Page 1
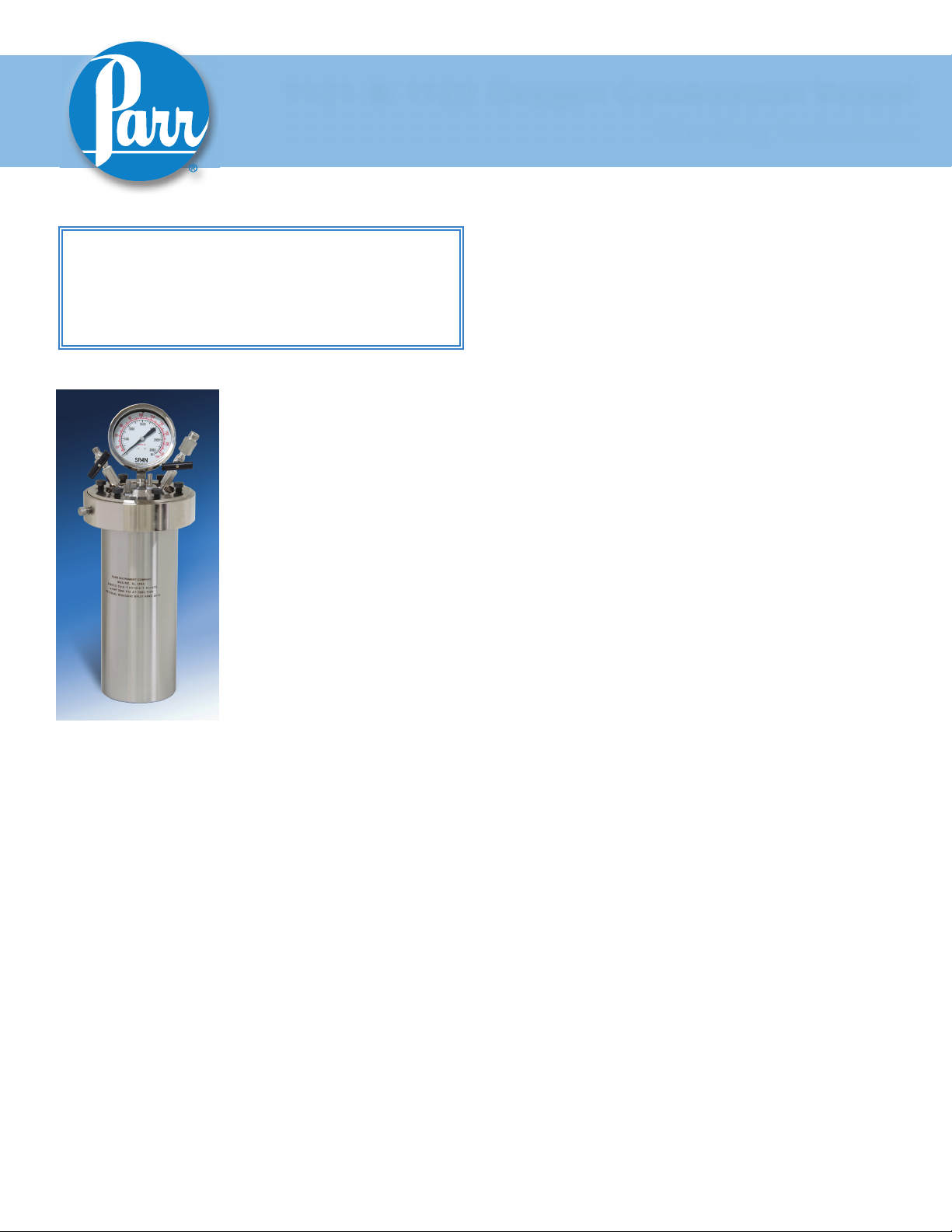
210M
1121 & 1122 Oxygen Combustion Vessel
Operating Instructions
An Oversized Special Purpose Combustion Vessel
Note About Nomenclature:
Historically, burning a sample enclosed in a high
pressure oxygen environment is known as Oxygen
Bomb Calorimetry and the vessel containing the
sample is known as an Oxygen Bomb. The terms
bomb and vessel are used interchangeably.
1121 Oxygen Combustion Vessel
The 1121 Oxygen Combustion
Vessel has been developed specifically to burn large samples
of slow burning materials such
as grain, wood fiber, paper and
other vegetable matter. Volatile liquids can also be burned
in this bomb provided special
techniques are used to retard
the burning rate. The ability of
the 1121 vessel to handle large
samples with complete recovery of all liquid and gaseous
combustion products makes it
particularly effective for determining trace elements in combustible materials and for preparing samples for tritium and
carbon-14 measurements.
1122 Oxygen Combustion Vessel
The 1122 Oxygen Combustion Vessel has been developed
specifically to burn samples liberating up to 0.1 MJ. This
bomb can safely accommodate sample sizes that are
significantly larger than the 350 ml 1108 vessel (33 kJ)
but less than the 1850 ml 1121 vessel (0.26 MJ). The 1122
style vessel is especially useful for preparing samples for
C-14 measurements where the volume of residual postcombustion gas must be minimized.
Allowable Sample Size
1121 Oxygen Combustion Vessel
Slow burning, combustible samples weighing up to 10
grams and releasing up to 0.26 MJ can be burned in the
1121 vessel using oxygen charging pressures up to 300
psig (20 atm), but these limits vary and must be checked
experimentally for each sample. The sample size must be
adjusted to an amount which will give complete combustion with peak pressures held in the range from 1000 to
1200 psig. The pressure should never exceed 1500 psig as
an absolute maximum. After a safe procedure has been
established for a specific material, the entrance to the
gage can be closed at the underside of the bomb head
using the 440A plug which is furnished for this purpose.
This will prevent repeated stress on the gage and keep
combustion products out of the gage passage.
1122 Oxygen Combustion Vessel
Slow burning, combustible samples weighing typically up
to 4 grams can be burned in the 1122 Oxygen Combustion
Vessel using oxygen charging pressures up to 300 psig
(20 atm). This filling pressure and the volume of the cylinder yields ~0.8 moles of 02. Using more than half of the
available oxygen during a sample preparation increases
the risk of incomplete combustion. Larger samples can be
prepared in this bomb as long as the total energy released
in the bomb doesn’t exceed 0.1 MJ. For example, each
mole of cellulose [(C5H10O5)n (mwt 150), where n =1500 to
>6000] burned requires 10 moles of 02. The heat of combustion of cellulose is ~17kJ/g. Preparing 6 grams of cellulose in the 1122 vessel results in a total energy release of
0.1 MJ and uses (10*6/150) moles, or half of the available
oxygen.
Sample Preparation
Finely divided samples to be burned in this bomb must
be compressed into pellets or, if this is not convenient,
powdered samples must be packed firmly in the bottom
of the combustion cup. Cellulosic materials should be reasonably dry, but some moisture may be desirable since
bone-dry samples usually burn too fast, resulting in incomplete combustion. It may be necessary, therefore, to
add moisture to cellulosic materials to slow the burning
rate. The amount of water added will have to be determined experimentally, with some materials such as paper
accepting up to 40% water by weight to obtain good combustions. In all cases, compress the sample into the cup
as firmly as possible.
Preparing the Charge
When loading the bomb, set the head in the tripod support ring and adjust the capsule support so that the top of
the cup is positioned 6 to 7 inches from the underside of
the bomb head. Fasten a 10 cm length of 45C10 fuse wire
to the electrode hooks and bend the wire so that it touches
the sample but not the capsule. If the wire does not reach
the sample, its length can be increased to 14 or 15 cm, but
longer lengths should not be used because the wire will
not get hot enough to ignite the sample. If the wire does
not reach the sample, add an auxiliary fuse made from a
strip of filter paper or a length of cotton or nylon thread.
It is always well to place from 25 to 50 ml of water in the
bottom of the cylinder before closing this bomb. This is
not absolutely necessary, but it is an excellent safeguard
against damage which might be caused if any of the metal
parts should ignite and drop to the bottom of the bomb.
Note: The bottom of the A445A Cup Support Bracket in
the 1122 vessel must be 5 mm from the end of the dip
tube. This positions the combustion cup a safe distance
from the head of the bomb. Attach 15 cm of the 45C10
fuse wire between the two electrode hooks. Refer to
the assembly drawing on page 4.
Page 2

1121 & 1122 Oxygen Combustion Vessel
Closing the Bomb
Before closing the bomb, check the head gasket to be
sure that it is in good condition and moisten it with a
few drops of water; then push the head firmly into the
cylinder. Slide the clamping rings into position and raise
the band from the bottom of the bomb to encircle the
rings. Position the band so that its cone pointed screw
enters the dimple drilled in the outer face of one of the
ring sections, then tighten the screw. Seal the bomb by
tightening each of the cap screws, applying a firm, hard
pull to the wrench supplied with the bomb. Or, if a torque
wrench is available, apply 25 ft-lbs to each screw. Tightening should proceed in a criss-cross pattern rather than
progressively around the circle.
Filling the Bomb
All of the fittings needed to charge the bomb with oxygen from a commercial oxygen cylinder are provided in
a Parr 1825A Oxygen Filling Connection. As an alternate,
the bomb can be filled by purchasing only the A19A7
hose assembly used on the 1825 filling connection and
attaching this hose to a standard pressure regulator on
the oxygen tank. The threaded coupling needed for the
bomb inlet connection is furnished with the A19A7 oxygen hose.
To fill the bomb, attach the hose to the inlet valve, open
the valve on the filling connection slowly and watch the
gage as the bomb pressure rises to the desired filling
pressure. The bomb should not be filled to more than 300
psig (20 atm.) at room temperature. Do not overfill the
bomb. If too much oxygen should accidentally be introduced, do not proceed with the combustion. Detach the
filling hose; exhaust the bomb and refill with oxygen before igniting the charge.
Firing the Bomb
Recommended practice is to immerse the bomb in water when it is fired, however this is not required. It must
always be placed behind a heavy shield or barricade to
protect the operator in case of an accidental explosion.
Set the ignition unit outside the barricade and arrange
the bomb so that the operator will be protected by the
barricade but with the pressure gage visible so that he
can observe the bomb pressure. The bomb must stay behind the barricade during firing and remain there until
there is a definite indication that the peak pressure has
been reached and the pressure is definitely going down.
The pressure will increase rapidly during the first 3 to 5
seconds after firing, after which it may drop slightly, only
to rise again during the next 30 seconds. Most combustions are complete after the first minute, but it is well to
wait at least 2 minutes after firing before handling the
bomb. In all cases, be sure that the pressure is definitely
going down before opening the discharge valve. Always
open the valve slowly and be sure that the operator’s
hand or face are not in line with the discharge.
Firing the 1122 Oxygen Combustion Vessel
The mass of the 1122 Oxygen Combustion Vessel is approximately 11.2 kg. An energy release of 0.1MJ will raise
the temperature of the unit ~20C. This assumes the heat
capacity of the bomb is about 1/10 that of an equal mass
of water and the heat has sufficient time to distribute uniformly.
CAUTION! During the combustion process and
shortly thereafter, the midsection of the cylinder immediately above the sample holder will
become quite warm. Take care when handling
the unit after the combustion is complete.
Opening the Bomb
To open the bomb: loosen the cap screws in the split ring
sections then loosen the knurled screw in the outer band
and push the band downward to rest on the table. The
ring sections can now be removed and the head lifted
from the cylinder. Transfer the head to the tripod support
ring, being careful not to bend or disturb the electrode
or sample holder. Normally the 441HC sealing ring will
come out with the head, but if the bomb has been under
pressure for some time the ring may have absorbed oxygen and become swollen, in which case it will drop away
from the head. The gasket will gradually return to its normal size after the absorbed gas dissipates.
WARNING: Do not overcharge this bomb and do
not fi re it if there is any evidence of a gas leak.
The sample weight must not exceed 10 grams
and the charging pressure should not exceed
300 psig, unless cautious experiments show that
a higher charging pressure is required to obtain
complete combustion, and the higher charge
does not produce a peak pressure in excess of
1500 psig at any time during a test. If there is
any reason to suspect that the bomb is leaking,
immerse it in water and check for leaks before
fi ring. Inspect the bomb regularly and replace
any parts which are no longer serviceable or
which show signs of weakness.
Special Procedures
Suggested procedures for handling volatile liquids or
other unusual materials can be obtained by contacting
Parr Instrument Company direct, either by telephone or
correspondence.
Customer Service
Questions concerning the installation or operation
of this instrument can be answered by the Parr
Customer Service Department:
1-309-762-7716 • 1-800-872-7720
Fax: 1-309-762-9453 • E-mail: parr@parrinst.com
2
Parr Instrument Company
Page 3

1121 & 1122 Oxygen Combustion Vessel
1121 Parts List
Item Part No. Description Item Part No. Description
1 A232HC SPLIT RING W/ 232HCFDE CAP SCREWS 16 SC1932SC10 10-32 X 5/8 SHSS
2 A233HC DROP BAND W/ 233HCF SCREW 17 388A SPACER
3 599HC2 CYLINDER, 1850mL, 316SS 18 A122VB NEEDLE VALVE, 1/8" NPTM, 316SS
4 4A5AD STRAIGHT ELECTRODE, 5', 316SS 19 590HC ADAPTER FOR FILLING CONNECTION
5 68AC2 LOCK NUT, 303SS 20 234HC COMPRESSION RING
6 402A2 ELECTRODE, CORE 21 432A3 HEAD, 316SS
7 96AC CERAMIC WASHER 22 712HC 0-RING, NBR
8 441HC 0-RING, 4' O.D., NEOPRENE 23 440A GAGE PLUG
9 401A SLEEVE INSULATOR 24 SN3724HX 3/8-24 JAM NUT
10 238A 0-RING, BUNA-N 25 523A INLET TUBE W/NUT SS
11 143AC INSULATOR 26 446A COMBUSTION CUP
12 411A TERMINAL NUT (2) 27 23AC HOOK ELECTRODE
13 260HC2 VALVE EXTENSION (2) 28 A445A CUP SUPPORT BRACKET
14 A133VB NEEDLE VALVE, 1/8' NPTM, 316SS 29 358HC2F SET SCREW
15 593HCP30YE GAGE, 3-1/2", 3000 PSI, SS
www.parrinst.com
3
Page 4

1121 & 1122 Oxygen Combustion Vessel
1122 Parts List
The following table illustrates parts that are unique to the 1122 combustion vessel. The corresponding part for the
larger 1121 combustion vessel is also provided for reference purposes.
1122 Oxygen Combustion Vessel 1121 Oxygen Combustion Vessel
Part No. Description Part No. Description
599HC 920 ml Cylinder 599HC2 1850 ml Cylinder
4A6 Electrode 4A5 Straight Electrode, 5”
QW3191B Dip Tube 257HC14 Dip Tube
441HCJV 0-ring, FKM 441HC 0-ring, Neoprene
Revision 05/13/13
P
arr Instrument Company
211 53rd Street • Moline, Illinois 61265 USA 1-309-762-7716 • 1-800-872-7720 • Fax: 1-309-762-9453
E-mail: parr@parrinst.com • http://www.parrinst.com
 Loading...
Loading...