Invacare Perfecto User manual
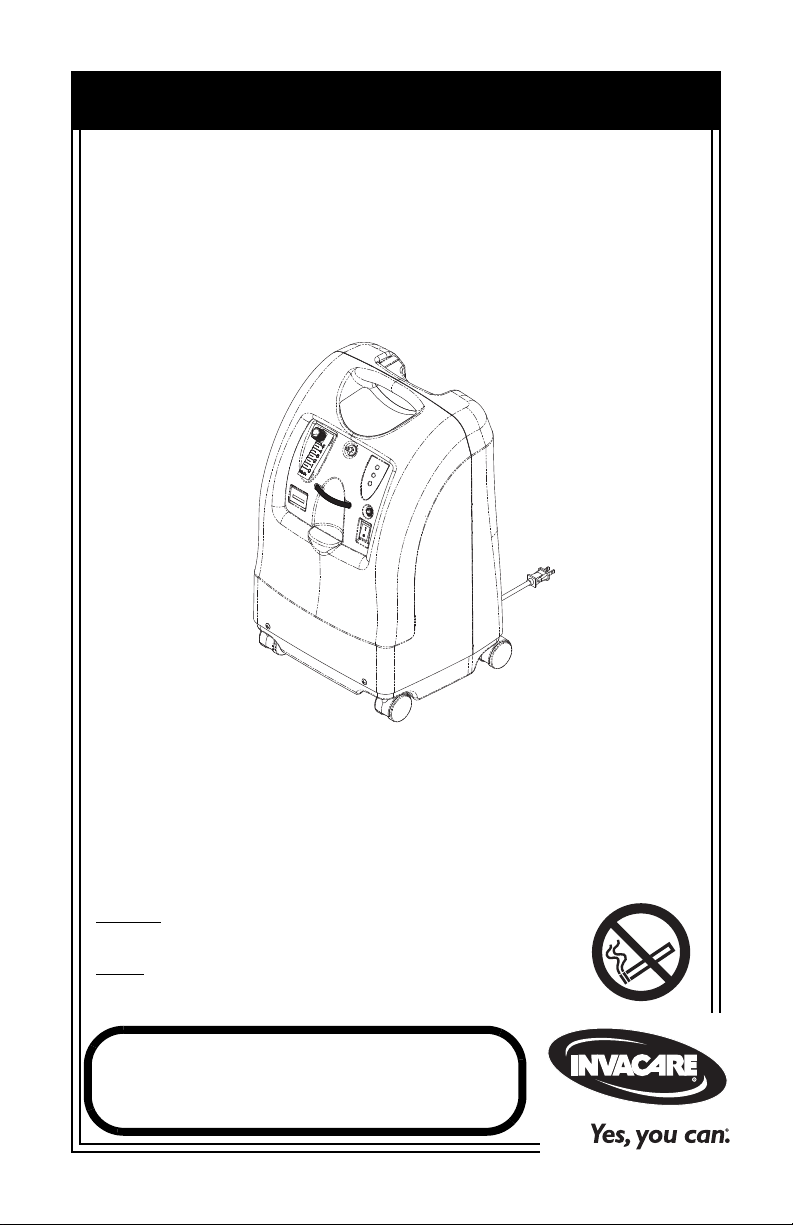
User Manual
Perfecto
™
Series
2
Oxygen Concentrators without SensO2®,
Oxygen Concentrators with SensO
HomeFill®Compatible
2
Perfecto2 with SensO
Model IRC5PO2
Model IRC5PO2W
Model IRC5PO2V
Dealer: This manual MUST be given to the end
user of this product.
User:
BEFORE using this product, read this
manual and save for future reference.
For more information regarding
Invacare products, parts, and services,
please visit www.invacare.com
2
Model IRC5P
Model IRC5PW
Perfecto
2
WARNING
WARNING
DO NOT USE THIS PRODUCT OR ANY AVAILABLE
OPTIONAL EQUIPMENT WITHOUT FIRST
COMPLETELY READING AND UNDERSTANDING
THESE INSTRUCTIONS AND ANY ADDITIONAL
INSTRUCTIONAL MATERIAL SUCH AS OWNER’S
MANUALS, SERVICE MANUALS OR INSTRUCTION
SHEETS SUPPLIED WITH THIS PRODUCT OR
OPTIONAL EQUIPMENT. IF YOU ARE UNABLE TO
UNDERSTAND THE WARNINGS, CAUTIONS OR
INSTRUCTIONS, CONTACT A HEALTHCARE
PROFESSIONAL, DEALER OR TECHNICAL
PERSONNEL BEFORE ATTEMPTING TO USE THIS
EQUIPMENT - OTHERWISE, INJURY OR DAMAGE
MAY OCCUR.
ACCESSORIES WARNING
Invacare products are specifically designed and manufactured
for use in conjunction with Invacare accessories. Accessories
designed by other manufacturers have not been tested by
Invacare and are not recommended for use with Invacare
products.
ACCESSORIES
There are many different types of humidifiers, oxygen tubing,
cannulas and masks that can be used with this device. You
should contact your local home care provider for
recommendations on which of these devices will be best for
you. They should also give you advice on the proper usage,
maintenance, and cleaning.
The supply accessories (nasal cannula, mask, tubing,
humidifier, etc.) used to deliver oxygen to the patient may
include a means to reduce the propagation of fire in the
accessories for the safety of the patient and others. If a
commercially available, fire-activated flow stop device is used
in the accessories setup, it should be placed as close to the
patient as practicable.
NOTE: Updated versions of this manual can be found at
www.invacare.com.
Perfecto
™
Series 2 Part No 1143482
2

Table of Contents
TABLE OF CONTENTS
ACCESSORIES .................................................. 2
SPECIAL NOTES ............................................... 4
LABEL LOCATION ............................................ 6
SECTION 1—GENERAL GUIDELINES .....................7
To Reduce the Risk of Burns, Electrocution, Fire or Injury to
Persons .................................................................................................. 7
Operating Information ....................................................................... 8
Radio Frequency Interference.......................................................... 8
SECTION 2—FEATURES ......................................10
SECTION 3—HANDLING ....................................11
Unpacking............................................................................................11
Inspection............................................................................................ 11
Storage................................................................................................. 11
SECTION 4—TYPICAL PRODUCT PARAMETERS .12
SECTION 5—OPERATING INSTRUCTIONS ..........14
Introduction........................................................................................ 14
Select a Location ...............................................................................14
Set Up .................................................................................................. 15
Power Switch .....................................................................................17
Flowrate...............................................................................................18
Front Panel Indicators - Units with SensO2................................19
Front Panel Indicators - Units without SensO2..........................20
SECTION 6—MAINTENANCE .............................22
Routine Maintenance........................................................................22
SECTION 7—TROUBLESHOOTING GUIDE ............25
LIMITED WARRANTY .................................... 27
Part No 1143482 3 Perfecto
™
Series
2
SPECIAL NOTES
SPECIAL NOTES
Signal words are used in this manual and apply to hazards or
unsafe practices which could result in personal injury or
property damage. Refer to the following table for definitions
of the signal words.
SIGNAL WORD MEANING
Danger indicates an imminently hazardous situation
DANGER
WARNING
CAUTION
which, if not avoided, will result in death or serious
injury.
Warning indicates a potentially hazardous situation
which, if not avoided, could result in death or
serious injury.
Caution indicates a potentially hazardous situation
which, if not avoided, may result in property
damage or minor injury or both.
NOTICE
The information contained in this document is subject to
change without notice.
DANGER
DO NOT SMOKE while using this device. Keep all
matches, lighted cigarettes or other sources of ignition
out of the room in which this product is located and away
from where oxygen is being delivered.
NO SMOKING signs should be prominently displayed.
Textiles and other materials that normally would not
burn are easily ignited and burn with great intensity in
oxygen enriched air. Failure to observe this warning can
result in severe fire, property damage and cause physical
injury or death.
Perfecto
™
Series 4 Part No 1143482
2
SPECIAL NOTES
CAUTION
“Caution: Federal law restricts this device to sale or rental by
or on order of a physician, or any other practitioner licensed
by the law of the State in which he/she practices to use or
order the use of this device.”
Invacare recommends an alternate source of supplemental
oxygen in the event of a power outage, alarm condition or
mechanical failure. Consult your physician or equipment
provider for the type of reserve system required.
This equipment is to be used as an oxygen supplement and is
not considered life supporting or life sustaining.
Part No 1143482 5 Perfecto
™
Series
2
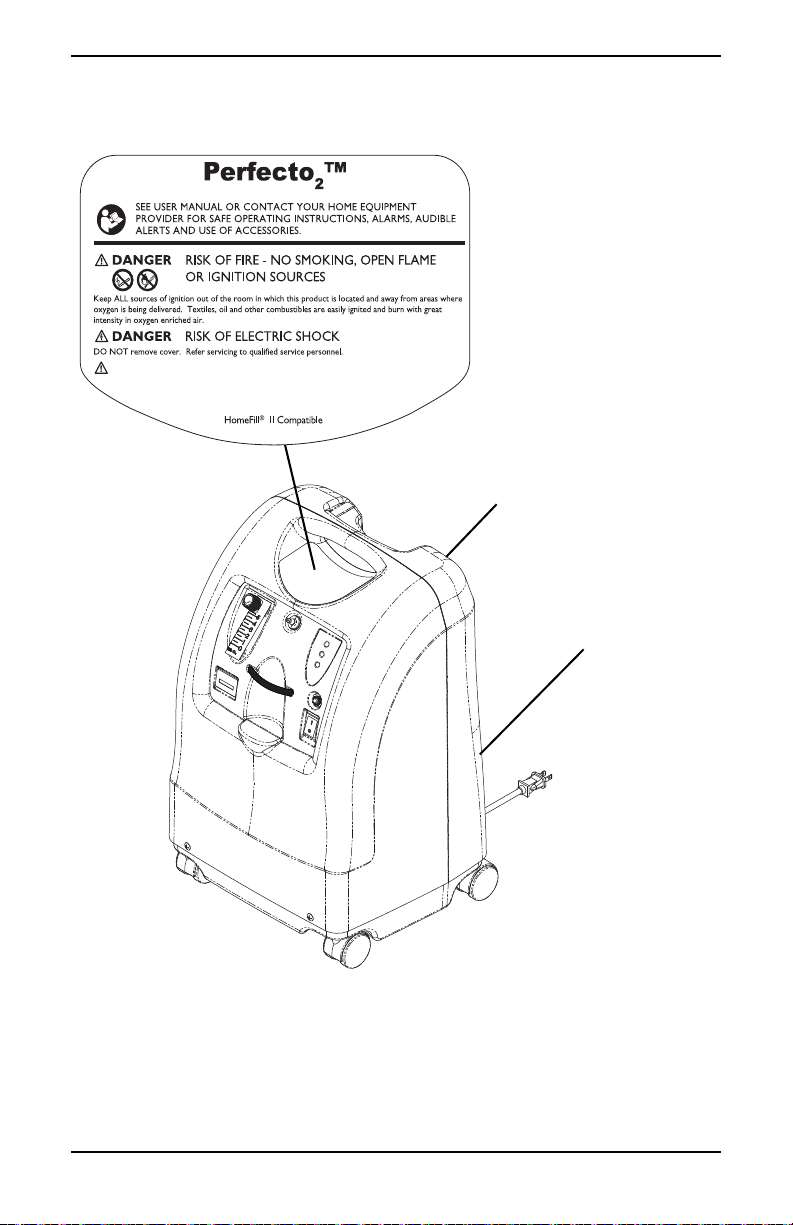
LABEL LOCATION
P
/
N
1
1
4
7
9
2
0
R
e
v
.
B
CAUTION
Federal (USA) law restricts this device to sale by or on the order of a
physician.
Serial Number Label is
located on the resonator
intake assembly
Specification Label is
located on the back
near the bottom
LABEL LOCATION
Perfecto
™
Series 6 Part No 1143482
2
SECTION 1—GENERAL GUIDELINES
SECTION 1—GENERAL GUIDELINES
In order to ensure the safe installation, assembly and
operation of the Perfecto2 concentrator these instructions
MUST be followed.
WARNING
This section contains important information for the safe
operation and use of this product.
To Reduce the Risk of Burns,
Electrocution, Fire or Injury to Persons
DANGER
Risk of electric shock. DO NOT disassemble. Refer servicing
to qualified service personnel. No user serviceable parts.
Avoid using while bathing. If continuous usage is required
by the physician’s prescription, the concentrator MUST be
located in another room at least 7 ft from the bath.
DO NOT come in contact with the concentrator while wet.
DO NOT place or store product where it can drop into
water or other liquid.
DO NOT reach for product that has fallen into water.
Unplug IMMEDIATELY.
If the concentrator has a damaged cord or plug, if it is not
working properly, if it has been dropped or damaged, or
dropped into water, call qualified technician for
examination and repair.
A spontaneous and violent ignition may occur if oil, grease
or greasy substances come in contact with oxygen under
pressure. These substances MUST be kept away from the
oxygen concentrator, tubing and connections, and all
other oxygen equipment. DO NOT use any lubricants
unless recommended by Invacare.
Avoid creation of any spark near medical oxygen
equipment. This includes sparks from static electricity
created by any type of friction.
Part No 1143482 7 Perfecto
™
Series
2

SECTION 1—GENERAL GUIDELINES
Operating Information
For optimum performance, Invacare recommends that each
concentrator be on and running for a minimum of 30 minutes
at a time. Shorter periods of operation may reduce maximum
product life.
Keep the oxygen tubing, cord, and unit out from under such
items as blankets, bed coverings, chair cushions, clothing and
away from heated or hot surfaces, including space heaters,
stoves and similar electrical appliances.
DO NOT move or relocate concentrator by pulling on the
power cord.
NEVER drop or insert any object or liquid into any opening.
Invacare recommends that Crush-Proof oxygen tubing be
used with this product and not exceed 50 ft in length.
There are no user serviceable parts. This does not include
normal maintenance items. See maintenance section for user
maintenance items.
A product should NEVER be left unattended when plugged
in. Make sure the Perfecto2 is Off when not in use.
Close supervision is necessary when this product is used near
children or physically-challenged individuals.
Additional monitoring or attention may be required for
patients using this device who are unable to hear or see
alarms or communicate discomfort.
DO NOT connect the concentrator in parallel or series with
other oxygen concentrators or oxygen therapy devices.
Radio Frequency Interference
This equipment has been tested and found to comply with
EMC limits specified by IEC/EN 60601-1-2. These limits are
designed to provide a reasonable protection against
electromagnetic interference in a typical medical installation.
Perfecto
™
Series 8 Part No 1143482
2

SECTION 1—GENERAL GUIDELINES
Other devices may experience interference from even the low
levels of electromagnetic emissions permitted by the above
standards. To determine if the emissions from the Perfecto2 is
causing the interference, turn the Perfecto2 Off. If the
interference with the other device(s) stops, then the Perfecto2
is causing the interference. In such rare cases, interference
may be reduced or corrected by one of the following
measures:
• Reposition, relocate, or increase the separation
between the equipment.
• Connect the equipment into an outlet on a circuit
different from that to which the other device(s) is
connected.
Part No 1143482 9 Perfecto
™
Series
2
 Loading...
Loading...