/105
Ethylene gas C2H4 100%
Ethylene dichloride
(anhydrous)
dry C2H2Cl2 100% X 221/105 221/105
Ethyl Acetate CH3 COOC2H5 100% 320/160 320/160 221/105
Ferric Chloride FeCl3 100% X X 248/120
Ferric Hydroxide Fe(OH)3 6% 70/21 80/27 68/20
Ferric Hydroxide Fe(OH)3 100% 70/21 80/27 nd
Ferric Sulphate Fe2(SO4)3 15% 221/105 104/40 221/105
Ferric Sulphate Fe2(SO4)3 35% X 104/40 221/105
Ferric Sulphate Fe2(SO4)3 100% X X 221/105
Ferrous Sulphate FeSO4 100% X X 248/120
Fluoboric Acid HBF4 30% 80/27 200/93 X
Fluorine (anhydrous) dry F any X X X
Fluosilicic Acid H2SiF6 any X X X
Formaldehyde CH2O 100% 221/105 X 221/105
Formic Acid (aerated) HCOO4 50% X 221/105 221/105
Formic Acid (non-aerated) HCOO4 85% X 221/105 X
Heptane C6H12 100% 221/105 221/105 221/105
Hydrazine (NH2)2 100%
Hydrobromic Acid HBO3 100% X 140/60 X
Hydrochloric Acid HCL + water any X X X
Hydrofluoric Acid HF + water any X X X
Hydrogen gas H
Hydrogen Chloride gas HCL any X X X
Hydrogen Cyanide NCN 100% 88/31 88/31 88/31
Hydrogen Fluoride gas HF any X X X
Hydrogen Peroxide H2O2 50% 194/90 194/90 X
Hydrogen Peroxide H2O2 90% 118/48 118/48 X
Iodine Liquid I2 100% X
77/25
= Compatible
X = Not Compatible nd = No Data Available
Page 8

8
Corrosion Guide Rev 1.05 Jan 2006
Fluid Chem. Formula Concentration
Stainless Steel
Max oF/oC
Hastelloy C-22
Max oF/oC
Titanium
Max oF/oC
Lactic Acid CH3CHOHCOOH 25% 122/50 122/50 122/50
Lithium Chloride LiCl 50% X 212/100 212/100
Magnesium Chloride MgCl2 50% X 280/138 248/120
Magnesium Hydroxide Mg(OH)2 100% 212/100 212/100 167/75
Magnesium Sulphate MgSO4 50% nd 200/93 200/93
Maleic Acid CH2(COOH)2 100% X 176/80 248/120
Manganese Chloride MnCl2 45% X 212/100 212/100
Mercuric Chloride HgCl2 10% X X 266/130
Mercuric Cyanide Hg(CN)2 10% 95/35 X 95/35
Methanol + > 2% H2O C2H4OH 98%
Methanol + > 50ppm H20 C2H40H 99.99%
X
Methanol pure C2H40H 100%
X
Methyl Ethyl Ketone CH3CH2COCH3 100% 200/93 200/93 200/93
Methyl Methacrylate C5H8O2 100%
Methylene Chloride CH2Cl2 100% X X 239/115
Mono Sodium
Orthophosphate in water
NaPO3 200 millimoles nd nd 86/30
Monochloacetic Acid CH2ClCOOH 100% nd nd
N-Butyric Acid CH3(CH2)2COOH 100% X 212/100 212/100
Nickel Chloride NiCl2 100% X 194/90 194/90
Nickel Nitrate Ni(NO3)2 50% X
77/25
Nickel Sulphamate 50%
Nitric Acid HNO3 100% “red fuming” 75/24 75/24 140/60
Nitric Acid HNO3 70% 75/24 75/24 140/ 60
Nitric Acid HNO3 50% 100/38 75/24 140/ 60
Nitric Acid HNO3 40% 125/52 75/24 140/ 60
Nitric Acid HNO3 30% 130/66 75/24 140/ 60
Nitric Acid HNO3 20% 176/80 130/66 140/ 60
Oxalic Acid (COOH)2 100% X 212/100 X
Oxygen rich gas mixture O2 > 35%
X
“Passivation” fluid Mixture HNO3 + HF X X X
Pentane C5H10 100% X
Perchloric Acid HClO4 any X 212/100 nd
Phenol C6H5OH 95% 131/55
X
Phosphoric Acid H3PO4 100% X X X
Phosphoric Acid H3PO4 98% X 77/25 X
Phosphoric Acid H3PO4 20% X 77/25 77/25
Phosphoric Acid H3PO4 5% 77/25 77/25 77/25
Potassium Bromide KBr 100% X X
Potassium Bromide KBr 30% X 95/35
Potassium Chloride KCI 99% X
Potassium Hydroxide KOH 40% 170/77 170/77 170/77
Potassium Iodide KI 75% 212/100 212/100 212/100
Potassium Nitrate KNO3 100% X 212/100 240/115
Potassium Permanganate KMnO4 100% X
Potassium Sulphate K2SO4 25% 212/100 X 240/115
Propanol (Propyl Alcohol) C3H6OH 100%
Seawater (Brine) C3H6OH 100% X
Silver Nitrate AgNO3 70% 85/30 85/30 212/70
Sodium Bisulphate NaHSO4 20% X X 85/30
Sodium Carbonate Na2CO3 100% 212/100 212/100 212/100
Sodium Chlorate NaCIO3 100% X 302/150 302/150
Sodium Chloride NaCI 100% X 212/100 212/100
Sodium Cyanide NaCN 50% 77/25 nd 266/130
Sodium Dichromate Na2Cr 100% X 80/27 77/25
Sodium Formate HCOONa 50% 131/55 131/55 nd
= Compatible
X = Not Compatible nd = No Data Available
Page 9

9
Corrosion Guide Rev 1.05 Jan 2006
Fluid Chem. Formula Concentration
Stainless Steel
Max
oF/o
C
Hastelloy C-22
Max
oF/o
C
Titanium
Max
oF/o
C
Sodium Hydroxide NaOH 45% X 104/40 131/55
Sodium Hydroxide NaOH 75% X 104/40 X
Sodium Hypochlorite NaOCl any X X X
Sodium Iodide NaI 65% X nd 212/100
Sodium Nitrate NaNO3 60% 233/112 233/112 248/120
Sodium Nitrate NaNO3 100% X X 248/120
Sodium Nitrite NaNO2 50% 212/100 212/100 266/130
Sodium Peroxide (Na)2O2 15% 212/100 X X
Sodium Phosphate NaPO4 15% X 212/100 212/100
Sodium Silicate Na2SiO3 100% 212/100 212/100 212/100
Sodium Sulphate Na2SO4 20% 212/100 212/100 X
Sodium Sulphide Na2S 50% X 212/100 X
Sodium Sulphite NaSO3 25% 212/100 212/100 212/100
Sodium Sulphite NaSO3 50% nd nd 212/100
Stannic Chloride SnCl2 any X X X
Stearic Acid CH2(CH2)16COOH 100%
212/100 X
Succinic Acid (CH2 COOH)2 50% X X
Sulphamic Acid NH2 SO2 OH any X nd X
Sulphur (molten) S 100%
Sulphuric Acid H2SO4
10%velocity
< 3 m/s
100/38 100/38 X
Sulphuric Acid H2SO4
25%velocity
< 3 m/s
75/24 100/38 X
Sulphuric Acid H2SO4
40%velocity
< 3 m/s
X 100/38 X
Sulphuric Acid H2SO4
55%velocity
< 3 m/s
X 86/30 X
Sulphurous Acid H2SO3 10% X X 175/80
Tannic Acid C76H52O46 95% 212/100 X 212/100
Tartaric Acid (CHOHCOOH) 2 50% 212/100 X 122/50
Terephthalic Acid C8H6O4 77%
Tetrachlorethane C2H2Cl4 100% X 160/71 160/71
Tetrachlorethylene
(Perchloroethylene)
Cl2C:CCl2 100% X 285/140 X
Thionyl Chloride SO2Cl2 100% X nd 104/40
Toluene C6H5CH3 100%
Trichlorethane C2H3Cl3 100% X X 77/25
Tricloroacetic Acid CCl3COOH 50% X 212/100 nd
Trisodium Phosphate (Na)3PO4 90% X
nd
Urea (NH2)2CO 100% 194/90 194/90 194/90
Zinc Chloride ZnCL2 75% X
212/100
Zinc Chloride ZnCL2 100% X
X
Zinc Sulphate ZnSO4 35% X 212/100 212/100
= Compatible
X = Not Compatible nd = No Data Available
Page 10

10
Corrosion Guide Rev 1.05 Jan 2006
Chem. Formula Fluid Concentration
Stainless Steel
Max oF/oC
Hastelloy C-22
Max oF/oC
Titanium
Max oF/oC
Air
Carbonic Acid Saturated X
Nickel Sulphamate 50%
(CH2 COOH)2 Succinic Acid 50% X X
(CH2)5(COOH)2 Adipic Acid 100% 50/10 200/93 428/220
(CHOHCOOH) 2 Tartaric Acid 50% 212/100 X 122/50
(COOH)2 Oxalic Acid 100% X 212/100 X
(Na)2O2 Sodium Peroxide 15% 212/100 X X
(Na)3PO4 Trisodium Phosphate 90% X
nd
(NH2)2 Hydrazine 100%
(NH2)2CO Urea 100% 194/90 194/90 194/90
(NH4)2CO3 Ammonium Carbonate 50% X X
(NH4)2SO4 Ammonium Sulphate 10% X 220/104 248/120
(NH4)2SO4 Ammonium Sulphate 100% X X 248/120
(NH4)3PO4 Ammonium Phosphate 10% X 140/60 248/120
(NH4)3PO4 Ammonium Phosphate 100% X 140/60 140/60
(NH4)C2O4 Ammonium Oxalate 10% X 75/24 75/24
(NH4)ClO3 Ammonium Chlorate 30% nd nd 122/50
AgNO3 Silver Nitrate 70% 85/30 85/30 212/70
Al2SO4 Aluminium Sulphate 6.50% X 285/140 220/105
Al2SO4 Aluminium Sulphate 40% X 285/140 220/105
Al2SO4 Aluminium Sulphate 100% X nd 200/93
AlCl3 Aluminium Chloride 10% X 220 /105 200/93
AlCl3 Aluminium Chloride 40% X 220 / 105 X
AlCl3 Aluminium Chloride 100% X 158/70 X
AlCl3 Aluminium Fluoride 20% X X X
AlNO3 Aluminium Nitrate 100% 302/150 X 208/98
Ar Argon 100%
Ba(NO3)2 Barium Nitrate 20% 150/65 X 77/25
Ba(OH)2 Barium Hydroxide 10% 225/107 225/107 77/25
BaCl2 Barium Chloride 25% X 158/70 122/50
BaCO3 Barium Carbonate 100% X X 77/25
BH3 Boric Acid 10% X 320/160 320/160
BH3 Boric Acid 100% nd nd 77/25
Br Bromine Liquid 100% X X X
C12H14O4 Diethyl Phthalate 100% 221/105 nd 221/105
C2H2Cl2 dry
Ethylene dichloride
(anhydrous)
100% X 221/105 221/105
C2H2Cl4 Tetrachlorethane 100% X 160/71 160/71
C2H3Cl3 Trichlorethane 100% X X 77/25
C2H4 Ethylene gas 100%
C2H40H Methanol + > 50ppm H20 99.99%
X
C2H40H Methanol pure 100%
X
2H4OH Methanol + > 2% H2O 98%
C2H5OH Ethyl Alcohol (Ethanol) 100% 221/105 221/105 221/105
C3H6OH Propanol (Propyl Alcohol) 100%
C3H6OH Seawater (Brine) 100% X
C5H10 Pentane 100% X
C5H8O2 Methyl Methacrylate 100%
C6H12 Heptane 100% 221/105 221/105 221/105
C6H5CH3 Toluene 100%
C6H5COOH Benzoic Acid 10% X 180/82 248/120
C6H5COOH Benzoic Acid 100% X 180/82 248/120
C6H5NH2xHCl Aniline hydro chloride 25% X nd 212/100
C6H5OH Phenol 95% 131/55
X
C6H6 Benzene 100% X X 240/116
C6H6NH2 Aniline 100% 509/265 248/120 230/110
= Compatible
X = Not Compatible nd = No Data Available
Page 11

11
Corrosion Guide Rev 1.05 Jan 2006
Chem. Formula Fluid Concentration
Stainless Steel
Max oF/oC
Hastelloy C-22
Max oF/oC
Titanium
Max oF/oC
C6H8O7 Citric Acid 25% X 221/105 212/100
C6H8O7 Citric Acid 60% X 221/105 X
C76H52O46 Tannic Acid 95% 212/100 X 212/100
C7H7Cl Benzyl Chloride 100% X X 140/60
C8H6O4 Terephthalic Acid 77%
Ca(Ocl)2 Calcium Hypochlorite 15% X 122/50 221/105
Ca(Ocl)2 Calcium Hypochlorite 95% X X 221/105
Ca(OH)2 Calcium Hydroxide 50% X 212/100 171/77
CaCl2 Calcium Chloride 75% X 221/105 221/105
CaCl2 Calcium Chloride 100% X 392/200 X
CaCl2+6H2O Calcium Hypochlorite 15% X 122/50 221/105
CaCl2+6H2O Calcium Hypochlorite 95% X X 221/105
CaCO3 Calcium Carbonate 100%
CaSO4 Calcium Sulphate 10% nd X nd
CaSO4 Calcium Sulphate 100% X nd 140/60
CCl3COOH Tricloroacetic Acid 50% X 212/100 nd
CCl4 dry
Carbon Tetrachloride
(anhydrous)
100% 140/60 140/60 248/120
CH2 Acetylene 100% 400/204 220/105 79/26
CH2(CH2)16COOH Stearic Acid 100%
212/100 X
CH2(COOH)2 Maleic Acid 100% X 176/80 248/120
CH2CICOOH Chloroacetic Acid 85% X X 221/105
CH2Cl2 Methylene Chloride 100% X X 239/115
CH2ClCOOH Monochloacetic Acid 100% nd nd
CH2O Formaldehyde 100% 221/105 X 221/105
CH3 COOC2H5 Ethyl Acetate 100% 320/160 320/160 221/105
CH3(CH2)2COOH Butyric Acid 100% X 260/127 225/107
CH3(CH2)2COOH N-Butyric Acid 100% X 212/100 212/100
CH3CH2COCH3 Methyl Ethyl Ketone 100% 200/93 200/93 200/93
CH3CHO Acetaldehyde 100% 200/93 200/93 300/149
CH3CHOHCOOH Lactic Acid 25% 122/50 122/50 122/50
CH3CO2CH3 Acetate 100% 400/204 400/204 400/204
CH3COCH3 Acetone 100% 400/204 140/60 200/93
CH3COCI Acetyl Chloride 100% 70/21 99/37 220/105
CH3COOH Acetic Acid 20% 400/204 300/149 260/127
CH3COOH Acetic Acid 50% 160/71 210/99 260/127
CH3COOH Acetic Acid 80% 160/71 210/99 260/127
CH3COOH Acetic Acid 95% X 200/93 210/99
CH3COOH Acetic Acid 100% X 200/93 200/93
CHCl2COOH Dichloroacetic Acid any nd nd X
CHCl3 Chloroform 100% 80/27 80/21 221/105
Cl2 + water Chlorine (aqueous) any X X X
Cl2 dry Chlorine (anhydrous) any X X X
Cl2C:CCl2
Tetrachlorethylene
(Perchloroethylene)
100% X 285/140 X
Cu(NO3)2 Copper Nitrate 100% 280/138 X 77/25
CuCl Cuprous Chloride 55% nd nd 194/90
CuCl2 Cupric Chloride any X X X
CuCN Cupric Cyanide 100% X X 77/25
CuSO4 Copper Sulphate 100% X X 221/105
F dry Fluorine (anhydrous) any X X X
Fe(OH)3 Ferric Hydroxide 6% 70/21 80/27 68/20
Fe(OH)3 Ferric Hydroxide 100% 70/21 80/27 nd
Fe2(SO4)3 Ferric Sulphate 15% 221/105 104/40 221/105
Fe2(SO4)3 Ferric Sulphate 35% X 104/40 221/105
Fe2(SO4)3 Ferric Sulphate 100% X X 221/105
= Compatible
X = Not Compatible nd = No Data Available
Page 12

12
Corrosion Guide Rev 1.05 Jan 2006
Chem. Formula Fluid Concentration
Stainless Steel
Max oF/oC
Hastelloy C-22
Max oF/oC
Titanium
Max oF/oC
FeCl3 Ferric Chloride 100% X X 248/120
FeSO4 Ferrous Sulphate 100% X X 248/120
H Hydrogen gas
H2O2 Hydrogen Peroxide 50% 194/90 194/90 X
H2O2 Hydrogen Peroxide 90% 118/48 118/48 X
H2SiF6 Fluosilicic Acid any X X X
H2SO3 Sulphurous Acid 10% X X 175/80
H2SO4 Sulphuric Acid
10%
velocity < 3 m/s
100/38 100/38 X
H2SO4 Sulphuric Acid
25%
velocity < 3 m/s
75/24 100/38 X
H2SO4 Sulphuric Acid
40%
velocity < 3 m/s
X 100/38 X
H2SO4 Sulphuric Acid
55%
velocity < 3 m/s
X 86/30 X
H3PO4 Phosphoric Acid 100% X X X
H3PO4 Phosphoric Acid 98% X 77/25 X
H3PO4 Phosphoric Acid 20% X 77/25 77/25
H3PO4 Phosphoric Acid 5% 77/25 77/25 77/25
H8CrO5 Chromic Acid 25% X 167/75 221/105
H8CrO5 Chromic Acid 50% X 75/24 221/105
H8CrO5 Chromic Acid 100% X X 221/105
HBF4 Fluoboric Acid 30% 80/27 200/93 X
HBO3 Hydrobromic Acid 100% X 140/60 X
HCISO3 Chlorosulphonic Acid 100% X 185/85 X
HCL Hydrogen Chloride gas any X X X
HCl / H2SO4 Aqua Regia 100% X X X
HCL + water Hydrochloric Acid any X X X
HClO4 Perchloric Acid any X 212/100 nd
HCOO4 Formic Acid (aerated) 50% X 221/105 221/105
HCOO4 Formic Acid (non-aerated) 85% X 221/105 X
HCOONa Sodium Formate 50% 131/55 131/55 nd
HF Hydrogen Fluoride gas any X X X
HF + water Hydrofluoric Acid any X X X
Hg(CN)2 Mercuric Cyanide 10% 95/35 X 95/35
HgCl2 Mercuric Chloride 10% X X 266/130
HNO3 Nitric Acid 100% “red fuming” 75/24 75/24 X
HNO3 Nitric Acid 75% 75/24 75/24 140/ 60
HNO3 Nitric Acid 70% 75/24 75/24 140/ 60
HNO3 Nitric Acid 50% 100/38 75/24 140/ 60
HNO3 Nitric Acid 40% 125/52 75/24 140/ 60
HNO3 Nitric Acid 30% 130/66 75/24 140/ 60
HNO3 Nitric Acid 20% 176/80 130/66 140/ 60
HNO3 + HF Mixture “Passivation” fluid X X X
I2 Iodine Liquid 100% X
77/25
K2SO4 Potassium Sulphate 25% 212/100 X 240/115
KBr Potassium Bromide 100% X X
KBr Potassium Bromide 30% X 95/35
KCI Potassium Chloride 99% X
KI Potassium Iodide 75% 212/100 212/100 212/100
KMnO4 Potassium Permanganate 100% X
KNO3 Potassium Nitrate 100% X 212/100 240/115
KOH Potassium Hydroxide 40% 170/77 170/77 170/77
LiCl Lithium Chloride 50% X 212/100 212/100
Mg(OH)2 Magnesium Hydroxide 100% 212/100 212/100 67/75
MgCl2 Magnesium Chloride 50% X 280/138 248/120
MgSO4 Magnesium Sulphate 50% nd 200/93 200/93
MnCl2 Manganese Chloride 45% X 212/100 212/100
Na2CO3 Sodium Carbonate 100% 212/100 212/100 212/100
= Compatible
X = Not Compatible nd = No Data Available
Page 13

13
Corrosion Guide Rev 1.05 Jan 2006
Chem. Formula Fluid Concentration
Stainless Steel
Max oF/oC
Hastelloy C-22
Max oF/oC
Titanium
Max oF/oC
Sodium Dichromate 100% X 80/27 77/25
Na2S Sodium Sulphide 50% X 212/100 X
Na2SiO3 Sodium Silicate 100% 212/100 212/100 212/100
Na2SO4 Sodium Sulphate 20% 212/100 212/100 X
NaCI Sodium Chloride 100% X 212/100 212/100
NaCIO3 Sodium Chlorate 100% X 302/150 302/150
NaCN Sodium Cyanide 50% 77/25 nd 266/130
NaHSO4 Sodium Bisulphate 20% X X 85/30
NaI Sodium Iodide 65% X nd 212/100
NaNO2 Sodium Nitrite 50% 212/100 212/100 266/130
NaNO3 Sodium Nitrate 60% 233/112 233/112 248/120
NaNO3 Sodium Nitrate 100% X X 248/120
NaOCl Sodium Hypochlorite any X X X
NaOH Sodium Hydroxide 45% X 0/40 0/55
NaOH Sodium Hydroxide 75% X 0/40 X
NaPO3
Mono Sodium
Orthophosphate in water
200 millimoles nd nd O86/30
NaPO4 Sodium Phosphate 15% X 212/100 212/100
NaSO3 Sodium Sulphite 25% 212/100 212/100 212/100
NaSO3 Sodium Sulphite 50% nd nd 212/100
NCN Hydrogen Cyanide 100% 88/31 88/31 88/31
NH2 SO2 OH Sulphamic Acid any X nd X
NH3 + water Ammonia (aqueous) 30% 158/70 158/70 X
NH3 + water Ammonia (aqueous) 50% 86/30 302/150 X
NH3 dry Ammonia (anhydrous) always 100% 248/120 248/120 77/25
NH4CH3COO Ammonium Acetate 15% 220/105 220/105 77/25
NH4CH3COO Ammonium Acetate 55% 170/77 nd nd
NH4Cl Ammonium Chloride 40% X 221/105 221/105
NH4ClO4 Ammonium Perchlorate 20% 170/77 X 285/140
NH4F Ammonium Fluoride 25% X 170/77 nd
NH4HCO3 Ammonium Bicarbonate 50% X nd 212/100
NH4NO3 Ammonium Nitrate 28% 280/137 80/27
NH4OH Ammonium Hydroxide 45% X X 170/77
Ni(NO3)2 Nickel Nitrate 50% X
77/25
NiCl2 Nickel Chloride 100% X 194/90 194/90
O2 Oxygen rich gas mixture > 35%
X
S Sulphur (molten) 100%
SnCl2 Stannic Chloride any X X X
SO2Cl2 Thionyl Chloride 100% X nd 104/40
ZnCL2 Zinc Chloride 75% X
212/100
ZnCL2 Zinc Chloride 100% X
X
ZnSO4 Zinc Sulphate 35% X 212/100 212/100
= Compatible
X = Not Compatible nd = No Data Available
If you do not find the required fluid in the table, please contact your nearest sales office.
Page 14

14
Corrosion Guide Rev 1.05 Jan 2006
Flow
Flange Bolt
Centralising
Sleeves
Gaskets
Transition
Piece
Pipe Work
Flow Meter
Flange Bolt
Typical Transition Piece Application
2. Abrasion Guidelines & information.
2.1 Introduction
For almost 10 years, the Krohne Corimass G Class
and Optimass 7000 series Coriolis mass flow meters
have been successfully used on abrasive fluids such as
slurries (mineral and metal mining) as wella as sand &
water mixtures.
In these applications, a single straight measuring tube
will always offer clear advantages over other designs
with tube geometries that suffer erosion and premature
failure of flow dividers and bends in the abrasive fluid
stream. Notwithstanding this, even a meter with a single straight tube will suffer some erosion unless simple
precautions are taken.
Other specific problems with abrasive fluids are typified
by their tendency to separate out with the heavier particles falling to the bottom of the pipeline and the carrier
fluid flowing above in a stratified flow.
The purpose of this application guidance is to highlight
potential problems so that they can be mitigated
against during the planning and installation of the
meter.
2.2 Protecting Flanges with Transition Pieces
Since the meter measuring tube will typically have a
different internal diameter than the process pipework, a
“step change” will occur where the flanges are connected. This edge presents a very obvious erosion point
and after a period, the weld between the flange raised
face and measuring tube could fail causing a leak path.
Transition pieces are stainless steel (although Hastelloy
can be used) discs that are sandwiched between the
two flanges, secured by through bolts and centred by
rubber sleeves around the bolts.
The taper on the internal diameter of the disc is manufactured to correct the difference between the meter
and process pipe, thus providing a gradual transition
for the abrasive fluid into the meter.
They must be considered as “sacrificial wear parts”,
and should be removed periodically for inspection of
the internal taper dimension and replacement if necessary.
Prices for these parts are available from Krohne.
2.3 Managing fluid velocity
There are two considerations here based on the fluid
flow rate and density:
Maximum velocity
To prevent excessive erosion this should be never be
more that 4 m/sec (12 ft/sec).
Minimum velocity
So the particles and carrier are homogeneous mixed
this should be at least 1 m/s (3 ft/sec).
The Optimass sizing software will assist you in calculating these limits. For abrasive slurries always size
according to these velocity limits, and not lowest measuring error which tends towards a smaller meter size
and hence a higher velocity.
2.4 Titanium as the standard material
Titanium should always be used as the standard material for measuring tubes. This is because size-for-size,
a titanium measuring tube has a greater wall thickness
than either stainless steel or Hastelloy. Simply put, this
tube has more material to erode before failure, so
extending the working lifetime of the meter.
Optimass Sizing Software Screen Shot
Page 15

15
Corrosion Guide Rev 1.05 Jan 2006
Meter
size
Material Outside dia.
Wall
Thickness
Internal
dia.
40
T 38.10 ± 0.13 0.91 ± 0.09 36.28
H 38.10 ± 0.1 0.71 ± 0.07 36.68
S 38.10 ± 0.13 0.71 ± 0.07 36.68
50
T 50.80 ± 0.15 1.24 ± 0.01 48.32
H 50.80 ± 0.15 1.00 ± 0.01 48.80
S 48.26 ± 0.13 1.00 ± 0.01 46.26
80
T 73.00 ± 0.254 2.10 ± 0.02 68.80
H 73.03 ± 0.254 1.04 ± 0.01 70.95
S 73.00 ± 0.13 1.40 ± 0.01 70.20
All dimensions in mm
It should be noted that the exception to this is when
stainless steel or Hastelloy are required for fluid compatibility (corrosion resistance).
2.5 Conditioning of the fluid flow profile
(as it enters the meter)
Firstly remember that a Coriolis mass flow meter directly measures mass flow and density of the fluid. It does
not measure velocity, so from a measuring principle
standpoint there is no need for flow profile conditioning.
However with an abrasive fluid, there is the requirement to condition the flow so that the abrasive particles
enter the meter parallel to the tube wall. This minimises
the probability that any given abrasive particle will
strike the tube wall and remove (erode) the tube material.
If the flow is “tumbling” or “swirling” as it enters the
meter, there is a risk that erosion will occur at the specific point where the particles preferentially impact onto
the tube wall, so causing premature failure. These
problems are always associated with using a pipework
bend or elbow very close to the meter inlet.
Therefore we recommend a straight length of inlet
process pipe equal to at least 10, or preferably 20 x
pipe internal diameters.
2.6 Installation of the flow meter
In order to keep the heavy abrasive particles evenly
dispersed in the carrier fluid as a homogenous mixture
that is required for correct meter operation, we would
recommend a vertical meter installation. Otherwise
there is the tendency, especially at lower flow velocities, for the fluid to separate out and become stratified.
Further, a flow direction vertically upwards is normally
preferred to ensure that meter is always full of liquid,
and does not “siphon” empty.
2.7 Inclusion of air or gas in the fluid
High density fluids such as mineral slurries typically
require a high drive energy due to their tendency
towards being inhomogeneously mixed.
Entrained air or gas will cause a further increase in the
required energy to drive (vibrate) the tube system. If
the inclusion is too great then the meter will not operate
correctly. This problem is particularly pronounced on
larger sized meters (T 50 & T 80), which typically are
used for abrasive fluid applications in order to reduce
the fluid velocity to acceptable levels.
So we would recommend that all efforts be made to
prevent the inclusion of air or gas in the fluid as part of
the process design, since from our experience once
entrained in the fluid it is virtually impossible to remove
prior to the metering point
2.8 Consider installing the meter in a by-pass
If the application is for density measurement only, then
often a more cost-effective solution is to install a smaller sized meter (although size 25 is smallest recommended) in a by-pass line off the main pipeline.
2.9 Conclusions
We are confident that if the seven steps above are followed correctly, then the probability of application related problems are greatly reduced and the operating lifetime of the meter greatly increased.
3. Environmental Protection
The materials exposed to the environment as standard
are:
MFM x050 Ex
MFM x051 Ex
MFM xo50 Ex
MFM x051 Ex
MFC 050 Ex
MFC 051 Ex
•
304L Stainless Steel for the sensor body with optional
316LStainless Steel for offshore
applications.
•
Electronics housing is die cast
aluminium, powder coated.
•
The aluminium has a low copper
content (see specification table).
All above housings will be powder coated in silver and
All covers (screw-on type) will be painted in blue RAL
5005
MFC 300C
MFC 300F
MFC 300W
•
304L Stainless Steel for the sensor body with optional
316LStainless Steel for offshore
applications.
•
Electronics housing in Stainless
Steel 316L.
Page 16

16
Corrosion Guide Rev 1.05 Jan 2006
Alloy type Si Fe Cu Mn Mg Cr Ni Zn Ti Pb
ADC 1
AlSi12Fe
11.0...13.0 1.3 1.0 0.3 0.3 - 0.5 0.5 - -
ADC 3
AlSi9Mg0,5Fe
9.0...10.0 3 1.0 0.3 0.4...0.6 - 0.5 0.5 - -
AlSi10Mg [Fe] 9.0...11.0 1.0 0.1 0.55 0.20...0.50 - 0.15 0.15 0.20 0.15
ALZn10Si8Mg 8.5...9.3 0.4 0.02 0.4 0.3...0.5 - - 9.0...10.0 0.1 -
For aggressive coastal or off shore based environments, KROHNE can provide an optional salt water resistant
coating. (see specifications below)
This is not in the standard price list. Please contact Product Management in Wellingborough, UK.
3.1 Housing Specifications
The MFC 050 and MFC 051 Range of Converters are available with KD(W) housings
KD Housing
This housing is prepared for long term applications in coastal and offshore environments.
KDW
The “W” suffix denotes the same specification as the standard KD housing but with the following additions:
•
Ingress protection IP 67
•
Special surface finish to:
•
Aluminium housing
•
Chromatized with (Henkel) Alodine 1200 S
•
Coated with Brillux 5910 Polyester powder
•
All outside threads have stainless steel inlays
•
Blue (RAL 5005) housing colour
•
1000 h spray test with sodium chloride solutions to:
•
DIN 50021:1988
•
ISO 1456:1974
•
ASTM B 117:1973
The MFC 300 Range of Converters are available with a SS 316L Housing
ADC 1
AlSi12Fe
ADC 3
AlSi9Mg0,5Fe
AlSi10Mg [Fe] ALZn10Si8Mg SS 316L
MFC050
MFC 051
MFC050 Ex
MFC051 Ex
MFC 300
MFC 300 Ex
Page 17

17
Corrosion Guide Rev 1.05 Jan 2006
Sources of information:
ATI Wah Chung, Albany Oregon, Technical Data Sheet: TitAly-052
Haynes International, Kokomo, Indiana, USA
Sandvick AB, SE-811 81 Sandviken, Sweden
Page 18

18
Corrosion Guide Rev 1.05 Jan 2006
Australia
KROHNE Australia Pty Ltd
Quantum Business Park
10/287 Victoria Rd
Rydalmere NSW 2116
TEL.: +61 2 8846 1700
FAX: +61 2 8846 1755
e-mail: krohne@krohne.com.au
Austria
KROHNE Austria Ges.m.b.H.
Modecenterstraße 14
A-1030 Wien
TEL.: +43(0)1/203 45 32
FAX: +43(0)1/203 47 78
e-mail: info@krohne.at
Belgium
KROHNE Belgium N.V.
Brusselstraat 320
B-1702 Groot Bijgaarden
TEL.: +32(0)2-4 66 00 10
FAX: +32(0)2-4 66 08 00
e-mail: krohne@krohne.be
Brazil
KROHNE Conaut
Controles Automaticos Ltda.
Estrada Louis Pasteur, 230 C.P. 56
06835 - 080 EMBU - SP
TEL.: +55(0)11-4785-2700
FAX: +55(0)11-4785-2768
e-mail: conaut@conaut.com.br
China
KROHNE Measurement Instruments
(Shanghai) Co. Ltd.
9th Floor, Xujiahui International Building
1033 Zhaojiabang Road
Shanghai 200030
P. R. China
TEL.: +86 21 6487 9611
FAX: +86 21 6438 7110
e-mail: info@krohne-asia.com
CIS
Kanex KROHNE Engineering AG
Business-Centre Planeta, Office 403
Marxistskaja-Street 3
109147 Moscow/Russia
TEL.: +7(0)095-9117 165
FAX: +7(0)095-9117 231
e-mail: krohne@dol.ru
Czech Republic
KROHNE CZ, spol. s r.o.
Sobìšická 156
63800 Brno
TEL.: +420 545 532 111
FAX: +420 545 220 093
e-mail: brno@krohne.cz
France
KROHNE S.A.S.
Les Ors
BP 98
F-26103 ROMANS Cedex
TEL.: +33(0)4-75 05 44 00
FAX: +33(0)4-75 05 00 48
e-mail: info@krohne.fr
Germany
KROHNE Messtechnik
GmbH & Co. KG
Ludwig-Krohne-Str.
D-47058 Duisburg
TEL.: +49(0)203-301-0
FAX: +49(0)203-301-10 389
e-mail: info@krohne.de
India
KROHNE Marshall Ltd.
A-34/35, M.I.D.C.
Industrial Area, H-Block,
Pimpri Poona 411018
TEL.: +91(0)202-7442020
FAX: +91(0)202-7442020
e-mail: pcu@vsnl.net
Iran
KROHNE Liaison Office
North Sohrevardi Ave.
26, Sarmad St., Apt. #9
Tehran 15539
TEL.: ++98-21-874-5973
FAX: ++98-21-850-1268
e-mail: krohne@krohneiran.com
Italy
KROHNE Italia Srl.
Via V. Monti 75
I-20145 Milano
TEL.: +39(0)2-4 30 06 61
FAX: +39(0)2-43 00 66 66
e-mail: info@krohne.it
Korea
KROHNE Korea
Room 508 Miwon Bldg
43 Yoido-Dong
Youngdeungpo-Ku
Seoul, Korea
TEL.: 00-82-2-780-1743
FAX: 00-82-2-780-1749
e-mail: krohnekorea@krohnekorea.com
Netherlands
KROHNE Altometer
Kerkeplaat 12
NL-3313 LC Dordrecht
TEL.: +31(0)78-6306300
FAX: +31(0)78-6306390
e-mail: postmaster@krohne-altometer.nl
Netherlands
KROHNE Nederland B.V.
Kerkeplaat 14
NL-3313 LC Dordrecht
TEL.: +31(0)78-6306200
FAX: +31(0)78-6306405
Service Direkt: +31(0)78-6306222
e-mail: info@krohne.nl
Norway
KROHNE Norway A.S.
Ekholtveien 114
NO-1526 Moss
P.O. Box 2178, NO-1521 Moss
TEL.: +47(0)69-264860
FAX: +47(0)69-267333
e-mail: postmaster@krohne.no
Internet: www.krohne.no
Singapore
Tokyo Keiso - KROHNE Singapore Pte. Ltd.
14, International Business Park,
Jurong East
Chiyoda Building #01-01/02
Singapore 609922
Singapore
TEL.: ++65-65-67-4548
FAX: ++65-65-67-9874
South Africa
KROHNE Pty. Ltd.
163 New Road
Halfway House Ext. 13
Midrand
TEL.: +27(0)11-315-2685
FAX: +27(0)11-805-0531
e-mail: midrand@krohne.co.za
Spain
I.I. KROHNE Iberia, S.r.L.
Poligono Industrial Nilo
Calle Brasil, n°. 5
E-28806 Alcalá de Henares-Madrid
TEL.: +34(0)91-8 83 21 52
FAX: +34(0)91-8 83 48 54
e-mail: krohne@krohne.es
Switzerland
KROHNE AG
Uferstr. 90
CH-4019 Basel
TEL.: +41(0)61-638 30 30
FAX: +41(0)61-638 30 40
e-mail: info@krohne.ch
United Kingdom
KROHNE Ltd.
Rutherford Drive
Park Farm Industrial Estate
Wellingborough,
Northants NN8 6AE, UK
TEL.: +44(0)19 33-408 500
FAX: +44(0)19 33-408 501
e-mail: info@krohne.co.uk
USA
KROHNE Inc.
7 Dearborn Road
Peabody, MA 01960
TEL.: +1-978 535-6060
FAX: +1-978 535 -1720
e-mail: info@krohne.com
Other Representatives
Algeria
Argentina
Belarus
Cameroon
Canada
Chile
Colombia
Croatia
Denmark
Ecuador
Egypt
Finland
Gabon
Ghana
Greece
Hong Kong
Hungary
Indonesia
Ivory Coast
Iran
Ireland
Israel
Japan
Jordan
Other Countries
KROHNE Messtechnik
GmbH & Co. KG
Ludwig-Krohne-Str.
D-47058 Duisburg
TEL.: +49(0)203-301-0
FAX: +49(0)203-301-389
e-mail: export@krohne.de
Kuwait
Libya
Lithuania
Malaysia
Morocco
Mauritius
Mexico
New Zealand
Peru
Poland
Portugal
Romania
Saudi Arabia
Senegal
Slovakia
Slovenia
Sweden
Taiwan
Thailand
Turkey
Tunisia
Venezuela
Yugoslavia
Subject to change without notice
 Loading...
Loading...