Page 1

MicroPulser
™
Electroporation Apparatus
Operating Instructions
and Applications Guide
Catalog Number
165-2100
For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723)
Page 2

Table of Contents
Page
Section 1 Safety Information .......................................................................................1
1.1 Electrical Hazards.......................................................................................................1
1.2 Mechanical Hazards ...................................................................................................1
1.3 Other Safety Precautions............................................................................................1
Section 2 Introduction..................................................................................................2
2.1 Overview of electroporation theory ...........................................................................2
2.2 Manipulation of instrument parameters.....................................................................4
Section 3 Factors Affecting Electroporation .............................................................5
3.1 Cell growth .................................................................................................................6
3.2 DNA............................................................................................................................6
3.3 Electroporation Media................................................................................................7
Section 4 MicroPulser Operating Instructions..........................................................9
4.1 Setting up the MicroPulser System............................................................................9
4.2 Operation of the MicroPulser.....................................................................................9
4.3 Electroporation using the MicroPulser ...................................................................11
Section 5 High Efficiency Electrotransformation of E. coli...................................12
5.1 Preparation of electrocompetent cells......................................................................12
5.2 Electroporation .........................................................................................................12
5.3 Solutions and reagents for electroporation ..............................................................13
Section 6 Electroporation of Staphylococcus aureus...............................................13
6.1 Preparation of electrocompetent cells......................................................................13
6.2 Electroporation .........................................................................................................14
6.3 Solutions and reagents for electroporation ..............................................................14
Section 7 Electroporation of Agrobacterium tumefaciens .....................................15
7.1 Preparation of electrocompetent cells......................................................................15
7.2 Electroporation .........................................................................................................15
7.3 Solutions and reagents for electroporation ..............................................................16
Section 8 Electroporation of Saccharomyces cerevisiae..........................................16
8.1 Preparation of electrocompetent cells......................................................................16
8.2 Electroporation .........................................................................................................16
8.3 Solutions and reagents for electroporation ..............................................................17
Section 9 Electroporation of Schizosaccharomyces pombe.....................................17
9.1 Preparation of electrocompetent cells......................................................................17
9.2 Electroporation .........................................................................................................18
9.3 Solutions and reagents for electroporation ..............................................................18
Section 10 Electroporation of Dictyostelium discoideum ..........................................18
10.1 Preparation of electrocompetent cells......................................................................18
10.2 Electroporation .........................................................................................................19
10.3 Solutions and reagents for electroporation ..............................................................19
Section 11 Electroporation of Pichia pastoris ............................................................19
11.1 Preparation of electrocompetent cells......................................................................19
11.2 Electroporation .........................................................................................................20
11.3 Solutions and reagents for electroporation ..............................................................20
Page 3

Appendix I References .................................................................................................21
Appendix II Troubleshooting Guide for the MicroPulser ........................................22
Appendix III Product Information ................................................................................25
Page 4

Warranty
Bio-Rad Laboratories warrants the MicroPulser against defects in materials and workmanship for
1 year. If any defects occur in the instrument during this warranty period, Bio-Rad Laboratories will,
at Bio-Rad's option, repair or replace the defective parts free of charge. The following defects, however,
are specifically excluded:
1. Defects caused by improper operation.
2. Repair or modification done by anyone other than Bio-Rad Laboratories or an authorized
agent.
3. Use of fittings or other spare parts supplied by anyone other than Bio-Rad Laboratories.
4. Damage caused by accident or misuse.
5. Damage caused by disaster.
6. Corrosion due to use of improper solvent or sample.
For any inquiry or request for repair service, contact Bio-Rad Laboratories after confirming the
model, serial number, invoice number, and purchase order number of your instrument.
Model
Catalog No.
Date of Delivery
Serial No.
Invoice No.
Purchase Order No.
Page 5

Section 1
Safety Information
Read This Information Carefully Before Using The MicroPulser.
The MicroPulser meets the safety requirements of EN61010 and the EMC requirements
of EN61326 (for Class B, including flicker and harmonics).
1.1 Electrical Hazards
The MicroPulser produces voltages up to 3,000 volts and is capable of passing very high
currents. When charged to maximum voltage, the instrument stores about 50 joules. A certain
degree of respect is required for energy levels of this order. Safety system features prevent
operator access to the recessed input jacks and to the recessed electrode contacts inside the
sample chamber. These mechanical interlocks should never be circumvented.
There is high voltage present whenever the yellow pulse button is depressed and "PLS"
is shown in the light emitting diode display on the front of the instrument. If the capacitor
has been partially charged but not fired (for example, when the charging cycle has been
interrupted before the pulse is delivered), some charge may remain on the internal capacitor.
However, the user cannot make contact due to the system safety features.
1.2 Mechanical Hazards
The MicroPulser contains a patented arc-protection circuit that dramatically reduces the
incidence of arcing in the cuvette when high voltage is delivered into the sample. The unit
incorporates a circuit which senses the beginning of an arc and diverts current from the sample
within ~5 µsec, preventing, or greatly reducing mechanical, visual, and auditory phenomena
at the shocking chamber. Should an arc occur, the sample chamber is effective in containing
these small discharges, but nonetheless we strongly recommend wearing safety glasses when
using the instrument.
Do not use the MicroPulser with samples suspended in conductive media (refer to Section 3.3
for information on sample resistance).
1.3 Other Safety Precautions
Turn the unit off when not attended.
Avoid spilling any liquids onto the apparatus. Use only a paper towel or a cloth wet with
either water or alcohol to clean the outside surfaces of the MicroPulser.
Use only the Bio-Rad cables supplied with the MicroPulser.
Only use the shocking chamber in the assembled condition. Do not attempt to circumvent
the protection of the shocking chamber or use it while disassembled.
Verify the display segments periodically.
Do not use the MicroPulser if obvious case damage exists that exposes part of the inside
of the unit.
No user-serviceable parts are contained within the MicroPulser; the case should only be
opened by properly trained personnel.
1
Page 6

2
Warning: The MicroPulser generates, uses, and radiates radio frequency energy. If it is not
used in accordance with the instructions given in this manual, it may cause interference with
radio communications. The MicroPulser has been tested and found to comply with the limits
for Class A computing devices (pursuant to Subpart J of Part 15 of FCC Rules) which provide
reasonable protection against such interference when operated in a commercial environment.
Operation of this equipment in a residential area is likely to cause interference. In this case the
user will be required, at their own expense, to take whatever measure may be required to correct
the interference.
Section 2
Introduction
2.1 Overview of Electroporation Theory
The MicroPulser system is used for the electroporation of bacteria, yeast, and other
microorganisms where a high voltage electrical pulse is applied to a sample suspended in a
small volume of high resistance media. The system consists of a pulse generator module, a
shocking chamber, and a cuvette with incorporated electrodes (Figure 1). The sample is placed
between the electrodes in the cuvette. The MicroPulser module contains a capacitor, which is
charged to a high voltage; the module then discharges the current in the capacitor into the
sample in the cuvette.
Fig. 1. MicroPulser consisting of Pulse Generator Module, Shocking Chamber and Cuvette.
Page 7
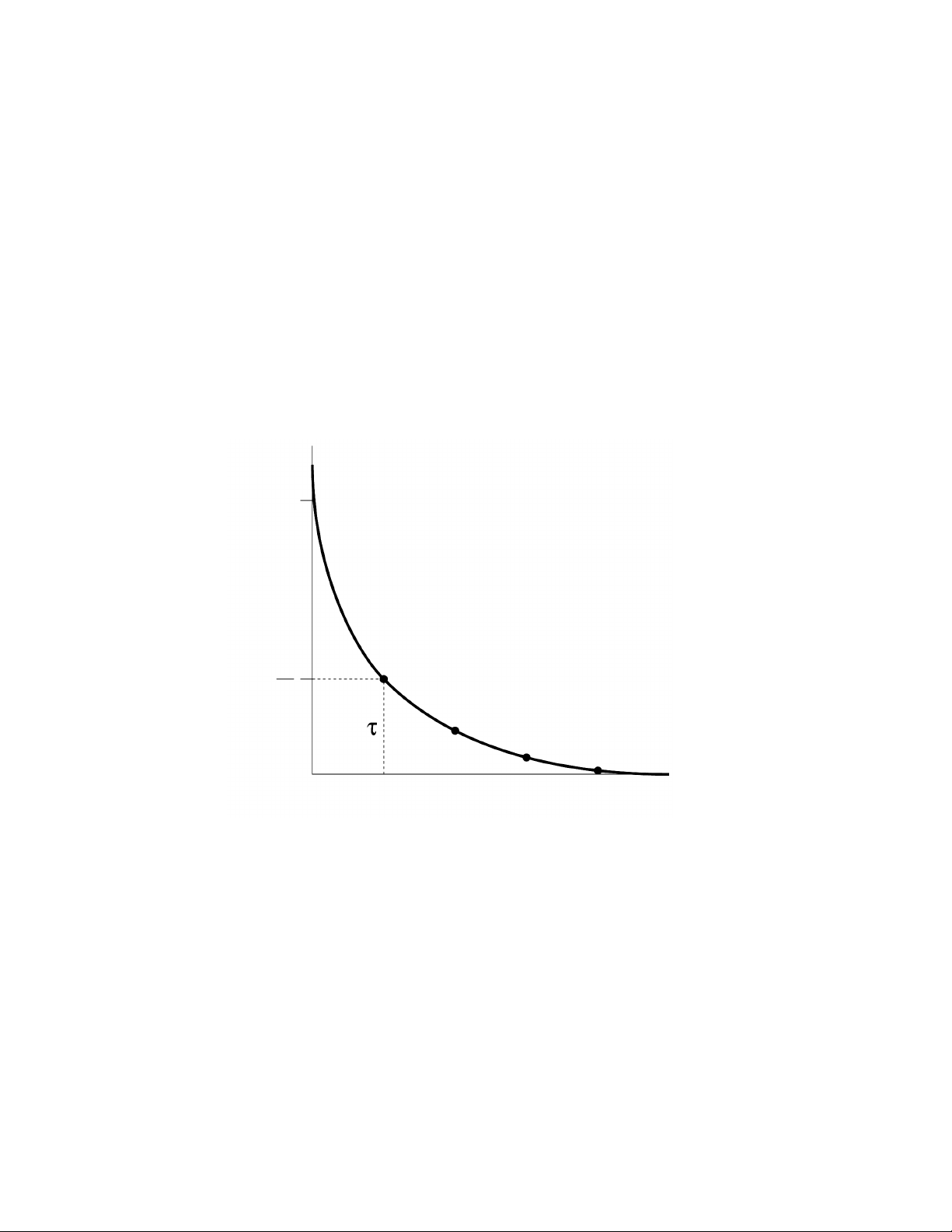
The capacitance discharge circuit of the MicroPulser generates an electrical pulse with an
exponential decay waveform (Figure 2). When the capacitor is discharged into the sample, the
voltage across the electrodes rises rapidly to the peak voltage (also known as the initial voltage,
V0), and declines over time, t, as follows,
Vt= V0[e
-(t/τ)
] Equation 1
where τ = R x C, the time constant, a convenient expression of the pulse length. The resistance
of the circuit, R, is expressed in ohms, and the capacitance of the apparatus, C, is expressed
in microfarads. According to Equation 1, τ is the time over which the voltage declines to 1/e
(~37%) of the peak value. The internal circuitry of the MicroPulser is designed to provide
optimum electroporation of E. coli and S. cerevisiae, as well as many other microorganisms,
in which the optimum transformation efficiency occurs at a time constant of approximately
5 msec. These electroporation conditions are achieved by using a 10 microfarad capacitor
and by placing a 600 ohm resistor in parallel with the sample cuvette along with a 30 ohm
resistor in series with the sample cuvette.
Fig. 2. Exponential decay pulse from a capacitance discharge system. When the capacitor,
charged to an initial voltage, Vois discharged into cells, the voltage applied to the cells decreases over
time so that at time t = τ, the voltage is (1/e) x Voof the initial value.
In addition to the time constant, the electric field strength is the other instrument param-
eter that is important in determining transformation efficiency. The electric field strength, E,
is the voltage applied between the electrodes and is described by
E = V/d Equation 2
where V is the voltage applied and d is the distance (cm) between the electrodes. The strength
of the electric field and the size of the cells determine the voltage drop across each cell, and
it is this voltage drop that may be the important manifestation of the voltage effect in electroporation.
3
V
0
V
0
e
Time (msec)
Voltage (V)
Page 8

The purpose of the 30 ohm series resistor in the MicroPulser is to protect the instrument
circuitry should arcing occur. Under normal operation, when samples are in high resistance
media, this resistor will not affect the voltage applied to the sample. However, this resistor will
significantly decrease the voltage applied to the sample if the resistance of the sample is low.
The fractional drop in voltage applied to the sample is given by
R30/ (R30+ R
sample
)
When R
sample
is 600 ohms, there is a 5% voltage drop to the sample [30 / (30 + 600) =
0.048]. For this reason, electroporation with the MicroPulser should not be performed in
solutions with a resistance of less than ~600 ohms. This includes samples in which the growth
medium was not adequately removed from the cells, DNA samples containing salt contributed
by residual sodium chloride, or ligation mixtures. The MicroPulser is able to measure the
resistance of the sample and will not pulse into very low resistance media.
2.2 Manipulation of Instrument Parameters
Several parameters on the MicroPulser may be altered to achieve maximum
transformation efficiency. These include the field strength, E, the time constant, τ, and the
width of a truncated exponential decay pulse. The field strength may be manipulated in two
ways. First, voltages between 200 and 3000 V may be set directly on the MicroPulser. This
parameter is the most easily controlled. The process of varying the voltage while keeping all
other conditions unchanged is the basis for most electroporation optimization procedures.
Second, using cuvettes with different electrode gap widths permits a means of changing the
field strength. For electroporation of microorganisms, 0.1 and 0.2 cm gap cuvettes are most
often used. Electroporation of E. coli is generally carried out at a voltage of 1.8 kV
(E = 18 kV/cm) when electroporating cells in 0.1 cm cuvettes and at a voltage of 2.5 kV
(E = 12.5 kV/cm) when electroporating cells in 0.2 cm cuvettes. These electroporation
conditions are pre-programmed into the MicroPulser as programs Ec1 (V = 1.8 kV) and Ec2
(V = 2.5 kV) in the bacterial settings menu. In addition, a third program, Ec3 in the bacterial
settings menu, delivers a voltage of 3.0 kV (E = 15 kV/cm in 0.2 cm cuvettes) which we have
found results in even higher transformation efficiency compared to electroporation at 2.5 kV.
The time constant may be altered by changing the sample resistance. The sample
resistance may be manipulated in two ways. First, increasing the salt or buffer concentration
of the electroporation media decreases the resistance of the sample, and vice versa, resulting
in a change in the time constant. Second, the volume of the sample in the cuvette is inversely
proportional to the resistance of the sample; decreasing the sample volume increases the
sample resistance. This effect of volume on sample resistance is most noticeable in low
resistance media. These effects are discussed further in Section 3.3.
4
Page 9
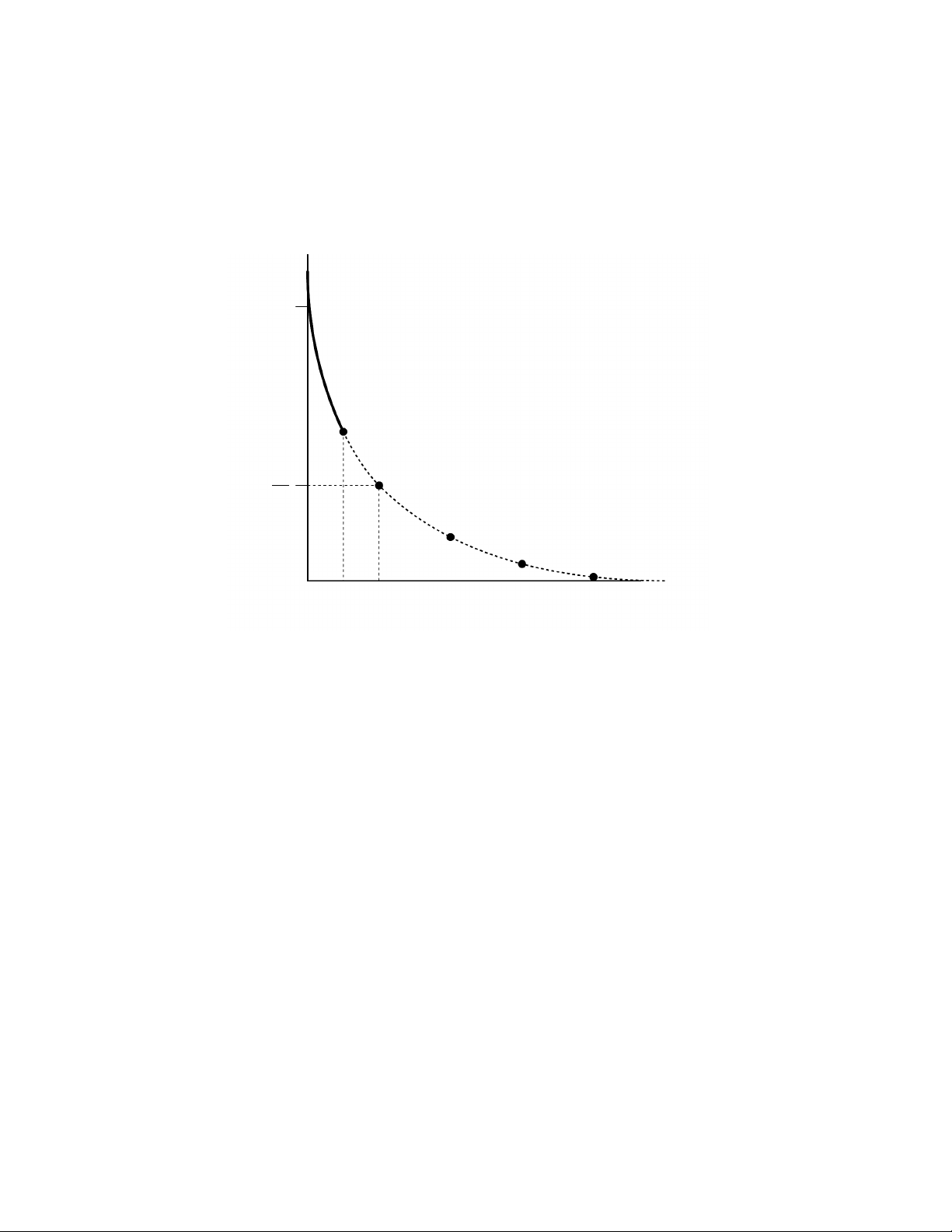
The MicroPulser also includes a means to truncate the exponential decay pulse sooner
than the expected time constant as long as the voltage is greater than 600 V. When the pulse
is terminated by the MicroPulser, voltage is applied to the sample only for the specified time,
which may be between 1.0 and 4.0 msec. Figure 3 shows how this waveform differs from
the true exponential decay pulse.
Fig. 3. Truncation of an exponential decay pulse by the MicroPulser. The solid line shows the voltage applied to the cells as a function of time during a pulse terminated after 2.5 msec. The dashed line
shows the voltage that would normally be applied to the cells during a true exponential decay pulse.
Section 3
Factors Affecting Electroporation
The electrical conditions for the electroporation of microorganisms have been verified
through years of research (see Chang, et al., 1992, and Nickoloff, 1995, for overviews as well
as for protocols on electroporation of numerous species). For many microorganisms, optimum electrotransformation occurs under electrical conditions relatively similar to those used
for E. coli and S. cerevisiae, two species that are most commonly used in research today. For
electroporation of E. coli, conditions reported as being used most often are 0.2 cm cuvettes containing 40 µl of cells at a voltage of 2.5 kV and a time constant of ~5 msec. For
electroporation of S. cerevisiae, conditions reported as being used most often are 0.2 cm
cuvettes containing 40 µl of cells at a voltage of 1.5 kV and a time constant of ~5 msec. For
many bacterial species, including Salmonella, Pseudomonas, Helicobacter, Borrelia,
Streptococcus, Lactococcus, and Enterococcus, the conditions for electroporation are
identical to those used for E. coli. For many other bacterial species, altering the field strength
will often result in higher electrotransformation. A similar case is found with other species of
yeast.
The MicroPulser is designed to deliver precisely those pulse parameters needed for the
highest transformation efficiency of E. coli and S. cerevisiae. The time constant has been set
5
Voltage (V)
V
0
V
0
e
2.5
5
Time (msec)
Page 10

at 5 milliseconds when working with high-resistance samples. For these organisms, the
MicroPulser has pre-programmed settings for delivery of the correct voltage when
electroporating E. coli in either 0.1 or 0.2 cm cuvettes, or when electroporating S. cerevisiae
in either 0.2 or 0.4 cm cuvettes.
3.1 Cell Growth
For most bacterial species, the highest transformation efficiencies are obtained when cells
are harvested in early to mid-log growth. For E.coli, as the cells reach stationary phase, the
transformation efficiency will decline precipitously (Dower, 1990). In contrast, most yeast
species are generally harvested in mid- to late-log growth. For S. cerevisiae, the
transformation efficiency increases as much as 60-fold from early to late-log cultures (Becker
and Guarente, 1991). The optimal portion of the growth phase to harvest cells is generally
dependent on the cell type. When preparing competent cells of a new species it is generally
best to employ conditions worked out for use with the same genus. Suggestions for factors to
consider and general methods for producing electrocompetent cells are discussed in the
articles by Dower et al. (1992) and Trevors et al. (1992).
3.2 DNA
While the majority of electroporation applications involve delivery of plasmid DNA to
cells, it should be mentioned that nearly any type of molecule can be introduced into cells by
electroporation, including RNA, proteins, carbohydrates, and small molecules. With few
exceptions, when delivering autonomously replicating plasmids, the highest transformation
efficiencies are obtained when electroporating supercoiled plasmid. However, electroporating
plasmid that will integrate into the host genome is usually most efficient using linear
plasmid. For example, Candida, Pichia, and Tetrahymena are transformed more efficently
when transformed with linearized than with supercoiled integrating plasmids.
In both E. coli and Listeria monocytogenes, the transformation efficiency of relaxed
circular plasmid is only slightly lower than that of supercoiled plasmid (Leonardo and Sedivy,
1990, Park and Stewart, 1990). However, linear plasmid is about 103- 104-fold less efficient
than the corresponding circular plasmid in both E. coli and Streptococcus pyogenes
(Shigekawa and Dower, 1988, Simon and Ferretti, 1991). Electroporation efficiency per mole
of plasmid generally decreases as the plasmid size increases in numerous species, including
E. coli (Leonardo and Sedivy, 1990, Siguret et al., 1994), Pseudomonas aeruginosa (Dennis
and Sokol, 1995), and Streptococcus thermophilus (Somkuti and Steinberg, 1988). However,
in some species, including Lactococcus lactis (Holo and Nes, 1995), Enterococcus faecalis
(Cruz-Rodz and Gilmore, 1990), and Clostridium perfringens (Allen and Blaschek, 1990),
transformation efficiency appears to be independent of plasmid size up to 20–30 kb.
Although transformation of most microorganisms has been accomplished using plasmid
DNA isolated by a variety of methods, the plasmid purity has an effect on transformation
efficiency. Significantly lower transformation efficiencies are generated with unpurified
miniprep plasmid DNA than with plasmid DNA purified by a variety of procedures. Plasmid
produced using the Bio-Rad Quantum matrix is as efficient as CsCl-purified plasmid for
transformation of microorganisms.
Generally, for all types of microorganisms, the frequency of transformation increases
with inceasing DNA concentration in the electroporation buffer. For E. coli, the frequency of
transformation (transformants/survivor) is dependent on DNA concentration over at least six
orders of magnitude (10 pg/ml to 7.5 µg/ml); within this range the DNA concentration
determines the probablility that a cell will be transformed. At the higher DNA concentrations,
up to 80% of the survivors are transformed (Dower et al., 1988). Because the number of
6
Page 11

transformants recovered is the product of the transformation frequency and the number of
cells present, the transformation efficiency (transformants/µg DNA) increases with cell
concentration over the range of 109to at least 3 x 1010cells/ml. Therefore, to obtain a high
transformation frequency, use high DNA concentration. To obtain high transformation
efficiency, use high cell concentration (and low DNA concentration to avoid
cotransformations). In each case, a small sample volume (20–50 µl) allows economical use
of DNA and cells (see Dower et al., 1988, for a detailed discussion of these factors).
3.3 Electroporation Media
The MicroPulser is designed for use with samples in high resistance media (>600 ohms).
For this reason, when preparing electrocompetent cells, it is important to wash cells
thoroughly to remove all traces of growth media. Failure to thoroughly remove the growth
media from the cells may result in the sample arcing during electroporation. Cells should be
washed at least three times with water or with non-ionic solutions, such as glucose, glycerol,
sucrose, sorbitol, or polyethylene glycol. For many microorganisms, glycerol is a convenient
electroporation medium, since it is recommended as a cryoprotectant for storage of cell cultures.
7
Page 12

Fig. 4. Resistance of solutions of (A) NaCl and MgCl2 and of (B) buffers of NaPO4 at pH 6.1 and 7.3
and HEPES at pH 7.5. Resistance was measured in 0.2 cm cuvettes containing either 40 µl or 200 µl of
solution at room temperature.
Figures 4A and B show the effect of concentration of several biologically important ionic
solutions on sample resistance. Note that: (1) volume has a significant effect on sample
resistance—for ionic solutions, sample resistance is inversely proportional to the volume of
solution in the cuvette; (2) the resistance of a solution containing divalent ions is lower than
a solution containing the same concentration of monovalent ions; (3) the resistance of a
buffered solution is affected by its pH.
The addition of even small concentrations of ionic compounds significantly reduces the
resistance of the sample and may cause arcing. Residual salt from ethanol precipitation of
DNA should be reduced by washing the DNA pellet prior to dissolving it in either water or
Tris-EDTA. Table 1 shows that, although adding a solution of plasmid in 10 mM Tris,
pH 8.0–1 mM EDTA to water does reduce the sample resistance, this should not result in the
inability to electroporate a sample in the MicroPulser. DNA may be used directly from enzyme
reactions for transformation, but the final salt concentration in the electroporation sample
should be kept below ~5 meq for high voltage operation. Finally, ligation mixtures may be
used for transformation, but only in very low quantities or when the ionic strength is reduced
by dilution (Willson and Gough, 1988), dialysis (Heery and Dunican, 1989; Jacobs et al.,
1990), or ethanol precipitation (Böttger, 1988; Zabarovsky and Winberg, 1990).
Table 1. Resistance of Water in 0.2 cm Cuvettes To Which TE
Has Been Added
1
.
SAMPLE R
sample
R
sample
(40 µl volume) (200 µl volume)
Water > 6 x 10
5
> 6 x 10
5
Water + 1 µl TE > 6 x 10
5
35,000
Water + 5 µ TE 11,200 8,700
Water + 10 µl TE 4,850 4,700
1
The resistance of 0.2 cm cuvettes containing either 40 or 200 µl water and the indicated
volume of TE (10 mM Tris, pH 8.0, 1 mM EDTA) was measured at 1000 V.
8
Page 13

Fig. 5. MicroPulser control panel.
Section 4
MicroPulser Operating Instructions
Refer to Figure 1 for a view of the components of the MicroPulser system and to Figure 5
for a definition of the buttons and LEDs.
4.1 Setting Up The MicroPulser System
1. Connect the black power cord to the rear panel of the MicroPulser Pulse Generator
Module. Plug the cord into a wall outlet or power strip.
2. Pull down the fold-down foot on the underside of the MicroPulser. Insert this foot into the
track on the base of the shocking chamber. Insert the shocking chamber slide into the
shocking chamber.
3. Connect the leads from the shocking chamber to the output jacks on the front panel of the
MicroPulser; polarity is not important to the electroporation process.
4. Turn on the apparatus using the power switch on the right rear panel. The light emitting
diode (LED) display should illuminate and read “Ec1” and the LED next to the Bacteria
Settings should be illuminated.
4.2 Operation of the MicroPulser
1. Selecting Pre-Programmed Settings
The MicroPulser is pre-programmed with settings for electroporation of a number of
commonly used organisms. Included under the Bacteria Settings program are the following:
Mnemonic Organism Parameters
Voltage Number of Time constant
(kV) pulses (msec)
Ec1
E. coli
(0.1 cm cuvette) 1.8 1 -
Ec2
E. coli
(0.2 cm cuvette) 2.5 1 -
9
Page 14

StA
S. aureus
(0,2 cm cuvette) 1.8 1 2.5
Agr
A. tumefaciens
(0.1 cm cuvette) 2.2 1 -
Ec3
E. coli
(0.2 cm cuvette) 3.0 1 -
Included under the Fungi Settings program are the following:
Mnemonic Organism Parameters
Voltage Number of Time constant
(kV) pulses (msec)
Sc2
S. cerevisiae
(0.2 cm cuvette) 1.5 1 -
Sc4
S. cerevisiae
(0.2 cm cuvette) 3.0 1 -
ShS
S. pombe
(0.2 cm cuvette) 2.0 1 -
dic
D. discoideum
(0.4 cm cuvette) 1.0 2 1.0
Pic
P. pastoris
(0.2 cm cuvette) 2.0 1 -
Pressing the "Settings" button cycles the Settings LED between "Bacteria", "Fungi", and
"Manual". When the LED next to Fungi is lit, the mnemonic for the fungi programs are
displayed. Pressing the "Raise" and "Lower" buttons to the left of the display LED cycles
between the different fungi programs. When the mnemonic is displayed, the parameters
associated with the mnemonic are automatically selected.
To change from one bacteria setting to another, while the Settings LED next to Bacteria
is lit, press the "Raise" and "Lower" buttons to the left of the display LED to cycle between
the different programs. When the mnemonic is displayed, the parameters associated with the
mnemonic are automatically selected.
While a program mnemonic is displayed for either a Bacteria or Fungi Setting,
simultaneously pressing both the "Raise" and "Lower" buttons shows the program
parameters selected on the display LED. The display LED first shows the voltage value, then
displays a "t" followed by the time in msec, then, if time and multiple pulses are associated
with a program, display "P" followed by "2", indicating that two successive pulses are given.
If no "t" is given the pulse is not truncated, and if no "P" is given there is a single pulse.
2. Using The Micropulser in Manual Mode
A. To change the voltage.
Press the "Settings" button to illuminate the LED next to "Manual". The display LED
now shows the voltage (in kV). Pressing the "Raise" and "Lower" buttons to the left of the
display LED allows selection of the desired voltage in the range from 0.20 kV to 3.00 kV.
If the instrument was just turned on, the display LED shows "0.00"
B. To truncate the pulse.
Press both "Raise" and "Lower" buttons simultaneously while the "Manual" LED is
illuminated. The display LED now shows “t—” and indicates the pulse time that has been
selected for the pulse. The default setting when the power is turned on is the standard
exponential decay pulse, or no pulse truncation, which is indicated by the two dashes.
Releasing only the "Lower" button results in the display LED showing the time of the
truncated pulse in milliseconds. The display LED initially changes to "t1.0" and rises in
0.1 msec increments to "t4.0". This permits truncating the exponential decay pulse between
1–4 msec. Simultaneously pressing both "Raise" and "Lower" buttons, then releasing
only the "Raise" button, results in lowering the indicated truncation time.
10
Page 15

3. Pulse Function
Pressing the "Pulse" button results in the capacitor charging to the set voltage; during this
time "PLS" is shown on the display LED. A tone will sound to indicate that the pulse has
been delivered. When multiple pulses are delivered by one of the built-in programs, "PLS" is
shown on the display LED during the entire time and a tone sounds each time a pulse is
delivered. To manually deliver multiple pulses, after the tone sounds from the first pulse,
press the pulse button again.
If a lower pitched tone sounds, accompanied by "Arc" being shown on the display LED,
the arc prevention and quenching (ARQ) system has been actuated and the pulse has been
terminated. This is usually an indication of attemped cuvette arc-over, but may also occur if
the sample resistance is too low. Since the energy delivered during such an ARQ event is
low, it is usually possible to pulse the sample again at parameters which will not result in an
arc and still produce acceptable results. However, it is not advisable to use a sample in which
two arc events have occurred.
4. Measurements
Pressing the "Measurements" button results in illumination of the "Actual kV" LED. This
indicates that the display LED shows the actual voltage delivered (in kV) during the last pulse.
If the instrument was just turned on and no pulse has been given, the display LED shows
"0.00". Pressing the "Measurements" button again results in illumination of the "Time ms"
LED. This indicates that the display LED shows the time constant (in msec) of the last pulse.
If the instrument was just turned on and no pulse has been given, the display LED shows
"0.00". Holding the "Measurements" button toggles the display LED between the Actual
voltage and the Time constant.
4.3 Electroporation Using The MicroPulser
1. Place the cell suspension in an electroporation cuvette and tap the liquid to the bottom of
the cuvette. Up to 0.4 ml (400 µl) of solution may be placed in the 0.2 cm cuvette, and up
to 80 µl may be placed in the 0.1 cm cuvette. Note that temperature may have a significant
influence on transformation frequency. Electroporation of some organisms, including
E. coli and S. cerevisiae, is more efficient in chilled cuvettes.
2. Insert the cuvette into the slide of the shocking chamber. Push the slide into the chamber
until the cuvette makes firm contact with the chamber electrodes.
3. To charge the capacitor and deliver a pulse, press the yellow "Pulse" button; the display
LED will show "PLS" until a tone sounds indicating that the pulse has been given. The
display LED will then show the program, the time constant, or the actual volts delivered,
depending on the LED selected.
4. Withdraw the slide from the chamber, remove the cuvette, and process the sample.
5. The time constant and the actual voltage delivered to the sample are shown on the display
LED by pressing the "Measurements" button. When the LED next to "Actual kV" is
illuminated, the voltage is displayed in kilovolts. The time constant can be displayed by
pressing the "Measurements" button again. The LED next to "Time ms" will be
illuminated; the time constant is displayed in milliseconds.
6. To turn the unit off press the power switch on the right rear panel. The sample chamber
may now be safely disconnected, if desired. Never remove the sample chamber cover
until the leads are disconnected.
11
Page 16

Section 5
High Efficiency Electrotransformation of
E. coli
Electroporation provides a method of transforming E. coli at efficiencies as high as 10
9
to 1010transformants/µg, which is greater than is possible with the best chemical methods. The
following protocol describes a method for for preparing and electrotransforming E. coli to
high efficiencies. We are interested in hearing of additional strains transformed by
electroporation and including this information in subsequent versions in our Electroprotocols
manual. Please contact your local Bio-Rad representative, access our web site at
www.bio-rad.com, or, in the U.S., call our Technical Services at (800) 424-6723 with any
comments or questions.
5.1 Preparation of Electrocompetent Cells
See Ausubel et al. (1987) and Miller and Nickoloff (1995) for additional information.
1. Inoculate 500 ml of L-broth with 1/100 volume of a fresh overnight E. coli culture.
2. Grow the cells at 37 °C shaking at 300 rpm to an OD
600
of approximately 0.5–0.7 (the best
results are obtained with cells that are harvested at early- to mid-log phase; the appropriate
cell density therefore depends on the strain and growth conditions).
3. Chill cells on ice for ~20 min. For all subsequent steps, keep the cells as close to 0 °C as
possible (in an ice/water bath) and chill all containers in ice before adding cells. To harvest,
transfer the cells to a cold centrifuge bottle and spin at 4000 x g for 15 minutes at 4 °C.
4. Carefully pour off and discard the supernatant. It is better to sacrifice the yield by pouring
off a few cells than to leave any supernatant behind.
5. Gently resuspend the pellet in 500 ml of ice-cold 10% glycerol. Centrifuge at 4000 x g for
15 minutes at 4 °C; carefully pour off and discard the supernatant.
6. Resuspend the pellet in 250 ml of ice-cold 10% glycerol. Centrifuge at 4000 x g for
15 minutes at 4 °C; carefully pour off and discard the supernatant.
7. Resuspend the pellet in ~20 ml of ice-cold 10% glycerol. Transfer to a 30 ml sterile
Oakridge tube. Centrifuge at 4000 x g for 15 minutes at 4 °C; carefully pour off and
discard the supernatant.
8. Resuspend the cell pellet in a final volume of 1–2 ml of ice-cold 10% glycerol. The cell
concentration should be about 1–3 x 1010 cells/ml.
This suspension may be frozen in aliquots on dry ice and stored at -70 °C. The cells are
stable for at least 6 months under these conditions.
5.2 Electroporation
1. Thaw the cells on ice. For each sample to be electroporated, place a 1.5 ml microfuge
tube and either a 0.1 or 0.2 cm electroporation cuvette on ice.
2. In a cold, 1.5 ml polypropylene microfuge tube, mix 40 µl of the cell suspension with
1 to 2 µl of DNA (DNA should be in a low ionic strength buffer such as TE). Mix well
and incubate on ice for ~1 minute. (Note: it is best to mix the plasmids and cells in a
microfuge tube since the narrow gap of the cuvettes prevents uniform mixing.)
3. Set the MicroPulser to “Ec1” when using the 0.1 cm cuvettes. Set it to "Ec2" or "Ec3"
when using the 0.2 cm cuvettes. See Section 4 for operating instructions.
12
Page 17

4. Transfer the mixture of cells and DNA to a cold electroporation cuvette and tap the
suspension to the bottom. Place the cuvette in the chamber slide. Push the slide into the
chamber until the cuvette is seated between the contacts in the base of the chamber. Pulse
once.
5. Remove the cuvette from the chamber and immediately add 1 ml of SOC medium to the
cuvette. Quickly but gently resuspend the cells with a Pasteur pipette. (The period between
applying the pulse and transferring the cells to outgrowth medium is crucial for recovering
E. coli transformants (Dower et al., 1988). Delaying this transfer by even 1 minute causes
a 3-fold drop in transformation. This decline continues to a 20-fold drop by 10 minutes.
6. Transfer the cell suspension to a 17 x 100 mm polypropylene tube and incubate at 37 °C
for 1 hour, shaking at 225 rpm.
7. Check and record the pulse parameters. The time constant should be close to 5 milliseconds. The field strength can be calculated as actual volts (kV) / cuvette gap (cm).
8. Plate on selective medium.
5.3 Solutions and Reagents For Electroporation
1. L-Broth: 10 g Bacto tryptone, 5 g Bacto yeast extract, 5 g NaCl; dissolve in 1.0 L water.
Autoclave.
2. 10% (v/v) Glycerol: 12.6 g glycerol (density = 1.26 g/cc) in 90 ml of water. Autoclave
or filter sterilize.
3. TE: 10 mM Tris-HCl pH 8.0, 1 mM EDTA.
4. SOC: 2% Bacto tryptone, 0.5% Bacto yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM
MgCl2, 10 mM MgSO4, 20 mM glucose.
Section 6
Electroporation of
Staphylococcus aureus
6.1 Preparation of Electrocompetent Cells
See Lee (1995) for additional information.
1. Inoculate 3 ml of B2 broth in a 17 x 100 mm tube with a colony from a fresh
S. aureus plate.
2. Incubate at 37 °C overnight, shaking at 250 rpm.
3. Inoculate 1.5 ml of the overnight culture into 150 ml of fresh B2 broth in a 1 liter flask.
Incubate at 37 °C, shaking at 250 rpm, to ~2 x 108cells/ml. The doubling time of
S. aureus is about 30 min at 37 °C.
4. Chill the cells in an ice water bath for 15 min to stop growth. Decant the cells into a sterile
500 ml centrifuge bottle. Harvest the cells by centrifugation at 12,000 x g for 15 min at
4 °C.
5. Carefully pipette off the supernatant, keeping the cell pellet on ice.
6. Resuspend the cell pellet in 500 ml of sterile, ice-cold water. Pellet the cells by
centrifugation at 12,000 x g for 15 min at 4 °C; carefully remove the supernatant. Wash
the cells 2 more times in 500 ml of sterile, ice-cold water.
13
Page 18

7. Resuspend the cell pellet in 25 ml of sterile, ice-cold 10% glycerol. Transfer to a 30 ml
sterile Oakridge tube. Pellet the cells by centrifugation at 12,000 x g for 15 min at 4 °C;
carefully remove the supernatant..
8. Resuspend the cell pellet in 2 ml of 10% glycerol; the final cell concentration should be
~1 x 1010 cells/ml.
9. Dispense 250 µl aliquots of the electrocompetent cells into sterile 1.5 ml microfuge tubes;
freeze the cells in an isopropanol-dry ice bath, then store at -70 °C. The cells are stable for
several months under these conditions.
6.2 Electroporation
1. Pipette the DNA samples (5 ng - 2 µg in a volume ~ 3 µl) to be electroporated into
sterile 1.5 ml microfuge tubes.
2. Thaw the competent cells at room temperature for several minutes. Add 50 µl of cells to
each DNA sample; gently pipette up and down to mix.
3. Incubate the samples at room temperature for 30 min.
4. Set the MicroPulser to "StA". See Section 4 for operating instructions.
5. Transfer the mixture of cells and DNA to a 0.2 cm electroporation cuvette and tap the
suspension to the bottom of the tube. Place the cuvette in the chamber slide. Push the
slide into the chamber until the cuvette is seated between the contacts in the base of the
chamber.
6. Pulse once.
7. Remove the cuvette from the chamber and immediately add 1 ml of SMMP medium
containing a subinhibitory concentration of antibiotic; gently transfer the cells to a sterile
17 x 100 mm tube using a Pasteur pipette. Incubate 1 hr at 37 °C, shaking at 250 rpm.
8. Check and record the pulse parameters. The time constant should be close to
2.5 milliseconds. The field strength can be calculated as actual volts (kV) / cuvette gap (cm).
9. Plate aliquots of the electroporated cells on trypticase soy agar containing selective antibiotic.
Incubate plates for 36–48 hrs at 37 °C.
6.3 Solutions and Reagents For Electroporation
1. B2 medium: 10 g casein hydrolysate, 25 g yeast extract, 5 g glucose, 25 g NaCl, 1 g
K2HPO4; dissolve in 900 ml water and adjust pH to 7.5; bring volume to 1.0 L. Autoclave
2. SMMP: 55 ml 2X SMM, 40 ml 4X Penassay broth, 5 ml 10% (w/v) bovine albumin;
adjust pH to 7.0; filter sterilize
3. Trypticase soy agar: 40 g trypticase soy agar (Becton Dickinson, Sparks, MD) in 1 L of
water. Autoclave.
4. 2X SMM: 25 ml 0.2 M sodium hydrogen maleate, 40 ml 0.1 N NaOH; adjust the pH to
6.5. Add 5 ml 1 M MgCl2, 42.7 g sucrose; dissolve and bring volume to 125 ml. Filter
sterilize.
5. 4X Penassay broth: 17.5 g Antibiotic Medium 3 (Becton Dickinson) dissolved in
250 ml water. Autoclave.
6. 0.2 M sodium hydrogen maleate: 11.6 g maleic anhydride or 13.7 g maleic acid, 4 g
NaOH; dissolve in 500 ml water. Autoclave.
14
Page 19

Section 7
Electroporation of
Agrobacterium tumefaciens
7 .1 Preparation of Electrocompetent Cells
See Lin (1995) for additional information.
1. Inoculate 1.5 L of YM broth in a 2.8 L Fernbach flask with an aliquot from log phase
culture of A. tumefaciens.
2. Incubate at 30°C overnight, shaking at 300 rpm to a density of 5–10 x 107cells/ml.
3. Decant the cells into sterile 500 ml centrifuge bottles and pellet the cells by centrifugation
at 3000 x g for 10 min at 4 °C.
4. Carefully pour off and discard the supernatant; place the centrifuge bottles with the cell
pellets on ice.
5. Add ~50 ml of sterile, ice-cold 10% glycerol to each of the bottles and vortex to
resuspend the cell pellets; bring the volume in each of the centrifuge bottles to 500 ml
with sterile, ice-cold 10% glycerol. Pellet the cells by centrifugation at 3000 x g for
10 min at 4 °C; pour off and discard the supernatant.
6. Wash the cells again as in step 5.
7. Resuspend each of the cell pellets in 5 ml of sterile, ice-cold 10% glycerol and transfer to
a chilled 30 ml Oakridge tube. Pellet the cells by centrifugation at 3000 x g for 5 min at
4 °C; pour off and discard the supernatant.
8. Resuspend the cell pellet in 0.5 ml of sterile, ice-cold 1 M sorbitol; the final cell volume
should be ~1.5 ml and the cell concentration should be ~ 5 x 1010 cells/ml. Dispense
200 µl aliquots of the electrocompetent cells in sterile 1.5 ml microfuge tubes; freeze the
cells in an isopropanol-dry ice bath, then store at -70 °C. The cells are stable for about
6 months under these conditions.
7.2 Electroporation
1. Pipette the DNA samples (up to 5 µl) to be electroporated into sterile 1.5 ml microfuge
tubes; the DNA should be in either water or TE. Place tubes on ice.
2. For each DNA sample to be electroporated, add 1 ml of YM broth to a 17 x 100 tube at
room temperature, and place a 0.1 cm electroporation cuvette on ice.
3. Thaw the electrocompetent A. tumefaciens cells on ice. For each DNA sample to be
electroporated, add 20 µl of electrocompetent cells to each DNA sample; gently tap the
tubes to mix.
4. Set the MicroPulser to "Agr". See Section 4 for operating instructions.
5. Transfer the DNA—cell samples to the electroporation cuvettes and tap the suspension
to the bottom of the tube. Place the cuvette in the chamber slide. Push the slide into the
chamber until the cuvette is seated between the contacts in the base of the chamber. Pulse
once.
6. Remove the cuvette from the chamber and immediately use the YM broth in the
17 x 100 mm tube to transfer the cells from the cuvette to the tube.
7. Check and record the pulse parameters. The time constant should be about 5 milliseconds.
The field strength can be calculated as actual volts (kV) / cuvette gap (cm).
15
Page 20

8. Incubate the cells 3 hr at 30 °C, shaking at 250 rpm. Plate aliquots of the electroporated
cells on YM agar plates containing the appropriate selective media. Incubate plates for
48 hrs at 30 °C.
7.3 Solutions and Reagents For Electroporation
1. YM broth: 0.4 g yeast extract, 10 g mannitol, 0.1 g NaCl, 0.1 g MgSO4, 0.5 g
K2HPO4.3H20, dissolve in 1.0 L of water and adjust to pH 7.0. Autoclave. For YM plates,
add 15 g agar/1 L of YM broth.
Section 8
Electroporation of
Saccharomyces cerevisciae
8.1 Preparation of Electrocompetent Cells
See Becker & Guarantee (1991) and Ausubel et al. (1987) for additional information.
1. Inoculate 500 ml of YPD in a 2.8 L Fernbach flask with an aliquot from an overnight
culture of S. cerevisiae. The doubling time of S. cerevisiae is approximately 2 hrs at 30 °C.
2. Incubate at 30 °C overnight, shaking at 250 rpm, to a density of ~1 x 108cells/ml.
3. Chill the cells in an ice water bath for 15 min to stop growth.
4. Decant the cells into two sterile 250 ml centrifuge bottles and pellet the cells by
centrifugation at 3000 x g for 5 min at 4 °C.
5. Carefully pour off and discard the supernatant; place the centrifuge bottles with the cell
pellets on ice.
6. Add ~50 ml of sterile, ice-cold water to each of the bottles and vortex to resuspend the cell
pellets; bring the volume in each of the centrifuge bottles to 250 ml. Pellet the cells by centrifugation at 3000 x g for 5 min at 4 °C; pour off and discard the supernatant.
7. Wash the cells again as in step 6 with a total of 250 ml sterile, ice-cold water.
8. Resuspend the cell pellet in 20 ml of sterile, ice-cold 1 M sorbitol and transfer to a chilled
30 ml Oakridge tube. Pellet the cells by centrifugation at 3000 x g for 5 min at 4 °C; pour
off and discard the supernatant.
9. Resuspend the cell pellet in 0.5 ml of sterile, ice-cold 1 M sorbitol; the final cell volume
should be ~1.3 ml and the cell concentration should be ~1 x 1010cells/ml. Keep the cells
on ice and use as soon as possible for electroporation.
8.2 Electroporation
1. Pipette the DNA samples (5–100 ng in a volume of 5 µl) to be electroporated into sterile
1.5 ml microfuge tubes. Place tubes on ice.
2. If 0.2 cm cuvettes are used, add 40 µl of the competent cells to each DNA sample; if
0.4 cm cuvettes are used, add 80 µl of the competent cells to each DNA sample. Mix
gently and incubate on ice for ~5 min.
3. Set the MicroPulser to “Sc2” when using 0.2 cm cuvettes or to “Sc4” when using 0.4 cm
cuvettes. See Section 4 for operating instructions.
16
Page 21

4. Transfer the DNA-cell samples to the appropriate electroporation cuvettes that have been
chilled in ice and tap the suspension to the bottom of the tube. Place the cuvette in the
chamber slide. Push the slide into the chamber until the cuvette is seated between the
contacts in the base of the chamber. Pulse once.
5. Remove the cuvette from the chamber and immediately add 1 ml of ice cold 1 M
sorbitol to the cuvette; gently transfer the diluted cells into a sterile tube.
6. Check and record the pulse parameters. The time constant should be close to 5 milliseconds.
The field strength can be calculated as actual volts (kV) / cuvette gap (cm).
7. Plate aliquots of the electroporated cells on selective agar plates containing 1 M sorbitol.
Incubate plates for 48–72 hrs at 30°C.
8.3 Solutions and Reagents For Electroporation
1. YPD: 10 g yeast extract, 20 g peptone, dissolve in 900 ml water. Autoclave. Add 100 ml
sterile 20% glucose.
2. 1M sorbitol: 182.2 g sorbitol, dissolve in 800 ml water. Bring volume to 1.0 L with water.
Autoclave.
3. 20% glucose: 20 g glucose, dissolve in 60 ml water. Adjust volume to 100 ml with water.
Sterilize through a 0.22 µ filter.
Section 9
Electroporation of
Schizosaccharomyces pombe
9.1 Preparation of Electrocompetent Cells
See Prentice (1991) for additional information.
1. Inoculate 500 ml of YCD in a 2.8 L Fernbach flask with an aliquot from an overnight
culture of S. pombe. The doubling time of S. pombe is approximately 2 hrs at 30 °C.
2. Incubate at 30 °C overnight, shaking at 250 rpm, to a density of 1 x 107 cells/ml (OD
600
~0.7).
3. Chill the cells in an ice water bath for 15 min to stop growth.
4. Decant the cells into two sterile 250 ml centrifuge bottles and pellet the cells by
centrifugation at 3000 x g for 5 min at 4 °C.
5. Carefully pour off and discard the supernatant; place the centrifuge bottles with the cell
pellets on ice.
6. Add ~50 ml of sterile, ice-cold 1.2 M sorbitol to each of the bottles and vortex to resuspend
the cell pellets; bring the volume in each of the centrifuge bottles to 250 ml. Pellet the cells
by centrifugation at 3000 x g for 5 min at 4 °C; pour off and discard the supernatant.
7. Wash the cells again as in step 6 with a total of 250 ml sterile, ice-cold 1.2 M sorbitol.
8. Resuspend the cell pellet in 20 ml of sterile, ice-cold 1.2 M sorbitol and transfer to a
chilled 30 ml Oakridge tube. Pellet the cells by centrifugation at 3000 x g for 5 min at 4 °C;
pour off and discard the supernatant.
9. Resuspend the cell pellet in 0.5 ml of sterile, ice-cold 1.2 M sorbitol; the final cell volume
should be ~1.3 ml and the cell concentration should be ~1 x 109cells/ml. Keep the cells
on ice and use as soon as possible for electroporation.
17
Page 22

9.2 Electroporation
1. Pipette the DNA samples (up to 1 µg) to be electroporated into sterile 1.5 ml microfuge
tubes. Place tubes on ice.
2. Add 200 µl of the competent cells to each DNA sample and mix gently.
3. Set the MicroPulser to "ScS". See Section 4 for operating instructions.
4. Transfer the DNA-cell samples to 0.2 cm electroporation cuvettes that have been chilled
in ice and tap the suspension to the bottom of the tube. Place the cuvette in the chamber
slide. Push the slide into the chamber until the cuvette is seated between the contacts in
the base of the chamber. Pulse once.
5. Remove the cuvette from the chamber and immediately add 0.8 ml of ice cold 1.2 M
sorbitol to the cuvette; gently transfer the diluted cells to a sterile tube.
6. Check and record the pulse parameters. The time constant should be close to 5 milliseconds.
The field strength can be calculated as actual volts (kV) / cuvette gap (cm).
7. Incubate the tubes at room temperature for 40–60 min. Plate aliquots of the electroporated
cells on minimal agar plates containing 1.2 M sorbitol. Incubate plates for 60–96 hrs at
30 °C.
9.3 Solutions and Reagents for Electroporation
1. YCD media: 10 g yeast extract, 2 g casamino acids, dissolve in 900 ml water. Autoclave.
Add 100 ml 20% glucose
2. 1.2 M sorbitol: 218.6 g sorbitol, dissolve in 700 ml water. Add water to 1.0 L.
Section 10
Electroporation of
D. discoideum
10.1 Preparation of Electrocompetent Cells
See Howard et al. (1988) and Knecht & Pang (1995) for additional information.
1. Inoculate D. discoideum cells at a concentration of 5–7 x 105cells/ml into 40 ml of HL5
media in a 500 ml flask. The cells may either be scraped from a plate or transferred from
liquid media. The doubling time of D. discoideum is approximately 12 hrs at 21 °C.
2. Incubate the culture at 21 °C for about 24 hrs, shaking at 125 rpm. About 16–20 hrs prior
to preparing the competent cells, dilute the cells to 7 x 105cells/ml with HL5 media.
Incubate at 21 °C overnight, shaking at 125 rpm.
3. Transfer 100 ml of the cells into two sterile, disposable, 50 ml centrifuge tubes and incubate on ice for 15 min to stop growth.
4. Pellet the cells by centrifugation at 400 x g for 5–7 min at room temperature.
5. Carefully pour off and discard the supernatant; place the centrifuge bottles with the cell
pellets on ice.
6. Pool the cell pellets and resuspend in 50 ml of sterile, ice-cold E buffer. Pellet the cells
by centrifugation at 400 x g for 5–7 min at room temperature.
7. Carefully pour off and discard the supernatant; place the centrifuge bottles with the cell
pellets on ice and resuspend the cells at a concentration of 1 x 107cells/ml. Keep the cells
on ice and use as soon as possible for electroporation.
18
Page 23

10.2 Electroporation
1. Pipette the DNA samples (up to 50 µg) to be electroporated into sterile 1.5 ml microfuge
tubes. Place tubes on ice.
2. Add 800 µl of the competent cells to each DNA sample and pipette up and down to mix;
incubate on ice ~1 min.
3. Set the MicroPulser to "dic". See Section 4 for operating instructions.
4. Transfer the DNA-cell samples to 0.4 cm electroporation cuvettes that have been chilled
in ice and tap the suspension to the bottom of the tube. Place the cuvette in the chamber
slide. Push the slide into the chamber until the cuvette is seated between the contacts in
the base of the chamber. Pulse once (the program delivers two pulses approximately 5 sec
apart).
5. Remove the cuvette from the chamber and immediately dilute the cells to 10 ml with the
appropriate media.
6. Check and record the pulse parameters. The time constant should be 1 millisecond. The
field strength can be calculated as actual volts (kV) / cuvette gap (cm).
7. When selecting for complementation of an auxotrophic mutant, the cells may be plated
immediately into selective media lacking the appropriate nutrient. When selecting for
antibiotic resistance, incubate the cells overnight at 21 °C prior to adding the selective
agent.
10.3 Solutions and Reagents For Electroporation
1. HL5 media: 17.8 g bacteriological peptone (Oxoid, Ogdensburg, NY), 7.2 g yeast extract,
0.54 g Na2HPO4, 0.4 g KH2PO4, 130 µl B12/Folic acid mix; bring to 1L with water and
adjust to pH 6.3–6.5. Autoclave for 25 min on two successive days. Prior to use, add
20 ml of 50% glucose and 10 ml of 100 X Antibiotic-Antimycotic (Life Technologies,
Gaithersburg, MD).
2. B12/Folic acid mix: 5 mg B12, 200 mg folic acid; add 95 ml water, then pH to 6.5–6.8
with 5N NaOH; bring to 100 ml with water. Filter sterilize and store at -20 °C protected
from light.
3. E buffer: 10 ml 100 mM NaH2PO4, adjusted to pH 6.1 with KOH, 10 ml 0.5 M sucrose,
80 ml water; autoclave.
Section 11
Electroporation of
Pichia pastoris
11.1 Preparation of Eelectrocompetent Cells
See Cregg & Russell (1998) for additional information.
1. Inoculate 500 ml of YPD in a 2.8 L Fernbach flask with an aliquot from a fresh overnight
culture of P. pastoris. The doubling time of P. pastoris is approximately 2 hrs at 30 °C.
2. Incubate at 30 °C overnight, shaking at 300 rpm, to a density of 5–7 x 107cells/ml.
3. Decant the cells into two sterile 250 ml centrifuge bottles and pellet the cells by centrifugation at 3000 x g for 5 min at 4 °C.
4. Carefully pour off and discard the supernatant.
19
Page 24

5. Add 50 ml of sterile YPD/HEPES to each of the bottles and vortex to resuspend the cell
pellets; add 1.25 ml of 1M DTT to each bottle; mix gently. Incubate the cells for 15 min
at 30 °C.
6. Add 200 ml of sterile, ice-cold 1 M sorbitol to each centrifuge bottle. Pellet the cells by
centrifugation at 3000 x g for 5 min at 4 °C; pour off and discard the supernatant.
7. Add ~50 ml of sterile, ice-cold 1 M sorbitol to each of the bottles and vortex to resuspend
the cell pellets; bring the volume in each of the centrifuge bottles to 250 ml with sterile,
ice-cold 1 M sorbitol. Pellet the cells by centrifugation at 3000 x g for 5 min at 4 °C; pour
off and discard the supernatant.
8. Resuspend each cell pellet in 10 ml of sterile, ice-cold 1 M sorbitol and pool in a chilled
30 ml Oakridge tube. Pellet the cells by centrifugation at 3000 x g for 5 min at 4 °C; pour
off and discard the supernatant.
9. Resuspend the cell pellet in 0.5 ml of sterile, ice-cold 1 M sorbitol; the final cell volume
should be ~1.3 ml and the cell concentration should be ~1 x 109cells/ml. Keep the cells
on ice and use as soon as possible for electroporation.
11.2 Electroporation
1. Pipette the DNA samples (up to 10 µg) to be electroporated into sterile 1.5 ml microfuge
tubes. Place tubes on ice.
2. Add 40 µl of the competent cells to each DNA sample and mix gently.
3. Set the MicroPulser to "Pic". See Section 4 for operating instructions.
4. Transfer the DNA—cell samples to 0.2 cm electroporation cuvettes that have been chilled
in ice and tap the suspension to the bottom of the tube. Place the cuvette in the chamber
slide. Push the slide into the chamber until the cuvette is seated between the contacts in
the base of the chamber. Pulse once.
5. Remove the cuvette from the chamber and immediately add 1.0 ml of ice cold 1.0 M
sorbitol to the cuvette when selecting for complementation of an auxotrophic mutant, or
1.0 ml of ice cold YPD/sorbitol when selecting for antibiotic resistance. Gently transfer
the diluted cells to a sterile tube.
6. Check and record the pulse parameters. The time constant should be close to 5 milliseconds.
The field strength can be calculated as actual volts (kV) / cuvette gap (cm).
7. When selecting for complementation of an auxotrophic mutant, the cells may be plated
immediately onto minimal agar plates containing 1 M sorbitol but lacking the appropriate
nutrient. When selecting for antibiotic resistance, incubate the cells at 30 °C for 1–2 hrs
without shaking; plate aliquots of the electroporated cells on YPD agar plates containing
1 M sorbitol with the appropriate antibiotic. Incubate the plates for 72–96 hrs at 30 °C.
11.3 Solutions and Reagents for Electroporation
1. YPD/HEPES: 100 ml YPD media, 20 ml 1 M HEPES, pH 8.0
2. 1M DTT: 1.55 g dithiothreitol, dissolve in 8 ml water. Bring the volume to 10 ml with
water. Filter sterilize.
3. YPD/sorbitol: 10 g yeast extract, 20 g peptone, 182.2 g sorbitol, dissolve in 700 ml water;
bring volume to 900 ml with water. Autoclave. Add 100 ml sterile 20% glucose.
20
Page 25

Appendix I
References
1. Allen, S.P. and Blaschek, H.P., Factors involved in the electroporation-induced transformation of
Clostridium perfringens, Appl. Environ. Microbiol., 54, 2322 (1990).
2. Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K.
(eds.) Current Protocols in Molecular Biology, John Wiley & Sons, NY (1987).
3. Becker, D.M. and Guarente, L., High-efficiency transformation of yeast by electroporation, Methods
Enzymol., 194, 182 (1991).
4. Böttger, E. C., High-efficiency generation of plasmid cDNA libraries using electro-transformation,
BioTechniques, 6, 878 (1988).
5. Chang, D.C., Chassy, B.M., Saunders, J.A., and Sowers, A.E. (eds.) Guide to Electroporation and
Electrofusion, Academic Press, Inc., San Diego (1992).
6. Cregg, J.M. and Russell, K.A., Transformation, in Methods in Molecular Biology, 103, Higgins,
D.R. and Cregg, J.M. (eds.), Humana Press, Totowa, NJ, 27 (1998).
7. Cruz-Rodz, A.L. and Gilmore, M.S., High efficiency introduction of plasmid DNA into glycine
treated Enterococcus faecalis by electroporation, Mol. Gen. Genet., 224, 152 (1990).
8. Dennis, J.J. and Sokol, P.A., Electrotransformation of Pseudomonas, in Methods in Molecular
Biology, 47, Nickoloff, J.A., ed., Humana Press, Totowa, NJ, 125 (1995).
9. Dower, W. J., Electroporation of bacteria: a general approach to genetic transformation, in Genetic
Engineering—Principles and Methods, 12, Plenum Publishing Corp., NY, 275 (1990).
10. Dower, W. J., Miller, J. F., and Ragsdale, C. W., High efficiency transformation of E. coli by high
voltage electroporation, Nuc. Acids Res., 16, 6127 (1988).
11. Dower, W.J., Chassy, B.M., Trevors, J.T., and Blaschek, H.P., Protocols for the transformation of
bacteria by electroporation, in Guide to Electroporation and Electrofusion, Chang, D. C., Chassy,
B. M., Saunders, J. A., and Sowers, A. E. (eds.), Academic Press Inc., 485, San Diego (1992).
12. Heery, D. M., and Dunican, L. K., Improved efficiency M13 cloning using electroporation, Nuc.
Acids Res., 17, 8006 (1989).
13. Holo, H. and Nes, I.F., Transformation of Lactococcus by electroporation, in Methods in Molecular
Biology, 47, Nickoloff, J.A. (ed.), Humana Press, Totowa, NJ, 195 (1995).
14. Howard, P.K., Ahern, K.G., and Firtel, R.A., Establishment of a transient expression system for
Dictyostelium discoideum, Nuc. Acids Res., 16, 2613 (1988).
15. Jacobs, M., Wnendt, S., and Stahl, U., High-efficiency electro-transformation of Escherichia coli
with DNA from ligation mixtures, Nuc. Acids Res., 18, 1653 (1990).
16. Knecht, D. and Pang, K.M., Electroporation of Dictyostelium discoideum, in Methods in Molecular
Biology, 47, Nickoloff, J.A., ed., Humana Press, Totowa, NJ, 321 (1995).
17. Lee, J.C., Electrotransformation of Staphylococci, in Methods in Molecular Biology, 47, Nickoloff,
J.A., ed., Humana Press, Totowa, NJ, 209 (1995).
18. Leonardo, E. D., and Sedivy, J. M., A new vector for cloning large eukaryotic DNA segments in
Escherichia coli, Bio/Technol., 8, 841 (1990).
19. Lin, J.-J., Electrotransformation of Agrobacterium, in Methods in Molecular Biology, 47, Nickoloff,
J.A., ed., Humana Press, Totowa, NJ, 171 (1995).
20. Miller, E.M. and Nickoloff, J.A., Escherichia coli electrotransformation, in Methods in Molecular
Biology, 47, Nickoloff, J.A., ed., Humana Press, Totowa, NJ, 105 (1995).
21. Nickoloff, J.A. (ed.) Methods in Molecular Biology, 47, Humana Press, Totowa, NJ, (1995).
22
. Park, S.F. and Stewart, G.S.A.B., High efficiency transformation of Listeria monocytogenes by
electroporation of penicillin-treated cells, Gene, 94, 129 (1990).
23. Prentice, H.L., High efficiency transformation of Schizosaccharomyces pombe by electroporation,
Nuc. Acids Res., 20, 621 (1991).
24. Shigekawa, K. and Dower, W.J., Electroporation of eukaryotes and prokaryotes: A general approach
to the introduction of macromolecules into cells, Biotechniques, 6, 742 (1988).
21
Page 26

25. Siguret, V., Ribba, A.-S., Cherel, G., Meyer, D., and Pieru, G., Effect of plasmid size on transformation efficiency by electrtoporation of Escherichia coli DH5α, Biotechniques, 16, 422 (1994).
26. Simon, D. and Ferretti, J.J., Electrotransformation of Streptococcus pyogenes with plasmid and
linear DNA, FEMS Micobiol. Lett., 82, 219 (1991).
27. Somkuti, G.A. and Steinberg, Genetic transformation of Streptococcus thermophilus by electroporation, Biochimie, 70, 579 (1988).
28. Summers, D. K., and Withers, H. L., Electrotransfer: direct transfer of bacterial plasmid DNA by
electroporation, Nuc. Acids Res., 18, 2192 (1990).
29. Taketo, A., DNA transfection of Escherichia coli by electroporation, Biochim. Biophys. Acta, 949,
318 (1988).
30. Taketo, A., RNA transfection of Escherichia coli by electroporation, Biochim. Biophys. Acta, 1007,
127 (1989).
31. Trevors, J.T., Chassy, B.M., Dower, W.J., and Blaschek, H.P., Electrotransformation of bacteria by
plasmid DNA, in Guide to Electroporation and Electrofusion, Chang, D. C., Chassy, B. M.,
Saunders, J. A., and Sowers, A. E. (eds.), Academic Press Inc., 265, San Diego (1992).
32. Willson, T. A., and Gough, N. M., High voltage E. coli electro-transformation with DNA following ligation, Nuc. Acids Res., 16, 11820 (1988).
33. Zabarovsky, E. R., and Winberg, G., High efficiency electroporation of ligated DNA into bacteria,
Nuc. Acids Res., 18, 5912 (1990).
Appendix II
Troubleshooting Guide for the Micropulser
Operational Problem Possible cause and solution
1. Display does not light Power is not supplied to electronics.
when unit is turned on. Check power cord and wall outlet power source.
Check that power switch is on.
Check/replace fuse. Two 2 A, 250 V Type T fuses
are located on the back of the instrument immediately above the power cord.
2. When the buttons are pressed, No pulse delivery.
the unit does not indicate “PLS”. Pulse button is not depressed hard enough
Verify instrument operation by the following tests:
Turn on the power switch.
Verify the LED next to “Bacteria” is illuminated.
Press the “Settings” button.
Verify the LED next to “Fungi” is illuminated.
Press the “Settings” button again.
Verify the LED next to “Manual” is illuminated.
Press the “Settings” button again.
The LED next to “Bacteria” should be lit.
Press the “Raise” button several times to
change the display from “Ec1” to “Ec3”.
Press the “Raise” and “Lower” buttons simultaneaously to verify the display reads “3.00”
volts.
Press the “Pulse” button until “PLS” is displayed; a beep tone should be heard within
6 sec.
Press the “Measurements” button.
Verify the “Actual kV” LED illuminates.
Verify the display LED shows a reading
between “2.95” and “3.05”.
Press the “Measurements” button again.
Verify the “Time ms” LED illuminates.
22
Page 27

Electrical Problem Possible cause and solution
Verify the display LED shows a reading
between “5.50” and “6.50”.
If problems persists, contact Bio-Rad.
1. Instrument shows “Arc” on Arcing in the cuvette is the result either of an
display LED actual arc occurring or of medium that is too
conductive. An actual arc will occur usually only
at high voltage (>1500V). If media is too
conductive, the display LED may show “Arc”
even though an actual arc has not occurred. The
limit of conductivity depends on the voltage,
electrode gap, and sample volume, but under
standard conditions, solutions of 10 meq or higher
(< 600 ohms resistance) will certainly indicate
“Arc”.
To determine whether an actual arc has occurred
or the media is too conductive, look at the cuvette
while pulsing the sample again. If a small spark
occurs across the electrodes, an arc has
occurred. If no spark is observed, lower the voltage
and re-pulse the sample. Continue lowering and
re-pulsing the sample until a pulse occurs.
Check the time constant; if the time constant is
low (< 3 msec), the sample is too conductive.
There are several causes of excessive conductivity:
1. Washing and resuspending cells in a buffer too
high in ionic strength.
2. Insufficient washing of the cells—salts from the
growth medium are not completely removed;
the cells should be washed at least three to
four times with non-conductive solution.
3. Lysed cells in the preparation—cell contents
contribute to conductivity.
4. DNA solution too high in salt; for example, CsCl
carried over from plasmid preparation, or
residual salts from ethanol precipitation or ligation.
Electroporation with cuvettes above 0 °C: reducing
sample temperature increases sample resistance.
2. Wrong time constant. Samples electroporated in the Micropulser
should have a time constant close to 5 msec. If
the time constant is much shorter than the
expected value (
e.g.
, 3 msec instead of 5 msec),
the sample is too conductive. The
probable reasons for this are listed above under
“arcing”. Correct the problem of high conductivity
by additional washing of the cells, or by removal
of salts from the DNA preparation.
3. Sample does not “twitch”. This may mean that the pulse is not reaching the
sample. Check the connections between the
MicroPulser and sample chamber. Check to see
that the contacts in the base of the sample
chamber are not broken.
4. Instrument displays “no” on The manual setting is used and the voltage is set
front panel. to 0.00. Use the “Raise” button to select a voltage
between 0.02 and 3.00 kV.
5. Instrument displays “err” on Turn instrument off, then on again.
front panel. If problem persists, contact Bio-Rad.
23
Page 28

Biological
The general symptom addressed in this section is transformation efficiencies that are too
low to detect or too low to be useful. The following is a list of the areas of possible problems
and some suggested solutions.
Problem Possible cause and solution
1. The pulse. Is the pulse actually applied to the sample? At high
voltage with a small-volume (40 µl) sample this is
easy to check. The sample will "twitch" when
pulsed. If you don’t see a twitch, refer to the electrical
troubleshooting section for information on electrical
problems. Also make sure that the cuvette is making
contact with the electrodes at the back of the sample
chamber. If electrodes are broken or corroded call
Bio-Rad for replacements.
Are the amplitude and length of the pulse sufficient?
E. col
i requires pulses of approximately 5
msec with field strengths of 12 to 18 kV/cm.
S.
cerevisiae
requires pulses of approximately 5 msec
with a field strength of ~7.5 kV/cm. There is usually
some cell death with electrical conditions producing
transformation. Survival rates of 20 to 80% are typical.
If no cell death occurs, the pulse is probably too
weak. Conversely if too many cells are killed
(>80%), the pulse is probably too intense and
transformation will probably be poor. To find the
optimum pulse characteristics, use a pulse length
of ~5 msec and test for transformation over a range
of field strengths.
2. The DNA. Check the quantity and quality of the DNA on a gel.
Often, mini-preps contain less DNA than expected.
DNA stored improperly for long periods may be
degraded and lack transforming activity.
Some preparations of DNA may contain substances
that inhibit transformation or are toxic to the cells.
Try to use DNA free of SDS, phenol, etc.
Is the selection appropriate for the marker (and its
level of expression)?
3. The cells. Were the cells harvested at the correct stage in the
growth phase? Bacterial cells generally show the
highest transformation efficiencies when
harvested in the early to mid-log growth phase.
Yeast cells generally show the highest transformation
effiencies when harvested in late log phase.
Different growth conditions may improve
transformation.
Are too many cells killed? The pulse is too intense,
toxic substances are present in DNA or cell
preparations, wrong temperature of electroporation
are all possibilities.
Are the cells transferred to outgrowth medium
immediately after the pulse? For
E. coli
this is very
important.
Is the correct selection applied after the recovery
period?
4. The temperature. Are the cuvettes cold?
Is the cuvette holder (slide) prechilled?
If frozen, have the cells been stored properly
(usually in 10-15% glycerol at -70 °C)?
24
Page 29

Appendix III
Product Information
Specifications
Input voltage 100–120 V RMS, 50/60 Hz;
automatic mains voltage
switching
220–240 V RMS, 50/60 Hz
Input current 2 amp RMS (100–120 V),
1 amp RMS (220–240 V)
Maximum output voltage and current 3000 V peak into > 3.3 kohm load
limited to 100 amp peak
maximum
Output waveform Decaying exponential waveform
with RC time constant of 5 msec,
assuming loads of ~3.3 kohm
Output voltage adjustment Voltage adjustable in 200–3000
V range with 10 V display
resolution; 10 pre-programmed
voltage steps
Ambient operating temperature 3.5–35 °C
Dimensions (H x W x D) 8 x 21 x 31cm
Weight 2.9 kg (6.4 lbs)
Related products
Cuvettes
Catalog
Number Product Description
Mini packs
165-2083 MicroPulser/Gene Pulser Cuvettes, 0.1cm gap, 5 sterile
165-2082 MicroPulser/Gene Pulser Cuvettes, 0.2cm gap, 5 sterile
165-2081 MicroPulser/Gene Pulser Cuvettes, 0.4cm gap, 5 sterile
Standard Packs
165-2089 MicroPulser/Gene Pulser Cuvettes, 0.1cm gap, 50 sterile
165-2086 MicroPulser/Gene Pulser Cuvettes, 0.2cm gap, 50 sterile
165-2088 MicroPulser/Gene Pulser Cuvettes, 0.4cm gap, 50 sterile
Jumbo Packs
165-2093 MicroPulser/Gene Pulser Cuvettes, 0.1cm gap, 500 sterile
165-2092 MicroPulser/Gene Pulser Cuvettes, 0.4cm gap, 500 sterile
165-2091 MicroPulser/Gene Pulser Cuvettes, 0.2cm gap, 500 sterile
25
Page 30

Catalog
Number Product Description
Plasmid purification
732-6100 Quantum Prep Miniprep Kit, 100 preps, includes 20 ml cell
resuspension buffer, 25 ml cell lysis buffer, 25 ml neutralization
buffer, 20 ml Quantum Prep matrix, 63 ml wash buffer, 100 mini spin
filters, instructions
732-6120 Quantum Prep Midiprep Kit, 20 preps, includes 110 ml cell
resuspension buffer, 110 ml cell lysis buffer, 110 ml neutralization
buffer, 20 ml Quantum Prep matrix, 125 ml wash buffer, 20 midi spin
filters, instructions
732-6130 Quantum Prep Maxiprep Kit, 10 preps, includes 165 ml cell
resuspension buffer, 250 ml cell lysis buffer, 165 ml neutralization
buffer, 110 ml Quantum Prep matrix, 270 ml wash buffer, 10 midi
spin filters, instructions
26
Page 31

Bio-Rad
4006174 Rev B
Laboratories
Life Science
Group
Website www.bio-rad.com Bio-Rad Laboratories Main Office 2000 Alfred Nobel Drive, Hercules, CA 94547, Ph. (510) 741-1000, Fx. (510)741-5800
Also in: Australia Ph. 02 9914 2800, Fx. 02 9914 2889 Austria Ph. (01) 877 89 01, Fx. (01) 876 56 29 Belgium Ph. 09-385 55 11, Fx. 09-385 65 54
Canada Ph. (905) 712-2771, Fx. (905) 712-2990 China Ph. 86-10-62051850/51, Fx. 86-10-62051876 Denmark Ph. 45 39 17 99 47, Fx. 45 39 27 16 98
Finland Ph. 358 (0)9 804 2200, Fx. 358 (0)9 804 1100 France Ph. 01 43 90 46 90, Fx. 01 46 71 24 67 Germany Ph. 089 318 84-0, Fx. 089 318 84-100
Hong Kong Ph. 852-2789-3300, Fx. 852-2789-1257 India Ph. (91-11) 461-0103, Fx. (91-11) 461-0765 Israel Ph. 03 951 4127, Fx. 03 951 4129
Italy Ph. 39-02-216091, Fx.39-02-21609-399 Japan Ph. 03-5811-6270, Fx. 03-5811-6272 Korea Ph. 82-2-3473-4460, Fx. 82-2-3472-7003
Latin America Ph. 305-894-5950, Fx. 305-894-5960 Mexico Ph. 514-2210, Fx. 514-2209 The Netherlands Ph. 0318-540666, Fx. 0318-542216
New Zealand Ph. 64-9-4152280, Fx. 64-9-4152284 Norway Ph. 22-74-18-70, Fx. 22-74-18-71 Russia Ph. 7 095 979 98 00, Fx. 7 095 979 98 56
Singapore Ph. 65-2729877, Fx. 65-2734835 Spain Ph. 34-91-661-7085, Fx. 34-91-661-9698 Sweden Ph. 46 (0)8-55 51 27 00, Fx. 46 (0)8-55 51 27 80
Switzerland Ph. 01-809 55 55, Fx. 01-809 55 00 United Kingdom Ph. 0800-181134, Fx. 01442-259118
00-000 0099 Sig 031799Bulletin 0000 US/EG Rev A
 Loading...
Loading...