Page 1

For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723)
Helios Gene Gun
System
Instruction
Manual
Catalog Numbers
165-2431 and 165-2432
Page 2

Warranty and Regulatory Notices
Warranty Statement
This warranty may vary outside of the continental United States. Contact your local Bio-Rad
office for the exact terms of your warranty.
Bio-Rad Laboratories warrants to the customer that the Helios Gene Gun System (catalog
number 165-2431 and 165-2432) will be free from defects in material and workmanship, and will
meet all performance specifications for the period of one year from the date of shipment. This
warranty covers all parts and labor.
In the event that the instrument must be returned to the factory for repair under warranty,
the instrument must be packed for return in the original packaging.
Bio-Rad shall not be liable for any incidental, special or consequential loss, damage, or
expense directly or indirectly arising from the use of the Helios Gene Gun System. Bio-Rad
makes no warranty whatsoever in regard to products or parts furnished by third parties, such
being subject to the warranty of their respective manufacturers. Service under this warranty
shall be requested by contacting your nearest Bio-Rad office.
The following items are considered customer-installable consumables: tubing, desiccant
pellets, and microcarriers. The battery, razor blade, O-rings, barrel liner, cartridge holders, and
syringe are replacement parts (see Section 9.2). These consumables and replacement parts are
not covered by this warranty and are warranted only to be free from defects in workmanship.
This warranty does not extend to any instruments or parts that have been subject to misuse, neglect, or accident, or that have been modified by anyone other than Bio-Rad, or that have
been used in violation of Bio-Rad instructions.
The foregoing obligations are in lieu of all other obligations and liabilities including negligence and all warranties, of merchantability, fitness for a particular purpose or otherwise,
expressed or implied in fact or by law, and state Bio-Rad’s entire and exclusive liability and
buyer’s exclusive remedy for any claims or damages in connection with the furnishing of goods
or parts, their design, suitability for use, installation or operation. Bio-Rad will in no event be
liable for any special, incidental or consequential damages whatsoever, and Bio-Rad’s liability
under no circumstances will exceed the contract price for the goods for which liability is claimed.
Bio-Rad is not responsible for any injury caused by the use of this instrument for purposes other
than those for which it is intended.
Regulatory Notices
Important: This Bio-Rad instrument is designed and certified to meet EN55011,
EN50082-1, and EN61010 requirements, which are internationally accepted electromagnetic compliance and electrical safety standards. Certified products are safe to use when operated
in accordance with the instruction manual. This instrument should not be modified or altered
in any way. Alteration of this instrument will result in the following:
Void the manufacturer’s warranty.
Void the regulatory certifications.
Create a potential safety hazard.
Page 3

Note: This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to Part 15 of the FCC rules. These limits are designed to provide reasonable protection against harmful interference when the equipment is operated in a
commercial environment. This equipment generates, uses, and can radiate radio frequency
energy and, if not installed and used in accordance with the instruction manual, may cause
harmful interference to radio communications. Operation of this equipment in a residential area
is likely to cause harmful interference in which case the user will be required to correct the
interference at his own expense.
Patent License and Usage
Particle bombardment technology is covered by several patents which are held by
E. I. duPont de Nemours & Co. and Auragen, Inc. Particle bombardment may be used for
research purposes for gene delivery. Use of particle bombardment for commercial purposes
requires a commercial license from the appropriate patent holder. The Helios Gene Gun is
designed for research purposes only and is not intended for human or veterinary use. Licensed
only for research use.
Page 4

Table of Contents
Page
Section 1 General Safety Information....................................................................1
1.1 Helios Gene Gun Safety........................................................................................1
1.2 Pressurized Helium and Nitrogen Safety..............................................................1
1.3 Power Safety..........................................................................................................1
1.4 Ear and Eye Protection..........................................................................................2
Section 2 Introduction to Particle Delivery...........................................................2
2.1 Particle Delivery Technology ...............................................................................2
2.2 The Helios Gene Gun............................................................................................3
2.3 Operating Principle of the Helios Gene Gun System...........................................3
2.4 Requirements for System Operation.....................................................................4
Section 3 Product Description ................................................................................7
3.1 Packing List...........................................................................................................7
3.2 Identification of System Components and Controls.............................................8
Section 4 Setting up the Helios Gene Gun System.............................................12
4.1 Inserting the Battery into the Helios Gene Gun .................................................12
4.2 Connecting the Helios Gene Gun to a Helium Source.......................................13
4.3 Connecting the Tubing Prep Unit to a Nitrogen Source ....................................14
Section 5 Operation of the Helios Gene Gun System.........................................17
5.1 Quick Guide to Operation...................................................................................17
5.2 Preparation of System Components Prior to Bombardment..............................18
5.3 Particle delivery using the Helios Gene Gun......................................................23
5.4 Removing Used Cartridges, Depressurization, and Shut Down........................28
Section 6 Preparation of Mammalian Cell Targets............................................29
6.1 In vitro Delivery to Adherent Cells ....................................................................29
6.2 In vitro Delivery to Suspension Cultures............................................................30
6.3 in vivo Delivery to Epidermis .............................................................................31
Section 7 Optimization of Gene Gun Parameters..............................................32
7.1 Overview .............................................................................................................32
7.2 Parameters for in vitro Delivery..........................................................................34
7.3 Parameters for in vivo Delivery ..........................................................................35
Section 8 Troubleshooting.....................................................................................35
8.1 DNA/Microcarrier Preparation...........................................................................35
8.2 Cartridge Preparation ..........................................................................................35
8.3 Helios Gene Gun Operation................................................................................36
8.4 In Vitro and in Vivo Targeting ............................................................................36
Section 9 Product Information.............................................................................37
9.1 Helios Gene Gun System....................................................................................37
9.2 Spare Parts...........................................................................................................38
9.3 Specifications ......................................................................................................38
Section 10 Appendices .............................................................................................39
10.1 Precipitation of RNA onto Microcarriers ...........................................................39
10.2 Replacing the O-rings and Inner Sleeve on the Helios Gene Gun.....................40
10.3 Replacing the O-ring on the Tubing Prep Unit...................................................41
10.4 Replacing Tubing Cutter Razor Blade and Unit Disassembly...........................42
10.5 Cleaning and Sterilizing the Helios Gene Gun...................................................43
10.6 Testing Cartridges for Microcarrier Penetration and Density............................43
10.7 Quantitation of DNA in Cartridges.....................................................................45
10.8 References ...........................................................................................................46
10.9 Quick Guide ........................................................................................................47
Page 5
Section 1
General Safety Information
Caution: In particle bombardment DNA-coated microparticles are accelerated to
velocities in excess of 1,000 ft/sec in order to penetrate the cell membrane and through multiple layers of cells in tissues and organs. In the Helios Gene Gun, this accelerating force is
supplied by a high pressure helium pulse. Numerous safety features have been designed into this
instrument to protect both the user and bystanders. The parts used in manufacturing the Helios
Gene Gun have been chosen because they are designed to work at the pressures required for operation and have a wide safety margin. General safety principles are indicated below. Specific
safety recommendations are indicated in the appropriate sections throughout the manual.
1.1 Helios Gene Gun Safety
Caution: While the Helios Gene Gun has a trigger button which is time-activated by
a safety interlock switch, accidental or unintentional discharge is still possible. Do not point
the gun at people. The Helios Gene Gun is for research use only.
Refer to Section 4.2 for connecting the Helios Gene Gun to a helium source, to Section 5.3
for use of the Gene Gun, and to Section 5.4 for depressurization and shut down of the Gene Gun.
1.2 Pressurized Helium and Nitrogen Safety
Caution: Although helium and nitrogen are neither toxic nor flammable, all gases
under pressure are potentially dangerous if used improperly. Always be sure pressurized tanks
are properly secured. This may be accomplished by placing the tank in a floor stand or by using
a wall-mounted or bench-mounted strap. Please follow the instructions provided with the
helium cylinder from the supplier and those that are applicable for your institution (see your
Site Safety Officer). Bio-Rad has supplied tubing, fittings, a control valve, and a pressure
regulator capable of safely handling the high pressure helium gas used in the Helios Gene
Gun. These components have been carefully selected and are the only parts to be used with
the Helios Gene Gun System.
Refer to Section 2.4 for a description of the helium and nitrogen gases required for the
Helios Gene Gun System.
1.3 Power Safety
Figure 1 shows the serial number certification label which is found underneath the
molded case of the Helios Gene Gun. This label provides the manufacturing data about the
instrument. This instrument is operational using a standard 9 volt battery. Change the battery only after detaching the Gene Gun from the helium hose.
Refer to Section 2.4 for a description of the battery required for the Gene Gun and to
Section 4.1 for information on replacing the battery in the Gene Gun.
1
Page 6
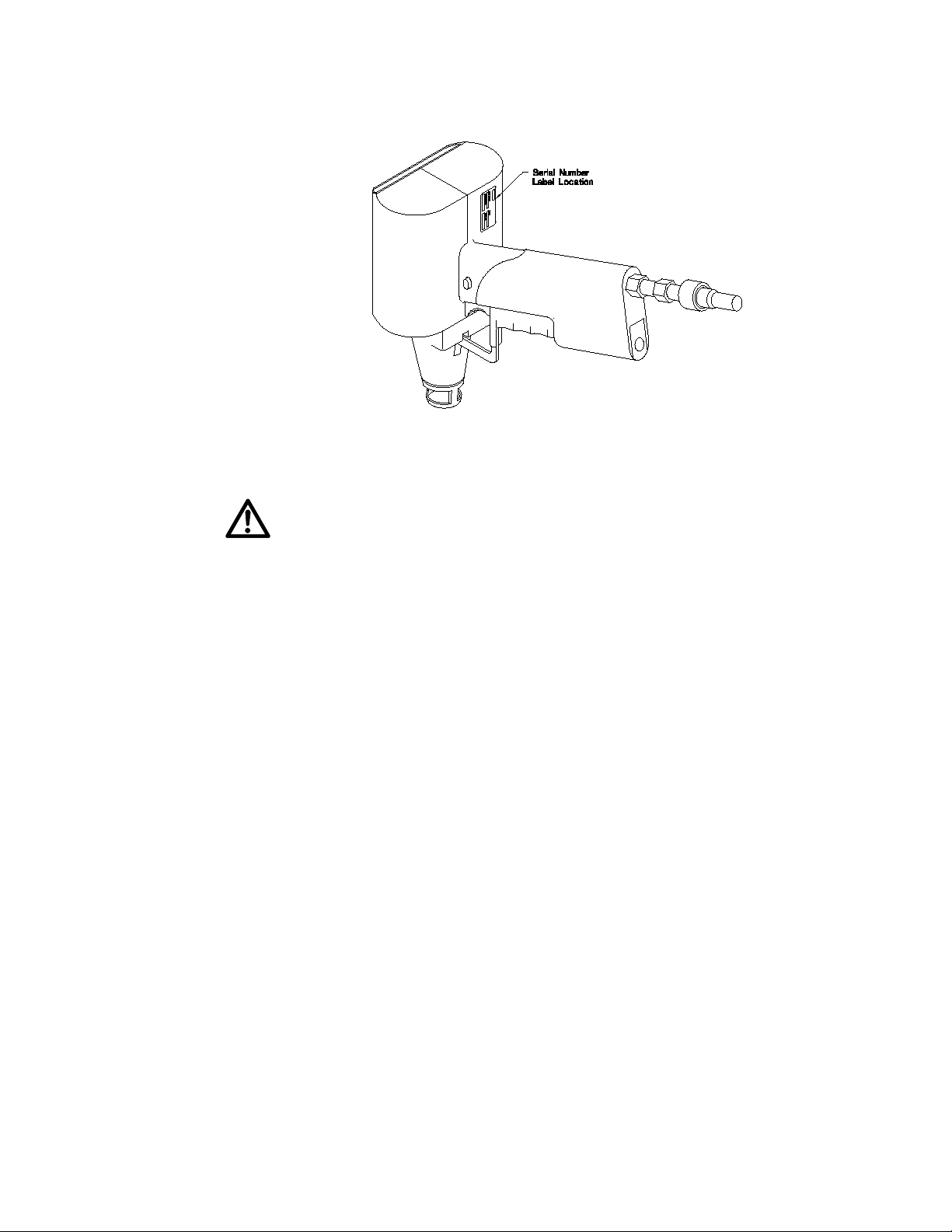
Fig. 1. Location of the instrument serial number label on the Helios Gene Gun.
1.4 Ear and Eye Protection
Caution: Expansion of gas from high pressure to low pressure produces a sound
wave, the intensity of which is a function of the gas pressure. The intensity of the sound generated by discharging the Helios Gene Gun is ~108 decibels (db) at 400 psi; sustained noise
levels of 85 db or brief noise levels of 110 db may lead to permanent hearing damage. Hearing
protection should be worn by all those in the immediate vicinity when discharging the Helios
Gene Gun. Earmuffs or ear plugs provide equivalent protection against hearing damage.
Refer to Section 2.4 for suggestions on ear protection. Eye protection should always be
worn when working with high pressure gases.
Section 2
Introduction to Particle Delivery
2.1 Particle Delivery Technology
Particle bombardment is a physical method of cell transformation in which high density,
sub-cellular sized particles are accelerated to high velocity to carry DNA into cells. The technique was first described as a method of gene transfer into plants (Klein et al., 1987, 1988;
McCabe et al., 1988) and subsequently shown to be applicable to mammalian experimental
systems (Zelenin et al., 1989; Yang et al., 1990; Williams et al., 1991). Because it does not
depend on specific ligand-receptors and/or the biochemical features of structural components
typically present on cell surfaces, particle-mediated gene transfer can be readily applied to a
variety of biological systems. Consequently, this procedure can be used to transform such
diverse targets as bacteria (Shark et al., 1991; Smith et al., 1992), fungi (Armaleo et al., 1990),
and intracellular organelles (Johnston et al., 1988; Boynton et al., 1988). Since it is a physical method of gene delivery, particle bombardment also overcomes physical barriers to
effective gene transfer, such as the stratum corneum of the epidermis and the cell wall of
plants. Particle bombardment is a convenient method for transforming intact cells in culture
since minimal pre- or post-bombardment manipulation is necessary. In addition, this technique is much easier and faster to perform than the tedious task of microinjection. Both
transient and stable expression are possible with particle bombardment. In addition to DNA,
RNA may also be transferred to cells by particle bombardment (Qiu, et al., 1996). Table 1 lists
some of the advantages of using particle bombardment for in vitro and in vivo transformation.
2
Page 7

T able 1. Advantages of particle bombardment for
in vitro
and
in vivo
gene transfer.
• Easy to use, rapid, versatile gene delivery system
• Independent of target cell type
• Useful for both transient and stable expression
• Requires only small amounts of DNA
• No carrier DNA is needed
• Requires only small numbers of cells
• May obtain high levels of co-transformation
• Large DNA fragments may be transferred
• Direct intracellular delivery to many cells in the target area
• Applicable to both in vitro and in vivo transformation
• No extraneous genes or proteins are delivered
2.2 The Helios Gene Gun
The Helios Gene Gun is the second instrument in Bio-Rad’s particle delivery product
line. In contrast to the PDS-1000/He instrument where the overall size of the target to be
transformed is limited by the size of the chamber and the target tissue is subjected to a vacuum during bombardment, the Helios Gene Gun requires no vacuum and any target accessible
to the barrel can be transformed. Consequently, the Helios Gene Gun may be used in a much
wider variety of gene transfer applications and provides a tool for both in vitro and in vivo
transformations in the research lab. Essentially any type of cells which can be made accessible to its nozzle may be transformed.
Gene gun models have also been developed by Auragen, Inc., a Bio-Rad collaborator. Cell penetration, gene expression and other measures of performance vary with
the model of gene gun used. Users must be careful to select operating parameters
optimized for their particular model. The Accell®model used by Auragen Inc. may
include modifications to be included in the future Helios models. The current Helios
Gene Gun has been designed to serve a wide range of research uses.
In vertebrates, the epidermal cells of the skin are the most obvious target (Yang et al.,
1990; Williams et al., 1991). In vivo experimental systems have targeted the skin for vaccination studies (Tang et al., 1992; Fynan et al., 1993; Eisenbraun et al., 1993), and wound
healing (Andree, et al., 1994), and cytokine gene therapy studies in mouse tumor models
(Sun et al., 1995; Keller et al., 1996; Rakhmilevich et al., 1996). In addition to skin, muscle
and internal organs, including liver, pancreas, spleen, kidney, etc., when appropriately exposed
surgically, can also be targeted in vivo (Yang et al., 1990; William et al., 1991; Cheng et al.,
1993). Using the Accell Gene Gun, both primary and established cultures of mammalian cells
have been transfected in vitro and ex vivo (Albertini et al., 1996; Mahvi et al., 1996;
Rakhmilevich et al., 1996). Additionally, transgenic expression of β-galactosidase, luciferase,
IL-12, granulocyte macrophage-colony stimulating factor and a nuclear papillomavirus protein has been demonstrated following in vivo transformation (Sundaram et al., 1996; Keller
et al., 1996; Rakhmilevich et al., 1996). Meristematic tissues and leaves are obvious target cells
for in vivo transformation of plants.
2.3 Operating Principle of the Helios Gene Gun System
The Helios Gene Gun System consists of all of the components needed to prepare DNAcoated microcarriers, coat the DNA-microcarrier suspension onto the inner surface of the
Gold-Coat™tubing, cut the tubing into cartridges which are used in the Helios Gene Gun, and
finally propel the microcarriers and their associated DNA into cells.
3
Page 8
Prior to transfection, the plasmid DNA must be attached to the gold particles. This is accomplished by precipitation of the DNA from solution in the presence of gold microcarriers and the
polycation spermidine by the addition of CaCl2. The particles are then washed extensively with ethanol
to remove the water and resuspended in ethanol. Using the Tubing Prep Station, the DNA/microcarrier solution is coated onto the inner wall of Gold-Coat tubing and dried. The tubing is then cut into 0.5"
length cartridges using the Tubing Cutter. These cartridges, when inserted into the cartridge holder of
the Helios Gene Gun are the source of the DNA which enters the target cells by the helium discharge.
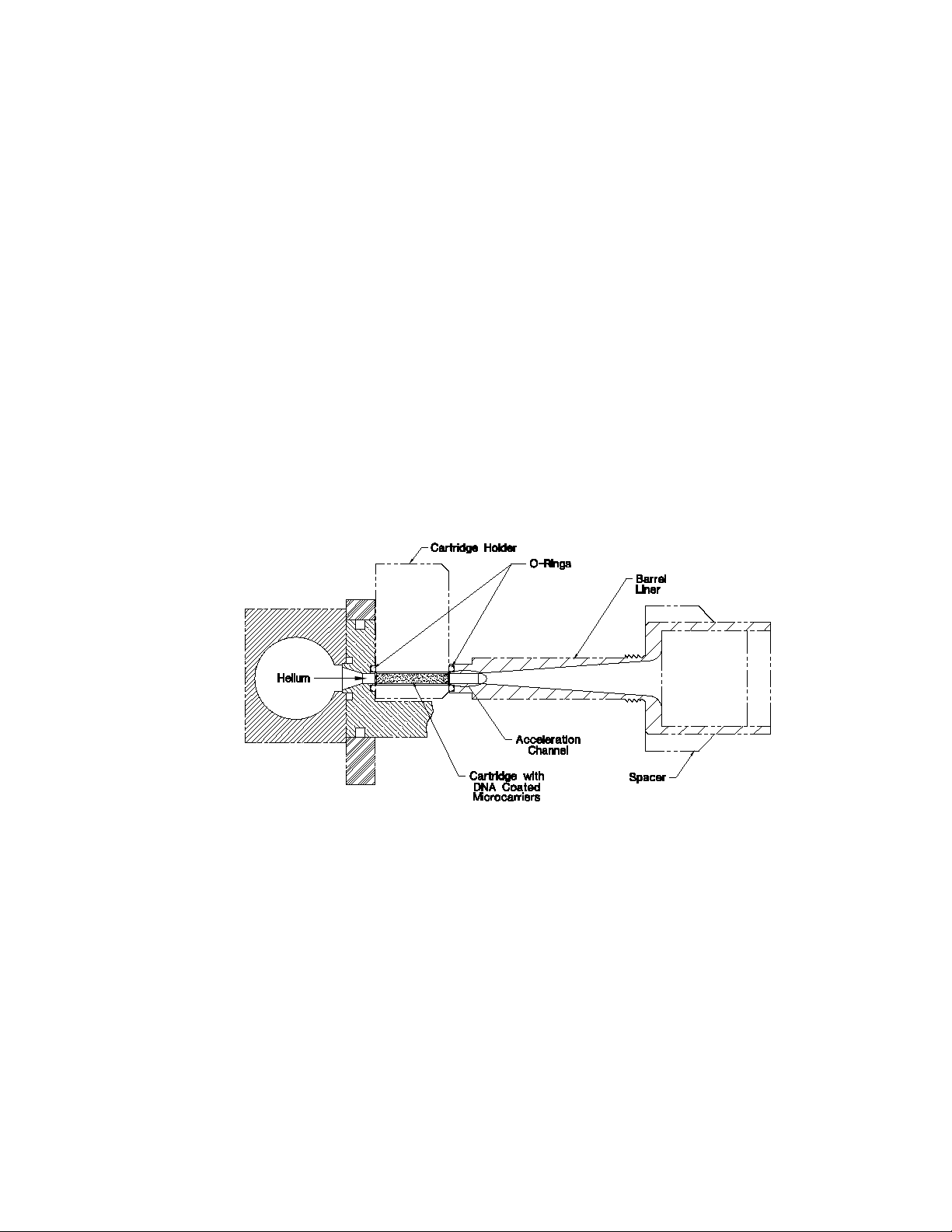
The Helios Gene Gun employs a high velocity stream of helium to accelerate gold particles
coated with plasmids or RNA to velocities sufficient to penetrate and transform cells, both in vitro
and in vivo (Figure 2). The discharge is initiated by pressing the trigger buttons which activates the
main valve, causing helium to travel down the bore of the particle delivery device. When the helium enters one of the bores of the cylinder containing the cartridge, the gold particles on the inside
of the tubing are pulled from the surface, become entrained in the helium stream, and begin to
pick up speed. Immediately past the acceleration channel, the barrel begins to open as a cone. The
slope of the cone causes the gas to be pulled outward, a process known as the Coanda effect (Reba,
1966), expanding the high pressure jet into a less destructive low velocity pulse, while the gold particles maintain a high velocity. The expansion also helps spread the microcarriers from their original
1/16" diameter to an area approximately 1/2" in diameter at the target site.
Fig. 2. How the samples are delivered. Helium gas is pulsed through the cartridge loaded with DNA-coated microcarriers. This pulse sweeps the microcarriers from the inside wall of the cartridge. As the microcarriers
enter the barrel liner they pick up speed in the acceleration channel then spread out as they travel down the
barrel; the increased cross-sectional area of the barrel from the acceleration chamber to the spacer also
moderates the helium shock wave so it is less intense when it reaches the target cells. The O-rings on each
side of the cartridge holder direct the flow of helium through the cartridge and the acceleration channel. The
spacer maintains optimal target distance and permits venting of the helium gas away from the target.
2.4 Requirements for System Operation
Selecting a Site for Operation
The Helios Gene Gun is a portable particle bombardment device. The range of its use is
limited by its requirement for a supply of pressurized helium and the 6 foot length of pressurized helium hose. When using the Gene Gun, only a small area is needed for setting down
the gun during an experiment, for loading the cartridges into the cartridge holders and exchanging cartridge holders during experiments. In addition, a clean and dry area is needed for
working with the tissue samples.
4
Page 9

Preparation of the gold/DNA tubes used in the Gene Gun requires an area approximately 1 m2for the Tubing Prep Station, for manipulating the tubing, precipitating the DNA onto
the gold, and processing the tubing into cartridges. Additionally, the Tubing Prep Station
requires an electrical outlet and a tank of pressurized nitrogen for evaporating the ethanol from
the DNA-coated gold particles from the inner surface of the tubing.
User Supplied Components
Helium Supply
Only helium gas is to be used with the Helios Gene Gun. The low atomic weight of helium results in maximum gas expansion when the high pressure helium is released through the
valve opening and enters the cartridge at atmospheric pressure. Thus, sufficient acceleration
of the DNA-coated microcarriers is generated for penetration of the target cell membrane.
Compressed helium of grade 4.5 (99.995%) or higher should be used; lower grades may
contain contaminating material which can obstruct gas flow within the Helios Gene Gun as
well as contaminate the biological sample. A helium tank pressurized to 2,600 psi [approximately 5 ft (1.7 m) high, 291 cu ft standard in the United States] is recommended, although
a smaller tank [~2.5 ft (~0.8 m) high] may be used. Follow all safety instructions provided by
the helium supplier for helium tank installation.
The helium pressure regulator (supplied) has a CGA 580 female fitting (standard in the United
States) for attachment to the user-supplied helium tank. An adaptor to this fitting may be required
outside of the United States. Contact your local Bio-Rad office for information on the helium pressure regulator adaptor requirements in your location. The regulator supplied with the Helios Gene
Gun is the only one that should be used with this instrument because of its three safety features: (1)
a self-venting valve that permits decreasing the pressure in the Helios Gene Gun System in the
event of battery failure or when it is necessary to reduce the pressure during an experiment; (2) an
over-pressure relief valve that prevents the helium pressure in the Helios Gene Gun System from
being adjusted above 700 psi ± 10%; and (3) a check valve that shuts off pressure if the helium hose
is disconnected while the system is still pressurized (Note: a check valve is also present at the
female connector of the helium hose where it connects to the Gene Gun to shut off pressure to the
gun if it is disconnected while the system is still pressurized.) Refer to Section 4.2 for proper use
of the helium regulator and to Section 5.4 for a description on shutting down the Gene Gun System.
A user supplied 10" or 12" (~25 cm) adjustable wrench or a 1 1/8" open end wrench is
required for attachment of the helium regulator to the helium tank.
Nitrogen Supply
Compressed nitrogen of grade 4.8 (99.998%) or higher is required for cartridge preparation using the Tubing Prep Station. Nitrogen is used for this purpose because it is relatively
inexpensive and provides a water-free atmosphere for evaporating the ethanol from the
DNA/gold sample inside the tubing. As with the helium tank, the nitrogen tank should be
properly secured on a floor stand or with a strap for safety.
A nitrogen regulator must be attached to the tank. A single stage regulator with an output gauge
that registers a maximum of 30 psi is recommended since an output pressure of no more than
1–2 psi is needed to produce the 0.4 liters per minute (LPM) flow rate necessary for using the Tubing
Prep Station. A regulator especially designed for this use, including a self-venting valve, an overpressure relief valve, and a hose barb for attaching the nitrogen hose is available from Bio-Rad
(catalog number 165-2425). Other regulators which are adjustable to give a low pressure output
may also be used. Manufacturers of regulators include Victor and Matheson; examples of regulators which may be used include Victor Model No. SR250A-580 and Matheson Model No. 3537-580.
Large scientific supply houses (e.g., VWR, Fisher, CMS, etc.) are also good sources for regulators.
The nitrogen line provided for use with the Cartridge Prep Unit is 3/16" diameter Tygon tubing.
5
Page 10

Battery
One battery is provided with the Helios Gene Gun System. Under normal use, it should
provide approximately 1,000 discharges. For maximum life, only alkaline batteries should
be used.
Laboratory Equipment
The following materials should be available before beginning any work with the Helios system.
Ultrasonic cleaner (e.g., Fisher FS3, Branson 1210)
Vortex mixer
Analytical balance
Microfuge
Peristaltic pump capable of pumping 5–8 ml/min (e.g. Bio-Rad Econo Pump,
catalog number 731-8140)
1.5 ml microfuge tubes
20 µl, 200 µl, and 1,000 µl micropipettors and tips
5 ml and 10 ml pipettes and pipettors
Lab timer
Ear protection (e.g., VWR catalog number 56610-728 (earmuffs) or catalog number
56610-680 (ear plugs)
1 1/8" open end or 10" or 12" (~25 cm) adjustable wrench
Helium tank (grade 4.5 or higher)
Nitrogen tank (grade 4.8 or higher)
Nitrogen regulator (e.g., Bio-Rad, catalog number 165-2425)
Scissors
Marking pen
Laboratory reagents
The following chemicals will be needed for coating plasmid onto the gold and for preparing
the tubing:
Gold microcarriers
Polyvinylpyrollidone, MW = 360,000
100% ethanol (e.g., Spectrum Chemical, catalog numberET-107; it is extremely important
that this be free of water; an unopened bottle should be used daily)
Spermidine (e.g., Sigma, catalog numbers S-0266 or S-4139)
Calcium chloride (CaCl2)
Plasmid DNA (for most applications, this should be at a concentration of ~1 µg/µl)
Plasmid DNA of high purity suitable for the Helios Gene Gun can be obtained through
use of any of Bio-Rad’s Quantum Prep®Plasmid Prep kits.
Catalog
Number Description
732-6100 Quantum Prep Plasmid Miniprep Kit, 100 preps
732-6120 Quantum Prep Plasmid Miniprep Kit, 20 preps
732-6130 Quantum Prep Plasmid Maxiprep Kit, 10 preps
732-6150 Quantum Prep HT/96 ClearVac Plasmid Miniprep Kit,
2 x 96 preps
6
Page 11

Section 3
Product Description
3.1 Packing List
The Helios Gene Gun System (see Figures 3 and 4) is shipped with the following components. If items are missing or damaged, contact your local Bio-Rad office.
Helios Gene Gun Kit
Instruction manual
Warranty card (please complete and return)
Helios Gene Gun
5 cartridge holders
5 barrel O-rings
5 barrel liners (four plus one installed in Gene Gun)
9 volt battery
Cartridge extractor tool
Helium hose assembly
Helium regulator
Tubing Cutter and 10 razor blades
Tubing Prep Station (see Figure 4)
Tubing Prep Unit (base, tubing support cylinder and power cord)
Nitrogen hose [12 ft, (~4m), Nalgene tubing 8000-0030, 3/16" ID, 5/16 " OD]
3/16" barb-to-male Luer-Lok fitting
10 cc syringe sleeve
5 O-rings, Tubing Prep Station
2 1/8" barb-to-male Luer fittings
5/64” Allen wrench
Syringe Kit
5 10 cc syringes
5 1/8" barb to female Luer fittings
1 syringe adaptor tubing [silicone, 5ft, (~2.6 m), 0.104" ID, 0.192" OD]
Optimization Kit
Gold-Coat Tubing [50 ft, (~26 m)]
1.6 µ gold microcarriers, 0.25 g
1.0 µ gold microcarriers, 0.25 g
0.6 µ gold microcarriers, 0.25 g
Polyvinylpyrrolidone, 360,000 MW (0.5 g)
5 desiccant pellets (store tightly sealed)
5 cartridge collection/storage vials
Note: If any of the system components (Helios Gene Gun, Tubing Prep Station, Tubing
Cutter, Helium Regulator, or Helium Hose) are dropped, check them for proper operation
before use.
7
Page 12
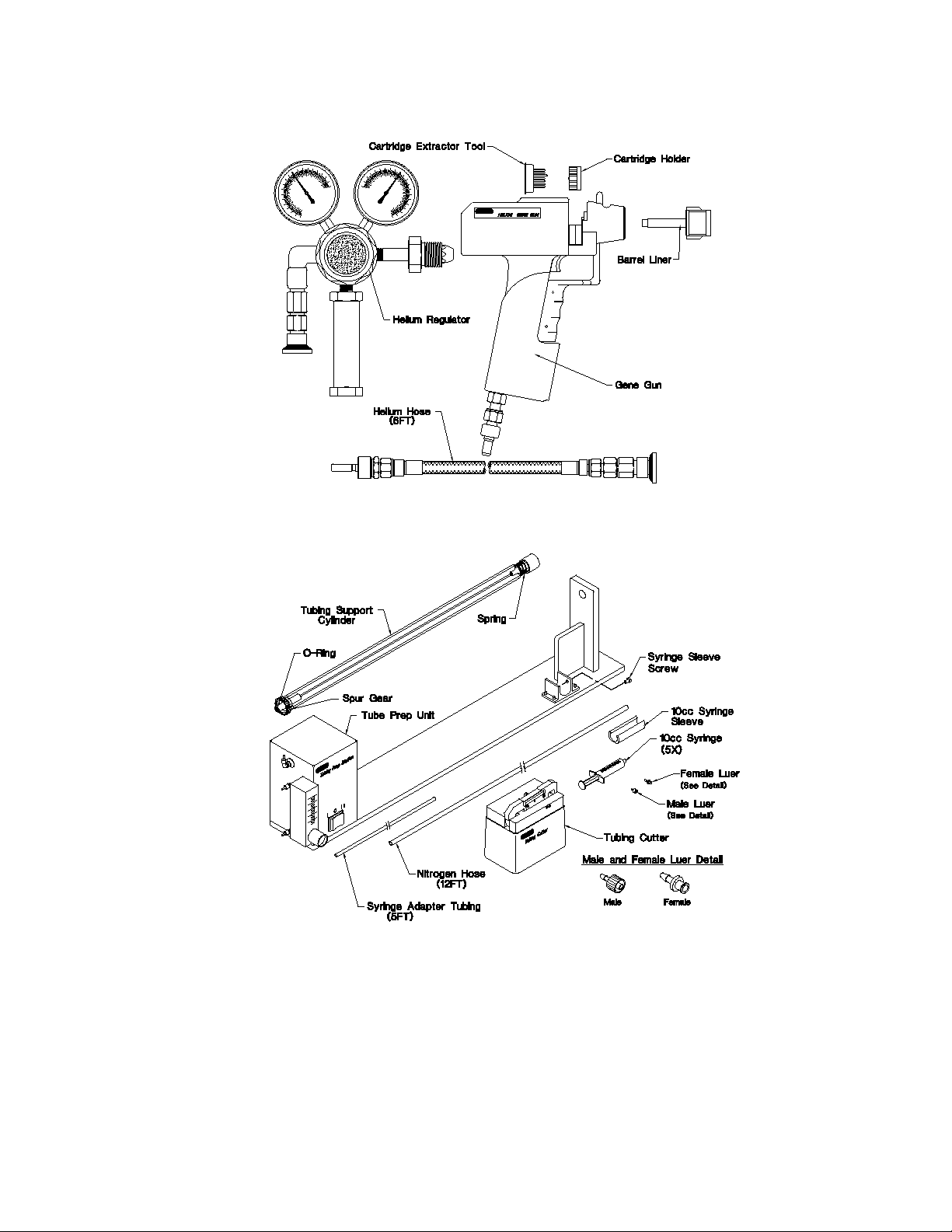
Fig. 3. Major components used for sample delivery with the Helios Gene Gun.
Fig. 4. Components of the Tubing Prep Station.
3.2 Identification of System Components and Controls
Helios Gene Gun
The locations front and back refer to the barrel end and LED display end of the Helios
Gene Gun, respectively. The locations left and right refer to the left and right sides of the
Gene Gun from the viewpoint of the user holding the device. Top and bottom refer to the
side of the gun that the cartridge holder is on and the side of the gun that the helium hose
connects, respectively (see Figure 5).
8
Page 13
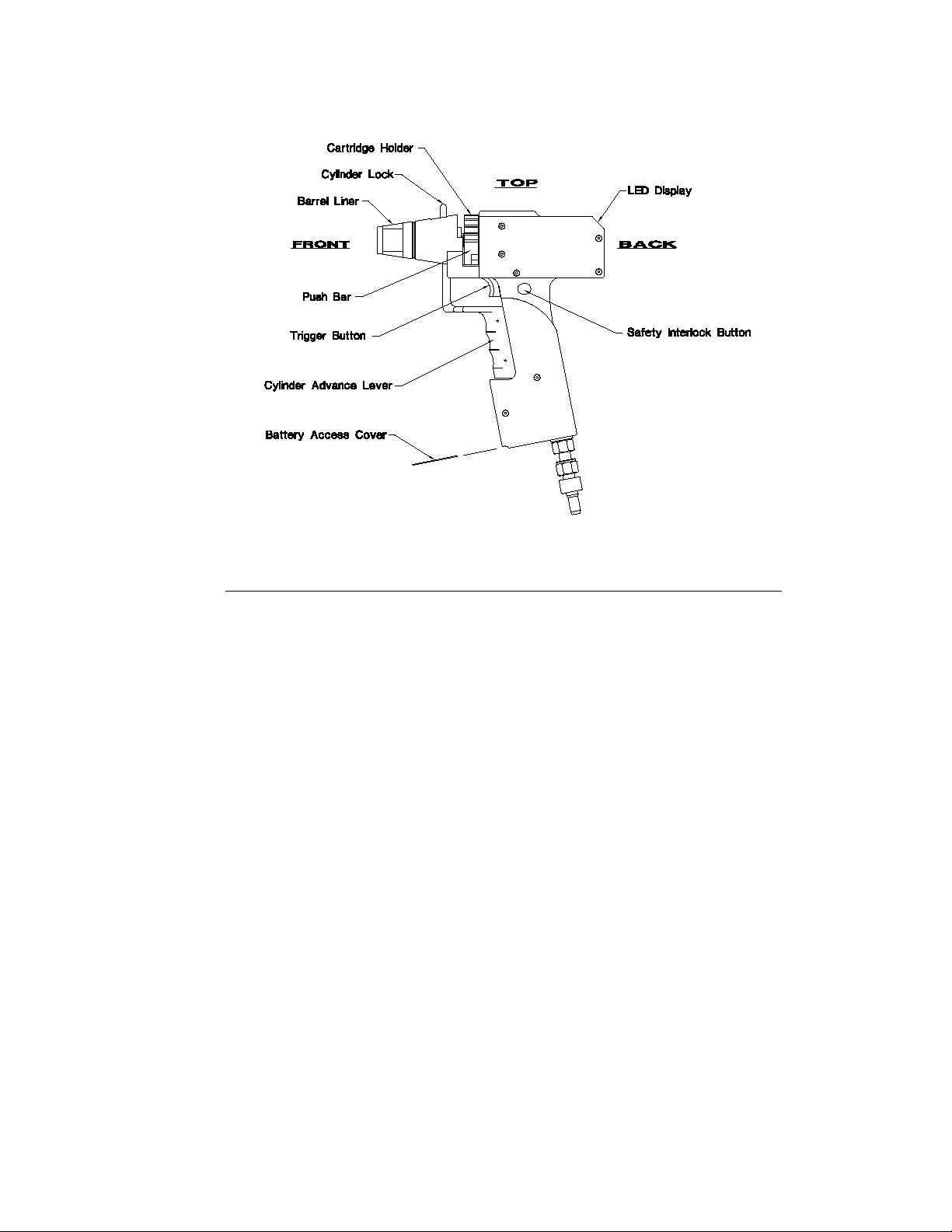
Fig. 5. Components and controls on the Helios Gene Gun.
Gene Gun Controls Description
Cylinder Lock Controls movement of the barrel pin. The cylinder lock is
spring-loaded; its natural position is in the backward (locked)
position so the barrel pin is inserted in the hole in the cartridge
holder; this keeps the cartridge holder in its proper position for
firing. Moving the cylinder lock forward disengages the barrel
pin from the cartridge holder to permit removing the cartridge
holder from the gun. Moving the cylinder lock forward and to
the right latches the cylinder lock to permit removal of the
cartridge holder; however, to prevent damage to the O-rings,
the cartridge holder should only be removed after compressing
the cylinder advance lever (see below).
Safety Interlock Switch Switch that must be held down to permit the trigger button to
be operational. Once this switch is depressed, the trigger button
is functional for 30 sec; the LED ARMED display flashes
quickly during this time. If the trigger button is not pressed
within the alloted time, this safety interlock switch must be
released and pressed again to re-activate the trigger button.
Trigger Button Controls the flow of helium gas through the Gene Gun. This switch
activates the solenoid, momentarily (for ~40 msec) opening the main
valve, and permitting helium to enter the cartridge and barrel. The
trigger button is only active for 30 sec after the safety interlock switch
is depressed.
Cylinder Advance Lever A multi-functional lever which is spring-activated by pulling the
lever backwards. When inserting or removing a cartridge holder,
pull back and hold in the cylinder advance lever; this moves the
barrel liner forward to provide additional room for maneuvering
the cartridge holder. After discharging the microcarriers from one
cartridge, pull back and release the cylinder advance lever; this
ratchets the cartridge holder, bringing the next cartridge into firing
position—the number visible on the very top of the cartridge rim
indicates the active sample position.
9
Page 14

Push Bar A metal bar that ratchets the cartridge holder from one position
to the next when the Cylinder Advance Lever is pressed. Move
this bar to the left (outward) prior to inserting a cartridge
holder to provide additional room for maneuvering the cartridge
holder.
LED Display An 11 light display.The display is normally off; inserting a
cartridge holder in the Gene Gun and advancing to position 1
activates the display. The left-most 7 LEDs act to indicate charging and ready status of the gun. After each firing of the gun and
at reset, the CHARGING LEDs turn-on in bargraph fashion,
left-to-right, throughout the 5 second charging time. Once the
Gene Gun is fully charged, the CHARGED LED will flash, indicating the safety interlock switch can be pressed. Upon pressing
the safety interlock, the ARMED LED’s sequentially flash.
When the trigger is pressed during the 30 sec armed period, the
gun fires and the FIRED LED turns-on for 1 sec. The change
bargraph then operates as described. The last light indicates
battery status : good battery (steady green light) or low battery
(flashing red light). The Gene Gun can be fired when the green
light is illuminated. If the battery is low, neither the safety interlock switch or the trigger button is active and an alarm will beep
three times every 15 sec.
Tubing Prep Station (see Figure 6)
Tubing Prep
Unit Controls Description
3 Position Switch Located on the motor housing and controls rotation of the tubing
support cylinder. At position (I), the tubing support cylinder turns
continuously at 30 revolutions per minute (rpm). At position (II),
the tubing support cylinder rotates only while the switch is
depressed. At position (O), no rotation occurs and the unit is off.
Flow Meter Registers the rate of nitrogen flow in liters per min (LPM) into the
tubing support cylinder. The valve on the flowmeter is used to
control the rate of nitrogen flow.
Tubing Support Cylinder A 28" aluminum cylinder with an opening on the right side
leading to a channel which holds the Gold-Coat tubing. The
left side of the channel has a replaceable O-ring into which the
tubing must be inserted. The tubing support cylinder can be
removed by pushing it to the right/left to compress the spring
which holds it in position.
Tubing Cutter (see Figure 7)
An instrument for rapid preparation of cartridges from Gold-Coat tubing. It cuts the tubing into
the exact length and shape required by the Gene Gun.
Tubing Cutter Part Description
Arm A spring-loaded piece that holds a razor blade (used for cutting
the tubing). The razor blade is held in place by the locking knob
on the lock block.
Base The support for the arm. It positions a storage vial under the
tubing channels so that the cut tubing pieces fall into the vial.
10
Page 15
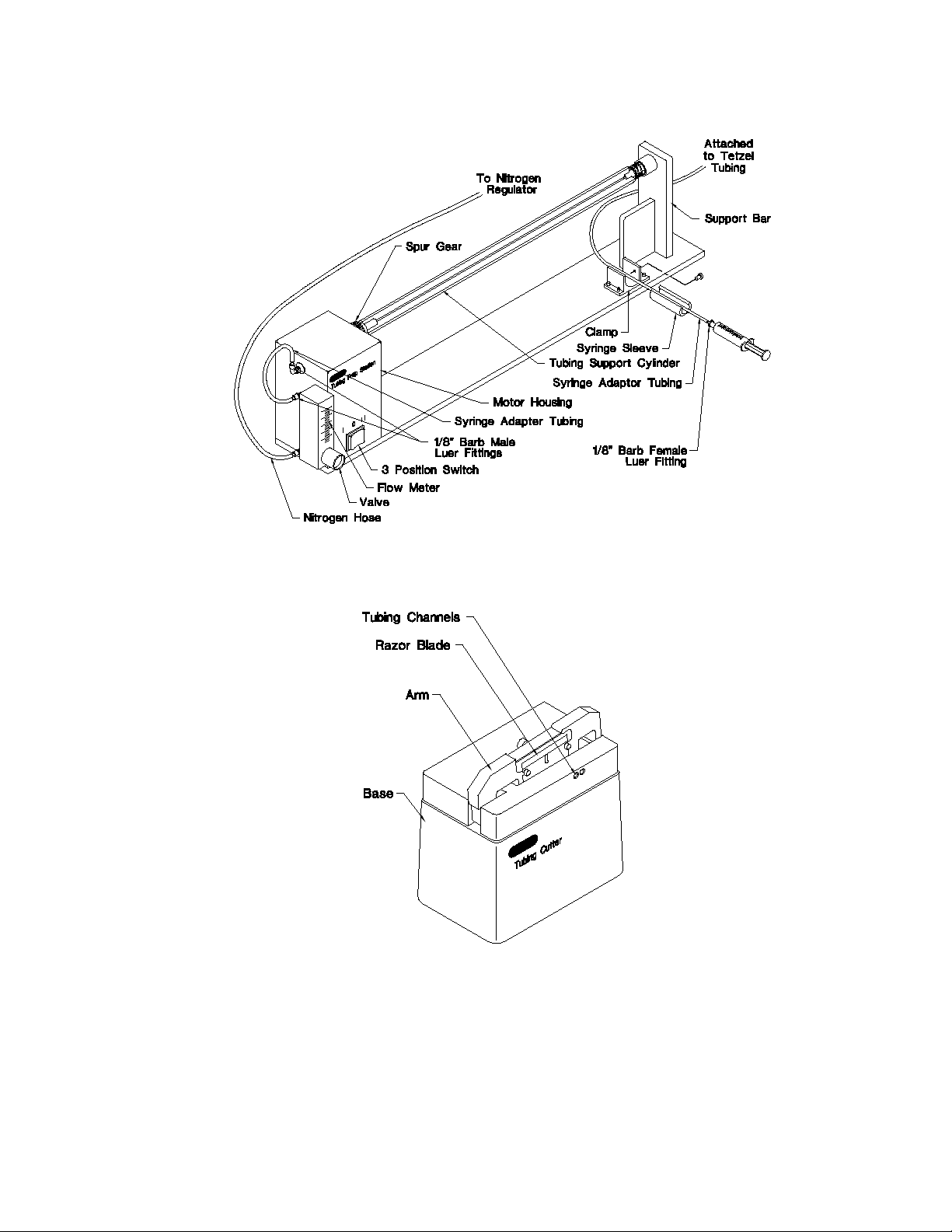
Fig. 6. Components and controls on the Tubing Prep Station, fully assembled.
Fig. 7. The Tubing Cutter.
Cartridge Extractor Tool (see Figure 8)
A 12-prong tool for removal of tubes from the cartridge holder. One prong is longer than
the others so it can be easily inserted into one of the bores of the cartridge holder to orient the
remaining 11 prongs.
11
Page 16
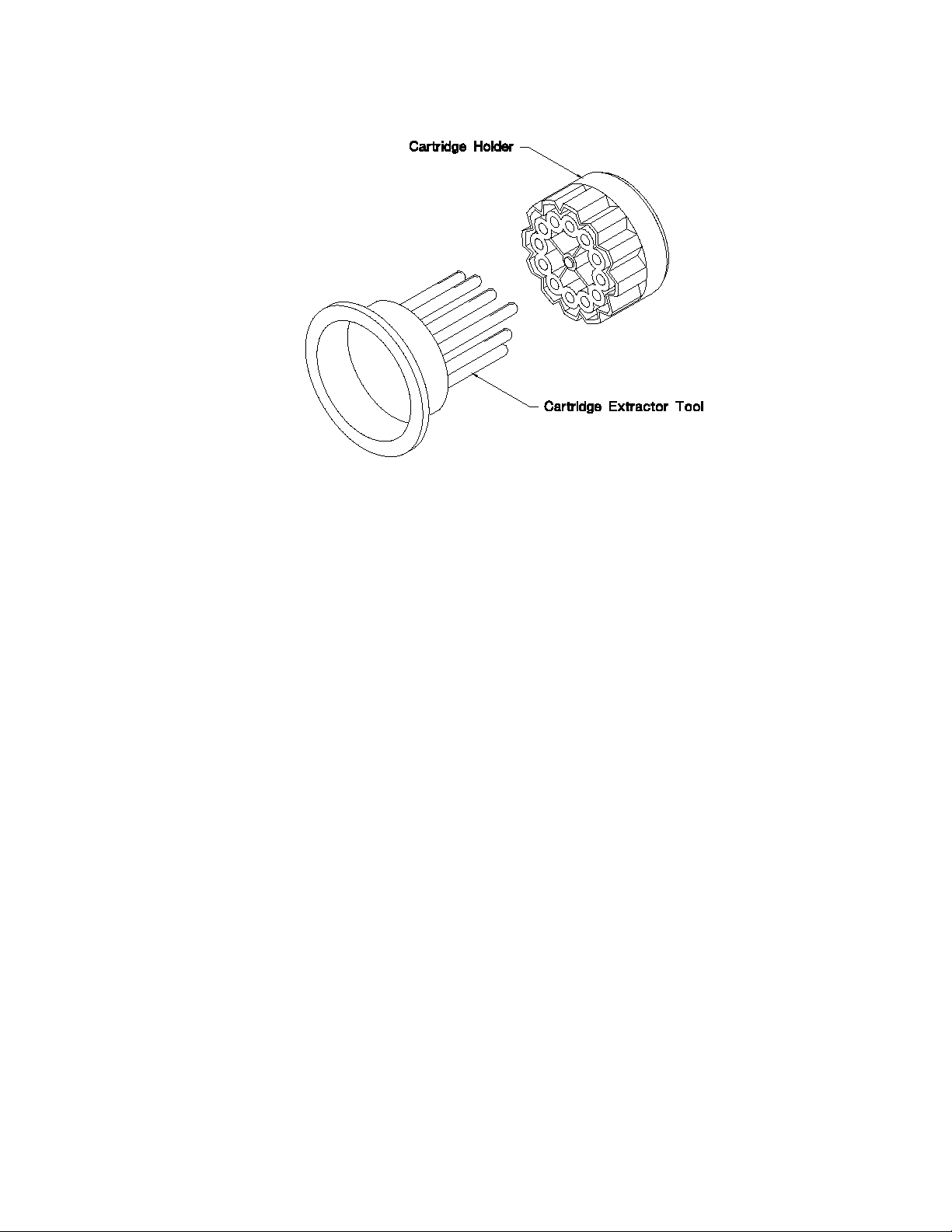
Fig. 8. Cartridge Holder and Cartridge Extractor Tool.
Section 4
Setting up the Helios Gene Gun System
4.1 Inserting the Battery into the Helios Gene Gun
The electrical system of the Helios Gene Gun is powered by a 9 volt battery. Under normal use, this should provide sufficient energy for 1,000 shots. The battery compartment is in
the base of the handle near the attachment fitting for the helium hose (Figure 9).
To insert the battery, first remove the battery cover by sliding it toward the front of the
Gene Gun. Slide the battery into the opening as shown in Figure 9. The battery must be oriented with the positive terminal toward the front of the Gene Gun indicated by the "+" symbol
inside the battery compartment. (Note: If the battery is inserted correctly, a tone will be audible for ~5 sec and the battery status LED will light; if the battery is inserted backward, the
electrical system will not operate and the Helios Gene Gun will be non-operational.) The battery is held in place by the battery cover.
12
Page 17

Fig. 9. Battery compartment. The battery compartment is located at the base of the handle of the Gene
Gun next to the connection for the helium hose and is protected by a battery cover that slides forward.
The battery is inserted with the positive terminal (the smaller of the two terminals) facing forward.
4.2 Connecting the Helios Gene Gun to a Helium Source
Refer to Section 1, Safety Information, and Section 3.2, Identification of System
Components and Controls, prior to system installation.
Helium Pressure Regulator Installation
Components needed
Pressure regulator for helium cylinder (with pressure relief valve, check valve, and female
Swagelok®Quick-Connect fitting), provided with unit (Figure 10).
Helium cylinder of grade 4.5 or higher (minimum 99.995% pure); maximum pressure of
2,600 psi, user supplied.
1 1/8" open-end wrench or a 10" or 12" adjustable wrench, user supplied.
Note: The regulator is intended for use only with helium gas with a maximum pressure
of 2,600 psi. The outlet on pressurized helium cylinders used in the United States is com-
patible with the fitting supplied on the pressure regulator with the Helios Gene Gun
System (CGA 580, female fitting). Outside the US, contact your local Bio-Rad office for
information regarding the proper cylinder/regulator fitting in your area.
Procedure
1. Secure the cylinder in a floor stand or to a wall or lab bench with a strap so it will not tip
or fall during use.
2. Inspect the cylinder valve for dirt, dust, oil, grease or damaged threads. Remove dust and
dirt with a clean cloth. Do not attach the regulator if you determine that the valve port is
damaged or cannot be cleaned. Inform your gas supplier of this condition and request a
replacement cylinder.
3. Clear the valve port of any foreign matter by standing to the side of the cylinder and
quickly opening and closing the cylinder valve.
4. Attach the regulator to the cylinder valve and tighten securely with a 1 1/8" open-end
wrench or a 10" or 12" adjustable wrench.
13
Page 18

Attaching the Helios Gene Gun to the Helium Regulator
Components needed
Helium regulator attached to a helium cylinder
Helium hose assembly
Helios Gene Gun
Procedure
1. Insert the stem of the Swagelok Quick-Connect fitting on the helium hose into the opening in
the body of the Swagelok Quick-Connect fitting on the helium regulator and push until it
clicks. The helium hose will be locked into the helium regulator (see Figure 10). Note: If the
helium regulator has been pressurized, the stem and body will not lock. Turning the regulator
valve counter-clockwise will depressurize the system.
2. In a similar manner, insert the stem of the Swagelok Quick-Connect fitting on the Helios
Gene Gun into the opening in the body of the Swagelok Quick-Connect fitting on the
helium hose until it clicks. The Gene Gun will be locked into the helium hose.
Fig. 10. Connecting the helium hose to the helium regulator.
4.3 Setting up the Tubing Prep Station
Refer to Section 3.2, Identification of System Components and Controls, prior to system
installation. See Figure 11 for a diagram of the assembled Tubing Prep Station.
The Tubing Prep Station is shipped disassembled. The following sections describe assembly of the Tubing Prep Station, attachment of the syringes and tubing, installation of the nitrogen
pressure regulator, and connection of the Tubing Prep Station to the nitrogen regulator.
A peristaltic pump is recommended for removal of the ethanol from the Gold-Coat tubing
after the microcarriers have been loaded (see Section 5.1). If a peristaltic pump is not available,
this may be done manually using a syringe. Assembly of the syringe and tubing is described
in step 5 in the following section.
14
Page 19

Assembly of the Tubing Prep Station and Syringes
Components needed
Tubing Prep Station, base
Tubing Prep Station, tubing support cylinder
Tubing Prep Station, power cord
O-rings, for tubing support cylinder
Syringe adapter tubing (silicone, 5 ft, 0.104" ID, 0.192" OD)
10 cc syringes (2)
10 cc syringe sleeve
1/8" barb-to-male Luer fittings (2)
1/8" barb-to-female Luer fittings (2)
Scissors, user supplied
Fig. 11. Components and controls on the Tubing Prep Station, fully assembled.
Procedure
1. Place an O-ring in the stainless steel end of the tubing support cylinder next to the spur gear.
2. Insert the end of the tubing support cylinder with the spring into the hole in the support bar
on the base of the Tubing Prep Station. Push the tubing support cylinder to compress the
spring, then slide the end of the tubing support cylinder containing the O-ring into the sup-
port on the motor housing. (Note: Carry the Tubing Station by the base, not by the tubing
support cylinder.)
3. Cut a 4–5" piece of syringe adapter tubing; attach each end to the barb of the 1/8" barb-to-
male Luer fittings. Attach one of the male Luer-Lok fittings to the female Luer on the top of
the flowmeter. Attach the other male Luer-Lok fitting to the female Luer on the side of the
motor housing on the Tubing Prep Station.
15
Page 20

4. Cut a 12–13" piece of syringe adapter tubing; attach one end of the tubing to the barb of a 1/8"
barb to female Luer fitting; attach the female Luer to a 10 cc syringe. This syringe and tubing
will be used to load the DNA/microcarrier suspension into the Gold-Coat tubing (Section 5.1).
5. (Optional—if peristaltic pump not used) Cut a 16-18" piece of syringe adapter tubing; attach
one end of the tubing to the barb of a 1/8" barb-to-female Luer fitting; attach the female Luer
to a 10 cc syringe. Slide the syringe into the 10 cc syringe sleeve; fasten the sleeve (with the
open side facing up) to the clamp on the base of the Tubing Prep Station using the plastic
screw; the screw should be tightened sufficiently to hold the sleeve and syringe stationary but
allow free movement of the plunger. This syringe and tubing will be used to remove the
ethanol from the DNA/microcarrier suspension in the Gold-Coat tubing if a peristaltic pump
is not available.
6. Attach the power cord to the three pronged receptacle on the back of the base of the Tubing
Prep Station and plug into an appropriate electrical outlet.
Nitrogen Pressure Regulator Installation
Components needed
Pressure regulator for nitrogen cylinder, user supplied (see Section 2.4). A regulator is avail-
able from Bio-Rad (catalog number 165-2425) which is ready for use in the Helios Gene
Gun System.
Nitrogen tank of grade 4.8 or higher (99.998% pure); maximum pressure of 2,600 psi, user
supplied.
1 1/8" open-end wrench or a 10" or 12" (~25 cm) adjustable wrench, user supplied.
Procedure
1. Secure the cylinder in a floor stand or to a wall or lab bench with a strap so it will not tip or
fall during use.
2 Inspect the cylinder valve for dirt, dust, oil, grease or damaged threads. Remove dust and
dirt with a clean cloth. Do not attach the regulator if you determine that the valve port is dam-
aged or cannot be cleaned. Inform your gas supplier of this condition and request a
replacement cylinder.
3. Clear the valve port of any foreign matter by standing to the side of the cylinder and quick-
ly opening and closing the cylinder valve.
4. If necessary, connect a male hose barb to the nitrogen regulator.
5. Attach the regulator to the cylinder valve and tighten securely with a 1 1/8" open-end wrench
or a 10" or 12" (~25 cm) adjustable wrench.
Connecting the Tubing Prep Station to the Nitrogen Regulator
Components needed
Nitrogen regulator attached to a nitrogen cylinder
Nitrogen hose [12 ft (~4 m), of 3/16" ID Nalgene tubing]
3/16" barb-to-male Luer fitting
Tubing Prep Station
Procedure
1. Determine the length of nitrogen hose needed to connect the Tubing Prep Station to the the
nitrogen regulator and cut it if necessary.
2. Connect the 3/16" barb-to-male Luer fitting to one end of the nitrogen hose.
3. Push the other end of the nitrogen hose onto the male hose barb on the nitrogen regulator. Do
not use hose clamps to secure the nitrogen hose on the Tubing Prep Station.
16
Page 21

4. The nitrogen regulator should be turned on and adjusted to the correct pressure prior to con-
necting the nitrogen line to the Tubing Prep Station. Close the nitrogen pressure regulator by
turning the regulator adjustment screw counterclockwise until the adjusting spring pressure is
released and the screw moves without resistance. Release nitrogen into the pressure regulator
by carefully and slowly opening the cylinder valve on the nitrogen tank. The cylinder pressure
in the tank is indicated on the high pressure gauge (the gauge closest to the cylinder). Hold the
nitrogen line in your hand and slowly turn the regulator adjustment screw clockwise until
nitrogen can just be heard flowing from the nitrogen line. Clamp the nitrogen line with your
fingers; the pressure on the output (low pressure) gauge should register no more than 1–2 psi.
If the pressure is too high, turn the regulator screw counterclockwise to reduce the pressure.
5. Connect the male Luer-Lok fitting on the the nitrogen line to the female Luer fitting on the
side of the Tubing Prep Station. The flow of nitrogen into the Tubing Prep Station can be
adjusted with the valve on the flowmeter.
Section 5
Operation of the Helios Gene Gun System
5.1 Quick Guide to Operation
Before the Bombardment
1. Coat microcarriers with DNA, load into tubes, and prepare cartridges prior to day of exper-
iment.
2. Check helium supply (50 psi in excess of desired delivery pressure).
3. Clean and/or sterilize the Gene Gun, tube holders, and barrel liners as appropriate.
4. Connect the Gene Gun to a helium source.
5. Activate the Gene Gun: turn on the flow of helium to the desired pressure and with an empty
cartridge holder in place, make 2–3 “pre-shots” by engaging the safety interlock and firing
the trigger.
Firing the Device
1. Load cartridges into the cartridge holder and place in Gene Gun.
2. Prepare/position target cells for bombardment.
3. Bombard sample: engage safety interlock and press the firing trigger
After the Bombardment
1. Remove cartridge holder from Gene Gun.
2. Remove cartridges from cartridge holder.
3. Turn off the helium pressure to the system.
4. Turn the regulator value counterclockwise to de-pressurize the system.
5. Disconnect the helium hose and Gene Gun.
17
Page 22

5.2 Preparation of System Components Prior to Bombardment
Calculating the Amounts of Gold and Plasmid Required
Prior to precipitating DNA onto the gold particles and loading them into the Gold-Coat
tubing, it is necessary to calculate the amount of DNA and gold required for each transformation. Points to consider in making these calculations are presented below. The amount of
DNA loaded per mg of microcarriers is referred to as the DNA Loading Ratio (DLR). Typical
DLRs range between 1 and 5 µg DNA/mg gold. Adding more DNA tends to cause agglomeration of the gold particles, probably as a result of DNA binding to more than one particle.
The amount of microcarriers delivered per target is referred to as the Microcarrier Loading
Quantity (MLQ). Typical MLQs range from 0.25 to 0.5 mg/cartridge for in vivo delivery to
epidermal cells, but may be slightly lower for in vitro delivery to mammalian cells. Refer to
Table 2 for representative starting amounts of microcarriers and plasmid to use for different
MLQs and DLRs. Refer to Section 7 for suggestions on parameter optimization and starting
conditions for using the Helios Gene Gun to deliver DNA to mammalian cells
Procedure 1: Determining the Microcarrier Loading Quantity (MLQ)
1. For most systems, delivering 0.5 mg of gold per target is a good starting point.
2. A 1 ml suspension will fill an 8.5" length of tubing; one cartridge is 0.5" long. Each 30"
length of tubing can be filled with approximately 25" (3.0 ml) of DNA/gold suspension.
(There will be a void space at each end.)
3. For delivering 0.5 mg of microcarriers per target (MLQ=0.5), resuspend the DNA/micro-
carrier sample at 8.5 mg of gold/ml ethanol. A 25" length of tubing will require 25 mg of
gold resuspended in a volume of 3 ml of ethanol.
Procedure 2: Determining the DNA Loading Ratio (DLR)
1. For many applications, delivery of 1 µg of plasmid per target is a good starting point.
2. At a MLQ of 0.5 mg/cartridge, a DLR of 2 µg DNA/mg gold results in loading 1 µg of
DNA/cartridge and in delivery of 1 µg of DNA per target. Preparation of two lengths of
Gold-Coat tubing requires 100 µg of DNA and 50 mg of gold. The concentration of
DNA should be approximately 1 µg/µl and the volume of DNA should not exceed the
volume of spermidine in Section 5.2, Precipitation of DNA onto Microcarriers, Step 3.
If the DNA is too dilute, concentrate it by ethanol precipitation. If a high DLR is desired,
increase the volume of spermidine and CaCl2so that equal volumes of each component
are added (spermidine, DNA, and CaCl2) up to a total volume of 1,200 µl.
3. For a detailed description on determining which MLQs and DLRs will work best for sev-
eral mammalian targets, refer to Section 7.
18
Page 23

T able 2. Microcarriers and DNA Required for Various Microcarrier
Loading Quantities (MLQ) and DNA Loading Ratios (DLR)
1
Calculated Particle Materials Required for Selected
Delivery Conditions MLQ’s and DLR’s
MLQ DLR Final
(µg/ (µg/ Gold DNA volume Tubing
(mg/shot) mg gold) shot) (mg) (µg) (ml)
2
(total in)
3
0.5 2 1 50 100 6.0 50
0.125 8 1 12.5 100 6.0 50
0.25 4 1 25 100 6.0 50
0.75 1.33 1 75 100 6.0 50
1.0 1 1 100 100 6.0 50
0.5 0.002 0.001 50 0.1 6.0 50
0.5 0.02 0.01 50 1 6.0 50
0.5 0.2 0.1 50 10 6.0 50
0.5 10 5 20 200 2.4 20
1 For most applications with mammalian cells, in initial experiments, use an MLQ of 0.5 and a DLR of 2.
2 Based on loading 1 ml of the DNA-coated microcarriers suspended in ethanol in 8.5 inches (22 cm) of Gold-Coat tubing.
3 Various lengths of tubing may be prepared. Adjust amounts of gold, DNA volume of ethanol in proportion to the change from the
length of tubing listed above for each desired MLQ and DLR. Approximately 25 inches of tubing can be prepared in the Tubing
Prep Station at one time; 50 inches of tubing will usually yield 80–90 cartridges.
Precipitation of DNA onto Microcarriers
It is important to use an unopened bottle of 100% ethanol each day this step is performed.
Opened bottles of ethanol absorb water and the presence of water in the tubing while drying
will lead to streaking, clumping, and uneven coating of the microcarriers over the inner surface of the Gold-Coat tubing, resulting in poor or unusable cartridges. All ethanol solutions
should be opened only briefly when in use and kept tightly capped when not in use.
Polyvinylpyrrolidone (PVP) serves as an adhesive during the cartridge preparation process. At higher discharge pressures, preparing cartridges with PVP can increase the total
number of particles delivered. The optimum amount of PVP to be used must be determined
empirically. Typical PVP concentrations range from 0.01 to 0.1 mg/ml. For recommendations on the amount of PVP to use in initial experiments, refer to Section 7.
Materials
Provided
Gold microcarriers
Polyvinylpyrrolidone (PVP), 360,000 MW
To be supplied by user
Fresh 100% ethanol
15 ml disposable polypropylene centrifuge tubes
1.5 ml microfuge tubes
0.05 M spermidine
1 M CaCl
2
200 µl and 500 µl pipettors and tips
5 ml, 10 ml pipettes and pipette-aid
Purified plasmid DNA resuspended in distilled water or 10 mM Tris (pH 8.0), 1 mM EDTA
Ultrasonic cleaner (e.g., Fisher FS3, Branson 1210)
Analytical balance capable of weighing microgram quantities
Microfuge
19
Page 24

Procedure
Time considerations: preparation of the DNA/gold suspension requires approximately 30 min.
Several samples may be prepared simultaneously without a significant increase in time.
1. Prepare a stock solution of 20 mg/ml PVP in ethanol in a small screw-cap container.
Dilute this solution with ethanol to prepare PVP solutions at the desired concentration
(generally 0.01–0.1 mg/ml); prepare 3.5 ml of the dilute solution for each 30" length of
Gold-Coat tubing, (25” to be coated) in the Tubing Prep Station. Keep these solutions
tightly capped when not in use. Prepare solution daily.
2. In a 1.5 ml microfuge tube, weigh out gold microcarriers. (Refer to Procedure 1 for a
detailed description on determining MLQ. Refer to Table 2 for suggestions on the rela-
tive amounts of gold and microcarriers required and on the length of tubing produced.)
3. To the measured gold, add 100 µl of 0.05 M spermidine. (However, if the volume of plasmid
to be added in step 5 is greater than 100 µl, refer to the discussion above for Procedure 2:
Determining the DNA Loading Rate, and add the appropriate volume of spermidine.
4. Vortex the gold and spermidine mixture for a few seconds, then sonicate for 3–5 seconds
using an ultrasonic cleaner to break up gold clumps.
5. To the gold and spermidine mixture, add the required volume of plasmid to achieve the
desired DLR. (Refer to Procedure 2 for a detailed description on determining DLR. Refer to
Table 2 for suggestions on the relative amounts of gold and microcarriers required and on the
length of tubing produced.) For co-transfection of multiple plasmids, add each of the plasmids
at this step. DNA does not associate with the microcarriers prior to addition of CaCl2.
6. Mix DNA, spermidine and gold by vortexing ~5 sec.
7. While vortexing the mixture at moderate rate on a variable speed vortexer, add 100 µl of
1 M CaCl2dropwise to the mixture. The volume added should equal that of the spermidine
in Step 3.
8. Allow the mixture to precipitate at room temperature for 10 min.
9. Most of the gold will now be in the pellet, but some may be on the sides of the tube.
The supernatant should be relatively clear. Spin the microcarrier solution in a microfuge
~15 sec to pellet the gold. Remove the supernatant and discard.
10. Resuspend the pellet in the remaining supernatant by vortexing briefly. Wash the pellet
three times with 1 ml of fresh 100% ethanol each time; spin ~5 sec in a microfuge between
each wash. Discard the supernatants.
11. After the final ethanol wash, resuspend the pellet in 200 µl of the ethanol solution containing the appropriate concentration of PVP prepared in step 1. Transfer this suspension
to a 15 ml disposable polypropylene centrifuge tube with a screw cap. Rinse the microfuge
tube once with 200 µl with the same ethanol/PVP solution and add to the centrifuge tube.
Add the necessary volume of the ethanol/PVP solution to the centrifuge tube to bring the
DNA/microcarrier solution to the desired MLQ.
12. The suspension is now ready for tube preparation. Alternatively, the DNA/microcarrier
suspensions can be stored for up to 2 months at -20 °C. Prior to freezing, tighten the cap
securely and put Parafilm®around the cap of the tube. After storage at -20 °C, allow the
particle suspension to come to room temperature prior to breaking the Parafilm seal.
20
Page 25

Loading the DNA/Microcarrier Suspension into Gold-Coat Tubing Using the
Tubing Prep Station
Materials
Supplied
Tubing Prep Station (see Section 4.3)
Gold-Coat tubing
To be Supplied by User
Microcarrier/DNA suspension(s) from Section 5.1, Precipitation of DNA onto
Microcarriers, at room temperature
Ultrasonic cleaner
Vortexer
100% ethanol
Peristaltic pump
Minute timer
Nitrogen tank (see Section 2.4)
Nitrogen regulator (see Section 2.4)
Scissors
Procedure
Time considerations: Since only one piece of Gold-Coat tubing can be coated at a time,
this procedure may take 15–30 min for the first sample and 15–20 min for additional samples.
1. Set up the Tubing Prep Station and connect to a nitrogen tank as described in Section 4.3.
2. Prior to using the Tubing Prep Station, a peristaltic pump should be set up and calibrated to be used later for removing ethanol from the tubing at the rate of 0.5–1.0"/sec.
[Note: 1 ml of liquid occupies 8.5" (21.5 cm) of tubing, removing liquid at 0.5–1.0"/sec
is the same as 0.06–0.12 ml/sec or 3.6–7.2 ml/min.] Using a 10 ml graduated cylinder, calibrate a peristaltic pump at 5.5–6.0 ml/min. The tubing at the end of the peristaltic pump
which will be connected to the Gold-Coat tubing should have an inside diameter of 1/8".
3. If a peristaltic pump is not available, a syringe fitted with a 16–18" piece of silicone adaptor tubing and inserted into the syringe sleeve and clamped onto the base of the Tubing Prep
Station may be used to remove the liquid from the Gold-Coat tubing (see Section 4.3).
Liquid should be removed from the Tefzel tubing at 0.5–1.0"/sec. Practice removing liquid at this rate from the Tefzel tubing by cutting a ~30" piece of tubing, loading it with
~3.0 ml of ethanol (~24" (~60 cm) of tubing), inserting it into the Tubing Prep Station,
and removing the liquid at 0.5–1.0"/sec. Marking the tubing at several points and using a
timer which measures time in seconds should make removal more accurate. It should take
25–45 sec to draw the liquid from the entire length of tubing. (Note: use ethanol rather
than water for practicing this step because, if water leaks into the Tubing Prep Station, it
may contaminate subsequent pieces of Tefzel tubing leading to poorly coated tubes
[see Section 8.2]).
4. Prior to preparing cartridges, ensure that the Gold-Coat tubing is completely dry by purging with nitrogen. Insert an uncut piece of tubing into the opening on the right side of the
Tubing Prep Station. The edge of the hole is beveled to permit easier insertion. Push the
tubing into the hole and into the tubing support cylinder. At the opposite end of the tubing support cylinder is an O-ring. There will be slight resistance as the tubing is pushed
into the O-ring; insert the tubing another 1/2" (1 cm).
5. Using the knob on the flowmeter, turn on the nitrogen and adjust the flow to 0.3–0.4 LPM.
Allow nitrogen to flow through the Gold-Coat tubing for at least 15 min immediately prior
to using it in the following steps.
21
Page 26

6. Remove the Gold-Coat tubing from the Tubing Prep Station. Turn off the flow of nitrogen to the Tubing Prep Station using the knob on the flowmeter.
7. From the dried Gold-Coat tubing cut a 29–30" (~75 cm) length of tubing for each 3 ml sample of Microcarrier/DNA suspension. (Note: Cutting the tubing with a scissors may distort
the shape of the end; the tubing may be easier to insert into the tubing support cylinder if the
end is subsequently cut in the tubing cutter.) Insert one end of the Gold-Coat tubing into the
end of the adaptor tubing fitted to the 10 cc syringe (see Section 4.3, step 5).
8. Vortex the microcarrier suspension and, if necessary, sonicate briefly to achieve an even
suspension of gold. Invert the tube several times to resuspend the gold; immediately
remove the cap and quickly draw the gold suspension into the Gold-Coat tubing approximately 22–24", approximately 58 cm, (6–8" (~17 cm) from the end). AVOID DRAWING
BUBBLES INTO THE GOLD-COAT TUBING: Do not vortex the microcarrier suspension while drawing it into the tubing. Do not try to remove all of the liquid from the
container with the suspension. Remove the tubing from the suspension and continue drawing the suspension into the tubing another 2–3" (~6 cm) to leave some space at each end.
9. Immediately bring the Gold-Coat tubing to a horizontal position and slide the loaded
tube, with syringe attached, into the tubing support cylinder in the Tubing Prep Station
until the tubing passes through the O-ring.
10. Allow the microcarriers to settle for 3–5 min. Detach the Gold-Coat tubing from the adaptor tubing and attach to the tubing on the peristaltic pump or on the 10 cc syringe.
(Be careful not to rotate the Gold-Coat tubing.) Remove ethanol at the rate of 0.5–1.0"/sec
(this should require 30–45 sec).
11. Detach the peristaltic pump or syringe from the Gold-Coat tubing. Immediately turn the
Gold-Coat tubing 180° while in the groove and allow the gold to begin coating the inside
surface of the tubing for 3–4 sec.
12. Turn the switch on the Tubing Prep Station to ON (I) to start rotating the Tubing Prep
Station. (Warning: Keep objects away from the gears of the Tubing Prep Station while in
operation.)
13. Allow the gold to smear in the tube for 20–30 sec, then open the valve on the flowmeter
to allow 0.35–0.4 LPM of nitrogen to dry the Gold-Coat tubing, while it continues to rotate.
14. Continue drying the Gold-Coat tubing while turning for 3–5 min.
15. Turn the motor on the Tubing Prep Station to OFF (O). Turn off the nitrogen by closing
the valve on the flowmeter. Remove the tubing from the tubing support cylinder.
Preparing 0.5" Cartridges Using the Tubing Cutter
This procedure requires only a few minutes and should be performed as soon after load-
ing the DNA/microparticle suspension into the tubing as possible. It is important to store the
coated tubes in a desiccated environment. Tubes stored at 4 °C are stable for at least 8 months.
Materials
Supplied
Tubing Cutter
Razor blade
Cartridge storage vial
Desiccant pellets
To be Supplied by User
Gold-Coat tubing coated with microcarriers from Section 5.2
Scissors
Marking pen
22
Page 27

Procedure
1. Examine the coated the Gold-Coat tubing to verify that the microcarriers are evenly distributed over the length of the tubing. Ideally, the gold should be spread uniformly over the
entire inside surface of the tubing; however, while drying, it may polarize to one side of the
tubing. As long as there are no clumps or bare sections, the tubing can be used for cartridges.
2. Using scissors, cut off and discard sparsely and unevenly coated tubing from one of the
ends. With a marking pen mark any sections of tubing to be discarded. This is usually
limited to the outer 1–2" where the gold has settled, but may also include internal sections
of tubing which are un unevenly coated with gold.
3. Use the Tubing Cutter to cut the remaining tubing into 0.5" pieces as follows:
a. Place a cartridge storage vial containing a desiccant pellet inside the base of the Tubing
Cutter.
b.Insert the cut ends of one or two pieces of tubing into the tubing channels on the front
face of the Tubing Cutter (Figure 7); be sure to push them in until they make contact
with the rear plate.
c. Push down sharply on the handle; the tubes will drop into the storage vial.
d.Repeat the process of inserting the uncut tubing and cutting the cartridges until the
entire length of usable tubing is cut.
4. Cap the vial tightly, label, wrap with Parafilm, and store at 4 °C.
5.3 Particle Delivery Using the Helios Gene Gun
This section describes the procedure for preparing the Helios Gene Gun for firing, discharg-
ing the device, loading cartridges into the cartridge holder, and delivering DNA to target cells.
(Warning: Use the Helios Gene Gun in a well ventilated area.)
Materials
Supplied
Helios Gene Gun
Barrel liner (see Section 10.5 on sterilization)
Cartridge holder
Helium hose
Helium regulator (see Section 4.2)
To be Supplied by User
Helium tank (see Section 2.4)
Ear protection
Cartridges with coated microcarriers (from Section 5.2)
Activating the Helios Gene Gun for Gene Delivery
When first setting up the system and prior to discharging the Gene Gun into a target, it is
important to pressurize the helium hose and the internal reservoirs of the gun with the correct
helium pressure. This is accomplished by discharging the device prior to delivering loaded
tubes to the target cells. It is important to have a cartridge holder in place during these
“pre-shots” to keep the O-rings from being blown out (see Section 10.2).
1. Insert an empty cartridge holder into the Helios Gene Gun as follows:
a. Move the cylinder lock on the Gene Gun so it is latched in the forward position and the
barrel pin does not protrude behind the barrel (Figure 12).
b.Unlatch the push bar by pulling it outward (Figure 12).
23
Page 28

Fig. 12. Positioning the cylinder lock and the push bar in preparation for loading a cartridge holder into the Gene Gun. The cylinder lock has been pushed forward and into the slot on the right to move
the locking pin away from the opening occupied by the cartridge holder. Likewise, the push bar has
been pulled to the left to increase the space for inserting the cartridge holder.
Fig. 13. Inserting a cartridge holder into the Gene Gun. Pulling the cylinder advance lever in moves
the barrel forward to increase the space for inserting the cartridge holder behind the barrel liner. The
cartridge holder is inserted with the labeled position number 12 (visible at the top) in line with the center
of the Gene Gun (see also Fig 12).
24
Page 29

c. Pull back and hold the cylinder advance lever to retract the inner barrel sleeve into the
gun barrel (Figure 13).
d. Place the empty cartridge holder into the Gene Gun with the position 12 label facing up
and the numbered side of the cartridge holder facing the exit nozzle of the barrel
(Figure 12).
e. When the cartridge holder is in its correct position, the knob on the backside of the car-
tridge holder will slip into the notch on the barrel plate and the cartridge holder will
be flush with the barrel plate.
f. Release the cylinder advance lever; the O-ring on the inner barrel sleeve should hold the
cartridge holder in position.
g.Unlatch the cylinder lock; it should snap into position and the barrel pin should be
inserted into the center hole in the cartridge holder.
h.Push the push bar in; it should snap back into position and engage the cartridge holder
in one of the deep crevices (see Figure 14).
Fig. 14. Correct assembly of the cartridge holder in the Gene Gun in preparation for discharge.
Releasing the cylinder advance lever moves the barrel liner backward, bringing the O-ring on the back
of the liner in contact with the cartridge holder. Releasing the cylinder lock inserts the locking pin into the
center hole in the cartridge holder.
i. Push in and release the cylinder advance lever to ratchet the cartridge holder one position,
bringing the first cartridge into firing position. The number 12 should be seen at the top
point of the cartridge holder. The Gene Gun is now ready for pressurizing with helium.
Note: do not wiggle the cartridge holder after the cartridge is in firing position. The
Helios Gene Gun has a self-centering mechanism which places the cartridge in the proper
position for firing.
2. Set up the Helios Gene Gun and connect to a helium source as described in Section 4.2.
Refer to Section 3.2 for identification of controls on the Gene Gun.
25
Page 30

Fig. 15. LED display of the Helios Gene Gun. Using a pressurized system, once the cartridge holder of
the Gene Gun is correctly inserted and engaged in postion 1, the five CHARGING lights will be sequentially illuminated. Once the unit is fully charged (~5 sec), the CHARGED light will flash. If the safety interlock
is then pressed, the ARMED lights will alternately flash. If the safety interlock continues to be pressed (in
this ARMED state) and the trigger button is pressed, the helium will discharge and the FIRED light will
then be illuminated for one second. The process of charging and arming the gun occurs automatically
after it is fired. The green battery light in the BATTERY STATUS window indicates that the battery is good.
3. Start the flow of helium as follows: (Note: If a continous gas leak is observed after opening either the cylinder valve or the regulator adjustment screw, close the appropriate valve
and check the tightness of the fitting. Contact Bio-Rad if a system leak persists.)
a. Close the helium pressure regulator by turning the regulator adjustment screw counter-
clockwise until the adjusting spring pressure is released and the screw moves without resistance.
b.Release helium into the pressure regulator by slowly opening the cylinder valve on the
helium tank; open the valve fully. The helium pressure in the tank is indicated on the
high pressure gauge (the one closest to the cylinder). Verify that the pressure in the
tank is at least as high as the desired discharge pressure.
c. Open the helium pressure regulator using the regulator adjustment screw and set the
discharge pressure to the desired setting (100–600 psi). The pressure to the system is
indicated by the low pressure gauge on the regulator. Do not set the discharge pressure above 600 psi; the helium regulator has an over-pressure relief valve that will vent
the system at 700 ± 50 psi. Additionally, the regulator is self-venting: turning the the
regulator adjustment screw counterclockwise will reduce pressure to the system.
4. Put on hearing and eye protection, point the device away from any by-standers, and
depress the trigger 2–3 times to discharge the device as described below. If this step is
performed without a cylinder in place, the O-rings may be blown out.
a. For right handed users (see Figure 16):
Engage the safety interlock switch by pushing in with the thumb.
Push the firing trigger with the index finger to discharge the cartridge.
b.For left handed users:
Engage the safety interlock switch by pushing it in with the lower part of the index finger.
Push the firing trigger with the index finger to discharge the cartridge.
26
Page 31

Notes: (1) The firing trigger is functional only while the safety interlock switch is pushed
in. The safety interlock switch activates the trigger button for 30 sec; if the Gene Gun is
not fired within the 30 sec period, the safety interlock switch must be released, then
pressed again to reactivate it. (2) After firing the gun, there is a 5 sec delay before the
gun may be fired again. (3) Advance the cartridge holder by squeezing the cylinder
advance lever between each shot.
Fig. 16. Position of hand over the safety interlock switch in preparation for firing.
5. Removing the cartridge holder from the Helios Gene Gun is accomplished as follows:
a. Move the cylinder lock on the Gene Gun forward and to the right so it is latched and
the barrel pin does not protrude behind the barrel (Figure 12).
b.Unlatch the push bar by pulling it outward.
c. Pull back and hold the cylinder advance lever to retract the inner barrel sleeve into the
gun barrel (Figure 13).
d.Pull the cartridge holder up and out of the Gene Gun. The gun will turn-off after 3 min.
Loading Cartridges into the Helios Gene Gun
Each cartridge holder has slots for 12 cartridges. Each slot is numbered along the edge of
the cartridge holder, as seen when loading. The numbers correspond to the firing order when
the cartridge holder is properly positioned in the Gene Gun. The cartridge holder should be
loaded with cartridges beginning with position 1, then clockwise through position 12 (see
Figure 17). Note, however, that the cartridge that is in firing position is at the bottom of the
cartridge holder when it is inserted in the Gene Gun. Therefore, the cartridge in firing position will not be visible to the user but will be indicated by the number at the top of cartridge
holder and visible along the cartridge rim.
1. Place the cartridge holder on a flat surface with the numbered edge facing up.
2. Starting with position 1, load up to 12 cartridges into the cartridge holder (Figure 17).
We recommend loading only one type of DNA per cylinder to avoid possible confusion.
3. Invert the cartridge holder and push the cartridges against a flat surface so that they are
flush with the numbered side of the cylinder.
4. Insert the loaded cartridge holder into the Helios Gene Gun as described above. When the
LED on the back of the Gene Gun indicates that the first cartridge is in firing position
(Figure 15) the device is ready to deliver DNA.
27
Page 32

Fig. 17. Loading cartridges into the cartridge holder. The numbers located on the outer rim indicates
sample number when delivered by the Gene Gun.
DNA delivery to target tissue (see Section 6 for suggestions on preparing mammalian
target cells)
1. Touch the target area with the spacer so that the spacer is flush and the Gene Gun is perpendicular to the target surface. Activate the safety interlock switch and press the trigger
button to deliver the DNA/microcarriers to the target.
2. Ratchet to the next cartridge by pulling in and releasing the cylinder advance lever. After
approximately 5 sec, the Gene Gun is ready to deliver the next cartridge.
3. After all tubes have been discharged, remove the cartridge holder as described below.
5.4 Removing Used Cartridges, Depressurization, and Shut Down
Materials
Supplied
Cartridge extractor tool
Removing Cartridges from the Cartridge Holder
1. Hold the cartridge holder containing the cartridges to be extracted with the back side (the
side with the knob in the center) facing up.
2. Insert the long prong of the Cartridge Extractor Tool into one of the bores of the cartridge
holder; turn the Cartridge Extractor Tool until all of the prongs mesh with the bores in the
cartridge holder.
3. Push the prongs of the tool into the cartridge holder until the cartridges are ejected.
Depressurizing and Shutting Down the Helios Gene Gun
1. After discharging the last cartridge, turn off the helium pressure to the system by closing
the valve on the helium tank.
28
Page 33

2. Turn the regulator valve counterclockwise until both the high and low pressure gauges on
the helium regulator register 0 psi. Several increase/decrease adjustments on the regulator
may be necessary. Listen for venting to ensure complete depressurization. The system is now
depressurized and can be safely disassembled.
3. Disconnect the helium hose from the regulator by pulling the sleeve on the Swagelok
Quick-Connect coupling toward the helium hose and pulling the fittings apart.
4. Disconnect the helium hose from the Gene Gun by pulling the sleeve on the Swagelok
Quick-Connect coupling toward the Gene Gun and pulling the fitting apart. (Warning:
For safety, do not leave the Helios Gene Gun unattended while attached to the helium
regulator.)
Section 6
Preparation of Mammalian Cell Targets
Time Considerations: Setting up the device requires no more than 5 min, while the deliv-
ery process requires approximately 30–60 sec per cell target. If necessary, sterilize parts as
described in Section 10.5 before starting.
6.1
In vitro
Delivery to Adherent Cells
Method 1
Day 1
1. Trypsinize cells from flasks. Resuspend the cells in tissue culture media at a density so that
inoculating 2 ml into a 35 mm tissue culture plate will produce a monolayer 60–80%
confluent after 24 hr.
2. Inoculate 2 ml of cells into 35 mm tissue culture plates.
3. Incubate overnight under the appropriate conditions.
Day 2
1. Prepare the Helios Gene Gun for operation as described in Section 5.3.
2. Immediately prior to DNA delivery, aspirate the media from the dish.
3. Hold the dish perpendicular to the spacer and touch the end of the plastic spacer (if the spacer is sterile), or as close as possible to the target, and discharge the Gene Gun (Figure 18).
4. Add 1.5–2 ml of media to the plate and return to the incubator.
Method 2
1. Trypsinize cells from flasks. Resuspend the cells in tissue culture media buffered with
25 mM HEPES (pH 7.3) and count viable cells.
2. Dilute cells to 2 x 106cells/ml.
3. Using a sterile pipet tip, spread 100 µl of the cell suspension in a 1.5 cm diameter circle
in the center of a 35 mm dish, being careful not to break the meniscus. It is useful to draw
a 1.5 cm diameter circle on an index card to use as a template under the culture dish.
4. Allow the plates to incubate undisturbed for 30 min to permit the cells to attach.
5. Gently add 1 ml of media to each plate and place in the incubator. Incubate the cells for
at least 4 hr.
6. Prepare the Helios Gene Gun for operation as described in Section 5.3.
29
Page 34

7. Immediately prior to DNA delivery, aspirate the media from the dish.
8. Hold the dish perpendicular to the spacer and touch the end of the plastic spacer (if the spacer is sterile), or as close as possible to the target, and discharge the Gene Gun (Figure 18).
9. Add 1.5–2 ml of media to the plate and return to the incubator.
6.2
In vitro
Delivery to Suspension Cultures
Method 1
1. Prepare the Helios Gene Gun for operation as described in Section 5.3.
2. Transfer the cells to a centrifuge tube and pellet in a table top centrifuge for 5 min at 250 x g.
3. Resuspend the cells in tissue culture media containing 25 mM HEPES (pH 7.3) at a concentration of 5 x 107cells/ml.
4. Inoculate 20 µl (1 x 106cells) of the cell suspension into a 1.5 cm circle in the center of
a 35 mm dish. It is useful to draw a 1.5 cm diameter circle on an index card for use as a
template under the culture dish.
5. Hold the dish perpendicular to the spacer and touch the end of the plastic spacer (if the spacer is sterile), or as close as possible to the target, and discharge the Gene Gun (Figure 18).
6. Add 1.5–2 ml of media to the plate and return to the incubator.
Fig. 18. Correct placement of the Gene Gun when transfecting cells
in vitro
(photo courtesy of
Auragen, Inc.).
Method 2
1. Prepare 35 mm tissue culture plates with Cell-Tak (Collaborative Research, Cambridge,
MA) using manufacturer’s instructions.
2. Transfer cells to a centrifuge tube and pellet in a table top centrifuge for 5 min at 250 x g.
3. Resuspend cells in tissue culture media without serum at a concentration such that inoculating 2 ml of cells into the 35 mm tissue culture plates treated with Cell-Tak will produce
a monolayer 60–80% confluent.
4. Inoculate 2 ml of cells into 35 mm tissue culture plates.
30
Page 35

5. Incubate 30 min to 4 hr under the appropriate conditions.
6. Prepare the Helios Gene Gun for operation as described in Section 5.3.
7. Immediately prior to DNA delivery, aspirate the media from the dish.
8. Hold the dish perpendicular to the spacer and touch the end of the plastic spacer (if the spacer is sterile), or as close as possible to the target, and discharge the Gene Gun (Figure 18).
9. Add 1.5–2 ml of media to the plate and return to the incubator.
6.3
In vivo
Delivery to Epidermis
Animal Preparation
1. Anesthetize the animal if necessary for safe handling.
2. Clip fur as closely as possible over the desired target area using Oster clippers with a
#40 surgical blade and brush or vacuum fur off.
3. (Optional) After clipping, a commercial depilatory such as Nair can be used to completely
remove the animal’s fur. This treatment removes the stratum corneum from the skin, completely exposing the epidermis. Carefully rinse the skin with warm water following
depilatory treatment.
4. If the target site is wet or dirty, clean and dry with 70% ethanol.
Helios Gene Gun Operation
1. Prepare the Helios Gene Gun for operation as described in Section 5.3.
2. Hold the spacer directly against the target site (Figure 19) and discharge the device.
(Discomfort following Helios Gene Gun bombardment of the skin is minimal. No apparent macroscopic disruption of the skin, external bleeding or hematoma should be observed.
Some animals may show transient erythema or inflammation at the treatment site.)
Fig. 19.
In vivo
bombardment of murine epidermis (photo courtesy of Auragen, Inc.).
31
Page 36

Section 7
Optimization of Gene Gun Parameters
7.1 Overview
The flexibility of the particle delivery system allows fine-tuning of experimental param-
eters; however, it is necessary for each laboratory to determine the optimal parameters for
their particular instrument and in vivo or cell culture system. Any quantitative assay may be
used to determine the optimum combination of critical parameters for the particular biological system under investigation. Important parameters to evaluate include the helium pressure,
the PVP concentration, the Microcarrier Loading Quantities (MLQ), and the DNA loading
ratio (DLR). It should be noted that absolute transgene expression levels are only a part of the
processes leading to immune or other biological responses, thus, each researcher must identify those parameters that result in the appropriate level, location, and duration of transgene
expression following particle-mediated delivery. The representative results shown below
reveal a commonly observed and useful trait of the Helios Gene Gun: a broad, bell shaped distribution of effective delivery parameters.
Scientists are advised to optimize bombardment parameters for their particular gene gun
and biological system. The experimental approach which appears to be most effective in
determining the optimum bombardment conditions is the following.
Expt 1: Optimize helium pressure: Coat plasmid onto gold particles at 2 µg plasmid/mg gold.
Prepare tubes with 0.05 mg/ml PVP and 500 µg gold/shot (1 µg plasmid/shot).
Bombard the target tissue at 50, 100, 200 and 400 psi helium to determine the optimum
helium pressure.
Expt 2:
Optimize PVP concentration: Use gold particles coated with 2 µg plasmid/mg gold
as in Experiment 1. Prepare tubes with 0, 0.05, and 0.1 mg/ml PVP. Bombard the target
tissue using the optimum helium pressure determined in Experiment 1.
Expt 3:
Optimize MLQ: Coat plasmid onto gold particles at 2 µg, 4 µg, and 8 µg plasmid/mg
gold; prepare tubes with each DNA/gold sample at 1 µg plasmid/shot (this is 500 µg
gold/shot, 250 µg gold/shot, and 125 µg gold/shot, respectively), and containing
the optimum amount of PVP determined in Experiment 2. Bombard the target tissue
at the optimum helium pressure determined in Experiment 1 or 2.
Expt 4:
Optimize DLR: The lowest amount of DNA that has been found to give a detectable
level of gene expression is 1 ng of plasmid, but this is dependent on the vector, the
target, and the assay system. For most experimental systems, bombardment with
1 µg of plasmid produces near maximal expression. Increasing the amount of plasmid per bombardment does not result in a proportional increase in gene expression.
However, bombardment with higher amounts of DNA may be important when using
several expression vectors. To verify the optimum amount of plasmid per bombardment precipitate different amounts of plasmid onto gold particles–depending on
how extensive a study desired, this may be as simple as a single two-fold dilution
to multiple dilutions using between 1 ng and 5 µg of plasmid per bombardment;
the other bombardment parameters should be as determined in the previous experiments.
For example, Figure 20 shows a test of discharge pressure on transient gene expression,
in mouse skin transfected in vivo as described in Section 6.3. Optimum pressure was determined to occur at 200 psi. This pressure resulted in adequate penetration of the particles
without excessive tissue damage, and deposition of the majority of the particles in the epithelial layer rather than the relatively acellular underlying dermal tissue.
32
Page 37

Fig. 20. Luciferase expression in mouse skin transfected
in vivo
using the Helios Gene Gun.
Skin homogenates were assayed 24 hours post-transfection.
As illustrated in Figure 21, the optimum MLQ is determined by adjusting the concentration
of the DNA/microcarrier suspension loaded into the Gold-Coat tubing. For suspension cells,
higher MLQs (0.5–1.0 mg gold/cartridge) are often needed due to the high cell concentrations in
the aqueous cell smear. Adherent cell monolayers and intact tissues may require reduced MLQs
(0.06–0.25 mg gold/cartridge) to minimize tissue damage while maximizing transfection.
Fig. 21. Effect of the amount of accelerated gold particles on
in vitro
expression levels. Suspension
cultures of CHO cells were bombarded as described in Section 6.2 using the Accell Gene Gun. After 18 hours,
the CHO cells that had reattached to the tissue culture plate were lysed and assayed for luciferase expression
(Thompson
et al.
1993).
33
0.0
0.5
1.0
1.5
2.0
2.5
3.0
mg gold per cartridge
Luciferase relative light units x 10
-8
1.0 0.5 0.25 0.12 0.06
-6
100 200 400
Helium pressure (psi)
80
60
40
20
0
hGH activity (ng/ml)
Luciferase activity (relative light units x 10
-8
)
Page 38

Another variable parameter, DNA loading rate (DLR), is determined by varying the con-
centration of DNA precipitated on to the gold particles. Table 3 shows DNA dosage results
for transfection of CHO cells using the method described in Section 6.2. Secretion of murine
granulocyte macrophage colony stimulating factor (mGM-CSF) was greater at higher DNA
dosages. Similar results have been found for several in vivo and in vitro systems, with maximal expression observed for 1–5 µg DNA/mg gold particles. At dosages above ~5 µg/mg, the
DNA and gold can form a single clump which is unsuitable for cartridge preparation.
Table 3. Effect of DNA concentration on mGM-CSF expression
in CHO cells
a
Expression
c
DNA loading rate
b
(ng/106cells)
2.5 515
0.25 239
0.025 25
0.0025 0.9
a CHO cells were transfected as suspension cultures using the Accell Gene Gun.
b Irrelevant plasmid DNAwas added to each preparation to bring the total amount of DNA precipitated constant.
c mGM-CSF in the media was assayed 24 hours post-bombardment by ELISA as described by Mahvi et al. (1996).
7.2 Parameters for
in vitro
Delivery
The following conditions have been found to be effective for transformation of mam-
malian cell lines. However, each laboratory should determine the optimum parameters for
their particular application. (See previous section.)
34
Page 39

T able 4. Suggested starting conditions for
in vitro
transformation
of tissue culture cells using the Helios Gene Gun.
Parameter Conditions
Helium pressure: 50–200 psi
PVP: 0–0.015 mg/ml
MLQ: 0.125–0.5 mg/tube
Microcarriers: 1.0 or 1.6 µ gold
DLR: 0.5–2.5 µg DNA/mg gold (0.06–1.25 µg DNA/cartridge)
7.3 Parameters for
in vivo
Delivery
The following are suggested starting conditions for optimizing DNA delivery to the skin of
various species. Each laboratory should determine the optimum parameters for their particular
application (see Section 7.1 and Table 5). Bombardment conditions are those used in references
cited in Section 10.8 using the Accell Gene Gun. Use this information as a starting point when
optimizing bombardment conditions with the Helios Gene Gun. Lower helium pressures should
be tested early in the optimization process.
T able 5. Examples of parameters for delivery into skin of various
species
Species Site MLQ(mg/target) DLR(µg/mg) PVP(mg/ml) psi
Dog
9
Dorsum 0.5 1–2 0.1 300
Monkey
7, 8, 9
Abdomen 0.25 2 0–0.05 300–400
Mouse
17, 19
Abdomen* 0.25–0.5 1–2.5 0–0.1 300–500
Pig
7, 8, 10
Inner thigh 0.25–0.5 0.5–2.5 0.05 500
(clipping not
necessary)
Rabbit
27
Back 0.5 1–2 None 350–400
* Do not use a depilatory agent to remove the fur (Section 6.3, step 3)
Section 8
Troubleshooting
8.1 DNA/Microcarrier Preparation
Problem Microcarriers agglomerate after coating with DNA.
Possible solutions Lower DNA Loading Rate.
8.2 Cartridge Preparation
Problem Gold does not spread evenly in the Gold-Coat tubing (rings,
clumps, uncoated sections, streaks).
Possible solutions Eliminate any potential sources of water in the tube and in the
final DNA/Microcarrier preparation.
35
Page 40

1. Rewash remaining microcarrier prep 2–3 times using a
fresh bottle of 100% ethanol.
2.Flush Gold-Coat tubing with nitrogen gas for 15 min prior to
loading with DNA/gold microcarrier solution.
3.If using PVP, make a new stock of PVP in ethanol using a
fresh bottle of alcohol.
4.Resuspend gold/DNA pellet prior to first ethanol wash.
5.After drawing off the ethanol, quickly turn the Gold-Coat
tubing with your fingers prior to rotating.
8.3 Helios Gene Gun Operation
Problem LED display not lit.
Possible solutions Battery missing or discharged; replace battery.
Problem Device does not fire (helium burst does not occur).
Possible solutions 1. Check that the helium tank and regulator knobs are opened
and that there is sufficient helium in the tank.
2.Check that the helium hose is properly connected.
3.Check that the battery is good and that the LED display
indicates that a cartridge is in firing position.
Problem The first cartridge fires well but gold is not delivered in
subsequent cartridges.
Possible solutions Check that the O-rings are in place and are not worn (see Section 10.2).
Check that the cartridge holder is properly positioned in the Gene
Gun (see Section 5.2).
Problem The device fires but gold is not delivered.
Possible solutions 1. Check that the O-rings are in place and are not worn.
2.Check that the barrel liner is tight (see Section 10.2).
3.Check that the cartridge holder is properly positioned in the
Gene Gun (see Section 5.2).
4.Helium pressure is too low; increase the discharge pressure.
5.Cartridge preparation is bad; make new cartridges.
6.Too much PVP in tubes; increase helium pressure or reduce
PVP concentration.
Problem A discharge of helium occurs from the gun after first turning on
the helium regulator and pressurizing the system.
Possible solutions Open the helium regulator quickly one or two turns and pressurize the
system to > 100 psi. The pressure can then be re-adjusted to another setting.
If continuous helium discharge is observed after this, contact Bio-Rad.
8.4
In Vitro
and
in Vivo
Targeting
Problem Gold does not penetrate skin or organ.
Possible solutions 1. Check that the helium pressure has not dropped below the
desired delivery pressure.
2.Increase the PVP concentration in the cartridge preparations.
3.Increase the delivery pressure.
4.Check that the gold has been discharged from the cartridge
after firing.
5.Use larger gold particles.
Problem Poor or no expression.
36
Page 41

Possible solutions 1. Make sure DNA is resuspended in TE or distilled water, not saline.
2.Check that DNA has been precipitated onto the microcarriers
(see Section 10.7).
Problem Attached cells are lifting off the center of the plate after delivery
of gold particles, or there is a dead zone in the center of the target.
Possible solutions 1. Lower the helium pressure and/or lower the microcarrier
loading rate.
Problem Contamination of the cell culture.
Possible solution Wipe the end of the barrel with ethanol or dilute bleach.
Section 9
Product Information
9.1 Helios Gene Gun System
The two catalog numbers for the two Helios Gene Gun Systems (100/120V and 220/240V)
and component descriptions are as follows:
Catalog
Number Product Description
165-2431 Helios Gene Gun System, 100/120 V includes
165-2411 Helios Gene Gun Kit, that includes
Gene Gun Unit, 1
165-2416 O-rings, Helios Gene Gun, 5
165-2417 Barrel Liner, 5
165-2426 Cartridge Holder, white, 5
165-2435 Cartridge Extractor Tool, 1
165-2436 Battery, 9 V, 1
165-2412 Helium Hose Assembly, with Swagelock Quick-Connect fittings, 1
165-2413 Helium Regulator, CGA 580 female fitting, with pressure relief
valve. Maximum pressure 2,600 psi., 1
165-2418 Tubing Prep Station, includes Tubing Prep Station, 100/120 V,
Tubing Support Cylinder, 1 Power Cord, 1, O-rings, Tubing Prep
Unit, 5 (165-2419), Nitrogen Regulator Hose, Nalgene, 12 ft,
3/16” Barb to Male Luer-Lock Fitting, 2, Nitrogen Flow Meter Fitting,
1/8” Barb-to-Male Luer-Lock Fitting, 2, Allen wrench, 5/64”, 1,
Syringe Holder, 10 cc, 1
165-2421 Syringe Kit, 1, includes: Syringe Adaptor Tubing, Silicone, 5 ft,
0.104” ID x 0.192” OD, 1, Syringe 10 cc, 5, Syringe Adaptor
Fitting, 1/8” Barb-to-Female Luer-Lock Fitting, 5
165-2422 Tubing Cutter, Helios Gene Gun, that includes Tubing Cutter Unit
and Razor blades, 10 (165-2423)
165-2424 Helios Gene Gun Optimization Kit, that includes
165-2262 0.6 micron gold, 0.25 g
165-2263 1.0 micron gold, 0.25 g
165-2264 1.6 micron gold, 0.25 g
165-2440 Cartridge Kit, Helios Gene Gun, 1, that contains PVP
37
Page 42

(polyvinylpyrrolidone, MW 360,000), 0.5 g, Cartridge Collection/
Storage Vials, 5, Dessicant Pellets, 5, Gold-Coat Tubing, 50 ft.
165-2432 Helios Gene Gun System, 220/240 V, as above for 165-2431, except
165-2420 Tubing Prep Station, 220/240 V, 1
9.2 Spare Parts
Part
Number Product Description
9204987 Door, Battery Compartment, Gene Gun
9204988 Cover, Molded Nose
9204995 External Barrel Handle
9101503 Screw, 6-32 x 1/4, FH
9100453 Thumbscrew, Nylon, 1/4 x 20 x 1”
9100620 Thumbscrew, Nylon, 1/4 x 20 x 1/2”
8004478 Assembly, C-shaft (Tube Support Assembly)
9009712 Fuse, 5 x 20 mm, 0 .125A
9007296 Fuse, 5 x 20 mm, 0.25A
9400359 Sealant, Pipe
9109223 Fitting, Elbow, Female Luer
9301185 Kit, Helium regulator, soft goods (O-rings), version A
165-2436 9 V Battery
165-2423 Razor Blades, 10
165-2416 O-rings, Gene Gun
165-2417 Barrel Liner
165-2426 Cartridge Holder
9.3 Gene Gun Specifications
Physical
Dimensions Approximately 8” x 10”
Weight 3.15 lbs
Construction Super Epoxy or Polycarbonate
Cylinder Acetal
Barrel Liner Ryton
O-rings Viton
Electrical
Maximum Current 10 mA peak
Voltage Input 9 VDC alkaline battery, replaceable
Battery Life 1,000 discharges in continuous use
Functional
Input Pressure 600 psi maximum Helium
Regulator Relief Pressure 700 psi ± 35 psi at regulator assembly
Regulator Adjustment 800 psi limit maximum
Discharges 12 per cylinder revolution
Indexing Manual
38
Page 43

Environmental
Operating 50 °F ( 10 °C) to 90 °F (32 °C) temp.
30–80 % humidity
Storage 32 °F (0 °C) to 140 °F (60 °C) temp.
10–90 % humidity
Tubing Prep Station Specifications
Physical
Dimensions Approximately 33.5” x 4”
Weight 11.2 lbs
Construction Aluminum and Acrylic
Electrical
Maximum Current 62 mA/125 mA peak
Voltage Input 220–240 Vac/100–120 Vac
Input Frequency 50/60 Hz
Functional
Input Pressure 30 psi N
2
Relief Pressure 30 psi ± 1.5 psi at regulator assembly
Regulator Adjustment TDB psi limit maximum
Speed 30 RPM nominal
Tubing Fill manual
Environmental
Operating 50 °F (10 °C) to 90 °F (32 °C) temp
30–80 % humidity
Storage 32 °F (0 °C) to 140 °F (60 °C) temp
10–90 % humidity
Section 10
Appendices
10.1 Precipitation of RNA onto microcarriers
Primary RNA transcripts and mRNA may be effectively delivered in vivo and in vitro
using the Helios Gene Gun System (Qiu et al., 1996). The procedure is similar to that for
DNA, but the precipitation step is performed with ammonium acetate and 2-propanol.
Materials
In addition to those identified in Section 5.2:
Purified RNA preparation (e.g. capped, polyadenylated, or oligo dT-selected mRNA).
5 M ammonium acetate
2-propanol
Experiments involving RNA require careful technique to prevent RNA degradation. In the studies published to date, it was not necessary to pre-treat the gold particles to prevent RNase contamination.
Procedure (sufficient for one length of Gold-Coat tubing):
1. In a 1.5 ml microfuge tube, weigh out 25 mg gold particles.
39
Page 44

2. To measured gold, add aqueous solution of mRNA preparation.
The ratios of RNA to gold used are similar to those used for DNA (1–15 µg RNA/mg particles).
3. Add 1/10 volume of 5 M ammonium acetate and 2 volumes of 2-propanol, mix by vor-
texing. Place the microfuge tube at -20 °C for 1 hour.
4. Proceed with Section 5.2, steps 9–12.
10.2 Replacing the O-rings and Barrel Liner on the Helios Gene
Gun
Fig. 22. Location of barrel liner and O-rings in the Gene Gun. The barrel (cover) is removed for visual clar-
ity but is not required for barrel liner removal/insertion.
The Helios Gene Gun has three user-serviceable parts: the barrel liner and two O-rings
(Figure 22). The barrel liner screws into the barrel of the Gene Gun. It is a molded plastic
piece with the correct shape to permit optimum spread and penetration of the microcarriers at
the target site. It may be autoclaved between experiments or replaced.
To remove the barrel liner:
Hold the exposed part of the barrel liner (the spacer) and turn counterclockwise 4–5 turns
(see Figure 5).
To insert the barrel liner:
1. Remove the cartridge holder from the Gene Gun.
2. Place the barrel liner into the Gene Gun and turn clockwise until the threads just bind.
Do not overtighten.
The O-rings function to keep the helium from expanding around the tube in the firing
position in the cartridge holder. One O-ring is inserted at the end of the barrel liner that makes
contact with the cartridge holder; the other O-ring surrounds the exit port of the main valve.
Both O-rings fit in grooves with center supports.
40
Page 45

To replace the O-ring on the end of the barrel liner:
1. Remove the barrel liner.
2. Remove the old O-ring from the barrel liner.
3. Place a new O-ring in the groove and press it in gently. Do not use grease or adhesive.
To replace the O-ring around the exit port of the main valve:
1. Using a pin or syringe needle, poke the O-ring from the edge to pop it out. Be careful
not to scratch the surface of the valve.
2. Place a new O-ring in the groove and press it in gently. Do not use grease or adhesive.
10.3 Replacing the O-ring on the Tubing Prep Station
The Tubing Prep Station has two user-serviceable parts: O-rings at the end of the tubing
support cylinder nearest the motor. If the O-rings are worn, nitrogen may leak around them and
the amount of nitrogen indicated on the flowmeter will not be passing through the tubing. Under
normal use, the lifetime of the O-rings should be longer than 1 year. The O-rings may be changed
as follows (see Figure 24):
1. Remove the tubing support cylinder by pushing it to the right to contract the spring on the sup-
port bar, then raise the left side up and away from the base of the unit.
2. The outer O-ring should be located in the slot at the end of the tubing support cylinder,
although it may remain in the support on the motor housing. It may be removed with a fin-
gernail or a blunt object, such as a toothpick. If worn, replace the O-ring with a new one.
3. The inner O-ring is at the end of the tubing support cylinder next to the stem. To replace
it, remove the stainless steel stem at the end of the tubing support cylinder by loosening
the hex nut on the lower surface of the tubing support cylinder one-eighth turn using a
5/64" Allen wrench. The stem and spur gear can be pulled out of the tubing support cylin-
der. Remove the O-ring from the slot in the stem with a fingernail or blunt object and
replace if worn. Insert the stem into the end of the tubing support cylinder; press gently
on the end to hold the O-ring in place and tighten the hex nut. Replace the tubing support
cylinder in the base of the Tubing Prep Station.
Fig. 24. Replacing the O-rings on the Tubing Prep Station. The O-rings are located in the tubing support
cylinder on the side next to the motor housing. One O-ring (A) fits into a slot on the outside edge of the stem
at the end of the tubing support cylinder. The other O-ring (B) is on the inside surface of the stem and can
be removed only after loosening the hex nut and removing the stem from the tubing support cylinder.
41
Page 46

10.4 Replacing the razor blade on the Tubing Cutter and disassembly of the unit
The cutting edge of the Tubing Cutter is a standard, single edge razor blade. Because cutting is done only on one end of the blade at once, it can be rotated after the first end becomes dull.
The first sign of a dull blade will be incomplete cutting of two adjacent cartridges. Front refers
to the side of the Tubing Cutter with the tubing channels. Right, left, and back refer to the sides
of the Tubing Cutter when the user faces the front of the unit (see Figure 23).
1. Loosen the red locking knob on the back of the Tubing Cutter by turning it approximately one
full turn until the lock block on the rear of the cutting arm is free to move.
2. Move the lock block to the right to free the razor blade retaining screw on the right side.
3. Lift up on the right side of the razor blade until it can be lifted free of the Tubing Cutter.
4. Insert a new blade (or rotate the old blade 180˚) by inserting the notch on the left side of the
blade into the retaining screw on the left side of the cutting arm.
5. Hold the razor blade so that the notch on the blade is next to retaining screw on the right side
of the cutting arm. Using the red locking knob, move the retaining screw into the notch in the
razor blade and turn the locking knob clockwise to lock the razor blade in place.
Fig. 23. The Tubing Cutter.
The Tubing Cutter may be disassembled for cleaning. For safety, remove the razor blade
prior to disassembly.
1. Place the Tubing Cutter in front of you on a solid surface so that you are facing the front of
the unit.
2. Hold the base between the thumb and index finger of your right hand. At the same time,
push the left side of the Tubing Cutter arm to the right with the index finger of your left hand.
This will disengage the arm from the pivot pin on the left side of the base and from the retain-
ing pin on the right side of the base — the tension on the spring will push the arm up.
42
Page 47

3. Raise the arm up and away from the base.
4. The Tubing Cutter may be cleaned with soap and water and /or with 70% or 100% ethanol.
Do not autoclave the Tubing Cutter.
5. Assembly is the reverse of disassembly. With the spring in place, lay the cutting arm, correctly
oriented, on top of the base. With the index and middle fingers of your left hand, press down
on the left side of the arm to compress the spring about 3/16”. Simultaneously, with the index
and middle fingers of your right hand, push the arm to the left to engage the cutting arm
under the stainless steel pivot pin on the left side of the base and under the stainless steel
retaining pin on the right side of the base.
10.5 Cleaning and Sterilizing the Helios Gene Gun
In situations where sterility is a concern, such as in vitro targeting, the spacer may be
brought as close to the target surface as possible without actually touching the surface.
Alternatively, by wiping down the end of the barrel liner with 70% alcohol or dilute bleach
followed by a rinse with distilled water, the spacer may touch the target surface.
It is possible to sterilize certain parts of the instrument by autoclaving. The following
parts may be autoclaved:
Barrel liner
Cartridge holder
Cartridge extractor tool
The following items should never be autoclaved or imersed in liquid:
Gene Gun
Tubing Prep Station
Helium Regulator
These parts, along with the Tubing Cutter, may be cleaned using a cloth and a mild soap
solution. Do not autoclave the Tubing Cutter.
Although Gold-Coat tubing may be autoclaved, this is not recommended since it may
result in water condensation inside the tubing which will lead to poor quality cartridges.
10.6 Testing Cartridges for Microcarrier Penetration and Density
(Optional)
Either of these procedures may be used to roughly determine the quality of the cartridge
preparation. These procedures give only qualitative results and are therefore best when they
can be compared against cartridges that have been shown to produce positive results in a biological assay. The results are very dependent on the size of the microcarriers and on the amount
of PVP used in loading the microcarriers into the Gold-Coat tubing.
Fig. 25. Representative bombardment showing penetration of 1.6 µ gold particles into Parafilm at
(left to right) 100, 200, 300, 400, 500, and 600 psi using the Helios Gene Gun.
43
Page 48

Discharge into Parafilm
Materials
Parafilm laboratory film (American Can Company)
Glass plate
Procedure
1. Cut a piece of Parafilm from the roll; a 1" length is required for each cartridge to be
assayed.
2. Smooth and press the Parafilm laboratory film (waxy surface down) onto the glass plate.
Fold the edges around the sides then peal away the protective paper.
3. Connect the helium regulator, helium hose, and Helios Gene Gun as described in Section 4.2.
4. Pressurize the system as described in Section 5.3.
5. Discharge a cartridge at the test psi into a strip of Parafilm laboratory film. At this point,
one can roughly determine the quality of the microcarrier preps by examining the pat-
tern of gold particles. When examining the resulting pattern, it should appear circular
with a dark center (Figure 25). The discharged cartridges should be completely empty.
Spot darkness Proportional to particle penetration
Spot width Proportional to particle spread
Spot shape Uniformity of shot
Discharge into 3% Water Agar
Materials
Slides, coverslips and mounting solution
Microscope with 10X eyepiece micrometer
3% water agar in 60 mm petri dishes
Procedure
1. Discharge a cartridge at the test psi into 3% water agar. At this point, one can roughly
determine the quality of the microcarrier preps by examining the pattern of gold parti-
cles. When examining the resulting pattern, it should appear circular with a dark center.
The discharged cartridges should be completely empty.
Additionally, one can also examine microcarrier depth penetration into the water agar
microscopically. Cut a thin slice approximately 0.5 cm long through the center of the
agar target. Mount the slice onto a slide with mounting solution and a cover slip and ana-
lyze for microcarrier depth and concentration.
2. A band of microcarriers will be visible in the agar with increasing particle sizes at
increased depths. The researcher will be able to determine areas of high, medium, and
low microcarrier density in each slice (Figure 26).
3. To determine the depth of penetration, align the 0 of an eyepiece micrometer at the upper
surface of the agar and measure the distance to the arbitrary floor of the microcarrier dis-
tribution. This value is the microcarrier depth.
The distribution pattern of the microcarriers is bell-curve shaped with respect to depth
and area. The highest microcarrier density is concentrated at the center of the shot and at the
surface of the target site. For this reason, in order to obtain a truly representative microcarrier distribution cross-section, be careful to examine the center and full length of your target site.
44
Page 49

Fig. 26. Representative cross-section showing penetration of gold particles into water agar after
discharge with a helium pulse.
10.7 Quantitation of DNA in Cartridges
This procedure can be used for estimating the amount of DNA in cartridges when 0.5 µg
or more of DNA has been loaded per tube.
Materials
10 mM Tris, pH 8.0, 1 mM EDTA 1,000 µl micropipettor and tips
Ultrasonic cleaner UV spectrophotometer
Vortexer Quartz cuvettes
Microfuge
Procedure
1. Place five 0.5" cartridges in a microfuge tube and add 500 µl of TE.
2. Sonicate and/or vortex to dislodge gold and solubilize the DNA. Spin for 5 min in a
microfuge to pellet gold.
3. On a spectrophotometer determine the A
260
by reading against a TE blank. If 100% of the
DNA were precipitated onto the microcarriers, the A
260
readings should be as follows:
Plasmid/cartridge A
260
0.5 µg 0.1
1.0 0.2
2.0 0.4
4. DNA sample may also be analyzed by restriction enzyme digestion and analysis by agarose
gel electrophoresis.
45
Page 50

10.8 References
1. Albertini, M. R., Emler, C. A., Schell, K., Tans, K. J., King, D. M. and Sheeby, M. J., Cancer Gene
Ther., 3, In press (1996).
2. Andree, C., Swain, W. F., Page, C. P., Macklin, M. D., Slama, J., Hatis, D. and Eriksson, E., Proc.
Natl. Acad. Sci. USA, 91, 12188-12192 (1994).
3. Armaleo, D., Ye, G. N, Shark, K. B., Sanford, J. C. and Johnston, S. A., Curr. Genet. 17, 97-103
(1990).
4. Boynton, J. E., Gillham, N. W., Harris, E. H., Hosler, P. J., Johnson, A. M., Jones, A. R., Randolph-
Anderson, B. L., Robertson, D., Klein, T. M., Shark, K. B. and Sanford, J. C., Science 240,
1534-1538 (1988).
5. Cheng, L., Ziegelhoffer, P. R. and Yang, N. S., Proc. Natl. Acad. Sci. USA, 90, 4455-4459 (1993).
6. Eisenbraun, M. D., Fuller, D. H. and Haynes, J. R., DNA Cell Biol., 12, 791-797 (1993).
7. Fuller, J. T., Fuller, D. H., McCabe, D., Haynes, J. R., and Widera, G., Ann. N. Y. Acad. Sci., 772,
282-284 (1995).
8. Fuller, J. T., Fuller, D. H., McCabe, D., Haynes, J. R., and Widera, G., Vaccines, 96,87-90 (1996).
9. Fynan, E. F., Webster, R. G., Fuller, D. H., Haynes, J. R., Santaro, J. C. and Robinson. H. L., Proc.
Natl. Acad, Sci. USA, 90, 11478-11482 (1993).
10. Haynes, J. R., McCabe, D. E., Swain, W. F., Widera, G., Fuller J. T., J. Biotechnol., 44, 37-42
(1996)
11. Johnston, S. A., Anziano, P. Q., Shark, K., Sanford, J. C. and Butow, R. A., Science240, 1538-1541
(1988).
12. Keller, E. T., Burkholder, J. K., Shi, F., Pugh, T. D., McCabe, D., Malter, J. S., MacEwen. E. G.,
Yang, N. S. and Ershler, W. B., Cancer Gene, 3, In press (1996).
13. Klein, T, M., Fromm, M., Weissinger, A., Tomes, D., Schaaf, S., Sletten, M., and Sanford, J. C.,
Proc. Natl. Acad. Sci. USA, 85, 4305-4309 (1988).
14. Klein, T. M., Wolf, E. D., Wu, R., Sanford, J. C., Nature 327, 70-73 (1987).
15. Mahvi, D. M., Burkholder, J. K., Turner, J., Culp, J., Malter, J. S., Sondel, P. M., and Yang, N. S.,
Human Gene Ther., In press (1996).
16. McCabe, D. E., Swain, W. F., Martinell, B. J. and Christou, P., Bio/Technology, 6, 923-926 (1988).
17. Pertmer, T. M., Eisenbraun, M. D., McCabe, D., Prayaga, S. K., Fuller, D. H., and Haynes, J. R.,
Vaccine, 13, 1427-1430 (1995)
18. Qiu, P., Ziegelhoffer, P., Sun, J. and Yang, N. S., Gene Therapy, 3, 262-268 (1996).
19. Rakhmilevish, A. L., Turner, J., Ford, J. M., McCabe, D., Sun, W. H., Sondel, P. M., Grote, K.
and Yang, N. S., Proc. Natl. Acad. Sci. USA, 93, In press (1996).
20. Reba, I., Scientific American,214, 84-92 (1966).
21. Shark, K. B., Smith, F. D., Harpending, P. R., Rasmussen. J. L. and Sanford, J. C., Appl. Environ.
Microbiol., 57, 480-485 (1991).
22. Smith, F. D., Harpending, P. R., and Sanford, J. C.,J. Gen. Microbiol., 138, 239-248 (1992).
23. Sun, W. H., Burkholder, J. K., Sun, J., Culp, J., Turner, Joel, Lu, X. G., Pugh, T. D., Ershler, W. B.
and Yang, N. S., Proc. Natl. Acad. Sci. USA, 92, 2889-2893 (1995).
24. Sundaran, P., Xiao, W. and Brandsma, J. L., Nuc. Acids Res., 24, 1375-1377 (1996).
25. Tang, D. C., DeVit, M., and Johnston, S. A., Nature356, 152-154 (1992).
26. Williams, R. S., Johnston, S. A., Riedy, M., DeVit, M. J., McElligott, S. G. and Sanford, J. C.,
Proc. Natl. Acad. Sci. USA, 88, 2726-2730 (1991).
27. Xiao, W. and Brandsma, J.L., Nuc. Acids Res., 24, 2620-2622 (1996).
28. Yang, N. S., Burkholder, J., Roberts, B., Martinell, B. and McCabe, D., Proc. Natl. Acad. Sci. USA,
87, 9568-9572 (1990).
29. Zelenin, A. V., Titomirov A. V. and Kolesnikov, V. A., FEBS Lett., 244, 65-67 (1989).
The instruments used in these experiments vary. Use the above information as a starting point when optimizing bombardment conditions
with the Helios Gene Gun. Lower helium pressures should be tested early in the optimization process.
46
Page 51

10.9 Quick Guide to Operation
Before the Bombardment
1. Coat microcarriers with DNA, load into tubes, and prepare cartridges prior to day of exper-
iment.
2. Check helium supply (50 psi in excess of desired rupture pressure).
3. Clean and/or sterilize the Gene Gun, tube holders, and barrel liner as appropriate.
4. Connect the Gene Gun to a helium source.
5. Activate the Gene Gun: turn on the flow of helium to the desired pressure and with an empty
cartridge holder in place, make 2–3 “pre-shots” by engaging the safety interlock and firing
the trigger.
Firing the Device
1. Load cartridges into the cartridge holder and place in Gene Gun.
2. Prepare/position target cells for bombardment.
3. Bombard sample: engage safety interlock and press the firing trigger
After the Bombardment
1. Remove cartridge holder from Gene Gun.
2. Remove cartridges from cartridge holder.
3. Turn off the helium pressure to the system.
4. Turn the regulator value counterclockwise to de-pressurize the system.
5. Disconnect the helium hose and Gene Gun.
47
Page 52

Bio-Rad
M1652411 Rev C
Laboratories
Life Science
Group
Website www.bio-rad.com Bio-Rad Laboratories Main Office 2000 Alfred Nobel Drive, Hercules, CA 94547, Ph. (510) 741-1000, Fx. (510)741-5800
Also in: Australia Ph. 02 9914 2800, Fx. 02 9914 2889 Austria Ph. (01) 877 89 01, Fx. (01) 876 56 29 Belgium Ph. 09-385 55 11, Fx. 09-385 65 54
Canada Ph. (905) 712-2771, Fx. (905) 712-2990 China Ph. 86-10-62051850/51, Fx. 86-10-62051876 Denmark Ph. 45 39 17 99 47, Fx. 45 39 27 16 98
Finland Ph. 358 (0)9 804 2200, Fx. 358 (0)9 804 1100 France Ph. 01 43 90 46 90, Fx. 01 46 71 24 67 Germany Ph. 089 318 84-0, Fx. 089 318 84-100
Hong Kong Ph. 852-2789-3300, Fx. 852-2789-1257 India Ph. (91-11) 461-0103, Fx. (91-11) 461-0765 Israel Ph. 03 951 4127, Fx. 03 951 4129
Italy Ph. 39-02-216091, Fx.39-02-21609-399 Japan Ph. 03-5811-6270, Fx. 03-5811-6272 Korea Ph. 82-2-3473-4460, Fx. 82-2-3472-7003
Latin America Ph. 305-894-5950, Fx. 305-894-5960 Mexico Ph. 514-2210, Fx. 514-2209 The Netherlands Ph. 0318-540666, Fx. 0318-542216
New Zealand Ph. 64-9-4152280, Fx. 64-9-4152284 Norway Ph. 22-74-18-70, Fx. 22-74-18-71 Russia Ph. 7 095 979 98 00, Fx. 7 095 979 98 56
Singapore Ph. 65-2729877, Fx. 65-2734835 Spain Ph. 34-91-661-7085, Fx. 34-91-661-9698 Sweden Ph. 46 (0)8-55 51 27 00, Fx. 46 (0)8-55 51 27 80
Switzerland Ph. 01-809 55 55, Fx. 01-809 55 00 United Kingdom Ph. 0800-181134, Fx. 01442-259118
00-000 0099 Sig 031799Bulletin 0000 US/EG Rev A
 Loading...
Loading...