
Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and
Accessories
Cleaning, Disinfection, and Sterilization Instructions
Mat’l 103251 Ver. B
(This document is made from 713512 Version B).
Welch Allyn, Inc.
4341 State Street Road
Skaneateles Falls, NY 13153
Tel. +1 800 535-6663 or +1 315 685 4560 • Fax +1 315 685 3361
www.welchallyn.com
The CE mark on these products indicates that they are in conformity
with the provisions of Council Directive 93/42/EEC.
European Regulatory Manager, Medical Division, Welch Allyn Ltd,
Navan Business Park, Dublin Road, Navan, County Meath, Republic
of Ireland
Tel: +353 46 90 67700 Fax: +353 46 90 67755
© 2009 Welch Allyn, Inc. All rights reserved. No one is permitted to
reproduce or duplicate, in any form, this manual or any part thereof
without permission from Welch Allyn. Welch Allyn assumes no
responsibility for any injury to anyone, or for any illegal or improper
use of the product, that may result from failure to properly use the
product in accordance with the instructions, cautions, warnings, or
statement of intended use published in this manual. Welch Allyn®is
a trademark of Welch Allyn, Inc. Metricide®is a registered
trademark of Metrex. CaviWipes™ is a trademark of Metrex.
CIDEX®OPA, Sterrad®, CIDEZYME®and ENZOL®are registered
trademarks of Advanced Sterilization Products, a division of
Johnson and Johnson Company.
USA +1 800 535 6663
+1 315 685 4560
Canada +1 800 561 8797 China +86 21 6327 9631
European Call Center +353 46 90 67790 France +33 1 60 09 33 66
Germany +49 7477 9271 70 Japan +81 3 3219 0071
Latin America +1 305 669 9003 Netherlans +31 157 505 000
Singapore +65 6419 8100 South Africa +27 11 777 7555
United Kingdom +44 207 365 6780 Sweden +46 85 853 6551
Australia +61 2 9638 3000

ii Cleaning, Disinfection, and Sterilization Instructions

Rigid Reusable & Single use Sigmoidoscopes, Anoscopes, and Accessories ENGLISH 1
ENGLISH
Intended Use
The Welch Allyn Rigid Reusable & Single Use Sigmoidoscopes and Anoscopes are used
to illuminate and facilitate the examination of the rectal and anal cavity. The instrument
also provides a means of entry for surgical instruments into the rectal and anal cavity.
Endoscopes and the insufflation system are categorized as semi-critical devices requiring
high level disinfection and the illumination system is categorized as a non-critical device
requiring low-level disinfection according to the Multi-society guideline for reprocessing
flexible gastrointestinal endoscopes published by Gastrointestinal Endoscopy (volume 58,
No. 1, 2003) and simultaneously in Infection Control and Hospital Epidemiology. Optional
higher level processes have been included in these instructions.
Refer to ASTM F1518-00, Standard Practice for Cleaning and Disinfection of Flexible
Fiberoptic and Video Endoscopes Used in the Examination of the Hollow Viscera, for
additional guidance on reprocessing of these devices/accessories. Although this standard
is for flexible endoscopes, it contains information that can be applied to rigid endoscopes
as well.
Caution U.S. federal law restricts this device to sale by or on the order of a
physician or licensed healthcare practitioner. This device should only be used by
trained personnel.
Caution Perform reprocessing immediately after each use.
Caution Consult your facility's procedures and the reprocessing agents
manufacturer's instructions for the agents reuse, recommended Personal
Protective Equipment and other safety precautions such as ventilation, etc.
Caution Examine surfaces of all components for wear or damage after
cleaning, disinfection, and/or sterilization.
Caution For cleaning and/or disinfection, use brushes appropriate for the size of
the endoscope channel parts, connectors, and orifices (e.g. bristles should
contact all surfaces). Cleaning/disinfection tools (brushes, etc.) should be single
use or thoroughly cleaned, disinfected, and/or sterilized between uses.
Caution Unless otherwise specified, perform all reprocessing procedures at
ambient (room) temperature.
Caution Clean and disinfect all devices or accessories per these instructions
prior to returning to Welch Allyn (e.g. for repair and service).
Caution Users are responsible to qualify any deviations from these procedures.
2 ENGLISH Cleaning, Disinfection, and Sterilization Instructions
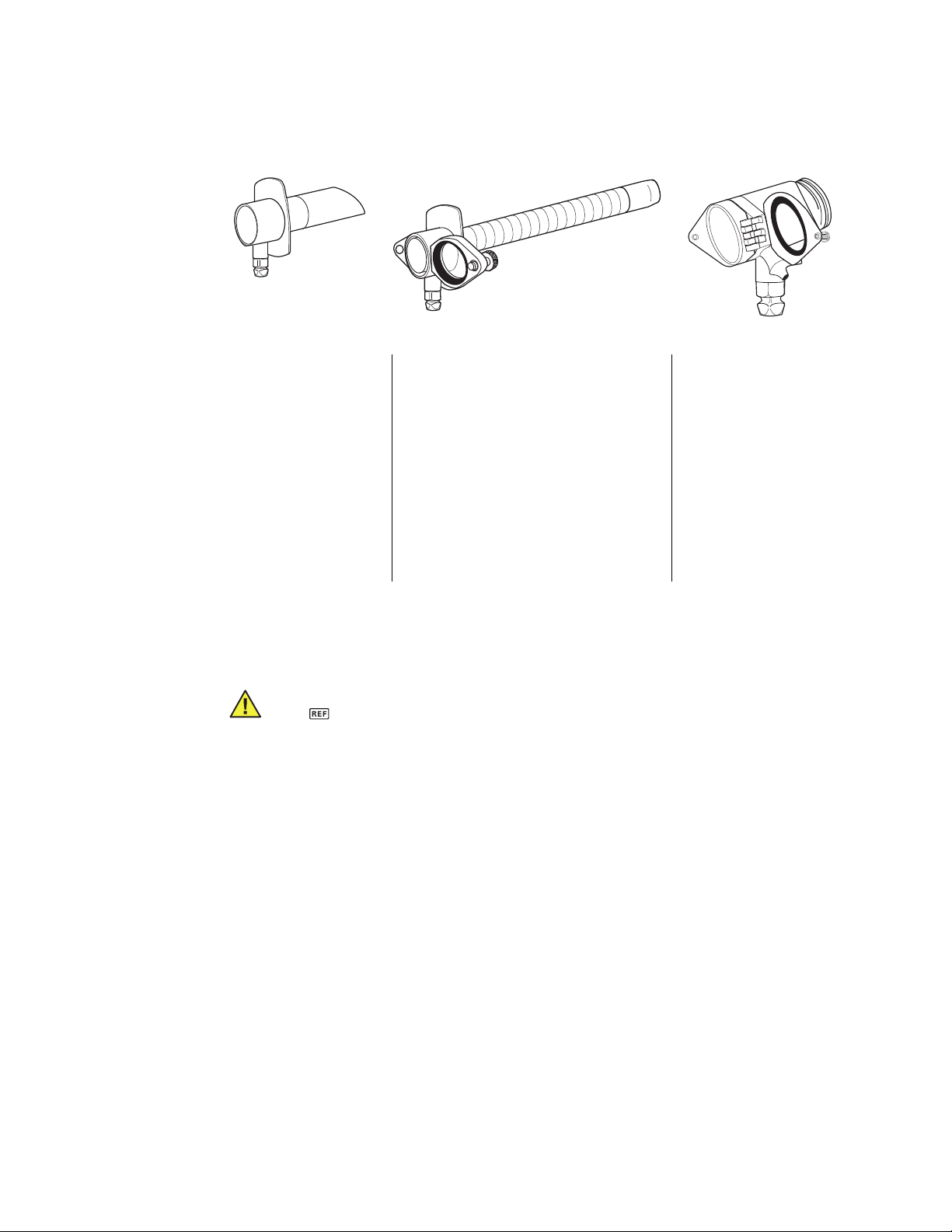
Rigid Sigmoidoscopes, Anoscopes, and Light Carrier
37023 32820 36019
Cleaning
Anoscope
Part Numbers:
37019 38614 32010 32820 36019
37023 38619 32020 32830 38700
37027 38622 32410 33220 Suction Tube*
38108 38900 32420 33620
38114 39614 32810 33830 30130
38119 39619 30140
38122 39622
*Suction Tubes not illustrated
Note
For all steps, remove obturator for separate cleaning and open viewing window.
Caution Welch Allyn recommends the use of the Single Use Insufflation Bulb
Filter ( 30210) for all applicable endoscopic procedures. The filter does not
eliminate the need to properly process the insufflation bulb with each use, but
restricts internal contamination and aspiration of fluids.
Sigmoidoscope
Part Numbers:
Light Carrier
Part Numbers:
Part Numbers:
1. Prepare an enzymatic detergent formulated for endoscopic instruments according to
the manufacturer’s instructions.These instructions were validated using ENZOL®/
CIDEZYME®.
2. Submerge the item in the cleaning solution.
3. Using a soft bristle brush, scrub the item submerged in the cleaning solution for a
minimum of 5 minutes then rinse with sterile water for 5 minutes.
High Level Disinfection
1. Follow the Cleaning instructions above.
2. Using CIDEX®OPA (0.55% OPA, 14-day maximum reuse), soak the item for a
minimum of 12 minutes.
3. Perform 3 sequential rinses of the item for a minimum of 5 minutes each with sterile
water.
 Loading...
Loading...