Page 1

Gene Transfer
Gene Pulser MXcell
Electroporation Guide
™
Page 2
To choose a starting
set of protocols,
search our library of
electroprotocols at
www.bio-rad.com/
genetransferprotocols/
http://www.ncbi.nlm.
* Note: The parameters
entered into the preset
on proven conditions
used for electroporation
of mammalian cells and
take advantage of the
Gene Pulser MXcell’s
ability to apply multiple
conditions simultaneously.
The preset protocols
or search
nih.gov/PubMed/.
protocols are based
can be edited and
stored in your user
file for future use.
Gene Pulser MXcell Electroporation Guide
The Gene Pulser MXcell
Electroporation Guide
The Gene Pulser MXcell electroporation system is filled with features that will enable
you to quickly optimize conditions for efficient deliver y of molecules into most eukaryotic
cells, including mammalian cells and plant protoplasts. This guide will assist you in
determining the parameters for obtaining efficient transfection of molecules into your
favorite cells while maintaining high cell viability.
Transfection efficiency and cell viability
Transfection and cell viability are affected by:
n
Cell type and its physiological condition and state prior to electroporation:
cells should be actively growing, healthy, and free of contamination
n
Temperature of buffer during electroporation: when using Gene Pulser® electroporation
buffer, electroporation should be performed at room temperature
n
Cell density: for most experiments using the 96-well plate, we recommend using
150 µl of cells/well at a density of 1 x 106 to 2 x 106 cells/ml in Gene Pulser
electroporation buffer
n
Concentration of the transfected molecule (RNA, DNA): ideally a concentration
of 5–40 µg/ml should be used for plasmid DNA and 10–100 nM for siRNA (only
Bio-Rad’s siLentMer™ Dicer-substrate siRNA duplexes)
n
The characteristics of the electric pulse
Electroporation protocols
Electroporation applies an electric pulse to cells to promote uptake of molecules (RNA,
DNA) into the cells. The electric pulse is defined by parameters that can be programmed
as protocols in the Gene Pulser MXcell. Two type of pulses can be delivered by the
Gene Pulser MXcell: the exponent ial waveform which is defined by voltage,
capacitance, and resistance or the square waveform which is defined by voltage,
pulse duration, and resistance.
The Gene Pulser MXcell can be programmed three ways:
n
Manually, under “Protocol Setup”
n
By creating a voltage gradient under “Gradient Protocol”
n
By using preprogrammed protocols under “Pre-Set Protocols”*
This guide will help you program the Gene Pulser MXcell whether you have prior
knowledge of optimal electroporation conditions for your cells or not.
2
Visit us on the Web at discover.bio-rad.com | Electroporation Optimization Overview
Page 3

Schematic of 96-well
electroporation plate
One well set
Gene Pulser MXcell Electroporation Guide
Glossary
Electroporation plate: a plate in which electroporation is performed; available in
12-, 24-, and 96-well formats
Mini protocol: a preprogrammed set of 4–6 different protocols delivered to 4–6 well sets
Parameter s: the physical constants (waveform, voltage, capacitance, duration,
resistance) that define the electric pulse
Preset protocols: a set of preprogrammed protocols designed for rapid screening of
multiple parameters that can be used as a template
Protocol/electroporation conditions: parameters defining the electric pulse
that will be delivered to specific well sets or wells on an electroporation plate
Waveform: defines the type of electric pulse delivered to the cells
n
The exponential waveform builds up a charge in a capacitor and when the charge
is applied to the sample the voltage delivered decays exponentially, until the charge
remaining is about 37% of the original pulse
n
The square waveform relies on a charge being applied to the cells for a set time
Well set: a set of four adjacent wells in a column on a 96-well plate; the same
electroporation conditions or protocol are applied to all the cells of a well set
Whole plate protocol: a preprogrammed set of 24 protocols delivered across
the entire plate
Abbreviations
Cgrad: capacitance gradient
D: duration of pulse
Dgrad: duration gradient
Exp: exponential
NP: number of pulses
P: pulse
Sqr: square
Vgrad: voltage gradient
Visit us on the Web at discover.bio-rad.com | Electroporation Optimization Glossary
3
Page 4
Gene Pulser MXcell Electroporation Guide
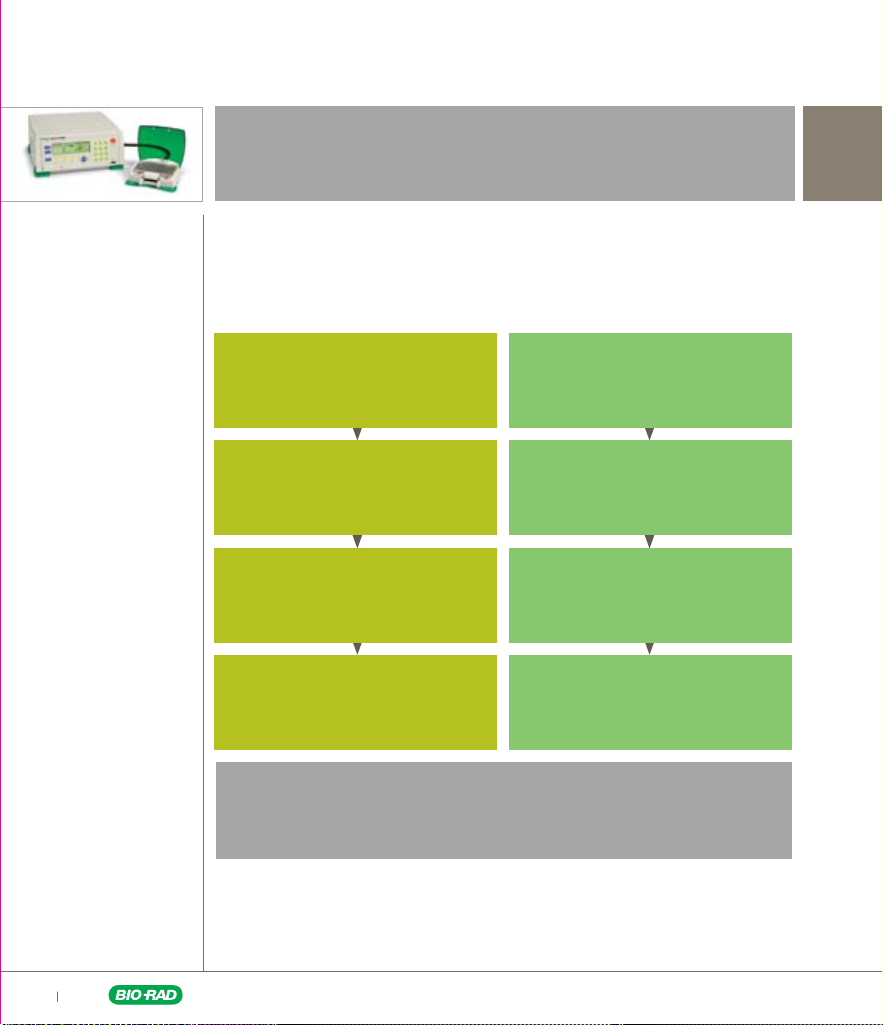
Electroporation Parameters Selection Pathways
The first parameter to identify is the waveform. This guide will take you down two
possible paths to identify other parameters that will yield the best transfection results.
These parameters can be identified in parallel using the MXcell system.
Identify Pulse Waveform
Exponential Waveform Square Waveform
Optimize Capacitance Optimize Voltage
Optimize Voltage Optimize Pulse Duration
Optimize Resistance Optimize Number of Pulses
Path taken to determine optimal electroporation conditions
4
Visit us on the Web at discover.bio-rad.com | Electroporation Optimization Pathways
Page 5
Gene Pulser MXcell Electroporation Guide
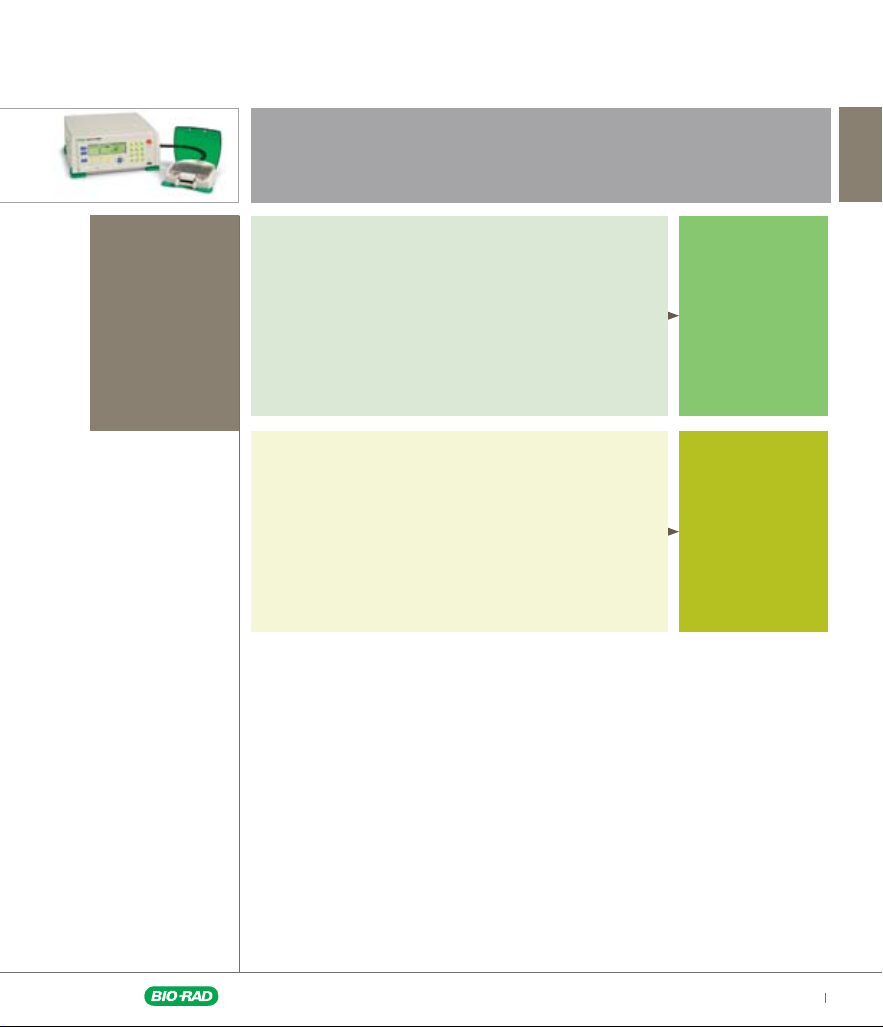
Start Your Protocols Selection Here
To choose a starting
set of protocols,
search our library of
electroprotocols at
www.bio-rad.com/
genetransferprotocols/
or search
http://www.ncbi.nlm.
nih.gov/PubMed/.
Known electroporation conditions
You know the electroporation conditions or found
a suitable set of protocols in our library
You need a quick confirmation that your existing
protocol is optimal
You know the waveform
Unknown electroporation conditions
You are working with a new cell line or cell type
You have no information about electroporation conditions
Go to page 6
Go to page 7
Visit us on the Web at discover.bio-rad.com | Protocols Selection
5
Page 6
Gene Pulser MXcell Electroporation Guide
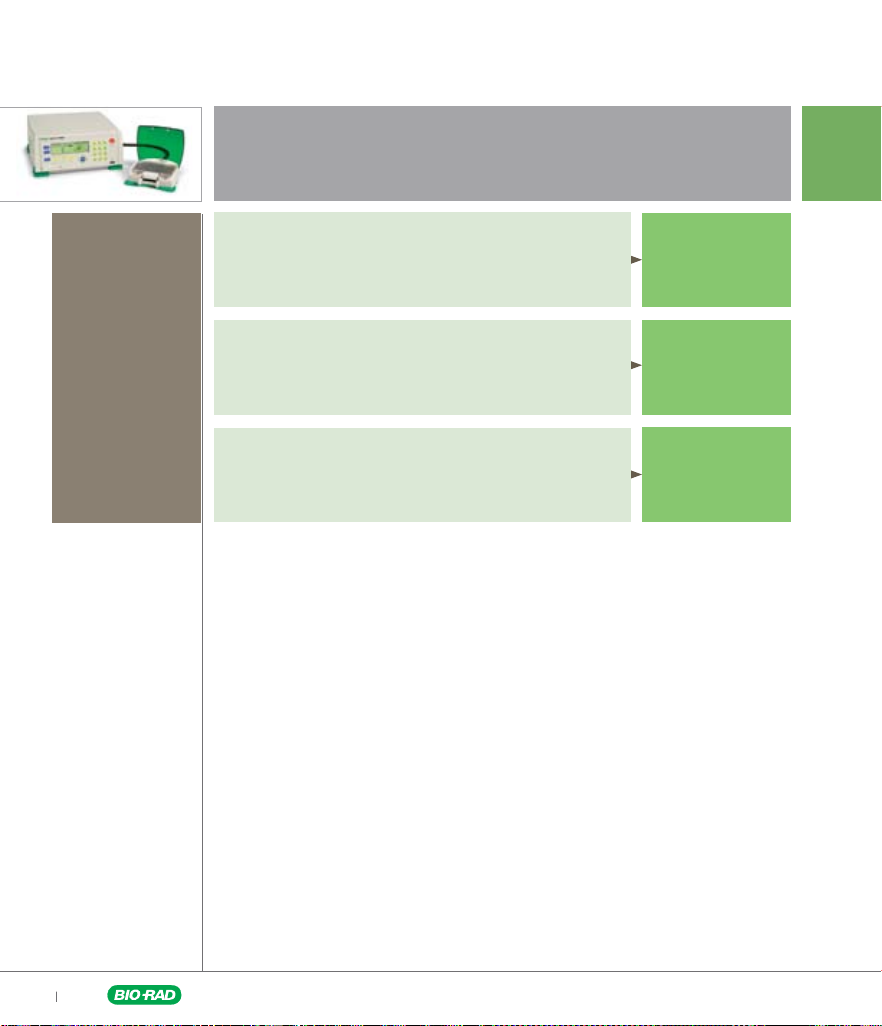
Known Electroporation Conditions
Cell number
Based on the
number of cells
availabl e for your
experime nt, you
will use eithe r a
whole or a partia l
electro poratio n
plate (4– 6 well sets).
Cell number <5 x 10
6
Use Mini Protocols
Cell number >5 x 10
6
Use Whole Plate Protocols
Multiple cell lines
Use Whole Plate Protocols
Go to pages
9–10
Go to pages
11–12
Go to page 13
6
Visit us on the Web at discover.bio-rad.com | Protocols: Known Conditions
Page 7

Gene Pulser MXcell Electroporation Guide
Unknown Electroporation Conditions
Cell number
Based on the
number of cells
availabl e for your
experime nt, you
will use eithe r a
whole or a partia l
electro poratio n
plate (4– 6 well sets).
Cell number <5 x 10
6
Use Mini Protocols
Cell number >5 x 10
6
Use Whole Plate Protocols
Go to page 8
Go to page 8
Visit us on the Web at discover.bio-rad.com | Protocols: Unknown Conditions
7
Page 8

Gene Pulser MXcell Electroporation Guide
Unknown Electroporation Conditions
Check all well
sets after each
electroporation for
transfection efficiency
and cell viability.
For an overview of
common analytical
methods, go to
pages 15–17.
Cell number <5 x 10
6
Use preset protocols Opt mini 96-well /Sqr, Ex p to identify waveforms and
other initial conditions.
Square waveform Exponential waveform
1 2 3 4 5 6
200 V
A
Good for
first time
users!
2,000 µF
20 ms
B
C
D
Cell number >5 x 10
250 V
2,000 µF
20 ms
6
300 V
2,000 µF
20 ms
250 V
350 µF
1,000 W
250 V
500 µF
1,000 W
Use preset protocols Opt 96-well/Exp, Sqr to identify waveforms and
other initial conditions.
1 2 3 4 5 6 7 8 9 10 11 12
150 V
200 V
250 V
300 V
350 V
400 V
250 V
250 V
250 V
250 V
A
350 µF
B
C
D
150 V
E
20 ms
F
Square Exponential
G
H
350 µF
200 V
20 ms
350 µF
250 V
20 ms
350 µF
300 V
20 ms
350 µF
350 V
20 ms
350 µF
450 V
20 ms
200 µF
250 V
5 ms
250 µF
250 V
10 ms
350 µF
250 V
15 ms
500 µF
250 V
20 ms
250 V
750 µF
250 V
25 ms
250 V
750 µF
1,000 W
250 V
1,000 µF
250 V
30 ms
Would you l ike to fur ther op timi ze? Use the p reset p rotoco ls on pages 9–10 ( if <5 x 106 cells)
or pages 11–12 (if >5 x 106 cells); for manual programming, go to pag e 14.
Good result s? Congratula tions! Be reward ed for your work and share your protoc ol
with scient ists worldw ide. Visit ww w.bio-ra d.com/genet ransfer protocols/.
8
Visit us on the Web at discover.bio-rad.com | Unknown Conditions: Setup
Page 9

Gene Pulser MXcell Electroporation Guide
Choose the preset
mini protocols that
best match your
known conditions and
edit parameters as
needed, following the
instructions.
Check all well
sets after each
elect ropora tion
for transfecti on
effici ency and
cell viabilit y.
For an overview of
common analytical
methods, go to
pages 15–17.
Electroporation Conditions Are Known
300 V
350 µF
6
250 V
200 µF
250 V
350 µF
Keep the preset values for capacitance
and resistance:
250 V
n
500 µF
Enter the known voltage value
(median voltage) in well set ABCD 2
n
Enter the median voltage decreased
by 50 V in well set ABCD 1
n
Enter the median voltage increased
by 50 V in well set ABCD 3
n
Enter the median voltage value in well
sets ABCD 4–6
n
Save the protocols under a new name
Cell number <5 x 10
Exponential Waveform Protocols
Use preset protocols Opt mini 96-well/Exp to identify optimal voltage
and capacitance.
1 2 3 4 5 6
200 V
350 µF
250 V
350 µF
A
B
C
D
Would you l ike to fur ther op timi ze? For manual programming, go to page 14.
Good result s? Congratula tions! Be reward ed for your work and share your protoc ol
with scient ists worldw ide. Visit ww w.bio-ra d.com/genet ransfer protocols/.
Visit us on the Web at discover.bio-rad.com | Known Conditions: Exponential Setup — Mini
9
Page 10

Gene Pulser MXcell Electroporation Guide
Choose the preset
mini protocols that
best match your
known conditions and
edit parameters as
needed, following the
instructions.
Check all well
sets after each
elect ropora tion
for transfecti on
effici ency and
cell viabilit y.
For an overview of
common analytical
methods, go to
pages 15–17.
Electroporation Conditions Are Known
300 V
20 ms
10 ms
2 P
6
250 V
15 ms
7 ms
3 P
250 V
20 ms
Keep the preset values for pulse duration,
capacitance, and resistance:
250 V
n
25 ms
Enter the known voltage value
(median voltage) in well set ABCD 2
n
Enter the median voltage decreased
by 50 V in well set ABCD 1
n
Enter the median voltage increased
by 50 V in well set ABCD 3
n
Enter the median voltage value in well
sets ABCD 4–6
n
Save the protocols under a new name
Keep the preset values:
n
Enter the voltage that yielded the
best results from the Opt mini
96-well/Sqr experiment
n
Save the protocols under a new name
Cell number <5 x 10
Square Waveform Protocols
Use preset protocols Opt mini 96-well/Sqr to identify optimal voltage
and pulse duration.
1 2 3 4 5 6
200 V
20 ms
250 V
20 ms
A
B
C
D
For further optimization of your experiment, identify the optimal number of pulses.
Square Waveform Protocols
Use preset protocols Opt 96-well/Sqr, NP, D to identify optimal number of pulses.
1 2 3 4
20 ms
A
B
C
15 ms
1 P
2 P
D
Would you l ike to fur ther op timi ze? For manual programming, go to page 14.
Good result s? Congratula tions! Be reward ed for your work and share your protoc ol
with scient ists worldw ide. Visit ww w.bio-ra d.com/genet ransfer protocols/.
10
Visit us on the Web at discover.bio-rad.com | Known Conditions: Square Setup — Mini
Page 11

Gene Pulser MXcell Electroporation Guide
Choose whole plate
preset protocols that
best match your
known conditions
and edit parameters
as needed, following
the instructions.
Check all well
sets after each
elect ropora tion
for transfecti on
effici ency and
cell viabilit y.
For an overview of
common analytical
methods, go to
pages 15–17.
Electroporation Conditions Are Known
Cell number >5 x 10
Exponential Waveform Protocols
Use whole plate preset protocols 96-well/Exp, Vgrad, Cgrad to identif y
optimal voltage and capacitance.
1 2 3 4 5 6 7 8 9 10 11 12
A
B
C
D
E
F
G
H
Use the top half of the electroporation plate to identify optimal voltage.
n
n
n
n
Use the bottom half of the electroporation plate to identify optimal capacitance.
n
n
n
n
Save the protocols under a new name.
Would you l ike to fur ther op timi ze? For manual programming, go to page 14.
100 100 100 200 200 200 300 300 300 400 400 400
DV
(V)
200 200 200 350 350 350 500 500 500 1,000 1,000 1,000
DC
(µF)
Enter the known capacitance value in well sets ABCD 1–12, or keep preset values
if unknown
Enter the known voltage (median voltage) value in well sets ABCD 4–6
Vary the voltage by 100 V increments around the median value in other well sets
Keep the preset values for all other parameters
Enter the known voltage value in well sets EFGH 1–12
Enter the known capacitance (median capacitance) value in well sets EFGH 4–6
Vary capacitance by 5–10% around the median value in other well sets or use
preset values
Keep the preset values for all other parameters
Good result s? Congratula tions! Be reward ed for your work and share your protoc ol
with scient ists worldw ide. Visit ww w.bio-ra d.com/genet ransfer protocols/.
6
Visit us on the Web at discover.bio-rad.com | Known Conditions: Exponential Setup — Whole Plate
11
Page 12

Gene Pulser MXcell Electroporation Guide
Choose whole plate
preset protocols that
best match your
known conditions
and edit parameters
as needed, following
the instructions.
Check all well
sets after each
elect ropora tion
for transfecti on
effici ency and
cell viabilit y.
For an overview of
common analytical
methods, go to
pages 15–17.
Electroporation Conditions Are Known
Cell number >5 x 10
Square Waveform Protocols
Use whole plate preset protocols 96-well/Sqr, Vgrad, Dgrad to identify
optimal voltage and pulse duration.
1 2 3 4 5 6 7 8 9 10 11 12
A
100 100 100 200 200 200 300 300 300 400 400 400
B
DV
(V)
C
D
E
F
G
H
Use the top half of the electroporation plate to identify optimal voltage.
n
n
n
n
Use the bottom half of the electroporation plate to identify optimal pulse duration.
n
n
Save the protocols under a new name.
10 10 10 15 15 15 20 20 20 30 30 30
DD
(ms)
Enter the known capacitance value in well sets ABCD 1–12, or keep preset values
if unknown
Enter the known voltage (median voltage) value in well sets ABCD 4–6
Vary the voltage by 100 V increments around the median value in other well sets
Keep the preset values for all other parameters
Enter the known voltage value in well sets EFGH 1–12
Keep the preset values for all other parameters
6
Would you l ike to fur ther op timi ze? For manual programming, go to page 14.
Good result s? Congratula tions! Be reward ed for your work and share your protoc ol
with scient ists worldw ide. Visit ww w.bio-ra d.com/genet ransfer protocols/.
12
Visit us on the Web at discover.bio-rad.com | Known Conditions: Square Setup — Whole Plate
Page 13

You work with multiple
cell lines that require
both waveforms.
Use whole plate preset
protocols to deliver
exponential and
square waveforms.
Edit parameters as
needed, following the
instructions.
Check all well
sets after each
electroporation for
transfection efficiency
and cell viability.
For an overview of
common analytical
methods, go to
pages 15–17.
Gene Pulser MXcell Electroporation Guide
Multiple Cell Lines
Exponential and Square Waveforms Protocols
Use preset protocols Uniform 96 -well/Exp, Sqr to identif y optimal
conditions for the different cell lines.
Expo nent ial wa vefor m Squa re wave form
1 2 3 4 5 6 7 8 9 10 11 12
250 V
A
350 µF
B
C
D
E
F
G
H
Use the lef t half of the plate to deliver exponential-decay pulses.
n
Enter your known voltage (median value) and vary capacitance around the known
value in well sets ABCD 1–6
n
Enter your known capacitance value and vary voltage around the median voltage
value in well sets EFGH 1–6
n
Use the preprogrammed setting for all other values
Use the right half of the plate to deliver square-wave pulses.
n
Enter your known median voltage value and vary pulse duration around the preset
value in well sets ABCD 7–12
n
Enter your known pulse duration or preset value and vary voltage around the median
voltage value in well sets EFGH 7–12
Save the protocols under a new name.
250 V
20 ms
Would you l ike to fur ther op timi ze? For manual programming, go to page 14.
Good result s? Congratula tions! Be reward ed for your work and share your protoc ol
with scient ists worldw ide. Visit ww w.bio-ra d.com/genet ransfer protocols/.
Visit us on the Web at discover.bio-rad.com | Known Conditions: Multiple Cell Lines
13
Page 14

Gene Pulser MXcell Electroporation Guide
Electroporation Protocols Decision Tree
Once the best waveform is identified, use this tree to program the MXcell system to
improve other parameters.
Initial Experimental Results
Exponential Waveform Square Waveform
Optimize Capacitance
Manually program a capacitance
gradient (5–10% increments)
around the value that yielded the
best results, keeping all other
parameters constant.
Optimize Voltage
Using the optimized capacitance
value, program a voltage gradient
(5–10% increments) around the value
that yielded the best results,
keeping all other parameters constant.
Use the optimized voltage and keep all
other parameters constant. Vary pulse
Optimize Voltage
Program a voltage gradient around
the value that yielded the best
results, keeping all other
parameters constant.
Optimize Pulse Duration
values at 5 ms intervals around the
pulse that yielded the best
results (see protocols on page 10).
Optimize Resistance
Using the optimized capacitance and
voltage values, manually program a
resistance gradient (5–10% increments)
around the values that yielded the
best results, keeping all other
parameters constant.
14
Visit us on the Web at discover.bio-rad.com | Electroporation Decision Tree
Optimize Number of Pulses
Use 2–3 shorter pulses that add
up to the pulse duration that yielded
the best results (see protocols
on page 10).
Page 15

Gene Pulser MXcell Electroporation Guide
Electroporation Protocol
Materials
Needed
n
Actively growing,
freshly passaged
mammalian cells
— For whole
plate protocols:
16 x 106 adherent
cells or 48 x 106
cells in suspension
— For mini protocols
(6 well sets): 4 x 106
adherent cells or
12 x 106 cells
in suspension
n
Cell growth medium
n
PBS
n
Gene Pulser MXcell
electroporation system
n
96-well
electroporation plate
n
Gene Pulser
electroporation
buffer or other
buffer suitable for
electroporation
n
Molecule to
electroporate: siRNA,
such as siLentMer
Dicer-substrate
siRNA duplexes,
or DNA
n
24-well tissue
culture plates
Programming
the Instrument
n
Turn on the Gene
Pulser MXcell
n
Select “Pre-Set
Protocols”
n
Select appropriate
protocols and edit if
necessary
Setting Up the Instrument
n
If the cells are adherent, trypsinize the cells and add medium to
inactivate the trypsin; if the cells are in suspension, skip this step
n
Pellet the cells, remove the medium, and resuspend cells in
PBS by gentle pipeting; count the cells
1.
For whole plate protocols: transfer 16 x 106 adherent
cells or 48 x 106 cells in suspension to a new tube, pellet the
cells, and remove PBS by aspiration.
For mini protocols (6 well sets): transfer 4 x 106 adherent
cells or 12 x 106 cells in suspension to a new tube, pellet the
cells, and remove PBS by aspiration.
2. Resuspend the cells in 16 ml (4 ml for mini protocol) of Gene
Pulser electroporation buffer (this provides 1x106 cells/ml of
adherent cells or 3x106 cells/ml of cells in suspension).
3. Add the molecule (siRNA, DNA) to the cell suspension.
4. Pipet 150 µl of cell suspension into the appropriate wells
of an electroporation plate.
5. Place the lid on the plate and gently rock the plate back
and forth to wet the electrodes.
6. Place the electroporation plate securely into the plate
chamber, close the lid, and press the PULSE button.
7. Transfer all or 100 μl of the cells from each electroporation
well to the wells of a 24-well tissue culture plate containing
500 µl of grow th medium.
Note: using thi s method a llows re plication of th e exper iment, p rovidi ng
two dup licate 24-we ll tissue cultu re dishes.
8. Incubate cells at 37°C in a humidified CO2 incubator until
they are ready to be assayed.
Visit us on the Web at discover.bio-rad.com | Electroporation Protocol
15
Page 16

Gene Pulser MXcell Electroporation Guide
Electroporation Evaluation Methods
There are many techniques available to examine cells for transfection efficiency and cell
viability. We have briefly summarized three commonly used methods. It is critical to the
success of your experiment to evaluate all cells that were electroporated.
Fluorescence Microscopy for Adherent Cells
Fluorescence microscopy can be used to visualize a fluorescent signal within the cell.
This method is commonly used when expressing a GFP-tagged protein.
Materials
n
PBS
n
Fixation buf fer: 2–4% formaldehyde in PBS
n
70% glycerol
Method
1. Remove the medium from the wells of the electroporation plate and wash the cells
once with 500 –700 μl of PBS.
2. Add 300 μl of fixation buffer to each well and incubate at room temperature for 10 min.
3. Remove the fixative, and perform 2 washes with PBS.
4. Add 70% glycerol, and store the cells at 4°C until they are ready to be analyzed by
fluorescence microscopy using the appropriate filters.
Flow Cytometry
Flow cytometr y can be used to measure the number of cells containing a fluorescent
tag, such as a fluorescent siRNA or a GFP-tagged protein.
Materials
n
Fixation buf fer: 2–4% formaldehyde in PBS
n
PBS
n
70% glycerol
Method
1. For adherent cells, add 100 μl trypsin per well to detach cells and add medium
(200–300 μl) to inactivate the trypsin. For cells in suspension, skip this step.
2. Transfer the cell suspension to a 1.5 ml centrifuge tube and pellet the cells (300 RCF).
3. Remove the medium and resuspend the cells in 500 μl PBS.
4. Transfer the resuspended cells to a flow cytometer tube for analysis.
16
Visit us on the Web at discover.bio-rad.com | Electroporation Evaluation Methods
Page 17

Gene Pulser MXcell Electroporation Guide
Electroporation Evaluation
Methods, continued
Fluorometer and Scanner Analysis for Adherent Cells
Fluorometric analysis of cell lysates can be used to examine lysed cells for the
presence of a fluorescence signal. This approach can be used for detecting
expression of a GFP-tagged protein.
Materials
n
Lysis buffer (0.5% NP-40, 10 mM Tris pH 8.0, and 1 mM EDTA)
n
PBS
n
96-well dark plate with flat, clear bottom
Method
1. Remove the medium and wash the cells once with 500–700 μl of PBS.
2. Add 100 μl lysis buffer to each well and evenly distribute the lysis
buffer by gently rocking the plate.
3. Incubate the plate at –80°C for 10 min.
4. Remove the plate from –80°C and allow the lysate to thaw on ice.
5. Pipet each sample 4–5 times to wash cells off the bottom of the plate.
6. Transfer the sample to a 96-well dark plate with flat, clear bottom for
fluorometer or scanner analysis.
Visit us on the Web at discover.bio-rad.com | Electroporation Evaluation Methods
17
Page 18

Gene Pulser MXcell Electroporation Guide
See the Gene Pulser MXcell
Electroporation System
manual for more information.
Preset Protocols
96-well/E xp 96 9 6 Use for initial optimal protocol Applies the sam e waveform
identification for many cell types across the whole plate
96-well/Sqr 96 9 6 Use for initial optimal protocol Applies the sam e waveform
identification for many cell types across the whole plate
Mixed 96-well/Exp, Sqr 96 96 Use for mixing different waveforms Exp: 250 V, 350 μF
by alternating rows of exponential Sqr: 250 V, 20 ms
(250 V/350 μF) and square waveforms
(250 V/20 ms)
Mixed 24-well/Exp, Sqr 24 24 Use for mixing diffe rent waveforms by Exp: 250 V, 350 μF, 1,000 Ω
alternating rows of exponential Sqr: 250 V, 20 ms, 1,000 μF, 1,000 Ω
(250 V/350 μF) and square waveforms
(250 V/20 ms)
24-well/Exp 24 24 Use for initial protocol setup for Same conditions for the
many cell types whole plate
24-well/Sqr 24 24 Use for initial protocol setup for Same conditions for the
many cell types whole plate
Opt 24-well/Exp, Sqr 24 24 Use with cell line with no protocol Exp: 150–450 V, 200–1,000 μF
reference; this protocol includes a Sqr: 150–450 V, 5–30 ms
range of common starting conditions
Uniform 24-well/E xp, Sqr 24 24 Use with a set of defined conditions Exp: 250 V, 350 μF, 1,000 Ω
to compare dif ferent cell lines and Sqr: 250 V, 20 ms, 1,000 μF, 1,000 Ω
electroporation of different molecules
within the same or different cell lines
12-well/Exp 12 12 Use for initial protocol setup for many Same conditions for the
cell ty pes whole plate
12- well/Sqr 12 12 Use for initial protocol setup for many Same conditions for the
cell ty pes whole plate
Opt 12-well/Exp, Sqr 12 12 Use with cell line with no protocol reference; Exp: 150–400 V, 200–500 μF
this protocol includes a range of common Sqr: 150–300 V, 15–25 ms
star ting conditions
Uniform 12-well/Exp, Sqr 12 12 Use with a set of defined conditions to Exp: 250 V, 350 μF, 1,000 Ω
compare different cell lines and Sqr: 250 V, 20 ms, 1,000 μF, 1,000 Ω
electroporation of different molecules
within the same or different cell lines
Mixed 12-well/Exp, Sqr 12 12 Use for mixing different waveforms by Exp: 250 V, 350 μF, 1,000 Ω
alternating rows of exponential Sqr: 250 V, 20 ms, 1,000 μF, 1,000 Ω
(250 V/350 μF) and square waveforms
(250 V/20 ms)
Additional Preset Protocols Available
# of
Plate
Type
Wells
Used
Application Parameters
18
Visit us on the Web at discover.bio-rad.com | Additional Preset Protocols
Page 19

Life Science
Group
08-0394 0608 Sig 0308
Bulletin 5700 Rev A US/EG
Bio-Rad
Laboratories, Inc.
Web site www.bio-rad.com USA 800 4BIORAD
Australia 61 02 9914 2800 Austria 01 877 89 01 Belgium 09 385 55 11
Brazil 55 21 3237 9400 Canada 905 364 3435 China 86 21 6426 0808
Czech Republic 420 241 430 532 Denmark 44 52 10 00
Finland 09 804 22 00 France 01 47 95 69 65 Germany 089 318 84 0
Greece 30 210 777 4396 Hong Kong 852 2789 3300
Hungary 36 1 455 8800 India 91 124 4029300 Israel 03 963 6050
Italy 39 02 216091 Japan 03 6361 7000 Korea 82 2 3473 4460
Mexico 52 555 488 7670 The Netherlands 0318 540666
New Zealand 0508 805 500 Norway 23 38 41 30 Poland 48 22 331 99 99
Portugal 351 21 472 7700 Russia 7 495 721 14 04
Singapore 65 6415 3188 South Africa 27 861 246 723
Spain 3491 590 5200 Sweden 08 555 12700 Switzerland 061717 95 55
Taiwan 886 2 2578 7189 United Kingdom 020 8328 2000
The siLentMer products are manufactured by Integrated DNA Technologies, Inc. (IDT) and are for research use
only. For custom siRNA synthesis, contact IDT.
 Loading...
Loading...