Page 1

MAX 30 Oxygen Concentrator
Installation & Maintenance Manual
Nidek Medical Products, Inc.
3949 Valley East Industrial Dr, Birmingham, AL 35217, USA
Tel: (205) 856-7200 Toll Free: (800) 822-9255 Fax: (205) 856-0533
E-mail:info@nidekmedical.com
Website: www.nidekmedical.com
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 1 of 28
Page 2
Table of Contents
Topic Page
Using this Manual 3
Initial Inspection / Typical Application 4
Warranty Information and Liabilities 5
General Safety Guidelines
Product Information
>
Features and Applications
>
PSA Technology
>
Components
>
Process Flow Diagram & Description
>
Specifications
Safety Precautions
Pre – Installation
Required Operating Conditions
Set-up and Installation
Operating Instructions
Troubleshooting Guide
Preventive Maintenance
6
7
7
8-14
15-16
17
18
19
20
21
22
23
24
Technical Service Assistance
Appendix
A
Spare Parts List
B
Maintenance Log
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 2 of 28
25
26-27
28
Page 3

USING THIS MANUAL
This guide is intended for operators and users of NIDEK Medical Products. It includes
information on our warranty, policy, features, functions, proper set-up and installation,
operation and preventive maintenance of our device.
The following symbols are used throughout this guide.
ON (Mains Power switched on)
OFF (Mains Power switched off)
Type B Device
Class I Electrical Protection
DO NOT EXPOSE TO OPEN FIRE
DO NOT USE OIL OR GREASE
Technical Information
Consult the accompanying documents
Keep in a vertical position
Fragile – Handle with care
Sound-Listen for Sound
General Warning
Timer-
QMS certified to Annex II of 93/42/EEC by
the approved organization 0413
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 3 of 28
Page 4

Initial Inspection
The crate should be opened and inspected immediately upon delivery. Unpack the device
at once and perform a visual inspection to determine if it is dented, bent or scratched. Also check
to make sure the power cord is attached and that the control panel has not been damaged in any
way during shipment.
At Nidek Medical Products (NMP), we are committed to using shipping
companies with good reputations for taking care in the handling of freight and providing
service in the event of damage.
TYPICAL APPLICATIONs
Oxygen Concentrator Small Clinic
Fish Farms Ozone Production
Glass Blowing Welding
Laboratory Use Aquaculture
Veterinary Clinic
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 4 of 28
Page 5

Warranty
Nidek Medical Products, Inc. (NMP). warrants to the original dealer-purchaser of a NMP MAX 30 Oxygen concentrator,
that it shall: 1) Conform to Nidek Medical’s specifications, subject to ANSI tolerances, at the time of manufacture and 2) be
free of defects in material and workmanship for a period of twelve (12) months from the date of delivery.
To make claim under this warranty, the Purchaser must: 1) Give Nidek Medical written notice of the breach of warranty,
within ten (10) days after discovery of such breach; 2) immediately upon discovery of the claimed breach, discontinue all
use of the enricher; and 3) upon the request of Nidek Medical, return the concentrator or the applicable component part,
freight prepaid, to Nidek Medical’s plant of manufacture or such other location as designated by Nidek Medical. If it is
determined by Nidek Medical that the concentrator or the applicable component is in breach of warranty, Nidek Medical, at
its option, will repair or replace it without charge.
The cost of returning the concentrator or component part to the Purchaser after repair or replacement will be paid by Nidek
Medical. If, however, any concentrator or component part returned by the Purchaser because of an alleged breach of
warranty is found by Nidek Medical not to be in breach of warranty, then the concentrator or component part will be
returned to the Purchaser, shipping charges collect, and the Purchaser agrees to pay a service charge to Nidek Medical to
cover the cost of handling and testing the concentrator or component part. Dealer labor costs for removal and replacement
of parts under warranty are not covered and are the responsibility of the dealer.
This warranty is void if the concentrator or any component part thereof has been damaged by accident, abuse, misuse,
neglect, alteration, improper service, repair by other than authorized personnel or other causes not arising out of defects in
material or workmanship. Wear of components in normal operation, and failures resulting there from, as determined by
Nidek Medical, are excluded from this warranty.
This warranty is not assignable by the Purchaser.
NIDEK MEDICAL MAKES NO OTHER WARRANTIES OF ANY KIND WHATSOEVER, EXPRESS OR IMPLIED,
WITH RESPECT TO THE CONCENTRATOR OR ITS COMPONENT PARTS AND ALL IMPLIED WARRANTIES,
INCLUDING, BUT NOT LIMITED TO, WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A
PARTICULAR PURPOSE ARE HEREBY EXPRESSLY DISCLAIMED AND EXCLUDED BY NIDEK MEDICAL.
Nidek Medical’s non-exclusive liability with respect to the concentrator shall be to repair or replace (at Nidek Medical’s
sole option) the concentrator or any of its component parts that prove to be defective in materials or workmanship during the
warranty period. Normal maintenance required during the warranty period is not included in this warranty. No claim of any
kind whatsoever against Nidek Medical with respect to the concentrator or its component parts whether or not based in
contract, warranty, negligence, strict liability in tort, or any other theory of law, shall be greater in amount that the purchase
price of the concentrator. Without limiting the generality of any of the foregoing, Nidek Medical shall in no event be liable
for any special, indirect, incidental, or consequential damages.
Nidek Medical Oxygen Concentrator products shall not be used for breathable or medical oxygen applications; unless they
are assembled with the appropriate support equipment, tested, and operated in compliance with either American, Canadian
or ISO norms for hospital oxygen systems
If the Nidek Medical Oxygen Concentrator product is planned to be used to supply oxygen to a high pressure filling station,
please refer to:
• CGA publications that can be found at http://www.cganet.com
• ISO 10083 that can be found at http://www.iso.org
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 5 of 28
Page 6
GENERAL SAFETY GUIDELINES
Only persons who have read and understood this entire manual should be allowed to
install and operate the Max 30 Oxygen Concentrator (hereafter known as the device).
The WARNINGS below indicate a potentially hazardous situation. If conditions are not
avoided a situation could occur that results in serious injury or death.
• Oxygen is not a flammable gas, but it accelerates the combustion of materials. Do not use in
explosive atmosphere. To avoid risk of fire and explosion the concentrator should be kept
away from Flames, Heat sources, Incandescent sources, Smoking Materials, Matches, Oil,
Grease, Solvents, Aerosols, etc. Do not allow oxygen to accumulate on upholstery or other
fabric such as bedding or personal clothing. If concentrator is operating while not connected
to patient, position cannula so that the gas flow is diluted in the ambient air.
• Improper patient connection to and use of the cannula may result in injury including
strangulation. Avoid situations that might cause the cannula or hose to become entangled
about the patient’s neck.
• Use of other accessories not described in this User's Guide are not recommended. Patient
benefit may be diminished.
• No modification to the equipment is allowed. To do so may affect patient benefit.
• Contraindications; those who continue to smoke (because of the increased fire risk and the
probability that the poorer prognosis by smoking will offset the treatment benefit).
• Device must have power to operate. In the event of power loss and for continued operation a
backup source is recommended.
• DO NOT disassemble due to danger of electrical shock. Refer servicing to qualified service
personnel.
• To avoid the risk of electric shock, this equipment must only be connected to a supply
mains with protective earth. If not available, contact a qualified electrician. Do not defeat
this safety feature.
The CAUTIONS below indicate a potentially hazardous situation. If conditions are not
avoided a situation could occur that results in property damage or minor injury or
both.
• Use the power cord provided, and check that the electrical characteristics of the power
socket used match those indicated on the manufacturer’s plate on the rear panel of the
device.
• We recommend against the use of extension cords and adapters, as they are potential
sources of sparks and fire.
• The device has an audible alarm to warn the user of problems. In order that the alarm may
be heard, the maximum distance that the user can move away from it must be determined to
suit the surrounding noise level.
• The device must only be used for oxygen therapy and only on a medical prescription. The
indicated daily duration and flow must be followed, otherwise it may present a risk to the
health of the patient.
• Do not position device so that it is difficult to access the mains power cord, so that it
accessible for disconnect.
• Do not use in a specifically magnetic environment (MRI, X-ray, etc.). May cause device malfunction.
• Note: Medical Device Regulations require users and service providers to report to the manufacturer
any incident that could, if repeated, result in injury to any person.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 6 of 28
Page 7

Product Information
Features and Applications
The NMP Model MAX 30 extracts oxygen from the atmosphere using Pressure Swing
Adsorption (PSA) technology. It concentrates oxygen up to 93% (± 3 %) purity which
can be applied in various ways.
Features
Easy to use
Just connect to an electrical outlet, turn the Master Switch ON/OFF power switch to the ON
position and press the START button on the front display panel and set the desired flow rate.
Dependable
Its internal air compressors, filtration system, molecular sieve, storage tanks and flow control
system are designed for 24/7 operation.
Durable
With oxygen-clean brass tubing and valves, the MAX 30 can operate even in environments as
described under the specifications page.
Safe
A built-in oxygen pressure regulator maintains oxygen outlet pressure at 50 psi (3.4 bar). Each
of the compressors on the MAX 30 has 0.38 hp and have a built-in safety relief valve to prevent
excessive pressures in each compressor.
Pressure Swing Adsorption (PSA) Technology
An NMP Oxygen Concentrator is an on-site oxygen generating machine capable of producing oxygen on
demand in accordance with your requirements. In effect, it separates the oxygen (21%) from the air it is
provided and returns the nitrogen (78%) to the atmosphere through a waste gas muffler. The separation
process employs a technology called Pressure Swing Adsorption (PSA). At the heart of this technology
is a material called Molecular Sieve (synthetic zeolite). This sieve is an inert, ceramic-like material that is
designed to adsorb nitrogen more readily than oxygen. Each of the two beds that make up each of the
enricher contains this sieve. The process is described below.
Stage 1 Compressed air is fed into the first molecular sieve bed. Nitrogen is trapped, while oxygen is allowed
to flow through.
Stage 2 When the sieve in the first bed becomes full of nitrogen, the airflow is then directed into the second
bed.
Stage 3 As the second bed separates the oxygen from the nitrogen, the first bed vents its nitrogen into the
atmosphere.
Stage 4 Compressed air is once again fed into the first bed and the process is repeated continuously. A
constant flow of oxygen is produced
This air separation process is reliable and virtually maintenance-free.
The molecular sieve will last indefinitely, as long as it does not become contaminated with water or oil
vapors. This is why regular filter element replacement is crucial to trouble-free operation. The filter
elements are inexpensive and require semi-annual maintenance.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 7 of 28
Page 8

External Components Drawing
Front View
1. Human Machine Interface (HMI) 9. Filter Holder
2. Control Buttons 10. Power Cord
3. Flow Meters
4. Handles
5. Casters
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 8 of 28
Page 9
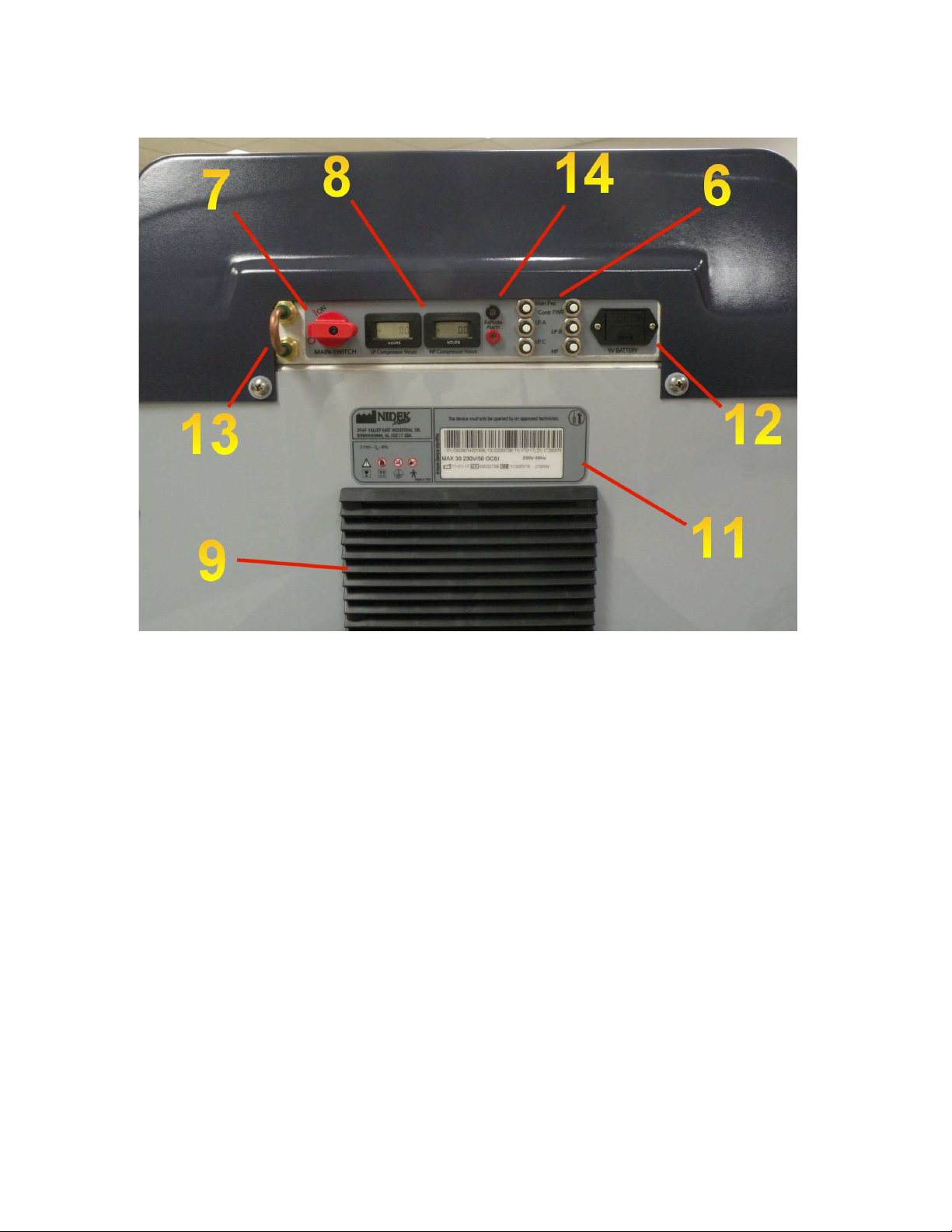
External Components Drawing
6 Circuit Breakers 7. Master Power Switch
Back View
8. Hour meters 9. Filter Holder
11. Manufacturers Device Label 12. 9V Battery Holder
13. Output to Remote Tank 14. Remote Alarm Contacts
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 9 of 28
Page 10

External Components Drawing
Human Machine Interface
15. Start Button 16. Stop Button
17. Utility Screen Button 18. Home Button
Utility Screen
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 10 of 28
Page 11

External Components Description
Oxygen Concentrator
Controls & Display(1 &2)
Flow Meters (3)
Handles (4)
Casters (5)
Circuit Breakers (6)
Master Power Switch (7)
Hour meter (8)
Filter Holder (9)
This display provides the controls on how to operate the unit and shows
pertinent information about unit operation.
This flow meter shows the output of the unit while it is operating. The
oxygen outlet is located on the bottom of the Flow Meter
Used to facilitate moving unit.
Used to relocate unit
The circuit breakers that opens if there is an electrical overload in the system.
The main system reset is on the back of the machine. There is also a circuit
breaker for each of the individual compressors.
This switch controls master power to machine. The display will illuminate
when the master power switch is in the ON position.
The two hour meter shows how long the unit and the high pressure
compressor has been operating. This helps indicate when service intervals
are due. It is resettable to identify time between service intervals, but the
accumulated time can not be modified.
The filter holders are located on each side and the rear of the unit and the
filter elements should be cleaned every two weeks or sooner if in a dusty
and dirty environment. Replacement Filter Element part # 9600-1053.
Power Cord (10)
Device UDI Label (11)
9V Battery Holder (12)
Output to Remote Tank (13)
Remote Alarm Contacts (14)
Start Button (15)
Stop Button (16)
Utility Button (17)
Home Button (18)
The power cord used on 230 VAC 50 Hz or 60 Hz electrical systems comes
with a three-pronged grounded plug, (EURO or other as requested).
Disconnection of this power cord from the mains source is used to isolate the
mains power from the device if needed.
Provides Identifying information on the unit.
Provides alarm for loss of power, should sound when ever unit is turned on
to show that the battery is good.
Output and Return connection to auxiliary tank.
Contact closure in Unit to drive external alarm signal.
Press to starts the unit, there will be a short delay until units starts.
Press to stop the unit.
Displays the monitoring screen to allow troubleshooting
Returns to the main screen.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 11 of 28
Page 12

Internal Components Drawing
Top Internal View
Front Internal View Back Internal View
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 12 of 28
Page 13

Top View Parts Description
A. PLC I. Booster Safety Relief Valve
B. OCSI Board J. Booster Isolation Valve
C. Power Supply K. Oxygen Storage Tank
D. Temperature Switch L. Hourmeters (2)
E. Pressure Switch (2) M. Bypass to Remote Tank
F. Terminal Blocks N. Master Power Switch
G. Control Relays (3) O. Circuit Breakers (6)
H. Booster Pressure Regulator P. Booster Unloader Valve
Q. Buzzer R. Pressure Sensor (2)
Front View Parts Description
AA. B Sieve Bed Silencer AG. A Sieve Bed Waste Valve
AB. B Sieve Bed Waste Valve AH. A Sieve Bed Silencer
AC. B Sieve Bed AI. Supply Hoses (2)
AD. B Sieve Bed Supply Valve AJ. Equalizing Valve
AE. A Sieve Bed Supply Valve AK. Discharge Check Valve (2)
AF. A Sieve bed AL. Balancing Orifice
AM. Unloading Valve
Back View Parts Description
BA. Booster Pressure Compressor BE. Low Pressure Compressor B
BB. Low Pressure Storage Tank BF. Cooling Fans (8)
BC. Moisture Separator/ HX (3) BG. Low Pressure Compressor A
BD. Low Pressure Compressor C BH. Compressor Inlet Filter (3)
BI. Low Pressure Storage Tank
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 13 of 28
Page 14

Internal Components Description
Sieve Beds (AC & AF)
Pressure Regulator (H)
These sieve beds contain the molecular sieve that performs the air
separation process, as well as the process control valves and oxygen
storage tank. They are spring loaded to prevent settling and should never
need to be opened. If the sieve becomes contaminated, the beds can be
easily replaced.
The pressure regulator controls the pressure delivered to the oxygen
outlet. It should be set in a way so that the pressure does not exceed 50
psi (3.4 bar).
Oxygen Monitoring
Circuit Board (B)
Compressor Inlet Filter
(BH)
Air Compressor (BD, BE
& BG)
Heat Exchanger/
Moisture Separator (BC)
PLC (A)
Terminal Strip
Assembly (F)
This circuit board monitors the operation of the unit. It continuously
monitors the output of the unit to ensure it is operating within an
acceptable range.
The compressor inlet filter keeps dust and dirt from entering the
compressor and needs to be changed twice a year in normal
environments to maintain performance. In especially dirty and oily
areas, it should be changed more often. Four times a year is
recommended.
The air compressor supplies the feed air to the sieve beds
work as designed for a minimum of 10,000 hours and may last 20,000
hours in some cases. It is suspended by four springs to dampen vibration
that should not require replacement.
The heat exchanger moisture separator delivers the feed air from the air
compressor to the modular bed. Significant moisture removal occurs
before the air enters the sieve beds, improving performance.
The Programmable Logic Controller (PLC) provides the logic that
operates the unit.
The terminal strip distributes electrical power as required to the
compressors and control components of the machine.
.
It should
Cooling Fans (BF)
Oxygen Pressure Switch
(E)
Oxygen Pressure Sensor
(R)
Oxygen Storage Tank (K)
Multiple cooling fans pull air thru 3 cabinet filters to
provide overall cooling to the unit.
The oxygen pressure switches provide a safety function to
shut the unit off if pressure exceeds maximum values.
The oxygen pressure sensor controls operation of the
booster compressor.
The oxygen storage tank, provides a small buffer to allow the unit to
operate smoothly on high demand applications, it provides product equal
to approximately 6 seconds of operation.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 14 of 28
Page 15

Process Flow Diagram
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 15 of 28
Page 16

Process Flow Description
Once the incoming air is filtered and compressed in the MAX 30 unit, it is directed into
one of the two sieve beds. As the air enters the bed, the nitrogen is adsorbed by the sieve and the
oxygen passes through as product gas to the storage tank at a pressure around 20 psi (1.3 bar)
Each bed produces oxygen until the sieve in that bed is saturated with nitrogen. When this occurs,
the feed airflow is directed to the other bed, which continues the production process. While the
second bed is producing oxygen, the first bed is releasing into the atmosphere the nitrogen it
adsorbed, under very low pressure through a waste gas silencer.
From the storage tank, the oxygen product gas passes through a booster compressor
designed to raise the operating pressure. Oxygen at the higher pressure passes into the booster
storage tank. This storage tank serves as a reservoir for the oxygen prior to entering flow meter. A
regulator maintains the oxygen output at 50 psi (3.4 bar). From the storage tank the oxygen passes
through a bypass OCSI monitor where a digital display of the concentration is produced. The
booster compressor will automatically de-energize when the maximum pressure is reached.
OCSI Display:
.
The OCSI board monitors the output of the machine to make sure the oxygen concentration is
within acceptable conditions. Output of the machine will be up to 30LPM of dry oxygen at 50psi
(3.4 bar) discharge pressure. The board will use an ultrasonic sensor to determine the purity of the
oxygen as it exits the sieve beds. The board will monitor the purity level and alarm if the purity
falls below 90%. If the purity falls below the set point the red indicator on the display will be
illuminated and the buzzer will alarm continuously until the either the purity returns to above the
set point or the unit/machine is turned off. On startup the indicator will show green when purity
has exceeded the set point. The board should never require calibration.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 16 of 28
Page 17

[United States Pharmacopeia (USP) XXII oxygen 93% Monograph]
Physical
Unit Specifications
Performance
63 SCFH @ 50 psi
Oxygen Volume/Pressure
30 LPM or @ 3.4 bar
Oxygen Purity
Oxygen Dew point
Feed Air Requirement
Response Time
Oxygen Outlet Fitting
Sound Levels
Dimensions
Weight
93% (± 3%)
- 60° F (-51° C)
None, compressors included
Approximately 5 minutes to attain maximum purity after
initial start-up or extended shut-down, or longer if a
supplemental tank is used.
1/8" NPT Male Insert
60 dBA @ 1 m
24 x 21 x 44 in (W x D x H)
610 x 530 x 1120 mm (W x D x H)
200 lb (91 kg)
Power Requirement
Oxygen Flow Rate
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 17 of 28
Standard (International)
230 VAC, 50/60 Hz, Single Phase, 11 A
63 SCFH / 30LPM
Page 18

Safety Precautions
It is very important that you read the precautions below and make yourself aware of the
hazards of oxygen in general. While it can be handled and used very safely, it can also
be mishandled or applied incorrectly causing dangerous situations.
Oxygen is a fire hazard. It can be very dangerous as it vigorously accelerates the
burning of combustible materials. To avoid fire and/or the possibilities of an explosion,
oil, grease or any other easily combustible materials must not be used on or near the
oxygen concentrator. DO NOT SMOKE NEAR THE UNIT. The unit should be kept
away from heat and flames. Individuals who have experience handling oxygen systems
should become the designated operators of the oxygen concentrator within your facility.
In sensitive applications, it is important to have a backup supply of oxygen since the
concentrator does not come with any reserve storage tank and requires electrical power
to operate. Therefore, during power outages oxygen will not be produced.
Do not use extension cords to bring power to the concentrator. The current drawn into
the unit is high and could overheat some extension cords. It is also important to use only
a properly grounded outlet.
High pressure oxygen may present a hazard. Always follow proper operating
procedures, and open valves slowly. Rapid pressurization may result in personal injury
Safety glasses and hearing protection are required when venting oxygen under high
pressure.
Ensure that the oxygen outlet stream is not directed toward anyone’s clothing.
Oxygen will embed itself in the material and one spark or hot ash from a cigarette
could ignite the clothing vigorously.
There are several onboard storage locations that might remain pressurized after
the unit is shutoff, Ensure that this pressure is released prior to performing any
service on the unit.
.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 18 of 28
Page 19

Pre-Installation
MAX 30
2
000
Before installing the NMP Oxygen Concentrator, it is necessary to consider the
location, space available and power supply for the concentrator.
1) Locating the MAX 30:
The oxygen concentrator should be located in an area that is indoors and remains
between 40 F (5 C) and 100 F (38 C). Setting the machine outdoors or in an
area that is not normally within this temperature range will void the NMP
Warranty.
There should be a distance of at least 12 in (20 cm) between the unit and any side
or back wall in the room that it will be located. It should also not be located any
closer than 24 in. (60 cm) from the discharge of any other operating units. This
ensures proper airflow into the concentrator and minimizes any restriction.
2) Space Available for the MAX 30:
If the
ft3or 56.6 m3), that room should be well ventilated (at least 8 air changes in the
room per hour). The concentrator will be discharging nitrogen into the atmosphere
of the room and a nitrogen build up could be dangerous to people entering the
room. If the concentrator is placed in a small closet, the air in that closet will
become enriched with nitrogen. As the concentrator continues to run, it would
become more and more difficult for it to separate the oxygen from the air because
oxygen will make up a smaller and smaller fraction of the air that is fed into the
unit is going to be set up in a room that is small, (less than
3) Power Supply for the MAX 30:
The oxygen concentrator should be positioned within 8 ft (2.2 m) of the electrical
outlet that will power it. The reason for this is that the motor draws a large current
during the first few seconds of start-up. It is also very important for this reason
NOT to use any extension cords with the unit. They could overheat and melt,
possibly causing a fire. Caution should be exercised to ensure the mains power
cord is accessible in the event the unit needs to be disconnected from the mains
supply.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 19 of 28
Page 20

Required Operating Conditions
Location of Machine:
The standard oxygen concentrator is intended for use indoors in a commercial or light
industrial setting. The enclosure meets NEMA 1 protection guidelines, which provides a
degree of protection against dust and falling dirt. It is classified as IPX1 in accordance with
60529-1:2001, which provides for a degree of protection from spillage and falling water.
Feed Air/Ambient Air Quality:
The life of any PSA oxygen concentrator is directly related to the air quality that is fed into it.
Hot, humid, dirty, oily air deteriorates and degrades the performance of the molecular sieve.
In order to preserve the effectiveness and extend the life of the concentrator, precautions must
be taken to ensure that the air provided is cool, dry, clean and oil-free. Changing the inlet air
filter is a simple and easy way to provide the unit with some protection. It is advisable to set
up the unit in an air-conditioned or a well-ventilated area. The room should also be free of
toxic gases and high concentrations of hydrocarbons, especially carbon monoxide. Humid,
oily areas should be avoided as installation sites as much as possible.
Ambient Air Temperature:
The machine is designed for use over a temperature range of 40 F to 104 F (5 C to 40 C).
Since hot air has the ability to hold much more water in the form of humidity than cool air,
operating the units in hot areas will reduce the effective life of the molecular sieve.
Acceptable humidity is between 15 % and 95 % for both operations and storage.
Note: Operation outside of this temperature range will not be warranted by NMP. The device
may be stored at between -20° C and 60° C
Electrical Power:
The power for the control circuitry of the oxygen concentrator is a single-phase electrical
supply of 230 VAC and about 11 A at a frequency of 50 Hz or 60Hz depending on model.
This equates to approximately 2100 W of power. It is required that a 15 A circuit be dedicated
to each MAX 30 unit. Additionally, the unit must be connected to this circuit using only the
supplied power cord, and without additional extension cords.
Positioning:
The unit must be stored, transported and operated in an upright position only, with no
obstruction blocking airflow around the unit.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 20 of 28
Page 21

Set-up & Installation
Although every MAX 30 unit is thoroughly tested and checked before it is shipped from
our facility, the following checks are necessary to ensure that none of the internal
components have been damaged in shipment. This check should take less than five
minutes to perform. (Refer to ‘Initial Inspection’ on Page 2 before reading the
instructions below)
Make a visual inspection of the machine and make sure all parts are properly attached.
(Refer to ‘Components’ section)
Connect the unit into an electrical outlet. A receptacle plug of local configuration will
need to be attached first if the supplied plug is not acceptable.
Turn the ON/OFF switch to the ON position and make sure that the display light is
illuminated. Press the START Button on the display unit.
After a brief delay, listen for the sound of multiple compressors to start operating, if you
do not hear it within ten minutes, shut the machine down immediately and call NMP for
assistance.
The oxygen flow will continue to increase on the flow meter until the unit is up to
operating pressure at which time the flow meter will indicate correctly. If this does not
occur, check to make sure that none of the hose connections have come loose. Call NMP
Technical Service Department at +1(205) 856-7200, if no loose connections are found
and trouble persists.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 21 of 28
Page 22

Operating Instructions
Start-up
• Once the system has been installed in accordance with the set-up and installation
instructions, it may be operated. The following steps should provide some direction.
• Connect the oxygen outlet to the application
• After connecting it to an electrical outlet and making sure the master switch is in the ON
position, press the start button on the display unit on the machine to the, wait for 5 to 10
minutes for the unit to come up to rated purity.
• As the unit is coming up to pressure and the correct purity the panel will remain “red”
indicating unacceptable output. Once the purity and pressure are acceptable the display will
change to “green” indicating it is ready for use. Once flow is established the digital display
will indicate the purity of the output Oxygen and the flow meters will indicate the amount
of Oxygen flowing to the output.
• Begin using Oxygen.
Shut-down
• To shut off the machine, press the stop button on the display unit. The compressors will
quit immediately and the display will continue to show the unit status. If the unit will be off
for an extended period then the master switch on the back of the unit can be placed in the
off position.
• To shut off the machine, press the stop button on the display unit. The compressors will
quit immediately and the display will continue to show the unit status. If the unit will be off
for an extended period then the master switch on the back of the unit can be placed in the
off position
Caution: After unit is turned to off the oxygen flow will continue as the pressure in the
unit bleeds down.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 22 of 28
Page 23

Troubleshooting Guide
Use a leak testing solution to locate
Use a leak testing solution to locate
Problem Sign Cause Solution
Machine not starting
Pressure Switch not
Working
Low Oxygen Pressure
Machine not
turning ON/OFF
at target pressures
Machine not
plugged in
Machine not
turned on
No power to the
machine
Circuit breaker has
tripped
Compressor under
pressure
Loose wire
Faulty switch Remove switch and return for
This may be a result of a
leak in the system.
Ensure that machine is
plugged in.
Ensure that switch is in the
ON position.
Ensure that there is power
supply to the machine.
Push in the reset button on the
right hand side of the cabinet.
Remove the head pressure that
exists in the compressor outlet
stream.
Check that all wiring
connections are secure.
replacement.
and repair any air leaks.
Oxygen purity has fallen
below acceptable limits
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 23 of 28
This may be a result of a
leak in the system.
Beds Are Hydrated
Dirty Filters
and repair any air leaks.
Replace Beds
Replace Filters
Page 24

Preventive Maintenance
Air Filter Cleaning
The air filter elements (3) should be removed and cleaned in soapy water every two weeks or 20 hours
of operation to reduce the dust and dirt contamination for inside of the unit.
Compressor Filter Element Replacement:
The air filter element provided with the MAX 30 must be replaced every six (6) months on
an average and more frequently in dusty environments. This element helps to maintain the
quality of the feed air supply, preserve the molecular sieve inside the oxygen enricher and
extend the life of the air compressor.
Failure to replace the filter element on schedule will result in the warranty becoming
invalid.
Cabinet & Power Cord:
The cabinet and power cord should be occasionally wiped down with a sponge or clean rag
and some soapy water. Avoid the use of ammonia or other strong chemical based cleaning
solvents. This prevents dust and dirt from building up on the machine.
Air Compressor:
You should consider your air compressors an important part of your oxygen generating
system. In addition to changing the air filter element, maintenance is relatively simple. The
fans on either end should remain free of debris/dust. The air compressors should last five (5)
or six (6) years or longer under normal operating conditions. The low pressure compressors
should be rebuilt after 15,000 hours of operation. The booster compressor should be rebuilt
after 6,000 hours of operation. Hour meters on the rear of the unit indicated hours on the low
pressure (LP) compressors and booster (HP) compressor. As indicated by use, both will need
to be rebuilt or replaced. Oxygen purity and flow rate along with feed air pressure delivered
to the sieve beds will all be indicators that the air compressor has expended its life.
Replacement in the field is possible, but returning the unit to NMP or an authorized service
center is recommended.
OCSI Display Board
The OCSI board should never require calibration and can not be calibrated in the field.
Calibration can be verified if needed periodically. Remove the back of the unit disconnect the
hoses from the sensor on the large board, supply the board with calibration quality oxygen
(99.99%) and check the display, if the display is reading 90.2% +/- 3% then it is within the
calibration specifications, if it is outside the range it should be replaced
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 24 of 28
Page 25

Technical Service Assistance
It is our intention to provide complete customer satisfaction. This manual is one way in which
we hope to provide you with technical assistance.
If you do not find what you need in this manual or you have other questions about this
equipment, please feel free to contact us directly. We look forward to serving your oxygen
needs and invite your inquiries. We will respond to you as promptly as possible.
You can reach NMP through the following means:
By Telephone (Outside the United States):
Your local International Access Code (usually 0 or 00), followed by
The Country Code for the U.S. which is (1), followed by Our Area
Code and Number (205) 856-7200
By Fax (Within or outside the United States): +1(205) 856-0533
By E-Mail or Website:
info@nidekmedical.com
http://www.nidekmedical.com
By Mail:
Nidek Medical Products
3949 Valley East Industrial Dr
Birmingham, Alabama 35071 USA
By UPS, FedEx or Common Carrier: (Address to return shipments)
Nidek Medical Products
3949 Valley East Industrial Dr
Birmingham, Alabama 35071 USA
Technical service personnel are available from 7:00 AM to 4:00 PM CST (GMT - 6).
We also have a list of Distributors and Authorized Service Agents available upon request.
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 25 of 28
Page 26

Appendix A
Spare Parts List
PART NAME
Power Cord 16A Euro 9600-1038 1
Strain Relief for Power Cord 9600-1080 1
Hourmeter - Resettable 8400-5028 1
Master On-Off Switch 9800-1008 1
15 A Circuit Breaker 9800-1519 1
5 A Circuit Breaker 8400-1019 5
Pressure Switch/Sensor 9800-1521 1
Temperature Switch 9800-1507 1
Programmable Logic Controller 9800-1500 1
HMI 9600-1505 1
PART NUMBER QUANTITY
OCSI Board 9800-1810 3
24VDC Power Supply 9800-1505 1
24VDC Control Relay 4 Pole 9800-1509 3
24VDC Control Relay 2 Pole 9800-1512
Sieve Bed Control Valves 9800-1200 4
Inline Check Valves 9800-1114 2
High Pressure Oxygen Regulator 9800-1157 1
Low Pressure Oxygen Regulator 8400-1060 2
Compressor Assembly (230 VAC, 50 Hz) 9251-1632 3
Compressor Assembly (230 VAC, 60 Hz)
Thomas Compressor Rebuild Kit
Booster Compressor Assembly (230VAC, 50/60Hz)
9251-1532
7355-3670
9800-1632
1/4" NPT 3-Way Isolation Valve 24VDC
1/4" OD Blue Oxygen Polyurethane Tubing - Per Foot 7854-6109
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 26 of 28
9800-1205 3
Page 27

PART NAME
PART NUMBER QUANTITY
1/4" ID Braid Reinforced PVC Tubing - Per Foot
3/8" OD Green Nylon Tubing - Per Foot (Low Temp Air) 7854-6107
7854-6105
3/8” OD Clear FEP Tubing - Per Foot (High Temp Air) 7854-6106
Inlet Air Filter Element
Exhaust Fan 230 V
Air Compressor filter (Change every 6 months)
9600-1053 2
8400-1024 10
9800-1012 2
Compressor Capacitor 9250-1322 3
Air Compressor Filter Element 9800-1027 3
Flowmeter (1-15LPM) 9800-1047 1
Product Filter 9250-1053 3
Caster 4”, Swivel & Locking
Caster 4”, Swivel
9800-1013 2
9800-1018 2
Moisture Separator
Compressor Outlet Fitting
Moisture Separator Inlet Fitting
Moisture Separator Outlet Fitting
Inline Orifice
Replacement Sieve Bed
Manual-Available Free on Website
9251-1911 3
9251-1052 3
9250-1163 3
9250-1167 3
9800-1121 3
0600-0500 2
2010-9800
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 27 of 28
Page 28

Appendix B
Maintenance Log
Da t e Pa r t Reason for Maintenance
Authorized Service
Technician Signature
2010-9800 Rev B, Installation and Maintenance Guide ECC1052 6/13/17 Page 28 of 28
 Loading...
Loading...