Schiller FRED EASY User manual
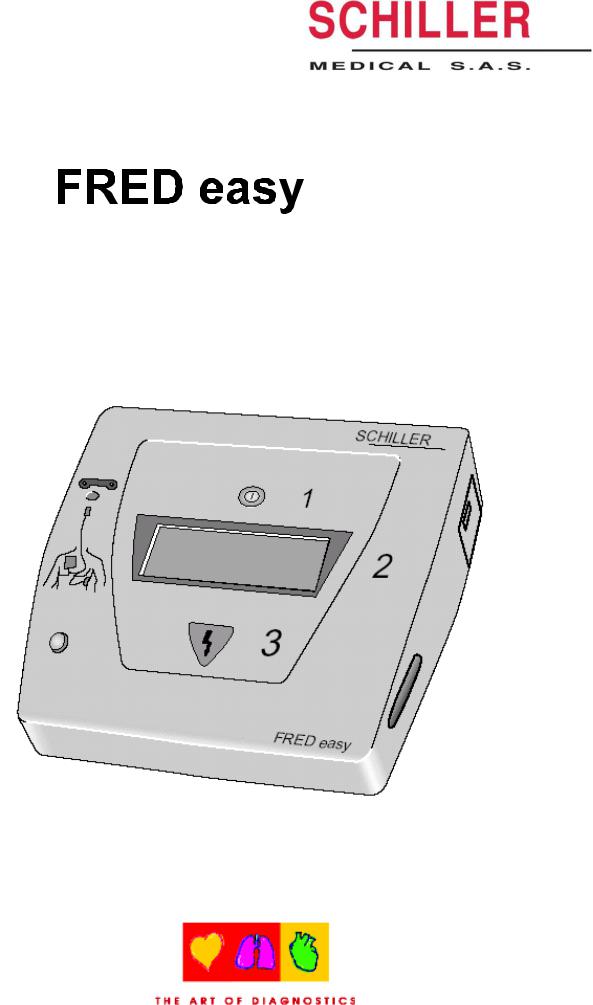
Semiautomatic defibrillator (PAD)
Service Manual
Version 01.00
Part No. 0-48-0029

FRED EASY
Revision history of the service manual
Version 01.00: |
May 2003 |
Part no. 0-48-0029 |
Page I |
May 2003 |

FRED EASY
WARNING
This manual shall be considered to form an integral part of the device described.
This technical manual is intended for qualified personnel and describes the operating, maintenance and troubleshooting procedures for FRED
EASY.
Compliance with its content is a prerequisite for proper device performance and for the safety of the patient and operator.
The manufacturer shall only be liable for the safety, reliability and performance of the device if:
-assembly, extensions, adjustments, modifications or repairs are performed by the manufacturer or by persons authorised by the manufacturer.
-the electrical installation of the facility of use complies with the requirements applicable in the country.
-the device is used in accordance with its instructions for use.
-the spare parts used are original parts from SCHILLER.
This manual describes the device at the time of printing.
The supply of this manual does not in any event constitute permission or approval to modify or repair a device.
The manufacturer agrees to supply all the spare parts for a period of ten years.
All rights reserved for the devices, circuits, processes and names appearing in this manual.
The FRED EASY device shall be used as described in the UserNs Manual. The device may not be used for any purpose that has not been specifically described in the manual, as such use could be hazardous.
Part no. 0-48-0029 |
Page II |
May 2003 |

FRED EASY
SAFETY INFORMATION
!The product is marked as follows:
CE0459
in accordance with the requirements of Council Directive 93/42/EEC relating to medical equipment, based on the essential requirements of annex I of the directive.
!It fully meets the electromagnetic compatibility requirements of standard IEC 60601-1-2/EN 60601-2 \Electromagnetic compatibility of medical electrical devices].
!The device has undergone interference suppression in accordance with the requirements of standard EN 50011, class B.
!In order to optimise patient safety, electromagnetic compatibility, accurate measurement indication and proper device performance, users are advised to use only original spare parts supplied by SCHILLER. Any use of accessories other than original accessories shall be at the exclusive risk of the user. The manufacturer shall not be liable for any damage due to the use of incompatible accessories or consumable supplies.
!The manufacturer shall only be liable for the safety, reliability and performance of the device if:
-assembly, configuration, modifications, extensions or repairs are made by personnel from SCHILLER MEDICAL or personnel duly authorised by SCHILLER MEDICAL.
-the device is used in accordance with its instructions for use.
!Any use of the device other than as described in the instructions for use shall be made at the exclusive risk of the user.
!This manual covers the device version and the safety standards applicable at the time of printing. All rights reserved for the circuits, processes, names, software and devices appearing in this manual.
!The quality assurance system in use in the facilities of SCHILLER meets international standards EN ISO 9001 and EN 46001.
!Unless otherwise agreed in writing by SCHILLER, no part of the manufacturerNs literature may be duplicated or reproduced.
Part no. 0-48-0029 |
Page III |
May 2003 |

FRED EASY
Safety symbols used on the device
Danger! High voltage
Conventions used in the manual
|
|
|
Danger: |
indicates an imminent hazard which, if not avoided, will result in |
|
! |
|
|
death or serious injury to the user (and/or others). |
|
|
|
|
|
|
|
|
|
|
|
|
|
Caution: |
Warning indicating conditions or actions that could lead to device |
|
" |
|
|
or software malfunctioning. |
|
|
|
|
|
|
|
|
|
|
|
# |
|
Note: |
Useful information for more effective and practical device |
|
|
|
operation. |
|
|
|
|
|
Additional information or explanation relating to the paragraphs |
|
|
|
|
preceding the note. |
|
|
|
||
Manufacturer: |
|
|||
SCHILLER |
|
|||
MEDICAL SA |
|
|||
4, rue Louis Pasteur ZAE sud |
|
|||
F- 67 162 Wissembourg |
|
|||
Tel. |
: **33 / (0) 3.88.63.36.00 |
|
||
Fax |
: **33 / (0) 3.88.94.12.82 |
|
||
Part no. 0-48-0029 |
Page IV |
May 2003 |

FRED EASY
PRECAUTIONS WHILE TESTING THE DEVICE
While testing the FRED EASY defibrillator, the patient may only be simulated with fixed high-voltage and high-power resistors that are well insulated from the ground or earth. Poorly insulated devices or devices with loose contacts or devices containing components such as spark arresters or electronic flash lamps may never be used as they could irremediably destroy the device.
Part no. 0-48-0029 |
Page V |
May 2003 |

FRED EASY
CONTENTS
1.Operation_________________________________________________________ 1-1
1.1Display and controls _____________________________________________________ 1-1
1.2Explanation of symbols used ______________________________________________ 1-2
1.3Device operation ________________________________________________________ 1-3
1.4Defibrillation procedure ___________________________________________________ 1-3
1.5Recording (optional) _____________________________________________________ 1-6
1.6Technical specifications __________________________________________________ 1-7
2.Testing and maintenance ____________________________________________ 2-1
2.1Functional testing _______________________________________________________ 2-1
2.2Systematic verification before use __________________________________________ 2-3
2.3Cleaning and disinfecting _________________________________________________ 2-3
3.Troubleshooting____________________________________________________ 3-1
4.Replacement of parts _______________________________________________ 4-1
4.1Device disassembly procedure _____________________________________________ 4-2
4.2Working on the CPU circuit________________________________________________ 4-3
4.3Working on the LCD display _______________________________________________ 4-3
4.4Working on the DEFI circuit _______________________________________________ 4-3
4.5Replacing the high-voltage capacitor ________________________________________ 4-3
4.6Reassembling the device _________________________________________________ 4-5
4.7Replacement of parts ____________________________________________________ 4-5
5.Technical description of boards________________________________________ 5-1
5.1FRED easy ____________________________________________________________ 5-1
5.2CPU (part no. WSM0005A)________________________________________________ 5-3
5.3Defibrillator, part no. WSM0008A __________________________________________ 5-28
5.3.1Driving the high-voltage capacitor charge_______________________________________ 5-33
5.3.2Chronograms ____________________________________________________________ 5-40
5.3.3Description of signals ______________________________________________________ 5-43
5.3.4ECG preamplifier _________________________________________________________ 5-43
5.3.5Defibrillator control circuit ___________________________________________________ 5-44
5.3.6High-voltage circuit ________________________________________________________ 5-48
5.3.7IGBT control circuit ________________________________________________________ 5-50
5.3.8Fault detection circuit ______________________________________________________ 5-52
6.Device modifications ________________________________________________ 6-1
6.1Definition ______________________________________________________________ 6-1
6.2CPU circuit ____________________________________________________________ 6-1
6.3DEFI circuit ____________________________________________________________ 6-1
7.Diagrams and layout drawings ________________________________________ 7-1
7.1CPU circuit WSM0005A __________________________________________________ 7-1
7.2Defi circuit WSM0008A ___________________________________________________ 7-4
Part no. 0-48-0029 |
Page VI |
May 2003 |
Operation
1. Operation
This section briefly outlines the operating of the device. For more detailed information, please refer to the User^s Manual.
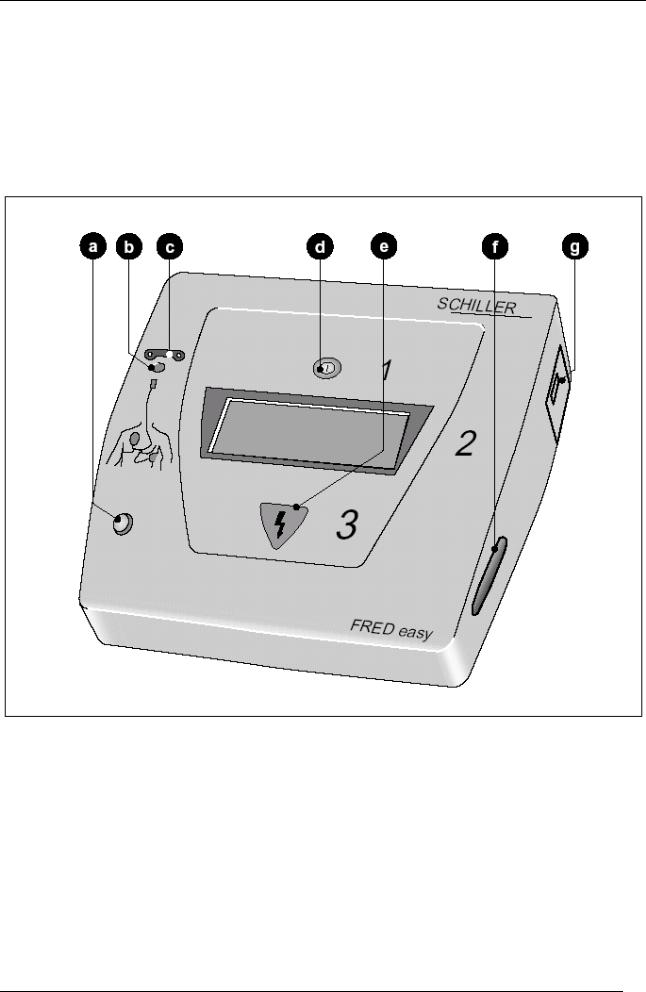
1.1Display and controls
aThe green indicator lamp flashes when the device is ready to operate.
b The yellow indicator lamp flashes as long as the electrodes are not in place.
cConnection of adhesive electrodes
d Green  key to switch the device on and off and start analysing e Key for triggering the defibrillation shock
key to switch the device on and off and start analysing e Key for triggering the defibrillation shock
f Memory card (optional)
gBattery
Part no. 0-48-0029 |
Page 1-1 |
May 2003 |
Operation
1.2Explanation of symbols used
Symbols on the device or accessories
BF type signal input, protected from defibrillation
Caution! High voltage!
Expiry date for the use of defibrillation electrodes
Follow the instructions for use
Open the electrode packaging
Remove the protective film
Single use only. Do not reuse.
Do not fold the packaging
Storage temperature range
Symbols displayed on the screen
Number of shocks given since starting up
Battery capacity
Memory card
Memory card not found
Adult electrode detected
Child electrode detected
Time since the machine was started up (minutes and seconds)
Part no. 0-48-0029 |
Page 1-2 |
May 2003 |
Operation
1.3Device operation
FRED easy® is a battery-operated semiautomatic defibrillator that provides biphasic defibrillation pulses.
Defibrillation takes place by means of single-use adhesive electrodes through which the ECG signals required for the analysis are also collected. Adhesive electrodes are available for children and adults. The device recognises the type of electrode applied and selects the defibrillation energy values accordingly.
The user is provided with written and audio instructions (display and loudspeaker) relating to the use of the device.
The power is supplied by plug-in disposable lithium batteries. Their capacity is sufficient for
-200 shocks at the maximum power rating or
-use of the monitor for seven hours (cyclical, 30 minutes on, 30 minutes off) or
-5 years standing by.
As soon as a battery is put in place in the FRED easy® device, it runs a self test to check the status of the device and battery. If the device does not find any fault, the green indicator begins to flash to show that it is ready to operate and the display disappears. Likewise, the device runs a self test each time it is switched on.
If, during the test, the device finds an error:
-it issues an audio alarm,
-the green indicator does not flash
The alarm signal rings till the battery is completely discharged. Key  is used to repeat the self test and the error message is displayed.
is used to repeat the self test and the error message is displayed.
Also, the device runs a self test after every seven days. The test is announced by a beep. If, during the test, the device finds an error:
-it issues an audio alarm,
-the green indicator does not flash
If you press key  the corresponding error message is displayed.
the corresponding error message is displayed.
In that case, you must replace the battery and repeat the test. Depending on the test result, the error message will disappear or another message will be displayed.
1.4Defibrillation procedure
All the stages are explained orally to the user at the same time as they are displayed on the screen. Once key  is pressed, the introductory text tells the user what is to be done if the patient has the following symptoms:
is pressed, the introductory text tells the user what is to be done if the patient has the following symptoms:
-the patient has ceased to breathe,
-there is no sign of circulation.
The introductory text is repeated till the FRED easy® device recognises the application of the adhesive electrodes. Depending on the configuration, the introductory text may be deleted and the device directly requests the application of the adhesive electrodes.
After that, the FRED easy® device asks the user to start an ECG analysis and to no longer touch the patient. The analysis lasts approximately 10 s. Depending on the configuration, the device starts the ECG analysis automatically.
Note
-With the signals from the AHA (American Heart Association) database, the detection accuracy of FRED easy® was found to be 98.43 % (sensitivity), with a specificity rate of 99.80 %.
-The device may be configured so that it automatically starts the ECG analysis.
If the analysis program recognises a heart rate that calls for defibrillation, it automatically charges the energy required for defibrillation and asks the user to deliver the shock after the energy is charged.
The following are considered to warrant defibrillation:
-ventricular fibrillation or
-ventricular tachycardia with a heart rate of over 180 bpm.
Part no. 0-48-0029 |
Page 1-3 |
May 2003 |

Operation
If the device finds a heart rate that calls for defibrillation, defibrillation is only authorised if the patient is found to have no pulse or if there are no signs of circulation.
If the defibrillation shock has no effect, the device automatically charges the energy required for a second or even a third shock.
Note
The energy values are set by default as follows (the technical assistance of SCHILLER can configure other default values if needed).
Shock |
Adult |
Child |
1 |
90 J |
15 J |
2 |
130 J |
30 J |
3 |
150 J |
50 J |
If the third shock is also ineffective, the FRED easy® device asks to user to alternately administer artificial respiration and heart massage. After a minute, an ECG analysis will be requested once again. Depending on the configuration, the new analysis may be automatic.
After a successful defibrillation shock, the FRED easy® device asks the user to check the patient^s breathing and blood circulation. If there is no sign of circulation, the device asks the user to alternately administer artificial respiration and heart massage. If there is any sign of circulation, the patient must be laid on his or her side.
If the analysis program does not recognise a heart rate calling for defibrillation,
-FRED easy® informs the user that defibrillation is not necessary and
-asks the user to look for breathing and signs of circulation.
If there is no sign of circulation, the FRED easy® device asks the user to alternately administer artificial respiration and heart massage. If there are any signs of circulation, the user is asked to lay the patient on his or her side.
After a minute, the FRED easy® device repeats the request for an ECG analysis. Depending on the configuration, the new analysis may be automatic.
The following values can be configured by the technical assistance department of SCHILLER:
-upon starting up: introductory text or immediate request to apply the adhesive electrodes,
-voice volume,
-energy levels of shocks 1, 2 and 3, with adult and child separate
-starting of the ECG analysis via the keypad or automatically.
Part no. 0-48-0029 |
Page 1-4 |
May 2003 |
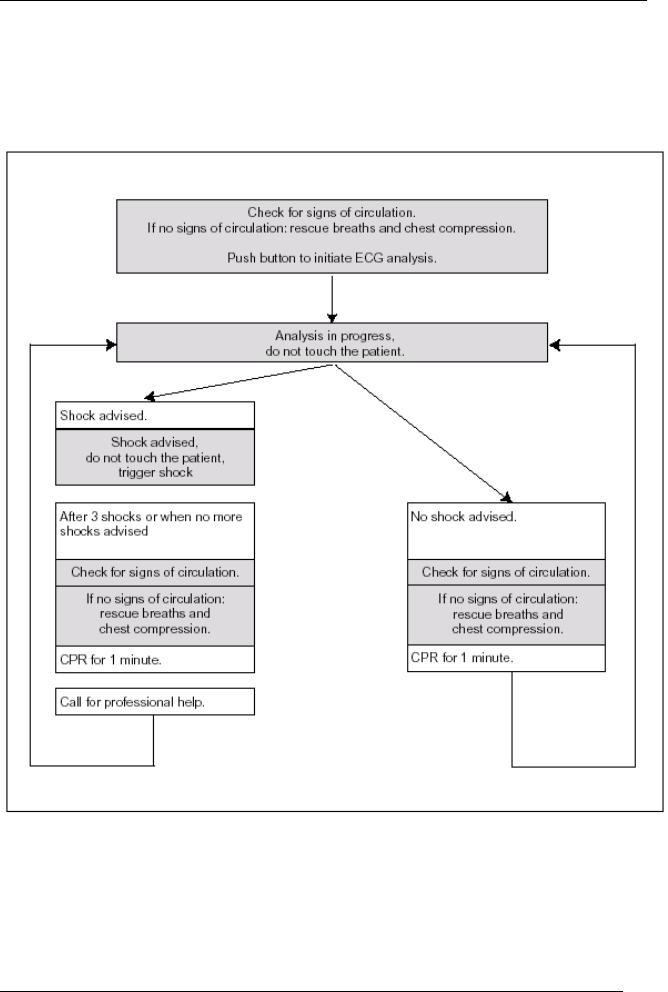
Operation
Procedure chart
Part no. 0-48-0029 |
Page 1-5 |
May 2003 |

Operation
1.5Recording (optional)
For information, the memory board can save the following:
-half an hour of ECG
-half an hour of speech,
-500 events relating to the procedure (see overview opposite).
Plugging in a memory card activates the memory function and the display includes the icon  .
.
The memory card is analysed by means of a PC, with the SAED Reader software.
Icon  flashes when the memory card is full.
flashes when the memory card is full.
Important! The device must be switched off when you insert the card. Otherwise, the device will not recognise the card and will display the following symbol: 
If no symbol is displayed even after the card has been put in place, you must make sure that the card is indeed a card designed for such devices by SCHILLER.
Part no. 0-48-0029 |
Page 1-6 |
May 2003 |
Operation
1.6Technical specifications
!Form of the defibrillation pulse
-Modulated defibrillation pulse with two pulse phases and physiologically optimised phase duration of 4 ms.
-Constant value of the average current delivered and the energy discharged depending on patient impedance, using pulse modulations in the pause position in the two phases.
-Modulation of the second phase (negative phase) so that the residual charges of the first phases are degraded in the heart.
!Standard energy settings:
-Adult (discharge in 50 .): 90 -130 -150 J
-Child (automatic switch when child electrodes are connected): 15 -30 -50 J
-The energy levels can be configured by the technical assistance department of Schiller if the standard values need to be changed:
1 - 2 - 4 - 6 - 8 - 15 - 30 - 50 - 70 - 90 - 110 - 130 - 150 J (Adult) 1 - 2 - 4 - 6 - 8 - 15 - 30 - 50 - 70 (Child)
-Tolerance at 50 ȍ: ± 3 J or ± 15 % (whichever is greater).
!Automatic charge control after a shock is recommended following an analysis
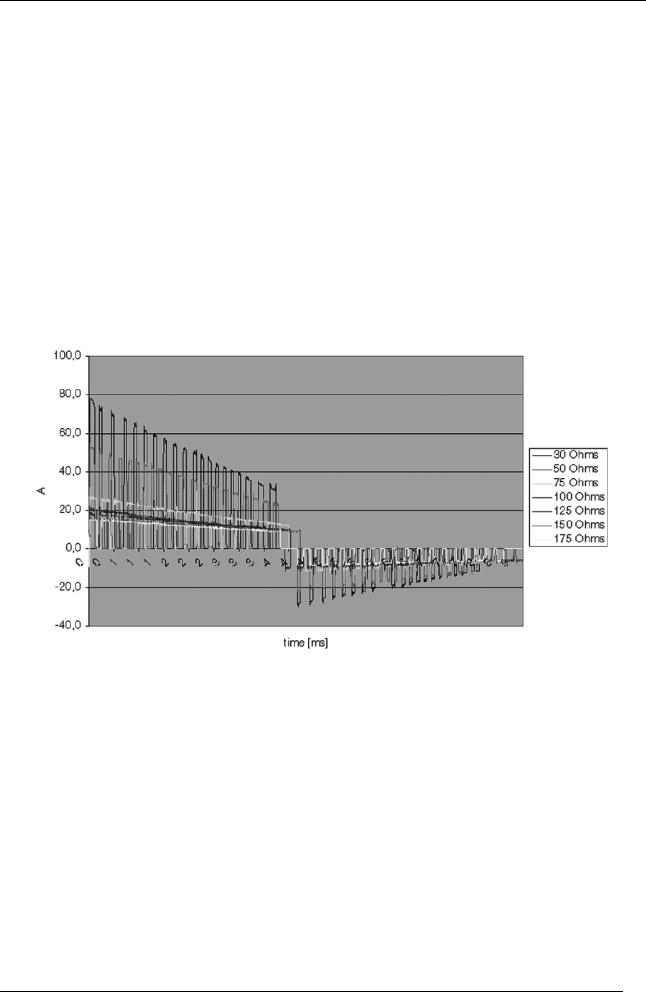
!Patient resistance 30 - 175 .
!Charge duration, from the time a shock is recommended up to the time when the device is ready:
<10 s
!Cycle time between two shocks:
<20 s
!Indication that the devices is ready to deliver a shock: key  goes on.
goes on.
!The shock is delivered with key
!Internal safety discharge if:
-the heart rate does not call for defibrillation
-after 20 seconds of the device indicating its readiness for a shock, no shock is delivered
-there is an electrode fault
-the battery voltage is insufficient
-the device is faulty
-the device is switched off
!The shock is delivered with single-use adhesive electrodes applied in the anterior / anterior-lateral positions
!BF type defibrillation electrode connector.
Part no. 0-48-0029 |
Page 1-7 |
May 2003 |

Operation
!Defibrillation electrodes:
-Adult electrodes: Active area 78 cm²
-Child electrodes: Active area 28 cm²
-Electrode cable length: 2 m
!VT / VF recognition:
-Shock recommendation: for VF and VT (VT > 180 bpm)
-Sensitivity: 98.43 %
Specificity: 99.8 %. These values have been found with the AHA database, which contains cases of VF and VT with and without artefacts.
- Conditions required for ECG analysis:
Minimum amplitude for the signals used > 0.15 mV , signals of < 0.15 mV are considered to show asystole.
- Definition:
Sensitivity: Correct detection of heart rates for which defibrillation shocks are recommended Specificity: Correct detection of heart rates for which defibrillation shocks are not recommended
!Display:
-LCD, 100 x 37 mm, high definition, with EL backlighting, display of text and icons.
!Recording of the use of the device (optional)
-ECG recording (half an hour)
-Voice recording (half an hour)
-Event recording (500 events)
!Lithium battery capacity
-200 shocks at the maximum power rating or
-Use of the monitor for seven hours (cyclical, 30 minutes on, 30 minutes off)
-Five years standing by.
!Environment conditions:
-Transport / storage:
Temperature - 30 to + 50 °C
Relative humidity of air 0 to 95 %, non condensing Atmospheric pressure 500 - 1060 hPa
- Use:
Temperature - 30 to + 50 °C
Relative humidity of air 0 to 95 %, non condensing Atmospheric pressure 700 - 1060 hPa
!Electromagnetic compatibility:
-The FRED easy® device only uses radio frequency range energy for its internal functions. It is treated against interference in accordance with standard CISPR 11 class B
-The FRED easy® device can be subjected to the following interference without any adverse effect on its functioning:
!electrostatic discharges of up to 8 kV.
!energy in the radio frequency range up to 20 V/m (80 - 2500 MHz, 5 Hz modulated).
!magnetic fields of 100 A/m, 50 Hz
!Dimensions and weight:
- Width |
: 220 mm |
|
- Depth |
: 230 mm |
|
- Height |
: 70 mm |
|
- Approximate weight |
: 1.5 kg (with battery) |
|
Part no. 0-48-0029 |
Page 1-8 |
May 2003 |

Testing and maintenance
2. Testing and maintenance
This section describes the testing and maintenance procedures recommended for FRED easy.
2.1Functional testing
The device runs an automatic functional test. In order to ensure proper performance, tests are performed when the device starts in the nominal mode (SAD mode), once a week and each time a battery is inserted. The Test mode defines specific device behaviour and is used to test its vital functions. The functioning of the Test mode is not redundant with the self test phase. The aim of the self test is to check the vital functions of the device when it is switched on. The aim of the Test mode is to regularly check the vital parts of the device in order to ensure that it will continue to perform even when it is used occasionally.
The device is also in the Test mode when a battery is inserted. In that case, specific tests are defined.
Specific case of self tests:
Self tests are performed automatically when the device is switched on and require no action by the operator. No message describing the test under way is displayed when the device runs its self tests. A message is displayed to merely show that the device is about to start n oSTARTINGpq.
The software version of the device is also displayed during the self test.
The self test consists in checking the following functions:
!ADC converter test n it is used to validate the functioning of the analogue to digital converter used to acquire battery parameters (voltage and temperature) and patient parameters (ECG 250Hz, MVTS, impedance variation, patient impedance)
!Defibrillator test, level 1 n used to validate the status of the defibrillator hardware and particularly to ensure that communication with the defibrillator is operational and that the defibrillation hardware module has not found any error.
!EEPROM configuration test n it is used to make sure that access to the EEPROM containing device configuration information is valid and that the information is consistent
!RTC test n used to validate the operating of the real time clock of the device
!Battery test n used to validate the battery level (the test shows an error if the remaining battery charge is 0%)
!LCD test n used to validate the display
!OKI test n used to validate the speech prompt system
!Encoder test n used to validate the operating of the sound environment acquisition system
If any of the tested peripherals shows an error, the device cannot be used. In that case, the device status display (flashing LED) is changed to indicate that the device may not be used n the LED goes off n and a message indicates the test that has failed. When the LED goes off, a buzzer is triggered to indicate a device fault.
If the tests succeed, the device executes the SAD operating mode.
Part no. 0-48-0029 |
Page 2-1 |
May 2003 |

Testing and maintenance
Specific case of periodic tests:
Because the device is used in emergency situations, it must be ready to operate when it is needed. As a result, self tests are insufficient. That is why periodic testing is performed to maximise the chances of detecting any error in the device, including when it is not used.
Periodic testing includes the following operations:
!Self tests
!Level 2 hardware test of the defibrillator module n test of a battery test command by the defibrillator module. This test is used to check that the power source of the device (lithium cell or battery) makes it possible to charge the device.
!ECG acquisition circuit test n checking the functioning of the ECG signal acquisition chain, which acts as the basis for analysing the patient^s heart signal. This test is used to make sure that the data on which the diagnostic is based are consistent.
The tests are performed automatically from time to time. Every week at 12.00 hours, the tests are performed if the device is off. The clock wakes the device for the requisite operations. The alarm date is configurable by means of the configuration PC.
Periodic tests are logged in order to be able to determine the nature of any failure and the time when the failure occurred. The last thirty results of periodic testing are logged in the memory. The memory can be read simply by means of a PC, in the configuration and transfer mode. That makes it possible to identify the origin of the problem found.
The logged data include the following:
!test date
!test time
!test result
!origin of the problem found, if the result shows an error
The detection of a problem during a periodic test makes the device impossible to use. The Status display is changed in order to indicate that a problem has been found. Nothing is displayed during the periodic tests in order to maximise the use of machine power. That is why the Status display is the only means of indication.
When the device is switched on again, an error message indicates the problem found during periodic testing. Each time the device is switched on, the error message is displayed. The error disappears when the problem is corrected and when new tests succeed after battery insertion.
If the tests are successful, the device is switched off.
Specific case of battery insertion tests:
Given that periodic tests cannot be performed when the device no longer has a battery, the device must be tested as soon as a new battery is inserted, in order to indicate any fault as soon as possible. Tests are triggered as soon as a battery is inserted in the device.
In that case, the test and the operations performed are:
!Self tests if no error is found during previous periodic tests
!Self tests, level-2 hardware test of the defibrillator module and ECG acquisition circuit tests, if an error has been found during the previous periodic tests.
If any of the tested peripherals shows an error, the device cannot be used. In that case, the device status display (flashing LED) is changed to indicate that the device may not be used n the LED goes off n and a message indicates the test that has failed.
Part no. 0-48-0029 |
Page 2-2 |
May 2003 |
Testing and maintenance
If the battery insertion test is successful and if the last logged periodic test has failed, the battery insertion test is logged. The logged data include:
!test date
!test time
!test result - OK
!reset information, to indicate that a problem had been found during periodic tests and has been corrected.
If the tests are completed successfully, the device goes off automatically at the end of the operation. Otherwise, the device stays on to indicate the failed test.
2.2Systematic verification before use
Before each use of the device, the device, cables, connectors and electrodes must undergo a visual inspection.
If you find any faults or malfunctioning that could impact the safety of the patient or the user, do not use the device before it is repaired.
Systematic verification before each use
!Verification of the device housing
!No mechanical damage
!No penetration of liquid in the device
!Check the condition of the control buttons and the connectors.
2.3Cleaning and disinfecting
Caution: Switch the device off before cleaning it. Remove the cell " before you start cleaning the device in order to eliminate the risk of the device starting up accidentally. Also disconnect
the defibrillation electrodes of the device before cleaning.
No liquid shall be allowed to enter into the device. However, if that does happen, the device may not be used before it is verified by the after-sales service department.
The device or electrodes may never be cleaned with substances such as ether, acetone, esters, aromatic chemicals etc.
Never use phenol-based cleaners or cleaners containing peroxide derivatives to disinfect the surfaces of the housing of the device.
!Dispose of all single-use electrodes immediately after use in order to eliminate the risk of accidental reuse (disposal with hospital waste).
!Before cleaning the electrode cables of sensors, disconnect them from the device. For cleaning and disinfecting, wipe the cables with a gauze cloth moistened with cleaner or disinfectant. Never immerse the connectors in liquid. The cleaning solution used may be any cleaning or disinfecting solution that is commonly used in hospitals.
!Proceed likewise with the device housing, with a cloth moistened with cleaner or disinfectant. No liquid may be allowed to penetrate into the device during the operation.
Part no. 0-48-0029 |
Page 2-3 |
May 2003 |

Troubleshooting
3. Troubleshooting
This section addresses the troubleshooting procedures for FRED easy®. If you have trouble locating or correcting the problem, contact the after-sales service department of Schiller.
Precautions during troubleshooting
While testing the FRED EASY defibrillator, the patient may only be simulated with fixed high-voltage and high-power resistors that are well insulated from the ground or earth. Poorly insulated devices or devices with loose contacts or devices containing components such as spark arresters or electronic flash lamps may never be used as they could irremediably destroy the device.
The user^s manual contains a troubleshooting table intended for the user.
ERROR |
FINDING |
|
POSSIBLE CAUSES |
|
CORRECTIVE ACTION |
|
|
|
|
|
|
|
|
1. |
Cell fault |
1. |
Replace the cell |
The device is not switched on when the battery is |
2. |
Incorrect insertion |
2. |
Repeat the insertion |
|
inserted. |
|
3. |
Problem with contacts |
3. |
Check contacts |
|
|
4. |
Problem with fuses |
4. |
Check fuses |
|
|
|
|
|
|
When the cell is inserted, the self test succeeds |
1. |
Fuse F1 on CPU board |
1. |
Replace fuse |
|
but the OK indicator does not flash. |
faulty |
|
|
||
|
|
|
|
|
|
No display of message: |
|
1. |
Check the button cell on |
1. |
Replace the button cell |
FRED IS TESTING |
|
the CPU (out of order or |
2. |
Replace fuse |
|
|
|
down) |
|||
|
|
2. |
Check if F1 is faulty on |
|
|
|
|
DEFI board |
3. |
Replace CPU |
|
|
|
3. |
CPU board faulty |
4. |
Replace DEFI board |
|
|
4. |
DEFI board faulty |
|
|
|
|
|
|
|
|
When the cell is inserted, no self test but the OK |
1. |
CPU board faulty |
1. |
Replace CPU |
|
indicator flashes. |
|
|
|
|
|
|
|
|
|
|
|
The self test succeeds, but the OK indicator does |
1. |
CPU board faulty |
1. |
Replace CPU |
|
not flash. The screen stays on for 5 s |
|
|
|
|
|
|
|
|
|
|
|
The On/Off key does not |
No display and/or no |
1. |
CPU board faulty |
1. |
Replace CPU |
respond. |
voice messages |
|
|
|
|
|
|
|
|
|
|
Message: |
|
1. |
DEFI board |
1. |
Replace DEFI board |
DEFIBRILLATOR |
|
|
|
|
|
CPU ERROR |
|
|
|
|
|
|
|
|
|
|
|
Part no. 0-48-0029 |
Page 3-1 |
May 2003 |

Troubleshooting
Message: |
|
1. DEFI board |
1. Replace DEFI board |
|
DEFIBRILLATOR |
|
|
|
|
INTEGRITY ERROR |
|
|
|
|
PROGRAM |
|
|
|
|
|
|
|
|
|
Message: |
|
1. DEFI board |
1. Replace DEFI board |
|
DEFIBRILLATOR |
|
|
|
|
DETECTOR ERROR |
|
|
|
|
|
|
|
|
|
Message: |
|
1. DEFI board |
1. Replace DEFI board |
|
DEFIBRILLATOR ERROR |
|
|
||
VOLTAGE REFERENCE |
|
|
|
|
|
|
|
|
|
Message: |
|
1. DEFI board |
1. Replace DEFI board |
|
DEFIBRILLATOR |
|
|
|
|
CONVERTER ERROR |
|
|
|
|
ADC |
|
|
|
|
|
|
|
|
|
Message: |
|
1. DEFI board |
1. Replace DEFI board |
|
DEFIBRILLATOR |
|
|
|
|
CHARGE TRANSISTOR |
|
|
|
|
ERROR |
|
|
|
|
|
|
|
|
|
Message: |
|
1. DEFI board |
1. Replace DEFI board |
|
DEFIBRILLATOR |
|
|
|
|
SAFETY DISCHARGE |
|
|
|
|
ERROR |
|
|
|
|
|
|
|
|
|
Message: |
|
1. CPU board |
1. Replace CPU board |
|
DEFIBRILLATOR |
|
|
|
|
EPROM ERROR |
|
|
|
|
|
|
|
|
|
Message: |
|
1. CPU board |
1. Replace CPU board |
|
DEFIBRILLATOR |
|
2. DEFI board |
2. Replace DEFI board |
|
SHOCK BUTTON ERROR |
|
|
||
|
|
|
|
|
Message: |
|
1. CPU board |
1. Replace CPU board |
|
DEFIBRILLATOR |
|
2. DEFI board |
2. Replace DEFI board |
|
COMMUNICATION ERROR |
|
|
||
|
|
|
|
|
Message: |
|
1. CPU board |
1. Replace CPU board |
|
SYSTEM ERROR |
|
|
|
|
|
|
|
|
|
Fault detected during |
|
Monitor on, but the |
1. CPU board |
1. Replace CPU board |
the self test |
|
OK LED does not |
|
|
|
|
flash |
|
|
|
|
|
|
|
Part no. 0-48-0029 |
Page 3-2 |
May 2003 |

Troubleshooting
Cell symbol flashing, but no remaining % |
1. |
Cell contact fault |
1.1 Check cell contact |
|||
|
|
|
|
|
1.2 Replace cell |
|
Electrodes not connected and LED under |
1. |
DEFI board faulty |
1. |
Replace DEFI board |
||
electrode connection off |
|
2. |
CPU board faulty |
2. |
Replace CPU board |
|
|
|
|
|
|
|
|
Electrodes connected to simulator with 50-ohm |
1. |
DEFI board faulty |
1. |
Replace DEFI board |
||
impedance, but the LED does not go off |
2. |
CPU board faulty |
2. |
Replace CPU board |
||
|
|
|
|
|
|
|
No cell voice message |
|
1. |
Speaker fault |
1. |
Replace speaker |
|
|
|
|
2. |
CPU board fault |
2. |
Replace CPU board |
|
|
|
|
|
|
|
Device off, OK LED off |
|
Audio alarm |
1. |
Buzzer fault |
1. |
Replace buzzer |
and no buzzer |
|
|
2. |
CPU board fault |
2. |
Replace CPU board |
|
|
|
|
|
|
|
Green Analysis key or orange Shock key not on |
1. |
CPU board fault |
1. |
Replace CPU board |
||
|
|
|
|
|
|
|
Abnormal internal discharge |
|
1. |
DEFI board faulty |
1. |
Replace DEFI board |
|
|
|
|
|
|
|
|
No shock delivered |
|
1. |
DEFI board faulty |
1. |
Replace DEFI board |
|
|
|
|
2. |
CPU board fault |
2. |
Replace CPU board |
|
|
|
|
|
|
|
No record on memory card |
|
1. |
Memory card |
1. |
Replace memory board |
|
|
|
|
2. |
CPU board fault |
2. |
Replace CPU board |
|
|
|
|
|
|
|
Loss of date and time |
|
1. |
Button cell |
1. |
Replace button cell |
|
|
|
|
2. |
CPU board fault |
2. |
Replace CPU board |
|
|
|
|
|
|
|
Part no. 0-48-0029 |
Page 3-3 |
May 2003 |

Replacement of parts
4. Replacement of parts
This section addresses the issue of how to dismantle FRED easy in order to replace faulty parts. The warnings below apply to all work inside the device.
!
"
"
Warning: |
FRED easy is a defibrillator with a high-voltage capacitor that |
|
can be charged to a fatal voltage. The device may only be |
|
dismantled by specially authorised and trained personnel. |
|
|
|
|
Caution: |
Before dismantling the device, remove the battery or the cell |
|
from its slot. |
|
|
|
|
Caution: |
The device contains circuits sensitive to electrostatic |
|
discharge. All work on the FRED! easy device shall be |
|
performed in accordance with ESD rules. The repairs shall be |
|
performed on an antistatic mat connected to the earth and the |
|
operator shall wear an antistatic strap also connected to the |
|
mat. In the event of any work on the high-voltage part of the |
|
defibrillator, remove the antistatic strap. |
|
|
"
"
Caution : |
During the replacement of one of the boards, it should |
|
absolutely be seen whether the versions are compatible by |
||
|
||
|
consulting the table available at Schiller Medical. Only the |
|
|
versions are compatible which comprise a HARDWARE |
|
|
number in the table of the versions. |
|
|
On your device, the HARDWARE version number perhaps |
|
|
consulted only with the module of remote loading. For each |
|
|
replacement of chart, it is necessary to charge the good |
|
|
number of HARDWARE version given speaks table. |
|
|
|
|
Caution: |
A general device test shall be performed each time the device |
|
|
is opened. |
|
|
|
Part no. 0-48-0029 |
Page 4-1 |
May 2003 |

Replacement of parts
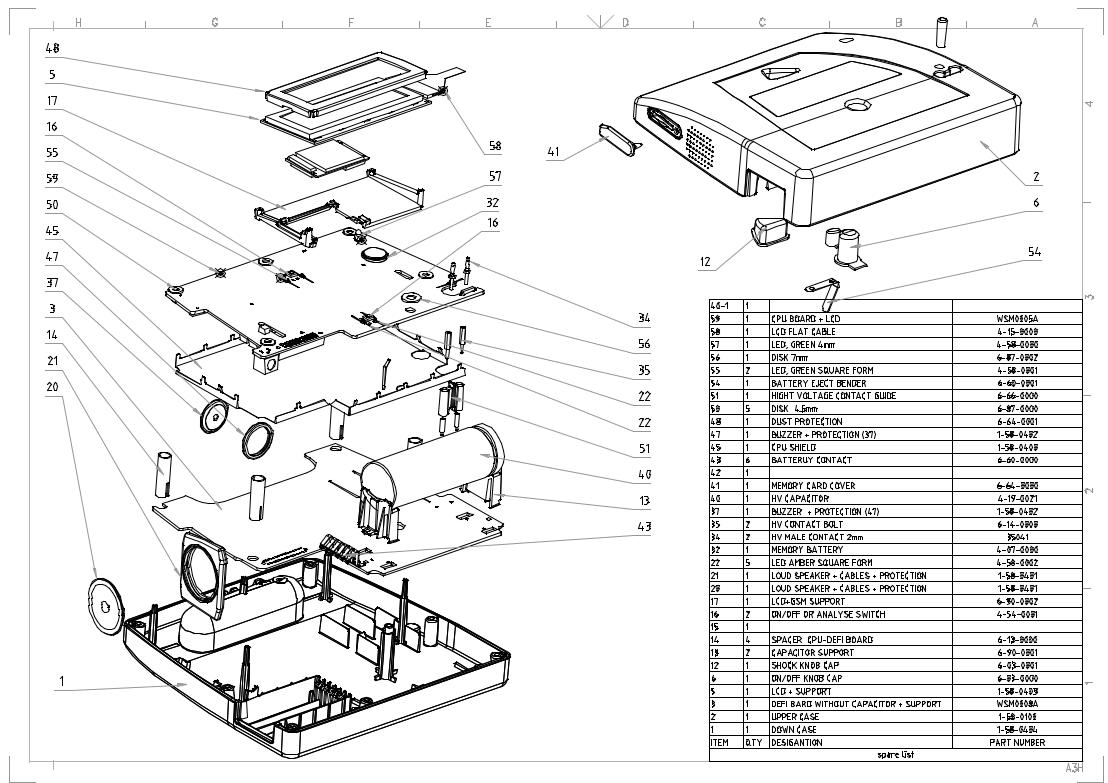
4.1Device disassembly procedure
Follow the points below while disassembling the device:
1.Remove the Lithium cell from its slot.
2.Disconnect the electrode cables.
3.Turn the device over (LCD screen down) and unscrew the nine assembly screws of the two (upper and lower) halves of the housing.
4.After removing the nine screws turn the device over (LCD screen facing you).
5.The upper half can now be removed by pulling it up gently. The electrode connections to the left and the speaker to the right of the device may offer some resistance. In order not to pull off the speaker wires, hold it in the lower part while removing the upper part.
" |
Caution: |
Take care not to lose the caps of the control buttons placed |
|
in the upper part. |
|
|
|
|
Part no. 0-48-0029 |
Page 4-2 |
May 2003 |

Replacement of parts
4.2Working on the CPU circuit
Follow the instructions below to remove the CPU:
1.Remove the six rubber washers.
2.Disconnect the CPU from the connector to the rear of the device near the battery slot.
3.Take off the buzzer and the speaker of the lower housing and carefully pull the connectors.
" |
Caution: |
The circuit includes components that are sensitive to |
|
electrostatic discharge. The work described above shall be |
|
|
|
performed in accordance with ESD rules. |
|
|
|
4.3Working on the LCD display
After removing the CPU PCB, the LCD display can be removed by following the instructions below:
1.Carefully remove the connector of the backlighting circuit connected to the CPU.
2.Remove the connector of the flat jumper.
3.Unscrew the four screen fastening screws.
The liquid crystal display is a version with backlighting. The two functions cannot be dissociated.
If the display is to be replaced, make sure the new screen is clean. Never clean the surface of an LCD screen with cloth or paper that could be very slightly abrasive and scratch the screen. Any dust should be removed by blowing compressed air.
4.4Working on the DEFI circuit
To remove the DEFI circuit, remove the spacers and pull out the circuit, which is just placed inside the housing.
" |
Caution: |
The circuit includes components that are sensitive to |
|
electrostatic discharge. After the PCB is disconnected from |
|
|
|
the device, comply with ESD rules. |
|
|
|
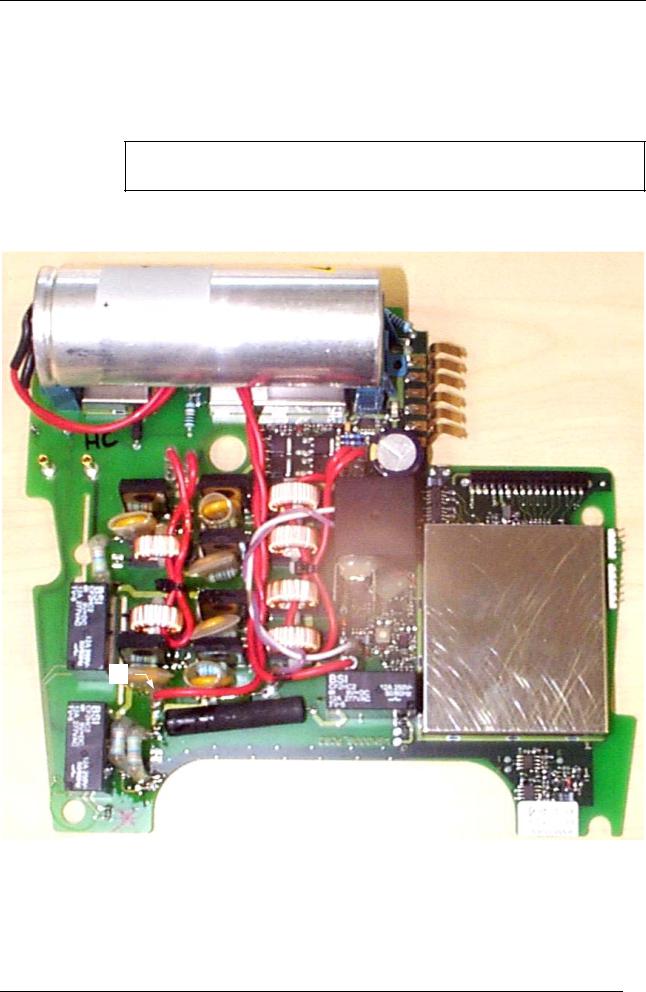
4.5Replacing the high-voltage capacitor
! |
Warning: |
This operation relates to the high-voltage capacitor, which |
|
can carry fatal charges. Before starting to work, take care to |
|
|
|
discharge the high-voltage capacitor completely. The |
|
|
terminals of the high-voltage capacitor must never be |
|
|
touched directly. The high-voltage capacitor may never be |
|
|
replaced by people other than specially authorised and |
|
|
trained personnel. |
|
|
|
Part no. 0-48-0029 |
Page 4-3 |
May 2003 |
Replacement of parts
The replacement of the high-voltage capacitor is an extremely rare operation, as the life of the capacitor is very long. However, if needed, the high-voltage capacitor may be replaced in accordance with the following instructions:
1.Disconnect the two Faston lugs
2.Take the capacitor off its support
After removing the (fully discharged) high-voltage capacitor from the lower
# part, short the three terminals of the capacitor with conducting wire.
While replacing the high-voltage capacitor, glue it to the support, twist the wires and wire them in accordance with the polarity requirements. The wires are to be routed as required.
Make sure that nothing has been forgotten before the device is started up again.
+
" |
Caution: |
This operation relates to an essential component of the high- |
|
voltage part. It may only be performed by specially authorised |
|
|
|
personnel who have been trained in repairing FRED! easy |
|
|
devices. |
|
|
The delivered energy must undergo testing. |
|
|
|
Part no. 0-48-0029 |
Page 4-4 |
May 2003 |
Replacement of parts
4.6Reassembling the device
To put back the device, reverse the procedures.
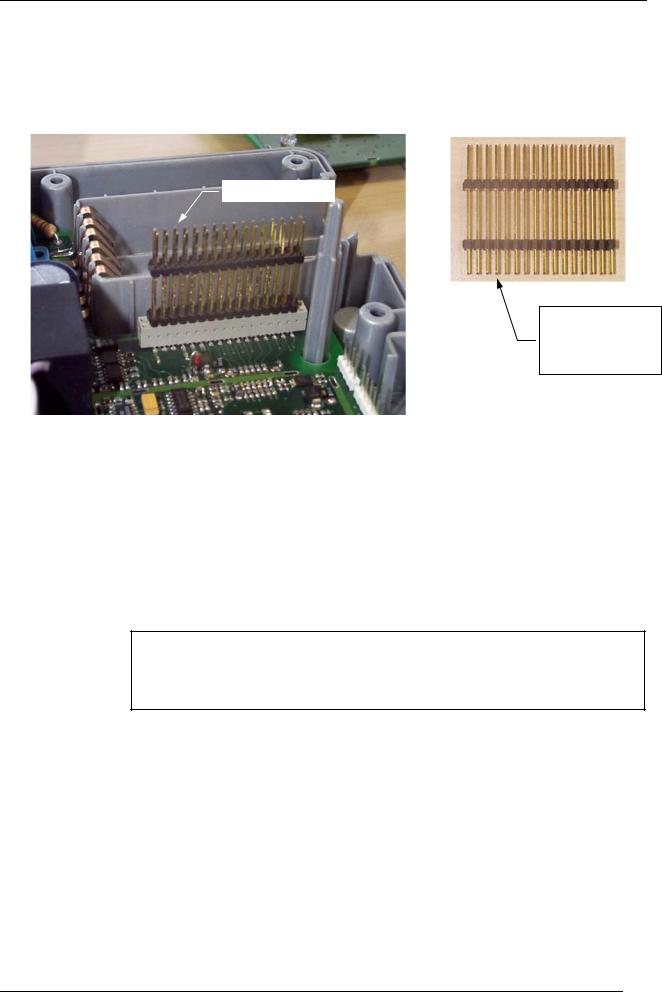
Instructions for assembling the connector between boards:
4-21-0003
Short connections on the DEFI circuit
4.7Replacement of parts
! |
Warning: |
Parts may only be replaced by personnel who have been |
|
specially trained and authorised by Schiller Medical. |
|
|
|
Besides, the replacement parts shall be original Schiller |
|
|
Medical parts. |
|
|
|
Note: To order a new part from Schiller Medical, provide the part number and
# the serial number of the device located under the device. After that, specify the item code of the part, the part number, the description provided in the list of parts and the ECL of the replaced part.
Part no. 0-48-0029 |
Page 4-5 |
May 2003 |
|
|
Device modifications |
Part no. 0-48-0029 |
Page 4-1 |
May 2003 |

Technical description of boards
5. Technical description of boards
5.1FRED easy
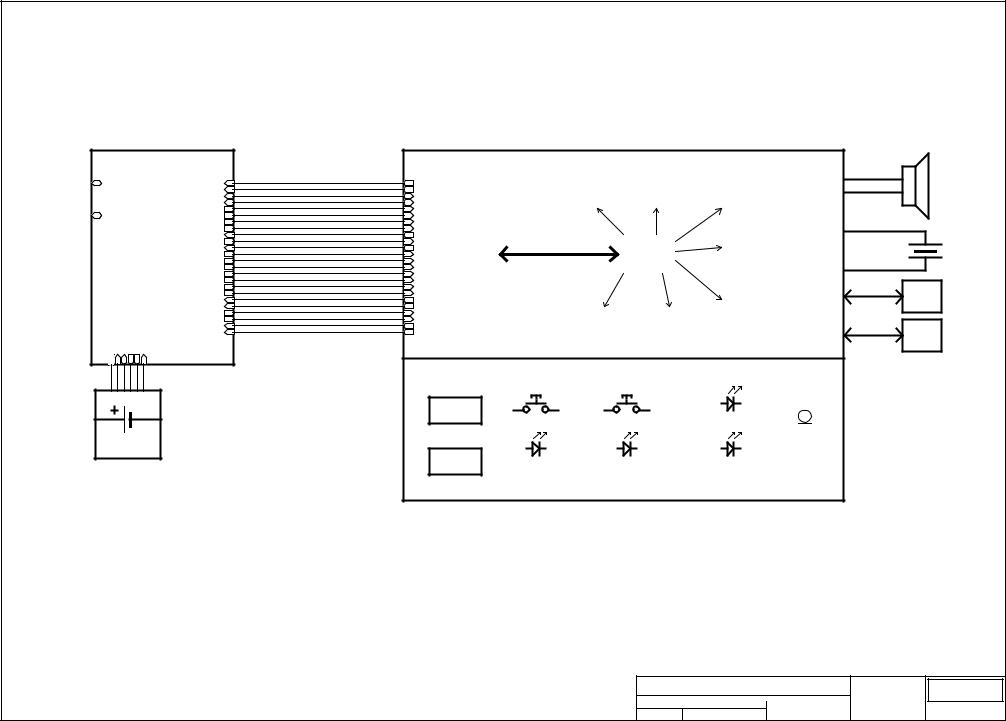
General description of FRED easy:
FRED easy is technically divided into two subassemblies including:
!The DEFI board includes the various digital processing functions specific to the defibrillator, the analogue processing functions and the high-voltage circuit of the defibrillator.
!The CPU board includes the various functions relating to digital processing, analogue processing, data saving, additional power supplies and controls and displays.
The two boards communicate electrically between each other through a 32-pin connector JP(4).
Controls, power supply, display and recording:
The various controls and display elements of the CPU board are as follows:
!An LCD screen that acts as the visual interface between FRED easy and the user.
!A dual-function key (On/Off and Analysis) for controlling device switching on and off. Its other function consists in enabling the starting of a patient signal analysis.
!Two green LEDs indicative of the On/Off n Analysis keys offer an additional visual interface for the user.
!The SHOCK key controls the delivering of a defibrillation shock.
!The orange LEDs indicative of the Shock key offer an additional visual interface for the user.
!Status LED that continuously indicates the success of the last device self test
!A buzzer to indicate any anomaly found at the last self test
!An Electrode Fault LED to indicate where the defibrillation electrodes are to be connected. Also provides information about the continuity of the electrical circuit.
!A speaker for the messages intended for the user.
!A microphone for recording the sound in the environment.
!An MMCard for recording the ECG signal, the events of the operation and the environment sounds.
!A Mini DIN 7 connector for the serial link with a PC in order to download programs and configure FRED easy. During these operations, the board is powered by an external power source through the same connector.
Part no. 0-48-0029 |
Page 5-1 |
May 2003 |
|
|
|
Central Process Unit |
|
|
|
|
|
|
|
|
|
|
|
|||
|
DEFIBRILLATOR BOARD |
|
|
|
|
CPU BOARD |
|
|
|
|
|
|
|
|
|||
|
WSM0008 |
|
|
|
|
WSM0005 |
|
|
|
|
|
|
|
|
SPEAKER |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ts (Soldered |
APEX |
UBAT_FUSED_CPU |
UBAT_FUSED_CPU |
|
Analog Process |
Serial |
|
ADPCM |
|
|
JP1 |
|
|
|
|
||
|
Of |
|
Encode/Decode |
|
|
|
|
||||||||||
|
|
DISPO_DEFI_3 |
DISPO_DEFI_3 |
|
CPUMMC |
|
|
|
|
|
|||||||
|
|
BATT_NC |
BATT_NC |
|
|
|
|
|
|
|
|||||||
|
|
|
ECG / ADC |
|
+ Amplification |
|
|
|
|
|
|
||||||
|
|
SDA |
SDA |
|
|
|
|
|
|
|
|
|
|
||||
|
|
SCL |
SCL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STERNUM |
CHOC_KEY |
CHOC_KEY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ONOFF_ANALYSE_KEY |
ONOFF_ANALYSE_KEY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RST_DEFI |
RST_DEFI |
|
|
|
|
|
|
|
|
|
|
|
|
|
BUZZER |
|
|
TXD_DEFI |
TXD_DEFI |
|
|
|
Microcontroller |
|
Led Status |
|
|
|
|
|
|||
|
|
RXD_DEFI |
RXD_DEFI |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
Alarm Buzzer |
|
|
|
|
|
|
|||||
|
|
ECG_DEFI |
ECG_DEFI |
JP4 |
|
|
Process |
|
|
JP2 |
|
|
|
|
|||
|
|
INH_PACE/ |
INH_PACE/ |
|
|
|
|
|
|
|
|
|
|
||||
|
|
-5V |
-5V |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
+3.3V |
+3.3V |
|
|
|
Unit |
|
|
|
|
|
|
|
|
|
|
|
|
+5V |
+5V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TYPE_ELECTR |
TYPE_ELECTR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BACK_005HZ |
BACK_005HZ |
|
|
|
|
|
|
|
|
|
|
|
|
LCD |
|
|
|
10HZ |
10HZ |
|
|
|
|
|
|
LCDInterface |
|
JP3 |
|
|
|
||
|
|
ECG_LB |
ECG_LB |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
Z_ELEC_DEFI |
Z_ELEC_DEFI |
|
Serial Com |
|
|
|
|
|
|
|
|
|
|
||
|
|
DISPO_DEFI_1 |
DISPO_DEFI_1 |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
DISPO_DEFI_2 |
DISPO_DEFI_2 |
|
|
RS232 |
|
Start/Stop |
|
|
|
|
|
|
|
|
|
|
GND BATT NC SDA SCL +3.3V UBATT |
DELTA_Z |
DELTA_Z |
|
|
|
|
|
|
JP6 |
|
|
GSM |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
INHIBITION/ |
INHIBITION/ |
|
|
I2C |
|
Power |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SOLDERED ON PCB BOARD |
|
|
|
|
|
|
|
|
||||
|
BATTERY PACK |
|
Minidin 7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Connector |
|
CHOC_KEY |
ONOFF_ANALYSE_KEY |
LED ELECTRODES |
|
|
|
|
|
|
||||
|
Li-MnO2 |
|
|
|
|
|
|
|
|
|
|
|
ELECTRET |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MICROPHONE |
|
|
|
|
||
|
12V - 2.8Ah |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
SDCARD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LED CHOC |
|
LED ANALYSE |
|
|
LED STATUS |
|
|
|
|
|
|
|
|
|
|
|
Connector |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Central Process Unit |
|
|
|
Schema No. : |
|
|
||||
|
|
|
|
|
|
|
|
|
|
WSM0005_SYN2 |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Error : Schiller Med.S.A.Logo.bmp f |
|||
|
|
|
|
|
|
|
Project : 01.58.E.09 |
|
PCB No.: WSM0005_PCB2 |
Date: |
08/01/03 |
||||||
|
|
|
|
|
|
|
|
4, rue Louis Pasteur |
|||||||||
Part no. 0-48-0029 |
Page 2 |
|
|
May 2003Size: |
A3 |
Drawn by : JME/NF |
Art. No. : WSM0005A |
Sheet |
1 of |
9 |
ZAE Sud BP50 |
||||||
|
|
67162 WISSEMBOURGCEDEX |
|||||||||||||||
 Loading...
Loading...