Page 1

X Series® Operator’s Guide
9650-001355-01 Rev. H
Page 2
The issue date for the X Series Operator's Guide (REF 9650-001355-01 Rev. H) is May, 2014.
0123
ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA USA
01824-4105
ZOLL International Holding B.V.
Newtonweg 18
6662 PV ELST
The Netherlands
If more than 3 years have elapsed since the issue date, contact ZOLL Medical Corporation to det ermine if
additional product information updates are available.
Copyright © 2014 ZOLL Medical Corporation. All rights reserved. CPR-D-padz, Pedi-padz, OneStep, Real
CPR Help, Rectilinear Biphasic , RescueNet, See-Thru CPR, Stat-padz, SurePower, X Series, and ZOLL are
trademarks or registered trademarks of ZOLL Medical Corporation in the United States and/or other countries.
All other trademarks are the property of their respective owners.
Masimo, Rainbow, SET, SpCO, and SpMet are trademarks or registered trademarks of Masimo Corporation in
the United States and/or other countries.
IC Model: XSCP-1
Page 3

Table of Content s
Ch a p t e r 1 General Information
Product Description ............................................................................................................1-1
X Series Optional Features .........................................................................................1-2
How to Use This Manual.....................................................................................................1-3
Operator’s Guide Updates..................................................................................................1-3
Unpacking...........................................................................................................................1-3
Symbols Used on the Equipment .......................................................................................1-4
Conventions........................................................................................................................1-7
X Series Indications for Use ...............................................................................................1-7
Manual Defibrillation ...................................................................................................1-8
Semiautomatic Operation (AED) ................................................................................1-8
ECG Monitoring ..........................................................................................................1-9
CPR Monitoring ..........................................................................................................1-9
External Transcutaneous Pacing ................................................................................1-9
Non-Invasive Blood Pressure Monitoring ...................................................................1-9
Temperature Monitoring .............................................................................................1-9
SpO
Monitoring .......................................................................................................1-10
2
Respiration Monitoring ..............................................................................................1-10
Monitoring .........................................................................................................1-10
CO
2
Invasive Pressure Monitoring ...................................................................................1-10
12-Lead Analysis ......................................................................................................1-10
X Series Product Functions..............................................................................................1-11
Defibrillator Function .................................................................................................1-11
Defibrillator Output Energy .......................................................................................1-11
External Pacemaker .................................................................................................1-11
ECG Monitoring ........................................................................................................1-11
Electrodes .................................................................................................................1-12
Batteries ....................................................................................................................1-12
Ready For Use (RFU) Indicator ................................................................................1-13
Safety Considerations.......................................................................................................1-15
Warnings...........................................................................................................................1-15
General ..................................................................................................................... 1-15
ECG Monitoring ........................................................................................................1-16
Defibrillation .............................................................................................................. 1-17
Pacing ....................................................................................................................... 1-18
CPR ..........................................................................................................................1-19
Pulse Oximeter .........................................................................................................1-19
Noninvasive Blood Pressure .....................................................................................1-20
IBP ............................................................................................................................1-20
CO
........................................................................................................................... 1-21
2
Respiration ................................................................................................................1-21
Ferromagnetic Equipment ........................................................................................1-21
9650-001355-01 Rev. H X Series Operator’s Guide i
Page 4

TABLE OF CONTENTS
Battery ......................................................................................................................1-21
Operator Safety ........................................................................................................1-22
Patient Safety ...........................................................................................................1-23
Cautions............................................................................................................................1-24
Restarting the Defibrillator................................................................................................1-25
FDA Tracking Requirements.............................................................................................1-25
Notification of Adverse Events ..................................................................................1-26
Software License..............................................................................................................1-26
Service..............................................................................................................................1-27
The ZOLL Serial Number..................................................................................................1-28
Ch a p t e r 2 Product Overview
Defibrillator Controls and Indicators....................................................................................2-1
The Front Panel ..........................................................................................................2-2
Display Screen ............................................................................................................2-4
Battery Status and Auxiliary Power Indicators ............................................................2-6
Patient Cables and Connectors ..................................................................................2-7
External Paddles .......................................................................................................2-10
AC Auxiliary Power Adapter .....................................................................................2-13
DC Auxiliary Power Supply (optional) .......................................................................2-14
Connecting the DC Auxiliary Power Supply to a Suitable Vehicle Power Source ....2-14
Connecting AC Auxiliary Power Adapter or DC Auxiliary Power Supply to X Series
Unit ......................................................................................................................... 2-15
Navigating the Display Screen..........................................................................................2-15
Quick Access Keys ...................................................................................................2-16
Navigation Keys .......................................................................................................2-19
Display Brightness ....................................................................................................2-19
Common Tasks.................................................................................................................2-19
Changing the Display Brightness ..............................................................................2-19
Replacing a Battery Pack on the X Series ................................................................2-20
Using Treatment Buttons ..........................................................................................2-21
Ch a p t e r 3 Monitoring Overview
X Series Monitoring Functions............................................................................................3-1
ECG ............................................................................................................................3-2
Heart Rate ..................................................................................................................3-2
Respiration Rate .........................................................................................................3-2
Temperature ...............................................................................................................3-2
Invasive Pressures (IBP) ............................................................................................3-2
Non-Invasive Blood Pressure (NIBP) .........................................................................3-2
Capnography (CO
Pulse Oximetry (SpO
Monitoring Display Options.................................................................................................3-3
Configuring the Waveform Display.....................................................................................3-7
ii www.zoll.com 9650-001355-01 Rev. H
) .................................................................................................... 3-3
2
) ...............................................................................................3-3
2
Page 5

Chapter 4 Trends
Displaying the Trends Status Window ................................................................................4-1
Displaying and Printing Trend Information..........................................................................4-2
Changing the Trends Status Window Display.....................................................................4-3
Continuous Waveform Recording ...............................................................................4-3
Chapter 5 Alarms
Visual Alarm Indicators.......................................................................................................5-1
Audible Alarm Indicators.....................................................................................................5-2
Alarm Indicator Self-Test.....................................................................................................5-2
Patient Alarm Display .........................................................................................................5-3
Life Threatening Rhythm Alarms........................................................................................5-4
Equipment Alert Display .....................................................................................................5-4
Responding to Active Alarms -- Silencing the Alarm..........................................................5-5
Re-enabling an Alarm .................................................................................................5-5
Suspending Alarms.............................................................................................................5-5
The Alarm Suspension Timer .....................................................................................5-6
Alarm Options..................................................................................................................... 5-7
Selecting Default Alarm Limits ....................................................................................5-8
Setting Alarm Limits Relative to the Patient -- Stat Set Option ...................................5-8
Ch a p t e r 6 Monitoring ECG
ECG Monitoring Setup........................................................................................................6-2
Preparing the Patient for Electrode Application ..........................................................6-2
Applying Electrodes to the Patient ..............................................................................6-3
Connecting the ECG Cable To the X Series Unit .......................................................6-5
Selecting ECG Waveforms for Display .......................................................................6-6
Selecting the Waveform Trace Size ...........................................................................6-8
ECG Monitoring and Pacemakers......................................................................................6-9
ECG System Messages......................................................................................................6-9
Ch a p t e r 7 Monitoring Respiration (Resp) and Heart Rate (HR)
Respiration/Breath Rate Meter...........................................................................................7-2
Using Impedance Pneumography to Measure Respiration ........................................7-2
Configuring Respiration (RR/BR) Alarms and Settings ......................................................7-3
Enabling/Disabling RR/BR Alarms and Setting Alarm Limits ......................................7-3
Using the Resp Parameter Control Panel ...................................................................7-4
Heart Rate Meter........ .......... ........... .......... ........... ..............................................................7-5
Configuring Heart Rate (HR) Meter Alarms........................................................................7-5
Enabling/Disabling HR Alarms and Setting Alarm Limits ............................................7-6
Life Threatening Rhythm Alarms ................................................................................7-7
Using the Heart Rate Parameter Control Panel ..........................................................7-9
RESP System Message ...................................................................................................7-10
9650-001355-01 Rev. H X Series Operator’s Guide iii
Page 6

TABLE OF CONTENTS
Ch a p t e r 8 Monitoring Non-Invasive Blood Pressure (NIBP)
How does NIBP Work?.......................................................................................................8-2
The NIBP Numeric Display.................................................................................................8-3
NIBP Setup and Use...........................................................................................................8-3
Selecting the NIBP Cuff......................................................................................................8-4
Connecting the NIBP Cuff...................................................................................................8-5
Applying the Cuff to the Patient..........................................................................................8-7
Ensuring Correct Cuff Inflation Settings..............................................................................8-8
Configuring NIBP Alarms and Settings...............................................................................8-9
Enabling/Disabling NIBP Alarms and Setting Alarm Limits ........................................8-9
Using the NIBP Parameter Control Panel .................................................................8-11
NIBP System Messages...................................................................................................8-14
Ch a p t e r 9 Monitoring CO
2
Overview.............................................................................................................................9-1
CO
Monitoring Setup and Use..........................................................................................9-2
2
Selecting the CO
Connecting the CO
Sampling Line ...............................................................................9-3
2
Sampling Lines ..........................................................................9-4
2
Applying a FilterLine Set .............................................................................................9-5
Applying a Smart CapnoLine Nasal or Nasal/Oral Cannula .......................................9-6
Measuring CO
Setting CO
Enabling/Disabling Alarms and Setting CO
Using the CO
CO
System Messages ....................................................................................................9-11
2
...................................................................................................................9-7
2
and Respiration Rate Alarms..........................................................................9-8
2
Alarm Limits ..........................................9-8
2
Parameter Control Panel ..................................................................9-10
2
Patents..............................................................................................................................9-12
Chapter 10 Pulse CO-Oximetry (SpO2, SPCO, and SpMet)
Warnings -- SpO2, General...............................................................................................10-2
Warnings -- SpO
SpO
Setup and Use ........................................................................................................10-4
2
Selecting the SpO
Applying the SpO
Applying a Two-Piece Single-Use Sensor/Cable ......................................................10-5
Applying a Reusable SpO
Cleaning and Reuse of Sensors ...............................................................................10-8
Connecting the SpO
Displaying SpO
Enabling/Disabling SpO
Setting Upper and Lower SpO
Setting Upper and Lower SpCO and SpMet Alarm Limits ......................................10-10
, Oximeter Sensor.................................................................................10-3
2
Sensor...............................................................................................10-4
2
Sensor................................................................................................10-4
2
Sensor/Cable ................................................................10-7
2
Sensor............................................................................................10-8
2
, SpCO, and SpMet Measurements........................................................10-8
2
Alarms and Setting Alarm Limits..............................................10-9
2
Alarm Limits ...........................................................10-9
2
iv www.zoll.com 9650-001355-01 Rev. H
Page 7

Using the SpO2 Parameter Control Panel ......................................................................10-10
Selecting the SpCO and SpMet Monitoring ............................................................10-10
Specifying the SpO
Selecting the SpO
Selecting the Heart Rate/ Pulse Rate (HR/PR) Tone .............................................10-11
SpO
System Messages................................................................................................. 10-11
2
Functional Testers and Patient Simulators .....................................................................10-13
Averaging Time .....................................................................10-11
2
Sensitivity ................................................................................10-11
2
Chapter 11 Monitoring Invasive Pressures (IBP)
Invasive Pressure Transducers........................................................................................ 11-1
IBP Setup..........................................................................................................................11-2
Attaching the Invasive Pressure Transducer ....................................................................11-2
Zeroing the Transducer.............................................................................................. .......11-3
Rezeroing a Transducer...................................................................................................11-4
Displaying IBP Measurements.......................................................................................... 11-5
Conditions Affecting IBP Measurements ..................................................................11-5
Enabling/Disabling IBP Alarms and Setting Alarm Limits.................................................11-6
Setting Upper and Lower Systolic (SYS) Alarm Limits .............................................11-6
Setting Upper and Lower Diastolic (DIA) Alarm Limits .............................................11-7
Setting Upper and Lower Mean Arterial Pressure (MEAN) Alarm Limits ..................11-7
Setting IBP Source Label ..........................................................................................11-8
IBP System Messages...................................................................................................... 11-9
Ch a p t e r 1 2 Monitoring Temperature
Temperature Monitoring Setup .........................................................................................12-1
Selecting and Applying Temperature Probes....................................................................12-1
Connecting the Temperature Probe..................................................................................12-2
Displaying Temperature....................................................................................................12-2
Enabling/Disabling Temperature Alarms and Setting Alarm Limits...................................12-3
Setting Upper and Lower Temperature Alarm Limits........................................................12-3
Setting Upper and Lower Temperature Alarm Limits........................................................12-4
Selecting the Temperature Label......................................................................................12-4
Temperature System Messages .......................................................................................12-5
Chapter 13 Automated External Defibrillator (AED) Operation
AED Operation..................................................................................................................13-2
Determine Patient Condition Following Medical Protocols .......................................13-2
Begin CPR Following Medical Protocols ..................................................................13-2
Prepare Patient .........................................................................................................13-3
1 Turn on unit ............................................................................................................13-3
2 Analyze ..................................................................................................................13-5
3 Press SHOCK ........................................................................................................13-7
9650-001355-01 Rev. H X Series Operator’s Guide v
Page 8

TABLE OF CONTENTS
Operating Messages .................................................................................................13-8
Audio and Display Messages ...................................................................................13-8
Switching to Manual Mode Operation.............................................................................13-10
Chapter 14 12-Lead ECG Interpretive Analysis
Entering Patient Information.............................................................................................14-2
Entering the Patient Name and ID ............................................................................14-3
Entering Patient Age and Gender .............................................................................14-4
12-Lead ECG Monitoring Setup........................................................................................14-4
Preparing the Patient for Electrode Application ........................................................14-4
Applying Electrodes to the Patient ............................................................................14-5
Connecting the 12-Lead Cable .................................................................................14-7
Observing the 12-Lead Waveform Traces ................................................................14-8
12-Lead Interpretive Analysis ...................................................................................14-8
Fault Conditions Affecting 12-Lead Interpretive Analysis .......................................14-11
Printing 12-Lead Waveform Traces ................................................................................14-12
12-Lead Print and Display Options.................................................................................14-13
Selecting 12-Lead Acquire ......................................................................................14-14
Specifying the Number of 12-Lead Print Copies .....................................................14-14
Specifying the 12-Lead Print Format ......................................................................14-14
Printing 10 Seconds of the Lead ll Waveform Trace ..............................................14-14
Specifying the 12-Lead Frequency Response ........................................................14-14
Enabling 12-Lead Analysis .....................................................................................14-15
Chapter 15 Manual Defibrillation
Emergency Defibrillation Procedure with Paddles............................................................15-1
Determine the Patient’s Condition Following Local Medical Protocols ..................... 15-2
Begin CPR Following Local Medical Protocols .........................................................15-2
Turn On Unit .............................................................................................................15-2
1 Select Energy Level ...............................................................................................15-2
2 Charge Defibrillator ................................................................................................15-3
3 Deliver Shock .........................................................................................................15-5
Emergency Defibrillation Procedure with Hands-Free Therapy Electrodes......................15-6
Determine the Patient’s Condition Following Local Medical Protocols ..................... 15-6
Begin CPR Following Medical Protocols ..................................................................15-6
Prepare Patient .........................................................................................................15-6
Turn On Unit .............................................................................................................15-7
1 Select Energy Level ...............................................................................................15-7
2 Charge Defibrillator ................................................................................................15-8
3 Deliver Shock .........................................................................................................15-9
Internal Paddles................................................................................................................15-9
Verification Prior to Use ..........................................................................................15-10
Synchronized Cardioversion........................................................................................... 15-11
Synchronized Cardioversion Procedure.........................................................................15-12
Determine the Patient’s Condition and Provide Care Following Local Medical
Protocols ..............................................................................................................15-12
vi www.zoll.com 9650-001355-01 Rev. H
Page 9

Prepare Patient .......................................................................................................15-12
Turn On Unit ...........................................................................................................15-12
Press the Sync Key ................................................................................................15-12
1 Select Energy Level .............................................................................................15-13
2 Charge Defibrillator ..............................................................................................15-13
3 Deliver Shock .......................................................................................................15-14
Chapter 16 Advisory Defibrillation
Advisory Defibrillation Procedure......................................................................................16-2
Determine the Patient’s Condition Following Local Medical Protocols ..................... 16-2
Begin CPR Following Local Medical Protocols .........................................................16-2
Prepare Patient .........................................................................................................16-2
1 Turn on unit ............................................................................................................16-3
2 Press ANALYZE Button .........................................................................................16-4
3 Press SHOCK button .............................................................................................16-5
Ch a p t e r 1 7 Advisory/CPR Protocol Defibrillation
Advisory/CPR Protocol Defibrillation Procedure...............................................................17-2
Determine the Patient’s Condition Following Local Medical Protocols ..................... 17-2
Begin CPR Following Local Medical Protocols .........................................................17-2
Prepare Patient .........................................................................................................17-2
1 Turn on unit ............................................................................................................17-3
2 Press ANALYZE Button .........................................................................................17-4
3 Press SHOCK button .............................................................................................17-5
Chapter 18 External Pacing
External Pacing.................................................................................................................18-2
Pacer Modes .............................................................................................................18-2
Pacing in Demand Mode ..................................................................................................18-2
Determine Patient Condition and Provide Care Following Local Medical Protocols .18-2
Prepare the Patient ...................................................................................................18-2
1 Turn On Unit ..........................................................................................................18-2
2 Apply ECG Electrodes/Hands-Free Therapy Electrodes .......................................18-3
3 Press Pacer button ................................................................................................18-3
4 Set Mode ...............................................................................................................18-4
5 Set Pacer Rate ......................................................................................................18-4
6 Turn On Pacer .......................................................................................................18-4
7 Set Pacer Output ...................................................................................................18-4
8 Determine Capture ................................................................................................18-4
9 Determine Optimum Threshold ..............................................................................18-5
Pacing in Fixed Mode .......................................................................................................18-6
1 Turn On Unit ..........................................................................................................18-6
2 Apply ECG Electrodes/Hands-Free Therapy Electrodes .......................................18-6
3 Press Pacer button ................................................................................................18-7
4 Set Mode ...............................................................................................................18-7
9650-001355-01 Rev. H X Series Operator’s Guide vii
Page 10

TABLE OF CONTENTS
5 Set Pacer Rate ......................................................................................................18-7
6 Turn On Pacer .......................................................................................................18-7
7 Set Pacer Output ...................................................................................................18-7
8 Determine Capture ................................................................................................18-8
9 Determine Optimum Threshold ..............................................................................18-8
Pediatric Pacing ........................................................................................................18-9
Chapter 19 Real CPR Help
CPR Voice Prompts..........................................................................................................19-2
CPR Metronome...............................................................................................................19-2
CPR Dashboard................................................................................................................19-3
Rate and Depth Measurements ................................................................................19-3
CPR Release Indicator .............................................................................................19-3
Chest Compression Indicator ...................................................................................19-4
CPR Countdown Timer .............................................................................................19-4
CPR Idle Time Display ..............................................................................................19-4
FULLY RELEASE Prompt ........................................................................................19-5
CPR Compression Bar Graph ..........................................................................................19-5
Chapter 20 See-Thru CPR
Using See-Thru CPR........................................................................................................20-2
Examples .................................................................................................................. 20-2
Chapter 21 Patient Data
Storing Data......................................................................................................................21-1
Log Capacity Indicator ..............................................................................................21-2
Capturing a Data Snapshot...............................................................................................21-2
Reviewing and printing snapshots ............................................................................21-2
Treatment Summary Report.............................................................................................21-3
Printing Treatment Summary Report ........................................................................21-3
Transferring Data to a USB Device...................................................................................21-4
Clearing the Log .......................................................................................................21-4
Chapter 22 Wireless Communications
The Wireless Icon.............................................................................................................22-2
The Wireless Menu...........................................................................................................22-3
Creating a Temporary Access Point Profile ..............................................................22-3
Bluetooth Device Pairing ..........................................................................................22-8
Setting up Communications in the Supervisor Menu......................................................22-10
Setting up Wireless Profiles ....................................................................................22-10
WiFi Access Point Profiles ......................................................................................22-12
Setting up Cellular Communications .......................................................................22-18
Setting up an Ethernet Connection .........................................................................22-22
viii www.zoll.com 9650-001355-01 Rev. H
Page 11

Configuring Report Transmissions .........................................................................22-23
Sending a 12-lead report................................................................................................22-23
Communications System Messages...............................................................................22-25
Transmission Status of 12 Lead Snapshots....................................................................22-26
Chapter 23 Printing
Printing Patient Data.........................................................................................................23-1
Printer Setup .............................................................................................................23-2
Automatic Prints ........................................................................................................23-2
Printing Waveforms ..................................................................................................23-2
Printing Reports ...................................................................................... ........... .......23-3
Printing Trends ....................................................................................... ........... .......23-4
Chapter 24 Maintenance
Daily/Shift Check Procedure.............................................................................................24-2
Inspection .................................................................................................................24-2
Defibrillator/Pacing Test with Hands-Free Therapy Electrodes.........................................24-3
Defibrillator Testing with External Paddles........................................................................24-5
Recommended Minimum Preventive Maintenance Schedule..........................................24-7
Annually .................................................................................................................... 24-7
Guidelines for Maintaining Peak Battery Performance.....................................................24-7
Cleaning instructions ........................................................................................................24-8
Cleaning the X Series unit ........................................................................................24-8
Cleaning the NIBP Blood Pressure Cuff ...................................................................24-8
Cleaning SpO
Cleaning Cables and Accessories ............................................................................24-9
Loading Recorder Paper ...........................................................................................24-9
Cleaning the Print Head ..........................................................................................24-10
Sensors ............................................................................................24-9
2
Ap p e n d i x A Specifications
Defibrillator..........................................................................................................................A-2
Monitor/Display.................................................................................................................A-14
Impedance Pneumography...............................................................................................A-15
Alarms...............................................................................................................................A-16
Recorder...........................................................................................................................A-17
Battery ..............................................................................................................................A-17
General.............................................................................................................................A-18
Pacer ................................................................................................................................A-19
CO
...................................................................................................................................A-19
2
Pulse Oximeter.................................................................................................................A-20
Non-Invasive Blood Pressure...........................................................................................A-22
Invasive Pressures ...........................................................................................................A-23
9650-001355-01 Rev. H X Series Operator’s Guide ix
Page 12

TABLE OF CONTENTS
Temperature......................................................................................................................A-24
Clinical Trial Results for the Biphasic Waveform ..............................................................A-25
Randomized Multicenter Clinical Trial for Defibrillation of Ventricular Fibrillation (VF)
and Ventricular Tachycardia (VT) ...........................................................................A-25
Randomized Multi-Center Clinical trial for Cardioversion of Atrial Fibrillation (AF) ...A-26
Synchronized Cardioversion of Atrial Fibrillation ......................................................A-28
Electromagnetic Compatibility Guidance and Manufacturer’s Declaration.......................A-29
Electromagnetic Immunity (IEC 60601-1-2) ..............................................................A-30
Electromagnetic Immunity: Life-Supporting Functions .............................................A-31
Electromagnetic Immunity: Non Life-Supporting Functions ......................................A-32
ECG Analysis Algorithm Accuracy....................................................................................A-35
Clinical Performance Results ....................................................................................A-35
Wireless Output Guidance and Manufacturer’s Declaration.............................................A-36
RF Transmission Emitted (IEC 60601-1-2) ...............................................................A-36
FCC Notice ...............................................................................................................A-36
Canada, Industry Canada (IC) Notices .....................................................................A-36
Appendix B Accessories
x www.zoll.com 9650- 001355-01 Rev. H
Page 13

Product Description
The ZOLL® X Series® unit is an easy-to-use portable defibrillator that combines defibrillati on
and external pacing with the following monitoring capabilities: ECG, CO-Oximeter, Noninvasive Blood Pressure, IBP, CO
resuscitation situations and its rugged , compact, lightweight design makes it ideal for transport
situations. It is powered by auxiliary power and an easily replaced battery pack that is quickly
recharged in the device when it is connected to auxiliary power. In addition, the unit’s battery
may be recharged and tested using a ZOLL SurePower™ Battery Charger Station.
Note: The X Series has defibrillation and pacing functionality, but some of the monitoring
functions are optional features. See the complete list of options in Fig. 1-1. All features
are included in this manual, but only purchased features will be available on your unit.
The product is designed for use in both the hospital and the rugged EMS environment. The
device is a versatile automated external defibrillator with manual capabilities and may be
configured to operate in Manual, Advisory or Semiautomatic modes. It can be configured to
start up in Semiautomatic (AED) mode or manual mode.
When operating in manual configuration, the device operates as a conventional defibrillator
where the device’ s char ging and disc harging i s fully controlled by the operator. In Advisory and
AED modes, some features of the device are automated and a sophisticated detection algorithm
is used to identify ventricular fibrillation and determine the appropriateness of defibrillator
shock delivery. Units may be configured to automatically charge, analyze, recharge, and
prompt the operator to “PRESS SHOCK”, depending on local protocols. The unit is switched
from AED mode to Manual mode for ACLS use by pressing the appropriate key on the front
panel.
Chapter 1
General Information
, Temperature, and Respiration. It has been designed for all
2
9650-001355-01 Rev. H X Series Operator’s Guide 1-1
Page 14

CHAPTER 1GENERAL INFORMATION
The X Series unit assists caregivers during cardiopulmonary resusc itatio n (CPR) by evaluat ing
the rate and depth of chest compressions and providing feedback to the rescuer.
Real CPR Help® requires the use of ZOLL OneStep™ CPR electrodes, OneStep™ Complete
electrodes, or CPR-D-padz
adaptively filtered, using the See-Thru C PR
compressions.
The unit has a large colorful LCD display of numerics and waveform data that provides easy
visibility from across the room and at any angle. ECG, plethysmograph, and respi ration
waveform traces can be displayed simultaneously, giving easy access to all patient monitoring
data at once. The display screen is configurable, so you can choose the best visual layout to fit
your monitoring needs. The X Series includes a transcutaneous pacemaker consisting of a pulse
generator and ECG sensing circuitry. Pacing supports both demand and fixed noninvasive
pacing for adult patients and adolescent, child, and infant pediatric patients.
The X Series has a patient data review and collection system that allow s you to view, store, and
transfer patient data. The X Series unit contains a printer and USB port, which you can use to
print the data and transfer it to a PC.
The X Series unit can send data through a wireless connection to remote locations. The
X Series unit can send 12-lead report snapshots (including trend data) to a recipient via a ZOLL
server. Ful l disclosure case s, which also contai n trend data, can be automatically retrieved from
the X Series unit using ZOLL RescueNet or ePCR software.
®
. When using these pads, the displayed ECG waveforms can be
®
feature, to reduce the artifact caused by chest
X Series Optional Features
The following features are optional in the X Series unit.
Note: All features are included in this manual, but only purchased features will be avai lable
on your unit.
Figure 1-1 X Series Optional Features
Optional Feature
12-Lead ECG with Interpretation
(Masimo®) with SpCO® and SpMet
SpO
2
NIBP (with Smartcuf® and SureBPTM)
(Oridion® Microstream®)
EtCO
2
Temperature (2 Channels)
Invasive Pressures (3 Channels)
Advanced CPR Help
Pacing
Microphone
®
1-2 www.zoll.com 9650-001355-01 Rev. H
Page 15

How to Use This Manual
The X Series Operator's Guide provides information operators need for the safe and effective
use and care of the X Series product. It is important that all persons using this device read and
understand all the information contained within.
Please thoroughly read the safety considerations and warnings section.
Procedures for daily checkout and unit care are located in the Chapter 24, "Maintenance".
Operator ’s Guide Updates
An issue or revision date for this manual is shown on the front cover. If more than three years
have elapsed since this date, contact ZOLL Medical Corporation to determine if additional
product information updates are available.
All users should carefully review each manual update to understand its significance and then
file it in its appropriate section wi thin this manual for subsequent referenc e.
Product documentation is available through the ZOLL website at www.zoll.com. From the
Products menu, choose Product Manuals.
Unpacking
How to Use This Manual
Carefully inspect each container for damage. If the shipping container or cushion material is
damaged, keep it until the contents have been checked for completeness and the instrument has
been checked for mechanical and electrical integrity. If the contents are incomplete, if there is
mechanical damage, or if the defibrillator does n ot pass its electrical self-t est, U.S.A. customers
should call ZOLL Medical Corporation (1-800-348-9011). Customers outside of the U.S.A.
should contact the nearest ZOLL authorized representative. If the shipping container is
damaged, also notify the carrier.
9650-001355-01 Rev. H X Series Operator’s Guide 1-3
Page 16
CHAPTER 1GENERAL INFORMATION
Symbols Used on the Equipment
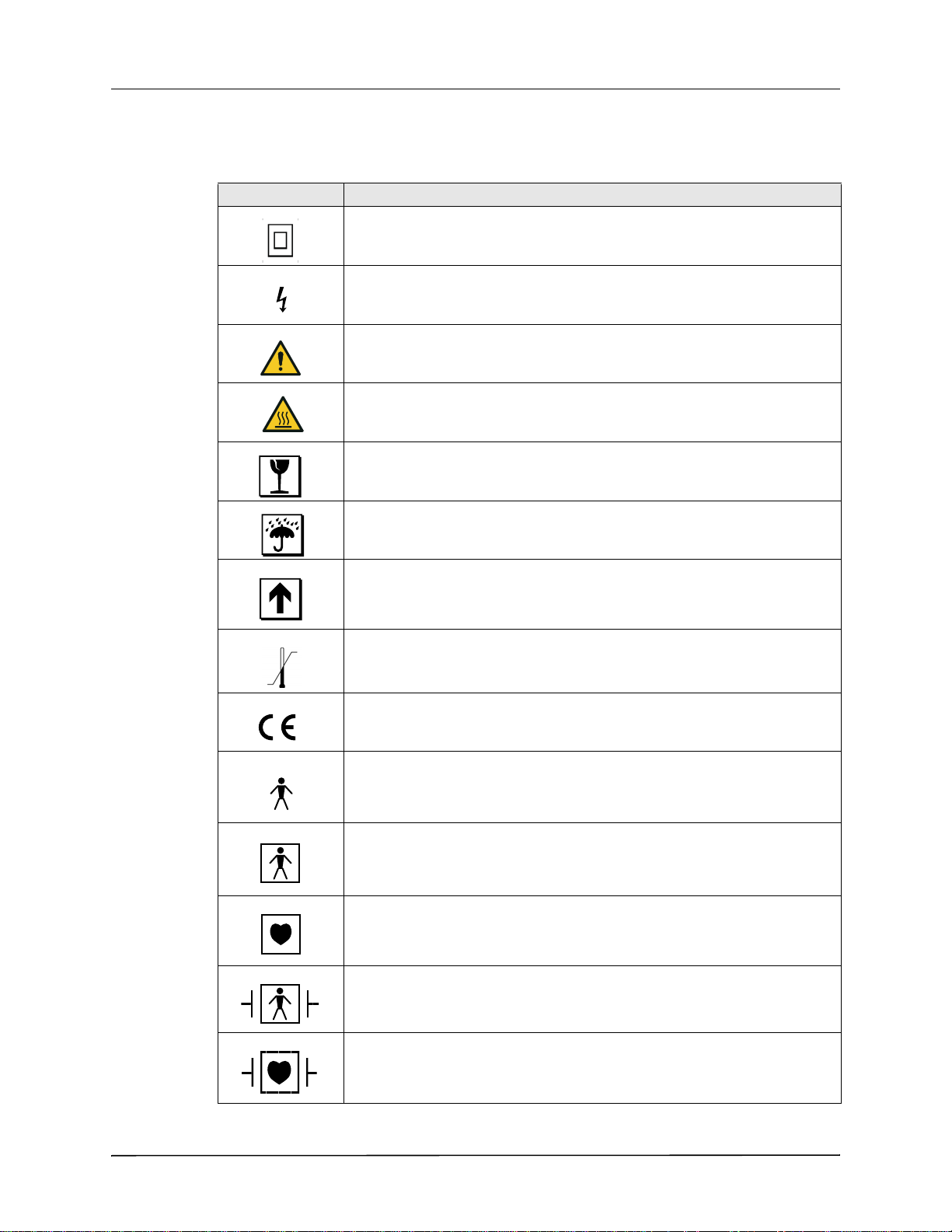
Any or all of the following symbols may be used in this manual or on this equipment:
Symbol Description
Class II equipment.
Dangerous voltage.
General warning: Observe and follow all safety signs.
Hot surface.
Fragile, handle with care.
Keep dry.
This end up.
Temperature limitation.
Conformité Européenne Complies with medical device directive 93/42/EEC.
Type B patient connection.
Type BF patient connection.
Type CF patient connection.
Defibrillator-proof type BF patient connection.
Defibrillator-proof type CF patient connection.
1-4 www.zoll.com 9650-001355-01 Rev. H
Page 17
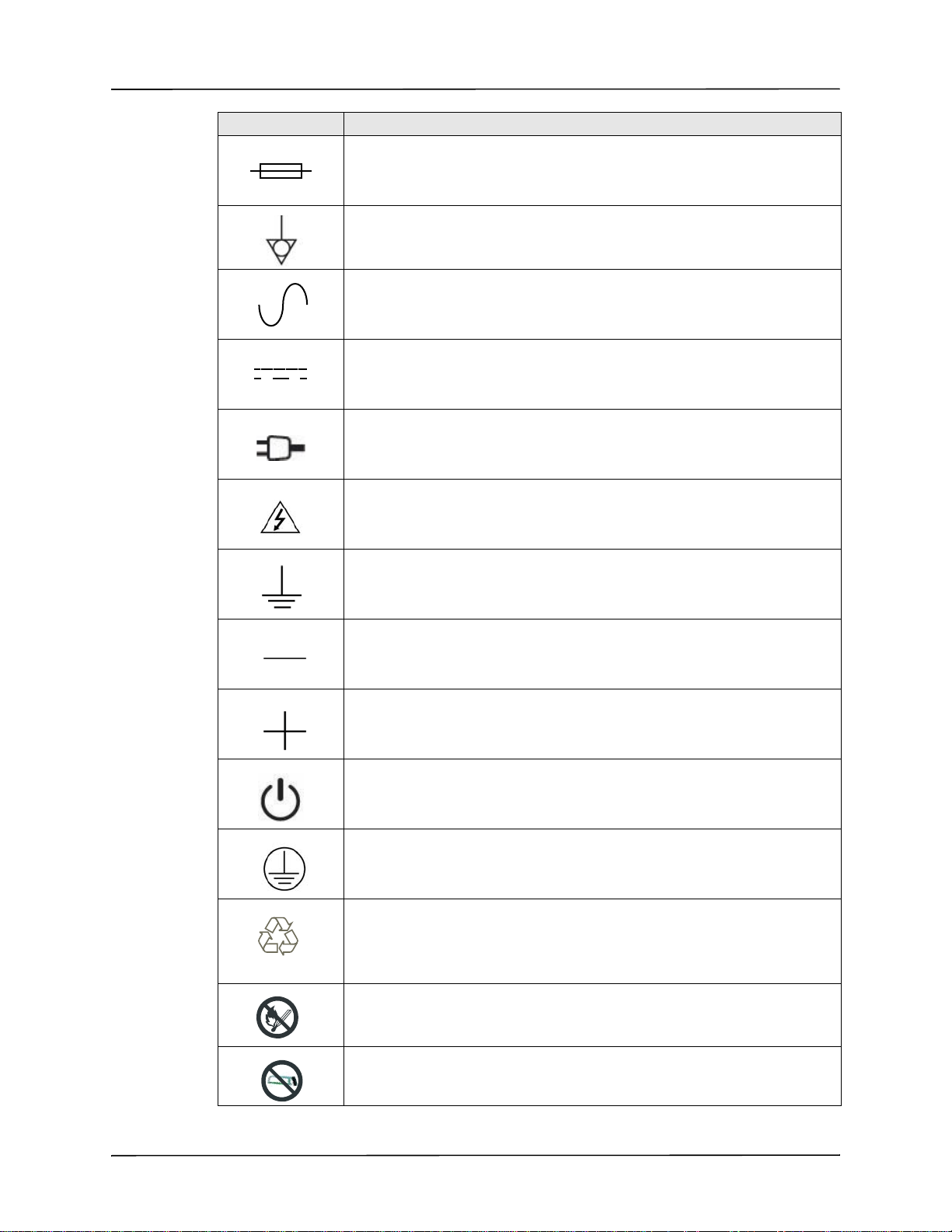
Symbol Description
2%452.
,I)/.
RECYCLE
,I)/.
Fusible link.
Equipotentiality.
Alternating current (ac).
Direct current (dc).
Auxiliary power adapter operation.
Caution, high voltage.
Symbols Used on the Equipment
Earth (ground).
Negative input terminal.
Positive input terminal.
Power On/Off
Protective earth (ground).
Contains lithium. Recycle or dispose of properly.
Keep away from open flame and high heat.
Do not open, disassemble, or intentionally damage.
9650-001355-01 Rev. H X Series Operator’s Guide 1-5
Page 18

CHAPTER 1GENERAL INFORMATION
Symbol Description
Do not crush.
Do not discard in trash. Recycle or dispose of properly.
Return to a collection site intended for waste electrical and electronic
equipment (WEEE). Do not dispose of in unsorted trash.
Date of manufacture.
Use by.
Latex-free.
Do not reuse.
Do not fold.
Not sterile.
Manufacturer.
Authorized representative in the European Community.
Serial Number.
Catalogue number.
Consult instructions for use.
Refer to instruction manual/booklet.
1-6 www.zoll.com 9650-001355-01 Rev. H
Page 19
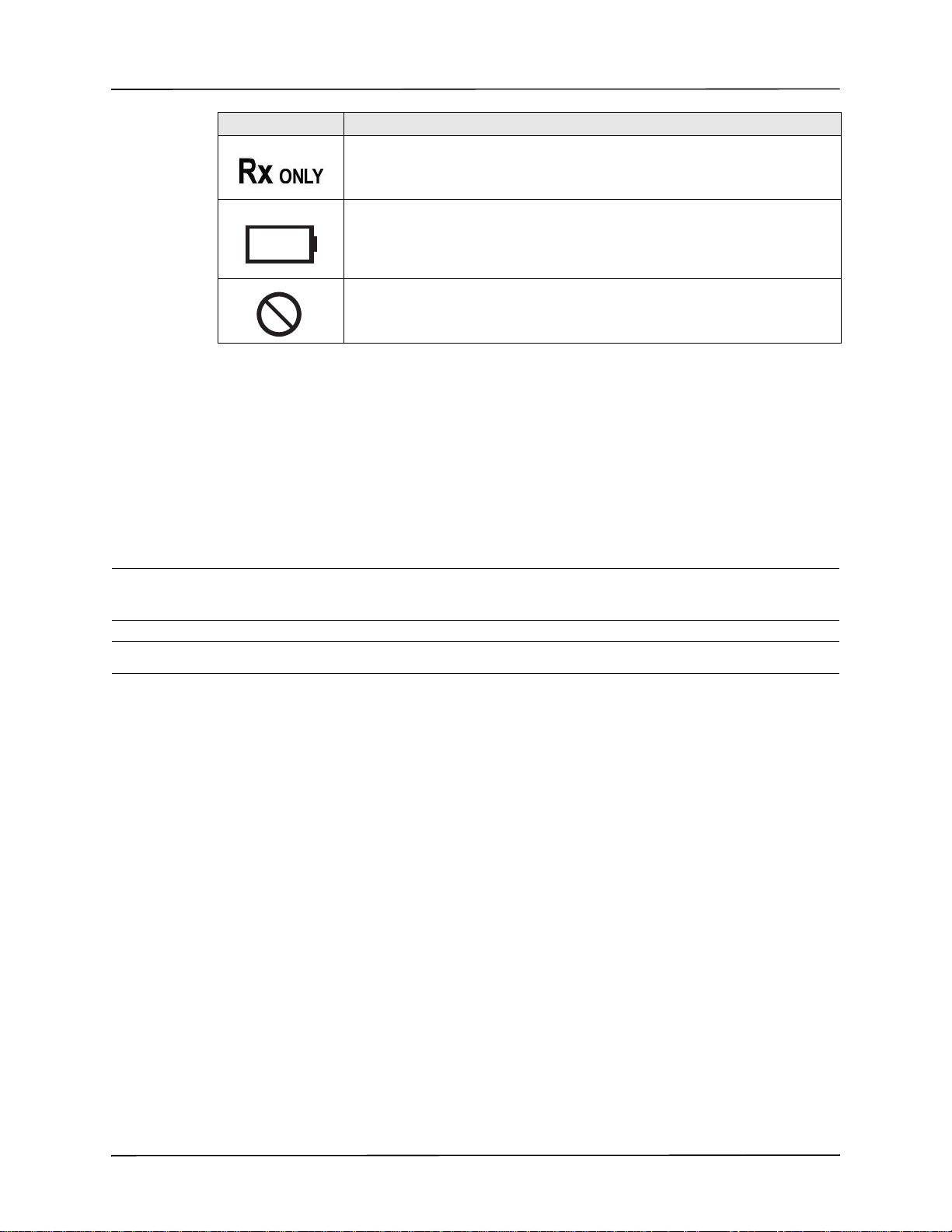
Symbol Description
MR
Conventions
This guide uses the following conventions:
Within text, the names and labels for physical buttons and softkeys appear in boldface type (for
example, “Press the Charge button or press the Pacer button”).
This guide uses uppercase italics for audible prompts and for text messages displayed on the
screen (for example, LEAD FAULT).
Conventions
Prescription only.
Battery charging status.
Do not use device, cables, or probes in an MRI environment.
War ning ! Warning statements alert you to conditions or actions that can result in personal injury
or death.
Caution Caution statements alert you to conditions or actions that can result in damage to the unit.
X Series Indications for Use
The X Series is intended for use by trained medical personnel who are familiar with basic
monitoring, vital sign assessment, emergency cardiac care, and the use of the X Series.
The X Series is also intended for use by (or on the order of) physicians at the scene of an
emergency or in a hospital emergency room, intensive care unit, cardiac care unit, or other
similar areas of a hospital. The usage may be in an ambulance or at the scene of an emergency.
It is also intended to be used during the transport of patients. The X Series will be used
primarily on patients experiencing symptoms of cardiac arrest or in post trauma situation. It
may also be used whenever it is required to monitor any of th ose functions that are included (as
options) in the device. The X Series unit can be used on pediatric patients (as described in the
following table) and on adult patients (21 years of age or older) with and without heart
dysfunction.
9650-001355-01 Rev. H X Series Operator’s Guide 1-7
Page 20
CHAPTER 1GENERAL INFORMATION
Pediatric Patient Subpopulation Approximate Age Range
Newborn (neonate) Birth to 1 month of age.
Infant 1 month to 2 years of age.
Child 2 to 12 years of age.
Adolescent 12 to 21 years of age.
When the pediatric patient is less than 8 years of age or weighs less than 55 lbs. (25 kg.), use
ZOLL Pedi-padz
®
pediatric defibrillation electrodes. Do not delay therapy to determine the
patient’s exact age or weight. AED mode and ALS rescue protocol are not intended for use on
neonatal patients.
Manual Defibrillation
Use of the X Series in the manual mode for external and internal defibrillation is indicated on
victims of cardiac arrest where there is apparent lack of circulation as indicated by:
• Unconsciousness.
• Absence of breathing.
• Absence of pulse.
This product should be used only by qualified medical personnel for converting ventricular
fibrillation and rapid ventricular tachycardia to sinus rhythm or other cardiac rhythms capable
of producing hemodynamically significant heart beats.
The unit can also be used for synchronized cardioversion of certain atrial or ventricular
arrhythmias. Qualified medical personnel must decide when synchronized cardioversion is
appropriate.
The patient population will range from newborn (neonate) to adult.
Semiautomatic Operation (AED)
X Series products are designed for use by emergency care personnel who have completed
training and certification requirements applicable to the use of a defibrillator where the device
operator controls delivery of shocks to the patient.
They are specifically designed for use in early defibrillation programs where the delivery of a
defibrillator shock during resuscitation involving CPR, transportation, and definitive care are
incorporated into a medically-approved patient care protocol.
Use of the X Series in the Semiautomatic mode for defibrillation is indicated on victims of
cardiac arrest where there is apparent lack of circulation as indicated by:
• Unconsciousness.
• Absence of breathing.
• Absence of pulse.
1-8 www.zoll.com 9650-001355-01 Rev. H
Page 21

Specifications for the ECG rhythm analysis functi on are provided in the section “ECG Analysis
Algorithm Accuracy” on page A-35.
When the patient is less than 8 years of age or weighs less that 55 lbs. (25 Kg), you must use
ZOLL pediatric defibrillation electrodes. Do not de lay t herapy to det ermine pat ient’s exact age
or weight.
ECG Monitoring
The X Series is intended for use to monitor and/or record 3-, 5-, or 12-lead ECG waveform and
heart rate, and to alarm when heart rate is above or below limits set by the operator. The patient
population will range from newborn (neonate) to adult, with and without heart dysfunction.
CPR Monitoring
The CPR monitoring function provides visual and audio feedback designed to encourage
rescuers to perform chest compressions at the AHA/ERC recommended rate of 100
compressions per minute. Voice and visual prompts encourage a minimum compression depth
of at least 1.5 (3.8 cm) or 2.0 inches (5.0 cm), depending on the configuration, for adult
patients. The CPR monitoring function is not intended for use on patients under 8 years of age.
X Series Indications for Use
External Transcutaneous Pacing
This product can be used for temporary external cardiac pacing in conscious or unconscious
patients as an alternative to endocardial stimulation.
The purposes of pacing include:
• Resuscitation from standstill or bradycardia of any etiology:
• As a standby when standstill or bradycardia might be expected:
• Suppression of tachycardia.
• Pediatric pacing.
Non-Invasive Blood Pressure Monitoring
The X Series is intended for use to make non-invasive measurements of arterial pressure and
heart rate, and to alarm if either parameter is outside of the limits set by the user. Measurements
are made using an inflatable cuff on the patient's arm or leg. The patient population will range
from newborn (neonate) to adult.
Temperature Monitoring
The X Series is intended for use to make continuous temperature measurements of rectal,
esophageal, or surface temperatures, and to alarm if the temperature is outside of the limits set
by the user. The patient population will range from newborn (neonate) to adult.
9650-001355-01 Rev. H X Series Operator’s Guide 1-9
Page 22

CHAPTER 1GENERAL INFORMATION
SpO2 Monitoring
The X Series pulse CO-oximeter, with Masimo Rainbow® SET technology and the Rainbow
series of sensors, is intended for use for continuous noninvasive monitoring of functional
oxygen saturation of arterial hemoglobin (SpO
(SpCO), and/or methemoglobin saturation (SpMet). The pulse CO-oximeter and accessories
are indicated for use on adult, pediatric, and neonatal patients during both no motion and
motion conditions, and for patients who are well or poorly perfused, in hospitals, hospital-type
facilities, or in mobile environments.
Respiration Monitoring
The X Series is intended for use to continuously monitor respiration ra te and to alarm if the rate
falls outside of the range set by the operator. Because the measurement method actually
measures respiratory effort, apnea episodes with continued respiratory effort (such as
obstructive apnea) may not be detected. It is not intended to be used as an apnea monitor. The
patient population will range from newborn (neonate) to adult.
CO2 Monitoring
), pulse rate, carboxyhemoglobin saturation
2
The X Series is intended for use to make cont inuo us nonin vasive measurem ent an d moni tori ng
of carbon dioxide concentration of the expired and inspired breath and breath rate. The patient
population will range from newborn (neonate) to adult.
Invasive Pressure Monitoring
The X Series is intended for use to display and make continuous invasive pressure
measurements from any compatible pressure transducer. The primary intended uses are arterial
blood pressure, central venous pressure and intracranial pressure monitoring. Any contraindications of the particular transducer selected by the user shall apply. The patient population
will range from newborn (neonate) to adult.
12-Lead Analysis
The 12-lead ECG Analysis is intended for use in acquiring, analyzing and reporting ECG data,
and to provide interpretation of the data for consideration by caregivers. The interpretations of
ECG data offered by the device are only significant when used in conjunction with caregiver
overread as well as consideration of all other relevant patient data. The 12-lead ECG Analysis
is intended for use on adults (> 18 years of age).
1-10 www.zoll.com 9650-001355-01 Rev. H
Page 23

X Series Product Functions
Defibrillator Function
The X Series contains a direct current (dc) defibrillator capabl e of delivering up to 200 jo ules. It
may be used in synchronized mode to perform synchronized cardioversion using the patient’s
R-wave as a timing reference. The unit uses paddles or disposable, pregelled electrodes for
defibrillation.
Defibrillator Output Energy
X Series defibrillators can deliver biphasic energy from 1 joule to 200 joules. The energy
delivered through the chest wall, however, is determined by the patient’s transthoracic
impedance. An adequate amount of e lectrolyte gel mu st be applied t o the paddles and a force of
10 to 12 kilograms (22 to 26.4 pounds) must be applied to each paddle in order to minimize this
impedance. If hands-free therapy electrodes are used, make sure that they are properly applied.
(Refer to the instructions on the electrode package).
X Series Product Functions
External Pacemaker
X Series defibrillators include a transcutaneous pacemaker consist in g of a pulse generator and
ECG-sensing circuitry. Noninvasive transcutaneous pacing (NTP) is an established and proven
technique. This therapy is easily and rapidly applied in both emergency and nonemergency
situations when temporary cardiac stimulation is indicated.
The output current of the pacemaker is continuously variable from 10 to 140 mA (the output
current is 0 mA when paused). The rate is continuously variable from 30 to 180 pulses per
minute (ppm), by increments of 5 ppm (10 bpm when greater than 100 ppm).
The pacing output pulse is delivered to the heart via ZOLL hands-free defibrillation/pacing
electrodes placed on the patient’s back and the precordium.
Proper operation of the device, together with correct electrode placeme nt, is critical to
obtaining optimal results. Every operator must be thoroughly familiar with these operating
instructions.
ECG Monitoring
The patient’s ECG is monitored by connecting the patient to the unit via a 3-, 5-, or 12-lead
patient cable or hands-free therapy electrodes. The ECG waveform is presented on the display
along with the following information:
• averaged heart rate, derived by measuring R to R intervals
• lead selection - I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6 (with ECG cable),
PADDLES, or PADS.
• ECG size - 0.125, 0.25, 0.50, 1.0, 2.0, 4.0 cm/mV, AUTO
• status messages
The ECG bandwidth is user selectable.
9650-001355-01 Rev. H X Series Operator’s Guide 1-11
Page 24
CHAPTER 1GENERAL INFORMATION
Electrodes
The X Series units will defibrillate, cardiovert, and monitor ECG using hands-free therapy
electrodes. The X Series unit will pace using ZOLL hands-free therapy electrodes.
Energy Select, Charge and Shock controls are located on the paddles and front panel. When
using hands-free therapy electrodes, you must use the controls on the front panel of the unit. To
switch between paddles and hands-free therapy electrodes, remove the multifunction cable
(MFC or OneStep) from the apex paddle and connect the hands-free therapy electrodes to the
cable.
You should always check the expiration date on the electrode packaging. Do not use expired
electrodes, which might result in false patient impedance readings and affect the level of
delivered energy, or cause burns.
This symbol on the electrode package is accompanied by the expiration date.
For Stat-padz
lower right corner of the label, below the lot number.
Note: ZOLL electrodes contain no hazardous materials and may be disposed of in general
trash unless contaminated with pathogens. Use appropriate precautions when
disposing of contaminated electrodes.
When the patient is less than 8 years old or weighs less than 55 lb. (25 kg), use ZOLL OneStep
Pediatric defibrillation electrodes. Do not delay therapy to determine the patient’s exact age or
weight.
®
II, this symbol does not appear; the expiration date appears on the
Batteries
X Series models use an easily replaced rechargeable li thium-ion battery pack (the Su r ePower II
Battery Pack). A new, fully charged battery pack typically delivers more than 6 hours of ECG
monitoring. Use of other functions (such as the defibrillator, printer, or pacemaker) reduces this
time.
When a LOW BATTERY icon appears on the display and the unit emits three beeps in
conjunction with the displayed battery icon, the battery must be replaced and recharged.
You can charge the battery by either of the following methods:
• Internal charging — plug the X Series into an auxiliary power adapter to automatically
begin charging the installed battery pack. The front panel battery indicator operates as
follows:
When the indicator is: It means:
Steady yellow Battery is charging.
Steady green Battery is charged.
Alternating yellow and
green
Not lit No battery in device.
The charge state cannot be
determined or a battery charging
fault has been detected.
Note: Upon power up, it takes approximately 45 seconds for the LEDs on the battery to
accurately display run time.
1-12 www.zoll.com 9650-001355-01 Rev. H
Page 25
• External charging — use the ZOLL SurePower Battery Charger with the X Series battery
adapter to charge the battery pack and test the battery’s capacity. For details, refer to the
SurePower II Battery Pack Guide.
The Recalibration LED icon ( ) lights for approximately 10 seconds (after you press and
release the Display button) if the battery ne eds to be calibrated . If the Recalibration LED lights,
the runtime indicator will not display run time for that battery. For best performance of the
battery, you should recalibrate the battery as soon as possible.
To manually recalibrate the SurePower II Battery Pack, you can insert the battery into the
SurePower Charger Station and perform a Manual Test (for mo re information, see the ZOLL
SurePower Charger Station Operator’s Guide).
After you recalibrate the battery, the Recalibration LED will only flash when you press the
Display button.
Ready For Use (RFU) Indicator
The X Series has an RFU indicator on the front panel that indicates if the device is ready for
use. The RFU indicator has three states which are described in the following table.
State Description Action
X Series Product Functions
Ready for Use The device is ready for use. Patient
monitoring, defibrillation, and
pacing parameters are functional
and the battery is above the low
battery capacity.
Note: If the device is plugged into
the auxiliary power adapter, the
Ready for Use indicator may display
even if the battery is depleted.
Check the status of the battery
before removing the device from the
auxiliary power adapter.
None required.
9650-001355-01 Rev. H X Series Operator’s Guide 1-13
Page 26
CHAPTER 1GENERAL INFORMATION
State Description Action
Flashing One or more of the following has
occurred:
• The battery is not properly
installed.
• A low battery is installed.
• A battery fault has occurred.
• There is no battery installed
while connected to auxiliary
power.
• One or more patient monitoring
parameters have failed self-test
(NIBP, SpO
Temp).
• The front panel button self-test
failed.
• The speech database self-test
failed.
Do Not Use One or more of the following has
occurred:
• The battery is not properly
installed.
• No battery is installed and
auxiliary power is not present.
• A very low battery (below
software shutdown limit) was
installed.
• ECG, defibrillator, or pacer selftests have failed, or other critical
self-tests have failed.
, CO2, IBP, or
2
Install a fully charged battery in the
unit and check the RFU indicator
again. If the RFU indicator
continues to flash, remove the unit
from service and contact the
appropriate technical personnel or
the ZOLL Technical Service
Department.
Install a fully charged battery in the
unit and check the RFU indicator
again. If the RFU indicator
continues to display the Do Not
Use symbol, remove the unit from
service and contact the appropriate
technical personnel or the ZOLL
Technical Service Department.
1-14 www.zoll.com 9650-001355-01 Rev. H
Page 27

Safety Considerations
All operators should review these safety considerations before using the X Series unit.
X Series units are high-energy defibrillators capable of delivering 200 joules. To completely
deactivate the unit, press the power switch to turn the unit off.
To manually disarm a charged (or charging) defibrillator, do one of the following:
• Press the Disarm quick access key .
• Change the selected energy.
• Press the power switch to turn the unit off.
For safety, the X Series automatically disarms if left charged for more than 60 seconds if the
shock button ( ) is not pressed.
Warnings
General
Federal (U.S.A.) law restricts this defibrillator to sale by or on the order of a physician.
Safety Considerations
Only appropriately trained, skilled personnel who are familiar with equipment operation should
perform emergency defibrillation. The prescribing physician should determine what training,
such as Advanced Cardiac Life Support (ACLS) or Basic Life Support (BLS) certification, is
appropriate.
Only skilled personnel trained in Advanced Cardiac Life Support (ACLS) and who are familiar
with equipment operation should perform synchronized cardioversion. The precise cardiac
arrhythmia must be determined before attempting defi bril lat io n.
These operating instructions describe the functions and proper operation of the X Series
products. They are not a substitute for a formal patient care training course. Operators should
obtain formal training from an appropriate authority before using this defibrillator for patient
care.
Proper operation of the unit and correct electrode placement is critical to obtaining optimal
results. Operators must be thoroughly familiar with proper device operation.
The use of external pacing/defibrillation electrodes, accessories, or adapter devices from
sources other than ZOLL is not recommended. ZOLL makes no representations or warranties
regarding the performance or effectiveness of its products when used with pacing/defibrillation
electrodes or adapter devices from other sources. Defibrillator failures attributable to the use of
pacing/defibrillation electrodes or adapters not manufactured by ZOLL might void ZOLL’s
warranty.
At receipt of shipment, check pacing/defibrillation electrodes to ensure compatibility.
Allow ample slack in cables to make sure that cables do not tug at electrodes.
Do not disassemble the unit. A shock hazard exists. Refer all problems to authorized service
personnel.
Follow all recommended maintenance instructions. If a problem occurs, obtain service
immediately. Do not use the defibrillator until it has been inspected by appropriate personnel.
9650-001355-01 Rev. H X Series Operator’s Guide 1-15
Page 28

CHAPTER 1GENERAL INFORMATION
The X Series unit might not perform to specifications when stored at the upper or lower
extreme limits of storage temperature and then immediately put into use. The X Series unit
should not be stored or used outside of the environmental limits p rovided in Appendix A of this
manual.
A void using the X Series adjacent to, or stacked on, other equipment. If unavoidable, verify that
the unit operates normally in this configuration before clinical use.
The X Series unit should be installed and put in to service a ccordi ng to the EM C informati on in
Appendix A of this manual.
Do not use internal paddles while the X Series unit’s auxiliary power source is connected to an
aircraft AC power operating at a frequency of 400 Hz.
The use of accessories, transducers, and cables other than those specified in this manual and
related X Series option manual inserts may result in increased emissions or decreased immunity
of the X Series.
Perform functional test of internal paddles prior to use.
Do not use or place the unit in service if the Ready For Use indicator (at the upper right of the
front panel) displays a red circle with a line through it.
Carefully route patient cabl es to avoid tri pping over the m, or inadverte ntly pulli ng the unit onto
the patient.
Always inspect the unit for damage if it has been dropped.
Only authorized personnel should use the Supervisor menus.
If uncertain about the accuracy of any measurement, first check the patient’s vital signs by
alternate means, and then make sure the monitor is functioning correctly.
ECG Monitoring
Implanted pacemakers might cause the heart rate meter to count the pacemaker rate during
incidents of cardiac arrest or other arrhythmias. Dedicated pacemaker detection circuitry may
not detect all implanted pacemaker spikes. Check the patient's pulse; do not rely solely on heart
rate meters. Patient history and physical examination are important factors in determining the
presence of an implanted pacemaker. Pacemaker patients should be carefully observed. See
“Pacemaker Pulse Rejection:” on page A-15 of this manual for disclosure of the pacemaker
pulse rejection capability of this instrument.
Use only ECG electrodes that meet the AAMI standard for electrode performance
(AAMI EC-12). Use of electrodes not meeting this AAMI standard could cause the ECG tr ace
recovery after defibrillation to be significantly delayed.
Prior to attempting synchronized cardioversion, ensure the ECG signal quality is good and that
sync markers are displayed above each QRS complex.
Do not place electrodes directly over an implanted pacemaker.
The X Series unit detects ECG electrical signals only. It does not detect a pulse (effective
circulatory perfusion). Always verify pulse and heart rate by physical assessment of the patient.
Never assume that the display of a nonzero heart rate means that the pat ient has a pulse.
Excessive artifact can result due to improper skin preparation of th e electrode site s. Follow skin
preparation instructions in Chapter 6: “Monitoring ECG.”
1-16 www.zoll.com 9650-001355-01 Rev. H
Page 29

Equipment such as electrocautery or diathermy equipment, RFID readers, electronic art icle
surveillance (EAS) systems, or metal detectors that emit strong radio frequency signals can
cause electrical interference and distort the ECG signal displayed by the monitor, thereby
preventing accurate rhythm analysis. Ensure adequate separation between such emitters, the
device, and the patient when performing rhythm analysis.
Shock Hazard: Use of accessories, other than those specified in the operating instructions, may
adversely affect patient leakage currents.
Certain line-isolation monitors may cause interference on the ECG display and may inhibit
heart rate alarms.
Monitoring ECG through the paddles may result in inaccurate heart rate display due to artifact.
Defibrillation
The ZOLL X Series can deliver 200 joules o f electrical energy. If this electrical energy is not
discharged properly, as described in the this manual, the electrical energy could cause persona l
injury or death to the operator or bystander.
To avoid possible damage to the X Series unit, turn off pacing before defibrillating the patient
with a second defibrillator.
Warnings
After a synchronized cardioversion, the SYNC mode may be cleared after each shock or
disarm. The user may have to reselect (press) the SYNC button after each synchronized
cardioversion shock performed on a patient. In Defib/Pacer Default sett ings in the Supervisor
Setup menu, the X Series can be configured to remain in the SYNC mode after each
synchronized cardioversion.
Synchronized cardioversion can be performed in the paddle monitoring mode. However, it is
possible that artifact can be produced by the moving paddles, which could cause t he
defibrillator to trigger on the artifact. It is recommended that monitoring in leads I, II or III be
used during synchronized cardioversion. Paddle monitoring should not be used for elective
cardioversions procedures.
To avoid stress to the defibrillator or the tester, never attempt to repeatedly charge and
discharge the defibrillator in rapid succession. If a need for repetitive testing arises, allow a
waiting period of at least 2 minutes after every third discharge.
In the SYNC mode, the defibrillator does not discharge without a command signal (R-w ave
detection) from the ECG monitor indicate d by a SYNC marker on the trace and a flashing
SYNC indicator.
If conductive gel forms a continuous path between the defibrillator electrodes, delivered energy
may be dramatically reduced to zero. In this case, reposition the electrodes to eliminate the
shunting path before attempting additional shocks.
Improper defibrillation technique can cause skin burns. To limit possible skin burns, use only
ZOLL defibrillation gel on paddles, ensure the gel covers the entire paddle surface and press
firmly against patient’s chest.
If a new energy level is selected after the CHARGE button is pushed and while the defibrillator
is charging or charged, the defibrillator will disarm. The CHARGE button will need to be
pressed again to charge to the new energy level.
Prior to defibrillation, disconnect from the patient any medical electronic device that is not
labeled “defibrillation protected.”
9650-001355-01 Rev. H X Series Operator’s Guide 1-17
Page 30

CHAPTER 1GENERAL INFORMATION
Before charging the defibrillator, verify that the energy selected on the display is the desired
output.
Defibrillation takes priority over external pacing. Should the defibrillator be charged during the
administration of external pacing, the pacer turns off and the defibrillator charges to the
selected energy.
Pacing
Ventricular fibrillation does not respond to pacing and requires immediate defibrillation.
Therefore, the patient’s dysrhythmia must be determined immediately, so that you can employ
appropriate therapy . If t he patient is in ventricula r fibrillation and defibrilla tion is successful but
cardiac standstill (asystole) ensues, you should use the pacemaker.
Ventricular or supraventricular tachycardias can be interrupted with pacing, but in an
emergency or during circulatory collapse, synchronized cardioversion is faster and more
certain.
Pulseless electrical activity (PEA) can occur following prolonged cardiac arrest or in other
disease states with myocardial depression. Pacing might then produce ECG responses wi th out
effective mechanical contractions, making other effective treatment necessary.
Pacing can evoke undesirable repetitive resp onses, tachycardia, or fibrillation in the presence of
generalized hypoxia, myocardial ischemia, cardiac drug toxicity, electrolyte imbalance, or
other cardiac diseases.
Pacing by any method tends to inhibit intrinsic rhythmicity. Abrupt cessation of pacing,
particularly at rapid rates, can cause ventricular standstill and should be avoided.
Noninvasive temporary pacing can cause discomfort of varying intensity, which occasionally
can be severe and preclude its continued use in conscious patients.
Similarly, unavoidable skeletal muscle contraction might be troublesome in very sick patients
and might limit continuous use to a few hours. Erythema or hyperemia of the skin under the
hands-free therapy electrodes often occurs; this effect is usually enhanced along the perimeter
of the electrode. This reddening should lessen substantially withi n 72 hours.
There have been reports of burns under the anterior electrode when pacing adult patients with
severely restricted blood flow to the skin. Prolonged pacing should be avoided in these cases
and periodic inspection of the underlying skin is advised.
There are reports of transient inhibition of spontaneous respiration in unconscious patients with
previously available units when the anterior electrode was placed too low on the abdomen.
The pacing rate determination can be adversely affected by artifact. If the patient’s pulse and
the heart rate display are sig nificantly different, external pacing pulses may not be delivered
when required.
Artifact and ECG noise can make R-wave detection unreliable, affecting the HR meter and the
demand mode pacing rate. Always observe the patient closely during pacing operations.
Consider using asynchronous pacing mode if a reliable ECG trace is unob tai nab le .
Transcutaneous pacing should not be used to treat V FIB (ventricular fibrillation). In cases of
V FIB, immediate defibrillation is advised.
1-18 www.zoll.com 9650-001355-01 Rev. H
Page 31

Warnings
Transcutaneous pacing may cause discomfort ranging from mild to severe, depending on the
patient’s tolerance level, muscle contractions and electrode placement. In certain cases,
discomfort may be decreased by slightly relocating the pacing pads.
It is important to monitor the patient closely to verify that both mechanical and electrical
capture are occurring. Electrical capture can be verified by observing the presence of a large
ectopic beat after the pacing pulse is delivered. The size and morphology of the beat are
dependent on the patient. In some instances the be at may appe ar as a rel ati vel y normal looking
QRS pulse. Mechanical capture can be verified by checking for signs of increased blood flow
i.e., reddening of the skin, palpable pulses, increased blood pressure, etc. Continuously observe
the patient during pacing administration , to insure capture retention. Do not leave the patient
unattended when administering external pacing therapy.
Warning! This device can only be used for external pacing of patients and cannot be used for
internal pacing. Do not connect internal pacing lead wires to the X Series defibrillator.
CPR
The CPR monitoring function is not intended for use on patients under 8 years of age.
Place the patient on a firm surface before performing CPR.
The patient must be motionless during CPR for accurate CPR measurements.
Pulse Oximeter
Keep the ZOLL finger probe clean and dry.
SpO2 measurements may be affected by certain patient conditions: severe right heart failure,
tricuspid regurgitation or obstructed venous return.
SpO
2
vasoconstriction or hypovolemia or under conditions where there is no pulsating arterial
vascular bed.
SpO
2
devices, IR lamps, bright lights, improperly applied sensors; the use of non-ZOLL sensors, or
damaged sensors; in patients with smoke inhalation, or carbon monoxide poisoning, or with
patient movement.
Tissue damage can result if sensors are applied incorrectly, or left in the same location for an
extended period of time. Move sensor every 4 hours to reduce possibility of tissue damage.
Do not use any oximetry sensors during MRI scanning. MRI procedures can cause conducted
current to flow through the sensors, causing patient burns.
measurements may be affected when using intravascular dyes, in extreme
measurements may be affected in the presence of strong EMI fields, electrosurgical
Do not apply SpO
when the arterial circulation is cut off during NIBP measurements, and may affect SpO
sensor to the same limb that has an NIBP cuff. The SpO2 alarm may sound
2
2
measurements.
9650-001355-01 Rev. H X Series Operator’s Guide 1-19
Page 32

CHAPTER 1GENERAL INFORMATION
In some instances, such as obstructed airway, the patient's breathing attempts may not produce
any air exchange. These breathing attempts can still produce chest size changes, creating
impedance changes, which can be detected by the respiration detector. It is best to use the pulse
oximeter whenever monitoring respirations, to accurately depict the patient's respiratory
condition.
Noninvasive Blood Pressure
Only a physician can interpret pressure measurements.
Blood pressure measurement results may be affected by the position of the patient, his or her
physiological condition and other factors.
Substitution of a component different from that supplied by ZOLL (e.g., cuff, hoses, etc.) may
result in measurement error. Use only ZOLL-approved cuffs and hoses. To avoid the risk of
intravenous line misconnection and possible introduction of air into a patient’s blood, do not
modify the NIBP system or hoses with Luer Lock adapters.
IBP
Do not use a blood pressure cuff on the limb being used for IV infusion or for SpO
Accurate pressure readings may not be achieved on a person experiencing arrhythmias,
shaking, convulsions or seizures. Medication may also affect pressure readings. The correct
size cuff is essential for accurate blood pressure readings.
Blood pressure hoses must be free of obstructions and crimps.
If the patient’s cuff is not at heart level, an error in measurement may result.
When monitoring blood pressure at frequent intervals, observe the cuffed extremity of the
patient for signs of impeded blood flow.
Do not monitor one patient’s NIBP while monitoring another patient’s ECG.
Blood pressure measurement may be inaccurate if tak en while acc elera ting or decelera ting in a
moving vehicle.
If an NIBP measurement result is questionable or “motion” indication is displayed, repeat the
measurement. If the repeated measurement result is still questionable, use another blood
pressure measurement method.
Do not use the NIBP on cardiopulmonary bypass patients.
To ensure compatibility and electrical safety, accessory pressure sensors should comply with
ANSI/AAMI BP-22 and IEC 60601-2-34 for IBP or ANSI/AAMI NS28 for ICP.
monitoring.
2
Follow instructions supplied with any accessory pressure sensor regar di ng calibration and
removal of trapped air.
Avoid touching metal parts of any transducer while it is in contact with the patient.
Do not reuse any components that are labeled for single use only.
Transducers should be rated to withstand an accidental drop of at least a meter onto a hard
surface.
Transducers that are subject to immersion in liquids should be rated as watertight.
1-20 www.zoll.com 9650-001355-01 Rev. H
Page 33

CO
Warnings
2
During MRI scanning, the monitor must be placed outside the MRI suite. When the monitor is
used outside the MRI suite, EtCO
which permits placement of the monitor outside the MRI suite.
When using the monitor with anesthetics, nitrous ox ide or high concentrations of oxygen,
connect the gas outlet to a scavenger system.
monitoring can be implemented using a long FilterLine®
2
Use only Oridion Microstream CO
Microstream CO
lines.
If using the CO
or when it becomes occluded.
CO
readings and respiratory rate can be affected by sensor application errors, certain ambient
2
environmental conditions, and certain patient co nd itions.
sampling lines are labeled for single patient use onl y. Do not reuse sampling
2
Monitor for extended critical care, replace the airway adapter every 24 hours
2
Respiration
Do not operate the X Series with any other monitor with respiration measurements on the same
patient. The two devices could affect the respiration accuracy.
The device should not be used as an apnea monitor.
Ferromagnetic Equipment
Biomedical equipment and accessories, such as ECG electrodes, cables, and oximeter probes
contain ferromagnetic materials. Ferromagnetic equipment mu st no t be used in the presence of
high magnetic fields created by magnetic resonance imaging (MRI) equipm ent.
sampling lines.
2
The large magnetic fields generated by an MRI device can attract ferromagnetic equipment
with an extremely violent force, which could cause serious personal injury or death to persons
between the equipment and the MRI device.
Battery
Although the device can opera te with auxiliary power alone, ZOLL strongly recommends that
you operate the unit with a battery installed at all times. Operating the unit with a battery
provides a backup in case of AC power shortage, and results in faster charge time. The battery
can be automatically recharged while it is installed in the unit. Keep a fully charged spare
battery pack with the defibrillator at all times.
Test battery packs regularly. A battery that does not pass the ZOLL charger’s capacity test
might cause the X Series unit to shut down unexpectedly.
If the Low Battery indication occurs at any time during operation, immediately replace the
battery pack.
9650-001355-01 Rev. H X Series Operator’s Guide 1-21
Page 34

CHAPTER 1GENERAL INFORMATION
If the LOW BATTERY icon appears, plug the X Series unit into a power source or install a fully
charged battery pack. When the warning low battery shutdown prompt appears, immediately
replace the battery pack with a fully charged pack or plug the X Series unit i nto a power source,
as unit shut down due to a low battery condition is imminent.
If mistreated, a battery pack might explode. Do not disassemble a battery pack or dispose of it
in fire.
Operator Safety
The X Series can deliver 200 joules of electrical energy. If this electrical energy is not
discharged properly, as described in this manual, the electrical energy could cause personal
injury or death to the operator or bystanders.
Do not use the X Series in the presence of oxygen-rich atmospheres, flammable anesthetics, or
other flammable agents (such as gasoline). Usin g the unit in such envi ronments might cause an
explosion.
Do not use the unit near or within standing water. Electrical safety might be compromised when
the defibrillator is wet.
Never discharge the unit with the defibrillation electrodes or paddles shorted together or in
open air.
Do not discharge the defibrillator except as indicated in the instructions. Discharge the
defibrillator only when defibrillation electrodes or paddles are properly applied to the patient.
To avoid risk of electrical shock, do not touch the gelled area of the hands-free therapy
electrodes during pacing or defibrillation.
T o avoid risk of electrical shock, do not allow electrolyte gel to accumulate on hands or paddle
handles.
To avoid risk of electrical shock, do not allow patient connectors to contact other conductive
parts, including earth.
For defibrillation using paddles, use only high-conductivi ty electrolyte gel specified for such
use by the manufacturer.
When using paddles for defibrillation, use your th umb s to operat e th e SHOCK buttons. Doing
so avoids inadvertent shock to the operator.
The use of accessory equipment that does not comply with the equivalent safety requirements
of the X Series defibrillator could reduce the level of safety of the combined system. When
choosing accessory equipment, consider the following:
• Use of the accessory in the patient vicinity.
• Evidence that the safety certification of the accessory has been performed in accordance
with the appropriate IEC (EN) 60601-1 and/or IEC (EN) 60601-1-1 harmonized national
standards.
Always check that the equipment functions properly and is in proper condition before use.
Disconnect all electro-medical equipment that is not defibrillation-protected from the patient
prior to defibrillation.
Before discharging the defibrillator, warn everyone to STAND CLEAR of the patient.
1-22 www.zoll.com 9650-001355-01 Rev. H
Page 35

Do not touch the bed, patient, or any equipment connected to the patient during defibrillation.
A severe shock can result. To avoid hazardous pathways for the defibrillation current, do not
allow exposed portions of the patient's body to touch any metal objects, such as a bed frame.
T o avoid risk of electric al shock, do not allow printer to come into contact with other condu cive
parts, such as equipment connected to the USB port.
Patient Safety
Inappropriate defibrillation or cardioversion of a patient (for example, with no malignant
arrhythmia) may precipitate ventricular fibrillation, asystole, o r other dangerous arrhythmias.
Defibrillation without proper application of electrodes or paddle electrolyte gel might be
ineffective and cause burns, particularly when repeated shocks are necessary. Erythema or
hyperemia of the skin under the paddles, or electrodes often occurs; this effect is usually
enhanced along the perimeter of the paddles or electrodes. This reddening should diminish
substantially within 72 hours.
This equipment should be connected to only one patient at a time.
Use only OneStep Pediatric electrodes to defibrilla te patients under 8 year s of age. Use of adult
electrodes, or pediatric electrodes other than OneStep Pediatric electrodes, can result in the
delivery of excessive energy doses.
Warnings
Neonatal and pediatric defibrillation energy level settings should be based on site-specific
clinical protocols.
To ensure patient safety, do not place the monitor in any position that might cause it to fall on
the patient.
To ensure patient safety, connect the X Series only to equipment with circuits that are
electrically isolated.
Use only high-quality ECG electrodes. ECG electrodes are for rhythm acquisition only; you
cannot use ECG electrodes for defibrillation or pacing.
Do not use therapy or ECG electrodes if the gel is dried, separated, torn or split from the foil;
patient burns may result from using such electrodes. Poor adherence and/or air pockets under
therapy electrodes can cause arcing and skin burns.
Check the expiration date on the electrode packaging. Do not use electrodes after their
expiration date.
Excessive body hair or wet, diaphoretic skin can inhibit electro de co up lin g to th e skin. Clip
excess hair and dry any moisture from the area where an electrode is to be attached.
Therapy electrodes should be replaced periodically during continuous pacing. Consult
electrode directions for proper replacement instructions.
Prolonged pacing (more than 30 minutes), particularly in neonates or adults with severely
restricted blood flow, may cause burns. Periodically inspect the skin under the electrodes.
Carefully route the patient cables away from the patient’s neck to reduce the possibility of
patient entanglement or strangulation.
T o avoid electrosurgery burns at monitoring sites, ensure proper connection of the
electrosurgery return circuit so that a return path cannot be made through monit oring electrodes
or probes.
9650-001355-01 Rev. H X Series Operator’s Guide 1-23
Page 36

CHAPTER 1GENERAL INFORMATION
During electrosurgery, observe the following guidelines to minimize electrosurgery unit (ESU)
interference and provide maximum operator and patient safety:
• Keep all patient monitoring cables away from earth ground, ESU knives, and ESU return
wires.
• Use electrosurgical grounding pads with the largest practical contact area.
Always ensure proper application of the electrosurgical return electrode to the patient.
Check electrical leakage levels before use. Leakage current might be excessive if more than one
monitor or other piece of equipment is connected to the patient.
Cautions
If the unit is to be stored longer than 30 days, remove the battery pack.
Do not sterilize the defibrillator, or its accessories unless the accessories are labelled as
sterilizable.
Do not immerse any part of the defibrillator in water.
Do not use the defibrillator if excessive condensation is visible on the device. Wipe only the
outside with a damp cloth.
Do not use ketones (such as acetone or MEK) on the defibrillator.
Avoid using abrasives (including paper towels) on the display window.
To achieve the specified level of protection against spilled or splashed liquids, thoroughly dry
all exposed surfaces of this device prior to operation or connections to auxiliary power.
If liquids enter the device connectors, remove all liquid from the connectors and allow the
device to dry thoroughly prior to use.
Grounding reliability can be achieved only when the equipment is connected to a receptacle
marked “HOSPITAL ONL Y,” “HOSPITAL GRADE,” or equivale nt. If the grou ndin g inte grity
of the line cord or ac receptacle is questionable, operate the defibrillator using battery power
only.
Do not connect to an electrical outlet controlled by a wall switch or dimmer.
To protect the unit from damage during defibrillation, for accurate ECG information, and to
protect against noise and other interference, use only intern al current-limiting ECG cables
specified or supplied by ZOLL.
For continued safety and EMI performance, use only the line cord supplied by ZOLL.
Electrical installation of the room or the building in which the monitor is to be used must
comply with regulations specified by the country in which the equipment is to be used.
Dispose of battery packs in accordance with national, regional and local regulations. Battery
packs should be shipped to a reclamation facility for recovery of metal and plastic compounds
as the proper method of waste management.
Do not place the device where the controls can be changed by the patient.
1-24 www.zoll.com 9650-001355-01 Rev. H
Page 37

Restarting the Defibrillator
Certain events require the X Series products to be restarted after they shut off or become
inoperative (for example, when the battery runs down and the unit shuts off).
In such a case, always try to restore defibrillator operation as follows:
1. Press the power switch on the top of the unit to turn it off.
2. If necessary , replace a depleted battery with a fully charge d pack, or connect the de fibrillator
to auxiliary power.
3. Press the power switch on the top of the unit to turn it back on.
This sequence is necessary to restart the defibrillator and can also be used to clear some fault
messages when immediate use of the defibrillator is required.
If the X Series unit is powered off for less than 2 minutes, all patient monitoring parameter
settings will be retained. If the unit has been powered off for at least two minutes, it will be
considered a New Patient and all of the patient-specific parameters (alarm limits, defibrillator
energy, etc.) will be reset to their default values.
FDA Tracking Requirements
Restarting the Defibrillator
U.S. Federal Law (21 CFR 821) requires the tracking of defibrillators. Under this law, owners
of this defibrillator must notify ZOLL Medical Corporation if this product is
• received
• lost, stolen, or destroyed
• donated, resold, or otherwise distributed to a different organization
If any such event occurs, contact ZOLL Medical Corporation in writing with the following
information:
1. Originator's organization – Company name, address, contact name, and contact phone
number
2. Model number, and serial number of the defibrillator
3. Disposition of the defibrillator (for example, received, lost, stolen, destroyed, distrib uted to
another organization), new location and/or organization (if known and different from
originator’s organization) – company name, address, contact name, and contact phone
number
4. Date when the change took effect
Please address the information to:
ZOLL Medical Corporation
Attn: Tracking Coordinator
269 Mill Road
Chelmsford, MA 01824-4105
Fax: (978) 421-0025
Telephone: (978) 421-9655
9650-001355-01 Rev. H X Series Operator’s Guide 1-25
Page 38

CHAPTER 1GENERAL INFORMATION
Notification of Adverse Events
As a health care provider, you may have responsibilities under the Safe Medical Devices Act
(SMDA), for reporting to ZOLL Medical Corpora tion, and possibl y to the FDA, the occurrence
of certain events.
These events, described in 21 CFR Part 803, include device-related death and serious injury or
illness. In addition, as part of our Quality Assurance Program, ZOLL Medical Corporation
requests to be notified of device failures or malfunctions. This information is required to ensure
that ZOLL Medical Corporation provides only the highest quality products.
Software License
Note: Read this Operator’s Guide and License agreement carefully before operating any of
the X Series products.
Software incorporated into the system is protected by copyright laws and international
copyright treaties as well as other intellectual property laws and treaties. This software is
licensed, not sold. By taking delivery of and using this system, the Purchaser signifies
agreement to and acceptance of the following terms and conditions:
1. Grant of License: In consideration of payment of the software license fee which is part of
the price paid for this product ZOLL Medical Corporation grants the Purchaser a nonexclusive license, without right to sublicense, to use the system software in object-code
form only.
2. Ownership of Software/Firmware: Title to, ownership of and all rights and interests in the
system software and all copies thereof remain at all times vested in the manufacturer, and
Licensors to ZOLL Medical Corporation and they do not pass to purchaser.
3. Assignment: Purchaser agrees not to assign, sublicense or otherwise transfer or share its
rights under the license without the express written permission of ZOLL Medical
Corporation.
4. Use Restrictions: As the Purchaser, you may physically transfer the products from one
location to another provided that the software/firmware is not copied. You may not disclose,
publish, translate, release or distribute copies of the software/firmware to others. You may
not modify, adapt, translate, reverse engineer, decompile, crosscompile, disassemble or
create derivative works based on the software/firmware.
NO IMPLIED LICENSE
Possession or purchase of this device does not convey any express or implied license to use the
device with replacement parts which would, alone, or in combination with this device, fall
within the scope of one or more of the patents relating to this device.
1-26 www.zoll.com 9650-001355-01 Rev. H
Page 39

Service
Service
The X Series only requires recalibration of the CO2 module. Service is required after 20,000
hours of use of the CO
however, perform periodic tests of the defibrillator functionality to verify proper operation.
If a unit requires service, contact the ZOLL Technical Service Department.
For customers In the U.S.A. For customers outside the U.S.A.
Telephone:
Fax:
1-800-348-9011
1-978-421-9655
1-978-421-0010
When requesting service, please provide the following information to the service
representative:
module. Appropriately trained and qualified person nel should,
2
Call the nearest authorized ZOLL Medical Corporation
representative.
To locate an authorized service center, contact the
International Sales Department at
ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA 01824-4105
Telephone: 1-978-421-9655
• Unit serial number
• Description of the problem
• Department using the equipment and name of the person to contact
• Purchase order to allow tracking of loan equipment
• Purchase order for a unit with an expired warranty
• Sample ECG or other stripcharts demonstrating the problem (if available and applicable),
less any confidential patient information.
Returning a unit for service
Before sending a unit to the ZOLL Technical Service Department for repair, obtain a service
request (SR) number from the service representative.
Remove the battery pack from the unit. Pack the unit with its cables and battery in the original
containers (if available) or equivalent packaging. Be sure the assigned service request number
appears on each package.
For customers Return the unit to
In the U.S.A. ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA 01824-4105
Attention: Technical Service Department (SR number)
Telephone: 1-800-348-9011
9650-001355-01 Rev. H X Series Operator’s Guide 1-27
Page 40

CHAPTER 1GENERAL INFORMATION
For customers Return the unit to
In Canada ZOLL Medical Canada Inc.
1750 Sismet Road, Unit #1
Mississauga, ON L4W 1R6
Attention: Technical Service Department (SR number)
Telephone: 1-866-442-1011
In other locations The nearest authorized ZOLL Medical Corporation representative.
To locate an authorized service center, contact the International Sales
Department at
ZOLL Medical Corporation
269 Mill Road
Chelmsford, MA 01824-4105
Telephone: 1-978-421-9655
The ZOLL Serial Number
Each ZOLL product displays a serial number that contains information about t hat product.
From left to right, ZOLL serial numbers are structured as follows:
• A two-character product code
• A three-character date-of-manufacture code
• A product serial number of six or more alphanumeric characte rs
The first two characters of the date-of-manufacture code give the last two digi ts of the year (for
example, “06” appears for products manufactured in 2006). The last character of the date-ofmanufacture code gives the month in which the product was manufactured. The month appears
in the form of a single alphanumeric character: “A” for January, “B” for February, “C” for
March, and so on through “L” for December.
The product serial number is a unique set of alphanumeric c haracters that ZOLL assigns to each
individual unit.
1-28 www.zoll.com 9650-001355-01 Rev. H
Page 41

Product Overview
SHOCK
123
¸
0!#%2
2
1
6
8
9
4
6
3
6
7
10
5
Defibrillator Controls and Indicators
Chapter 2
9650-001355-01 Rev. H X Series Operator’s Guide 2–1
Page 42

CHAPTER 2PRODUCT OVERVIEW
SHOCK
123
¸
0!#%2
Seven quick
Display screen
RFU indicator
Auxiliary power LED
Battery charge LED
Silence/reset
Display
Navigation keys
Snapshot
NIBP
Shock
Charge
Pacer
Analyze
Energy Select
access keys
Power button
Visual Alarm Indicators
Microphone (optional)
Table 2-1. X Series Unit Features
Item Description
1 Handle Integrated carrying handle.
2 Front panel Includes the display screen and primary controls.
3 Microphone (optional) Records audio activity in the vicinity of the X Series unit.
4 Speaker Emits R-wave detection beeps and alarm tones.
5 Paper Compartment Holds the paper for the printer.
6 Patient connectors For details, refer to “Patient Cables and Connectors” on
7 USB device connector For connecting the X Series defibrillator to a USB device.
8 Battery compartment Holds a rechargeable lithium ion battery pack.
9 Auxiliary power connector For connecting the device to an auxiliary power adapter.
10 Dock connector For connecting the device to a docking station.
The Front Panel
page 2-7.
For details, refer to “Transferring Data to a USB Device” on
page 21-4“.
The front panel of the X Series device includes the display screen, quick access keys, battery
and auxiliary power indicators, Ready For Use (RFU) indicator, and the defibrillation front
panel buttons: Pacer, Analyze, Energy Select, Charge, and Shock ( ). See Figure 2-1.
Refer to Table 2-2 on page 2-3 for informati on about the controls and indicators.
Figure 2-1. X Series Front Panel
2–2 www.zoll.com 9650-001355-01 Rev. H
Page 43

Defibrillator Controls and Indicators
PACER
Table 2-2. X Series Controls and Indicators
Control or Indicator Description
Display screen Shows therapeutic settings, physiological waveforms and other
information for each monitored parameter, messages, time, and quick
access key labels.
Quick access keys Seven buttons control different functions of the unit. Labels for the quick
access keys appear on the monitor display to the right of each key.
Auxiliary power LED Illuminated when the unit is plugged in to an auxiliary power adapter.
Battery charge LED Indicates battery status:
Steady yellow: Battery is charging.
Steady green: Battery is charged.
Alternating green and yellow: The charge state cannot be determined
or a battery charging fault has been
detected.
No light: Batte ry is not installed.
Visual alarm
indicators
Pacer button Displays pacer settings window to start/stop pacing activity or change
Red, yellow, and green lights located on the top of the unit that flash on
and off when the unit is powered up and are used to indicate a patient
alert, equipment alert, and data transfer.
the rate, output, or mode settings.
ANALYZE button Displays in Manual mode only. Initiates ECG analysis to determine
whether or not a shockable rhythm is present.
ENERGY SELECT
buttons
CHARGE Button Charges the defibrillator to the selected energy. In addition to the
Shock Button The front panel Shock button is only active when using hands-free
NIBP button Starts/stops an NIBP measurement.
Snapshot button Records 24 seconds of numeric and waveform data.
Two sets of up-down arrow buttons control the selection of defibrillator
energy; one set is located on the front panel and the other set is located
on the STERNUM paddle.
CHARGE button on the front panel, there is one located on the APEX
paddle handle.
therapy electrodes or internal defibrillation paddles without a discharge
button. The Shock button illuminates when the device is charged and
ready.
To discharge the defibrillator when using paddles (internal or external)
with discharge buttons, press and hold the SHOCK buttons on the
paddles.
9650-001355-01 Rev. H X Series Operator’s Guide 2–3
Page 44

CHAPTER 2PRODUCT OVERVIEW
Table 2-2. X Series Controls and Indicators (continued)
Control or Indicator Description
Navigation keys
Display/Home button Cycles through three available display modes or functions as a Home
Silence/Reset button Silences the current alarm tone for 90 seconds or resets a silenced
The up (clockwise) arrow will cause the cursor to travel in an
upward direction if the cursor is being used to navigate through a
vertical list or in a clockwise direction if the cursor is being used to
navigate around the full screen. Likewise, the down (counterclockwise)
arrow will cause the cursor to travel in a downward direction if the cursor
is being used to navigate through a vertical list or in a counterclockwise
direction if the cursor is being used to navigate around the full screen.
The up (clockwise) and down (counterclockwise) arrows may also be
used to modify parameter settings.
The Select button acts based on what is highlighted.
button when in a menu.
alarm tone.
RFU indicator Shows the status of the unit, based on its most recent readiness check.
Ready
Power button Located on the top of the unit, this button turns the unit on and off.
Microphone (optional) Records audio activity in the vicinity of the X Series unit.
Charge Indicator Light
(not shown)
Display Screen
The front panel includes a color display which shows:
• Date and time
• Patient mode
• Battery status indicator
• Time elapsed (since unit was turned on)
• Quick access keys
• Waveform source
• Color-coded waveforms and ECG lead identifiers
• SpO
• Heart rate numeric data
• Respiration rate numeric data
• Temperature numeric data
Do Not Use
numeric data
2
A red circle with a line through it indicate s that the unit’s readiness has
been compromised and that it may not be ready for therapeutic use.
Located on the APEX paddle, this light turns on when the defibrillator is
charged and ready.
2–4 www.zoll.com 9650-001355-01 Rev. H
Page 45

Defibrillator Controls and Indicators
Date and time Patient mode
Battery status Time elapsed
Quick
access
keys
Message
Waveform
SpO
2
data
CO2 data
Respiration rate
NIBP data
Heart rate
Current temp
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
79
(96)
NIBP mmHg
38
12
BR
CO2 mmHg
SpO2 %
97
T1 ºF
98.6
EtCO2 Low AlarmSpO2 Check Sensor
Some alarm limits disabled.
Status queue
• Non-invasive blood pressure numeric data
• EtCO
• Invasive pressure numeric data
• The selected energy, charging status, and delivered energy for defibrillation and
numeric data
2
synchronized cardioversion
• The output current and stimulus rate for pacing
• Messages and prompts
Figure 2-2 shows the layout of parameter values, waveforms, system dat a, and quick access key
labels.
12
CO
2
R
Figure 2-2. X Series Display Screen
Color coding
To differentiate information for various parameters, the unit displays each type of information
in a specific user-configurable color.
9650-001355-01 Rev. H X Series Operator’s Guide 2–5
Page 46

CHAPTER 2PRODUCT OVERVIEW
Low
1:00+
2:00+
3:00+
Battery Status and Auxiliary Power Indicators
The battery status indicator displays various battery icons to indicate the approximate
remaining unit run time based on the charged state of the battery. Additionally, these icons
provide indications of the status of the battery connection and communication with the unit.
The auxiliary power indicator indicates that the unit is being powered by the auxiliary power
adapter.
Note: Upon powering up the X Series unit, the battery capacity will be displayed within
approximately 15 seconds under normal conditions. Under some circumstances, such
as activating the defibrillator immediately after the unit is turned on, the battery icon
may display less than one hour battery capacity for up to two minutes after exiting the
defibrillation mode.
Icon Status Indication/Action
Auxiliary power adapter is
connected
No battery detected Either there is no battery in the unit
Low battery capacity Replace the battery soon.
The unit is being powered by the
auxiliary power adapter.
while it is being powered by the
auxiliary power adapter, or the
device cannot detect that the
battery is connected.
Communication failure The unit is unable to establish
communication with the battery
and the battery capacity is
unknown. Check the battery
contacts.
Battery fault A battery fault has been detected.
Replace the battery.
Battery Level 1 The battery has less than one hour
of remaining battery capacity.
Battery Level 2 The battery has greater than one
hour of remaining battery capacity .
Battery Level 3 The battery has greater than two
hours of remaining battery
capacity.
Battery Level 4 The battery has greater than three
hours of remaining battery
capacity.
Battery Level 5 The battery is fully charged.
2–6 www.zoll.com 9650-001355-01 Rev. H
Page 47

Patient Cables and Connectors
ECG
SpO
2
NIBP
CO
2
CO
2
Exhaust
USB
IBP
Temp
MFC
The left and right sides of the unit include sets of connectors for patient cables.
Note: The SPO2, NIBP, CO2, Temperature, and IBP functions are optional. If your unit does
not include these options, it does not have the applicable connectors.
Defibrillator Controls and Indicators
Figure 2-3. Patient Cable Connectors on Left Side of Unit
Figure 2-4. Patient Cable Connectors on Right Side of Unit
9650-001355-01 Rev. H X Series Operator’s Guide 2–7
Page 48

CHAPTER 2PRODUCT OVERVIEW
2. Connector is locked
into place.
1. Insert MFC into unit.
Connector Description
ECG For connecting 3- or 5-lead ECG cable (12-lead monitoring is
SpO
2
NIBP For connecting NIBP hose.
CO
2
Temp For connecting temperature probe(s).
Multifunction Cable (MFC) For connecting paddles or ZOLL hands-free therapy and pacing
USB For connecting the X Series defibrillator to a USB device.
IBP For connecting IBP cable(s).
Multifunction Cable (MFC)
The unit ships with an MFC that is used to defibrillate the patient. Any other cables that ship
with your unit depend on the options you have purchased.
optional).
For connecting Masimo SpO2/CO cable.
For connecting CO2 sampling line.
electrodes.
Figure 2-5. MFC
Inserting MFC into Unit
Plug the MFC connector into the therapy input connector on the right side of the unit. Push the
connector in with the arrows aligned. The connector will click when it locks into place.
2–8 www.zoll.com 9650-001355-01 Rev. H
Page 49

Defibrillator Controls and Indicators
1. Twist MFC connector
2. Pull out connector.
to the left.
Latch
Removing MFC from Unit
Twist the connector to the left to unlock it, and pull out the MFC connector.
OneStep Cable (optional)
The OneStep™ cable is used with OneStep ele ctrodes for ECG monitoring and for use with
Real CPR Help.
Figure 2-6. OneStep Cable
When connecting a OneStep electrode to the OneStep cable, push the two connectors together
until the latch clicks, as shown.
9650-001355-01 Rev. H X Series Operator’s Guide 2–9
Page 50

CHAPTER 2PRODUCT OVERVIEW
Latch
1. Align MFC as shown.
2. Insert MFC into APEX handle.
MFC connected to
APEX paddle
MFC Connected to
APEX Paddle
When disconnecting the OneStep electrode and OneStep cable, press down the latch with your
thumb as shown.
External Paddles
Paddles are defibrillation-proof Type BF equipment.
The external paddles on the X Series device are used for defibrillation and synchronized
cardioversion.
Caution You cannot use paddles for external transcutaneous pacing.
Attaching the MFC cable
Attach the MFC from the X Series unit to the connector at the base of the APEX paddle.
Figure 2-7. Attaching the MFC to the APEX Paddle
Figure 2-8. MFC Connected to APEX Paddle
2–10 www.zoll.com 9650-001355-01 Rev. H
Page 51

Defibrillator Controls and Indicators
Latch
Latch
1. Align OneStep cable as shown. 2. Insert OneStep cable into APEX paddle.
If you need to detach the MFC from the APEX paddles, push the RELEASE button (see Figure
2-11) in the direction of the arrow and unplug the MFC.
Attaching the OneStep Cable
When connecting a OneStep electrode to the OneStep cable, push the two connectors together
until the latch clicks, as shown.
When disconnecting the OneStep electrode and OneStep cable, press down the latch with your
thumb as shown.
When attaching the OneStep cab les to p addles, at tac h the OneS te p cable from the X Se ries unit
to the connector at the base of the apex paddle.
Figure 2-9. Attaching the OneStep Cable to the APEX Paddle
9650-001355-01 Rev. H X Series Operator’s Guide 2–11
Page 52

CHAPTER 2PRODUCT OVERVIEW
OneStep cable connected
to APEX paddle
SHOCK
Buttons
ENERGY
SELECT
Buttons
CHARGE
Button
Charge Ready
Indicator
STERNUM Paddle
APEX Paddle
Connector
and RELEASE
button for
MFC or
RECORDER
Button
OneStep cable
Figure 2-10. OneStep Cable Connected to APEX Paddle
If you need to detach the OneStep cable from the APEX paddl es, push the RELEASE button
(see Figure 2-11) in the direction of the arrow and unplug the OneStep cable.
Refer to Chapter 15, "Manual Defibrillation" before using paddles for defibrillation. The
paddles include controls for selecting defibrillation energy, charging, and delivering a shock.
2–12 www.zoll.com 9650-001355-01 Rev. H
Figure 2-11. External Paddles
Page 53

Defibrillator Controls and Indicators
To expose the pediatric plate, press the PEDI button at
the top of the paddle, then slide the Adult plate upward.
Before replacing the Adult plate, be sure to clean the
pediatric plate and surrounding area thoroughly.
Slide the Adult plate onto the paddle until it locks into
place.
PEDI button
Pediatric-size electrodes are built in to the paddle assembly beneath the standard electrode
plates. The user must manually adjust energy settings to pediatric levels consistent with their
institution’s protocols.
Figure 2-12. Pediatric Plate
Note: The X Series defibrillator also supports ZOLL autoclavable internal handles for use
during open chest defibrillation procedures.
AC Auxiliary Power Adapter
The AC auxiliary power adapter is used as backup power to operate the X Series unit. When it
is connected to the unit, it powers the unit and charges the battery that is installed inside it.
When the power cord is plugged in and the auxiliary power connector is inserted into the back
of the X Series unit, the auxiliary power LED on the front panel illuminates and the auxiliary
power icon ( ) displays at the top of the display screen. See “Connecting the AC Auxiliary
Power Adapter or DC Auxiliary Power Supply” on page 2-15 for instructions on connecting the
adapter to the X Series unit.
Figure 2-13. AC Auxiliary Power Adapter
Caution To ensure continuous operation, always keep a battery installed in the device that is being
powered by the AC Auxiliary Power Adapter.
9650-001355-01 Rev. H X Series Operator’s Guide 2–13
Page 54

CHAPTER 2PRODUCT OVERVIEW
Power Supply
Vehicle Power Input Plug
Output Connector
DC Auxiliary Power Supply (optional)
The DC auxiliary power supply is used as backup power to operate the X Series unit. When it is
connected to the unit, it powers the unit and char ge s the ba tte ry tha t is install ed insid e it. When
the vehicle power input plug is connected to vehicle power and the power supply output
connector is inserted into the back of the X Series device, the auxiliary power LED on the front
panel illuminates and the auxiliary power icon ( ) displays at the top of the display screen.
Figure 2-14. DC Auxiliary Power Supply
Warning! Do not connect the DC Auxiliary Power Supply to any power source other than DC
voltage, within the range 12-24 VDC.
Do not perform any unauthorized modification to the DC Auxiliary Power Supply.
Caution To prevent heat buildup while in use, place the DC Auxiliary Power Supply in a location that
allows unrestricted air circulation.
When the DC Auxiliary Power Supply is in use, assess electromagnetic compatibility with
surrounding devices.
To ensure continuous operation, always keep a battery installed in the device that is being
powered by the DC Auxiliary Power Supply.
Connecting the DC Auxiliary Power Supply to a Suitable Vehicle Power Source
Connect the input plug (Hubbell part number HBL7545C) of the DC Auxiliary Power Supply
to the vehicle power source. When the input plug is connected to the vehicle, the brass blade
(red wire) of the Hubbell connector connects to the DC positive (+) vehicle supply terminal,
and the silver blade (black wire) connects to the DC negative (-) vehicle supply terminal.
2–14 www.zoll.com 9650-001355-01 Rev. H
Page 55

Navigating the Display Screen
Connecting AC Auxiliary Power Adapter or DC Auxiliary Power Supply to X Series Unit
To connect the AC auxiliary power adapter or the DC auxiliary power supply to the X Series
unit, align the white arrow of the auxiliary power connector with the arrow on the input
connector on the back of the unit and push it in. To disconnect the auxiliary power adapter/
power supply, grasp the connector and pull it out.
Figure 2-15. Connecting the AC Auxiliary Power Adapter or DC Auxiliary Power Supply
Navigating the Display Screen
You can access the X Series functions using the quick access keys that are located on the left
side of the display screen, and the navigation keys that are located on the right side of the front
panel.
9650-001355-01 Rev. H X Series Operator’s Guide 2–15
Page 56

CHAPTER 2PRODUCT OVERVIEW
I, II,
III...
SYNC
IBP
First level keys
Second level keys
I, II,
III...
SYNC
Quick Access Keys
The seven quick access keys on the left side of the display screen are an easy way to access the
functionality of the X Series. When you press the last key (left arrow), five more keys are
displayed.
12
CO
2
R
Table 2-3. X Series Quick Access Keys
Quick access key Description
Lead Selects the ECG input source for the first waveform trace.
12 lead Displays the 12-lead monitoring screen.
12
CO
2
Turns CO2 on and off.
LOG
Treatment Displays the current clinical treatment options.
R
Sync Activates the synchronized cardioversion mode.
Print Starts or stops a continuous chart print.
2–16 www.zoll.com 9650-001355-01 Rev. H
Page 57

Navigating the Display Screen
IBP
Manual
Mode
Pause
Print
Trends
STOP
Table 2-3. X Series Quick Access Keys
Quick access key Description
More/Back Goes to the next or previous level of quick access keys.
Brightness Changes the brightness se tting -- toggles through high contrast
display (white background), color display (black background), and
night vision goggle (NVG) friendly display.
IBP Displays IBP setup and zero buttons.
Alarms Displays the Limits option to allow the user to view/set all parameter
alarm limits and the alarm suspend button.
Log Opens the Log Control panel.
LOG
Setup Displays the Setup menu to allow the user to configure settings such
as ECG, display/volume, printer, trends, operational checklist, and
supervisor.
Treatment Summary Displays treatment summary cases, which you can print.
Manual Mode Allows user to change from AED Mode to Manual Mode.
Pause
Allows user to Pause the rescue cycle
.
Print Trends Prints the trends that are displayed in the Trend Summary window.
Trend Settings Displays settings for trend display format, trend on interval, and trend
on alarm.
Transfer Log Transfers the current data in the log to a USB drive.
Clear Log Deletes the current data in the log.
Acquire Collects 10 seconds of 12-lead data for print.
12
Stop Acquisition Stops acquisition of 12-lead data.
Patient Information Allows you to enter information to accompany 12-lead data: patient
name, age, gender, and ID.
9650-001355-01 Rev. H X Series Operator’s Guide 2–17
Page 58

CHAPTER 2PRODUCT OVERVIEW
Row
Row
Exit
Stat
Set
Limits
Disarm
Disarm
Disarm
Table 2-3. X Series Quick Access Keys
Quick access key Description
Row Up Allows you to move to the previous row when entering patient
Row Down Allows you to move to the next row when entering patient information.
12-Lead Review Reviews all your 12-lead captured data.
12-Lead Review Next Goes to the next page of the 12-lead snapshot you are reviewing.
Transmit Transmits 12-lead data.
Exit 12-Lead Exits the 12-lead monitoring screen.
12
Stat Set Sets all alarm limits relative to the patient’s current vital signs.
information.
Alarm Cancel Suspends the current alarm.
Limits Displays the current alarm settings.
Disarm Safely discharges the defibrillator internally. No energy is delivered to
the patient.
IBP Setup Brings up the IBP Control Panel for the corresponding channe l (P1,
P2, or P3).
IBP Zero Zeroes the IBP transducer for the corresponding channel (P1, P2, or
P3).
2–18 www.zoll.com 9650-001355-01 Rev. H
Page 59

Navigation Keys
Use the navigation keys (up/clockwise arrow, down/counterclockwise arrow , an d select button)
to navigate through windows and make selections .
Using Up/Clockwise and Down/Counterclockwise Arrows
Use the up/clockwise down/counterclockwise arrows to do the following:
• Move clockwise and counterclockwise through the main display windows.
• Move up and down in a window.
• Change parameter settings.
Using the Select Button
Use the Select button to do the following:
• Display the settings window while a parameter is highlighted in the main window.
• Select options from a window.
Display Brightness
Common Tasks
The monitor can display in two different brightness modes:
• high contrast with white background (for optimal display in bright sunlight)
• color with black background (numerics and waveforms are easy to read)
Common Tasks
The section contains procedures for the following tasks:
• “Changing the Display Brightness” on page 2-19.
• “Replacing a Battery Pack on the X Series” on page 2-20.
• “Using Treatment Buttons” on page 2-21
Changing the Display Brightness
The following procedure shows how to select the different brightness options.
1. Press the power switch to turn the unit on.
2. Press the Brightness quick access key ( ) repeatedly to toggle through the brightness
options until you find your selection.
Note: Selecting a higher brightness setting (such as 70%) will deplete the battery pack at a
faster rate than when choosing a lower brightness setting (such as 30%). To select the
brightness setting, go to the Setup>Display/Volume>Display Brightness menu to
adjust the display percentage.
9650-001355-01 Rev. H X Series Operator’s Guide 2–19
Page 60

CHAPTER 2PRODUCT OVERVIEW
Replacing a Battery Pack on the X Series
This section describes how to replace a battery pack on the X Series.
Replacing a Battery Pack on the X Series
T o remove a battery pack, use your fingers to grasp and raise the latch and pull the battery pack
out of the compartment.
Figure 2-16. Removing a Battery Pack
To install a battery pack:
1. Line up the battery so it will slide into the battery well.
2. Push the battery into place.
Figure 2-17. Installing a Battery Pack
2–20 www.zoll.com 9650-001355-01 Rev. H
Page 61

Using Treatment Buttons
Pressing the Treatment qu ick acc ess key ( ) causes the unit to display preconfigured b uttons
that contain clinical actions. These buttons allow you to add a treatment snapshot (which
itemizes drugs or treatments administered to the patient) to a Treatment Summary Report.You
can do this by selecting Print on Treatment Snapshot from Setup>Supervisor>Printer. The
following is a list of preconfigured treatment buttons:
• O2
• ASA
• Nitro
• Morph
• IV
• B Block
• Lido
• MgSO4
• Valium
• Sedate
Customizing Tr eatment But tons
You can also customize up to 9 treatment buttons by pressing the Setup quick acce ss key ( ),
and then selecting Supervisor>Log>T reat ment Options. Highli ght De fine C ustom Labels , and
then can customize up to 9 buttons.
Common Tasks
R
9650-001355-01 Rev. H X Series Operator’s Guide 2–21
Page 62

CHAPTER 2PRODUCT OVERVIEW
2–22 www.zoll.com 9650-001355-01 Rev. H
Page 63

Monitoring Overview
This chapter provides an overview of the X Series unit’s monitoring functions. It describes the
types of vital sign monitoring that X Series provides, and the flexibility that the X Series unit
gives you in displaying a patient’s vital signs information.
X Series Monitoring Functions
The X Series unit provides an array of standard, a nd optional, mo nitoring functio ns, and allows
you to view the vital signs measurements that these functions provide in a variety of formats.
The X Series unit also allows you to set alarm limits for each monitoring function. Should a
patient’s vital signs measurements go outside of these limits, the X Series issues an audible
alarm tone and displays visual alarm indications to alert you.
If the X Series unit is powered off for less than 2 minutes, all patient monitoring parameter
settings are retained. If the X Series unit is powered off for 2 minutes or longer, the unit
operates as if there is a New Patient and all patient-specific parameters (alarm limits,
defibrillator energy, etc.) are reset to their default values.
Chapter 3
The X Series unit can monitor the following patient vital signs:
• ECG
• Heart Rate
• Respiration Rate
• Temperature
• Invasive Pressures (IBP)
• Non-invasive Blood Pressure (NIBP)
• Capnography (CO
• Pulse Oximetry (SpO
9650-001355-01 Rev. H X Series Operator’s Guide 3-1
)
2
)
2
Page 64

CHAPTER 3MONITORING OVERVIEW
ECG
An ECG waveform trace appears at the top of the display area. You can specify that the unit
display the waveform trace of any available ECG source, such as
and so on, in this area. You can configure the X Series unit to display up to four ECG waveform
traces. In addition to being able to specify the ECG source for each waveform trace, you can
adjust the display scale of those traces to make them easier to view.
Heart Rate
A Heart Rate meter gives the patient’s heart rate in Beats Per Minute (bpm). By default, the X
Series unit derives the heart rate from the patient’s ECG, but can be config ured to use other
monitoring functions to derive the patient’s heart rate.
Respiration Rate
A Respiration Rate meter gives the patient’s respiration rate in Breaths Per Minute (br/min).
The X Series unit can be configured to derive the respiration rate from the patient’s ECG or
from the optional CO
monitoring function.
2
Pads, ECG Leads l, ll, or lll,
Temperature
The Temperature (Temp) meter can display temperature measurements fr om up to two
temperature probes. The X Series unit provides two separate temperature monitoring channels
and, if both are used, displays the monitored temperatures, in degrees F or C, one after the
other, followed by the difference between those temperatures.
Invasive Pressures (IBP)
The X Series unit provides three separate channels for monitoring arterial, venous, or
intracranial pressure using internal probes. The pressure measurements for each pressure
channel appear in a labeled (
P1, P2, P3) numeric display.
Non-Invasive Blood Pressure (NIBP)
The X Series unit provides patented Smartcuf motion-tolerant technology for NIBP monitoring.
NIBP monitoring measures the patient’s systolic, diastolic, and mean blood pressure through an
inflatable blood pressure cuff that the X Series unit inflates/deflates. NIBP measurements can
be taken automatically or on-demand by p ressing the NIBP butto n ( ) on the front panel of the
X Series unit. The blood pressure measurements appear in a labeled (
You can also specify that the X Series unit display non-invasive pressure waveforms in the
waveform trace area.
NIBP) numeric display.
3-2 www.zoll.com 9650-001355-01 Rev. H
Page 65

Capnography (CO2)
CO2 monitoring measures the CO2 concentration in a patient’s exhaled breath (End Tidal
Carbon Dioxide --EtCO
CO
concentration in the gasses supplied to intubated patients (Fractional Inspired Carbon
2
Dioxide -- FiCO
serves as an indicator for rebreathing in non-intubated pati ents. CO
both intubated and non-intubated patients.
Monitoring Display Options
). CO2 monitoring can also measure a patient’s breath rate and the
2
). Since FiCO2 represents the amount of CO2 present during inhalation, it also
2
monitoring can be used for
2
The EtCO
display . The EtCO
, breath rate, and FiCO2 measurements appear in a labeled (EtCO2) numeric
2
and FiCO2 measurements can appear as values given in millimeters of
2
mercury (mmHg). You can also specify that the X Series unit display a CO
waveform trace display area.
Pulse Oximetry (SpO2)
Pulse Oximetry monitoring measures the oxygen saturation (SpO2) of arterial blood at a
peripheral site such as a finger or toe. If optional features SpCO and Sp Met are installe d, it also
monitors carboxyhemoglobin saturation (SpCO), and methemoglobin saturation (SpMet).
monitoring determines the ratio of oxygenated hemoglobin to total hemoglobin in arterial
SpO
2
blood and displays this ratio as percent SpO
features SpCO and SpMet are installed, these values alternate and display as
under the SpO
display. You can also specify that the X Series unit display an SpO
2
plethysmograph in the waveform trace display area.
Monitoring Display Options
The X Series unit gives you great flexibility in how you display a patient’s vital signs
information. By pressing the Display/Home button ( ) on the front panel, you can
successively display the patient’s vital signs information in these three windows:
capnogram in the
2
in a labeled (SpO2) numeric display. If optional
2
SpCO and SpMet
2
• Waveform Display window, which initially displa ys an ECG waveform trace and numeric
displays for each monitoring function.
• Trends Status window, which displays a report listing vital signs measurements that the X
Series unit logs automatically, and the primary ECG waveform trace.
• Large Numerics Display window, on which large numeric displays of all vital signs
measurements appear.
The Waveform Display window appears when you power on the X Series unit. Initially, the
Waveform Display window displays a single ECG waveform trace. All other monitored values
appear in numeric display areas at the bottom of the screen:
9650-001355-01 Rev. H X Series Operator’s Guide 3-3
Page 66

CHAPTER 3MONITORING OVERVIEW
I, II,
III...
SYNC
06/06/2011
12
CO
2
R
You can display up to four waveform traces that you specify on the Waveform Display window .
You will determine how to add waveform traces to this window later in this chapter.
Press the Home/Display button when viewing the Waveform Display window, and the unit
displays the Trends Status window. The Trends Status window reports the patient’s vital sign
measurements, which the X Series logs automatically at a configurable interval (see the
following chapter, Trends, for more detailed information about the Trends Status window). The
primary ECG waveform trace appears above the Trends
report:
3-4 www.zoll.com 9650-001355-01 Rev. H
Page 67

06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
NIBP mmHg
121
79
(96)
EtCO2 mmHg
12
BR
38
SpO2 %
97
T1 ºF
NIBP Trends
Time HR/PR
bpm
NIBP
mmHg
SpO2
%
RR/BR
br/min
12:20:21 81
122/60 (85) 1597
12:15:21 73
124/63 (86) 1397
12:30:21 72
122/60 (85) 1297
12:25:21 80
122/60 (85) 1496
12
CO
2
R
Monitoring Display Options
98.6
9650-001355-01 Rev. H X Series Operator’s Guide 3-5
Page 68

CHAPTER 3MONITORING OVERVIEW
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
T1 ºF
98.6
P3
mmHg
12.4
P2
mmHg
P1
mmHg
121 79
(96)
25 9
(15)
HR bpm
80
NIBP mmHg
121
79
(96)
23:45
SDM
SYNC
EtCO2 mmHg
38
BR
12
SpO2 %
97
Press the Home/Display button when viewing the Trends Status window and the Large
Numerics Display window appears. The patient’s vital signs measurements appear in large
labeled numeric displays; no waveform trace appears on this screen:
12
CO
2
R
Press the Home/Display button to redisplay the Primary Display window.
Note: When the X Series unit is displaying the Defibrillation or Pacing Control panels, the
unit will not allow the display the Large Numerics Display window.
3-6 www.zoll.com 9650-001355-01 Rev. H
Page 69

Configuring the Waveform Display
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
79
(96)
NIBP mmHg
38
12
BR
CO2 mmHg
SpO2 %
97
T1 ºF
98.6
Pads
III
aVL
aVF
V
Insert
Source
II
aVR
I
Cascade
You can display up to four waveform traces on the Waveform Display window. The first
waveform trace always uses an ECG lead as its source (such as
on. The default is
automatically default to another ECG lead for the first trace. As you insert the remaining three
traces, you can specify that the traces use an ECG lead as the waveform source, or that the trace
derive its waveform from other available monitoring functions (such as
IBP channels
If configured, the unit can display four ECG traces on startup, when no other monitoring
devices are attached.
The X Series unit can also cascade a trace onto the adjoining trace area to double the duration
of the trace display.
Pads). If Pads are not connected, the unit can be configured to
P1, P2, or P3).
Configuring the Waveform Display
Pads or Leads l, ll, or lll, and so
Resp, CO2, SpO2 or
On the Waveform Display window, to insert a new trace (
displayed trace, highlight and select the trace label above the trace. In the following example,
the unit is configured to cascade the ECG Lead l trace:
Insert) or cascade (Cascade) a
12
CO
2
R
Note: The X Series unit automatically inserts a new waveform when you turn on a parameter
) or a new sensor signal is present (SPO2, IBP). The X Series unit automatically
(CO
2
removes a waveform when you turn off a parameter or remove a sensor and the unit
displays the resulting equipment alert.
9650-001355-01 Rev. H X Series Operator’s Guide 3-7
Page 70

CHAPTER 3MONITORING OVERVIEW
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
79
(96)
NIBP mmHg
38
12
BR
CO2 mmHg
SpO2 %
97
T1 ºF
98.6
When the unit cascades the ECG Lead lI trace, the Waveform Display window appears as
follows:
12
CO
2
R
The following screens demonstrate how t o insert two more waveform traces into th e window. A
third trace is inserted for ECG lead
that when the third trace is inserted, the numeric displays move to the right side of the window
to allow more room for the waveform traces.
aVR, and fourth trace for EtCO2 (a capnogram). Notice
3-8 www.zoll.com 9650-001355-01 Rev. H
Page 71

Inserting a third waveform trace for ECG lead aVR:
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
79
(96)
NIBP mmHg
38
12
BR
CO2 mmHg
SpO2 %
97
T1 ºF
98.6
Pads
III
aVL
aVF
V
Insert
Source
II
I
Cascade
aVR
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mVaVR
1 cm/mV
II
HR bpm
80
NIBP mmHg
121
79
(96)
EtCO2 mmHg
12
BR
38
SpO2 %
97
T1 ºF
98.6
12
CO
2
R
Configuring the Waveform Display
9650-001355-01 Rev. H X Series Operator’s Guide 3-9
12
CO
2
R
Page 72

CHAPTER 3MONITORING OVERVIEW
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
1 cm/mV aVR
1 cm/mV
II
HR bpm
80
NIBP mmHg
121
79
(96)
EtCO2 mmHg
12
BR
38
SpO2 %
97
T1 ºF
98.6
Pads
I
II
III
aVR
aVL
aVF
V
SpO2
Source
P1
P2
P3
Insert
Cascade
Remove
Resp
CO2
SYNC
Adult
00:17:43
I, II,
III...
0
30
60
CO2
0 to 60 mmHg
1 cm/mV
avR
SYNC
1 cm/mV
II
HR bpm
80
NIBP mmHg
121
79
(96)
EtCO2 mmHg
12
BR
38
SpO2 %
97
T1 ºF
Inserting a capnogram (CO2) into the fourth trace area:
12
CO
2
R
3-10 www.zoll.com 9650-001355-01 Rev. H
06/06/2011 12:34:56
12
CO
2
R
98.6
Page 73

The X Series unit accumulates a patient’s trend information by logging all monitored vital sign
measurements to memory at a user-configurable interval. It also logs all monitored vital sign
measurements when the following occurs:
• An NIBP measurement is taken and the Trend on NIBP option is on
• You press the Snapshot button ( ) on the front panel
• A patient alarm occurs and the Trend on Alarm option is on
The X Series unit can store at least 24 hours of trend information when logged at a 1 minute
trend interval. You can view, print, or save to external memory all logged trend information.
Displaying the T rends Status Window
The X Series unit displays the logged trend information in the Trends status window. Press the
Display/Home button ( ) to display the Trends window, the primary ECG trace, and the
small numeric displays for each monitoring function:
Chapter 4
T rends
9650-001355-01 Rev. H X Series Operator’s Guide 4-1
Page 74

CHAPTER 4TRENDS
Navigate Here to Scroll Through Trends
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
(96)
NIBP mmHg
38
BR
CO2 mmHg
SpO2 %
97
T1 ºF
NIBP Trends
Time HR/PR
bpm
NIBP
mmHg
SpO2
%
RR/BR
br/min
12:20:21 81
122/60 (85) 1597
12:15:21 73
124/63 (86) 1397
Navigate Here to Scroll Through Trends
12:30:21 72
122/60 (85) 1297
12:25:21
Print
Trends
06/06/2011 12:34:56
12
CO
2
R
98.6
Figure 4-1 Trends Status window
The Trends status wi ndow displays the lo gged trend information and the time at which the trend
measurements were logged. While trend measurements are logged to memory at a userconfigurable interval, the Trends status window can display the logged information at an
interval that you specify, with the exception of NIBP measurements, which are logged and
reported at the times they are taken. The Trends status window reports the trends information at
5-minute intervals.
Displaying and Printing Trend Information
To navigate in the Trends status window:
1. Use the navigation keys to highlight the
press Select.
2. Press the Up/Down buttons ( ) to scroll through the trend information.
3. T o print all trends for the current patient, highlight the NIBP Trends field, then press Select.
Press the Print Trend Summary button in the
4. T o select which trends to print for the current patient, press the Log quick access key ( ),
then press the Print Trends button ( ).
79
Navigate Here to Scroll Through Trends bar, then
12
Trend Settings menu.
LOG
4-2 www.zoll.com 9650-001355-01 Rev. H
Page 75

Changing the Trends Status Window Display
Changing the Trends Status Window Display
By default, the Trends status window displays all logged trend information. It displays the
numeric information for all monitoring functions, which the unit logs at a user-configurable
interval, and when you take NIBP measurements, when a patient alarm occurs, and when you
press .
To configure the display of the Trends status window, press ( ) and press the Trend Settings
quick access key ( ) to display the Trends Settings control panel. On the Trends Settings
control panel, select
Trend Display Format to specify the following monitored vital signs that
appear in the Trends status window:
Trend Fo rmat Vital Signs Displayed
Resp HR, SpO2, RR, EtCO2, FiCO2
NIBP HR, SpO2, NIBP, RR
IBP1 HR, SpO2, IBP1, RR
IBP2 HR, SpO2, IBP2 RR
IBP3 HR, SpO2, IBP3, RR
Temp
HR, SpO2, T1, T2, T
Continuous Waveform Recording
Continuous waveform recording allows you to record continuous waveforms to a full
disclosure case log for the patient being monitored. This feature needs to be enabled by a
supervisor. The supervisor can access this feat ure by pressing the Setup quick access key ( ),
and then selecting Supervisor>Log>Waveform Recording. In this menu, the following settings
can be customized:
LOG
• Record Displayed Waveforms – Record only the top waveform that is displayed or record
all waveforms that are displayed.
• Record Additional Waveforms – Always record the CO
IBP and the CO
waveforms.
2
waveform or always record the
2
There is an additional waveform that can be enabled in the Waveform Recording menu. This
option, Record Pads Imp. Waveform, measures the patient’s impedance between
defibrillation electrodes.
Note: When Continuous waveform recording is disabled, no waveforms (except snapshots)
are recorded.
The full disclosure case can, at a minimum, concurrently store the following information:
• 32 monitor snapshots
• 500 non-ECG events
• 24 hours of continuous ECG (4 waveforms), Capnography, IBP (3 channels), and Pads
Impedance
The actual information that is stored depends on usage. Also, the specific combination of stored
continuous waveform data depends on how the waveform recording settings are configured in
the Supervisor menu.
9650-001355-01 Rev. H X Series Operator’s Guide 4-3
Page 76

CHAPTER 4TRENDS
4-4 www.zoll.com 9650-001355-01 Rev. H
Page 77

The X Series unit supports the detection and indication of patient alarms and tec hn ica l ale r ts .
A patient alarm is any alarm condition that is caused by a monitored patient-related variable,
such as a measured vital sign that falls outside of a configured alarm limit. You can configure
patient alarm limits for each of the physiologic monitoring functions.
A technical alert is monitored equipment-related vari able that the X Series unit can detect, such
as a disconnected sensor, internal diagnostics failures, and so on. Technical alerts are always
enabled and are not user-configurable.
Patient alarms are always classified as high-priority alarms. Technical alerts are classified as
medium or low priority alarms.
Alarm conditions from patient alarms and technical alerts are stored in the Event Log and
retained with normal power down or total loss of power.
Visual Alarm Indicators
Chapter 5
Alarms
In addition to status messages that appear on the display, the X Series unit lights the red or
yellow LED on the front panel to indicate the priority level of th e highest-p riority acti ve alarm.
The X Series LEDs indicate the priority level of the highest-priority active alarm as shown in
the following table.
Active Alarm Priority Visual Alarm Indicator
High Priority -- Patient Alarm Flashing Red LED
Medium Priority -- Technical Alert Flashing Yellow LED
Low Priority -- Technical Alert Continuous Yellow LED
9650-001355-01 Rev. H X Series Operator’s Guide 5-1
Page 78

CHAPTER 5ALARMS
Audible Alarm Indicators
The X Series unit sounds an audible alarm to indicate the priority level of highest-priority
active alarm. The X Series indicates the priority level of the highest-priority active alarm by
sounding the audible alarm tones described in the following table.
Active Alarm Priority Audible Alarm Indicator Alarm/Alert Volume
High Priority -Patient Alarm
Medium Priority -Technical Alert
Low Priority -Technical Alert
Two sets of five short beep tones,
repeated at 15-second intervals
One set of three longer beep tones,
repeated at 30-second intervals
A single short beep tone, not
repeated
Audible alarms can be silenced or suspended. More detailed information about how to silence
and suspend audible alarms is included later in this chapter.
Alarm Indicator Self-Test
The X Series unit performs a self-test of the audio and visual alarm indicators upon power-up.
To ensure that the alarms and alerts are functioning properly, verify that two alarm tones are
heard and the green, yellow, and red LEDs are illuminated upon power up.
Volume is adjustable, up to a
maximum sound pressure of at
least 70 dBA (measured at 1m)
Volume is 3 to 12 dBA below high
priority alarm
Volume is 3 to 6 dBA below the
medium priority alert
5-2 www.zoll.com 9650-001355-01 Rev. H
Page 79

Patient Alarm Display
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
79
(96)
NIBP mmHg
38
12
BR
CO2 mmHgCO2 mmHg
BR
33
22
SpO2 %
97
T1 ºF
98.6
EtCO2 Low Alarm
When a patient’s vital signs measurements trigger an alarm, in addition to sounding the patient
alarm, the X Series unit displays an alarm message, and changes the display characteristics of
the monitoring function’s numeric display (the alarming parameter appears in red against a
white background).
Patient Alarm Display
In the following example, the EtCO
alarm limit (
EtCO2 Low Alarm):
12
CO
2
R
measurement (22 mmHg) has dropped below the lower
2
9650-001355-01 Rev. H X Series Operator’s Guide 5-3
Page 80

CHAPTER 5ALARMS
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
(96)
NIBP mmHg
38
BR
CO2 mmHg
SpO2 %
Check
Sensor
T1 ºF
SpO2 Check Sensor
Life Threatening Rhythm Alarms
When LTA monitoring is enabled, the X Series will monitor fo r the following life threatening
ECG rhythms: asystole, ventricular fibrillation, ventricular tachycardia, extreme bradycardia,
and extreme tachycardia.
Depending upon configuration of the unit, it generates visible and audible alarms as needed.
Note: LTA monitoring is not available in AED mode.
Equipment Alert Display
When a problem with the X Series unit or an attached sensor triggers an alert, in addition to
sounding an equipment alert, the X Series unit displays an alert message (yellow background,
black text).
Warning! Always respond immediately to a system alarm since the patient may not be monitored
during certain alert conditions.
In the following example, an equipment alert message indicates that the SpO2 sensor has
become unattached (
SpO2 Check Sensor) from the unit:
06/06/2011 12:34:56
12
CO
2
R
98.6
79
12
5-4 www.zoll.com 9650-001355-01 Rev. H
Page 81

Responding to Active Alarms -- Silencing the Alarm
Responding to Active Alarms -- Silencing the Alarm
When a patient alarm is triggered and the alarm tone sounds
1. Check the patient and provide appropriate care.
2. Press the Alarm Silence/Reset button ( )on the X Series unit’s front panel to silence the
alarm briefly (90 seconds).
3. After caring for the patient, check that the appropriate alarms are set (for more information
about setting and enabling alarms, see appropriate monitoring chapters later in this manual).
Note: Pressing suspends the alarm tone for all active alarms. If the patient’s vital signs
measurements trigger another, different alarm, the patient alarm tone will sound, even
if the first alarm silence period hasn’t expired.
Re-enabling an Alarm
To re-enable an alarm before the alarm silence period has expired, press the Alarm Silence/
Reset button.
Warning! Do not silence the audible alarm if patient safety may be compromised.
Suspending Alarms
When caring for a patient, you may want to suspend potential or current patient alarms and
equipment alerts for a period of time. To suspend patient alarms
1. Press the More quick access key ( )to access the second set of quick access keys and
press the Alarms quick access key.
2. Press the Alarms Suspend quick access key ( ).
No alarms will sound while alarms are suspended; however, if an alarm occurs during the
suspension period, the X Series unit will display visual alarm indicators -- alarm messages in
the message area (white text on a red background) and red/white numeric displays).
The duration of the alarm suspension can be configured to be for 2, 4, or 15 minutes, or for an
indefinite period of time. The ability to suspend alarms can also be disabled.
Warning! When audible alarms are disabled, make sure that the patient is closely observed.
9650-001355-01 Rev. H X Series Operator’s Guide 5-5
Page 82

CHAPTER 5ALARMS
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
(96)
NIBP mmHg
38
BR
CO2 mmHgCO2 mmHg
BR
22
SpO2 %
97
T1 ºF
EtCO2 Low Alarm
1:30
Alarm Suspension Timer
The Alarm Suspension Timer
During an alarm suspension, the window displays an alarm suspension timer at the top of the
display next to the message area:
06/06/2011 12:34:56
12
CO
2
R
98.6
79
12
33
5-6 www.zoll.com 9650-001355-01 Rev. H
Page 83

Alarm Options
Setup > Supervisor > Alarms
Default Neonate
General
Default Adult
Default Pediatric
The X Series unit provides alarm options that you can specify through the Supervisor parameter
control panel (access to Supervisor is passcode-controlled).
Press the More quick access key ( ), press the Setup quick access key ( ), and select
Supervisor
SAVE when you are finished. Once you have entered your supervisor passcode, you will be
able to access the configurable options in the Supervisor menu.
Select Alarms to display the alarms parameter control panel:
Alarm Options
. Using the navigation keys, select the four digits in the Supervisor passcode. Press
Figure 5-1 Alarms Parameter Control Panel
9650-001355-01 Rev. H X Series Operator’s Guide 5-7
Page 84

CHAPTER 5ALARMS
Stat
Set
Selecting Default Alarm Limits
The first three options -- Default Adult, Default Pediatric, Default Neonate -- allow you to set all
alarm limits to the X Series unit’s factory-specified default values, by patient type.
Warning! • A potential hazard exists if different alarm limits are used for the same or similar
equipment in any single area.
• Confirm the alarm limits are appropriate for the patient each time there is a new
patient case.
• Do not set alarm limits to such extreme values that render the alarm system useless.
Setting Alarm Limits Relative to the Patient -- Stat Set Option
The X Series unit also allows you to set all alarm limits relative to the patient’s current vital
signs measurements by performing the following actions:
1. Press .
2. Press the Alarm quick access key ( ).
3. Press the Stat Set quick access key ( ). The X Series unit sets all parameters to a new value
based on the current values as follows:
Parameter
(units)
HR/PR
(bpm)
IBP
(mmHg)
NIBP
(mmHg)
RR/BR
(/min)
Range Upper Limit Calculation Lower Limit Calculation
Numeric < 26 Limit is unchanged Limit = 25
26 Numeric 99
100 Numeric 250
Numeric > 250 Limit = 250 Limit is unchanged
Numeric < 26 Limit = Numeric + 5 Limit = Numeric – 5
26 Numeric 99
Numeric > 99 Limit = Numeric + 20 Limit = Numeric – 20
Numeric < 26 Limit = Numeric + 5 Limit = Numeric – 5
26 Numeric 99
Numeric > 99 Limit = Numeric + 20 Limit = Numeric – 20
Numeric < 26 Limit = Numeric + 5 Limit = Numeric – 5
26 Numeric 99
Numeric > 99 Limit = Numeric + 20 Limit = Numeric – 20
Limit = Numeric x 1.2 Limit = Numeric x 0.8
Limit = Numeric + 20 Limit = Numeric – 20
Limit = Numeric x 1.2 Limit = Numeric x 0.8
Limit = Numeric x 1.2 Limit = Numeric x 0.8
Limit = Numeric x 1.2 Limit = Numeric x 0.8
5-8 www.zoll.com 9650-001355-01 Rev. H
Page 85

Alarm Options
SpO
2
(%)
SpCO
(%)
SpMet
(%)
EtCO
(mmHg)
FiCO
2
(mmHg)
Temp
(°C)
Temp
(°F)
Entire range Limit = 100 (Adult and
Limit = Numeric – 5
Pediatric)
Limit = Numeric + 5 (Neonate)
Entire range Limit = Numeric + 2
Upper limit 40
Entire range Limit = Numeric + 2
Upper limit 15
Entire range Limit = Numeric + 10 Limit = Numeric - 5 mmHg
2
Limit = Numeric - 2
Lower limit 0
Limit = Numeric - 2
Lower limit 0
unless the numeric falls
below the lower alarm limit
range, in which case Stat
Set sets the lower limit to
15 mmHg.
Entire range Limit = Numeric + 5 N/A
Entire range Limit = Numeric + 0.5 Limit = Numeric – 0.5
Entire range Limit = Numeric + 0.9 Limit = Numeric – 0.9
9650-001355-01 Rev. H X Series Operator’s Guide 5-9
Page 86

CHAPTER 5ALARMS
5-10 www.zoll.com 9650-001355-01 Rev. H
Page 87

Chapter 6
Monitoring ECG
This chapter describes how to use the X Series unit to monitor ECG.
X Series units can perform ECG monitoring through 3-, 5-, or 12-lead ECG patient cables,
Multi-Function Pads, or standard defibrillation paddles. The use of an ECG patient cable and
electrodes is required, however, to monitor ECG during pacing.
OneStep cables and electrodes cannot be used for ECG monitoring.
You can use a 3-lead, 5-lead, or 12-Lead wire configuration for ECG monitoring (see Running
H/F 1 for more information on 12-lead monitoring).
Warning! • Excessive body ha ir or wet, sweaty skin may interfere with electrode adhesion.
Remove the hair and/or moisture from the area where the electrode is to be attached.
• Use only electrodes that are well within the expi ration date ind icated on th e p ackage.
• Remove ECG electrodes from their sealed package immediately prior to use. Using
previously opened or out-of- date electrodes may degrade the ECG signal quality.
• Monitoring electrodes may become polarized during defibrillator discharge, causing
the ECG waveform to briefly go off screen. ZOLL Medical Corporation recommends
the use of high quality silver/silver chloride (Ag/AgCl) electrodes to minimize this
effect; the circuitry in the instrument returns the trace to the monitor display within
a few seconds.
• To assure protection against the effects of defibrillator discharge, use only
ZOLL-approved accessories.
9650-001355-01 Rev. H X Series Operator’s Guide 6-1
Page 88

CHAPTER 6MONITORING ECG
• To avoid a shock hazard and interference from nearby electrical equipment, keep
electrodes and patient cables away from grounded metal and other electrical
equipment.
• To avoid electrosurgery burns at monitoring sites, ensure proper connection of the
electrosurgery return circuit so that the return paths cannot be made through
monitoring electrodes or probes.
• Check the operation and integrity of the X Series unit and ECG cable regularly by
performing the Daily Operational Verification Test.
• Implanted pacemakers may cause the heart rate meter to count the pacemaker rate
during incidents of cardiac arrest or other arrhythmias. Carefully observe
pacemaker patients. Check the patient's pulse; do not rely solely on heart rate
meters. Dedicated pacemaker detection circuitry may not detect all implanted
pacemaker spikes. Patient history and physical exam are important in determining
the presence of an implanted pacemaker.
ECG Monitoring Setup
The proper application and placement of electrodes is essential for high quality ECG
monitoring. Good contact between the electrode and skin minimizes motion artifact and signal
interference.
The following procedure describes how to monitor a patient’s ECG using 3- and 5-Lead ECG
cables. For information on the application and use on MultiFunction Pads and External
Paddles, which you can also use to monitor ECG, see Chapter 15, Manual Defibrillation.
To monitor a patient’s ECG using 3- and 5-Lead ECG cables, perform the following steps:
1. Prepare the patient's skin for electrode application:
2. Apply the electrode pads to the patient.
3. Connect each lead of the ECG cable to the appropriate electrode.
4. Insert the patient cable plug into the ECG input connector on the X Series unit.
5. Select the ECG waveforms to be displayed on the waveform trace display screen.
6. Observe the patient‘s electrocardiogram on the display , and adjust size of the ECG wave form
trace, as necessary.
Preparing the Patient for Electrode Application
The proper application of electrodes is essential for high quality ECG moni toring. Good cont act
between the electrode and skin minimizes motion artifact and signal interference.
Before applying electrodes, prepare the patient’s skin, as necessary:
• Shave or clip excess hair at electrode site.
• Clean oily skin with an alcohol pad.
• Rub site briskly to dry.
6-2 www.zoll.com 9650-001355-01 Rev. H
Page 89

Applying Electrodes to the Patient
The following sections show where to place electrodes when using 3- and 5-Lead cables to
perform ECG monitoring. For 3-Lead ECG cables, apply electrodes as in Figure 4-1, 3-Lead
Electrode Placement. For 5-Lead ECG cables, apply electrodes as in Figure 4-2, 5-Lead
Electrode Placement.
Avoid placing electrodes over tendons and major muscle masses.
Make sure that the ECG electrodes are placed to allow defibrillation, if necessary.
3-Lead Electrode Placement
Depending upon local usage, the ECG leads are marked either RA, LA, and LL (or R, L, and
F). The following table shows the markings and color codes for the different lead sets.
AHA Color Coding IEC Color Coding Placement of Electrodes
RA/White Electrode R/Red Electrode Place near patient’s right mid-clavicular line,
LA/Black Electrode L/Yellow Electrode Place near patient’s left mid-clavicular line,
ECG Monitoring Setup
directly below clavicle.
directly below clavicle.
LL/Red Electrode F/Green Electrode Place between 6th and 7th intercostal
space on patient’s left mid-clavicular line.
Figure 6-1 3-Lead Electrode Placement
9650-001355-01 Rev. H X Series Operator’s Guide 6-3
Page 90

CHAPTER 6MONITORING ECG
5-Lead Electrode Placement
Depending upon local usage, th e ECG leads are marked either RA , LA, LL , RL, and V or R, L,
F, N and C. The following table shows the markings and color codes for the diff e re nt lead set s .
AHA Color Coding IEC Color Coding Placement of Electrodes
RA/White Electrode R/Red Electrode Place near patient’s right mid-clavicular line,
LA/Black Electrode L/Yellow Electrode Place near patient’s left mid-clavicular line,
LL/Red Electrode F/Green Electrode Place between 6th and 7th intercostal
RL/Green* Electrode N/Black* Electrode Place between 6th and 7th intercostal
V/Brown* Electrode C/White* Electrode Single movable chest electrode. Place this
directly below clavicle.
directly below clavicle.
space on patient’s left mid-clavicular line.
space on patient’s right mid-clavicular line.
electrode in one of the positions, V1 - V6,
as shown in the following figure.
V1 -- 4th intercostal space at right sternal
margin.
V2 -- 4th intercostal space at left sternal
margin.
V3 -- Midway between V2 and V4 leads.
V4 -- 5th intercostal space at mid-clavicular
line.
V5 -- Same transverse level as V4 at left
anterior-axillary line.
V6 -- Same transverse level as V4 at left
mid-axillary line.
6-4 www.zoll.com 9650-001355-01 Rev. H
Page 91

ECG Monitoring Setup
Figure 6-2 5-Lead Electrode Placement
Connecting the ECG Cable To the X Series Unit
The X Series unit accepts Welch Allyn Propaq® ECG cables as well as ZOLL
X Series ECG cables. Connect the ECG cable to the ECG connector on the left side of the X
Series unit as follows:
Figure 6-3 Connecting ECG Cable to X Series Unit
9650-001355-01 Rev. H X Series Operator’s Guide 6-5
Page 92

CHAPTER 6MONITORING ECG
06/06/2011
I, II,
III...
SYNC
I, II,
III...
Selecting ECG Waveforms for Display
You can fit up to four waveforms on the X Series display. The first waveform at the top of the
display is always an ECG waveform. (If
automatically default to another ECG lead.) In the following example, Lead
source of the ECG waveform trace:
12
CO
2
R
Pads are not connected, the unit can be configured to
II (RA-LL), is the
The X Series unit is configured to display
Pads by default for the top trace. If configured, the
unit can also display four ECG traces on startup, when no other monitoring devices are
attached.
The are two ways to specify which ECG lead is the source of the prima ry waveform trace. One
way is to press the ECG lead selection quick access key to display the available ECG
waveform sources. The available waveform sources are determined by the type of ECG cable
connected to the unit.
The other way to specify the source of the primary waveform trace is to navigate to and select
the source label for the primary ECG waveform (Lead
l in the screen below). The X Series unit
then displays the available ECG waveform sources. The following example illustrates the
waveform source list that the X Series unit displays when a 5-lead ECG cable is connecte d to it.
The list of available ECG waveform sources i ncludes Leads
can select Lead
lI (the default), or use the navigation keys to highlight and select another
I, II, III, aVR, aVL, aVF, and V. You
displayed ECG lead as the source for the waveform trace.
6-6 www.zoll.com 9650-001355-01 Rev. H
Page 93

06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
12
SYNC
1 cm/mV
I
HR bpm
80
121
79
(96)
NIBP mmHg
38
12
BR
CO2 mmHg
SpO2 %
97
T1 ºF
98.6
Pads
III
aVL
aVF
V
Insert
Source
II
I
Cascade
aVR
CO
R
ECG Monitoring Setup
2
Select a current waveform source, and the unit displays the waveform immediately. If you
select a waveform source that is not currently available, the unit displays the message, LEAD
FAULT.
For more information on how to configure the display of waveforms on the X Series unit, see
Chapter 3, Monitoring Overview.
9650-001355-01 Rev. H X Series Operator’s Guide 6-7
Page 94

CHAPTER 6MONITORING ECG
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
(96)
NIBP mmHg
38
BR
CO2 mmHg
SpO2 %
97
T1 ºF
0.125 cm/mV
1.0 cm/mV
4.0 cm/mV
Auto
Lead II Size
0.50 cm/mV
2.0 cm/mV
0.25 cm/mV
Selecting the Waveform Trace Size
The X Series unit allows you to select the waveform trace size to adjust the size of displayed
the ECG waveform.
To select the waveform size, use the navigation keys to highlight and select the trace size that
appears to the right of the electrode label:
06/06/2011 12:34:56
12
CO
2
R
The default trace size is
0.125, 0.25, 0.50 cm/mV) trace size. You can also specify that the X Series unit select a best-fit
(
trace size (
AUTO).
98.6
79
1cm/mV. You can select a larger (2.0, 4.0 cm/mV) or smaller
12
6-8 www.zoll.com 9650-001355-01 Rev. H
Page 95

ECG Monitoring and Pacemakers
When the unit performs ECG monitoring on a patient with an implantable pacemaker, the unit’s
Pacer indicator feature can indicate the occurrence of pacemaker signals.
ECG Monitoring and Pacemakers
If the Pacer Indicator setting is
• detects the implantable pacemaker pulses
• blanks the pacemaker pulses from the waveform—preventing them from disturbing the
ECG waveform and allowing for an accurate QRS detection
• displays and prints vertical dashed lines to indicate the detected pacemaker signals
If the Pacer Indicator setting is
• detect the implantable pacemaker pulses
• blank the pacemaker pulses from the waveform
• display or print the vertical dashed line pacer markers
You can turn the Pacer Indicator
There are situations where ECG artifact could simulate pacemaker signals which could cause
false pacemaker detection and blanking. These situations may cause inaccurate QRS detection
and it may be desirable to turn the Pacer Indicator
setting is
OFF, implantable pacemaker signals may cause inaccurate QRS detection and it may
be desirable to turn the Pacer Indicator on.
ECG System Messages
When monitoring ECG, the X Series unit may display the following message s:
System Message Cause
ON, the X Series performs the following actions:
OFF, the X Series does not perform the following actions:
ON/OFF from the Setup>ECG menu.
off. Inversely, when the Pacer Indicator
LEAD FAULT The current ECG source lead is defective (check
lead and replace, if necessary).
-- OR --
An unavailable waveform source has been specified
for the trace display (check specified waveform
source and correct, if necessary).
PADDLE FAULT or CA BLE FAULT Check the pad, paddle or cable and replace if
necessary.
9650-001355-01 Rev. H X Series Operator’s Guide 6-9
Page 96

CHAPTER 6MONITORING ECG
6-10 www.zoll.com 9650-001355-01 Rev. H
Page 97

Chapter 7
06/06/2011 12:34:56
Adult
00:17:43
I, II,
III...
SYNC
1 cm/mV
II
HR bpm
80
121
79
(96)
NIBP mmHg Resp br/min
16
SpO2 %
97
T1 ºF
98.6
Respiration Meter
Monitoring Respiration (Resp) and Heart Rate (HR)
This chapter describes how to use the X Series unit to monitor Respiration (Resp) and Heart
HR).
Rate (
The X Series unit displays Respiration (Resp) and Heart Rate (HR) mete rs. The Respiration and
Heart Rate meters display values that the X Series unit derives from measurements taken by
other X Series monitoring functions.
12
CO
2
9650-001355-01 Rev. H X Series Operator’s Guide 7-1
R
Heart Rate
Meter
Page 98

Chapter 7 Monitoring Respiration (Resp) and Heart Rate (HR)
Note: The respiration rate is disabled during defibrillation. During defibrillation, the
respiration rate will display ??? on the snapshot.
Respiration/Breath Rate Meter
If enabled, the X Series unit displays the patient’s respiration in the Respiration/Breath Rate
Meter.
The respiration
monitoring function. If CO2 monitoring is not available, the unit derives the respiration
CO
2
rate through impedance pneumography, using a specifi ed ECG electrode co nfiguratio n. If ECG
monitoring isn’t functioning, the Resp/BR meter will not display a respiration rate.
meter displays the respiration rate that it derives, by default, from the unit’s
Using Impedance Pneumography to Measure Respiration
Impedance pneumography detects respiration by applying a high-frequency, low-current AC
signal to the patient and measuring the changes in impedance through ECG electrode Lead
(RA-LA) or Lead
increases; as the patient exhales, impedance decreases.
Warning! • Impedance pneumograp hy detects respiratory effort through changes in chest
volume. However, No Breath episodes with continued respiratory effort may go
undetected. Always monitor and set alarms for SpO
pneumography to monitor respiratory function.
• With any monit or that de tects r espir atory effort thr ough impeda nce pneumography,
artifact due to patient motion, apnea mattress shaking, or electrocautery use may
cause apnea episodes to go undetected. Always monitor and set alarms for SpO
when using impedance pneumography to monitor respiratory functio n.
ll (RA-LL). As the patient inhales and chest volume expands, impedance
when using impedance
2
l
2
• When using impedance pneumography, don’t use the X Series unit with another
respiration monitor on the same patient, because the respiration measurement
signals may interfere with one another.
• Impedance pneumography is not recommended for use on paced patients, because
pacemaker pulses may be falsely counted as breaths.
• Impedance pneumography is not recommended for use with high frequency
ventilation.
• Since impedance pneumography uses the same leads as the ECG channel, the
X Series unit determines which s i gn als are cardiovascular artifact and which signals
are the result of respiratory effort. If the breath rate is within five percent of the
heart rate, the monitor may ignore breaths and trigger a respiration alarm.
7-2 www.zoll.com 9650-001355-01 Rev. H
Page 99

Configuring Respiration (RR/BR) Alarms and Settings
Limits
Configuring Respiration (RR/BR) Alarms and Settings
The X Series unit allows you to enable and disable the Respiration (RR/BR) Rate alarm, to set
alarm limits, and to specify the ECG monitoring source for the Respiration rate.
Enabling/Disabling RR/BR Alarms and Setting Alarm Limits
When enabled, the X Series unit sounds alarms whenever the patient’s respiration rate is above
or below the specified respiration rate alarm limits.
To enable (or disable) Resp alarms and set Upper and Lower alarm limits, you can either do so
through the Alarms quick access key ( ), or the Resp Parameter Control Panel.
To configure
RR/BR alarms through the Alarms quick access key:
1. Press .
2. Press .
3. Press the Limits quick access key ( ). Use the navigation keys to highlight and select the
RR/BR Alarm menu selection.
4. On the RR/BR Alarm Setti ngs menu, use the navigation keys to select the fields that you want
to change:
• Upper Limit Enable
• Lower Limit Enable
• Upper Limit
• Lower Limit
5. When you are finished changing values on the alarm menu, navigate to the Backarrow key
to confirm your choices and exit the menu.
9650-001355-01 Rev. H X Series Operator’s Guide 7-3
Page 100

Chapter 7 Monitoring Respiration (Resp) and Heart Rate (HR)
Resp
Lower Upper
RR/BR Alarm 10
30
No Breath Alarm 30 sec
Resp Monitoring On
Resp Lead
Lead II (RA-LL)
CO2/Resp Sweep Speed
12.5 mm/s
Respiration Rate Alarm Limits
Initially, the Resp Alarm Settings menu specifies that Resp alarms are enabled (ON) or
disabled (OFF), and displays the default Upper and Lower respiration rate alarm limits. The
upper and lower limits can be
ON or OFF (default is OFF).The following table lists the default
respiration rate alarm limits for adult, pediatric, and neonate patients, and gives the range in
which you can set these limits:
Patient Type Respiration Rate Default Respiration Rate Range
Adult Lower: 3 br/min
Upper: 50 br/min
Pediatric Lower: 3 br/min
Upper: 50 br/min
Neonate Lower: 12 br/min
Upper: 80 br/min
Using the Resp Parameter Control Panel
To configure alarms through the Resp Parameter Control Panel, use the navigation keys to
highlight and select the Respiration Rate meter and display the
Lower: 0 to 145 br/min
Upper: 5 to 150 br/min
Lower: 0 to 145 br/min
Upper: 5 to 150 br/min
Lower: 0 to 145 br/min
Upper: 5 to 150 br/min
Resp Parameter Control Panel:
Figure 7-1 Respiration Parameter Control Panel
The Resp Parameter Control Panel allows you to set the following parameters:
• RR/BR Alarm -- enable/disable the Resp alarm and set high/low alarm limits.
• No Breath Alarm -- sets the duration of the No Breath alarm, or disables alarm by selecting
“Off”.
• Resp Monitoring -- enable/disable respiration monitori ng .
• Resp Lead -- selects the Resp lead, Lead I (RA-LA) or Lead II (RA-LL), from which the X
Series unit calculates the respiration rate. Resp Lead selection is independent of ECG Lead
selection.
• CO2/Resp Sweep Speed -- sets the respiratory sweep speed on the display.
7-4 www.zoll.com 9650-001355-01 Rev. H
 Loading...
Loading...