Page 1

Mini-PROTEAN®3 Cell
Instruction Manual
Catalog Numbers
165-3301
165-3302
For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723)
Page 2

Table of Contents
Page
Section 1 General Information....................................................................................1
1.1 Introduction ................................................................................................................1
1.2 Components................................................................................................................2
1.3 Specifications .............................................................................................................4
1.4 Chemical Compatibility .............................................................................................4
1.5 Safety ..........................................................................................................................5
Section 2 Set Up and Basic Operation........................................................................5
2.1 Gel Cassette Preparation ............................................................................................5
2.2 Mini-PROTEAN 3 Cell Assembly and Sample Loading .........................................8
2.3 Gel Electrophoresis ....................................................................................................9
Section 3 Separation Theory and Optimization......................................................10
3.1 Introduction ..............................................................................................................10
3.2 SDS-PAGE (Laemmli) Buffer System....................................................................11
3.3 Native PAGE............................................................................................................12
Section 4 Reagent Preparation and Stock Solutions ..............................................13
4.1 Volumes Required Per Gel ......................................................................................13
4.2 SDS-PAGE (Laemmli) Buffer System....................................................................13
4.3 Discontinuous Native PAGE (Ornstein-Davis).......................................................15
4.4 Continuous Native PAGE ........................................................................................16
Section 5 References ...................................................................................................18
Section 6 Maintenance ...............................................................................................18
Section 7 Troubleshooting..........................................................................................19
Section 8 Product Information and Accessories .....................................................21
Section 9 Warranty Information...............................................................................23
Page 3

Section 1
General Information
1.1 Introduction
The Mini-PROTEAN 3 cell runs both hand cast gels and Ready Gel precast gels
interchangeably. The Mini-PROTEAN 3 system includes a casting stand and glass plates with
permanently bonded gel spacers that simplify hand casting and eliminate leaking during
casting. The cell can run one or two gels, and the mini tank is compatible with other Bio-Rad
electrode modules for tank blotting, 2-D electrophoresis, and electro-elution.
Fig. 1. Mini-PROTEAN 3 system components.
1
Page 4

2
1.2 Components
To get the best performance from your Mini-PROTEAN 3 cell, familiarize yourself with
the components by assembling and disassembling the cell before using it (refer to
Figures 1 and 2).
Spacer Plate The Spacer Plate is the taller glass plate with gel spacers
permanently bonded. Spacer Plates are available in 0.5 mm, 0.75 mm,
1.0 mm, and 1.5 mm thicknesses, which are marked directly on
each Spacer Plate.
Short Plate The Short Plate is the shorter, flat glass plate that combines with the
Spacer Plate to form the gel cassette sandwich.
Casting Frame The Casting Frame, when placed on the benchtop, evenly aligns
and secures the Spacer Plate and the Short Plate together to form the
gel cassette sandwich prior to casting.
Gel Cassette Assembly One Casting Frame, a Spacer Plate, and a Short Plate form one Gel
Cassette Assembly.
Casting Stand The Casting Stand secures the Gel Cassette Assembly during gel
casting. It contains pressure levers that seal the Gel Cassette
Assembly against the casting gaskets.
Gel Cassette Sandwich A Spacer Plate and Short Plate with polymerized gel form a Gel
Cassette Sandwich after casting.
Combs A selection of molded combs is available.
Buffer Dam The molded, one-piece buffer dam is used when running only one
gel.
Electrode Assembly The Electrode Assembly holds the Gel Cassette Sandwich. It houses
the sealing gasket, the upper and lower electrodes and the
connecting banana plugs. The anode (lower electrode) banana plug
is identified with a red marker and the cathode (upper electrode)
banana plug with a black marker.
Clamping Frame The Clamping Frame holds the Electrode Assembly and Gel
Cassette Sandwich in place. Its pressure plates and closure cams
seal the Gel Cassette Sandwich against U-shaped gaskets on the
Electrode Assembly to form the inner buffer chamber.
Inner Chamber The Electrode Assembly, two Gel Cassette Sandwiches or one gel
cassette sandwich and a buffer dam, and the Clamping Frame form
the Inner Chamber.
Mini Tank and Lid The Mini Tank and Lid combine to fully enclose the inner chamber
during electrophoresis. The lid cannot be removed without
disrupting the electrical circuit. The Mini Tank and Lid are also
compatible with other Bio-Rad electrode modules for blotting, first
dimension 2-D, and electro-elution.
Page 5
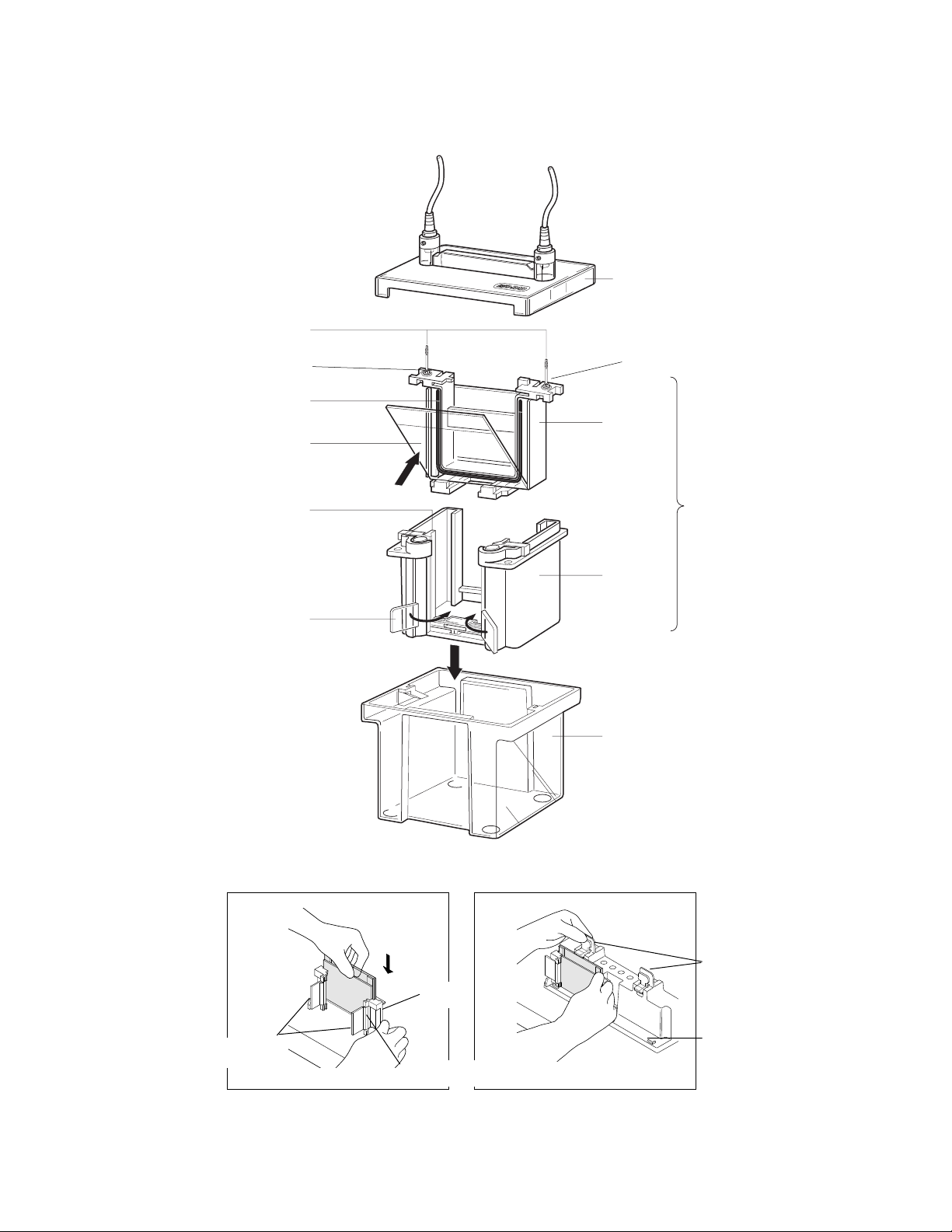
Fig. 2. Assembling the Mini-PROTEAN 3 cell.
Fig. 3. Assembling the Mini-PROTEAN 3 Casting Frame and Casting Stand.
3
Lid
Electrode
Assembly
Clamping
Frame
Mini Tank
Spring loaded levers
Casting Stand without
gaskets. Gaskets must be
used for proper seal.
Casting
Frame
Pressure cam
pivot point
Pressure cams in
"open position"
Inner
Chamber
Assembly
Cams
Pressure Plate
Gel Cassette
Sandwich
Notch
on U-Shaped Gasket
Banana Plugs
Anode banana plug
(red)
Cathode banana plug
(black)
Page 6
1.3 Specifications
Casting Stand* Polycarbonate
Pin, Retaining Ring, and Spring Stainless Steel
Casting Frames* Polysulfone
Gray Gaskets Silicone Rubber (gray)
Clamping Frame** Glass-filled liquid crystal polymer (Vectra
™
)
Pressure Plate and Cams Polycarbonate
Electrode Assembly Glass-filled liquid crystal polymer
Electrodes Platinum wire, 0.010 inches diameter
Gasket, electrode inner core Silicone Rubber (green)
Mini Tank and Lid Molded Polycarbonate
Sample Loading Guides
†
Delrin
™
Combs* Polycarbonate
Maximum Sample Volume Per Well
# wells Well width 0.5 mm 0.75 mm 1.0 mm 1.5 mm
5 12.7 mm — 70 µl 105 µl 160 µl
9 5.08 mm — 33 µl 44 µl 66 µl
10 5.08 mm 22 µl 33 µl 44 µl 66 µl
15 3.35 mm 13 µl 20 µl 26 µl 40 µl
IPG 76.2 mm — — 420 µl 730 µl
Prep/2-D
Reference well 3.1 mm — 13 µl 17 µl 30 µl
Sample well 71.7 mm — 310 µl 400 µl 680 µl
Overall Size of cell 16cm(L)x12cm(W)x18cm(H)
Gel Size 8 cm (W) x 7.3 cm (H)
Inner Plate 10.1 cm (W) x 7.3 cm (H)
Outer Plater 10.1 cm (W) x 8.3 cm (H)
Precast Gel Compatibility Ready Gels
Voltage Limit 600 VDC and 15 watts
Shipping Weight 2.0 kg
1.4 Chemical Compatibility
Mini-PROTEAN 3 components are not compatible with acetone, ethanol, or butanol. Use of
organic solvents voids all warranties. Call 1-800-4-BIORAD or your local Bio-Rad
representative for technical information regarding additional chemical compatibility of the
Mini-PROTEAN 3 cell with various laboratory reagents.
The Mini-PROTEAN 3 combs are not compatible with repeated exposure to 100%
TEMED. Rubbing the combs with TEMED prior to casting will destroy the structural
integrity of the combs over time.
* US patent No. 6,162,342
** US patent No. 5,632,877
†
US patent No. 5,656,145
4
Page 7

1.5 Safety
Power to the Mini-PROTEAN 3 cell is supplied by an external DC voltage power supply
(not included). The output of this power supply must be isolated from external ground to
insure that the DC voltage output floats with respect to ground. All Bio-Rad power supplies
meet this important safety requirement. Regardless of the power supply used, the maximum
specified operating parameters for the Mini-PROTEAN 3 cell are as follows:
• 600 VDC maximum voltage limit
• 15 watts maximum power limit
• 50 °C maximum ambient temperature limit
The current to the cell enters the unit through the lid assembly which provides a safety
interlock to the user. The current to the cell is broken when the lid is removed. Always turn
off the power supply before removing the lid. Do not attempt to use the cell without the
safety lid.
Important: This Bio-Rad product is designed and certified to meet *EN61010-1 safety
standards. Certified products are safe to use when operated in accordance with the instruction
manual. This instrument should not be modified or altered in any way. Alteration of this
instrument will
• Void the warranty
• Void the EN61010-1 certification, and
• Create a potential safety hazard.
Bio-Rad is not responsible for any injury or damage caused by use of this instrument for
purposes other than those for which it is intended or by modifications of the instrument not
performed by Bio-Rad or an authorized agent.
*
EN61010-1 is an internationally accepted electrical safety standard for laboratory instruments.
Section 2
Set Up and Basic Operation
2.1 Gel Cassette Sandwich Preparation
Hand Cast Gels
1. Glass Cassette and Casting Stand Assembly
Note: Ensure the casting stand, casting frames, and glass plates are clean and dry before
setting up the casting stand assembly. During regular use, a powder residue may build
up behind the pressure cams of the casting frame at the pivot point. This powder should
be removed before each use.
a. Place the Casting Frame upright with the pressure cams in the open position and
facing forward on a flat surface.
b. Select a Spacer Plate of the desired gel thickness and place a Short Plate on top of
it (see Figure 4a).
c. Orient the Spacer Plate so that the labeling is "up". Slide the two glass plates into the
Casting Frame, keeping the Short Plate facing the front of the frame (side with
pressure cams) (see Figure 4b).
Note: Ensure both plates are flush on a level surface and labeling on the Spacer Plate is
oriented correctly. Leaking may occur if the plates are misaligned or oriented incorrectly.
d. When the glass plates are in place, engage the pressure cams to secure the glass
cassette sandwich in the Casting Frame (see Figure 4c). Check that both plates are
flush at the bottom.
5
Page 8
e. Engage the spring loaded lever and place the gel cassette assembly on the gray cast-
ing stand gasket. Insure the horizontal ribs on the back of the Casting Frame are
flush against the face of the Casting Stand and the glass plates are perpendicular to
the level surface. The lever pushes the Spacer Place down against the gray rubber
gasket (see Figure 4d).
f. Repeat steps a–e for a second gel.
4a. Place a Short Plate on top of the 4b. Slide the two plates into the Casting
Spacer Plate. Frame keeping the Short Plate facing front.
4c. Lock the pressure cams to secure 4d. Secure the Casting Frame in the Casting
the glass plates. Stand by engaging the spring loaded lever.
Fig. 4. Assembling the Mini-PROTEAN 3 casting stand and frame.
2. Gel Casting
a. Discontinuous Polyacrylamide Gels
i. Place a comb completely into the assembled gel cassette. Mark the glass plate
1 cm below the comb teeth. This is the level to which the resolving gel is
poured. Remove the comb.
ii. Prepare the resolving gel monomer solution by combining all reagents except
APS and TEMED. (Refer to Section 4 for gel formulations.) Degas the
solution under vacuum for at least 15 minutes. Do not use a sink water aspirator.
iii. Add APS and TEMED to the degassed monomer solution and pour to the mark
using a glass or disposable plastic pipette. Pour the solution smoothly to
prevent it from mixing with air.
iv. Immediately overlay the monomer solution with water or t-amyl alcohol.
Note: If water is used, add it slowly and evenly to prevent mixing. Do not overlay
w/butanol or isobutanol.
v. Allow the gel to polymerize for 45 minutes to 1 hour. Rinse the gel surface
completely with distilled water. Do not leave the alcohol overlay on the gel
for more than 1 hour because it will dehydrate the top of the gel.
6
Page 9

Note: At this point the resolving gel can be stored at room temperature overnight. Add 5 ml
of a 1:4 dilution of 1.5 M Tris-HCl, pH 8.8 buffer (for Laemmli System) to the resolving
gel to keep it hydrated. If using another buffer system, add 5 ml 1x resolving gel buffer
to the resolving gel surface for storage.
vi. Prepare the stacking gel monomer solution. Combine all reagents except APS
and TEMED. Degas under vacuum for at least 15 minutes.
vii. Before casting the stacking gel, insert a piece of filter paper to dry the area in
between the glass plates above the resolving gel. Take care not to touch the
surface of the gel.
viii. Add APS and TEMED to the degassed stacking gel monomer solution and pour
the solution between the glass plates. Continue to pour until the top of the short
plate is reached.
ix. Insert the desired comb between the spacers starting at the top of the Spacer
Plate, making sure that the tabs at the ends of each comb are guided between the
spacers. It is easiest to insert the combs starting at an angle and insert well 1
first, then 2, 3, and so on until the combs is completely inserted. Seat the comb
in the gel cassette by aligning the comb ridge with the top of the Short Plate.
x. Allow the stacking gel to polymerize for 30–45 minutes.
xi. Gently remove the comb and rinse the wells thoroughly with distilled water or
running buffer.
xii. Rinse the Casting Frame(s) and Stand with distilled, deionized water after use.
b. Continuous Polyacrylamide Gels
i. Prepare the monomer solution by combining all reagents except the APS and
the TEMED. Degas under vacuum for 15 minutes (Refer to Section 4 for gel
formulations).
ii. Add APS and TEMED to the degassed monomer solution and pour the solution
between the glass plates. Continue to pour until the top of the Short Plate is reached.
iii. Insert the desired comb between the spacers starting at the top of the Spacer
Plate, making sure that the tabs at the ends of each comb are guided between the
spacers. It is easiest to insert the combs starting at an angle and insert well 1
first, then 2, 3, and so on until the combs is completely inserted. Seat the comb
in the gel cassette by aligning the comb ridge with the top of the Short Plate.
iv. Allow the gel to polymerize for 45 minutes to 1 hour.
v. Gently remove the comb and rinse the wells thoroughly with distilled water or
running buffer.
vi. Rinse the Casting Frame(s) and Stand with distilled, deionized water after use.
Ready Gel Precast Gels
1. Ready Gel Cassette Preparation
Note: The Mini-PROTEAN 3 cell is guaranteed for use only with Bio-Rad's Ready Gel
precast gels.
a. Remove the Ready Gel from the storage pouch.
b. Gently remove the comb and rinse the wells thoroughly with distilled water or
running buffer.
c. Cut along the dotted line at the bottom of the Ready Gel Cassette with a razor blade.
d. Pull the clear tape at the bottom of the Ready Gel Cassette to expose the bottom
edge of the gel.
e. Repeat for second Ready Gel.
Note: If only one gel is to be run, use the mini cell buffer dam.
7
Page 10

2.2 Mini-PROTEAN 3 Electrophoresis Module Assembly and
Sample Loading
Mini-PROTEAN 3 Electrophoresis Module Assembly
1. Remove the Gel Cassette Assemblies from the Casting Stand. Rotate the cams of the
Casting Frames inward to release the Gel Cassette Sandwich (see Figure 5a).
2. Place a Gel Cassette Sandwich into the slots at the bottom of each side of the Electrode
Assembly. Be sure the Short Plate of the Gel Cassette Sandwich faces inward toward the
notches of the U-shaped gaskets (see Figure 5b).
3. Lift the Gel Cassette Sandwich into place against the green gaskets and slide into the
Clamping Frame (see Figure 5c).
4. Press down on the Electrode Assembly while closing the two cam levers of the Clamping
Frame to form the Inner Chamber and to insure a proper seal of the short plate against the
notch on the U-shaped gasket. (see Figure 5d). Short plate must align with notch in gasket.
5a. Remove the Gel Cassette Sandwich 5b. Place Gel Cassette Sandwich into the Electrode
from the Casting Frame. Assembly with the Short Plate facing inward.
5c. Slide Gel Cassette Sandwiches and 5d. Press down on the Electrode Assembly
Electrode Assembly into the clamping while closing the two cam levers of the
frame. Clamping Frame.
5e. Lower the Inner Chamber into the Mini Tank.
Fig. 5. Mini-PROTEAN 3 assembly.
8
Page 11

Note: Gently pressing the top of the Electrode Assembly while closing the Clamping
Frame cams forces the top of the Short Plate on each Gel Cassette Sandwich to seat against
the rubber gasket properly and prevents leaking.
5. Lower the Inner Chamber Assembly into the Mini Tank. Fill the inner chamber with ~125 ml
of running buffer until the level reaches halfway between the tops of the taller and shorter
glass plates of the Gel Cassettes.
Note: Do not overfill the Inner Chamber Assembly. Excess buffer will cause the siphoning
of buffer into the lower chamber which can result in buffer loss and interruption of
electrophoresis.
6. Add ~200 ml of running buffer to the Mini Tank (lower buffer chamber).
Sample Loading
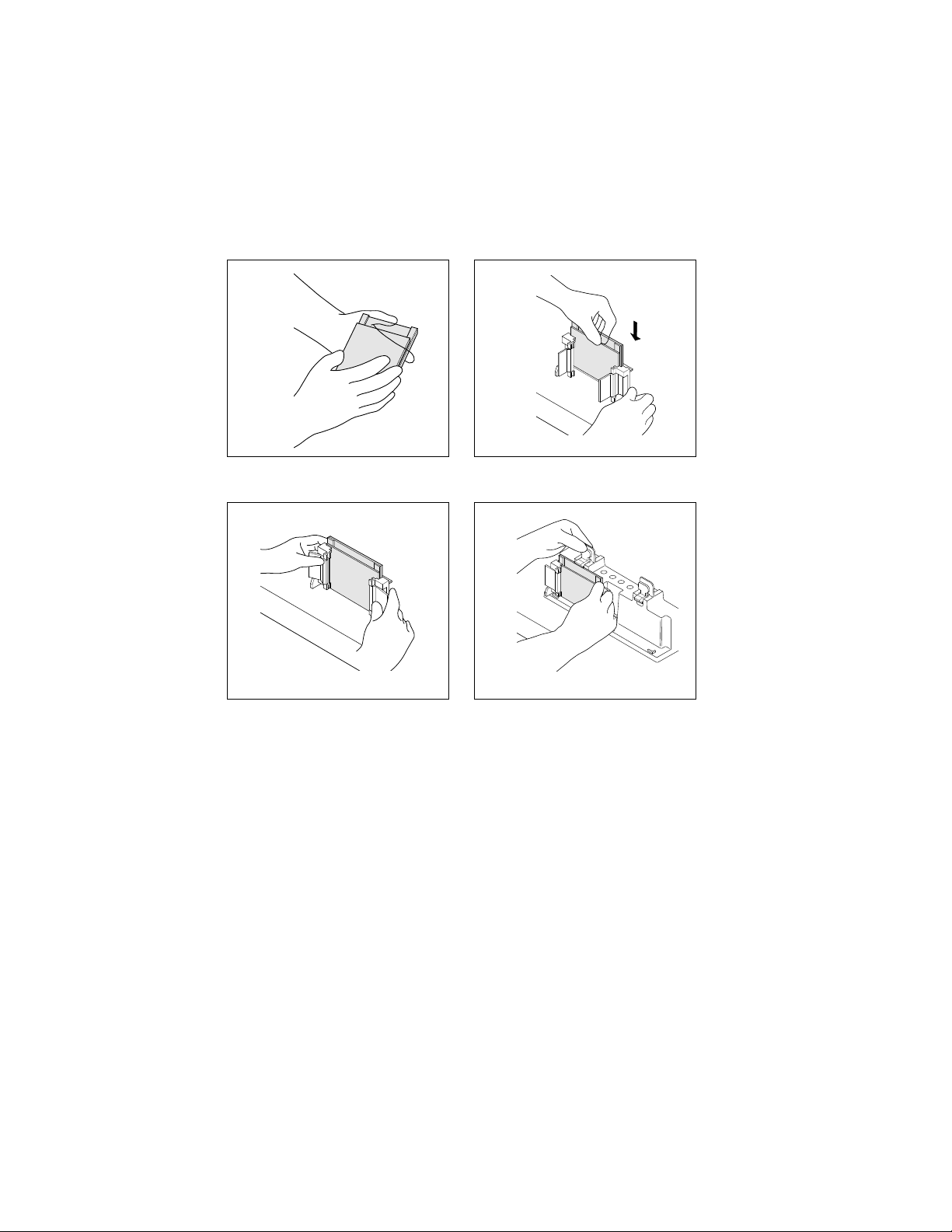
1. Load the samples into the wells with a Hamilton syringe or a pipette using gel loading tips.
2. If using Bio-Rad's patented sample loading guide, place it between the two gels in the
Electrode Assembly. Sample loading guides are available for 9, 10, 12, and 15 well formats.
Fig. 6. Using the Sample Loading Guide (patent #5,656,145).
3. Use the Sample Loading Guide to locate the sample wells. Insert the Hamilton syringe or
pipette tip into the slots of the guide and fill the corresponding wells.
Note: Load samples slowly to allow them to settle evenly on the bottom of the well. Be
careful not to puncture the bottom of the well with the syringe needle or pipette tip.
2.3 Gel Electrophoresis
Mini Tank Assembly
1. Place the Lid on the Mini Tank. Make sure to align the color coded banana plugs and
jacks. The correct orientation is made by matching the jacks on the lid with the banana
plugs on the electrode assembly. A stop on the lid prevents incorrect orientation.
9
Pipet Tip
Sample
Loading
Guide
Page 12

Power Conditions
1. Insert the electrical leads into a suitable power supply with the proper polarity.
2. Apply power to the Mini-PROTEAN 3 cell and begin electrophoresis; 200 volts constant
is recommended for SDS-PAGE and most native gel applications. Run time is approxi-
mately 35 minutes at 200 volts for SDS-PAGE.
Gel Removal
1. After electrophoresis is complete, turn off the power supply and disconnect the electrical leads.
2. Remove the tank lid and carefully lift out the Inner Chamber Assembly. Pour off and
discard the running buffer.
Note: Always pour off the buffer before opening the cams to avoid spilling the buffer.
3. Open the cams of the Clamping Frame. Pull the Electrode Assembly out of the Clamping
Frame and remove the Gel Cassette Sandwiches.
4. Remove the gels from the Gel Cassette Sandwich by gently separating the two plates of the
gel cassette. The green, wedgeshaped, plastic Gel Releaser may be used to help pry the glass
plates apart.
Note: To remove the gel from a Ready Gel Cassette, first slice the tape along the sides
of the Ready Gel Cassette where the inner glass plate meets the outer plastic plate.
5. Run the sharp edge of the Gel Releaser or a razor blade along each spacer to separate the
gel from the spacer. Remove the gel by floating it off the glass plate by inverting the gel
and plate under fixative or transfer solution, agitating gently until the gel separates from
the plate.
6. Rinse the Mini-PROTEAN 3 cell electrode assembly, Clamping Frame and Mini Tank
with distilled, deionized water after use.
Section 3
Separation Theory and Optimization
3.1 Introduction
Polyacrylamide gel electrophoresis separates molecules in complex mixtures according
to size and charge. During electrophoresis there is an intricate interaction of samples, gel
matrix buffers, and electric current resulting in separate bands of individual molecules. Hence
the variables that must be considered in electrophoresis are gel pore size, gel buffer systems,
and the properties of the molecule of interest.
Gel Pore Size
Gel pores are created by the crosslinking of polyacrylamide with bis-acrylamide (bis) to
create a network of pores. This structure allows the molecular sieving of molecules through
the gel matrix. Gel pore size is a function of the acrylamide monomer concentration used
(%T). By convention, polyacrylamide gels are characterized by %T which is the weight
percentage of the total monomer including the crosslinker. The %T gives an indication of the
relative pore size of the gel. In general, pore size decreases with increasing %T.
%T is calculated using the following equation.
%T =
g acrylamide + g crosslinker x 100%
total volume (ml)
10
Page 13

%C is the crosslinker:acrylamide monomer ratio of the monomer solution. %C is calculated using the following equation.
%C =
g crosslinker x 100%
g acrylamide + g crosslinker
2.67% C is traditionally used for most analytical gels.
Gels can be made as a single continuous percentage throughout the gel, or can be cast as
a gradient %T through the gel. Typical compositions are from 7.5% up to 20% for single
percentage gels, or gradients ranging from 4–15% to 10–20%.
The total monomer concentration for optimal separation is referred to as optimal %T.
Optimal %T will vary depending on the molecular weight of the molecule of interest.
Empirically the pore size providing optimum resolution for proteins is that which results in a
relative mobility (Rf) value between 0.55–0.6. Rfvalues for specific proteins are
calculated as follows.
Rf=
Distance migrated by the protein of interest
Distance migrated by the ion front
Gel Buffer System
The buffer system determines the power requirements and affects separation. The buffer
system is composed of the buffer used in the gel and the running buffer. There are
continuous and discontinuous buffer systems.
Continuous Buffer Systems
In continuous buffer systems the same buffer ions are present, at constant pH and
concentration throughout the system. The gel is typically made of one continuous %T and the
sample is loaded directly into the part of the gel where separation will occur. The band width
is determined in part by the height of the sample load in the well, so samples should be
concentrated and volumes small for best results.
Discontinuous Buffer Systems
In discontinuous buffer systems different buffer ions are present in the gel and electrode
reservoirs. By using different buffers in the gel and in the electrode solutions and adding a
stacking gel to the resolving gel, samples are compressed into a thin starting band and
individual proteins are finely resolved and separated. Discontinuous buffer systems were
devised initially for use with undenatured, or native proteins; however the most popular
discontinuous system employed is the SDS-PAGE buffer system by Laemmli.lFormulations
for this system are included in Section 4.1.
3.2 SDS-PAGE (Laemmli) Buffer System
The Laemmli buffer system is a discontinuous buffer system that incorporates SDS in
the buffer. In this system, proteins are denatured by heating them in buffer containing
sodium dodecyl sulfate (SDS) and a thiol reducing agent such as 2-mercaptoethanol (βME.)
The resultant polypeptides take on a rod-like shape and a uniform charge-to-mass ratio
proportional to their molecular weights. Proteins separate according to their molecular weight,
making this system extremely useful for calculating molecular weights.
11
Page 14

3.3 Native PAGE
Native PAGE is a technique for separating biologically active proteins. In contrast to
SDS-PAGE, the mobilities of proteins in a Native PAGE system depend on both size and
charge. There is no single electrophoresis buffer system that will optimally separate all
proteins in a native gel. Key parameters for separating proteins in a Native PAGE system are
pI of the protein of interest and the pH of the electrophoresis buffer
pH and pI
The pH of the electrophoresis buffer must be within the pH range over which the protein
of interest is stable and retains biological activity. In addition, the pH of the buffer must impart
sufficient charge to the protein for it to move through the gel. Changes in pH will affect both
the charge and size (hydrodynamic volume) of the protein of interest and will affect
migration rates. For example, a buffer with a pH greater than the pI of the protein will impart
a negative charge on the protein and it will migrate toward the positive electrode (anode).
Conversely, a buffer with a pH lower than the pI of the protein will impart a positive charge
and the protein will migrate to the negative electrode (cathode). A pH equal to the pI will
result in no net charge in the protein and it will not migrate in an electric field.
Protein mobilities are best modified by the buffer's pH. Buffers with a pH closer to the pI
will provide the best resolution. However run times may be lengthy. Conversely, buffers with
a pH further from the pI will allow faster migration but resolution may be compromised. The
choice of pH becomes a tradeoff between separation and speed.
How to Choose a Native PAGE system
1. Discontinuous Buffer Systems (Ornstein-Davis2)
A discontinuous buffer system should be the first non-denaturing gel system tried.
Detailed protocols are provided in Section 4.2. The advantage of a discontinuous system
is the use of a stacking gel to concentrate dilute protein samples. However, the stacking
phenomena can also cause aggregation of some proteins and interfere with resolution. If
protein aggregation occurs, a continuous buffer system should be used.
Note: The pH attained in the resolving gel of the Ornstein-Davis system approaches
pH 9.5, which may be outside the range of stability for some proteins, causing denaturation.
Additionally, the pI of the protein of interest may be too close to or above the Ornstein-
Davis buffer pH (9.5), which may result in a very low net charge or a positive net charge
that may significantly reduce or even prohibit migration to the anode. Alternative
discontinuous systems can be found in an article by Chrambach and Jovin.
3
Note: It is very desirable to know the pI of the protein of interest before selecting a buffer
system.
2. Continuous Buffer Systems
A continuous buffer system will be required if discontinuous systems cannot be used due
to stacking-induced protein aggregation. In a continuous system the same buffer is used
in the upper and lower electrode chambers as in the gel. Since stacking does not occur,
proteins migrate in bands at least as wide as the height of the applied sample in the well.
Consequently, sample volumes should be minimized. The mobility of proteins in a
continuous system is dictated by pH rather than by sieving through the polyacrylamide
gel. For this reason, 6% polyacrylamide gels are recommended for most applications.
For very large proteins, 4% or 5% gels may be used. McLellan describes various continuous
buffer systems from pH 3.8–10.2.4Detailed protocols are provided in Section 4.3.
12
Page 15

Section 4
Reagent Preparation and Stock Solutions
4.1 Volumes Required Per Gel
The volumes listed are required to completely fill a gel cassette. Amounts may be adjusted
depending on the application (with or without comb, with or without stacking gel, etc.).
Gel Thickness (mm) Volume (ml)
0.5 2.8
0.75 4.2
1.0 5.6
1.5 8.4
Note: 10 ml of monomer solution is sufficient for two stacking gels of any thickness.
4.2 SDS-PAGE (Laemmli)1Buffer System
Stock Solutions and Buffers
1. Acrylamide/Bis (30% T, 2.67% C)
87.6 g acrylamide (29.2 g/100 ml)
2.4 g N'N'-bis-methylene-acrylamide (0.8 g/100 ml)
Make to 300 ml with deionized water. Filter and store at 4 °C in the dark (30 days
maximum.)
or, use:
Preweighed Acrylamide/Bis, 37.5:1 mixture (30%T, 2.67% C)
(Bio-Rad catalog number 161-0125, 150 g)
30% Acrylamide/Bis Solutions, 37.5:1 mixture (30%T, 2.67% C)
(Bio-Rad catalog number 161-0158, 500 ml)
(Bio-Rad catalog number 161-0159, 2 x 500 ml)
2. 10% (w/v) SDS
Dissolve 10 g SDS in 90 ml water with gentle stirring and bring to 100 ml with deionized
water. Alternatively 10% SDS solution (250 ml) can be used (Bio-Rad catalog number
161-0416).
3. 1.5 M Tris-HCl, pH 8.8
27.23 g Tris base (18.15 g/100 ml)
80 ml deionized water
Adjust to pH 8.8 with 6 N HCl. Bring total volume to 150 ml with deionized water and
store at 4 °C. Alternatively 1.5 M Tris-HCl, pH 8.8 (1 L) premixed buffer can be used
(Bio-Rad catalog number 161-0798).
4. 0.5 M Tris-HCl, pH 6.8
6 g Tris base
60 ml deionized water
Adjust to pH 6.8 with 6 N HCl. Bring total volume to 100 ml with deionized water and
store at 4 °C. Alternatively 0.5 M Tris-HCl, pH 6.8 (1 L) premixed buffer can be used
(Bio-Rad catalog number 161-0799).
13
Page 16

5. Sample Buffer (SDS Reducing Buffer)
3.55 ml deionized water
1.25 ml 0.5 M Tris-HCl, pH 6.8
2.5 ml glycerol
2.0 ml 10% (w/v) SDS
0.2 ml 0.5%(w/v) bromophenol blue
9.5 ml Total Volume
Store at room temperature.
Use: Add 50 µl β-Mercaptoethanol to 950 µl sample buffer prior to use. Dilute the
sample at least 1:2 with sample buffer and heat at 95 °C for 4 minutes.
6. 10x Electrode (Running) Buffer, pH 8.3 (makes 1 L)
30.3 g Tris base
144.0 g Glycine
10.0 g SDS
Dissolve and bring total volume up to 1,000 ml with deionized water. Do not adjust pH
with acid or base. Store at 4 °C. If precipitation occurs, warm to room temperature before
use. Alternatively, electrophoresis running buffer 10x Tris/Glycine/SDS, 5 L cube
(Bio-Rad catalog number 161-0772) can be used.
Use: Dilute 50 ml of 10x stock with 450 ml deionized water for each electrophoresis
run. Mix thoroughly before use.
7. 10% APS (fresh daily)
100 mg ammonium persulfate
Dissolved in 1 ml of deionized water.
Gel Formulations (10 ml)
1. Prepare the monomer solution by mixing all reagents except the TEMED and 10% APS.
Degas the mixture for 15 minutes.
30% Degassed
DDI H2O Acrylamide/Bis *Gel Buffer 10% w/v SDS
Percent Gel (ml) (ml) (ml) (ml)
4% 6.1 1.3 2.5 0.1
5% 5.7 1.7 2.5 0.1
6% 5.4 2.0 2.5 0.1
7% 5.1 2.3 2.5 0.1
8% 4.7 2.7 2.5 0.1
9% 4.4 3.0 2.5 0.1
10% 4.1 3.3 2.5 0.1
11% 3.7 3.7 2.5 0.1
12% 3.4 4.0 2.5 0.1
13% 3.1 4.3 2.5 0.1
14% 2.7 4.7 2.5 0.1
15% 2.4 5.0 2.5 0.1
16% 2.1 5.3 2.5 0.1
17% 1.7 5.7 2.5 0.1
*
Resolving Gel Buffer - 1.5 M Tris-HCl, pH 8.8
*
Stacking Gel Buffer - 0.5 M Tris-HCl, pH 6.8
14
Page 17

2. Immediately prior to pouring the gel, add:
For 10 ml monomer solution:
Resolving Gel: 50 µl 10% APS and
5 µl TEMED
Stacking Gel: 50 µl 10% APS and
10 µl TEMED
Swirl gently to initiate polymerization.
Note: Prepare any desired volume of monomer solution by using multiples of the 10 ml
recipe. The volumes of APS and TEMED must be adjusted accordingly.
Warning: The catalyst concentration is very important! Webbing and incomplete well
formation can result from inaccurate catalyst concentration.
4.3 Discontinuous Native PAGE (Ornstein-Davis)
2
Stock Solutions and Buffers
1. Acrylamide/Bis (30% T, 2.67% C)
87.6 g acrylamide (29.2 g/100 ml)
2.4 g N'N'-bis-methylene-acrylamide (0.8 g/100 ml)
Make to 300 ml with deionized water. Filter and store at 4 °C in the dark (30 days
maximum).
or, use:
Preweighed Acrylamide/Bis, 37.5:1 mixture
(Bio-Rad catalog number 161-0125, 150 g)
30% Acrylamide/Bis Solutions, 37.5:1 mixture
(Bio-Rad catalog number 161-0158, 500 ml)
(Bio-Rad catalog number 161-0159, 2 x 500 ml)
2. 1.5 M Tris-HCl, pH 8.8
27.23 g Tris base (18.15 g/100 ml)
80 ml deionized water
Adjust to pH 8.8 with 6 N HCl. Bring total volume up to 150 ml with deionized water and
store at 4 °C. Alternatively 1.5 M Tris-HCl, pH 8.8 (1 L) premixed buffer can be used
(Bio-Rad catalog number 161-0798).
3. 0.5 M Tris-HCl, pH 6.8
6 g Tris base
60 ml deionized water
Adjust to pH 6.8 with 6 N HCl. Bring total volume up to 100 ml with deionized water and
store at 4 °C. Alternatively 0.5 M Tris-HCl, pH 6.8 (1 L) premixed buffer can be used
(Bio-Rad catalog number 161-0799).
4. Sample Buffer
5.55 ml deionized water
1.25 ml 0.5 M Tris-HCl, pH 6.8
3.0 ml glycerol
0.2 ml 0.5% (w/v) bromophenol blue
10.0 ml Total Volume
Store at room temperature.
Use: Dilute the sample at least 1:2 with sample buffer and heat at 95 °C for 4 minutes.
15
Page 18

5. 10x Electrode (Running) Buffer, pH 8.3
30.3 g Tris base (15 g/l)
144.0 g Glycine (72 g/l)
Bring total volume up to 1,000 ml with deionized water. Do not adjust pH. Alternatively
electrophoresis running buffer 10x Tris/Glycine, 1 L (Bio-Rad catalog number 161-0734)
can be used.
Usage: Dilute 50 ml of 10x stock with 450 ml deionized water for each electrophoresis run.
Gel Formulations (10 ml)
1. Prepare the monomer solution by mixing all reagents except the TEMED and 10% APS.
Degas the mixture for 15 minutes.
30% Degassed
Percent DDI H2O Acrylamide/Bis *Gel Buffer
Gel (ml) (ml) (ml)
4% 6.2 1.3 2.5
5% 5.8 1.7 2.5
6% 5.5 2.0 2.5
7% 5.2 2.3 2.5
8% 4.8 2.7 2.5
9% 4.5 3.0 2.5
10% 4.2 3.3 2.5
*
Resolving Gel Buffer - 1.5 M Tris-HCl, pH 8.8
*
Stacking Gel Buffer - 0.5 M Tris-HCl, pH 6.8
2. Immediately prior to pouring the gel, add:
50 ml APS and
TEMED (5 µl for Resolving Gels; 10 µl TEMED for stacking gels)
Swirl gently to initiate polymerization.
Note: Prepare any desired volume of monomer solution by using multiples of the 10 ml
recipe. The volumes of APS and TEMED must be adjusted accordingly.
4.4 Continuous Native PAGE
Stock Solutions and Buffers
1. Acrylamide/Bis (30% T, 2.67% C)
87.6 g acrylamide (29.2 g/100 ml)
2.4 g N'N'-bis-methylene-acrylamide (0.8 g/100 ml)
Make to 300 ml with deionized water. Filter and store at 4 °C in the dark (30 days
maximum.)
or, use:
Preweighed Acrylamide/Bis, 37.5:1 mixture
(Bio-Rad catalog number 161-0125, 150 g)
30% Acrylamide/Bis Solutions, 37.5:1 mixture
(Bio-Rad catalog number 161-0158, 500 ml)
(Bio-Rad catalog number 161-0159, 2 x 500 ml)
16
Page 19

2. Sample Buffer
1.0 ml Electrophoresis Buffer
3.0 ml Glycerol
0.2 ml 0.5% Bromophenol Blue
5.8 ml Deionized water
10.0 ml Total Volume
3. Continuous Buffers (McLellan)
4
McLellan describes various continuous buffer systems from pH 3.8 to pH 10.2. Use the
table below to prepare 5x continuous non-denaturing PAGE electrophoresis buffers. Add
both the acidic and basic component to 1 liter of water. Do not adjust the pH. If the final pH
is outside the listed range discard the buffer and remake.
Basic Acidic
pH Component 5x Solution Component 5x Solution
3.8 Beta-Alanine 13.36 g/L Lactic Acid 7.45 ml/L
(89.09 MW) 85% Solution
4.4 Beta-Alanine 35.64 g/L Acetic Acid 11.5 ml/L
(89.09 MW) 17.4 M
4.8 GABA 41.24 g/L Acetic Acid 5.75 ml/L
(103.1 MW) 17.4 M
6.1 Histidine 23.28 g/L MES 29.5 g/L
(155.2 MW) (195.2 MW)
6.6 Histidine 19.4 g/L MOPS 31.4 g/L
(155.2 MW) (209.3 MW)
7.4 Imidazole 14.64 g/L HEPES 41.7 g/L
(68.08 MW) (238.33 MW)
8.1 Tris 19.38 g/L EPPS 37.85 g/L
(121.14 MW) (252.2 MW)
8.7 Tris 30.29 g/L Boric Acid 7.73 g/L
(121.14 MW) (61.83 MW)
9.4 Tris 36.34 g/L CAPS 44.26 g/L
(121.14 MW) (221.3 MW)
10.2 Ammonia 12.5 ml/L CAPS 22.13 g/L
(14.8 M) (221.3 MW)
Dilute 200 ml of 5x buffer with 800 ml deionized water to prepare 1x electrophoresis
buffer. The final concentrations of buffer components will be.
pH Basic Component Acidic Component
3.8 30 mM Beta-Alanine 20 mM Lactic Acid
4.4 80 mM Beta-Alanine 40 mM Acetic Acid
4.8 80 mM GABA 20 mM Acetic Acid
6.1 30 mM Histidine 30 mM MES
6.6 25 mM Histidine 30 mM MOPS
7.4 43 mM Imidazole 35 mM HEPES
8.1 32 mM Tris 30 mM EPPS
8.7 50 mM Tris 25 mM Boric Acid
9.4 60 mM Tris 40 mM CAPS
10.2 37 mM Ammonia 20 mM CAPS
17
Page 20

Gel Formulations (10 ml)
1. Prepare the monomer solution by mixing all reagents except the TEMED and 10% APS.
Degas the mixture for 15 minutes.
30% Degassed Continuous
Percent DDI H2O Acrylamide/Bis Buffer
Gel (ml) (ml) (ml)
4% 6.7 1.3 2.0
5% 6.3 1.7 2.0
6% 6.05 2.0 2.0
Note: Prepare any desired volume of monomer solution by using multiples of the 10 ml
recipe.
2. Immediately prior to pouring the gel, add:
For 10 ml monomer solution:
50 µl 10% APS
10 µl TEMED
Swirl gently to initiate polymerization.
Note: Below pH 6, TEMED becomes a less effective catalyst. Increase the concentration
of TEMED 5-fold to polymerize gels with a pH range between 4 and 6.
Section 5
References
1. Laemmli, U. K., Nature, 227, 680 (1970).
2. Ornstein, L. and Davis, B. J., Anal. NY Acad. Sci., 121, 321 (1964).
3. Chrambach, A. and Jovin, T. M., Electrophoresis, 4, 190–204 (1984).
4. McLellan, T., Analytical Biochemistry, 126, 94–99 (1982).
Section 6
Maintenance
Mini-PROTEAN 3 tank and lid, Rinse thoroughly with distilled water after
electrode assembly, clamping frame every use.
Casting stand and frame Rinse thoroughly with distilled water after
every use.
Glass plates and combs Wash with a laboratory detergent, then rinse
thoroughly with distilled water.
Limit submersion of Spacer Plates in strongly
basic solutions, such as >100 mM NaOH, to
less than 24 hours. Limit submersion in
chromic-sulfuric acid glass cleaning solution
to 2–3 hours. Prolonged submersion
compromises the integrity of the adhesive.
To preserve the longevity of the adhesive
bond, avoid extended submersion (>5 days) in
cleaning solutions made from Bio-Rad
cleaning concentrate (161-0722) or other
strongly basic detergents.
18
Page 21

Section 7
Troubleshooting Guide
Problem Cause Solution
1. "Smile effect" - band pattern a. Center of the gel running a. Buffer not mixed well or buffer
curves upward at both sides hotter than either end. in upper chamber too
of the gel. concentrated. Remake buffer,
insuring thorough mixing,
especially when diluting 5x or
10x stock.
b. Power conditions excessive. b. Decrease power setting from
200 V to 150 V or fill lower
chamber to within 1 cm of top
of Short Plate.
2. Vertical streaking of protein. a. Sample overload. a. Dilute sample, selectively
remove predominant protein
in the sample, or reduce
voltage by about 25% to
minimize streaking.
b. Sample precipitation. b. Centrifuge sample before
addition of SDS sample
buffers, or decrease%Tof
resolving gel.*
c. The ratio of SDS to protein
should be enough to coat
each protein molecule with
SDS, generally 1.4:1. It may
require more SDS for some
membrane protein samples.
For example, SDS in sample
can be increased to 4% and/or
in running buffer increased to
0.4%.
3. Lateral band spreading. a. Diffusion out of the wells a. Minimize the time between
prior to turning on the sample application and
current power start up.
b. Ionic strength of sample b. Use same buffer in sample as
lower than that of gel. in gel or stacking gel.
4. Skewed or distorted bands. a. Poor polymerization around a. Degas stacking gel solution
sample wells. thoroughly prior to casting;
increase ammonium persulfate and TEMED concentrations by 25%; for stacking gel
or low%T, leave APS the
same and double the TEMED
concentration.
b. Salts in sample. b. Remove salts by dialysis,
desalting column, Micro BioSpin columns, etc.
c. Uneven gel interface. c. Decrease the polymerization
rate. Overlay gels very
carefully.
5. Lanes constricted at bottom a. Ionic strength of sample a. Desalt sample and neighboring
of gel. higher than that of samples.
surrounding gel.
6. Run taking unusually long a. Running buffer too a. Check buffer protocol, dilute
time. concentrated. if necessary.
b. Excessive salt in sample. b. Desalt sample.
19
Page 22

Problem Cause Solution
7. Run too fast, poor resolution. a. Running or reservoir a. Check buffer protocol,
buffer too dilute. concentrate if necessary.
b. Voltage too high. b. Decrease voltage by
25–50%.
8. Doublets observed where a. A portion of the protein a. Prepare fresh sample buffer
a single protein species may have been solutions if over 30 days old;
is expected (SDS-PAGE) reoxidized during the run or increase 2-mercaptoethanol
may not have been fully concentration in the sample
reduced prior to run. buffer; substitute DTT for BME.
9. Observe fewer bands than a. Protein(s) migrating at the a. Increase%Tofresolving
expected and one heavy dye front. gel.*
band at dye front.
b. Protein degradation. b. Use protease inhibitors,
e.g.
PMSF, etc.
10. Upper buffer chamber leaks. a. Upper buffer chamber over a Keep level of buffer below
filled. the top of the Spacer Plates.
b. Improper assembly. b. Be sure u-shaped electrode
core gasket is clean, free of
cuts, and lubricated with buffer.
Be sure Short Plate is
under
the
notch on the gasket, not on top
of it and press down on electrode assembly when closing
cams of the frame.
11. Leaking during gel casting. a. Chipped glass plates. a. Insure glass plates are free of
flaws.
b. Spacer Plate and Short b. Insure cassette is aligned
Plate not level. correctly.
c. Casting Stand gasket is c. Replace casting stand gaskets.
flawed or worn out.
12. Poor end well formation. a. Incorrect catalyst a. Prepare fresh catalyst solution,
concentration. or increase catalyst concentra-
tion of stacking gel to
0.06% APS and 0.12% TEMED.
b. Monomer solution not b. Degas monomer solution
degassed. Oxygen inhibits immediately prior to casting the
polymerization. stacking gel.
13. Webbing/excess acrylamide a. Incorrect catalyst a. Prepare fresh catalyst solution,
behind the comb. concentration. or increase catalyst concentra-
tion of stacking gel to
0.06% APS and 0.12% TEMED.
14. The pressure cams on the a. A build up of a powder a. Rinse or wipe off the powder
casting frame are difficult residue at the pivot point residue before each use.
to close or make a noise of the pressure cams.
when closed.
*Polyacrylamide gels are described by reference to two characteristics:
1) The total monomer concentration, (%T) and
2) The crosslinking monomer concentration (%C).
g acrylamide + g bis-acrylamide
Total Volume
g bis-acrylamide
g acrylamide + g bis-acrylamide
20
x 100%
x 100%
Page 23

Section 8
Product Information and Accessories
Catalog
Number Description
Mini PROTEAN 3 Systems
165-3301 Mini-PROTEAN 3 Electrophoresis System, 10 well, 0.75 thick-
ness, complete system includes 2 combs, 5 sets of glass plates,
casting stand, 2 casting frames, sample loading guide, 2 gel
releasers, and Electrophoresis Module
165-3302 Mini-PROTEAN 3 Electrophoresis Module, for Ready Gel precast
gel applications, includes electrode assembly, clamping frame,
tank, lid with power cables, mini cell buffer dam, 2 gel releasers
165-3375 Mini-PROTEAN II Upgrade Kit, includes Mini-PROTEAN 3
Clamping Frame and Electrode Assembly
165-3314 Mini-PROTEAN 3 Cell/PowerPac 300 System, 100/120 V
165-3315 Mini-PROTEAN 3 Cell/PowerPac 300 System, 220/240 V
165-3316 Mini-PROTEAN 3 Cell/PowerPac Junior System, 100–240 V
165-3317 Mini-PROTEAN 3 Cell and Mini Trans-Blot®module
Casting Modules
Each casting module includes 2 combs, 5 sets of glass plates, casting stand,
2 casting frames, and the appropriate Sample Loading Guide.
0.5 mm spacer 0.75 mm spacer 1.0 mm spacer 1.5 mm spacer
5 well comb NA 165-3327 165-3332 165-3338
9 well comb NA 165-3328 165-3333 165-3339
10 well comb 165-3325 165-3329 165-3334 165-3340
15 well comb 165-3326 165-3330 165-3335 165-3341
Prep/2D comb NA 165-3331 165-3336 165-3342
IPG comb NA NA 165-3337 165-3343
Hand Cast Gel Accessories and Replacement Parts
165-3303 Mini-PROTEAN 3 Casting Stand, 1
165-3304 Mini-PROTEAN 3 Casting Frame, 1
165-3305 Mini-PROTEAN 3 Casting Stand Gaskets (replacement), 2
165-3308 Mini-PROTEAN 3 Short Plates, 5
165-3309 Mini-PROTEAN 3 Spacer Plates with 0.5 mm spacers, 5
165-3310 Mini-PROTEAN 3 Spacer Plates with 0.75 mm spacers, 5
165-3311 Mini-PROTEAN 3 Spacer Plates with 1.0 mm spacers, 5
165-3312 Mini-PROTEAN 3 Spacer Plates with 1.5 mm spacers, 5
21
Page 24

Catalog
Number Description
Other Replacement Parts
165-3306 Mini-PROTEAN 3 Clamping Frame,1
165-3307 Mini-PROTEAN 3 Electrode Assembly,1
165-3201 Sample Loading Guide, 9 well (red),1
165-3146 Sample Loading Guide, 10 well (yellow),1
165-3203 Sample Loading Guide, 12 well (green),1
165-3132 Sample Loading Guide, 15 well (blue),1
165-3130 Buffer Dam,2
165-3320 Mini PROTEAN 3 Gel Releaser,5
165-3149 Replacement Electrode Assembly Gaskets,2
165-3157 Gaskets, for precast carbohydrate gels,2
161-0990 Empty Cassettes, 1.0 mm Ready Gel,10
165-2975 Buffer Tank and Lid, replacement
165-2948 Replacement Power Cables
165-2949 Cell Lid with Power Cables
900-7680-8 Replacement Platinum Wire, cathode, 8 inches
900-7680-13 Replacement Platinum Wire, anode, 13 inches
Combs
0.5 mm spacer 0.75 mm spacer 1.0 mm spacer 1.5 mm spacer
5 well comb NA 165-3352 165-3357 165-3363
9 well comb NA 165-3353 165-3358 165-3364
10 well comb 165-3350 165-3354 165-3359 165-3365
15 well comb 165-3351 165-3355 165-3360 165-3366
Prep/2D comb NA 165-3356 165-3361 165-3367
IPG comb NA NA 165-3362 165-3368
22
Page 25

Section 9
Warranty Information
The Mini-PROTEAN 3 cell is warranted for 1 year against defects in materials and
workmanship. If any defects should occur during this warranty period, Bio-Rad Laboratories
will replace the defective parts without charge. However the following defects are specifically excluded.
1. Defects caused by improper operation.
2. Repairs or modifications done by anyone other than Bio-Rad Laboratories or their
authorized agent.
3. Damaged caused by accidental misuse.
4. Damage caused by disaster.
5. Common consumable replacement parts including platinum wire, the rubber gaskets, and
glass plates.
6. Damage caused by the use of organic solvents.
For inquiry or request for repair service, contact your local Bio-Rad office.
Warranty Information
Model
Catalog Number
Date of Delivery
Serial Number
Invoice Number
Purchase Order No
23
Page 26

S
cience
eb site
a
a
4
k
dFrance
ael
yJap
ea
ds
9
Swede
8
1
US/EG
A
Bio-R
ad
.
4006157 Rev B
Laboratories,Inc
Life
Group
ulletin 0000
W
Alsoin: Australi
Brazil
Denmar
Isr
Kor
TheNetherlan
n Ph. 46(0)8-55 51 27 00, Fx. 46(0)8-55 51 27 80SwitzerlandPh. 061-717-9555,Fx. 061-717-9550 United Kingdom Ph. 0800-181134,Fx. 01442-25911
Rev
Ital
Finlan
Austri
Belgium Ph. 09-385 55 11,Fx. 09-385 65 5
Ph. 01 47 95 69 65,Fx. 01 47 41 9133
an
Ph. 47-23-38-41-30,Fx. 47-23-38-41-3
00-000 0000 Sig 010
 Loading...
Loading...