Bio-Rad Bio-Plex Pro Rat Cytokine, Chemokine, and Growth Factor Assays User Manual

Bio-Plex Pro
™
Cytokine,
Chemokine, and Growth
Factor Assays
Instruction Manual
For technical support, call your local Bio-Rad of fice, or in the U.S., call 1-800-424-6723.
For research use only. Not for diagnostic procedures.

Table of Contents
Introduction 1
Principle 2
Kit Contents and Storage 4
Recommended Materials 5
Assay Workflow 6
Important Considerations 7
Detailed Instructions
1. Plan Plate Layout 8
2. Prepare Instrument 9
3. Prepare Wash Method 10
4. Prepare Standards 11
5. Prepare Samples 16
6. Prepare Coupled Beads 19
7. Run Assay 22
8. Read Plate 28
Troubleshooting Guide 35
Plate Layout Template 40
Calculation Worksheet 41
Safety Considerations 45
Legal Notices 45
Ordering Information 46

Introduction
Cytokines, chemokines, and growth factors are a diverse group of cell
signaling proteins expressed and secreted by virtually all cell types,
including cells of endothelial, epithelial, and immune origin. These proteins
interact with specific receptors on target cells to mediate important
physiological responses such as growth, immunity, inflammation,
and hematopoiesis. Dysregulation of expression is associated with
pathological conditions ranging from cancer and diabetes to infection and
autoimmune disease.
Bio-Plex Pro
biomarkers in a single well of a 96-well plate in 3–4 hours. These robust
immunoassays require as little as 12.5 μl serum or plasma or 50 μl cell
culture supernatant or other biological fluid. The use of magnetic (MagPlex)
beads allows researchers to automate wash steps on a Bio-Plex Pro (or
similar) wash station. Magnetic separation offers greater convenience and
reproducibility compared to vacuum filtration.
For more information please visit www.bio-rad.com/bio-plex.
™
assays enable researchers to quantify multiple protein
1
1

Principle
Technology
The Bio-Plex® multiplex system is built upon the three core elements of
xMAP technology:
n
Fluorescently dyed microspheres (also called beads), each with a distinct
color code or spectral address to permit discrimination of individual
tests within a multiplex suspension. This allows simultaneous detection
of up to 500 different types of molecules in a single well of the 96-well
microplate on the Bio-Plex
molecules on the Bio-Plex
of molecules on the Bio-Plex
n
On the Bio-Plex 200 and Bio-Plex 3D systems, a dedicated flow
cytometer with two lasers and associated optics to measure the
different molecules bound to the surface of the beads. In the
Bio-Plex MAGPIX, the entire sample load volume is injected into a
chamber where the beads are imaged using LED and CCD technology
n
A high-speed digital signal processor that efficiently manages the
fluorescence data
Assay Format
Bio-Plex Pro™ assays are essentially immunoassays formatted on
magnetic beads. The assay principle is similar to that of a sandwich ELISA
(Figure 1). Capture antibodies directed against the desired biomarker are
covalently coupled to the beads. Coupled beads react with the sample
containing the biomarker of interest. After a series of washes to remove
unbound protein, a biotinylated detection antibody is added to create
a sandwich complex. The final detection complex is formed with the
addition of streptavidin-phycoerythrin (SA-PE) conjugate. Phycoerythrin
serves as a fluorescent indicator or reporter.
®
3D system, up to 100 different types of
®
200 system, and up to 50 different types
®
MAGPIX™ system
2
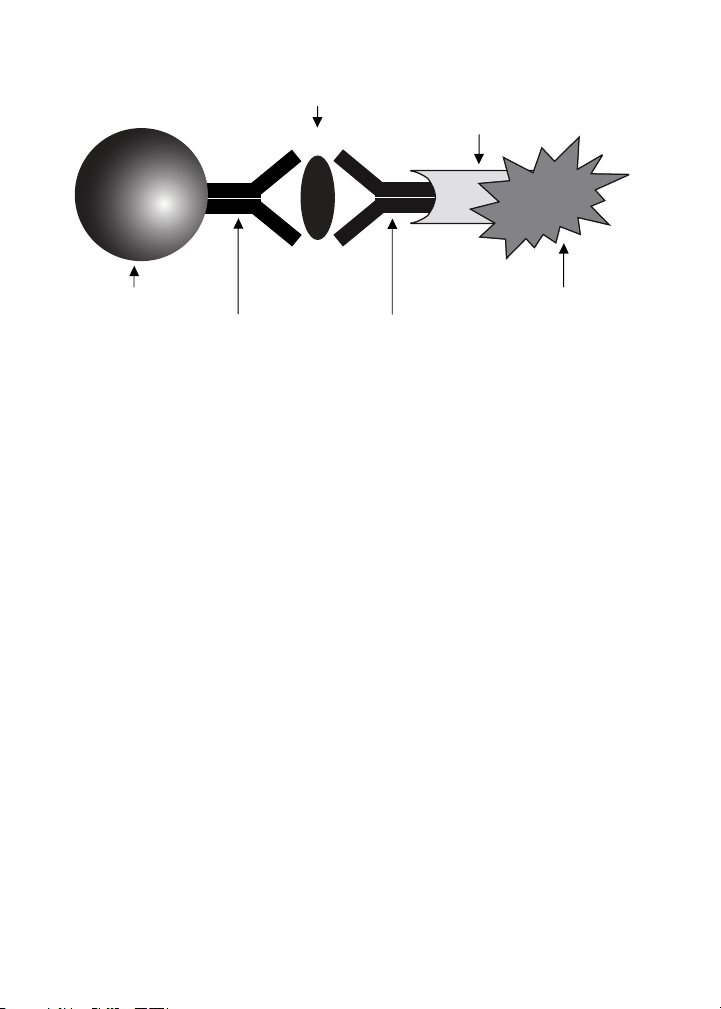
Biomarker
of Interest
Streptavidin
Magnetic Bead
Capture
Antibody
Fig. 1. Bio-Plex sandwich immunoassay.
Biotinylated
Detection
Antibody
Phycoerythrin
Fluorescent
Reporter
Data Acquisition and Analysis
Data from the reactions are acquired using a Bio-Plex system or similar
Luminex-based reader. When a multiplex assay suspension is drawn into
the Bio-Plex 200 reader, for example, a red (635 nm) laser illuminates the
fluorescent dyes within each bead to provide bead classification and thus
assay identification. At the same time, a green (532 nm) laser excites PE
to generate a reporter signal, which is detected by a photomultiplier tube
(PMT). A high-speed digital processor manages data output, and
Bio-Plex Manager
™
software presents data as median fluorescence
intensity (MFI) as well as concentration (pg/ml). The concentration of
analyte bound to each bead is proportional to the MFI of reporter signal.
Using Bio-Plex Data Pro
™
software, data from multiple instrument runs
can be combined into a single project for easy data management, quick
visualization of results, and simple statistical analysis.
33

Kit Contents and Storage
Reagents Supplied
Bio-Plex Pro
™
cytokine assays are offered in a convenient kit format that
includes assay, reagent, and diluent components in a single box (Table 1).
Table 1. Contents of Bio-Plex Pro cytokine, chemokine, and growth factor assays.*
1 x 96-Well 10 x 96-Well
Component Format Format
Standard diluent* 10 ml 100 ml
Sample diluent* 40 ml 80 ml
Assay buffer 50 ml 500 ml
Wash buffer 200 ml 1.5 L
Detection antibody diluent 5 ml 50 ml
Streptavidin-PE (100x) 1 tube 1 tube
Filter plate and/or flat bottom plate (96-well) 1 plate 10 plates
Sealing tape 1 pack of 4 10 packs of 4
Assay Quick Guide 1 booklet 1 booklet
Standard 1 vial 10 vials
Human and Mouse Cytokine (Group I and II)
Coupled magnetic beads (10x) 1 tube 1 tube
Detection antibodies (10x) 1 tube 1 tube
Mouse Cytokine (Group III) and Rat Cytokine (Group I)
Coupled magnetic beads (20x) 1 tube 1 tube
Detection antibodies (20x) 1 tube 1 tube
* Bio-Plex Pro high dilution reagent kit, 1 x 96-well, contains 70 ml serum-based diluent in lieu of
standard diluent and sample diluent.
Storage and Stability
Kit contents should be stored at 4°C and never frozen. Coupled magnetic
beads and streptavidin-PE should be stored in the dark. All components
are guaranteed for a minimum of six months from the date of purchase
when stored as specified.
4

Table 2. Recommended materials.
Item
Bio-Plex Pro Assays Quick Guide 4
Ordering Information
Bulletin #10024985 (download
at www.bio-rad.com/bio-plex)
®
Bio-Plex
Bio-Plex validation kit
200 system or Luminex system with HTF
Bio-Rad catalog #171-000205
Bio-Rad catalog #171-203001
Run the validation kit monthly to ensure optimal performance
of fluidics and optics systems
Bio-Plex calibration kit
Bio-Rad catalog #171-203060
Run the calibration kit daily to standardize
fluorescence signal
Bio-Plex Pro wash station
Bio-Rad catalog #300-34376
For use with magnetic bead-based assays only
Bio-Plex Pro II wash station
Bio-Rad catalog #300-34377
For use with both polystyrene (nonmagnetic) and magnetic
bead-based assays
Bio-Plex handheld magnetic washer
Bio-Rad catalog #170-20100
For use with magnetic bead–based assays only
Bio-Plex Pro flat bottom plates (40 x 96-well)
Bio-Rad catalog #171-025001
For magnetic separation on the Bio-Plex Pro wash station
Microtiter plate shaker
IKA MTS 2/4 shaker for 2 or 4 microplates
IKA catalog #320-8000
or
Barnstead/Lab-Line Model 4625 plate
VWR catalog #57019-600
shaker (or equivalent capable of 300–1,100 rpm)
®
Bio-Rad
Aurum™ vacuum manifold
Bio-Rad catalog #732-6470
For vacuum filtration
BR-2000 vortexer
Reagent reservoirs, 25 ml
For capture beads and detection antibodies
Reagent reservoir, 50 ml (for reagents and buffers)
Pall Life Science Acrodisc: 25 mm PF syringe filter
(0.8/0.2 µm Supor membrane)
Filter plate, 1 x 96-well, with clear plastic lid and tray
®
Titertube
micro test tube
Bio-Rad catalog #166-0610
VistaLab catalog #3054-1002
or
VistaLab catalog #3054-1004
VistaLab catalog #3054-1006
Pall Life Sciences
catalog #4187
Bio-Rad catalog #171-304502
Bio-Rad catalog #223-9390
Other: 15 ml polypropylene tubes for reagent dilutions, calibrated pipets, pipet tips, sterile
distilled water, aluminum foil, absorbent paper towels,1.5 or 2 ml microcentrifuge tubes, and
standard flat bottom microplate (for calibrating vacuum manifold).
5
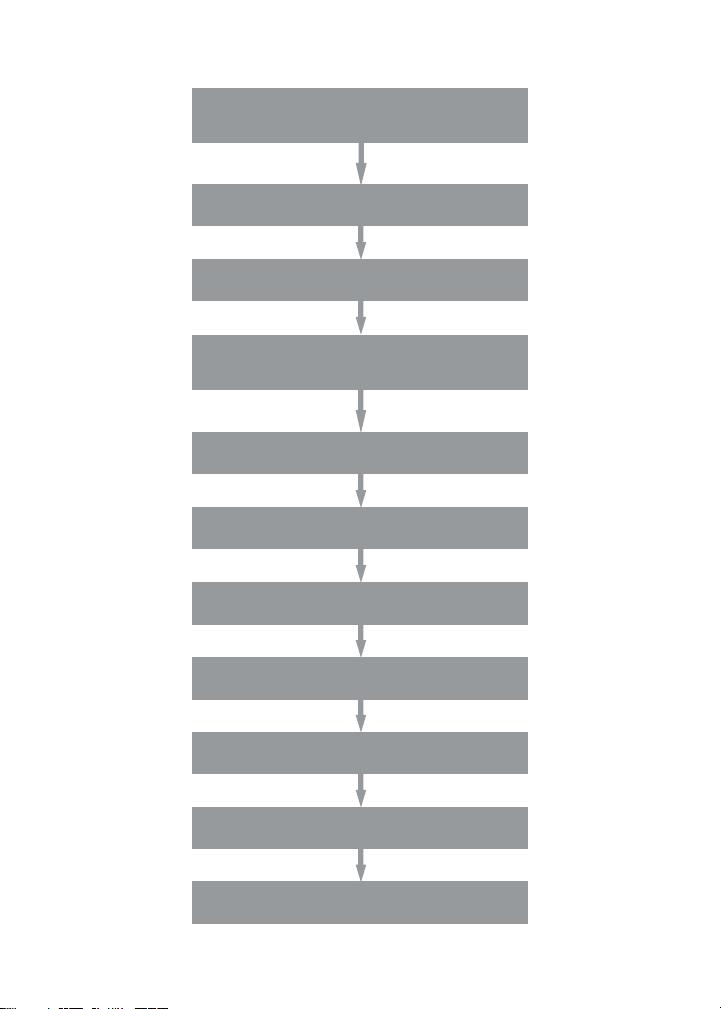
Assay Workflow
Prewet wells
(for lter plate only)
Add 50 μl 1x beads to wells
Wash 2 x 100 μl
Add 50 μl standards, blank, samples,
incubate at RT with shaking at 850 rpm
(incubation time varies by assay)
Wash 3 x 100 μl
Add 25 μl 1x detection antibody, incubate
30 min at RT with shaking at 850 rpm
Wash 3 x 100 μl
Add 50 μl 1x streptavidin-PE, incubate
10 min at RT with shaking at 850 rpm
Wash 3 x 100 μl
Resuspend in 125 μl assay buffer,
shake at 850 rpm for 30 sec
Read plate on Bio-Plex system
6

Important Considerations
Instruments and Software
The cytokine assays described in this manual are compatible with all
currently available Luminex-based life science research instruments.
Assays can be read and analyzed with either Bio-Plex Manager
or Luminex xPonent software.
Assay Procedures
Pay close attention to vortexing, shaking, and incubation times and to
Bio-Plex
specifically for each assay panel.
®
reader PMT (RP1) setting, as these have been optimized
Assay Quick Guide
Each assay kit includes a printed Bio-Plex Pro™ Assay Quick Guide (bulletin
#10024985), which can be used to prepare and run a full 1 x 96-well assay
plate. Users can also download a copy at www.bio-rad.com/bio-plex.
Bead Regions
Bead regions for all analytes are listed in the Read Plate section.
Multiplexing Compatibility
For human and mouse, the maximum number of singleplex diabetes
and cytokine analytes that may be mixed is limited by the 10x cytokine
antibody stock concentrations as shown in the table below.
™
software
Table 3. Maximum number of singleplex cytokine and diabetes analytes that may
be multiplexed.
Human, mouse, and rat diabetes
Mouse cytokine (group III) analytes (20x)
Human and mouse cytokine (groups I, II)
analytes (10x)
0 2 4 6 8 10
10 9 8 7 6 5
7
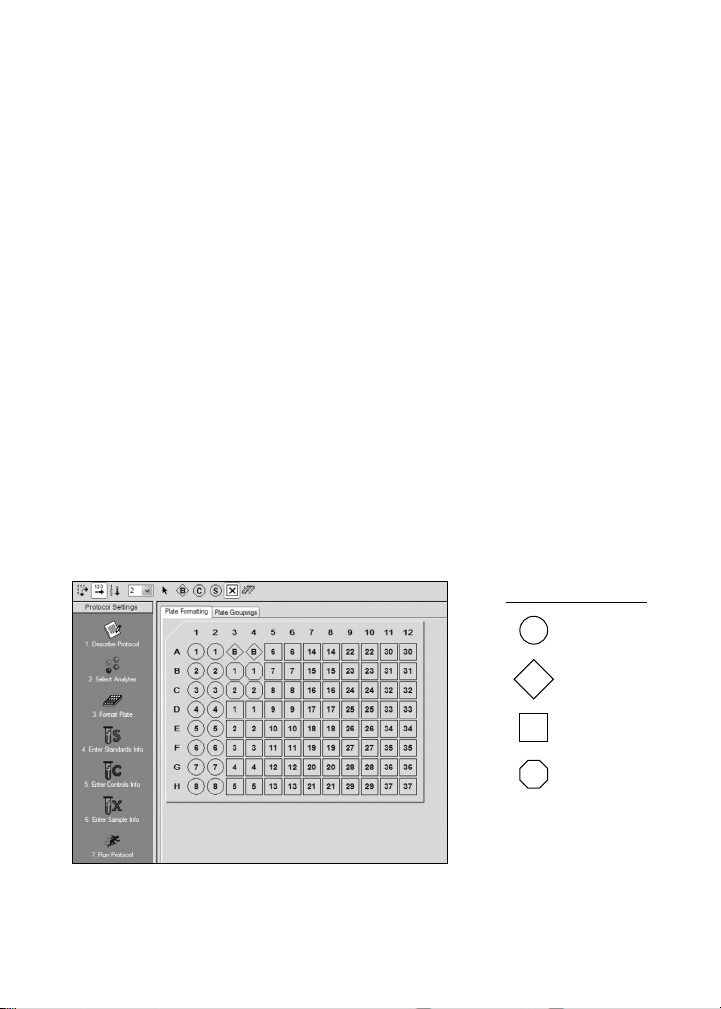
1. Plan Plate Layout
Prior to running the assay, determine the total number of wells in the
experiment using the Plate Layout Template on page 40 or the Plate
Formatting tab in Bio-Plex Manager
in Figure 2, with all conditions in duplicate.
1. Assign standards to columns 1 and 2, with the highest
concentration in row A and the lowest concentration in row H.
2. Assign the blank to wells A3 and A4. The blank should consist of your
chosen standard diluent. Note that Bio-Plex Manager automatically
subtracts the blank (B) MFI value from all other assay wells.
3. User-defined controls are assigned to wells in columns 3 and 4.
4. The remainder of the plate is available for samples.
5. Once the total number of wells is known, you can calculate the
required volumes of beads, detection antibody, and streptavidin-PE.
Use Tables 10–14, 18–22, and 23, respectively, or the Calculation
Worksheet on page 41.
Legend
S Standard
™
. A suggested plate layout is shown
B Blank
X Samples
C Controls
Fig. 2. Suggested plate layout. For detailed instructions on
plate formatting in Bio-Plex Manager, see section 8.
8
2. Prepare Instrument
Start up and calibrate the Bio-Plex® 100/200 or similar system with
Bio-Plex Manager
should be run daily or before each use of the instrument to standardize the
fluorescent signal. To prepare either a Bio-Plex 3D or Bio-Plex
reader, consult its respective user manual.
The validation kit should be run monthly to ensure performance of fluidics
and optics systems. Refer to either the software manual or online Help for
directions on how to conduct validation.
™
software prior to setting up the assay. The calibration kit
®
MAGPIX™
Start Up System (Bio-Plex 100, 200, or Similar)
1. Empty the waste bottle and fill the sheath fluid bottle before starting
if high throughput fluidics (HTF) are not present. This will prevent
fluidic system backup and potential data loss.
2. Turn on the reader, XY platform, and HTF (if included). Allow the
system to warm up for 30 min (if not already done).
3. Select Start up
for 4 hr without acquiring data, the lasers will automatically turn off.
To reset the 4-hr countdown, select Warm up
lasers/ optics to reach operational temperature.
and follow the instructions. If the system is idle
and wait for the
Calibrate System
1. Select Calibrate and confirm that the default values for CAL1
and CAL2 are the same as the values printed on the bottle of
Bio-Plex calibration beads. Use the Bio-Plex system low RP1
target value even if assays will be run at high RP1.
2. Select OK and follow the software prompts for step-by-step
instructions for CAL1 and CAL2 calibration.
Note: In Bio-Plex Manager version 6.1 and higher, startup, warm up,
and calibration can be performed together by selecting the “Start up and
calibrate” icon.
9

3. Prepare Wash Method
Bio-Plex Pro™ assays are compatible with both magnetic separation and
vacuum filtration methods. However, for best results, we recommend
performing the assays in a flat bottom plate with magnetic separation.
Table 4. Summary of compatible wash stations and plate types.
Wash Method Wash Station Assay Plate
Magnetic separation Bio-Plex Pro Flat bottom plate
Bio-Plex Pro II (use MAG programs)
Bio-Plex
Vacuum filtration Bio-Plex Pro II (use VAC programs) Filter plate
Vacuum manifold (manual)
Setting up the Bio-Plex Pro or Bio-Plex Pro II
Wash Station
The wash station does not require calibration; however, it should be primed
before use. For more information, refer to the Bio-Plex Pro and Pro II wash
station quick guide (bulletin #5826).
1. Install the appropriate plate carrier on the wash station.
2. Use the prime procedure to prime channel 1 with wash buffer.
Setting Up the Bio-Plex Handheld Magnetic Washer
Place an empty flat bottom plate on the magnetic washer by sliding
it under the retaining clips. Push the clips inward to secure the plate.
Make sure the plate is held securely. If needed, the clips can be adjusted
for height and tension. For detailed instructions, refer to the user guide
(bulletin #10023087).
®
handheld magnetic washer
Setting up a Vacuum Manifold
Calibrate the vacuum manifold by placing a standard 96-well flat bottom
plate on the unit and adjusting the pressure to –1 to –3" Hg. In general,
100 µl liquid should take 3–4 sec to clear the well. For more detailed
instructions, refer to bulletin #10005042.
10

4. Prepare Standards
General Instructions
n
It is essential to reconstitute and dilute standards exactly as
described in this section. Incorrect preparation may lead to low signal,
high background, or inconsistent measurements from plate to plate
n
The peel-off label provided with the standards lists the concentration
of the most concentrated dilution point, S1. Enter this information into
Bio-Plex Manager
n
For users who wish to mix assays from different panels, such as
diabetes assays with group I cytokines, guidance is provided here for
mixing 2 different lyophilized standards. Bead regions were chosen to
avoid overlap whenever possible. However, performance of multiplexes
containing assays from different groups have not been extensively
validated. Therefore, users must confirm that the assay performance is
still fit for purpose
Selecting a Diluent for Standards
Refer to Table 5 for recommended diluents based on different sample types.
As a general rule, reconstitute and dilute standards in a diluent similar to
the final sample type or sample matrix.
Table 5. Summary of recommended diluents for standards.
Sample Type Diluent for Standards Add BSA
Serum and plasma Standard diluent None
Culture media, with serum Culture media None
Culture media, serum-free Culture media To 0.5% final
Lavage, sputum, other fluids Bio-Plex
Lysate Bio-Plex sample diluent To 0.5% final*
* At least 0.5% final w/v BSA is recommended to stabilize analytes and reduce absorption
to labware.
™
software as instructed in Section 8
®
sample diluent To 0.5% final*
11
RP1 (PMT) Setting for Standard Curves
The Bio-Plex 200 and 3D systems have two RP1 (PMT or photomultiplier
tube) setting options, while the Bio-Plex
®
MAGPIX™ has no PMT and
therefore no PMT setting options. Instead, MAGPIX uses default instrument
settings similar to low PMT on the Bio-Plex 200 (Table 6).
Table 6. Overview of PMT setting options on Bio-Plex systems.
Instrument RP1 (PMT)
Bio-Plex 100, 200* Low, high
Bio-Plex 3D* Standard, enhanced
Bio-Plex MAGPIX* N/A, use default instrument settings
* Or similar Luminex-based system.
The Bio-Plex Pro™ human and mouse cytokine assays were developed
on the low PMT setting using the Bio-Plex 200 system, while the rat
cytokine assays were developed on the high PMT setting. Protocols using
alternative standard dilution series or different PMT settings should be
validated by the end user, for example when mixing the cytokine assays
with diabetes assays (Table 7 and Table 24).
Table 7. Settings for optimal sensitivity on the Bio-Plex 200 system*.
Assay Low RP1 (PMT) High RP1 (PMT)
Human cytokines (group l, ll)
Mouse cytokines (group l, ll, III)
Rat cytokines (group l) User validation required*
Low RP1 (PMT), broad High RP1 (PMT), narrow
Cross-panel mixing
Human cytokines + diabetes
Mouse cytokines + diabetes User validation required*
Rat cytokines + diabetes
* Contact Bio-Rad technical support for the most up to date recommendations on PMT
settings and cross-panel multiplexing compatibility.
range cytokine curve range cytokine curve
•
•
User validation required*
User validation required*
•
•
•
•
Reconstitute a Single Vial of Standards
This procedure prepares enough material to run each dilution in duplicate.
1. Gently tap the vial containing the lyophilized standard.
12
2. Add 500 μl of the appropriate diluent (see Table 5). Do not use assay
buffer to reconstitute the standards.
3. Gently vortex the reconstituted standard for 5 sec then incubate on
ice for 30 min. Be consistent with the incubation time in every
assay to ensure best results.
4. During the incubation period, prepare the samples as instructed in the
Prepare Samples section.
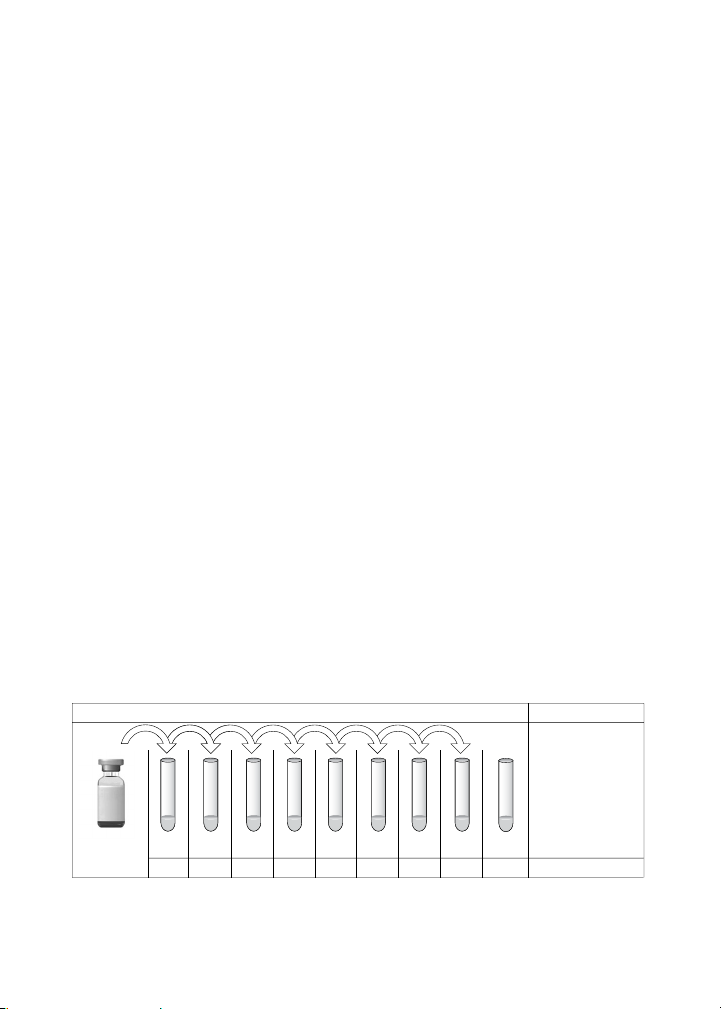
Prepare Standard Dilution Series From a Single
Antigen Vial
The following procedure produces an eight-point standard curve with a
fourfold dilution between each point. Pipet carefully using calibrated pipets
and use new pipet tips for every volume transfer.
1. Label nine 1.5 ml polypropylene tubes S1 through S8 and Blank.
2. Add the specified volume of standard diluent to each tube
(Figures 3 and 4).
3. Vortex the reconstituted standards gently for 5 sec before removing
any volume. Add 128 µl into the S1 tube containing 72 µl of standard
diluent. Vortex at medium speed for 5 sec, then use a new pipet tip
to transfer 50 µl from S1 tube to S2 tube. Vortex.
4. Continue with 1:4 (fourfold) serial dilutions from tube S2 to S8 as
shown in Figure 3. Use reconstituted and diluted standards
immediately. Do not freeze for future use.
128 50 50 50 50 50 50 50
Reconstituted
Standard
Fig. 3. Preparing a fourfold dilution series of cytokine standards.
72 150 150 150 150 150 150 150 150
S1 S2 S3 S4 S5 S6 S7 S8 Blank
13
Transfer Volume (µl)
Diluent (µl)
 Loading...
Loading...