Page 1

ENrich™ Q
ENrich
™
S
High-Resolution Ion
Exchange Columns
Instruction Manual
Catalog numbers
780-0001
780-0003
780-0021
780-0023
Please read these instructions before you use ENrich Q and S
high-resolution ion exchange media. If you have any questions or
comments regarding these instructions, please contact your
Bio-Rad Laboratories representative.
Page 2

Page 3

Table of Contents
Section 1: Characteristics of the ENrich Ion
Exchange (IEX) Columns ................................... 1
1.1 Introduction .......................................................1
1.2 The ENrich Separation Media ............................. 1
1.3 Connection to the NGC™ and Other
Chromatography Systems ................................. 2
Section 2 : Use of the ENrich IEX Columns ........................ 3
2.1 Preparation for Initial Use ................................... 3
2.2 Buffer Selection ................................................. 3
2.3 Sample Preparation ........................................... 5
2.4 Elution Conditions .............................................. 5
Section 3 : Care of the ENrich IEX Columns ....................... 7
3.1 Column Cleaning ............................................... 7
3.2 Bed Height Adjustment ...................................... 8
3.3 Frit Replacement ............................................... 9
3.4 Storage Conditions ...........................................12
Section 4 : Product Information .........................................13
Page 4

Page 5

Section 1: Characteristics of the
ENrich Ion Exchange Columns
1.1 Introduction
ENrich prepacked ion exchange columns meet the needs of the
biochromatographer for rapid and reproducible high-resolution
separations of proteins and other biomolecules, including
peptides and polynucleotides. Two column sizes (bed volumes
of 1 and 8 ml) provide predictable scale-up of high-resolution
separations without sacrificing capacity.
1.2 The ENrich Separation Media
Each column contains a spherical, rigid, and highly porous
polymeric support derivatized with the strongly basic –N+(CH3)3
quaternary ammonium group or the strongly acidic –SO
The 10 μm particle-size and narrow particle size distribution
provide excellent resolution of biomolecules at high flow rates
and with low backpressures. The hydrophilic ENrich media
demonstrate extremely low non-specific binding of biomolecules
accompanied by high recovery of biological activity.
Stability
The columns are stable over a 2–12 pH range, allowing easy
cleaning and regeneration.
The ENrich support is compatible with aqueous solutions of 6 M
guanidine-HCl and 8 M urea. Nonionic detergents and organic
solvents such as methanol, ethanol, and isopropanol may also
be used. Ionic detergents can be used only if the detergents
have the same charge as the support (that is, sodium dodecyl
sulfate (SDS), a negatively charged detergent, should not be
used with a positively charged anionic exchanger like ENrich Q).
–
group.
3
ENrich Instruction Manual 1
Page 6
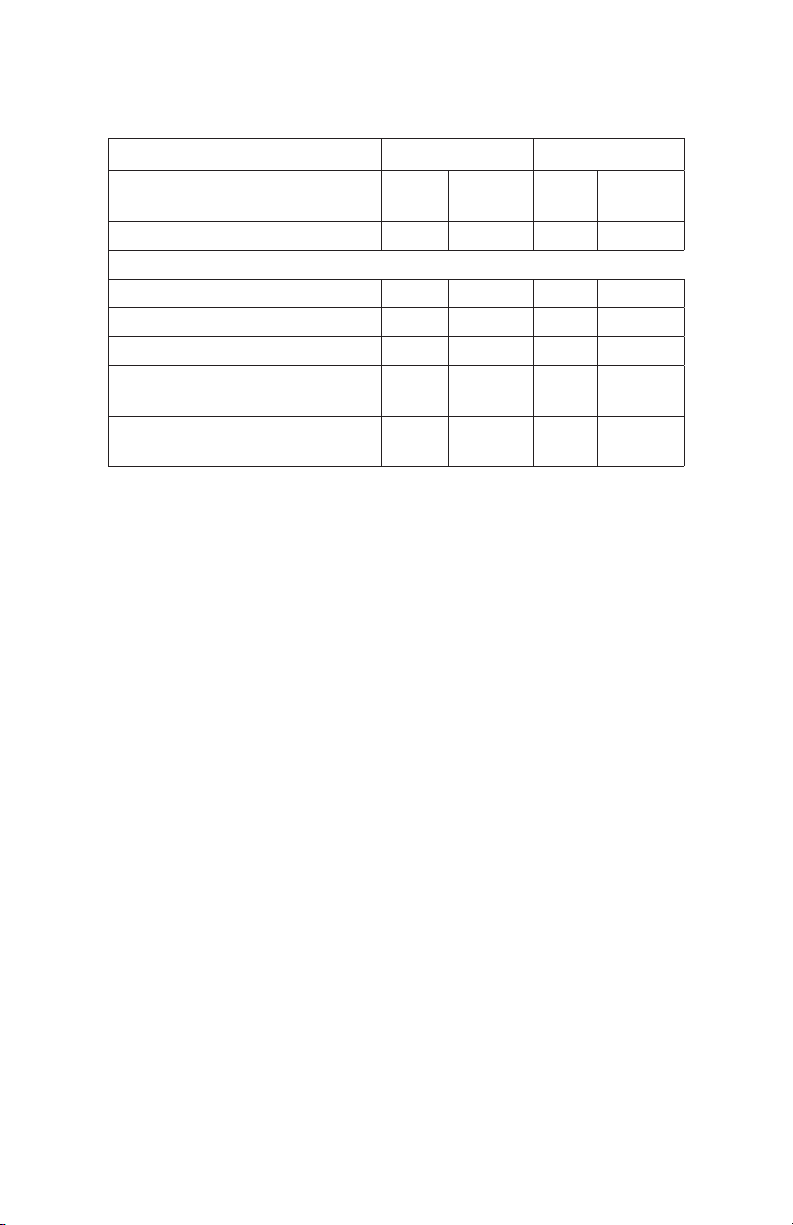
Table 1. Column characteristics.
Q S
Column dimensions
(diameter x height, mm)
Column volume, ml 1 8 1 8
Maximum protein capacity, mg
Bovine serum albumin 130 1000 – –
Human IgG – – 120 940
Average particle size, μm 10 10 10 10
Maximum operating pressure,
psi
Recommended flow
rate, ml/min*
* At room temperature. Viscosity may increase at lower temperatures, which will
reduce the recommended flow rates.
5 x 50 10 x 100 5 x 50 10 x 100
500 500 500 500
1 4 1 4
1.3 Connection to the NGC and Other
Chromatography Systems
The ENrich columns are fitted with 10-32 type female fittings on either
end. Standard 10-32 fittings can be used to plumb the column to
Bio-Rad’s NGC chromatography system or other vendors’ systems.
Adaptors are available for connection to systems that use 1/4-28
fittings (catalog # 750-0564).
2 ENrich Instruction Manual
Page 7

Section
: Use of the ENrich
2
Columns
2.1 Preparation for Initial Use
The columns are supplied in a storage solvent of 20% ethanol
in water. Prior to initial use and after extended storage periods,
each column should be conditioned as described in steps 1–4.
Always use HPLC-grade reagents and be sure to filter and degas
solvents. During these four steps do not exceed more than 50%
of the recommended flow rates (see Table 1).
1. Wash with 5 column volumes of water.
2. Wash with 5 column volumes of low ionic strength
equilibration buffer (such as 20 mM buffer salt).
3. Wash with 5 column volumes of high ionic strength limit
buffer (such as 1.0 M NaCl).
4. Wash with 5 column volumes of low ionic strength
equilibration buffer.
The column may now be further equilibrated in the start buffer at
the desired flow rate.
2.2 Buffer Selection
Table 2 lists commonly used buffers for ion exchange
chromatography. The buffers are specific to the type of ion
exchange. Therefore, it is important not to use anionic buffers
with the ENrich Q, which would interact with the anionic
exchange group on the support.
The choice of whether to use an anion or cation exchanger is
determined mainly by the isoelectric point (pI) and the relationship
between pH and the activity/stability of the protein(s) of interest.
When the type of ion exchanger is determined, the pH-activity
relationship also determines the choice of buffer. As a general
rule, the chosen buffer should be used within ±1.0 pH unit of
its pKa value. This permits use of the lowest possible buffer
concentration while maintaining maximum buffering capacity. We
recommend a minimum buffer concentration of 20 mM.
ENrich Instruction Manual 3
Page 8
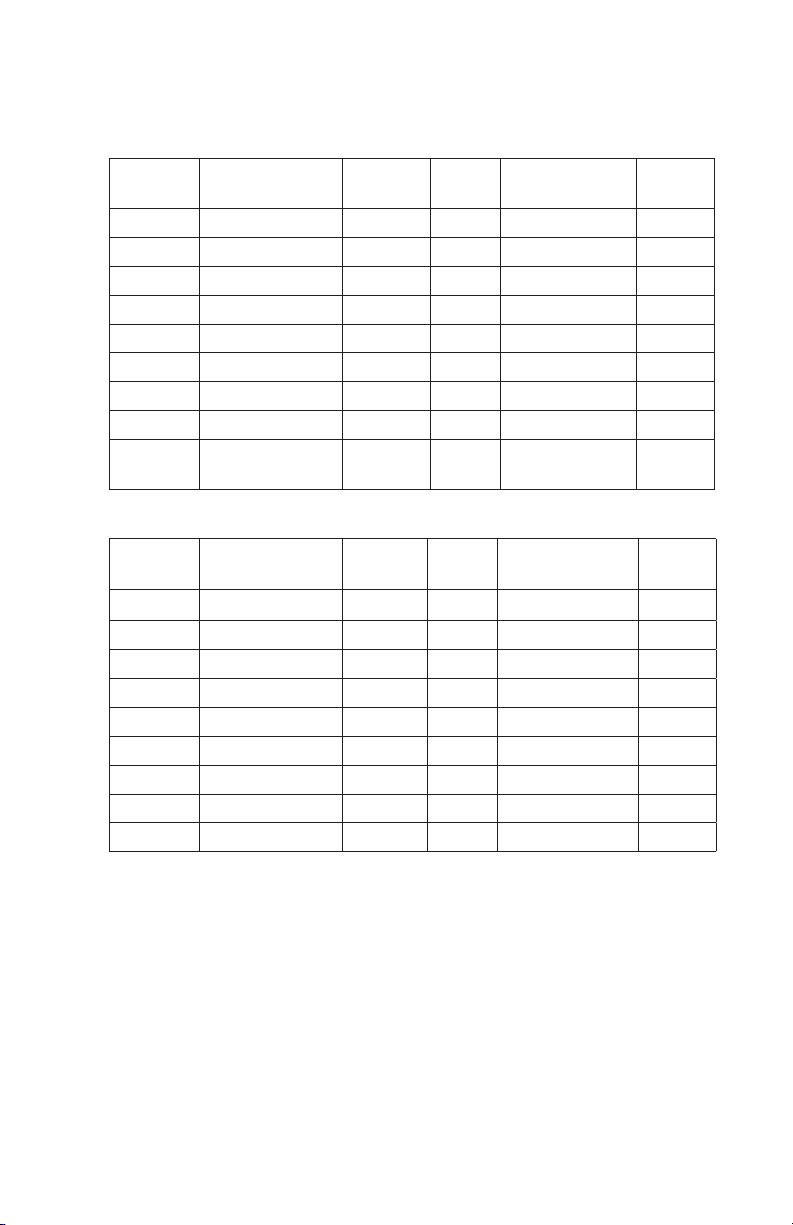
Table 2. Buffers for ion exchange chromatography.
Anion Exchange — ENrich Q
pH
Range
Buffer MW pKa @
25°C
Counter-Ion pKa/°C
5.0–6.0 Piperazine 86.1 5.7 Cl-/HCOO
5.0–6.0 L-histidine 155.2 6.15 CI
5.8–7.2 Bis-Tris 209.2 6.5 CI
6.4–7.3 Bis-Tris propane 282.3 6.8,9 CI
7.3–8.3 Triethanolamine 149.2 7.8 CI-/CH3COO
7.6–8.6 Tris 121.1 8.1 CI
8.4–8.8 Diethanolamine 105.1 8.9 CI
9.0–9.9 Ethanolamine 61.1 9.5 CI
9.8–10.3 1,3-diamino-
74.1 10.5 CI
propane
Cation Exchange — ENrich S
pH
Range
Buffer MW pKa @
25°C
Counter-Ion pKa/°C
3.6–4.3 Lactic acid 90.1 3.8 Na
4.2–5.2 Citric acid 192.1 3.1 Na
5.5–6.7 MES 195.2 6.1 Na
6.1–7.5 PIPES 302.4 6.8 Na
6.5–7.9 MOPS 209.3 7.2 Na
6.7–7.6 Phosphate 120/142 7.2 Na
6.8–8.2 TES 229.2 7.4 Na
6.8–8.2 HEPS 238.3 7.5 Na
7.4–8.8 Tricine 179.2 8.1 Na
–
–0.015
–
–
–
–
–
–
–
+
+
+
+
+
+
+
+
+
–
–
–0.017
–
–0.02
–0.031
–0.025
–0.029
–0.023
–
–
–0.011
–0.009
–0.006
–0.003
–0.002
–0.014
–0.021
Always use buffer components of the highest purity available,
as UV-absorbing impurities may cause baseline disturbances
and interfere with the detection of protein peaks. Always filter
and degas buffers.
4 ENrich Instruction Manual
Page 9

2.3 Sample Preparation
Proper adjustment of the sample pH and ionic strength is critical
for consistent and reproducible chromatography. For best
results, the sample should be exchanged into the start buffer or
diluted to the start buffer’s concentration. Buffer exchange can
be performed using Bio-Spin® 6 and Micro Bio-Spin™ 6 columns,
Econo-Pac® 10DG desalting columns, Bio-Gel® P-6DG gel, or
the Bio-Scale™ Mini desalting cartridges with Bio-Gel P-6. The
choice of product depends on sample volume. Centrifuge or filter
the sample
(0.2–0.45 μm filter) to remove particulates. Application of turbid
or lipid-containing samples may reduce the column lifetime.
Sample Load
The maximum sample load for each column is shown in
Table 1. This amount may vary somewhat depending on the
actual sample composition. We do not recommend overloading
the column as both resolution and column lifetime will decrease.
For larger loads, perform several chromatographic runs
with a reduced load. Ideally, samples should be bound in a
concentrated zone at the top of the column. Higher sample
loads produce a broad application zone in which components
with less charge are displaced by more highly charged
components. This may result in a shift of certain peaks to an
earlier elution position in the gradient. The recommended sample
load is approximately 20% of the maximum.
2.4 Elution Conditions
Separations by ion exchange are typically accomplished by
increasing the salt concentration of the eluent either as a step or
as a continuous gradient. Sodium chloride (NaCl) and potassium
chloride (KCl) are the most common elution salts. For many
separations, varying the pH of the elution buffer in addition to its
salt concentration may be advantageous.
ENrich Instruction Manual 5
Page 10

Gradient Volumes and Salt Concentrations
As a starting point for developing a separation, we recommend using
the ENrich column with a simple linear gradient over 15 column volumes
(15 ml).
Suggested protocol for ENrich 5 x 50 ion exchange columns:
• Use a flow rate of 1.0 ml/min. Equilibrate the column with 5 column
volumes (5 ml) of equilibration buffer. Then apply the sample.
Following sample application, wash unbound proteins from the
column with 3 column volumes (3 ml) of equilibration buffer A. For
elution, use a gradient volume of 15 column volumes (15 ml) to an
NaCl concentration of 0.5 M.
• Follow this segment of the gradient by stepping the salt
concentration to 1.0 M (100% B) and then hold at 1.0 M for 3 ml
before
re-equilibrating the column with 3 ml of start buffer A. This gradient
is shown schematically in Figure 1. When an initial separation has
been performed and the elution position of the protein of interest
determined, the gradient composition and volume are adjusted to
achieve maximum resolution. Normally, a gradient volume of 10 to
20 ml per ml of column bed volume is sufficient.
• The slope of the gradient will affect resolution. A steep gradient
will result in relatively small peak volumes but short peak-to-peak
distances. A shallower gradient normally gives greater resolution
but peak volumes are larger.
Fig. 1. Schematic gradient for separation on an ENrich IEX column. Volume,
column volume.
6 ENrich Instruction Manual
Page 11

Section 3
: Care of the ENrich Ion
Exchange Column
3.1 Column Cleaning
Careful preparation (especially filtration) of the sample and
the buffers will maintain the column performance and lifetime.
Normally, washing with 1.0 M NaCl or KCl will remove most
bound components. However, if there is a significant decrease in
column performance (increasing backpressures or a significant
drop in resolution), then a more extensive cleaning protocol such
as that described in steps 1–6 should be used. Always reverse
the flow during this procedure so tightly bound substances
at the top of the column are removed quickly.
During this operation do not exceed more than 50% of the
recommended flowrates (see Table 1).
1. Wash with 2 column volumes of 2.0 M NaCl or KCl followed
by 3 column volumes of water.
2. Wash with two 100 μl injections of 1.0 M NaOH followed by
3 column volumes of water.
3. Wash with two 100 μl injections of 50% acetic acid followed
by 3 column volumes of water.
4. If lipid contamination is suspected, wash with 1 column
volume of 20% ethanol followed by 3 column volumes of
water.
5. Wash with 2 column volumes of 2.0 M NaCl or KCl, or the
salt containing the desired counter-ion.
6. Wash with 3 column volumes of equilibration buffer.
ENrich Instruction Manual 7
Page 12

3.2 Bed Height Adjustment
Under certain conditions of buffer composition, high flow rates
or long-term use, the resin bed may compress, creating a void
between the frit and the top of the bed. Normally, the void can
be eliminated by turning the adjusting nut clockwise until the frit
just touches the top of the bed (Figure 2). If the bed compresses
at high flow rates, stop the pump and loosen the top fitting, then
use the adjusting nut to remove the void, retighten the top fitting
and resume pumping buffer. If the bed has compressed from
long use, the top frit should be replaced as a precaution.
Fig. 2. Turning the adjusting nut raises or lowers the adaptor.
8 ENrich Instruction Manual
Page 13

3.3 Frit Replacement
The top frit may need to be replaced after extensive column use
or if increasing backpressures are noticed. Always try cleaning
the column in the reverse direction (as described in Section 3.1)
before replacing the frit. A frit kit is available which contains a frit
removal tool, 2 O-rings and 2 frits.
Figure 3 shows a column diagram to assist in the replacement of
the top frit.
Fig. 3. Column diagram.
1. Start buffer through the column at a slow flow rate
(0.5 ml/min or less).
2. Remove the lower end tubing and fitting from the column.
Firmly hold the bottom of the column over a sink or
container.
3. Loosen the lock nut turning it clockwise (Figure 4).
Fig. 4. Loosen the lock nut by turning it clockwise.
ENrich Instruction Manual 9
Page 14

4. Raise the adaptor a few millimeters by slowly by turning the
adjusting nut counterclockwise (Figure 5).
Fig. 5. Raise the adaptor by turning the adjusting nut counterclockwise.
5. Unscrew the upper retainer. Let it rest on the bottom retainer
(Figure 6).
Fig. 6. Remove the retainer.
6. Slowly pull out the upper adaptor from the glass column.
Allow the buffer flow to continue in order to maintain the
integrity of the top of the bed.
10 ENrich Instruction Manual
Page 15

7. Stop the pump. Plug the bottom of the column. Set the
column upright in a beaker. Remove the tubing and fitting
from the top of the adaptor.
8. Remove the frit from the adaptor by hooking one end of the
frit removal tool/tweezer into the frit in a sideways motion
with slight downward pressure.
9. Push the new frit into the end of the adaptor.
10. Replace the O-rings if they appear worn or torn. If the
O-rings are replaced, wet them with buffer before the next
step.
11. Add a few drops of buffer to the top of the resin bed. Insert
the adaptor and push it down to the bed. Some buffer
should flow out of the top of the column.
12. Screw on the upper retainer (Figure 7).
Fig. 7. Reattach and hand-tighten the retainer.
ENrich Instruction Manual 11
Page 16

13. Lower the adaptor to the top of the bed by turning the
adjusting nut clockwise (Figure 8).
Fig. 8. Lower the adaptor by turning the adjusting nut clockwise.
14. Tighten the lock nut by turning it counterclockwise (Figure 9).
Fig. 9. Hand-tighten the lock nut by turning it counterclockwise.
3.4 Storage Conditions
Prior to long-term storage, the column should be cleaned as
previously described and then washed with 3 column volumes of
20% ethanol at a flow rate that is 50% of the recommended flow
rate (see Table 1). This will prevent microbial growth. Store the
column in a safe place at room temperature or 4°C. Never allow
the column to freeze.
12 ENrich Instruction Manual
Page 17

Section
Catalog
Number Product Description
780-0001 ENrich Q 5 x 50 Column
780-0003 ENrich Q 10 x 100 Column
780-0021 ENrich S 5 x 50 Column
780-0023 ENrich S 10 x 100 Column
780-0091 ENrich 5 Frit Kit, includes 2 frits, 1 frit remover,
780-0093 ENrich 10 Frit Kit, includes 2 frits, 1 frit remover,
780-0008 1/16” 10-32 Male Fittings, 2/pk
: Product Information
4
2 O-rings
2 O-rings
ENrich Instruction Manual 13
Page 18

Page 19

Page 20

Life Science
Group
Sig 121310027324 Rev E US/EG
Bio-Rad
Laboratories, Inc.
Web site www.bio-rad.com USA 80 0 424 6723
Australia 61 2 9914 2800 Austria 01 877 89 01 Belgium 09 385 55 11
Brazi l 55 11 3065 7550 Canada 905 364 3 435 China 86 21 6169 8500
Czech Republic 420 241 430 532 Denma rk 44 52 10 00
Finland 09 804 22 00 France 01 47 95 69 65 Ger many 089 31 88 4 0
Greece 30 210 9532 220 Hon g Kong 852 2789 33 00
Hungary 36 1 459 6100 India 91 124 4029300 Israel 03 963 6050
Italy 39 02 2160 91 Japan 81 3 6361 7000 Korea 82 2 3473 4460
Mexico 52 55 5 488 7670 The Netherlands 0318 540666
New Zealand 64 9 415 2280 No rway 23 38 41 30
Poland 48 22 331 99 99 Portugal 351 21 472 7700
Russia 7 495 721 14 04 Singapore 65 6415 3188
South Africa 27 861 246 723 Spain 34 91 59 0 5200
Sweden 08 555 1270 0 Switzerland 026 674 55 05
Taiwan 886 2 2578 7189 Thailand 1800 88 22 88
United Kingdom 020 8328 200 0
 Loading...
Loading...