Page 1

DC Protein Assay
Instruction
Manual
For Technical Service
Call Your Local Bio-Rad Office or
in the U.S. Call 1-800-4BIORAD
(1-800-424-6723)
Page 2

Table of Contents
Section 1 Introduction and Principle ……………………………… 1
Section 2 Product Description ……………………………………… 1
Section 3 Materials Required but Not Supplied…………………… 1
3.1 Safety Considerations …………………………………………… 2
Section 4 Reagent Compatibility …………………………………… 2
Section 5 Instructions ……………………………………………… 3
5.1 Standard Assay Protocol ………………………………………… 3
5.2 Microplate Assay Protocol ……………………………………… 3
Section 6 Response of Various Proteins …………………………… 4
Section 7 Storage …………………………………………………… 4
Section 8 Troubleshooting Guide…………………………………… 5
Section 9 Ordering Information …………………………………… 6
Section 10 Related Materials ………………………………………… 6
Section 11 References ………………………………………………… 7
Section 12 Safety Information ……………………………………… 8
Page 3

Section 1
Introduction and Principle
The Bio-Rad DC Protein Assay is a colorimetric assay for protein concen-
tration following detergent solubilization. The reaction is similar to the well-documented Lowry1assay, but with the following improvements: The reaction
reaches 90% of its maximum color development within 15 minutes thereby
saving valuable time, and the color changes not more than 5% in 1 hour or 10%
in 2 hours after the addition of reagents.
The assay is based on the reaction of protein with an alkaline copper tartrate
solution and Folin reagent. As with the Lowry assay, there are two steps which
lead to color development: The reaction between protein and copper in an alkaline
medium, and the subsequent reduction of Folin reagent by the copper-treated protein.1Color development is primarily due to the amino acids tyrosine and tryptophan, and to a lesser extent, cystine, cysteine, and histidine.
reduction of the Folin reagent by loss of 1, 2, or 3 oxygen atoms, thereby producing one or more of several possible reduced species which have a characteristic blue
color with maximum absorbance at 750 nm and minimum absorbance at 405 nm.
Section 2
Product Description
Reagent package (catalog number 500-0116) includes:
250 ml REAGENT A, an alkaline copper tartrate solution
2000 ml REAGENT B, a dilute Folin Reagent
5 ml REAGENT S
(Sufficient for 500 standard assays or 10,000 microplate assays)
1,2
Proteins effect a
2
The reagent package may be purchased as a kit with a bovine gamma globulin standard (kit catalog number 500-011 1) or bovine serum albumin standard
(kit catalog number 500-0112).
Section 3
Materials Required but Not Supplied
For standard assay:
13 x 100 mm test tubes
Reservoir for working reagent (size depends on amount of reagent that
will be prepared)
Pipets accurately delivering 100 µl, 500 µl, and 4.0 ml
Graduated cylinders or pipets for reagent preparation
Spectrophotometer set to 750 nm
1
Page 4

Vortex mixer
Plastic or glass cuvettes with 1 cm path length matched to laboratory spec-
trophotometer
Test tube rack to hold 13 x 100 mm test tubes
For microplate assay:
Microtiter plates
Reservoir for working reagent
Pipets for reagent preparation
Pipets accurately delivering 5 µl, 25 µl, and 200 µl
Microplate reader set to 750 nm
3.1 Safety Considerations
Eye protection and gloves should be worn while using this product. Consult
MSDS at the end of this manual for additional information.
Section 4
Reagent Compatibility
The listed compounds were tested and found to be compatible with the BioRad DC Protein Assay. In some cases, the presence of one or more of these
substances will effect a change in the response of the protein to the assay
reagents; therefore, the standard should always be prepared in the same buffer
as the sample.
10% SDS 1% CHAPS 2% NP-40
1% Triton†X-100 1% CHAPSO 1% Thesit
1% Tween†20 1% Octyl glucoside 1% Brij†-35
0.2% C12E8* 0.1 M Tris, pH 8 0.5 M NaOH
0.5 M HCl 0.5 M (NH4)2SO
0.05 M CaCl
0.05% Sodium azide 1 mM DTT (dithiothreitol)
2
4
0.025 M EDTA
0.4 M Guanidine HCl 4 M Urea
†
Note: The DC Protein Assay is incompatible with 2-mercaptoethanol (BME)
† BRIJ and TWEEN are registered trademarks of Atlas Chemical. THESIT is
a registered trademark of Desitin Arzneimittel GMBH. TRITON is a registered
trademark of Rohm and Haas.
*octaethyleneglycol dodecyl ether
2
Page 5

Section 5
Instructions
5.1 Standard Assay Protocol
1. Preparation of working reagent
Add 20 µl of reagent S to each ml of reagent A that will be needed for the run.
(This working reagent A' is stable for one week even though a precipitate
will form after one day. If precipitate forms, warm the solution and vortex.
Do not pipet the undissolved precipitate, as this will likely plug the tip of the
pipet, thereby altering the volume of reagent that is added to the sample.)
If samples do not contain detergent, you may omit step #1 and simply use
reagent A as supplied.
2. Prepare 3 - 5 dilutions of a protein standard containing from 0.2 mg/ml to
about 1.5 mg/ml protein. A standard curve should be prepared each time the
assay is performed. For best results, the standards should always be prepared
in the same buffer as the sample.
3. Pipet 100 µl of standards and samples into clean, dry test tubes.
4. Add 500 µl of reagent A' or A (see note from step 1) into each test tube.
Vortex.
5. Add 4.0 ml reagent B into each test tube and vortex immediately.
6. After 15 minutes, absorbances can be read at 750 nm. The absorbances will
be stable at least 1 hour. (See Troubleshooting Guide for recommendation
on using a wavelength other than 750 nm.)
5.2 Microplate Assay Protocol
1. Preparation of working reagent
Add 20 µl of reagent S to each ml of reagent A that will be needed for the run.
(This working reagent A' is stable for 1 week even though a precipitate
will form after 1 day. If precipitate forms, warm the solution and vortex. Do
not pipet the undissolved precipitate, as this will likely plug the tip of the pipet,
thereby altering the volume of reagent that is added to the sample.)
If samples do not contain detergent, you may omit step #1 and simply use
reagent A as supplied.
2. Prepare 3 - 5 dilutions of a protein standard containing from 0.2 mg/ml to about
1.5 mg/ml protein. A standard curve should be prepared each time the assay
is performed. For best results, the standar d should be prepar ed in the same
buffer as the sample.
3. Pipet 5 µl of standards and samples into a clean, dry microtiter plate.
3
Page 6
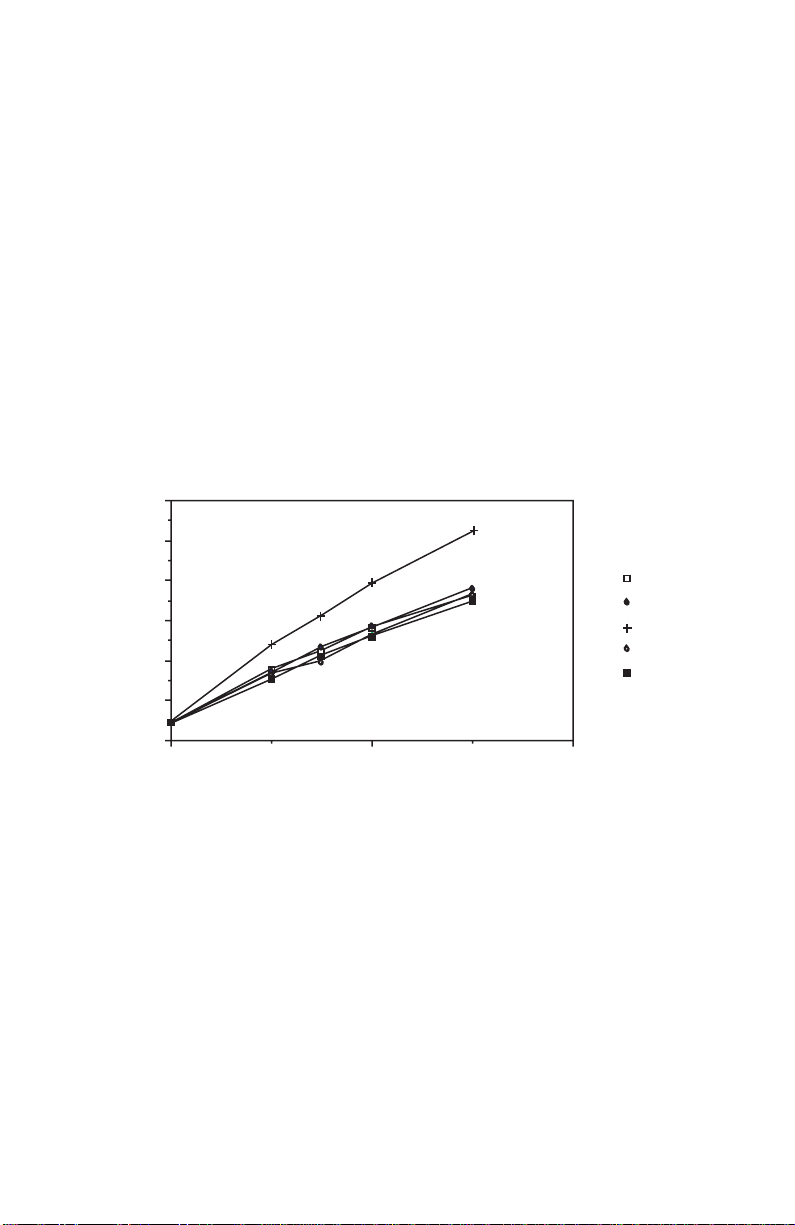
4. Add 25 µl of reagent A' or reagent A (see note from step 1) into each well.
protein concentration, mg/ml
absorbance
BSA
IgG
Ribonuclease A
Ovalbumin
Conalbumin
2.01.00.0
5. Add 200 µl reagent B into each well. If microplate reader has a mixing func-
tion available, place plate in reader and let the plate mix for 5 seconds. If not,
gently agitate the plate to mix the reagents. If bubbles form, pop them with a
clean, dry pipet tip. Be careful to avoid cross-contamination of sample wells.
6. After 15 minutes, absorbances can be read at 750 nm. The absorbances will
be stable for about 1 hour. (See Troubleshooting Guide for recommendation
on using a wavelength other than 750 nm.)
Section 6
Response of Various Proteins
As with any colorimetric assay , different proteins will elicit greater or less er color formation. The following proteins have been assayed with the protein
assay. As demonstrated by the graph, there is a slight variation in color development with different proteins.
Section 7
Storage
Lyophilized preparations of Protein Standard I (bovine gamma globulin) and
Protein Standard II (bovine serum albumin), if included, should be refrigerated upon
arrival. These lyophilized preparations have a shelf life of one year at 4 °C. Rehydrated
and stored at 4 °C, the protein solutions should be used within 60 days. Rehydrated
and stored at -20 °C, the protein solutions should be used within 6 months.
REAGENT A, REAGENT B, and REAGENT S should be stored away from
direct sunlight at room temperature (25-30 °C). (Reagents A and B may also be
stored in the refrigerator.) All reagents are good for 6 months from date of purchase.
4
Page 7

Section 8
Troubleshooting Guide
1. The buffer that I normally use
is not listed in the reagent
compatibility list. How will I
know if it interferes with the
assay?
2. My sample is a mixture of
proteins. Which standard
should I use for the standard
curve?
It is best to run two standard curves: One
with protein in the same buffer as your
sample and one with protein in water and
then plot a graph of protein concentration vs. absorbance. If the buffer does
not interfere, the two graphs of the standard curve will have identical slope.
Partial interference can be compensated
for by adding the buffer or interfering
component to the standard curve for the
actual protein assay.
In any protein assay, the best protein to
use as a standard is a purified preparation
of the protein being assayed. In the
absence of such an absolute reference
protein, one must select another protein
as a relative standard. The best relative
standard to use is one which gives a color
yield similar to that of the protein being
assayed.
Any purified protein can be selected as a
reference standard if only relative protein values are desired. Bio-Rad offers
two standards: bovine gamma globulin
(Standard I, catalog number 500-0005)
and bovine serum albumin (Standard II,
catalog number 500-0007).
3. Is any sample preparation
required?
4. May I use a wavelength other
than 750 nm?
5. May I store the reagents in the
refrigerator?
In general, no. However, the protein must
be solubilized. (The sample can not be a
suspension or an unfiltered homogenate.)
Yes. Absorbance can be measured at 650750 nm.
Yes, but all components must be warmed
to 25-30 °C prior to use. Reagent S will
develop a precipitate during cold room
storage. W arming to 37 °C for 5 minutes
will dissolve the precipitate.
5
Page 8

Section 9
Ordering Information
Catalog
Number Product Description
500-0111 Bio-Rad
Reagents Package and bovine gamma globulin standard
DC
Protein Assay Kit I, includes contents of
500-0112 Bio-Rad
Reagents Package and bovine serum albumin standard
500-0116 Bio-Rad
include a standard
Section 10
Related Materials
Catalog
Number Product Description
500-0001 Bio-Rad Protein Assay Kit I, 450 ml dye reagent concentrate
and bovine gamma globulin standard for general use, based on
Bradford method
500-0002 Bio-Rad Protein Assay Kit II, 450 ml dye reagent concentrate
and bovine serum albumin standard for general use, based on
Bradford method
500-0006 Bio-Rad Protein Assay Dye Reagent Concentrate, 450 ml dye
reagent concentrate supplied without a standard, based on
Bradford method
500-0005 Protein Standard I, bovine gamma globulin
500-0007 Protein Standard II, bovine serum albumin
223-9950 Disposable Polystyrene Cuvettes, 100 -3.5 ml cuvettes
DC
Protein Assay Kit II, includes contents of
DC
Protein Assay Reagents Package, does not
224-0096 Costar 96 Well Flat Bottom EIA Plate, polystyrene microtiter
plates, 5 per package, carton of 100
170-6601 Model 3550 Microplate Reader, 100/120 VAC
170-6602 Model 3550 Microplate Reader, 220/240 VAC
170-6621 Model 450 Microplate Reader, 100/120 VAC
170-6622 Model 450 Microplate Reader, 220/240 VAC
For information on Bio-Rad’s extensive Microplate Data Analysis Systems,
please call 800/4-BIORAD in the U.S., or contact your local Bio-Rad
Representative.
6
Page 9

Section 11
References
1. Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., “Protein Measurement
with the Folin Phenol Reagent,” Journal of Biological Chemistry, 193 (1951): 265-
275.
2. Peterson, Gary L., “Review of the Folin Phenol Protein Quantitation Method of
Lowry, Rosebrough, Farr , and Randall,” Analytical Biochemistry, 100 (1979): 201-
220.
7
Page 10

Section 12
Material Safety Data Sheets
I. PRODUCT IDENTIFICATION
TRADE NAME:
Catalog No.: Used in Kits: 500-0111, 500-0112, and 500-0116
Chemical identity, Common names: SODIUM HYDROXIDE, Caustic soda, lye.
Formula: NaOH, a caustic. M.W.: 40.00
MANUFACTURER’S NAME:
BIO-RAD LABORATORIES
LIFE SCIENCE GROUP
2000 ALFRED NOBEL DRIVE EMERGENCY PHONE No:
HERCULES, CA 94547 510/232-7000
DATE PREPARED OR REVISED: 2/3/95 NAME OF PREPARER:ROY WOOD
DC
PROTEIN ASSAY REAGENT A
II. HAZARDOUS INGREDIENTS
CHEMICAL NAMES CAS NUMBERS PERCENT EXPOSURE LIMITS IN AIR
ACGIH TLV OSHA PEL OTHER
Sodium Hydroxide 1310-73-2 1-5%* — 2 mg/m
*Aqueous solution also containing less than 1% sodium tartrate and less than .1% copper sulfate.
3
—
III. PHYSICAL/CHEMICAL CHARACTERISTICS
BOILING POINT: 100 °C SPECIFIC GRAVITY(H2O = 1): 1
VAPOR PRESSURE: N/A MELTING POINT: N/A
VAPOR DENSITY(AIR = 1): N/A EVAPORATION RATE (BUTYL ACETATE = 1): N/A
SOLUBILITY IN WATER: Infinite. APPEARANCE AND COLOR: Pale blue liquid.
IV. FIRE AND EXPLOSION HAZARD DATA
FLASH POINT: N/A FLAMMABLE LIMITS: N/A
(METHOD USED): N/A
EXTINGUISHING MEDIA: Not required. Use media suitable for surrounding materials.
SPECIAL FIRE FIGHTING PROCEDURES: In the event of a fire, wear full protective clothing
and NIOSH-approved self-contained breathing apparatus with full facepiece operated in the
pressure demand or other positive pressure mode.
UNUSUAL FIRE AND EXPLOSION HAZARDS: None expected .
8
Page 11

V. HEALTH HAZARD INFORMATION
SYMPTOMS OF OVEREXPOSURE (for each potential route of exposure):
INHALED: Corrosive! Inhalation not likely due to nature of the product. If product dries out, the
inhaled sodium hydroxide dust may be fatal as a result of spasm, inflammation and edema of the
larynx and bronchi, chemical pneumonitis and pulmonary edema.
CONTACT WITH SKIN OR EYES: Corrosive! Extremely destructive to tissue of the skin and
eyes. May cause irritation of eyes and with greater exposures, severe burns with possibility of
blindness resulting.
ABSORBED THROUGH SKIN: Corrosive! Destructive to skin on contact can cause irritation or
severe burns and scarring with greater exposures.
SWALLOWED: Corrosive! DANGER: May be fatal if swallowed. Causes severe burns. Sodium
hydroxide is classified as a poison under Federal Caustic Poison Act.
HEALTH EFFECTS OR RISKS FROM EXPOSURE
ACUTE: Corrosive! Causes severe burns. See above.
CHRONIC: Prolonged contact with dilute solutions or dust has a destructive effect upon tissue.
FIRST AID: EMERGENCY PROCEDURES
EYE CONTACT: Flush with large amounts of water for at least 15 minutes, lifting the upper and
lower lids occasionally. Get immediate medical attention.
SKIN CONTACT: Flush skin with large amounts of water for at least 15 minutes, while removing
contaminated clothing and shoes. Wash clothes before reuse. Get medical attention.
INHALED: Remove to fresh air. If not breathing, give artificial respiration. Get immediate medical
attention.
SWALLOWED: DO NOT INDUCE VOMITING! Give large quantities of water or milk if available.
Never give anything by mouth to an unconscious person. Get medical attention immediately.
SUSPECTED CANCER AGENT
X NO: THIS PRODUCT’S INGREDIENTS ARE NOT FOUND IN THE LISTS BELOW.
YES: _____FEDERAL OSHA ______NTP ______IARC
MEDICAL CONDITIONS AGGRAVATED BY EXPOSURE: Persons with pre-existing skin disorders or eye problems or impaired respiratory function may be more susceptible to the effects of
this substance.
VI. REACTIVITY DATA
STABLE X UNSTABLE
CONDITIONS TO AVOID: NA
INCOMPATIBILITY(Materials to avoid): Strong acid solutions, organic halogen compounds, and
nitromethane.
HAZARDOUS DECOMPOSITION PRODUCTS: Sodium oxide.
HAZARDOUS POLYMERIZATION MAY OCCUR______ WILL NOT OCCUR X
CONDITIONS TO AVOID: N/A
9
Page 12

VII. SPILL, LEAK, AND DISPOSAL PROCEDURES
SPILL RESPONSE PROCEDURES: CAUTION: Caustic material. Cover spill with adsorbent and
collect for solid waste disposal.
PREPARING WASTES FOR DISPOSAL: Comply with all applicable federal, state, and local regulations on spill reporting, waste handling, and waste disposal.
VIII. SPECIAL HANDLING INFORMATION
VENTILATION AND ENGINEERING CONTROLS: In general, dilution ventilation is a satisfactory
health hazard control for this substance.
RESPIRATORY CONTROLS: This product is a liquid and does not contain volatile organics and
should not require any special respiratory controls. Respiratory controls may be required for protection against other chemicals being used in the same area.
EYE PROTECTION: Use chemical safety goggles. Contact lenses should not be worn when
working with this material. Maintain eye wash fountain and quick-drench facilities in work area.
GLOVES: Chemical resistant gloves such as neoprene.
OTHER CLOTHING AND EQUIPMENT: Lab coat or apron.
WORK PRACTICES, HYGIENIC PRACTICES: Use good laboratory practices. Wash hands after
using and before eating. Do not eat, drink, or smoke in the work area.
OTHER HANDLING AND STORAGE REQUIREMENTS: Keep in a tightly closed container to
protect product quality. Store in a cool, dry, ventilated area.
PROTECTIVE MEASURES DURING MAINTENANCE OF CONTAMINATED EQUIPMENT:
Protective clothing, eyewear and appropriate NIOSH-approved respiratory protection should be
worn.
We believe that the information contained herein is current as of the date of this Material Safety
Data Sheet. Since the use of this information and conditions of use of the product are not within
the control of Bio-Rad, it is the user’s responsibility to handle the product under conditions of safe
use.
10
Page 13

I. PRODUCT IDENTIFICATION
TRADE NAME:
DC
PROTEIN ASSAY REAGENT B
Catalog No.: Used in Kits: 500-0111, 500-0112, and 500-0116
Chemical identity, Common names: FOLIN REAGENT.
Formula: MIXTURE M.W.: NA
MANUFACTURER’S NAME:
BIO-RAD LABORATORIES
LIFE SCIENCE GROUP
2000 ALFRED NOBEL DRIVE EMERGENCY PHONE No:
HERCULES, CA 94547 510/232-7000
DATE PREPARED OR REVISED: 2/3/95 NAME OF PREPARER:ROY WOOD
II. HAZARDOUS INGREDIENTS
CHEMICAL NAMES CAS NUMBERS PERCENT EXPOSURE LIMITS IN AIR
ACGIH TLV OSHA PEL
REAGENT B is a diluted FOLIN reagent containing
less than 1% each of the following ingredients:
Lithium Sulfate 10377-48-7 no data found
Tungstic acid,
sodium salt 10213-10-2 1 mg/m
Molybdic acid,
sodium salt 10102-40-6 5 mg/m
Hydrochloric acid 7647-01-0 7.5 mg/m
Phosphoric acid 7664-38-2 1 mg/m
3
3
3
3
1 mg/m3(TWA)
5 mg/m
7 mg/m
1 mg/m
3
3
3
III. PHYSICAL/CHEMICAL CHARACTERISTICS
BOILING POINT: 100 °C (water) SPECIFIC GRAVITY(H2O = 1): 1
VAPOR PRESSURE: NA MELTING POINT: NA
VAPOR DENSITY(AIR = 1): NA EVAPORATION RATE (BUTYL ACETATE = 1): NA
ODOR: odorless SOLUBILITY IN WATER: infinite
APPEARANCE AND COLOR: Clear liquid.
IV. FIRE AND EXPLOSION HAZARD DATA
FLASH POINT: NA FLAMMABLE LIMITS: NA
(METHOD USED): NA
EXTINGUISHING MEDIA: Nothing special required. Use media suitable for surrounding materials.
SPECIAL FIRE FIGHTING PROCEDURES: For surrounding fire wear self-contained breathing
apparatus and protective clothing to prevent contact with skin and eyes.
UNUSUAL FIRE AND EXPLOSION HAZARDS: No special hazards known.
11
Page 14

V. HEALTH HAZARD INFORMATION
SYMPTOMS OF OVEREXPOSURE (for each potential route of exposure):
INHALED: Not expected due to nature of product.
CONTACT WITH SKIN OR EYES: Would be irritating to eyes.
ABSORBED THROUGH SKIN: Could be mildly irritating to skin.
SWALLOWED: Large doses may cause gastrointestinal distress.
HEALTH EFFECTS OR RISKS FROM EXPOSURE
ACUTE: Contains dilute acids. Could be a mild irritant. The concentration of all the ingredients
are less than 1% each. There is no specific information available for diluted FOLIN reagent. The
diluted solution does contain dilute acids and heavy metals: SAX, 5th Ed. lists Molybdenum as
“slightly toxic” and tungsten is listed as somewhat more toxic than molybdenum but “does not
constitute an important health hazard”.
CHRONIC: To the best of our knowledge, the chemical, physical, and toxicological properties
have not been thoroughly investigated for FOLIN reagent.
FIRST AID: EMERGENCY PROCEDURES
EYE CONTACT: Flush with large volumes of water for at least 15 minutes, lifting the upper and
lower lids occasionally. Get medical attention.
SKIN CONTACT: Remove any contaminated clothing. Wash skin with plenty of flowing water for
at least 15 minutes. If irritation develops, get medical attention.
INHALED: Remove to fresh air. Get medical attention for any breathing difficulty.
SWALLOWED: Induce vomiting by giving two glasses of water, or milk if available and sticking
finger down throat. Call a physician immediately. Never give anything by mouth to an unconscious person.
SUSPECTED CANCER AGENT
X NO: THIS PRODUCT’S INGREDIENTS ARE NOT FOUND IN THE LISTS BELOW.
YES: _____FEDERAL OSHA ______NTP ______IARC
MEDICAL CONDITIONS AGGRAVATED BY EXPOSURE:
No information found.
VI. REACTIVITY DATA
STABLE X UNSTABLE _______
CONDITIONS TO AVOID: N/A
INCOMPATIBILITY (Materials to avoid): Strong oxidizers.
HAZARDOUS DECOMPOSITION PRODUCTS: Upon dehydration oxides of phosphorous and
hydrogen chloride gas may form.
HAZARDOUS POLYMERIZATION MAY OCCUR______ WILL NOT OCCUR X
CONDITIONS TO AVOID: N/A
12
Page 15

VII. SPILL, LEAK, AND DISPOSAL PROCEDURES
SPILL RESPONSE PROCEDURES: Cover spill with a sorbent and collect for solid waste disposal..
PREPARING WASTES FOR DISPOSAL: Comply with all applicable federal, state, and local reg-
ulations on spill reporting waste handling, and waste disposal.
VIII. SPECIAL HANDLING INFORMATION
VENTILATION AND ENGINEERING CONTROLS: In general, good ventilation is sufficient.
However, if conditions of use create discomfort to the worker a local exhaust system should be
considered.
RESPIRATORY CONTROLS: Should not be required for this product.
EYE PROTECTION: Chemical safety goggles.
GLOVES: Use chemical resistant gloves such as neoprene.
OTHER CLOTHING AND EQUIPMENT: Wear lab coat or apron.
WORK PRACTICES, HYGIENIC PRACTICES: Use good laboratory practices. Wash hands after
using and before eating. Do not eat, drink, or smoke in the work area.
OTHER HANDLING AND STORAGE REQUIREMENTS: Keep tightly sealed to protect quality.
Store in a cool, dry, ventilated area.
PROTECTIVE MEASURES DURING MAINTENANCE OF CONTAMINATED EQUIPMENT:
Proper protective clothing, eye protection, and respiratory equipment should be worn.
We believe that the information contained herein is current as of the date of this Material Safety
Data Sheet. Since the use of this information and conditions of use of the product are not within
the control of Bio-Rad, it is the user’s responsibility to handle the product under conditions of safe
use.
13
Page 16

1. PRODUCT IDENTIFICATION
TRADE NAME:
Catalog No.: Used in Kits: 500-0111, 500-0112, and 500-0116
Chemical identity, Common names: Sodium dodecyl sulfate.
Formula: C12H25NaO4S M.W.: 288.38
MANUFACTURER’S NAME:
BIO-RAD LABORATORIES
LIFE SCIENCE GROUP
2000 ALFRED NOBEL DRIVE EMERGENCY PHONE No:
HERCULES, CA 94547 510/232-7000
DATE PREPARED OR REVISED: 2/3/95 NAME OF PREPARER:ROY WOOD
DC
PROTEIN ASSAY REAGENT S
II. HAZARDOUS INGREDIENTS
CHEMICAL NAMES CAS NUMBERS PERCENT EXPOSURE LIMITS IN AIR
ACGIH TLV OSHA PEL OTHER
sodium dodecyl sulfate 151-21-3 5-10%
†Aqueous solution. Data given is for 100% SDS unless otherwise noted.
*orl-rat LD50: 1288 mg/kg
†
no data found *
III. PHYSICAL/CHEMICAL CHARACTERISTICS
BOILING POINT: 100 °C (water) SPECIFIC GRAVITY (H2O = 1): N/A
VAPOR PRESSURE: N/A MELTING POINT: 200 °C (362 °F) decomposes
VAPOR DENSITY(AIR = 1): N/A EVAPORATION RATE (BUTYL ACETATE = 1): N/A
ODOR: Slight fatty odor SOLUBILITY IN WATER: 0.1 g/ml
APPEARANCE AND COLOR: Clear liquid.
IV. FIRE AND EXPLOSION HAZARD DATA
FLASH POINT: N/A FLAMMABLE LIMITS: N/A
(METHOD USED): N/A
EXTINGUISHING MEDIA: Water spray, dry chemical, alcohol foam, or carbon dioxide.
SPECIAL FIRE FIGHTING PROCEDURES: As with most organic solids, fire is possible at elevat-
ed temperatures or by contact with an ignition source.
UNUSUAL FIRE AND EXPLOSION HAZARDS: Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust explosion hazard.
14
Page 17

V. HEALTH HAZARD INFORMATION
SYMPTOMS OF OVEREXPOSURE (for each potential route of exposure):
INHALED: Not expected due to nature of product. However, it dried out the powder causes irrita-
tion of mucous membranes, throat and respiratory tract. Symptoms of sore throat, coughing, and
choking can occur.
CONTACT WITH SKIN OR EYES: Irritating to eye tissue with redness and pain.
ABSORBED THROUGH SKIN: Mildly irritating to skin, causes a rash on continued exposure.
SWALLOWED: Large doses may cause gastrointestinal distress.
HEALTH EFFECTS OR RISKS FROM EXPOSURE
ACUTE: See above.
CHRONIC: To the best of our knowledge, the chemical, physical, and toxicological properties
have not been thoroughly investigated.
FIRST AID: EMERGENCY PROCEDURES
EYE CONTACT: Flush with large volumes of water for at least 15 minutes, lifting the upper and
lower lids occasionally. Get medical attention.
SKIN CONTACT: Remove any contaminated clothing. Wash skin with plenty of flowing water for
at least 15 minutes. If irritation develops, get medical attention.
INHALED: Remove to fresh air. Get medical attention for any breathing difficulty.
SWALLOWED: Induce vomiting by giving two glasses of water, or milk if available and sticking
finger down throat. Call a physician immediately. Never give anything by mouth to an unconscious person.
SUSPECTED CANCER AGENT
X NO: THIS PRODUCT’S INGREDIENTS ARE NOT FOUND IN THE LISTS BELOW.
YES: _____FEDERAL OSHA ______NTP ______IARC
MEDICAL CONDITIONS AGGRAVATED BY EXPOSURE:
No information found.
VI. REACTIVITY DATA
STABLE X UNSTABLE_______
CONDITIONS TO AVOID: N/A
INCOMPATIBILITY (Materials to avoid): Strong oxidizers.
HAZARDOUS DECOMPOSITION PRODUCTS: Oxides of sulfur and hydrogen sulfide.
HAZARDOUS POLYMERIZATION MAY OCCUR______ WILL NOT OCCUR X
CONDITIONS TO AVOID: N/A
15
Page 18

VII. SPILL, LEAK, AND DISPOSAL PROCEDURES
SPILL RESPONSE PROCEDURES: Caution, may be slippery. Small spill may be cleaned up
with a sponge and water washed down the drain.
PREPARING WASTES FOR DISPOSAL: Comply with all applicable federal, state, and local regulations on spill reporting waste handling, and waste disposal.
VIII. SPECIAL HANDLING INFORMATION
VENTILATION AND ENGINEERING CONTROLS: In general, good ventilation is sufficient.
However, if conditions of use create discomfort to the worker a local exhaust system should be
considered.
RESPIRATORY CONTROLS: Should not be required for this product.
EYE PROTECTION: Chemical safety goggles.
GLOVES: Use chemical resistant gloves such as neoprene.
OTHER CLOTHING AND EQUIPMENT: Wear lab coat or apron.
WORK PRACTICES, HYGIENIC PRACTICES: Use good laboratory practices. Wash hands after
using and before eating. Do not eat, drink, or smoke in the work area.
OTHER HANDLING AND STORAGE REQUIREMENTS: Keep tightly sealed to protect quality.
Store in a cool, dry, well-ventilated place away from incompatible materials.
PROTECTIVE MEASURES DURING MAINTENANCE OF CONTAMINATED EQUIPMENT:
Proper protective clothing, eye protection, and respiratory equipment should be worn.
We believe that the information contained herein is current as of the date of this Material Safety
Data Sheet. Since the use of this information and conditions of use of the product are not within
the control of Bio-Rad, it is the user’s responsibility to handle the product under conditions of safe
use.
16
Page 19

Life Science
Group
Website www.bio-rad.com U.S. (800) 4BIORAD Australia 02 9914 2800
Austria (01) 877 89 01 Belgium 09-385 55 11 Canada (905) 712-2771
China 86-10-62051850 86-10) 62051850 Denmark 45 39 17 99 47
Finland 358 (0)9 804 2200 France (01) 43 90 46 90 Germany 089 318 84-0
Hong Kong 852-2789-3300 India (91-11) 461 0103 Israel 03 951 4127
Italy 02 21609.1 Japan 03-5811-6270 Korea 82-2-3473-4460
The Netherlands 31 318-540666 New Zealand 64-9-4152280
Singapore 65-2729877 Spain (91) 661 70 85 Sweden 46 (0)8 627 50 00
Switzerland 01-809 55 55 United Kingdom 0800-181134
000097 Sig 120197Bulletin 0000 US/EG Rev A
Bio-Rad
Laboratories
LIT448 Rev D
 Loading...
Loading...