Page 1

M6/M6T/M6 Exp/M6s/M6 Pro/M55/M58/M5 Exp
Diagnostic Ultrasound System
Operator’s Manual
[Basic Volume]
Page 2

Page 3

Contents
Contents ............................................................................................................................... i
Intellectual Property Statement .......................................................................................................... I
Responsibility on the Manufacturer Party ......................................................................................... II
Warranty ............................................................................................................................................ II
Exemptions ................................................................................................................................... II
Customer Service Department .................................................................................................... III
Important Information ....................................................................................................................... IV
About This Manual ........................................................................................................................... IV
Notation Conventions ........................................................................................................................ V
Operator’s Manuals ........................................................................................................................... V
Hardcopy Manuals ............................................................................................................................ V
Software Interfaces in this Manual ................................................................................................... VI
Conventions ..................................................................................................................................... VI
1 Safety Precautions .................................................................................................... 1-1
1.1 Safety Classification ............................................................................................................. 1-1
1.2 Meaning of Signal Words ..................................................................................................... 1-2
1.3 Meaning of Safety Symbols ................................................................................................. 1-2
1.4 Safety Precautions ............................................................................................................... 1-3
1.5 Latex Alert .......................................................................................................................... 1-11
1.6 Warning Labels .................................................................................................................. 1-11
2 System Overview ...................................................................................................... 2-1
2.1 Intended Use ........................................................................................................................ 2-1
2.2 Contraindication ................................................................................................................... 2-1
2.3 Product and Model Code ..................................................................................................... 2-1
Product Differences ........................................................................................................................ 2-1
2.4 Product Specifications .......................................................................................................... 2-2
2.4.1 Imaging Modes ............................................................................................................. 2-2
2.4.2 Power supply ................................................................................................................ 2-2
2.4.3 Environmental Conditions ............................................................................................. 2-3
2.4.4 External Dimensions and Weight ................................................................................. 2-3
2.5 System Configuration ........................................................................................................... 2-3
2.5.1 Standard Configuration ................................................................................................. 2-3
2.5.2 Options ......................................................................................................................... 2-4
2.6 Introduction of Each Unit ...................................................................................................... 2-9
2.7 Mobile trolley ...................................................................................................................... 2-10
2.8 Extend Modules.................................................................................................................. 2-14
i
Page 4

2.8.1 Probe Extend Module ................................................................................................. 2-14
2.8.2 I/O extend module ...................................................................................................... 2-15
2.8.3 V/A Extend Module ..................................................................................................... 2-16
2.8.4 ECG module ............................................................................................................... 2-17
2.9 Control Panel ...................................................................................................................... 2-18
2.10 Symbols .............................................................................................................................. 2-22
3 System Preparation .................................................................................................. 3-1
3.1 Move/Posit the System ........................................................................................................ 3-1
3.2 Power Supply ....................................................................................................................... 3-1
3.2.1 Connecting the External Power Supply ........................................................................ 3-1
3.2.2 Powered by Batteries ................................................................................................... 3-2
3.3 Power ON /OFF .................................................................................................................... 3-2
3.3.1 Powering ON the System ............................................................................................. 3-2
3.3.2 Powering OFF the System ........................................................................................... 3-4
3.3.3 Standby ......................................................................................................................... 3-5
3.4 Connecting /Disconnecting a Probe ..................................................................................... 3-6
3.4.1 Connecting a Probe ...................................................................................................... 3-6
3.4.2 Disconnecting a Probe ................................................................................................. 3-6
3.5 Connecting the Footswitch ................................................................................................... 3-7
3.6 Connecting/ Removing a USB Memory Device ................................................................... 3-7
3.7 Graph /Text printer................................................................................................................ 3-8
3.8 Video Printer ......................................................................................................................... 3-9
3.9 Basic Screen and Operation .............................................................................................. 3-11
3.9.1 Basic Screen ............................................................................................................... 3-11
3.9.2 Basic Operations of Screens ...................................................................................... 3-16
4 Exam Preparation ..................................................................................................... 4-1
4.1 To Start an Exam .................................................................................................................. 4-1
4.2 Patient Information ............................................................................................................... 4-1
4.2.1 New Patient Information ............................................................................................... 4-2
4.2.2 Retrieve Patient Information ......................................................................................... 4-6
4.3 Select an Exam Mode and Probe. ....................................................................................... 4-8
4.3.1 Supported Exam Modes ............................................................................................... 4-8
4.3.2 Selecting Exam Modes and Probes ............................................................................. 4-8
4.4 Select the Imaging Mode ................................................................................................... 4-10
4.5 Activate & Continue an Exam ............................................................................................ 4-10
4.5.1 Activate an Exam ........................................................................................................ 4-10
4.5.2 Continue an Exam ...................................................................................................... 4-10
4.6 Pause Exam and End Exam .............................................................................................. 4-10
4.6.1 Pause an Exam .......................................................................................................... 4-10
ii
Page 5

4.6.2 End an Exam .............................................................................................................. 4-11
4.7 Cancel an Exam ................................................................................................................. 4-11
4.8 Anonymous Patient Exam .................................................................................................. 4-12
5 Image Optimization ................................................................................................... 5-1
5.1 Switching Between Imaging Modes ..................................................................................... 5-1
5.2 Image Adjustment................................................................................................................. 5-2
5.3 B Mode Image Optimization ................................................................................................. 5-4
5.3.1 B Mode Exam Protocol ................................................................................................. 5-4
5.3.2 B Mode Parameters ...................................................................................................... 5-4
5.3.3 B Mode Image Optimization ......................................................................................... 5-4
5.4 M Mode Image Optimization .............................................................................................. 5-11
5.4.1 M Mode Exam Protocol .............................................................................................. 5-11
5.4.2 M Mode Parameters ................................................................................................... 5-11
5.4.3 M Mode Image Optimization ....................................................................................... 5-12
5.5 Color Mode Image Optimization ......................................................................................... 5-15
5.5.1 Color Mode Exam Protocol ......................................................................................... 5-15
5.5.2 Color Mode Image Optimization ................................................................................. 5-15
5.5.3 Color Mode Image Optimization ................................................................................. 5-16
5.6 Power Mode Image Optimization ....................................................................................... 5-21
5.6.1 Power Mode Exam Protocol ....................................................................................... 5-21
5.6.2 Power Mode Image Parameters ................................................................................. 5-21
5.6.3 Power Mode Image Optimization ............................................................................... 5-22
5.7 PW/CW Doppler Mode Optimization .................................................................................. 5-23
5.7.1 PW / CW Mode Exam Protocol .................................................................................. 5-23
5.7.2 PW/CW Mode Image Parameters .............................................................................. 5-23
5.7.3 PW/CW Doppler Mode Optimization .......................................................................... 5-24
5.8 Anatomical M Mode............................................................................................................ 5-30
5.8.1 Free Xros M Mode ...................................................................................................... 5-30
5.8.2 Free Xros CM (Curved Anatomical M Mode) ............................................................. 5-32
5.9 TDI ...................................................................................................................................... 5-33
5.9.1 TDI Exam Protocol...................................................................................................... 5-33
5.9.2 TDI Image Parameters ............................................................................................... 5-34
5.9.3 TDI Image Optimization .............................................................................................. 5-35
5.9.4 TDI Quantitative Analysis (QA) ................................................................................... 5-35
5.10 Color M Mode ..................................................................................................................... 5-39
5.10.1 Enter Color M Mode ................................................................................................... 5-39
5.10.2 Exit Color M Mode ...................................................................................................... 5-39
5.10.3 Image Parameters ...................................................................................................... 5-39
5.11 3D/4D ................................................................................................................................. 5-41
iii
Page 6

5.11.1 Note before Use.......................................................................................................... 5-41
5.11.2 Overview ..................................................................................................................... 5-42
5.11.3 3D/4D Preset .............................................................................................................. 5-47
5.11.4 Smart 3D ..................................................................................................................... 5-49
5.11.5 4D ............................................................................................................................... 5-61
5.11.6 Static 3D ..................................................................................................................... 5-63
5.12 iScape ................................................................................................................................ 5-65
5.12.1 Basic Procedures for iScape Imaging ........................................................................ 5-65
5.12.2 iScape Preset ............................................................................................................. 5-66
5.12.3 Image Acquisition........................................................................................................ 5-66
5.12.4 iScape Viewing ........................................................................................................... 5-67
5.12.5 Cine Review ................................................................................................................ 5-68
5.12.6 Save Image ................................................................................................................. 5-69
5.13 Stress Echo ........................................................................................................................ 5-70
5.13.1 About the Stress Echo Feature ................................................................................... 5-70
5.13.2 Acquisition of Stress Echo Loops ............................................................................... 5-70
5.13.3 Navigation Toolbar ...................................................................................................... 5-72
5.13.4 Selecting Preferred Stress Echo Loops (Select Mode) .............................................. 5-74
5.13.5 Review Mode .............................................................................................................. 5-75
5.13.6 Wall Motion Scoring and Reports ............................................................................... 5-78
5.13.7 Maintenance and Protocol .......................................................................................... 5-80
5.13.8 Saving Stress Echo Data ............................................................................................ 5-83
5.13.9 Exiting the Stress Echo Feature ................................................................................. 5-84
5.13.10 Measurement .............................................................................................................. 5-84
5.14 Contrast Imaging ................................................................................................................ 5-85
5.14.1 Basic Procedures for Contrast Imaging ...................................................................... 5-85
5.14.2 Contrast Image Parameters ....................................................................................... 5-86
5.14.3 Measurement, Comments and Body Marks ............................................................... 5-87
5.15 Elastography ...................................................................................................................... 5-88
5.15.1 Basic Procedure for Elastography .............................................................................. 5-88
5.15.2 Pressure Hint Curve ................................................................................................... 5-88
5.15.3 Mass Measurement .................................................................................................... 5-89
5.15.4 Cine Review ................................................................................................................ 5-89
5.16 Image Preset ...................................................................................................................... 5-90
5.16.1 Image Preset .............................................................................................................. 5-90
5.16.2 Soft Menu and Menu Preset ....................................................................................... 5-92
6 Display & Cine Review.............................................................................................. 6-1
6.1 Image Display ....................................................................................................................... 6-1
6.1.1 Splitting Display ............................................................................................................ 6-1
iv
Page 7

6.1.2 Image Magnification ..................................................................................................... 6-1
6.1.3 Spot ............................................................................................................................... 6-1
6.1.4 Pan ............................................................................................................................... 6-2
6.1.5 iZoom (Full-screen Zooming) ....................................................................................... 6-2
6.1.6 Freeze/ Unfreeze the Image ......................................................................................... 6-2
6.2 Cine Review ......................................................................................................................... 6-3
6.2.1 Entering/ Exiting Cine Review ...................................................................................... 6-4
6.2.2 Cine Review in 2D Mode .............................................................................................. 6-4
6.2.3 Cine Review in M or D Mode ........................................................................................ 6-5
6.2.4 Linked Cine Review ...................................................................................................... 6-6
6.2.5 Review Play .................................................................................................................. 6-6
6.3 Image Compare .................................................................................................................... 6-6
6.3.1 Cine Compare ............................................................................................................... 6-6
6.3.2 Frame Compare ............................................................................................................ 6-7
6.4 Cine Saving .......................................................................................................................... 6-7
6.5 Live Capture ......................................................................................................................... 6-7
6.6 Cine Memory ........................................................................................................................ 6-8
6.6.1 Cine Memory Setting .................................................................................................... 6-8
6.7 Cine Settings ........................................................................................................................ 6-8
7 ECG ............................................................................................................................ 7-1
7.1 ECG Operation Basic Procedures ....................................................................................... 7-2
7.2 ECG Setting ......................................................................................................................... 7-2
7.3 Parameter Description ......................................................................................................... 7-3
7.4 ECG Review ......................................................................................................................... 7-4
8 Measurement ............................................................................................................. 8-1
8.1 Basic Operations .................................................................................................................. 8-1
8.2 General Measurements ........................................................................................................ 8-2
8.2.1 2D General Measurements .......................................................................................... 8-2
8.2.2 M General Measurements ............................................................................................ 8-3
8.2.3 Doppler General Measurements .................................................................................. 8-3
8.3 Application Measurement ..................................................................................................... 8-4
8.4 Measurement Accuracy ........................................................................................................ 8-5
9 Comments and Body Marks ..................................................................................... 9-1
9.1 Comments (Annotations) ...................................................................................................... 9-1
9.1.1 To Add Comments ........................................................................................................ 9-1
9.1.2 Comment Menu ............................................................................................................ 9-1
9.1.3 Adding Comments ........................................................................................................ 9-2
9.1.4 Moving Comments ........................................................................................................ 9-4
v
Page 8

9.1.5 Modifying (Editing) Comments ..................................................................................... 9-4
9.1.6 Deleting Comments ...................................................................................................... 9-4
9.2 Body Marks (Pictograms) ..................................................................................................... 9-5
9.2.1 Soft Menu for Body Marks ............................................................................................ 9-5
9.2.2 Adding Body Marks ...................................................................................................... 9-5
9.2.3 Moving Body Marks ...................................................................................................... 9-6
9.2.4 Deleting Body Marks .................................................................................................... 9-6
10 Patient Data Management ...................................................................................... 10-1
10.1 Patient Information Management ....................................................................................... 10-1
10.1.1 Enter Patient Information ............................................................................................ 10-1
10.1.2 Patient Information Setting ......................................................................................... 10-1
10.2 Image File Management .................................................................................................... 10-2
10.2.1 Memory Media ............................................................................................................ 10-2
10.2.2 Image File Formats ..................................................................................................... 10-2
10.2.3 Image Storage Preset ................................................................................................. 10-2
10.2.4 Saving Images to the System ..................................................................................... 10-3
10.2.5 Quickly Saving Images to USB Flash Drive ............................................................... 10-4
10.2.6 Quickly Saving Full Screen Image to the System ...................................................... 10-4
10.2.7 Thumbnails ................................................................................................................. 10-4
10.2.8 Image Review and Analysis ........................................................................................ 10-5
10.2.9 iVision ......................................................................................................................... 10-7
10.2.10 Sending Image File ..................................................................................................... 10-8
10.3 Report Management .......................................................................................................... 10-9
10.4 Patient Data Management (iStation) ................................................................................ 10-10
10.4.1 Viewing Patient Information ...................................................................................... 10-11
10.4.2 Searching a Patient .................................................................................................. 10-12
10.4.3 Patient Data Management ........................................................................................ 10-12
10.4.4 Examinations ............................................................................................................ 10-13
10.5 Network Storage ............................................................................................................... 10-13
10.6 Print Job Management ..................................................................................................... 10-14
10.7 Backing Up and Erasing Files through DVD Drive ........................................................... 10-14
10.8 Patient Task Management ................................................................................................ 10-16
10.9 Administration ................................................................................................................... 10-17
10.9.1 Access Setting .......................................................................................................... 10-17
10.9.2 Setting Access Control ............................................................................................. 10-17
10.9.3 System Login ............................................................................................................ 10-17
10.9.4 Add/ Delete a User ................................................................................................... 10-18
10.9.5 Modify Password ...................................................................................................... 10-20
11 DICOM ...................................................................................................................... 11-1
vi
Page 9

11.1 DICOM Preset .................................................................................................................... 11-1
11.1.1 Local TCP/IP Setting .................................................................................................. 11-1
11.1.2 DICOM Local Setting .................................................................................................. 11-3
11.1.3 DICOM Server Setting ................................................................................................ 11-4
11.1.4 DICOM Service Setting .............................................................................................. 11-5
11.2 Verify Connectivity ............................................................................................................ 11-16
11.3 DICOM Service ................................................................................................................ 11-16
11.3.1 DICOM Storage ........................................................................................................ 11-16
11.3.2 DICOM Print ............................................................................................................. 11-18
11.3.3 DICOM Worklist ........................................................................................................ 11-19
11.3.4 MPPS ........................................................................................................................ 11-20
11.3.5 Storage Commitment ................................................................................................ 11-20
11.3.6 Query/ Retrieve......................................................................................................... 11-21
11.4 DICOM Media Storage ..................................................................................................... 11-22
11.5 Structured Report (SR) ..................................................................................................... 11-24
11.6 Showcase Recording ....................................................................................................... 11-24
11.7 DICOM Task Management ............................................................................................... 11-24
12 Probes and Biopsy ................................................................................................. 12-1
12.1 Probe .................................................................................................................................. 12-1
12.1.1 Name and Function of Each Part of the Probe ........................................................... 12-4
12.1.2 Orientation of the Ultrasound Image and the Probe Head ......................................... 12-5
12.1.3 Procedures for Operating ........................................................................................... 12-6
12.1.4 Wearing the Probe Sheath ......................................................................................... 12-8
12.1.5 Probes Cleaning and Disinfection .............................................................................. 12-9
12.1.6 Storage and Transportation ...................................................................................... 12-13
12.2 Biopsy Guide .................................................................................................................... 12-14
12.2.1 Basic Procedures for Biopsy Guiding ....................................................................... 12-16
12.2.2 Needle-guided Brackets ........................................................................................... 12-17
12.2.3 Biopsy Preset ............................................................................................................ 12-25
12.2.4 Needle-guided Bracket Inspection and Installation .................................................. 12-26
12.2.5 Biopsy Menu ............................................................................................................. 12-32
12.2.6 iNeedle ...................................................................................................................... 12-33
12.2.7 Verifying the Biopsy Guide Line ................................................................................ 12-35
12.2.8 Removing the Needle-guided Bracket ...................................................................... 12-36
12.2.9 Clean and Sterilize the Needle-guided Bracket ........................................................ 12-39
12.2.10 Storage and Transportation ...................................................................................... 12-40
12.2.11 Disposal .................................................................................................................... 12-40
12.3 Middle Line ....................................................................................................................... 12-41
13 Recording ................................................................................................................ 13-1
vii
Page 10

13.1 DVR .................................................................................................................................... 13-1
13.1.1 DVR Recording ........................................................................................................... 13-1
13.1.2 DVR Video Replay ...................................................................................................... 13-1
14 Setup........................................................................................................................ 14-1
14.1 System Preset .................................................................................................................... 14-2
14.1.1 Region ........................................................................................................................ 14-3
14.1.2 General ....................................................................................................................... 14-3
14.1.3 Image Preset .............................................................................................................. 14-5
14.1.4 Meas ........................................................................................................................... 14-5
14.1.5 OB ............................................................................................................................... 14-5
14.1.6 Comment .................................................................................................................... 14-5
14.1.7 Key Config .................................................................................................................. 14-6
14.1.8 Biopsy ......................................................................................................................... 14-9
14.1.9 Option ....................................................................................................................... 14-10
14.1.10 Admin ........................................................................................................................ 14-10
14.2 Exam Preset ..................................................................................................................... 14-10
14.2.1 Exam Selection ......................................................................................................... 14-11
14.2.2 Exam Configuration .................................................................................................. 14-11
14.2.3 User-defined Exam Modes ....................................................................................... 14-12
14.3 Image Preset .................................................................................................................... 14-13
14.4 Measure Preset ................................................................................................................ 14-13
14.5 Body Mark Preset ............................................................................................................. 14-13
14.5.1 Preset Body Mark for Exam Mode............................................................................ 14-13
14.5.2 User-defined Body Marks ......................................................................................... 14-14
14.5.3 Body Mark Softkey Preset ........................................................................................ 14-17
14.6 Comment Preset .............................................................................................................. 14-17
14.6.1 Custom Comments ................................................................................................... 14-18
14.6.2 Comment Softkey Preset .......................................................................................... 14-18
14.7 Peripheral Preset.............................................................................................................. 14-19
14.8 Network Preset ................................................................................................................. 14-20
14.9 Manage Settings .............................................................................................................. 14-21
14.9.1 Exporting Setup Data................................................................................................ 14-21
14.9.2 Importing Setup Data ................................................................................................ 14-21
14.10 Maintenance ..................................................................................................................... 14-22
14.11 System Information .......................................................................................................... 14-22
15 Batteries .................................................................................................................. 15-1
15.1 Overview ............................................................................................................................ 15-1
15.2 Precautions ........................................................................................................................ 15-2
15.3 Installing and Removing the Batteries ................................................................................ 15-2
viii
Page 11

15.4 Battery Status Indicator ...................................................................................................... 15-3
15.5 One Full Discharge /Charge Cycle..................................................................................... 15-3
15.6 Checking Battery Performance .......................................................................................... 15-4
15.7 Battery Disposal ................................................................................................................. 15-4
16 Acoustic Output ...................................................................................................... 16-1
16.1 Concerns with Bioeffects .................................................................................................... 16-1
16.2 Prudent Use Statement ...................................................................................................... 16-1
16.3 ALARA Principle (As Low As Reasonably Achievable) ...................................................... 16-1
16.4 MI/TI Explanation ............................................................................................................... 16-2
16.4.1 Basic Knowledge of MI and TI .................................................................................... 16-2
16.4.2 MI/TI Display ............................................................................................................... 16-3
16.5 Acoustic Power Setting ...................................................................................................... 16-3
16.6 Acoustic Power Control ...................................................................................................... 16-4
16.7 Acoustic Output .................................................................................................................. 16-5
16.7.1 Derated Ultrasonic Output Parameters ...................................................................... 16-5
16.7.2 Limits of Acoustic Output ............................................................................................ 16-5
16.7.3 Differences between Actual and Displayed MI and TI ................................................ 16-5
16.8 Measurement Uncertainty .................................................................................................. 16-6
16.9 References for Acoustic Power and Safety ........................................................................ 16-6
17 EMC Guidance and Manufacturer’s Declaration ................................................... 17-1
18 System Maintenance .............................................................................................. 18-1
18.1 Daily Maintenance .............................................................................................................. 18-1
18.1.1 Cleaning the System .................................................................................................. 18-1
18.1.2 Checking the Probe .................................................................................................... 18-5
18.1.3 Checking the Power Cable and Plug .......................................................................... 18-5
18.1.4 Checking Appearance ................................................................................................ 18-5
18.1.5 Backup of the System Hard Drive .............................................................................. 18-5
18.2 Troubleshooting .................................................................................................................. 18-5
Appendix A
Appendix B
Appendix C
Appendix D
Appendix E
Wireless LAN ........................................................................................A-1
To Use the Lock ....................................................................................B-1
Barcode Reader ....................................................................................C-1
iScanHelper ...........................................................................................D-1
Electrical Safety Inspection ................................................................. E-1
ix
Page 12

Page 13

©2017 Shenzhen Mindray Bio-Medical Electronics Co., Ltd. All rights Reserved.
For this Operator’s Manual, the issue date is 2017-11.
The MindrayVNC ver. 1.0 contained in this product is revised by MINDRAY on Aug, 2009,
based on the UltraVNC ver. 1.0.5.5, and it complies with GNU General Public License.
SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD. owns the intellectual
property rights to the revised part. Please contact Ultrasound1.rd@mindray.com.cn for
MindrayVNC ver. 1.0.
Intellectual Property Statement
SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD. (hereinafter called Mindray)
owns the intellectual property rights to this Mindray product and this manual. This manual
may refer to information protected by copyright or patents and does not convey any license
under the patent rights or copyright of Mindray, or of others.
Mindray intends to maintain the contents of this manual as confidential information.
Disclosure of the information in this manual in any manner whatsoever without the written
permission of Mindray is strictly forbidden.
Release, amendment, reproduction, distribution, rental, adaptation, translation or any other
derivative work of this manual in any manner whatsoever without the written permission of
Mindray is strictly forbidden.
, and are the trademarks, registered or otherwise, of
Mindray in China and other countries. All other trademarks that appear in this manual are
used only for informational or editorial purposes. They are the property of their respective
owners.
This posting serves as notice under 35 U.S.C. §287(a) for Mindray patents:
http://www.mindrayna.com/patents
I
Page 14
Responsibility on the Manufacturer Party
Contents of this manual are subject to change without prior notice.
All information contained in this manual is believed to be correct. Mindray shall not be liable
for errors contained herein or for incidental or consequential damages in connection with the
furnishing, performance, or use of this manual.
Mindray is responsible for the effects on safety, reliability and performance of this product,
only if:
all installation operations, expansions, changes, modifications and repairs of this
product are conducted by Mindray authorized personnel;
the electrical installation of the relevant room complies with the applicable national
and local requirements; and
the product is used in accordance with the instructions for use.
MindrayVNC ver. 1.0 is free open source software, the performance of MindrayVNC
ver. 1.0 is not guaranteed by MINDRAY.
Note
This equipment must be operated by skilled/trained clinical professionals.
Warning
It is important for the hospital or organization that employs this equipment to carry out a
reasonable service/maintenance plan. Neglect of this may result in machine breakdown or
personal injury.
Warranty
THIS WARRANTY IS EXCLUSIVE AND IS IN LIEU OF ALL OTHER WARRANTIES,
EXPRESSED OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY OR
FITNESS FOR ANY PARTICULAR PURPOSE.
Exemptions
Mindray's obligation or liability under this warranty does not include any transportation or
other charges or liability for direct, indirect or consequential damages or delay resulting from
the improper use or application of the product or the use of parts or accessories not approved
by Mindray or repairs by people other than Mindray authorized personnel.
This warranty shall not extend to:
Malfunction or damage caused by improper use or man-made failure.
Malfunction or damage caused by unstable or out-of-range power input.
Malfunction or damage caused by force majeure such as fire and earthquake.
Malfunction or damage caused by improper operation or repair by unqualified or
unauthorized service people.
Malfunction of the instrument or part whose serial number is not legible enough.
Others not caused by instrument or part itself.
II
Page 15

Customer Service Department
Manufacturer: Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Address: Mindray Building,Keji 12th Road South,High-tech industrial
park,Nanshan,Shenzhen 518057,P.R.China
Website:
E-mail Address: service@mindray.com
Tel:
Fax:
Manufacturer: Mindray DS USA, Inc.
Address: 800 MacArthur Blvd.
Tel: +1(201) 995-8000
Toll Free: +1 (800) 288-2121
Fax: +1 (800) 926-4275
www.mindray.com
+86 755 81888998
+86 755 26582680
Mahwah, NJ 07430-0619 USA
III
Page 16

Important Information
1. It is the customer’s responsibility to maintain and manage the system after delivery.
2. The warranty does not cover the following items, even during the warranty period:
(1) Damage or loss due to misuse or abuse.
(2) Damage or loss caused by Acts of God such as fires, earthquakes, floods, lightning,
etc.
(3) Damage or loss caused by failure to meet the specified conditions for this system,
such as inadequate power supply, improper installation or environmental conditions.
(4) Damage or loss due to use of the system outside the region where the system was
originally sold.
(5) Damage or loss involving the system purchased from a source other than Mindray or
its authorized agents.
3. This system shall not be used by persons other than fully qualified and certified medical
personnel.
4. Do not make changes or modifications to the software or hardware of this system.
5. In no event shall Mindray be liable for problems, damage, or loss caused by relocation,
modification, or repair performed by personnel other than those designated by Mindray.
6. The purpose of this system is to provide physicians with data for clinical diagnosis. It is
the physician’s responsibility for diagnostic procedures. Mindray shall not be liable for the
results of diagnostic procedures.
7. Important data must be backed up on external memory media.
8. Mindray shall not be liable for loss of data stored in the memory of this system caused by
operator error or accidents.
9. This manual contains warnings regarding foreseeable potential dangers, but you shall
always be alert to dangers other than those indicated as well. Mindray shall not be liable
for damage or loss that results from negligence or from ignoring the precautions and
operating instructions described in this operator’s manual.
10. If the manager for this system is changed, be sure to hand over this operator’s manual to
the new manager.
About This Manual
This operator’s manual describes the operating procedures for this diagnostic ultrasound
system M6/M6T/M6 Exp/M6s/M6 Pro/M55/M58/M5 Exp and the compatible probes. To
ensure safe and correct operations, carefully read and understand the manual before
operating the system.
IV
Page 17
Notation Conventions
In this operator’s manual, the following words are used besides the safety precautions (refer
to "Safety Precautions"). Please read this operator’s manual before using the system.
CAUTION:
U.S.A. Federal Law restricts this device to sale by or on the
The diagnostic ultrasound system is not intended for
ophthalmic use. Its use in this clinical specialty is
contraindicated.
order of a physician.
Operator’s Manuals
Please read the operator’s manuals carefully before operating the system.
You may receive multi-language manuals in compact disc or paper. Please refer to English
manual for latest information and register information.
The content of the operator manual, such as screens, menus or descriptions, may be different
from what you see in your system. The content varies depending upon the software version,
options and configuration of the system.
Hardcopy Manuals
Operator’s Manual [Basic Volume]: Describes the basic functions and operations of
the system, safety precautions, exam modes, imaging modes, preset, maintenance
and acoustic output, etc.
Operator’s Manual [Advanced Volume]: Describes measurement preset,
measurements and calculations, etc.
Operator’s Manual [Acoustic Power Data and Surface Temperature Data]: Contains
data tables of acoustic output for transducers.
Operation Note: Contains quick guide for basic operations of the system.
NOTE: 1. The manuals in CD are the manuals translated into languages other than
English according to English manuals.
2. When you find that the contents of the manuals in CD are NOT consistent with
the system or English manuals, please ONLY refer to the corresponding
English manuals.
3. The accompanying manuals may vary depending upon the specific system you
purchased. Please refer to the packing list.
V
Page 18

Software Interfaces in this Manual
Depending on the software version, preset settings and optional configuration, the actual
interfaces may be different from those in this manual.
Conventions
In this manual, these conventions are used to describe the buttons on the control panel, the
items in menu, buttons in dialog box and some basic operations:
<Buttons>: The angular bracket indicates buttons, knobs and other controls on
control panel.
[Items in menu and buttons in dialog box]: The square bracket indicates items in
menu or the soft menu, or buttons in dialog box.
Click [Items or Button]: Move the cursor to the item or button and press <Set>, or
click it on the soft menu.
[Items in Menu][Items in Submenu]: Selects a submenu item following the path.
[Dyn Rng (Value)]: Indicates menu items with parameter, (value) shows the current
value of the item.
VI
Page 19

1
Safety Precautions
1.1 Safety Classification
According to the type of protection against electric shock:
CLASS I EQUIPMENT
According to the degree of protection against electric shock:
Type-BF applied part
According to the degree of protection against harmful ingress of water:
The main unit belongs to IPX0, and the probes belong to IPX7
Footswitch: 971 SWNOM belongs to IP68
Footswitch: SP-997-350 (3-pedal) belongs to IPX8
According to the degree of safety of application in the presence of a FLAMMABLE
ANESTHETIC MIXTURE WITH AIR or WITH OXYGEN OR NITROUS OXIDE:
EQUIPMENT not suitable for use in the presence of a FLAMMABLE ANESTHETIC
MIXTURE WITH AIR or WITH OXYGEN OR NITROUS OXIDE
According to the mode of operation:
CONTINUOUS OPERATION
According to the installation and use:
PORTABLE EQUIPMENT
MOBILE EQUIPMENT (when the system is installed on the mobile trolley)
Safety Precautions 1-1
Page 20
1.2 Meaning of Signal Words
In this manual, the signal words"
“
CAUTION
instructions. The signal words and their meanings are defined as follows. Please
understand their meanings clearly before reading this manual.
Signal word Meaning
DANGER
WARNING
CAUTION
NOTE
Tips
”, “NOTE” and "Tips" are used regarding safety and other important
Indicates an imminently hazardous situation that, if not avoided, will
result in death or serious injury.
Indicates a potentially hazardous situation that, if not avoided, could
result in death or serious injury.
Indicates a potentially hazardous situation that, if not avoided, may
result in minor or moderate injury.
Indicates a potentially hazardous situation that, if not avoided, may
result in property damage.
Important information that helps you to operate the system more
effectively.
DANGER
”, “
WARNING
”,
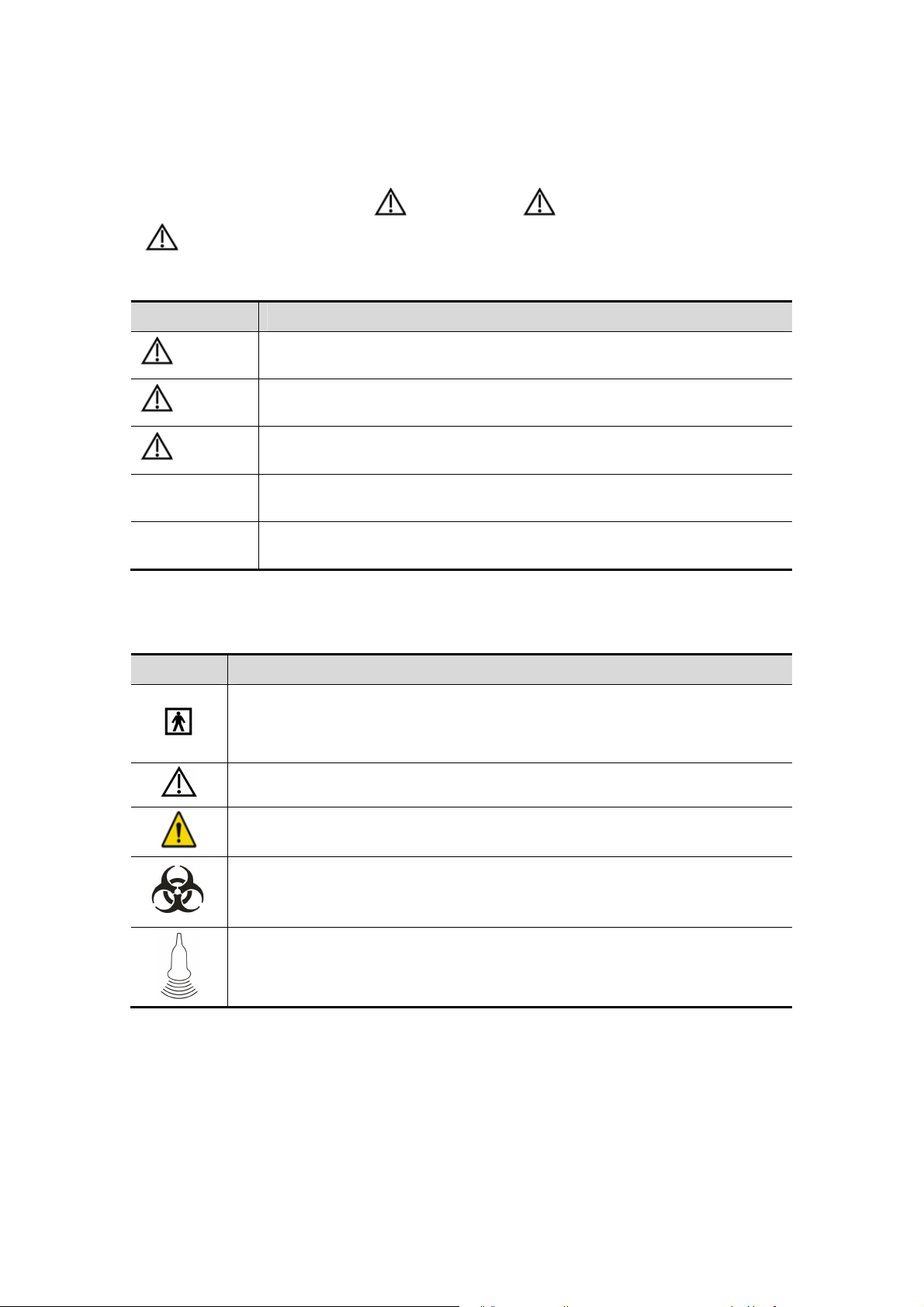
1.3 Meaning of Safety Symbols
Symbol Description
Type-BF applied part.
The ultrasound probes connected to this system are type-BF applied parts.
The ECG module connected to this system is Type-BF applied part.
Caution!
General warning sign.
Patient/user infection due to contaminated equipment. Be careful when
performing the cleaning, disinfection and sterilization.
Patient injury or tissue damage from ultrasound radiation. It is required to
practice ALARA when operating ultrasound system.
1-2 Safety Precautions
Page 21
1.4 Safety Precautions
Please observe the following precautions to ensure patient and operator’s safety when
using this system.
DANGER:
WARNING:
DO NOT use flammable gasses, such as anesthetic gas or
hydrogen, or flammable liquids such as ethanol, near this
system, because there is danger of explosion.
1. Do connect the adapter power plug of this system and
power plugs of the peripherals to wall receptacles that
meet the ratings indicated on the rating nameplate.
Using a multifunctional receptacle may affect the
system protective grounding performance, and
cause the leakage current to exceed safety
requirements.
Use the cable provided with this system to connect the
printer. Other cables may result in electric shock.
You must use the power adapter provided with the
system; otherwise electric shock may result.
You can only adopt the power supply method provided
by Mindray, other power supply modes (e.g. using a
UPS) may result in electric shock.
2. Connect the protective grounding conductor before
turning ON the system. Disconnect the grounding cable
after turning OFF the system. Otherwise, electric shock
may result.
3. For the connection of power and grounding, follow the
appropriate procedures described in this operator’s
manual. Otherwise, there is risk of electric shock. DO
NOT connect the grounding cable to a gas pipe or
water pipe; otherwise, improper protective grounding
may result or a gas explosion may occur.
4. Before cleaning the system, disconnect the power cord
from the outlet. System failure and electric shock may
result.
5. This system is not water-proof designed. DO NOT use
this system in any place where water or any liquid
leakage may occur. If any water is sprayed on or into
the system, electric shock or device malfunction may
result. If water is accidentally sprayed on or into the
system, power off the system immediately and contact
Mindray Customer Service Department or sales
representative.
Safety Precautions 1-3
Page 22

6. DO NOT use a probe that has a damaged, scratched
surface, or exposed wiring of any kind. Immediately
stop using the probe and contact Mindray Customer
Service Department or sales representative. There is
risk of electric shock if a damaged or scratched probe
is used.
7. DO NOT allow the patient to contact the live parts of the
ultrasound system or other devices, e.g. signal I/O
ports. Electric shock may occur.
8. Do not use an aftermarket probe other than those
specified by Mindray. The probes may damage the
system, causing a profound failure, e.g. a fire in the
worst case.
9. Do not subject the probes to knocks or drops. Use of a
defective probe may cause an electric shock.
10. Do not open the covers and front panel of the system.
Short circuit or electric shock may result when the
system hardware is exposed and powered on.
11. DO NOT use this system simultaneously with
equipment such as an electrosurgical unit,
high-frequency therapy equipment, or a defibrillator,
etc. Otherwise, there is a risk of electric shock to the
patient.
12. Only use the ECG leads provided with the ECG module;
otherwise electric shock may result.
13. When moving the system, you should first fold the LCD
display, disconnect the system from other devices
(including probes) and disconnect the system from the
power supply.
14. Accessory equipment connected to the analog and
digital interfaces must comply with the relevant IEC
standards (e.g., IEC 60950 information technology
equipment safety standard and IEC 60601-1 medical
equipment standard). Furthermore, all configurations
must comply with the standard IEC 60601-1-1. It is the
responsibility of the person, who connects additional
equipment to the signal input or output ports and
configures a medical system, to verify that the system
complies with the requirements of IEC 60601-1-1. If you
have any questions regarding these requirements,
consult your sales representative.
15. Prolonged and repeated use of keyboards may result in
hand or arm nerve disorders for some individuals.
Observe the local safety or health regulations
concerning the use of keyboards.
16 The operator SHOULD NOT touch SIP/SOP and the
patient at the same time.
17 If the battery cannot be automatically maintained in a
fully usable condition, please conduct periodic
checking of the battery.
1-4 Safety Precautions
Page 23
18 The ultrasound system use a mains plug as isolation
means to the mains power supply. Please do not set the
ultrasound system in a place difficult to operate the
mains plug.
CAUTION:
19 Do not modify this equipment without authorization of
the manufacture.
1. Precautions concerning clinical examination
techniques:
This system must be used only by qualified medical
professionals.
This operator’s manual does not describe clinical
examination techniques. The clinician should select the
proper examination techniques based on specialized
training and clinical experience.
2. Malfunctions due to radio wave:
If a radio wave emitting device is used in the
proximity of this system, it may interfere with
operations. DO NOT use or take any devices
transmitting RF signals (such as cellular
phones, transceivers and radio controlled
products) in the room placing the system.
If a person brings a device that generates radio
waves near the system, ask him / her to
immediately turn OFF the device.
3. Precautions concerning movement of the system:
When you place the system on the mobile trolley
and move them together, you must secure all
objects on the mobile trolley to prevent them
from falling. Otherwise you should separate the
system from the mobile trolley and move them
individually.
When you have to move the system with the
mobile trolley upward or downward the stairs,
you must separate them first and then move
them individually.
Object placed on the monitor may fall and injure
an individual when moving.
Confirm that there is no peripheral device
connected to the system before moving the
system. Otherwise, peripheral device may fall
and injure an individual.
4. DO NOT expose the system to excessive vibration
through transportation. Mechanical damage may result.
Safety Precautions 1-5
Page 24

5. Do not connect this system to outlets with the same
circuit breakers and fuses that control the current of
devices such as life-support systems. If this system
malfunctions and generates over current, or when there
is an instantaneous current at power ON, the circuit
breakers and fuses of the building’s supply circuit may
be tripped.
6. Always keep the system dry. Avoid transporting this
system quickly from a cold place to a warm place;
otherwise condensation or water droplets may form
allowing a short circuit and possible electric shock.
7. If the circuit protector is tripped, it indicates that the
system or a peripheral device was improperly shut down
and the system is unstable. You cannot repair the
system under this circumstance and must call the
Mindray Customer Service Department or sales
representative.
8. There is no risk of high-temperature burns during
normal ultrasound examinations. It is possible for the
surface temperature of the probe to exceed the body
temperature of a patient due to environmental
temperature and exam mode combinations. Apply the
probe only for a period of time required for the purpose
of diagnosis.
9. The system and its accessories are not disinfected or
sterilized prior to delivery. The operator is responsible
for the cleaning and disinfection of probes and
sterilization of biopsy brackets according to the
manuals, prior to the use.
All items must be thoroughly processed to completely
remove harmful residual chemicals, which will not only
harmful to the human body, but also damage the
accessory.
1-6 Safety Precautions
10. It is necessary to press [End Exam] to end the current
scan that is in progress and clear the current Patient
Information field. Otherwise, new patient data may be
combined with the previous patient data.
11. DO NOT connect or disconnect the system’s power cord
or its accessories (e.g., a printer or a recorder) without
turning OFF the power first. This may damage the
system and its accessories or cause electric shock.
12. If the system is powered off improperly during
operation, it may result in data damage of the system’s
hard disk or system failure.
13. Do not use the system to examine a fetus for a long
period of time.
14. Do not use a USB memory device (e.g., a USB flash
drive, removable hard disk) which has unsafe data.
Otherwise, system damage may result.
Page 25
15. It is recommended to only use the video devices
specified in this manual.
NOTE: 1. DO NOT use the system in the vicinity of strong electromagnetic field (such
as a transformer), which may affect the performance of the system.
2. DO NOT use the system in the vicinity of high-frequency radiation source,
which may affect the performance of the system or even lead to failure.
3. To avoid damaging the system, DO NOT use it in following environment:
(1) Locations exposed to direct sunlight.
(2) Locations subject to sudden changes in environmental temperature.
(3) Dusty locations.
(4) Locations subject to vibration.
(5) Locations near heat generators.
(6) Locations with high humidity.
16. Do not use gel, disinfectant, probes, probe sheath or
needle-guided brackets that are not compatible with the
system.
17. Read the Acoustic Output Principle in the operation
manual carefully before operate this system on clinical
examination.
18. Please use the ultrasound gel compliant with the
relevant local regulations.
4. Turn ON the system only after the power has been turned OFF for a while.
If the system is turned ON immediately after being turned OFF, the system
may not be rebooted properly and could malfunction.
5. When using or placing the system, keep the system horizontal to avoid
imbalance.
6. Remove ultrasound gel from the face of a probe when the examination is
complete. Water in the gel may enter the acoustic lens and adversely affect
the performance and safety of the probe.
7. You should properly back up the system to a secure external storage
media, including system configuration, settings and patient data. Data
stored to the system’s hard drive may be lost due to system failure,
improper operation or accident.
8. Do not apply external force to the control panel; otherwise, the system may
be damaged.
9. If the system is used in a small room, the room temperature may rise.
Please provide proper ventilation and free air exchange.
10. To dispose of the system or any part, contact Mindray Customer Service
Department or sales representative. Mindray is not responsible for any
system content or accessories that have been discarded improperly.
11. Electrical and mechanical performance may be degraded due to long
usage (such as current leakage or distortion and abrasion); the image
sensitivity and precision may become worse too. To ensure optimal system
operations, it is recommended that you maintain the system under a
Mindray service agreement.
Safety Precautions 1-7
Page 26
12.
13. Ensure that the current exam date and time are the same as the system
14. DO NOT turn OFF the power supply of the system during printing, file
Please read the following precautions carefully to ensure the safety of the patient and the
operator when using the probes.
WARNING:
The iScape feature constructs a single extended image from a series of
individual image frames. The quality of the final image is user-dependent
and requires skill to efficiently apply the feature and technique. Exercise
caution when measurements are performed from an iScape image.
date and time.
storage or invoking other system operations. An interrupted process may
not be completed, and can become lost or corrupted.
The ultrasonic probe is only for use with the specified
1.
ultrasonic diagnostic system. Please refer to the 2.5
System Configuration to select the proper probe.
Confirm that the probe and cable are normal before and
2.
after each examination. A defective probe may cause
electric shock to the patient.
Do not subject the probe to shock. A defective probe may
3.
cause electric shock to the patient.
CAUTION:
Do not disassemble the probe to avoid the possibility of
4.
electric shock.
Never immerse the probe connector into liquids such as
water or disinfectant because the connector is not
5.
waterproof. Immersion may cause electric shock or
malfunction.
A probe sheath must be installed over the probe before
6.
performing intra-cavity or intra-operative examination.
When using the probe, wear sterile gloves to prevent
1.
infection.
Be sure to use sterile ultrasound gel. Please use the
ultrasound gel compliant with the relevant local
2.
regulations. And manage the ultrasound gel properly to
ensure that it does not become a source of infection.
In normal diagnostic ultrasound mode, there is no
danger of a normal-temperature burn; however, keeping
3.
the probe on the same region of the patient for a long
time may cause such a burn.
Do not use the carrying case for storing the probe. If the
carrying case is used for storage, it may become a
4.
source of infection.
It is required to practice ALARA when operating
5.
1-8 Safety Precautions
ultrasound system. Minimize the acoustic power without
compromising the quality of images.
Page 27
The probe and accessories supplied with it are not
6.
delivered disinfected or sterilized. Sterilization (or
high-level disinfect) before use is required.
Disposable components (for example the probe sheath,
the sterile gloves) are packaged sterile and are
single-use only. Do not use if integrity of packaging
7.
violated or if expiration date has passed. Please use the
disposable components compliant with the relevant
local regulations.
Please use the disinfection or sterilization solution that
recommended in this operator’s manual; otherwise
Mindray will not be liable for damage caused by other
8.
solutions. If you have any questions, please contact
Mindray Customer Service Department or sales
representative.
The probe sheath contains natural rubber that can cause
9.
allergic reactions in some individuals.
Do not use pre-lubricated condoms as a sheath.
10.
Lubricant may not be compatible with the transducer
material and damage may result.
Transducer damage may be caused by inappropriate gel,
detergent or cleanser:
Do not soak or saturate transducers with solutions
containing alcohol, bleach, ammonium chloride
11.
compounds, acetone or formaldehyde.
Avoid contact with solutions or coupling gels containing
mineral oil or lanolin.
Safety Precautions 1-9
Page 28

NOTE:
1. Read the following precautions to prevent the probe from malfunction:
Clean and disinfect the probe before and after each examination.
After the examination, wipe off the ultrasound gel thoroughly.
Otherwise, the ultrasound gel may solidify and the image quality
would be degraded.
2. Ambient conditions:
To prevent the probe from being damaged, do not use it where it will be
exposed to:
Direct sunlight or X-rays
Sudden changes in temperature
Dust
Excessive vibration
Heat generators
Use the probes under the following ambient conditions:
ambient temperature: 0℃~ 40℃
relative humidity:30% ~ 85% (no condensation)
atmospheric pressure:700 hPa ~ 1060 hPa.
Use the L16-4Hs and D7-2s probe under the following ambient conditions:
ambient temperature: 10°C ~ 40°C
relative humidity: 30% ~ 85% (no condensation)
atmospheric pressure: 700 hPa ~ 1060 hPa
Use the DE11-3s probe under the following ambient conditions:
ambient temperature: 18°C ~ 30°C
relative humidity: 20% ~ 85% (no condensation)
atmospheric pressure: 700 hPa ~ 1060 hPa
Use the C6-2Gs probe under the following ambient conditions:
ambient temperature: 0°C ~ 40°C
relative humidity: 20% ~ 85% (no condensation)
atmospheric pressure: 700 hPa ~ 1060 hPa
3. Repeated disinfection will eventually damage the probe, please check the
probe's performance periodically.
1-10 Safety Precautions
Page 29
1.5 Latex Alert
When choosing a probe sheath, it is recommended that you directly contact CIVCO for
obtaining probe sheath, pricing information, samples and local distribution information.
For CIVCO information, please contact the following:
CIVCO Medical Instruments Tel: 1-800-445-6741
WWW.civco.com
WARNING:
Allergic reactions in latex (natural rubber) sensitive patients
may range from mild skin reactions (irritation) to fatal
anaphylactic shock, and may include difficulty in breathing
(wheezing), dizziness, shock, swelling of the face, hives,
sneezing or itching of the eyes (FDA Medical Alert on latex
products, “Allergic Reactions to Latex-containing Medical
Devices”, issued on March 29, 1991).
1.6 Warning Labels
The warning labels are attached to this system in order to call your attention to potential
hazards. The symbol on the warning labels indicates safety precautions.
The warning labels use the same signal words as those used in the operator’s manual.
Read operator’s manual carefully before using the system.
The name, pattern and meaning of each warning label are described as follows:
No. Warning Labels Meaning
1
Read this information carefully before using
the system.
The following labels are available
when the system works with the
mobile trolley.
2
a. Do not place the system on a sloped
surface. Otherwise the system may slide,
resulting in personal injury or the system
malfunction. Two persons are required to
move the system over a sloped surface.
b. Do not sit on the system.
c. DO NOT push the system when the
casters are locked.
Safety Precautions 1-11
Page 30

No. Warning Labels Meaning
CONFORMS TO AAMI STD 60601-1, IEC
3
STD 60601-2-37,IEC STD 60601-2-18;
CERTIFIED TO CSA STD C22.2 NO.
60601-1, 60601-2-37, 60601-2-18
1-12 Safety Precautions
Page 31

2
System Overview
2.1 Intended Use
The M6/M6T/M6 Exp/M6s/M6 Pro/M5 Exp/M55/M58 Diagnostic Ultrasound System is
applicable for adults, pregnant women, pediatric patients and neonates. It is intended for
use in fetal, abdominal, Intra-operative, pediatric, small organ (breast, thyroid, testes),
neonatal cephalic, adult cephalic, trans-rectal, trans-vaginal, musculo-skeletal
(conventional), musculo-skeletal (superficial), cardiac adult, cardiac pediatric,
Trans-esoph. (Cardiac), peripheral vessel and urology exams
2.2 Contraindication
None.
2.3 Product and Model Code
M □
Model code;
Product code.
Product Differences
Product
Model
Feature
B-Profile
B-Hist
Color Velocity
NOTE: The functions described in the operator’s manual may vary depending upon the
specific system you purchased.
M6 M6T M6 Exp M6s M6 Pro M55 M58 M5 Exp
√ × √ × × √ × √
√ √ √ × × × √ ×
√ √ × √ × × × √
System Overview 2-1
Page 32

2.4 Product Specifications
2.4.1 Imaging Modes
B Mode B
M Mode M
Anatomical M: Free Xros M, Free Xros CM
Color M Mode (CM)
C Mode Color
D Mode PW Doppler
CW Doppler
Special Imaging Smart 3D
Static 3D
4D
iScape
TDI
Color M
Stress Echo
Contrast Imaging
Elastography
Power(Dirpower)
2.4.2 Power supply
Voltage
100-240V~ (AC adapter)
220-240V~, 50/60Hz (when configured with mobile trolley UMT-300)
Frequency 50/60Hz (AC adapter)
Input Power 1.5 – 0.6A (AC adapter)
600 VA (when configured with mobile trolley UMT-300)
Fuse 250V~ T3.15AL
NOTE:
2-2 System Overview
The CE region applied voltage is 220-240V~.
Page 33

2.4.3 Environmental Conditions
Operating conditions Storage and transportation conditions
Ambient
temperature
Relative
humidity
Atmospheric
pressure
WARNING:
0℃~40℃ -20℃~55℃
30%~85% (no condensation) 30%~95% (no condensation)
700hPa~1060hPa 700hPa~1060hPa
Do not use this system in the conditions other than those
specified.
2.4.4 External Dimensions and Weight
External dimensions: 361mm(H)×357mm(L)×75mm(W)
Net weight: 6.5Kg (including batteries and 4D board, no power adapter)
2.5 System Configuration
2.5.1 Standard Configuration
Main unit
Accessories
Operator's manuals
Ultrasound gel
Power adapter and connecting cable
Battery Pack
Multilingual controls overlay
System Overview 2-3
Page 34

2.5.2 Options
2.5.2.1 Probes and Needle-guided Brackets Available
Probe
model
C5-2s Convex
7L4s Linear
L7-3s Linear
L12-4s Linear
7L5s Linear
L14-6s Linear
Type Intended Use Region Applied
Fetal, abdominal, pediatric, peripheral
vessel
Abdominal, pediatric, small organ (breast,
thyroid, testes), neonatal cephalic,
musculo-skeletal (conventional,
superficial), peripheral vascular
Abdominal, pediatric, small organ (breast,
thyroid, testes), musculo-skeletal
(conventional, superficial), peripheral
vascular
Abdominal, pediatric, small organ (breast,
thyroid, testes), mulo-skeletal
(conventional and superficial), peripheral
vessel
Pediatric, small organ (breast, thyroid,
testes), musculo-skeletal (conventional,
superficial), peripheral vascular
Pediatric, small organ (breast, thyroid,
testes), neonatal cephalic,
musculo-skeletal (conventional,
superficial), peripheral vascular
Body surface
Body surface
Body surface
Body surface
Body surface
Body surface
Abdominal, pediatric, neonatal cephalic,
P4-2s Phased
P7-3s Phased
P12-4s Phased
V10-4s Transvaginal
V10-4Bs Transvaginal Transvaginal
4CD4s 4D convex Fetal, abdominal, pediatric Body surface
6C2s Convex
L14-6Ns Linear
adult cephalic, cardiac adult, cardiac
pediatric
Abdominal, pediatric, neonatal cephalic,
adult cephalic, musculo-skeletal
(conventional), cardiac adult, cardiac
pediatric
Abdominal, pediatric, neonatal cephalic,
adult cephalic, cardiac adult, cardiac
pediatric
Fetal, trans-rectal, trans-vaginal, urology
Abdomen, pediatrics, transcranial,
vascular, small parts, musculoskeletal
Pediatric, small organ (breast, thyroid,
testes), mulo-skeletal (conventional and
superficial), peripheral vessel
Body surface
Body surface
Body surface
Transvaginal
Body surface
Body surface
2-4 System Overview
Page 35

Probe
model
Type Intended Use Region Applied
CW2s Pencil probe
7LT4s Linear
P7-3Ts
CW5s Pencil probe
6LB7s Linear Trans-rectal, urology Transvaginal
3C5s Convex Fetal, abdominal, pediatric Body surface
2P2s
6CV1s Convex Fetal, trans-rectal, trans-vaginal, urology Body surface
L16-4Hs Linear
Phased
probe
Phased
probe
Pediatric, adult cephalic, adult cardiac,
pediatric cardiac
Abdominal, Intraoperative (abdominal,
thoracic, and vascular), Pediatric, Small
Organ (breast, thyroid, testes), Neonatal
Cephalic, Musculo-skeletal (Conventional
and Superficial), Peripheral Vascular
Trans-esoph.(Cardiac) Transesophageal
Pediatric, adult cephalic, cardiac adult,
cardiac pediatric, peripheral vessel
Abdominal, pediatric, neonatal cephalic,
adult cephalic, cardiac adult, cardiac
pediatric,
Abdominal, intra-operative (abdominal,
thoracic, and vascular), pediatric, small
organ (breast, thyroid, testes), neonatal
cephalic, musculo-skeletal (conventional,
superficial), peripheral vessel
Body surface
Intra-operative
Body surface
Body surface
Body surface,
Intra-operative
C6-2Gs Convex
C11-3s Convex
DE11-3s Convex Fetal, trans-rectal, trans-vaginal Transvaginal
D7-2s Convex Fetal, abdominal Body surface
Some of the probes have matched needle-guided brackets for biopsy, the available
probes and the corresponding needle-guided brackets are listed as follows:
Probe Model
V10-4s/
V10-4Bs/ 6CV1s
6C2s
3C5s
NGB-004
Metal/needle un-detachable
NGB-005 (metal/needle
un-detachable)
NGB-006
Plastic/needle detachable;
Metal/needle detachable
Fetal, abdominal, pediatric, cardiac adult,
cardiac pediatric
Abdominal, pediatric, neonatal cephalic,
cardiac pediatric, peripheral vessel
Needle-guided Bracket
Model
Biopsy
Angle/Depth
(±1°)
/ 16G, 17G, 18G
12.7°, 24.2°
25°, 35°, 45°
Body surface
Body surface
Applicable Biopsy
Needle
13G, 15G, 16G, 18G,
20G
Metal: 14G, 16G, 18G,
20G, 22G
Plastic: 13G, 15G,
16G, 18G, 20G
System Overview 2-5
Page 36

Probe Model
L7-3s/ 7L4s/
L12-4s/ 7L5s/
L14-6Ns
6LB7s
7LT4s
P4-2s/2P2s
C5-2s
L14-6s
C11-3s
C6-2Gs
DE11-3s
Needle-guided Bracket
Model
NGB-007
Plastic/needle detachable;
Metal/needle detachable
NGB-009
Metal/needle detachable
NGB-010 (metal/needle
detachable)
NGB-011
Metal/needle un-detachable
NGB-015
Metal/needle detachable
NGB-016
Metal/needle detachable
NGB-018
metal/needle detachable
NGB-024
Metal/needle detachable
NGB-027
Metal/needle un-detachable
Biopsy
Angle/Depth
(±1°)
40°, 50°, 60°
0°
30°, 40°, 50°
11°, 23°
25°, 35°, 45°
30°, 40°, 50°
15°, 25°, 35°
7°, 25°, 35°
1.7° 16G, 17G, 18G
Applicable Biopsy
Needle
Metal: 14G, 16G, 18G,
20G, 22G
Plastic: 13G, 15G,
16G, 18G, 20G
13G, 15G, 16G, 18G,
20G
13G, 15G, 16G, 18G,
20G
13G, 15G, 16G, 18G,
20G
14G, 16G, 18G, 20G,
22G
14G, 16G, 18G, 20G,
22G
14G, 16G, 18G, 20G,
22G
14G, 16G, 18G, 20G,
22G
2.5.2.2 Options
No.
1. iClear module
2. CW module (requires to be configured in factory)
3. iScape View
4. Free Xros M module
5. Free Xros CM module (Curved Free Xros M)
6. Smart 3D module
7. 4D module (requires to be configured in factory)
8. TDI (Tissue Doppler imaging) module
9. TDI Quantitative Analysis
10. IMT module (Only can be applied with the Vascular Package configured)
11. Smart OB
12. Stress Echo (only can be applied when Cardiac Package is configured)
13. Contrast Imaging
14. Strain Elastography
Item
15. iNeedle
Abdomen General Package(including relevant exam mode, comments,
16.
measurements, body marks and report)
2-6 System Overview
Page 37

No.
Obstetrics Package(including relevant exam mode, comments, measurements,
17.
body marks and report)
Gynecology Package(including relevant exam mode, comments, measurements,
18.
body marks and report)
Cardiology Package(including relevant exam mode, comments, measurements,
19.
body marks and report)
Small Parts Package(including relevant exam mode, comments, measurements,
20.
body marks and report)
Urologiy Package(including relevant exam mode, comments, measurements, body
21.
marks and report)
Vascular Package(including relevant exam mode, comments, measurements, body
22.
marks and report)
Pediatrics Package(including relevant exam mode, comments, measurements,
23.
body marks and report)
Nerve Package(including relevant exam mode, comments, measurements, body
24.
marks and report)
Emergency& Critical Package(including relevant exam mode, comments,
25.
measurements, body marks and report)
Item
DICOM Basic module (including: task management, DICOM storage, DICOM print,
26.
DICOM storage commitment, DICOM media storage (including DICOM DIR) and
etc.)
DICOM Worklist module (only can be applied with the DICOM basic function module
27.
configured)
DICOM MPPS (only can be applied with the DICOM basic function module
28.
configured)
DICOM OB/GYN structured report (only can be applied with the DICOM basic
29.
function module configured)
DICOM Vascular structured report (only can be applied with the DICOM basic
30.
function module configured)
DICOM Cardiac structured report (only can be applied with the DICOM basic
31.
function module configured)
DICOM Query/Retrieve (only can be applied with the DICOM basic function module
32.
configured)
DVD R/W Drive:
33.
Model: SE-208GB/CHBS (SUMSUNG)
34. I/O Extend Module
Probe Extend Module:
35.
PEM-21, support all optional probes except pencil probe.
36. V/A Extend Module
37. ECG module (ECG-21: only can be applied with I/O Extend Module configured)
38. ECG Lead
System Overview 2-7
Page 38

No.
Footswitch:
39.
971-SWNOM (2-pedal)
971-SWNOM (3-pedal)
Mobile Trolley(configured with a notebook combination lock):
UMT-200, UMT-300
Options for UMT-300: power module, probe extend module PEM-21, built-in DVD
40.
R/W drive, display module SMM-11; where, SMM-11 can be applied only when I/O
extend module and power module are configured, and the built-in DVD R/W driver
can be applied only when power module is configured.
41. Pack
42. Dust-proof cover
43. Wireless adapter
Item
2.5.2.3 Peripherals Supported
Item Model
Black /white video
printer
Color video printer SONY UP-D25MD
Bar code reader
WARNING:
SONY UP-D897, SONY UP-X898MD, MITSUBISHI P95DW-N,
SONY UP-D898MD
SYMBOL LS2208(1-D)
SYMBOL DS6707(2-D)
This system complies with IEC60601-1-2:2007, and its RF
emission meets the requirements of CISPR11 Class B. In a
domestic environment, the customer or the user should
guarantee to connect the system with Class B peripheral
devices; otherwise RF interference may result and the
customer or the user must take adequate measures
accordingly.
2-8 System Overview
Page 39

2.6 Introduction of Each Unit
No.
1 Monitor Displays the images and parameters during scanning.
2 Control Panel Operator-system interface or control.
3 CW pencil probe port Connects the pencil probe to the main unit
4 Handle Used for carrying the system.
5
Probe port
6 Transducer locking lever
7
I/O extend port
Power input port
8
9 USB port Connects USB devices.
Name Function
Connects a probe to the main unit; or connects a probe
extend module.
Locks or unlocks the probe connected with the main
unit.
: locked symbol
: unlocked symbol
Connects the I/O extend module.
Connects the power adapter.
10
Network port
S-Video separate video
11
output
Connects the network.
Connects DVR recorder or video printer.
System Overview 2-9
Page 40

2.7 Mobile trolley
The system can be configured with 2 models of mobile trolley: UMT-200 and UMT-300.
Mobile Trolley is used for placing the ultrasound system, extend modules and etc.
WARNING:
1. Be sure to connect the equipotential wire before
inserting the power plug into the receptacle; be sure to
remove the power plug from the receptacle before
disconnecting the equipotential wire; otherwise electric
shock may result.
2. When you connect another device to the trolley, you
should use the equipotential wire to connect each of
equipotential terminals; otherwise electric shock may
result.
3. Connect the earth cable before powering ON. Disconnect
the earth cable after powering OFF. Otherwise, electric
shock may result.
4. DO NOT connect the trolley to the outlets with the same
circuit breakers and fuses that control the current to
devices such as life-support systems. If the trolley or the
system malfunctions and generates over-current, or
when there is an instantaneous current at power ON, the
circuit breakers and fuses of the building’s supply circuit
may be tripped.
5. If UMT-300 has configured with display module, please
take care of the display module position to leave enough
space below when adjusting the display support arm, so
that the display module will not hit into the monitor of the
ultrasound system.
CAUTION:
2. Maximum output power of the outlet in the trolley is
NOTE 1. If UMT-300 has configured with display module, please take care of the
display module position in case of hurting one’s head.
2. If probe extend module is configured, please do not place any objects under
the module.
2-10 System Overview
1. When moving the trolley with mounted system, please
take care of the connector of the power adapter in case of
damage.
350VA.
Page 41

UMT-200
UMT-300
System Overview 2-11
Page 42

No. Name Function
1. Display module
2. Probe cable hook Probe cable hanger
3. Ultrasound system /
4. Probe holder Used for placing probes temporarily
5. Operating platform To place the ultrasound system
6.
7. Built-in DVD RW drive DVD-RW drive
8.
9.
10. Caster Used for securing or moving the system
11. Display support arm
12. I/O extend module To extend I/O ports
Adjustable telescopic column
switch
Compartment for placing ECG
module or B/W printer
Compartment for placing color
printer
Displays the image and parameters during
scanning
Raise the handle to adjust height of the
platform
/
/
Supports the display module, for adjusting
the height and position of the display
module.
13. Probe port To connect probe or probe extend module.
14. Adjustable telescopic column To adjust height of the operating platform
15. Probe extend module To extend ports for connecting probes
16. Power adapter /
Power supply input/output interface,
17. Power module
equipotential terminal, breaker and power
indicator
2-12 System Overview
Page 43

Power Panel
No.
Name Function
1. Circuit breaker Switch on or off the mains power supply
2. Power inlet AC power inlet
Indicates if the trolley is connected to the mains power supply:
If connected with mains power and the breaker is on, the
3. Power indicator
indicator illuminates in green color
If not connected with mains power or the breaker is off, the
indicator does not illuminate
4. Power outlet Supply power for optional peripheral devices (e.g. DVR)
Equipotential
5
terminal
Used for equipotential connection, to balance the protective
earth potentials between the system and other electrical
equipment
System Overview 2-13
Page 44

2.8 Extend Modules
There are four extend modules available for the system:
Probe extend module
I/O extend module
V/A extend module
ECG module
2.8.1 Probe Extend Module
Overview
To connect the module:
a) Insert the module connector into the probe port with its bulge facing to the
right side of the system (when you are facing to the front of the system).
b) Toggle the lock lever to the top position.
CAUTION:
You must turn off the ultrasound system before connecting or
disconnecting the probe extend module. Otherwise the
system may be damaged.
NOTE: 1. Before connecting/disconnecting the probe extend module, please freeze
the image first to keep the module in a good condition.
2. If you use the probe extend module to connect a probe, the image quality
may be degraded.
2-14 System Overview
Page 45

2.8.2 I/O extend module
I/O Extend Module
Overview
No. Symbol Function
<1>, <2>
<3> ECG Port Connects the ECG module
<4>
<5>, <6>
<7>
<8>
<9>
<10>
To connect the module:
Connect the I/O extend module to the main unit via the I/O extend port, thus the data port
is extended. As shown in the following figure.
USB port
Serial port
Audio output port
Mic In port
Remote control port
Composite video output port
DVI-I output port
Connects USB devices.
Connects serial port devices
Used for audio signals of Doppler mode sound
from DVD output or audio comments
Reserved.(Connects a microphone used for
receiving audio comments when a recorder is
used to record images)
Connects the control port of the video printer
Used for receiving the output image signal of
the video printer or DVD recorder
Connects a display or projector
CAUTION:
When connects serial port devices (via port <4>) or the ECG
module (via port <3>), you should first freeze the image or power
off the machine, or malfunction may result.
System Overview 2-15
Page 46

2.8.3 V/A Extend Module
< >1< >2< >3< >
Overview
The module is connected to the USB port of the main unit via a USB cable.
4
No. Name Function
<1>, <2> Audio input port Used for audio signal input
<3> Composite video input port
<4> Separate video input port
Connection
Connect the V/A extend module to the main unit via a USB port. As shown in the following
figure.
Used for composite signal video input
(Reserved)
Used for separate signal video input
(Reserved)
2-16 System Overview
Page 47

2.8.4 ECG module
Overview
To connect the ECG module, the system should also be configured with the I/O extend
module.
The ECG module is connected to main unit via connection to the I/O extend module.
Name Function
ECG lead port
Connection
Connect the ECG module to the main unit via the I/O extend module, see "2.8.2 I/O
extend module" for details). As shown in the following figure.
NOTE: You must turn off the ultrasound system before connecting or disconnecting the
ECG module. Otherwise the system or the module may be damaged.
Used for ECG signal input
System Overview 2-17
Page 48

2.9 Control Panel
<1> <2> <3> <4> <5> <6>
<8> <9> <10> <11> <12> <13> <14> <15> <16>
<18>
<20> <21> <22> <23>
<25>
<26>
<27>
<28>
<29>
<30>
<31>
<45>
<46>
4
3
<
>
3
3
<
>
2
3
<
<35>
>
<49>
<36>
<37>
<50>
<47>
<19>
<39>
<38>
<40>
<17>
<43>
<41>
<7>
<24>
<42>
<44>
<48>
<51>
<52>
No. Name Description Function
Press to select the soft menu items displayed on
<1> /
Soft menu
controls 1
the bottom of the screen.
Refer to the subsequent contents for specific
functions.
Press to select the soft menu items displayed on
<2> /
Soft menu
controls 2
the bottom of the screen.
Refer to the subsequent contents for specific
functions.
Press to select the soft menu items displayed on
<3> /
Soft menu
controls 3
the bottom of the screen.
Refer to the subsequent contents for specific
functions.
Up/ down controls are used to turn pages
up/down when there is more than one page for
<4> /
Soft menu
controls 4
the soft menu.
Left/ right keys are used to switch among the
different modes.
2-18 System Overview
Page 49

No. Name Description Function
Press to select the soft menu items displayed on
<5> /
<6> /
<7> / Power button
Soft menu
controls 5
Soft menu
controls 6
the bottom of the screen.
Refer to the subsequent contents for specific
functions.
Press to select the soft menu items displayed on
the bottom of the screen.
Refer to the subsequent contents for specific
functions.
It does not illuminate when the system is turned
off.
Press the button to turn on the system, the
system enters work status and the indicator
lights on and becomes green.
When the system enters the standby status, the
indicator turns orange.
<8> Esc Exit
<9> Help Help Press to enter help status.
<10> Report Report Press to open or close the diagnosis reports.
<11> iStation /
<12> F1-F4
<13> Quad
<14> Biopsy Biopsy Press to show or hide the biopsy guide line.
<15> Setup Setup Press to show the Setup menu.
<16> Del / Press to delete the comment, etc.
<17> TGC / Move to adjust time gain compensation.
<18> /
<19> Menu Main menu
User-defined
key
Quad-split
screen
Alphanumeric
keys
Press to exit the current status to the previous
status.
Press to enter or exit the patient information
management system.
You can assign a function to the key.
Press to enter Quad mode.
Switch image windows in the Quad mode.
Same as those of PC.
Press to display or hide a mode-specific
parameter menu.
<20> Comment
<21> Arrow Arrow Press to enter or exit the arrow comment status.
<22> Clear Clear
<23>
Direction
keys
ABC
(Comments)
Press to enter or exit the character comment
status.
Press to clear the comments or measurement
calipers on the screen.
Press “Fn+/” to adjust volume or monitor
brightness
System Overview 2-19
Page 50

No. Name Description Function
Rotate to increase or decrease the image gain.
<24> iTouch /
Press to optimize the image, serving as a
one-key optimization.
<25> Patient
<26> Exam Exam Type Press to select an exam mode and a probe.
<27> Review Review To review the stored images.
<28> End Exam End Exam Press to end an exam.
<29> Body Mark Body Marks Press to enter or exit the Body Mark status.
<30> Cine Cine Review Press to enter or exit the Cine Review status.
<31> Zoom Zoom Press to enter or exit the Zoom status.
<32> Cursor Cursor Press to show the cursor.
<33> F5
<34> Measure Measurement Press to enter or exit the measurement state.
<35> Update /
<36> Set Set
<37> B / Press to enter B mode
Patient
Information
User-defined
key
Press to enter the [Patient Info] screen.
You can assign a function to the key.
Press to change the currently active window.
Or start/stop image acquisition in iScape or
3D/4D.
Press to confirm an operation, same as the
left-button of the mouse.
Press to enter Dual mode from another modes.
<38> Dual /
<39> Color / Press to enter Color mode.
<40> Power / Press to enter Power mode.
<41> CW / Press to enter CW mode.
<42> M / Press to enter M mode.
<43> PW / Press to enter PW mode.
<44> F6
<45> Print Print Press to print; user-defined key.
<46> Save Save Press to save; user-defined key.
<47> Depth Depth
<48> Freeze Freeze Press to freeze or unfreeze the image.
<49> / Trackball Roll the trackball to change the cursor position.
User-defined
key
Press to switch between the two windows in
Dual mode.
You can assign a function to the key.
Press to increase or decrease the imaging depth
in scanning mode.
2-20 System Overview
Page 51

No. Name Description Function
Rotate to adjust image parameters or direction of
<50> /
Multifunctional
knob
comment arrows.
Press to show the menu, rotate to select the
item.
Indicates if the main unit is connected to the
power supply.
<51> / Indicator 1
If not connected, the indicator does not
illuminate.
If connected, the indicator illuminates in green
color.
Indicates the current status of the batteries.
When the system is supplied with power by the
batteries and the power capacity is lower than
30%, the indicator is yellow and flashes.
<52> / Indicator 2
When the batteries are being charged, the
indicator light is on and in yellow color.
When the battery capacity is charged to the full
capacity, the indicator color turns green.
In other statuses, the indicator light is off.
Tip: “/” means the key / knob has no silk-printed name.
System Overview 2-21
Page 52

2.10 Symbols
This system uses the symbols listed in the following table, and their meanings are
explained as well.
Symbol Description
Type-BF applied part
Caution
AC (Alternating current)
Battery Status Indicator
Power button
Probe port
Probe connection unlocked symbol
Probe connection locked symbol
I/O extend port
USB port
Network port
S-VIDEO signal interface; VIDEO signal interface
Pencil probe port (reserved)
2-22 System Overview
Battery installation position indicator
ECG function
No user serviceable parts (applied to the power adapter)
Indoor, dry location use only (applied to the power adapter)
Connects serial port devices
ECG function
Connects a display or projector
Audio signal
Microphone input jack
Remote control port
Product serial number
Manufacture date
Page 53

3
System Preparation
3.1 Move/Posit the System
Please read and understand the safety precautions before placing the system to ensure
safety for both operator and device.
1. Turn off the power and disconnect the system from all peripherals.
2. Carry the system by holding the handle.
3. Place the system in a desired location.
4. Leave at least 20cm at the back and both sides of the system.
CAUTION:
Maintain a generous–free air flowing space around the back
and both sides of the system; failure may result due to
increased rise in system operating temperature.
3.2 Power Supply
This system can work normally only when it is connected to the external power supply or
the battery capacity is sufficient.
3.2.1 Connecting the External Power Supply
1. Connect the connector of the power adapter to the adapter port in the system.
2. Use a three-wire cable to connect the adapter with the external power supply.
3. The external power supply system must meet the following requirements:
Power supply voltage:100-240V~
Power supply frequency: 50/60Hz
Input current: 1.5- 0.6A
The model of the power adapter: ADP1210-01.
If you have any question about the power adapter, please contact your sales
representative.
NOTE: 1. You must use the specified power adapter.
2. Do not use this power adapter in the conditions other than those specified.
System Preparation 3-1
Page 54

3.2.2 Powered by Batteries
When connected to the external power supply, the system is powered by the external
power. The lithium ion batteries inside it are in the charging status.
When disconnected from the external power supply, the system is powered by the
lithium ion batteries. Refer to chapter “15 Batteries” for the detailed operations and
precautions.
3.3 Power ON /OFF
3.3.1 Powering ON the System
CAUTION:
Checking before Power ON
To check the system before the system is turned on:
No.
The temperature, relative humidity and atmospheric pressure shall meet the
<1>
<2> There shall be no condensation.
<3>
<4> There shall be no loose screws on the monitor or control panel.
requirements of operating conditions. See "2.4.3 Environmental Conditions" for
details.
There shall be no distortion, damage or dirt on the system and peripheral devices.
If any dirt is found, cleaning shall be performed as defined in section “18.1.1
Cleaning the System”.
To ensure safe and effective system operation, you must
perform daily maintenance and checks.
If the system begins to function improperly – immediately
stop scanning. If the system continues to function improperly
– fully shut down the system and contact Mindray Customer
Service Department or sales representative.
If you use the system in a persistent improperly functioning
state – you may harm the patient or damage the equipment.
Check Item
<5>
<6>
<7>
<8>
<9>
<10> The overall scanning environment and field must be clean.
3-2 System Preparation
There shall be no cable damage (e.g. power cord). Maintaining secure
connections to the system at all times.
The probes and probe cables shall be free of damage or stains.
See Probe chapter for details on probe cleaning and disinfection.
No miscellaneous odds and ends are allowed to be attached or affixed to the
control panel.
Ensure that all connections are free from damage and remain clear of foreign
object blockages.
There shall be no obstacles around the system and its air vent.
Probe cleaning and disinfection. (Please refer to chapter “12 Probes and Biopsy”
for details)
Page 55

Turning on the Power
To turn on the system:
1. Press the power button in the upper right corner on the control panel
2. The system enters the work status.
3. The indicator lights on and becomes green.
To check the system after it is turned on
Press the power button in the upper right corner on the control panel to turn on the
system.
To check the system after the system is turned on:
No.
<1> There shall be no unusual sounds or smells indicating possible overheating.
<2> There shall be no persistently displayed system error message.
<3>
<4>
<5> The control panel keys and knobs are fully functional.
<6>
There shall be no evident excessive noise, discontinuous, absent or black artifacts
in the B mode image.
Check if there is abnormal heat on the surface of the probe during an ultrasound
procedure.
The exam date and time are the same as the system date and time, and are
displayed correctly.
WARNING:
1. If you use a probe giving off excessive heat, it may burn
the patient.
2. If you find anything not functioning properly, this may
indicate that the system is defective. In this case, shut
down the system immediately and contact Mindray
Customer Service Department or sales representative.
Check Item
NOTE: When you start the system or switch between probes, you will hear clicking
sounds – this is expected behavior.
System Preparation 3-3
Page 56

3.3.2 Powering OFF the System
You need to follow the correct procedures to power off the system. In addition, after you
upgrade the software or when the system is down, you need to power off and restart it.
If you will not use the system for a long period of time, you shall:
1. Disconnect the power adapter.
2. Disconnect the mains power.
3. Turn off powers of all peripherals connected to the system.
To power off your system normally:
Gently press the power button once on the upper right corner of the control panel. The
[Shutdown Confirm] screen appears.
Shut down: To power off the system normally.
Standby Click to enter the standby status.
Cancel: to cancel the operation.
To shut down the system in a direct way if you cannot do it normally:
Press and hold the power button for a long time and the system will power off without
displaying the [Shutdown Confirm] screen. However, shutting down the system this way
may destroy the data.
NOTE: 1. DO NOT rush shutdown of the system in a direct way. It may destroy the
data.
2. After the software upgrade, please shut down the system in the normal way
(using “Shut down” method) so as to guarantee a fully update.
3-4 System Preparation
Page 57

3.3.3 Standby
Definition of standby: in a normal environmental temperature and humidity condition, the
system is connected with only one probe while no other external devices or extend
modules are connected, only 3V3_STB and 5V_STB power supply is available and in the
meantime, the standby indicator is in orange.
When the battery capacity is charged to the full capacity, the standby time of the system is
no less than 64 hours.
To enter standby:
Fully fold the LCD display and wait for 30 sec, then the system enters the standby
status.
Open [Setup]->[System Preset]->“General” to set the time for screensaver and
standby. If there is no operation, the system enters screensaver after the waiting
time set for screensaver, and if there is still no operation, the system enters
standby after the waiting time for standby.
Press the power button and select “Standby”.
When the system enters the standby status, the power button backlight turns orange.
To exit standby
Unfold the LCD display.
Press the power button.
When the system enters the standby status, if need to power off:
Long press the power button for several seconds.
Press the power button to exit the standby status and then power off the system.
NOTE: 1. Power off the system if you will not use the system for a long period of time
(including storage/ transportation condition), and you should not allow the
system in standby status, otherwise the batteries will be out of power and
permanently damaged.
2. If you will not use the system for a long period of time, DO NOT leave the
system in the standby status, you should shut down the system, disconnect
power adapter, mains power, and turn off powers of all connected
peripherals.
System Preparation 3-5
Page 58

3.4 Connecting /Disconnecting a Probe
1.
CAUTION:
3.4.1 Connecting a Probe
When connecting or disconnecting a probe, place it in a
proper position, to prevent the probe from falling off or
becoming damaged.
2. Only use the probes provided by Mindray. Aftermarket
probes may result in damage or cause a fire.
WARNING:
1. Keep the cable end of the probe to the right side of the system, and insert the
connector into the port of the system, and then press in fully. See the figure below.
2. Toggle the locking lever to the top position.
3. Place the cable properly to avoid being treaded or wrapping with other devices. DO
NOT allow the probe head to hang free.
The probes, cables and connectors are in proper operating
order and free from surface defects, cracks and peeling.
Using a defective probe may cause electric shock.
Locking lever
3.4.2 Disconnecting a Probe
1. Toggle the locking lever to the bottom position to unlock the connector of the probe.
2. Pull the probe connector straight out as shown in the figure below.
3-6 System Preparation
Page 59

3.5 Connecting the Footswitch
Connect the footswitch to the main unit via a USB port. As shown in the following figure.
Set the functions of the footswitch in the [Key Config] page. Refer to "14.1.7 Key Config"
for details.
3.6 Connecting/ Removing a USB
Memory Device
WARNING:
When connecting a USB memory device to the ultrasound system via a USB port:
1. You can hear a sound if it is connected successfully.
2. You can see the symbol in the lower right corner of the imaging screen.
To remove the USB memory device:
1. Click to open the “Remove USB Device” dialogue box.
2. Select the memory device to be removed.
3. Click [OK] and you can hear a sound.
4. Remove the USB memory device. There will be sound feedback when remove the
USB memory device.
DO NOT directly remove a USB memory device; otherwise,
the USB memory device and /or the system may be damaged.
System Preparation 3-7
Page 60

3.7 Graph /Text printer
Connecting a local printer
As shown in the figure below, a graph /text printer has a power cord and data cable. The
power cord shall be directly connected to a protective grounding wall receptacle as
required.
Power cord
Data cable
USB connector
1. Connect the two USB ports of the printer and the system with the USB cable of the
printer.
2. Power on the system and the printer.
3. Install the printer driver: "[Setup]-> [Peripheral Preset]->[Printer]->[Printer Driver]" and
click [Add Printer], as shown in the figure:
4. Select "Add Local Printer" and click [Next] to enter the screen of browsing driver;
select the desired driver and click [OK] to install the driver.
3-8 System Preparation
Page 61

Printers listed in "2.5 System Configuration" have drivers installed already.
Click [Printer Attribute] to see the printer attribute.
5. Click [OK] to finish the installation.
Add Network Printer
After the system is connected into a LAN, enter the "[Setup] -> [Peripheral Preset] ->
[Printer] -> [Printer Driver]" screen.
1. Click [Add Printer] and select the types of adding network printer
Search Network Printer
Select "Search Network Printer" and click [Next] to search for the printer; select
the domain and server in the screen to find the printer.
Connecting to the specified printer
Select "Connect to this Printer" and enter the printer address in the field box.
2. When the network printer is successfully connected, you can see the printer in the list.
Tips: the network printer functions depending on the configured network environment in
the hospital, please consult the network configuration manager in case of failure.
NOTE: When you install the printer’s driver, you must specify the specific path for
installation; otherwise, vague path may result in longer time for searching.
Report print
You can use a graph/ text printer to print report or images.
To set the default report printer and properties:
In "[Setup] -> [Print Service]" screen, select the "Report Print" column in the
service list, set the items in the "Property" box.
To print a report:
Click [Print] in the report dialog box to print a report; or, use the user-defined key
to print, see "14.1.7 Key Config" for details.
Please refer to the accompanying manuals of the printers for more details.
3.8 Video Printer
The system support digital video printers, including B/W printers and color printers.
USB cable
Power cord
Connecting a local printer
1. Place the printer in the proper position.
2. Connect the power cord of the printer to a receptacle. Use a USB cable to connect the
USB port of the system with the USB port of the printer.
3. Load a paper roll, and turn on the system and printer.
System Preparation 3-9
Page 62

4. Install the printer driver (steps are the same as of graph/text printers; please refer to
relevant chapters for details). Printers listed in "2.5.2.3 Peripherals Supported" have
drivers installed already.
5. Add a print service:
(1) Open "[Setup] → [Peripheral Preset] → [Print Service]" screen.
(2) Click [Add Service] to enter the following page.
(3) Select the service type as "Digital Image Print" and enter the service name
manually.
(4) Click [OK] to return to the Printer Service page.
(5) Set the items in the Property box and click [Save] to save the settings.
Image print
Video printers are mainly used for image print; refer to DICOM chapter for DICOM
image print.
Modify print service:
a) Select a printer service in the list.
b) Select the printer type in the Property box.
c) Set print properties: Paper size, Paper orientation, Rows*Columns, Vertical
Align, Page margin etc.
d) Click [Save] to confirm.
Video output settings:
Enter "[Setup] → [Peripheral Preset] → [Input & Display]"; set the output size.
Image print
Select the image to be printed on the iStation or Review screen, and click
[Send To] to select the printer to print.
To print by the user-defined key (<Print> as an example):
a) Enter "[Setup] → [System Preset] → [Key Config]".
b) Select "Print" in the "Key Function" list, and select the desired print service in
the right "Print" list.
Please refer to the accompanying manuals of the printers for more details.
3-10 System Preparation
Page 63

3.9 Basic Screen and Operation
3.9.1 Basic Screen
The following diagram maps out the different areas in the screen:
Hospital Name
Logo
Patient Information Probe
Image
Parameter and
Menu Area
Image-in-image
Thumbnail
Exam
Time
Help information area Cursor area
Acoustic
Power
Exam
Mode
Image area
Cine Review
Body Mark Area
MI/TI
Operator
ECG
icon
Accession#
Thumbnail Area of
Images Stored
Freeze
icon
Soft Menu Area System status icon
Information Area
The information area displays manufacturer logo, hospital name, exam date & time,
acoustic power & MI/TI, freeze icon, patient information, probe model, current exam
mode, ECG icon (if ECG module is connected), and accession #, etc.
To preset whether gender, age or operator is displayed: Enter "[Setup]→[System
Preset]→[General Preset]" and check "Gender", "Age" or "Operator" in the [Patient
Info] box in the upper left corner of the screen.
Logo
Manufacture logo, displayed in the upper left corner of the screen.
Hospital name
Display the hospital name. Hospital name can be set via "[Setup] → [System
Preset] → [Region]".
Exam time
Displays the exam time, including date and time. Exam time can be set via
"[Setup]→[System Preset]→[Region]". Exam time will be frozen with the frozen
image.
System Preparation 3-11
Page 64

Acoustic power & MI/TI
Display the acoustic power, including the acoustic power, MI (Mechanical Index)
and TI (Thermal Index).
Freeze icon
The freeze icon means the image is frozen.
Patient Information
Displays patient name, ID, gender and age etc. Enter the patient information
through the "Patient Info" screen. Or, import the saved patient data from iStation
or the DICOM Worklist server.
Probe Model
Display the currently-used probe model, or the default model.
Exam Mode
Displays the currently used exam type, e.g. A-Abdomen, is displayed.
Operator
Displays the operator’s name on the screen. This information is entered through
the “Patient Info” screen.
ECG icon
Displays ECG icon, which consists of a heart icon and heart rate, e.g. " 75bpm"
Accession#
Serial number used in DICOM or the manually entered serial number in “Patient
Info” screen.
Image Parameter and Menu Area
The image parameter and menu are both displayed in this area. When no menu is
available; this area displays the image parameters of the current mode.
Menu area
When an image menu is displayed, the imaging parameters will be covered by
the menu.
Include image menu, measurement menu, comment menu, body mark menu and
so on. Use the trackball or the multifunctional knob to operate on the menu.
Use the <Menu> to show or hide the menu. You can also show the menu by
pressing the multifunctional knob.
The menu area consists of menu title, menu items and page-turning button. As
shown in figure below.
3-12 System Preparation
Page 65

Menu title
Tab
Page-turning
button
Drop-down list
button
Items
Page-turning
button
Menu title
Displays the menu name.
Tab
Divide the menu items according to measurement conditions.
Page-turning button
When there are too many items in a menu, the items will be divided into more
than one page.
You can turn pages by page-turning buttons, and .
Items
Refers to the items on a menu. For item that is applicable for more than one
mode, the item appears as shared item in the certain mode. Items of image
modes and measurement can be preset.
Down-drop list button
If there are several options available for one item, you can choose the options
through the button.
yThe multifunctional knob can be used to operate the menu. Press the knob to
open the menu; rotate the knob to navigate through the items one by one, and
press it to select the item.
Depending on the item types, the multifunctional knob can:
For a commanding item or command optional item: press the knob to directly
activate the item.
For a parameter item or ON/OFF item: press the knob to lock and select the
item, then rotate the knob to switch among the available values; press the
knob again to release and deselect the item.
For an item with sub-menu: press the knob to extend the sub-menu, and the
cursor navigates to the first sub-item. Operate the sub-menu through the
multifunctional knob. At the same time, you can exit from the sub-menu to the
previous item by clicking “Exit” in the sub-menu.
System Preparation 3-13
Page 66

For tab items in the menu: e.g. the “Right” and “Left” items in the
measurement menu. Rotate the multifunctional knob to navigate the tab, and
press the knob to select the tab.
Parameter Area
Display the image parameters for the activated image window. If there are more
than one imaging modes, the system displays the parameters of each image
mode respectively. For details, please refer to the corresponding imaging
mode(s).
Image area
The image area displays the ultrasound images, ECG waveforms, probe mark (or
activating window mark), time line (in M or PW mode), coordinate axis (including
depth, time, velocity/frequency), focal position (located at depth axis in the form of
), besides, the annotation, body mark, measurement calipers, color bar/grayscale
bar are also displayed here.
Tips: Amplitude and position of ECG waveform can be preset, for more details, please
refer to “7.2 ECG Setting”.
Image-in-Image Area
In the zoom status, this area displays the thumbnail of a complete image, and a
rectangular frame is used to highlight the currently magnified area. This feature is
called image-in-image.
Cursor area
The status icon area indicates the current cursor status, such as in cineloop
status.
Help information area
The help information area displays various help information or progress bar in the
current status.
Besides, the system can provide help for each key. Press <Fn>+<Help> to enter
key-help status, you can see the cursor changes into a arrow with a question mark on
it. Press any key on the control panel, the screen displays the key-relevant
information, meanwhile, the system exits the key-help status.
Tips: in terms of help information, “TB” refers to “Trackball”; “Knob” refers to
“Multifunctional knob”.
Thumbnail area of images stored
Displays the thumbnail images stored under the current patient.
Image manage area
Page up/down: when there are more than one page images have been stored,
you can turn to the next or preview page by or respectively.
Delete: select a thumbnail image, and click to delete it.
Send to: select a thumbnail image, and click to send it to external devices.
Soft Menu Area
The soft menu items displayed are corresponding to functions of soft menu controls in
the control panel. The soft menu items are related to image mode and settings of
each mode in preset.
The soft menu controls are located at the top of the control panel, shown in the
following figure.
3-14 System Preparation
Page 67

Page-turning:
Use the up/down keys in soft menu controls <4> to turn pages up or down; you
can operate the items only when they are highlighted.
Mode switching:
Left / right keys in <4> are used to switch among the modes. The soft menu items
vary depending on the mode. The menu navigation will cause the change of the
menu, while the change of the menu will cause the menu navigation.
Soft menu control:
The soft menu items are operated respectively through the five groups of soft
menu controls <1>, <2>, <3>, <5> and <6>.
The soft menu items can also be adjusted by <Set> on the control panel. Move
the cursor onto a soft menu item and press <Set> to change the parameter of the
item.
System status area
This area displays the relevant system icons, such as USB memory device, printer,
network connection, and current system time, etc.
Click to see the hidden status icons.
None-fixing Area
Areas illustrated here are position-changeable; you can move them by the trackball
within a certain area on the monitor.
Result window
The measurement result window displays the results of recently performed
measurements.
To move the result window:
a) Place the cursor on the title of result window (you can see the cursor
changes into ).
b) Press <Set> and move the trackball, the window moves together with the
cursor.
c) Move the cursor to the target position, press <Set> again to anchor the result
window in the target position.
Comment area
For details, please refer to “9 Comments and Body Marks”.
Body mark area
For details, please refer to “9 Comments and Body Marks”.
System Preparation 3-15
Page 68

3.9.2 Basic Operations of Screens
A screen consists of title, page tabs, contents and buttons, as shown in the following
figure:
Title
Tab
Content
Control
button
Composition Description
Title Bar
Page Tab
Contents
[OK] and [Cancel]
To reposition a dialogue box:
1. Roll the trackball to move the cursor onto the title bar of the dialogue box. At this time
the cursor becomes a ; press <Set>.
2. Roll the trackball and reposition the rectangular graphic to the new desired location.
3. Press the <Set>, and the dialogue box is moved to the desired position.
The title bar is used to give a description for the content and
function of the screen.
For some screens, contents are distributed across several
pages. Click the tab by pressing <Set> to open/close the
available pages.
Radio button: click to select the item.
Check box: click to check or uncheck the item.
Entry box: enter characters manually via the keyboard.
Position the cursor into the box, and then enter the letters
or characters.
Drop-down list box: click “▼” to show the list and select
an item.
When the operation of a screen is complete, click [OK] or
[Cancel] to save or cancel the operation, and close the
screen.
3-16 System Preparation
Page 69

4
Exam Preparation
CAUTION:
Before examining a new patient, press <End Exam> to end the
exam of the previous patient, update the patient ID and
information, to avoid mixing data of the next new patient.
4.1 To Start an Exam
You can start a patient exam in the following situations:
New patient information: Enter the patient information, if it is a new patient, refer
to "4.2.1 New Patient Information" for details; however, the system also supports
anonymous patient exam, refer to "4.8 Anonymous Patient Exam" for details.
New exam: to start a new exam for an already registered patient, the recorded
information can be obtained either through iStation or Worklist, refer to "4.2.2.1
iStation" and "4.2.2.2 DICOM Worklist" for details.
Activate an exam: select an exam that is ended within 24 hours, and continue the
exam. For details, please refer to “4.5 Activate & Continue an Exam”.
Continue an exam: select an exam that is paused within 24 hours, and continue
the exam. For details, please refer to “4.5 Activate & Continue an Exam”.
General procedure for an exam: Enter the patient information -> Select an exam mode
and probe -> Choose an imaging mode -> Start the exam.
To start a new patient exam, it is better to enter the detailed patient information. The
system will set up a unique information database for each patient based on the patient
information entered, so that the information of one patient will not be confused with that of
another patient.
4.2 Patient Information
To enter the "Patient Info" screen
Press <Patient>, or,
Move the cursor onto the patient information area on the monitor and press <Set>
to open the screen.
To exit the "Patient Info" screen
Click [OK] on the "Patient Info" screen; or, press <Patient> on the control panel
again, to save the settings and exit the screen.
Click [Cancel] or press <Esc> to exit the screen without saving any of the entered
patient data.
Press <B> or <Freeze> to return to the current exam mode with the entered
information being saved.
Click [Basic] or [Detail] on the screen to show or hide the detailed patient information.
Exam Preparation 4-1
Page 70

4.2.1 New Patient Information
The "Patient Info" screen is shown as follows (Take abdomen exam for example):
Place the cursor onto the targeted box. The field box is highlighted and a flashing cursor
appears. Information can be entered or selected from the options.
You can also change the cursor position by <Tab>, <Enter> or direction keys.
1
2
3
4
Detailed information is described as follows:
1. General information
Patient ID
Once you enter the ID and confirm it, you are not allowed to change it. There are 2
ways to generate the patient ID. “\” is not permitted.
Tips: Patient ID of an activated exam (within 24 hours) can be modified.
Auto generate ID
Select "Auto generated ID" through the path: [Setup] -> [System Preset] -> [General].
System will automatically populate the (patient) ID field with a unique time-stamp
identification code. The auto generated ID can be edited manually.
Enter the ID
If you deselect "Auto Generated ID", you need to enter an ID.
If an ID that is already existed in the system is entered, the system prompts "The ID
existed, load data", you can choose to import the data.
Name
Enter the patient name directly through the keyboard. Characters of A through Z and 0
through 9 and “.” are allowed.
“\”, “^”, “=” and “,” are not permitted.
Gender
Select Male, Female or Unknown for patient gender in the drop down list.
4-2 Exam Preparation
Page 71

DOB (Date of birth):
You can either enter the birth date of a patient manually according to the format
displayed in the field, or click to select the date. In the table, you can select the
desired year (or enter it manually); month and day, then click [OK] to finish it.
Age:
Auto generated age: once the DOB is entered, the system can display an
auto-generated age in the field box, the unit can be “Years”, “Months” or
“Days”. If the age is less than one year, the system will automatically
calculate the age in months or days.
Also, you can manually enter the age.
NOTE: When you enter the date manually, please enter it in the format as that of the
system.
2. Exam Type
Exam application type
You can select among 8 types: ABD (Abdomen), OB (Obstetrics), GYN (Gynecology),
CARD (Cardiac), VAS (Vascular), URO (Urology), SMP (Small Part), and PED
(Pediatrics).
Select the exam type tab to enter the exam-specific information.
General information:
Study description: To enter description for each exam.
Primary indications: To enter the primary indications (reason to perform the exam.)
Secondary indications: To enter the secondary indications.
CPT4 code: To enter the CPT4 code.
CPT4 description: To enter the CPT4 description.
Exam specified information:
Exam Type Information
Description
Height /
ABD
(Abdomen)
Weight /
BSA (body
surface
area)
After the height and weight are entered, the system will
automatically calculate the BSA based on the formula
which is set via "[Setup] -> [System Preset] -> [General]".
Exam Preparation 4-3
Page 72

Exam Type Information
Calculation
index
OB
(Obstetric)
Gravida Times of pregnancy.
Ectopic Times of abnormal pregnancy. e.g. extrauterine pregnancy
Gestations Number of embryos (1, 2, 3; 1 is default)
Description
According to the entered index (can be selected in the
drop-down list); including LMP (last menstrual period), IVF
(in vitro fertilization), PRV (previous exam date), BBT
(basic body temperature)), the system can automatically
calculate GA and EDD (estimated delivery date); or,
calculates GA and LMP according to the EDD and entered
date.
LMP: After you enter LMP, the system will
calculate and display GA and EDD.
IVF: After you enter IVF, the system will calculate
GA and EDD.
PRV: input the date and GA of the last exam, the
system will calculate a new GA and EDD.
BBT: input BBT, the system will calculate the GA
and EDD.
EDD: after you enter EDD, the system will
calculate and display GA and LMP.
GYN
(Gynecology)
Cardiology
VAS
(Vascular)
Para Times of delivery
Aborta Times of abortion
LMP Last menstrual period
Gravida Times of pregnancy.
Para Times of delivery
Ectopic Times of abnormal pregnancy. e.g. extrauterine pregnancy
Aborta Times of abortion
Height /
Weight /
BSA (body
surface
area)
After the height and weight are inputted, the system will
automatically calculate the BSA based on the formula
which is set via "[Setup] -> [System Preset] ->[General]".
BP Blood pressure.
HR /
RA Press Right Atrium Pressure
BP(L) Input left blood pressure.
BP(R) Input right blood pressure.
URO
Serum PSA /
(Urology)
SMP (Small
Parts)
PPSA
coefficient
None /
/
4-4 Exam Preparation
Page 73

Exam Type Information
Description
PED
(Pediatrics)
None /
3. Operating Information
Accession #: exam number used in DICOM. It should be entered within 16 letters
or characters; “\” is not permitted.
Diagnostician: people who is responsible for the exam. "\", "^","=" and "," are not
permitted.
The system remembered automatically the diagnostician information that is
inputted. Click the Diagnostician drop-down list, select “Del Single” or “Del All” to
delete the information of the diagnostician.
Operator: people who is responsible for images acquisition and scanning. "\", "^",
"=" and "," are not permitted.
The system remembered automatically the operator information that is inputted.
Click the operator drop-down list, select “Del single” or “Del All” to delete the
information of the operator.
Ref. Physician: the people who requires the ultrasound exam. "\", "^", "=" and ","
are not permitted.
The system remembered the Ref.Physician information that is inputted. Click the
Ref. Physician drop-down list, select “Del single” or “Del All” to delete the
Ref.Physician information.
Comment: exam-specific explanation or remarks.
4. Functional key
[Pause Exam]: to pause the current exam.
[Cancel Exam]: to cancel the current exam.
[New Patient]: click to clear the current patient information in the patient
information screen in order to input information of a new patient.
[New Exam]: click to clear the entered exam information to start a new exam for
the current patient.
[OK]: click to save the patient data entered and exit the screen.
[Cancel]: click to cancel the patient data entered and exit the screen.
Exam Preparation 4-5
Page 74

4.2.2 Retrieve Patient Information
4.2.2.1 iStation
The patient data can be obtained in iStation from the system hardware or USB memory
device. You can enter the searching conditions for the patient.
1. To enter iStation screen (the screen is shown as follows):
Press <iStation> on the control panel; or,
Click [iStation] in the "Patient Info" screen; or
Press the <Review> on the control panel and click [iStation] in the screen.
2. Select the data source:
Select the data source in the drop-down list of "Data Source". The occupying space
percentage of the selected data source will be displayed.
3. Set the searching condition
Define the searching item: Name, ID, DOB or Exam Date; and then enter the related
keyword.
Select “Find in results”, the system will search the keyword based on the existed
searched results.
Click [Reset], the system will clear the information you entered, and all the
recorded patient information of the system will be listed out.
4. Select the desired patient information from the list.
[New Exam]: click to enter “Patient Info” screen, meanwhile, the corresponding
patient information is also imported to the new exam. After you edit the patient
information in the [Patient Info] screen, select [OK] to start a new exam.
[Activate Exam]: click to continue an exam that is finished within 24 hours.
4-6 Exam Preparation
Page 75

[Continue Exam]: click to continue an unfinished exam that is carried out within
24 hours.
[Review]: click to switch to the Review screen.
[Exit]: click to exit to iStation dialogue box.
4.2.2.2 DICOM Worklist
When the DICOM basic package is configured and the Worklist server has been set, click
[Worklist] in the "Patient Info" screen to query or import the patient data. (For the setting of
Worklist server, please refer to “11.1.4.3 DICOM Worklist Setting”.)
Procedure:
1. Select data source: choose a worklist server in the drop-down list of “Worklist Server”,
and then all the patient exam records in the server are listed out.
2. Set the searching condition:
Enter the period which includes the exam date, and click [Query] to search.
Enter patient ID, patient name, accession #, the system provides the result in
real-time.
Or select the keyword type, enter the keywords and then click [Query] to search.
To reset the criteria, click [Clear] button.
3. Select the desired patient from the list, and:
Click [Start Exam], the patient information is imported into the system and then an
exam is started.
Click [Transfer], the patient information is imported into the "Patient Info" screen.
After you edit the patient information in the "Patient Info" screen, click [OK] to
start a new exam.
Click [Show Detail] to see details of patient data.
4. Click [Exit] to exit the Worklist.
Exam Preparation 4-7
Page 76

4.3 Select an Exam Mode and Probe.
CAUTION:
If the exam mode is changed during a measurement, all
measurement calipers on the image will be cleared. The data
of general measurements will be lost, but the data of
application measurements will be stored in the reports.
4.3.1 Supported Exam Modes
The system can be configured with applications including abdominal, gynecology,
obstetrics, cardiac, vascular, small parts, urological, pediatrics, EM and nerve use, and the
system will support corresponding exam modes related to the application software (refer
to “2.5.2 Options” for details); the system also supports user-defined exam modes.
The system supports to preset application type, measurements, comments, body marks,
image parameters for each exam mode (including user-defined exam modes). For details,
please refer to “14.2 Exam Preset”.
You can select exam modes for the probes. For details, please refer to “14.2.1 Exam
Selection”.
4.3.2 Selecting Exam Modes and Probes
Select the exam mode(when connecting with one probe):
(1) Press <Exam> to open the following dialog box.
(2) Roll the trackball and press <Set> to select the exam mode.
4-8 Exam Preparation
Page 77

Select the exam mode (when more than one probe are connected):
(1) Press <Exam> to open the following dialog box.
(2) Roll the trackball and press <Set> to select the exam mode, and use the left/right
keys of the soft menu controls to switch the probe.
To save the current settings for the current exam mode quickly:
(1) Press <Exam> to open the dialog box.
(2) Click [Save], to save the image parameters of the current image mode as setup
data. A dialogue box pops up to prompt you that the operation will cover the
current image preset data.
(3) Click [Save as] to save the current image parameter, measurement, comments
and body mark settings to the designated exam mode.
Click [Exam Preset] to enter the exam preset screen. For exam preset, please refer to
“14.2 Exam Preset”.
Exit:
Click [Exit] or press <Exam> to exit, press <B>, <Freeze> or <ESC> can also exit the
screen.
Exam Preparation 4-9
Page 78

4.4 Select the Imaging Mode
Use the corresponding keys in the control panel to enter the imaging modes.
For the detailed operations in each imaging mode, please refer to “5 Image Optimization”.
4.5 Activate & Continue an Exam
4.5.1 Activate an Exam
Select an exam that is finished within 24 hours, click [Activate Exam] in “iStation” or
“Review” screen to activate the exam.
Tips:
The system can automatically load the patient information and exam data to
continue the exam.
If you want to activate an exam with data in an external memory database, you
have to first import the patient data to the system’s patient database.
4.5.2 Continue an Exam
Select an exam that is paused within 24 hours, click [Continue Exam] in “iStation” or
“Review” screen to continue the exam.
If you want to continue an exam with data in an external memory database, you have to
first allow the system to load the patient data to the system’s patient database.
4.6 Pause Exam and End Exam
4.6.1 Pause an Exam
Sometimes, you have to stop an uncompleted exam due to some special causes or
system power off/ when the exam is paused, the system can begin other exams.
1. Press <Patient> to enter "Patient Info" screen.
2. Click [Pause Exam].
If the system is powered off during scanning, the exam status turns "paused" after the
system restart.
Only one exam can be paused every time.
When an exam is paused, the system will do the following:
1. Save the exam-related images, reports and measurement data, and modify the status
as “Paused”.
2. Save the exam information, including report, imaging mode, exam mode, image
parameters, operation mode, imaging /measurement data and so on.
3. If the system has been configured with a MPPS server, it will send the status
information to the server. For details, please refer to the DICOM chapter.
4-10 Exam Preparation
Page 79

4.6.2 End an Exam
Before examining a new patient, press <End Exam> to end the exam of the previous
patient, update the patient ID and information, to avoid mixing data of the next new
patient.
To end an exam, you can do one of the following:
Press <End Exam> on the control panel.
Click [New Patient] on the Patient Info screen to end the last patient exam and
clear the patient information.
Click [New Exam] on the Patient Info screen (or iStation screen, or Review screen)
to end the last exam and clear the exam data.
4.7 Cancel an Exam
An undergoing exam can be stopped by clicking [Cancel Exam] in the Patient Info screen.
1. Press <Patient> to enter "Patient Info" screen.
2. Click [Cancel Exam].
3. The system prompts the confirmation message, and requires to select the reason for
canceling the exam as the following figure:
4. Select the reason and click [OK].
5. After the above operations being taken, the exam is cancelled, and images,
measurements and report are saved with the status of the exam to be "Canceled".
If the system has been configured with a MPPS server, it will send the status
information "Cancelled" to the server. For details, please refer to relevant description
in the DICOM chapter.
Exam Preparation 4-11
Page 80

4.8 Anonymous Patient Exam
The system can also carry out image scanning and measurement even though no patient
information is available:
Deselect "Save image without patient info" in "[Setup] -> [System Preset] ->
[General]".
When saving an image, the system pops up the Patient Info screen to ask for the
patient information.
Enter the information and click [OK] to save the image and data.
Select [Cancel] and the system will not save the images.
If "Save image without patient info" is selected.
The system will automatically generate an ID, and save the images in the ID.
4-12 Exam Preparation
Page 81

5
Image Optimization
WARNING:
1. The images displayed in this system are only reference
for diagnosis. Mindray is not responsible for the
correctness of diagnostic results. It is the responsibility
of the clinician, who performs the exam, to capture the
correct diagnostic results.
2. In Dual-B imaging mode, the measurement results of the
merged image may be inaccurate. Therefore, the results
are provided for reference only, not for confirming a
diagnosis.
5.1 Switching Between Imaging Modes
Key Description
B Mode key: press to enter B Mode.
M Mode key: press to enter M Mode.
PW Mode key: press to enter PW Mode.
In 3D/4D mode, press to enter 3D image in single window.
User-defined key
CW Mode key: press to enter CW Mode.
Color Mode key: press to enter Color Mode.
Power mode key: press to enter Power Mode.
Dual-split display key: press to enter the Dual-split display mode, and
to switch between the windows.
Press to change the currently active window.
Press to start or end image capture in 3D/4D or iScape.
Press to enter the quad-split screen display, or switches image
window in the Quad mode.
Click to enter different modes: 3D/4D, iScape, TDI, Free Xros M,
Stress Echo.
Define buttons for Contrast Imaging mode and Elastography;
Press to enter.
Image Optimization 5-1
Page 82

5.2 Image Adjustment
Before optimizing the image by adjusting image parameters, adjust the brightness and
contrast of the monitor to the best.
Requirement Available Operations
Adjust gain
To modify the brightness
To modify gray scale image
effect
To increase frame rate of gray
scale imaging
Adjust TGC
Adjust [A. power] (do try to adjust gain first before
increasing the acoustic power)
Adjust [Dyn Ra.]
Adjust [Gray Map]
Adjust [Persistence]
Adjust [iClear] (optional)
Decrease depth
Decrease the [Focus Number] in B mode
Decrease the [FOV] in B mode
Decrease [Line Density]
Turn on [High FR] in harmonic mode
Decrease ROI in Color/Power mode
To increase frame rate of color
imaging
To modify flow images effect
(Resolution and sensitivity)
Adjustment through image menu
Press <Menu> or multifunctional knob to open the menu; use the multifunctional knob
to adjust.
Adjustment through soft menu controls
The soft menu locates at the bottom of the screen, items of which are dependent
upon image modes and preset. Take B mode adjustment as an example.
Turn on [B/C Wide] in Color/Power mode
Decrease [Packet Size] in Color mode
Decrease [Line Density]
Adjust [Freq]
Adjust [Scale]
Adjust [Packet Size]
Adjust [Line Density]
Adjust [Smooth]
Image mode switching
5-2 Image Optimization
Page 83

Use left/ right controls in soft menu controls <4> to switch among the modes. The
soft menu items vary depending on the mode. The menu navigation will cause
the change of the menu, while the change of the menu will cause the menu
navigation.
Page up/down
Use the up/down keys in soft menu controls <4> to turn pages up or down; you
can operate the items only when they are highlighted.
Soft menu controls operation
a) The soft menu items are operated respectively through the five groups of soft
menu controls <1>, <2>, <3>, <5> and <6>.
b) You can also move the cursor to the certain soft menu item, and then press
<Set> or rotate the multifunctional knob to adjust.
Adjustment through image parameter area
The image parameter area is located in the upper left corner of the screen. The image
parameters are displayed while the image menu is not available.
(1) Move the cursor to the item on the parameter area, and a frame will be around the
certain item, as .
(2) Press <Set> to adjust, and rotate the multifunctional knob to select the values.
Adjustment through control panel
Trackball, control panel key, knob or sliders.
Adjustment through grayscale/ color bar
Move the cursor onto the grayscale bar, and press <Set> to switch among the
gray maps.
Move the cursor onto the color bar, and press <Set> to switch among the color
maps.
Image Optimization 5-3
Page 84

5.3 B Mode Image Optimization
B mode is the basic imaging mode that displays real-time images of anatomical tissues
and organs.
5.3.1 B Mode Exam Protocol
1. Enter the patient information, and select the appropriate probe and exam mode.
2. Press <B> on the control panel to enter B mode.
3. Adjust parameters to optimize the image.
4. Perform other operations (e.g. measurement and calculation) if necessary.
In real-time scanning of all imaging modes, press <B> on the control panel to return to
B mode.
5.3.2 B Mode Parameters
In B Mode scanning, the image parameter area in the upper left corner of the screen
will display the real-time parameter values as follows:
Display F 5.0 D 17.6 G 50 FR 40 IP 4 DR 60
Parameter Frequency Depth Gain Frame Rate B IP B Dynamic Range
Items that appear in the menu or the soft menus are dependent upon preset, which
can be changed or set through "[Setup] -> [Image Preset]"; please refer to "5.16
Image Preset" for details.
5.3.3 B Mode Image Optimization
Gain
Description To adjust the gain of the whole receiving information in B mode. The
real-time gain value is displayed in the image parameter area in the upper
left corner of the screen.
Operation Rotate the <iTouch> knob clockwise to increase the gain, and
anticlockwise to decrease.
Or adjust it in the image parameter area.
Effects Increasing the gain will brighten the image and you can see more received
signals. However, noise may also be increased.
5-4 Image Optimization
Page 85

Depth
Description This function is used to adjust the display depth of sampling, the real-time
value of which is displayed on the image parameter area in the upper left
corner of the screen.
Operation To change depth, press keys in the lower right corner of the control panel.
Press key to decrease the depth; Press key to increase the
depth. The adjustable depth values vary depending upon the probe types.
Effects Increase the depth to see tissue in deeper locations, while decrease the
depth to see tissue in shallower locations.
Impacts Depth increase will cause a decrease in the frame rate.
TGC
Description The system compensates the signals from deeper tissue by segments to
optimize the image.
Operation To increase the gain compensation at an area of interest, move the TGC
slider to the right.
To decrease the gain compensation at the corresponding area of interest,
move the TGC slider to the left.
About 1.5s after the adjustment is finished, the TGC curve disappears.
Effects Adjust the signal gain for the certain image area to get a balanced image.
Frequency
Description This function is used to select the operating frequency of the current probe,
the real-time value of which is displayed in the image parameter area in the
upper left corner of the screen, where “F” represents B mode frequency,
and “FH” represents harmonic frequency.
Operation You can select a harmonic frequency or a B mode frequency.
Adjust the frequency value through the [Frequency] item in the soft menu
or menu, “H” indicates harmonic frequency.
Or adjust it in the image parameter area.
Values of frequency vary depending upon the probe types.
Select the frequency according to the detection depth and current tissue
characteristics.
Effects The higher the frequency the better the near field resolution but the worse
the force of penetration.
Harmonic imaging enhances near field resolution and reduces
low-frequency and large amplitude noise, so as to improve small parts
imaging.
Acoustic Power
Description Refers to the power of ultrasonic wave transmitted by the probe, the
real-time value of which is displayed in the image parameter area in the
upper left corner of the screen.
Image Optimization 5-5
Page 86

Operation Adjust through the [A. power] item in the soft menu or menu.
The adjusting range vary depending upon probe types.
Effects Generally, increasing the acoustic power will increase the brightness and
contrast of the image as well as the force of penetration.
Impacts You should perform exams according to actual situation and follow the
ALARA Principle.
Focus
Description
Operation Adjust the focus number through the [Focus Number] in the soft menu or
Effects The area that is focused will be of a higher contrast and resolution to
Impacts The greater the number of focus, the slower the frame rate to image.
Refers to adjustment of focus of the ultrasonic beams, symbols as " " of
which are displayed on the right of the image.
menu.
Adjust the focus position through the [Focus Position] in the soft menu or
menu.
provide a much clearer image.
Imaging Display Adjustment
Description More information can be obtained without moving the probe or changing
the sampling position.
FOV (Field
of View)
FOV
Position
Change the scan range through the [FOV] item in the soft menu or menu.
You can get a much larger field of view when selecting a larger FOV, but
the frame rate will decrease.
Change the scan position through the [FOV Position] item in the soft menu
or menu.
When the scan range is "W”, the FOV position cannot be changed.
B Steer This function is to steer the beam transmitted by the probe.
Steer the transmitting beam through the [B Steer] item in the soft menu or
menu.
The steering angles vary depending upon the probe types.
Trapezoid Turn on or off the function through [Trapezoid] item in the soft menu or
menu.
You can define user-defined key for trapezoid function in "[Setup] ->
[System Preset] -> [Key Config]".
ExFOV Turn on or off the function through [ExFOV] item in the soft menu or menu.
You can define user-defined key for ExFOV function in "[Setup] -> [System
Preset] -> [Key Config]".
Impacts The FOV position is available only for the convex and phased probes.
The B Steer and Trapezoid function are available only for linear probes.
The ExFOV function is available only for convex probes.
5-6 Image Optimization
Page 87

Line Density
Description The function determines the quality and information of the image.
Operation Adjust through the [Line Density] item in the soft menu or menu.
Effects The higher the line density, the higher the resolution, and the lower the
frame rate.
Dynamic Range
Description This function is used to adjust the B image resolution to compress or
expand the gray display range.
The real-time dynamic range value is displayed on the image parameter
area in the upper left corner of the screen.
Operation Adjust through the [Dyn Ra.] item in the soft menu or menu.
Or adjust it in the image parameter area.
Effects The more the dynamic range, the more specific the information, and the
lower the contrast with more noise.
iClear
Description This function is used to increase image profile, so as to distinguish the
image boundary.
Operation Adjust through the [iClear] item in the soft menu or menu.
Effects The bigger the value the more clearly the profile of the image.
Impacts This function is optional.
Smooth
Description This function is to reject image noise and to make images smooth.
Operation Adjust through the [Smooth] item in the soft menu or menu.
Persistence
Description This function is used to superimpose and average adjacent B images, so
as to optimize the image and remove noises.
Operation Adjust through the [Persistence] item in the soft menu or menu.
Effects Persistence can remove image noise to make details to be clearer.
Impacts Persistence increase may lead to signal missing.
Invert /Rotation
Description This function provides a better observation for image display.
Invert(U/D
Flip and
L/R Flip)
To invert the image horizontally or vertically.
Click [L/R Flip] or [U/D Flip] in the menu or the soft menu to invert the
image.
Rotation Rotate the image through the [Rotation] item in the menu or the soft menu.
When the image is rotated at the angle of 90° or 270°, the depth scale is
displayed on the upper part of the screen.
Image Optimization 5-7
Page 88

When you invert or rotate an image, the “M” mark will change its position correspondingly
on the screen; the M mark is located in the upper left corner of the imaging area by
default.
iBeam
Description This function is used to superimpose and average images of different steer
angles to obtain image optimization.
Operation Turn on or off the function through the [iBeam] item in the soft menu or
menu.
Effects Images after iBeam processing can be optimized with less spot noise and
higher resolution, so that more details for the structure are revealed.
Impacts When linear probe is being used, iBeam function is not available when
trapezoid function is turned on or B steer is adopted.
Image Merge
Description In the Dual-split mode, when the images of the two windows use the same
probe type, depth, invert status, rotation status and magnification factor,
the system will merge the two images so as to extend the field of vision.
Operation Turn on or off the function through the [Img Merge] item in the soft menu or
menu.
Impacts Image Merge is available only for linear probes.
Gray Map
Description This function applies the gray correction to obtain the optimum images.
Operation Select among the maps through the [Gray Map] item in the soft menu or
menu.
You can also adjust through the grayscale bar: move the cursor to the
grayscale bar and press <Set> on the control panel to adjust.
IP (Image Processing)
Description IP is a combination of several image processing parameters, which is used
for a fast image optimization, the real-time group of which is displayed in
the image parameter area in the upper left corner of the screen.
The IP combination parameters include dynamic range, iClear, persistence
and smooth.
Operation Select among the IP groups through the [IP] item in the soft menu or menu.
Or adjust it in the image parameter area.
Colorize and Colorize Map
Description Colorize function provides an imaging process based on color difference
rather than gray distinction.
Operation Turn on or off the function through the [Colorize] item in the soft menu or
menu.
Select the colorize map through the [Colorize Map] item in the soft menu or
menu.
5-8 Image Optimization
Page 89

TSI (Tissue Specific Imaging)
Description The TSI function is used to optimize the image by selecting acoustic speed
according to tissue characteristics.
Operation Select among the TSI modes through the [TSI] item in the soft menu or
menu.
The system provided 4 ways of optimization for specific tissues: general,
muscle, fluid and fat.
iTouch
Description To optimize image parameters as per the current tissue characteristics for
a better image effect.
Operation Press <iTouch> on the control panel to turn on the function, the symbol of
which will be displayed in the image parameter area of the screen; long
press <iTouch> to exit iTouch status.
Post Process
Description Post process is used to apply modifications to a gray map in order to
optimize overall image quality. Post process function includes 3
parameters: curve, gray rejection and γ.
Gray
Rejection
Curve To manually enhance or restrain the signal in the certain scale.
γ The γ correction is used to correct non-linear distortion of images.
Impacts The post process adjustment will not influence the cine review.
This function is to reject image signals less than a certain gray scale, then
the rejected signal corresponding area turns black.
Click [Gray Rejection] in the soft menu or menu to adjust.
The adjusting range is 0-5.
Click [Curve] in the soft menu or menu to open the dialogue box to adjust.
Drag the curve node to increase or decrease the gray scale information:
drag the node up to increase the information and down to decrease.
Click [γ] in the soft menu or menu to adjust.
The adjusting range is 0-3; the image turns dark when the value is
increased.
High FR
Description When the THI function is turned on, this function can provides you with
much higher frame rate.
Operation In single B mode when THI is turned on, click the [High FR] item in the soft
menu or menu to obtain images with high frame rates.
LGC
Description Adjust gain of the corresponding image areas to increase the image lateral
resolution.
Image Optimization 5-9
Page 90

Operation Adjust through the [LGC1]-[LGC8] item in menu.
The 8-segment LGC indicate the corresponding image areas on the main
screen.
Increasing the LGC value will increase the gain of the corresponding image
areas.
HScale
Description Display or hide the width scale (horizontal scale).
The scale of the horizontal scale is the same as that of vertical scale
(depth), they change together in zoom mode, or when the number of the
image window changes. When image is turned up/down, the HScale will
also be inverted.
Operation Click [HScale] on the soft menu to display or hide the scale.
Patient Temperature
Description If the current active probe is P7-3Ts, the parameter will display under
the B mode menu. You can enter the patient temperature by this
function.
Operation Enter the temperature by [Patient Temperature] on the soft menu.
WARNING
If the patient temperature is above 37°°°°C (98.6°°°°F) and the [Patient
Temperature] setting is below the actual reading, the system could
overestimate the temperature of TEE transducer’s distal tip. This
could trigger the Auto-Cool function. If the patient temperature
reaches or is above 37°°°°C (98.6°°°°F) and the [Patient Temperature]
setting is above the actual reading, the system could
underestimate the temperature of the TEE transducer's distal tip.
The patient is exposed to excessive temperatures.
5-10 Image Optimization
Page 91

5.4 M Mode Image Optimization
5.4.1 M Mode Exam Protocol
1. Select a high-quality image during B mode scanning, and adjust to place the area of
interest in the center of the B mode image.
2. Press <M> on the control panel, and roll the trackball to adjust the sampling line.
3. Press <M> on the control panel again or <Update> to enter M mode, and then you
can observe the tissue motion along with anatomical images of B mode.
During the scanning process, you can also adjust the sampling line accordingly when
necessary.
4. Adjust the image parameters to obtain optimized images.
5. Perform other operations (e.g. measurement and calculation) if necessary.
If you choose "Enter 1D Mode Directly" in "[Setup]->[Image Preset]->[Other]", then
the sampling line will be displayed at all times in B mode images, and pressing <M>
will directly enter M mode.
5.4.2 M Mode Parameters
In M mode scanning, the image parameter area in the upper left corner of the screen
displays the real-time parameter values as follows:
Display V 3 IP 6 DR 65 G 45
Parameter M Speed M IP M Dynamic Range M Gain
During M mode imaging, menus for B mode and M mode are displayed in the soft
menu at the same time, use the left/right keys of soft menu controls <4> to switch
between the menus of B mode and M mode.
During M mode scanning, frequency and acoustic power of the probe are
synchronous with that of B mode.
Adjustment of the depth, focus position or TGC to the B mode image will lead to
corresponding changes in M mode image.
Press <Update> to switch between the real-time B and freezing B images.
Items that appear in the menu or the soft menus are dependent upon preset, which
can be changed or set through "[Setup] -> [Image Preset]"; please refer to "5.16
Image Preset" for details.
Image Optimization 5-11
Page 92

5.4.3 M Mode Image Optimization
Gain
Description To adjust the gain of M mode image. The real-time gain value is displayed
in the image parameter area in the upper left corner of the screen.
Operation Rotate the <iTouch> knob clockwise to increase the gain, and
anticlockwise to decrease.
Or adjust it in the image parameter area.
The adjusting range is 0-100.
Effects Increasing the gain will brighten the image and you can see more received
signals. However, noise may also be increased.
Focus Position
Description
Operation Adjust the focus position through the [Focus Position] item in the soft menu
To change the focus position in M mode, symbols as " " of which are
displayed on the right of the image.
or menu.
Time Mark
Description To show the time mark in M mode image.
Operation Turn on or off the function through [Time Mark] item in the soft menu or
menu.
Effects When time mark is displayed on the M mode image, it's much easier to
identify the cardiac cycles and detect more details.
Display Format
Description To set the display format of M mode image with B mode image.
Operation Adjust through the [Display Format] item in the soft menu or menu.
There are 4 formats available for image display: L/R, V1:1, V1:2, Full.
Effects Adjust according to the actual situation and obtain a desired analysis
through comparison.
Speed
Description This function is used to set the scanning speed of M mode imaging, and
the real-time speed value is displayed in the image parameter area in the
upper left corner of the screen.
Operation Change the speed through the [Speed] item in the soft menu or menu.
Or adjust it in the image parameter area.
There are 6 levels of scan speed available, the smaller the value the faster
the speed.
Effects Speed changing makes it easier to identify disorders in cardiac cycles.
5-12 Image Optimization
Page 93

IP (Image Processing)
Description IP is a combination of several image processing parameters, which is used
for a fast image optimization. The IP combination number is displayed in
the image parameter area in the upper left corner of the screen.
The M IP combination parameters include dynamic range, edge enhance,
and M soften.
Operation Select among the IP groups through the [IP] item in the soft menu or menu.
Or adjust it in the image parameter area.
The system provides 8 groups of IP combinations, and the specific value of
each parameter can be preset.
Colorize and Colorize Map
Description Colorize function provides an imaging process based on color difference
rather than gray distinction.
Operation Turn on or off the function through the [Colorize] item in the soft menu or
menu.
Select the colorize map through the [Colorize Map] item in the soft menu or
menu.
The system provides 10 different maps to be selected among.
Post Process
Description Post process is used to apply modifications to the image in order to
optimize overall image quality. Post process function includes 3 parameters
to adjust: curve, gray rejection and γ.
Gray
Rejection
Curve To manually enhance or restrain the signal in the certain scale.
γ The γ correction is used to correct non-linear distortion of images.
Impacts The post process adjustment will not influence the cine review.
This function is to reject image signals less than a certain gray scale.
Click [Gray Rejection] in the soft menu or menu to adjust.
The adjusting range is 0-5.
Click [Curve] in the soft menu or menu to open the dialogue box to adjust.
Drag the curve node to increase or decrease the gray scale information:
drag the node up to increase the information and down to decrease.
Click [γ] in the soft menu or menu to adjust.
The adjusting range is 0-3.
Gray Map
Description This function applies the gray correction to obtain the optimum image
through adjusting 3 parameters: curve, gray rejection and γ.
Image Optimization 5-13
Page 94

Operation Select among the maps through the [Gray Map] item in the soft menu or
menu.
You can also adjust through the grayscale bar: move the cursor to the
grayscale bar and press <Set> on the control panel to adjust.
The system provides 8 groups of gray map.
Edge Enhance
Description This function is used to increase image profile, so as to distinguish the
image boundary.
Operation Adjust through the [Edge Enhance] item in the soft menu or menu.
The system provides 4 levels of edge enhance effects. The bigger the
value the stronger the effect.
Impacts Larger edge enhance may lead to noise increasing.
Dynamic Range
Description This function is used to adjust the B image resolution to compress or
expand the gray display range. The real-time dynamic range value will be
displayed in the image parameter area in the upper left corner of the
screen.
Operation Adjust through the [Dyn Ra.] item in the soft menu or menu.
Or adjust it in the image parameter area.
The adjusting range is 30-160dB in increments of 5.
Effects The more the dynamic range, the more specific the information, and the
lower the contrast with more noise.
M Soften
Description This feature is used to process the scan lines of M images to reject noise,
making the image details to be clearer.
Operation Adjust through the [M Soften] item in the soft menu or menu.
The system provides 5 levels of M Soften adjustment, the bigger the value
the stronger the effect.
5-14 Image Optimization
Page 95

5.5 Color Mode Image Optimization
The Color mode is used to detect color flow information, and the color is designed to judge
the direction and speed of blood flow.
Generally, the color above the color bar indicates the flow towards the probe, while the
color below the color bar indicates the flow away from the probe; the brighter the color, the
faster the flow speed; while the darker the color, the slower the flow speed.
5.5.1 Color Mode Exam Protocol
1. Select a high-quality image during B mode scanning, and adjust to place the area of
interest in the center of the image.
2. Press <Color> to enter B+Color mode. Use the trackball and <Set> to change
position and size of the Region of Interest (ROI).
3. Adjust the image parameters during scanning to obtain optimized images.
4. Perform other operations (e.g. measurement and calculation) if necessary.
5.5.2 Color Mode Image Optimization
In Color mode scanning, the image parameter area in the upper left corner of the
screen displays the real-time parameter values as follows:
Display F 3.3 G38 IP 1 WF 43 PRF 0.4k
Parameter Frequency Color
Gain
In Color mode, acoustic power is synchronous with that of B mode. Adjustment of the
depth or zoom to the B mode image will lead to corresponding changes in Color mode
image.
During Color mode imaging, menus for B mode and Color mode are displayed in the
soft menu at the same time, use the left/right keys of soft menu controls <4> to switch
the menus of B mode and Color mode.
Items that appear in the menu or the soft menus are dependent upon preset, which
can be changed or set through "[Setup] -> [Image Preset]"; please refer to "5.16
Image Preset" for details.
Color
IP
Color Wall
Filter
Pulse Repetition
Frequency PRF
Image Optimization 5-15
Page 96

5.5.3 Color Mode Image Optimization
Color Gain
Description Refers to the overall sensitivity to flow signals, and this function is used to
adjust the gain in Color mode. The real-time gain value is displayed in the
image parameter area in the upper left corner of the screen.
Operation Rotate the <iTouch> knob clockwise to increase the gain, and
anticlockwise to decrease.
Or adjust it in the image parameter area.
The adjusting range is 0-100.
Impacts Increasing the gain will increase the flow signal presented as well as noise,
while the signals may be missing when the gain is adjusted too low.
ROI Adjustment
Description To adjust the width and position of ROI in Color mode.
Operation When the ROI box is dotted line, roll the trackball to change its size.
When the ROI box is solid line, roll the trackball to change the position.
Press <Set> to switch between the solid line and the dotted line status.
You can set the default ROI position and size in "[System Preset]->[Image
Preset]-> Color".
Impacts The larger the ROI box, the lower the frame rate, and the lower the
resolution and color sensitivity.
Frequency
Description Refers to the operating frequency in Color mode of the probe, the real-time
value of which is displayed in the image parameter area in the upper left
corner of the screen.
Operation Select the frequency value through the [Frequency] item in the soft menu
or menu.
Or adjust it in the image parameter area.
Values of frequency vary by probes. Select the frequency value according
to the need of the detection depth and the current tissue characteristics.
Effects The higher the frequency, the worse the axial resolution, and the better the
force of penetration.
Focus Position
Description To adjust the color focus position against the ROI position.
Operation Change the position through the [Focus Position] item in the soft menu or
menu.
B Display
Description To turn on or off the B image display as Color imaging remains active.
5-16 Image Optimization
Page 97

Operation Turn on or off the function through the [B Display] item in the soft menu or
menu.
B/C Align
Description To set and constrain the maximum width of the B mode image to that of the
Color ROI.
Operation Turn on or off the function through the [B/C Align] item in the soft menu or
menu.
Impacts Frame rate will increase when the function is turned on.
Dual Live
Description This function is used to display B image and Color image synchronously.
Operation Click [Dual Live] in the soft menu or menu to turn on or off the function.
When the function is turned on, the window will be automatically switched
to the dual windows (one for B image, and the other for Color image).
Steer
Description The feature is used to adjust the ROI of color flow with different angles with
immobility of the linear probe.
Operation Adjust through the [Steer] item in the soft menu or menu, or rotate the
multifunctional knob to adjust.
Values of steering angles vary by probes.
Effects This function is used to adjust the scan angle of linear probes, so as to
change the angle between the transmitting beam and flow direction.
Impacts Steer is available only for linear probes.
Line Density
Description The function determines the quality and information of the image.
Operation Adjust through the [Line Density] item in the soft menu or menu.
There are 4 levels of line density provided: UH, H, M, L.
Effects The higher the line density, the higher the resolution.
Impacts The higher the line density, the smaller the frame rate.
Packet Size
Description This function is an indication of the ability to detect flow, which is used to
adjust the accuracy of color flow.
Operation Adjust through the [Packet Size] item in the soft menu or menu.
There are 4 levels of packet size provided, 0 represents no packet size
control and the bigger the value the higher the sensitivity.
Effects The higher the packet size, the more sensitive indication for low-velocity
flow.
Impacts Increasing the packet size will lead to frame rate decrease.
Image Optimization 5-17
Page 98

Flow State
Description This function is used for a fast image optimization by providing an
adjustment of 2 parameters, including scales and filters.
Operation Adjust through the [Flow State] item in the soft menu or menu.
There are 3 levels provided to adjust: L, M, H.
Persistence
Description This function is to adjust the temporal smooth in Color mode to optimize
the image.
Operation Adjust through the [Persistence] item in the menu or the soft menu.
The system provides 5 levels of persistence adjustment, 0 represents no
persistence and the bigger the value the stronger the effect.
Smooth
Description This feature is used to reject noise and smooth the image.
Operation Adjust through the [Smooth] item in the soft menu or menu.
The system provides 5 levels of smooth function, the bigger the value the
higher the smooth.
Scale
Description This function is used to adjust the speed range of color flow, which is
adjusted through PRF in the system. The real-time PRF value will be
displayed in the image parameter area in the upper left corner of the
screen.
Operation Adjust the speed range through the [Scale] item in the menu or the soft
menu.
The adjusting range varies by frequency, probe and depth; adjust
according to the actual situation.
Effects To provide a much clearer color flow image.
Use low PRF to observe low-velocity flows, and high PRF to observe
high-velocity flows.
Impacts Aliasing may occur if low velocity scale is used and high velocities are
encountered.
Low velocities may not be identified when a high velocity scale is used.
Baseline
Description Refers to the area where the velocity is zero in the scale. Adjust according
to the actual situation so as to get an optimum flow display.
Operation Adjust through the [Baseline] item in the soft menu or menu.
Positive value means to increase the signals above the baseline, and
negative value means to increase the signals below the baseline.
5-18 Image Optimization
Page 99

Invert
Description To set the display mode of the color flow, and the color scale will be
inverted when the function is turned on.
Operation Turn on or off the function through the [Invert] item in the soft menu or
menu.
Select "Auto Invert" in the "[Setup] -> [System Preset] -> [Image Preset]",
then the color bar can automatically invert when the color flow is steered to
a certain angle to accommodate operator’s habit of distinguishing flow
direction.
Map
Description This function is a combination of several image parameters, which
indicates the display effect of color image.
Operation Select among the maps through the [Map] item in the soft menu or menu.
You can also adjust through the color bar: move the cursor to the color bar
and press <Set> on the control panel to adjust.
The system provides 21 different maps to be selected among, where the V
group provides 11 ordinary maps and the VV group provides 10 2-D maps.
Color IP
Description Color IP is a combination of several image processing parameters, which is
used for a fast image optimization. The IP combination number is displayed
in the image parameter area in the upper left corner of the screen.
The Color IP combination parameters include persistence and smooth.
Operation Select among the maps through the [Color IP] item in the soft menu or
menu.
Or adjust it in the image parameter area.
The system provides 8 groups of IP combinations, and the specific value of
each parameter can be preset.
WF (Wall Filter)
Description It filters out low-frequency signals to provide effective information, and this
function is used to adjust the filtered frequency. The real-time value is
displayed in the image parameter area in the upper left corner of the
screen.
Operation Select through the [WF] item in the soft menu or menu.
Or adjust it in the image parameter area.
There are 8 levels of wall filter function, adjust according to the actual
situation and the probe.
Impacts Flow signals may be missing.
Priority
Description This function is used to set levels of the flow display, to display the
grayscale signal or color signal.
Image Optimization 5-19
Page 100

Operation Select the value through the [Priority] item in the soft menu or menu.
The adjusting range is 0-100%.
Effects The higher the value, color signals are prior to be displayed; while the
lower the value, grayscale signals are prior to be displayed.
iTouch
Description To optimize image parameters as per the current tissue characteristics for
a better image effect.
Operation Press <iTouch> on the control panel to turn on the function, the symbol of
which will be displayed in the image parameter area of the screen.
HR Flow (High Resolution Flow)
Description This function enhances the micro vessel imaging effect, and can be used to
analyze the tissue blood supply condition.
Operations Set [HR Flow] on the menu in the top-left corner of the screen to “On” to
turn on HR Flow status.
The parameters in HR Flow mode are independent from those in Color
mode.
To use HR Flow feature, you need to select probe L12-4s/ 7L4s in Thyroid
exam mode or C5-2s/ 3C5s in Fetal Cardiac exam mode.
Smart Track
Description To optimize image parameters as per the current tissue characteristics for
a better image effect. The angle and the position of the ROI are adjusted
after the function is enabled. The area is tracked without being affected by
the dynamic moves.
Operation Tap [Smart Track] on the soft menu under Color/Power mode. The vessels
lay in the middle of the ROI.
Impacts The probe L12-4s and 7L4s for Vascular, carotid and EM VAS exam modes
supports the smart track.
5-20 Image Optimization
 Loading...
Loading...