Page 1

/VS-900c
Vital Signs Monitor
Service Manual
Page 2

Page 3

Intellectual Property Statement
SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD. (hereinafter called Mindray)
owns the intellectual property rights to this product and this manual. This manual may refer
to information protected by copyrights or patents and does not convey any license under
the patent rights of Mindray, nor the rights of others. Mindray does not assume any liability
arising out of any infringements of patents or other rights of third parties.
Mindray intends to maintain the contents of this manual as confidential information.
Disclosure of the information in this manual in any manner whatsoever without the written
permission of Mindray is strictly forbidden. Release, amendment, reproduction, distribution,
rent, adaption and translation of this manual in any manner whatsoever without the written
permission of Mindray is strictly forbidden.
, , and are the registered trademarks or trademarks owned by
Mindray in China and other countries. All other trademarks that appear in this manual are
used only for editorial purposes without the intention of improperly using them. They are
the property of their respective owners.
This posting serves as notice under 35 U.S.C.§287(a) for Mindray patents:
http://www.mindrayna.com/patents.
The issued date for this manual is May 2019 (Version 11.0).
© Copyright 2013-2019 Shenzhen Mindray Bio-Medical Electronics Co., Ltd. All rights
reserved.
WARNING
Federal Law (USA) restricts this device to sale by or on the order of a
physician.
NOTE
This manual describes all features and options. The equipment may not have
all of them. Contact Mindray service department for any questions.
I
Page 4

Manufacturer’s Responsibility
Contents of this manual are subject to changes without prior notice.
All information contained in this manual is believed to be correct. Mindray shall not be
liable for errors contained herein nor for incidental or consequential damages in connection
with the furnishing, performance, or use of this manual.
Mindray is responsible for safety, reliability and performance of this product only in the
condition that:
All installation operations, expansions, changes, modifications and repairs of this
product are conducted by Mindray authorized personnel; and
The electrical installation of the relevant room complies with the applicable
national and local requirements; and
This product is operated under strict observance of this manual.
Return Policy
In the event that it becomes necessary to return a unit to Mindray, follow the instructions
below.
1. Obtain a return authorization.
Contact the Mindray Service Department and obtain a Mindray Customer Service
Authorization Number. The Mindray Customer Service Authorization Number
must appear on the outside of the shipping container. Return shipments will not
be accepted if the Mindray Customer Service Authorization Number is not clearly
visible. Please provide the model number, serial number, and a brief description of
the reason for return.
2. Freight policy
The customer is responsible for freight charges when this product is shipped to
Mindray for service (including any relevant customs fees or other freight related
charges).
3. Return address
Please send the part(s) or equipment to the address offered by Customer Service
Department.
II
Page 5

Contact Information
Manufacturer:
Address:
Tel :
Fax:
Website:
Distributor:
Address:
Tel :
Website:
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Mindray Building, Keji 12th Road South, Hi-tech Industrial Park,
Nanshan, Shenzhen 518057 P.R. China
+86 755 81888998
+86 755 26582680
www.mindray.com
Mindray DS USA, Inc.
800 MacArthur Boulevard Mahwah, New Jersey 07430 USA
1.800.288.2121, 1.201.995.8000
www.mindray.com
III
Page 6

Preface
Manual Purpose
This manual provides detailed information about the assembling, dissembling, testing and
troubleshooting of the equipment to support effective troubleshooting and repair. It is not
intended to be a comprehensive, in-depth explanation of the product architecture or
technical implementation.
Observance of the manual is a prerequisite for proper equipment maintenance and
prevents equipment damage and personnel injury.
Intended Audience
This manual is for biomedical engineers, authorized technicians or service representatives
responsible for troubleshooting, repairing and maintaining the equipment.
Passwords
A password may be required to access different modes of the following equipment. The
passwords are listed below:
User maintenance: 888888
Configuration mode: 315666
Demo mode : Contact Mindray service personnel for the password
Factory maintenace: Contact Mindray service personnel for the password
It is recommended that the user should change the passwords for user maintenance and
configuration mode once they take ownership of the equipment.
IV
Page 7

Contents
1 Introduction .............................................................................................................. 1-1
1.1 Manual Information ................................................................................................................. 1-1
1.2 Safety Information .................................................................................................................... 1-1
1.2.1 Warnings ......................................................................................................................... 1-2
1.2.2 Cautions .......................................................................................................................... 1-2
1.2.3 Notes ................................................................................................................................ 1-2
1.3 Equipment Symbols ................................................................................................................ 1-2
2 Theory of Operation ................................................................................................. 2-1
2.1 Overview ...................................................................................................................................... 2-1
2.2 Connectors for Peripheral Devices ..................................................................................... 2-2
2.3 Main Unit ..................................................................................................................................... 2-2
2.4 Front Housing Assembly ........................................................................................................ 2-4
2.5 Main Board .................................................................................................................................. 2-4
2.6 Rear Housing Assembly .......................................................................................................... 2-5
2.7 External Module ........................................................................................................................ 2-6
3 Equipment Installation ............................................................................................ 3-1
3.1 Unpacking the Equipment .................................................................................................... 3-1
3.2 Preparation for Installation ................................................................................................... 3-1
3.2.1 Preparation for Installation Site .............................................................................. 3-1
3.2.2 Environmental Requirements ................................................................................. 3-1
3.2.3 Electrical Requirements ............................................................................................ 3-2
3.3 Equipment Installation ........................................................................................................... 3-2
3.3.1 Preparation for Power on .......................................................................................... 3-3
4 Testing and Maintenance ......................................................................................... 4-1
4.1 Introduction ................................................................................................................................ 4-1
4.2 Performance Tests .................................................................................................................... 4-1
4.2.1 Performance Test Frequencies ................................................................................ 4-2
4.2.2 Visual Inspection .......................................................................................................... 4-2
4.2.3 SpO2 test ......................................................................................................................... 4-2
4.2.4 NIBP Test ......................................................................................................................... 4-4
4.2.5 CO2 Test (Only Available For Accutorr 7).............................................................. 4-7
4.2.6 Exergen TemporalScanner Thermometer Test .................................................. 4-9
4.2.7 SmarTempTM Module Test ......................................................................................... 4-9
4.3 Electrical Safety and Other Tests ...................................................................................... 4-10
4.3.1 Electrical Safety and Other Test Frequencies .................................................. 4-10
4.3.2 Electric Safety Tests .................................................................................................. 4-10
4.3.3 Power-on Test............................................................................................................. 4-10
4.3.4 Touchscreen Calibration ......................................................................................... 4-11
4.3.5 Recorder check .......................................................................................................... 4-11
4.3.6 Bar Code Scanner Test ............................................................................................. 4-11
4.3.7 Battery Check ............................................................................................................. 4-12
4.3.8 Nurse Call Reply Performance Test ..................................................................... 4-12
4.4 Factory Maintenance ............................................................................................................ 4-13
1
Page 8

4.4.1 Accessing Factory Maintenance Menu ............................................................ 4-13
4.4.2 Drawing Waves .......................................................................................................... 4-13
4.4.3 Software Version ....................................................................................................... 4-14
4.4.4 Monitor Information ............................................................................................... 4-15
5 Troubleshooting ....................................................................................................... 5-1
5.1 Overview ..................................................................................................................................... 5-1
5.2 Part Replacement ..................................................................................................................... 5-1
5.3 Checking Equipment Status ................................................................................................. 5-1
5.4 Checking Software Version ................................................................................................... 5-1
5.5 Checking Technical Alarms ................................................................................................... 5-2
5.6 Troubleshooting Guide .......................................................................................................... 5-2
5.6.1 Power On/Off Failure ................................................................................................. 5-2
5.6.2 Display Failures ............................................................................................................ 5-3
5.6.3 Alarm Lamp Failures ................................................................................................... 5-4
5.6.4 Button and Encoder Failures ................................................................................... 5-4
5.6.5 Sound Failures .............................................................................................................. 5-5
5.6.6 Battery Failures ............................................................................................................. 5-5
5.6.7 Recorder Failures ......................................................................................................... 5-6
5.6.8 Output Interface Failure............................................................................................ 5-7
5.6.9 Data Storage Failure ................................................................................................... 5-7
5.6.10 Wired Network Related Problems ....................................................................... 5-8
5.6.11 Wi-Fi Related Problems (Only Available for Accutorr 7) .............................. 5-9
5.6.12 Module defective ...................................................................................................... 5-9
5.6.13 Exergen TemporalScanner Thermometer Module Problems ................ 5-10
5.6.14 CO2 Module Problems (Only Available for Accutorr 7) ............................. 5-11
5.6.15 Technical Alarm Messages .................................................................................. 5-11
6 Disassembly and Repair .......................................................................................... 6-1
6.1 Tools Required ........................................................................................................................... 6-1
6.2 Preparations for Disassembly .............................................................................................. 6-1
6.3 Disassembling the Main Unit ............................................................................................... 6-2
6.3.1 Disassembling the Temperature Module (Optional) ...................................... 6-2
6.3.2 Removing the Recorder ............................................................................................ 6-3
6.3.3 Separating the Front and Rear Half of the Monitor ........................................ 6-3
6.3.4 Removing the Parameter Connector Panel Assembly ................................... 6-5
6.3.5 Disassembling the Main Bracket Assembly ....................................................... 6-6
6.3.6 Removing the Parameter Board (SpO2 Optional) and Power Management
Board .......................................................................................................................................... 6-8
6.3.7 Removing the Parameter Connector Panel Assembly (With CO2, Only
Available for Accutorr 7) ...................................................................................................... 6-9
6.3.8 Disassembling the CO2 Module Assembly (Only Available for Accutorr 7)6-10
6.3.9 Disassembling CO2 main Module (Only Available for Accutorr 7) ......... 6-10
6.3.10 Disassembling the Main Bracket Assembly (With CO2, Only Available for
Accutorr 7) ............................................................................................................................. 6-11
6.3.11 Disassembling Pumps and Valves ................................................................... 6-12
6.3.12 Disassembling AC/DC Power Board and Battery Converter Board ..... 6-12
6.4 Disassembling the Front Housing Assembly .............................................................. 6-13
6.4.1 Removing the Touchscreen Control Board ..................................................... 6-13
6.4.2 Removing the 2.4G Wi-Fi Module (Optional, Only Available for Accutorr 7)
................................................................................................................................................... 6-14
2
Page 9

6.4.3 Removing the 5G Wi-Fi Module (Optional, Only Available for Accutorr 7)6-15
6.4.4 Removing the Main Control Board .................................................................... 6-17
6.4.5 Removing SD Card ................................................................................................... 6-17
6.4.6 Removing the Touchscreen .................................................................................. 6-18
6.4.7 Disassembling the Display .................................................................................... 6-18
6.4.8 Removing the Keypad ............................................................................................ 6-19
6.4.9 Removing the Encoder ........................................................................................... 6-19
6.4.10 Removing the Alarm Lamp................................................................................. 6-19
6.5 Disassembling the SmarTempTM Module (Optional) ................................................. 6-20
6.5.1 Removing the SmarTempTM Module PCBA and Module Power Board PCBA
................................................................................................................................................... 6-20
6.5.2 Disassembling the Temperature On-Position Detection Board PCBA .. 6-21
6.6 Disassembling the Exergen TemporalScanner Themometer Module (Optional)6-22
7 Parts ........................................................................................................................... 7-1
7.1 Introduction ................................................................................................................................ 7-1
7.2 Main Unit ..................................................................................................................................... 7-1
7.2.1 Exploded View .............................................................................................................. 7-1
7.2.2 Parts List .......................................................................................................................... 7-2
7.3 Front Housing Subassembly (Touchscreen) ................................................................... 7-3
7.3.1 Exploded View .............................................................................................................. 7-3
7.3.2 Parts List .......................................................................................................................... 7-4
7.4 Rear Housing Assembly (without CO2 module, CO2 Only Available for Accutorr 7)
................................................................................................................................................................ 7-5
7.4.1 Exploded View .............................................................................................................. 7-5
7.4.2 Parts List .......................................................................................................................... 7-5
7.5 Rear Housing Assembly (with CO2 module, Only Available for Accutorr 7) ........ 7-7
7.5.1 Exploded View .............................................................................................................. 7-7
7.5.2 Parts List .......................................................................................................................... 7-7
7.6 Main Bracket Assembly .......................................................................................................... 7-9
7.6.1 Exploded View .............................................................................................................. 7-9
7.6.2 Parts List .......................................................................................................................... 7-9
7.7 Power Management Board Assembly ............................................................................ 7-10
7.7.1 Exploded View ........................................................................................................... 7-10
7.7.2 Parts List ....................................................................................................................... 7-10
7.8 Parameter Connector Panel Assembly (without CO2 module, CO2 Only Available for
Accutorr 7) ....................................................................................................................................... 7-11
7.8.1 Exploded View ........................................................................................................... 7-11
7.8.2 Parts List ....................................................................................................................... 7-11
7.9 Parameter Connector Panel Assembly (with CO2 module, Only Available for
Accutorr 7) ....................................................................................................................................... 7-12
7.9.1 Exploded View ........................................................................................................... 7-12
7.9.2 Parts List ....................................................................................................................... 7-12
7.10 Predictive Temperature Assembly ................................................................................ 7-13
7.10.1 Exploded View ......................................................................................................... 7-13
7.10.2 Parts List .................................................................................................................... 7-13
7.11 Exergen TemporalScanner Thermometer Assembly .............................................. 7-14
7.11.1 Exploded View ......................................................................................................... 7-14
7.11.2 Parts List .................................................................................................................... 7-15
7.12 Exergen frame Assembly .................................................................................................. 7-16
7.12.1 Exploded View ......................................................................................................... 7-16
3
Page 10

7.12.2 Parts List .................................................................................................................... 7-16
8 Hardware and Software Upgrade ........................................................................... 8-1
8.1 Hardware Upgrade .................................................................................................................. 8-1
8.1.1 Upgrade Package ........................................................................................................ 8-1
8.1.2 Upgrading Parameter Modules ............................................................................. 8-1
8.1.3 Upgrading Temperature ........................................................................................... 8-2
8.1.4 Upgrading Wi-Fi (Only Available for Accutorr 7) .............................................. 8-2
8.1.5 Enabling Parameter Functions ............................................................................... 8-3
8.2 Software Upgrade .................................................................................................................... 8-3
A Electrical Safety Inspection .................................................................................... A-1
A.1 Power Cord Plug ..................................................................................................................... A-1
A.2 Device Enclosure and Accessories ................................................................................... A-2
A.3 Device Labeling ...................................................................................................................... A-2
4
Page 11

1 Introduction
1.1 Manual Information
Version Revision History
1.0 New
2.0 Modify temperature module test method, update parts list
3.0 Modify the Wi-Fi content
4.0 Delete the equipment symbols
5.0
6.0 Add CO2 description
7.0 Update parts list for new cleaning and disinfecting agents
8.0 Add VS-900c
9.0 Add the VS-900c silicon button.
10.0 Delete the warranty and exemption information
11.0 Add Exergen TemporalScannerTM Themometer contents
1.2 Safety Information
Modify the description of equipment symbols, add the patent
information
WAR NIN G
Indicates a potential hazard or unsafe practice that, if not avoided, will result
in death or serious injury.
CAUTION
Indicates a potential hazard or unsafe practice that, if not avoided, could
result in minor personal injury or product/property damage.
NOTE
Provides application tips or other useful information to ensure that you get
the most from your product.
1-1
Page 12

1.2.1 Warnings
WAR NIN G
All installation operations, expansions, changes, modifications and repairs of
this product are conducted by Mindray authorized personnel.
There is high voltage inside the equipment. Never disassemble the
equipment before it is disconnected from the AC power source or the
battery.
When you disassemble/reassemble a parameter module, a patient leakage
current test must be performed before it is used again for monitoring.
The equipment must be connected to a properly installed power outlet with
protective earth contacts only. If the installation does not provide for a
protective earth conductor, disconnect it from the power line and operate it
on battery power, if possible.
Disposal of the packaging material should observe the applicable waste
control regulations and keeping it out of children’s reach.
1.2.2 Cautions
CAUTION
Make sure that no electromagnetic radiation interferes with the
performance of the equipment when preparing to carry out performance
tests. Mobile phone, X-ray equipment or MRI devices are a possible source of
interference as they may emit higher levels of electromagnetic radiation.
Before connecting the receiver to the power line, check that the voltage and
frequency ratings of the power line are the same as those indicated on the
unit’s label or in this manual.
Protect the equipment from damage caused by drop, impact, strong
vibration or other mechanical force during servicing.
1.2.3 Notes
NOTE
Refer to the operator’s manual for detailed operation and other information.
1.3 Equipment Symbols
See theapplicable Operator’s Manual for information about the symbols used on this
product and its packaging.
1-2
Page 13

y
2 Theory of Operation
2.1 Overview
The Monitor is intended for monitoring physiologic parameters, including SpO2, Pulse Rate,
NIBP and Temperature, on adult, pediatric, and neonatal patients in healthcare facilities by
clinical physicians or appropriate medical staff under the direction of physicians.
The equipment also:
Provides audible and visual alarm indications in case of patient or equipment
problems;
Provides display, review, storage and printing of monitored information;
Incorporates multiple input devices such as buttons, knob, and touchscreen; and,
Enables program upgrade over the network.
Monit or
Nurse Call
Reserved port
Other device
The above figure shows a system consisting of the vital signs monitor and its peripheral
Mindray Proprietary LAN
i
F
-
i
W
EMR Server
AP
CMS/Gatewa
devices. The vital signs monitor:
Can be used for monitoring the physiological parameters, giving alarms and
reviewing patient data, etc.
Supports recorder.
Supports nurse call function.
Supports Wi-Fi module, wired network, remote view, and communication with the
BeneVision Central Monitoring System (hereinafter called CMS) (only available for
the Accutorr 7).
Supports external AC power source and an internal battery.
Supports clinical data acquisition: by SD card
or by USB drive.
NOTE
Wi-Fi module is only available for the Accutorr 7.
2-1
Page 14
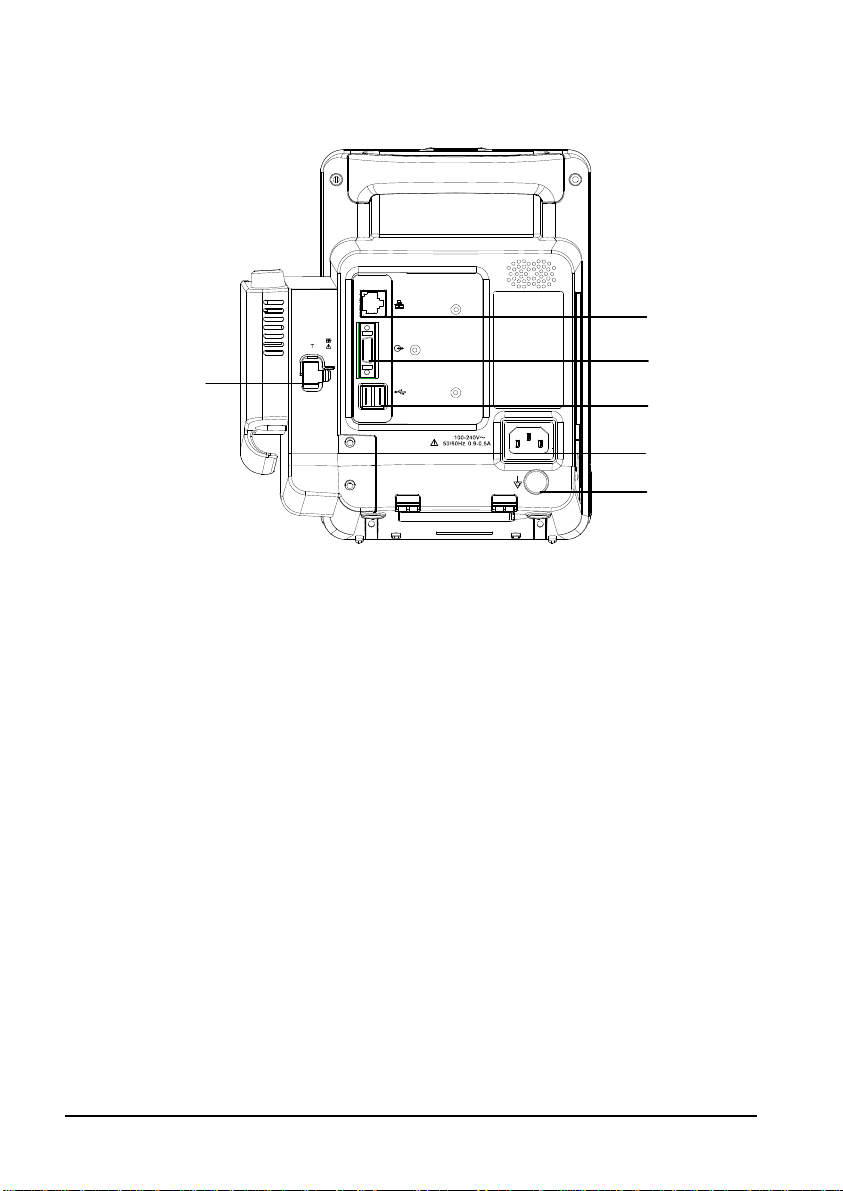
2.2 Connectors for Peripheral Devices
1
2
3
4
5
6
1. Connector for Temperature probe
2. Network connector: It is a standard RJ45 connector used to communicate with
external devices, such as central monitoring system, e-Gateway, or used to export
data or upgrade the system.
3. Multi-function connector: connects to the hospital's nurse call system, or connects
external devices through DIAP protocol.
4. USB connector: connects to barcode scanner or USB disk.
5. AC Power Input
6. Equipotential grounding terminal: When the equipment and other devices are to
be used together, their equipotential grounding terminals should be connected
together to eliminate the potential difference between them.
2.3 Main Unit
The main unit of the vital signs monitor consists of three parts:
Front housing assembly: main board, Wi-Fi module (only available for Accutorr 7),
keypad board assembly (knob), display, touchscreen, and alarm lamp board.
Rear housing assembly: power module (AC/DC), power management and
interface board (including SpO
module, and SpO
board.
2
External module:Temperature module.
isolation power), recorder, speaker, battery, NIBP
2
2-2
Page 15

The following figure shows the main unit architecture of the vital signs monitor.
Front Housing Assembly
Alarm lamp
board
Knob
Power
On/Off
keypad
(Indicator)
SD card
Main board
Wi-Fi module
I2C
LVD
S
Backlight
Touchscree
n control
board
LCD display
Touch
screen
Rear Housing Assembly
Speaker
Recorder
2600mAh
battery
15V
AC-DC power
module
AC-IN
Power
management
and interface
board
SpO
2
isolation power
External interface
RJ45/USB/Multifunctional
(nurse call, extended ports)
Pump
NIBP module
SpO2 board
(Standard
Mindray, OEM
optional)
NIBP socket
SpO2socket
External Module
Predictive
Temp
module
Temp
probe
2-3
Page 16
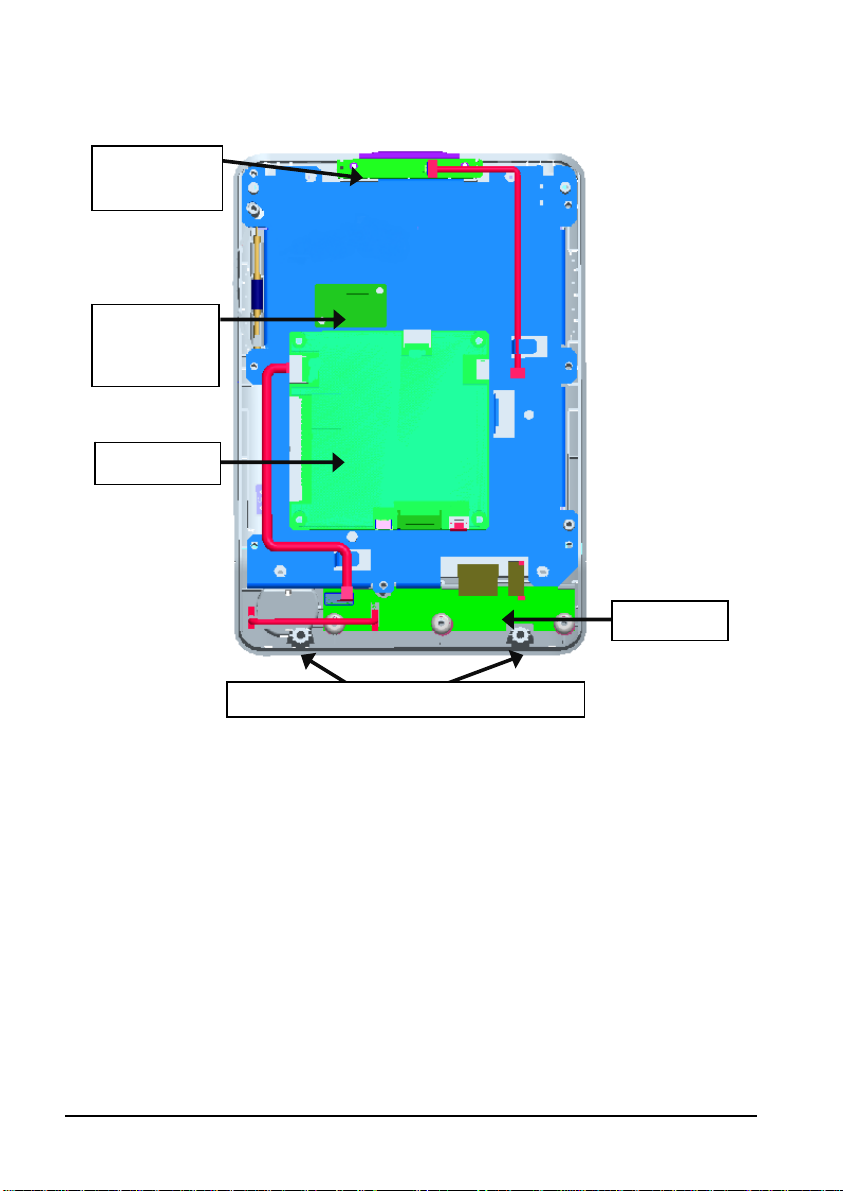
T
2.4 Front Housing Assembly
Alarm lamp
board
ouchscreen
control
board
Main board
Keypad
Screws securing the front and rear housing
2.5 Main Board
The main board is the control center of the equipment. It provides communication, display,
and data storage functions, including:
Communication with SpO
through connector;
Communication with power management board, keypad board, and recorder
through connectors;
Providing drive for display and backlight;
Providing 2 USB connectors, a network connector, and a multifunctional
connector;
Communication with touchscreen control board through I2C;
Providing drive for SD card; and,
Providing drive for Wi-Fi module(only available for Accutorr 7).
board, NIBP module, and Temperature module
2
2-4
Page 17
ypad
Ke
The keypad scans and detects the input of keys and encoder, integrates the power on/off
key, and connects AC and battery indicators.
Alarm Lamp Board
The alarm lamp board is located at the top of front housing. It has two-color indicators, red
and yellow. The alarm lamp board directly connects the main board through a cable. It is
controlled directly by the main board.
Touchscreen and Touchscreen Control Board
The touchscreen control board drives the touchscreen and implements communication
with the vital signs monitor.
Wi-Fi Module (Optional, Only Available for Accutorr 7)
The Wi-Fi Module enables the equipment to connect to an 802.11 a/b/g/n Mindray
proprietary network.
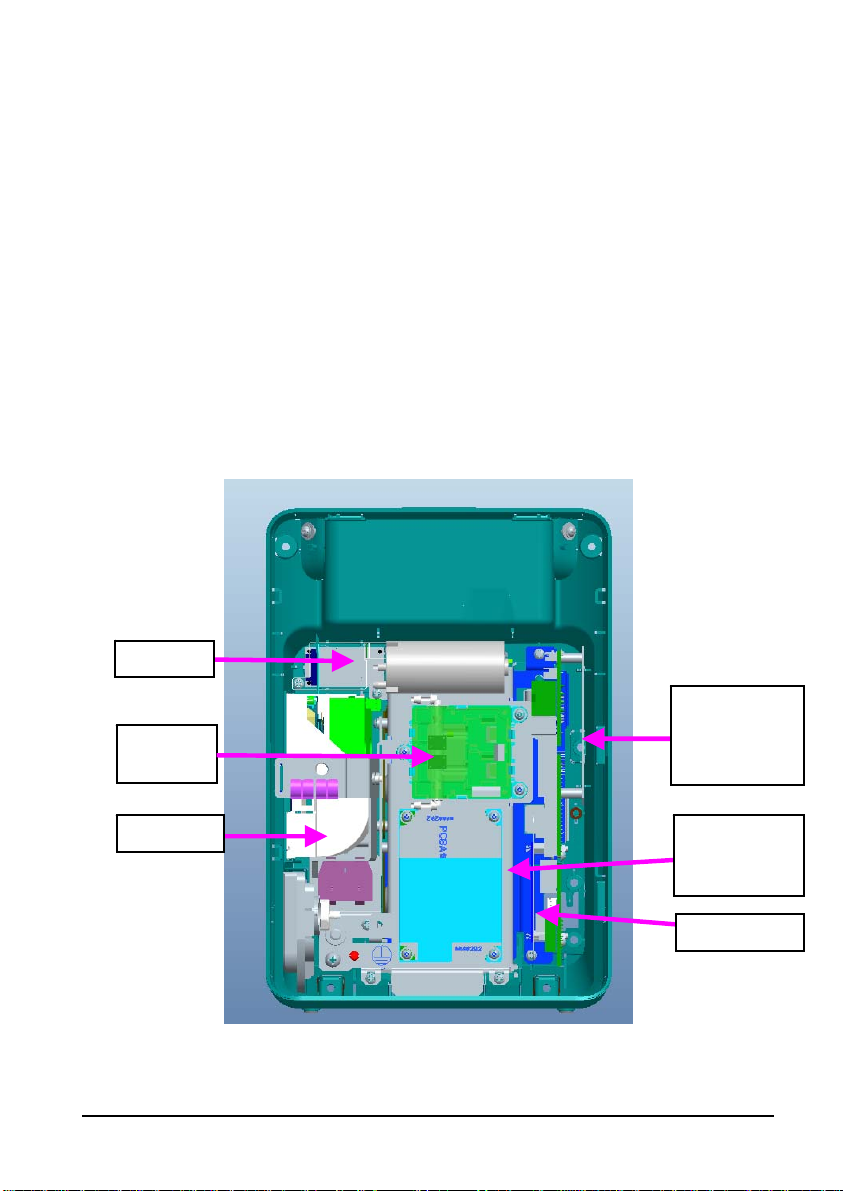
2.6 Rear Housing Assembly
Speaker
Power
NIBP
module
management
and interface
board
Recorder
AC/DC
power
module
SpO2 board
Rear housing assembly consists of power module (AC/DC), power management and
interface board (including SpO
and SpO
board.
2
isolation power), recorder, speaker, battery, NIBP module,
2
2-5
Page 18

AC/DC Power Module
The AC/DC power board transforms the input AC into DC power, which is the power source
for all voltages in the equipment.
Power Management
and Interface Board
The power management and interface board provides the following functions:
Charge and discharge of battery and charge detection;
DC/DC conversion: outputs 12V and 5V DC power;
Control over power on/off key and AC, BAT indicator;
Communication transmission among parameter modules;
Providing isolation power for SpO
Providing external connectors
module; and,
2
, filter and protection for these connectors.
Recorder
The recorder receives data from the main board and sends them to the thermal printhead
for printing.
NIBP Module
The NIBP module consists of blood pressure measurement board and pump and valve
assembly, providing measurement acquisition of blood pressure data. The main functions of
the NIBP module are:
NIBP measurement; and,
Data exchange with the main board.
Board
SpO
2
The SpO2 board collects SpO2 signals, processes SpO2 algorithm and sends measurement
results to the main board. The power management interface board provides isolation power
for it.
CO
Module (Only Available for Accutorr 7)
2
CO
concentration of CO
of specific wavelengths. The main functions of the CO
monitoring is a continuous, non-invasive technique for determining the
2
in the patient’ airway by measuring the absorption of infrared (IR) light
2
module are:
2
Provides a CO2 waveform, and EtCO2, FiCO2, awRR measurement; and
Data exchange with the main board through the serial ports.
2.7 External Module
An external Temp module can be attached to the monitor.
The independently developed Mindray Temperature module consists of an isolation power
board, Temperature measurement board, and probes. The Temperature measurement
board collects Temperature signals, processes algorithm and sends measurement results to
the main board.
2-6
Page 19
3 Equipment Installation
3.1 Unpacking the Equipment
Open the package and take out the packing list. Check that all the articles included in the
packing list are available and the quantity and specification are correct. Make sure that:
All the optional parts purchased by the customer
Notify Mindray North America if your order is incorrect or is incomplete.
In case of damage during transportation, keep the packing material and notify
Mindray North America immediately.
Keep the packing material until all equipment is checked and accepted.
3.2 Preparation for Installation
3.2.1 Preparation for Installation Site
1. Ensure that the site meets all safety, environmental and power requirements.
2. Ensure that a network connector is available if the equipment needs to be
connected to network.
3.2.2 Environmental Requirements
To avoid explosion hazard, do not use the equipment in the presence of flammable
anesthetics, vapors or liquids. The environment where the equipment will be used should
be reasonably free from vibration, dust and corrosive substances. If these conditions are not
met, the system may not function normally.
The environmental specification is as follows:
Main Unit
Item
Operating
environment
Storage
environment
NOTE
The environmental specifications of unspecified accessories are the same as
Temperature (℃)
0 to 40 (without Temp
module)
5 to 40 (with SmarTemp module)
16 to 40 (with Exergen Temp
module)
-30 to 70
-20 to 50 (with Exergen Temp
module)
erature
have been received.
Relative humidity
(noncondensing)
15% to 95% 427.5 to 805.5
10% to 95% 120.0 to 805.5
Altitude
(mmHg)
3-1
Page 20

those of the main unit.
3.2.3 Electrical Requirements
Check cables and power cords. Make sure that:
1. System cables, power cords, and power plugs are not damaged, and that the pins
are not loose. If damage is noted, discontinue use and replace.
2. Patient cables and connections are secure.
WARNING
Use only properly grounded power outlets.
Use the supplied power cord only!
Vol tage
Current
Frequency
100 to 240V AC
to 0.5A
0.9
50/60 Hz
3.3 Equipment Installation
Follow the procedure below to install the equipment:
1. Ensure the unit and accessories are not damaged.
2. Install the battery (optional). For detailed operations, please refer to the operator’s
manual.
3. Connect AC power.
4. Connect the accessories.
Installation Support
The vital signs monitor can be mounted on a wall bracket or on a rolling stand. The wall
bracket or rolling stand can be ordered optionally. Each type of mounting bracket is
delivered with a complete set of mounting hardware and instructions. For detailed
installation information, please refer to Wall-mount Bracket Instructions for Use (PN:
0010-20-42933) and Rollstand Instructions for Use (PN: 0010-20-42934).
CAUTION
Use only Mindray supplied or approved mounting solutions.
The mounting bracket should be installed by qualified service personnel.
3-2
Page 21

3.3.1 Preparation for Power on
1. Before using the equipment, check for any mechanical damage and make sure
that all external cables, plug-ins and accessories are properly connected.
2. Plug the power cord into the AC power source. If you run the equipment on
battery power, ensure that the battery is sufficiently charged.
3. Press the Power ON/OFF button (
equipment.
) on the front panel to turn on the
3-3
Page 22

FOR YOUR NOTES
3-4
Page 23
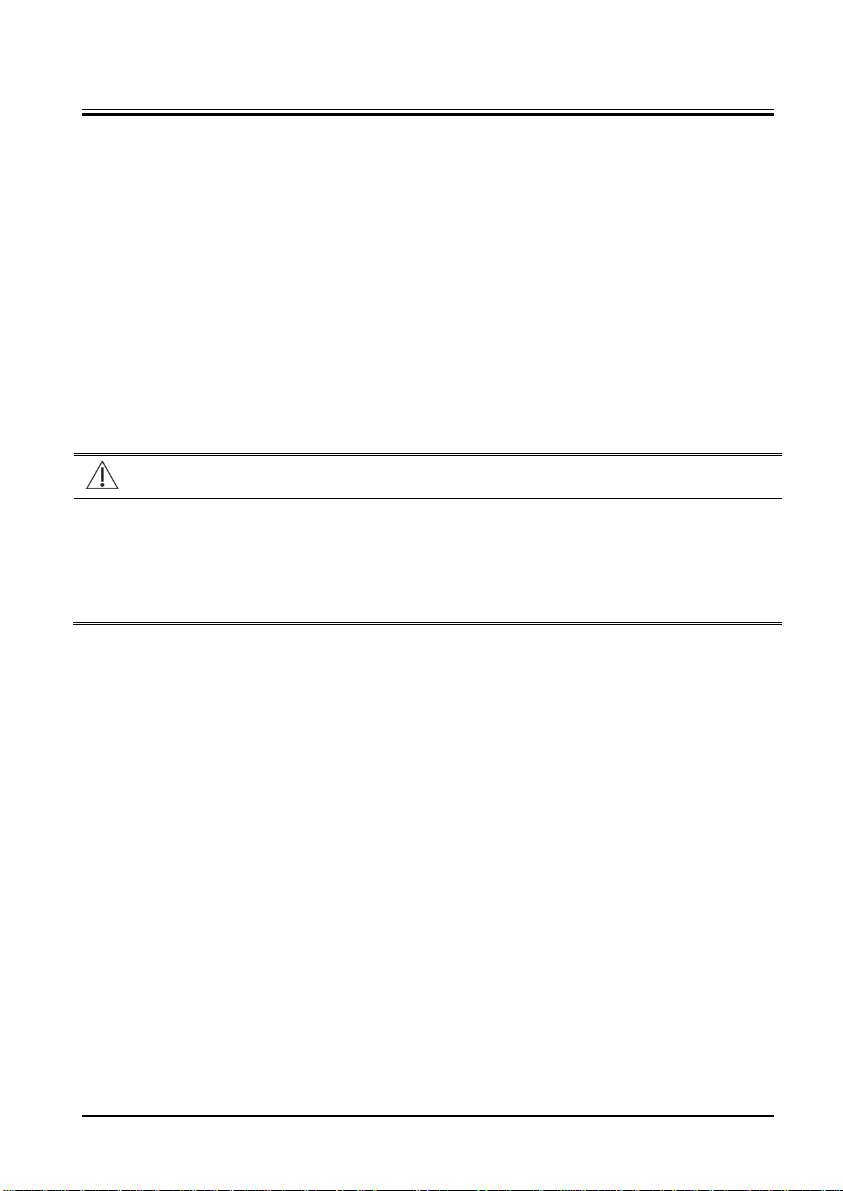
4 Testing and Maintenance
4.1 Introduction
The expected service life of the equipment is five years. To ensure the equipment always
functions normally, qualified service personnel should perform regular inspection,
maintenance and test. This chapter provides a checklist of the testing procedures for the
equipment with recommended test equipment and frequency. The service personnel
should perform the testing and maintenance procedures as required and use appropriate
test equipment.
The testing procedures provided in this chapter are intended to verify that the equipment
meets the performance specifications. If the equipment or a module fails to perform as
specified in any test, repairs or replacements must be done to correct the problem. If you
have any question, contact our Technical Support Department.
CAUTION
All tests should be performed by qualified service personnel only.
Care should be taken to avoid changing the settings in [User Settings >>] and
[Factory Maintenance >>] menus to avoid loss of data.
Service personnel should acquaint themselves with the test tools and make
sure that test tools and cables are available.
4.2 Performance Tests
Performance test are designed to ensure that measurement results are accurate. The
following sections provide a list of performance and accuracy tests and their recommended
frequencies.
4-1
Page 24
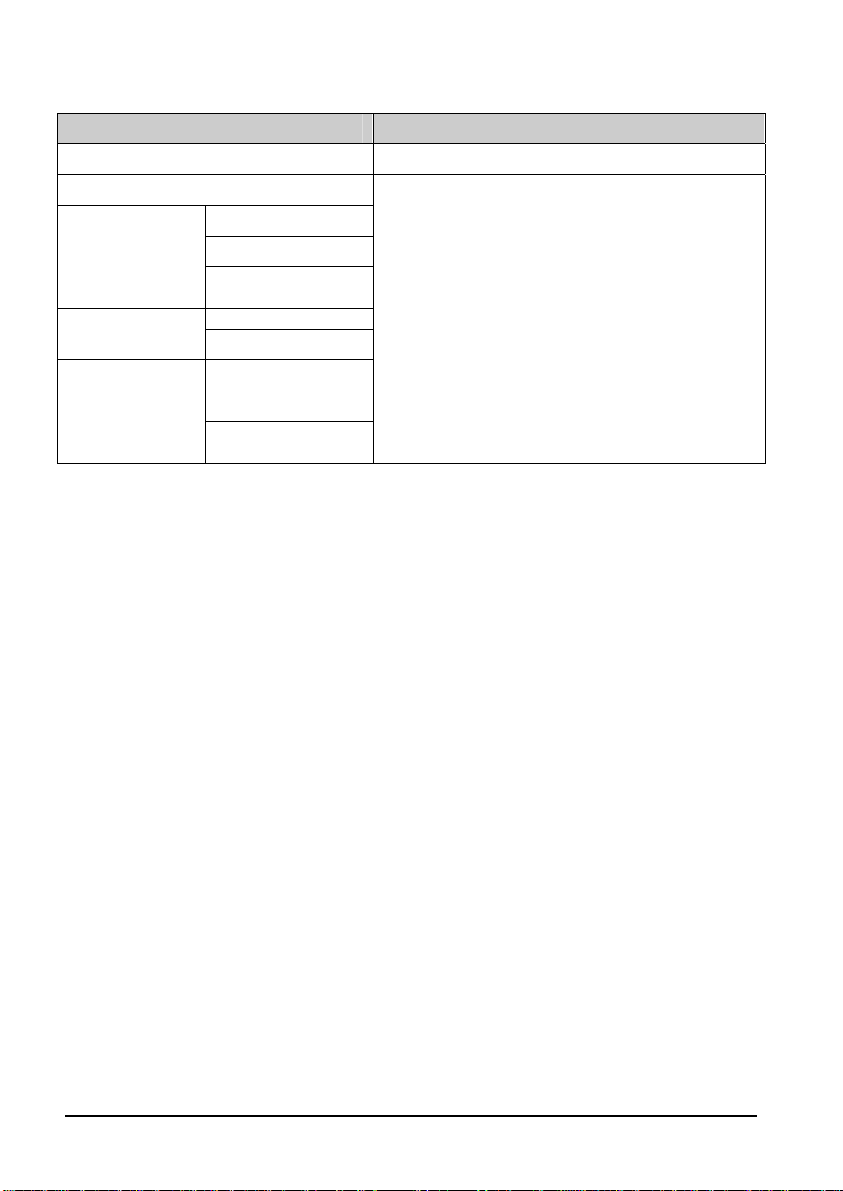
4.2.1 Performance Test Frequencies
Check/Maintenance Item Frequency
Visual inspection 1. When first installed or reinstalled.
SpO2test
Leakage test
NIBP test
CO2 test
(Accutorr 7 only)
TEMP test
Accuracy test
NIBP cuff
overpressure test
Performance test
Calibration
Exergen
TemporalScanner
thermometer
1. If the user suspects that the measurement is
incorrect.
2. Following any repairs or replacement of
relevant module.
3.
Every two years for SmarTemp
and SpO
4.
test.
2
Per year is recommended for NIBP and CO
TM
module test
2
tests.
SmarTempTM
module
4.2.2 Visual Inspection
Perform an overall inspection on the appearance of the equipment. The test is passed if the
equipment has no obvious signs of damage. Follow these guidelines when inspecting the
equipment:
Carefully inspect the case, display screen, buttons, and knob for obvious signs of
damage.
Inspect all external connections for loose connectors, bent pins or frayed cables.
Inspect all connectors on the equipment for loose connectors or bent pins.
Make sure that safety labels and data plates on the equipment are clearly legible.
4.2.3 SpO
Test Met ho d 1
Tool required:
None
Test procedure:
1. Connect SpO
2. Place the SpO
3. Verify the Pleth Wave and Pulse Rate are displayed on the screen.
4. Remove the SpO
test
2
sensor for adult to the SpO2 connector of the monitor. Set [Patient
2
Category
] to [Adult].
sensor on your finger.
2
alarm is triggered.
sensor from your finger and make sure that the SpO2 Sensor Off
2
4-2
Page 25

Measurement validation
The SpO2 accuracy has been validated in human studies against arterial blood sample
reference measured with a CO-oximeter. Pulse oximeter measurements are statistically
distributed, and only about two-thirds of the measurements can be expected to fall within
the specified accuracy compared to CO-oximeter measurements.
NOTE
The SpO2 simulator can only be used to verify that the pulse oximeter
operates properly. It cannot be used to verify the accuracy of the pulse
oximeter or the SpO2 sensor. To verify the accuracy, clinical tests are
required.
Test Met ho d 2
Tool required:
SpO
simulator, Index-2 recommended
2
Test procedure:
1. Connect the SpO
sensor to the SpO2 simulator.
2
2. Selected the model and manufacturer of the SpO2 module to be tested on the
simulator, and set the simulator as follows: SpO
to 96% and Pulse Rate to 80 bmp.
2
3. Set the patient type to [Adult], [Pediatric], and [Neonatal] respectively. Observe
the monitor and make sure the displayed SpO
and Pulse Rate value fall in the
2
following range.
Manufacturer SpO2 Pulse Rate
Nellcor
Masimo
96% ± 2% (Adult, pediatric)
96% ± 3% (Neonate)
96% ± 2% (Adult, pediatric)
96% ± 3% (Neonate)
80 ± 3 bpm
80 ± 3 bpm
4-3
Page 26
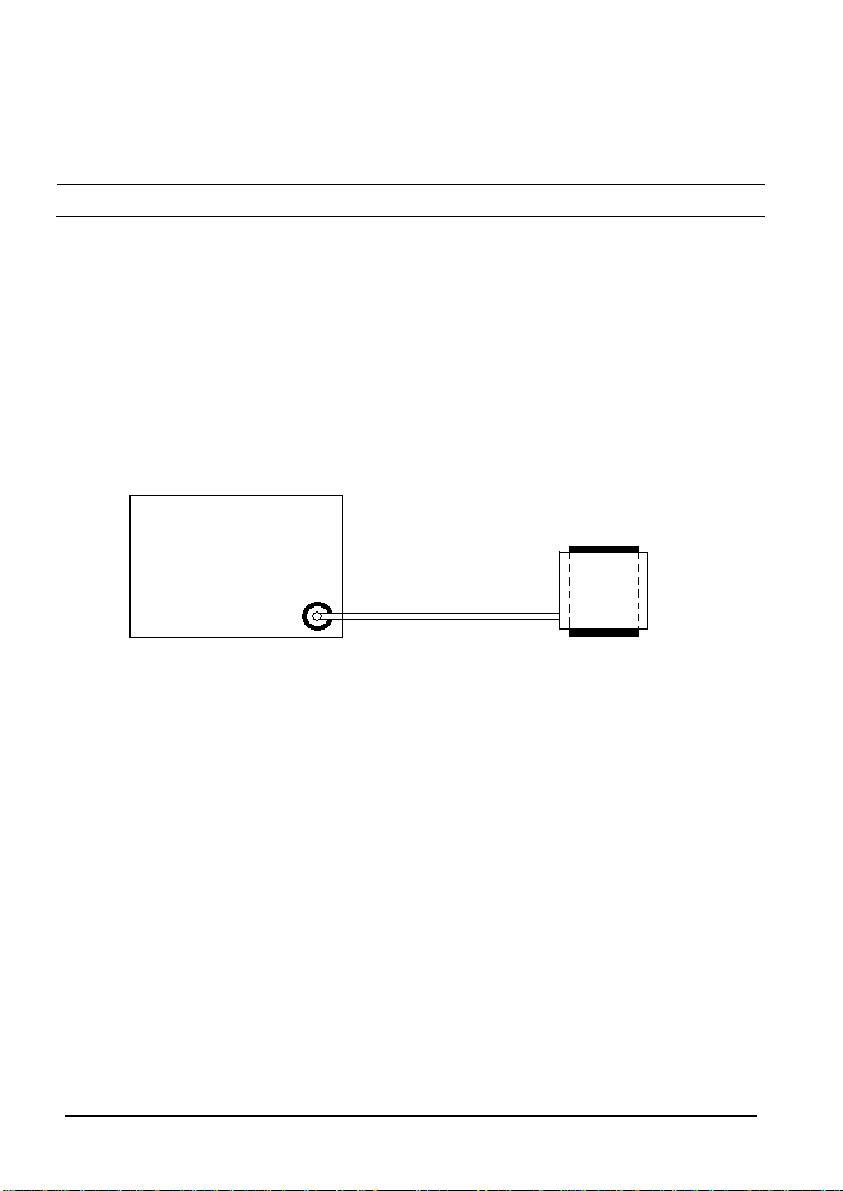
4.2.4 NIBP Test
Leakage Test
NOTE
Perform NIBP leakage test before any other NIBP test and calibration.
ools required:
T
Adult NIBP Cuff
NIBP Hose
Cylinder
Follow this procedure to perform the
leakage test:
1. In the [Patient Demographics] menu, set [Patient Category] to [Adult].
2. Connect the NIBP cuff to the NIBP connector on the monitor.
3. Apply the cuff to the cylinder as shown below.
Monitor
Connector for NIBP cuff
NIBP Hose
Cylinder
Cuff
4. Select [Main]→[Maintenance >>]→[User Settings>>]→Enter required
password→→[
Leakage Testing…] is displayed in the NIBP parameter area.
[
Module Maintenance >>]→[NIBP Leakage Test]. The message
After about 20 seconds, the monitor will automatically deflate. This means the test is
complete. If no message is displayed in the NIBP parameter area, it indicates that the system
has no leakage. If the message [
NIBP Pneumatic Leak] is displayed, it indicates that the
system may have a leak. Check the tubing and connections for leakages. Ensure that the
tubing and connections are all correct, perform a leakage test again. If the problem persists,
contact our Technical Support Department.
You may also perform a manual leakage test:
1. Perform steps 1 to 4 in the NIBP Accuracy Test section.
2. Raise the pressure in the rigid vessel to 250 mmHg with the manometer bulb.
Then, wait for 5 seconds to allow the pressure to stabilize.
3. Record the current pressure value, wait 60 seconds and record the pressure again.
4. Compare the two pressure values and verify the difference is 6mmHg or less.
4-4
Page 27
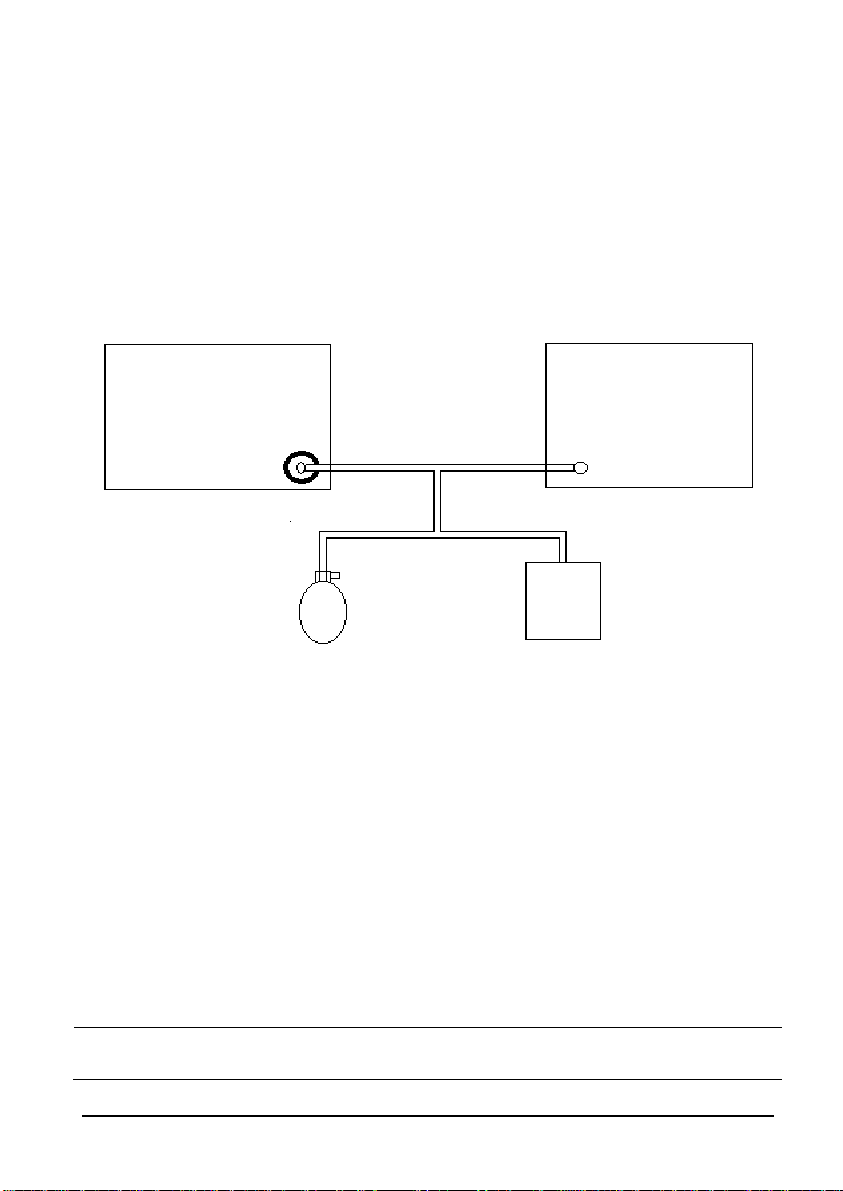
NIBP Accuracy Test
Tool required:
T-shape connector
Appropriate tubing
Manometer bulb
Rigid Vessel with volume 500 ± 25 ml
Reference manometer (calibrated with accuracy equal to or better than 0.75
mmHg)
Follow this procedure to perform the accuracy test:
1. Connect the equipment as shown below.
Monitor
Standard
sphygmomanometer
Connector for NIBP cuff
Manometer bulb
Appropriate tubing
Rigid Vessel
2. Before inflation, check that the reading of the manometer is 0. If not, turn off the
manometer bulb to let the whole airway open to the atmosphere. Turn on the
manometer bulb after the reading is 0.
3. Select [Main]→[Maintenance >>]→[User Settings>>]→Enter required
password→[
Module Maintenance >>]→[NIBP Accuracy Test].
4. Check the manometer values and the monitor values. Both should be 0 mmHg.
5. Raise the pressure in the rigid vessel to 50 mmHg with the manometer bulb. Then,
wait for 10 seconds to let the measured values become stable.
6. Compare the manometer values with the monitor values. The difference between
the manometer and displayed values should be ± 3 mmHg. If it is greater than ± 3
mmHg, contact Mindray Technical Support.
7. Raise the pressure in the rigid vessel to 200 mmHg with the manometer bulb.
Then, wait for 10 seconds to let the measured values become stable. Repeat step
6.
NOTE
You can use an NIBP simulator to replace the manometer bulb and the
reference manometer to perform the test.
4-5
Page 28

Cuff Overpressure Tes t
NIBP
Tools required:
T-shape connector
Appropriate tubing
Manometer bulb
Metal Vessel with volume 500 ± 25 ml
Reference manometer (calibrated, with accuracy equal to or better than 0.75
mmHg)
Follow this procedure to perform the calibration:
1. Perform steps 1 to 4 in the NIBP Accuracy Test section.
2. Select [
Main]→[Maintenance >>]→[Factory Maintenance >>]→enter the required
password→[
Calibrate NIBP >>]→[Overpressure Protection Circuit].
3. In [Overpressure Protection Circuit] menu, set [Patient Category] to [Adu/Ped].
Raise the pressure to 330 mmHg. After the pressure value is stabilized, select
[Calibrate] to start calibration.
4. In the [
Overpressure Protection Circuit] menu, set [Patient Category] to
Neonatal]. Raise the pressure to 165 mmHg. When the pressure value is stabilized,
[
select [
Calibrate] to start calibration.
5. All calibration results are displayed in the [Calibrate NIBP] menu. If the calibration
fails, check the test system for leakage and perform another calibration.
4-6
Page 29
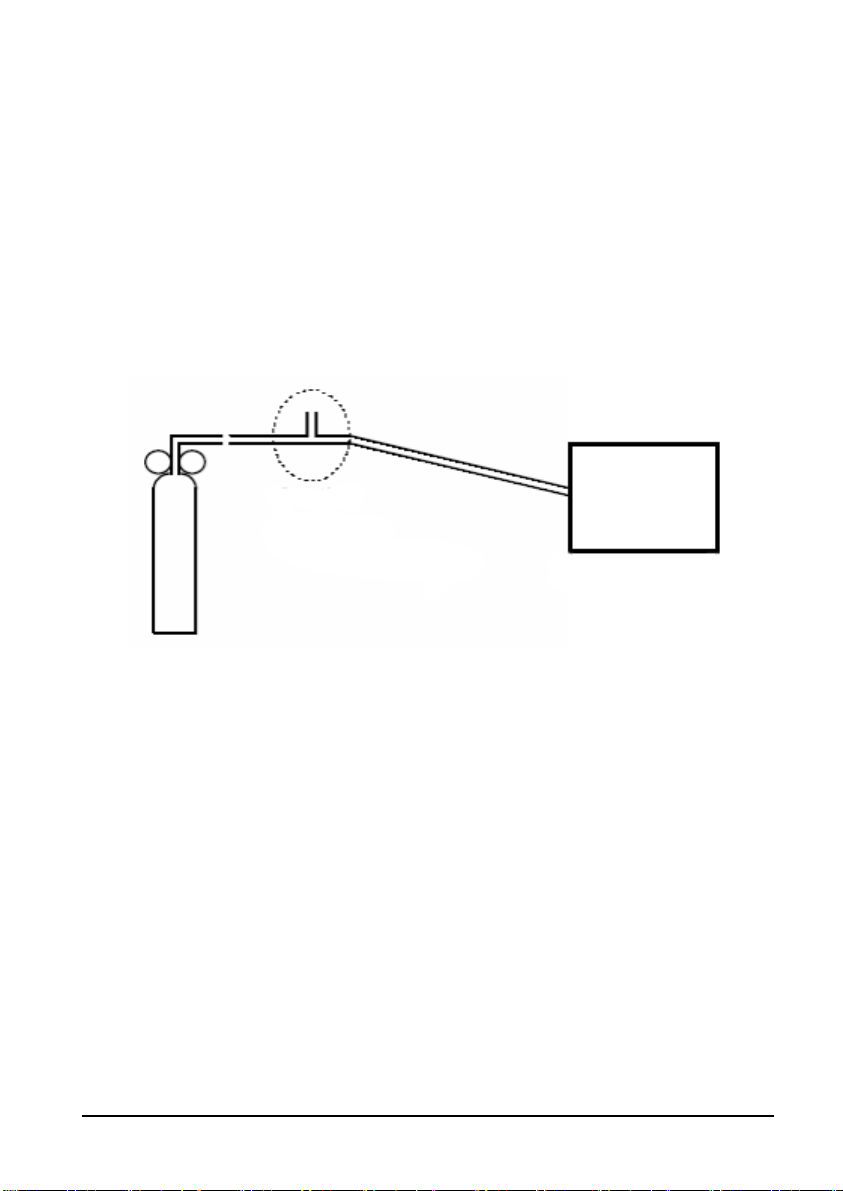
T
4.2.5 CO
Test (Only Available For Accutorr 7)
2
Accuracy Te s t
Tools required:
A steel gas cylinder with 5% CO
2 , 21% O
, and balance gas N2 (P/N 0075-00-0033-01)
2
T-shape connector
Tubing
1. Select [Main]→ [Maintenance >>]→[User Maintenance>>]→enter the required
password→ [Module Maintenance>>]→[Calibrate CO2>>]
2. Connect the test system as follows:
Relief valve
-shape connector
Cylinder
3. Open the valve to start flowing CO
to atmosphere.
4. Verify the realtime CO
2 value is within 5.0 ± 0.3% in the [Calibrate CO2] menu.
Tubing
Monitor
2 and make sure that there is sufficient flow to vent
4-7
Page 30
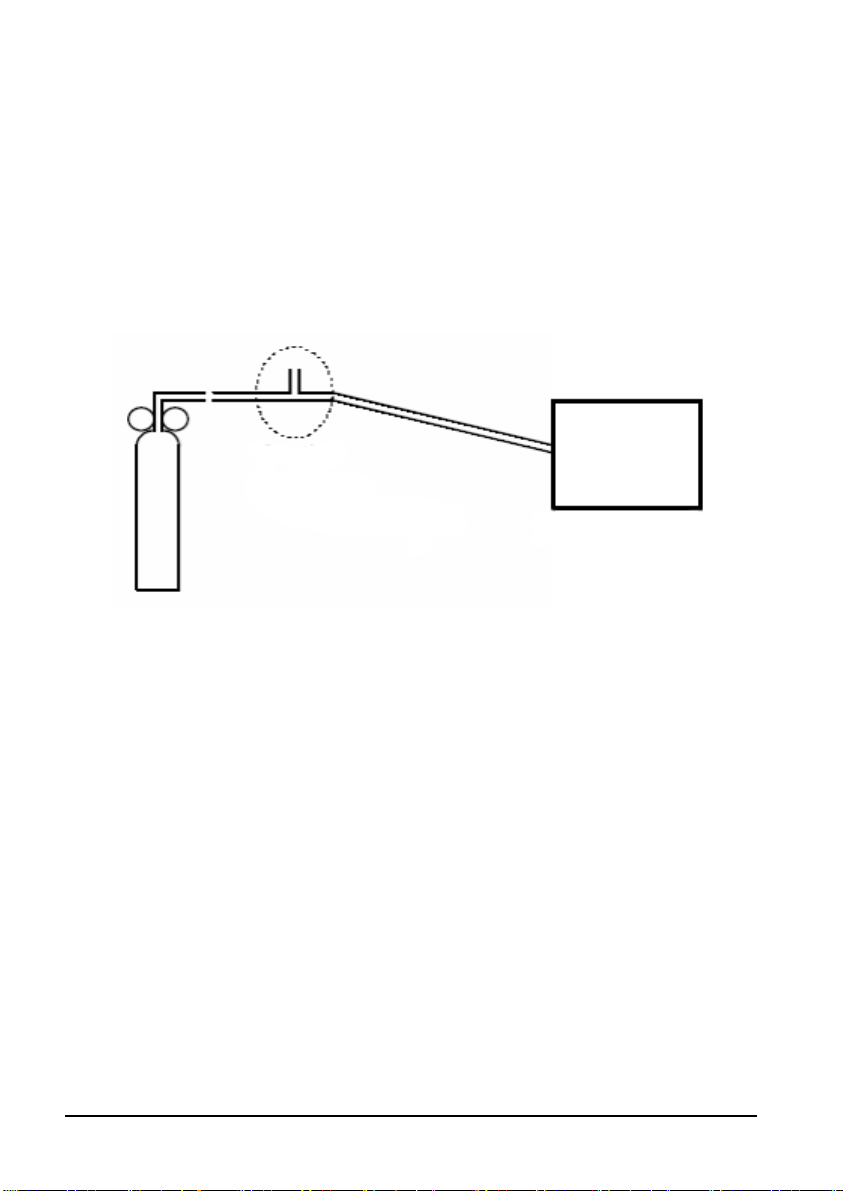
T
T
CO2 Calibration
Tools required:
A steel gas cylinder with 5% CO
2, 21% O2, and balance gas N2 (P/N 0075-00-0033-01)
T-shape connector
Tubing
1. Select [
Main]→ [Maintenance >>]→[User Maintenance>>]→enter the required
password→ [
Module Maintenance>>]→[Calibrate CO2>>].
2. In the [Calibrate CO2] menu, select [Zero].
3. After the zero calibration is finished successfully, connect the equipment as follows:
Relief valve
-shape connector
ubing
Monitor
Cylinder
4. Open the valve to start flowing CO
2 and make sure that there is sufficient flow to vent
to atmosphere.
5. In the [Calibrate CO2] menu, enter the CO
6. In the [Calibrate CO2] menu, the measured CO
measured CO
2 module.
CO
2 concentration becomes stable, select [Calibrate CO2] to calibrate the
2 concentration in the [CO2] field.
2 concentration is displayed. After the
If the calibration is completed successfully, the message [Calibration Completed!] is
displayed in the [Calibrate CO2] menu. If the calibration fails, the message [Calibration
Failed ] is displayed. In this case, attempt to calibrate the CO
2 module again.
4-8
Page 31

4.2.6 Exergen TemporalScanner Thermometer Test
Tool required:
Water bath
Blackbody as specified in EN 12470-5-2003
Four-channel thermometer (model: 1529-R)
1. Put the probe of four-channel thermometer into water bath, and then put the
blackbody into water bath, set the water bath to 37.0℃. allow the water bath
sufficient time to equilibrate.
2. Set the Exergen TemporalScanner Themometer to EAR mode and “℃”.
3. Install a new probe cover. Measure and record the target temperature of blackbody
with the Exergem thermometer and four-channel thermometer.
4. Verify accuracy of temperature on monitor(subtract temperature value on
four-channel thermometer from temperature value on monitor) is within±0.1℃.
5. Repeat sections 1 to 4. For water bath set to 42.0℃.
6. Verify accuracy of temperature on monitor is within ±0.2℃.
Please contact our service personnel if the Temp test fails.
4.2.7 SmarTemp
Required Tool:
Thermostatic oil tank (HART 7102 recommended) or Water Bath and Precision
Thermometer for reference
Test procedure:
1. Set the temperature of the oil tank or water bath to 37°C and conduct the test
after the temperature stabilizes.
2. Set temperature unit to °C.
3. Select temperature parameter area to access [
temperature type to [
4. In Monitor Mode, remove the Temperature probe from the probe sheath, insert a
probe cover, and place the probe into the oil tank or water bath.
5. Wait till the Temp value displayed on the monitor stabilizes. Verify that the
displayed value is ±0.1°C of the oil bath thermostat setting or reference
thermometer.
Contact our Technical Support Department if the Temperature test fails.
NOTE
Due to the different application environment and the test object in vivo and
vitro conditions, there are deviations in the measurement result. The
TM
Module Test
Monitor].
Temp Setup] menu, and then set
4-9
Page 32
maximum deviation of 2.5℃ may exist in predictive Temperature
measurement by liquid bath techniques.
4.3 Electrical Safety and Other Tests
4.3.1 Electrical Safety and Other Test Frequencies
Check/Maintenance Item Frequency
1. Following any repair or replacement of any
module
Electric Safety Tests
Power-on test
Touchscreen calibration
Recorder check
Bar code scanner test
Functional test
Battery check
Performance test
Nurse call function test
4.3.2 Electric Safety Tests
See Appendix A Electrical Safety Inspection for electrical safety tests.
4.3.3 Power-on Test
This test is to verify that the equipment can power up correctly. This test is passed if the
equipment starts up by following this procedure:
1. Insert the battery in the battery compartment, and connect the equipment to the
AC mains. The AC mains indicator and battery indicator light up.
2. If monitor is physically damaged.
3. Every two years.
For details, refer to A Electrical Safety Inspection.
1. When first installed or reinstalled.
2. Following any repairs or replacement of any
main unit parts.
1. When the touchscreen appears abnormal.
2. After the touchscreen is replaced.
Following any repair or replacement of the
recorder.
Following any repair or replacement of the
code scanner
1. When first installed.
2. Whenever a battery is replaced.
Once per year or if the battery run time reduced
significantly.
If the user suspects that the nurse call function
does not work well.
.
bar
2. Press the Power ON/OFF button (
equipment. The work status indicator lights up inside the Power ON/OFF button.
) on the front panel to turn on the
4-10
Page 33

3. The equipment gives a beep, which indicates that the selftest for alarm audio is
passed, and the alarm lamp turns yellow, then red, and then off, which indicates
that the selftest for alarm lamp has passed.
4. The welcome screen clears and the normal operation screen is displayed.
4.3.4 Touchscreen Calibration
Tool required:
None
Test procedure:
1. Select [
Main]→[Maintenance >>]→[Calibrate Touchscreen].
2. The
3. Select, in turn, the central point of the
4. After the calibration is completed, the message [Screen Calibration Completed!].
Select [
symbol will appear at different positions of the screen.
symbol.
Ok] to confirm the completion of calibration.
4.3.5 Recorder check
Tool required:
None
Test procedure:
1. Print SpO
should be clear.
2. Remove the paper from the recorder then try to print and verify that an error
message (recorder out of paper) appears. Reinstall the paper and verify the
recorder functions properly.
3. Set the recorder to print trend data. Verify the recorder prints trends correctly.
Contact our Technical Support Department if the recorder test fails.
Pleth waveform. The recorder should print correctly and the printout
2
4.3.6 Bar Code Scanner Test
Tool required:
None
Test procedure:
1. Aim the bar code scanner at the target bar code. Adjust the field of view to
capture the bar code.
2. Hold the trigger until the bar code scanner gives a beep, indicating the bar code is
successfully decoded. Meanwhile, the scanned characters are displayed on the
monitor.
Please contact our service personnel if the bar code scanner fails to work normally.
4-11
Page 34

4.3.7 Battery Check
Tool required:
None
Func tional Te st
1. Remove the battery (if equipped), then connect the equipment to AC power.
2. Verify that the equipment works correctly when powered form an AC source.
3. Disconnect AC power and reinstall the battery.
4. Verify the equipment functions properly on battery power.
Performance Test
Run the equipment on battery power and verify it performs in accordance to the
specifications stated in the Battery chapter in the operator’s manual (P/N: 046-004882-00 or
P/N: 046-013024-00).
4.3.8 Nurse Call Reply Performance Test
Tool required:
Oscilloscope
Test procedure:
1. Connect the nurse call cable to the Multi-function connector on the rear panel.
2. Select [
3. In the [
4. Click
5. Select [
6. Make the monitor to generate an alarm and check that the oscilloscope outputs
7. Quit the demo mode, and repeat step 2.
8. In the [
9. Repeat steps 4 and 5.
10. Create an alarm condition and verify that the oscilloscope displays positive pulses
Main]→[Maintenance >>]→[User Settings >>]→enter the required
password→[
set [
Conta ct Type] to [Normally Open, and [Signal Type] to [Continuo us].
Ok]→[Nurse Call >>].
Nurse Call Setup] menu, select the [Alarm Level] and [Alarm Category], and
to save the changes and quit the menu.
Main]→[Maintenance >>]→[Demo >>]→enter the required
password→[
Ok] to enter demo mode.
continuous high level when there is an alarm.
Nurse Call Setup] menu, select the [Alarm Level] and [Alarm Category], and
set [
Conta ct Type] to [Normally Open], and [Signal Type] to [Pulse].
of 1 second width when there is an alarm.
4-12
Page 35

4.4 Factory Maintenance
4.4.1 Accessing Factory Maintenance Menu
Select [Main]→[Maintenance >>]→[Factory Maintenance >>]→enter the required
password→[
Ok] to access the Factory Maintenance menu.
4.4.2 Drawing Waves
There are two methods for drawing waves: [Color] and [Mono].
Color: selecting Color will have smoother waveforms.
Mono: selecting Mono will have a wider viewing angle.
4-13
Page 36

4.4.3 Software Version
Selecting the [Software Version >>] will show software version information, as shown
below:
1
2
3
1. System software version
2. Power software version
3. Module software version
4-14
Page 37

4.4.4 Monitor Information
Selecting [Monitor Information >>] will show the status of the monitor as shown below:
NOTE
If the main board is replaced, the serial number of the monitor must be
entered on the new main board in the Electronic SN Setup menu.
4-15
Page 38

FOR YOUR NOTES
4-16
Page 39

5 Troubleshooting
5.1 Overview
In this chapter, equipment problems are listed along with possible causes and
recommended corrective actions. Refer to the tables to check the monitor, identify
and correct problems.
The tables provided are meant as a general guide. For more detailed troubleshooting
assistance, contact our Technical Support Department.
5.2 Part Replacement
Printed circuit boards (PCBs), major parts and components in the monitor are
replaceable. Once you isolate a PCB you suspect defective, follow the instructions in
chapter 6 Disassembly and Repair to replace the PCB with a known good one and
check that the trouble disappears or the equipment passes all performance tests.
To obtain information on replacement parts or order them, refer to chapter 7 Parts.
5.3 Checking Equipment Status
Some troubleshooting tasks may require you to identify the hardware version and
status of your monitor. To check equipment status:
1. Select [
2. Select [
5.4 Checking Software Version
Some troubleshooting may involve software compatibility. Thus it requires you to
know your monitor configuration and software version. For detailed information on
version compatibility, please contact our Customer Service Department. To view
information on the system configuration and system software version,
Select [
Main]→[Maintenance >>]→[Factory Maintenance >>]→Enter the required
password→[
system software version and module software version.
Main]→[Maintenance >>]→[Monitor Information >>]. Then you can
view the information on system start time, selfcheck, etc.
Main]→[Maintenance >>]→[Factory Maintenance >>]→enter the
required password→[
information on the monitor’s current status.
Ok]→[Software Version >>]. You can also view the information on
Ok]→[Monitor Information >>]. You can also view the
5-1
Page 40

5.5 Checking Technical Alarms
Before troubleshooting the monitor, check for technical alarm messages. Correct all
Technical Alarms before continuing.
For detailed information on technical alarm message, possible cause and corrective
action, refer to the monitor’s operation manual.
5.6 Troubleshooting Guide
5.6.1 Power On/Off Failure
Symptoms Possible Cause Troubleshooting
AC mains not
connected or battery
too low
Cable defective
The monitor
fails to start.
Power board
defective
Power management
board defective
The main board
failed.
Check that AC mains is properly connected
or battery capacity is sufficient.
1. Check that the cable between the keypad
board and main board is correctly
connected.
2. Check that the cable between the power
board and power management board is
correctly connected.
3. Check that the cable between the main
board and power management board is
correctly connected.
Replace the power board.
Replace the power management board.
Replace the main board.
5-2
Page 41

5.6.2 Display Failures
Symptoms Possible Cause Troubleshooting
1. Check if the cable between the display
and main board and the backlight cable are
The display is
blank or
black.
Images
overlapped
or distorted
Cable defective
Main board defective Replace the main board.
Display defective Replace the display.
Main board error
Cable defective
correctly connected.
2. Check that the cables and connectors are
not damaged.
Replace the main board, or upgrade the
main board with the upgrade software.
Check if the cable between the display and
main board and the backlight cable are
correctly connected.
Touchscreen
does not
respond.
Touch
position
invalid
Touchscreen
disabled
Cable defective
Touchscreen control
board defective
Touchscreen
defective
The main board
failed.
Touchscreen not
calibrated
Check if there is a
above the [
hold the [
Main] quickkey. If yes, press and
Main Menu] quickkey for more
symbol displayed
than 3 seconds to enable the touchscreen.
1. Check that the cable between the
touchscreen and touchscreen control board
is correctly connected.
2. Check that the cable between the
touchscreen control board and main board
is correctly connected.
Replace the touchscreen control board
.
Replace the touchscreen.
Replace the main board.
Calibrate the touchscreen.
5-3
Page 42

5.6.3 Alarm Lamp Failures
Symptoms Possible Cause Troubleshooting
1. Check that the cable between the alarm
lamp board and main board is correctly
The Alarm
Lamp does
not illuminate
properly or
not at all.
Cable defective
Alarm lamp board
defective
The main board
failed.
connected.
2. Check that the cables and connectors are
not damaged.
Replace the alarm lamp board
Replace the main board.
5.6.4 Button and Encoder Failures
Symptoms Possible Cause Troubleshooting
Check that the cable between the keypad
Buttons do
not work
Encoder does
not work
Cable defective
Keypad board failure Replace the keypad board.
Cable defective
Knob failure Replace the encoder.
Keypad board failure Replace the keypad board.
board and main board is correctly
connected.
Check that the cable between the knob
1.
and keypad board is correctly connected.
2. Check that the cable between the keypad
board and main board is correctly
connected.
5-4
Page 43

5.6.5 Sound Failures
Symptoms Possible Cause Troubleshooting
No hardkey
or encoder
sound, or
hardkey or
encoder
sound
abnormal
No alarm
sound or
alarm sound
abnormal
The key volume is set
to 0.
Cable defective
Speaker defective Replace the speaker.
The main board
failed.
Power management
board defective
The alarm volume is
set to 0.
Cable defective
Select [
Main]→[General Setup >>] to adjust
the key volume.
Check that the cable between the speaker
and interface board is properly connected.
Replace the main board.
Replace the power management board.
Select [
Main]→[Maintenance >>]→]User
Settings >>
password
the [
]→enter the required
→[Ok]→[Alarm Setup >>] and set
Minimum Alarm Volume] to a proper
level in the prompt menu. Select
Main]→[General Setup >>] to adjust the
[
key volume.
1. Check that the cable between the speaker
and interface board is properly connected.
Speaker defective Replace the speaker.
The main board
failed.
Power management
board defective
Replace the main board.
Replace the power management board.
5.6.6 Battery Failures
Symptoms Possible Cause Troubleshooting
Battery defective Replace the battery.
Check that the cable between the battery
Battery
cannot be
charged
Cable defective
Power management
board defective
Battery interface
board defective
interface board and power management
board is correctly connected.
Replace the power management board.
Replace the battery interface board.
5-5
Page 44

NOTE
If the Battery Module fails, it may affect other modules or boards in the
main unit.
Components of the main unit are powered by the power module. In the
event that a component malfunctions, check if the operating voltage is
correct.
5.6.7 Recorder Failures
Symptoms Possible Cause Troubleshooting
1. Check if the recorder status indicator lights.
2. If yes, select
[
Main]→[Maintenance >>]→[Factory
Maintenance >>
]→enter the required
password→[Ok]→[Device Config. >>] and
check the recorder box in the prompt menu to
enable the recorder. Otherwise, check for
other possible causes.
Check that the cable between the recorder
and main board is correctly connected.
Stop the recorder and re-install the paper roll.
1. Check the thermal print head and the paper
roller for foreign matter.
2. Clean the thermal print head with an
appropriate cleaning solution such as contact
cleaner or isopropal alcohol
.
No printout
Poor print
quality or
paper not
feeding
properly
Recorder module
disabled
Paper reversed Re-install the paper roll.
Cable defective
Recorder defective Replace the recorder.
Paper roll not
properly installed
Print head dirty
Recorder defective Replace the recorder.
5-6
Page 45

5.6.8 Output Interface Failure
Symptoms Possible Cause Troubleshooting
1. Check that the cable between the
power management board and main
board is correctly connected.
2. Check that the cable between power
management board and interface board
is correctly connected.
Replace the connector board.
Replace the power management board.
Main]→ [Transfer Data to USB]
Select [
Unable to use
the USB
devices; USB
drive data
transfer failure
Cable defective
The connector board
failed.
Power management
board defective
The main board failed. Replace the main board.
Improper setup
5.6.9 Data Storage Failure
Symptoms Possible Cause Troubleshooting
Fails to review
archived
patient data
SD card failure
Abnormal patient
admitting
SD card full;
unavailable for more
patient data
The main board failed. Replace the main board.
SD card not formatted Format the SD card.
SD card failure Replace the SD card.
SD card is locked Unlock the SD card.
Main board defective Replace the main board.
Admit the patient properly.
uneeded patient data, clear the
Delete
related alarm, and readmit the patient.
5-7
Page 46

5.6.10 Wired Network Related Problems
Symptoms Possible Cause Troubleshooting
Check LAN cable connection. LAN
cable shall not be longer than 50
m
eters.
Check for IP address conflict. If yes,
reconfigure the IP address.
1. Check that the cable between the
power management board and
main board is correctly connected.
2. Check that the cable between
power management board and
interface board is correctly
connected.
Replace the connector board.
Replace the power management
board.
Replace the main board.
Check LAN cable connection. LAN
cable shall not be longer than 50
eters.
m
Check LAN cable connection. LAN
cable shall not be longer than 50
m
eters.
A monitor can only be viewed by
four other monitors at the same time
under the View Others mode. The
excessive view requests system will
be ignored.
Check for IP address conflict. If yes,
reconfigure the IP address.
Unable to connect
the wired network
The monitor is
frequently off line or
disconnects from the
network.
The monitor is
connected to a LAN
but cannot view
other patients under
the View Others
mode
Incorrect LAN cable
connection
Incorrect IP address
configuration
Cable defective
The connector board
failed.
Power management
board defective
The main board
failed.
Incorrect LAN cable
connection
Incorrect LAN cable
connection
Excessive requests
for viewing the
monitor at the same
time
Incorrect IP address
configuration
5-8
Page 47

5.6.11 Wi-Fi Related Problems (Only Available for Accutorr 7)
Symptoms Possible Cause Troubleshooting
The monitor is
frequently off line or
disconnects from the
Wi-Fi network.
Unable to connect
the Wi-Fi network.
The Wi-Fi signal is unstable in
the operating area.
The monitor's Wi-Fi antenna
is detached or not properly
connects the Wi-Fi module.
Antenna damaged Replace the Wi-Fi antenna.
Wi-Fi module defective Replace the Wi-Fi module.
Incorrect IP address
configuration
The Wi-Fi signal is unstable in
the operating area.
The monitor's Wi-Fi antenna
is detached or not connected
to the Wi-Fi module.
Antenna damaged Replace the Wi-Fi antenna.
Wi-Fi module defective Replace the Wi-Fi module.
Main board defective Replace the main board.
Check the signal quality of
the
Mindray Wi-Fi network.
Disassemble the monitor
and fix the Wi-Fi antenna.
Check for IP address conflict.
If yes, reconfigure the IP
address.
Check the signal quality of
the
Mindray Wi-Fi network.
Fix the Wi-Fi antenna.
5.6.12 Module defective
Symptoms Possible Cause Troubleshooting
1. Check that the cable between the
external converter board inside the
module and the converter board is
correctly connected.
2. Replace the converter board.
1. Check that the cable between the
main board and power management
board is correctly connected.
2. Replace the power management
board.
3. Replace the main board.
Check the cables connecting the
converter board and corresponding
parameter module.
Failed to connect the
external parameter
modules
Module can be
loaded, but "XX
communication
Module defective
Main unit defective
Cable defective
inside the module
5-9
Page 48

Symptoms Possible Cause Troubleshooting
stopped" is reported
or some parameters
cannot be used
"XX" indicates the configured modules, such as NIBP, SpO2 and Temperature.
Parameter module
defective
Converter board
defective inside the
module
Replace the corresponding module.
Replace corresponding converter
board.
5.6.13 Exergen TemporalScanner Thermometer Module Problems
Symptoms Possible cause Troubleshooting
Dirty Lens
Releasing the button before
finished measuring
Measuring when an ice
pack
or wet compress is on the
forehead
Abnormally low
Temperature
Measuring a completely
diaphoretic patient
Improperly scanning down
the side of the face
Anything covering the area
to be measured would
Abnormally high
temperature
Processing Error Error
insulate and prevent heat
from dissipating, resulting
in
false high readings.
Clean lens of scanner every two
weeks.
Release the button after
finished measuring.
Remove ice pack or wet
compress, wait 2 minutes, and
re-take temperature.
Complete diaphoresis includes
diaphoresis of area behind the
ear and suggests that the
temperature is rapidly
dropping. Use an alternative
method of temperature
measurement in these cases
until the patient is dry and the
temporal artery measurement
can be repeated.
Scan straight across forehead.
The temporal artery is closest
to skin in that area.
Confirm measurement site has
not recently been in contact
with heat insulators such as
hats, blankets, and hair.
Scan the area not covered or
wait about 30 seconds for the
previously covered area to
equilibrate to the environment.
Restart. Return for repair if
error message persists
5-10
Page 49

5.6.14 CO
Symptoms Possible Cause Troubleshooting
CO2 Sensor High
Tem p
CO2 Zero Failed The zeroing failed.
CO2 No Filterline
CO2 FilterLine
Occluded
CO2 frequently
zeroes during the
first hour after
start-up.
Module Problems (Only Available for Accutorr 7)
2
Ambient temperature
is too high or there is a
module failure.
Lower the operating temperature. If
the alarm persists, the CO2 module
may have a failure. Change the CO
module.
Wait for the next successful zeroing,
or repower the module.
The mini water trap
disconnected.
The airway or mini
water tarp is occluded.
The CO
module
2
temperature is not
stable.
Make sure that the mini watertrap
and sampling line is connected.
Check the airway and remove the
occlusion.
No action is required. The zeroing
does not affect measurement
accuracy. The zeroing frequency will
decrease over time.
5.6.15 Technical Alarm Messages
Please refer to the operator’s manual.
2
5-11
Page 50

FOR YOUR NOTES
5-12
Page 51

6 Disassembly and Repair
6.1 Tools Required
To disassemble and replace the parts and components, the following tools may be required:
Philips screwdrivers
Tweezers
Sharp nose pliers
Clamp
Slot-type screwdriver
6.2 Preparations for Disassembly
Before disassembling the equipment, finish the following preparations:
Stop monitoring, turn off the equipment, and disconnect all the accessories and
peripheral devices.
Disconnect the AC power source and remove the battery.
WARNING
Before disassembling the equipment, be sure to eliminate the static charges
first. When disassembling the parts labeled with static-sensitive symbols,
make sure you are wearing electrostatic discharge protection such as
antistatic wristband or gloves to avoid damaging the equipment.
Properly connect and route the cables and wires when reassembling the
equipment to avoid short circuit.
Follow correct sequence to disassembly the equipment. Otherwise, the
equipment may be damaged permanently.
Be sure to disconnect all the cables before disassembling any parts. Be sure
not to damage any cables or connectors.
Place the screws and parts from the same module together to facilitate
reassembling.
Use care during reassembly to ensure cables are properly routed and all
gaskets are intact and correctly positioned.
6-1
Page 52

T
6.3 Disassembling the Main Unit
NOTE
The recorder can be disassembled separately.
To disassemble the equipment, place the equipment on a work surface free
from foreign material, avoiding damaging the antiglare screen, touchscreen
and the knob. Be careful not to break the two cotters on the front ends of
rear housing.
All repairs should be performed in an anti-static environment by qualified
service personnel.
6.3.1 Disassembling the Temperature Module (Optional)
1. Lay the monitor on a table as shown below. Unscrew the three M3×6 screws, pull
the Temperature module up, and disconnect the Temperature cable.
emperature
cable
6-2
Page 53

6.3.2 Removing the Recorder
Unscrew the two M3×6 screws and pull the clamps as indicated to remove the recorder.
Then disconnect the cable.
Clamps
NOTE
The recorder can be disassembled separately.
6.3.3 Separating the Front and Rear Half of the Monitor
1. Lay the monitor on a table as shown below. Unscrew the four M3 screws.
6-3
Page 54

2. Remove the recorder or recorder cover, and pull the rear housing out as indicated
below to separate the front and rear housing.
3. Stand the monitor and separate the front housing assembly and rear housing assembly
with caution. Disconnect the cable between the main board and power management board
and then take off the front panel.
Cable
NOTE
When reassembling the equipment, be sure to check if the front housing
waterproof strip is correctly placed.
6-4
Page 55

6.3.4 Removing the Parameter Connector Panel Assembly
Lay the rear housing assembly of the equipment on a table. Disconnect the cable for AC
receptacle and SpO2 cable, and then the silicon tube.
Cable for AC
input
receptacle
SpO2 signal
cable
Parameter connector panel assembly
Silicon tube
6-5
Page 56

6.3.5 Disassembling the Main Bracket Assembly
1. For monitors without a multi-function connector, use a flat blade screwdriver to
gently lift the cover release on the inside of the unit, and then remove the cover.
Cover
6-6
Page 57

2. Disconnect the speaker cable, recorder cable, power cord and battery interface
board cable.
3. Unscrew the five self-tapping screws and one grounding screw. Then remove the
main bracket assembly.
Battery interface
board cable
5 self-tapping
screws
Speaker cable
Recorder
cable
Grounding screw
Power cord
6-7
Page 58

6.3.6 Removing the Parameter Board (SpO
Optional) and Power
2
Management Board
1. Unscrew the three screws and remove the power management board assembly
from the main bracket assembly.
2. Unscrew the two M3 screws and remove the parameter board from the power
management board assembly.
Parameter Board
6-8
2 M3 screws
Page 59

3. Unscrew the two M3 screws and remove the power management board assembly.
2 M3 screws
Power
management
board
6.3.7 Removing the Parameter Connector Panel Assembly (With CO
Only Available for Accutorr 7)
1. Lay the rear housing assembly of the equipment on a table.
2. Pull out the AC receptacle, SpO
outlet tube and CO
connection cable.
2
Gas inlet tube
CO2 connection
cable
Gas outlet
Parameter connector panel assembly
cable, and then the silicon tube, gas inlet tube, gas
2
SpO2 signal
cable
Silicon tube
,
2
6-9
Page 60

6.3.8 Disassembling the CO
Module Assembly (Only Available for
2
Accutorr 7)
1. Pull out the AC receptacle, NIBP connection cable, CO2 connection cable.
2. Unscrew two M3X6 screws.
3. Remove the CO
module.
2
NIBP connection
cable
AC receptacle
Screw
CO
2
connection
cable
Screw
6.3.9 Disassembling CO2 main Module (Only Available for Accutorr 7)
3XM2.5 screws
6-10
Page 61

6.3.10 Disassembling the Main Bracket Assembly (With CO
, Only
2
Available for Accutorr 7)
1. Disconnect the speaker cable, recorder cable, power cable, and battery interface board
cable. Unscrew the five PT3×8 screws and M4 screw.
2. Take out the main bracket assembly.
Speaker cable
Recorder cable
Battery
interface board
Power cord
One
grounding
screw
Five self-tapping screws
6-11
Page 62

6.3.11 Disassembling Pumps and Valves
1. Cut the two cable ties and remove the pump.
Cable tie
2. Unscrew the three screws as indicated and remove the NIBP board.
6.3.12 Disassembling AC/DC Power Board and Battery Converter
Board
1. Unscrew the four screws as indicated and remove the AC/DC power board.
6-12
Page 63

T
2. Unscrew the two M3 nuts to remove the battery interface board.
6.4 Disassembling the Front Housing Assembly
NOTE
To disassemble the equipment, place the equipment on a work surface free
from foreign material, avoiding damaging the antiglare screen, touchscreen
and the knob.
Remember to install the screen support pad properly during reassembly.
Operations relating to optional parts may not apply to your equipment.
Position the touchscreen properly with the flexible cable facing down.
6.4.1 Removing the Touchscreen Control Board
Remove the two M3 screws as shown below. Disconnect the cable between main board and
touchscreen board, and the touchscreen control board cable, and then remove the
touchscreen control board.
ouchscreen
Control Board
cable
Cable between
the main board
and touchscreen
board
2 M3 screws
6-13
Page 64

6.4.2 Removing the 2.4G Wi-Fi Module (Optional, Only Available for
Accutorr 7)
1. Remove the two antennas on the front panel from the slots as shown below:
Antenna
2. Remove the antennas from the Wi-Fi module PCBA.
3. Push the clamps aside to remove the Wi-Fi module.
Antenna
Clamps
6-14
Page 65

6.4.3 Removing the 5G Wi-Fi Module (Optional, Only Available for
Accutorr 7)
1. Remove the antennas on the front panel from the slots as shown below:
Antenna
2. Remove the antennas from the Wi-Fi module PCBA.
Antenna
6-15
Page 66

3. Remove the antennas from antenna sleeve.
Antenna
Antenna
sleeve
4. Push the clamps aside to remove the Wi-Fi module.
5. Remove the Wi-Fi module.
Clamps
6-16
Page 67

Wi-Fi module
6.4.4 Removing the Main Control Board
Disconnect the alarm lamp cable, cable for display backlight, display cable, and the cable
between the main board and keypad board respectively. Unscrew the four M3×8 screws
and remove the main board, as shown below:
Cable
Cable
Cable
6.4.5 Removing SD Card
Remove the main board, and push the SD card as indicated below to remove the SD card.
6-17
Page 68

SD card
6.4.6 Removing the Touchscreen
Unscrew the seven PT3×8 screws as indicated below. Remove the touchscreen assembly
and then the touchscreen.
Touchscreen
6.4.7 Disassembling the Display
Unscrew the four M3×6 screws indicated below to remove the screen.
6-18
Page 69

6.4.8 Removing the Keypad
Unplug the encoder cable and unscrew the three PT3×8 screws indicated below. Remove
the keypad.
6.4.9 Removing the Encoder
Pull the knob off the encoder shaft. Remove the retaining nut. Then remove the encoder.
Knob
Encoder
shaft
6.4.10 Removing the Alarm Lamp
Unscrew the four PT2×6 screws indicated below and remove the alarm lamp board and
alarm lamp.
6-19
Page 70

T
T
Temp
T
6.5 Disassembling the SmarTemp
6.5.1 Removing the SmarTemp
TM
Module (Optional)
TM
Module PCBA and Module Power
Board PCBA
Unscrew the two M3×6 screws and the two M3 screws. Remove the cover board and metal
sheet.
Disconnect the Temperature board cable and the cable between Temperature isolation
power board and Temperature board. Unscrew the four M3×6 screws, you can remove the
Temperature module PCBA. Then unscrew the three M3×6 screws, you can remove the
Temperature module power board PCBA.
4 M3x6 screws
Screw for cover
Cover
emperature
module power
board PCBA
3 M3X6
Screw for cover
Metal sheet
emperature
board cable
Cable between
the isolation
power board and
board
emperature
module PCBA
Screw for metal
6-20
Page 71

T
6.5.2 Disassembling the Temperature On-Position Detection Board
PCBA
1. Unscrew the four M3×6 screws as indicated and remove the temperature module
housing.
2. Unscrew the four M2 screws as indicated below and remove the Temperature
on-position detection board PCBA.
emperature on-position detection board PCBA
NOTE
Remember to assemble the silicon button for the Temperature on-position
detection switch during reassembly.
6-21
Page 72

6.6 Disassembling the Exergen TemporalScanner Themometer
Module (Optional)
1. Unscrew three M3×6 screws, and then remove the panel.
M3×6 screws
2. Pull out the thermometer cable, unscrew two M3X6 screws, and then take out the
bottom panel.
M3×6 screws
Connection
cable
6-22
Page 73

3. Unscrew four M3X6 screws, and then take out the metal bracket.
M3×6 screws
4. Unscrew three M3X6 screws, and then take out the power isolation board.
6-23
Page 74

FOR Y
OUR NOTES
6-24
Page 75

7 Parts
7.1 Introduction
This section contains the exploded views and parts lists of the main unit to identify the
parts during disassembly of the monitor and replacing the parts.
This manual is based on a fully loaded configuration. Your equipment may not contain all
options and therefore may not be appear the same as the unit in this manual.
NOTE
For parts in the Parts List below that contain two part numbers, the first part
number is for patient monitors built with one version of plastic material, the
second part number is for patient monitors built with another version of
plastic material. The way to distinguish which material your monitor is built
from is if it contains the symbol
7.2 Main Unit
7.2.1 Exploded View
.
7-1
Page 76

7.2.2 Parts List
Item
No.
1 Accutorr 7 Front Housing Assembly
1 VS-900c Front Housing Assembly 115-057544-00 Touch screen
2 TR6F recorder for Accutorr 7
2 TR6F recorder for VS-900c 115-046892-00 N/A
3 Rear Housing Assembly
4
5 Screw, pan head Phillips, M3×8 M04-000605--- N/A
6 Temperature module
Description FRU part number Remarks
115-022922-00/
115-050858-00
115-001290-00/
115-046892-00
115-017698-00/
Touch screen
N/A
Cover assembly
115-048904-00
Screw, Pan head with washer, Phillips
M3×8
M04-004015--- N/A
115-017716-00/
115-048910-00
N/A
7-2
Page 77

7.3 Front Housing Subassembly (Touchscreen)
7.3.1 Exploded View
7-3
Page 78

7.3.2 Parts List
Item
No.
1 Accutorr 7 Front housing
Description FRU part number Remarks
3 Silicon water-proof strip
4 Alarm lamp
5 Silicon water-proof strip
115-022921-00/
115-050857-00
Accutorr 7
front
housing
service kit
8 Touchscreen position pad (8")
1 VS-900c Front housing
3 Silicon water-proof strip
4 Alarm lamp
5 Silicon water-proof strip
115-057546-00
VS-900c
front
housing
service kit
8 Touchscreen position pad (8")
2.4G: 0012-00-1730-01
2 Antenna cable
5G: 115-035362-00/
115-048914-00
N/A
6 Alarm Lamp Board 051-001362-00 N/A
7
9 Touch-panel, resistive-type, 8.4”
Cross recessed pan head self-tapping
screw PT2X6
M04-051003--- N/A
021-000058-00 or
021-000271-00
N/A
10 8” display-short
115-018259-00 N/A 12 8” display-long
13 LCD TFT 8.4”
11 Screw, pan head Phillips, M3×6 M04-002505--- N/A
14 Touchscreen control board PCBA 051-000881-00 N/A
15 Main board PCBA 115-023203-00 N/A
16
Screw, Pan Head W/Washer Phillips
M3X6
M04-004012--- N/A
17 ST3.3X8 screw 030-000338-00 N/A
18 Keypad board adjusting sleeve
115-020467-00 N/A
19 Keypad board cushion
7-4
Page 79

Item
No.
20 Keypad PCBA 051-001359-00 N/A
21 Encoder 801-0010-00010-00 N/A
22 Accutorr 7 silicon buttons 049-000607-00 Accutorr 7
22 Silicon buttons 049-000998-00 VS-900c
23 Knob 043-003372-01 Accutorr 7
23 Knob 043-010368-00 VS-900c
7.4 Rear Housing Assembly (without CO
Description FRU part number Remarks
module, CO2 Only
2
Available for Accutorr 7)
7.4.1 Exploded View
7.4.2 Parts List
Item
Description FRU part number Remarks
No.
1 Rear Housing Subassembly for Accutorr
1 Rear Housing Subassembly for VS-900c 115-048904-00 N/A
2 Battery door assembly
115-017698-00/
115-048904-00
043-003359-01/
7-5
N/A
N/A
Page 80

Item
Description FRU part number Remarks
No.
043-008878-01
3 AC socket assembly 115-017699-00 N/A
4 Parameter connector panel assembly
115-017711-00/
115-048908-00
115-017706-00/
115-048906-00
115-017707-00/
115-048907-00
Without SpO2
With Masimo SpO2
With Nellcor SpO2
5 Parameter connector board support 043-003168-00 N/A
6 Power management board 115-018262-00 With multi-IO
Screw, Pan head w
7
M3X6
ith washer, Phillips
M04-004012--- N/A
8 Valve assembly 115-063257-00 N/A
9 Pump
801-9261-0004000
NIBP pump service
kit
10 Shock absorption cushion for pump 047-005212-01 N/A
11 ST3.3X8 screw 030-000338-00 N/A
12 Speaker
13 Speaker pad
14 Handle
801-9261-0001000
043-003165-01/
N/A
N/A
043-008828-00
Cable between the power
/
management board and power board
Cable between the interface board and
/
main board
Cable between NIBP module and
/
power management board
009-003237-00 N/A
9211-20-87225 N/A
009-003238-00 N/A
/ Recorder cable 009-001969-00 N/A
/ multifunctional connector cap 043-003311-01 N/A
7-6
Page 81

7.5 Rear Housing Assembly (with CO
Accutorr 7)
7.5.1 Exploded View
module, Only Available for
2
7.5.2 Parts List
Item
Description FRU part number Remarks
No.
1 Rear Housing Subassembly
2 Battery door assembly
3 AC socket assembly 115-017699-00 N/A
Parameter connector panel assembly
4
Parameter connector panel assembly
5 Parameter connector board support 043-003168-00 N/A
115-017698-00/
115-048904-00
043-003359-01/
043-008878-00
115-038391-00/
115-051242-00
115-038392-00/
115-051243-00
7-7
N/A
N/A
Nellcor SpO2
Masimo SpO2
Page 82

Item
Description FRU part number Remarks
No.
6 Power management board 115-018262-00 With multi-IO
Screw, Pan head w
7
M3X6
ith washer, Phillips
M04-004012--- N/A
8 Valve assembly 115-063257-00 N/A
9 Pump
10 Shock absorption cushion for pump
801-9261-00040-00
NIBP pump
service kit
/ Fixing strip
11 M02D CO2 module 115-038402-00 /
12 Main bracket assembly 115-038394-00 N/A
13 Speaker bolster plate 042-008296-00 N/A
14 Speaker
15 Speaker pad
Cable between the power
/
management board and power board
Cable between the interface board and
/
main board
Cable between NIBP module and
/
power management board
020-000027-00 N/A
009-003237-00 N/A
9211-20-87225 N/A
009-003238-00 N/A
/ Recorder cable 009-001969-00 N/A
/ multifunctional connector cap 043-003311-01 N/A
7-8
Page 83

7.6 Main Bracket Assembly
7.6.1 Exploded View
7.6.2 Parts List
Item No. Description FRU part number
1 Screw, Pan head w/washer M04-004012---
2 Power board insulator 047-010575-00
3 Power board 022-000125-00
4 Knob, Battery latch 0380-00-0593
5 Nut with washer
115-018254-00 6 Battery spring
7 6301 battery interface PCBA
7-9
Page 84
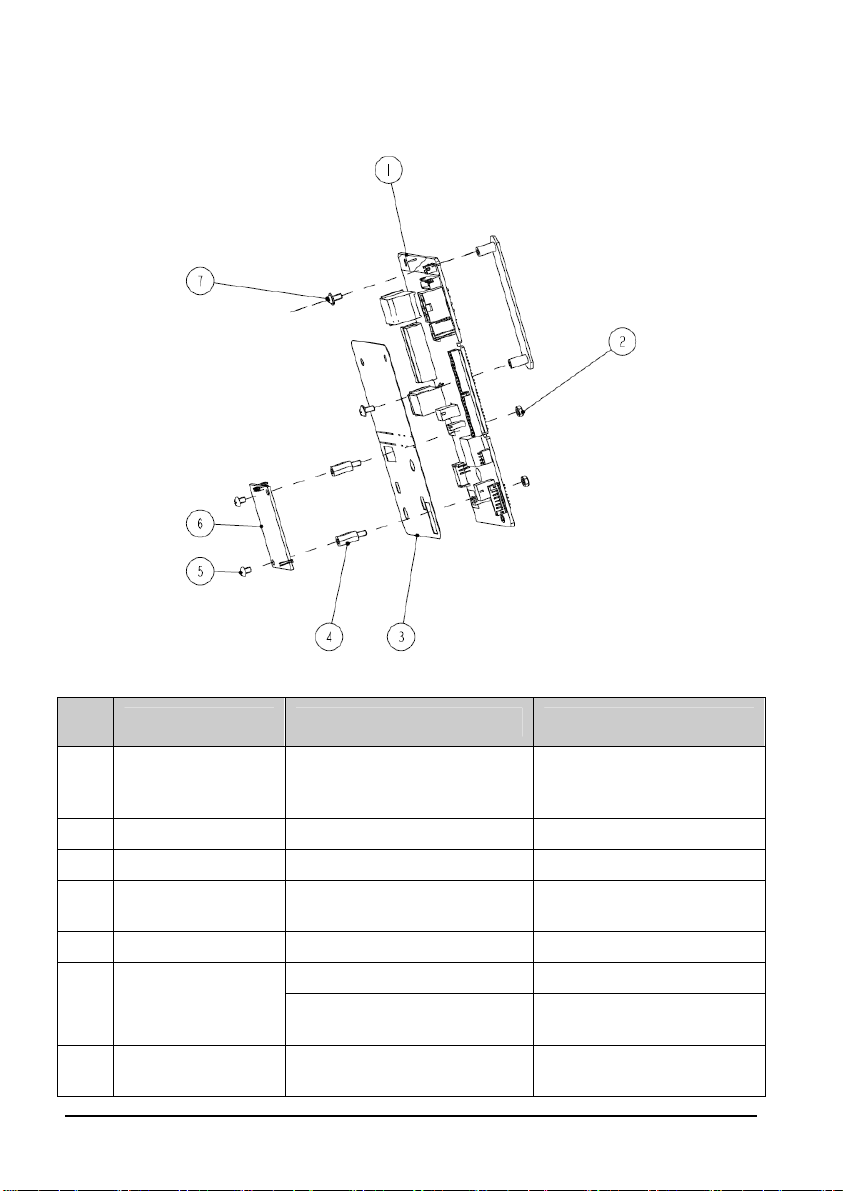
7.7 Power Management Board Assembly
7.7.1 Exploded View
7.7.2 Parts List
Item
Description FRU part number Remarks
No.
Power
1
management
board
service kit
115-018262-00
2 Plastic hexagon nut M90-000156--- N/A
3 SpO2 shield 047-010576-00 N/A
Plastic hexagon
4
bolt
099-000129-00 N/A
5 Screw, pan head M04-051001-01 N/A
100-000106-00 Nellcor SpO
6 SpO
7
board
2
Screw, Pan head
w/washer
040-000109-01
M04-004012--- N/A
7-10
with multifunctional
connector
SpO
board, Masimo
2
MS-2013
board (MDU)
2
Page 85

7.8 Parameter Connector Panel Assembly (without CO
Only Available for Accutorr 7)
CO
2
7.8.1 Exploded View
module,
2
7.8.2 Parts List
Item
Description FRU part number Remarks
No.
1 Parameter panel
2 NIBP fitting
1 Parameter panel
2 NIBP fitting
1 Parameter panel
2 NIBP fitting
3 SpO
signal cable
2
/ Decorative NIBP socket 043-003304-01 N/A
/ Decorative IBP socket
115-017711-00/
115-048908-00
115-017706-00/
115-048906-00
115-017707-00/
115-048907-00
without
Masimo SpO2
Nellcor SpO2
SpO2
009-003243-00 Masimo SpO
009-003244-00 Nellcor SpO2 module
043-001893-01/
043-008498-00
N/A
module
2
7-11
Page 86

7.9 Parameter Connector Panel Assembly (with CO
Only Available for Accutorr 7)
7.9.1 Exploded View
module,
2
7.9.2 Parts List
Item
Description FRU part number Remarks
No.
1 Parameter panel 043-006643-00
2 Hand feel spring 042-013555-00 N/A
3 NIBP fitting
115-010541-00/
115-046902-00
4 SpO
signal cable
2
009-003243-00 Masimo SpO
009-003244-00 Nellcor SpO2 module
5 1 slot CO2+O2 outlet nipple 041-017255-00 N/A
DRYLINE PRIME Receptacle
6
with no panel
115-036134-00 N/A
/ Decorative NIBP socket 043-003304-01 N/A
/ Decorative IBP socket
043-001893-0
043-008498-00
1/
Applicable for Nellcor or
Masimo SpO
N/A
N/A
module
2
module
2
7-12
Page 87

7.10 Predictive Temperature Assembly
7.10.1 Exploded View
7.10.2 Parts List
Item
No.
1 Screw, Pan head w/washer M04-004012---
2 Predictive Temperature module power board PCBA 801-6006-00043-00
3 Predictive Temperature housing
4 Temperature on-position detection board PCBA 051-001419-00
5 External compartment for Temperature module
6 Temperature cover 049-000547-01
7 Silicon buttons M09A-20-62064
8 Screw, Pan head Self-Tapping PT2X6 M04-051003---
Description FRU part number
043-003326-01/
043-008862-00
043-003312-01/
043-008842-00
7-13
Page 88

Item
No.
9 Screw, Flat Head Phillips, M3X6 M04-005005---
10 Predictive Temperature module PCBA 051-001435-00
/ Predictive Temperature board cable 009-003368-00
/
/
Description FRU part number
Cable between the Temp
management board
Cable between the isolation power board and
Temp
erature board
erature module and power
009-003239-00
009-003240-00
7.11 Exergen TemporalScanner Thermometer Assembly
7.11.1 Exploded View
7-14
Page 89

7.11.2 Parts List
SN Description FRU part number Remarks
1 Temp connection cable, 009-006361-00 /
2 Lower cover
3 Fixed screw 041-023435-00 /
4 Spacer gasket 047-016529-00 /
5 7 X 12 Base 047-010577-00 /
6 Pin buckle 041-022395-00 /
7 Cover 115-038403-00 /
8 Temp holder 042-016758-00 /
9 Screw, flat head Philips M3 X 6 M04-005005--- /
10 Stop cover 043-006888-00 /
11 Insulated sheet 047-017159-00 /
12 Insulated sheet connection cable 009-006362-00 /
13
Screw, pan head W/Washer
Philips M3 X 6
043-008823-00
M04-004012--- /
/
7-15
Page 90

7.12 Exergen frame Assembly
7.12.1 Exploded View
7.12.2 Parts List
S
1 Exergen frame cover
2 Lower cover /
3 spring holder /
4 Exergen frame base /
5 7 X 12 Base /
6 tail bed antiskid pad /
N
Description
FRU part
045-003420-00
7-16
number
Remar
/
ks
Page 91

8 Hardware and Software Upgrade
8.1 Hardware Upgrade
The monitor supports upgrade of the following functions:
SpO2 measurement
Tem p
8.1.1 Upgrade Package
Upgrade package Description of upgrade package PN of upgrade
SpO2 Masimo SpO2 upgrade kit 115-027698-00
Temp(SmarTempTM
module)
Tem p (Exe rge n
TemporalScanner™
Thermometer
module)
Note: measurement accessories are not included in the above upgrade packages.
8.1.2 Upgrading Parameter Modules
8.1.2.1 Upgrading Nellcor SpO
Contents of upgrade package:
A Nellcor SpO2 board;
An SpO
A connector panel assembly for Nellcor SpO
Two M3×6 screws
1. Remove the power management board and connector panel assembly as
2. Assemble the SpO
3. Install the power management board assembly with the SpO
erature measurement
package
Nellcor SpO2 upgrade kit 115-027699-00
SmarTemp
TM
module 115-027700-00
Exergen module (FDA) 115-062403-00
2
insulator;
2
board; and,
2
described in section 6.3 Disassembling the Main Unit.
board and insulator onto the power management board as
2
described in section 6.3.6 Removing the Parameter Board (SpO2 Optional) and
Power Management Board.
board and the
2
connector panel assembly in the service kit into the main unit as described in
section 6.3 Disassembling the Main Unit.
8-1
Page 92

8.1.2.2 Upgrading Masimo SpO
2
Contents of upgrade package:
A power management board assembly with Masimo SpO2; and,
A Masimo SpO
connector panel assembly.
2
1. Remove the power management board, connector panel assembly and the
stopple of multifunctional connector (if there is one) as described in section 6.3
Disassembling the Main Unit.
2. Install the power management board assembly with the SpO2 board and the
connector panel assembly in the service kit into the main unit as described in
section 6.3 Disassembling the Main Unit.
8.1.3 Upgrading Temperature
Contents of upgrade package:
A Temp
erature module with cables; and,
Two M3×6 screws.
Remove the decorative cover from the Temperature module connector. Install the
Temperature module onto the main unit as described in section 6.5 Disassembling the
SmarTempTM Module (Optional).
8.1.4 Upgrading Wi-Fi (Only Available for Accutorr 7)
Contents of upgrade package:
Antenna sleeve
Wi-Fi antenna
Carrier board of wireless module (PCBA)
Radio module support IEEE 802.11a/b/g/n
Wi-Fi label
Three cross pan head screw M2X4
Follow this procedure to upgrade:
1. Install the Wi-Fi module onto the main unit as described in section 6.4
Disassembling the Front Housing Assembly.
2. Paste the Wi-Fi label.
8-2
Page 93

3. Upgrade software.
4. Test that the wireless network can be connected. Refer to the operator’s manual
for wireless network connection.
8.1.5 Enabling Parameter Functions
1. Select [Main]→[Maintenance >>]→[Factory Maintenance >>]→enter the required
password→[
2. In the prompt menu, check the upgraded functions.
3. Click to save the changes and quit the menu.
4. Restart the monitor and the software for upgraded parameters are enabled.
Ok]→[Device Config. >>].
8.2 Software Upgrade
Software upgrades must be performed by Mindray, NA authorized service personnel. Call
Service Dispatch 1 800 288-2121 ext: 7875.
NOTE
The software upgrading could result in clearing the historical patient data. It
is recommended to export patient data before upgrading software.
8-3
Page 94

FOR YOUR NOTES
8-4
Page 95

A Electrical Safety Inspection
The following electrical safety tests are recommended as part of a comprehensive
preventive maintenance program. They are a proven means of detecting abnormalities that,
if undetected, could prove dangerous to either the patient or the operator. Additional tests
may be required according to local regulations.
All tests can be performed using commercially available safety analyzer test equipment.
Follow the instructions of the analyzer manufacturer.
The consistent use of a safety analyzer as a routine step in closing a repair or upgrade is
emphasized as a mandatory step if an approved agency status is to be maintained. The
safety analyzer also proves to be an excellent troubleshooting tool to detect abnormalities
of line voltage and grounding, as well as total current loads.
A.1 Power Cord Plug
A.1.1 The Power Plug
Tes t Item Acceptance Criteria
The power plug pins No broken or bent pin. No discolored pins.
The plug body No physical damage to the plug body.
The power plug
The power cord
The strain relief
The power plug No loose connections.
No physical damage to the strain relief. No plug
warmth for device in use.
No physical damage to the cord. No
deterioration to the cord.
For devices with detachable power cords,
inspect the connection at the device.
For devices with non-detachable power cords,
inspect the strain relief at the device.
A-1
Page 96

A.2 Device Enclosure and Accessories
A.2.1 Visual Inspection
Tes t Item Acceptance Criteria
No physical damage to the enclosure and accessories.
The enclosure and
accessories
No physical damage to meters, switches, connectors, etc.
No indication of exposure to fluid spills (e.g., water, coffee,
chemicals, etc.).
No loose or missing parts (e.g., knobs, dials, terminals, etc.).
A.2.2 Physical Inspection
Tes t Item Acceptance Criteria
No unusual noises (e.g., a rattle inside the case).
No unusual smells (e.g., burning or smoky smells,
The enclosure and accessories
particularly from ventilation holes).
No taped notes that may suggest device deficiencies or
operator concerns.
A.3 Device Labeling
Check the labels provided by the manufacturer or the healthcare facility are present and
legible.
Main unit label
Integrated warning labels
A-2
Page 97

Page 98

PN: 046-005296-00 (11.0)
 Loading...
Loading...