Page 1

3800 Pulse Oximeter
TruTrak
®
+
User’s Manual
SpO
–
+
♥
2
–
+
85
+
♥
–
+
–
PI
1.25
r
130
40
3800
TruTrak®
6050-0006-380
Printed in USA
Page 2

Important
This device performs as described in this manual, and in accompanying labels
and inserts, when assembled, operated, maintained, and repaired in accordance
with the instructions provided.
Attention! Consult the accompanying instructions before using this
device.
The safety, reliability, and performance of this device can be assured only under
the following conditions:
• If it is used according to the accompanying operating instructions.
• If fittings, extensions, readjustments, changes, or repairs are carried out by
agents authorized by Datex-Ohmeda.
• If it is used in buildings having ground equalization wiring that complies with
relevant local standards and regulations.
CAUTION: US Federal law restricts this device to sale by or on the order of a licensed medical
practitioner. Outside the USA, check local laws for any restriction that may apply.
This device must be cleaned and checked periodically. Do not use a defective
device. Parts that are broken, missing, plainly worn, distorted, or contaminated
should be replaced immediately. If repair or replacement becomes necessary,
request service advice from Datex-Ohmeda (information is listed on the back
cover). Do not repair this device or any of its parts other than in accordance with
written instructions provided by Datex-Ohmeda.
The user of this device shall have the sole responsibility for any malfunction that
results from improper use, faulty maintenance, improper repair, unauthorized
service, damage, or alteration by anyone other than Datex-Ohmeda.
Trademarks
Datex®, Ohmeda®, OxyTip®, PerfTrak®, TruTrak®, and PIr™ are the property of
Instrumentarium Corp. or its subsidiaries.
Cidex is a registered trademark of Johnson & Johnson.
Microsoft Windows Terminal is a trademark of Microsoft Corporation.
ProComm is a trademark of DataStorm Technologies.
All other product and company names are the property of their respective owners.
Text revised: October 2002
© 2002 Datex-Ohmeda, Inc. All rights reserved.
Page 3

Table of Contents
1/Overview
Product description.....................................................................................................................................1-1
Intended use........................................................................................................................................1-1
TruTrak
PIr pulsatile value............................................................................................................................1-2
Other features.....................................................................................................................................1-2
Functional components................................................................................................................1-3
Principles of operation..................................................................................................................1-4
Front panel.......................................................................................................................................................1- 6
Alarm silence button......................................................................................................................1-7
Numeric display...............................................................................................................................1-7
Graphic display .................................................................................................................................1-8
SpO2 alarm limits, high and low............................................................................................1-8
Pulse rate alarm limits, high and low..................................................................................1-9
Display contrast adjuster..............................................................................................................1-9
Power/Standby button/AC power light..............................................................................1-9
Carrying handle ................................................................................................................................1-9
Sensor connector...........................................................................................................................1-10
Pulse beep volume button .......................................................................................................1-10
Alarm volume button..................................................................................................................1-10
Rear panel......................................................................................................................................................1-11
Power entry module .................................................................................................................... 1-11
Equipotential ground connector........................................................................................... 1-11
Product information label ........................................................................................................ 1-11
Mode Switch ....................................................................................................................................1-11
RS-232 serial connector..............................................................................................................1-12
Precautions....................................................................................................................................................1-12
Warnings............................................................................................................................................1-12
Cautions..............................................................................................................................................1-14
+ technology......................................................................................................................1-1
Calibration..............................................................................................................................1-5
Alarm silence ........................................................................................................................1-7
All mute....................................................................................................................................1-7
Battery operation.................................................................................................................1-9
Failure of operation........................................................................................................1-12
Explosion hazard..............................................................................................................1-12
Electrical shock and flammability hazard........................................................1-12
Electrical shock hazard ................................................................................................ 1-12
Data validity........................................................................................................................1-13
Patient safety.......................................................................................................................1-13
Patient safety (sensors)..................................................................................................1-13
RS-232 system interconnection................................................................................1-13
Handle the monitor with care.................................................................................. 1-14
Cleaning................................................................................................................................1-14
Battery.....................................................................................................................................1-14
Sensors...................................................................................................................................1-14
Disposal.................................................................................................................................. 1-14
Miscellaneous.....................................................................................................................1-14
i
Page 4

Table of Contents
2/Setup and Operations
Powering the oximeter...............................................................................................................................2-1
Setup....................................................................................................................................................................2- 2
Factory settings and default settings .....................................................................................2-2
Mode switch settings......................................................................................................................2-3
Checkout procedure...................................................................................................................................2- 5
Signal and data validity............................................................................................................................2- 8
Plethysmographic waveform.....................................................................................................2-8
Numeric display...............................................................................................................................2-9
Trend data......................................................................................................................................................2-10
Before powering on the oximeter ..............................................................................2-2
After powering on the oximeter .................................................................................2-2
Language..................................................................................................................................2-3
Averaging mode...................................................................................................................2-4
Patient mode..........................................................................................................................2-4
PIr pulsatile value display.............................................................................................2-4
EMI line frequency ............................................................................................................2-4
Low perfusion.......................................................................................................................2-8
Signal noise............................................................................................................................2-9
SpO2...........................................................................................................................................2-9
Pulse rate ..............................................................................................................................2-10
PIr pulsatile value............................................................................................................2-10
3/Messages and Troubleshooting
Messages.............................................................................................................................................................3-1
Alarm categories............................................................................................................................................3-4
High priority........................................................................................................................................3-4
Medium priority................................................................................................................................3-4
Low priority.........................................................................................................................................3-5
System failure.....................................................................................................................................3-5
Troubleshooting.............................................................................................................................................3-6
4/Maintenance and Service
Cleaning.............................................................................................................................................................4-1
Oximeter................................................................................................................................................4-1
Recharging the battery..............................................................................................................................4-2
Replacing the battery.................................................................................................................................4-2
Replacing the fuses.....................................................................................................................................4-3
Repair policy and procedure.................................................................................................................4-4
Packaging and return procedure............................................................................................4-4
Parts list ..............................................................................................................................................................4-5
ii
Page 5

Table of Contents
A/Compliance and Specifications
Compliance with standards...................................................................................................................A-1
General safety requirements.....................................................................................................A-1
Electromagnetic compatibility (EMC) .................................................................................A-2
Electromagnetic effects...................................................................................................A-2
Safety checks for software..........................................................................................................A-2
Specifications.................................................................................................................................................A-3
Circuitry................................................................................................................................................A-3
Audio indicators...............................................................................................................................A-3
Audible alarms.................................................................................................................................A-3
Alarm limits ..........................................................................................................................A-3
Displays.................................................................................................................................................A-4
Numeric display (Light-Emitting Diodes–LEDs) ..............................................A-4
Graphic display (Liquid Crystal Display–LCD)................................................A-4
Mode switch.......................................................................................................................................A-4
SpO2.......................................................................................................................................................A-4
Interfering substances.....................................................................................................A-4
Pulse rate..............................................................................................................................................A-5
PIr pulsatile value...........................................................................................................................A-5
Sensor emitter wavelength ranges........................................................................................A-5
Environmental...................................................................................................................................A-5
Electrical...............................................................................................................................................A-5
Battery.......................................................................................................................................A-5
Power........................................................................................................................................A-6
Current leakage...................................................................................................................A-6
Fuses..........................................................................................................................................A-6
Serial output, RS-232.....................................................................................................................A-6
Dimensions and weight ...............................................................................................................A-6
B/Communications
Serial device communications..............................................................................................................B-1
Requirements....................................................................................................................................B-1
RS-232 interface cable—serial pinout...................................................................................B-2
Connection..........................................................................................................................................B-2
Serial communication output................................................................................................................B-3
Auto-output mode............................................................................................................................B-3
Trend-output mode......................................................................................................................... B-4
Warranty
iii
Page 6

List of Figures
Name Page
Figure 1-1. Signal processing block diagram ...........................................................................1-3
Figure 1-2. Comparative light absorption...................................................................................1-4
Figure 1-3. Extinction versus wavelength graph....................................................................1-4
Figure 1-4. 3800 Pulse Oximeter front panel............................................................................1-6
Figure 1-5. 3800 Pulse Oximeter rear panel..........................................................................1-11
Figure 2-1. Typical adult plethysmographic waveform .....................................................2-8
Figure 2-2. Typical neonate plethysmographic waveform...............................................2-8
Figure 2-3. Low perfusion waveform............................................................................................2-8
Figure 2-4. Noisy plethysmographic waveform ......................................................................2-9
iv
Page 7

1/Overview
This chapter
• Introduces the product, including the principles of its operation.
• Describes the oximeter’s controls and features.
• Lists the precautions you must take when using the oximeter.
Product description
The Datex-Ohmeda Model 3800 pulse oximeter with TruTrak
features two easy-to-read displays that present patient data and status information.
• The numeric display shows the SpO2 and pulse rate values.
• The graphic display shows the plethysmographic waveform, messages, the
Relative Perfusion Index (PIr™) pulsatile value, and the high and low alarm
limit settings for SpO2 and pulse rate.
Intended use
The 3800 pulse oximeter with TruTrak+ technology is indicated for spot-checking
and continuous monitoring of functional oxygen saturation and pulse rate,
including monitoring during conditions of clinical patient motion.1 This device is
intended for use with adult, pediatric, and neonatal patients in both hospital and
non-hospital environments.
Important: Only Datex-Ohmeda OxyTip
TruTrak+ technology
TruTrak+ technology improves pulse oximetry performance during conditions of
clinical patient motion. In the clinical environment, oximetry readings are affected
by several types of patient motion. The types of motion include clenching,
pressing, and rubbing as well as extending, flexing, and kicking. Unlike motion
technologies that use only a single method to correct for motion, TruTrak+ selects
one of many proprietary motion-correction algorithms, depending on the type and
intensity of the motion.
®
+ technology
®
+ sensors can be used with this monitor.
TruTrak+ technology employs a patented five-step process that consists of
1) high-speed data sampling; 2) motion identification, quantification, and
1
Anesthesia & Analgesia. 2002;94,1S, S54-S60
1-1
Page 8
3800 User’s Manual
PIr pulsatile value
correction; 3) calculation of the SpO2 value; 4) weighting and averaging of the
SpO2 value; and 5) the display of an improved SpO2 value. The result of this
process is a more accurate and stable displayed SpO2 value, with fewer false
alarms or dashed displays.
Important: For TruTrak+ performance, the averaging mode must be set to Long.
See Setup in chapter 2.
The PIr pulsatile value indicates the strength of the pulse signal at the sensor site:
the higher the PIr value, the stronger the pulse signal. A strong pulse signal
increases the validity of SpO2 and pulse rate data.
PIr is a relative value that varies from patient to patient. Clinicians can use the PI
value to compare the strength of the pulse signal at different sites on a patient in
order to locate the best site for the sensor (the site with the strongest pulse signal).
You can choose to display or not display the PIr value (see Setup in chapter 2).
Other features
• PerfTrak® waveform display, an automatic scale of the plethysmographic
• Large SpO2 digital display for clear differentiation from the pulse rate value.
• Backlit display and contrast control for excellent visibility in subdued lighting
• Direct access to user-selectable high and low alarm limits for SpO2 and pulse
• An audible pulse indicator with an adjustable volume; the automatic pitch
• Visual and audible (adjustable volume) alarms.
• An alarm-silence feature that silences audible alarms for 120 seconds.
• An all-mute feature that silences audible alarms until deactivated.
• Automatic tiered alarm messages.
• Short, medium, or long SpO2 response averaging modes.
• Adult or neonatal patient modes for default pulse rate alarm settings.
• Automatic storage of up to 12 hours of SpO2, pulse rate, and alarm limit
• An automatic self-test and calibration check at start-up. After start up, the
• Rechargeable, sealed, lead-acid battery operation, including battery status
r
waveform to provide a relative indication of the sensor site perfusion level.
conditions; adjustable viewing angle, using the pull-down feet under the
monitor.
rate.
modulation reflects changing SpO2 level.
violations data in trend memory, which can be output through the RS-232
serial connector.
oximeter continuously performs background self-tests.
reporting.
1-2
Page 9
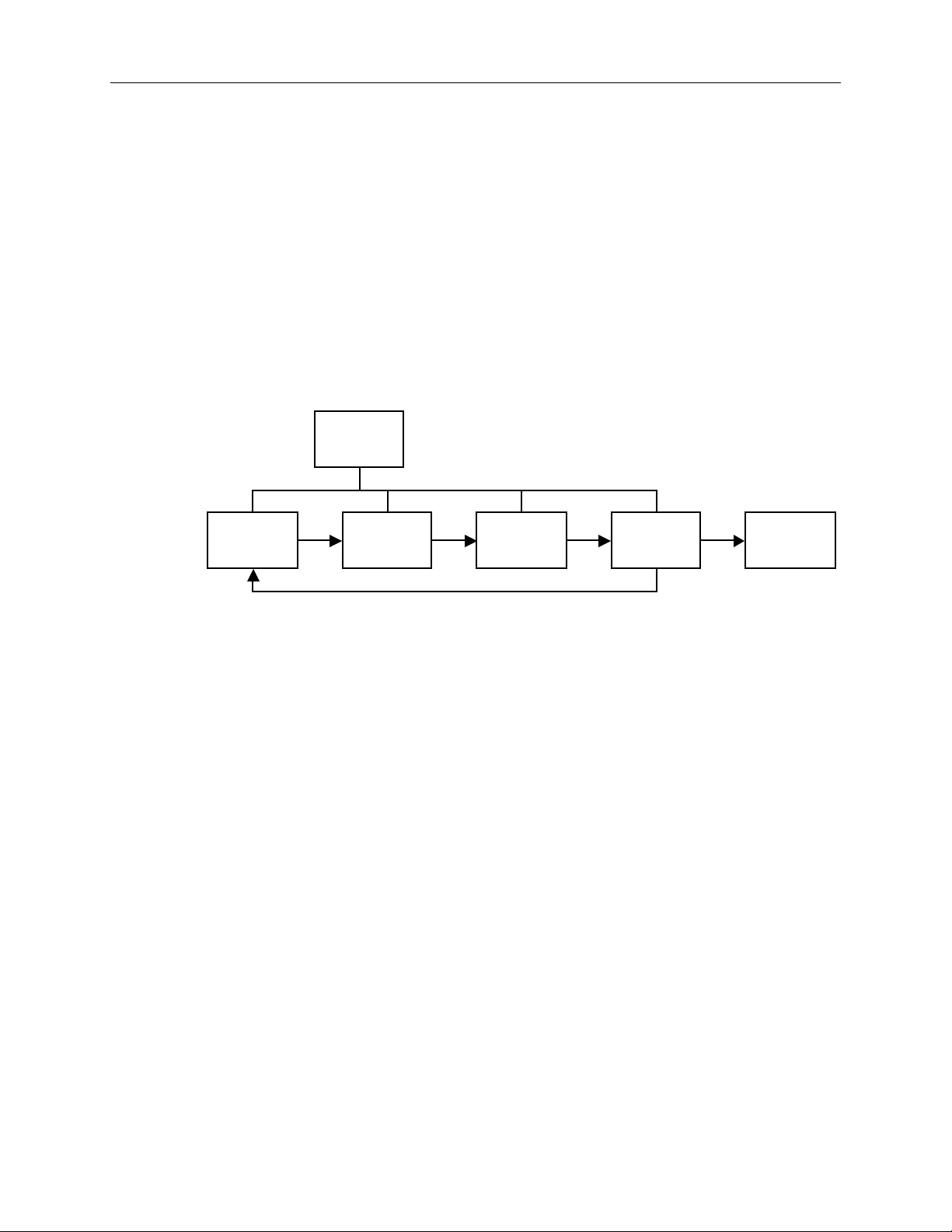
Functional components
The 3800 oximeter uses the following key electrical component elements to
determine SpO2, pulse rate, and PIr pulsatile values:
• The sensor
• Sensor-signal processing
• Microprocessor calculations
The sensor consists of
• The light source—red and infrared light-emitting diodes (LEDs)
• The photodetector—an electronic device that produces an electrical current
proportional to incident light intensity
Timing
Control
1/Overview
Sensor
Figure 1-1. Signal processing block diagram
The two light wavelengths generated by the LEDs pass through the tissue at the
sensor site. The photodetector collects this light (partially absorbed and modulated) and converts it into an electronic signal that is sent to the oximeter for
further processing.
The electronic circuitry receives the photodetector’s electronic signal, processes it,
and passes it on to the microprocessor for calculation of the SpO2, pulse rate, and
PIr pulsatile value.
Analog
Processing
A/D
Converter
Digital
Processing
Input/
Output
1-3
Page 10
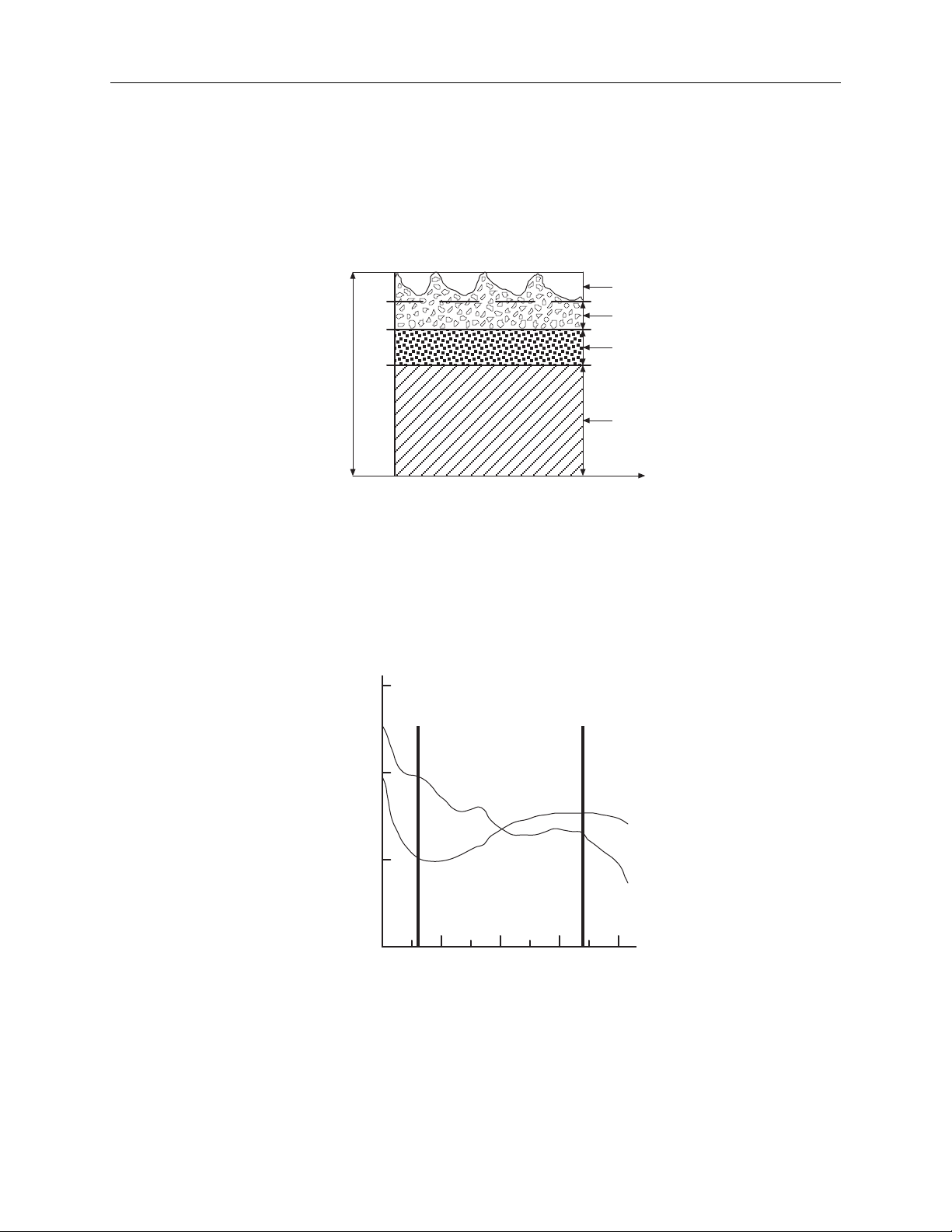
3800 User’s Manual
T
Principles of operation
The 3800 pulse oximeter uses a two-wavelength pulsatile system—red and infrared
light—to distinguish between oxygenated (O2Hb) and reduced (HHb) hemoglobin,
each of which absorbs different amounts of light emitted from the oximeter
sensor. The system then calculates the relative percentage of these two
constituents and displays SpO
.
2
Variable absorption
(due to arterial pulse)
Arterial blood absorption
Venous blood absorption
Absorption
Other tissue absorption
ime
Figure 1-2. Comparative light absorption
Arterial blood pulsation at the test site modulates transmission of the oximeter
sensor’s light. Since other fluids and tissues present generally don’t pulsate, they
don’t modulate the light passing through that location. The attenuation of light
energy due to arterial blood flow is detected and isolated by using the pulsatile
portion of the incoming signal. PIr pulsatile value is a measure of the relative size
of this portion of the signal.
1
(Red)
660 nm
0
(Infrared)
940 nm
Extinction (10x)
O2Hb
-1
HHb
-2
600 700 800 900 1000
Wavelength (nm)
Figure 1-3. Extinction versus wavelength graph
The sensor’s photodetector converts the light, which is partially absorbed and
modulated as it passes through the tissue sample, into an electronic signal. Since
O2Hb and HHb allow different amounts of light to reach the photodetector at the
selected wavelengths, the electronic signal varies according to the light source that
1-4
Page 11

1/Overview
is “on” and the oxygenation of the arterial hemoglobin. Analog and digital signal
processing then converts the light-intensity information into SpO2, pulse rate, and
PIr pulsatile values for display on the monitor.
Calibration
A CO-oximeter typically uses four or more wavelengths of light and calculates
reduced hemoglobin (HHb), oxyhemoglobin (O2Hb), carboxyhemoglobin (COHb),
and methemoglobin (MetHb). Datex-Ohmeda pulse oximeters use two
wavelengths ranges, 650 nm - 665 nm and 930 nm - 950 nm, both with an average
power of less than 1 mW. These wavelengths are used to calculate the presence of
O2Hb and reduced HHb. Because of this, pulse oximetry readings will be
different than CO-oximetry readings in situations where a patient’s COHb or
MetHb are increased.
Two different methods of calibration are currently used by manufacturers of pulse
oximeters: fractional and functional.
Important: This pulse oximeter uses the functional calibration method. The user
cannot change the calibration method to fractional.
• Fractional saturation is represented mathematically as the percentage of the
total amount of hemoglobin carrying oxygen. It is determined by dividing the
oxyhemoglobin by the total hemoglobin.
Fractional SpO2 = x 100 = x 100
• Functional saturation is represented mathematically as the percentage of
hemoglobin capable of carrying oxygen that is carrying oxygen.
Functional SpO2 = x 100 = x 100
The calculation of SpO2 assumes 1.6% carboxyhemoglobin (COHb), 0.4%
methemoglobin (MetHb), and no other pigments. Appreciable variation from these
values will influence SpO2 accuracy. These values are based on the DatexOhmeda Pulse Oximeter Empirical Calibration Study.
O2Hb
Hb
Hb
TOTAL
O2Hb
- COHb - MetHb O2Hb + HHb
TOTAL
O2Hb + HHb + COHb + MetHb
O2Hb
O2Hb
1-5
Page 12

3800 User’s Manual
Front panel
11
10
12
+
♥
–
+
–
9
PI
1.25
r
8
3
Figure 1-4. 3800 Pulse Oximeter front panel
1 Alarm silence button
2 Numeric display (LED)
3 Graphic display (LCD)
4 SpO2 alarm limits, high/low setting buttons
5 Pulse rate alarm limits, high/low setting buttons
6 Display contrast adjust slide
7 Power/Standby button
8 Carrying handle
9 Sensor connector
10 Pulse beep volume button
11 Alarm volume button
3800
TruTrak®
85
130
40
SpO
–
+
♥
2
–
+
4
5
6
7
1-6
Page 13

1/Overview
1 Alarm silence button
This button has two functions:
• 120-second alarm silence—activated by a single press.
• Continuous all mute—activated by three quick presses (if the all-mute
feature is enabled). Press once to deactivate.
Alarm silence
When an active alarm condition exists, press this button to silence the audible
portion of the alarm for 120 seconds. The flashing red or yellow alarm light
becomes a steady light. If an alarm condition still exists after 120 seconds, the
audible tone and flashing light resume.
Exceptions: Both NO SENSOR and SENSOR OFF audible alarms will not be
activated until after the unit obtains a valid signal. The same conditions apply
to an active audible alarm for NO SENSOR, SENSOR OFF, or SENSOR
FAILURE that has been silenced; i.e., once the sensor alarm condition is
acknowledged by silencing the audible alarm, a new audible alarm will not
sound until the condition has been cleared and the unit obtains a valid signal.
NOTE: Pressing the alarm silence button produces 120 seconds of silence,
regardless of other alarm conditions that may occur during this 120-second
interval, except for the SYSTEM FAILURE, CONNECT UNIT TO LINE
POWER, and BUTTON STUCK alarms.
All mute
To continuously silence any alarm that can be silenced, press the alarm
silence button three times within three seconds. After you have activated all
mute, the all mute icon flashes between the SpO2 and pulse rate alarm limit
settings on the right side of the screen display. When an alarm condition
occurs, the alarm button light flashes and the alarm message appears on the
waveform display but no audible alarm sounds.
When all mute is active, press the alarm silence button once to deactivate this
feature and enable all audible alarms.
2 Numeric display
SpO2 numeric area—calculated SpO
Pulse rate numeric area—calculated pulse rate
2
1-7
Page 14

3800 User’s Manual
3 Graphic display
21
85
3
PI
r
4
1 Plethysmographic waveform
2 Message area
3 SpO2 and pulse rate alarm limits
1.25
The PerfTrak waveform display appears after the monitor has detected
data from the sensor. It represents the blood volume change of the
hemodynamic system, assuming no other factors (e.g., motion artifact) are
present. This waveform scales automatically to the perfusion level or
strength of the signal being received at the patient monitoring site.
Status and alarm messages appear above the waveform area on the
waveform display (the height of the waveform is reduced while messages
are displayed). Status messages give you information about the oximeter's
operating condition. Alarm messages alert you to conditions that need
your attention. See chapter 3 for complete alarm and status message
information.
The high and low alarm limit settings appear here. If a limit is set to OFF,
three dashes appear in the location for that limit.
When an SpO2 or pulse rate limit is violated, the LED on the numeric
display and the LCD’s limit value flashes for that alarm.
130
40
1-8
SpO
4 PIr pulsatile value
Dashes (- - -) appear if the following conditions exist: no sensor is
connected to the unit, the sensor is not attached to the patient, the sensor
has failed, there is insufficient light penetrating the tissue site, or there is
too much ambient light.
4 SpO2 alarm limits, high and low
2
+
–
The top button sets the high alarm limit and the bottom button sets the low
alarm limit. For either limit, press the + side of the button to raise the value or
the – side to lower it. As you press one of these buttons, the values do not
cycle through the available settings; e.g., when you reach 100, the value does
not cycle (or wrap) to 50 or OFF.
Page 15
♥
y
1/Overview
5 Pulse rate alarm limits, high and low
+
–
The top button sets the high alarm limit, the bottom button sets the low alarm
limit. For either limit, press the + side to raise the value or the – side to lower
it. As you press one of these buttons, the values do not cycle through the
available settings; e.g., when you reach 235, the value does not cycle (or wrap)
to 30 or OFF.
6 Display contrast adjuster
Use this sliding lever to adjust the vertical viewing angle of the graphic
display Slide the lever to the left to reduce the contrast and to the right to
increase it.
7 Power/Standby button/AC power light
This button toggles between On (operational mode) and Off (standby mode).
The battery recharges as long as the unit is plugged into the AC power supply.
No displays are visible in the Off/Standby mode.
The green light to the right of the button is lit when the unit is connected to
an AC power supply.
85
130
40
Battery operation
The oximeter runs for at least five and one-half hours on a new, fully charged
battery at normal operating temperatures. LOW BATTERY appears when
between 5 and 15 minutes of battery operation time remain. Plug the
monitor into AC power to continue monitoring and recharge the battery. The
unit will operate with a dead or defective battery when it is connected to the
AC power supply.
When the CONNECT UNIT TO LINE POWER message appears, you must
immediately plug the oximeter into the AC power supply or the unit turns
itself off after 10 seconds.
When operating on battery power, an icon appears between the two pairs of
alarm limit values on the right side of the LCD. This icon indicates the batter
condition as follows:
Charged/not low
Low
If the all mute condition exists, the display of this icon alternates with the
display of the all mute icon.
This icon appears on the status screen:
Depleted, not installed, or defective
8 Carrying handle
The lower front portion of the oximeter’s case is designed to be a carrying
handle for ease of moving the unit from one place to another.
1-9
Page 16

3800 User’s Manual
9 Sensor connector
The sensors for this oximeter plug into this nine-contact connector. Use only
Datex-Ohmeda sensors compatible with this oximeter (see Parts list in
chapter 4).
♥
+
–
+
–
10 Pulse beep volume button
This button adjusts the volume level for the pulse indicator in incremental
steps from OFF to level 5 (default is 2). Press the + side of the button to
increase the volume or the – side to decrease it; you will hear the volume
level as you press the button. As you press one of these buttons, the values do
not cycle through the available settings; e.g., when you reach 5, the value does
not cycle (or wrap) to OFF.
As you adjust the volume, the volume setting is shown in the message area
above the waveform.
NOTE: The pitch of the pulse tone changes as the SpO2 value increases or
decreases—the higher the SpO2 value, the higher the pitch of the pulse tone.
11 Alarm volume button
This button adjusts the audible alarm volume level in incremental steps from
1 to 5 (default is 3). You cannot set the alarm volume to OFF. Press the + side
of the button to increase the alarm volume or the – side to decrease it; you
will hear the volume level as you press the button. As you press one of these
buttons, the values do not cycle through the available settings; e.g., when you
reach 5, the value does not cycle (or wrap) to 1.
As you adjust the volume, the volume setting is shown in the message area
above the waveform.
1-10
Page 17

Rear panel
1/Overview
100 – 240V~ 50–60 Hz 0.45 A T2.0AH/250V
Mode Switch
1315 West Century Drive
Louisville, CO 80027-9560 USA Made in USA
12 3 45
Figure 1-5. 3800 Pulse Oximeter rear panel
WARNING: Electrical shock hazard. Because the unit is not grounded when it is operating on
battery power, do not connect any equipment to the RS-232 connector on the rear panel
unless the unit is connected to the AC power supply.
RS 232
1 Power entry module
This module contains
• Fuses
• Power connector for the power cord that connects the oximeter to the
AC power supply for continuous operation and/or battery recharging.
2 Equipotential ground connector
In locations where this type of ground is required, connect your grounding
system here.
Mode Switch
3 Product information label
The following symbols may appear on the monitor, on labels affixed to the
monitor, and/or on shipping materials. Refer to Compliance with standards in
Appendix A for definitions of symbols that indicate compliance with
standards set by regulatory agencies.
CAUTION: US Federal law restricts this device to sale by or on
the order of a licensed medical practitioner. Outside the USA,
check local laws for any restriction that may apply.
The serial number for this product appears as SN AAAYxxxxx.
The first three letters are specific to the product type, the fourth
letter indicates the year of manufacture (F=2002, G=2003, etc. “I”
and “O” are not used.). The last five digits are the sequential
number for the unit as produced in the indicated year.
G
The warranty expiration date for this product is printed near
this symbol.
4 Mode Switch
This symbol identifies the two-position switches that set the display language,
the averaging mode, the patient mode, the display of the PIr pulsatile value,
and the EMI line frequency. See chapter 2 for instructions.
1-11
Page 18

3800 User’s Manual
RS 232
Precautions
Warnings
5 RS-232 serial connector
This 9-pin connector provides serial digital information on SpO2, pulse rate,
alarm limit violations, and alarm messages. It is compatible with most RS-232
devices capable of accepting a 9600 baud input. See Appendix B for
instructions.
Two types of precautions appear in this manual: warnings and cautions.
• A WARNING indicates the possibility of injury to the patient or operator.
• A CAUTION indicates a condition that may lead to equipment damage or
malfunction.
Failure of operation
If the oximeter fails any part of the checkout procedures or current leakage test,
remove it from operation until qualified service personnel have corrected the
situation.
It is possible for any device to malfunction; therefore, always verify unusual data
by performing a formal patient assessment.
Explosion hazard
Do not use the monitor in the presence of any flammable anesthetic mixture.
Electrical shock and flammability hazard
Before cleaning or servicing the oximeter, always turn it off and disconnect the
power cord from the AC power supply.
Electrical shock hazard
Do not remove the monitor cover. An operator may perform only maintenance
procedures specifically described in this manual. Refer servicing to qualified
service personnel trained in the repair of this equipment.
Measure the oximeter’s leakage current whenever an external device is connected
to the RS-232 port. Forward and reverse polarity = 100 microamperes maximum.
This equipment must be properly grounded.
• Electrical safety specifications (e.g., current leakage and ground resistance) can
be assured only when the monitor is connected to a three-wire, grounded
receptacle without the use of extension cords or adapters.
• If there is any doubt about the integrity of the AC power supply protective
earth conductor, operate the monitor on internal battery power.
• Because the unit is not grounded when it is operating on battery power, do not
connect any equipment to the RS-232 connector on the rear panel unless the
unit is connected to the AC power supply.
1-12
Page 19

1/Overview
Data validity
Conditions that may cause inaccurate readings and impact alarms include
interfering substances, excessive ambient light, electrical interference, excessive
motion, low perfusion, low signal strength, incorrect sensor placement, poor sensor
fit, and movement of the sensor on the patient.
To prevent erroneous readings, do not use an inflated blood pressure cuff or arterial
blood pressure measurement device on the same limb as the oximeter sensor.
Patient safety
The correct use of the oximeter is to measure only arterial oxygen saturation
(SpO2), pulse rate, and Relative Perfusion Index pulsatile value.
• A pulse oximeter does not measure respiration and should never be used as a
substitute for an apnea monitor or as the primary monitor for infants being
monitored for apnea.
• A pulse oximeter may be used during sleep studies of adults only to gather
information regarding SpO2, pulse rate, and PIr pulsatile value.
This device is not intended for use in a magnetic resonance imaging (MRI)
environment.
Patient safety (sensors)
Patient conditions (such as reddening, blistering, skin discoloration, ischemic skin
necrosis, and skin erosion) may warrant changing the sensor site frequently or
using a different style of sensor.
To prevent patient injury or equipment damage, use only Datex-Ohmeda oximeter
sensors approved for use with this oximeter. For complete information about the
safe and appropriate use of a sensor, consult the instructions for that sensor.
Discard a damaged sensor immediately. Do not repair a damaged sensor or use a
sensor repaired by others.
RS-232 system interconnection
Accessory equipment connected to the RS-232 serial connector must be certified
according to the current version of the respective IEC/EN standards (e.g., IEC
60950 for data processing equipment and IEC/EN 60601-1 for medical equipment).
All configurations shall also comply with IEC/EN 60601-1-1. Anyone who connects
additional equipment to the RS-232 serial connector configures a medical system,
and is therefore responsible that the system complies with the requirements of
IEC/EN 60601-1-1. If in doubt, call your local authorized service office, as listed on
the back cover of this manual. The 3800 is referred to as an IEC/EN 60601/F
device in the summary of situations table contained in IEC/EN 60601-1-1.
1-13
Page 20

3800 User’s Manual
Cautions
Handle the monitor with care
Cleaning
Battery
Improper handling can cause damage or inaccurate results.
Do not autoclave, pressure sterilize, or gas sterilize the oximeter.
Use cleaning solution sparingly. Do not soak or immerse the monitor in liquid.
Excessive solution can flow into the monitor and damage internal components.
When cleaning the display area, do not use abrasive cleaning compounds or other
materials that could damage the screen.
Do not use petroleum-based solutions, acetone solutions, or other harsh solvents to
clean the oximeter. These substances may damage the oximeter and cause a
malfunction.
The 3800 internal battery, containing lead and acid, is a hazardous waste. Dispose
of the battery through an approved hazardous materials disposal facility or return
it to Datex-Ohmeda for disposal.
To prevent damage to the lead-acid battery, do not turn the monitor on after the
LOW BATTERY message appears without first plugging it in to the AC power
supply.
Sensors
Do not apply tension to the sensor cable; sensor damage may result.
Disposal
Dispose of this medical device and its packing materials according to local
requirements.
Miscellaneous
US Federal law restricts this device to sale by or on the order of a licensed
medical practitioner.
1-14
Page 21

2/Setup and Operations
This chapter provides the following information and instructions:
• Powering the oximeter.
• Selecting the language, averaging mode, patient mode, PIr pulsatile value
display, and EMI (electromagnetic interference) line frequency.
• Checkout procedure—to determine that all functions of the oximeter are
working properly.
• Signal and data validity guidelines.
• Trend data.
To operate the oximeter effectively, you must
• Know how the oximeter derives its readings (see Principles of operation in
chapter 1).
• Be familiar with its controls and components (see chapter 1).
• Understand its messages (see chapter 3).
Powering the oximeter
The 3800 pulse oximeter is designed to operate on battery power and on all
commonly available voltage supplies. Your oximeter was shipped with the correct
power cord for your local AC power supply. Any hospital-grade power cord,
however, with the female connector end that fits into the power module (IEC-320
type) on the 3800 can be used; the male connector that plugs into the grounded
“wall” outlet may be whatever is needed locally. The oximeter accepts a range of
AC mains power; see Appendix A for details.
To protect data validity in cases of possible electromagnetic interference, make
sure the EMI line frequency mode switch is set to the same frequency as your
local AC power supply before using the unit for patient monitoring; see EMI line
frequency under Mode switch settings later in this chapter.
A battery does not need to be installed for the oximeter to operate on the AC
power supply.
2-1
Page 22

3800 User’s Manual
Setup
Factory settings and default settings
Before powering on the oximeter
When you turn on the oximeter, the following settings are in effect and remain in
operation until you change them.
Use the mode switches in the oximeter’s rear panel to set the language, averaging
mode, patient mode, PIr pulsatile value display, and EMI line frequency.
Parameter Factory Setting Range
Language English English, French, German,
Italian, Japanese, Portuguese,
Spanish, or Swedish
Averaging mode
Patient mode Adult Adult or Neonate
PIr pulsatile value display Yes Yes (display PIr value) or No
EMI line frequency 60 Hz 50 Hz or 60 Hz
Long / TruTrak+
(12 seconds)
Long / TruTrak+ (12 seconds),
Medium (6 seconds) or
Short (3 seconds)
After powering on the oximeter
Changes you make to the parameters shown below take effect immediately.
Parameter Default Setting Range
High SpO2 limit OFF
(appears as: – – –)
Low SpO2 limit 85% 50% to 100% or OFF
High pulse rate 130 bpm*
(adult mode)
200 bpm
(neonatal mode)
Low pulse rate 40 bpm
(adult mode)
50% to 100% or OFF
30 to 235 bpm or OFF
30 to 235 bpm or OFF
2-2
100 bpm
(neonatal mode)
Alarm volume 3 1 to 5
Pulse volume 2 1 to 5 or OFF
* bpm= beats-per-minute
Page 23

Mode switch settings
A bank of eight numbered, two-position switches is accessed through the rear
panel. The up position is ON and the down position is OFF.
2/Setup and Operations
=
Important: If you change the switch settings while the oximeter is on, the new
settings do not take effect until you power off, then on again.
=
Language
Switches 1, 2, and 3 set the language used for the display and data transmitted
through the RS-232 port. (For Japanese, data transmissions are in English only.)
Switches Language
123
English
123
French
123
German
123
Italian
123
Japanese
123
123
123
Portuguese
Spanish
Swedish
2-3
Page 24

3800 User’s Manual
Averaging mode
Switches 4 and 5 set the averaging mode. The averaging mode selects the time
period of data used to calculate a weighted average SpO2 value to be displayed by
the oximeter.
Switches SpO2 and Pulse Rate Averaging
45
45
45
45
Long (12 seconds) Yes (enabled)
Medium (6 seconds) No
Short (3 seconds) No
Patient mode
Switch 6 sets the patient mode.
Switch Patient mode Low pulse rate alarm limit High pulse rate alarm limit
6
Adult 40 bpm 130 bpm
6
Neonate 100 bpm 200 bpm
PIr pulsatile value display
Switch 7 sets the display of the PIr pulsatile value.
TruTrak+
2-4
Switch Display PIr value
7
Yes
7
No
EMI line frequency
Switch 8 sets the EMI line frequency. To optimize EMI (electromagnetic
interference) immunity, make sure switch 8 is in the correct position for the AC
power line frequency in use.
Switch EMI Line Frequency
8
60 Hz
8
50 Hz
Page 25

Checkout procedure
WARNING: Failure of operation. If the oximeter fails any part of the checkout procedures or
current leakage tests, remove it from operation until qualified service personnel have
corrected the situation.
WARNING: Explosion hazard. Do not use the monitor in the presence of any flammable
anesthetic mixture.
WARNING: Electrical shock hazard. This equipment must be properly grounded.
• Electrical safety specifications (e.g., current leakage and ground resistance) can be
assured only when the monitor is connected to a three-wire, grounded receptacle without
the use of extension cords or adapters.
• If there is any doubt about the integrity of the AC power supply protective earth conductor,
operate the monitor on internal battery power.
• Because the unit is not grounded when it is operating on battery power, do not connect
any equipment to the RS-232 connector on the rear panel unless the unit is connected to
the AC power supply.
2/Setup and Operations
If you plan to send serial data to another device, make sure the connection
between the device and the rear panel connector is made before you power on
the monitor and make sure the monitor is connected to the AC power supply.
Important: For TruTrak+ performance, the averaging mode must be set to Long.
1. Inspect the oximeter case for damage. Make sure the display windows are
clean.
WARNING: Sensors
• Discard a damaged sensor immediately. Do not repair a damaged sensor or use a
sensor repaired by others.
• To prevent patient injury or equipment damage, use only Datex-Ohmeda oximeter
sensors approved for use with this oximeter. For complete information about the safe
and appropriate use of a sensor, consult the instructions for that sensor.
CAUTION: Do not apply tension to the sensor cable; sensor damage may result.
2. Check that the sensor is a compatible model before connecting it to the
oximeter. Only Datex-Ohmeda OxyTip+ sensors can be used with this monitor.
If you’re using a reusable sensor, make sure it opens and closes smoothly.
Remove substances that may interfere with the transmission of light between
the sensor's light source and detector.
3. Connect the sensor cable to the sensor connector on the monitor. Make sure it
is a firm connection and that the cable is not twisted, sliced, or frayed.
4. Attach the sensor to a finger or an ear, depending on the sensor you are using.
2-5
Page 26

3800 User’s Manual
5. To turn on the oximeter, press the power button.
The first screen shows the Datex-Ohmeda logo and the model name
(Model 3800).
The next screen shows the averaging mode in effect, the patient mode, the
progress of the self-test, and the status of the battery charge.
Averaging Mode: Long
Patient Mode: Adult
Self-test in progress …
(indicates battery-charge status)
Below the bar graph, the version number of the unit’s system and oximetry
software appears as Version X.XXX/YY.YYY, where X’s represent the system
software version and Y’s the oximetry software version.
Diagnostic self-test
During this time, the system performs a diagnostic self-test (electronics,
battery status, analog signal path integrity, calibration check) and sets the
default parameters. This self-test takes approximately 10 seconds.
• A start-up tone sequence tests the audio circuit; all display LEDs and
the LCD backlight are illuminated, then blanked.
• The alarm LED toggles between red and yellow while a numeric
countdown from 9 to 0 occurs on each seven-segment LED display
ending with a decimal point.
• A battery icon is displayed to indicate the battery condition as either
charged, depleted, or defective/missing (see chapter 3).
Upon successful completion of all diagnostic self-tests, the unit is
considered to be in calibration and begins normal operation. This message
is displayed:
Test passed. In calibration.
If the unit does not pass the self-test, an error message is displayed and the
unit is inoperable.
6. On the displays, verify
• The high and low alarm limits for SpO2 and pulse rate.
• Dashes (– – –) appear for any limit set to OFF.
• The readings for SpO2, pulse rate, and PIr pulsatile value.
Dashes may appear on the display until the SpO2, pulse rate, and PI
pulsatile value readings have stabilized (approximately 12 seconds).
r
2-6
Page 27

2/Setup and Operations
NOTE: The audible alarm feature for all alarm conditions is silenced for the first
two minutes after powering on.
7. If two minutes have elapsed since you powered on, verify that the patient
alarms are functional by setting the high and low SpO2 and pulse rate alarm
limits beyond the current readings. Make sure
• An alarm tone sounds.
• The violated alarm limit and reading flash on the display.
• Depending on the priority of the alarm, a red or yellow alarm light flashes.
8. Verify the sensor alarms are functional by removing the sensor from the
sensor site. Make sure
• SENSOR OFF or CHECK SENSOR SITE appears in the message area of
the graphic display.
• The alarm tone sounds; the alarm light flashes.
9. Unplug the sensor from the oximeter. Make sure
• NO SENSOR appears.
• The alarm tone sounds; the alarm light flashes.
10. Press the alarm silence button. Make sure
• The alarm tone ceases.
• The alarm light is steady.
11. To begin patient monitoring, connect the desired Datex-Ohmeda sensor to the
oximeter. Attach that sensor to the patient.
To verify the sensor is on correctly and that the data are verifiable, see Signal and
data validity in this chapter.
WARNING: Patient safety. Patient conditions (such as reddening, blistering, skin discoloration,
ischemic skin necrosis, and skin erosion) may warrant changing the sensor site frequently or
using a different style of sensor.
2-7
Page 28

3800 User’s Manual
Signal and data validity
Plethysmographic waveform
The oximeter’s PerfTrak waveform display provides a visual indicator of the
validity of the values that appear on the display. The waveform is scaled to
correspond to the perfusion level or strength of the signal being received at the
patient monitoring site.
NOTE: When a message appears in the upper portion of the LCD, the waveform
rectangle becomes smaller but the correspondence between signal strength and
waveform height is maintained.
You should be able to easily identify three complete passes of the
plethysmographic waveform. Although the waveform shape may vary from patient
to patient, under normal conditions it corresponds to the arterial pressure
waveform. Use Figures 2-1 (adult) and 2-2 (neonate) as guidelines to determine a
sensor placement that generates the fewest noise spikes.
Figure 2-1. Typical adult plethysmographic waveform
Figure 2-2. Typical neonate plethysmographic waveform
The “typical” neonate waveform differs from that of an adult, including the
absence of a dicrotic notch (a notch on the descending limb of the normal arterial
pulse tracing that corresponds to aortic valve closure).
Low perfusion
As the perfusion at the patient monitoring site decreases, so will the height of the
waveform. (The PIr pulsatile value is a numeric representation of the relative
height of the waveform.) The height will decrease to the point where the signal
quality becomes too small or too poor for accurate, reliable readings. At that point,
the message CHECK SENSOR SITE appears in the message area and an alarm is
generated. The waveform will be similar to Figure 2-3.
CHECK SENSOR SITE
2-8
Figure 2-3. Low perfusion waveform
Page 29

2/Setup and Operations
Signal noise
The following conditions can cause noisy waveforms:
• Poor sensor placement.
• Motion at the sensor site.
• Electrical interference.
Figure 2-4. Noisy plethysmographic waveform
If three easily identifiable passes of a “typical” waveform do not occur,
• Make sure the sensor’s detector is flush with the sensor site (for sensor
application, see the instructions for the sensor you are using).
• Make sure the light source and detector are directly opposite each other.
• Select a site where the distance between the light source and the detector is
less.
• Make sure the patient site is stable; minimize movement of the sensor site.
• Massage the sensor site with a 70% isopropyl alcohol pad or rubefacient cream
(10-30% methyl salicylate and 2-10% menthol) for 20-30 seconds. Strong
vasodilator creams, such as nitroglycerin paste, are not recommended.
• If possible, remove electrical noise sources such as electrosurgery or
electrical/electronic devices (e.g., fans). If these solutions are not possible,
operate the oximeter on battery power, or try plugging the oximeter into a
different electrical outlet.
• If artificial nails or excessive fingernail polish are present, select another site
or remove the polish/artificial nails.
Numeric display
SpO
2
Stability of the SpO2 readings is a good indicator of signal validity. Although
“stability” is a relative term, with practice you’ll get a good feeling for changes that
are artifactual or physiological and the speed of each. The stability of the readings
over time is affected by which averaging mode you're using. In Long / TruTrak+
mode (12-second averaging), the readings tend to be more stable because the
signal is averaged over a longer period of time than the Short (3 seconds) or
Medium (6 seconds) modes.
Too great a distance between the sensor emitter and detector can reduce signal
strength and result in a poor signal. When the value is too low, the message
CHECK SENSOR SITE may appear to alert you that the SpO2 value may not be
accurate. Perfuse the sensor site or relocate the sensor to a site with higher blood
flow.
2-9
Page 30

3800 User’s Manual
Pulse rate
PIr pulsatile value
Trend data
Compare the displayed pulse rate to the patient’s palpated pulse rate. If the unit’s
rate varies significantly from the palpated rate, the data may be less accurate due
to motion artifact or other noise.
A cough or other hemodynamic pressure disturbance can disrupt the pulse rate,
which is determined from the plethysmographic waveform. The time span
between the waveform’s peaks determines the pulse rate. The unit uses the same
averaging mode (long, medium, or short) as that selected for SpO2.
The PIr pulsatile value is a measurement of the strength of the photoplethysmographic signal read by the oximeter. The greater the number, the greater the
pulsatility and the validity of the SpO2 and pulse rate data. This value is useful
when determining that the sensor is correctly attached and that the data are
verifiable. It is also an indicator of relative perfusion at the sensor site.
The oximeter stores a maximum of 12 hours of trend data. The process maintains
the lowest SpO2 value that occurs during each 6-second interval along with the
corresponding pulse rate, and highest-priority error message and all alarm limit
violations. You can access the trend data stored in the 3800 through the serial port
on the rear of the oximeter. See Appendix B for details.
The trend data are maintained as long as the unit’s battery is connected and
charged to minimum operating level.
To clear trend data:
1. Hold down the alarm silence button while you power on the oximeter.
2. At the Clear Trend Data? prompt,
• To select YES to clear all data from the trend buffer, press the high SpO
button (+ or –).
• To select NO to retain the data, press the low pulse rate limit button (+ or –).
If you don’t press either button within 10 seconds, the trend data are retained and
the unit proceeds with the power-on sequence.
2
2-10
Page 31

3/Messages and Troubleshooting
This chapter contains
• Descriptions of the messages and indicators that appear on the screen.
• Alarm categories and their characteristics.
• A chart for troubleshooting situations that may occur while using the oximeter.
Become thoroughly familiar with this information before using the oximeter to
monitor a patient.
Messages
The pulse oximeter acknowledges your actions and the monitor’s conditions by
displaying messages in the waveform screen message display area. Alarm
messages appear when any alarm condition occurs.
The following chart alphabetically lists the messages that may appear on the
oximeter, why the message appears, and the action(s) to take if the message
indicates a problem.
Message Possible cause(s) Recommended action(s)
The all mute feature is activated. No action required. (Press the
Appears between the alarm
limit settings.
Indicates a fully charged battery.
alarm silence button once to
deactivate.)
No action required.
Appear on status screen
during power-on sequence
and on right side of LCD
during battery operation.
Appears on status screen
during power-on sequence.
Appears in message area on
waveform screen.
Indicates a low-charged battery.
Indicates battery failure, or a
depleted or missing battery.
The alarm or pulse tone volume
is being adjusted.
To recharge, plug the unit into AC
mains power.
To recharge, plug the unit into AC
mains power. If the condition
persists, the unit requires service.
No action required. To adjust
audio volume, see Front panel in
chapter 1.
3-1
Page 32

3800 User’s Manual
y
Message Possible cause(s) Recommended action(s)
AMBIENT LIGHT Excessive ambient light. Relocate the sensor to a site more
BUTTON STUCK Appears if something is pressing
against the buttons on the
monitor.
shielded from light or reduce the
amount of light shining on the
sensor.
Make sure nothing is pressing
against the front of the unit.
Appears when the last button
you pressed has not released
properly or has been pressed for
more than 30 seconds.
CHECK SENSOR SITE Appears when SpO2 readings ma
be invalid due to motion, an
unacceptable sensor site, poor
placement, low perfusion, or
because the sensor is off the
patient.
Clear Trend Data?
YES/NO
CONNECT UNIT TO LINE POWER The battery needs immediate
INSUFFICIENT LIGHT Dirt on the sensor emitter or
Appears when the alarm silence
button is held down while you’re
turning on the oximeter.
recharging.
detector. Sensor detector failure.
Press that button again or turn the
power off and then on. If the
condition persists, the unit
requires service.
For all causes, reposition or
relocate the sensor, and/or
increase perfusion (see the sensor
user instructions).
Press the high SpO2 alarm limit
button (+ or –) to clear trend data
from memory (YES).
Press the low pulse rate alarm
limit button (+ or –) to retain trend
data in memory (NO).
If you take no action within 10
seconds, the trend data are
retained in memory.
Plug the oximeter into the AC
power supply; otherwise the unit
will turn itself off in 10 seconds.
Clean the sensor (if reusable) or
replace it.
3-2
Test site dirty. Misaligned or
poorly positioned sensor.
Insufficient light penetrating the
tissue site. Dark pigmentation.
Fingernail polish present.
Clean the test site. Reposition the
sensor or select another test site.
Reposition the sensor or select
another test site.
Remove polish or select another
test site.
Page 33

Message Possible cause(s) Recommended action(s)
INTERFERENCE DETECTED Appears when the signal Is too
erratic to be processed due to
proximity of other electrical
equipment generating highfrequency electromagnetic noise.
LOW BATTERY Appears when 5 to 15 minutes of
battery operation remain.
LOW QUALITY SIGNAL
(appears only in serial
communication output)
NO SENSOR
(also see
CHECK SENSOR SITE)
Sensor off patient.
Perfusion not sufficient for valid
readings. Motion at sensor site,
electrical noise, or incorrect
sensor placement.
Sensor not connected or not fully
inserted into the sensor
connector.
3/Messages and Troubleshooting
No action required. May be
caused by strong radio frequency
(RF) interference possibly
generated by electrosurgery.
SpO2 and PR readings do not
change during detected interference (or become dashes if
interference persists). When
interference ceases, signal
processing resumes.
Plug the oximeter into the AC
power supply to recharge the
battery and continue monitoring.
Important: To prevent permanent
damage to the battery, recharge a
discharged battery within eight
hours after the LOW BATTERY
message is displayed.
Reattach the sensor.
Check patient and oximeter setup.
Insert sensor cable into the
connector.
May be an incorrect sensor.
SENSOR FAILURE The connected sensor is not an
OxyTip+ sensor.
Oximeter can’t identify the
connected sensor.
Broken sensor cable wire,
inoperative LEDs, or faulty
detector; the sensor has failed.
SENSOR OFF Sensor off patient. Reattach the sensor.
SYSTEM FAILURE #XXX:
SERVICE UNIT
TruTrak+ OFF TruTrak+ technology is not
An internal component of the
unit has failed. XXX represents
the error code.
active; the averaging mode is not
set to Long.
Refer to the instructions for the
sensor you are using.
Connect a Datex-Ohmeda
OxyTip+ sensor.
Replace sensor. Refer to the
instructions for the sensor you are
using.
Replace sensor.
Unit requires service.
For TruTrak+ performance, set the
averaging mode to Long (see
Setup in chapter 2).
3-3
Page 34

3800 User’s Manual
Alarm categories
3800 oximeter tiered alarms fall into three priority categories: high, medium and
low. Depending on what is occurring at the time, an alarm may fall into more
than one category.
NOTE: The audible alarm feature for all alarm conditions is silenced for the first
two minutes after powering on.
High priority
Requires immediate operator response.
Red alarm button light flashes.
Two five-tone sequences (beep-beep-beep, beep-beep) sound every 10 seconds
until the condition is removed or the alarm is silenced
• A violation of the low or high SpO2 limit (violated limit flashes).
• BUTTON STUCK
• CONNECT UNIT TO LINE POWER
If the following alarms occur during active monitoring, they also fall into the high
priority category.
• AMBIENT LIGHT
• CHECK SENSOR SITE
• INSUFFICIENT LIGHT
• NO SENSOR
• SENSOR FAILURE
• SENSOR OFF
3-4
NO SENSOR and SENSOR OFF alarm conditions are not active until after the
oximeter displays an initial valid reading.
Medium priority
Requires prompt operator response.
Yellow alarm button light flashes.
One three-tone sequence (beep-beep-beep) every 20 seconds until the condition is
removed or the alarm is silenced.
• A violation of the low or high pulse rate limit (violated limit flashes).
• INTERFERENCE DETECTED
• LOW BATTERY
Page 35

Low priority
3/Messages and Troubleshooting
If the following alarms occur before active monitoring, these alarms are
considered to be of medium priority:
• AMBIENT LIGHT
• CHECK SENSOR SITE
• INSUFFICIENT LIGHT
• NO SENSOR
• SENSOR FAILURE
• SENSOR OFF
Requires operator awareness.
Yellow alarm button light illuminates continuously.
One tone (beep) sounds; no repetition.
• INTERFERENCE DETECTED
Requires operator awareness.
Yellow alarm button light illuminates continuously.
• LOW QUALITY SIGNAL
System failure
A special category of alarms exists for system failure and the imminent failure of
pulse oximeter operation.
Requires immediate operator response.
Red alarm button light continuously flashes.
A continuous tone sounds; overrides all mute condition.
• SYSTEM FAILURE #XXX, SERVICE UNIT
For more information, refer to the 3800/3900/3900P Technical Reference Manual.
3-5
Page 36

3800 User’s Manual
Troubleshooting
The following chart list some conditions that may occur with the oximeter along
with the cause(s) and recommended action(s) for correcting them.
Condition Possible cause(s) Recommended action(s)
Unit does not power on. The battery is fully discharged or
disconnected and/or the unit is
not plugged into the AC power
supply.
To charge the battery and begin
monitoring, plug the unit into the
AC power supply.
If the condition persists, the unit
requires service.
One or both of the fuses have
blown.
Unit powers on but the
graphic display is blank.
Continuous speaker tone. Internal failure. Unit requires service.
Buttons don’t work when
pressed.
Unit doesn’t beep when
powered on.
Dashed display, waveform
may appear erratic; various
alarm messages
The viewing contrast is not
correct.
Internal failure. Unit requires service.
Disconnected or failed speaker. Unit requires service.
Sensor failure. Replace sensor.
Replace the fuse(s). See Replacing
the fuses in chapter 4.
If the new fuse blows shortly after
installation, the unit requires
service.
Use the display contrast adjust
slide to adjust the viewing angle.
If the condition persists, the unit
requires service.
See CHECK SENSOR SITE under
Messages in this chapter.
3-6
Page 37

4/Maintenance and Service
This chapter covers
• Maintenance procedures:
Cleaning the oximeter, as necessary.
Recharging the battery, as necessary.
Replacing the fuses in the power module, as necessary.
• The Datex-Ohmeda repair policy.
• A list of items you may order for the oximeter.
Cleaning
To clean a reusable sensor, refer to the instructions for the sensor.
Oximeter
WARNING: Electrical shock and flammability hazard. Before cleaning the oximeter, always turn
it off and disconnect the power cord from the AC power supply.
CAUTION:
• Do not autoclave, pressure sterilize, or gas sterilize this oximeter.
• Use cleaning solution sparingly. Do not soak or immerse the monitor in liquid. Excessive
solution can flow into the monitor and damage internal components.
• When cleaning the display area, do not use abrasive cleaning compounds or other materials
that could damage the screen.
• Do not use petroleum-based solutions, acetone solutions, or other harsh solvents to clean
the oximeter. These substances may damage the oximeter and cause a malfunction.
Be sure that the oximeter is turned off and unplugged from the AC power supply
before cleaning and that the unit is completely dry before use.
To clean the display panel, use a cotton swab moistened with 70% isopropyl
alcohol and gently wipe the panel.
4-1
Page 38

3800 User’s Manual
To clean the outer surface of the oximeter, use a soft cloth dampened with a mild
soap and water solution or one of the following solutions:
70 vol% isopropyl or ethyl alcohol
quaternary ammonia
3 vol% hydrogen peroxide in water
100:1 bleach solution
Cidex® plus activator (ready solution contains 2 vol% glutaraldehyde)
Recharging the battery
The oximeter's internal battery (a sealed pack of 8V lead-acid batteries) provides
the following operation times when it is new, used at normal temperatures, and
charged to full capacity: at least 5 -1/2 hours of continuous operation.
A LOW BATTERY message appears when 5 to 15 minutes of battery operation
remain. When the alarm message CONNECT UNIT TO LINE POWER appears
during operation on battery power, an audible alarm sounds and the oximeter
automatically shuts off in approximately 10 seconds.
Important: To prevent permanent damage to the battery, recharge a discharged
battery within eight hours after the LOW BATTERY message is displayed.
To recharge the battery, plug the oximeter into the AC power supply. The
oximeter is operational while recharging the battery.
The battery charging times are approximately
• 4 hours for 80% battery capacity.
• 8 hours for 100% battery capacity.
Under normal conditions, the battery lasts for several hundred charge/discharge
cycles. The battery will not overcharge.
Batteries stored for extended periods of time should be recharged every six
months to maintain the charging capacity of the battery.
Replacing the battery
If the battery will no longer recharge with AC power connected, it should be
replaced. Battery replacement should be performed by authorized service
personnel only.
4-2
Page 39

Replacing the fuses
Should a power problem blow one or both of the fuses in the power input module
on the rear panel, you’ll need to replace them.
Tool required
Small flat-blade screwdriver, 5 mm (3/16 inch)
WARNING: To protect against fire hazard, replace only with fuses of the same type and voltage
rating.
1. Power off the oximeter and unplug the power cord from the back of the
oximeter.
2. Insert the small flat-blade screwdriver into the center slot of the fuse holder.
Gently pry loose and remove the fuse holder.
Fuse holder
4/Maintenance and Service
3. Note how the fuses are placed in the fuse holder for installation of the new
fuses.
Fuses
4. To remove the fuses from the fuse holder, use the edge of the screwdriver
blade to pry against the bottom of the metal portion of the fuse where it is
secured to the glass portion of the fuse.
5. Place the new fuse(s) (T2.0AH/250V) in the fuse holder, properly orienting
them as shown above.
6. Slide the fuse holder back into the power entry module and press firmly to
make sure it is fully inserted.
NOTE: If the fuses blow shortly after replacement, the unit requires service.
4-3
Page 40

3800 User’s Manual
Repair policy and procedure
Contact Datex-Ohmeda or your authorized service office (see the back cover of this
manual) to order parts or for assistance.
Do not use malfunctioning equipment. Have the unit repaired by Datex-Ohmeda.
After repair, perform the Checkout procedure (in chapter 2) to verify the unit is
fully functional.
WARNING: Do not remove the cover of the monitor. An operator may only perform
maintenance procedures specifically described in this manual. Refer servicing to qualified
service personnel trained in the repair of this equipment.
Packaging and return procedure
If you are instructed to ship the monitor to Datex-Ohmeda or to an authorized
service office for repair, follow these steps:
1. Clean the monitor. Make sure it is completely dry before you pack it for
shipment.
2. Package the monitor carefully for shipment (in the original shipping container
if possible).
3. You may be required to enclose the following items (when you call for
assistance, verify the shipping requirements):
• A letter describing the problem in detail.
• Person (name, telephone/fax number, and country) to contact for
questions about necessary repairs.
• Ship-to and bill-to information.
• Purchase order number for tracking purposes or to cover repairs if the
oximeter is not under warranty.
4. Ship the monitor as directed by your service office.
4-4
Page 41

Parts list
4/Maintenance and Service
Description REF
Sensors
Refer to the sensor chart that accompanies this manual for a
list of the sensors you can use with the 3800. Only Datex-
Ohmeda OxyTip
Battery pack 6050-0004-277
Carrying case, 3800 6050-0004-610
3800 User’s Manual
Danish
+ sensors can be used with this monitor.
English
Finnish
French
German
Italian
Japanese
Polish
Portuguese
Spanish
Swedish
6050-0006-382
6050-0006-380
6050-0006-384
6050-0006-386
6050-0006-388
6050-0006-390
6050-0006-392
6050-0006-394
6050-0006-396
6050-0006-398
6050-0006-400
3800/3900/3900P Technical Reference Manual, English
Service kit, PCA drawings
Power cord
Socket Type: Commonly Used In:
Australia, China
Canada, Japan, Latin America, USA
Continental Europe
Italy
United Kingdom
6050-0006-404
6050-0006-476
6030-0000-001
0208-0943-300
6030-0000-006
6030-0000-002
6050-0002-259
4-5
Page 42

3800 User’s Manual
4-6
Page 43

A/Compliance and Specifications
This chapter contains
• Information about the tests that were conducted and the regulations with
which the oximeter complies to assure its safe use.
• Performance specifications for the oximeter.
Compliance with standards
The presence on the monitor of any symbol described below indicates
compliance with the standard represented by that symbol.
Medical Device Directive 93/42/EEC of the European Union for a
0197
class I (with a measuring function), IIa, IIb, or III device.
Medical electrical equipment classified in the US and Canada
with respect to electric shock, fire, and mechanical hazards only,
in accordance with the Canadian Standards Association
CAN/CSA C22.2 No. 601.1 and Underwriters Laboratories Inc.
UL 2601-1.
General safety requirements
The 3800 complies with the requirements of IEC/EN 60601-1 Part 1: General
requirements for safety of medical electrical equipment.
Type BF applied part.
Type of protection against electric shock: Class I/Internal electrical power source
Degree of protection against ingress of liquids: Ordinary (IPX0)
Mode of operation: Continuous
The monitor also complies with the following:
EN 865 Pulse oximeters – Particular requirements
EN 475 Medical devices – Electrically-generated alarm signals
A-1
Page 44

3800 User’s Manual
Electromagnetic compatibility (EMC)
Electromagnetic effects
The 3800 complies with the requirements of IEC/EN 60601-1-2: Electromagnetic
compatibility – Requirements and tests.
Emissions IEC/EN 55011 Group I, Class B
The 3800 pulse oximeter was tested with an RS-232 cable attached when
operating on AC power. It was tested with no peripheral devices when operating
on battery power.
When installing and using this monitor, take precautions to ensure electromagnetic
compatibility. For more information, refer to the 3800/3900/3900P Technical
Reference Manual.
Electromagnetic interference, including interference from portable and mobile
radio frequency (RF) communications equipment, can affect this monitor.
Indications that the 3800 is experiencing electromagnetic interference include the
following:
• Variations in the PerfTrak waveform display.
• Sudden increases or decreases in the waveform height that do not correlate to
the physiological condition of the patient.
• Sensor-related messages that are not resolved by the instructions found in this
manual.
• The display of dashes on numeric LEDs when a valid physiological signal is
present.
This interference may be intermittent and careful correlation between the effect
and its possible source is important. Indications of interference should not occur if
the monitor is used within its intended electromagnetic environment.
Safety checks for software
The Datex-Ohmeda software design controls include performance of a risk
analysis using methods consistent with EN 1441 Medical devices – Risk analysis.
To ensure proper operation of the software, the 3800 employs three separate
watchdog circuits for the microprocessors, power-on self-tests (including memory
checksum and calibration verification), and memory tests during monitoring. The
software continuously monitors the patient sensor and, if a failure is detected,
discontinues power to the sensor.
A-2
Page 45

Specifications
Circuitry
Audio indicators
A/Compliance and Specifications
Unless otherwise indicated, all specifications are nominal and are subject to
change without notice.
Microprocessor-controlled
Automatic self-test of oximeter when powered on
Automatic setting of default parameters
Automatic alarm messages
Trend data output of SpO2, pulse rate, and alarm messages via RS-232 serial
port—up to 12 hours of stored data
Adjustable-volume audible pulse
Adjustable-volume audible alarm tone
Pitch modulation reflects changing SpO2 levels
Alarm silence (120 seconds); all mute (continuous silence)
Pulse rate out-of-limits alarm
SpO2 level out-of-limits alarm
Sensor-condition alarms
System-failure and recharge-battery alarms
Audible alarms
Setting levels available:
Intensity at 1-meter distance:
Alarm limits
SpO2 alarm limit range:
Pulse rate alarm limit range in beats per minute (BPM):
Alarm: 1 through 5
Pulse beep: OFF and 1 through 5
Volume setting of 1: 55 decibels (minimum)
Volume setting of 5: 85 decibels (maximum)
High = 50 to 100% or OFF
Low = OFF or 50 to 100%
High = 30 to 235 or OFF
Low = OFF or 30 to 235
A-3
Page 46

3800 User’s Manual
Displays
Numeric display (Light-Emitting Diodes–LEDs)
Graphic display (Liquid Crystal Display–LCD)
The displayed SpO2, pulse rate, and PIr values are updated every second. The
plethysmographic waveform sweep is updated every 4 seconds.
Arterial oxygen saturation (SpO2) reading
Pulse-rate reading
Plethysmographic waveform
PIr pulsatile value
High and low SpO2 alarm limits settings
High and low pulse rate alarm limits settings
Sensor condition alarms
System operational status messages
Alarm messages
Contrast adjustment for best viewing
Mode switch
SpO
2
Interfering substances
Language: English (factory setting), French, German, Italian, Japanese, Portuguese,
Spanish, or Swedish
Averaging mode: Long / TruTrak+ (12 seconds–factory setting),
Medium (6 seconds), or Short (3 seconds)
Patient mode: Adult (factory setting) or Neonate
PIr pulsatile value display: Yes (factory setting) or No
EMI line frequency: 60 Hz (factory setting) or 50 Hz
Range: 0 to 100%
Accuracy, A
70 to 100% ± 2 digits
70 to 100% ± 3 digits during conditions of clinical patient motion
(with TruTrak+ enabled)
Below 70% unspecified
Resolution: 1%
Carboxyhemoglobin may erroneously increase readings. The level of increase is
approximately equal to the amount of carboxyhemoglobin present. Dyes, or any
substances containing dyes, that change usual arterial pigmentation may cause
erroneous readings.
(previously represented by 1 Standard Deviation):
rms
A-4
Page 47

Pulse rate
Range: 30 to 250 bpm
Accuracy assuming a constant pulse rate: ± 2% or ± 2 bpm (whichever is greater)
Accuracy during conditions of clinical patient motion: unspecified
Resolution: 1 bpm
PIr pulsatile value
Range: 0.00 to 9.99
Averaging interval: 12 seconds
Resolution: 0.01
Sensor emitter wavelength ranges
Red LED peak wavelength range: 650 to 665 nm
Infrared (IR) LED peak wavelength range: 930 to 950 nm
Average power: ≤ 1 mW
A/Compliance and Specifications
Environmental
Parameter Operating Transport and Storage
Temperature 0 to 50 ºC
Relative humidity 5% to 95% noncondensing 5% to 95% condensing
Pressure
Approximate elevation
Electrical
Battery
Type: pack of 8-volt, sealed lead-acid
Capacity: 3.2 ampere hours
Operation time for a new battery at normal operating temperatures: at least 5 1/2
Low battery indicator (LOW BATTERY): when the battery has between 5 and 15
Charge time:
Life: several hundred charge/discharge cycles
–40 to 70 ºC
(32 to 122 ºF)
106 to 47 kPa
–378 to 6000 m
(–1240 to 19,686 ft.)
hours (with all functions operative from a fully charged battery)
minutes remaining capacity
4 hours = 80% capacity
8 hours = 100% capacity
(–40 to 158 ºF)
106 to 47 kPa
–378 m to 6000 m
(–1240 to 19,686 ft.)
A-5
Page 48

3800 User’s Manual
Power
Current leakage
Fuses
Serial output, RS-232
Consumption (typical): 15 watts
Input voltage range: 90 to 264 VAC at 47-63 Hz
Current (typical): 0.45 A(rms) at 100 V, 0.37 A(rms) at 120 V,
0.25 A(rms) at 220/ 230/240 V
With power on, forward or reverse polarity: 100 microamperes maximum
Ground resistance: less than 0.1 Ω
T2.0AH/250V, 5 mm (OD) x 20 mm (length)
Data output every 2 seconds (auto-output mode) or 6 seconds (trend-output mode):
SpO2, pulse rate, alarm limit violation messages, and displayed alarm/error
messages.
9600 baud
Full duplex
Number of bits per character: 8
Parity: none
Bits: 1 start, 1 stop
Handshaking: CTS/RTS
Connector type: 9-pin standard D, female
Connector pin functions:
2 = oximeter receives data
3 = oximeter transmits data
5 = signal ground
7 = RTS
8 = CTS
Dimensions and weight
Height: 9.4 cm (3.7 in.)
Width: 24.4 cm (9.5 in.)
Height: 22.5 cm (8.9 in.)
Weight: 2.9 kg (6.5 lb.)
A-6
Page 49

B/Communications
This appendix covers serial device connections for computer/oximeter
communication.
WARNINGS: Electrical shock hazard
• Measure the oximeter’s leakage current whenever an external device is connected to the
RS-232. Forward and reverse polarity: 100 microamperes maximum.
• Because the unit is not grounded when it is operating on battery power, do not connect
any equipment to the RS-232 connector on the rear panel unless the unit is connected to
the AC main power supply.
WARNING: RS-232 system interconnection. Accessory equipment connected to the RS-232
connector must be certified according to the current version of the respective IEC/EN
standards (e.g., IEC 60950 for data processing equipment and IEC/EN 60601-1 for medical
equipment). All configurations shall also comply with IEC/EN 60601-1-1. Anyone who connects
additional equipment to the RS-232 connector configures a medical system, and is therefore
responsible that the system complies with the requirements of IEC/EN 60601-1-1. If in doubt,
call your local authorized service office, as listed on the back cover of this manual. The 3800 is
referred to as an IEC/EN 60601/F device in the summary of situations table contained in
IEC/EN 60601-1-1.
Serial device communications
Requirements
Connect the oximeter only to computers with
• An RS-232 interface.
• The ability to accept ASCII-formatted data at a baud rate of 9600.
The settings on the computer must be:
9600 baud
8-bit data
No parity
1 stop bit
1 start bit
Full duplex
Handshaking, CTS/RTS
B-1
Page 50

3800 User’s Manual
RS-232 interface cable—serial pinout
Important: Use only a cable designed to interface directly between your
computer’s connector and the RS-232 connector on the oximeter.
Configure the RS-232 interface cable as follows:
Connection
RS 232
Pins 1, 4, 6, and 9 are not used.
To acquire trend data on the computer, the computer must be running a thirdparty communications program, such as ProComm™ or Microsoft Windows
Terminal™, set to receive at 9600 baud.
Pin 2 Oximeter receives data
1
Pin 3 Oximeter transmits data
Pin 5 Signal ground
Pin 7 RTS
Pin 8 CTS
Equipment needed
• A board for the computer that supports serial communication with the same
serial port connections as the oximeter.
• A male (DB-9P) to female (DB-9P) interface cable with the proper pin
connections.
• A third-party communication program.
Procedure
1. Locate the serial connector on the rear panel of the oximeter and connect the
male end of the RS-232 interface cable to it.
2. Locate the RS-232 interface connector on the rear panel of the computer and
connect the female end of the RS-232 interface cable to it.
3. Make sure that the RS-232 interface cable is securely connected on both ends.
4. Make sure the computer’s communication program is running and ready to
receive data at the correct baud rate.
B-2
Page 51

Serial communication output
The oximeter is capable of two-way communication with computers:
• Auto-output mode (default)—current data.
• Trend-output mode—trend data stored in the oximeter's memory.
To use these modes,
• The oximeter must be connected to the computer.
• The computer must be running a communications program.
SpO2, pulse rate, and alarm conditions are transmitted and updated every two
seconds in auto-output mode or every six seconds in trend-output mode. One line
of ASCII data is output to the computer every two seconds in auto-output mode, or
every six seconds in trend-output mode.
The information sent to the computer screen is formatted similarly to the
following example:
Model 3800 Pulse Oximeter
TREND DATA OUTPUT
6 SECONDS PER DATA POINT
: 1 ** POWER ON **
: 2 SpO2= --- PR= --: 3 SpO2= --- PR= --- SENSOR OFF
: 4 SpO2= 95 PR= 0 LOW PR
: 5 SpO2= 95 PR= 0 LOW PR
: 6 SpO2= 95 PR= 93
: 7 SpO2= 95 PR= 92
: 8 SpO2= 94 PR= 96 HIGH PR
: 9 SpO2= 89 PR= 96 LOW SPO2, HIGH PR, BUTTON STUCK
: 10 SpO2= 92 PR= 100 HIGH PR, BUTTON STUCK
: 11 SpO2= 95 PR= 99
: 12 SpO2= 95 PR= 98
: 13 SpO2= --- PR= --- NO SENSOR
:
::
END TREND DATA
B/Communications
Auto-output mode
This is the default mode, which transmits monitoring data being currently
collected. It is present when the oximeter begins communication with a computer,
and is the mode the oximeter returns to when exiting from other modes.
Messages relating to SpO2, pulse rate, and alarm limits violations that appear on
the oximeter also appear on the computer.
B-3
Page 52

3800 User’s Manual
Trend-output mode
This mode allows up to 12 hours of trend data to be output to a computer.
NOTE: 12 hours of trend data are output in approximately 5 minutes.
To enter trend-output mode:
Enter: <esc> CJ <enter>
While trend data are being output, messages that appear on the oximeter do not
appear on the computer but they are stored in trend.
To exit trend-output mode while trend data are being output:
Enter: <esc> CK <enter>
After trend data are output, auto-output mode automatically resumes. Trend data
are still in memory and can be output again without turning the oximeter off and
on again.
NOTE: No other modes can be activated through the computer interface while in
trend-output mode; only the trend output exit command is recognized.
To clear-trend data in the oximeter’s memory using the computer:
Enter: <esc> CP <enter>
B-4
Page 53

Warranty
The 3800 Pulse Oximeter (the product) is sold by Datex-Ohmeda, Inc. only under the warranties
set forth in the following paragraphs. Such warranties are extended only with respect to the
purchase of the product directly from Datex-Ohmeda Authorized Dealers as new merchandise
and are extended to the first Buyer thereof, other than for resale.
Limited warranty
Datex-Ohmeda warrants that the product meets the published specifications at the time of
shipment from the factory.
Products not under warranty
The following items are not covered under this warranty: disposable items, accessories, service
kits, and replacement parts. These items may be covered under a separate warranty. Consult
Datex-Ohmeda for details.
Duration
The product is warranted against defects in materials and workmanship for a period of three (3)
years from the date of delivery to the user (in no event for a period of more than four [4] years
from the date of original delivery by Datex-Ohmeda to an Authorized Dealer). The battery is
warranted against defects in materials and workmanship for one (1) year from the date of delivery
to the user.
If any part of the product proves defective under proper and normal use within the warranty
period, as the purchaser's exclusive remedy, Datex-Ohmeda will repair or replace, at its sole
discretion, the product or any defective part provided it is returned to Datex-Ohmeda Service
within 30 days of the failure.
Limitation
Datex-Ohmeda may at any time discharge its warranty obligation by repairing and returning the
product to original factory performance. This may be accomplished by installing new or
remanufactured assemblies or by other repairs deemed appropriate by Datex-Ohmeda. The choice
of repair or replacement by Datex-Ohmeda shall be the sole remedy of the buyer or user.
Conditions
This warranty is valid only when qualified personnel have performed installation and service on
the product and when all recommended planned maintenance procedures have been completed
during the warranty period. Damage caused by the abuse or misuse of the product is not covered
by this warranty. Datex-Ohmeda shall not be liable for damage resulting from the improper
installation or the misuse of the product.
Exclusion of warranties
Oral statements about the product do not constitute warranties, shall not be relied on by the buyer
or user, and are not part of any warranty extended by Datex-Ohmeda.
Except as set forth in this limited warranty, Datex-Ohmeda makes no warranties, expressed or
implied, including the implied warranty of merchantability and the implied warranty of fitness
for a particular purpose. Except for the obligations under this limited warranty, Datex-Ohmeda
shall not have any obligation or liability for any incidental or consequential damages (including
those from commercial loss) or other loss, damage, or injury resulting directly or indirectly from
the product.
 Loading...
Loading...