Page 1

Bio-Plex Pro™
Cell Signaling Assays
Instruction Manual
For technical support, call your local Bio-Rad office, or in the U.S., call 1-800-424-6723.
For research use only. Not for diagnostic procedures.
Page 2

Table of Contents
Introduction 1
Principle 2
Kit Components and Storage 4
Recommended Materials 5
Assay Workflow 6
lmportant Considerations 7
Detailed Instructions 8
11. Prepare the Samples 8
22. Plan the Plate Layout 12
33. Prepare the Wash Method 14
44. Run the Assay 16
55. Read the Plate 21
Troubleshooting Guide 28
Plate Layout Template 31
Calculation Worksheet 32
Safety Considerations 34
Legal Notices 34
Ordering Information 35
Page 3

Introduction
Bio-Plex Pro™ Cell Signaling Assays
Cell signaling is a complex process through which cells receive and
respond to stimuli from the surrounding environment. For example,
circulating cytokines and chemokines elicit a response from lymphocytes
by binding to cell surface receptors and activating intracellular
phosphoprotein signaling cascades. This turns on and off specific
genes in the nucleus — thus regulating protein expression, cell growth,
proliferation, motility, and survival (Chang and Karin 2001). Aberrant
signaling can lead to serious pathologies including cancer, autoimmune
diseases, cardiovascular disease, and neurological disorders.
Understanding which cell types and signaling pathways are involved in a
disease allows researchers to develop more precisely targeted therapies
with better efficacy and safety.
Bio-Plex Pro cell signaling assays are magnetic bead-based
immunoassays for the detection of intracellular phosphoproteins and
total target proteins in cell and tissue lysates. The assays are available as
singleplex sets, which researchers can combine on their own to make a
multiplex assay, or as premixed, all-in-one, multiplex kits. Phosphoprotein
detection and total target detection are carried out in separate wells of a
96-well assay plate, with just 1–10 μg of sample per well.
The assays have been optimized for exceptional sensitivity, high
specificity and improved performance over western blotting. The use
of magnetic (MagPlex) beads allows automation of wash steps on a
Bio-Plex Pro or similar wash station, which greatly simplifies assay
processing and improves assay precision.
For a complete list of all Bio-Plex Pro cell signaling targets, please visit
www.bio-rad.com/bio-plex.
References
Chang L and Karin M (2001). Mammalian MAP kinase signalling
cascades. Nature 410, 37–40.
1
Page 4

Principle
Technology
The Bio-Plex
®
suspension array system is built upon the three core
elements of xMAP technology:
n
Fluorescently dyed microspheres (also called beads), each with a distinct
color code or spectral address to permit discrimination of individual tests
within a multiplex suspension. This allows simultaneous detection of up to
500 different types of molecules in a single well of the 96-well microplate
on the Bio-Plex
Bio-Plex
Bio-Plex
n
A dedicated plate reader. The Bio-Plex 200 and Bio-Plex 3D systems are
®
®
®
3D system, up to 100 different types of molecules on the
200 system, and up to 50 different types of molecules on the
MAGPIX™system
flow cytometry–based instruments with two lasers and associated optics
to measure the different molecules bound to the surface of the beads. In
the Bio-Plex MAGPIX system, the entire sample load volume is injected into
a chamber where the beads are imaged using LED and CCD technology
n
A high-speed digital signal processor that efficiently manages the
fluorescence data
Assay Format
Bio-Plex Pro
™
assays are essentially immunoassays formatted on
magnetic beads. The assay principle is similar to that of a sandwich
ELISA (Figure 1). Capture antibodies directed against the desired
biomarker are covalently coupled to the beads. Coupled beads react
with the sample containing the biomarker of interest. After a series of
washes to remove unbound protein, a biotinylated detection antibody
is added to create a sandwich complex. The final detection complex is
formed with the addition of streptavidin-phycoerythrin (SA-PE) conjugate,
which serves as a fluorescent indicator or reporter.
2
Page 5
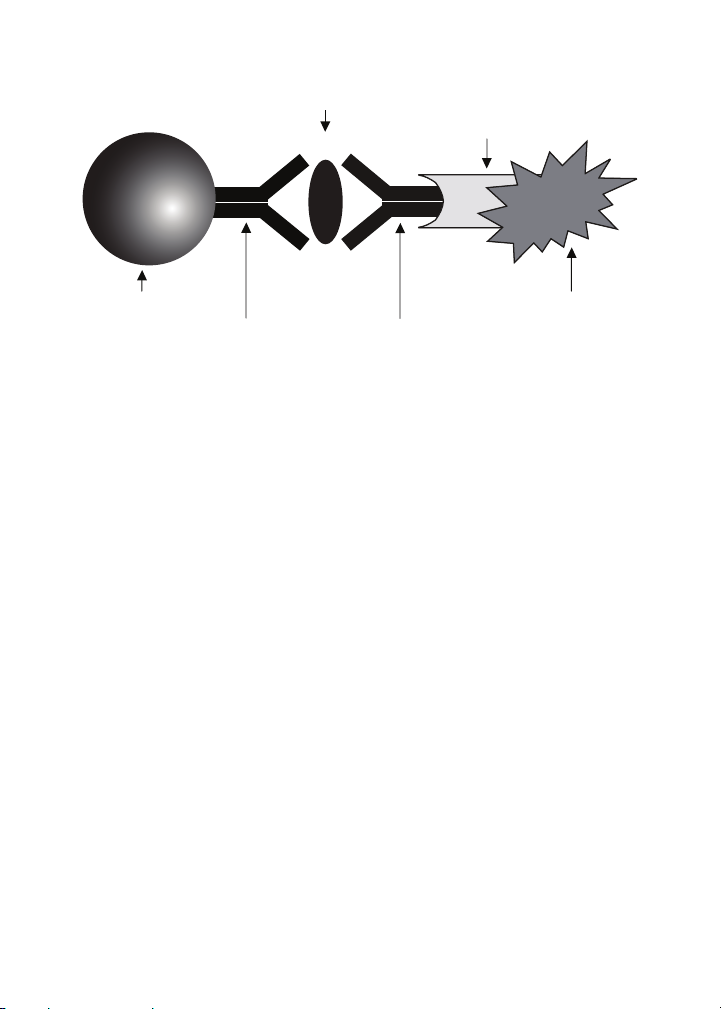
Biomarker
of Interest
Streptavidin
Magnetic Bead
Capture
Antibody
Fig. 1. Bio-Plex sandwich immunoassay.
Biotinylated
Detection
Antibody
Phycoerythrin
Fluorescent
Reporter
Data Acquisition and Analysis
Data from the reactions are acquired using a Bio-Plex system or similar
Luminex-based reader. When a multiplex assay suspension is drawn into
the Bio-Plex 200 reader, for example, a red (635 nm) laser illuminates
the fluorescent dyes within each bead to provide bead classification
and thus assay identification. At the same time, a green (532 nm)
laser excites PE to generate a reporter signal, which is detected by a
photomultiplier tube (PMT). A high-speed digital processor manages
data output, and Bio-Plex Manager
™
software presents data as median
fluorescence intensity (MFI). The relative concentration of analyte bound
to each bead is proportional to the MFI of the reporter signal.
Using Bio-Plex Data Pro
™
software, data from multiple instrument runs
can be combined into a single project for easy data management, quick
visualization of results, and simple statistical analysis.
3
Page 6

Kit Components and Storage
Table 1. Components required for a complete 1 x 96-well cell signaling assay.
Note that custom x-Plex
detection antibodies in an all-in-one kit.
Component
Singleplex* or Multiplex Set
Coupled magnetic beads (20x)
Detection antibodies (20x)
Cell Signaling Reagent Kit (catalog #171-304006M)
Cell wash buffer
Cell lysis buffer
Cell lysis factor QG
Wash buffer
Detection antibody diluent
Resuspension buffer
Streptavidin-PE (100x)
Flat bottom plate
Sealing tape
Assay Quick Guide
Bio-Rad Cell Lysate Controls (optional)**
Positive control (treated or untreated)
Negative control (phosphatase treated)
* Users can mix compatible singleplex sets to create their own multiplex assays.
** Refer to Table 3 to identify the appropriate controls for your phosphoprotein or total target
of interest.
*** Volumes shown are approximate.
™
assays include a premixed (multiplex) set of beads and
Quantity
1 tube
1 tube
1 bottle (50 ml)
1 bottle (50 ml)
1 vial
1 bottle (330 ml)
1 bottle (10 ml)
1 bottle (40 ml)
1 tube
1 plate
1 pack of 4
1 booklet
50 μg per vial
50 μg per vial
***
Storage and Stability
Kit contents should be stored at 4°C and never frozen. Coupled magnetic
beads and streptavidin-PE should be stored in the dark. All components
are guaranteed for a minimum of six months from the date of purchase
when stored as specified.
4
Page 7

Table 2. Recommended materials.
Item
™
Bio-Plex Pro
Bio-Plex
Assays Quick Guide 3
®
200 system or Luminex system with HTF
Bio-Plex validation kit
Run monthly
Bio-Plex calibration kit
Run daily to standardize fluorescence signal
Bio-Plex Pro wash station
Ordering Information
Bulletin #10024930 (download
at www.bio-rad.com/bio-plex)
Bio-Rad catalog #171-000205
Bio-Rad catalog #171-203001
Bio-Rad catalog #171-203060
Bio-Rad catalog #300-34376
For use with magnetic bead-based assays only
Bio-Plex handheld magnetic washer
Bio-Rad catalog #171-020100
For magnetic bead–based assays only
Bio-Plex Pro flat bottom plates (forty 96-well plates)
Bio-Rad catalog #171-025001
For magnetic separation on the Bio-Plex Pro wash station
Microtiter plate shaker
IKA MTS 2/4 shaker for 2 or 4 microplates
or
Barnstead/Lab-Line Model 4625 plate
IKA catalog #320-8000
VWR catalog #57019-600
shaker (or equivalent capable of 300–1,100 rpm)
™
vacuum manifold
Aurum
Bio-Rad catalog #732-6470
For vacuum filtration
BR-2000 vortexer
Reagent reservoirs, 25 ml
For capture beads and detection antibodies
Reagent reservoir, 50 ml (for reagents and buffers)
Pall Life Science Acrodisc: 32 mm PF syringe filter
(0.45 µm Supor membrane)
™
protein assay kit II
DC
Phenylmethylsulfonyl fluoride (PMSF)
Dimethyl sulfoxide (DMSO)
Kontes tissue grinder
Bio-Rad catalog #166-0610
VistaLab catalog #3054-1002
or
VistaLab catalog #3054-1004
VistaLab catalog #3054-1006
Pall Life Sciences
catalog #4654
Bio-Rad catalog #500-0112
Sigma catalog #P7626
Sigma catalog #D2650
VWR catalog #KT885000-0002
Other: Polypropylene tubes for reagent dilutions, calibrated pipets, pipet tips, sterile distilled
water, aluminum foil, paper towels, 1.5 or 2 ml microcentrifuge tubes, and a standard flat
bottom microplate (for calibrating vacuum manifold).
5
Page 8
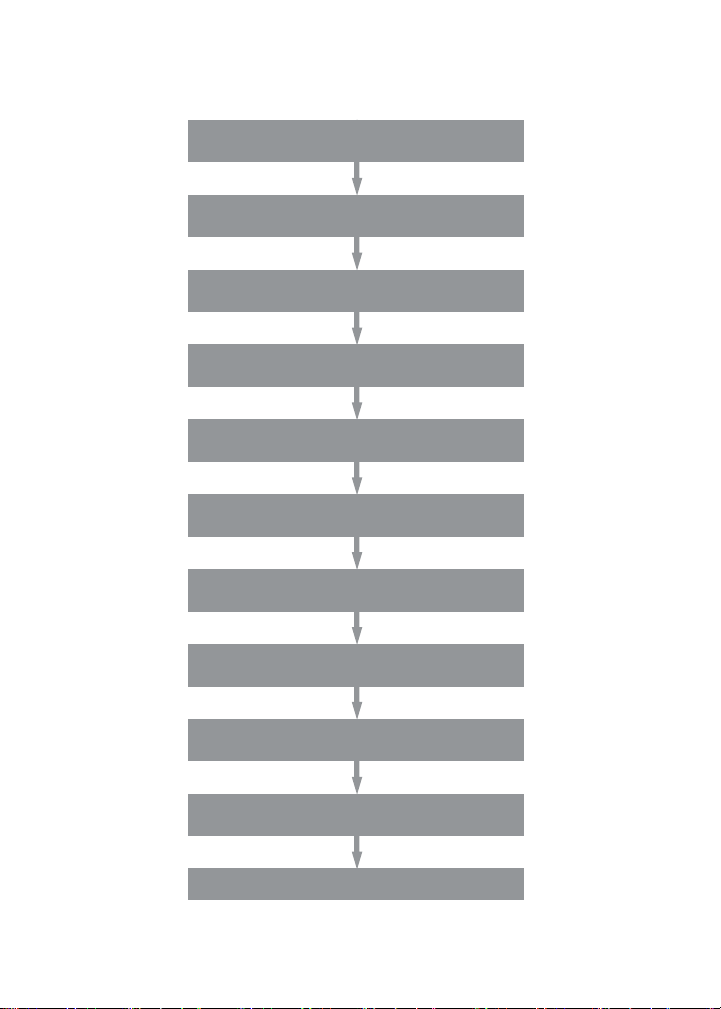
Assay Workflow
Prewet wells (for lter plate only)
Add 50 μl 1x beads to wells
Wash 2x
Add 50 μl samples, controls, and blanks;
incubate overnight at RT with shaking
Wash 3x
Add 25 μl 1x detection antibody,
incubate 30 min at RT with shaking
Wash 3x
Add 50 μl 1x s treptavidin- PE,
incubate 10 min at RT with shaking
Wash 3x
Add 125 μl of res uspens ion buffer,
shake for 30 sec
Acquire data
6
Page 9

lmportant Considerations
Assay Procedures
n
Please pay close attention to vortexing, shaking, and incubation
times and to Bio-Plex
optimized specifically for the cell signaling assays
n
For optimal performance, use only reagents specific for Bio-Plex Pro™
cell signaling assays. Reagents in other Bio-Plex assay panels have not
been validated for use in the cell signaling assays
n
Do not reuse diluted (1x) coupled beads, detection antibodies, or
streptavidin-PE
n
Wash as outlined in Table 5. Incomplete washes may cause
assay variation
n
If the data are not acquired immediately, the assay plate may be stored
at 4°C for up to 24 hr protected from light
Assay Quick Guide
Each assay kit comes complete with a printed Bio-Plex Pro Assay Quick Guide
(bulletin 10024930), which can be used to prepare and run a full 1 x 96-well
assay plate. Users can also download a copy at www.bio-rad.com/bio-plex.
Bead Regions and Multiplexing Compatibility
n
Bead regions for all analytes are listed in Table 12
n
Compatible singleplex assays may be mixed to create a multiplex assay
n
Do not mix phosphoprotein assays with corresponding total target
assays (for example, phospho-Akt and total Akt)
®
reader PMT (RP1) setting, as these have been
Instruments and Software
The Bio-Plex Pro cell signaling assays described in this manual are
compatible with all currently available Luminex-based life science
research instruments. Assays can be read and analyzed with either
Bio-Plex Manager
™
software or Luminex xPONENT software (Section 5,
Read the Plate, under Detailed Instructions).
7
Page 10

Detailed Instructions
The following pages provide detailed instructions for each step of the assay
procedure, including sample preparation, running the assay, and reading
the plate with Bio-Plex Manager
™
and Luminex xPONENT software.
1. Prepare the Samples
Considerations
n
The degree of phosphorylation of a given analyte is highly dependent
on the cell type and cell stimulation or treatment conditions
n
Cell lines may vary in their signaling responses to the same stimulation
n
The suggested final protein concentration range in the assay is
3–200 μg/ml (0.15–10 μg per assay well) except for Pl3K p85 (Tyr
which is 31–1,000 μg/ml (1.6–50 µg/well)
n
Optimization of cell lysate concentration may be needed based on
target protein expression levels
n
Cell lysate should be clear of particulate matter before use
Cell Lysates
The Bio-Plex Pro™ cell signaling reagent kit (catalog #171-304006M)
is required for preparing lysates derived from cell culture and tissue
samples. Just before use, prepare an adequate volume of cell lysis buffer
by adding PMSF and cell lysis factor QG.
n
Prepare 500 mM PMSF by dissolving 0.436 g PMSF in 5 ml DMSO.
(Store as aliquots at –20°C). Add PMSF to the cell lysis buffer at a final
concentration of 2 mM
n
Reconstitute cell lysis factor QG to 100x with 250 μl of diH2O and vortex
to mix. Add the reconstituted factor to the cell lysis buffer to a final 1x
working concentration
458
)
8
Page 11

Adherent Cells
1. Stop the treatment reaction by aspirating the culture medium and
quickly rinsing the cells with ice-cold cell signaling cell wash buffer
(bottle with the blue cap). The volume of buffer required is the same
as the volume of aspirated cell culture medium. Keep the cells on ice
during all steps when possible.
2. Completely remove the buffer before lysing the cells.
3. Immediately add the cell lysis buffer to the cells. The amount of
lysing solution needed depends on the cell density in the culture
vessel (for example, add 1.5–2 ml of lysis buffer to a 10 cm dish that
is ~80% confluent).
Note: It may be necessary to lyse the samples with different volumes of
cell lysis solution to obtain the specified protein concentration range.
4. Scrape the cells with a cell scraper, collect cell suspension into an
appropriately sized tube and gently rock for 20 min at 4°C.
5. Perform either of the following to remove insoluble cellular particulates:
n
Centrifuge the cell lysate solution at 4,500 x g for 20 min at 4°C,
and then filter the lysate using a 0.45 μm syringe filter
n
If the lysate volume is not adequate for filtration, centrifuge the lysate
at 15,000 x g for 10 min at 4°C using a benchtop microcentrifuge
6. Collect the filtered lysate or supernatant after centrifugation.
7. Measure protein concentration using Bio-Rad’s DC
™
protein assay
kit and if needed, adjust protein concentration with cell lysis buffer
containing PMSF and cell lysis factor QG.
8. The suggested working protein concentration range for Bio-Plex
®
cell signaling assays is 3–200 μg/ml (0.15–10 μg per assay well).
9. Store the aliquoted lysates at –70°C until ready to use.
9
Page 12

Suspension Cells
1.
Collect cell suspension and pellet the cells by spinning at 1,000 x g for
5 min at 4°C.
2. Aspirate off cell culture medium completely.
3. Wash by resuspending the cells with ice-cold cell wash buffer
(bottle with the blue cap)
.
4. Centrifuge the cells at 1,000 x g for 5 min at 4ºC.
5. Completely remove the buffer.
6. Immediately add the proper volume of cell lysis buffer and gently rock
for 20 min at 4°C.
7. Remove insoluble cellular particulates as described in Adherent Cells
step 5 above
.
8. Follow Adherent Cells steps 6–9 above.
Tissue Samples
1. Cut the tissue into small pieces (~3 x 3 mm) for ease of handling and
blood removal. If necessary, wash the tissue with ice-cold cell wash
buffer (bottle with the blue cap) to completely remove all blood. Then
transfer the tissue to a 2 ml tissue grinder.
2. Add an adequate volume of cell lysis solution and grind the tissue
sample on ice using approximately 20 strokes.
Note: It may be necessary to lyse the samples with different volumes of
cell lysis solution to obtain the specified protein concentration range.
3. Transfer the ground tissue to a clean microcentrifuge tube and freeze
sample at –70°C. Freezing and thawing samples helps increase cell
lysis effects.
4. Thaw the sample and sonicate on ice (for example, with a Sonifier
450: Duty Cycle = 40, Output = 1, Pulse Sonicating = 18x).
5. Remove insoluble cellular matter as in Adherent Cells step 5 above.
6. Follow Adherent Cells steps 6–9 above.
10
Page 13

Bio-Rad Cell Lysate Controls
The positive and negative cell lysate controls are used for qualitative
verification of assay performance. Refer to Table 3 to select the
appropriate controls for your phosphoprotein or total target of interest.
Table 3. Selection guide for Bio-Rad cell lysate controls.
Phosphoprotein of Interest Lysate Control Catalog #
473
Akt (Ser
Akt (Thr
Erk1/2 (Thr
ATF-2 (Thr
c-Jun (Ser
CREB (Ser
Btk (Tyr
Lyn (Tyr
c-AbI (Tyr
EGFR (Tyr
HER-2 (Tyr
HSP27 (Ser
IGF-1R (Tyr
IkB-a (Ser
NF-kB p65 (Ser
p70 S6 Kinase (Thr
BAD (Ser
IRS-1 (Ser
mTOR (Ser
Stat1 (Tyr
Stat3 (Ser
Src (Tyr
VEGFR-2 (Tyr
ZAP70 (Tyr
Negative control for all phosphoprotein assays Phosphatase-treated HeLa 171-YZB001
) GSK-3a/b (Ser21/Ser9)
308
) MEK1 (Ser
202
204
185
/Tyr
, Thr
71
) JNK (Thr
63
) p38 MAPK (Thr
133
) p53 (Ser15)
223
) Pl3K p85 (Tyr
507
) Syk (Tyr
245
) Untreated K-562 171-YZT003
1068
) EGFR (Tyr
1248
) p90 RSK (Ser
78
) S6 ribosomal protein EGF-treated SK-BR-3 171-YZ0003
1131
) IR-b (Tyr
32
/Ser36) Smad2 (Ser
536
)
421
136
) PDGFR-a (Tyr
636
639
/Ser
2448
) PTEN (Ser
701
) Stat3 (Tyr
727
)
416
) Src-transfected NIH3T3 171-YZ0013
1175
) VEGF-treated HUVEC 171-YZ0010
319
) H2O2-treated Jurkat 171-YZ0012
187
/Tyr
)
424
/Ser
) p70 S6 Kinase (Thr
) PDGFR-b (Tyr
217
221
/Ser
) EGF-treated HEK-293 171-YZ0001
183
185
/Tyr
)
180
182
/Tyr
) UV-treated HEK-293 171-YZ0009
458
)
352
)
1173
) EGF-treated HeLa 171-YZ0002
380
)
235
236
/Ser
(Ser
)
1146
) IGF-1-treated HEK-293 171-YZ0005
465
467
/Ser
389
754
)
751
) PDGF-treated NIH3T3 171-YZ0007
380
)
705
)
-treated Ramos 171-YZ0011
H
2O2
)
TNF-a–treated HeLa 171-YZ0008
) NGF-b–treated PC12 171-YZ0006
IFNa-treated HeLa 171-YZ0004
continues
11
Page 14

Table 3. Selection guide for Bio-Rad cell lysate controls (continued).
Total Target Lysate Control Catalog #
Total Akt Total mTOR
Total Erk 1/2 Total p38 MAPK
Total GSK-3b Total p70 S6 Kinase
Total IkB-a Total PTEN
Total JNK Total Smad2
Total MEK1 Total IGF-1R
Total Btk H
Total c-Jun
Total CREB
Total HER-2 EGF-treated SK-BR-3 171-YZ0003
Total Src Src-transfected NIH3T3 171-YZ0013
Total ZAP-70 H
Negative control for all total target assays Detection antibody diluent
House Keeping Protein
Human GAPDH b-Actin Untreated HeLa 171-YZT002
Negative control for all total target assays Detection antibody diluent
Untreated HeLa 171-YZT002
-treated Ramos 171-YZ0011
2O2
Untreated HEK-293 171-YZT001
-treated Jurkat 171-YZ0012
2O2
2. Plan the Plate Layout
Prior to running the assay, determine the total number of wells in the
experiment using the Plate Layout Template on page 30 or the Plate
Formatting tab in Bio-Plex Manager. A suggested plate layout is shown in
Figure 2, with all conditions in duplicate. Please note that the Bio-Plex 200
instrument reads the plate vertically.
12
Page 15

1. Assign blank, negative control, positive control, and samples
accordingly.
Note: When designating blank using
B
, Bio-Plex Manager
software will automatically subtract the blank MFI value from all other
assay wells.
2. Once the total number of wells is known, see Tables 6–9 or the
Calculation Worksheet on pages 32–33 to determine the required
volumes of beads, detection antibodies, and streptavidin-PE to use.
Note that 20–25% excess volume is included in the calculations to
compensate for transfer loss.
Legend
B Blank
X Samples
C Controls
Fig. 2. Suggested plate layout. For detailed instructions on
plate formatting in Bio-Plex Manager, see Section 5, Read the Plate.
13
Page 16

3. Prepare the Wash Method
The cell signaling assays are compatible with both magnetic separation
and vacuum filtration methods. However, for best results, we recommend
performing the assays in a flat bottom plate with magnetic separation.
Table 4. Summary of compatible wash stations and plate types.
Wash Method Wash Station Assay Plate
Magnetic separation Bio-Plex Pro Flat bottom plate
Bio-Plex handheld magnetic washer
Vacuum filtration Vacuum manifold (manual) Filter plate
Setting up the Bio-Plex Handheld Magnetic Washer
Place an empty flat bottom plate on the magnetic washer by sliding it
under the retaining clips. Push the clips inward to secure the plate. Make
sure the plate is held securely. For detailed instructions, refer to the user
guide (bulletin 10023087).
Setting up the Bio-Plex Pro
The wash station should be primed before use. See bulletin 5826.
1. Install the appropriate plate carrier on the wash station.
2. Use the Prime procedure to prime channel 1 with wash buffer.
Note:
n
Before using the Bio-Plex Pro wash station, make sure to define/edit a
program with the correct settings for cell signaling assays (Table 5)
n
Existing cytokine assay programs: MAGx2, MAGx3, VACx2, and VACx3
should not be used
Refer to the wash station instruction manual (bulletin 10013125), or
contact Bio-Rad Technical Support for more information on defining,
editing, or importing wash station programs.
14
Page 17

Table 5. Summary of wash steps and settings. After each assay incubation step, perform the
appropriate wash step as shown below.
Bio-Plex Pro Wash Station/Handheld Magnet/Vacuum Manifold*
Wash Program Settings and Manual Wash Steps
Assay Step # of Washes Volume, µl Magnetic Soak, min
1. Add beads to plate 2 200 1
2. Sample incubation 3 200 1
3. Detection Ab incubation 3 200 1
4. SA-PE incubation 3 200 1
* No magnetic soak for vacuum filtration.
Setting up a Vacuum Manifold
Calibrate the vacuum manifold by placing a standard 96-well flat bottom
plate on the unit and adjusting the pressure to between –1 and –3" Hg. In
general, 200 μl liquid should take 5–6 sec to clear the well. For detailed
instructions, refer to bulletin 10005042.
Using a Vacuum Manifold
n
After each incubation, place the filter plate on a calibrated vacuum
apparatus and remove the liquid by vacuum filtration
n
To wash, add 200 μl wash buffer to each well and remove the liquid as
before. Ensure that all wells are exposed to the vacuum
n
Thoroughly blot the bottom of the filter plate with a clean paper towel
between each vacuum step to prevent cross contamination
n
Place the assay plate on the plastic plate holder/tray as needed
n
Before each incubation, gently cover the plate with a new sheet of
sealing tape. Avoid pressing down over the wells to prevent leaking
from the bottom
15
Page 18

4. Run the Assay
Considerations
n
Bring all assay components and samples to room temperature before use
n
Use calibrated pipets and pipet carefully, avoiding bubbles
n
Assay incubations are carried out in the dark. Cover the plate with
aluminum foil or otherwise protect from extended exposure to light
DAY 1
Prepare Samples and Controls
1. Thaw sample lysates and keep on ice (see Section 1, Prepare the
Samples, for lysate preparation).
2. Reconstitute lyophilized cell lysate control with 250 μl of diH
vortex for 5 sec to mix, and incubate at room temperature for 20 min.
Protein concentration is now 200 μg/ml. Unused lysate can be stored
at –20°C for 3 months.
3. Centrifuge all samples and lysate controls at 15,000 x g for 10 min at
4°C before dispensing to wells.
Prepare and Add Coupled Beads and Samples
4. Use Tables 6 and 7 or the Calculation Worksheet on pages 32–33
as a reference to calculate the volume of coupled beads and wash
buffer needed.
2
O,
5. Add the required volume of wash buffer to an appropriately-sized
polypropylene tube. This will be used to dilute beads to 1x.
6. Vortex the 20x stock of coupled beads at mid speed for 30 sec.
Carefully open the cap and pipet any liquid trapped in the cap back into
the tube. This is important to ensure maximum bead recovery. Do not
centrifuge the vial; doing so will cause the beads to pellet.
16
Page 19

7. Dilute coupled beads to 1x by pipetting the required volume into the
tube containing wash buffer. Vortex.
Each well of the assay requires 2.5 μl of the 20x stock adjusted to a
final volume of 50 μl in wash buffer.
Note: To minimize volume loss, use a 200–300 μl capacity pipet to
remove beads from the 20x stock tube. If necessary, perform the volume
transfer in 2 steps. Do not use a 1,000 μl capacity pipet and/or wide
bore pipet tip.
8. Protect the beads from light with aluminum foil. Equilibrate to room
temperature prior to use.
Preparing 1x coupled beads from 20x stock (includes 20% excess volume):
Table 6. Premixed panel or one singleplex assay.
# of Wells 20x Beads, µl Wash Buffer, µl Total Volume, µl
96 288 5,472 5,760
48 144 2,736 2,880
Table 7. Mixing two singleplex assays.
20x Beads, µl 20x Beads, µl Wash
# of Wells Singleplex #1 Singleplex #2 Buffer, µl Total Volume, µl
96
48
288 288 5,184 5,760
144 144 2,592 2,880
9. Cover unused wells of the assay plate with sealing tape.
10. Prewet the filter plate. Skip this step if using a flat bottom plate.
a. Prewet the wells with 200 μl wash buffer and remove the liquid by
vacuum filtration. Dry the bottom of the filter plate thoroughly by
blotting on a clean paper towel.
11. Vortex the diluted (1x) beads for 15 sec at medium speed. Transfer
50 µl to each well of the assay plate.
12. Wash the plate two times with 200 µl wash buffer according to your
method of choice.
17
Page 20

13. Gather the samples, Bio-Rad cell lysate controls, and blank. Use
detection antibody diluent as the blank. Transfer 50 µl of each sample
or blank to the appropriate wells of the plate.
14. Cover with a new sheet of sealing tape and incubate in the dark
overnight (15–18 hr) at room temperature with shaking.
Note: Fully resuspend the beads/sample mixture by vigorously shaking at
900–1,100 rpm for 30 sec. Slowly ramp up to speed to avoid splashing.
Then turn down to 300–450 rpm for the specified incubation time.
DAY 2
Prepare Instrument and Wash Method
15. Start up, warm up, and calibrate the Bio-Plex system as described in
Section 5, Read the Plate. This may take up to 30 min.
16. Meanwhile bring all buffers and diluents to room temperature.
17. Prepare the wash method as described in Section 3, Prepare the Wash.
Prepare and Add Detection Antibodies
18. Use Tables 8 and 9 or the Calculation Worksheet on pages 32–33 to
calculate the volume of detection antibodies and Bio-Plex detection
antibody diluent needed. Detection antibodies should be prepared
10 min before use.
19. Add the required volume of Bio-Plex detection antibody diluent to an
appropriately sized polypropylene tube.
20. Vortex the 20x stock of detection antibodies for 15–20 sec at
medium speed, then perform a quick spin to collect the entire volume
at the bottom of the tube.
21. Dilute detection antibodies to 1x by pipetting the required volume into
the tube containing detection antibody diluent. Vortex.
Each well of the assay requires 1.25 μl of the 20x stock adjusted to a
final volume of 25 μl in detection antibody diluent.
18
Page 21

Preparing 1x detection antibodies from 20x stock (includes 25% excess volume):
Table 8. Premixed panel or one singleplex assay.
20x Detection Detection Antibody
# of Wells Antibodies, µl Diluent, µl Total Volume, µl
96 150 2,850 3,000
48 75 1,425 1,500
Table 9. Mixing two singleplex assays.
20x Detection 20x Detection Detection
Antibodies, µl Antibodies, µl Antibody
# of Wells Singleplex #1 Singleplex #2 Diluent, µl Total Volume, µl
96
48
150 150 2,700 3,000
75 75 1,350 1,500
22. After the overnight incubation, slowly remove and discard the
sealing tape.
23. Wash the plate three times with 200 µl wash buffer according to
your method of choice.
24. Vortex the diluted (1x) detection antibodies gently for 5 sec and
transfer 25 µl to each well of the assay plate.
25. Cover with a new sheet of sealing tape and incubate in the dark for
30 min at room temperature with shaking. Fully resuspend the beads/
detection antibody mixture by shaking at 900–1,100 rpm for 30 sec.
Then turn down to 300–450 rpm for the specified incubation time.
Prepare and Add Streptavidin-PE (SA-PE)
26. While detection antibodies are incubating, use Table 10 or the
Calculation Worksheet on pages 32–33 to calculate the volume of
SA-PE and detection antibody diluent needed. SA-PE should be
prepared 10 min before use.
19
Page 22

27. Add the required volume of detection antibody diluent to an appropriately
sized polypropylene tube. This will be used to dilute SA-PE to 1x.
28. Vortex the 100x stock of SA-PE for 5 sec at medium speed. Perform
a quick spin to collect the entire volume at the bottom of the vial.
29. Dilute SA-PE to 1x by pipetting the required volume into the tube
containing detection antibody diluent. Vortex and protect from light
until ready to use.
Each well of the assay requires 0.5 μl of the 100x stock adjusted to a
final volume of 50 μl in detection antibody diluent.
Table 10. Preparing 1x SA-PE from 100x stock (includes 25% excess volume).
# of Wells 100x SA-PE, µl Detection Antibody Diluent, µl Total Volume, µl
96 60 5,940 6,000
48 30 2,970 3,000
30. After detection antibody incubation, slowly remove and discard
the sealing tape.
31. Wash the plate three times with 200 µl wash buffer according to
your method of choice.
32. Vortex the diluted (1x) SA-PE at medium speed for 5 sec and
transfer 50 µl to each well of the assay plate.
33. Cover with a new sheet of sealing tape and incubate in the dark for
10 min at room temperature with shaking. Fully resuspend the beads/
SA-PE mixture by shaking at 900–1,100 rpm for 30 sec. Then turn
down to 300–450 rpm for the specified incubation time.
34. After the streptavidin-PE incubation step, slowly remove and discard
the sealing tape.
35. Wash the plate three times with 200 µl wash buffer according to
your method of choice.
20
Page 23

36. To resuspend beads for plate reading, add 125 µl resuspension
buffer to each well. Cover the plate with a new sheet of sealing tape
and shake at 900–1,100 rpm for 30 sec.
36. Slowly remove the sealing tape and place the plate on the reader to
acquire data.
Table 11. Read the plate using the appropriate instrument settings.
Inst rument RP1 (PMT ) DD Gates Bead Even ts
Bio-Plex 100, 200* High 5,000 (low), 25,000 (high) 50
Bio-Plex 3D* Enhanced Select MagPlex beads 50
Bio-Plex MAGPIX* N/A, use default instrument settings
* Or similar Luminex-based system.
5. Read the Plate
Bio-Plex Manager software is recommended for all Bio-Plex Pro assay
data acquisition and analysis. Instructions for Luminex xPONENT software
are also included. For instructions on using other xMAP system software
packages, contact Bio-Rad Technical Support or your regional Bio-Rad
field applications specialist.
Prepare Instrument
Start up and calibrate the Bio-Plex 100/200 or similar system with Bio-Plex
Manager software prior to setting up the assay. The calibration kit should
be run daily or before each use of the instrument to standardize the
fluorescent signal. To prepare either a Bio-Plex 3D or Bio-Plex
reader, consult its respective user manual. For instructions on using other
xMAP system software packages, contact Bio-Rad Technical Support.
®
MAGPIX™
The validation kit should be run monthly to ensure optimal performance
of fluidics and optics systems. Refer to either the software manual or
online Help for instructions on how to conduct validation.
21
Page 24

Start Up System (Bio-Plex 100, 200, or similar)
1. Empty the waste bottle and fill the sheath fluid bottle before starting if
high throughput fluidics (HTF) are not present. This will prevent fluidic
system backup and potential data loss.
2. Turn on the reader, XY platform, and HTF (if included). Allow the
system to warm up for 30 min (if not already done).
3. Select Start up
for 4 hr without acquiring data, the lasers will automatically turn off.
To reset the 4-hr countdown, select Warm up
lasers/optics to reach operational temperature.
and follow the instructions. If the system is idle
and wait for the
Calibrate System
4. Select Calibrate and confirm that the default values for CAL1
and CAL2 are the same as the values printed on the bottle of Bio-Plex
calibration beads. Use the Bio-Plex system low RP1 target value
even if the assays will be run at high RP1. Bio-Plex Manager version
6.1 and higher will automatically calibrate at both high and low RP1
settings although only the low RP1 value option is listed under CAL2.
5. Select OK and follow the software prompts for step-by-step
instructions for CAL1 and CAL2 calibration.
Note: In Bio-Plex Manager version 6.1 and higher, startup, warm up,
and calibration can be performed together by selecting the Start up and
calibrate icon.
Prepare Protocol in Bio-Plex Manager Software
Version 6.0 and Higher
The protocol should be prepared in advance so that the plate is read as
soon as the experiment is complete.
A protocol file specifies the analytes in the assay, the plate wells to be
read, sample information, and instrument settings.
Bio-Plex Manager software versions 6.0 and higher contain protocols for
most Bio-Plex assays. Choose from available protocols or create a new
22
Page 25

protocol. To create a new protocol, select File, then New from the main
menu. Locate and follow the steps under Protocol Settings.
6. Click Describe Protocol and enter information about the assay
(optional).
7. Click Select Analytes and create a new panel. Visually confirm the
selected analytes and proceed to step 8.
a. Click the Add Panel button
in the Select Analytes toolbar.
Enter a new panel name. Select Bio-Plex Pro Assay (Magnetic)
or MagPlex Beads (Magnetic) from the assay dropdown list.
If using Bio-Plex Manager version 5.0 or lower, select MagPlex
from the assay dropdown list.
b. Click the Add button. Enter the bead region number and name for
the first analyte. Click Add Continue to repeat for each analyte in
the assay. Refer to the bead regions listed in Table 12.
c. Click the Add button when the last analyte has been added and
click OK to save the new panel.
d. Highlight analytes from the Available list (left) and move to the
Selected list (right) using the Add button. To move all analytes at
once, simply click the Add All button.
e. If some of the analytes need to be removed from the Selected
list, highlight them and select Remove. If desired, it is possible to
rename the panel by clicking Rename Panel and entering a new
panel name.
8. Click Format Plate, and format the plate according to the plate
layout created in Section 2, Plan the Plate Layout. To modify the plate
layout, follow the steps below (see Figure 3).
a. Select the Plate Formatting tab.
b. Select the blank icon
that contain blanks. Repeat this process for Controls
Samples
X
. Note that Bio-Plex Manager automatically
B
and drag the cursor over all the wells
C
and
subtracts the blank MFl value from all other assay wells.
23
Page 26

Table 12. Bead regions for cell signaling assays.
Phosphoprotein Targets Bead Region
473
Akt (Ser
Akt (Thr
ATF-2 (Thr
BAD (Ser
Btk (Tyr
c-AbI (Tyr
c-Jun (Ser
CREB (Ser
EGFR (Tyr
EGFR (Tyr
Erk1/2 (Thr
GSK-3a/b (Ser
HER-2 (Tyr
HSP27 (Ser
IGF-IR (Tyr
IR-b (Tyr
IRS-1 (Ser
IkB-a (Ser
JNK (Thr
Lyn (Tyr
MEK1 (Ser
) 75
308
) 75
71
) 20
136
) 26
223
) 39
245
) 45
63
) 56
133
) 19
1068
) 44
1173
) 44
202
204
185
/Tyr
, Thr
21
/Ser9) 18
1248
) 30
78
) 51
1131
) 43
1146
) 43
636
639
/Ser
) 76
32
/Ser36) 67
183
185
/Tyr
) 34
507
) 33
217
221
/Ser
187
/Tyr
) 38
) 27
Total Targets Bead Region
Akt 75
Btk 39
c-Jun 56
CREB 19
Erk 1/2 38
GSK3b 18
HER-2 30
IGF-1R 43
IkBa 67
Housekeeping Proteins Bead Region
Human GAPDH 21
Phosphoprotein Targets Bead Region
mTOR (Ser
2448
NF-kB p65 (Ser
p38 MAPK (Thr
15
p53 (Ser
) 53
p70 S6 Kinase (Thr
p70 S6 Kinase (Thr
p90 RSK (Ser
PDGFR-a (Tyr
PDGFR-b (Tyr
Pl3K p85 (Tyr
PTEN (Ser
S6 ribosomal protein (Ser
Smad2 (Ser
Src (Tyr
Stat1 (Tyr
Stat3 (Ser
Stat3 (Tyr
Syk (Tyr
VEGFR-2 (Tyr
ZAP-70 (Tyr
380
465
416
) 42
701
) 61
727
) 52
705
) 52
352
) 65
319
Total Targets Bead Region
JNK 34
MEK1 27
mTOR 46
p38 MAPK 36
p70 S6 Kinase 55
PTEN 22
Smad2 14
Src 42
ZAP-70 64
Housekeeping Proteins Bead Region
b-Actin 47
) 46
536
) 37
180
182
/Tyr
) 36
389
) 55
421
424
/Ser
380
) 35
754
) 28
751
) 57
458
) 54
) 22
/Ser
1175
) 29
) 55
235
/Ser
467
) 14
236
) 74
) 64
9. Click Enter Controls Info, and for user-specified controls, select
an analyte from the dropdown menu, then enter a description and
concentration. Repeat for each additional analyte in the assay.
For Bio-Rad cell lysate controls, format the appropriate wells as controls,
enter descriptions, but leave the concentrations blank. Alternatively, both
blanks and controls can be formatted as samples with clear descriptions.
24
Page 27

Fig. 3. Plate formatting.
10. Click Enter Sample Info and enter sample information and the
appropriate dilution factor if any.
11. Click Run Protocol and confirm that the assay settings are correct.
a. Refer to Table 11 for the recommended RP1 (PMT) setting.
Protocols using alternative settings should be validated by the
end user.
b. Confirm that data acquisition is set to 50 beads per region.
In Advanced Settings, confirm that the bead map is set to
100 region, the sample size is set to 50 µl, and the doublet
discriminator (DD) gates are set to 5,000 (Low) and 25,000 (High).
In Bio-Plex Manager software versions 4.0, 4.1, 4.1.1, and 5.0,
check Override Gates and set the DD gate values as indicated.
c. Select Start, name and save the .rbx file, and begin data
acquisition. The Run Protocol pop-up screen will appear. Click
Eject/Retract to eject the plate carrier.
25
Page 28

Acquire Data
1. Shake the assay plate at 900–1,100 rpm for 30 sec, and visually inspect
the plate to ensure that the assay wells are filled with buffer. Slowly
remove the sealing tape before placing the plate on the plate carrier.
2. Click Run Protocol, and on the pop-up screen, select Load Plate
and click OK to start acquiring data.
3. Use the Wash Between Plates
to reduce the possibility of clogging the instrument.
4. If acquiring data from more than one plate, empty the waste bottle
and refill the sheath bottle after each plate (if HTF is not present).
Select Wash Between Plates and follow the instructions. Then
repeat the Prepare Protocol and Acquire Data instructions.
5. When data acquisition is complete, select Shut Down
follow the instructions.
command after every plate run
and
Reacquire Data
It is possible to acquire data from a well or plate a second time using the
Rerun/Recovery mode located below Start in the Run Protocol step.
Any previous data will be overwritten unless the second run is saved under
a different file name.
1. Check the wells from which data will be reacquired.
2. Aspirate the buffer with the wash method of choice, but do not
perform wash step.
3. Add 100 µl of resuspension buffer to each well. Cover the plate with a
new sheet of sealing tape and shake plate at 900–1,100 rpm for 30 sec.
4. Repeat the Acquire Data steps to reacquire data. The data acquired
should be similar to those acquired initially; however, the acquisition
time will be extended because the wells have fewer beads.
26
Page 29

Luminex xPONENT Software
Although guidelines are provided here, consult the xPONENT software
manual for more details. Perform a system initialization with Luminex’s
calibration and performance verification kit, as directed by Luminex. Select
Batches to set up the protocol and follow the information under Settings.
Note: The instrument settings described below apply to Luminex 100/200
and FLEXMAP 3D or Bio-Plex
®
3D instruments. For the Bio-Plex MAGPIX
reader, use the default instrument settings.
1. Select MagPlex as the bead type for magnetic beads. This
automatically sets the DD gates.
2. Volume = 50 µl.
3. Refer to Table 11 to select the appropriate PMT setting for
your instrument.
4. Plate name: 96-well plate.
5. Analysis type: Qualitative.
Select Analytes to set up the panel.
1. Enter 50 in the Count field.
2. Select the bead region and enter the analyte name.
3. Click Apply all for Units and Counts.
Select Stds and Ctrls.
1. Enter descriptions and other information as applicable.
After the assay is complete, select Results, then select Saved Batches.
27
Page 30

Troubleshooting Guide
This troubleshooting guide addresses problems that may be encountered
with Bio-Plex Pro
™
assays. If you experience any of the problems listed
below, review the possible causes and solutions provided. Suboptimal
assay performance may also be due to the Bio-Plex
®
suspension array
reader. To eliminate this possibility, use the Bio-Plex validation kit to assist
in determining if the array reader is functioning properly.
Table 13. Troubleshooting guide.
Problem Possible Causes Possible Solutions
Low signals for Sample protein concentration Verify sample protein concentration
experimental too low or too high is within assay range. Optimization
samples of protein concentration may be
Poor cell lysate quality Prepare fresh lysate accordingly
Low signals for Incorrect dilution of detection Check the calculations and be
experimental samples antibody or streptavidin-PE careful to add the correct volumes
and Bio-Rad cell
lysate controls
Expired Bio-Plex reagents Check that reagents have not
were used expired. Use new or non-expired
Incorrect incubation Incubations should be at room
temperature temperature (20–22°C)
needed based on targeted protein
expression level
components
Insufficient incubation time Adhere to the recommended
Beads lost Use recommended magnetic
continues
incubation time
washer settings with correct
magnet soaking time
28
Page 31

Table 13. Troubleshooting guide (continued).
Problem Possible Causes Possible Solutions
Low bead count Cell debris in lysate Centrifuge at 15,000 x g for 10 min
not cleared at 4°C to remove cellular debris
Assay plate not shaken Shake plate at 900–1,100 rpm
enough prior to reading for 30 sec before data acquisition
Clogged reader Refer to the troubleshooting
guide in the Bio-Plex system
hardware instruction manual
(bulletin 10005042)
Miscalculation of bead dilution Check the calculations and be
careful to add the correct volumes
Clumping of stock beads Vortex the stock vial at different
in vials angles for 30 sec at medium speed
before aliquoting beads
Vacuum setting too high Adjust pressure to –1 to –3" Hg.
during suction of assay plate Generally, 100 μl liquid should
take 3–4 sec to clear from the well
Reader needle height Adjust the needle height to coincide
incorrect with the plate type provided in the kit
Beads lost Use recommended magnetic
continues
washer settings with correct
soaking time
29
Page 32

Table 13. Troubleshooting guide (continued).
Problem Possible Causes Possible Solutions
High background Prolonged incubation of Follow the procedure incubation
detection antibodies and/or time precisely
streptavidin-PE
Wash steps performed Perform washes as described in
incorrectly or insufficient the assay instructions
washing volume
High assay CV Bottom of filter plate not dry Dry the bottom of the filter plate
with absorbent paper towel
(preferably lint-free) to prevent
cross-well contamination
Contamination with wash Be careful not to splash wash buffer
buffer during wash steps from well to well. Filter wells
Cell debris in lysate not cleared Centrifuge at 15,000 x g for 10 min
at 4°C to remove cellular debris
Shaking speed too high during Fully resuspend bead mixture at
assay incubation 900–1,100 rpm for 30 sec, then turn
Reagents and assay Bring all reagents and assay
components not equilibrated components to room temperature
to room temperature prior to dispensing
completely to remove residual
buffer if using filter plate. Reduce
microplate shaking speed to
minimize splashing
down to 300–450 rpm for the
specified incubation time
30
Page 33

Plate Layout Template
31
Page 34

Calculation Worksheet
If using either a premixed panel or one singleplex assay, follow these instructions.
Plan the plate layout and enter the number of wells to be used in the assay:_______
1
1. Determine the volume of 1x coupled beads needed.
a. Each well requires 50 µl of coupled beads (1x): _______ x 50 µl = _______ µl
b. Include 20% excess to ensure enough volume: _______ µl x 0.20 = _______ µl
c. Total volume of 1x coupled beads: _______ µl + _______ µl = _______ µl
d. Volume of 20x coupled beads stock: _______ µl/20 = _______ µl
e. Volume of wash buffer required: _______ µl – _______ µl = _______ µl
2 3 4
4 5 6
2. Determine the volume of 1x detection antibody needed.
a. Each well requires 25 µl detection antibodies (1x): ______ x 25 µl = _______ µl
b. Include 25% excess to ensure enough volume: _______ µl x 0.25 = _______ µl
c. Total volume of 1x detection antibodies: _______ µl + _______ µl = _______ µl
d. Volume of 20x detection antibodies required: _______ µl/20 = _______ µl
e. Volume of detection antibody diluent required: _____ µl – _____ µl = _____ µl
3. Determine the volume of 1x streptavidin-PE needed.
a. Each well requires 50 µl streptavidin-PE (1x): _______ x 50 µl = _______ µl
b. Include 25% excess to ensure enough volume: _______ µl x 0.25 = _______ µl
c. Total volume of 100x streptavidin-PE: ______ µl + ______ µl = ______ µl
d. Volume of 100x streptavidin-PE required: _______ µl/100 = _______ µl
e. Volume of detection antibody diluent required: _______ µl – _______ µl = _______ µl
12 13 14
1 2
2 3
4 5
1 7
7 8
7 8 9
9 10
9 10 11
1 12
12 13
14 15
14 15 16
32
Page 35

If mixing two or more singleplex assays, follow these instructions.
Enter the number of wells to be used in the assay:_______
1
1. Determine the volume of 1x coupled beads needed.
a. Each well requires 50 µl coupled beads (1x): _______ x 50 µl = _______ µl
b. Include 20% excess to ensure enough volume: _______ µl x 0.20 = _______ µl
c. Total volume of 1x coupled beads: _______ µl + _______ µl = _______ µl
d. Enter the number of singleplex sets (or analytes) that will be multiplexed: _______
2 3 4
1 2
2 3
5
e. Volume of 20x coupled beads required from each stock tube:
_______ µl/20 = _______ µl
4 6
f. Total volume of bead stock required: _______ x _______ µl = _______ µl
g. Volume of wash buffer required: _______ µl – _______ µl = _______ µl
5 6 7
4 7 8
2. Determine the volume of 1x detection antibody needed.
a. Each well requires 25 µl detection antibodies (1x): _______ x 25 µl = _______ µl
b. Include 25% excess to ensure enough volume: _______ µl x 0.25 = _______ µl
c. Total volume of 1x detection antibodies: _______ µl + _______ µl = _______ µl
d. Enter the number of singleplex sets (or analytes) that will be multiplexed: _______
9 10 11
1 9
9 10
5
e. Volume of 20x detection antibodies required from each stock tube:
_______ µl/20 = _______ µl
11 12
f. Total volume of combined detection antibody stock: _____ µl x _____ = _____ µl
g. Volume of detection antibody diluent required: ____ µl – ____ µl = ____µl
12 5 13
11 13 14
3. Determine the volume of 1x streptavidin-PE needed.
a. Each well requires 50 µl streptavidin-PE (1x): _______ x 50 µl = _______ µl
b. Include 25% excess to ensure enough volume: _______ µl x 0.25 = _______ µl
c. Total volume of 100x streptavidin-PE: ______ µl + ______ µl = _______ µl
d. Volume of 100x streptavidin-PE required: _______ µl/100 = _______ µl
e. Volume of detection antibody diluent required: _______ µl – _______ µl = _______ µl
1 15
15 16
15 16 17
17 18
17 18 19
33
Page 36

Safety Considerations
Eye protection and gloves are recommended while using this product.
Consult the MSDS for additional information.
Human source material. Treat as potentially infectious.
The lysates provided with Bio-Plex Pro
components of human origin. The components are known to contain
an agent that requires handling at Biosafety Level 2 containment as
defined by U.S. government publication, Biosafety in Microbiological
and Biomedical Laboratories (Centers for Disease Control 1999). These
agents have been associated with human disease. These components
have not been screened for hepatitis B, human immunodeficiency viruses,
or other adventitious agents. Handle Bio-Plex
and negative controls as potentially biohazardous material under at least
Biosafety Level 2 containment.
™
cell signaling assays contain
®
phosphoprotein positive
Legal Notices
Acrodisc and Supor are trademarks of Pall Corporation. FLEXMAP,
MagPlex, xMAP, and xPONENT are trademarks of Luminex Corporation.
Barnstead and Lab-Line are trademarks of Thermo Fisher Scientific.
Sonifier is a trademark of Branson Ultrasonics Corporation.
The Bio-Plex
microspheres and instrumentation licensed to Bio-Rad Laboratories, Inc.
by the Luminex Corporation.
®
suspension array system includes fluorescently labeled
CST antibodies developed and validated for
Bio-Plex cell signaling, phosphoprotein, and
total target assays.
34
Page 37

Ordering Information
Detailed ordering information can be found at www.bio-rad.com/bio-plex.
Catalog # Description
Individual Components and Accessories
Various Bio-Plex Pro
171-304006M Bio-Plex Pro cell signaling reagent kit, 1 x 96-well
Various Bio-Rad cell lysate controls, pkg of 1 vial
171-304515 Bio-Plex Pro cell signaling wash buffer, for 1 x 96-well assay, 330 ml
171-304502 Filter plate, 1 x 96-well with clear plastic lid and tray
®
Bio-Plex
Create a premium custom assay using the online Bio-Plex Assay Builder.
Go to www.bio-rad.com/bio-plex/assaybuilder to select analytes of interest. Assays are
supplied as premixed coupled beads and detection antibodies in the all-in-one kit format.
Bio-Rad cell lysate controls are included.
x-Plex™ Assays (We Mix)
™
cell signaling singleplex sets, 1 x 96-well
35
Page 38

Bio-Rad
Laboratories, Inc.
Life Science
Group
Web site ww w.bio-rad.com USA 800 424 6723 Australia 61 2 9914 2800
Austria 01 877 89 01 Belgium 09 385 55 11 Brazi l 55 11 5044 5699
Canada 905 364 3435 China 86 21 6169 8500
Czech R epubl ic 420 241 430 532 De nmark 4 4 52 10 00
Finland 09 804 22 00 France 01 47 95 69 65 Ger many 08 9 31 884 0
Greece 30 210 9532 220 Hon g Kong 852 2789 3300
Hungary 36 1 459 6100 India 91 124 4029300 Israel 03 963 6050
Italy 39 02 216091 Japan 03 6361 7000 Korea 82 2 3473 4460
Mexico 52 5 55 488 7670 The Netherlands 0318 540666
New Zealand 64 9 415 2280 No rway 23 38 41 30
Poland 48 22 331 99 99 Portugal 351 21 472 7700
Russia 7 495 721 14 04 Singapore 65 6415 3188
South Africa 27 861 246 723 Spain 3 4 91 590 5200
Sweden 08 555 1270 0 Switzerland 026 674 55 05
Taiwan 886 2 2578 7189 Thailand 800 88 22 88
United Kingdom 020 8328 200 0
10-0021 0913 Sig 121210024929 Rev C
 Loading...
Loading...