Page 1

................................................................................................................................
Macro-Prep®t-Butyl and Methyl
Hydrophobic Interaction
Chromatography Media
Instruction Manual
Please read these instructions prior to using Macro-Prep hydrophobic
interaction chromatography media. If you have any questions or
comments regarding these instructions, please contact your local
Bio-Rad Laboratories representative.
Page 2

Table of Contents
Section 1 Introduction ......................................................1
Section 2 Intended Use ....................................................1
Section 3 Media Screening...............................................1
Section 4 Technical Specifications
and Characteristics ..........................................2
Section 5 Preparation for Use ..........................................3
Section 6 Column Packing................................................3
Section 7 Column Packing Evaluation..............................5
Section 8 Operation and Maintenance .............................7
Section 9 Ordering Information ......................................10
Page 3

Section 1
Introduction
All Bio-Rad products are manufactured under an externally approved ISO 9001
quality system. Bio-Rad Laboratories guarantees product quality and performance
of unopened product for 1 year from date of shipment. Regulatory support files
are available for Macro-Prep hydrophobic interaction chromatography (HIC) media.
If you need assistance validating the use of Macro-Prep media in a production
process, contact your local Bio-Rad Process Chromatography representative.
Section 2
Intended Use
Macro-Prep HIC media are designed specifically for intermediate purification steps
that remove host-cell contaminants from partially purified targets. Due to their
rigidity and unique surface chemistry, these media are particularly suited for HIC
operations requiring high throughput and high recovery of target.
Both Macro-Prep t-butyl and Macro-Prep methyl HIC media operate on a
mechanism of interaction that is based on hydrophobicity and charge.
The t-butyl and methyl groups are mildly hydrophobic. Depending on the chosen
pH of loading and elution buffers, the carboxyl groups can be exploited to ionically
repel target molecules while the hydrophobic groups retain contaminants. This is
an ideal strategy to minimize product losses commonly experienced with HIC
media due to denaturation of proteins when exposed to hydrophobic surfaces.
Section 3
Media Screening
For ease of screening of Bio-Rad HIC media, both forms of Macro-Prep HIC
media are provided as prepacked 1 ml and 5 ml cartridges. In addition, these
media are part of the media sampler pack, which also includes ion exchangers
and ceramic hydroxyapatite media. See Ordering Information in Section 9 at the
end of this instruction manual.
With Macro-Prep HIC media it is important to test both salt and pH conditions.
Since HIC is usually used after an affinity or ion exchange step, it may be
necessary to adjust the salt concentration and/or the pH of the sample load to
obtain optimal purification and recovery with Macro-Prep HIC media.
1
Page 4
2
Section 4
Technical Specifications and Characteristics
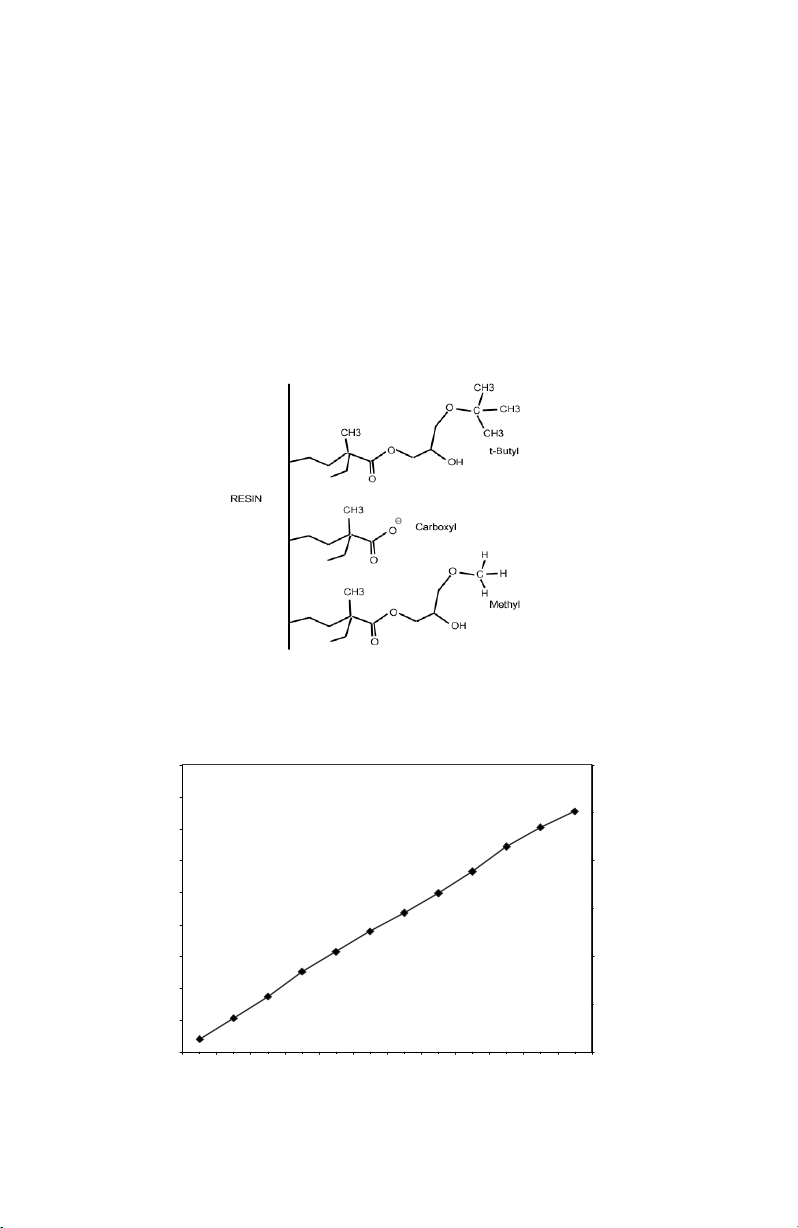
Macro-Prep t-butyl and Macro-Prep methyl media are based on rigid, epoxyactivated methacrylate beads derivatized with t-butyl or methyl groups,
respectively, and carboxyl groups (Figure 1). These products are shipped as a
slurry of 50% settled media in 20% ethanol. Table 1 lists the properties of the two
media. The rigidity of these media allows high flow rates without bed compression
(Figure 2). Testing in small laboratory-scale columns (10 mm in diameter x 100 mm
in height) showed no bed compression at a pressure of 7 bar and a flow rate of 40
ml/min (3,000 cm/hr). This property of the media provides for high throughput
during loading, column washing, equilibration, and cleaning.
0
5
10
15
20
25
30
35
40
45
37 75 114 159 195 233 271 313 359 409 458 487
Flow rate (cm/hr)
Pressure (psi)
0
1
2
3
Pressu re (bar)
Fig. 2. Pressure vs. flow for Macro-Prep media. Media bed dimensions were
140 mm diameter by 250 mm length.
Fig. 1. Macro-Prep HIC: Macro-Prep t-butyl contains t-butyl and carboxyl groups;
Macro-Prep methyl contains methyl and carboxyl groups.
Page 5

Table 1. Characteristics of Macro-Prep HIC Media
Functional groups COO- and t-butyl COO- and methyl
Ionic capacity Approximately 120 µeq/ml Approximately 120 µeq/ml
Shipping counterion Cl
Hydrophobicity Mild Mild
Median particle size (µm) 50 50
pH stability 1–10 1–10
Autoclavability (121°C, 30 min) Yes Yes
Shipping solution 20% ethanol 20% ethanol
Working flow-rate range 100–600 cm/hr 100–600 cm/hr
Sanitization 5 CV of 6 M guanidine-HCl, 5 CV of 6 M guanidine-HCl,
Storage at 4–40°C 20% ethanol 20% ethanol
Macro-Prep t-Butyl Macro-Prep Methyl
-
100 cm/hr 100 cm/hr
or or
5 CV of of 1% acetic acid 5 CV of 1% acetic acid
in 0.12 M phosphoric acid, in 0.12 M phosphoric acid,
pH 1.5, 100 cm/hr pH 1.5, 100 cm/hr
or or
1% acetic acid in 0.12 M 1% acetic acid in 0.12 M
phosphoric acid, pH 1.5 phosphoric acid, pH 1.5
-
Cl
Section 5
Preparation for Use
Macro-Prep HIC media are supplied fully hydrated in 20% ethanol as a 50% slurry.
Small volumes of Macro-Prep HIC media are easily washed in a Büchner funnel
with 4–5 column volumes (CV) of a low ionic strength buffer (<20 mM). For large
process-scale volumes, pack the column in a low ionic strength buffer (<20 mM)
or water and then rinse with at least 3 CV of the low ionic strength buffer or water
before transferring to the high-salt buffer. Alternatively, decant the ethanol shipping
solution and resuspend/decant 3–4 times in the low ionic strength buffer to be
used for column packing.
Section 6
Column Packing
Columns can be packed in different ways depending on the media and type of
column and equipment to be used. Macro-Prep HIC media can be packed using
pressure, flow, or suction packing methods. To pack highly efficient columns, it is
recommended to use a 50% slurry. Some general guidelines for packing small and
large columns are given below. Make sure to read and follow the instructions for
the column to be used. If unsure about a particular column, call your local Bio-Rad
Laboratories representative. Throughout this instruction, flow rate is expressed
linearly in centimeters per hour (cm/hr). The relationship between linear and
volumetric flow rate as delivered by a pump is:
Linear flow rate (cm/hr) X Cross-sectional area of the column (cm2) = Volumetric flow rate
Example: 300 cm/hr in a 14 cm diameter column = 300 cm/hr X πr2or 300 cm/hr X
(3.14 X 72) cm2or 46,158 cm3/hr or 769.3 ml/min.
3
Page 6

Packing Small Columns
The following slurry packing method was designed to pack 25 ml of Macro-Prep
HIC media into 5–15 mm ID, low- to medium-pressure lab columns. All buffers
should be degassed prior to packing. Use a packing reservoir that holds ~100 ml.
1. Prepare degassed buffer or water to be used as the packing solution.
2. Macro-Prep is shipped as a 50% slurry, so measure 50 ml of suspended
slurry into a 100 ml graduated cylinder. Allow the media to settle. Decant the
shipping solution from the settled media.
3. Add 25–50 ml of degassed packing buffer to the media.
4. Seal the cylinder and rotate it to suspend the media. Caution: Do not mix with
a magnetic stir bar, to avoid formation of small fines. Larger volumes of slurry
may be mixed with an overhead stirrer at low to moderate speed.
5. Fill the column with approximately 2 cm of packing buffer. Pour all of the
media slurry into the column with the packing reservoir attached.
6. Insert the column flow adaptor and start flow at ~100 cm/hr. Gradually
increase the flow rate from 100 cm/hr to the flow rate at which the maximum
allowable pressure for the column is reached, or to the flow rate at which the
maximum pressure for Macro-Prep is reached (7 bar or 102 psi). As the bed
compresses with increasing flow rate, stop the flow and adjust the flow
adaptor so it penetrates 1–2 mm into the media bed. Repeat the flow and
adjustment cycle until the bed no longer compresses.
7. Attach the column to your chromatography system and purge with starting
buffer at the maximum flow rate to be used during operation. You should not
see any further bed compression. If you do, adjust the flow adaptor 1–2 mm
into the media bed.
8. The column is now ready for use.
Note: For optimal long-term use, do not exceed 75% of the maximum
pressure attained during the column packing.
Packing Large Columns
In large columns, Macro-Prep should be packed using a 50% slurry at constant
pressure up to a maximum of 7 bar (102 psi). Pack with a constant flow rate that
is approximately 25% greater than the anticipated maximum to be used during
operation. Given the variety of industrial column hardware and packing skids, we
recommend use of standard operating procedures for packing provided by the
column vendor.
Bio-Rad EasyPack
™
and GelTec™columns are supplied with a movable piston.
They are packed by pumping the packing solution through the media bed at
constant pressure or flow.
4
Page 7

1. Packing begins with preparation of the tubing and the column. Clean and
rinse the column well with packing buffer before packing. The rinse is also
used to displace air from the column and its exit tubing. Make sure there is no
air trapped in the bottom flow cell. After removal of all the air from the bottom
flow cell and exit tubing, leave about 2 cm of liquid in the bottom of the
column. Close the outlet valve(s).
2. In a suitable container, mix the packing buffer with the media to form a 50%
slurry.
3. Pour or pump the slurry into the column.
4. Insert the movable piston and lower it to the surface of the liquid, making sure
no air is trapped under the piston.
5. Pressurize the inflatable seal to 2 bar (29 psi), so when lowering the piston
into the slurry, the buffer exits the top of the column through the inlet line. Be
sure the inlet line is completely filled with packing buffer to avoid air being
pumped back into the column.
6. Connect a pump and a pressure gauge, open all inlet and outlet valves, and
start packing at constant flow rate or pressure. Keep the flow rate or pressure
constant throughout the packing. Check the pressure at the column inlet. Do
not exceed the pressure limit of column or media.
7. Lower the piston as the bed compresses.
8. When the media bed no longer compresses, mark the bed height on the
column tube, close the outlet valve, and stop the pump. The bed will start to
expand in the column.
9. Lower the piston to within 1 cm of the surface of the media bed. Fully inflate
the seal (4 bar), start the pump, open the valves, and continue packing.
10. Repeat steps 8 and 9 until there is a maximum of 1 cm between media bed
surface and piston when the media bed is stable.
11. Close the bottom valve, stop the pump, disconnect the column inlet, and
lower the piston to the gel bed surface. The column is ready for testing of
column packing efficiency.
Section 7
Column Packing Evaluation
To check the packing efficiency and to monitor the column’s status over time,
packing efficiency and asymmetry should be tested immediately after packing and
at regular intervals to ensure reproducible performance. The values to be
determined for the packed column are the height equivalent theoretical plate
(HETP), number of theoretical plates (N), and the asymmetry factor (A
method used involves applying a test sample of a low molecular weight substance
5
). The
s
Page 8

that has no interaction with the media, e.g., 2 M NaCl. When using salt as the test
a b 10% of peak
Wh, width at half height
Bed height = 20 cm
Column Volume = 20 L
Flow rate = 100 cm/h
Ve = 10 L
Wh = 1 L
HETP = 0.036 cm
a = 1
b = 1.2
As = 1.2
substance, use a concentration of 2 M NaCl in water with 0.5 M NaCl in water as
the elution buffer. Concentrated buffer solutions, e.g., 10x buffer concentrate, is
also used. HETP varies depending on the test conditions. Since it is used as a
reference value, it is important to keep conditions and equipment constant so
results can be compared over time. Changes in solute, solvent, elution buffer,
sample volume, flow rate, flow path, temperature, etc. influence results. For
optimal results, ensure that the sample volume does not exceed 2.5% of the column volume, and maintain the flow rate between 75 and 150 cm/hr. If an acceptance limit is defined in relation to column performance, the column HETP and/or
calculated plate number, N, can be used as acceptance criteria for column use.
If a UV absorbing substance is used as the test sample, use a UV absorbance
monitor set at 280 nm. If 2 M NaCl is the probe, use a conductivity monitor; the
elution buffer should be 500 mM NaCl. Apply the sample as close to the column
inlet as possible to avoid dilution in the tubing and piping leading up to the column
inlet. Calculate HETP and the number of theoretical plates (N) as follows:
HETP = L/N
N = 5.54(V
e/W½h
L = Bed height (cm)
N = Number of theoretical plates
V
= Peak elution volume or time
e
W
= Peak width at peak’s half height in volume or time
½h
V
and W½hshould always be the same units
e
Bed height = 20 cm
Column volume = 20 L
Flow rate = 100 cm/h
= 10 L
V
e
W
½h
HETP = 0.036 cm
a = 1
b = 1.2
As= 1.2
Fig. 3. A simulated chromatography profile from which HETP and Asvalues are calculated.
)
= 1 L
2
V
e
W
width
½h
at half height
10% of peak
b
a
6
Page 9

7
Peaks should be symmetrical. The best achievable asymmetry factor is 1. Values
of 0.8 to 1.8 are excellent for large columns >30 cm in diameter. If functional
performance is acceptable, A
s
factors more distant from 1 put less demand on
the column packing procedure in production. A change in the shape of the peak is
usually the first indication of deteriorating performance. Peak asymmetry factor
calculation:
A
s
= b/a
a = Front section of peak width at 10% of peak height bisected by line denoting V
e
.
b = Latter section of peak width at 10% of peak height bisected by line denoting V
e
.
Section 8
Operation and Maintenance
Macro-Prep media were designed to remove trace contaminants while operating
at high throughput. Macro-Prep media should be run at the highest linear
velocities and loading capacities while staying within the pressure limits of the
column and chromatography system. For flow-through mode, a linear flow rate of
300 cm/hr in a 20 cm bed is a reasonable starting point. If the product is being
retained on the Macro-Prep, flow rates of 100–200 cm/hr are recommended.
Optimization can be achieved by changing the pH, the ionic strength, or additives
in the elution buffer.
All buffers commonly used for cation exchange chromatography can be used with
Macro-Prep HIC media (see Table 2).
Table 2. Common Buffers for Macro-Prep HIC.
Buffer Useful pH Range
Citric acid 2.6–3.6
Lactic acid 3.6–4.3
Formic acid 3.8–4.3
Acetic acid 4.8–5.2
MES 5.5–6.7
PIPES 6.1–7.5
MOPS 6.5–7.9
Phosphate 6.7–7.6
Bicine 8.2–8.7
HEPES 6.8–8.2
TES 6.8–8.2
Tricine 7.4–8.8
Column Equilibration
Higher salt concentrations are used to enhance hydrophobic interactions.
Normally, a column is equilibrated in a high-salt buffer, and the sample is loaded
onto the column at the same pH and salt concentration. At small scale, the
following salts are commonly used: ammonium sulfate (<pH 8), 1 M solutions of
sodium sulfate, sodium chloride, potassium chloride, or sodium citrate. At larger
scale, sodium citrate is a very practical salt. This salt can function as both a buffer
and a salt during HIC. Lower molar quantities are required to reach specific ionic
concentrations and the somewhat hydrophobic nature of the citrate ion often
Page 10

minimizes product loss due to overly strong product interaction with the media. If
the target molecule is in the flow-through, sodium citrate is often used at
concentrations of 500–600 mM for loading.
Sample Preparation
Adjust salt concentration and pH as necessary for desired binding of target or
contaminants. This is best done by mixing the correct amount of liquid
concentrate of salt into the sample load and then adjusting the pH.
Sample Load and Adsorption
The sample load is determined empirically by loading and evaluating breakthrough
of target or contaminants. Since HIC is an adsorption technique, the sample
volume is not a critical factor. Large volumes of very dilute feed such as cell
culture supernatant and clarified lysates can be loaded onto the media without
prior concentration. If the salt concentration of the sample load is lower than what
is used to equilibrate the column before loading, the protein of interest can show
low binding capacities, or can begin to elute from the column with unwanted
contaminants.
Wash through
After loading of the column, follow with 2 to 3 CV of the high ionic strength buffer.
This will wash out any unbound materials.
Elution
Elute target molecules with either a step or linear gradient. Usually, the salt
concentration at which the desired product binds is predetermined at small scale.
With this knowledge, the pH and salt concentration used in wash-through are
adjusted to eliminate the maximum amount of contamination before starting
elution of the target.
Regeneration
After each run, the packed bed should be washed with 3–5 CV of a low ionic
strength buffer such as 20 mM sodium citrate or 20 mM HEPES. This is followed
with 3–5 CV of water. If contamination remains, wash with 2–3 CV of 0.12 M
phosphoric acid in 1% acetic acid, pH 1.5. For very difficult cleaning, follow the
acid wash with a combination of nonionic detergents and then 30–70% ethanol.
Cleaning-in-Place (CIP)
If a column no longer yields reproducible results, the media may require a
thorough CIP to remove strongly bound contaminants. Acceptable CIP agents
include 25% acetic acid, 8 M urea, 1% Triton X-100, 70% ethanol, 30%
isopropyl alcohol, 1 N HCl, and 6 M guanidine-HCl. Cleaning with NaOH is not
recommended because the t-butyl and methyl groups are cleaved from the media
under strongly basic conditions. If it is absolutely necessary to use NaOH, use
0.1 N for 1 hr, and then wash immediately with a low pH, high-salt buffer such as
600 mM sodium citrate, pH 5.
8
Page 11

9
Sanitization and Long Term Storage
Sanitization is the reduction of bioburden, i.e. microorganisms and spores in the
column. Before long-term storage, Macro-Prep HIC media should be cleaned and
sanitized.
Procedure:
Cleaning: After elution, clean the column with 8 CV of 1% acetic acid in 0.12 M
phosphoric acid, pH 1.5, <100 cm/hr. If not satisfactorily cleaned, continue to
wash with 5 CV of 6 M guanidine-HCl, <100 cm/hr. If still not clean, wash the
column with 5 CV of 20% ethanol, <100 cm/hr.
To sanitize and store: Wash the column with 5 CV of 1% acetic acid in 0.12 M
phosphoric acid, pH 1.5. Store the column at 4–40°C.
Page 12

Section 9
Ordering Information
Macro-Prep HIC media are available in a variety of package sizes, as prepacked
Econo-Pac
media sampler pack.
Macro-Prep Methyl Products
Catalog # Description
158-0080 Macro-Prep Methyl HIC Support, 25 ml
156-0080 Macro-Prep Methyl HIC Support, 100 ml
156-0081 Macro-Prep Methyl HIC Support, 500 ml
156-0082 Macro-Prep Methyl HIC Support, 5 L
156-0083 Macro-Prep Methyl HIC Support, 10 L
732-0051 Macro-Prep Methyl HIC Support, 1 x 5 ml Econo-Pac cartridge
732-0053 Macro-Prep Methyl HIC Support, 5 x 1 ml Econo-Pac cartridges
732-0055 Macro-Prep Methyl HIC Support, 5 x 5 ml Econo-Pac cartridges
158-0100 Media Sampler Pack, contains one 25 ml bottle each of
Macro-Prep t-Butyl Products
Catalog # Description
158-0090 Macro-Prep t-Butyl HIC Support, 25 ml
156-0090 Macro-Prep t-Butyl HIC Support, 100 ml
156-0091 Macro-Prep t-Butyl HIC Support, 500 ml
156-0092 Macro-Prep t-Butyl HIC Support, 5 L
156-0093 Macro-Prep t-Butyl HIC Support, 10 L
732-0056 Macro-Prep t-Butyl HIC Support, 1 x 5 ml Econo-Pac cartridge
732-0057 Macro-Prep t-Butyl HIC Support, 5 x 5 ml Econo-Pac cartridges
732-0058 Macro-Prep t-Butyl HIC Support, 5 x 1 ml Econo-Pac cartridges
158-0100 Media Sampler Pack, as described above
®
cartridges for easy testing and small-scale purifications, and in the
UNOsphere
™
Q and S ion exchangers, Macro-Prep methyl and
t-butyl media, and Macro-Prep High Q, High S, DEAE, and CM
ion exchangers, one 10 g bottle each of CHT
™
ceramic
hydroxyapatite types I and II
Ask your representative for pricing details, larger volumes, and special packaging.
Triton is a trademark of Union Carbide Chemicals & Plastics Technology Corporation.
10
Page 13

Bio-Rad Laboratories, Inc.
2000 Alfred Nobel Dr., Hercules, CA 94547 USA
510-741-1000
LIT486 Rev C
 Loading...
Loading...