Ge Tonoport V User Manual

GE Healthcare
TONOPORT V
Ambulatory Blood Pressure System
Firmware Version 2.1
Operator’s Manual
2001589-085 ENG Revision F

Note
The information in this manual only applies to TONOPORT V, firmware version 2.1. It does not apply to earlier firmware versions.
Due to continuing product innovation, specifications in this manual are subject to change without notice.
CASE™ is a trademark owned by GE Medical Systems Information Technologies GmbH, a General Electric Company going to market as GE Healthcare.
© 2009–2016 General Electric Company. All rights reserved.
2 |
TONOPORT V |
2001589-085 Revision F |

Contents
1 |
Application, Safety Information |
6 |
2 |
Controls and Indicators |
10 |
3 |
Setup |
12 |
4 |
Application |
17 |
5 |
Data Output |
21 |
6 |
Error Codes |
22 |
7 |
Software Installation |
23 |
8 |
Cleaning, Maintenance, Disposal |
25 |
9 |
Technical Specifications |
27 |
10 |
Order Information |
28 |
11 |
Appendix - Electromagnetic Compatibility (EMC) |
29 |
Revision History
This manual is subject to the GE Healthcare change order service. The revision code, a letter that follows the document part number, changes with every update of the manual.
Part No./Revision |
Date |
|
2001589-085 |
Revision A |
2009-05 |
2001589-085 |
Revision B |
2010-04 |
2001589-085 Revision C |
2011-10-31 |
2001589-085 |
Revision D |
2014-01-31 |
2001589-085 |
Revision E |
2015-05-07 |
2001589-085 |
Revision F |
2016-07-12 |
Comment
Initial release
General Information: modifications in 3rd paragraph
Section 1.3: additional information concerning the ingress of liquids
Chapter 2: four symbols added
Chapter 3: additional information concerning alternative charger
CardioSys was removed globally
Chapters 1.1, 5 and 7: interface restrictions for CASE/CardioSoft were added
Chapter 2: relevant battery charger symbols were added
Chapter 7: CardioSoft version 6.7 for Windows 7 and reference to the “CASE-CS” folder were added Chapter 9: measuring range for mean pressure was corrected to '50 to 250 mmHg'
Changes on pages 4, 5, 11, 12, 23, and 24.
Changes on pages 8, 17, and 27.
Changes on pages 4, 11, and 28.
2001589-085 Revision F |
TONOPORT V |
3 |
General Information
General Information
The product TONOPORT V bears the CE marking CE-0482 (notified body MEDCERT GmbH) indicating its compliance with the provisions of the Council Directive 93/42/EEC about medical devices (including amendment 2007/47/EC) and fulfills the essential requirements of Annex I of this directive. It has an internal power source and is an MDD class IIa device. The device fulfills the requirements of the Directive 2011/65/EU of the European Parliament and of the Council.
It has a type BF applied part.
The product fulfills the requirements of the standard EN/IEC 60601-1 "Medical Electrical Equipment, Part 1: General Requirements for Basic Safety and Essential Performance" as well as the electromagnetic immunity requirements of the standard EN/IEC 60601-1-2 "Medical electrical equipment – Collateral standard: Electromagnetic compatibility – Requirements and tests" and applicable amendments.
The radio-interference emitted by this product is within the limits specified in CISPR11/EN 55011, class B.
The CE marking covers only the accessories listed in the "Order Information" chapter.
This manual is an integral part of the equipment. It should be available to the equipment operator at all times. Close observance of the information given in the manual is a prerequisite for proper equipment performance and correct operation and ensures patient and operator safety. Please note that information pertinent to several chapters is given only once. Therefore, carefully read the manual once in its entirety.
The symbol  means: Consult accompanying documents. It indicates points which are of particular importance in the operation of the equipment.
means: Consult accompanying documents. It indicates points which are of particular importance in the operation of the equipment.
This manual reflects the equipment specifications and applicable safety standards valid at the time of printing. All rights are reserved for devices, circuits, techniques, software programs, and names appearing in this manual.
On request GE Healthcare will provide a detailed Service Manual.
The safety information given in this manual is classified as follows:
Danger
indicates an imminent hazard. If not avoided, the hazard will result in death or serious injury.
Warning
indicates a hazard. If not avoided, the hazard can result in death or serious injury.
Caution
indicates a potential hazard. If not avoided, the hazard may result in minor injury and/or product/ property damage.
To ensure patient safety and interference-free operation and to guarantee the specified measuring accuracy, we recommend only original equipment accessories as available through GE Healthcare distribution. The user is responsible for the application of accessories from other manufacturers.
4 |
TONOPORT V |
2001589-085 Revision F |

General Information
PAR Medizintechnik GmbH & Co. KG
Sachsendamm 6
10829 Berlin
Germany
Tel. +49 30 235 07 00
Fax +49 30 213 85 42
Distributor:
GE Medical Systems
Information Technologies, Inc.
8200 West Tower Avenue
Milwaukee, WI 53223 USA
Tel: +1 414 355 5000
1 800 437 1171 (USA only)
1 800 668 0732 (Canada only)
Fax: +1 414 355 3790
The country of manufacture appears on the device label.
2001589-085 Revision F |
TONOPORT V |
5 |

Application, Safety Information
1 Application, Safety Information
1.1 Application
Intended Use
TONOPORT V is a small-size, patient-borne blood pressure monitor for ambulatory, non-invasive measurement of the patient’s blood pressure. If the blood pressure cuffs listed in chapter 10 "Order Information" fit the patient, it can be used on adults, children, and small children. TONOPORT V is not suitable for blood pressure measurements in neonates. Also it is not suitable for use in intensive-care medicine.
For periods of up to 30 hours, TONOPORT V records the patient's blood pressure at selectable intervals and saves the results. There is a choice of three different measurement protocols.
Using TONOPORT V with CASE™ / CardioSoft
TONOPORT V can be operated in conjunction with CASE™ (version 5.15 or later) or with the analysis program CardioSoft (version 4.14 or later) that is included with TONOPORT V. If the USB port is used (CardioSoft only), it is necessary to install the appropriate driver first (see “Software Installation” on page 23). With these systems, individual measurement protocols can be created and the stored data can be reviewed on-screen in tabular and graphic form. With V6.5 and subsequent versions, the patient ID used by the analysis program can be stored in TONOPORT V to allow the collected data to be downloaded without selecting the patient first (refer to the respective Operator Manuals; you will find the CardioSoft manual on the CardioSoft CD).
Biocompatibility
The parts of the equipment described in this manual, including all accessories, that come in contact with the patient during the intended use, fulfill the biocompatibility requirements of the applicable standards if used as intended. If you have questions in this matter, please contact GE Healthcare or its representatives.
Oscillometric Measuring Method
The blood pressure is measured by the oscillometric method. The criteria for this method are the pressure pulsations superimposed with every systole on the air pressure in the cuff.
The blood pressure cuff is wrapped around the upper arm and inflated to a pressure which must be clearly above the expected systolic pressure. A pressure transducer measures the cuff pressure as well as the superimposed pressure pulsations. During blood pressure measurements the cuff must be level with the heart. If this is not ensured, the hydrostatic pressure of the liquid column in the blood vessels will lead to incorrect results.
When the patient is sitting or standing during measurements, the cuff is automatically at the correct level.
Fig. 1-1 Waveform representing the pressure decrease in the cuff during a measurement: systolic pressure at 131 mmHg, diastolic pressure at 76 mmHg
6 |
TONOPORT V |
2001589-085 Revision F |

Application, Safety Information
1.2 Functional Description
The TONOPORT V monitor accommodates the blood pressure measuring system and a microprocessor for system control and data processing. The monitor is powered by two AA size batteries (either rechargeable NiMH batteries or alkaline batteries).
2001589-085 Revision F |
TONOPORT V |
7 |
Application, Safety Information
1.3 Safety Information
Danger
Risk to persons —
–The equipment is not designed for use in areas where an explosion hazard may occur. Explosion hazards may result from the use of flammable anesthetic mixtures with air or with oxygen, nitrous oxide, skin cleansing agents or disinfectants.
Warning
Risk to persons —
–Equipment may be connected to other equipment or to parts of systems only when it has been made certain that there is no danger to the patient, the operator, or the environment as a result. In those instances where there is any element of doubt concerning the safety of connected equipment, the user must contact the manufacturers concerned or other informed experts as to whether there is any possible danger to the patient, the operator, or the environment as a result of the proposed combination of equipment. Compliance with the standard IEC 60601-1 must always be ensured.
–TONOPORT V may be connected to CASE™ or to a PC with the CardioSoft program. While connected to any of these devices, TONOPORT V must be disconnected from the patient.
–Chemicals required for the maintenance of the equipment, for instance, must under all circumstances be prepared, stored, and kept at hand in their specific containers. Failure to observe this instruction may have severe consequences for the patient.
–The equipment has no protection against the ingress of liquids. Liquids must not enter the equipment. Equipment into which liquids have entered must be inspected by a service technician before use.
–Before cleaning, TONOPORT V must be disconnected from other equipment (CASE™,
PC).
–Dispose of the packaging material, observing the applicable waste-control regulations. Keep the packaging material out of children's reach.
8 |
TONOPORT V |
2001589-085 Revision F |

Application, Safety Information
Warning
Incorrect measurements —
–Magnetic and electrical fields are capable of interfering with the proper performance of the equipment. For this reason make sure that external equipment operated in the vicinity of TONOPORT V complies with the relevant EMC requirements. X-ray equipment, MRI devices, radio systems, etc. are possible sources of interference as they may emit higher levels of electromagnetic radiation.
Caution
Equipment damage, risk to persons —
–Before connecting the battery charger to the power line, check that the voltage ratings on the nameplate match those of your local power line.
–The battery charger is not a medical device. It must not be used in the patient environment.
–Before using the equipment, the operator is required to ascertain that it is in correct working order and operating condition.
–The operator must be trained in the use of the equipment.
–Only persons who are trained in the use of medical technical equipment and are capable of applying it properly are authorized to apply such equipment.
–There are no user-replaceable components inside the equipment. Do not open. For service or repair, please contact your local, authorized dealer (http://gehealthcare.com).
2001589-085 Revision F |
TONOPORT V |
9 |
Controls and Indicators
2 Controls and Indicators |
1 |
|
2 |
|
1 |
3 |
|
|
|
|
|
2 |
|
|
|
|
|
NBP |
|
4 |
|
|
|
|
|
|
|
11 |
|
|
TONOPORT |
|
|
|
|
|
|
||
|
|
|
|
V |
|
10 |
|
|
|
|
|
|
|
|
|
! |
3 |
9 |
off |
|
|
5 |
|
0 |
|
||||
|
|
|
|||
|
|
onI |
|
6 |
|
|
|
|
|
|
|
|
|
|
|
|
4 |
8 |
|
|
|
7 |
5 |
|
|
|
|
||
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
8 |
|
|
|
|
|
9 |
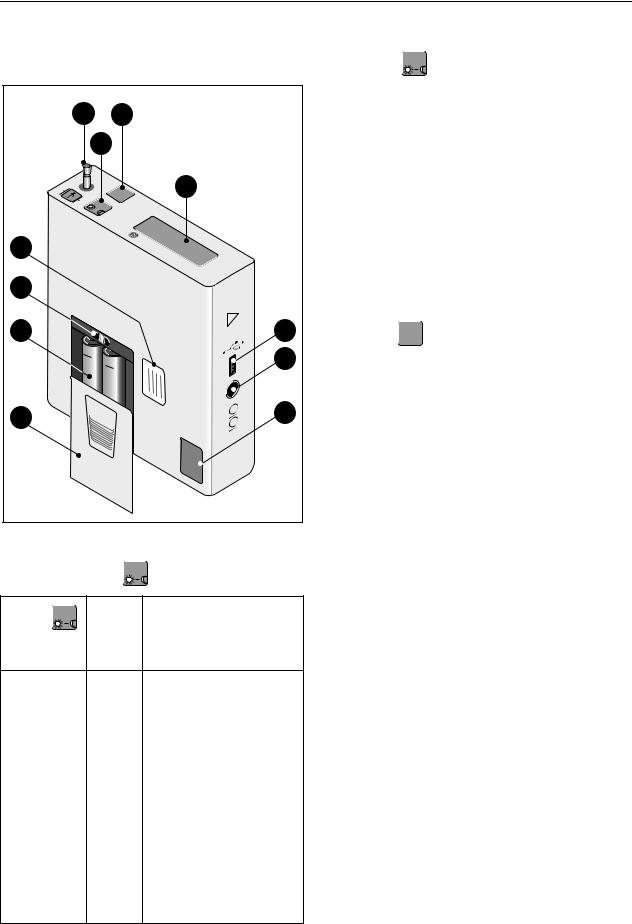
Fig. 2-1 TONOPORT V controls and indicators |
10 |
||||
|
|
|
|
|
|
Functions of the |
INFO |
Button |
11 |
||
Button |
INFO |
Message |
Function |
|
|
|
|
on |
|
|
|
|
|
display |
|
|
|
Push once |
H 1 |
clear memory |
|
|
|
Push twice |
H 2 |
set date and time |
|
|
|
Push 3 times |
H 3 |
select the measurement |
|
|
protocol |
|
|
|
Push 4 times |
H 4 |
activate calibration mode |
|
|
|
Push 5 times |
H 5 |
display firmware version |
|
|
|
Push 6 times |
H 6 |
select energy source |
|
|
|
Push 7 times |
H 7 |
enable/disable audio signal |
|
|
|
Push 8 times |
H 8 |
toggle pressure unit between |
|
|
mmHg and kPa |
Connection for blood pressure cuff
Button INFO : push to display the most recent parameter readings. Readings appear in the following order:
-systolic value "S" (unit mmHg or kPa shown on the display)
-diastolic value "D" (unit mmHg or kPa shown on the display)
-pulse rate "HR" (unit min-1)
The same button is used
-to toggle between the day phase and the night phase chapter 4, section "Toggle Manually Between Day and Night Phase") and
-to program the BP monitor (chapter 3 "Setup")
Button START : push to start and stop a
STOP
measurement, and to confirm entries
Liquid crystal display (LCD)
Port for connection to PC (USB)
Port for connection to PC (RS232)
Calibration mark
Lid covering battery compartment
(Rechargeable) batteries
ON/OFF switch
Nameplate
10 |
TONOPORT V |
2001589-085 Revision F |
Controls and Indicators
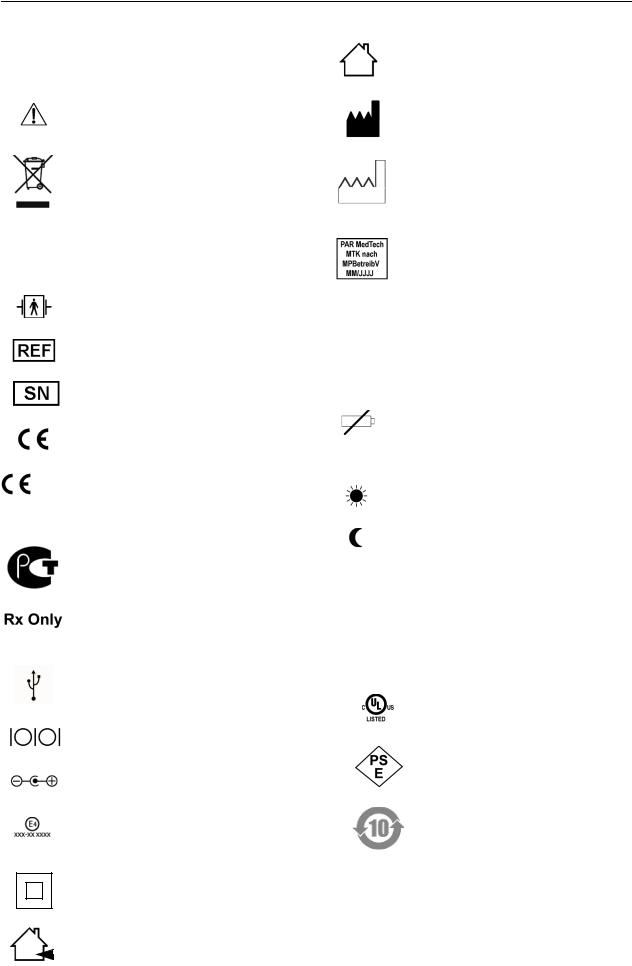
Explanation of Signs and Symbols
Symbols used on the equipment and on the packaging
Caution, consult accompanying documents
This symbol indicates that the waste of electrical and electronic equipment must not be disposed as unsorted municipal waste and must be collected separately. Please contact an authorized representative of the manufacturer for information concerning the decommissioning of your equipment.
Type BF applied part (defibrillation-proof)
Catalogue number
Serial number
CE marking
CE marked per the Medical Device 0482 Directive 93/42/EEC of the European
Union. The notified body is MEDCERT GmbH.
Gossudarstwenny Standart Russia (GOST)
In the USA, the product is only for use by or on the order of a physician, or persons licensed by U.S. law.
USB port, connection to PC
Serial port, connection to PC
Polarity of the DC input (charger only)
Approval mark for use of the equipment in a vehicle (charger only, xxx-xx xxxx alphanumeric characters)
Class II equipment
For indoor use only
For indoor use only
Manufacturer’s identification
Date of manufacture.
The number found under this symbol is the date of manufacture in the YYYYMM format.
Calibration mark, valid in Germany only (see section "Technical Inspections of the Measuring System" in chapter 8)
Symbols used on the display
Mblinks with each detected oscillation; is continuously lit when the monitor contains data
blinks when the batteries are almost depleted; is continuously displayed when batteries are discharged and no more BP measurements can be taken
day phase selected
night phase selected
Further relevant symbols used on the battery charger
TR15RA120 |
|
|
100-240V 0.4A |
Power supply type designation and |
|
47-63Hz |
ratings |
|
12V |
1.1A |
|
UL-certified product
Approval mark for Japan
Pollution control symbol according to the Chinese standard SJ/T113632006
|
|
|
|
11 |
2001589-085 Revision F |
TONOPORT V |
|||
Setup
3 Setup
Some Basic Facts on Battery Power
TONOPORT V is either powered by two rechargeable Nickel Metal Hydrid batteries (NiMH) or by two alkaline batteries. The device must be set to the power source used (see section "Insert Batteries" below). The device also contains a Lithium cell that powers the clock. The Lithium cell can only be replaced by a service technician.
The capacity of two fully charged or new batteries is sufficient for a minimum of 30 hours of operation or for 200 measurements.
The capacity of rechargeable batteries decreases with age. If the capacity of fully charged batteries is considerably less than 24 hours, the batteries must be replaced.
Caution
Equipment damage —
–Only use the original rechargeable, size AA Nickel Metal Hydrid batteries (from manufacturers such as Sanyo, Panasonic, Energizer, Duracell, Varta, or GP) with a capacity > 1500 mAh or size AA high-rate discharge alkaline batteries (such as Panasonic Evoia, Energizer Ultimate, Duracell Ultra, Duracell Power Pix, or Varta maxtech).
–Charge the NiMH batteries to capacity before using them for the first time.
–Recharge the NiMH batteries immediately after use and do not leave batteries uncharged.
–Use only the original charging unit to recharge the NiMH batteries.
–Do not attempt to recharge the alkaline batteries.
–If the TONOPORT V will not be used for one month or more, remove the (rechargeable) batteries from the device.
–Batteries must not be disposed as unsorted municipal waste and must be collected separately. Please contact an authorized representative of the manufacturer for information concerning the decommissioning of the batteries.
Insert Batteries
Note
Switch TONOPORT V off before inserting the batteries. To do so, slide the ON/OFF switch (10, Fig. 2-1) to the left while looking at the display.
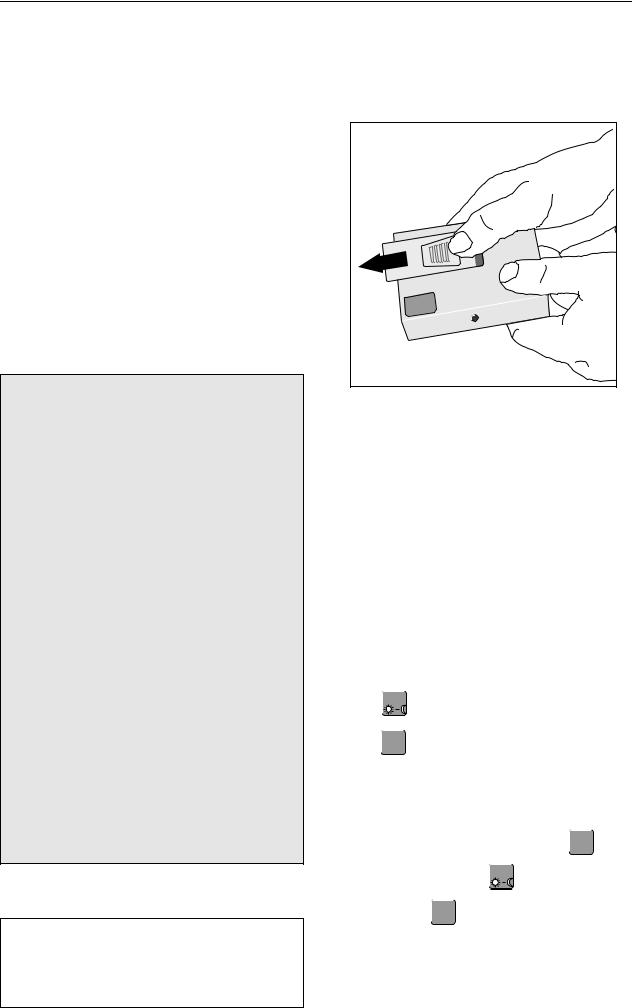
Hold TONOPORT V as shown in Fig. 3-1 and slide the lid of the battery compartment open (approx.
1 cm).
Fig. 3-1 Opening the battery compartment
It is not possible to open the lid more than about 1 cm which is just enough to reach the ON/OFF switch. To replace batteries, you must take off the lid (pull upward).
Place the two batteries in the compartment as indicated by the symbols.
Select Energy Source
Turn on the BP monitor. The switch is located inside the battery compartment. Slide the switch to the right, while looking at the display.
Wait for the time to be displayed.
Push |
INFO |
six times: the display shows "H 6". |
Push |
STOP |
: the display will show "AAAA" when the |
|
START |
|
BP monitor is set up for rechargeable NiMH batteries (as shipped) and "bbbb" when it is set up for alkaline batteries.
Confirm the displayed information with START or
STOP
change the selection with INFO and confirm the new
selection with START .
STOP
Next the BP monitor will briefly display the capacity of the inserted batteries. "A 100", for instance, means that the rechargeable batteries have a capacity of 100%, i.e., they are fully charged. "b 50" means that
12 |
TONOPORT V |
2001589-085 Revision F |
 Loading...
Loading...